SUMMARY
The gene CpSte11 of Cryphonectria parasitica, which encodes a yeast Ste11 homologue, was cloned and characterized. Gene replacement analysis revealed a high frequency of CpSte11 null mutants. When compared with the wild‐type parent strain, CpSte11 null mutants showed no difference in terms of growth rate or pigmentation. However, CpSte11 null mutants showed a marked decrease in both the number and size of stromal pustules on chestnut twigs. The virulence test showed that, in comparison with those of the wild‐type and virus‐infected hypovirulent strains, CpSte11 null mutants produced necrotic areas of intermediate size. Disruption of the CpSte11 gene also resulted in defects in female fertility. Down‐regulation of transcripts for the mating pheromone precursor gene, Mf2/2, and mating response transcription factors, such as cpst12 and pro1, was observed in CpSte11 null mutants. The down‐regulation of Mf2/2, cpst12 and pro1 was also observed in the mutant phenotype of Cpmk2, a mating response Fus3‐like mitogen‐activated protein kinase (MAPK) gene, but not in the mutant of Cpmk1, a high‐osmolarity glycerol Hog1‐like MAPK gene. These results indicate that the cloned CpSte11 gene is functionally involved in the mating response pathway and acts through downstream targets, including Cpmk2, cpst12, pro1 and Mf2/2. However, the characteristics of the CpSte11 null mutant were fully phenocopied only in the cpst12 null mutant, but not in other studied null mutants of components of the putative mating response pathway.
INTRODUCTION
Cryphonectria parasitica (Murrill) Barr, the causal agent of chestnut blight, was responsible for the almost complete disappearance of chestnut forests in North America at the beginning of the 20th century (Van Alfen, 1982; Van Alfen et al., 1975). Furthermore, disease severity has become more pronounced in other areas, including South Korea, where chestnut blight was once believed to cause only minor problems (Ju et al., 1999). However, strains containing double‐stranded RNA (dsRNA) Cryphonectria hypovirus 1 (CHV1) exhibit decreased virulence, a phenomenon referred to as ‘hypovirulence’ (Anagnostakis, 1982; Nuss, 1992; Van Alfen et al., 1975). In addition, strains containing CHV1 show diverse hypovirulence‐associated phenotypic changes, such as reduced sporulation, female fertility, pigmentation, laccase production and oxalate accumulation (Elliston, 1985; Havir and Anagnostakis, 1983; Rigling et al., 1989). The molecular basis for these phenotypic changes involves alterations in host transcriptional profiles in response to hypovirus infection (Allen et al., 2003; Allen and Nuss, 2004; Deng et al., 2007a; Kang et al., 2000; Kazmierczak et al., 1996). Considering the broad spectrum of phenotypic changes resulting from coordinated gene regulation, the perturbation of the fungal host signal transduction pathway on virus infection has been suggested. Accordingly, several fungal signal transduction pathways appear to be perturbed by hypovirus infection (Chen et al., 1996; Gao and Nuss, 1996; Kasahara and Nuss, 1997; Kim et al., 2002; Park et al., 2004; Turina et al., 2006).
Mitogen‐activated protein kinase (MAPK) signal transduction pathways are used by eukaryotic cells to transduce a wide variety of cellular signals through a stepwise phosphorylation relay. This cascade, which is conserved and occurs in a wide variety of organisms from yeast to humans (Herskowitz, 1995; Schaeffer and Weber, 1999), consists of three functionally interlinked protein kinases: MAPKKK (MAP kinase kinase kinase), MAPKK (MAP kinase kinase) and MAPK. Fungal MAPKs regulate numerous processes related to growth and differentiation (Xu, 2000). Moreover, MAPKs are involved in the pathogenicity of many plant pathogenic fungi (Zhao et al., 2007). Although five different MAPK pathways have been identified and studied in Saccharomyces cerevisiae, three MAPK homologues, involved in the pheromone response pathway (Fus3/Kss1), the cell wall integrity pathway (Slt2) and the osmoregulation/stress response pathway (Hog1), are generally found in other higher fungi (Xu, 2000; Zhao et al., 2007). Although the general functions of these three pathways are as listed above, they are anticipated to play more diverse roles in pathogenic fungi than their counterparts in S. cerevisiae. Indeed, depending on the fungus, different functions for the corresponding homologue in the MAPK pathway have been suggested. Thus, further studies on specific relationships between upstream inputs and downstream targets are needed to understand how extracellular stimuli are transduced to ultimately express a specific set of downstream target genes in fungi. Among three representative MAPK pathways, the MAPK signalling pathway, involved in the responsiveness to the mating pheromone, was of interest because the pheromone precursor genes were some of the initial genes shown to be down‐regulated by CHV1 (Powell and Van Alfen, 1987a). In addition, the homologue of the yeast Fus3/Kss1 pathway appears to be important in the pathogenicity of plant pathogenic fungi, but plays less of or no role in the virulence of human pathogens (Sakaguchi et al., 2010; Schamber et al., 2010; Zhao et al., 2007).
In C. parasitica, although several genes in MAPK pathways have been characterized (Choi et al., 2005; Park et al., 2004; Rostagno et al., 2009; Turina et al., 2006), no study on MAPKKK has been reported to date. Moreover, no study on the identification of signalling components in MAPK pathways to downstream transcription factors and target genes has been published. Compared with other MAPK pathways in C. parasitica, more is known about the putative mating response MAPK pathway. Accordingly, several putative downstream transcription factors, such as cpst12 and pro1, and downstream target genes, such as Mf2/1 and Mf2/2, encoding pheromone precursors have been analysed independently (Deng et al., 2007b; Sun et al., 2009; 1993, 1998). However, although CpMK2, the Fus3/Kss1 homologue from C. parasitica, has been characterized (Choi et al., 2005), no other component in the mating response MAPK pathway has been studied. In addition, the signalling components and downstream effectors of the mating response pathway are not yet fully understood.
In the current study, we cloned a yeast Ste11 homologue, a mating response MAPKKK, from C. parasitica and examined its biological functions. In addition, its relationships with possible downstream transcriptional factors and targets were examined to determine the pathway specificity and its role as a component in a specific regulatory hierarchy.
RESULTS
Characteristics of the CpSte11 gene
Three different MAPKKK genes were identified in the genome of C. parasitica by inspection of the draft genome sequence (http://genome.jgi‐psf.org/Crypa2/Crypa2.home.html). Polymerase chain reaction (PCR) amplification of the MAPKKK gene that showed high similarity to other MAPKKKs in the mating response pathway resulted in the expected 4.4‐kbp fragment. Southern blot analysis of total genomic DNA under high stringency with the cloned PCR amplicon as a probe suggested that a single copy of the cloned gene was present in the C. parasitica genome (data not shown). Based on the genomic sequence analysis, a near full‐length cDNA clone was obtained using reverse transcription‐polymerase chain reaction (RT‐PCR), with the primer pair CpSte11‐mF1 and CpSte11‐mR1, at nucleotide positions −6 to 12 and 2795 to 2813 (relative to the start codon), respectively, and the resulting 2752‐bp amplicon was cloned into the pGEM‐T Easy Vector (Promega, Madison, WI, USA). A sequence comparison with the corresponding genomic sequence revealed that the cloned gene contained two exons, with an intervening sequence of 67 bp.
Sequence analysis of the cloned cDNA showed an open reading frame (ORF) of 916 amino acids, with an estimated molecular mass of 101.6 kDa and a pI value of 8.72 (GenBank accession number for CpSte11 is AEC04750), named ‘CpSte11’ (a yeast Ste11 homologue in C. parasitica). Analysis of the deduced CpSte11 gene product (CpSTE11) revealed the presence of three conserved domains: an N‐terminal ‘sterile α motif’ (SAM) domain, related to a phosphorylation site between amino acids 66 and 128, a Ras‐association (RA) domain of the ubiquitin (UBQ) superfamily between amino acids 266 and 353, and the C‐terminal catalytic domain of serine/threonine protein kinases of the protein kinase C (PKC)‐like superfamily between amino acids 652 and 912 (Fig. 1).
Figure 1.
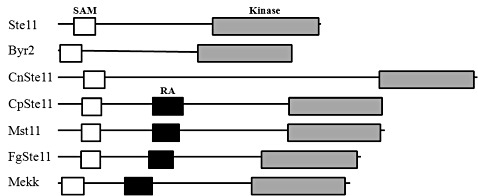
Characteristics of the CpSte11 gene from Cryphonectria parasitica. Schematic organization of CpSTE11 and its homologues from other fungi: Saccharomyces cerevisiae Ste11, Schizosaccharomyces pombe Byr2, Cryptococcus neoformans CnSte11, Magnaporthe grisea Mst11, Fusarium graminearum FgSte11 and Pneumocystis carinii Mekk. The sterile α motif (SAM) domain, Ras‐association (RA) domain and C‐terminal kinase domain are represented by white, black and grey boxes, respectively.
Regulation of CpSte11 gene expression
Northern blot analysis using RNA samples from standard liquid cultures revealed that CpSte11 was expressed at very low levels in virus‐free EP155/2 and its isogenic virus‐containing UEP1 (data not shown). Thus, quantitative real‐time RT‐PCR was used for the expression profile. As shown in Fig. 2, the expression of CpSte11 increased as growth proceeded in EP155/2, reached a peak at 3 days of growth, and then decreased gradually thereafter. However, significant down‐regulation in the expression level of CpSte11 was observed in the hypovirulent UEP1 strain, indicating that hypovirus infection altered the accumulation of the CpSte11 transcript in C. parasitica.
Figure 2.
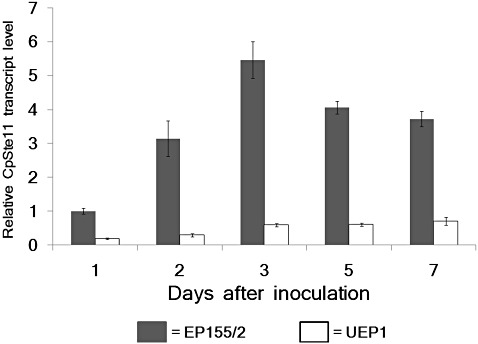
Quantitative real‐time reverse transcription‐polymerase chain reaction (RT‐PCR) analysis of CpSte11 transcript levels relative to levels of glyceraldehyde‐3‐phosphate dehydrogenase (gpd). The values on the y‐axis were normalized to the CpSte11 transcript levels in 1‐day cultured EP155/2 strain, with standard deviations, based on three independent measurements of two independent RNA preparations of the same sample, indicated by the error bars. The wild‐type EP155/2 strain and its isogenic hypovirus‐infected UEP1 strain are represented by filled and open bars, respectively.
Construction of a CpSte11 null mutant
To examine the effects of deleting the CpSte11 gene, a CpSte11 null mutant was constructed by site‐directed recombination during integrative transformation. A linear DNA containing a disrupted CpSte11 gene with 1095‐bp 5′ and 1322‐bp 3′ flanking regions was used to transform the virus‐free C. parasitica EP155/2 strain. In total, 54 stable single‐spored transformants were screened by PCR using the outer gene‐specific and inner hph primers, which corresponded to −1611 to −1591 and 1000 to 1017 (relative to the start codon of CpSte11 and hph, respectively). Six transformants showed a PCR amplicon of the expected size, 2.5 kb, corresponding to disrupted alleles of the CpSte11 gene. To confirm the pure replacement of the wild‐type allele with the disrupted allele, these six transformants were further examined by Southern blot analysis (Fig. 3). As shown in Fig. 3, the hybridization pattern of SalI‐digested genomic DNA of the CpSte11 null mutant with a probe prepared using the 1.1‐kb EcoRI‐digested CpSte11 fragment differed from the wild‐type, suggesting that the transforming vector had integrated at the CpSte11 locus by site‐directed homologous recombination. Moreover, SalI‐digested genomic DNA of the transformants showed a hybridizing band at 5.3 kb, corresponding to the expected size of the replaced allele, and the probe also hybridized to a 0.4‐kb SalI/BamHI‐hph fragment. In addition, the hybridization pattern of EcoRI‐ and SacI‐digested genomic DNA of the transformants with the 1.1‐kb probe showed hybridizing bands at 2.3 kb and 7.2 kb, instead of 8.7 kb and 5.2 kb, respectively, in the wild‐type (Fig. 3), in good agreement with the results of SalI digestion, indicating that the CpSte11 gene was replaced with part of the transforming vector, rather than being disrupted.
Figure 3.
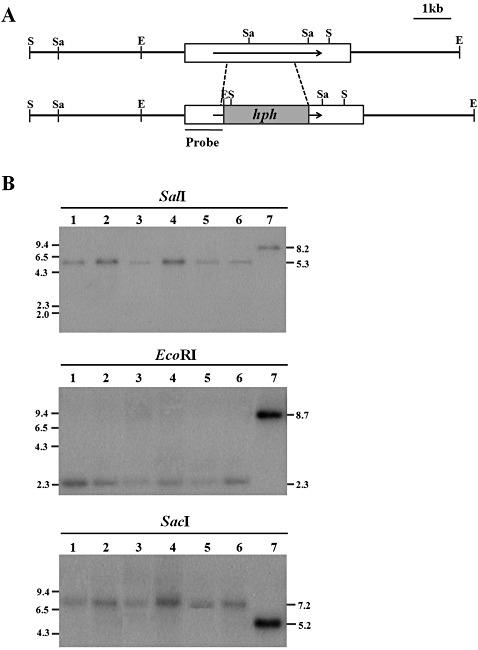
Restriction map and Southern blot analyses of CpSte11 null mutants and wild‐type EP155/2. (A) Restriction map of the CpSte11 genomic region and the expected replaced allele of the CpSte11 gene as a result of double homologous recombination. Boxes indicate the genomic region for the construction of the gene replacement vector pDSTE11, which replaced the internal 1932 bp with the hygromycin B resistance gene (hph), resulting in 1095‐ and 1322‐bp fragments as the 5′ and 3′ flanking regions, respectively. Arrows indicate the direction of transcription. E, EcoRI; S, SalI; Sa, SacI. (B) Southern blot analysis of the wild‐type EP155/2 strain (lane 7) and the CpSte11 null mutants TdSTE11‐3, ‐17, ‐56, ‐74, ‐78 and ‐85 (lanes 1–6). Enzymes used to digest the DNA samples are indicated above the lane numbers and the probe is indicated on the restriction map in (A). Numbers on the right and left indicate the size in kilobases. The CpSte11 null mutants underwent the desired replacement at CpSte11, as indicated by the expected changes in the sizes of the bands that hybridized with the probe.
As shown in Fig. 3, the six selected transformants exhibited identical hybridizing patterns, indicating that all transformants resulted from the same gene replacement of the CpSte11 gene with the hph gene in the transforming vector.
Phenotypic characteristics of CpSte11 null mutants
In the CpSte11 null mutants, no difference in radial growth was observed under standard growth conditions (under constant low‐level light at 25 °C; Fig. 4A). No change in pigmentation was observed as a result of a loss of function of the CpSte11 gene. However, asexual sporulation of the CpSte11 null mutants differed from that of the wild‐type strain EP155/2. The numbers of conidia per plate produced by the CpSte11 null mutants were significantly higher than those of EP155/2 (Table 1).
Figure 4.
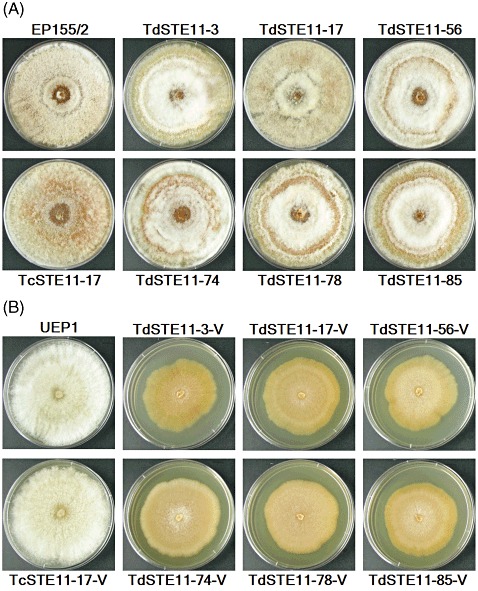
Colony morphology of the CpSte11 null mutant strains. Colonies after 15 days of culture are shown. The strains indicated are the wild‐type EP155/2, six CpSte11 null mutants (TdSTE11‐3, ‐17, ‐56, ‐74, ‐78 and ‐85) and the complemented strain of TdSTE11‐17 (TcSTE11‐17) (A). (B) Corresponding hypovirus‐transferred strains of (A) (UEP1, TdSTE11‐3‐V, ‐17‐V, ‐56‐V, ‐74‐V, ‐78‐V, ‐85‐V and TcSTE11‐17‐V).
Table 1.
Characteristics of null mutants compared with the wild‐type, complemented and hypovirulent strains.*
| Strain | Characteristic | Canker area (mm2)† | No. of conidiospores/mL‡ |
|---|---|---|---|
| EP155/2 | Wild‐type | 127.88 ± 20.26a§ | 2.37 × 108± 2.13 × 108a§ |
| TdSTE11‐3 | This study | 64.76 ± 6.24b | 9.01 × 108± 3.32 × 108b |
| TdSTE11‐17 | This study | 62.57 ± 11.30b | 6.43 × 108± 1.47 × 108b |
| TdSTE11‐56 | This study | 66.58 ± 14.87b | 7.28 × 108± 1.50 × 108b |
| TdSTE11‐74 | This study | 49.71 ± 16.07b | 1.06 × 109± 3.43 × 108b |
| TdSTE11‐78 | This study | 54.66 ± 21.75b | 7.98 × 108± 2.70 × 108b |
| TdSTE11‐85 | This study | 53.23 ± 8.27b | 9.58 × 108± 3.49 × 108b |
| TcSTE11‐17 | This study | 111.25 ± 22.55a | 1.53 × 108± 3.13 × 108a |
| UEP1 | Hypovirulent | 21.24 ± 2.55c | 2.23 × 106± 0.53 × 106c |
Culture conditions have been described previously (Kim et al., 1995).
The 5‐mm agar plugs with mycelium were placed on excised bark, and canker areas were measured 7 days after inoculation, as described previously (Lee et al., 1992). Data are the averages of replicates from four trials of each virulence test per strain.
The conidia from each plate were harvested with 10 mL of sterile water, and the number of conidia per plate was determined using a haemocytometer. Four replicates of each strain were used and the experiment was repeated three times.
Values are means ± standard deviation. Values followed by the same letter are not significantly different (P < 0.05), as determined by Duncan's multiple‐range test.
The laccase activity of the strains was examined on plates containing tannic acid. Compared with the wild‐type, no significant change in the dark‐brown‐coloured zones was observed in the CpSte11 null mutants, suggesting that laccase production was unaffected by the CpSte11 mutation (data not shown). No significant changes in temperature sensitivity, osmosensitivity and responses to oxidative stress were observed in the CpSte11 null mutants compared with the wild‐type (data not shown). These results suggest that responses to stress, including tannic acid, temperature, hypertonic conditions and oxidative stress, were not markedly changed by the loss of function of the CpSte11 gene.
To ensure that the phenotypic change attributed to the CpSte11 mutation was caused by the gene replacement event, the enhanced conidiation of two randomly selected CpSte11 null mutants was complemented in trans with a wild‐type allele of CpSte11. The geneticin‐resistant transformants that had received a wild‐type CpSte11 produced comparable numbers of conidia to those of the wild‐type EP155/2 (Fig. 4). PCR analyses revealed that the complemented transformants contained an additional wild‐type CpSte11 allele. Thus, in addition to multiple mutants showing the associated phenotypes, functional complementation using a wild‐type CpSte11 gene unequivocally confirmed that the phenotypic change in the mutant was caused by the disruption of CpSte11.
Effect of hypoviral infection on colony morphology in CpSte11 null mutants
To examine the biological functions of the CpSte11 gene related to hypovirus infection, we compared the phenotypic changes between virus‐free and virus‐containing isogenic CpSte11 null mutants. The transfer of hypovirus CHV1‐713 to the CpSte11 null mutants resulted in a lower growth rate, with less aerial mycelia showing dark brown rather than bright yellow pigmentation, as well as reduced conidiation (Fig. 4B). Moreover, the appearance of fast‐growing and normal‐pigmented sectors, indicating virus curing, as observed in the pro1 null mutant (Sun et al., 2009), did not occur in colonies of the virus‐containing CpSte11 null mutants. Stability in vertical inheritance of the hypovirus was examined by isolation of dsRNA from 20 randomly selected single‐spored progenies of the newly infected CpSte11 null mutants. All but one showed the presence of dsRNA on ethidium bromide‐stained gel (data not shown). These results suggest that the horizontal and vertical transmission of the hypovirus does not require the presence of the CpSte11 gene, and that CpSte11 is not implicated in the maintenance of hypovirus infection.
Impaired colonization of the CpSte11 null mutant on chestnut bark
The CpSte11 null mutants differed from the wild‐type EP155/2 in their ability to colonize chestnut twigs embedded in agar. When inoculated on the agar substrate adjacent to a sterile chestnut twig, all CpSte11 null mutants showed severely retarded colonization, characterized by a decreased number of stromal pustules and less extended stroma. Stromal pustules of the CpSte11 null mutants erupted primarily around broken bark or prominent lenticels (Fig. 5). Thus, the CpSte11 null mutants seemed to have difficulty in effectively colonizing and erupting through the bark of sterile chestnut twigs.
Figure 5.
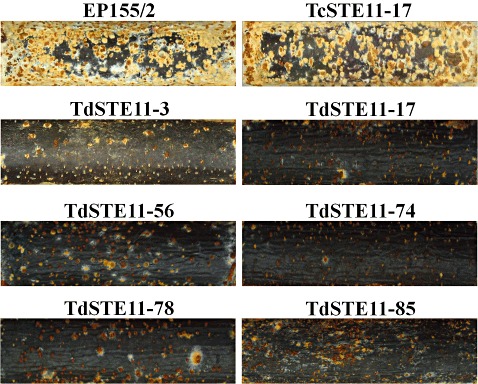
Stroma pustules on chestnut twigs. The wild‐type strain EP155/2 and the complemented strain of TdSTE11‐17 (TcSTE11‐17) were able to effectively colonize and erupt through the sterile chestnut twig, whereas the CpSte11 null mutant strains showed a decreased number of stromal pustules and less extended stroma.
The CpSte11 null mutants were inoculated on excised chestnut tree bark to test whether disruption of the CpSte11 gene affected virulence. The sizes of the necrotic areas on excised bark induced by the CpSte11 null mutants were smaller than those of the wild‐type strain EP155/2, indicating that the CpSte11 null mutants were less virulent than EP155/2 (Table 1). Thus, the CpSte11 null mutants had difficulty in effectively colonizing and growing under the bark of sterile chestnut twigs.
Mating ability of the CpSte11 null mutant
The CpSte11 null mutants were examined for their capability to serve as either male or female parent in sexual crosses with an opposite mating type strain EP6 (Mat‐1). Spermatization of strain EP6 with conidia from wild‐type EP155/2 or the two CpSte11 null mutants resulted in a similar number of perithecia and viable ascospores. However, when the CpSte11 null mutants were grown as a female and crossed with conidia from strain EP6, no mating product was observed. Although efficient colonization may be important for the mating process, it appears unlikely to be a necessary prerequisite for mating to occur, because the cryparin mutant, showing defects in eruption of stromal pustules, mated well as a female partner. Thus, the CpSte11 null mutants have defects in mating ability as a female partner.
Changes in the expression of other genes in the putative mating pathway of the CpSte11 null mutants
Because three different genes, Mf2/2, cpst12 and pro1, have been analysed and identified as important components in the putative mating pathway, the expression patterns of these genes were analysed in the CpSte11 null mutant by real‐time RT‐PCR. As shown in Fig. 6, all three genes were down‐regulated in the CpSte11 null mutant, indicating that the CpSte11 gene positively controls these three genes. In addition, the down‐regulation of two transcription factors, cpst12 and pro1, and a downstream target gene, Mf2/2, was observed in the Cpmk2 null mutant, a mutant in the mating response MAPK gene. However, no significant change in the expression of the two transcription factors or the Mf2/2 gene was observed in the null mutant of the Cpmk1 gene, which is another MAPK gene in the high osmotic response pathway (Park et al., 2004).
Figure 6.
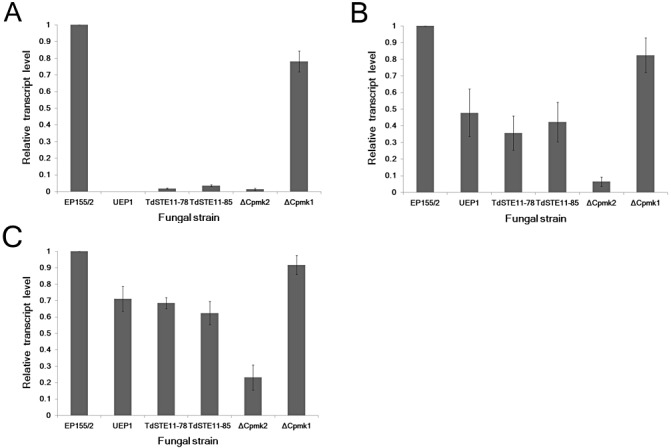
Changes in expression of Mf2/2, cpst12 and pro1 in the CpSte11 null mutants compared with the wild‐type (EP155/2), hypovirulent (UEP1), Cpmk1 and Cpmk2 mutant strains. Quantitative real‐time reverse transcription‐polymerase chain reaction (RT‐PCR) analysis of Mf2/2 (A), cpst12 (B) and pro1 (C) transcript levels relative to levels of glyceraldehyde‐3‐phosphate dehydrogenase (gpd). The values on the y‐axis were normalized to the transcript levels of the corresponding gene in 5‐day cultured EP155/2 strain, with standard deviations, based on three independent measurements of two independent RNA preparations, indicated by the error bars.
Western blot analysis using phospho‐specific and phospho‐nonspecific MAPK antibodies was conducted to determine changes in the phosphorylation of CpMK2. As CpMK2 contains the characteristic residues TEYVATR surrounding the dual phosphorylation site (italic), and the identical sequence is present in ERK1 (p44) and ERK2 (p42), the phospho‐p44/42 MAPK (Erk1 and Erk2) antibody was employed, as described previously (Choi et al., 2005). As shown in Fig. 7, phospho‐nonspecific antibodies revealed two major bands immunospecific to the p44/42 MAPK antibody, among which the 42‐kDa band was demonstrated to be specific to CpMK2 (Park et al., 2004). However, the level of the protein product of the Cpmk2 gene in the CpSte11 null mutant was lower than in the wild‐type. Furthermore, phosphorylation of CpMK2 was reduced significantly based on the near‐absence of phospho‐specific CpMK2 bands in the CpSte11 null mutant. Although differences in the levels of reduction of mRNA accumulation existed among two transcription factors and a downstream target gene, the specific down‐regulation of all three genes in both CpSte11 and Cpmk2 null mutants and Western blot analysis suggested that CpSte11 specifically affects a signalling cascade involving Cpmk2, and that the CpSte11 gene controls downstream target genes through the action of the Cpmk2 gene.
Figure 7.
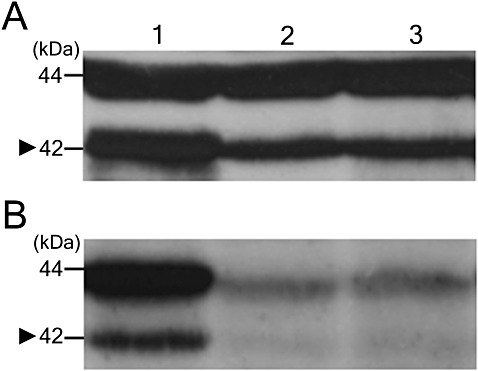
Immunoblot assay of phosphorylation of CpMK2 in the CpSte11 null mutant strains. Autoradiogram using the phospho‐nonspecific (A) and phospho‐specific (B) p44/42 mitogen‐activated protein kinase (MAPK) antibodies. Lanes 1, 2 and 3 contain protein samples from the wild‐type strain EP155/2, TdSTE11‐3 and TdSTE11‐17, respectively. The arrowhead indicates CpMK2.
DISCUSSION
In eukaryotic cells, including fungi, the MAPK cascade is well conserved and involved in the transduction of a variety of extracellular signals regulating different developmental processes. The sequential activation of the MAPK cascade eventually results in the activation of transcription factors and the expression of sets of downstream target genes in response to external stimuli. Although five different MAPK pathways have been identified in S. cerevisiae, three representative MAPK pathways, pheromone response, cell wall integrity and osmoregulation/stress response, exist in fungal pathogens (Zhao et al., 2007). The analysis of a C. parasitica genome database has revealed the presence of at least six MAPKKK‐, three MAPKK‐ and four MAPK‐related genes. blast searches have indicated that, among the six MAPKKKs, two show high similarity (E‐value < 1e‐177) to the known MAPKKKs involved in the pheromone response and cell wall integrity. The others show similarity to other MAPKKK‐related proteins with, as yet, uncharacterized functions. The three MAPKKs appear to be homologues for the components in the three known MAPK pathways (Rostagno et al., 2009). Among the four MAPK‐related genes, one has high similarity to Ime2, a meiosis induction MAPK‐related kinase of S. cerevisiae (Garrido et al., 2004). The other three MAPKs show high similarity to the corresponding homologues of the pheromone response, cell wall integrity and osmoregulation/stress response MAPK pathways. Like other filamentous fungi, C. parasitica appears to have only one pheromone response MAPK, which is homologous to yeast Fus3. In C. parasitica, homologues to Fus3 and Hog1 have been characterized previously (Choi et al., 2005; Park et al., 2004) and hypoviral regulation of MAPKK belonging to the cell wall integrity pathway has been demonstrated (Turina et al., 2006). However, no study at the level of MAPKKK has yet been reported. Among the different putative MAPKKKs, the deduced amino acid sequence of the CpSte11 gene shows the presence of highly conserved N‐terminal regulatory and C‐terminal serine/threonine protein kinase domains, hallmark features of this class of enzyme. In addition, the sequence shows high homology to the known pheromone response MAPKKKs, such as yeast Ste11 and Byr2, further suggesting that the cloned CpSte11 gene is the pheromone response MAPKKK. The preservation of these conserved domains in CPMKKK indicates that, although the response to external stimuli may differ from that of other fungi, homologues of the signalling pathway, via a MAPKKK cascade, exist and protein–protein interactions probably occur in a similar way.
In addition to the current study on the CpSte11 gene, female fertility has been hampered in mutants of the cpst12, pro1 and Mf2/2 genes (Deng et al., 2007b; Sun et al., 2009; Zhang et al., 1993). Moreover, molecular phenotypes showing the down‐regulation of all three genes in the CpSte11 null mutant, the down‐regulation of cpst12, pro1 and Mf2/2 genes in the Cpmk2 null mutant and a reduced level of phosphorylation of CpMK2 in the CpSte11 null mutant suggest that all four genes are under the control of the CpSte11 gene, acting through Cpmk2, and are likely to be important components in the same putative mating pathway. On the basis of comparative analyses of homologues in other fungi, the epistatic relationship of these fives genes is probably in the order CpSte11, Cpmk2, cpst12/pro1 and Mf2/2, resembling a regulatory cascade of a MAPK pathway, its downstream transcription factors and target genes. However, apart from mating incompetency as a female partner, phenotypic changes in colony morphology appear to be different depending on the gene. In terms of asexual conidiation, CpSte11 and cpst12 mutants reveal enhanced asexual sporulation, whereas Mf2/2 and pro1 mutants show reduced conidiation (Deng et al., 2007b; Sun et al., 2009; Zhang et al., 1993). The Cpmk2 null mutant shows abolished conidiation and phenotypic changes in the growth pattern related to culture conditions (liquid vs. solid media) (Choi et al., 2005). Enhanced conidiation is in sharp contrast with the function of MAPKKK ChSte11 of Cochliobolus heterostrophus (Izumitsu et al., 2009). Impaired colonization of the CpSte11 null mutants on chestnut bark has also been observed in the cpst12 null mutant. Because phenotypic changes, with fewer stromal pustules, have been observed in the cryparin mutant (Kazmierczak et al., 2005), we examined the expression pattern of the cryparin gene in the CpSte11 null mutants. No difference in the expression pattern of the cryparin gene was observed compared with wild‐type EP155/2 (data not shown), suggesting that the impaired colonization in this study is specifically caused by the loss of function of CpSte11. Neither viral instability nor growth defects on the plate, which occurred in the pro1 and Cpmk2 mutants, respectively, was observed in the CpSte11 null mutants.
The phenotypic changes, such as enhanced conidiation, impaired colonization on chestnut bark and mating incompetency as a female partner, in the CpSte11 null mutant were fully reproduced only in the cpst12 mutant (Deng et al., 2007b), and not in the pro1 mutant (Sun et al., 2009), even though both transcription factors are apparently under the control of the mating response pathways involving the CpSte11 and Cpmk2 genes (Choi et al., 2005). As summarized in Fig. 8, one possible explanation for this finding is that the protein product of the pro1 gene acts as a heterooligomeric complex with that of the cpst12 gene, whereas the cpst12 gene can act without pro1, reminiscent of yeast transcriptional regulation by transcription factors of Ste12 and Tec1 (Chen and Thorner, 2007). Accordingly, the expression of the downstream target genes of pro1 is dependent only on the heterodimeric complex, whereas the presence of cpst12 is essential for both heterodimeric and homodimeric complexes. Thus, a loss‐of‐function mutant in CpSte11 results in the simultaneous reduction of both transcription factors, which is reproduced in the cpst12 mutant. Likewise, the lack of a specific signalling component within the same putative mating pathway does not show the same phenotype, but rather different phenotypes, some of which may even appear to be opposite changes, i.e. enhanced conidiation in the CpSte11 null mutant versus abolished conidiation in the Cpmk2 null mutant. In addition, the mutation of a specific signalling component Cpmk2 has a more profound effect than CpSte11 on the expression of a putative target gene cpst12. However, the phenotypes of the Cpmk2 null mutant are more dissimilar from those of the cpst12 mutant than the CpSte11 null mutant. These results indicate that a given signalling component in the putative mating pathway of C. parasitica can act independently of other elements and can be used in more than one pathway within the same cell to regulate different developmental processes. Thus, specificity and cross‐pathway interactions may involve the modulation of the activity of a component by its association with different accessory proteins. It should be noted that the characteristic growth and developmental retardation of the Cpmk2 null mutant are observed only on agar plates and not in liquid culture. The wild‐type C. parasitica strain forms three morphologically distinct hyphal types on agar plates: feeding hyphae that appear as sparse hyphae with large diameters and penetrate the agar; an interwoven hyphal mat that forms at the agar surface with generally vertically oriented hyphae; and narrower, more cytoplasmically dense, aerial hyphae that locally differentiate into pycnidia formed above the mat. The growth and developmental retardation of the Cpmk2 null mutant are primarily a result of a defect in the development of feeding hyphae (Choi et al., 2005), reminiscent of defects in the invasive growth of the budding yeast.
Figure 8.
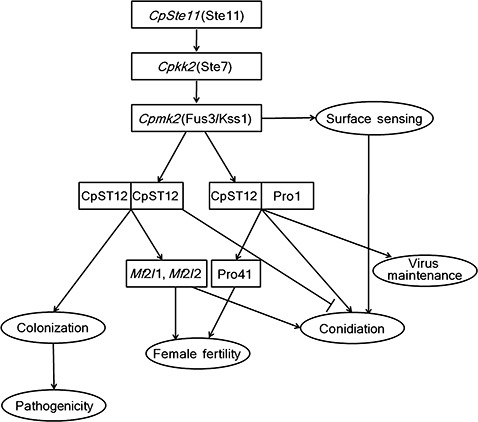
Schematic diagram of the pheromone response mitogen‐activated protein kinase (MAPK) signalling pathway in Cryphonectria parasitica. Rectangles, genes; ovals, phenotypes; arrows, activation; T‐bars, inhibition.
In C. parasitica, the mating process (perithecia formation) occurs only on chestnut bark and not on any axenic culture. Thus, colonization on chestnut bark is important for a successful mating process (female fertility). Pathogenicity also requires successful colonization of host tissue. However, recent studies on two downstream transcription factors cpst12 and pro1 have revealed that both are involved in female fertility, but show differences in pathogenicity between the two null mutants (Deng et al., 2007b; Sun et al., 2009), indicating that mating and pathogenicity can be somewhat independent of each other. In addition, female sterility appears to be under different downstream control mechanisms, because transcription factor pro1 regulates pro41 as in Sordaria macrospora, whereas cpst12 does not affect pro41 gene expression (Sun et al., 2009). The current study suggests the presence of downstream target genes of a pro1 heteromeric complex, which appears to be independent of the cpst12 heteromeric complex including pathogenicity. Thus, the characterization of the mechanism of MAPK‐directed developmental commitment and cell fate determination in C. parasitica will be of interest to understand colonization‐, mating‐ and pathogenicity‐specific pathways.
EXPERIMENTAL PROCEDURES
Fungal strains and growth
The CHV1‐713‐containing hypovirulent C. parasitica strain UEP1 and its isogenic virus‐free strain EP155/2 (ATCC 38755) were maintained on potato dextrose agar containing L‐methionine (100 mg/L) and biotin (1 mg/L) (PDAmb) plates under constant low light at 25 °C (Kim et al., 1995). Culture conditions and methods for preparing the primary inoculum for liquid cultures have been described previously (Kim et al., 1995). Radial growth on plates was assessed by measuring the diameter of the colonies. The mycelium was collected and lyophilized as described previously until use (Powell and Van Alfen, 1987b).
Cloning and characterization of a MAPKKK gene, CpSte11
The genome database of C. parasitica (Cryphonectria parasitica EP155 v2.0) was screened for the yeast Ste11 homologue and PCR amplification was performed with primers CpSte11‐gF1 (forward) (5′‐CTAGCGGAGACGGGCTGC‐3′) and CpSte11‐gR1 (reverse) (5′‐GGGTCTCTTGGTATGGTC‐3′). The resulting 4.4‐kb PCR amplicon was cloned into the pGEM‐T Easy Vector (Promega) and sequenced using the dideoxynucleotide method with universal and synthetic oligonucleotide primers.
To obtain a cDNA clone of CpSte11, RT‐PCR was performed with primers CpSte11‐cF1 (forward) (5′‐GTCGACAGGCGGGTGTTGT‐3′) and CpSte11‐cR1 (reverse) (5′‐GGTCTCGACCTGCTTGACTG‐3′). The resulting cDNA amplicon was cloned and sequenced.
Southern blot and Northern blot analysis
Genomic DNA from C. parasitica was extracted using the method of Churchill et al. (1990). DNA (10 µg) was digested with EcoRI, SalI and SacI, blotted onto a nylon membrane and hybridized with radioactive probes (Sambrook et al., 1989).
The temporal expression pattern of CpSte11 was examined by quantitative real‐time RT‐PCR using total RNA extracted from cultures at 1, 2, 3, 5 and 7 days after inoculation, as described previously (Kim et al., 2002). CpSte11 transcript levels were compared using glyceraldehyde‐3‐phosphate dehydrogenase (gpd) of C. parasitica as an internal control (Choi and Nuss, 1990).
Immunoblot analysis
To determine whether CpSte11 is specifically required for the phosphorylation of CpMK2, the levels of phosphorylated CpMK2 were examined by immunoblotting with an antibody specific for doubly phosphorylated p44/42 MAPK (phospho‐p44/42 MAPK antibody; Santa Cruz Biotechnology, Santa Cruz, CA, USA), as described previously (Choi et al., 2005). Total soluble protein was extracted from 5‐day cultured mycelia and a nonphosphorylated‐specific p44/42 MAPK antibody (Santa Cruz Biotechnology) was also used to verify the loading of equal amounts of protein.
Quantitative analysis of transcript accumulation using real‐time RT‐PCR
To examine the expression levels of target and internal control genes, quantitative RT‐PCR was performed using a GeneAmp 7500 sequence detection system (Applied Biosystems, Foster City, CA, USA) and a SYBR green mixture RT kit (Applied Biosystems). A single cycle to reverse transcribe 1 µg of RNase‐free RQ1 DNase‐treated RNA was followed by real‐time PCR using 2 µL of reverse‐transcribed cDNA and 150 nm each of forward and reverse primers. Analyses were conducted at least twice, in triplicate for each transcript, from at least two independent RNA preparations of the same sample with primers specific for gpd and the target genes. Primer pairs for each gene are indicated in Table 2. Transcript abundance, relative to the amount of gpd, in the sample was calculated using the comparative threshold cycle method, as described previously (Parsley et al., 2002). RNA was extracted from liquid cultures as described previously (Kim et al., 1995).
Table 2.
Primers for quantitative real‐time polymerase chain reaction (PCR) analysis.
| Target gene | Forward sequence (5′–3′) | Reverse sequence (5′–3′) | Product size (bp) |
|---|---|---|---|
| gpd | CCGTCAACGACCCCTTCAT | GTTGCCGTGTTGAGAGTCATACTT | 71 |
| Mf2/2 | TTCAAACCAACCATCAAAATGC | GCCGTTGACTCCCATGGA | 66 |
| cpst12 | GCATTCTCCAGATCAGACAACCT | AGGTTCAGTCCATCGACTCCAT | 77 |
| pro1 | CAATGCCAATCCGAAACAAA | GCACTTCTTCCTCCGGAGTCT | 85 |
| CpSte11 | CCTGAAGGAGATGGGTATTGACA | GAGTCCTCATTTCCGCTTCTTGTG | 111 |
Construction of a replacement vector and fungal transformation
The replacement vector pDSTE11, designed to favour double‐crossover integration events, was constructed as follows: a 4.4‐kb fragment containing the full‐length CpSte11 ORF was ligated into the pGEM‐T Easy Vector, and the resulting plasmid was used as template for inverted PCR employing the primers 5′‐GGTCTAGACTCAATCTTCGTATCAAATGCTTA‐3′ and 5′‐GGTCTAGACCTCACCTCGTACTTCCCAGACCA‐3′, which incorporate restriction sites for XbaI (italic). The PCR amplicon was digested with XbaI and religated. The resulting plasmid was further digested with XbaI and fused with a 2.3‐kb XbaI fragment of pBSIIhph harbouring the 2.4‐kb SalI fragment of pDH25 containing a hygromycin phosphotransferase gene cassette (hph). In the replacement vector, pDSTE11, the hph cassette was inserted between sites −492 and 1440 bp of the CpSte11 gene, relative to the start codon, and was flanked by approximately 1095 and 1322 bp of 5′ and 3′ sequences, respectively. NotI‐digested linear pDSTE11 was then used to transform the virus‐free EP155/2 strain.
Functional complementation of the CpSte11 null mutant was performed using a wild‐type allele. PCR amplification of the 5.6‐kb CpSte11 gene was performed with the primers 5′‐GGACTAGTACTGTTTCAACTAGCGGAGACG‐3′ and 5′‐CGGAATTCCAATGCTCACCCACGAAGACG‐3′ to amplify a fragment between sites −1591 and 3966 bp of the CpSte11 gene, relative to the start codon, incorporating SpeI and EcoRI sites (italic), respectively. The resulting 5.6‐kb PCR amplicon was cloned into the SpeI/EcoRI‐digested pBluescriptII KS (+) (Stratagene, La Jolla, CA, USA). The complementing vector, pCSTE11, was constructed by insertion of the 5.6‐kb CpSte11 gene into the SpeI/EcoRI‐digested recombinant pBluescriptII KS (+) carrying the 1.7‐kb XhoI fragment of pSD1 containing the geneticin resistance cassette (Nguyen et al., 2008). The resulting vector was then used to transform the CpSte11 null mutant.
Protoplast preparation and transformation were performed as described previously (Churchill et al., 1990). Transformants were selected from agar plates supplemented with 150 µg/mL hygromycin B (Calbiochem, San Diego, CA, USA) or 120 µg/mL geneticin (Invitrogen, Carlsbad, CA, USA), as appropriate, passaged three or four times on selective media and single spore isolated, as described previously (Churchill et al., 1990; Kim et al., 2004). PCR and Southern blot analyses were conducted with genomic DNA from the transformants to confirm the replacement and in trans complementation of the CpSte11 gene.
Characteristics of the CpSte11 null mutant
The phenotypic and molecular characteristics of the CpSte11 null mutant were compared with those of the wild‐type EP155/2 and hypovirulent UEP1 strains. Phenotypic changes in pigmentation, conidiation and mating capability were measured as described previously (Kim et al., 2002; Powell and Van Alfen, 1987b). A virulence test using excised chestnut tree bark was conducted according to Lee et al. (1992). Laccase activity was measured by growing the strains on Bavendamm's medium (0.7% tannic acid, 1.5% malt extract, 2.0% agar) and assessing the resulting coloration of the medium (Rigling et al., 1989). The laccase activity of the culture filtrate was determined as described previously (Kim et al., 2002).
To examine alterations in the expression levels of the putative target genes involved in the mating response pathway, including Mf2/2, cpst12, pro1 and Cpmk2, quantitative real‐time RT‐PCR was performed.
Transmission of the dsRNA virus
Virus transmission was performed as described previously (Cortesi et al., 2001). Briefly, mycelial plugs of the virus‐containing strain UEP1 were co‐cultured with adjacent mycelial plugs of virus‐free recipient transformants on PDAmb medium. After 5 days of co‐culture, putatively fused mycelia at the border between each pair of strains were transferred to hygromycin‐containing PDAmb and examined for the occurrence of a sector showing different phenotypes. Mycelia in the sector were successively transferred to fresh hygromycin‐containing medium, and strains were single spored to select virus‐infected recipient transformants. The presence of hypovirus was confirmed by purification of dsRNA from single‐spored isolates.
Isolation of dsRNA from C. parasitica
dsRNA was isolated according to a modified version of the procedure of Kim et al. (2008). The UEP1 and dsRNA‐transmitted transformants were grown on a cellophane membrane overlaying PDAmb for 7 days at 25 °C. Mycelia (0.2 g wet weight) were homogenized in extraction buffer [2 × STE: 0.2 m NaCl, 0.1 m tris(hydroxymethyl)aminomethane (Tris)‐HCl, pH 8.0, 2 mm ethylenediaminetetraacetic acid (EDTA), plus 2% sodium dodecylsulphate (SDS), 1% sodium bisulphate] using a bead beater (Biospec Products, Inc., Bartlesville, OK, USA). Following two successive phenol extractions, dsRNA was isolated by affinity chromatography using CC41 resin. Isolated dsRNA was analysed by electrophoresis in a 0.8% agarose gel.
ACKNOWLEDGEMENTS
This work was supported by a Korea Science and Engineering Foundation (KOSEF) grant, funded by the South Korean government (R11‐2008‐062‐02002‐0). We thank Alice C. L. Churchill (Cornell University, Ithaca, NY, USA) for providing the sequence information of a cloned CpSte11 gene fragment, which was used to initiate this study, prior to the availability of the genome sequence. We thank the Center for Fungal Pathogenesis and the Institute of Molecular Biology and Genetics at Chonbuk National University, Chonbuk, South Korea for kindly providing facilities for this research.
REFERENCES
- Allen, T.D. and Nuss, D.L. (2004) Specific and common alterations in host gene transcript accumulation following infection of the chestnut blight fungus by mild and severe hypoviruses. J. Virol. 78, 4145–4155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen, T.D. , Dawe, A.L. and Nuss, D.L. (2003) Use of cDNA microarrays to monitor transcriptional responses of the chestnut blight fungus Cryphonectria parasitica to infection by virulence‐attenuating hypoviruses. Eukaryot. Cell, 2, 1253–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anagnostakis, S.L. (1982) Biological control of chestnut blight. Science, 215, 466–471. [DOI] [PubMed] [Google Scholar]
- Chen, B. , Gao, S. , Choi, G.H. and Nuss, D.L. (1996) Extensive alteration of fungal gene transcript accumulation and elevation of G‐protein‐regulated cAMP levels by a virulence‐attenuating hypovirus. Proc. Natl. Acad. Sci. USA, 93, 7996–8000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, R.E. and Thorner, J. (2007) Function and regulation in MAPK signaling pathways: lessons learned from the yeast Saccharomyces cerevisiae . Biochim. Biophys. Acta, 1773, 1311–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi, E.S. , Chung, H.J. , Kim, M.J. , Park, S.M. , Cha, B.J. , Yang, M.S. and Kim, D.H. (2005) Characterization of the ERK homologue CpMK2 from the chestnut blight fungus Cryphonectria parasitica . Microbiology, 151, 1349–1358. [DOI] [PubMed] [Google Scholar]
- Choi, G.H. and Nuss, D.L. (1990) Nucleotide sequence of the glyceraldehyde‐3‐phosphate dehydrogenase gene from Cryphonectria parasitica . Nucleic Acids Res. 18, 5566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchill, A.C.L. , Ciuffetti, L.M. , Hansen, D.R. , Vanetten, H.D. and Van Alfen, N.K. (1990) Transformation of the fungal pathogen Cryphonectria parasitica with a variety of heterologous plasmids. Curr. Genet. 17, 25–31. [Google Scholar]
- Cortesi, P. , McCulloch, C.E. , Song, H. , Lin, H. and Milgroom, M.G. (2001) Genetic control of horizontal virus transmission in the chestnut blight fungus, Cryphonectria parasitica . Genetics, 159, 107–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng, F. , Allen, T.D. , Hillman, B.I. and Nuss, D.L. (2007a) Comparative analysis of alterations in host phenotype and transcript accumulation following hypovirus and mycoreovirus infections of the chestnut blight fungus Cryphonectria parasitica . Eukaryot. Cell, 6, 1286–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng, F. , Allen, T.D. and Nuss, D.L. (2007b) Ste12 transcription factor homologue CpST12 is down‐regulated by hypovirus infection and required for virulence and female fertility of the chestnut blight fungus Cryphonectria parasitica . Eukaryot. Cell, 6, 235–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliston, J.E. (1985) Characteristics of dsRNA‐free and dsRNA‐containing strains of Endothia parasitica in relation to hypovirulence. Phytopathology, 75, 151–158. [Google Scholar]
- Gao, S. and Nuss, D.L. (1996) Distinct roles for two G protein alpha subunits in fungal virulence, morphology, and reproduction revealed by targeted gene disruption. Proc. Natl. Acad. Sci. USA, 93, 14 122–14 127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrido, E. , Voss, U. , Muller, P. , Castillo‐Lluva, S. , Kahmann, R. and Perez‐Martin, J. (2004) The induction of sexual development and virulence in the smut fungus Ustilago maydis depends on Crk1, a novel MAPK protein. Genes Dev. 18, 3117–3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havir, E.A. and Anagnostakis, S.L. (1983) Oxalate production by virulent but not by hypovirulent strains of Endothia parasitica . Physiol. Plant Pathol. 23, 369–376. [Google Scholar]
- Herskowitz, I. (1995) MAP kinase pathways in yeast: for mating and more. Cell, 80, 187–197. [DOI] [PubMed] [Google Scholar]
- Izumitsu, K. , Yoshimi, A. , Kubo, D. , Morita, A. and Tanaka, C. (2009) The MAPKK kinase ChSte11 regulates sexual/asexual development, melanization, pathogenicity, and adaptation to oxidative stress in Cochliobolus heterostrophus . Curr. Genet. 55, 439–448. [DOI] [PubMed] [Google Scholar]
- Ju, Y.J. , Park, K.H. , Kim, D.H. and Cha, B.J. (1999) Survey on chestnut blight caused by Cryphonectria parasitica . In: Annual Meeting of the Korean Society of Plant Pathology, Andong, South Korea, p. 13.
- Kang, H.S. , Choi, J.W. , Park, S.M. , Cha, B.J. , Yang, M.S. and Kim, D.H. (2000) Ordered differential display from Cryphonectria parasitica . Plant Pathol. J. 16, 142–146. [Google Scholar]
- Kasahara, S. and Nuss, D.L. (1997) Targeted disruption of a fungal G‐protein beta subunit gene results in increased vegetative growth but reduced virulence. Mol. Plant–Microbe Interact. 10, 984–993. [DOI] [PubMed] [Google Scholar]
- Kazmierczak, P. , Pfeiffer, P. , Zhang, L. and Van Alfen, N.K. (1996) Transcriptional repression of specific host genes by the mycovirus Cryphonectria hypovirus 1 . J. Virol. 70, 1137–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazmierczak, P. , Kim, D.H. , Turina, M. and Van Alfen, N.K. (2005) A hydrophobin of the chestnut blight fungus, Cryphonectria parasitica, is required for stromal pustule eruption. Eukaryot. Cell, 4, 931–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, D.H. , Rigling, D. , Zhang, L. and Van Alfen, N.K. (1995) A new extracellular laccase of Cryphonectha parasitica is revealed by deletion of Lac1. Mol. Plant–Microbe Interact. 8, 259–266. [Google Scholar]
- Kim, M.J. , Choi, J.W. , Park, S.M. , Cha, B.J. , Yang, M.S. and Kim, D.H. (2002) Characterization of a fungal protein kinase from Cryphonectria parasitica and its transcriptional upregulation by hypovirus. Mol. Microbiol. 45, 933–941. [DOI] [PubMed] [Google Scholar]
- Kim, M.J. , Kwon, B.R. , Park, S.M. , Chung, H.J. , Yang, M.S. , Churchill, A.C.L. , Van Alfen, N.K. and Kim, D.H. (2008) Promoter analysis of the cell surface‐abundant and hypoviral‐regulated cryparin gene from Cryphonectria parasitica . Mol. Cells, 26, 496–502. [PubMed] [Google Scholar]
- Kim, M.J. , Park, S.M. , Kim, Y.H. , Cha, B.J. , Yang, M.S. and Kim, D.H. (2004) Deletion of a hypoviral‐regulated cppk1 gene in a chestnut blight fungus, Cryphonectria parasitica, results in microcolonies. Fungal Genet. Biol. 41, 482–492. [DOI] [PubMed] [Google Scholar]
- Lee, J.K. , Tattar, T.A. , Berman, P.M. and Mount, M.S. (1992) A rapid method for testing the virulence of Cryphonectria parasitica using excised bark and wood of American chestnut. Phytopathology, 82, 1454–1456. [Google Scholar]
- Nguyen, Q.B. , Kadotani, N. , Kasahara, S. , Tosa, Y. , Mayama, S. and Nakayashiki, H. (2008) Systematic functional analysis of calcium‐signalling proteins in the genome of the rice‐blast fungus, Magnaporthe oryzae, using a high‐throughput RNA‐silencing system. Mol. Microbiol. 68, 1348–1365. [DOI] [PubMed] [Google Scholar]
- Nuss, D.L. (1992) Biological control of chestnut blight: an example of virus‐mediated attenuation of fungal pathogenesis. Microbiol. Rev. 56, 561–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, S.M. , Choi, E.S. , Kim, M.J. , Cha, B.J. , Yang, M.S. and Kim, D.H. (2004) Characterization of HOG1 homologue, CpMK1, from Cryphonectria parasitica and evidence for hypovirus‐mediated perturbation of its phosphorylation in response to hypertonic stress. Mol. Microbiol. 51, 1267–1277. [DOI] [PubMed] [Google Scholar]
- Parsley, T.B. , Chen, B. , Geletka, L.M. and Nuss, D.L. (2002) Differential modulation of cellular signaling pathways by mild and severe hypovirus strains. Eukaryot. Cell, 1, 401–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell, W.A. and Van Alfen, N.K. (1987a) Differential accumulation of poly(A)+ RNA between virulent and double‐stranded RNA‐induced hypovirulent strains of Cryphonectria (Endothia) parasitica . Mol. Cell. Biol. 7, 3688–3693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell, W.A. and Van Alfen, N.K. (1987b) Two nonhomologus viruses of Cryphonectria (Endothia) parasitica reduce accumulation of specific virulence‐associated polypeptides. J. Bacteriol. 169, 5324–5326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigling, D. , Heiniger, U. and Hohl, H.R. (1989) Reduction of laccase activity in dsRNA‐containing hypovirulent strains of Cryphonectria (Endothia) parasitica . Phytopathology, 79, 219–223. [Google Scholar]
- Rostagno, L. , Crivelli, G. and Turina, M. (2009) Study of mRNA expression by real time PCR of Cpkk1, Cpkk2 and Cpkk3, three MEKs of Cryphonectria parasitica, in virus‐free and virus‐infected isogenic isolates. J. Phytopathol. 158, 409–416. [Google Scholar]
- Sakaguchi, A. , Tsuji, G. and Kubo, Y. (2010) A yeast STE11 homologue CoMEKK1 is essential for pathogenesis‐related morphogenesis in Colletotrichum orbiculare . Mol. Plant–Microbe Interact. 23, 1563–1572. [DOI] [PubMed] [Google Scholar]
- Sambrook, J. , Fritsch, E.F. and Maniatis, T. (1989) Molecular Cloning: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press. [Google Scholar]
- Schaeffer, H.J. and Weber, M.J. (1999) Mitogen‐activated protein kinases: specific messages from ubiquitous messengers. Mol. Cell. Biol. 19, 2435–2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schamber, A. , Leroch, M. , Diwo, J. , Mendgen, K. and Hahn, M. (2010) The role of mitogen‐activated protein (MAP) kinase signaling components and the Ste12 transcription factor in germination and pathogenicity of Botrytis cinerea . Mol. Plant Pathol. 11, 105–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, Q. , Choi, G.H. and Nuss, D.L. (2009) Hypovirus‐responsive transcription factor gene pro1 of the chestnut blight fungus Cryphonectria parasitica is required for female fertility, asexual spore development, and stable maintenance of hypovirus infection. Eukaryot. Cell, 8, 262–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turina, M. , Zhang, L. and Van Alfen, N.K. (2006) Effect of Cryphonectria hypovirus 1 (CHV1) infection on Cpkk1, a mitogen‐activated protein kinase kinase of the filamentous fungus Cryphonectria parasitica . Fungal Genet. Biol. 43, 764–774. [DOI] [PubMed] [Google Scholar]
- Van Alfen, N.K. (1982) Biology and potential for disease control of hypovirulence of Endothia parasitica . Annu. Rev. Phytopathol. 20, 349–362. [Google Scholar]
- Van Alfen, N.K. , Jaynes, R.A. , Anagnostakis, S.L. and Day, P.R. (1975) Chestnut blight: biological control by transmissible hypovirulence in Endothia parasitica . Science, 189, 890–891. [DOI] [PubMed] [Google Scholar]
- Xu, J.R. (2000) Map kinases in fungal pathogens. Fungal Genet. Biol. 31, 137–152. [DOI] [PubMed] [Google Scholar]
- Zhang, L. , Churchill, A.C. , Kazmierczak, P. , Kim, D.H. and Van Alfen, N.K. (1993) Hypovirulence‐associated traits induced by a mycovirus of Cryphonectria parasitica are mimicked by targeted inactivation of a host gene. Mol. Cell. Biol. 13, 7782–7792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, L. , Baasiri, R.A. and Van Alfen, N.K. (1998) Viral repression of fungal pheromone precursor gene expression. Mol. Cell. Biol. 18, 953–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, X. , Mehrabi, R. and Xu, J.R. (2007) Mitogen‐activated protein kinase pathways and fungal pathogenesis. Eukaryot. Cell, 6, 1701–1714. [DOI] [PMC free article] [PubMed] [Google Scholar]


