SUMMARY
Efficient and sustainable control of plant viruses may be achieved using genetically resistant crop varieties, although resistance genes are not always available for each pathogen; in this regard, the identification of new genes that are able to confer broad‐spectrum and durable resistance is highly desirable. Recently, the cloning and characterization of recessive resistance genes from different plant species has pointed towards eukaryotic translation initiation factors (eIF) of the 4E family as factors required for the multiplication of many different viruses. Thus, we hypothesized that eIF4E may control the susceptibility of melon (Cucumis melo L.) to a broad range of viruses. To test this hypothesis, Cm‐eIF4E knockdown melon plants were generated by the transformation of explants with a construct that was designed to induce the silencing of this gene, and the plants from T2 generations were genetically and phenotypically characterized. In transformed plants, Cm‐eIF4E was specifically silenced, as identified by the decreased accumulation of Cm‐eIF4E mRNA and the appearance of small interfering RNAs derived from the transgene, whereas the Cm‐eIF(iso)4E mRNA levels remained unaffected. We challenged these transgenic melon plants with eight agronomically important melon‐infecting viruses, and identified that they were resistant to Cucumber vein yellowing virus (CVYV), Melon necrotic spot virus (MNSV), Moroccan watermelon mosaic virus (MWMV) and Zucchini yellow mosaic virus (ZYMV), indicating that Cm‐eIF4E controls melon susceptibility to these four viruses. Therefore, Cm‐eIF4E is an efficient target for the identification of new resistance alleles able to confer broad‐spectrum virus resistance in melon.
INTRODUCTION
Viral diseases of cucurbit crops are major limiting factors for their production and may cause massive damage, particularly to melon and squash (Lovisolo, 1980), resulting in huge economic losses (Gaba et al., 2004). Major viruses that infect melon include: Cucurbit aphid‐borne yellows virus (CABYV, genus Polerovirus), Watermelon mosaic virus (WMV, genus Potyvirus), Zucchini yellow mosaic virus (ZYMV, genus Potyvirus), Papaya ringspot virus (PRSV, genus Potyvirus), Moroccan watermelon mosaic virus (MWMV, genus Potyvirus) and Cucumber mosaic virus (CMV, genus Cucumovirus), which are transmitted by aphids; Cucurbit yellow stunting disorder virus (CYSDV, genus Crinivirus), Beet pseudoyellows virus (BPYV, genus Closterovirus) and Cucumber vein yellowing virus (CVYV, genus Ipomovirus), which are transmitted by whiteflies (Abou‐Jawdah et al., 2000; Bananej and Vahdat, 2008; Gil‐Salas et al., 2009; Kassem et al., 2007; 2003, 2011; Papayiannis et al., 2005; Sevik and Arli‐Sokmen, 2003; Yakoubi et al., 2007); and Melon necrotic spot virus (MNSV, genus Carmovirus), which is transmitted by the soil fungus Olpidium bornovanus (Bananej and Vahdat, 2008; Cuadrado et al., 1993).
One of the most efficient and sustainable strategies for controlling plant virus infections is the use of genetically resistant plants. Around one‐half of the approximately 200 known resistance genes that target plant viruses are recessively inherited (Díaz‐Pendón et al., 2004; Kang et al., 2005b). A large number of recessive resistance genes have been cloned from crop species and shown to encode eukaryotic translation initiation factors (eIF) of the eIF4E or eIF4G family (Robaglia and Caranta, 2006; Truniger and Aranda, 2009). Thus, recessive resistance is often achieved through the absence of appropriate host factors that are essential for the virus to complete its biological cycle (hereafter referred to as susceptibility factors) (Truniger and Aranda, 2009), although this does not always seem to be the case (Gonzalez‐Ibeas et al., 2012). In plant cells, two eIF4F translation initiation complexes exist, eIF4F and eIF(iso)4F, which are formed by the cap‐binding proteins, eIF4E and eIF(iso)4E, that are bound to the scaffold proteins eIF4G and eIF(iso)4G, respectively (Browning, 2004). Both factors have been shown to play similar roles in mRNA translation in vitro (Browning, 1996) and to have redundant functions in vivo (Duprat et al., 2002; Ruffel et al., 2006; Sato et al., 2005; Yoshii et al., 2004), but additional unique roles have also been described (Gallie and Browning, 2001). In dicotyledons, more than one gene can code for proteins of the eIF4E or eIF(iso)4E subfamily. For example, in Arabidopsis, there are three genes that code for eIF4E proteins and, in tomato, two, and, in both species, only one gene encodes for eIF(iso)4E (Piron et al., 2010; Yoshii et al., 2004). In melon, only one gene encoding for eIF4E and one encoding for eIF(iso)4E have been identified (M. A. Aranda et al., unpublished data; Clepet et al., 2011; Gonzalez‐Ibeas et al., 2007). Resistances that are mediated by eIF4E or its isoform to several viruses belonging to the family Potyviridae have been described in different plant species (Hwang et al., 2009; Naderpour et al., 2010, reviewed in Truniger and Aranda, 2009). Outside of the family Potyviridae, two cases of eIF4E‐mediated resistance have been described, one in Arabidopsis to CMV (Yoshii et al., 2004) and one in melon to MNSV (Nieto et al., 2006; Truniger et al., 2008). The finding that different viruses have common susceptibility factors suggests that, as a biotechnological approach, transgenic resistance to a wide range of viruses may be achieved by a reduction in the expression levels of these factors through RNA silencing (Hwang et al., 2009, reviewed in Reddy et al., 2009).
The identification of eIF4E as a common susceptibility factor for different viruses led us to search for new Cm‐eIF4E alleles in the natural diversity of the melon (Cucumis melo L.) species by EcoTILLING, which is a variant of the TILLING strategy (Nieto et al., 2007). Unfortunately, no Cm‐eIF4E variants were identified, with the exception of one known single amino acid change that results in resistance to MNSV, but not to other viruses (Nieto et al., 2006). Before using other time‐consuming strategies to generate novel Cm‐eIF4E variants (e.g. by TILLING) (Comai and Henikoff, 2006), we decided to test whether Cm‐eIF4E controls the susceptibilities of plants to melon‐infecting viruses other than MNSV. For this assessment, we used an RNA silencing strategy. Here, we describe the generation of transgenic melon plants expressing a transcript that forms a RNA hairpin structure that is able to induce the specific silencing of Cm‐eIF4E. We demonstrated that the transgenic plants show down‐regulated Cm‐eIF4E expression, whereas the expression of its isoform Cm‐eIF(iso)4E is not affected. We challenged these transgenic melon plants with agronomically important melon‐infecting viruses, and identified resistances to CVYV, MNSV, MWMV and ZYMV, indicating that Cm‐eIF4E is a susceptibility factor that is required for these infections to take place.
RESULTS
Generation of transgenic melon plants
The aim of this study was to reduce the expression of the translation initiation factor Cm‐eIF4E in melon by post‐transcriptional gene silencing. This had to be performed carefully to avoid affecting indirectly the expression of its isoform, Cm‐eIF(iso)4E, because large regions of both genes share up to 75% of their nucleotide sequences. Thus, the fragment of Cm‐eIF4E that showed the lowest sequence identity relative to its isoform was chosen for the construction of the silencing vector. This fragment consisted of 175 nucleotides and is in the first 5′ quarter of the Cm‐eIF4E transcript, beginning exactly after the start codon (Fig. S1a, see Supporting Information). It was inserted as an inverted repeat into the pHANNIBAL vector (Wesley et al., 2001) on both sides of the pdk intron, so that expression results in an intron‐hairpin RNA (ihpRNA) able to induce RNA silencing of Cm‐eIF4E (Fig. S1b). Melon hypocotyl explants were transformed with this transgene using Agrobacterium tumefaciens. Transformed buds were selected with kanamycin. Over 30 putative independently transformed kanamycin‐resistant T0 plants were further analysed by polymerase chain reaction (PCR) to screen for the integrity of the inserted transgene and by flow cytometry to determine their ploidy levels. Most kanamycin‐resistant/PCR‐positive plants that were selected after transformation were tetraploid or chimeric. Eight diploid transgenic plants were self‐pollinated. These plants were often affected in growth and fertility; thus, we obtained abundant T2 seeds only from one transformed T0 line. These mainly segregating T2 generations were used for all the work presented in this article, as they are ideal to compare directly nontransformed and transformed plants. The segregation ratio for kanamycin resistance in the T1 generation of this transgenic line was 3 : 1 (resistant : susceptible), strongly suggesting the presence of a single transgene insertion.
Specific silencing of Cm‐eIF4E in transgenic melon plants
The effectiveness and specificity of the silencing induced by the ihpRNA, as expressed from the 35S promoter (Fig. S1b), was determined by the analysis of the accumulation levels of Cm‐eIF4E and Cm‐eIF(iso)4E mRNA using reverse transcription‐quantitative polymerase chain reaction (RT‐qPCR). The results showed a significant decrease in the accumulation of Cm‐eIF4E mRNA in transgenic T2 plants compared with nontransgenic T2 and wild‐type (WT) control plants (Fig. 1a, left), suggesting that the silencing was efficient. Importantly, the levels of Cm‐eIF(iso)4E mRNA remained unchanged, reaching very similar levels in the transgenic, nontransgenic and WT plants (Fig. 1a, right). Thus, the chosen sequence seemed to be able to induce the specific silencing of Cm‐eIF4E, but not of its isoform. To assess whether the presence of reduced Cm‐eIF4E mRNA levels in the transformed plants was truly the consequence of RNA silencing, its correlation with the production of transgene‐derived small interfering RNAs (siRNAs) was analysed by Northern blot. These experiments showed the accumulation of Cm‐eIF4E‐derived siRNAs in all transgenic plants, whereas none could be detected in the nontransgenic and WT plants (Fig. 1b), demonstrating that the low Cm‐eIF4E mRNA levels were the result of transgene‐induced RNA interference (RNAi).
Figure 1.
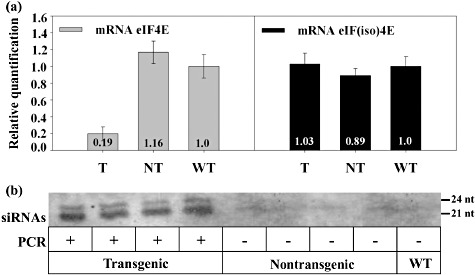
Cm‐eIF4E silencing in melon is specific and correlates with the appearance of Cm‐eIF4E‐derived small interfering RNAs (siRNAs). (a) Cm‐eIF4E and Cm‐eIF(iso)4E mRNAs were quantified by reverse transcription‐quantitative polymerase chain reaction (RT‐qPCR). Average Cm‐eIF4E (left) and Cm‐eIF(iso)4E (right) expression values of 16 noninoculated transformed (T) and 10 noninoculated nontransformed (NT) plants. NT corresponds to segregating T2 plants that did not carry the transgene. WT corresponds to wild‐type BGV130, used as reference in all RT‐qPCR quantifications. (b) Transgene‐derived siRNAs were detected by Northern blot. Digoxigenin‐labelled RNA and cRNA, complementary to both directions of the 175‐nucleotide Cm‐eIF4E fragment of the transgenic construct, were used as probes. The presence of the transgene was identified by PCR.
Transgenic plants are resistant to MNSV
We have demonstrated previously that Cm‐eIF4E is essential for the translation of MNSV RNAs and hence for its multiplication in melon (Nieto et al., 2006; Truniger et al., 2008). Thus, MNSV was used as a control for the susceptibility assays. Cotyledons of plants from a segregating T2 generation, together with WT plants as controls, were inoculated with MNSV. At 5 days post‐inoculation (dpi), inoculated cotyledons were sampled, and the following analyses were performed using the extracted DNA or RNA: (i) the determination of the presence of the transgene by PCR; (ii) the quantification of the viral and Cm‐eIF4E mRNAs by RT‐qPCR; and (iii) the identification of transgene‐derived siRNAs by Northern blot.
The results showed that viral multiplication was limited to almost undetectable levels in all of the plants that contained the transgene [PCR(+) plants, Fig. 2a, plants 1–10], suggesting that the down‐regulation of Cm‐eIF4E expression resulted in MNSV resistance. However, sister nontransgenic plants [PCR(−) plants, Fig. 2a, plants 11–13] that expressed large amounts of Cm‐eIF4E mRNA were susceptible to MNSV and showed similar values to WT plants. In addition, Northern blot analysis detected Cm‐eIF4E‐derived siRNAs in all of the PCR(+) but not PCR(−) plants, including WT and mock plants. Therefore, a perfect correlation of resistance to the Cm‐eIF4E‐dependent virus MNSV, with plants containing the transgene [PCR(+)] showing reduced Cm‐eIF4E expression and presenting siRNAs derived from the transgene, was found, suggesting that the virus resistance phenotype of this transgenic line was the result of Cm‐eIF4E silencing. Interestingly, although, at 5 dpi, the inoculated cotyledons of the transgenic melons showed no symptoms, at 12 dpi, very small lesions could be observed (Fig. 2b), but this infection did not progress to systemic infection.
Figure 2.
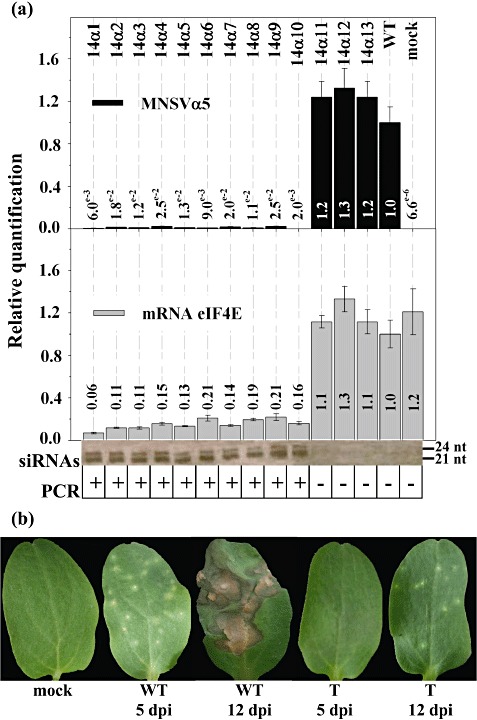
Cm‐eIF4E silencing in melon results in resistance to Melon necrotic spot virus (MNSV). (a) Analyses of plants of a segregating T2 generation inoculated with MNSV. The relative amounts of MNSV RNA and Cm‐eIF4E mRNA were quantified by reverse transcription‐quantitative polymerase chain reaction (RT‐qPCR). Transgene‐derived siRNAs were detected by Northern blot. The presence of the transgene was identified by PCR. (b) Inoculated cotyledons at 5 and 12 days post‐inoculation (dpi). WT (wild type), T (transformed).
Multiplication of different viruses in Cm‐eIF4E‐silenced plants
We tested the susceptibilities of the Cm‐eIF4E‐silenced plants to seven other agronomically important melon‐infecting viruses, including PRSV, WMV, CMV, ZYMV, CVYV, MWMV and CABYV; the first six were mechanically inoculated, whereas CABYV was agroinoculated (Prüfer et al., 1995). Cotyledons of T2 plants were inoculated with these viruses, and samples were obtained at the indicated dpi for further analyses. All of the plants were susceptible to PRSV, WMV, CMV and CABYV, whether or not they were transformed, and showed similar virus accumulation levels to those of WT plants (as detected by RT‐qPCR or Northern blot; see Fig. S2 in Supporting Information for examples). However, no virus accumulation was observed for ZYMV, CVYV or MWMV, as illustrated in the corresponding figures. All transgenic PCR(+) plants were symptomless after inoculation with ZYMV (Fig. 3b) and showed no virus RNA multiplication and low levels of Cm‐eIF4E mRNA accumulation, whereas transgene‐derived siRNAs were detected (Fig. 3a, plants 1–11). In contrast, the PCR(−) plants showed symptoms and high virus and Cm‐eIF4E mRNA accumulation values (Fig. 3a, plants 12–14), whereas no transgene‐derived siRNAs were detected. For T2 plants inoculated with CVYV, similar results were observed: only the PCR(+) plants were asymptomatic (Fig. 4b) and showed no accumulation of virus RNA and low levels of Cm‐eIF4E mRNA, correlating with the presence of transgene‐derived siRNAs (Fig. 4a). Similar results were obtained for inoculations with MWMV: all PCR(−) plants showed strong symptoms of viral infection at 4 dpi (Fig. 5, plants 1, 2, 6 and 7) and died a few days later, whereas the corresponding PCR(+) plants showed no symptoms (Fig. 5, plants 3–5, 8 and 9). Resistant plants remained asymptomatic after 30 dpi (Fig. 5). Because, for these three viruses, all resistant plants were PCR(+) and therefore transformed, and all susceptible plants were PCR(−) and therefore not transformed, we may conclude that Cm‐eIF4E is a susceptibility factor for MWMV, ZYMV and CVYV. Average Cm‐eIF4E mRNA levels were similar in mock‐inoculated (healthy) transgenic plants (Fig. 1; average value of relative quantification, 0.19), in transgenic plants showing resistance to CVYV, ZYMV or MWMV (3, 4, 5; average value of relative quantification, 0.16) and in transgenic plants showing susceptibility to PRSV, WMV, CABYV or CMV (Fig. S2; average value of relative quantification, 0.16).
Figure 3.
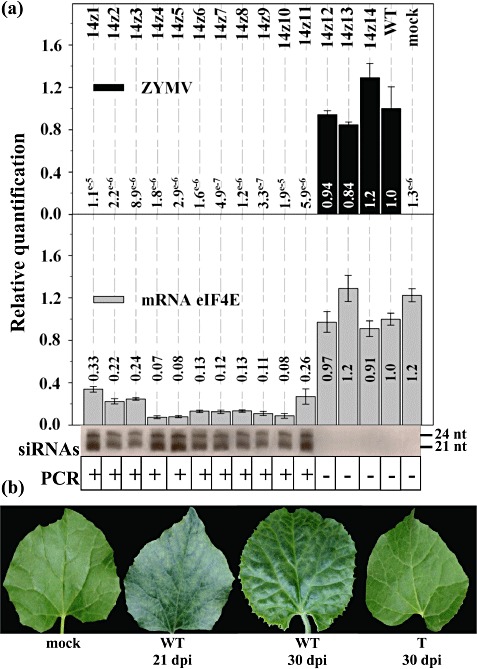
Cm‐eIF4E silencing in melon results in resistance to Zucchini yellow mosaic virus (ZYMV). (a) Analyses of plants from a segregating T2 generation inoculated with ZYMV. The relative amounts of ZYMV RNA and Cm‐eIF4E mRNA were quantified by reverse transcription‐quantitative polymerase chain reaction (RT‐qPCR). Transgene‐derived siRNAs were detected by Northern blot. The presence of the transgene was detected by PCR. (b) Disease symptoms on the leaves of wild‐type (WT) melon plants at 21 and 30 days post‐inoculation (dpi) and the symptomless leaves of transgenic (T) melon plants at 30 dpi.
Figure 4.
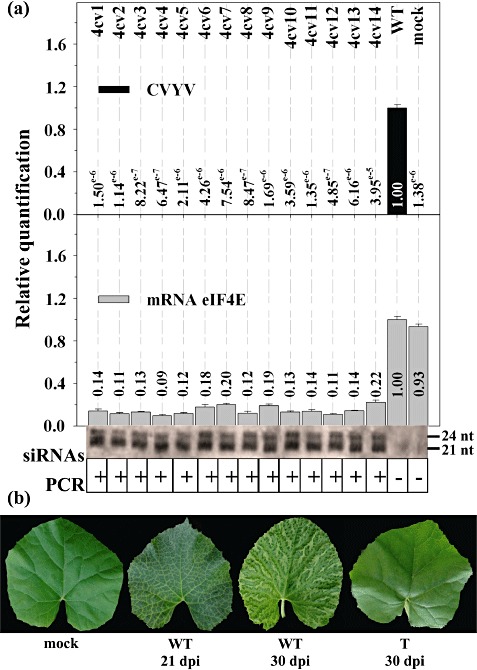
Cm‐eIF4E silencing in melon results in resistance to Cucumber vein yellowing virus (CVYV). (a) Analysis of homozygous plants from a T2 generation inoculated with CVYV. The relative levels of CVYV RNA and Cm‐eIF4E mRNA were quantified by reverse transcription‐quantitative polymerase chain reaction (RT‐qPCR). Transgene‐derived siRNAs were detected by Northern blot. The presence of the transgene was determined by PCR. (b) Disease symptoms on the leaves of wild‐type (WT) melon plants at 21 and 30 days post‐inoculation (dpi) and the symptomless leaves of transgenic (T) melon plants at 30 dpi.
Figure 5.
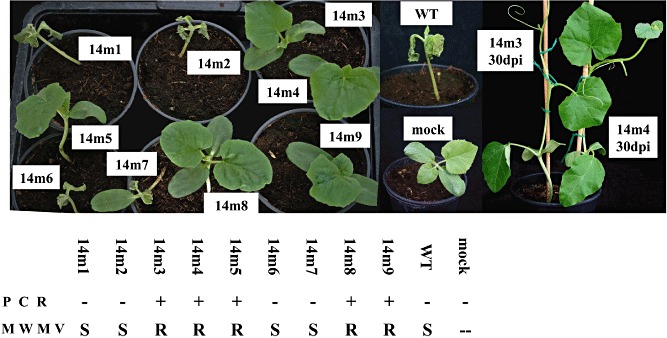
Cm‐eIF4E silencing in melon results in resistance to Moroccan watermelon mosaic virus (MWMV). Representative plants of a segregating T2 generation inoculated with MWMV at 5 days post‐inoculation (dpi). The presence of the transgene was determined by polymerase chain reaction (PCR). Susceptible plants died a few days later, whereas resistant plants remained symptomless, as shown at 30 dpi. WT, wild‐type.
DISCUSSION
In this study, melon transgenic plants expressing an ihpRNA that was able to induce the specific silencing of Cm‐eIF4E were generated and subjected to inoculations with eight agronomically important melon‐infecting viruses (Bananej and Vahdat, 2008; Desbiez et al., 2009; Kassem et al., 2007). We found that the expression of reduced levels of Cm‐eIF4E and increased levels of transgene‐derived siRNAs in these transgenic plants caused them to be resistant to CVYV, MNSV, MWMV and ZYMV, suggesting that Cm‐eIF4E is a susceptibility factor for these four viruses.
We were concerned that the silencing of Cm‐eIF4E could also result in the indirect silencing of Cm‐eIF(iso)4E because these two genes share 75% of their nucleotide sequences. Nonspecific silencing, which is based on partial sequence homology, has been described previously (Jackson and Linsley, 2004; Scacheri et al., 2004). Recently, it has been shown that the silencing of HC‐Pro from ZYMV in cucumber and melon resulted in resistance to ZYMV, but also to PRSV and WMV (Leibman et al., 2011). The HC‐Pro genes of these two viruses have sequence identities of 63% and 67% with that of ZYMV, respectively. We found that our transgenic plants were specifically silenced for Cm‐eIF4E, and that the levels of Cm‐eIF(iso)4E mRNA were not affected. This is possibly the result of having selected the fragment with the lowest sequence homology between the Cm‐eIF4E and Cm‐eIF(iso)4E genes for the silencing construct. In several cases, the reduced or absent expression of one gene from the 4E subfamily increased the expression of an isoform, suggesting that a homeostatic control mechanism exists that serves to maintain the essential cellular functions of eIF4E. For example, the tomato eif4e1 TILLING mutant, which did not affect growth, overexpressed eIF4E2 (Piron et al., 2010). In addition, in Arabidopsis mutants and transgenic tobacco plants, eIF(iso)4E depletion resulted in a compensatory increase in eIF4E levels (Combe et al., 2005; Duprat et al., 2002; Yoshii et al., 2004). However, the down‐regulation of eIF4E in tobacco did not trigger a reciprocal increase in eIFiso4E levels (Combe et al., 2005). We also did not observe a compensating increase in the expression of Cm‐eIF(iso)4E in our Cm‐eIF4E‐silenced transgenic melon plants, suggesting that, in melon, if partial compensation occurs, it is not regulated at the level of transcription or mRNA stability.
Our group has demonstrated previously that Cm‐eIF4E is essential for the translation and therefore multiplication of MNSV in melon (Nieto et al., 2006; Truniger et al., 2008). We have shown that eIF4E‐mediated resistance acts at the single‐cell level on translation, and thus no MNSV multiplication is observed in resistant melon protoplasts (Diaz et al., 2004). We first took advantage of this trait to utilize this virus as a control for the susceptibility assays to identify whether the levels of silencing that were obtained in the transgenic plants were sufficient to avoid viral multiplication. Our results showed that silencing decreased Cm‐eIF4E expression 6–10‐fold and that the reduced Cm‐eIF4E levels correlated perfectly with resistance to MNSV. Thus, the virus resistance phenotype of the transgenic line was the result of Cm‐eIF4E silencing. This was confirmed by the 100% correlation of resistance to CVYV, ZYMV and MWMV with Cm‐eIF4E silencing. Importantly, the successful infections of PRSV, WMV, CMV or CABYV did not affect the levels of Cm‐eIF4E mRNAs in the RNAi lines, suggesting that the silencing suppressors of these four viruses did not affect Cm‐eIF4E silencing. A closer inspection of the RT‐qPCR values that were obtained from the quantification of the virus RNAs of MNSV, CVYV and ZYMV in the transgenic plants compared with the mock‐inoculated plants (2, 3, 4) revealed that the transgenic plants showed higher resistances to CVYV and ZYMV than to MNSV (average values of relative quantification: 5.0e‐6, 2.5e‐6 and 1.4e‐2, respectively). Thus, the low levels of Cm‐eIF4E in transgenic plants seemed to still allow some residual multiplication of MNSV and, as a consequence, very small lesions on the inoculated cotyledons appeared after 12 dpi. This infection did not progress to systemic infection. However, no symptoms were observed in either the inoculated cotyledons or upper leaves until 30 dpi in the transgenic plants inoculated with CVYV, ZYMV and MWMV. These results suggest that the multiplication of potyvirus RNAs requires higher concentrations of eIF4E in the host cells than that of the MNSV genome, whose translation is independent of cap and VPg.
PRSV, WMV, ZYMV, CVYV and MWMV belong to the family Potyviridae. For several viruses of this family, eIF4E has been described as a susceptibility factor. Here, we found that three of these viruses depend on eIF4E for their multiplication in melon plants. This agrees with the previous finding that potyviruses demonstrate notable isoform specificity, with eIF4E and eIF(iso)4E usually having distinct and nonoverlapping functions in their infection cycles (Truniger and Aranda, 2009). In Arabidopsis, for example, the loss of eIF4E confers resistance to Clover yellow vein virus (ClYVV) (Sato et al., 2005), whereas the loss of eIF(iso)4E confers resistance to Turnip mosaic virus (TuMV), Tobacco etch virus (TEV) and Lettuce mosaic virus (LMV) (Duprat et al., 2002; Lellis et al., 2002). Although most potyviruses require one specific eIF4E isoform to multiply in a specific host, others are able to use both, as is the case for Pepper veinal mottle virus (PVMV) and Chilli veinal mottle virus (ChiVMV) in pepper (Hwang et al., 2009; Ruffel et al., 2006). Thus, in the case of PRSV and WMV, these viruses are either able to use both eIF4E and its isoform, or depend only on the eIF4E isoform, being eIF4E independent. The possibility that these viruses do not need any of the factors of the eIF4E family cannot be excluded. Our finding that ZYMV requires eIF4E for its multiplication in melon supports the work of Ling et al. (2009), who showed that a single nucleotide polymorphism in exon 1 of eIF4E in watermelon was closely associated with ZYMV resistance. Thus, it seems that eIF4E is a ZYMV susceptibility factor in both melon and watermelon. This is not always the case: for some potyviruses, it has been shown that the same virus requires different eIF4E isoforms to multiply in different hosts. For example, TEV and LMV require eIF(iso)4E to successfully infect Arabidopsis (Duprat et al., 2002; Lellis et al., 2002), whereas these same viruses require eIF4E in pepper and tomato (TEV) and in lettuce (LMV) (Kang et al., 2005a; Nicaise et al., 2003; Ruffel et al., 2005). Although CMV is not a potyvirus, this host‐dependent isoform specificity may explain why we found normal levels of CMV multiplication in transgenic melon plants, suggesting that its multiplication in melon is independent of eIF4E, although, in Arabidopsis, an eIF4E mutant, cum1, has been shown to be resistant to CMV (Yoshii et al., 2004).
From a practical point of view, we have identified that Cm‐eIF4E is required for the multiplication of three important emergent viruses, CVYV, ZYMV and MWMV, in melon. Because of its importance, CVYV has been included in the EPPO A2 Action List of pathogens that are recommended for regulation (Bananej and Vahdat, 2008; Lecoq et al., 2007; Yakoubi et al., 2007) (http://www.eppo.org/QUARANTINE/listA2.htm). However, MWMV has been reported previously in several countries (Lecoq et al., 2008; Yakoubi et al., 2008) and has recently been identified as a virus of increasing incidence in south‐eastern Spain (M. Juarez and M. A. Aranda, unpublished data). Finally, it has been established in several different countries that ZYMV is one of the most important cucurbit‐infecting viruses after CABYV, WMV and CMV (Bananej and Vahdat, 2008; Desbiez et al., 2009). These results suggest that eIF4E‐mediated resistance in melon could serve as a strategy for the control of these important viral diseases, either modifying the transformation strategy (Rommens et al., 2007) or searching for mutants in mutagenized melon populations using TILLING (Comai and Henikoff, 2006). In natural melon populations, no Cm‐eIF4E variants were identified using Eco‐TILLING (Nieto et al., 2007). By TILLING melon populations, several induced mutations in Cm‐eIF4E have been identified in two different melon cultivars, Piel de Sapo and Charentais, but their virus susceptibilities have not yet been tested (González et al., 2011).
EXPERIMENTAL PROCEDURES
Construction of the binary vector for RNAi
To obtain specific silencing of Cm‐eIF4E, a 175‐nucleotide fragment from the Cm‐eIF4E unigene (ICuGI accession no. MU21698) was chosen based on its low sequence identity with the Cm‐eIF(iso)4E unigene (ICuGI accession no. MU5578) (ICuGI, http://www.icugi.org/), as identified by sequence alignment using ClustalX (http://www.ebi.ac.uk/Tools/msa/clustalw2/). This Cm‐eIF4E fragment was PCR amplified using the melon expressed sequence tag (EST) clone MU21698 as the template and the primers CE‐79 (5′‐GGCCTCTAGA CTCGAGTAGTTGAAGATTCGATG‐3′), which contained XhoI (bold) and XbaI (italics) restriction sites, and CE‐80 (5′‐GGCCGAATTC GGATCCGAGGCTGATGCACTAG‐3′), which contained EcoRI (bold) and BamHI (italics) restriction sites, for the cloning in inverted directions into the same restriction sites of the pHANNIBAL plasmid. Primers CE‐163 (5′‐CCCCCACCCACGAGG‐3′) and CE‐164 (5′‐CCGGCGGTAAGGATC‐3′), corresponding to the Cauliflower mosaic virus (CaMV) 35S promoter and octopine synthase (OCS) terminator sequences, respectively, were used to verify the correct cloning by sequencing. The NotI fragment of this construct was further subcloned into the binary vector pART27 (CSIRO, Plant Industry, Clayton South, Victoria, Australia), and the resulting plasmid was verified by sequencing with the primers CE‐163/4.
Agrobacterium tumefaciens‐mediated transformation
The transformation protocol is available elsewhere (B. Gosalvez et al., unpublished data). Briefly, transformation was performed using hypocotyl explants from the melon accession BGV‐130. The explants were incubated with A. tumefaciens EHA105 culture. Incubated explants were cultured for 3 days before they were transferred to a selection medium containing 150 mg/L kanamycin (Duchefa, Haarlem, North Holland, Netherlands) for the selection of transformed cells. Once the elongated shoots produced roots, they were subcultured in the presence of 15 mg/L kanamycin to select out the transformed shoots that stably expressed the transgene. Once the root systems were well developed, the plants were transferred to soil and acclimatized in a confined glasshouse and multiplied by self‐pollination.
Seeds from melons that resulted from self‐pollination were peeled, sterilized and germinated in the dark for 48 h in a germination medium [Murashige and Skoog salts (Duchefa), 10 g/L sucrose, 8 g/L agar, pH 5.7] containing 50 mg/L kanamycin to select for the resistant transformed seeds.
Identification of transgenic plants by PCR
Genomic DNA was extracted from young leaves or the cotyledons of melon plants according to Dellaporta et al. (1983) and evaluated by PCR to detect the presence of the transgene. PCR was performed using 200 ng of genomic DNA in a final volume of 25 µL with 0.3 µm each of the primers CE‐404 (5′‐CGCACAATCCCACTATCCTTC‐3′, pHANNIBAL‐specific sequence) and CE‐409 (5′‐CCACGTCCTCTAGGGTTTTG‐3′, Cm‐eIF4E‐specific sequence) according to the Go‐Taq polymerase (Promega, Madison, WI, USA) protocol. DNA from the WT plants was used as a negative control. The amplification of a 157‐nucleotide fragment indicated the presence of the transgene (PCR+).
Virus inoculations
Treatments were performed on 10–15 plants from a T2 generation (homozygous or heterozygous). Cotyledons of 6–7‐day‐old plants were mechanically inoculated with fresh inoculum (in 0.03 m potassium phosphate buffer, pH 8.0) from cucurbits that had been infected with MNSV (isolate MNSV‐Mα5), PRSV (isolate PRSV‐WZ43), WMV (isolate WMV‐M116), CMV (isolate CMV‐fny), ZYMV (isolate ZYMV‐C71), CVYV (isolate CVYV‐AILM) and MWMV (isolate MWMV‐CE) following standard procedures (Hull, 2002). CABYV infection was achieved by the agroinoculation of cotyledons with an agroinfectious clone [pBINCAB (Prüfer et al., 1995) in A. tumefaciens strain C58C1]. Plants were grown in a glasshouse (16 h, 26 ± 2 °C/8 h, 18 ± 2 °C day/night, respectively). After inoculation, they were monitored daily for the appearance of symptoms. For the plants that had been inoculated with MNSV and CABYV, the inoculated cotyledons were harvested at 5 dpi. For the other systemically infecting viruses, the third leaf was harvested at approximately 21 dpi. Genomic DNA (see above) and total RNA (see below) for further analyses were extracted from the harvested cotyledons or leaves. For MWMV, only DNA was extracted from the cotyledons because this virus killed the susceptible plants, and no systemically infected leaves were able to be harvested. Viral accumulation was detected by RT‐PCR (see below), with the exception of the agroinoculated CABYV that was detected and quantified by Northern blot using a CABYV‐specific probe (Kassem et al., 2007)
Detection of siRNAs by Northern blot hybridization
Total RNA was isolated from melon plant tissues (leaves or cotyledons) using the TRI Reagent (Sigma, St. Louis, MO, USA) according to the manufacturer's instructions. Ten micrograms of RNA in 50% formamide were separated in a 20% polyacrylamide gel containing 7 m urea and 0.5 × Tris‐Borate‐EDTA (TBE) buffer, run in 0.5 × TBE buffer (180 V/90 min), transferred to a positively charged nylon membrane (Roche Applied Science, Indianapolis, IN, USA) in 0.5 × TBE buffer (80 mA/25 min) and fixed by UV exposure (1200 J × 100 min). The blotted membrane was hybridized with digoxigenin‐labelled (DIG RNA labelling mix, Roche Applied Science) RNA and cRNA probes corresponding to the selected 175‐nucleotide Cm‐eIF4E fragment at 42 °C overnight. After hybridization, the membrane was washed twice at 52 °C with 2 × standard saline citrate (SSC) and 2% sodium dodecylsulphate (SDS), once with 1 × SSC and 1% SDS, and once with 0.5 × SSC and 1% SDS for 10 min. The membrane was further developed according to the DIG labelling protocol (Roche Applied Science).
RT‐qPCR analysis to determine mRNA expression levels in melon plants
Prior to RT, the total RNA was treated with DNase I (New England Biolabs, Ipswich, MA, USA) and subsequently purified by phenol–chloroform extraction. RT was performed following the protocol of the transcriptase (Roche Applied Science) using an oligo‐dT(16) primer. Because neither MNSV nor CMV has poly(A) tails, the primers CE‐294 or CE‐169 (complementary to the 3′ end of MNSV or CMV, respectively, see below) were added to the corresponding RT reactions. Relative qPCRs to quantify Cm‐eIF4E and Cm‐eIF(iso)4E mRNAs and virus concentrations were performed using the ABI 7500 System (Applied Biosystems International, Foster City, CA, USA), as described in Gonzalez‐Ibeas et al. (2007), using SYBR green chemistry and cyclophilin expression (Melogen database, cCL3169) as internal reference. Data analyses were conducted using SDS‐7500 software (version 1.4, Applied Biosystems International). The following specific primer pairs were designed using Primer Express Software 3.0 (Applied Biosystems International): eIF4E‐CE‐147 (5′‐TTCGGTTCCTTCCCTTCCAT‐3′) and CE‐148 (5′‐CCGCCGATGTAGCTTTCATC‐3′); eIF(iso)4E‐CE‐149 (5′‐GGTATTTTCAGCGCCCAAGA‐3′) and CE‐150 (5′‐CCAATTTATGGGACTGCGGAT‐3′); MNSV‐CE‐293 (5′‐GCCCCAGGGAAATCCTAGAAT‐3′) and CE‐294 (5′‐CCGCTGTCACCACGTTCTTTA‐3′); CMV‐CE‐168 (5′‐ATGGGCAATGCGTTTCGTTA‐3′) and CE‐169 (5′‐CCGCTTACGATTCCCAACTGT‐3′); PRSV‐CE‐703 (5′‐CGGAGAGACACACAGTGGAAGAT‐3′) and CE‐704 (5′‐AACACACTAGCGCGAGTATTCAAT‐3′); WMV‐CE‐170 (5′‐TGTTGCTTCATGGAAGATTGGT‐3′) and CE‐171 (5′‐AAAATTGTGCCATCAGGTGCTA‐3′); CVYV‐CE‐638 (5′‐CGCAGCGCGACAACTTAAC‐3′) and CE‐639 (5′‐TTGTCTATGGCTTGGGATGGA‐3′); ZYMV‐CE‐172 (5′‐TGCCACCACTAGCGAAGACA‐3′) and CE‐173 (5′‐CGGCCTACCCTTTACTGCATT‐3′); and Cyclophilin‐CE‐155 (5′‐CGATGTGGAAATTGACGGAA‐3′) and CE‐156 (5′‐CGGTGCATAATGCTCGGAA‐3′).
NOTE ADDED IN PROOF
While this article was in proof a new publication appeared, that provides evidence that both eIF4E1 and eIF4E2 are involved in broad spectrum resistance in tomato against potyviruses.
Mazier, M., Flamain, F., Nicolaï, M., Sarnette, V. and Caranta, C. (2011) Knock‐down of both eIF4E1 and eIF4E2 genes confers broad‐spetrum resistance against potyviruses in tomato. PLoSone, 6, e29595.
Supporting information
Fig. S1 Construct for Cm‐eIF4E silencing. (a) Sequence alignments of Cm‐eIF4E (MU21698) and Cm‐eIF(iso)4E (MU5578) using CLUSTAL X. The selected Cm‐eIF4E sequence stretch (175 nucleotides) is shown in bold and italics, and the primer sequences that were used for the amplification of the fragment are additionally underlined; stars mark identical nucleotides. (b) Schematic representation of the intron‐hairpin‐structured RNA (ihp4E) expressed by the transgenic construct. The 175‐nucleotide inverted complementary sequence fragments from the Cm‐eIF4E gene (175‐4E) form the stem, which is separated by the pdk intron.
Fig. S2 Cm‐eIF4E silencing in melon does not affect the susceptibility of melon to Papaya ringspot virus (PRSV), Watermelon mosaic virus (WMV), Cucumber mosaic virus (CMV) and Cucurbit aphid‐borne yellows virus (CABYV). Results obtained in the analyses of plants from a segregating T2 generation inoculated with PRSV, WMV, CMV and CABYV; the relative amounts of virus RNA and Cm‐eIF4E mRNA were quantified by reverse transcription‐polymerase chain reaction (RT‐PCR) (with the exception of CABYV agroinoculated plants, for which virus accumulation was quantified by Northern blot), the transgene‐derived small interfering RNAs (siRNAs) were detected by Northern blot, and the presence of the transgene was detected by PCR.
Supporting info item
Supporting info item
ACKNOWLEDGEMENTS
This work was supported by the grant AGL2009‐07552/AGR (Ministerio de Ciencia e Innovación, Spain). A. Rodríguez‐Hernández was supported by grants from the National Council of Science and Technology (CONACyT, Mexico) and the Spanish Agency for International Development Cooperation (AECID). We also thank Mari Carmen Montesinos for technical assistance. The use of the selected nucleotide sequence used here for specific silencing of eIF4E and its use for obtention of broad range virus resistance, is covered by patent: Spain No. P201030821.
REFERENCES
- Abou‐Jawdah, Y. , Sobh, H. , Fayad, A. , Lecoq, H. , Delécolle, B. and Trad‐Ferré, J. (2000) Cucurbit yellow stunting disorder virus—a new threat to cucurbits in Lebanon. J. Plant Pathol. 82, 55–60. [Google Scholar]
- Bananej, K. and Vahdat, A. (2008) Identification, distribution and incidence of viruses in field‐grown cucurbit crops of Iran. Phytopathol. Medit. 47, 247–257. [Google Scholar]
- Browning, K.S. (1996) The plant translational apparatus. Plant Mol. Biol. 32, 107–144. [DOI] [PubMed] [Google Scholar]
- Browning, K.S. (2004) Plant translation initiation factors: it is not easy to be green. Biochem. Soc. Trans. 32, 589–591. [DOI] [PubMed] [Google Scholar]
- Clepet, C. , Joobeur, T. , Zheng, Y. , Jublot, D. , Huang, M. , Truniger, V. , Boualem, A. , Hernandez‐Gonzalez, M. , Dolcet‐Sanjuan, R. , Portnoy, V. , Mascarell‐Creus, A. , Cano‐Delgado, A. , Katzir, N. , Bendahmane, A. , Giovannoni, J. , Aranda, M. , Garcia‐Mas, J. and Fei, Z. (2011) Analysis of expressed sequence tags generated from full‐length enriched cDNA libraries of melon. BMC Genomics, 12, 252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comai, L. and Henikoff, S. (2006) TILLING: practical single‐nucleotide mutation discovery. Plant J. 45, 684–694. [DOI] [PubMed] [Google Scholar]
- Combe, J.P. , Petracek, M.E. , van Eldik, G. , Meulewaeter, F. and Twell, D. (2005) Translation initiation factors eIF4E and eIFiso4E are required for polysome formation and regulate plant growth in tobacco. Plant Mol. Biol. 57, 749–760. [DOI] [PubMed] [Google Scholar]
- Cuadrado, I.M. , Gómez, J. and Moreno, P. (1993) El virus de las manchas necróticas del melón (MNSV) en Almería. I. Importancia de MNSV como causa de la muerte súbita del melon. Bol. San. Veg. Plagas, 19, 96–106. [Google Scholar]
- Dellaporta, S. , Wood, J. and Hicks, J. (1983) A plant DNA minipreparation: version II. Plant Mol. Biol. Rep. 1, 19–21. [Google Scholar]
- Desbiez, C. , Joannon, B. , Wipf‐Scheibel, C. , Chandeysson, C. and Lecoq, H. (2009) Emergence of new strains of Watermelon mosaic virus in south‐eastern France: evidence for limited spread but rapid local population shift. Virus Res. 141, 201–208. [DOI] [PubMed] [Google Scholar]
- Diaz, J.A. , Nieto, C. , Moriones, E. , Truniger, V. and Aranda, M.A. (2004) Molecular characterization of a melon necrotic spot virus strain that overcomes the resistance in melon and nonhost plants. Mol. Plant–Microbe Interact. 17, 668–675. [DOI] [PubMed] [Google Scholar]
- Díaz‐Pendón, J.A. , Truniger, V. , Nieto, C. , Garcia‐Mas, J. , Bendahmane, A. and Aranda, M.A. (2004) Advances in understanding recessive resistance to plant viruses. Mol. Plant Pathol. 5, 223–233. [DOI] [PubMed] [Google Scholar]
- Duprat, A. , Caranta, C. , Revers, F. , Menand, B. , Browning, K.S. and Robaglia, C. (2002) The Arabidopsis eukaryotic initiation factor (iso)4E is dispensable for plant growth but required for susceptibility to potyviruses. Plant J. 32, 927–934. [DOI] [PubMed] [Google Scholar]
- Gaba, V. , Zelcer, A. and Gal‐on, A. (2004) Cucurbit biotechnology—the importance of virus resistance. In Vitro Cell. Dev. Biol. Plant 40, 346–358. [Google Scholar]
- Gallie, D.R. and Browning, K.S. (2001) eIF4G functionally differs from eIFiso4G in promoting internal initiation, cap‐independent translation, and translation of structured mRNAs. J. Biol. Chem. 276, 36951–36960. [DOI] [PubMed] [Google Scholar]
- Gil‐Salas, F.M. , Colyer, A. , Boonham, N. , Cuadrado, I.M. and Janssen, D. (2009) Resistance screening against Cucumber vein yellowing virus using a real‐time (Taqman) RT‐PCR assay in cucumber (Cucumis sativus). Crop Prot. 28, 109–112. [Google Scholar]
- González, M. , Xu, M. , Esteras, C. , Roig, C. , Monforte, A. , Troadec, C. , Pujol, M. , Nuez, F. , Bendahmane, A. , Garcia‐Mas, J. and Picó, B. (2011) Towards a TILLING platform for functional genomics in Piel de Sapo melons. BMC Res. Notes, 4, 289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez‐Ibeas, D. , Blanca, J. , Roig, C. , Gonzalez‐To, M. , Pico, B. , Truniger, V. , Gomez, P. , Deleu, W. , Cano‐Delgado, A. , Arus, P. , Nuez, F. , Garcia‐Mas, J. , Puigdomenech, P. and Aranda, M.A. (2007) MELOGEN: an EST database for melon functional genomics. BMC Genomics, 8, 306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez‐Ibeas, D. , Cañizares, J. and Aranda, M.A. (2012) Microarray analysis shows that recessive resistance to watermelon mosaic virus in melon is associated with the induction of defense response genes. Mol. Plant–Microbe Interact. 25, 107–118. [DOI] [PubMed] [Google Scholar]
- Hull, R. (2002) Matthews' Plant Virology. San Diego, CA: Academic Press. [Google Scholar]
- Hwang, J. , Li, J. , Liu, W. , An, S. , Cho, H. , Her, N. , Yeam, I. , Kim, D. and Kang, B. (2009) Double mutations in eIF4E and eIFiso4E confer recessive resistance to Chilli veinal mottle virus in pepper. Mol. Cells, 27, 329–336. [DOI] [PubMed] [Google Scholar]
- Jackson, A.L. and Linsley, P.S. (2004) Noise amidst the silence: off‐target effects of siRNAs? Trends Genet. 20, 521–524. [DOI] [PubMed] [Google Scholar]
- Kang, B.‐C. , Yeam, I. , Frantz, J.D. , Murphy, J.F. and Jahn, M.M. (2005a) The pvr1 locus in Capsicum encodes a translation initiation factor eIF4E that interacts with Tobacco etch virus VPg. Plant J. 42, 392–405. [DOI] [PubMed] [Google Scholar]
- Kang, B.‐C. , Yeam, I. and Jahn, M.M. (2005b) Genetics of plant virus resistance. Annu. Rev. Phytopathol. 43, 581–621. [DOI] [PubMed] [Google Scholar]
- Kassem, M.A. , Sempere, R.N. , Juarez, M. , Aranda, M.A. and Truniger, V. (2007) Cucurbit aphid‐borne yellows virus is prevalent in field‐grown cucurbit crops of southeastern Spain. Plant Dis. 91, 232–238. [DOI] [PubMed] [Google Scholar]
- Lecoq, H. , Dafalla, G. , Desbiez, C. , Wipf‐Scheibel, C. and Kheyr‐Pour, A. (2003) A 10‐year survey (1993–2002) of cucurbit viruses in Sudan. J. Plant Dis. Prot. 110, 68–69. [Google Scholar]
- Lecoq, H. , Dufour, O. , Wipf‐Scheibel, C. , Girard, M. , Cotillon, A.C. and Desbiez, C. (2007) First report of Cucumber vein yellowing virus in melon in France. Plant Dis. 91, 909. [DOI] [PubMed] [Google Scholar]
- Lecoq, H. , Justafré, I. , Wipf‐Scheibel, C. and Desbiez, C. (2008) Moroccan watermelon mosaic virus newly reported on zucchini squash in France. Plant Pathol. 57, 766. [Google Scholar]
- Lecoq, H. , Dafalla, G. , Delécolle, B. , Wipf‐Sheibel, C. and Desbiez, C. (2011) Snake melon asteroid mosaic virus, a tentative new member of the genus Sobemovirus infecting cucurbits. Plant Dis. 95, 153–157. [DOI] [PubMed] [Google Scholar]
- Leibman, D. , Wolf, D. , Saharan, V. , Zelcer, A. , Arazi, T. , Yoel, S. , Gaba, V. and Gal‐On, A. (2011) A high level of transgenic viral small RNA is associated with broad potyvirus resistance in cucurbits. Mol. Plant–Microbe Interact. 24, 1220–1238. [DOI] [PubMed] [Google Scholar]
- Lellis, A.D. , Kasschau, K.D. , Whitham, S.A. and Carrington, J.C. (2002) Loss‐of‐susceptibility mutants of Arabidopsis thaliana reveal an essential role for eIF(iso)4E during potyvirus infection. Curr. Biol. 12, 1046–1051. [DOI] [PubMed] [Google Scholar]
- Ling, K.‐S. , Harris, K. , Meyer, J. , Levi, A. , Guner, N. , Wehner, T. , Bendahmane, A. and Havey, M. (2009) Non‐synonymous single nucleotide polymorphisms in the watermelon eIF4E gene are closely associated with resistance to Zucchini yellow mosaic virus . Theor. Appl. Genet. 120, 191–200. [DOI] [PubMed] [Google Scholar]
- Lovisolo, O. (1980) Virus and viroid diseases of cucurbits. Acta Hortic. 88, 33–88. [Google Scholar]
- Naderpour, M. , Lund, O. , Larsen, R. and Johansen, E. (2010) Potyviral resistance derived from cultivars of Phaseolus vulgaris carrying bc‐3 is associated with the homozygotic presence of a mutated eIF4E allele. Mol. Plant Pathol. 11, 255–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicaise, V. , German‐Retana, S. , Sanjuan, R. , Dubrana, M.‐P. , Mazier, M. , Maisonneuve, B. , Candresse, T. , Caranta, C. and Le Gall, O. (2003) The eukaryotic translation initiation factor 4E controls lettuce susceptibility to the potyvirus lettuce mosaic virus. Plant Physiol. 132, 1272–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieto, C. , Morales, M. , Orjeda, G. , Clepet, C. , Monfort, A. , Sturbois, B. , Puigdomènech, P. , Pitrat, M. , Caboche, M. , Dogimont, C. , Garcia‐Mas, J. , Aranda, M.A. and Bendahmane, A. (2006) An eIF4E allele confers resistance to an uncapped and non‐polyadenylated RNA virus in melon. Plant J. 48, 452–462. [DOI] [PubMed] [Google Scholar]
- Nieto, C. , Piron, F. , Dalmais, M. , Marco, C. , Moriones, E. , Gomez‐Guillamon, M.L. , Truniger, V. , Gómez, P. , Garcia‐Mas, J. , Aranda, M.A. and Bendahmane, A. (2007) EcoTILLING for the identification of allelic variants of melon eIF4E, a factor that controls virus susceptibility. BMC Plant Biol. 7, 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papayiannis, L.C. , Ioannou, N. , Boubourakas, I.N. , Dovas, C.I. , Katis, N.I. and Falk, B.W. (2005) Incidence of viruses infecting cucurbits in cyprus. J. Phytopathol. 153, 530–535. [Google Scholar]
- Piron, F. , Nicolaï, M. , Minoïa, S. , Piednoir, E. , Moretti, A. , Salgues, A. , Zamir, D. , Caranta, C. and Bendahmane, A. (2010) An induced mutation in tomato eIF4E leads to immunity to two potyviruses. PLoS ONE, 5, e11313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prüfer, D. , Wipf‐Scheibel, C. , Richards, K. , Guilley, H. , Lecoq, H. and Jonard, G. (1995) Synthesis of a full‐length infectious cDNA clone of Cucurbit Aphid‐Borne Yellows Virus and its use in gene exchange experiments with structural proteins from other luteoviruses. Virology, 214, 150–158. [DOI] [PubMed] [Google Scholar]
- Reddy, D.V.R. , Sudarshana, M.R. , Fuchs, M. , Rao, N.C. and Thottappilly, G. (2009) Genetically engineered virus‐resistant plants in developing countries: current status and future prospects. Adv. Virus Res. 75, 185–220. [DOI] [PubMed] [Google Scholar]
- Robaglia, C. and Caranta, C. (2006) Translation initiation factors: a weak link in plant RNA virus infection. Trends Plant Sci. 11, 40–45. [DOI] [PubMed] [Google Scholar]
- Rommens, C.M. , Haring, M.A. , Swords, K. , Davies, H.V. and Belknap, W.R. (2007) The intragenic approach as a new extension to traditional plant breeding. Trends Plant Sci. 12, 397–403. [DOI] [PubMed] [Google Scholar]
- Ruffel, S. , Gallois, J. , Lesage, M. and Caranta, C. (2005) The recessive potyvirus resistance gene pot‐1 is the tomato orthologue of the pepper pvr2‐eIF4E gene. Mol. Genet. Genomics, 274, 346–353. [DOI] [PubMed] [Google Scholar]
- Ruffel, S. , Gallois, J.L. , Moury, B. , Robaglia, C. , Palloix, A. and Caranta, C. (2006) Simultaneous mutations in translation initiation factors elF4E and elF(iso)4E are required to prevent pepper veinal mottle virus infection of pepper. J. Gen. Virol. 87, 2089–2098. [DOI] [PubMed] [Google Scholar]
- Sato, M. , Nakahara, K. , Yoshii, M. , Ishikawa, M. and Uyeda, I. (2005) Selective involvement of members of the eukaryotic initiation factor 4E family in the infection of Arabidopsis thaliana by potyviruses. FEBS Lett. 579, 1167–1171. [DOI] [PubMed] [Google Scholar]
- Scacheri, P.C. , Rozenblatt‐Rosen, O. , Caplen, N.J. , Wolfsberg, T.G. , Umayam, L. , Lee, J.C. , Hughes, C.M. , Shanmugam, K.S. , Bhattacharjee, A. , Meyerson, M. and Collins, F.S. (2004) Short interfering RNAs can induce unexpected and divergent changes in the levels of untargeted proteins in mammalian cells. Proc. Natl. Acad. Sci. USA, 101, 1892–1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevik, M.A. and Arli‐Sokmen, M. (2003) Viruses infecting cucurbits in Samsun Turkey. Plant Dis. 87, 341–344. [DOI] [PubMed] [Google Scholar]
- Truniger, V. and Aranda, M.A. (2009) Recessive resistance to plant viruses. Adv. Virus Res. 75, 119–159, 231. [DOI] [PubMed] [Google Scholar]
- Truniger, V. , Nieto, C. , González‐Ibeas, D. and Aranda, M. (2008) Mechanism of plant eIF4E‐mediated resistance against a Carmovirus (Tombusviridae): cap‐independent translation of a viral RNA controlled in cis by an (a)virulence determinant. Plant J. 56, 716–727. [DOI] [PubMed] [Google Scholar]
- Wesley, S.V. , Helliwell, C.A. , Smith, N.A. , Wang, M. , Rouse, D.T. , Liu, Q. , Gooding, P.S. , Singh, S.P. , Abbott, D. , Stoutjesdijk, P.A. , Robinson, S.P. , Gleave, A.P. , Green, A.G. and Waterhouse, P.M. (2001) Construct design for efficient, effective and high‐throughput gene silencing in plants. Plant J. 27, 581–590. [DOI] [PubMed] [Google Scholar]
- Yakoubi, S. , Desbiez, C. , Fakhfakh, H. , Wipf‐Scheibel, C. , Marrakchi, M. and Lecoq, H. (2007) Occurrence of Cucurbit yellow stunting disorder virus and Cucumber vein yellowing virus in Tunisia. J. Plant Pathol. 89, 417–420. [Google Scholar]
- Yakoubi, S. , Desbiez, C. , Fakhfakh, H. , Wipf‐Scheibel, C. , Marrakchi, M. and Lecoq, H. (2008) Biological characterization and complete nucleotide sequence of a Tunisian isolate of Moroccan watermelon mosaic virus . Arch. Virol. 153, 117–125. [DOI] [PubMed] [Google Scholar]
- Yoshii, M. , Nishikiori, M. , Tomita, K. , Yoshioka, N. , Kozuka, R. , Naito, S. and Ishikawa, M. (2004) The Arabidopsis Cucumovirus Multiplication 1 and 2 loci encode translation initiation factors 4E and 4G. J. Virol. 78, 6102–6111. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 Construct for Cm‐eIF4E silencing. (a) Sequence alignments of Cm‐eIF4E (MU21698) and Cm‐eIF(iso)4E (MU5578) using CLUSTAL X. The selected Cm‐eIF4E sequence stretch (175 nucleotides) is shown in bold and italics, and the primer sequences that were used for the amplification of the fragment are additionally underlined; stars mark identical nucleotides. (b) Schematic representation of the intron‐hairpin‐structured RNA (ihp4E) expressed by the transgenic construct. The 175‐nucleotide inverted complementary sequence fragments from the Cm‐eIF4E gene (175‐4E) form the stem, which is separated by the pdk intron.
Fig. S2 Cm‐eIF4E silencing in melon does not affect the susceptibility of melon to Papaya ringspot virus (PRSV), Watermelon mosaic virus (WMV), Cucumber mosaic virus (CMV) and Cucurbit aphid‐borne yellows virus (CABYV). Results obtained in the analyses of plants from a segregating T2 generation inoculated with PRSV, WMV, CMV and CABYV; the relative amounts of virus RNA and Cm‐eIF4E mRNA were quantified by reverse transcription‐polymerase chain reaction (RT‐PCR) (with the exception of CABYV agroinoculated plants, for which virus accumulation was quantified by Northern blot), the transgene‐derived small interfering RNAs (siRNAs) were detected by Northern blot, and the presence of the transgene was detected by PCR.
Supporting info item
Supporting info item


