Summary
The protein–protein interaction between VPg (viral protein genome‐linked) of potyviruses and eIF4E (eukaryotic initiation factor 4E) or eIF(iso)4E of their host plants is a critical step in determining viral virulence. In this study, we evaluated the approach of engineering broad‐spectrum resistance in Chinese cabbage (Brassica rapa) to Turnip mosaic virus (TuMV), which is one of the most important potyviruses, by a systematic knowledge‐based approach to interrupt the interaction between TuMV VPg and B. rapa eIF(iso)4E. The seven amino acids in the cap‐binding pocket of eIF(iso)4E were selected on the basis of other previous results and comparison of protein models of cap‐binding pockets, and mutated. Yeast two‐hybrid assay and co‐immunoprecipitation analysis demonstrated that W95L, K150L and W95L/K150E amino acid mutations of B. rapa eIF(iso)4E interrupted its interaction with TuMV VPg. All eIF(iso)4E mutants were able to complement an eIF4E‐knockout yeast strain, indicating that the mutated eIF(iso)4E proteins retained their function as a translational initiation factor. To determine whether these mutations could confer resistance, eIF(iso)4E W95L, W95L/K150E and eIF(iso)4E wild‐type were over‐expressed in a susceptible Chinese cabbage cultivar. Evaluation of the TuMV resistance of T 1 and T 2 transformants demonstrated that the over‐expression of the eIF(iso)4E mutant forms can confer resistance to multiple TuMV strains. These data demonstrate the utility of knowledge‐based approaches for the engineering of broad‐spectrum resistance in Chinese cabbage.
Keywords: broad‐spectrum resistance, Chinese cabbage, eIF(iso)4E, plant transformation, potyvirus, resistance breeding, TuMV
Introduction
Plant viruses are among the most destructive pathogens for many agricultural crops, causing considerable yield losses throughout the world. More than 700 plant viruses have been reported to cause devastating diseases (Strange and Scott, 2005). Turnip mosaic virus (TuMV), a member of the Potyvirus genus, is one of the three major viruses found in Chinese cabbage (Brassica rapa), which is commonly cultivated in Asia, Europe and the USA. TuMV infects a wide range of cultivated plant species and causes significant economic losses in Brassica crops (Edwardson and Christie, 1991, Shattuck, 1992). The development of resistant plant cultivars by breeding methods is one of the most widely used approaches for the control of viral diseases, and most of the TuMV resistance genes known in Brassica crops are single dominant genes. These dominant resistance genes confer narrow‐spectrum resistance and can easily be overcome by new virus strains (Rusholme et al., 2007). For this reason, additional resources that can provide stable and broad‐spectrum resistance are needed in Chinese cabbage.
Several instances of resistance to potyviruses conferred by mutations in host factors have been reported (Lellis et al., 2002; Nicaise et al., 2003; Ruffel et al., 2002). Plant eukaryotic initiation factor 4E (eIF4E) family members are well‐known host factors that play a critical role in the infection of several potyviruses, and recessive resistance genes caused by mutations in this gene family have been deployed in breeding (Robaglia and Caranta, 2006; Wang and Krishnaswamy, 2012). The primary role of eIF4E is the control of the expression of proteins in the eukaryotic cell by binding 5′ cap (m7G(5′)ppp(5′)N) and other translation factors (Rhoads, 2009). This eIF4E‐mediated resistance often confers strong and broad‐spectrum resistance (Mazier et al., 2011; Piron et al., 2010; Provvidenti and Hampton, 1992; Rodríguez‐Hernández et al., 2012; Yeam et al., 2007). It has been suggested that interactions of eIF4E with the viral protein linked to the genome (VPg) are particularly important in viral RNA translation and replication (Beauchemin et al., 2007; Cotton et al., 2009; Grzela et al., 2006; Léonard et al., 2000; Miyoshi et al., 2006; Robaglia and Caranta, 2006; Wittmann et al., 1997). Substitutions of amino acids in eIF4E can affect the VPg–eIF4E interaction directly (Gao et al., 2004b; Kang et al., 2005b; Nicaise et al., 2003; Ruffel et al., 2002, 2005; Yeam et al., 2007), and it can be inferred that abolition of the VPg–eIF4E interaction is a key step in eIF4E‐mediated resistance.
Although eIF4E‐mediated resistance is a good resource for resistance breeding, the search for novel eIF4E alleles conferring virus resistance is time‐consuming and laborious. However, modern molecular biological tools can accelerate the discovery or engineering of novel alleles. TILLING (Targeting Induced Local Lesions in Genomes) or EcoTILLING has been used for high‐throughput screening of eIF4E mutants from germplasms or mutant populations (Ibiza et al., 2010; Jeong et al., 2012; Nieto et al., 2007; Piron et al., 2010). In addition, transgenic plants have been developed to silence host factors or over‐express mutated versions, and several studies have shown that virus resistance can be induced via the over‐expression of mutated eIF4E genes (Cavatorta et al., 2011; Duan et al., 2012; Kang et al., 2007; Yeam et al., 2007). Arabidopsis (Arabidopsis thaliana) eIF(iso)4E is a key host factor in TuMV resistance (Beauchemin et al., 2007; Duprat et al., 2002; Lellis et al., 2002; Léonard et al., 2000; Miyoshi et al., 2008). Consistent with this, the Brassica eIF(iso)4E gene has been shown to be strongly linked to the Brassica recessive resistance genes retr02 and trs (Kim et al., 2013; Qian et al., 2013). These results suggest that the eIF(iso)4E gene is an appropriate target for the engineering of TuMV resistance in Brassica species.
In this study, we describe a systematic knowledge‐based approach to engineer broad‐spectrum TuMV resistance in Chinese cabbage. Key amino acids in the cap‐binding pocket of eIF(iso)4E were chosen on the basis of previous research and a protein model, and these amino acids were mutated by site‐directed mutagenesis. The critical amino acids for interaction with TuMV VPg were identified through protein–protein interaction tests. Finally, the eIF(iso)4E genes harbouring critical mutations for VPg interaction were over‐expressed in a susceptible cultivar to induce resistance to TuMV.
Results
Site‐directed mutagenesis of cap‐binding pocket amino acids in eIF(iso)4E of Chinese cabbage
It was expected that key amino acid residues in the cap‐binding pocket of A. thaliana eIF(iso)4E would be conserved in Brassicaceae crops. To test this hypothesis, the eIF(iso)4E amino acid sequences of A. thaliana and B. rapa were aligned and their three‐dimensional protein structures were predicted using wheat (Triticum aestivum) eIF4E as a template (Protein Data Bank accession no. 2IDR). The predicted structures of the eIF(iso)4E proteins were quite similar (Fig. 1a) and the eIF(iso)4E amino acid sequence was highly conserved between these two Brassicaceae species (Fig. 1b). Based on a previous study (Miyoshi et al., 2006), seven candidate amino acids [tryptophan (Trp)49, Trp95, lysine (Lys)105, Lys150, threonine (Thr)107, serine (Ser)192 and aspartic acid (Asp)191] in the cap‐binding pocket were chosen (Fig. 1a,b). To determine their effects on the eIF(iso)4E–VPg interaction, these candidate amino acids were mutated to leucine by polymerase chain reaction (PCR)‐based, site‐directed mutagenesis. Seven single amino acid mutated eIF(iso)4Es (W49L, W95L, K105L, K150L, T107L, S192L and D191L) and one double‐mutated eIF(iso)4E (W95L/K150E) were prepared for further analysis.
Figure 1.
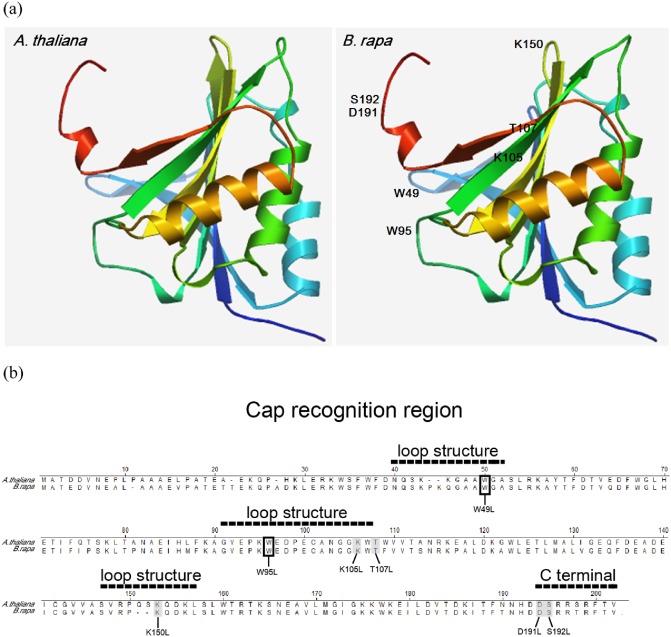
Positions of the mutated amino acids in the cap‐binding pocket of eIF(iso)4E [eukaryotic initiation factor(iso)4E]. (a) Comparison of predicted three‐dimensional structural models of Arabidopsis thaliana eIF(iso)4E and Brassica rapa eIF(iso)4E. The structures are shown in a ribbon representation. Amino acids of B. rapa eIF(iso)4E mutated in this work are labelled. The X‐ray crystal structure of wheat eIF4E (Protein Data Bank accession no. 2IDR) was used as the template structure. (b) Alignment of A. thaliana and B. rapa eIF(iso)4E amino acid sequences. Seven candidate cap‐binding pocket amino acids (Trp49, Trp95, Lys105, Thr107, Lys150, Asp191 and Ser192) from B. rapa that were mutated are indicated with grey boxes in the sequence alignment.
Interaction of eIF(iso)4E and TuMV VPg
To analyse the effect of each amino acid mutation, a yeast two‐hybrid system was used to assess the eIF(iso)4E–VPg interaction. Seven eIF(iso)4E mutants were transformed into yeast strain EGY48. The wild‐type (WT) eIF(iso)4E from B. rapa ‘Samjin’ was transformed into the yeast as a positive control, and empty vectors were transformed as negative controls. The eIF(iso)4E mutants that had single mutations in W49, W95, K150 or S192 showed weak interactions with VPg (Fig. 2a). However, the interaction with VPg of the K105L, T107L and D191L eIF(iso)4E single mutants remained strong (Fig. 2a). Quantitative β‐galactosidase assays [chlorophenolred‐β‐d‐galactopyranoside (CPRG) liquid assay] also showed the same results. The β‐galactosidase activity of the eIF(iso)4E W95L mutant was similar to that of the negative control (Fig. 2b). The W49L and K150L mutants also showed very low β‐galactosidase activity, although these activities were slightly higher than that of the negative control (Fig. 2b). The β‐galactosidase activities of the K105L, T107L and D191L eIF(iso)4E mutants were even higher than that of the WT positive control (Fig. 2b). The double amino acid mutant (W95L/K150E) of eIF(iso)4E was prepared to test whether these mutations could abolish the interaction with VPg. Qualitative and quantitative β‐galactosidase assays showed that the double‐mutated eIF(iso)4E W95L/K150E exhibited remarkably reduced interaction with TuMV VPg compared with that of the eIF(iso)4E WT (Fig. 2a,b). However, its β‐galactosidase activity was similar to that of the K150L single mutant. These results indicate that four amino acids, W49, W95, K150 and S192, in the cap‐binding pocket of B. rapa eIF(iso)4E are critical for interaction with TuMV VPg. Among the eIF(iso)4E mutants, the β‐galactosidase activities of W95L and K150L were the lowest. For this reason, these mutants were used for further studies in planta.
Figure 2.
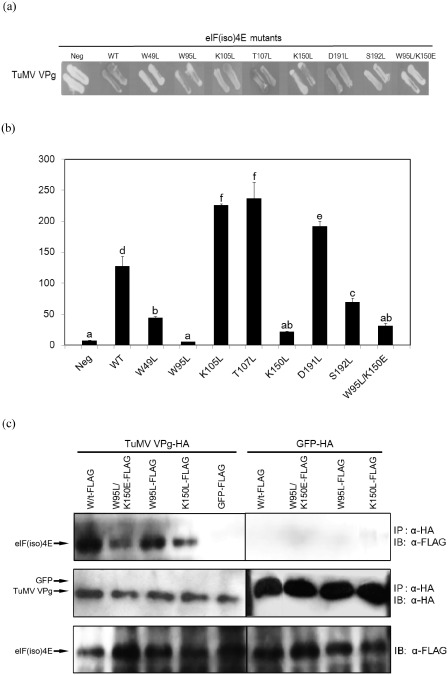
Interaction between Turnip mosaic virus (TuMV) VPg (viral protein genome‐linked) and Chinese cabbage eIF(iso)4E [eukaryotic initiation factor(iso)4E]. Bait plasmid pEG202 was used to express a TuMV VPg fusion protein, and prey plasmid pJG4‐5 was used to express wild‐type and mutated eIF(iso)4E proteins. (a) Yeast transformants were streaked on minimal medium agar plates containing 40 μg/mL X‐gal (5‐bromo‐4‐chloro‐3‐indolyl‐β‐d‐galactopyranoside) to assay the expression of the lacZ reporter gene, indicated by the development of a blue colour. (b) Quantitative liquid culture assay using chlorophenolred‐β‐d‐galactopyranoside (CPRG) as substrate was employed to calculate β‐galactosidase activity. One unit of β‐galactosidase equals the amount that hydrolyses 1 μmol of CPRG to chlorophenol red and d‐galactose per minute per cell. Neg, negative control; WT, susceptible WT Samjin eIF(iso)4E. Different letters denote statistically significantly different values [analysis of variance (ANOVA), P ≥ 0.05]. (c) Immunoblot of co‐immunoprecipitation assays between eIF(iso)4E (WT and mutants) and TuMV VPg. Immunoprecipitation (IP) was carried out with anti‐haemagglutinin (HA) agarose beads, and immunoblotting (IB) was performed with the indicated antibodies. Green fluorescent proteins (GFPs) (HA‐GFP and FLAG‐GFP) were used as negative controls.
In vivo interaction analysis of eIF(iso)4E and TuMV VPg
To confirm the yeast two‐hybrid results, co‐immunoprecipitation (co‐IP) was performed (Fig. 2c). Haemagglutinin (HA)‐tagged TuMV VPg was co‐expressed with FLAG‐tagged eIF(iso)4E proteins in Nicotiana benthamiana. Similar levels of expression of TuMV VPg were confirmed among all samples after immunoprecipitation (Fig. 2c). All of the FLAG‐tagged eIF(iso)4E proteins were co‐immunoprecipitated with HA‐TuMV VPg, although there were differences in the degree of interaction. According to the result, there was a strong interaction between WT eIF(iso)4E and VPg, whereas W95L/K150E and K150L mutants showed significantly reduced interaction with TuMV VPg (Fig. 2c). The interaction of the W95L mutant with VPg was also slightly reduced compared with the WT form, but not as much as that of the other two mutants. None of the FLAG‐tagged eIF(iso)4E proteins co‐immunoprecipitated with the negative control, green fluorescent protein (GFP)‐HA (Fig. 2c). Consistently, these co‐IP results also indicate in vivo interaction between the WT Brassica eIF(iso)4E protein and TuMV VPg, and that the mutations in K150 or W95 residues of eIF(iso)4E interrupt this interaction.
A bimolecular fluorescence complementation (BiFC) assay was also carried out to investigate the interaction between the eIF(iso)4E mutants and TuMV VPg in living plant cells. Arabidopsis bZIP protein, a transcription factor known to form homodimers and heterodimers via its C‐terminal leucine zipper domain (Siberil et al., 2001), was used as a positive control (Walter et al., 2004), and GFP was used as a negative control. TuMV VPg was fused to the N‐terminal fragment of yellow fluorescent protein (YFP) (TuMV VPg‐NE) and co‐expressed with a fusion of eIF(iso)4E to the C‐terminal fragment of YFP (eIF(iso)4E‐CE). The interaction between WT eIF(iso)4E and TuMV VPg was confirmed by the presence of YFP fluorescence throughout the cytoplasm and the nucleus (Fig. S1, see Supporting Information). There were significant signal reductions with the K150L and W95L/K150E mutants compared with the WT, but the W95L mutant signals were similar to those of the WT (Fig. S1). Thus, although the interaction signals were weak, the same trends of eIF(iso)4E–TuMV VPg interaction were observed as in the co‐IP assays (Figs S1 and 2c).
Yeast complementation assay
The candidate amino acids have different physical and chemical features from leucine (or glutamic acid) with which they were substituted, and we wondered whether the changes in these properties might affect the cap‐binding ability of the eIF(iso)4E mutants. Accordingly, a yeast complementation assay was performed to test the consequences of amino acid substitutions on yeast survival. Eight eIF(iso)4E mutants were introduced to the yeast strain JO55, in which the eIF4E gene is deleted. The WT eIF(iso)4E successfully complemented the loss of eIF4E in the yeast (Fig. 3). Furthermore, there were no differences in yeast growth among the eight mutants and the WT form (Fig. 3). These results indicate that none of the eIF(iso)4E mutants lost their cap‐binding ability in yeast, and retained their function as translation initiation factors.
Figure 3.

Complementation of yeast strain JO55 with eIF(iso)4E cDNAs. The yeast strain JO55 was transformed with plasmids encoding wild‐type (WT) or mutant eIF(iso)4E [eukaryotic initiation factor(iso)4E]. After transformation, yeast cells were grown in synthetic minimal medium containing 2% glucose. Each yeast clone was serially diluted, and growth on galactose‐ and glucose‐containing media was assessed. Yeasts were incubated at 30 °C for 5 days. WT, susceptible WT Samjin eIF(iso)4E; Gal/Raf, galactose/raffinose; Glu, glucose.
Development and analysis of transgenic Chinese cabbage over‐expressing mutated eIF(iso)4E genes
To test the hypothesis that eIF(iso)4E mutations which abolish the physical interaction with VPg could induce resistance in susceptible Chinese cabbage, WT, W95L and W95L/K150E versions of eIF(iso)4E proteins were over‐expressed in the susceptible Chinese cabbage cultivar ‘Seoul’. Our attempts to use K150L for transformation were unsuccessful, and so we could not analyse the effects of this mutation alone. Successful transgene insertion in the T1 transformants was assessed via PCR for the hygromycin phosphotransferase (HPT) gene and the eIF(iso)4E transgenes (Fig. 4a). The amplified eIF(iso)4E transgenes were then re‐sequenced (data not shown). T1 progenies that had a single copy of the transgene were selected for further assays (Table S1, see Supporting Information). None of the T1 transformants showed developmental defects compared with the untransformed plants.
Figure 4.
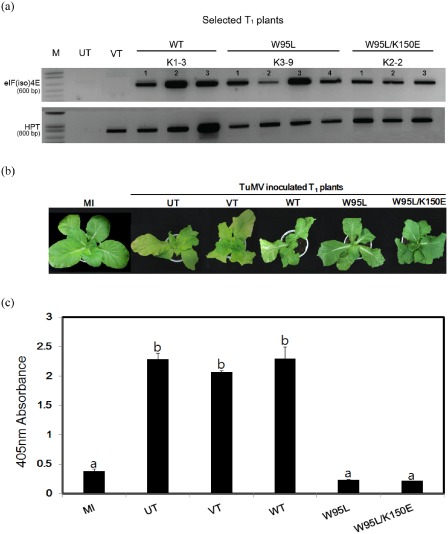
Turnip mosaic virus (TuMV) screening of T 1 transgenic Chinese cabbage over‐expressing mutated eIF(iso)4E [eukaryotic initiation factor(iso)4E] genes. (a) Polymerase chain reaction (PCR) analysis to confirm the presence of the eIF(iso)4E transgene and hygromycin phosphotransferase II (HPT II) using genomic DNA of T 1 plants that have a single copy of the transgene. M, DNA marker; UT, untransformed control; VT, vector‐transformed control; WT, susceptible wild‐type Samjin eIF(iso)4E transformant; W95L, eIF(iso)4E W95L mutant transformant; W95L/K150E, eIF(iso)4E W95L/K150E transformant. Transgenic T 1 plants selected on the basis of hygromycin (15 mg/mL) resistance were tested. (b) Phenotypes of TuMV CHN5‐inoculated eIF(iso)4E T 1 plants. The plants were photographed at 35 days post‐inoculation (dpi). (c) Enzyme‐linked immunosorbent analysis (ELISA) screening after TuMV inoculation. Double antibody sandwich (DAS)‐ELISA was performed at 35 dpi to assay virus accumulation using the fifth and sixth true leaves. MI, mock‐inoculated control; UT, untransformed ‘Seoul’ wild‐type control; VT, vector‐transformed control. Error bars represent standard deviation. Different letters denote statistically significantly different values [analysis of variance (ANOVA), P ≥ 0.05].
To test whether the over‐expressed eIF(iso)4E proteins affected the disease resistance of Chinese cabbage, T1 progenies were inoculated with TuMV CHN5. Typical TuMV symptoms were observed at 20 days post‐inoculation (dpi) in untransformed plants, vector‐transformed plants and plants over‐expressing eIF(iso)4E WT. At about 35 dpi, the symptoms on the WT eIF(iso)4E line became more severe, whereas the plants over‐expressing W95L or W95L/K150E did not show distinct symptoms (Fig. 4b). Double antibody sandwich enzyme‐linked immunosorbent analysis (DAS‐ELISA) confirmed the visual observations; virus particles accumulated in the transformants over‐expressing WT eIF(iso)4E, whereas there were significantly lower levels in W95L and W95L/K150E over‐expressing plants (Fig. 4c). These data demonstrate that the over‐expression of mutated eIF(iso)4E can induce TuMV resistance in susceptible Chinese cabbage.
T2 generation plants were obtained from the selected T1 plants by self‐pollination and accessed via PCR and hygromycin selection to confirm the presence of the transgene. Expression of the transgenic and endogenous eIF(iso)4E was tested by semi‐quantitative reverse transcription‐polymerase chain reaction (RT‐PCR) (Fig. 5a) and quantitative real‐time RT‐PCR (Fig. 5b). The expression of eIF(iso)4E in the transgenic plants was greatly enhanced relative to that in the untransformed control (Fig. 5a,b). By contrast, there was almost no difference in the expression of endogenous eIF(iso)4E among untransformed control and transgenic plants (Fig. 5b, left panel). The expression of endogenous eIF4E was also screened. Unexpectedly, eIF4E was also increased in the transgenic plants relative to that in the untransformed control (Fig. 5b, right panel). This result was similar to that of the eIF(iso)4E transgene (Fig. 5b, middle panel). This result indicates that the eIF(iso)4E transformation was successful, but may have affected endogenous eIF4E expression.
Figure 5.
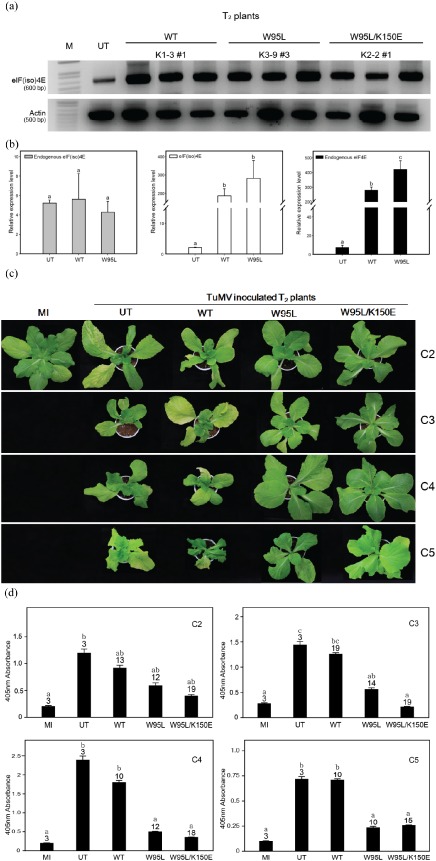
Turnip mosaic virus (TuMV) screening of T 2 transgenic Chinese cabbage over‐expressing eIF(iso)4E [eukaryotic initiation factor(iso)4E] mutants. (a) Reverse transcription‐polymerase chain reaction (RT‐PCR) analysis to test the expression of the eIF(iso)4E transgenes. M, DNA marker; UT, untransformed control; WT, Samjin eIF(iso)4E wild‐type transformant; W95L, eIF(iso)4E W95L mutant transformant; W95L/K150E, eIF(iso)4E W95L/K150E transformant. (b) Profiles of gene expression level of endogenous eIF(iso)4E, introduced eIF(iso)4E and endogenous eIF4E by quantitative real‐time RT‐PCR. The relative expression level of each gene, which was normalized using the actin reaction product, was defined by an arbitrary unit. Values represent mean ± standard errors. Different letters denote statistically significantly different values [analysis of variance (ANOVA), P ≥ 0.05]. UT, untransformed control; WT, Samjin eIF(iso)4E wild‐type transformant; W95L, eIF(iso)4E W95L mutant transformant. (c) Phenotypes of TuMV‐inoculated eIF(iso)4E T 2 plants. The plants were photographed at 45 days post‐inoculation (dpi). (d) Enzyme‐linked immunosorbent analysis (ELISA) screening after TuMV CHN2, CHN3, CHN4 and CHN 5 inoculation. Double antibody sandwich (DAS)‐ELISA was performed at 45 dpi to assess virus accumulation using the fifth and sixth true leaves. MI, mock‐inoculated; UT, untransformed ‘Seoul’ cultivar. The numbers on each bar indicate the total number of T 2 plants that were screened. Error bars represent standard deviation. Different letters denote statistically significantly different values (ANOVA, P ≥ 0.05).
T2 plants were inoculated with TuMV to determine whether the resistance was stable in the T2 progenies. To test for broad‐spectrum resistance, four different strains of TuMV, CHN2, CHN3, CHN4 and CHN5, were used. The screening results for the T2 plants were similar to those of the T1 plants. At about 45 dpi, symptoms in the T2 plants over‐expressing WT eIF(iso)4E became clear (Fig. 5c). By contrast, the T2 plants over‐expressing W95L or W95L/K150E did not show any TuMV symptoms (Fig. 5c). The virus accumulation in the transgenic plants was significantly reduced relative to that in the WT over‐expressing plants (Fig. 5d). The TuMV screening results also showed that over‐expression of the mutated eIF(iso)4E genes affected all tested TuMV strains (CHN2, CHN3, CHN4 and CHN5), although there were differences in virus accumulation depending on the strain: the conferred resistance was more effective against CHN3, CHN4 and CHN5 than CHN2. In addition, virus accumulation was lower in W95L/K150E double mutants than in the W95L mutant for TuMV CHN2, CHN3 and CHN5 strains (Fig. 5d). These results support the idea that the over‐expression of the mutated eIF(iso)4E gene of B. rapa can induce broad‐spectrum TuMV resistance in susceptible Chinese cabbage.
Discussion
In this study, we targeted eIF(iso)4E to generate Chinese cabbage with broad‐spectrum resistance to TuMV. We used a knowledge‐based approach to generate artificially mutated Chinese cabbage eIF(iso)4E proteins, first identifying key amino acids in the cap‐binding pocket that control the interaction with VPg. TuMV particle accumulation was clearly reduced when eIF(iso)4E proteins mutated in these amino acids were over‐expressed in plants. These results indicate that impaired interaction between eIF(iso)4E and TuMV VPg could underlie the conferred resistance.
The amino acids that were found to interrupt the eIF(iso)4E–VPg interaction in this study do not correspond to those mutated in known eIF4E/eIF(iso)4E alleles related to resistance. However, these amino acid residues are assumed to play an important role in the formation of the eIF4E cap‐binding pocket. The Trp95 residue may form a π–π bond to make an entrance to the cap‐binding pocket (Rhoads et al., 2007). The Lys150 residue stabilizes the cap‐binding structure through H‐bonds with the phosphate group of the 5′ cap (Okade et al., 2009). When this eIF(iso)4E binds to the 5′ cap of mRNA, translation is initiated.
VPg binds at or near the cap‐binding pocket of the eIF4E protein (Léonard et al., 2000; Miyoshi et al., 2006), and most of the amino acid substitutions in eIF4E/eIF(iso)4E conferring viral resistance are located in the cap‐binding pocket (Charron et al., 2008; Robaglia and Caranta, 2006; Truniger and Aranda, 2009). According to our results, it can be expected that the VPg‐ and 5′ cap‐binding sites overlap to some degree. Although the yeast two‐hybrid and in vivo interaction analysis data showed slight discrepancies, most probably as a result of differences between the yeast and plant systems, we conclude that the W95 and K150 residues very probably play important roles in TuMV resistance.
In this study, mutation of W95 and K150 together provided stronger resistance against TuMV strains CHN2, CHN3 and CHN4 than mutation of W95 alone. Consistent with this, natural resistance genes often contain several amino acid mutations in eIF4E (Andrade et al., 2009; Ashby et al., 2011; Bruun‐Rasmussen et al., 2007; Charron et al., 2008; Duan et al., 2012, Hart and Griffiths, 2013; Kang et al., 2005b; 2007; Naderpour et al., 2010; Ruffel et al., 2006; Stein et al., 2005). Mutations of more than one amino acid residue may lead to more dramatic protein alterations, which may increase the chances of abolishing interaction with VPg and make this kind of resistance gene more durable than one with a single amino acid mutation. Our study suggests that knowledge‐based in vitro mutagenesis facilitates the generation of such eIF4E variants that are more effective against TuMV in B. rapa.
A major problem with using transgenic plants in breeding is the phenotypic changes that can occur as a result of transformation. However, the mutated Brassica eIF(iso)4E proteins did not cause any differences in yeast growth compare with the WT form. In addition, we did not observe any phenotypic variation in transgenic Chinese cabbage. Previous research has shown that, even though a host factor may lose its function, redundancy of the host factors compensates for the mutated factor (Combe et al., 2005; Duprat et al., 2002; Lellis et al., 2002). Most of the known virus resistance genes that are derived from mutations in eIF4E do not alter plant development (Gao et al., 2004a, b; Kang et al., 2005b; Morales et al., 2005; Nicaise et al., 2003; Nieto et al., 2006; Piron et al., 2010). Together, these findings suggest that the transgenic lines developed in this study will be useful for breeding applications.
Engineered expression of naturally occurring eIF4E variants has been shown to induce resistance in other species. The ectopic expression of mutated pepper eIF4E induced strong resistance to multiple viral species in the tomato system (Kang et al., 2007). Another report has shown that transgenic expression of the pvr12 gene from pepper confers resistance to Potato virus Y (PVY) in potato (Cavatorta et al., 2011). In addition, when the Eva1‐eIF4E‐1 variant was over‐expressed in eIF4E‐1‐silenced potato, resistance against PVY was conferred (Duan et al., 2012). In contrast with the previous studies, the eIF(iso)4E mutants that were used for transformation in this study were obtained by site‐directed mutagenesis. Based on studies for eIF(iso)4E–TuMV VPg interaction, it was possible to target specific amino acids that may be critical in the interaction.
Although we did not silence the endogenous eIF(iso)4E in B. rapa, the transgenic expression of eIF(iso)4E variants showed TuMV resistance. Kang et al. (2007) first described this type of resistance in tomato, which is called ‘dominant negative’ interference. This ‘dominant negative’ term was introduced by Herskowitz (1987) for the first time. In the transgenic B. rapa, it appears that the over‐expressed eIF(iso)4E mutant protein overwhelmed the endogenous eIF(iso)4E. Real‐time PCR analysis showed that the expression levels of the introduced eIF(iso)4E were about 50 times greater than those of endogenous eIF(iso)4E in transgenic plants (Fig. 5b). Consequently, TuMV resistance seems to be induced by the interruption of virus–host interaction.
Interestingly, real‐time RT‐PCR of endogenous eIF4E showed that the expression of eIF4E was also increased in transgenic plants (Fig. 5b). It seems that the over‐expression of the transgene induced the expression of endogenous eIF4E. One report has shown that eIF4E protein may compensate for eIF(iso)4E when eIF(iso)4E is depleted in Arabidopsis and tobacco systems (Combe et al., 2005; Duprat et al., 2002). Although the expression patterns of eIF4E and eIF(iso)4E are different in our study, these two observations suggest that a common homeostatic control mechanism may exist in plants to control the cap‐binding protein levels (Combe et al., 2005). Although the expression of endogenous eIF(iso)4E was not reduced or depleted, the introduction of both mutant and WT eIF(iso)4E may somehow trigger the expression of native eIF4E. This result also suggests that TuMV prefers to use eIF(iso)4E rather than eIF4E in B. rapa. Although eIF4E was over‐expressed in plants transformed with WT and mutant eIF(iso)4E, resistance to TuMV was only induced by the eIF(iso)4E point mutants. The specificity of resistance in the point mutants indicates that increased expression of eIF4E does not cause TuMV resistance. Previous reports on TuMV host factors have suggested that TuMV is able to use both eIF4E and eIF(iso)4E of B. rapa in the Arabidopsis system (Jenner et al., 2010). However, according to our data, it seems that TuMV prefers to use eIF(iso)4E in B. rapa.
Here, we have developed TuMV‐resistant Chinese cabbage by over‐expressing mutated eIF(iso)4E. This is the first report of a transgenic TuMV‐resistant Chinese cabbage generated by eIF(iso)4E engineering. Our use of in vitro and in vivo interaction analysis to select target amino acids for mutation allowed us to streamline the process of searching for novel alleles that could provide resistance. Our study demonstrates that, if there is information available about key amino acids in a critical host factor which may affect the interaction between virus proteins, it may be easy to utilize broad‐spectrum resistance in breeding programmes. In addition, targeting the common host factor of many viruses addresses the problem of resistance‐breaking strains. This study thus provides important insight for broad‐spectrum resistance studies of Brassica crops, in which eIF4E genes have been exploited for resistance breeding. Despite much interest in ascertaining the mechanism of eIF4E‐mediated resistance, it remains unclear how the interaction between eIF4E and virus protein is linked to resistance. In addition, not many other host factors have been identified, and the pool of candidate resistance genes is too small to be utilized widely for various crops. More research concerning the interactomes of potyviruses and host factors is needed in order to apply candidate resistance gene approaches to many other crops.
Experimental Procedures
Plant and virus materials
Brassica rapa ssp. Pekinensis ‘Samjin’, a cultivar developed by Monsanto Korea (Chochiwon, South Korea), was used as a susceptible control and for cloning the susceptible eIF(iso)4E cDNA. Brassica rapa cultivar ‘Seoul’ was used for the transformation with eIF(iso)4E. TuMV CHN2, CHN3, CHN4 and CHN5 were provided by Namhan Huh (Nongwoo Bio, Yeojoo, South Korea). Virus inoculum was propagated in Chinese cabbage ‘Samjin’ (Monsanto Korea, Chochiwon, South Korea). The inoculum TuMV CHN4 was used for the cloning of TuMV VPg cDNA.
RT‐PCR and TA cloning of eIF(iso)4E and TuMV VPg
Total RNA was isolated from transformed Chinese cabbage using TRIzol (Invitrogen Life Technologies, Carlsbad, CA, USA). cDNA was synthesized using SuperScript II reverse transcriptase (Invitrogen Life Technologies) and oligo(dT). Full‐length eIF(iso)4E and TuMV VPg (CHN4) cDNA sequences were amplified with gene‐specific primers (Table 1). PCR was performed in reaction volumes of 50 μL with 50–100 ng of DNA as template, 1× PCR buffer (Takara Shuzo Co., Kyoto, Japan), 2.5 mm deoxynucleoside triphosphates (dNTPs), 1.25 units of EX‐Taq (Takara Shuzo Co.) and 5 pmol of each primer. The PCR conditions were 94 °C for 30 s, 55 °C for 30 s and 72 °C for 1 min for 30 cycles. DNA fragments were cut from the gel and DNA was recovered using a Zymo Gel Recovery kit (ZymoResearch, Orange, CA, USA). PCR products were cloned using a TOPO TA Cloning Kit (with pCR2.1‐TOPO vector, Invitrogen Life Technologies). The transgene sequences were confirmed at NICEM (Seoul National University, Seoul, South Korea) by the Sanger method. The primers used for cDNA synthesis of eIF(iso)4E and TuMV VPg are listed in Table 1.
Table 1.
Primer sequences used in the plasmid construction and eIF(iso)4E site‐directed mutagenesis
| Primer ID | Primer sequence | Direction |
|---|---|---|
| TuMV VPg (Nco1) | CCATGGATGGCGAAAGGCAAGAGGCA | F |
| TuMV VPg (Sal1) | GTCGACCTATCGTGGTCCACTGGGAC | R |
| eIF(iso)4E (EcoR1) | GAATTCATGGCGACAGAGGATGTGAA | F |
| eIF(iso)4E (Xho1) | CTCGAGTCAGACAGTGAACCGAGTTC | R |
| W49L | CAAGGCGCCGCCCTTGGAGCCTCCCTTCG | F |
| CGAAGGGAGGCTCCAAGGGCGGCGCCTTG | R | |
| W95L | GTTGAGCCTAAGCTTGAAGATCCTGAG | F |
| CTCAGGATCTTCAAGCTTAGGCTCAAC | R | |
| K105L | GCTAATGGCGGACTTTGGACTTTTGTT | F |
| AACAAAAGTCCAAAGTCCGCCATTAGC | R | |
| T107L | GGCGGAAAGTGGCTTTTTGTTGTTACC | F |
| GGTAACAACAAAAAGCCACTTTCCGCC | R | |
| K150L | AGTGTGCGCCCACTTCAGGACAAGCTC | F |
| GAGCTTGTCCTGAAGTGGGCGCACACT | R | |
| D191L | ACTAACCATGATCTTTCTAGAAGAACT | F |
| AGTTCTTCTAGAAAGATCATGGTTAGT | R | |
| S192L | AACCATGATGATCTTAGAAGAACTCGG | F |
| CCGAGTTCTTCTAAGATCATCATGGTT | R | |
| RT‐PCR eIF#1 | CTTCTGGGGTTTGCACGA | F |
| CCGACAAGAGCCATCAAAGTTTC | R | |
| HPT2 | CCTGAACTCACCGCGACG | R |
| AAGACCAATGCGGAGCATAT | F | |
| Brassica actin | GTGACAATGGAACTGGAATGG | F |
| AGACGGAGGATAGCGTGAGG | R |
eIF(iso)4E, eukaryotic initiation factor(iso)4E; HPT, hygromycin phosphotransferase; RT‐PCR, reverse transcription‐polymerase chain reaction; TuMV, Turnip mosaic virus; VPg, viral protein genome‐linked.
Real‐time RT‐PCR procedure
Using total RNAs from transformed Chinese cabbage, cDNA was synthesized by M‐MLV (Promega, Madison, WI, USA). Five plant samples from each T2 progeny were randomly selected and real‐time PCR was performed as described previously (Kang et al., 2012). The experiment was repeated three times. PCR was performed in reaction volumes of 20 μL with 5 μL of cDNA as template, 1× PCR buffer (Takara Shuzo Co.), 2.5 mm dNTPs (Takara Shuzo Co.), 1.25 μm SYTO9 (Invitrogen Life Technologies), 10 pmol of reverse and forward primers and 0.2 unit of Takara rTaq polymerase (Takara Shuzo Co.) using a Rotor‐gene TM 6000 thermocycler (Corbette Research, Sydney, Australia). Cycling conditions were 55 cycles of 95 °C for 20 s, 58 °C for 20 s and 72 °C for 20 s. Primers used for real‐time RT‐PCR are listed in Table 2.
Table 2.
Primer sequences used in real‐time reverse transcription‐polymerase chain reaction (RT‐PCR)
| Primer ID | Primer sequence | Direction | Target |
|---|---|---|---|
| Bra(iso)4E_3UTR | ACGGTCTTGGATGGTGGTTG | F | Endogenous eIF(iso)4E |
| GGCAGTCGGTCATACCTGTG | R | ||
| Bra_(iso)4E_RTc | AGCCTGCTGACAAGCTCGAA | F | eIF(iso)4E transgene |
| CCACTTTCCGCCATTAGCAC | R | ||
| Bra_eIF4E_RTb | TCCGTCTTCACCTTCTCCAC | F | Endogenous |
| CCAATCAAAGCAAGCAAGGT | R | eIF4E | |
| Bra‐actin‐b | TTCAATGTCCCTGCCATGTA | F | Brassica actin |
| CTCGGCAGTAGTGGTGAACA | R |
eIF(iso)4E, eukaryotic initiation factor(iso)4E.
Virus screening procedure
Plants were inoculated at the three‐to‐four‐leaf stages by mechanical inoculation. Before inoculation, virus inoculum was prepared by grinding TuMV‐inoculated plant leaves in 50 mm potassium phosphate buffer (pH 7.5). Mechanical inoculation was carried out by the application of virus inoculum with light carborundum dusting. At 20 dpi, plants were screened daily. Resistance or susceptibility was determined by the absence or presence, respectively, of visual symptoms, and confirmed by DAS‐ELISA. The ELISA antibodies were obtained from Kisan Biotech Co. Ltd., Seoul, South Korea.
Site‐directed mutagenesis and plasmid constructs
The eIF(iso)4E coding sequence from Chinese cabbage ‘Samjin’ was cloned into the vector pJG4‐5. eIF(iso)4E mutagenesis was carried out as described previously (Simionatto et al., 2009) with some modifications. The TuMV VPg coding sequence from inoculum (CHN4) was cloned into the vector pEG202. The coding regions of all eight produced mutants and VPg were cloned using the TOPO TA Cloning Kit with pCR2.1‐TOPO vector (Invitrogen Life Technologies), and then subcloned into the pJG4‐5 or pEG202 vector. The forward and reverse primers contained restriction sites for cloning. The primers used for cloning and site‐directed mutagenesis are listed in Table 1.
Protein modelling
Swiss Model Workspace, an automated protein‐modelling server (http://swissmodel.expasy.org/), was used to generate predicted three‐dimensional protein structures. The three‐dimensional structure of wheat eIF4E (Protein Data Bank accession no. 2IDR, Chain A) was used as a template. Models were evaluated by means of the Model Assessment package provided by SWISS‐MODEL (Arnold et al., 2006; Guex and Peitsch, 1997; Schwede et al., 2003).
Yeast two‐hybrid analysis
Yeast two‐hybrid analyses, using the yeast strain EGY48 and vectors pEG202, pJG4‐5 and pSH18‐34, were carried out as described previously (Kang et al., 2005a). The bait plasmid pEG202 was used for the fusion of TuMV VPg and the prey plasmid pJG4‐5 was used to express eIF(iso)4E from Chinese cabbage containing each substitution separately. Because the lacZ reporter plasmid pSH18‐34 was present in the yeast cells, interaction of the proteins was measured by β‐galactosidase assay. Yeast transformants were streaked onto minimal medium agar plates containing 40 μg/mL X‐gal (5‐bromo‐4‐chloro‐3‐indolyl‐β‐d‐galactopyranoside) to assay the expression of the lacZ reporter gene. The quantitative assay for the determination of β‐galactosidase activity using CPRG was performed as described in the manufacturer's protocol (Clontech, Palo Alto, CA, USA).
Yeast complementation analysis
Saccharomyces cerevisiae strain J055 [cdc33‐: LEU2 Leu2 ura3 his3 trp1 ade2 (YCp33supex‐h4E URA3)], containing a deletion of the chromosomal gene coding for eIF4E, was kindly provided by Dr Carole Caranta (INRA, Paris, France). The survival of the yeast depends on the presence of plasmid YCp33supex‐h4E URA3 containing a copy of the human eIF4E cDNA, under the control of the glucose‐repressible, galactose‐dependent GAL promoter. The Chinese cabbage coding sequences of the WT and mutant eIF(iso)4E alleles were cloned into the p424GBP/TRP1 glucose‐dependent vector and individually used to transform S. cerevisiae strain JO55. After transformation, yeast cells were grown in appropriate selective nutrient drop‐out medium containing 2% glucose, and tested at 30 °C for 4–5 days.
BiFC assay
The eIF(iso)4E and TuMV VPg cDNAs were cloned into pSPY‐CE and pSPY‐NE, respectively. For the N. benthamiana infiltration, the Agrobacterium tumefaciens strain GV2260 was infiltrated into the abaxial air space of leaves from 3‐week‐old plants. The p19 protein of Tomato bushy stunt virus was used to suppress gene silencing. Epidermal cell layers of tobacco leaves were assayed for fluorescence 3 days after infiltration. All images are projection stacks of multiple confocal sections produced with a Leica LCS‐SL confocal laser scanning microscope (Leica Microsystems, Heidelberg, Germany).
Co‐IP analysis
Purified plasmid DNA of pEG201‐HA and pEG202‐FLAG constructs was electroporated into A. tumefaciens GV2260. HA‐TuMV VPg and FLAG‐eIF(iso)4E (WT and mutants) were co‐expressed in N. benthamiana by Agrobacterium‐mediated transient expression. HA‐GFP and FLAG‐GFP were used as negative controls. Two leaves were infiltrated with Agrobacterium and collected after 72 h. The extraction buffer was GTEN (10% glycerol, 25 mM Tris (pH 7.5), 1 mM EDTA, 150 mM NaCl) buffer, 0.1% Triton X‐100, 10 mm dithiothreitol (DTT) (Sigma‐Aldrich, St. Louis, MO, USA), 2% polyvinylpolypyrrolidone (PVPP) (Sigma‐Aldrich), 0.5% plant protein protease inhibitor cocktail (Sigma‐Aldrich) and 0.5% protein phosphatase inhibitor cocktail (Sigma‐Aldrich). The IP buffer used for the immunoprecipitation reaction consisted of GTEN buffer, 0.15% Nonidet P‐40 and 2 mm DTT. Co‐IP was performed according to a previously described protocol (Hwang et al., 2013; Oh and Martin, 2011). Protein extracts were incubated for 4 h at 4 °C with anti‐HA agarose‐conjugated beads (Sigma‐Aldrich). The collected beads were washed eight times with IP buffer. The immunoprecipitants were washed and then boiled for 5 min in one volume of 5× sample buffer containing 1 m tris(hydroxymethyl)aminomethane (Tris)‐HCl (pH 6.8), 10% sodium dodecylsulphate (SDS), 50% glycerol, 5% β‐mercaptoethanol and 1% bromophenol blue. The proteins were separated on 15% sodium dodecylsulphate‐polyacrylamide gel electrophoresis (SDS‐PAGE) gels and immunoblotted with anti‐HA antibody (Sigma‐Aldrich) and anti‐FLAG antibody (Sigma‐Aldrich).
Vector construction and plant transformation
eIF(iso)4E cDNAs were cloned into the vector pMDC32, which contains a Cauliflower mosaic virus 35S promoter. pMDC32 constructs were electroporated into A. tumefaciens LBA4404. The presence and stability of the transgenes were verified by PCR. Chinese cabbage cultivar ‘Seoul’ was transformed using the leaf disc method at the National Institute of Horticultural and Herbal Science, Suwon, South Korea. The copy number of the transformants was tested at the National Institute of Horticultural and Herbal Science (Table S1). T1 lines that appeared to contain a single transgene and had a sufficient amount of seed were selected. For the T1 selection, 30–40 sterile seeds were grown on hygromycin (15 mg/mL) in half‐strength Murashige and Skoog medium. Three to four T1 plants for each construct were obtained, and selected T1 plants were self‐pollinated to produce T2 plants. Total genomic DNAs from transgenic and non‐transgenic plants were isolated from young green leaves of Chinese cabbage using the cetyltrimethylammonium bromide (CTAB) method of Hwang et al. (2009). The presence of the transgenes was verified by PCR.
Supporting information
Fig. S1 Bimolecular fluorescence complementation (BiFC) analysis of Turnip mosaic virus (TuMV) VPg (viral protein genome‐linked) and eIF(iso)4E [eukaryotic initiation factor(iso)4E]. (a) TuMV VPg was fused with the N‐terminal fragment of yellow fluorescent protein (YFP) (TuMV VPg‐NE) and co‐expressed with the fusion of eIF(iso)4E with the C‐terminal fragment of YFP (eIF(iso)4E‐CE). a, bZIP positive control; b, BiFC between TuMV VPg and eIF(iso)4E; c–e, BiFC between TuMV VPg and eIF(iso)4E mutants. (b) As negative controls, eIF(iso)4E‐CE was co‐expressed with NE (N‐terminal fragment of YFP) and TuMV VPg‐NE was co‐expressed with CE (C‐terminal fragment of YFP). f, BiFC between NE and eIF(iso)4E; g–i, BiFC between NE and eIF(iso)4E mutants; j, BiFC between TuMV VPg and CE; k, NE and CE were co‐expressed. All confocal images were taken 3 days post‐infiltration and 3‐week‐old Nicotiana benthamiana plants were used. All images are projection stacks of multiple confocal sections produced with a Leica LCS‐SL confocal laser scanning microscope. Bars, 50 μm. Chl, chlorophyll autofluorescence.
Table S1 T1 plants over‐expressing eIF(iso)4E [eukaryotic initiation factor(iso)4E] proteins and copy numbers of the transgene.
Acknowledgements
This study was supported by a grant (Project no. 609002‐5) from the Screening Center for Disease Resistance Vegetable Crops of the Technology Development Program, and a grant (Project no. 710001‐07) from the Vegetable Breeding Research Center through the R&D Convergence Center Support Program, Ministry of Agriculture, Food and Rural Affairs (MAFRA), South Korea. This research was also supported by Golden Seed Project (213002‐04‐1‐CG910), MAFRA, Ministry of Oceans and Fisheries (MOF), Rural Development Administration (RDA) and Korea Forest Service (KFS).
References
- Andrade, M. , Abe, Y. , Nakahara, K.S. and Uyeda, I. (2009) The cyv‐2 resistance to Clover yellow vein virus in pea is controlled by the eukaryotic initiation factor 4E. J. Gen. Plant Pathol. 75, 241–249. [Google Scholar]
- Arnold, K. , Bordoli, L. , Kopp, J. and Schwede, T. (2006) The SWISS‐MODEL Workspace: A web‐based environment for protein structure homology modelling. Bioinformatics, 22,195–201. [DOI] [PubMed] [Google Scholar]
- Ashby, J.A. , Stevenson, C.E. , Jarvis, G.E. , Lawson, D.M. and Maule, A.J. (2011) Structure‐based mutational analysis of eIF4E in relation to sbm1 resistance to Pea seed‐borne mosaic virus in pea. PLoS ONE, 6, e15873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchemin, C. , Boutet, N. and Laliberté, J.F. (2007) Visualization of the interaction between the precursors of VPg, the viral protein linked to the genome of Turnip mosaic virus, and the translation eukaryotic initiation factor iso 4E in planta . J. Virol. 81, 775–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruun‐Rasmussen, M. , Møller, I.S. , Tulinius, G. , Hansen, J.K.R. , Lund, O.S. and Johansen, I.E. (2007) The same allele of translation initiation factor 4E mediates resistance against two Potyvirus spp. in Pisum sativum . Mol. Plant–Microbe Interact. 20, 1075–1082. [DOI] [PubMed] [Google Scholar]
- Cavatorta, J. , Perez, K.W. , Gray, S.M. , Van Eck, J. , Yeam, I. and Jahn, M. (2011) Engineering virus resistance using a modified potato gene. Plant Biotech. J. 9, 1014–1021. [DOI] [PubMed] [Google Scholar]
- Charron, C. , Nicolaï, M. , Gallois, J.L. , Robaglia, C. , Moury, B. and Palloix, A. (2008) Natural variation and functional analyses provide evidence for co‐evolution between plant eIF4E and potyviral VPg. Plant J. 54, 56–68. [DOI] [PubMed] [Google Scholar]
- Combe, J.P. , Petracek, M.E. , van Eldik, G. , Meulewaeter, F. and Twell, D. (2005) Translation initiation factors eIF4E and eIFiso4E are required for polysome formation and regulate plant growth in tobacco. Plant Mol. Biol. 57, 749–760. [DOI] [PubMed] [Google Scholar]
- Cotton, S. , Grangeon, R. , Thivierge, K. , Mathieu, I. , Ide, C. and Wei, T. (2009) Turnip mosaic virus RNA replication complex vesicles are mobile, align with microfilaments, and are each derived from a single viral genome. J. Virol. 83, 10 460–10 471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan, H. , Richael, C. and Rommens, C.M. (2012) Overexpression of the wild potato eIF4E‐1 variant Eva1 elicits Potato virus Y resistance in plants silenced for native eIF4E‐1 . Trans. Res. 21, 929–938. [DOI] [PubMed] [Google Scholar]
- Duprat, A. , Caranta, C. , Revers, F. , Menand, B. , Browning, K.S. and Robaglia, C. (2002) The Arabidopsis eukaryotic initiation factor (iso) 4E is dispensable for plant growth but required for susceptibility to potyviruses. Plant J. 32, 927–934. [DOI] [PubMed] [Google Scholar]
- Edwardson, J. and Christie, R. (1991) Potyviruses. Fla. Agric. Exp. Stat. Monogr. Ser. 16, 1–4. [Google Scholar]
- Gao, Z. , Eyers, S. , Thomas, C. , Ellis, N. and Maule, A. (2004a) Identification of markers tightly linked to sbm recessive genes for resistance to Pea seed‐borne mosaic virus . Theor. Appl. Genet. 109, 488–494. [DOI] [PubMed] [Google Scholar]
- Gao, Z. , Johansen, E. , Eyers, S. , Thomas, C.L. , Noel Ellis, T. and Maule, A.J. (2004b) The potyvirus recessive resistance gene, sbm1, identifies a novel role for translation initiation factor eIF4E in cell‐to‐cell trafficking. Plant J. 40, 376–385. [DOI] [PubMed] [Google Scholar]
- Grzela, R. , Strokovska, L. , Andrieu, J.P. , Dublet, B. , Zagorski, W. and Chroboczek, J. (2006) Potyvirus terminal protein VPg, effector of host eukaryotic initiation factor eIF4E. Biochimie, 88, 887–896. [DOI] [PubMed] [Google Scholar]
- Guex, N. and Peitsch, M.C. (1997) SWISS‐MODEL and the Swiss‐PdbViewer: an environment for comparative protein modeling. Electrophoresis, 18, 2714–2723. [DOI] [PubMed] [Google Scholar]
- Hart, J.P. and Griffiths, P.D. (2013) A series of eIF4E alleles at the Bc‐3 locus are associated with recessive resistance to Clover yellow vein virus in common bean. Theor. Appl. Genet. 126, 2849–2863. [DOI] [PubMed] [Google Scholar]
- Herskowitz, I. (1987) Functional inactivation of genes by dominant negative mutations. Nature, 329, 219–222. [DOI] [PubMed] [Google Scholar]
- Hwang, J. , Li, J. , Liu, W.Y. , An, S.J. , Cho, H. , Her, N.H. , Yeam, I. , Kim, D. and Kang, B.C. (2009) Double mutations in eIF4E and eIFiso4E confer recessive resistance to Chilli veinal mottle virus in pepper. Mol. Cell, 27, 329–336. [DOI] [PubMed] [Google Scholar]
- Hwang, J. , Oh, C.S. and Kang, B.C. (2013) Translation elongation factor 1B (eEF1B) is an essential host factor for Tobacco mosaic virus infection in plants. Virology, 439, 105–114. [DOI] [PubMed] [Google Scholar]
- Ibiza, V. , Cañizares, J. and Nuez, F. (2010) EcoTILLING in Capsicum species: searching for new virus resistances. BMC Genom. 11, 631–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenner, C.E. , Nellist, C.F. , Barker, G.C. and Walsh, J.A. (2010) Turnip mosaic virus (TuMV) is able to use alleles of both eIF4E and eIF (iso) 4E from multiple loci of the diploid Brassica rapa . Mol. Plant–Microbe Interact. 23, 1498–1505. [DOI] [PubMed] [Google Scholar]
- Jeong, H.J. , Kwon, J.K. , Pandeya, D. , Hwang, J. , Hoang, N.H. , Bae, J.H. and Kang, B.C. (2012) A survey of natural and ethyl methane sulfonate‐induced variations of eIF4E using high‐resolution melting analysis in Capsicum . Mol. Breed. 29, 349–360. [Google Scholar]
- Kang, B.C. , Yeam, I. , Frantz, J.D. , Murphy, J.F. and Jahn, M.M. (2005a) The pvr1 locus in Capsicum encodes a translation initiation factor eIF4E that interacts with Tobacco etch virus VPg. Plant J. 42, 392–405. [DOI] [PubMed] [Google Scholar]
- Kang, B.C. , Yeam, I. and Jahn, M.M. (2005b) Genetics of plant virus resistance. Annu. Rev. Phytopathol. 43, 581–621. [DOI] [PubMed] [Google Scholar]
- Kang, B.C. , Yeam, I. , Li, H. , Perez, K.W. and Jahn, M.M. (2007) Ectopic expression of a recessive resistance gene generates dominant potyvirus resistance in plants. Plant Biotechnol. J. 5, 526–536. [DOI] [PubMed] [Google Scholar]
- Kang, W.H. , Seo, J.K. , Chung, B.N. , Kim, K.H. and Kang, B.C. (2012) Helicase domain encoded by Cucumber mosaic virus RNA1 determines systemic infection of Cmr1 in pepper. PLoS ONE, 7, e43136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, J. , Kang, W.H. , Yang, H.B. , Park, S. , Jang, C.S. , Yu, H.J. and Kang, B.C. (2013) Identification of a broad‐spectrum recessive gene in Brassica rapa and molecular analysis of the eIF4E gene family to develop molecular markers. Mol. Breed. 32, 385–398. [Google Scholar]
- Lellis, A.D. , Kasschau, K.D. , Whitham, S.A. and Carrington, J.C. (2002) Loss‐of‐susceptibility mutants of Arabidopsis thaliana reveal an essential role for eIF (iso) 4E during potyvirus infection. Curr. Biol. 12, 1046–1051. [DOI] [PubMed] [Google Scholar]
- Léonard, S. , Plante, D. , Wittmann, S. , Daigneault, N. , Fortin, M.G. and Laliberté, J.F. (2000) Complex formation between potyvirus VPg and translation eukaryotic initiation factor 4E correlates with virus infectivity. J. Virol. 74, 7730–7737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazier, M. , Flamain, F. , Nicolaï, M. , Sarnette, V. and Caranta, C. (2011) Knock‐down of both eIF4E1 and eIF4E2 genes confers broad‐spectrum resistance against potyviruses in tomato. PLoS ONE, 6, e29595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyoshi, H. , Suehiro, N. , Tomoo, K. , Muto, S. , Takahashi, T. and Tsukamoto, T. (2006) Binding analyses for the interaction between plant virus genome‐linked protein (VPg) and plant translational initiation factors. Biochimie, 88, 329–340. [DOI] [PubMed] [Google Scholar]
- Miyoshi, H. , Okade, H. , Muto, S. , Suehiro, N. , Nakashima, H. and Tomoo, K. (2008) Turnip mosaic virus VPg interacts with Arabidopsis thaliana eIF (iso) 4E and inhibits in vitro translation. Biochimie, 90, 1427–1434. [DOI] [PubMed] [Google Scholar]
- Morales, M. , Orjeda, G. , Nieto, C. , van Leeuwen, H. , Monfort, A. and Charpentier, M. (2005) A physical map covering the nsv locus that confers resistance to Melon necrotic spot virus in melon (Cucumis melo L.). Theor. Appl. Genet. 111, 914–922. [DOI] [PubMed] [Google Scholar]
- Naderpour, M. , Lund, O.S. , Larsen, R. and Johansen, E. (2010) Potyviral resistance derived from cultivars of Phaseolus vulgaris carrying bc‐3 is associated with the homozygotic presence of a mutated eIF4E allele. Mol. Plant Pathol. 11, 255–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicaise, V. , German‐Retana, S. , Sanjuán, R. , Dubrana, M.P. , Mazier, M. and Maisonneuve, B. (2003) The eukaryotic translation initiation factor 4E controls lettuce susceptibility to the potyvirus Lettuce mosaic virus . Plant Physiol. 132, 1272–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieto, C. , Morales, M. , Orjeda, G. , Clepet, C. , Monfort, A. and Sturbois, B. (2006) An eIF4E allele confers resistance to an uncapped and non‐polyadenylated RNA virus in melon. Plant J. 48, 452–462. [DOI] [PubMed] [Google Scholar]
- Nieto, C. , Piron, F. , Dalmais, M. , Marco, C.F. , Moriones, E. , Gómez‐Guillamón, M.L. , Truniger, V. , Ǵomez, P. , Garcia‐Mas, J. , Aranda, M.A. and Bendahmane, A. (2007) EcoTILLING for the identification of allelic variants of melon eIF4E, a factor that controls virus susceptibility. BMC Plant Biol. 7, 34–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh, C.S. and Martin, G.B. (2011) Tomato 14‐3‐3 protein TFT7 interacts with a MAP kinase kinase to regulate immunity‐associated programmed cell death mediated by diverse disease resistance proteins. J. Biol. Chem. 286, 14 129–14 136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okade, H. , Fujita, Y. , Miyamoto, S. , Tomoo, K. , Muto, S. and Miyoshi, H. (2009) Turnip mosaic virus genome‐linked protein VPg binds C‐terminal region of cap‐bound initiation factor 4E orthologue without exhibiting host cellular specificity. J. Biochem. 145, 299–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piron, F. , Nicolaï, M. , Minoïa, S. , Piednoir, E. , Moretti, A. , Salgues, A. , Zamir, D. , Caranta, C. and Bendahmane, A. (2010) An induced mutation in tomato eIF4E leads to immunity to two potyviruses. PLoS ONE, 5, e11313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provvidenti, R. and Hampton, R. (1992) Sources of resistance to viruses in the Potyviridae. Arch. Virol. Suppl. 5, 189–211. [DOI] [PubMed] [Google Scholar]
- Qian, W. , Zhang, S. , Zhang, S. , Li, F. , Zhang, H. , Wu, J. , Wang, X. , Walsh, J.A. and Sun, R. (2013) Mapping and candidate‐gene screening of the novel Turnip mosaic virus resistance gene retr02 in Chinese cabbage (Brassica rapa L.). Theor. Appl. Genet. 126, 179–188. [DOI] [PubMed] [Google Scholar]
- Rhoads, R.E. (2009) eIF4E: new family members, new binding partners, new roles. J. Biol. Chem. 284, 16 711–16 715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhoads, R.E. , Dinkova, T.D. and Jagus, R. (2007) Approaches for analyzing the differential activities and functions of eIF4E family members. Methods Enzymol. 429, 261–297. [DOI] [PubMed] [Google Scholar]
- Robaglia, C. and Caranta, C. (2006) Translation initiation factors: a weak link in plant RNA virus infection. Trends Plant Sci. 11, 40–45. [DOI] [PubMed] [Google Scholar]
- Rodríguez‐Hernández, A.M. , Gosalvez, B. , Sempere, R.N. , Burgos, L. , Aranda, M.A. and Truniger, V. (2012) Melon RNA interference (RNAi) lines silenced for Cm‐eIF4E show broad virus resistance. Mol. Plant Pathol. 13, 755–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruffel, S. , Dussault, M.H. , Palloix, A. , Moury, B. , Bendahmane, A. and Robaglia, C. (2002) A natural recessive resistance gene against Potato virus Y in pepper corresponds to the eukaryotic initiation factor 4E (eIF4E). Plant J. 32, 1067–1075. [DOI] [PubMed] [Google Scholar]
- Ruffel, S. , Gallois, J. , Lesage, M. and Caranta, C. (2005) The recessive potyvirus resistance gene pot‐1 is the tomato orthologue of the pepper pvr2‐eIF4E gene. Mol. Gen. Genet. 274, 346–353. [DOI] [PubMed] [Google Scholar]
- Ruffel, S. , Gallois, J.L. , Moury, B. , Robaglia, C. , Palloix, A. and Caranta, C. (2006) Simultaneous mutations in translation initiation factors eIF4E and eIF (iso) 4E are required to prevent Pepper veinal mottle virus infection of pepper. J. Gen. Virol. 87, 2089–2098. [DOI] [PubMed] [Google Scholar]
- Rusholme, R.L. , Higgins, E.E. , Walsh, J.A. and Lydiate, D.J. (2007) Genetic control of broad‐spectrum resistance to Turnip mosaic virus in Brassica rapa (Chinese cabbage). J. Gen. Virol. 88, 3177–3186. [DOI] [PubMed] [Google Scholar]
- Schwede, T. , Kopp, J. , Guex, N. and Peitsch, M.C. (2003) SWISS‐MODEL: an automated protein homology‐modeling server. Nucleic Acids Res. 31, 3381–3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shattuck, V. (1992) The biology, epidemiology and control of Turnip mosaic virus . Hortic. Rev. 14, 199–238. [Google Scholar]
- Siberil, Y. , Doireau, P. and Gantet, P. (2001) Plant bZIP G‐box binding factors. Eur. J. Biochem. 268, 5655–5666. [DOI] [PubMed] [Google Scholar]
- Simionatto, S. , Marchioro, S.B. , Galli, V. , Luerce, T.D. , Hartwig, D.D. and Moreira, Â.N. (2009) Efficient site‐directed mutagenesis using an overlap extension‐PCR method for expressing Mycoplasma hyopneumoniae genes in Escherichia coli . J. Microbiol. Methods, 79, 101–105. [DOI] [PubMed] [Google Scholar]
- Stein, N. , Perovic, D. , Kumlehn, J. , Pellio, B. , Stracke, S. , Streng, S. , Ordon, F. and Graner, A. (2005) The eukaryotic translation initiation factor 4E confers multiallelic recessive Bymovirus resistance in Hordeum vulgare (L). Plant J. 42, 912–922. [DOI] [PubMed] [Google Scholar]
- Strange, R.N. and Scott, P.R. (2005) Plant disease: a threat to global food security. Phytopathology, 43, 83–116. [DOI] [PubMed] [Google Scholar]
- Truniger, V. and Aranda, M. (2009) Recessive resistance to plant viruses. Adv. Virus Res. 75, 119–231. [DOI] [PubMed] [Google Scholar]
- Walter, M. , Chaban, C. , Schütze, K. , Batistic, O. , Weckermann, K. and Näke, C. (2004) Visualization of protein interactions in living plant cells using bimolecular fluorescence complementation. Plant J. 40, 428–438. [DOI] [PubMed] [Google Scholar]
- Wang, A. and Krishnaswamy, S. (2012) Eukaryotic translation initiation factor 4E‐mediated recessive resistance to plant viruses and its utility in crop improvement. Mol. Plant Pathol. 13, 795–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittmann, S. , Chatel, H. , Fortin, M.G. and Laliberté, J.F. (1997) Interaction of the viral protein genome linked of Turnip mosaic potyvirus with the translational eukaryotic initiation factor (iso) 4E of Arabidopsis thaliana using the yeast two‐hybrid system. Virology, 234, 84–92. [DOI] [PubMed] [Google Scholar]
- Yeam, I. , Cavatorta, J.R. , Ripoll, D.R. , Kang, B.C. and Jahn, M.M. (2007) Functional dissection of naturally occurring amino acid substitutions in eIF4E that confers recessive potyvirus resistance in plants. Plant Cell, 19, 2913–2928. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 Bimolecular fluorescence complementation (BiFC) analysis of Turnip mosaic virus (TuMV) VPg (viral protein genome‐linked) and eIF(iso)4E [eukaryotic initiation factor(iso)4E]. (a) TuMV VPg was fused with the N‐terminal fragment of yellow fluorescent protein (YFP) (TuMV VPg‐NE) and co‐expressed with the fusion of eIF(iso)4E with the C‐terminal fragment of YFP (eIF(iso)4E‐CE). a, bZIP positive control; b, BiFC between TuMV VPg and eIF(iso)4E; c–e, BiFC between TuMV VPg and eIF(iso)4E mutants. (b) As negative controls, eIF(iso)4E‐CE was co‐expressed with NE (N‐terminal fragment of YFP) and TuMV VPg‐NE was co‐expressed with CE (C‐terminal fragment of YFP). f, BiFC between NE and eIF(iso)4E; g–i, BiFC between NE and eIF(iso)4E mutants; j, BiFC between TuMV VPg and CE; k, NE and CE were co‐expressed. All confocal images were taken 3 days post‐infiltration and 3‐week‐old Nicotiana benthamiana plants were used. All images are projection stacks of multiple confocal sections produced with a Leica LCS‐SL confocal laser scanning microscope. Bars, 50 μm. Chl, chlorophyll autofluorescence.
Table S1 T1 plants over‐expressing eIF(iso)4E [eukaryotic initiation factor(iso)4E] proteins and copy numbers of the transgene.


