Summary
Sugar metabolism and sugar signalling are not only critical for plant growth and development, but are also important for stress responses. However, how sugar homeostasis is involved in plant defence against pathogen attack in the model crop rice remains largely unknown. In this study, we observed that the grains of gif1, a loss‐of‐function mutant of the cell wall invertase gene GRAIN INCOMPLETE FILLING 1 (GIF1), were hypersusceptible to postharvest fungal pathogens, with decreased levels of sugars and a thinner glume cell wall in comparison with the wild‐type. Interestingly, constitutive expression of GIF1 enhanced resistance to both the rice bacterial pathogen Xanthomonas oryzae pv. oryzae and the fungal pathogen Magnaporthe oryzae. The GIF1‐overexpressing (GIF1‐OE) plants accumulated higher levels of glucose, fructose and sucrose compared with the wild‐type plants. More importantly, higher levels of callose were deposited in GIF1‐OE plants during pathogen infection. Moreover, the cell wall was much thicker in the infection sites of the GIF1‐OE plants when compared with the wild‐type plants. We also found that defence‐related genes were constitutively activated in the GIF1‐OE plants. Taken together, our study reveals that sugar homeostasis mediated by GIF1 plays an important role in constitutive and induced physical and chemical defence.
Introduction
Plants have developed a sophisticated defence machinery against pathogen attack during the long co‐evolution with pathogens, which provides them with both pre‐existing, or constitutive, and inducible defence responses. The pre‐existing defence mainly consists of physical barriers, such as pectin, cellulose and hemicellulose, lignin and proteins, which affect cell wall integrity during the defence response against pathogen invasion (Hématy et al., 2009; Hückelhoven, 2007; Vorwerk et al., 2004). The activation of induced defence depends on a more complicated signalling network through which plants recognize pathogens and activate immune responses (Boller and He, 2009; Chisholm et al., 2006; Dodds and Rathjen, 2010; Jones and Dangl, 2006). It has been recognized that sugars not only serve as energy resources, but are also required as substrates and signals during plant defence responses (Roitsch and González, 2004; Rolland et al., 2006). However, pathogens have also evolved adaptive strategies to suppress host immunity and gain access to sugar supply (Chen L.Q. et al., 2010, 2012). Most recently, it has been reported that the disaccharide trehalose is required for the pathogenesis of Pseudomonas aeruginosa in Arabidopsis, which probably functions to promote the acquisition of nitrogen‐containing nutrients, thereby allowing P. aeruginosa to replicate in the intercellular spaces in the host (Djonović et al., 2013). Therefore, sugar metabolism/homeostasis plays an important role in plant defence responses and pathogen pathogenicity.
The plant cell wall is a matrix of polysaccharides, and provides more than just a physical barrier to invading microbes by playing a crucial role in the sensing or transmission of signals from the external stimuli of pathogen attack to establish basal defence, which is accompanied by various defence responses, such as the production of reactive oxygen species (ROS), activation of pathogenesis‐related (PR) proteins, generation of signal molecules (e.g. salicylic acid, jasmonic acid and ethylene), formation of cell wall appositions (CWAs) or papilla, callose deposition and the hypersensitive response (HR) (Hückelhoven, 2007). It has been reported that the polysaccharide composition of the plant cell wall plays an important role in defence to diverse pathogens (Vorwerk et al., 2004). Changes in cell wall composition result in altered immune responses to diverse pathogens in Arabidopsis (Ellis and Turner, 2001; Ramírez et al., 2011; Sánchez‐Rodríguez et al., 2009; Vogel et al., 2002, 2004). Interestingly, impairment of cellulose biosynthesis results in enhanced resistance to different types of pathogen in Arabidopsis (Hernández‐Blanco et al., 2007). The Arabidopsis agg1agg2 double‐mutant and agb1 mutant plants show decreased xylose levels and increased susceptibility to the necrotrophic fungus Plectosphaerella cucumerina (Delgado‐Cerezo et al., 2012). The overexpression of pectin methylesterase inhibitors restricts fungal infection by Botrytis cinerea in Arabidopsis (Lionetti et al., 2007). Moreover, the hydrolysis of cell wall polysaccharides during pathogen infection may also generate sugar or oligosaccharide signals which stimulate intracellular defence responses (Bolouri‐Moghaddam et al., 2010; Rolland et al., 2006). Thus, cell wall defence, which is associated with polysaccharide composition, is essential in the plant defence machinery.
Cell wall invertases (CW‐Invs) catalyse the irreversible hydrolysis of sucrose into glucose and fructose, the hexoses that play important roles in sucrose partitioning, cell differentiation, plant development and responses to biotic and abiotic stresses (Roitsch and González, 2004). The expression of CW‐Invs and enzymatic activity are inducible by pathogens (Berger et al., 2007; Roitsch and González, 2004). RNA interference (RNAi)‐mediated repression of CW‐Inv results in reduced resistance of transgenic tobacco plants (Essmann et al., 2008), and the expression of the yeast invertase in tobacco and Arabidopsis leads to the accumulation of sugars and increased defence gene expression and enhanced resistance (Herbers et al., 1996; von Schaewen et al., 1990). Interestingly, the activity of CW‐Inv is strongly dependent on post‐translational regulation by proteinaceous invertase inhibitors, whose expression and activity have been shown to be strongly repressed on pathogen infection in Arabidopsis, resulting in the release of invertase activity (Bonfig et al., 2010). Therefore, CW‐Invs and CW‐Inv–inhibitor complexes may affect plant defence responses through sugar homeostasis.
As a monocot, rice (Oryza sativa L.) has been commonly accepted as a model to study defence responses in cereal crops. Several studies have shown that sugar transportation plays an important role in the rice defence response against pathogens (Chen L.Q. et al., 2010, 2012; Chu et al., 2006; Yang et al., 2006). Recently, d‐allose has been found to enhance resistance to rice bacterial blight caused by Xanthomonas oryzae pv. oryzae (Xoo) by up‐regulating defence‐related genes (Kano et al., 2010). However, the role and underlying mechanism(s) of sugar homeostasis in rice defence are not yet fully understood. Previously, we have reported that the rice CW‐Inv GRAIN INCOMPLETE FILLING 1 (GIF1) plays an important role in sucrose partitioning during the grain‐filling process; sucrose unloaded by GIF1 in the ovular and stylar vascular tissues is critical for the stimulation of starch synthesis in the developing endosperm (Wang E.T. et al., 2008, 2010). Here, we report that gif1 mutant grains with a weakened cell wall barrier are hypersusceptible to postharvest fungal pathogens, whereas constitutive expression of GIF1 enhances resistance to both bacterial blight and fungal blast with enhanced physical and induced defence responses.
Results
The gif1 mutant is hypersusceptible to postharvest fungal pathogens
The grains of the rice gif1 mutant accumulated low levels of sugars with significantly lower CW‐Inv activity than that in wild‐type plants (Wang E.T. et al., 2008). When grown in paddy fields, we found that the gif1 grains were hypersusceptible to postharvest fungal pathogens (fungi rapidly covered the whole mutant glume surface under humid conditions), whereas the wild‐type grains were fully resistant to the pathogens (Fig. 1A,B,D). We isolated the pathogens from the gif1 glumes and discovered that the majority of the fungi were Alternaria sp. and Rhizopus sp. (Fig. 1C,E), both postharvest pathogenic fungi in cereals (Narayanasamy, 2006). Thus, we speculated that the loss of GIF1 function probably disrupts rice basal defence.
Figure 1.
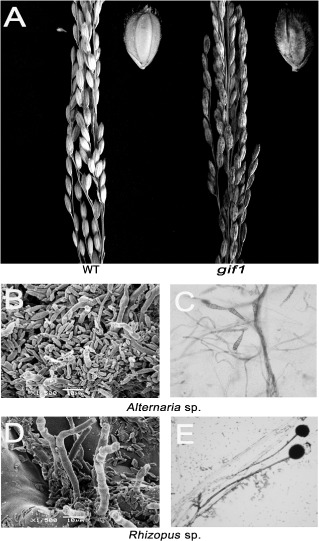
Hypersusceptibility of gif1 grains to postharvest fungal pathogens. (A) Panicles of the wild‐type (Zhonghua11) (WT) and gif1 mutant grown in the field under high humidity conditions. Insets are the WT and gif1 seeds. Note that the surface of the gif1 seeds is covered by mould, whereas no infection by the pathogen is observed on WT grains. (B, C) Scanning electron microscopy (SEM) images of Alternaria sp. on the gif1 glume surface. Bar, 10 μm. (D, E) SEM images of Rhizopus sp. on the gif1 glume surface. Bar, 10 μm.
Constitutive expression of GIF1 enhances resistance to bacterial and fungal pathogens
Because Alternaria sp. and Rhizopus sp. are not established pathogen models in rice, we investigated two established rice pathosystems, rice leaf bacterial blight caused by Xoo and fungal blast caused by Magnaporthe oryzae, two of the most devastating rice diseases in China. We did not find obvious differences in resistance to Xoo and M. oryzae between the gif1 mutant and the wild‐type control, because the wild‐type control is already highly susceptible to the pathogens (Fig. S1, see Supporting Information). One possibility is that GIF1 functions only in the filling grains (Wang E.T. et al., 2008), therefore not affecting the defence responses in leaves where the pathogens initiate infection. However, we could not exclude the possibility that other CINs expressed in leaves might function redundantly in disease resistance against leaf infection by Xoo and blast. Therefore, we investigated the expression of other CIN genes in infected leaves, and found that OsCIN1, OsCIN4 and OsCIN7 were induced in leaves infected by Xoo (Fig. S2, see Supporting Information). However, whether the additional CINs contribute to rice disease resistance and redundantly complement the possible GIF1 function in leaves should be confirmed by additional extensive genetic and biochemical studies.
To investigate the role of GIF1 in rice defence, we generated transgenic plants constitutively expressing GIF1 (GIF1‐OE), in which the expression and activity of CW‐Inv was increased significantly in comparison with wild‐type plants (Wang E.T. et al., 2008, 2010). Two‐month‐old plants were inoculated with Xoo virulent strains PXO99 and DY89031. Surprisingly, GIF1‐OE plants showed greatly enhanced resistance to both Xoo virulent strains, compared with the wild‐type control (Fig. 2A), with a significant decrease in bacterial growth (Fig. 2B,C). Therefore, GIF1 positively regulates bacterial disease resistance. We further assayed disease resistance to rice blast by inoculating 2‐week‐old seedlings with M. oryzae virulent races ZB1 (strain 2000‐2) and ZB15 (strain 09‐31‐1). The GIF1‐OE plants were more resistant, with significantly reduced lesion size when compared with the wild‐type control (Fig. 3A,B). Disease severity (score) on the GIF1‐OE plants averaged 1–2 compared with 6–7 on the wild‐type plants. We also investigated fungal penetration and growth in planta and quantified infection type frequency as described previously (Nakao et al., 2011). The results showed that Type III and IV infections were decreased significantly and Type I and II infections were increased significantly in GIF1‐OE plants compared with the wild‐type control at 48 h post‐inoculation (hpi) (Fig. 3C). Therefore, we concluded that the overexpression of GIF1 also increased blast resistance probably through the prevention of the penetration of fungal hyphae and restriction of hyphal growth.
Figure 2.
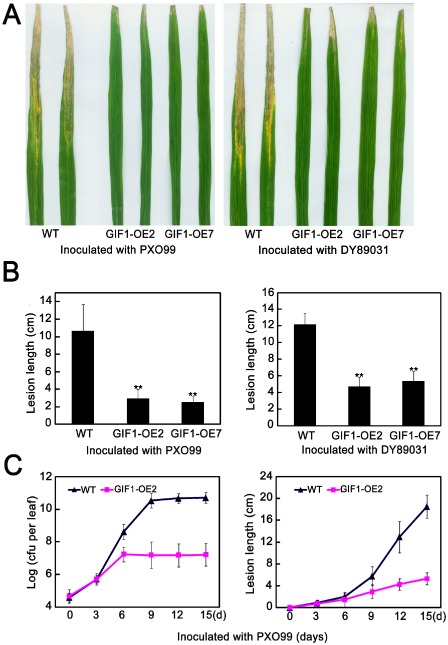
Enhanced disease resistance to bacterial blight in GRAIN INCOMPLETE FILLING 1‐overexpressing (GIF1‐OE) plants. (A) Lesions of the representative GIF1‐OE lines (GIF1‐OE2 and GIF1‐OE7) and the wild‐type (TP309) (WT) infected with Xanthomonas oryzae pv. oryzae (Xoo) for 2 weeks. (B) The lesion lengths of the GIF1‐OE plants inoculated with PXO99 and DY89031 for 2 weeks were decreased significantly. **Significant difference in comparison with the WT control (P ≤ 0.001). (C) Bacterial growth and corresponding lesion lengths in the GIF1‐OE and WT plants infected with PXO99 during a 15‐day inoculation assay. The data are given as means ± standard error (SE) (n = 3).
Figure 3.
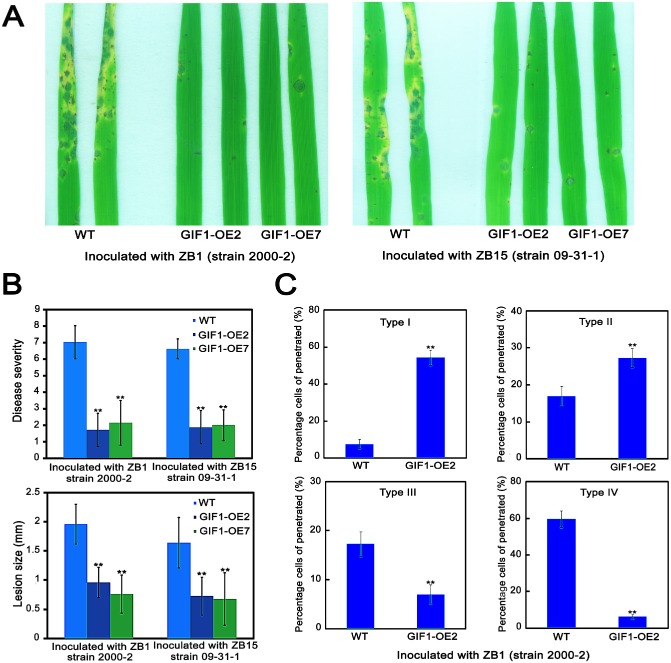
Enhanced disease resistance to fungal blast in GRAIN INCOMPLETE FILLING 1‐overexpressing (GIF1‐OE) plants. (A) Blast lesions were less developed in GIF1‐OE plants compared with the wild‐type (WT) plants. Leaves were spray inoculated with virulent race ZB1 (left) and ZB15 (right), and disease symptoms were recorded at 7 days post‐inoculation. (B) Disease severity and lesion size were decreased significantly in GIF1‐OE plants relative to the WT. (C) Average infection types in the GIF1‐OE and WT plants at 48 h post‐inoculation (hpi). Note that Types I and II were increased significantly, whereas Types III and IV were decreased significantly in GIF1‐OE. At least 100 lesions from 30 representative diseased leaves of 30 plants were measured. Data presented are the means ± standard error (SE) (n = 3). **Significant difference in comparison with the WT control (P ≤ 0.001).
We have shown that GIF1 and another CW‐Inv gene OsCIN1 constitute a pair of duplicate genes which experience subfunctionalization with different expression patterns and different kinetic parameters of enzymatic activity: GIF1 transcripts have been detected in roots, elongating internodes, shoots and panicles, but not in leaves. In contrast, OsCIN1 is expressed strongly in leaves (Wang et al., 2010). However, we did not observe any obvious difference in Xoo resistance between the OsCIN1‐overexpressing (OsCIN1‐OE) plants and wild‐type plants (Fig. S3, see Supporting Information), probably because OsCIN1 has low invertase activity and does not affect rice defence significantly, in addition to unchanged growth when overexpressed (Wang et al., 2010), even though it is inducible by Xoo (Fig. S2). Therefore, GIF1 probably modulates rice basal defence through its strong activity on sugar metabolism.
GIF1 modulates cell wall physical defence
Because the cell wall mainly consists of carbohydrates, we first postulated that GIF1 might contribute to cell wall‐based defence through sugar homeostasis. To explore this possibility, we analysed the cell wall composition and structure of the wild‐type, mutant and GIF1‐OE plants. We found that the levels of sucrose, glucose and fructose were decreased significantly in the gif1 glume (Fig. 4A). Interestingly, we found that the cell wall was thinner in the gif1 mutant than in the wild‐type (Fig. 4B), probably resulting from the decreased levels of sugars (Fig. 4A,C). In contrast, we detected higher levels of glucose and fructose in GIF1‐OE plant leaves; we also found the accumulation of sucrose in transgenic leaves (Fig. 4C). We did not find changes in sugar levels in apoplastic fluid (Fig. S4, see Supporting Information), suggesting that sugars were mainly accumulated inside the GIF1‐OE cell. Similar higher sugar levels were also observed in transgenic tobacco leaves that expressed a yeast invertase (von Schaewen et al., 1990). One explanation for the increased sugar accumulation could be that the overexpression of invertase blocks sucrose export from source leaves into the phloem (von Schaewen et al., 1990). In support of this postulation, GIF1‐OE grains were poorly filled (Wang E.T. et al., 2008, 2010). We did not observe significant differences in the cell wall thickness between uninfected GIF1‐OE and wild‐type plants (Fig. 4D). Interestingly, the cell wall of GIF1‐OE plants was much thicker than that of wild‐type plants after inoculation with Xoo and M. oryzae (Fig. 4D). Consequently, levels of xylose and cellulose, which are important cell wall constituents, were increased significantly in GIF1‐OE leaf cell walls compared with the wild‐type. Galactose levels were decreased and increased in gif1 and GIF1‐OE, respectively, compared with their corresponding wild‐type controls (Fig. 5). These results together indicate that GIF1 mediates rice defence partly through the reinforcement of cell wall‐based physical defence.
Figure 4.
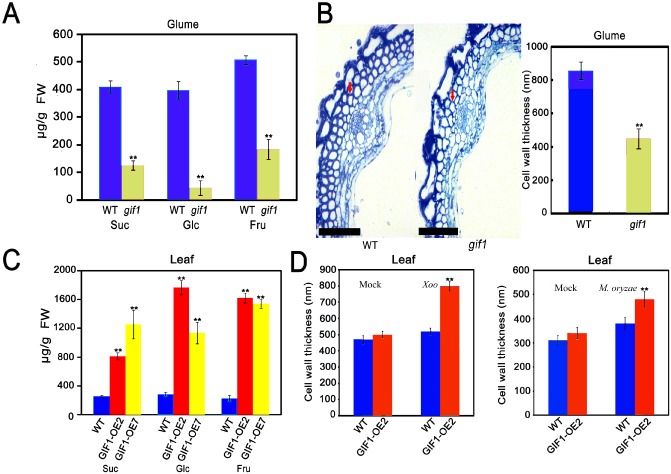
Comparison of cell wall composition and thickness in GRAIN INCOMPLETE FILLING 1‐overexpressing (GIF1‐OE), gif1 mutant and wild‐type (WT) plants. (A) Decreased levels of sucrose (Suc), glucose (Glc) and fructose (Fru) in the gif1 glume compared with the WT control. Data presented are means ± standard error (SE) (n = 3) with units of μg/g fresh weight (FW). **Significant difference in comparison with the WT control (P ≤ 0.001). (B) Cell wall thickness of the gif1 glume was decreased significantly in comparison with the WT. Red arrows indicate the cell wall. Bar, 50 μm. Glume sections from six individual plants were observed and at least 20 measurements were carried out for each sample. Data presented are the means ± SE (n = 3). **Significant difference in comparison with the WT control (P ≤ 0.001). (C) Suc, Glc and Fru were highly accumulated in the GIF1‐OE leaves compared with the WT control. Data presented are the means ± SE (n = 3) with units of μg/g FW. **Significant difference in comparison with the WT control (P ≤ 0.001). (D) Cell wall thickness was increased significantly in GIF1‐OE relative to WT plants after infection with PXO99 (left) and ZB1 (right). Leaf sections from six individual plants were observed and at least 20 measurements were carried out for each sample. Data presented are the means ± SE (n = 3). **Significant difference in comparison with the WT control (P ≤ 0.001).
Figure 5.
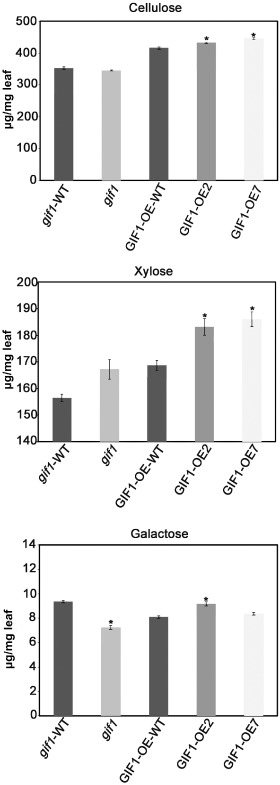
Levels of xylose, cellulose and galactose in leaf cell walls of the gif1 mutant, overexpression lines and wild‐types (WT) for gif1 (gif1‐WT) and overexpression line (GIF1‐OE‐WT). The data are given as means ± standard error (SE) (n = 3). *Significant difference in comparison with the WT control (P ≤ 0.01).
GIF1 affects callose deposition during pathogen infection
Callose deposition deploys a rapid defence barrier on pathogen attack (Hématy et al., 2009; Hückelhoven, 2007). To assay whether the GIF1‐mediated change in sugar homeostasis affects callose deposition during pathogen infection, leaves infected with Xoo were stained with aniline blue at 24 hpi. The results showed that callose deposition was more obvious in infected GIF1‐OE cells than in wild‐type cells (Fig. 6A). Consistent with this observation, the callose biosynthesis genes, OsGSL1, OsGSL3 and OsGSL5 (Hao et al., 2008), were more strongly up‐regulated in transgenic than in wild‐type plants, and reached a peak at 12 hpi (OsGSL1 and OsGSL3) or 48 hpi (OsGSL5) in GIF1‐OE plants (Fig. 6B). Similar callose deposition and gene induction were also observed in the rice–M. oryzae interaction (Fig. S5, see Supporting Information). We also observed similar results in another GIF1‐OE line (Fig. S6, see Supporting Information). Therefore, the GIF1‐mediated sugar homeostasis regulates callose induction, which probably limits pathogen penetration and proliferation.
Figure 6.
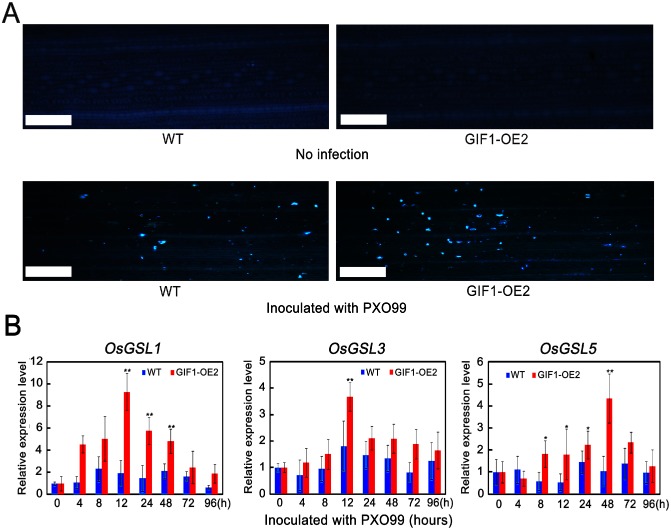
GRAIN INCOMPLETE FILLING 1 (GIF1) overexpression stimulates callose deposition. (A) Callose deposition was obviously increased in the GIF1‐overexpressing (GIF1‐OE) cells compared with the wild‐type (WT) cells at 24 h post‐inoculation (hpi) with PXO99. Bar, 100 μm. (B) Stronger up‐regulation of the callose biosynthesis genes OsGSL1, OsGSL3 and OsGSL5 in GIF1‐OE plants after PXO99 infection, in comparison with the WT. The data are given as means ± standard error (SE) (n = 3). Significant difference in comparison with the WT control: *P ≤ 0.01; **P ≤ 0.001.
GIF1 affects ROS accumulation and the HR
The plant cell wall is associated with defence responses, including ROS accumulation and the HR, when plants counteract pathogen infection (Hückelhoven, 2007). Soluble sugars have been proposed to modulate oxidative stress in plants (Couée et al., 2006), and increased levels of ROS may be a consequence of altered sugar levels in plants (Bolouri‐Moghaddam et al., 2010). To determine the involvement of ROS and the HR in the stimulated defence of GIF1‐OE plants, we analysed hydrogen peroxide (H2O2) accumulation and the HR in plants infected with bacterial and fungal pathogens. We observed that higher levels of H2O2 were accumulated in infected GIF1‐OE leaves when compared with the wild‐type control (Figs 7A, S6 and S7, see Supporting Information). We also observed stronger HR cell death in GIF1‐OE plants than in the wild‐type (Figs 7B, S6 and S7). In support of this, the peroxidase genes, POX8.1 and POX22.3, were more strongly induced in GIF1‐OE plants after pathogen infection (Figs 7C and S7), which have been suggested to be involved in H2O2 production/scavenging in rice (Ning et al., 2010). These results indicate that the GIF1‐mediated sugar homeostasis also enhances inducible chemical defence responses in rice.
Figure 7.
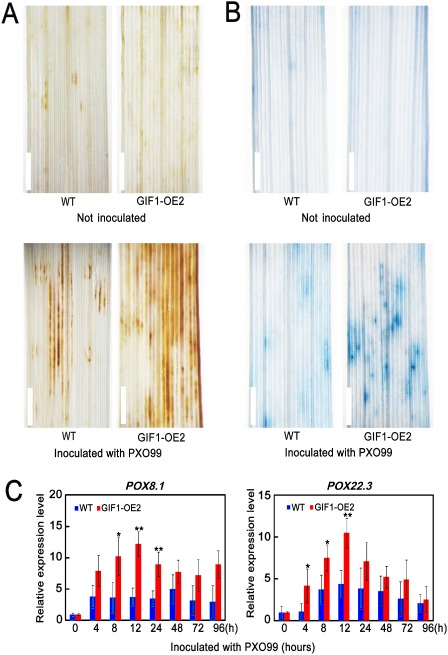
H2O2 accumulation and localized cell death (hypersensitive response, HR) after Xanthomonas oryzae pv. oryzae (Xoo) inoculation. (A) H2O2 accumulation was obviously increased in the GRAIN INCOMPLETE FILLING 1‐overexpressing (GIF1‐OE) leaves compared with the wild‐type (WT) control after infection with PXO99 at 24 h post‐inoculation (hpi). Bar, 100 μm. (B) Stronger HR cell death occurred in GIF1‐OE than in the WT after infection with PXO99 at 24 hpi. Bar, 100 μm. (C) The peroxidase genes POX8.1 and POX22.3 were more strongly induced in GIF1‐OE plants compared with the WT after inoculation with PXO99. The data are given as means ± standard error (SE) (n = 3). Significant difference in comparison with the WT control: *P ≤ 0.01; **P ≤ 0.001.
GIF1 regulates the expression of defence‐related genes
It has been shown that sugars regulate the expression of PR genes (Rolland et al., 2006). Our previous microarray assay revealed that the expression of some defence‐related genes was altered in uninfected gif1 grains [see the microarray data in the National Center for Biotechnology Information GEO database (http://www.ncbi.nlm.nih.gov/geo/) under accession codes GSE9498 and GSM240994–GSM240999]. Interestingly, the transcript levels of the rice defence‐related genes, PR1a, PR1b, PR3, PR10, WRKY45 and NONEXPRESSOR OF PATHOGENESIS‐RELATED GENES 1 (NPR1), were constitutively elevated in GIF1‐OE plants when compared with the wild‐type control (Fig. 8). We thus conclude that sugar homeostasis mediated by GIF1 activates the rice defence signalling network at the molecular level.
Figure 8.
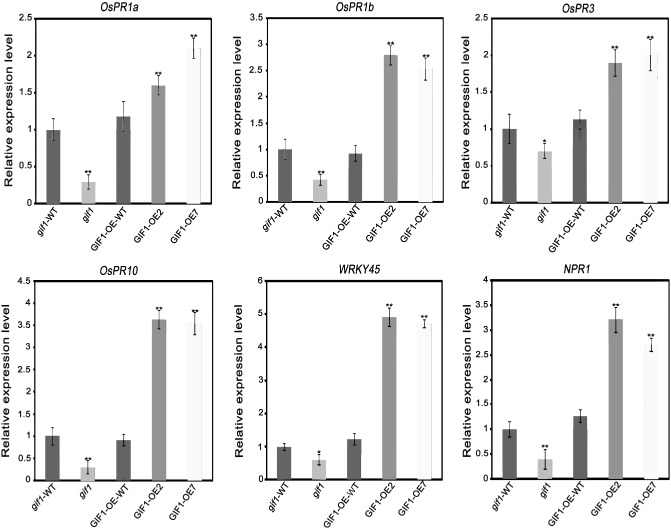
Constitutive activation of defence‐related genes in GRAIN INCOMPLETE FILLING 1‐overexpressing (GIF1‐OE) plants. The transcript levels of the rice defence‐related genes, PATHOGENESIS‐RELATED 1a (PR1a), PR1b, PR3, PR10, WRKY45 and NONEXPRESSOR OF PATHOGENESIS‐RELATED 1 (NPR1), were assayed by quantitative real‐time polymerase chain reaction (qRT‐PCR) in gif1, GIF‐OE and wild‐type (WT) controls (gif1‐WT for gif1 and GIF1‐OE‐WT for the overexpression lines). The data are given as means ± standard error (SE) (n = 3). Significant differences in comparison with the WT control: *P ≤ 0.01; **P ≤ 0.001.
Discussion
Sugars have been recognized as important signalling molecules that affect a variety of physiological responses and, in particular, regulate the expression of genes involved in photosynthesis, sink metabolism and defence responses (Rolland et al., 2006; Smeekens, 2000). In addition, plant defence against pathogens is costly in terms of energy and carbohydrates (Berger et al., 2007). In particular, most plant pathogens actively penetrate the plant cell wall to access intracellular nutrients; whereas plants, in turn, strength the cell wall and secrete antimicrobial components into the cell wall space to stop pathogen invasion. CW‐Invs are inducible by pathogen infection, and can increase local respiratory sink activity and are involved in plant defence against various pathogens (Roitsch and González, 2004; Rolland et al., 2006). However the mechanism by which CW‐Invs modulate defence remains largely unknown. Arabidopsis contains three predicted ‘defective invertases’ and three genuine CW‐Invs in its CW‐Inv family (Van den Ende et al., 2009). Our previous study has also shown that OsCIN1 displays low invertase activity (Wang et al., 2010), probably resulting from being a ‘defective invertase’ (Le Roy et al., 2013), lacking the aspartic acid (Asp)/lysine (Lys) or Asp/arginine (Arg) couple that is needed to catalyse the breakdown of sucrose (Le Roy et al., 2007). Our current experimental evidence indicates that GIF1, as a genuine CW‐Inv, plays a role in pre‐existing/constitutive and inducible defence responses. The overexpression of GIF1 strongly increases resistance to both bacterial and fungal diseases.
The crucial role of the plant cell wall in disease resistance has been highlighted by many studies (Hückelhoven, 2007). Cell wall mechanical strength can increase plant resistance to diverse pathogens (Hückelhoven, 2007; Schulze‐Lefert, 2004). Our previous study has also reported that the rice E3 ligase OsBBI1 confers broad‐spectrum resistance to blast fungus through the enhancement of cell wall defence responses (Li et al., 2011). In addition to forming a mechanical barrier to physical fungal penetration, cell wall reinforcements can also decrease susceptibility to cell wall‐degrading enzymes, impede nutrient diffusion to pathogens and possibly restrict the diffusion of toxins (Asselbergh et al., 2007). Interestingly, the cell wall of the gif1 mutant glume is thinner than that of the wild‐type control, a possible explanation of the increased susceptibility to postharvest fungal pathogens (Fig. 1). Cellulose is the main load‐bearing polymer and a potentially important factor in cell wall integrity, which mechanically strengthens the plant cell wall, and cellulose‐deficient mutant plants are often less resistant to pathogens (Hématy et al., 2009; Hückelhoven, 2007; Vorwerk et al., 2004). Similarly, we also observed that the cellulose content was reduced and increased significantly in the gif1 mutant and GIF1‐OE plants, respectively. Moreover, callose deposition is a well‐known plant defence reaction requiring a large amount of sugars (Hématy et al., 2009; Hückelhoven, 2007), GIF1 also positively regulates callose deposition during pathogen infection, forming an additional inducible physical barrier against pathogen invasion through cell wall reinforcement.
H2O2 has long been recognized as an important signalling molecule leading to plant defence responses. Recent studies have also proposed that sugar‐like molecules can counteract oxidative stress by acting as genuine ROS scavengers, and the absence of alkaline/neutral (A/N) invertases has been associated with higher oxidative stress defence gene expression, whereas overexpression of A/N invertases in Arabidopsis leaf mesophyll protoplasts down‐regulates ascorbate peroxidase 2 (APX2) (Xiang et al., 2011). In our study, the finding that GIF1 overexpression stimulated H2O2 accumulation and activated defence‐related genes, including POX and PR genes, is probably a consequence of the higher hexose levels or hexose/sucrose ratios, suggesting a ‘primed’ defensive response. We propose that the differential localization of invertases (cell wall, cytosol or organelle) may have a significant effect on ROS generation and/or scavenging, and thus the expression of defence‐related genes. Similarly, in transgenic rice plants expressing a fungal‐inducible PR gene from maize, high concentrations of sucrose acted as a signal to prime defence responses against both bacterial and fungal pathogens (Gómez‐Ariza et al., 2007). Similarly, the induction of defence by hexoses generated by increased invertase activity was also observed in Arabidopsis (Chou et al., 2000; Fotopoulos et al., 2003), tobacco (Herbers et al., 1996; Scharte et al., 2005), wheat (Sutton et al., 2007; Wright et al., 1995), tomato (Kocal et al., 2008) and pepper (Sonnewald et al., 2012). Silencing of the tobacco CW‐Inv impaired basal defence and hypersensitive cell death with significantly reduced sucrose export and apoplastic carbohydrate levels, revealing that CW‐Inv is important for the acquisition of carbohydrates during pathogen infection, and the availability of these carbohydrates can ensure successful defence responses (Essmann et al., 2008). Therefore, GIF1‐mediated sugar homeostasis also plays a role in inducible chemical defence in rice.
It is well known that most plant pathogens actively penetrate the plant cell wall to access intracellular nutrients; whereas plants strengthen the cell wall and secrete antimicrobial components into the cell wall space to stop pathogen invasion. Interestingly, the accumulation of sugars during the later stages of infection might serve as nutrients for invading pathogens, resulting in disease (Seo et al., 2007). Moreover, pathogens can induce the sucrose efflux transporter genes SWEETs, and the sugar efflux function of SWEET transporters is probably targeted by pathogens for nutritional gain (Chen F. et al., 2010).
Sucrose also acts as a signalling molecule in plant immunity to induce defence responses (Bolouri‐Moghaddam and Van den Ende, 2012). Interestingly, such ‘Sweet Immunity’ also affects circadian rhythms in plants (Bolouri‐Moghaddam and Van den Ende, 2013a, b). Moreover, in transgenic rice expressing a fungal glucose oxidase, the increased induction of H2O2 leads to cell death and enhanced resistance to both bacterial and fungal pathogens (Kachroo et al., 2003). It has also been suggested that the restriction of intercellular transport to the interface of adjacent phloem cells may be an effective mechanism to limit the availability of sucrose in the leaf apoplasm to prevent pathogen infection (Chen F. et al., 2010). With this scenario in mind, it would be worth dissecting in more detail the systemic allocation and subcellular partitioning of sugars during plant–pathogen interactions. In addition, further investigation of the involvement and underlying mechanisms of GIF1‐associated invertase inhibitors and defence genes in GIF1‐modulated defence activation might provide deeper insights into sugar‐triggered immunity in plants.
Experimental Procedures
Plant materials and growth conditions
The gif1 mutant (in the japonica Zhonghua 11 background), GIF1‐ and OsCIN1‐OE transgenic lines (in the japonica TP309 background) and their respective wild‐type controls were used (Wang E.T. et al., 2008, 2010). Rice plants were grown in a glasshouse or growth chamber with a 12‐h/12‐h day/night cycle at 26–28 °C and 80%–90% humidity.
Identification of postharvest fungal pathogens
The gif1 seeds infected by fungi in the field were harvested and washed with sterile water, and the glumes were then incubated on potato dextrose agar (PDA) at 28 °C to allow fungal growth. Fungal colonies were isolated and incubated on PDA at 28 °C for 5–7 days and maintained at 4 °C. The isolated fungi were observed under a microscope (Olympus SZX7, Tokyo, Japan). The pathogenic fungi Alternaria sp. and Rhizopus sp. were characterized by morphology and re‐inoculation of the gif1 grains in the field during filling.
Pathogen inoculation and disease assay
For Xoo resistance assay, 2‐month‐old rice plants were inoculated with the Xoo virulent races P6 (strain PXO99) and K1 (strain DY89031) by the leaf‐clipping method; the lesion length was measured after 2 weeks, and bacterial growth was also recorded (Wang Y.L. et al., 2008; Yang et al., 2008). For M. oryzae resistance, 2‐week‐old seedlings were spray inoculated with the virulent races ZB1 (strain 2000‐2) and ZB15 (strain 09‐31‐1), and disease severity was evaluated as described by Li et al. (2011). The average infection type was recorded for ZB1 after 48 hpi as described by Nakao et al. (2011). At least 40 plants of each line were evaluated for infection type.
RNA analysis
Total RNA was extracted using TRIzol Reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's instructions. cDNAs were synthesized from 3 μg of total RNAs using oligo(dT) primer and SuperScript III reverse transcriptase (Invitrogen). Quantitative real‐time polymerase chain reaction (qRT‐PCR) analysis was performed on an Eppendorf realplex2 Mastercycler (Eppendorf, Hamburg, Germany) using SYBR Premix Ex Taq (TaKaRa, Tokyo, Japan) and gene‐specific primers (Table S1, see Supporting Information). For pathogen induction of CIN genes, 2‐week‐old seedlings of wild‐type TP309 were inoculated with PXO99. Leaves were harvested at 0, 6, 24, 28 and 72 h after inoculation. Total RNA was prepared using TRIzol Reagent and the first cDNA strand was synthesized with a PrimeScript™ RT Reagent Kit with gDNA Eraser (TaKaRa). Gene‐specific primers (Table S1) were designed and RT‐PCR analysis was performed as described previously (Cho et al., 2005), using PBZ1 as a defence activation marker.
Callose deposition analysis
For the detection of callose deposition, 2‐week‐old seedlings were inoculated with Xoo PXO99 and M. oryzae ZB1 for 24 h, and the aniline blue method was used to detect callose deposition as described previously (Li et al., 2011).
3,3′‐Diaminobenzidine (DAB) and trypan blue staining
For the detection of H2O2 accumulation in rice, the DAB uptake method was adopted as described previously (Chen F. et al., 2010; Li et al., 2011). Two‐week‐old seedlings were inoculated with Xoo PXO99 and M. oryzae ZB1 for 24 h, and HR cell death was visualized by the trypan blue exclusion assay as described previously (Chen F. et al., 2010).
Scanning electron microscopy (SEM) and transmission electron microscopy (TEM) assays
For SEM observation, seed glumes were fixed in formalin–acetic acid–alcohol (FAA) overnight at 4 °C and observed as described previously in Lin et al. (2012). For TEM assay, leaf pieces (0.5–1.0 cm) were vacuum fixed in 2.5% glutaraldehyde in 0.1 m cacodylate buffer (pH 7.4) overnight at 4 °C and visualized as described by Li et al. (2011). In addition, leaf pieces after pathogen infection were fixed as depicted in TEM analysis. Serial 150‐nm‐thick sections were cut using a rotary microtome.
Cell wall composition and sugar content assays
Cell wall composition was analysed according to Zhang et al. (2011). Briefly, 1 g glume and flag leaf (fresh weight) were ground into a powder, washed with 70% ethanol and a mixture of chloroform and methanol, and then overnight in sodium acetate buffer. The released sugar and derivatives were assayed. Sugar contents were determined by gas chromatography‐mass spectrometry (GC‐MS) as described previously (Wang E.T. et al., 2008).
Statistical analyses
All statistical analyses were performed using SPSS (SPSS Inc., Chicago, IL, USA). Data from three independent assays were analysed using analysis of variance (ANOVA) by least‐significant difference test at P ≤ 0.01.
Supporting information
Fig. S1 Resistance of the gif1 mutant and the wild‐type control to Xanthomonas oryzae pv. oryzae (Xoo) and Magnaporthe oryzae.
Fig. S2 Induction of OsCIN genes by Xanthomonas oryzae pv. oryzae (Xoo).
Fig. S3 No difference in Xanthomonas oryzae pv. oryzae (Xoo) resistance between OsCIN1‐overexpressing (OsCIN1‐OE) and wild‐type plants.
Fig. S4 Sugar concentrations in apoplastic fluid of the wild‐type and GIF1‐overexpressing (GIF1‐OE) leaves.
Fig. S5 Callose deposition and callose gene expression during Magnaporthe oryzae infection.
Fig. S6 Callose deposition, H2O2 accumulation and localized cell death during Xanthomonas oryzae pv. oryzae (Xoo) and Magnaporthe oryzae infection in GIF1‐OE7.
Fig. S7 H2O2 accumulation and localized cell death (hypersensitive response, HR) during Magnaporthe oryzae infection.
Table S1 Primers used for quantitative real‐time polymerase chain reaction (qRT‐PCR).
Acknowledgements
We thank Yihua Zhou (Institute of Genetics and Development Biology, Chinese Academy of Sciences) for cell wall composition analysis. This work was supported by a National Key Basic Research and Development Program Grant (2011CB100700) and the National Natural Science Foundation of China (30730064).
References
- Asselbergh, B. , Curvers, K. , Francxa, S.C. , Audenaert, K. , Vuylsteke, M. , Van Breusegem, F. and Höfte, M. (2007) Resistance to Botrytis cinerea in sitiens, an abscisic acid‐deficient tomato mutant, involves timely production of hydrogen peroxide and cell wall modifications in the epidermis. Plant Physiol. 144, 1863–1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger, S. , Sinha, A.K. and Roitsch, T. (2007) Plant physiology meets phytopathology: plant primary metabolism and plant pathogen interactions. J. Exp. Bot. 58, 4019–4026. [DOI] [PubMed] [Google Scholar]
- Boller, T. and He, S.Y. (2009) Innate immunity in plants: an arms race between pattern recognition receptors in plants and effectors in microbial pathogens. Science, 324, 742–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolouri‐Moghaddam, M.R. and Van den Ende, W. (2012) Sugars and plant innate immunity. J. Exp. Bot. 63, 3989–3998. [DOI] [PubMed] [Google Scholar]
- Bolouri‐Moghaddam, M.R. and Van den Ende, W. (2013a) Sugars, the clock and transition to flowering. Front. Plant Sci. 4, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolouri‐Moghaddam, M.R. and Van den Ende, W. (2013b) Sweet immunity in the plant circadian regulatory network. J. Exp. Bot. 64, 1439–1449. [DOI] [PubMed] [Google Scholar]
- Bolouri‐Moghaddam, M.R. , Le Roy, K. , Xiang, L. , Rolland, F. and Van den Ende, W. (2010) Sugar signalling and antioxidant network connections in plant cells. FEBS J. 277, 2022–2037. [DOI] [PubMed] [Google Scholar]
- Bonfig, K.B. , Gabler, A. , Simon, U.K. , Luschin‐Ebengreuth, N. , Hatz, M. , Berger, S. , Muhammad, N. , Zeier, J. , Sinha, A.K. and Roitsch, T. (2010) Post‐translational derepression of invertase activity in source leaves via down‐regulation of invertase inhibitor expression is part of the plant defense response. Mol. Plant, 3, 1037–1048. [DOI] [PubMed] [Google Scholar]
- Chen, F. , Gao, M.J. , Miao, Y.S. , Yuan, Y.X. , Wang, M.Y. , Li, Q. , Mao, B.Z. , Jiang, L.W. and He, Z.H. (2010) Plasma membrane localization and potential endocytosis of constitutively expressed XA21 proteins in transgenic rice. Mol. Plant, 3, 917–926. [DOI] [PubMed] [Google Scholar]
- Chen, L.Q. , Hou, B.H. , Lalonde, S. , Takanaga, H. , Hartung, M.L. , Qu, X.Q. , Guo, W.J. , Kim, J.G. , Underwood, W. , Chaudhuri, B. , Chermak, D. , Antony, G. , White, F.F. , Sommerville, S.C. , Mudgett, M.B. and Frommer, W.B. (2010) Sugar transporters for intercellular exchange and nutrition of pathogens. Nature, 468, 527–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, L.Q. , Qu, X.Q. , Hou, B.H. , Sosso, D. , Osorio, S. , Fernie, A.R. and Frommer, W.B. (2012) Sucrose efflux mediated by SWEET proteins as a key step for phloem transport. Science, 335, 207–211. [DOI] [PubMed] [Google Scholar]
- Chisholm, S.T. , Coaker, G. , Day, B. and Staskawicz, B.J. (2006) Host–microbe interactions: shaping the evolution of the plant immune response. Cell, 124, 803–814. [DOI] [PubMed] [Google Scholar]
- Cho, J.I. , Lee, S.K. , Ko, S. , Kim, H.K. , Jun, S.H. , Lee, Y.H. , Bhoo, S.H. , Lee, K.W. , An, G. , Hahn, T.R. and Jeon, J.S. (2005) Molecular cloning and expression analysis of the cell‐wall invertase gene family in rice (Oryza sativa L.). Plant Cell Rep. 24, 225–236. [DOI] [PubMed] [Google Scholar]
- Chou, H.M. , Bundock, N. , Rolfe, S.A. and Scholes, J.D. (2000) Infection of Arabidopsis thaliana leaves with Albugo candida (white blister rust) causes a reprogramming of host metabolism. Mol. Plant Pathol. 1, 99–113. [DOI] [PubMed] [Google Scholar]
- Chu, Z. , Yuan, M. , Yao, J. , Ge, X. , Yuan, B. , Xu, C. , Li, X. , Fu, B. , Li, Z. , Bennetzen, J.L. , Zhang, Q. and Wang, S.P. (2006) Promoter mutations of an essential gene for pollen development result in disease resistance in rice. Genes Dev. 20, 1250–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couée, I. , Sulmon, C. , Gouesbet, G. and El Amrani, A. (2006) Involvement of soluble sugars in reactive oxygen species balance and responses to oxidative stress. J. Exp. Bot. 57, 449–459. [DOI] [PubMed] [Google Scholar]
- Delgado‐Cerezo, M. , Sánchez‐Rodrígueza, C. , Escuderoa, V. , Miedesa, E. , Fernándezc, P.V. , Jordáa, L. , Hernández‐Blancoa, C. , Sánchez‐Valleta, A. , Bednareke, P. , Schulze‐Leferte, P. , Somervilleg, S. , Estevezc, J.M. , Perssonb, S. and Molinaa, A. (2012) Arabidopsis heterotrimeric G‐protein regulates cell wall defense and resistance to necrotrophic fungi. Mol. Plant, 5, 98–114. [DOI] [PubMed] [Google Scholar]
- Djonović, S. , Urbach, J.M. , Drenkard, E. , Bush, J. , Feinbaum, R. , Ausubel, J.L. , Traficante, D. , Risech, M. , Kocks, C. , Fischbach, M.A. , Priebe, G.P. and Ausubel, F.M. (2013) Trehalose biosynthesis promotes Pseudomonas aeruginosa pathogenicity in plants. PLoS Pathog. 9, e1003217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodds, P.N. and Rathjen, J.P. (2010) Plant immunity: towards an integrated view of plant–pathogen interactions. Nat. Rev. Genet. 11, 539–548. [DOI] [PubMed] [Google Scholar]
- Ellis, C. and Turner, J.G. (2001) The Arabidopsis mutant cev1 has constitutively active jasmonate and ethylene signal pathways and enhanced resistance to pathogens. Plant Cell, 13, 1025–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essmann, J. , Schmitz‐Thom, I. , Schön, H. , Sonnewald, S. , Weis, E. and Scharte, J. (2008) RNA interference‐mediated repression of cell wall invertase impairs defense in source leaves of tobacco. Plant Physiol. 147, 1288–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fotopoulos, V. , Gilbert, M.J. , Pittman, J.K. , Marvier, A.C. , Buchanon, A.J. , Sauer, N. , Hall, J.L. and Williams, L.E. (2003) The monosaccharide transporter gene, AtSTP4, and the cell wall invertase, AtBfruct1, are induced in Arabidopsis during infection with the fungal biotroph Erysiphe cichoracearum . Plant Physiol. 132, 821–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez‐Ariza, J. , Campo, S. , Rufat, M. , Estopà, M. , Messeguer, J. , San Segundo, B. and Coca, M. (2007) Sucrose‐mediated priming of plant defense responses and broad‐spectrum disease resistance by overexpression of the maize pathogenesis‐related PRms protein in rice plants. Mol. Plant–Microbe Interact. 20, 832–842. [DOI] [PubMed] [Google Scholar]
- Hao, P.Y. , Liu, C.X. , Wang, Y.Y. , Chen, R.Z. , Tang, M. , Du, B. , Zhu, L.L. and Hong, G.C. (2008) Herbivore‐induced callose deposition on the sieve plates of rice: an important mechanism for host resistance. Plant Physiol. 146, 1810–1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hématy, K. , Cherk, C. and Somerville, S. (2009) Host–pathogen warfare at the plant cell wall. Curr. Opin. Plant Biol. 12, 406–413. [DOI] [PubMed] [Google Scholar]
- Herbers, K. , Meuwly, P. , Frommer, W.B. , Metraux, J.P. and Sonnewald, U. (1996) Systemic acquired resistance mediated by the ectopic expression of invertase: possible hexose sensing in the secretory pathway. Plant Cell, 8, 793–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández‐Blanco, C. , Feng, D.X. , Hu, J. , Sánchez‐Vallet, A. , Deslandes, L. , Llorente, F. , Berrocal‐Lobo, M. , Keller, H. , Barlet, X. , Sánchez‐Rodríguez, C. , Anderson, L.K. , Somerville, S. , Marco, Y. and Molina, A. (2007) Impairment of cellulose synthases required for Arabidopsis secondary cell wall formation enhances disease resistance. Plant Cell, 19, 890–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hückelhoven, R. (2007) Cell wall‐associated mechanisms of disease resistance and susceptibility. Annu. Rev. Phytopathol. 45, 101–127. [DOI] [PubMed] [Google Scholar]
- Jones, J.D. and Dangl, J.L. (2006) The plant immune system. Nature, 444, 323–329. [DOI] [PubMed] [Google Scholar]
- Kachroo, A. , He, Z. , Patkar, R. , Zhu, Q. , Zhong, J. , Li, D. , Ronald, R. , Lamb, C. and Chattoo, B.B. (2003) Induction of H2O2 in transgenic rice leads to cell death and enhanced resistance to both bacterial and fungal pathogens. Transgenic Res. 12, 577–586. [DOI] [PubMed] [Google Scholar]
- Kano, A. , Gomi, K. , Yamasaki‐Kokudo, Y. , Satoh, M. , Fukumoto, T. , Ohtani, K. , Tajima, S. , Izumori, K. , Tanaka, K. , Ishi, Y. , Tada, Y. , Nishizawa, Y. and Akimitsu, K. (2010) A rare sugar, d‐allose, confers resistance to rice bacterial blight with upregulation of defense‐related genes in Oryza sativa . Phytopathology, 100, 85–90. [DOI] [PubMed] [Google Scholar]
- Kocal, N. , Sonnewald, U. and Sonnewald, S. (2008) Cell wall‐bound invertase limits sucrose export and is involved in symptom development and inhibition of photosynthesis during compatible interaction between tomato and Xanthomonas campestris pv. vesicatoria . Plant Physiol. 148, 1523–1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Roy, K. , Lammens, W. , Verhaest, M. , De Coninck, B. , Rabijns, A. , Van Laere, A. and Van den Ende, W. (2007) Unraveling the difference between invertases and fructan exohydrolases: a single amino acid (Asp‐239) substitution transforms Arabidopsis cell wall invertase1 into a fructan 1‐exohydrolase. Plant Physiol. 145, 616–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Roy, K. , Vergauwen, R. , Struyf, T. , Yuan, S. , Lammens, W. , Mátrai, J. , De Maeyer, M. and Van den Ende, W. (2013) Understanding the role of defective invertases in plants: tobacco Nin88 fails to degrade sucrose. Plant Physiol. 161, 1670–1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, W. , Zhong, S.H. , Li, G.J. , Li, Q. , Mao, B.Z. , Deng, Y.W. , Zhang, H.J. , Zeng, L.J. , Song, F.M. and He, Z.H. (2011) Rice RING protein OsBBI1 with E3 ligase activity confers broad‐spectrum resistance against Magnaporthe oryzae by modifying the cell wall defence. Cell Res. 21, 835–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, L. , Zhong, S.H. , Cui, X.F. , Li, J.M. and He, Z.H. (2012) Characterization of temperature‐sensitive mutants reveals a role for receptor‐like kinase SCRAMBLED/STRUBBELIG in coordinating cell proliferation and differentiation during Arabidopsis leaf development. Plant J. 72, 707–720. [DOI] [PubMed] [Google Scholar]
- Lionetti, V. , Raiola, A. , Camardella, L. , Giovane, A. , Obel, N. , Pauly, M. , Favaron, F. , Cervone, F. and Bellincampi, D. (2007) Overexpression of pectin methylesterase inhibitors in Arabidopsis restricts fungal infection by Botrytis cinerea . Plant Physiol. 143, 1871–1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakao, M. , Nakamura, R. , Kita, K. , Inukai, R. and Ishikawa, A. (2011) Non‐host resistance to penetration and hyphal growth of Magnaporthe oryzae in Arabidopsis . Sci. Rep. 1, 171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanasamy, P. (2006) Post Harvest Pathogens and Disease Management. Hoboken, NJ: John Wiley and Sons, Inc. [Google Scholar]
- Ning, J. , Li, X.H. , Hicks, L.M. and Xiong, L.Z. (2010) A Raf‐like MAPKKK gene DSM1 mediates drought resistance through reactive oxygen species scavenging in rice. Plant Physiol. 152, 876–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramírez, V. , Agorio, A. , Coego, A. , García‐Andrade, J. , Hernández, M.J. , Balaguer, B. , Ouwerkerk, P.B. , Zarra, I. and Vera, P. (2011) MYB46 modulates disease susceptibility to Botrytis cinerea in Arabidopsis . Plant Physiol. 155, 1920–1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roitsch, T. and González, M.C. (2004) Function and regulation of plant invertases: sweet sensations. Trends Plant Sci. 9, 606–613. [DOI] [PubMed] [Google Scholar]
- Rolland, F. , Baena‐Gonzalez, E. and Sheen, J. (2006) Sugar sensing and signaling in plants: conserved and novel mechanisms. Annu. Rev. Plant Biol. 57, 675–709. [DOI] [PubMed] [Google Scholar]
- von Schaewen, A. , Stitt, M. , Schmidt, R. , Sonnewald, U. and Willmitzer, L. (1990) Expression of a yeast‐derived invertase in the cell wall of tobacco and Arabidopsis plants leads to accumulation of carbohydrate and inhibition of photosynthesis and strongly influences growth and phenotype of transgenic tobacco plants. EMBO J. 9, 3033–3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez‐Rodríguez, C. , Estévez, J.M. , Llorente, F. , Hernández‐Blanco, C. , Jordá, L. , Pagán, I. , Berrocal, M. , Marco, Y , Somerville, S. and Molina, A. (2009) The ERECTA receptor‐like kinase regulates cell wall‐mediated resistance to pathogens in Arabidopsis thaliana . Mol. Plant–Microbe Interact. 22, 953–963. [DOI] [PubMed] [Google Scholar]
- Scharte, J. , Schön, H. and Weis, E. (2005) Photosynthesis and carbohydrate metabolism in tobacco leaves during an incompatible interaction with Phytophthora nicotianae . Plant Cell Environ. 28, 1421–1435. [Google Scholar]
- Schulze‐Lefert, P. (2004) Knocking on the heaven's wall: pathogenesis of and resistance to biotrophic fungi at the cell wall. Curr. Opin. Plant Biol. 7, 377–383. [DOI] [PubMed] [Google Scholar]
- Seo, Y.S. , Cho, J.I. , Lee, S.K. , Ryu, H.S. , Han, M. , Hahn, T.R. , Sonnewald, U. and Jeon, J.S. (2007) Current insights into the primary carbon flux that occurs in plants undergoing a defense response. Plant Stress, 1, 42–49. [Google Scholar]
- Smeekens, S. (2000) Sugar‐induced signal transduction in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 51, 49–81. [DOI] [PubMed] [Google Scholar]
- Sonnewald, S. , Priller, J.P. , Schuster, J. , Glickmann, E. , Hajirezaei, M.R. , Siebig, S. , Mudgett, M.B. and Sonnewald, U. (2012) Regulation of cell wall‐bound invertase in pepper leaves by Xanthomonas campestris pv. Vesicatoria type three effectors. PLoS ONE, 7, e51763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton, P.N. , Gilbert, M.J. , Williams, L.E. and Hall, J.L. (2007) Powdery mildew infection of wheat leaves changes host solute transport and invertase activity. Plant Physiol. 129, 787–795. [Google Scholar]
- Van den Ende, W. , Lammens, W. , Van Laere, A. , Schroeven, L. and Le Roy, K. (2009) Donor and acceptor substrate selectivity among plant glycoside hydrolase family 32 enzymes. FEBS J. 276, 5788–5798. [DOI] [PubMed] [Google Scholar]
- Vogel, J.P. , Raab, T.K. , Schiff, C. and Somerville, S.C. (2002) PMR6, a pectate lyase‐like gene required for powdery mildew susceptibility in Arabidopsis . Plant Cell, 14, 2095–2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel, J.P. , Raab, T.K. , Somerville, C.R. and Somerville, S.C. (2004) Mutations in PMR5 result in powdery mildew resistance and altered cell wall composition. Plant J. 40, 968–978. [DOI] [PubMed] [Google Scholar]
- Vorwerk, S. , Somerville, S. and Somerville, C. (2004) The role of plant cell wall polysaccharide composition in disease resistance. Trends Plant Sci. 9, 203–209. [DOI] [PubMed] [Google Scholar]
- Wang, E.T. , Wang, J.J. , Zhu, X.D. , Hao, W. , Wang, L.Y. , Li, Q. , Zhang, L.X. , He, W. , Lu, B.R. , Ling, H.X. , Ma, H. , Zhang, G.Q. and He, Z.H. (2008) Control of rice grain‐filling and yield by a gene with potential signature of domestication. Nat. Genet. 40, 1270–1274. [DOI] [PubMed] [Google Scholar]
- Wang, E.T. , Xu, X. , Zhang, L. , Zhang, H. , Lin, L. , Wang, Q. , Li, Q. , Ge, S. , Lu, B.R. , Wang, W. and He, Z.H. (2010) Duplication and independent selection of cell‐wall invertase genes GIF1 and OsCIN1 during rice evolution and domestication. BMC Evol. Biol. 10, 108–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Y.L. , Gao, M.J. , Li, Q. , Wang, L.Y. , Wang, J.J. , Jeon, J.S. , Qu, N. , Zhang, Y.L. and He, Z.H. (2008) OsRAR1 and OsSGT1 physically interact and function in rice basal disease resistance. Mol. Plant–Microbe Interact. 21, 294–303. [DOI] [PubMed] [Google Scholar]
- Wright, D.P. , Baldwin, B.C. , Shephard, M.C. and Scholes, J.D. (1995) Source–sink relationships in wheat leaves infected with powdery mildew. I: alterations in carbohydrate metabolism. Physiol. Mol. Plant Pathol. 47, 237–253. [Google Scholar]
- Xiang, L. , Le Roy, K. , Bolouri‐Moghaddam, M.R. , Vanhaecke, M. , Lammens, W. , Rolland, F. and Van den Ende, W. (2011) Exploring the neutral invertase–oxidative stress defence connection in Arabidopsis thaliana . J. Exp. Bot. 62, 3849–3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, B. , Sugio, A. and White, F.F. (2006) Os8N3 is a host disease‐susceptibility gene for bacterial blight of rice. Proc. Natl. Acad. Sci. USA, 103, 10 503–10 508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, D.L. , Li, Q. , Deng, Y.W. , Lou, Y.G. , Wang, M.Y. , Zhou, G.X. , Zhang, Y.Y. and He, Z.H. (2008) Altered disease development in the eui mutants and Eui overexpressors indicates that gibberellins negatively regulate rice basal disease resistance. Mol. Plant 1, 528–537. [DOI] [PubMed] [Google Scholar]
- Zhang, S.J. , Song, X.Q. , Yu, B.S. , Zhang, B.C. , Sun, C.Q. , Knox, J.P. and Zhou, Y.H. (2011) Identification of quantitative trait loci affecting hemicellulose characteristics based on cell wall composition in a wild and cultivated rice species. Mol. Plant 5, 162–175. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 Resistance of the gif1 mutant and the wild‐type control to Xanthomonas oryzae pv. oryzae (Xoo) and Magnaporthe oryzae.
Fig. S2 Induction of OsCIN genes by Xanthomonas oryzae pv. oryzae (Xoo).
Fig. S3 No difference in Xanthomonas oryzae pv. oryzae (Xoo) resistance between OsCIN1‐overexpressing (OsCIN1‐OE) and wild‐type plants.
Fig. S4 Sugar concentrations in apoplastic fluid of the wild‐type and GIF1‐overexpressing (GIF1‐OE) leaves.
Fig. S5 Callose deposition and callose gene expression during Magnaporthe oryzae infection.
Fig. S6 Callose deposition, H2O2 accumulation and localized cell death during Xanthomonas oryzae pv. oryzae (Xoo) and Magnaporthe oryzae infection in GIF1‐OE7.
Fig. S7 H2O2 accumulation and localized cell death (hypersensitive response, HR) during Magnaporthe oryzae infection.
Table S1 Primers used for quantitative real‐time polymerase chain reaction (qRT‐PCR).


