Summary
Plant viruses, possessing a bacilliform shape and containing double‐stranded DNA, are emerging as important pathogens in a number of agricultural and horticultural crops in the tropics. They have been reported from a large number of countries in African and Asian continents, as well as from islands from the Pacific region. The viruses, belonging to two genera, Badnavirus and Tungrovirus, within the family Caulimoviridae, have genomes displaying a common plan, yet are highly variable, sometimes even between isolates of the same virus. In this article, we summarize the current knowledge with a view to revealing the common features embedded within the genetic diversity of this group of viruses.
Taxonomy
Virus; order Unassigned; family Caulimoviridae; genera Badnavirus and Tungrovirus; species Banana streak viruses, Bougainvillea spectabilis chlorotic vein banding virus, Cacao swollen shoot virus, Citrus yellow mosaic badnavirus, Dioscorea bacilliform viruses, Rice tungro bacilliform virus, Sugarcane bacilliform viruses and Taro bacilliform virus.
Microbiological properties
Bacilliform in shape; length, 60–900 nm; width, 35–50 nm; circular double‐stranded DNA of approximately 7.5 kbp with one or more single‐stranded discontinuities.
Host range
Each virus generally limited to its own host, including banana, bougainvillea, black pepper, cacao, citrus species, Dioscorea alata, rice, sugarcane and taro.
Disease symptoms
Foliar streaking in banana and sugarcane, swelling of shoots in cacao, yellow mosaic in leaves and stems in citrus, brown spot in the tubers in yam and yellow–orange discoloration and stunting in rice.
Useful websites
Introduction
Viruses belonging to the Genera Badnavirus and Tungrovirus are emerging as important pathogens of various crops in the tropics. Both genera form part of the family Caulimoviridae, whose members have a double‐stranded DNA (dsDNA) genome of 7.5–8.0 kb displaying features suggestive of a reverse transcription‐based replication step, namely a highly conserved initiator transfer RNA (tRNAmet) binding site, together with a gene arrangement [coat protein (CP)–protease–reverse transcriptase (RT)/ribonucleaseH (RNaseH)] resembling the gag–pol–env pattern of animal retroviruses. According to the latest release of the International Committee on the Taxonomy of Viruses (ICTV) (2011), within the family Caulimoviridae, genus Badnavirus has the largest number of species (25), the majority of which (19) are found in the tropics. Genus Tungrovirus has a single species, Rice tungro bacilliform virus (RTBV), which resembles badnaviruses in having a dsDNA and bacilliform shape, but differs in the genome organization [an extra open reading frame (ORF)] and vector transmission (by leafhopper, as opposed to mealybugs in the case of badnaviruses).
Over the past few years, reports of the analysis of nucleotide sequences of the members of these two viral genera have been accumulating steadily. This review attempts to synthesize the above information, focusing mainly on the badnaviruses found in the tropics (Table 1), and attempts to find commonalities within the diversity exhibited in their genomes. As these viruses are emerging as important pathogens of agricultural and horticultural crops in the tropics, their common features may point towards new strategies for their management.
Table 1.
Hosts, vectors and genomic information on badnaviruses and tungrovirus
| Name | Genome size (kb) | Number of ORFs | Host(s) | Mode of transmission | Country(ies) reported from | Accession numbers of complete sequences | Reference(s) |
|---|---|---|---|---|---|---|---|
| Banana streak virus | 7.4 | 4 | Banana | Aphid, mealybug (Dysmicoccus brevipes, Planococcus citri and P. ficus), pink sugarcane mealybug (Saccharicoccus sacchari), pineapple mealybug (D. brevipes) | Uganda, Australia, Vietnam, Nigeria, India | NC_008018, DQ859899, NC_007003, NC_003381, DQ451009, NC_007002, DQ092436, AF214005, AY493509, AY750155 | Geering et al. (2005); Harper and Hull, (1998); Lockhart and Jones, (2000); Lheureux et al. (2007) |
| Bougainvillea vein banding‐associated badnavirus | 8.8 | 4 | Bougainvillea spectabilis | – | Brazil, Taiwan, India | NC_011592, EU034539 | Baranwal et al. (2010); Rivas et al. (2005) |
| Cacao swollen shoot virus | 7.1 | 5 | Theobroma cacao | Mealybug (P. citri, Ferrisia virgata and P. njalensis) | Togo, Ghana, Ivory Coast, Nigeria | NC_001574, AJ609019, AJ781003, AJ609020, JN606110, L14546, AJ534983, AJ608931 | Hagen et al (1993); Kouakou et al. (2012); Muller and Sackey (2005); Quainoo et al. (2008) |
| Citrus yellow mosaic badnavirus | 7.5 | 6 | Citrus (sweet orange, Rangpur lime, acid lime, pummelo) | Mealybug (P. citri) | India | NC_003382, DQ875213, JN006806, JN006805, AF347695, FJ617224, EU489744, EU489745, EU708316, EU708317, EU884191 | Borah et al. (2009); Huang and Hartung (2001); Johnson et al. (2012) |
| Pineapple bacilliform comosus virus and Pineapple bacilliform erectifolius virus | 7.6 | 3 | Pineapple | Mealybug (D. brevipes) | Australia | NC_014648, GQ398110, GU121676 | Gambley et al. (2008) |
| Piper yellow mottle virus | – | – | Piper nigrum | Mealybug (F. virgata, P. citri) and black pepper lace bug (Diconocoris distanti). Also by grafting |
Brazil, Malaysia, Thailand, Philippines, Sri Lanka, India |
Complete sequences are not available | Bhat et al. (2003); Lockhart et al. (1997) |
| Rice tungro bacilliform virus | 8.0 (South‐East Asian), 7.9 (South Asian) | 4 | Oryza sativa | Green leafhopper (Nephotettix virescens, N. cincticeps, Resilia dorsalis) | Bangladesh, India, Indonesia, Malaysia, Philippines, Vietnam | JX255736, NC_001914, HQ385226, FN377814, AF113831, AF113830, AF113832, AF220561, AF076470, M65026, X57924, D10774, AJ314596, JX255736 | Banerjee et al. (2012); Cabauatan et al. (1999); Hay et al. (1991); Kano et al. (1992); Marmey et al. (1999); Mathur and Dasgupta (2007, 2013); Nath et al. (2002); Qu et al. (1991); Sharma and Dasgupta (2012) |
| Sugarcane bacilliform virus | 7.6 | 3 | Sugarcane Saccharum officinarum, S. barberi, S. sinense, S. robustum and Saccharum L. interspecific hybrids | – | Morocco, USA, India | NC_008017, NC_003031, AJ277091, NC_013455, FJ824814, FJ439817, FJ824813, M89923 | Bouhida et al. (1993); Lockhart and Autrey (1988); Muller et al. (2011) |
| Taro bacilliform virus | 7.5 | 4 | Taro (Colocasia esculenta) | Mealybug | Pacific Island countries | NC_004450, AF357836 | Yang et al. (2003) |
| Dioscorea bacilliform virus | 7.3 | 3 | D. cayenensis, D. bulbifera, D. japonica | – | Caribbean, Ivory Coast, Japan, France, Benin, Ghana, Togo, Nigeria | NC_009010, DQ822074, DQ822073 | Seal and Muller (2007) |
Diversity, Distribution and Host Range
Badnaviruses have been reported from across all tropical regions of the African, American and Asian continents, and in the Pacific Islands (Fig. 1). They are regarded as a highly diverse and heterogeneous group of viruses (Al‐Kaff and Covey, 1994; Geering et al., 2000; Harper et al., 2004, 2005; Jaufeerally‐Fakim et al., 2006; Kenyon et al., 2008; Lockhart and Olszewski, 1993). They share relatively low nucleotide identities, even within the same genus, when compared with other virus genera. The number of ORFs also varies from virus to virus; whereas most contain three ORFs (Sugarcane bacilliform virus, SCBV), some may have four (Taro bacilliform virus, TaBV), five (Cacao swollen shoot virus, CSSV), six (Citrus yellow mosaic badnavirus, CMBV) or seven (Dracaena sanderiana badnavirus) ORFs.
Figure 1.
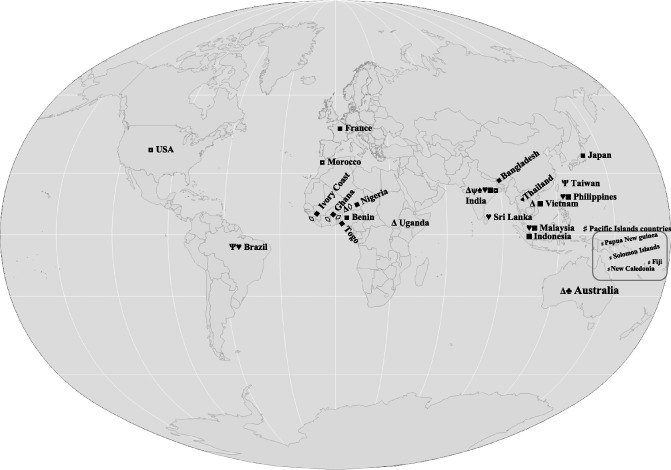
Occurrence of badnaviruses and tungrovirus across the tropical regions of the world. Symbols given for each virus are as follows: Δ, Banana streak virus; ψ, Bougainvillea vein banding‐associated badnavirus; ◊, Cacao swollen shoot virus; ♠, Citrus yellow mosaic badnavirus; ♣, Pineapple bacilliform comosus virus and Pineapple bacilliform erectifolius virus; ♥, Piper yellow mottle virus;  , Rice tungro bacilliform virus;
, Rice tungro bacilliform virus;  , Sugarcane bacilliform virus; ♯, Taro bacilliform virus; ■, Dioscorea bacilliform virus.
, Sugarcane bacilliform virus; ♯, Taro bacilliform virus; ■, Dioscorea bacilliform virus.
One of the most well‐studied badnavirus groups is that infecting banana: Banana streak viruses (BSVs). Viral leaf streak of banana was first described in Ivory Coast, Africa in 1968 (Lassoudiere, 1974), and the causal agent, BSV, was identified in Morocco in 1985 (Lockhart, 1986). Countries affected by this disease include Malaysia, China, Indonesia, India, Philippines, Vietnam, Thailand, Sri Lanka and Australia (Jones and Lockhart, 1993; Vuylsteke et al., 1996). In Africa, BSVs from Uganda were found to show a very high degree of variability, possibly as a result of a series of introductions of banana into Uganda, each with a different complement of infecting viruses (Tushmereirwe et al., 1996). Initially, four isolates of BSV, namely BSV‐RD (from cultivar Red Decca), BSV‐Cav (from cultivar Williams), BSV‐Mys (from cultivar Mysore) and BSV‐GF (from cultivar Goldfinger), were described by Lockhart and Olszewski (1993) based on DNA hybridization analysis. Two more isolates, BSV‐OL (from cultivar Obino l'Ewai; Harper et al., 1999) and, more recently, BSV‐IM (Geering et al., 2011) have been reported.
Variation in the nucleotide sequences of BSV isolates is considerable. For example, Geering et al. (2000) observed 21.8%–33.6% sequence differences in the RT/RNaseH coding regions of three BSV isolates in Australia. In another report, 140 partial sequences of the RT/RNaseH region (representing 49 infected banana samples) were reported to belong to as many as 15 different species (Harper et al., 2005). Interestingly two isolates of BSV cloned from southern India showed 99% and 97% identity to BSV‐OL in the RT/RNaseH domain (Cherian et al., 2004).
SCBV is another group of relatively well‐studied badnaviruses, widely reported from a number of species of sugarcane (Saccharum officinarum, S. barberi, S. sinense, S. robustum and Saccharum L. interspecific hybrids). It was first reported in 1985 (Rodriguez‐Lema et al., 1985) and was later purified in 1988 (Lockhart and Autrey, 1988). SCBVs have since been found to be distributed throughout all major sugarcane‐growing regions worldwide causing considerable yield reductions in some varieties (Autrey et al., 1995; Comstock and Lockhart, 1990; Lockhart and Autrey, 1988; Viswanathan et al., 1996). The first complete sequence of SCBV‐Mor (from Morocco; NC_008017) was reported in 1993 (Bouhida et al., 1993). Presently, there is one more complete SCBV sequence in the database, known as SCBV‐IM (isolate Ireng Maleng, from Australia; NC_003031). Although separated by a large geographical distance, SCBV‐IM was found to share quite high sequence identities with SCBV‐Mor (with amino acid sequence identities of 91.4%, 83.8% and 85.3% in ORF I, II and III, respectively). However, all SCBVs were more closely related to each other than to other badnaviruses within their ORF III amino acid sequence. The significant serological heterogeneity (Autrey et al., 1995) and sequence variability reported in SCBVs (Braithwaite et al., 1995; Smith et al., 1996) suggest that the viral populations are complex and variable, even within the group, a view further supported by reports by Geijskes et al. (2002) and Muller et al. (2011). The latter group reported three more complete sequences of SCBV, presumably representing a new species, Sugarcane bacilliform Guadeloupe A virus/Sugarcane bacilliform Guadeloupe D virus. The variability has been exemplified further in the latest ICTV report, in which SCBV‐Mor and SCBV‐IM have been described as two different species (Fauquet et al., 2005).
SCBV is an exception among the badnaviruses with its special ability to infect plants of two different families. SCBV‐Mor has been found to infect banana (Musaceae), and rice (Bouhida et al., 1993), Sorghum halepense and Brachiaria extensa (Frison and Putter, 1993), all three of the family Poaceae, through agroinoculation (cloned viral DNA in a binary plasmid introduced into the plant through Agrobacterium). Considering the fact that BSV can also multiply in sugarcane, SCBV and BSV are sometimes considered as strains of the same virus (Lockhart and Autrey, 1988).
CMBV, the causative agent for the mosaic disease of citrus, has been reported from a few districts of the state of Andhra Pradesh, India (Ahlawat et al., 1996; Dakshinamurti and Reddy, 1975) in at least four important citrus species: sweet orange (Ahlawat et al., 1996), pummelo (C. grandis L., Osbeck; Ahlawat, 1997; Ahlawat et al., 1996), acid lime (C. aurantifolia; Johnson et al., 2012) and rough lemon (C. jambhiri; Johnson et al., 2012). The virus is graft transmissible to 13 citrus cultivars. Five complete CMBV sequences from four different hosts [sweet orange, rangpur lime (Citrus × limonia, Osbeck), acid lime and pummel], all four sequences sharing more than 90% identity to each other (Borah et al., 2009; Johnson et al., 2012), are available in the database.
The association of a flexuous badnavirus with internal brown spot symptoms was first reported in Dioscorea alata and D. cayenensis‐rotundata in the Caribbean islands (Harrison and Roberts, 1973; Mantelle and Haque, 1979). Thereafter, the virus was partially characterized from D. alata (Phillips et al., 1999). The complete nucleotide sequence (7.4 kb) of another badnavirus infecting D. alata, which is not associated with the internal brown spot, was also reported and was named Dioscorea alata bacilliform virus (DaBV; Briddon et al., 1999). By immunosorbent electron microscopy, badnavirus‐like particles were reported from yam (D. sansibarensis), which was later further characterized by sequence analysis of the viral DNA. The sequence shared 61.9% identity with DaBV, and the virus was named Dioscorea sansibarensis bacilliform virus (DsBV; Seal and Muller, 2007). A study on 14 yam‐infecting DaBV and DsBV isolates from West Africa showed that they shared between 75% and 96% amino acid identities with each other (Eni et al., 2008).
TaBV is restricted to its host and is widespread throughout the Pacific Island countries (Yang et al., 2003). Analysis of the putative RT/RNaseH regions from 22 TaBV isolates collected from Fiji, French Polynesia, New Caledonia, Papua New Guinea, Samoa, Solomon Islands and Vanuatu showed a variability of 22.9% and 13.6% at the nucleotide and amino acid levels, respectively. The diversity was higher within the CP coding region (30.7% and 19.5% at the nucleotide and amino acid levels, respectively) in 13 of the 22 isolates mentioned above. Among all the isolates, those from the Solomon Islands showed the greatest variability, whereas those from New Caledonia and Papua New Guinea were the least variable (Yang et al., 2003).
CSSV was first reported on cocoa in West Africa and Nigeria in 1936 and 1944, respectively (Adegbola, 1971; Murray, 1945), but it was not until 1993 that the complete nucleotide sequence was reported (Hagen et al., 1993). Subsequently, five new isolates have been sequenced (sharing a maximum of 29.4% identity), two from Togo and three from Ghana (Muller and Sackey, 2005).
Bougainvillea spectabilis chlorotic vein banding virus (BsCVBV), listed as Bougainvillea chlorotic vein banding virus by the ICTV, infecting Bougainvillea spectabilis Willd, was reported for the first time from Brazil (Rivas et al., 2005). The same authors also generated a partial sequence (465 bp) of the viral DNA. Simultaneously, another badnavirus from Taiwan was also reported to cause mottling, chlorosis and vein banding in bougainvillea; the partial (676 bp) sequence of the latter shared 82% identity to the former (Tsai et al., 2005). Two new isolates of BsCVBV were partially characterized from southern and northern India (Baranwal et al., 2010). A polymerase chain reaction (PCR)‐amplified 600‐bp fragment from the RT/RNaseH region from both viruses showed more than 70% identity to BsCVBV.
There are several reports of badnaviruses associated with plant diseases in the tropics, whose identities are still unclear. For example, two badnaviruses, Pineapple bacilliform comosus virus (PBCoV) and Pineapple bacilliform erectifolius virus (PBErV), and an endogenous badnavirus (Endogenous pineapple pararetrovirus‐1, ePPRV‐1) were suspected to be involved in Pineapple mealybug wilt disease from partial sequence analyses (Gambley et al., 2008). However, there is no information regarding the complete genome sequence and infectivity of the isolated viruses in their natural and/or experimental hosts. Some other reports of the occurrence of badnaviruses associated with disease symptoms include Piper yellow mottle virus (PYMV), reported from Brazil, Malaysia, Thailand, Philippines, Sri Lanka (Duarte et al., 2001; Lockhart et al., 1997) and India (Bhat et al., 2003), and a badnavirus‐like sequence amplified from Yucca elephantipes from Costa Rica and Guatemala (Clover et al., 2003).
In the genus Tungrovirus, RTBV infection, together with Rice tungro spherical virus (RTSV; genus Waikavirus; family Secoviridae), causes rice tungro disease. At present, 12 complete sequences of RTBV are available in the database, eight of which are from South‐East Asia and four from India. The genome sequences fall into two distinct groups, the South Asian and South‐East Asian groups, sharing approximately 75% sequence identity, the nucleotide identities within a group being about 95% (Fan et al., 1996; Mathur and Dasgupta, 2007, 2013; Nath et al., 2002).
Disease Incidence and Economic Losses
Diseases caused by badnaviruses are widespread in tropical regions of the world. The symptoms are mostly expressed in the aerial parts of the plants and yield losses in crop plants caused by badnavirus infections can reach 90% (for example, in BSV; Dahal et al., 2000; Daniells et al., 2001; Lassoudiere, 1974). CMBV is one of the major factors of citrus decline (Aparna et al., 2002), which can sometimes lead to total failure of production from large citrus plantations by causing the premature death of plants (Ahlawat et al., 1996). Trees affected by CMBV produce significantly fewer and poorer quality fruits containing less juice and ascorbic acid (Ahlawat et al., 1996). Cacao swollen shoot disease causes serious crop losses in many cacao‐growing areas of West Africa (Thresh, 1991). CSSV and Phytophthora pod‐rot are the two most important diseases of cacao in Nigeria (Adejumo, 2005). Thresh (1959) reported that large areas in Nigeria (approximately 70 000 ha) had to be abandoned because of devastation by CSSV. Although SCBV is known to occur in all sugarcane‐growing regions of the world, details regarding the extent of yield losses caused by the virus are not available (Autrey et al., 1995). The virus was detected in around 50% of samples of S. officinarum L. clones in the USA (Comstock and Lockhart, 1990), and in the majority of samples from Morocco and Hawaii (Lockhart and Autrey, 1988).
In certain parts of the province of Kerala in India, up to 100% incidence of pepper mosaic disease, which is associated with PYMV, has been reported in black pepper plantations (Bhat et al., 2003), and causes considerable seed yield losses (Sivaraman et al., 2002). Similarly, the presence of badnaviruses in yam in the South Pacific islands poses serious implications for the international movement of yam germplasm (Kenyon et al., 2001).
Rice tungro disease, caused by the tungrovirus species RTBV (Azzam and Chancellor, 2002), is one of the most devastating viral diseases affecting food production in the world, and is the most important viral disease of rice in South and South‐East Asia (Hull, 1996). The annual loss caused by rice tungro disease has been estimated to be in excess of $US109 (Herdt, 1991). In India, although losses in rice production caused by the disease have been estimated to be about 1% at the national level, the figure could be substantial in specific regions of the country and may result in grave food shortages (Muralidharan et al., 2003).
Symptoms
Various types and degrees of symptoms, ranging from mild leaf distortion to death, have been reported to be associated with badnavirus infection. The severity and types of symptoms also depend on the species as well as the developmental stage of the host. Most of the badnaviruses, however, cause some kind of malformation in the foliage, or parts thereof. Symptoms of badnaviruses may also vary depending on the virus strains/isolates. For example, BSV isolates differ widely in their symptoms, varying from faint broken chlorotic lines to the necrosis of emerging leaves. There are, nevertheless, extreme cases of total and/or premature death of plants in badnavirus infections, such as BSVs (Geering et al., 2005), TaBV (Jackson, 1978; Rodoni et al., 1994), CSSV (Longworth, 1963), SCBVs (Viswanathan et al., 1996), etc. However, sometimes, certain badnaviruses may also produce symptomless infection, for example, SCBV and CSSV on certain sugarcane and cacao varieties (Geijskes et al., 2002; Muller et al., 2001). Virus‐specific symptoms are sometimes observed during the infection of certain badnaviruses, such as irregularly shaped outgrowths on the petioles in infections of TaBV (Jackson, 1978; Rodoni et al., 1994).
Several badnaviruses are also known to produce symptoms in plant parts in addition to the foliage. Examples of such viruses include BSVs producing streak symptoms in the pseudostem, which vary in colour from yellow to brown to black (Lockhart, 1986), DaBV causing discoloration of the flesh of the yam tuber (described as ‘internal brown spot’; Harrison and Roberts, 1973) and some CSSV isolates inducing swelling on shoots (Longworth, 1963) and roots (Muller and Sackey, 2005; Posnette, 1947). In addition to chlorotic speckles starting at the leaf tip, plants infected with viruses, such as SCBV, display various other symptoms, such as stunted growth in severely infected plants, poor or no tillering, reduction in internodal elongation, bunchy top, etc. (Viswanathan et al., 1996). Symptoms of CMBV in citrus plants include mosaic on various parts of the plant, including the leaves, branches, fruits, etc., and irregular yellow or bright green patches against a dark green background and yellow flecking along the veins.
In rice tungro disease, RTBV is the symptom determinant and produces stunting and yellow–orange foliar coloration in tungro‐affected patches in rice fields. As a result of the existence of RTBV and RTSV together under natural conditions, and the mechanical transmission of RTBV alone not being successful, it was difficult, until recently, to assign symptoms to the individual viruses. However, this issue has been resolved with agroinoculation, showing that plants infected with RTBV only display mild stunting and yellowing symptoms, whereas the symptoms are accentuated on dual infection with RTBV and RTSV (Dasgupta et al., 1991).
Transmission
Although several biotic agents are known to be involved in the transmission of badnaviruses, they do not multiply in vectors and there is no trans‐ovarial transmission. All life stages of vectors can acquire and transmit the virus (ICTV description; http://ictvdb.bio‐mirror.cn/ICTVdB). Vectors include mealybugs, leafhoppers, aphids and nematodes. Some badnaviruses are transmitted mechanically and by dodder.
Fourteen species of mealybugs of the family Pseudococcidae (order Hemiptera; Roivainen, 1976) transmit badnaviruses in a semipersistent manner. One of the earliest reports on the mealybug transmission of badnaviruses was in TaBV (Brunt et al., 1990; Gollifer et al., 1977; James et al., 1973). Experiments with specific species of mealybug showed that, although certain badnaviruses are transmitted by multiple species, others are more specific with regard to the species of mealybug vector. For example, CSSV is transmitted by mealybug species Planococcus citri, Ferrisia virgata and P. njalensis (Dufour, 1988), but CMBV is known to be transmitted only by P. citri (Reddy and Ahlawat, 1997). Mealybug transmission of PYMV [transmitted by P. citri (De Silva et al., 2002) and F. virgata (Bhat et al., 2003)] and BSV‐OL, BSV‐Mys and BSV‐GF (by P. citri and P. ficus) (Geering et al., 2005; Meyer et al., 2008) has been reported. SCBV and DaBV are transmitted by S. sacchari (Lockhart and Autrey, 1988) and P. citri (Kenyon et al., 2008), respectively.
The rice tungro virus complex is transmitted by various species of green leafhopper (GLH; Nephotettix virescens, N. cincticeps and Resilia dorsalis) in a semipersistent manner (Hibino et al., 1979). Although the transmission of RTBV is dependent on the presence of RTSV, the nature of the ‘helper factor’ is unknown. Evidence suggests that the helper factor is possibly not physically a part of RTSV, but a diffusible entity, synthesized by the rice plant in response to infection (Hibino and Cabauatan, 1987).
CMBV could be transmitted experimentally by Myzus persicae Sulz and Aphis craccivora Koch in a nonpersistent manner (Ahlawat et al., 1985). Similarly, PYMV has been shown to be transmitted by lace bug vectors (Diconocoris distanti; De Silva et al., 2002). Soil‐inhabiting nematodes have been reported as active transmitting agents of CSSV (Afolami, 1980; Lana and Adegbola, 1977). There are, however, no other reports to date of any other badnavirus or tungrovirus being transmitted by nematodes.
Some badnaviruses show mechanical transmissibility of various degrees to natural and experimental hosts, such as CMBV to citrus (Ahlawat et al., 1996; Aparna et al., 2002) and several noncitrus species (Aparna et al., 2002; Pant and Ahlawat, 1997), an uncharacterized black pepper‐associated badnavirus onto healthy pepper (Bhat et al., 2003), DaBV to several Dioscorea species (Kenyon et al., 2008), etc. However, efforts to mechanically transmit PYMV to healthy hosts (De Silva et al., 2002) and BsCVBV to several experimental hosts (Rivas et al., 2005) have failed.
PYMV was transmitted by grafting to a healthy host (De Silva et al., 2002). The graft transmission of an uncharacterized badnavirus in black pepper plants showed typical symptoms of pepper yellow mottle disease (Bhat et al., 2003). Vegetative propagation is the major mode of transmission of BSVs (Harper et al., 2004). CMBV has been demonstrated to be graft and dodder transmissible to at least 13 citrus cultivars (Ahlawat et al., 1996). Indeed, the major mode of dissemination of CMBV is believed to be through the infected rootstocks of rangpur lime during grafting (Baranwal et al., 2005).
Genome Organization and Gene Functions
All members of the family Caulimoviridae replicate their DNA genomes by reverse transcribing their more‐than‐genome‐length terminally redundant transcript. This step has prompted the coining of the name ‘pararetrovirus’, based on their similarity to RNA‐containing retroviruses. The proposed mechanism of pararetroviral replication (Hohn et al., 1985; Pfeiffer and Hohn, 1983) involves the production of a more‐than‐genome‐length terminally redundant transcript by host DNA‐dependent RNA polymerase. The transcript serves as a template for both the translation of viral proteins and reverse transcription for replication of the genome. The ubiquitous tRNAmet serves as the primer for the reverse transcription (Boeke and Corces, 1989), and the opposite strand is primed from a purine‐rich region (Franck et al., 1980; Hull et al., 1986; Richards et al., 1981; Verver et al., 1987). The reverse transcription is polymerized by the virally encoded RT, and the positive strand by the same enzyme and the virally encoded RNaseH. The use of RT in the replication of badnaviruses may be the reason for the high degree of sequence variation in badnaviral genomes (Harper and Hull, 1998). The dsDNA genome of badnaviruses has two or more site‐specific discontinuities, one in each strand marking the tRNA binding site, the DNA existing primarily in an open circular form (Lot et al., 1991; Medberry et al., 1990).
The genome of badnaviruses is approximately 7.5 kb in length, which typically contains three ORFs. Whereas ORFs I and II code for proteins with a molecular mass of approximately 10 kDa, ORF III codes for a much larger polyprotein (approximately 250 kDa), which is post‐translationally cleaved into smaller functional units by the viral aspartic protease (AP, encoded by the same ORF) to produce proteins required for virus movement, assembly and replication (Hohn and Futterer, 1997). Peptide motifs have been identified within the viral proteins, which have limited consensus sequences across badnaviruses (Fig. 2). This general arrangement has been observed in TaBV (Yang et al., 2003), SCBVs (Bouhida et al., 1993), CSSV (Hagen et al., 1993), BSVs (Harper and Hull, 1998) and CMBV (Borah et al., 2009; Huang and Hartung, 2001; Johnson et al., 2012). However, several badnaviruses have additional ORFs, often overlapping the above three. For example, in TaBV, a fourth ORF (ORF IV) has been reported, coding for a putative protein of 13 kDa (Yang et al., 2003). In CSSV, two extra ORFs (ORF X, 3 kDa; ORF IV, 14 kDa; overlapping ORF III) have been reported; ORF X was found to be diverse among different isolates (Hagen et al., 1993). Similarly, six ORFs have been reported in CMBV (Borah et al., 2009; Huang and Hartung, 2001; Johnson et al., 2012). The locations of ORF IV of TaBV and CMBV and ORF X of CSSV are similar (Fig. 3).
Figure 2.
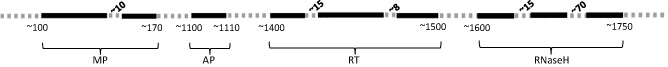
Arrangement of motifs present within open reading frame (ORF) III polyprotein. Bold continuous lines indicate the motifs and broken lines indicate the gaps. The numbers above the lines indicate the approximate lengths of nonhomologous amino acid residues, and those below the lines indicate the approximate start and end positions. AP, aspartic protease; MP, movement protein; RNaseH, ribonucleaseH; RT, reverse transcriptase.
Figure 3.
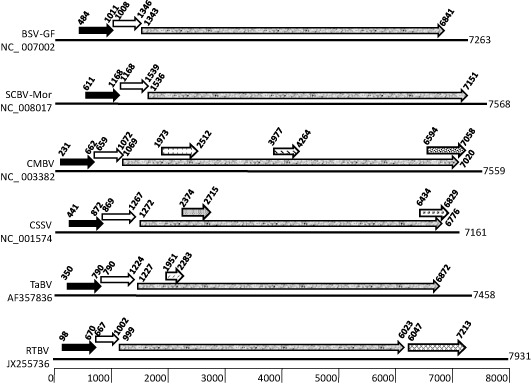
Organization of open reading frames (ORFs) in badnaviruses and tungrovirus. The homologous ORFs (arrows) are shaded in the same pattern, and their nucleotide positions along the genome are also indicated. The name of the virus and the National Center for Biotechnology Information (NCBI) accession number used for the analysis are shown on the left‐hand side, and the lengths of the genomes in nucleotides are indicated on the right‐hand side. The approximate nucleotide positions are scaled at the bottom. BSV‐GF, Banana streak virus cultivar Goldfinger; CMBV, Citrus yellow mosaic badnavirus; CSSV, Cacao swollen shoot virus; RTBV, Rice tungro bacilliform virus; SCBV‐Mor, Sugarcane bacilliform virus from Morocco; TaBV, Taro bacilliform virus.
Attempts to determine the functions of badnaviral ORFs have been reported mainly from CSSV. The carboxyl terminus of ORF II (132 amino acids) of CSSV is rich in lysine and proline, and also contains alanine residues, a composition somewhat similar to the histone‐like proteins. Partially purified full‐length and C‐terminal truncated versions of the CSSV ORF II proteins were able to interact with CSSV and other dsDNAs and with CSSV and other single‐stranded RNA (ssRNA) transcripts in a sequence‐nonspecific manner (Churchill and Travers, 1991). ORF II contains a ‘coiled‐coil’ self‐interaction domain to form a tetramer and a short, basic domain in the C‐terminus, providing sequence nonspecific ssRNA and dsDNA binding properties (Jacquot et al., 1996, 1997; Stavolone et al., 2001). This binding activity was progressively lost as the C‐terminus was gradually deleted (Churchill and Travers, 1991). In the cytoplasm, this protein could condense the CSSV DNA genome to permit the assembly of the bacilliform nucleocapsid. The CSSV ORF II‐derived protein has been proposed to be involved in viral DNA replication, viral RNA transport and protection (Jacquot et al., 1996). ORF III of CSSV encodes a polyprotein of 211 kDa which can be divided into three regions: region 1 coding for a putative viral movement protein, region 2 for a putative protein involved in cell‐to‐cell spread and region 3 for the viral capsid protein. Region 3 contains the consensus sequences for viral aspartate protease, RT and RNaseH (Hagen et al., 1993).
ORF I and ORF II of BSVs potentially encode two proteins of approximately 21 and 15 kDa, respectively. ORF III encodes a polyprotein of 208 kDa with functional motifs similar to other badnaviruses (Harper and Hull, 1998). Among BSV isolates, BSV‐Mys is exceptional with a nonconventional start codon for its ORF I (Geering et al., 2005), a feature also observed in the tungrovirus species RTBV. Recently, ORF I of BSV‐Cav from China was also found to have a non‐AUG start codon. The intergenic region (IR) of the isolate contains a large stem‐loop, which was presumed to contribute towards the proposed ribosome shunt in the virus (Zhuang et al., 2011), a feature common in all members of the family Caulimoviridae.
ORF III of CMBV codes for a 226‐kDa polypeptide and ORFs IV and V completely, and ORF VI partially, overlap ORF III (Borah et al., 2009; Huang and Hartung, 2001). The length of the IR varies from 600 to 731 bp and contains sequences such as the plant tRNAmet binding site and a TATAA consensus. Part of the 5′ untranslated region (nucleotide residues 122–231) contains short ORFs (sORFs) which can form stem‐loop structures bringing one sORF and the start codon of ORF I within five to ten nucleotides of each other, thereby possibly facilitating the translation of the latter by the ribosome shunt mechanism (Johnson et al., 2012). By computational analysis, the ORF II protein is predicted to have a nucleic acid binding and transposase‐like activity. Computational analyses also suggest that ORF IV is a component of the viral core complex, ORF V has a transcriptional regulator activity and ORF VI has a membrane localization function (Borah et al., 2009). In SCBV‐Mor, an additional intercellular transport domain was predicted in ORF III (Bouhida et al., 1993).
The only member of the genus tungrovirus, RTBV, has two site‐specific discontinuities in its genome, one on each strand of the circular dsDNA genome (Bao and Hull, 1992), which are the result of reverse transcription‐based DNA replication (Bao and Hull, 1994). The RTBV genome has four ORFs, the functions of a majority of which have not yet been completely elucidated. The first three ORFs partially overlap one another (Hay et al., 1991), whereas the fourth ORF is separated by an IR and is expressed from a spliced RNA (Futterer et al., 1994). The ORF II‐encoded protein has predicted nucleic acid binding (Jacquot et al., 1997) and CP binding domains at its C‐terminus (Herzog et al., 2000). The ORF III‐derived polyprotein is cleaved to generate four putative functional proteins, namely a movement protein, a CP, a protease and a RT/RNaseH (Hay et al., 1991). The CP has been localized between the amino acid residues 477 and 791 of the ORF III‐derived polyprotein (P194; Marmey et al., 1999). The viral protease, which cleaves P194, is associated with virions and is responsible for the formation of the capsid protein from the polyprotein (Marmey et al., 2005). Between the end of ORF IV and the beginning of ORF I, an IR harbours the single RTBV promoter, the polyadenylation signal, several sORFs (coding for 2–34 amino acids) and the splice donor site (Qu et al., 1991).
Genome Integration and Evolutionary Pathway
Genome sequencing efforts of various plants have revealed sequences resembling portions of integrated plant pararetroviruses (Harper et al., 1999; Jakowitsch et al., 1999; Lockhart and Jones, 2000; Richert‐Pöggeler and Shepherd, 1997; Staginnus and Richert‐Pöggeler, 2006 ). These integrated sequences, also called endogenous pararetroviruses (EPRVs), have been identified in the nuclear genomes of petunia, tobacco, potato, rice and tomato (Budiman et al., 2000; Gregor et al., 2004; Hansen et al., 2005; Jakowitsch et al., 1999; Kunii et al., 2004; Lockhart and Jones, 2000; Mao et al., 2000; Richert‐Pöggeler et al., 2003). It has been shown recently that some EPRVs have the potential to produce functional viruses (Provost and Iskra‐Caruana, 2006). The search for integrated BSV sequences producing episomal viral infection was promoted by the observation that several hybrids containing Musa acuminata (with A genome) and M. balbisiana (with B genome) genomes have a propensity to produce BSV infection after micropropagation (even when virus‐free source plants were used as explants), especially in AAAB tetraploids. Every banana species examined to date contains two different classes of integrated BSV DNAs: the first class consists of partial sequences (Geering et al., 2005; Ndowora et al., 1999) and the second class consists of multiple tandem copies of complete BSV genomes, interrupted by host genomic sequences, each complete copy having a similar, if not identical, structure (Geering et al., 2005). The latter class of integrated sequences is the source of BSV infections arising in tissue culture (Harper et al., 1999; Ndowora et al., 1999) mentioned above. Fluorescence in situ hybridization has revealed that integration occurs at two loci, one being approximately 150 kb and the other approximately 50 kb (Harper et al., 1999).
Although the exact mechanism of the integrated BSV sequences causing infection has not been elucidated, a speculative model can be suggested from the observed repeat structures (Ndowora et al., 1999). Because the ends of the BSV array harbour a pair of direct duplications and the region of rearranged BSV sequences of the array is also flanked by a pair of direct duplications, it is possible that an infectious genome is produced by recombination among these repeats. If this is the case, recombination between the repeats can delete the inserted endogenous sequences. A second recombination event between the terminal repeats can produce the free episomal circular BSV genome. As tissue culture is known to stimulate chromosomal aberrations probably involving recombination (Phillips et al., 1994), this model is consistent with tissue culture being a causal factor in the induction of infections from endogenous integrated EPRVs. An alternative model involving a recombination removing the inserted sequences, followed by transcription of the resulting integrated BSV genome, and reverse transcription of the transcript, has also been proposed (Ndowora et al., 1999).
It has been proposed that the integrated sequences confer a selective advantage to the plant by contributing towards plant virus resistance through the induction of transcriptional or post‐transcriptional gene silencing of homologous sequences of the invading virus (Hull et al., 2000). Supporting this hypothesis, Mette et al. (2002) showed that a reporter gene cloned under a tobacco EPRV promoter sequence was expressed in stably transformed Arabidopsis, but silenced in allotetraploid tobacco containing integrated sequences.
In order to determine the evolutionary pathway of integration of BSV DNA, Geering et al. (2005) sequenced such integrated DNA from several species and cultivars of banana. However, no commonality was observed between the sequences amplified from M. acuminata and M. balbisiana, suggesting that integration occurred following their speciation. The analysis of nucleotide substitution rates suggested that the integrated sequences evolved under a high degree of selective constraint, and that each distinct sequence resulted from an independent integration event (Geering et al., 2005).
The ability of some endogenous sequences of BSV to induce infections was demonstrated by Gayral et al. (2008). Subsequently, the distribution, insertion site polymorphism and evolution of integrated sequences of BSV‐GF and BSV‐IM were studied in 60 wild banana genotypes, and it was suggested that both BSV species integrated approximately 640 000 years ago. The analyses also indicated that the integrated sequences of these two isolates had experienced different selective pressures and therefore showed distinct patterns of rearrangement. In another study involving sequence analysis, at least 27 independent integration events were identified (Gayral et al., 2010).
A sequence showing approximately 50% nucleotide identity to TaBV in the RT/RNaseH coding region was detected in many virus‐free taro samples. This indicated a possibility of host genome integration of badnavirus infecting taro (Yang et al., 2003). A nonencapsidated caulimovirus sequence was amplified from pineapple, which was probably derived from an endogenous virus (Gambley et al., 2008).
Integrated RTBV‐like sequences have been reported from the rice genome. These sequences do not represent any intact ORF and are generally rearranged versions of the RTBV genome. Interestingly, rice varieties carrying low copy numbers of these sequences show high susceptibility to RTBV (Kunii et al., 2004). More recently, these EPRVs in rice have been found preferably in AT dinucleotide repeats, indicating a possible preference for such sites during integration (Liu et al., 2012).
Management
No serious attempts have been made for the management of badnaviruses. Conventional control measures (chemical, cultural and physical) against the vectors are often the only methods used. However, they are not effective in those cases in which the transmission is not well understood and/or occurs by more than one means, as well as in the case of the occurrence of integrated virus genomes. A major handicap in the development of control strategies against badnaviruses is the absence of comprehensive information on vectors or other modes of transmission, on the relationship between different strains, and their virulence and distribution.
With regard to nonconventional measures of management of badnaviruses, reports exist only on BSVs. BSV was eradicated from banana (cv. Williams BSJ, ITC.0579, AAA, Cavendish subgroup) through cryopreservation. Cryoprotective treatment resulted in 87% BSV‐free plants from meristematic lumps (Helliot et al., 2002, 2003). Later, the same group reported that three antiviral compounds [adefovir, tenofovir and 9‐(2‐phosphonomethoxyethyl)‐2,6‐diaminopurine] efficiently eradicated the episomal form of BSV from banana; up to 90% of plants regenerated from the meristems of BSV‐infected plants were found to be virus free following a 6‐month treatment with 10 μg/mL of any of the above compounds (Helliot et al., 2003).
Attempts to obtain resistance against rice tungro disease using classical breeding have not been durable (Dahal et al., 1990; Khush et al., 2004), mainly because of the absence of well‐characterized resistance genes in the rice germplasm (Azzam and Chancellor, 2002). Several attempts have been reported to obtain resistance against rice tungro disease by targeting RTBV, RTSV or GLH. Transgenic approaches against RTBV include the overexpression of two rice transcription factors, RF2a and RF2b (sequestered by the RTBV promoter during infection), leading to the amelioration of the shortage of these transcription factors (proposed to cause symptoms on RTBV infection), resulting in resistance (Dai et al., 2008). Similarly, although an earlier attempt to obtain CP‐mediated resistance against RTBV (Philippines isolate) produced disappointing results (Azzam et al., 1999), a later report using the CP gene derived from an Indian isolate of RTBV provided tolerance against the virus (Ganesan et al., 2009). Transgenic rice plants designed to express dsRNA corresponding to ORF IV of RTBV have been developed using RNA interference technology. These plants accumulate small RNAs against the RTBV transcript, indicating an effective RNA interference, and show a modest resistance against RTBV (Tyagi et al., 2008). This gene construct has now been diversified to two popular rice varieties by backcross breeding, effectively enhancing their value by adding the virus tolerance character (Roy et al., 2012).
Conclusions
The extreme heterogeneity of badnaviral and, to a lesser extent, tungroviral nucleotide sequences (Al‐Kaff and Covey, 1994; Geering et al., 2000; Harper et al., 2004, 2005; Jaufeerally‐Fakim et al., 2006; Kenyon et al., 2008; Lockhart and Olszewski, 1993; Nath et al., 2002) seems to suggest that this group of viruses, although ancient in origin, adapted so early and deeply to their respective hosts that most of the ancestral genes have been shed and new ones have been acquired for adaptation to lives restricted by the host. An extreme example is RTBV, which has been reported from only one species of rice (Oryza sativa), but between isolates from two rice‐growing regions of the world (South Asia and South‐East Asia) shows less than 70% overall sequence identity (Banerjee et al., 2012; Mathur and Dasgupta, 2013; Nath et al., 2002). Probably another consequence of the above heterogeneity is the varying numbers of ORFs in badnaviruses, all within their rather narrow genomic size range. Although most badnaviruses have three ORFs, some can have up to seven. Outside the common, rather short motifs of ORF III (CP, protease, RT/RNaseH), no regions share any significant homologies within the group. For example, CSSV, the closest relative of CMBV, shares only a maximum of 66.5% nucleotide sequence identity, even within ORF III, the most conserved ORF.
One of the major problems faced by badnaviral researchers is the lack of infectious clones for most members. Infectivity has been demonstrated only for CMBV (Huang and Hartung, 2001) amongst the tropical badnaviruses, and for RTBV (Dasgupta et al., 1991). Neither badnaviruses nor the badnaviral DNA can be mechanically inoculated onto the respective hosts, and hence agroinoculation has been the only successful method for the re‐introduction of cloned badnaviral genomes back to the host plant. Although the reason behind this lack of infectivity has not yet been addressed, it is probably because of the limitation and, possibly, adaptation of the virus particles to the vascular tissue of the host plant into which the vector delivers the viruses. Such possible adaptation, if any, should form the subject of active research in future.
Although information on the possible functions of some badnaviral and tungroviral ORFs exists, generalizations cannot be made on the possible functions of the corresponding ORFs of other badnaviruses for which such information is not available. The lack of infectious clones has also severely handicapped efforts to determine gene functions and their roles in pathogenesis by site‐directed mutagenesis, an approach which otherwise can be very fruitful. Moreover, the high degree of variability makes the detection and taxonomic designation of badnaviruses difficult, as exemplified by BSVs (Lheureux et al., 2007).
A majority of tropical badnaviruses infect plants which are vegetatively propagated. Hence, their importance as pathogens transmitted by infected plant propagules is an important consideration for quarantine purposes. Methods of detection of badnaviruses need to be made much more robust because of the atypical symptoms produced and the dependence of the results on the method used in sample preparation (Borah et al., 2008). More understanding of the molecular biology of the badnaviruses is therefore needed to achieve the ultimate goal of producing efficient and workable management strategies for the important diseases that they cause.
Acknowledgements
BKB, SS and AMAJ are grateful to the Council of Scientific and Industrial Research, New Delhi, and RK to the Indian Council of Medical Research, New Delhi, for research fellowships. Research in the laboratories of ID and DVRSG is supported by grants from the Department of Biotechnology (DBT), Government of India and the University of Delhi. There are no associated conflicts of interest of any author.
References
- Adegbola, M.O.K. (1971) Advances in the study of cacao swollen shoot virus disease in Nigeria In: Progress in Tree Crop Research In Nigeria (Cocoa Research Institute of Nigeria , ed.), pp. 134–143. Ibadan: Cocoa Research Institute of ; Nigeria. [Google Scholar]
- Adejumo, T.O. (2005) Crop protection strategies for major diseases of cocoa, coffee and cashew in Nigeria. Afr. J. Biotechnol. 4, 143–150. [Google Scholar]
- Afolami, S.O. (1980) Nematode survey of cocoa fields and nurseries in major growing areas of Nigeria and GES plots In: Annual Report Cocoa Research Institute of Nigeria (Cocoa Research Institute of Nigeria , ed.), pp. 49–51. Ibadan: Cocoa Research Institute of ; Nigeria. [Google Scholar]
- Ahlawat, Y.S. (1997) Viruses, greening bacterium and viroids associated with Citrus (Citrus species) decline in India. Indian J. Agr. Sci. 67, 51–57. [Google Scholar]
- Ahlawat, Y.S. , Chenulu, V.V. , Viswanath, S.M. , Pandey, P.K. and Bhagawati, K.N. (1985) Mosaic disease of citrus in India. Curr. Sci. 54, 873–874. [Google Scholar]
- Ahlawat, Y.S. , Pant, R.P. , Lockhart, B.E. , Srivastava, M. , Chakraborty, N.K. and Varma, A. (1996) Association of a badnavirus with citrus mosaic disease in India. Plant Dis. 80, 590–592. [Google Scholar]
- Al‐Kaff, N. and Covey, S.N. (1994) Variation in biological properties of cauliflower mosaic virus clones. J. Gen. Virol. 75, 3137–3145. [DOI] [PubMed] [Google Scholar]
- Aparna, G.S. , Gopal, K. , Subbaiah, K.V. , Reddy, M.N. and Sreenivasulu, M. (2002) First report of herbaceous hosts for Citrus yellow mosaic badnavirus from India. Plant Dis. 86, 920. [DOI] [PubMed] [Google Scholar]
- Autrey, L.J.C. , Boolell, S. , Lockhart, B.E.L. , Jones, P. and Nadif, A. (1995) The distribution of sugarcane bacilliform virus in various geographical regions In: Proceedings of XXI International Society of Sugarcane Technologists (Napompeth B. and Wisarath P., eds), pp. 527–541. Bangkok: Kasetsart University Press. [Google Scholar]
- Azzam, O. and Chancellor, T.B. (2002) The biology, epidemiology, and management of Rice tungro disease in Asia. Plant Dis. 86, 88–100. [DOI] [PubMed] [Google Scholar]
- Azzam, O. , Klöti, A. , Sta, C.F. , Fütterer, J. , Coloquio, E.L. , Potrykus, I. and Hull, R. (1999) Genetic engineering of rice for tungro resistance In: Proceedings of the Workshop on Rice Tungro Disease Management (Chancellor T.C.B., Azzam O. and Heong K.L., eds), pp. 39–44. Los Baños, Laguna, Philippines: International Rice Research Institute. [Google Scholar]
- Banerjee, A. , Roy, S. and Tarafdar, J. (2012) The large intergenic region of Rice tungro bacilliform virus evolved differentially amongst geographically distinguished isolates. Virus Genes, 44, 312–318. [DOI] [PubMed] [Google Scholar]
- Bao, Y. and Hull, R. (1992) Characterization of the discontinuities in rice tungro bacilliform virus DNA. J. Gen. Virol. 73, 1297–1301. [DOI] [PubMed] [Google Scholar]
- Bao, Y. and Hull, R. (1994) Replication intermediates of Rice tungro bacilliform virus DNA support a replication mechanism involving reverse transcription. Virology, 204, 626–633. [DOI] [PubMed] [Google Scholar]
- Baranwal, V.K. , Singh, J. , Ahlawat, Y.S. , Gopal, K. and Charaya, M.U. (2005) Citrus yellow mosaic virus is associated with mosaic disease in Rangpur lime rootstocks of citrus. Curr. Sci. 89, 1596–1599. [Google Scholar]
- Baranwal, V.K. , Arya, M. and Singh, J. (2010) First report of two distinct badnaviruses associated with Bougainvillea spectabilis in India. J. Gen. Plant Pathol. 76, 236–239. [Google Scholar]
- Bhat, A.I. , Devasahayam, S. , Sharma, Y.R. and Pant, R.P. (2003) Association of a badnavirus in black pepper (Piper nigrum L.) transmitted by mealybug (Ferrisia virgata) in India. Curr. Sci. 84, 1547–1550. [Google Scholar]
- Boeke, J.D. and Corces, V.G. (1989) Transcription and reverse transcription of retrotransposons. Annu. Rev. Microbiol. 43, 403–434. [DOI] [PubMed] [Google Scholar]
- Borah, B.K. , Johnson, A.M.A. , Sai Gopal, D.V.R. and Dasgupta, I. (2008) A comparison of four DNA extraction methods for the detection of Citrus yellow mosaic badnavirus from two species of citrus using PCR and dot‐blot hybridization. J. Virol. Methods, 151, 321–324. [DOI] [PubMed] [Google Scholar]
- Borah, B.K. , Johnson, A.M.A. , Sai Gopal, D.V.R. and Dasgupta, I. (2009) Cloning and computational analysis of complete genome sequences of Citrus yellow mosaic badna virus (CMBV) from acid lime and pummelo. Virus Genes, 39, 137–140. [DOI] [PubMed] [Google Scholar]
- Bouhida, M. , Lockhart, B.E.L. and Olszewski, N.E. (1993) An analysis of the complete sequence of a Sugarcane bacilliform virus genome infectious to banana and rice. J. Gen. Virol. 74, 15–22. [DOI] [PubMed] [Google Scholar]
- Braithwaite, K.S. , Egeskov, N.M. and Smith, G.R. (1995) Detection of Sugarcane bacilliform virus using the polymerase chain reaction. Plant Dis. 79, 792–796. [Google Scholar]
- Briddon, R.W. , Phillips, S. , Brunt, A. and Hull, R. (1999) Analysis of the sequence of Dioscorea alata bacilliform virus; comparison to other members of the badnavirus group. Virus Genes, 18, 277–283. [DOI] [PubMed] [Google Scholar]
- Brunt, A.A. , Crabtree, K. and Gibbs, A.J. (1990) Viruses of Tropical Plants, pp. 242–243. Wallingford, Oxfordshire: CABI. [Google Scholar]
- Budiman, M.A. , Mao, L. , Wood, T.C. and Wing, R.A. (2000) A deep‐coverage tomato BAC library and prospects towards development of an ATC framework for genome sequencing. Genome Res. 10, 129–136. [PMC free article] [PubMed] [Google Scholar]
- Cabauatan, P.Q. , Melcher, U. , Ishikawa, K. , Omura, T. , Hibino, H. , Koganezawa, H. and Azzam, O. (1999) Sequence changes in six variants of rice tungro bacilliform virus and their phylogenetic relationships. J. Gen. Virol. 80, 2229–2237. [DOI] [PubMed] [Google Scholar]
- Cherian, A.K. , Baranwal, V.K. , Malathi, V.G. , Pant, R.P. and Ahlawat, Y.S. (2004) Banana streak virus from India and its detection by polymerase chain reaction. Indian J. Biotechnol. 3, 409–413. [Google Scholar]
- Churchill, M.E.A. and Travers, A.A. (1991) Protein motifs that recognize structural features of DNA. Trends Biochem. Sci. 16, 92–97. [DOI] [PubMed] [Google Scholar]
- Clover, G.R.G. , Pearson, M.N. , Elliott, D.R. , Tang, Z. , Smales, T.E. and Alexander, B.J.R. (2003) Amplification of badnavirus‐like sequences from Yucca elephantipes . Australas. Plant Pathol. 32, 563–564. [Google Scholar]
- Comstock, J.C. and Lockhart, B.E.L. (1990) Widespread occurrence of Sugarcane bacilliform virus in U.S. sugarcane germplasm collection. Plant Dis. 74, 530. [Google Scholar]
- Dahal, G. , Hibino, H. , Cabunagan, R.C. , Tiongco, E.R. , Flores, Z.M. and Aguiero, V.M. (1990) Changes in cultivar reaction to tungro due to changes in ‘virulence’ of the leafhopper vector. Phytopathology, 80, 659–665. [Google Scholar]
- Dahal, G. , Ortiz, R. , Tenkouano, A. , Hughes, J.D.A. , Thottappilly, G. , Vuylsteke, D. and Lockhart, B.E.L. (2000) Relationship between natural occurrence of banana streak badnavirus and symptom expression, relative concentration of viral antigen, and yield characteristics of some micropropagated Musa spp. Plant Pathol. 49, 68–79. [Google Scholar]
- Dai, S. , Wei, X. , Alfonso, A.A. , Pei, L. , Duque, U.G. , Zhang, Z. , Babb, G.M. and Beachy, R.N. (2008) Transgenic rice plants that overexpress transcription factors RF2a and RF2b are tolerant to Rice tungro virus replication and disease. Proc. Natl. Acad. Sci. USA, 105, 21 012–21 016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dakshinamurti, V. and Reddy, G.S. (1975) Mosaic: a transmissible disorder of sweet oranges. Indian Phytopath. 28, 398–399. [Google Scholar]
- Daniells, J.W. , Geering, A.D.W. , Bryde, N.J. and Thomas, J.E. (2001) The effect of Banana streak virus on the growth and yield of dessert banana in tropical Australia. Ann. Appl. Biol. 139, 51–60. [Google Scholar]
- Dasgupta, I. , Hull, R. , Eastop, S. , Poggi‐pollini, C. , Blakebrough, M. , Boulton, M.I. and Davies, J.W. (1991) Rice tungro bacilliform virus DNA independently infects rice after Agrobacterium‐mediated transfer. J. Gen. Virol. 72, 1215–1221. [DOI] [PubMed] [Google Scholar]
- De Silva, D.P.P. , Jones, P. and Shaw, M.W. (2002) Identification and transmission of Piper yellow mottle virus and Cucumber mosaic virus infecting black pepper (Piper nigrum) in Sri Lanka. Plant Pathol. 51, 537–545. [Google Scholar]
- Duarte, M.L.R. , Albuquerque, F.C. and Chu, E.Y. (2001) New diseases affecting black pepper crop in Brazil. Int. Pepper Bull. Apr–Dec, 51–57. [Google Scholar]
- Dufour, B. (1988) Utilisation d'une méthode de transmission pour caractériser les forms togolaises du virus du cacao swollen shoot du cacaoyer. Café Cacao Thé, 32, 219–228. [Google Scholar]
- Eni, A.O. , Lava Kumar, P. , Asiedu, R. , Alabi, O.J. , Naidu, R.A. , Hughes, J.A. and Rey, M.E.C. (2008) First report of Cucumber mosaic virus in yams (Dioscorea spp.) in Ghana, Togo, and Republic of Benin in West Africa. Plant Dis. 92, 833. [DOI] [PubMed] [Google Scholar]
- Fan, Z. , Dahal, G. , Dasgupta, I. , Hay, J. and Hull, R. (1996) Variation in the genome of Rice tungro bacilliform virus: molecular characterization of six isolates. J. Gen. Virol. 77, 847–854. [DOI] [PubMed] [Google Scholar]
- Fauquet, C.M. , Mayo, M.A. , Maniloff, J. , Desselberger, U. and Ball, L.A. (2005) Virus Taxonomy: 8th Report of the International Committee of the Taxonomy of Viruses. Amsterdam: Elsevier Academic Press. [Google Scholar]
- Franck, A. , Guilley, H. , Jonard, G. , Richards, K.E. and Hirth, L. (1980) Nucleotide sequence of Cauliflower mosaic virus DNA. Cell, 21, 285–294. [DOI] [PubMed] [Google Scholar]
- Frison, E.A. and Putter, C.A.J. (1993) FAO/IBPGR Technical Guidelines for the Safe Movement of Cocoa Germplasm. Rome: Food and Agriculture Organization of the United Nations/International Board for Plant Genetic Resources. [Google Scholar]
- Futterer, J. , Potrykus, I. , Valles Brau, M.P. , Dasgupta, I. , Hull, R. and Hohn, T. (1994) Splicing in a plant pararetrovirus. Virology, 198, 663–670. [DOI] [PubMed] [Google Scholar]
- Gambley, C.F. , Geering, A.D.W. , Steele, V. and Thomas, J.E. (2008) Identification of viral and non‐viral reverse transcribing elements in pineapple (Ananas comosus), including members of two new badnavirus species. Arch. Virol. 153, 1599–1604. [DOI] [PubMed] [Google Scholar]
- Ganesan, U. , Suri, S.S. , Rajasubramaniam, S. , Rajam, M.V. and Dasgupta, I. (2009) Transgenic expression of coat protein gene of Rice tungro bacilliform virus in rice reduces the accumulation of viral DNA in inoculated plants. Virus Genes, 39, 113–119. [DOI] [PubMed] [Google Scholar]
- Gayral, P. , Noa‐Carrazana, J.‐C. , Lescot, M. , Lheureux, F. , Lockhart, B.E.L. , Matsumoto, T. , Piffanelli, P. and Iskra‐Caruana, M.‐L. (2008) A single Banana streak virus integration event in the banana genome as the origin of infectious endogenous pararetrovirus. J. Virol. 82, 6697–6710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gayral, P. , Blondin, L. , Guidolin, O. , Carreel, F. , Hippolyte, I. , Perrier, X. and Iskra‐Caruana, M.‐L. (2010) Evolution of endogenous sequences of Banana streak virus: what can we learn from Banana (Musa sp.) evolution? J. Virol. 84, 7346–7359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geering, A.D.W. , McMichael, L.A. , Dietzgen, R.G. and Thomas, J.E. (2000) Genetic diversity among Banana streak virus isolates from Australia. Phytopathology, 90, 921–927. [DOI] [PubMed] [Google Scholar]
- Geering, A.D.W. , Pooggin, M.M. , Olszewski, N.E. , Lockhart, B.E.L. and Thomas, J.E. (2005) Characterisation of Banana streak Mysore virus and evidence that its DNA is integrated in the B genome of cultivated Musa . Arch. Virol. 150, 787–796. [DOI] [PubMed] [Google Scholar]
- Geering, A.D.W. , Parry, J.N. and Thomas, J.E. (2011) Complete genome sequence of a novel badnavirus, Banana streak IM virus . Arch. Virol. 156, 733–737. [DOI] [PubMed] [Google Scholar]
- Geijskes, R.J. , Braithwaite, K.S. , Dale, J.L. , Harding, R.M. and Smith, G.R. (2002) Sequence analysis of an Australian isolate of Sugarcane bacilliform badnavirus . Arch. Virol. 147, 2393–2404. [DOI] [PubMed] [Google Scholar]
- Gollifer, D.E. , Jackson, G.V.H. , Dabek, A.J. , Plumb, R.T. and May, Y.Y. (1977) The occurrence and transmission of viruses of edible aroids in the Solomon Islands. PANS, 23, 171–177. [Google Scholar]
- Gregor, W. , Mette, M.‐F. , Staginnus, C. , Matzke, M.‐A. and Matzke, A.J.M. (2004) A distinct endogenous pararetrovirus family in Nicotiana tomentosiformis, a diploid progenitor of polyploid tobacco. Plant Physiol. 134, 1191–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagen, L.S. , Jacquemond, M. , Lepingle, A. , Lot, H. and Tepfer, M. (1993) Nucleotide sequence and genomic organization of Cacao swollen shoot virus . Virology, 196, 619–628. [DOI] [PubMed] [Google Scholar]
- Hansen, C.N. , Harper, G. and Heslop‐Harrison, J.S. (2005) Characterisation of pararetrovirus‐like sequences in the genome of potato (Solanum tuberosum). Cytogenet. Genome Res. 110, 559–565. [DOI] [PubMed] [Google Scholar]
- Harper, G. and Hull, R. (1998) Cloning and sequence analysis of Banana streak virus . Virus Genes, 17, 271–278. [DOI] [PubMed] [Google Scholar]
- Harper, G. , Osuji, J.O. , Heslop‐Harrison, J.S. and Hull, R. (1999) Integration of Banana streak badnavirus into the Musa genome: molecular and cytogenetic evidence. Virology, 255, 207–213. [DOI] [PubMed] [Google Scholar]
- Harper, G. , Hart, D. , Moult, S. and Hull, R. (2004) Banana streak virus is very diverse in Uganda. Virus Res. 100, 51–56. [DOI] [PubMed] [Google Scholar]
- Harper, G. , Hart, D. , Moult, S. , Hull, R. , Geering, A. and Thomas, J. (2005) The diversity of Banana streak virus isolates in Uganda. Arch. Virol. 150, 2407–2420. [DOI] [PubMed] [Google Scholar]
- Harrison, B.D. and Roberts, I.M. (1973) Association of virus‐like particles with internal brown spot of yam (Dioscorea alata). Trop. Agric. 50, 335–340. [Google Scholar]
- Hay, J.M. , Jones, M.C. , Blakebrough, M.L. , Dasgupta, I. , Davies, J.W. and Hull, R. (1991) An analysis of the sequence of an infectious clone of Rice tungro bacilliform virus, a plant pararetrovirus. Nucleic Acids Res. 19, 2615–2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helliot, B. , Panis, B. , Poumay, Y. , Swennen, R. , Lepoivre, P. and Frison, E. (2002) Cryopreservation for the elimination of Cucumber mosaic or Banana streak viruses from banana (Musa spp.). Plant Cell Rep. 20, 1117–1122. [Google Scholar]
- Helliot, B. , Panis, B. , Frison, E. , Clercq, E.D. , Swennen, R. , Lepoivre, P. and Neyts, J. (2003) The acyclic nucleoside phosphonate analogues, adefovir, tenofovir and PMEDAP, efficiently eliminate Banana streak virus from banana (Musa spp.). Antiviral Res. 59, 121–126. [DOI] [PubMed] [Google Scholar]
- Herdt, R.W. (1991) Research priorities for biotechnology In: Rice Biotechnology (Khush G.S. and Toennissen G.H., eds), pp. 19–54. Wallingford, Oxfordshire: CABI. [Google Scholar]
- Herzog, E. , Guerra‐Peraza, O. and Hohn, T. (2000) The Rice tungro bacilliform virus gene II product interacts with the coat protein domain of the viral gene III polyprotein. J. Virol. 74, 2073–2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibino, H. and Cabauatan, P.Q. (1987) Infectivity neutralization of Rice tungro‐associated viruses acquired by vector leafhoppers. Phytopathology, 77, 473–476. [Google Scholar]
- Hibino, H. , Saleh, H. and Roechan, M. (1979) Transmission of two types of Rice tungro‐associated viruses by insect vectors. Phytopathology, 69, 1266–1268. [Google Scholar]
- Hohn, T. and Futterer, J. (1997) The proteins and functions of plant pararetroviruses: knowns and unknowns. Crit. Rev. Plant Sci. 16, 133–161. [Google Scholar]
- Hohn, T. , Hohn, B. and Pfeiffer, P. (1985) Reverse transcription in CamV. Trends Biochem. Sci. 10, 205–209. [Google Scholar]
- Huang, Q. and Hartung, J.S. (2001) Cloning and sequences analysis of an infectious clone of Citrus yellow mosaic virus that can infect sweet orange via Agrobacterium‐mediated inoculation. J. Gen. Virol. 82, 2549–2558. [DOI] [PubMed] [Google Scholar]
- Hull, R. (1996) Molecular biology of Rice tungro viruses . Annu. Rev. Phytopathol. 34, 275–297. [DOI] [PubMed] [Google Scholar]
- Hull, R. , Sadler, J. and Longstaff, M. (1986) Sequence of Carnation etched ring virus DNA: comparison with Cauliflower mosaic virus and retroviruses . EMBO J. 5, 3083–3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull, R. , Harper, G. and Lockhart, B. (2000) Viral sequences integrated into plant genomes. Trends Plant Sci. 5, 362–365. [DOI] [PubMed] [Google Scholar]
- International Committee on Taxonomy of Viruses (ICTV) (2011) ICTV Master Species List 2009 Version 10 . Available at: http://talk.ictvonline.org/files/ictv_documents/m/msl/1231.aspx [accessed on June 3, 2013].
- Jackson, G.V.H. (1978) Alomae and bobone diseases of taro. South Pacific Commission Advisory Leaflet 8, 1–6. [Google Scholar]
- Jacquot, E. , Hagen, L.S. , Jacquemond, M. and Yot, P. (1996) The open reading frame 2 product of Cacao swollen shoot badnavirus is a nucleic acid‐binding protein. Virology, 225, 191–195. [DOI] [PubMed] [Google Scholar]
- Jacquot, E. , Keller, M. and Yot, P. (1997) A short basic domain supports a nucleic acid‐binding activity in the Rice tungro bacilliform virus open reading frame 2 product. Virology, 239, 352–359. [DOI] [PubMed] [Google Scholar]
- Jakowitsch, J. , Mette, M.F. , van der Winden, G. , Matzke, M.A. and Matzke, A.J.M. (1999) Integrated pararetroviral sequences define a unique class of dispersed repetitive DNA in plants. Proc. Natl. Acad. Sci. USA, 96, 13 241–13 246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James, M. , Kenten, R.H. and Woods, R.D. (1973) Virus‐like particles associated with two diseases of Colocasia esculenta (L.) Schott in the British Solomon Islands. J. Gen. Virol. 21, 145–153. [Google Scholar]
- Jaufeerally‐Fakim, Y. , Khorugdharry, A. and Harper, G. (2006) Genetic variants of Banana streak virus in Mauritius. Virus Res. 115, 91–98. [DOI] [PubMed] [Google Scholar]
- Johnson, A.M.A. , Borah, B.K. , Sai Gopal, D.V.R. and Dasgupta, I. (2012) Analysis of full‐length sequences of two Citrus yellow mosaic badnavirus isolates infecting Citrus jambhiri (rough lemon) and Citrus sinensis L. Osbeck (sweet orange) from a nursery in India. Virus Genes, 45, 600–605. [DOI] [PubMed] [Google Scholar]
- Jones, D.R. and Lockhart, B.E.L. (1993) Banana streak disease . Musa Fact Sheet No. 1. Montpellier: International Network for Improvement of Banana and Plantain. [Google Scholar]
- Kano, H. , Koizumi, M. , Noda, H. , Hibino, H. , Ishikawa, K. , Omura, T. , Cabauatan, P.Q. and Koganezawa, H. (1992) Nucleotide sequence of capsid protein gene of rice tungro bacilliform virus. Arch. Virol. 124, 157–163. [DOI] [PubMed] [Google Scholar]
- Kenyon, L. , Shoyinka, S.A. , Hughes, J.D.A. and Odu, B.O. (2001) An overview of viruses infecting yams in Sub‐Saharan Africa In: 1st Symposium of Plant Virology for Sub‐Saharan Africa (PVSSA), IITA, Ibadan, Nigeria, 4–8. [Google Scholar]
- Kenyon, L. , Lebas, B.S.M. and Seal, S.E. (2008) Yams (Dioscorea spp.) from the South Pacific Islands contain many novel badnaviruses: implications for international movement of yam germplasm. Arch. Virol. 153, 877–889. [DOI] [PubMed] [Google Scholar]
- Khush, G.S. , Angeles, E. , Virk, P.S. and Brar, D.S. (2004) Breeding rice for resistance to tungro virus at IRRI. SABRAO J. Breed. Genet. 36, 101–106. [Google Scholar]
- Kouakou, K. , Kebe, B.I. , Kouassi, N. , Ake, S. , Cilas, C. and Muller, E. (2012) Geographical distribution of Cacao swollen shoot virus molecular variability in Cote d'Ivoire. Plant Dis. 96, 1445–1450. [DOI] [PubMed] [Google Scholar]
- Kunii, M. , Kanda, M. , Nagano, H. , Uyeda, I. , Kishima, Y. and Sano, Y. (2004) Reconstruction of putative DNA virus from endogenous Rice tungro bacilliform virus‐like sequences in the rice genome: implications for integration and evolution. BMC Genomics, 5, 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lana, A.F. and Adegbola, M.O.K. (1977) Important virus diseases in West African crops. Rev. Plant Pathol. 56, 849–868. [Google Scholar]
- Lassoudiere, A. (1974) La mosaïque dite ‘à tirets’ du bananier ‘Poyo’ en Côte d'Ivoire. Fruits, 29, 347–349. [Google Scholar]
- Lheureux, F. , Laboureau, N. , Muller, E. , Lockhart, B.E.L. and Iskra‐Caruana, M.‐L. (2007) Molecular characterization of Banana streak acuminata Vietnam virus isolated from Musa acuminata siamea (banana cultivar). Arch. Virol. 152, 1409–1416. [DOI] [PubMed] [Google Scholar]
- Liu, R. , Koyanagi, K.O. , Chen, S. and Kishima, Y. (2012) Evolutionary force of AT‐rich repeats to trap genomic and episomal DNAs into the rice genome: lessons from endogenous pararetrovirus. Plant J. 72, 817–828. [DOI] [PubMed] [Google Scholar]
- Lockhart, B.E.L. (1986) Purification and serology of a bacilliform virus associated with banana streak disease. Phytopathology, 76, 995–999. [Google Scholar]
- Lockhart, B.E.L. and Autrey, L.J.C. (1988) Occurrence in sugarcane of a bacilliform virus related serologically to Banana streak virus . Plant Dis. 72, 230–233. [Google Scholar]
- Lockhart, B.E.L. and Jones, D.R. (2000) Banana streak virus In: Diseases of Banana, Abaca and Enset (Jones D.R., ed.), pp. 263–274. Wallingford, Oxfordshire: CABI Publishing. [Google Scholar]
- Lockhart, B.E.L. and Olszewski, N.E. (1993) Serological and genomic heterogeneity of banana streak badnavirus: implications for virus detection in Musa germplasm In: Breeding Banana and Plantain for Resistance to Diseases and Pests (Ganry J., ed.), pp. 105–113. Montpellier: CIRAD/INIBAP. [Google Scholar]
- Lockhart, B.E.L. , Kiratiya‐Angul, K. , Jones, P. , Eng, L. , De Silva, P. , Olszewski, N.E. , Lockhart, N. , Deema, N. and Sangalang, J. (1997) Identification of Piper yellow mottle virus, a mealybug‐transmitted badnavirus infecting Piper spp. in Southeast Asia. Eur. J. Plant Pathol. 103, 303–311. [Google Scholar]
- Longworth, J.F. (1963) Field trials on the effect of a Nigerian swollen shoot virus on the different cacao types. Ann. Appl. Biol. 52, 217–224. [Google Scholar]
- Lot, H. , Djiekpor, E. and Jacquemond, M. (1991) Characterisation of the genome of Cacao swollen shoot virus . J. Gen. Virol. 72, 1735–1739. [DOI] [PubMed] [Google Scholar]
- Mantelle, S.H. and Haque, S.Q. (1979) Internal brown spot disease of yams In: CARDI/ODM, St Augustine, Yam Virus Project, Bulletin No. 3, pp. 1–13. St. Augustine, Trinidad and Tobago: Caribbean Agricultural Research and Development Institute. [Google Scholar]
- Mao, L. , Wood, T.C. , Yu, Y. , Budiman, M.A. , Tomkins, J. , Woo, S.S. , Sasinowski, M. , Presting, G. , Frisch, D. , Goff, S. , Dean, R.A. and Wing, R.A. (2000) Rice transposable elements: a survey of 73,000 sequence tags connectors. Genome Res. 10, 982–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marmey, P. , Bothner, B. , Jacquot, E. , de Kochko, A. , Ong, C.A. , Yot, P. , Siuzdak, G. , Beachy, R.N. and Fauquet, C.M. (1999) Rice tungro bacilliform virus open reading frame 3 encodes a single 37‐kDa coat protein. Virology, 253, 319–326. [DOI] [PubMed] [Google Scholar]
- Marmey, P. , Rojas‐Mendoza, A. , deKochko, A. , Beachy, R.N. and Fauquet, C.M. (2005) Characterization of the protease domain of Rice tungro bacilliform virus responsible for the processing of the capsid protein from the polyprotein. Virol. J. 2, 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathur, S. and Dasgupta, I. (2007) Downstream promoter sequence of an Indian isolate of Rice tungro bacilliform virus alters tissue‐specific expression in host rice and acts differentially in heterologous system. Plant Mol. Biol. 65, 259–275. [DOI] [PubMed] [Google Scholar]
- Mathur, S. and Dasgupta, I. (2013) Further support of genetic conservation amongst isolates of Rice tungro bacilliform virus by sequence analysis of an isolate from north‐western India. Virus Genes, 46, 387–391. [DOI] [PubMed] [Google Scholar]
- Medberry, S.L. , Lockhart, B.E.L. and Olszewski, N.E. (1990) Properties of Commelina yellow mottle virus's complete DNA sequence, genomic discontinuities and transcript suggest that it is a pararetrovirus. Nucleic Acids Res. 18, 5505–5513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mette, M.F. , Kanno, T. , Aufsatz, W. , Jakowitsch, J. , van der Winden, J. , Matzke, M.A. and Matzke, A.J.M. (2002) Endogenous viral sequences and their potential contribution to heritable virus resistance in plants. EMBO J. 21, 461–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer, J.B. , Kasdorf, G.G.F. , Nel, L.H. and Pietersen, G. (2008) Transmission of activated‐episomal Banana streak OL (badna)virus (BSOLV) to cv. Williams Banana (Musa sp.) by three mealybug species. Plant Dis. 92, 1158–1163. [DOI] [PubMed] [Google Scholar]
- Muller, E. and Sackey, S. (2005) Molecular variability analysis of five new complete Cacao swollen shoot virus genomic sequences. Arch. Virol. 150, 53–66. [DOI] [PubMed] [Google Scholar]
- Muller, E. , Jacquot, E. and Yot, P. (2001) Early detection of Cacao swollen shoot virus using the polymerase chain reaction. J. Virol. Methods, 93, 15–22. [DOI] [PubMed] [Google Scholar]
- Muller, E. , Dupuya, V. , Blondina, L. , Bauffea, F. , Daugroisb, J.‐H. , Nathaliea, L. and Caruanaa, M.‐L. (2011) High molecular variability of Sugarcane bacilliform viruses in Guadeloupe implying the existence of at least three new species. Virus Res. 160, 414–419. [DOI] [PubMed] [Google Scholar]
- Muralidharan, K. , Krishnaveni, D. , Rajarajeshwari, N.V.L. and Prasad, A.S.R. (2003) Tungro epidemic and yield losses in paddy fields in India. Curr. Sci. 85, 1143–1147. [Google Scholar]
- Murray, D.F. (1945) First progress report of the Nigeria cocoa survey, April 1944–March 1945 (mimeographed).
- Nath, N. , Mathur, S. and Dasgupta, I. (2002) Molecular analysis of two complete Rice tungro bacilliform virus sequences from India. Arch. Virol. 147, 1173–1187. [DOI] [PubMed] [Google Scholar]
- Ndowora, T. , Dahal, G. , LaFleur, D. , Harper, G. , Hull, R. , Olsewski, N.E. and Lockhart, B. (1999) Evidence that badnavirus infection in Musa can originate from integrated pararetroviral sequences. Virology, 255, 214–220. [DOI] [PubMed] [Google Scholar]
- Pant, R.P. and Ahlawat, Y.S. (1997) Partial characterization of Citrus mosaic virus . Indian Phytopath. 50, 557–564. [Google Scholar]
- Pfeiffer, P. and Hohn, T. (1983) Involvement of reverse transcription in the replication of Cauliflower mosaic virus: a detailed model and test of some aspects. Cell, 33, 781–789. [DOI] [PubMed] [Google Scholar]
- Phillips, R.L. , Kaeppler, S.M. and Olhoft, P. (1994) Genetic instability of plant tissue cultures: breakdown of normal controls. Proc. Natl. Acad. Sci. USA, 91, 5222–5226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips, S. , Briddon, R.W. , Brunt, A.A. and Hull, R. (1999) The partial characterization of badnavirus infecting the Greater Asiatic or water yam (Dioscorea alata). J. Phytopathol. 147, 265–269. [Google Scholar]
- Posnette, A.F. (1947) Virus disease of cacao in West Africa. I. Cacao viruses 1A, 1B, 1C, 1D. Ann. Appl. Biol. 34, 388–402. [DOI] [PubMed] [Google Scholar]
- Provost, G.L. and Iskra‐Caruana, M.‐L. (2006) Improved detection of episomal Banana streak viruses by multiplex immunocapture PCR. J. Virol. Methods, 137, 7–13. [DOI] [PubMed] [Google Scholar]
- Quainoo, A.K. , Wetten, A.C. and Allainguillaume, J. (2008) Transmission of cocoa swollen shoot virus by seeds. J. Virol. Methods, 150, 45–49. [DOI] [PubMed] [Google Scholar]
- Qu, R.D. , Bhattacharyya, M. , Laco, G.S. , De Kochko, A. , Suba Rao, B.L. , Kaniewska, M.B. , Elmer, J.S. , Rochester, D.E. , Smith, C.E. and Beachy, R.N. (1991) Characterization of the genome of Rice tungro bacilliform virus: comparison with Commelina yellow mottle virus and Caulimoviruses . Virology, 185, 354–364. [DOI] [PubMed] [Google Scholar]
- Reddy, B.V.B. and Ahlawat, Y.S. (1997) Detection of the Citrus mosaic badnavirus by dot‐blot hybridization In: National Symposium on Citriculture, Nagpur, India. Abstract, p. 82. [Google Scholar]
- Richards, K.E. , Guilley, H. and Jonard, G. (1981) Further characterization of the dicontinuities in Cauliflower mosaic virus DNA (caulimovirus group). FEBS Lett. 134, 67–70. [DOI] [PubMed] [Google Scholar]
- Richert‐Pöggeler, K.R. and Shepherd, R.J. (1997) Petunia vein‐clearing virus: a plant pararetrovirus with the core sequences for an integrase function. Virology, 236, 137–146. [DOI] [PubMed] [Google Scholar]
- Richert‐Pöggeler, K.R. , Noreen, F. , Schwarzacher, T. , Harper, G. and Hohn, T. (2003) Induction of infectious Petunia vein clearing (pararetro) virus from endogenous provirus in petunia. EMBO J. 22, 4836–4845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivas, E.B. , Duarte, L.M.L. , Alexandre, A.V. , Fernandes, F.M.C. , Harakava, R. and Chagas, C.M. (2005) A new badnavirus species detected in Bougainvillea in Brazil. J. Gen. Plant Pathol. 71, 438–440. [Google Scholar]
- Rodoni, B.C. , Dale, J.L. and Harding, R.M. (1994) Review of alomae disease of taro. Papua New G. J. Agr. Forest Fish. 37, 14–18. [Google Scholar]
- Rodriguez‐Lema, E. , Rodriguez, D. , Fernandez, E. , Acevedo, R. and Lopez, D. (1985) Reporte de un nuevo virus de la cana de azucar. Cien. Inv. Agr. 23, 130. [Google Scholar]
- Roivainen, O. (1976) Transmission of cocoa viruses by mealy bugs (Homoptera: Pseudococcidae). J. Sci. Agric. Soc. Finland, 48, 433–453. [Google Scholar]
- Roy, S. , Banerjee, A. , Tarafdar, J. , Senapati, B.K. and Dasgupta, I. (2012) Transfer of transgenes for resistance to rice tungro disease into high yielding rice cultivars through gene based marker‐assisted selection. J. Agri. Sci. 150, 610–618. [Google Scholar]
- Seal, S. and Muller, E. (2007) Molecular analysis of a full‐length sequence of a new yam badnavirus from Dioscorea sansibarensis . Arch. Virol. 152, 819–825. [DOI] [PubMed] [Google Scholar]
- Sharma, S. and Dasgupta, I. (2012) Development of SYBR Green I based real time PCR assays for quantitative detection of Rice tungro bacilliform virus and Rice tungro spherical virus . J. Virol. Methods, 181, 86–92. [DOI] [PubMed] [Google Scholar]
- Sivaraman, K. , Madan, M.S. and Tarni Selvan, M. (2002) Black Pepper Guide . Calicut, Kerala, India: Directorate of Arecanut and Spices Development, Ministry of Agriculture.
- Smith, G.R. , Handley, J.A. , Harding, R.M. , Dale, J.L. , Gambley, C.F. and Braithwaite, K.S. (1996) Variability in sugarcane bacilliform and mosaic viruses and consequences for diagnosis In: Proceedings XXIII International Society of Sugarcane Technologists, Cartagena (Cock J.H. and Brekelbaum T., eds), pp. 390–396. Columbia, NY: Technica. [Google Scholar]
- Staginnus, C. and Richert‐Pöggeler, K.R. (2006) Endogenous pararetroviruses: two‐faced travelers in the plant genome. Trends Plant Sci. 11, 485–491. [DOI] [PubMed] [Google Scholar]
- Stavolone, L. , Herzog, E. , Leclerc, D. and Hohn, T. (2001) Tetramerization is a conserved feature of the virion‐associated protein in plant pararetroviruses. J. Virol. 75, 7739–7743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thresh, J.M. (1959) The control of cocoa swollen shoot disease in Nigeria. Trop. Agric. 36, 35–44. [Google Scholar]
- Thresh, J.M. (1991) The ecology of tropical plant viruses. Plant Pathol. 40, 324–339. [Google Scholar]
- Tsai, C. , Su, H. , Liao, Y. and Hung, T. (2005) First report of Bougainvillea spectabilis chlorotic vein‐banding virus infecting bougainvillea plants in Taiwan. Plant Dis. 89, 1363. [DOI] [PubMed] [Google Scholar]
- Tushmereirwe, W.K. , Karamura, E.B. and Karyeija, R. (1996) Banana streak virus (BSV) and an associated filamentous virus (unidentified) disease complex of Highland bananas in Uganda. Info. Musa, 5, 9–12. [Google Scholar]
- Tyagi, H. , Rajasubramaniam, S. , Rajam, M.V. and Dasgupta, I. (2008) RNA‐interference in rice against Rice tungro bacilliform virus results in its decreased accumulation in inoculated rice plants. Transgenic Res. 17, 897–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verver, J. , Schuns, P. , Hibi, T. and Goldbach, R. (1987) Characterization of the genome of Soybean chlorotic mottle virus . J. Gen. Virol. 68, 159–167. [Google Scholar]
- Viswanathan, R. , Alexander, K.C. and Garg, I.D. (1996) Detection of Sugarcane bacilliform virus in sugarcane germplasm. Acta Virol. 40, 5–8. [PubMed] [Google Scholar]
- Vuylsteke, D.R. , Chizala, C.T. and Lockhart, B.E.L. (1996) First report of banana streak virus disease in Malawi. Plant Dis. 80, 224. [Google Scholar]
- Yang, I.C. , Hafner, G.J. , Revill, P.A. , Dale, J.L. and Harding, R.M. (2003) Sequence diversity of South Pacific isolates of Taro bacilliform virus and the development of a PCR‐based diagnostic test. Arch. Virol. 148, 1957–1968. [DOI] [PubMed] [Google Scholar]
- Zhuang, J. , Wang, J. , Zhang, X. and Liu, Z. (2011) Molecular characterization of Banana streak virus isolate from Musa acuminata in China. Virol. Sin. 26, 393–402. [DOI] [PMC free article] [PubMed] [Google Scholar]


