Summary
Fungal histidine kinases (HKs) are involved in osmotic and oxidative stress responses, hyphal development, fungicide sensitivity and virulence. Members of HK class III are known to signal through the high‐osmolarity glycerol mitogen‐activated protein kinase (HOG MAPK). In this study, we characterized the Shk1 gene (SS1G_12694.3), which encodes a putative class III HK, from the plant pathogen Sclerotinia sclerotiorum. Disruption of Shk1 resulted in resistance to phenylpyrrole and dicarboximide fungicides and increased sensitivity to hyperosmotic stress and H2O2‐induced oxidative stress. The Shk1 mutant showed a significant reduction in vegetative hyphal growth and was unable to produce sclerotia. Quantitative real‐time polymerase chain reaction (qRT‐PCR and glycerol determination assays showed that the expression of SsHOG1 (the last kinase of the Hog pathway) and glycerol accumulation were regulated by the Shk1 gene, but PAK (p21‐activated kinase) was not. In addition, the Shk1 mutant showed no change in virulence. All the defects were restored by genetic complementation of the Shk1 deletion mutant with the wild‐type Shk1 gene. These findings indicate that Shk1 is involved in vegetative differentiation, sclerotial formation, glycerol accumulation and adaption to hyperosmotic and oxidative stresses, and to fungicides, in S. sclerotiorum. Taken together, our results demonstrate, for the first time, the role of two‐component HKs in Sclerotinia.
Introduction
Two‐component histidine kinases (HKs) regulate responses to environmental stimuli in bacteria and eukaryotes, including yeasts, plants, slime moulds and filamentous fungi (Bahn, 2008; Nemecek et al., 2006; Tanaka and Izumitsu, 2010). Most eukaryotic and all fungal HKs are of the hybrid type, in that the HK domain and the response regulator (RR) domain are present in a single protein. In hybrid‐type HKs, the phosphate group on the aspartate residue of the HK RR domain is transferred to a histidine residue of a histidine phosphotransfer (HPT) protein, and the RR protein is phosphorylated by the phosphorylated HPT protein (West and Stock, 2001). In eukaryotic cells, HKs are generally found at the head of intracellular signalling pathways that recruit more conventional downstream signalling modules, such as mitogen‐activated protein kinase (MAPK) cascades (West and Stock, 2001). In the yeast Saccharomyces cerevisiae, one HK, Sln1, is involved in responses to high osmolarity (Posas et al., 1996). In filamentous fungi, HKs are classified into 11 groups based on the protein sequence (Catlett et al., 2003), and only members of HK class III have been associated with responses to high osmolarity; these HKs include Os‐1 in Neurospora crassa (Ochiai et al., 2001), NikA in Aspergillus nidulans (Hagiwara et al., 2007), Bos1 in Botrytis cinerea (Cui et al., 2002; Viaud et al., 2006), Nik1 in Alternaria brassicicola and Cochliobolus heterostrophus (Avenot et al., 2005; Yoshimi et al., 2004) and Hik1 in Magnaporthe oryzae (Motoyama et al., 2005a). In addition, mutations in class III HKs can result in fungicide resistance and morphological defects (Hagiwara et al., 2007; Motoyama et al., 2005a; Ochiai et al., 2001; Viaud et al., 2006; Yoshimi et al., 2004). Class III HKs are also involved in the virulence of phytopathogenic fungi. Deletion of class III HKs strongly reduces the virulence of Fusarium oxysporum, B. cinerea and A. brassicicola (Cho et al., 2009; Liu et al., 2008; Rispail and Pietro, 2010; Viaud et al., 2006), but not of M. oryzae (Motoyama et al., 2005a). Similarly, point mutations in the class III HK Mf‐os1 decreased the virulence of Monilinia fructicola (Ma et al., 2006), but not of naturally occurring fungicide‐resistant field isolates of B. cinerea or A. brassicicola (Avenot et al., 2005; Cui et al., 2002). These results indicate that the roles of two‐component HKs vary significantly in different fungi.
Sclerotinia sclerotiorum is a necrotrophic, phytopathogenic, filamentous ascomycete with a broad host range and a worldwide distribution. Over 400 species of plant are susceptible to this pathogen, including many agronomic and horticultural crops (Boland and Hall, 1994; Duan et al., 2012; Purdy, 1979; Tu, 1997). Owing to inadequate levels of host resistance, the application of fungicides is the principal tool for the control of Sclerotinia diseases on most crops (Steadman, 1979). Although the dicarboximide fungicides iprodione and dimetachlone have been used for many years to control Sclerotinia diseases worldwide, and although the phenylpyrrole fludioxonil has strong fungicidal activity against S. sclerotiorum, the modes of action of these fungicides are not well known (Kuang et al., 2011; Ma et al., 2009; Shi et al., 2000; Zhang et al., 2002). Interestingly, dicarboximide fungicide‐resistant strains of B. cinerea, N. crassa and M. fructicola show increased sensitivity to osmotic stress (Cui et al., 2002; Ma et al., 2006; Oshima et al., 2002). These studies suggest a relationship between osmoregulation and dicarboximide resistance in phytopathogenic fungi.
In Fusarium graminearum, N. crassa and C. heterostrophus, all deletion mutants of Os1, Os2, Os4 and Os5 genes, which are involved in osmoregulation, were highly resistant to dicarboximide fungicides (Fujimura et al., 2003; Ochiai et al., 2007; Yoshimi et al., 2004; Zhang et al., 2002). In B. cinerea, however, BcOs1 deletion mutants were resistant to dicarboximide fungicides, and the BcOs2, BcOs4, BcOs5 and BcRrg1 deletion mutants remained sensitive to these fungicides (Liu et al., 2008; Segmüller et al., 2007; Viaud et al., 2006; Yan et al., 2010, 2011; Yang et al., 2012). These results suggest that the components in the osmotic signalling pathway have different roles in different phytopathogenic fungi. In the current research, we used a gene deletion and complementation strategy to characterize the class III HK Shk1, which is a key component of the osmotic signalling pathway in S. sclerotiorum.
Results
Characterization of the Shk1 gene
The Shk1 gene was identified by a search of the S. sclerotiorum genome sequence deposited in the Broad Institute (http://www.broadinstitute.org) using the program blast. The Shk1 nucleotide sequence was 4324 bp in length and was predicted to have five introns of 54, 64, 186, 54 and 36 bp, located at positions 712, 911, 2342, 3794 and 4277 of the sequence, respectively. The existence of these introns was verified by reverse transcription‐polymerase chain reaction with the primer pair P1/P2 (Table 1), which amplified 3930‐ and 4324‐bp fragments from cDNA and genomic DNA, respectively. Sequencing of the 3930‐bp product obtained from cDNA verified the predicted position and size of the introns. The predicted 1310‐amino‐acid sequence of Shk1 has 78% identity to Os1 of N. crassa (NCU02815.5) and 98% identity to BcOs1 (BC1G_00374) of B. cinerea. Analysis of the Shk1 sequence with the InterProScan prediction server (http://www.ebi.ac.uk/Tools/InterProScan/) detected all the characteristic domains of class III HKs, including six HAMP repeats (IPR003660) (Aravind and Ponting, 1999), the HK a signal transducer domain (HK; IPR003661), the HK‐like ATPase domain (HATPase; IPR003594) and the RR domain (REC; IPR001789) (Fig. 1G).
Table 1.
Primers and probes of Shk1 used in this study
| Primer code* | Sequence (5′→3′)† | Relevant characteristic |
|---|---|---|
| P1 | ATGGGGGACACTACGATAGC | Amplify the full cDNA sequence of the Shk1 gene |
| P2 | CAGAACTGCTTAGTACAGGTT | |
| P3 | AAAAGGTTGAACGGGCTTGTGTC | Amplify the left homologous arm of the Shk1 gene of S. sclerotiorum (1450 bp) |
| P4 | CCACCAGCCAGCCAACAGCTCCCATCGCAGTCGTGTGAGCTATCG | |
| P5 | CAATACGCAAACCGCCTCTCCCCGTCCTTCACTTGTAACGGCAG | Amplify the right homologous arm of the Shk1 gene of S. sclerotiorum (1541 bp) |
| P6 | ATGACATCTTGCGGACTTGGGGC | |
| P7 | GGGAGCTGTTGGCTGGCTGGTGG | Amplify the hph gene (1764 bp) |
| P8 | GGGGAGAGGCGGTTTGCGTATTG | |
| P9 | AGAACACTGACTGATGAAAGG | Amplify the knockout vector of the Shk1 gene of S. sclerotiorum (4260 bp) |
| P10 | CAGGGTGAAAGATAGGATACT | |
| P11 | AACGGAGGGTATTCTCGGGG | Amplify a partial fragment of the Shk1 gene of S. sclerotiorum (482 bp) |
| P12 | TGTCTTTCAGCGAAGCGATT | |
| P13 | CAAAGCATCAGCTCATCGAGAG | Amplify a partial fragment of the hph gene (503 bp) |
| P14 | GAAAAGTTCGACAGCGTCTCC | |
| P15 | GAGTAAAAACCGTTGATG | Confirm whether the hph genes homologously replaced the Shk1 gene of S. sclerotiorum (2255 bp) |
| P16 | GTCTGGACCGATGGCTGT | |
| P17 | TCATTTGGATGCTTGGGTAG | Confirm whether the hph genes homologously replaced the Shk1 gene of S. sclerotiorum (2333 bp) |
| P18 | CCTTAAACCAGATTCCGTAC | |
| P19 | CTGCAGAAccaccatgttggGTCGACAGAAGATGATATTG | Amplify the NEO cassette containing a trpC promoter (1181 bp) |
| P20 | CCGctcgagTCAGAAGAACTCGTCAAGAAGGCG | |
| P21 | TAAgcggccgcGCTGTACGAGTGATGCGA | Amplify the Shk1 gene (include the control region of the Shk1 gene) (6598 bp) |
| P22 | TAActgcagATAGTAGCAAACACTGCCAG | |
| P23 | TGAAGGGAAAGAGGAGAAGA | Amplify a probe for Southern blotting (560 bp) |
| P24 | GATTGGGAGCAGGATAAGAA | |
| P25 | TCAAGCCAAGCAACATCCTCGT | Amplify the SsHOG1 gene for quantitative real‐time PCR |
| P26 | ATCCCGCACTCCAAACATCAAC | |
| P27 | GAGTTTGTCGGTGCTCCCTTTG | Amplify the PAK gene for quantitative real‐time PCR |
| P28 | ATCATACGCTGCCATTCCTTCG | |
| P29 | CCCCAGCGTTCTACGTCT | Amplify the reference gene actin for quantitative real‐time PCR |
| P30 | CATGTCAACACGAGCAATG |
*The number code refers to the primer binding sites shown in Fig. 1A.
†The respective restriction enzyme sites included in the primers are listed in lowercase letters in the sequence.
Figure 1.
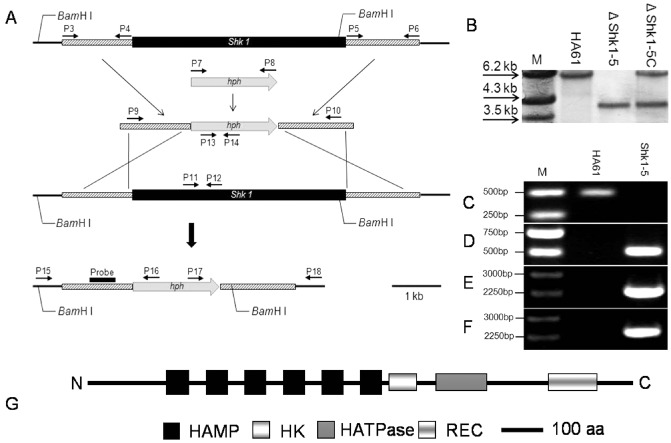
Generation and identification of Shk1 gene deletion mutants and the domain structure of Shk1 in Sclerotinia sclerotiorum. (A) Gene replacement strategy for Shk1. The hygromycin resistance cassette (hph) is denoted by the large grey arrow. Primer (P3–P18) binding sites are indicated by arrows (see Table 1 for the primer sequences). (B) Southern blot hybridization analysis of strains using the 5′‐flanking region of Shkl as a probe. Genomic DNA of the wild‐type progenitor HA61, Shk1 deletion mutant ΔShk1‐5 and complemented strain ΔShk1‐5C were digested with Bam HI. (C) Primer pair P11/P12 was used to specifically amplify the partial Shk1 (482 bp). (D) Primer pair P13/P14 was used to validate the selectable marker hph (503 bp). (E, F) Primer pairs P15/P16 and P17/P18 were used to amplify the two homologous arms with a partial fragment of the connecting area (2255 bp in E and 2333 bp in F). (G) Schematic representation of the domain structure of the two‐component histidine kinase gene (Shk1) in S. sclerotiorum. aa, amino acid.
Targeted knockout of the S. sclerotiorum Shk1 gene and complementation
To explore the biological role of Shk1 in S. sclerotiorum, a ΔShk1 null allele was generated by replacing the Shk1 gene with the hygromycin resistance cassette, using a fusion polymerase chain reaction (PCR) method (Fig. 1A). Among 212 hygromycin‐resistant transformants, three Shk1 deletion mutants were identified by PCR with different combinations of gene‐specific primers. Primer pair P11/P12 (Table 1, Fig. 1A), which amplifies a partial region in the Shk1 gene, generated a 482‐bp fragment in wild‐type strain HA61, but not in the mutant ΔShk1‐5 (Fig. 1C). Primer pair P13/P14 (Table 1, Fig. 1A) was used to amplify a 503‐bp fragment of the hph gene, which was only detected in ΔShk1‐5 (Fig. 1D). Then, primer pairs P15/P16 and P17/P18, which amplified the left and right flanking regions, generated 2255‐ and 2333‐bp fragments from ΔShk1‐5 (Fig. 1E, F), respectively. These amplification products indicated that the knockout fragment had replaced the Shk1 gene locus after double‐crossover homologous integration. As shown in Fig. 1B, when probed with the 560‐bp 5′‐flanking region of Shk1, the Shk1 deletion mutant ΔShk1‐5 had a 3.7‐kb band, but lacked a 6.1‐kb band, which was present in the wild‐type progenitor. Southern blot analysis confirmed that the transformant ΔShk1‐5 was the result of a single homologous replacement event at the Shk1 locus, and the complemented strain ΔShk1‐5C had integrated an intact copy of the Shk1 gene into the genome of the mutant ΔShk1‐5.
Involvement of Shk1 in vegetative growth and sclerotial formation
To test whether Shk1 was required for vegetative hyphal growth, colony growth was investigated on potato dextrose agar (PDA) plates. The growth rate of ΔSkh1‐5 showed a significant reduction compared with that of the wild‐type progenitor, but the growth rate did not differ between the complemented strain ΔShk1‐5C and the wild‐type progenitor (Fig. 2A). After incubation on PDA for 15 days, ΔSkh1‐5 was unable to produce sclerotia and showed a distinct colony morphology (Fig. 2B). Microscopic examination of hyphae grown on PDA was observed. Compared with the wild‐type progenitor, the Shk1 deletion mutant had more hyphal branches and grew in close proximity to each other at the extension zone (Fig. 2C). These results indicate that Skh1 plays a role in hyphal growth, branching and sclerotial formation in S. sclerotiorum.
Figure 2.
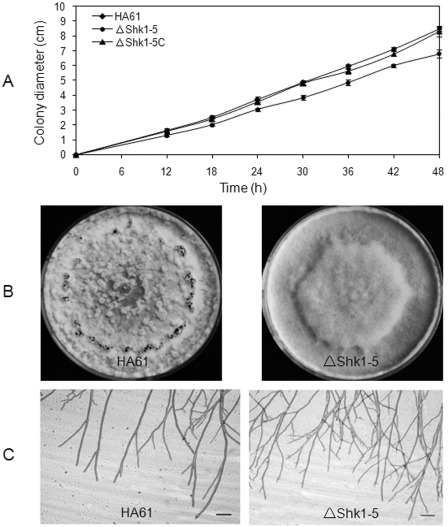
Biological characteristics of the Shk1 deletion mutant. (A) Mycelial growth rate of the wild‐type strain HA61, the Shk1 deletion mutant ΔShk1‐5 and the complemented strain ΔShk1‐5C on potato dextrose agar (PDA). Bars denote standard errors from three repeated experiments. (B) Colonies of the wild‐type strain HA61 and the mutant ΔShk1‐5 grown on PDA for 10 days. (C) Hyphal tip branching patterns of HA61 and ΔShk1‐5 on PDA for 1 day. Bar, 100 μm.
Shk1 is required for the sensitivity to fungicide, osmotic and oxidative stresses
Fungicide sensitivity tests showed that ΔShk1‐5 was highly resistant to both dicarboximide and phenylpyrrole fungicides and grew well on PDA amended with 1 μg/mL fludioxonil, 5 μg/mL iprodione or 5 μg/mL dimetachlone, whereas the complemented strain ΔShk1‐5C restored fungicide sensitivity to the wild‐type level (Fig. 3). HA61, ΔShk1‐5 and ΔShk1‐5C exhibited similar sensitivity to the benzimidazole fungicide carbendazim (Fig. 3). Compared with the wild‐type progenitor and the complemented strain, ΔShk1‐5 exhibited significantly reduced mycelial growth on PDA supplemented with NaCl, KCl, glucose and d‐sorbitol at the concentrations indicated in the figure legends (Fig. 4), and was more sensitive to salt stress than to d‐sorbitol (Fig. 4). In addition, the mutant ΔShk1‐5 showed increased sensitivity to oxidative stress generated by H2O2 on PDA plates. ΔShk1‐5 was unable to grow on PDA amended with 50 mm H2O2, which inhibited only 30%–40% of mycelial growth of the wild‐type progenitor and the complemented strain. These results indicate that Shk1 is involved in the response of S. sclerotiorum to phenylpyrrole and dicarboximide fungicides, and hyperosmotic and oxidative stresses.
Figure 3.
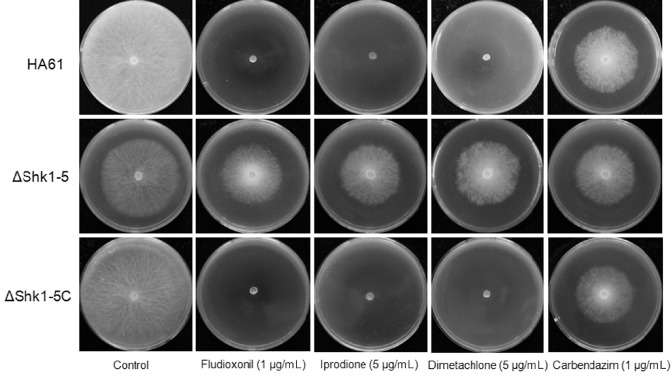
Sensitivity of the wild‐type strain HA61, the Shk1 deletion mutant ΔShk1‐5 and the complemented strain ΔShk1‐5C of Sclerotinia sclerotiorum to different fungicides. Cultures were grown for 2 days on potato dextrose agar (PDA) amended with fungicide as indicated.
Figure 4.
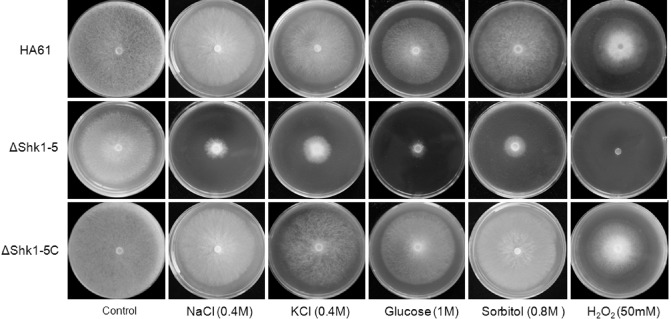
Sensitivity of the wild‐type strain HA61, the Shk1 deletion mutant ΔShk1‐5 and the complemented strain ΔShk1‐5C to osmotic stress (generated by NaCl, KCl, glucose or sorbitol) and oxidative stress (generated by H2O2). Cultures were grown on potato dextrose agar (PDA).
Shk1 regulates glycerol accumulation
Glycerol accumulation induced by fungicides and osmotic stress has been reported in S. cerevisiae and N. crassa via the activation of the high‐osmolarity glycerol (HOG) pathway (Lew, 2010; San‐Jose et al., 1996; Wojda et al., 2003). We therefore analysed glycerol accumulation in the mycelia of ΔShk1‐5 after 1, 2 and 4 h of treatment with 0.1 μg/mL fludioxonil, 1 μg/mL dimetachlone and 0.4 m NaCl. As shown in Fig. 5, in the absence of fungicides and osmotic stress, very little glycerol was detected in the wild‐type progenitor or the ΔShk1‐5 mutant. When treated with fungicides and NaCl, glycerol concentration in the wild‐type progenitor increased gradually with time, but that in the ΔShk1‐5 mutant was not obvious. This suggests that fungicides and osmotic stress failed to induce glycerol accumulation in the ΔShk1‐5 mutant (Fig. 5). In the complemented strain ΔShk1‐5C, glycerol accumulation with or without fungicides and NaCl was similar to that of the wild‐type (data not shown). These results show that the accumulation of glycerol in response to fungicides and osmotic stress is under the control of Shk1 in S. sclerotiorum.
Figure 5.
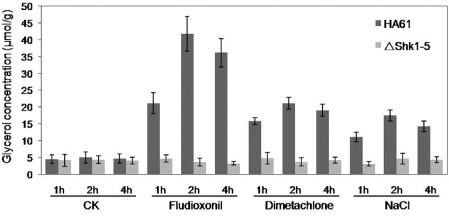
Intracellular glycerol concentration (in micromoles per gram of dried mycelia) of the wild‐type strain HA61 and the Shk1 deletion mutant ΔShk1‐5. The mycelia of each strain were treated with 0.1 μg/mL fludioxonil, 1 μg/mL dimetachlone or 0.4 m NaCl for 1, 2 and 4 h after growth in YEPD (10 mg/mL peptone, 3 mg/mL yeast extract, 20 mg/mL glucose) for 2 days. The cultures without treatment were used as the controls (CK). Bars denote standard errors from three repeated experiments.
Regulation of SsHOG1 and p21‐activated kinase (PAK) expression
The SS1G_07590 gene is orthologous to the MAPK gene of the HOG pathway in the budding yeast (Saito and Tatebayashi, 2004), and is named as SsHOG1 in this study. SsHOG1 is the last kinase of the Hog pathway in S. sclerotiorum; we were therefore interested in analysing the effects of Shk1 deletion on the expression of SsHOG1. Quantitative real‐time PCR (qRT‐PCR) assays showed that the expression of SsHOG1 was up‐regulated by various treatments in the wild‐type strain and ΔShk1‐5 (Fig. 6). The expression levels of SsHOG1 in ΔShk1‐5 were significantly lower than those in the wild‐type strain under various stress conditions (Fig. 6). In addition to the osmoregulating MAPK HOG1, PAK is also involved in osmotic stress responses (Leberer et al., 1992; Liu et al., 1993; O'Rourke and Herskowitz, 1998; Raitt et al., 2000; Ramer and Davis, 1993; Roberts and Fink, 1994). Therefore, the expression of PAK in ΔShk1‐5 was determined. The results indicated that the expression of PAK did not differ between the wild‐type and ΔShk1‐5, even in various stress conditions (Fig. 6). This indicates that PAK may act in independent and additive pathways, but not in the Hog MAPK pathway.
Figure 6.
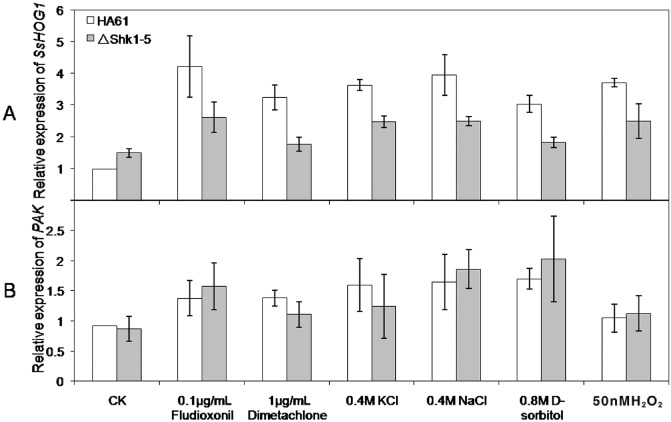
Relative expression levels of the SsHOG1 (A) and PAK (B) genes in the wild‐type strain HA61 and the Shk1 deletion mutant ΔShk1‐5. RNA samples were extracted from the mycelia of each strain untreated or treated with the fungicide fludioxonil (0.1 μg/mL), dimetachlone (1 μg/mL), 0.4 m NaCl, 0.4 m KCl, 0.8 M d‐sorbitol or 50 mm H2O2 for 3 h after being grown in YEPD (10 mg/mL peptone, 3 mg/mL yeast extract, 20 mg/mL glucose) for 2 days. The cultures without treatment were used as the controls (CK). The relative expression levels of SsHOG1 and PAK in the wild‐type strain or ΔShk1‐5 under different stress conditions were calculated as the amount of SsHOG1 and PAK mRNA divided by the amount in the wild‐type strain without treatment. Vertical bars in each column denote the standard errors of three repeated experiments.
Shk1 is not required for pathogenicity
The pathogenicity of the Shk1 deletion mutant was evaluated on several plant leaves. On wounded leaves of rapeseed, strawberry, tomato and cucumber plants, HA61, ΔShk1‐5 and ΔShk1‐5C caused serious disease lesions of similar size 2 days after inoculation (Fig. 7A–E). Studies of the infection process of S. sclerotiorum on bean hypocotyls and pea pods have documented the formation of infection cushion structures which may participate in the penetration and pathogenicity process (Huang and Kokko, 1992; Lumsden and Dow, 1973). To determine whether the Shk1 deletion mutant confers a change in the production of infection cushions, we placed agar plugs colonized with the wild‐type or the Shk1 deletion mutant on a transparent hydrophobic surface (empty Petri dishes) (Harel et al., 2006). The wild‐type and Shk1 deletion mutant both developed typical infection cushions after 24 h (Fig. 7F). These results indicate that, unlike certain other class III HKs (Rispail and Pietro, 2010), Shk1 is not required for pathogenicity on plant leaves.
Figure 7.
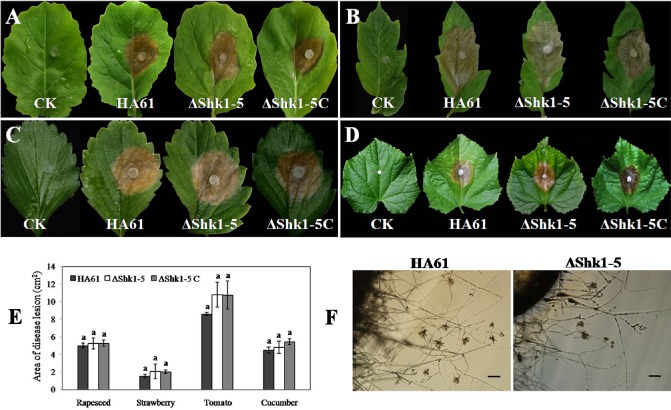
Pathogenicity assays on detached leaves of four host plants and infection cushion formation. Photographs of leaves of rapeseed (A), tomato (B), strawberry (C) and cucumber (D) were taken after culture for 2 days. (E) Area of disease lesions. Bars denote the standard errors of four repeated experiments. The lowercase letters above the bars indicate that the lesion size for each host was unaffected by the strain (P > 0.05). (F) Infection cushion formation assay. Potato dextrose agar plugs colonized with the wild‐type (left) or ΔShk1‐5 (right), 1 day after transfer to a hydrophobic surface. Bar, 100 μm.
Discussion
Fungal HKs have essential functions in osmotic and oxidative stress responses, cell cycle control and virulence (Motoyama et al., 2005b). In particular, class III HKs mediate the cellular responses to osmotic and oxidative stresses (Motoyama et al., 2005a; Viaud et al., 2006), conidiation and asexual morphology (Viaud et al., 2006), the mould‐to‐yeast transition of dimorphic fungi (Nemecek et al., 2006), fungicide resistance (Motoyama et al., 2005a; Ochiai et al., 2001; Viaud et al., 2006; Yoshimi et al., 2004) and pathogenicity (Liu et al., 2008; Nemecek et al., 2006; Viaud et al., 2006; Yamada‐Okabe et al., 1999). However, the function of class III HKs in these physiological processes differs greatly according to the type of mutation and fungal species. In this study, we characterized Shk1, a class III HK from the plant pathogen S. sclerotiorum, and demonstrated that Shk1 controls the resistance of S. sclerotiorum to phenylpyrrole and dicarboximide fungicides (Fig. 3). This result is consistent with that reported in N. crassa, M. grisea, C. heterostrophus, B. cinerea, F. oxysporum, and other fungi (Liu et al., 2008; Motoyama et al., 2005a; Ochiai et al., 2001; Viaud et al., 2006; Yoshimi et al., 2004).
Higher sensitivity to osmotic stress, including stress generated by salts and polyols, and to H2O2 has been reported in most mutants lacking class III HKs (Alex et al., 1996; Hagiwara et al., 2007; Motoyama et al., 2005a; Ochiai et al., 2001; Viaud et al., 2006; Yoshimi et al., 2004). In addition, the sensitivity of these mutants to osmotic stress differs depending on the osmoticum. For instance, mutants of M. oryzae and F. oxysporum show a strong growth reduction on polyols, but not on salt (Motoyama et al., 2005a; Rispail and Pietro, 2010), whereas mutants of N. crassa, C. heterostrophus and B. cinerea show a higher sensitivity to salt (NaCl or KCl) than to polyols (glycerol, sorbitol) (Alex et al., 1996; Viaud et al., 2006; Yoshimi et al., 2004). Sclerotinia sclerotiorum showed a similar trend, i.e. ΔShk1 mutants were more sensitive to salt stress than to sorbitol.
In the filamentous fungus F. graminearum, the HOG MAPK pathway is involved in the regulation of hyphal growth and branching (Zheng et al., 2012). Therefore, we determined the effects of Shk1 deletion on vegetative hyphal growth in S. sclerotiorum. In addition to the reduced growth rate, the Shk1 deletion mutant had more hyphal branches and grew in close proximity to each other at the extension zone. Surprisingly, ΔSkh1‐5 was unable to produce sclerotia and showed a distinct colony morphology on PDA plates (Fig. 2B). Glycerol production and accumulation are well known to be regulated by the HOG pathway in S. cerevisiae. Our results indicated that glycerol production was also regulated by Shk1 in S. sclerotiorum, and glycerol accumulation was also induced by fungicides and NaCl treatment in the wild‐type but not in the Shk1 mutant (Fig. 5). In summary, these data showed that the HOG pathway activated by Shk1 regulates hyphal growth and branching in S. sclerotiorum. It also plays a critical role in sclerotial formation, glycerol production and accumulation. In eukaryotic cells, HKs are found to act at the head of the HOG pathway (West and Stock, 2001). Therefore, it is important to characterize further the functions of the HOG pathway in hyphal growth, sclerotial formation and glycerol accumulation.
Ste20 activates distinct MAPK cascades that control filamentous growth, mating and osmotic stress responses (Leberer et al., 1992; O'Rourke and Herskowitz, 1998). The PAK gene (SS1G_02450) is orthologous to Ste20 and is involved in osmoadaptation in S. sclerotiorum (Duan Y.B., Ge C.Y. and Zhou M.G., unpublished data). SsHOG1 (SS1G_07590) is orthologous to the Fghog1, BcOs2 and Os2 genes of F. graminearum, B. cinerea and N. crassa, respectively, and the last kinase of the Hog pathway in S. sclerotiorum. In the current study, we found that the expression of SsHOG1 was regulated by the Shk1 gene, but PAK was not involved in the HOG pathway in S. sclerotiorum. To further explore relationships between the HOG pathway and the pathways controlled by PAK, side‐by‐side comparisons of various phenotypes among the mutants involved in the pathways could provide clearer information.
The role of class III HKs in virulence has been studied in plant pathogens, and phenotypes of mutants range from highly virulent to completely avirulent, depending on the type of mutation, genetic background and fungal species. For instance, class III HKs are required for full virulence in F. oxysporum, B. cinerea and A. brassicicola, but not in M. oryzae (Cho et al., 2009; Liu et al., 2008; Motoyama et al., 2005a; Rispail and Pietro, 2010; Viaud et al., 2006). It was therefore necessary to determine the role of class III HKs in the virulence of S. sclerotiorum. The results obtained with four species of host plant indicated that Shk1 is not required for virulence in S. sclerotiorum. We conclude that the function of HKs in pathogenicity differs among phytopathogenic fungi.
That fludioxonil‐resistant isolates of several filamentous fungi are also resistant to dicarboximide fungicides indicates that the mechanism of resistance is similar for both groups of fungicide (Avenot et al., 2005). Mutations in class III HKs increase dicarboximide resistance, indicating that class III HKs could be a primary target of the dicarboximides. This inference, however, must be confirmed with direct binding studies. In addition to involving the os‐1 gene that encodes the class III HK, resistance to dicarboximides in N. crassa also involves os‐2, os‐4 and os‐5 (Fujimura et al., 2000; Yang et al., 2012). Therefore, it will be interesting to isolate and characterize such genes from S. sclerotiorum in order to fully understand the resistance of S. sclerotiorum to dicarboximide fungicides. The findings from this study and previous reports have shown that the roles of osmotic signalling systems differ among fungi. An understanding of these differences should aid in the development of new fungicides and other methods of fungal control.
Experimental Procedures
Strains and culture conditions
Strain HA61 of S. sclerotiorum was used for transformation in this study. It was isolated from rapeseed in Jiangsu Province, China, and is sensitive to fludioxonil and dicarboximide (Duan et al., 2013). The hyphal structure of each strain growing on PDA was examined using an Olympus microscope (Olympus Optical Co., Ltd, Tokyo, Japan).
Autoclaved PDA was used to culture the wild‐type progenitor HA61 and its derived mutants in routine assays for in vitro sensitivity to fludioxonil, dimetachlone, iprodione, carbendazim, NaCl, KCl, H2O2, glucose and d‐sorbitol at the concentrations indicated in the figure legends (Chen et al., 2012). Each plate was inoculated with a plug (diameter, 5 mm) of mycelium from the leading edge of a 2‐day‐old colony. After the plates had been incubated at 25 °C for 2 days, the mean colony diameters (minus the diameter of the inoculation plug) were measured. The percentage of radial growth inhibition (RGI) was calculated using the formula RGI (%) = [(C − N)/(C − 5)] × 100, where C is the colony diameter of the control and N is the colony diameter of the treatment. The experiment was performed three times.
Identification of the kinase gene Shk1 in S. sclerotiorum
The Shk1 (SS1G_12694.3) gene was originally identified through a homology search of the S. sclerotiorum genome sequence (available at http://www.broadinstitute.org/annotation/genome/sclerotinia_sclerotiorum/MultiHome.html) using blast with the Os1 gene from N. crassa (Alex et al., 1996; Schumacher et al., 1997) and the BcOs1 gene from B. cinerea (Liu et al., 2008; Viaud et al., 2006) as queries. The autocalled gene Shk1 has five predicted introns. To verify the existence and size of the introns, RNA was extracted from the mycelia of the wild‐type progenitor HA61 with the RNAsimple Total RNA Kit (Tiangen Biotech. Co., Beijing, China) and was used for reverse transcription with the PrimeScript RT Reagent Kit (TaKaRa, Dalian, China). Reverse transcription PCR amplification of the cDNA was conducted with the primers P1/P2 (Table 1). The resultant PCR product was purified, cloned and sequenced.
Construction of the ΔShk1 null allele and fungal transformation
The knockout construct Shk1up‐hph‐dn of S. sclerotiorum Shk1 was generated by fusion PCR (Yu et al., 2004). First, 1.4‐kb upstream and 1.5‐kb downstream flanking fragments of Shk1 were amplified from the genomic DNA of S. sclerotiorum strain HA61 with the primer pairs P3/P4 and P5/P6 (Table 1, Fig. 1A), respectively. The 5′ regions of P4 and P5 contained the complementary sequences of primers P7 and P8, respectively, which were used to amplify a 1.8‐kb cassette containing the hygromycin B resistance gene under the control of the Aspergillus nidulans trpC promoter (Mullins et al., 2001). Then, the two Shk1 flanking fragments were mixed with the hygromycin B resistance cassette in a molar proportion of 1:1:3 and fused using the external P9 and P10 primers (Table 1, Fig. 1A). The construct generated was used to transform protoplasts of the wild‐type strain HA61.
Polyethylene glycol (PEG)‐mediated protoplast transformation was performed as described previously, but with some modifications (Maier et al., 2005). To generate protoplasts, plugs from the edge of a 2‐day‐old colony on PDA were placed in 250‐mL flasks containing 100 mL of liquid YEPD (10 mg/mL peptone, 3 mg/mL yeast extract, 20 mg/mL glucose), and the flasks were placed on a rotary shaker (175 rpm, 25 °C) for 36 h. Mycelia were collected on a sterile filter and washed twice with distilled water to remove medium. Then, 0.1 g of mycelia was resuspended in 20 mL of protoplast solution (10 mg/mL of lysing enzymes from Tichoderma harzianum; Sigma, St. Louis, MO, USA) and digested for 2.5 h at 30 °C and 85 rpm. Protoplasts were transformed and plated on regeneration medium (0.5 g/L yeast extract, 0.5 g/L casein hydrolysate, 0.7 m sucrose and 16 g/L agar powder). After 12 h, regeneration plates were overlaid with 10 mL of selective medium (0.5 g/L yeast extract, 0.5 g/L casein hydrolysate, 1 m sucrose and 12 g/L agarose) containing 100 μg/mL hygromycin B; the plates were incubated at 25 °C. After 4–10 days, hygromycin‐resistant colonies appeared, and individual transformants were transferred onto PDA plates amended with hygromycin B at 100 μg/mL. The plates were incubated at 25 °C for 1 day, and single hyphal tips from each transformant were picked with a sterile needle, transferred to PDA containing hygromycin B (100 μg/mL) and used in subsequent experiments. Transformants showing the homologous insertion of the construct were detected by PCR amplification of genomic DNA with four pairs of primers, P11/P12, P13/P14, P15/P16 and P17/P18 (Table 1, Fig. 1A), and confirmed by Southern analysis of genomic DNA digested with BamHI and hybridized with a labelled probe obtained by PCR amplification with primers P23/P24 (Table 1, Fig. 1A).
To confirm that the phenotype of the Shk1 deletion mutant resulted from deletion of the gene, a Shk1 deletion mutant was complemented with the full‐length Shk1 gene. The Shk1 complement plasmid pNEO‐Shk1‐Com was constructed using the backbone of pCAMBIA 1300 (CAMBIA, Canberra, Australia). First, a BstXI‐XhoI NEO cassette containing a trpC promoter (resistance to neomycin) was amplified from plasmid PII99‐Pro(DOHH)GFP with primers P19/P20 (Table 1) and cloned into the BstXI‐XhoI site of pCAMBIA 1300 to create plasmid pNEO. Then, the full‐length Shk1 gene, including the 1891‐bp upstream and 383‐bp terminator regions, was amplified from genomic DNA of the wild‐type strain with primers P21/P22 (Table 1), and subsequently cloned into the SacI‐SmaI site of pNEO to generate the complement plasmid pNEO‐Shk1‐Com. Before plasmid pNEO‐Shk1‐Com was transformed into strain ΔShk1‐5, Shk1 in this plasmid was sequenced to ensure sequence correctness. Transformation of ΔShk1‐5 with the full‐length Shk1 gene was conducted as described above, except that neomycin (100 μg/mL) was used as a selection agent.
Determination of intracellular glycerol accumulation
Glycerol accumulation in the mycelia of each strain was measured as described previously with some modifications (Henkel and Stoltz, 1982). Briefly, each strain was grown in YEPD for 2 days at 25 °C in a shaker. After treatment with 0.1 μg/mL fludioxonil, 1 μg/mL dimetachlone or 0.4 m NaCl for 1, 2 and 4 h, the mycelia of each strain were harvested and ground in liquid nitrogen. Then, the mycelial powders (0.1 g) were transferred to a 2‐mL microcentrifuge tube containing 1 mL glycerol extraction buffer (Applygen Technologies Inc., Beijing, China). After vortexing for 5 min, the tubes were centrifuged at 5000 g for 20 min; 5 μL of supernatant in each tube was mixed with 195 μL of detection buffer (Applygen Technologies Inc.). After the mixture had been incubated at 37 °C for 20 min, the glycerol concentration was determined at a wavelength of 550 nm using a SpectraMax M5 microplate reader (Molecular Devices Inc., Sunnyvale, CA, USA). The experiment was repeated twice.
Determination of the expression of SsHOG1 and PAK in the Shk1 deletion mutant
To extract total RNA, the mycelial plugs of the wild‐type strain HA61 or the Shk1 deletion mutant ΔShk1‐5 were transferred to YEPD and cultured for 2 days at 25 °C in the dark. Before the total RNA was extracted, the culture was treated for 3 h with fludioxonil, dimetachlone, NaCl, KCl, d‐sorbitol or H2O2 at the concentrations indicated in the legend of Fig. 5. Extraction of total RNA and reverse transcription were performed as described above. The expression of SsHOG1 and PAK was determined by qRT‐PCR. The qRT‐PCR amplifications were performed in a 7500 ABI PRISM Sequence Detector System (Applied Biosystems, Foster City, CA, USA) using SYBR Green I fluorescent dye detection. Amplification was conducted in a 20‐μL volume containing 10 μL of iTaq™ Universal SYBR® Green Supermix (Bio‐Rad Laboratories, Hercules, CA, USA), 2 μL of the reverse transcription product and 1 μL each of the forward and reverse primers (500 nm each) (Table 1). There were three replicates for each sample. For each sample, PCR amplifications with primers P29/P30 (Table 1) for the quantification of the expression of the actin gene were performed as a reference. The experiment was repeated twice. The expression of SsHOG1 and PAK in the wild‐type progenitor or ΔShk1‐5 under fungicide, osmotic or oxidative stress conditions, relative to that in the wild‐type strain without treatment, was calculated using the 2−ΔΔCt method (Aravind and Ponting, 1999).
Plant infection assays
To determine whether the pathogenicity of the Shk1 deletion mutant varied on different host plants, healthy, fully expanded leaves of rapeseed, strawberry, tomato and cucumber plants were inoculated with 5‐mm‐diameter plugs of 2‐day‐old cultures growing on PDA; for controls, leaves were inoculated with plugs that lacked fungi. Before inoculation, the leaves were wounded with a sterile needle tip to facilitate the penetration of the fungus into plant tissue. Inoculated plant leaves were incubated in a growth chamber (25 °C; relative humidity, 85%; 16 h daylight). The diameter of the lesion on each leaf was recorded 2 days after inoculation. There were 10 replicate leaves for each strain, and the experiment was repeated three times.
Infection cushion formation assay
Five‐millimetre‐diameter plugs of 2‐day‐old cultures from the wild‐type and the Shk1 deletion mutant growing on PDA were placed on the surface of empty Petri dishes (nine per dish). The Petri dishes were maintained at 100% relative humidity and 25 °C for 24 h. The formation of infection cushions was monitored by light microscopy using an Olympus microscope (Olympus).
Acknowledgements
This research was supported by the Special Fund for Agro‐scientific Research in the Public Interest (201103016 and 201303023).
References
- Alex, L. , Borkovich, K. and Simon, M. (1996) Hyphal development in Neurospora crassa: involvement of a two‐component histidine kinase. Proc. Natl. Acad. Sci. USA, 93, 3416–3421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aravind, L. and Ponting, C.P. (1999) The cytoplasmic helical linker domain of receptor histidine kinase and methyl‐accepting proteins is common to many prokaryotic signaling proteins. FEMS Microbiol. Lett. 176, 111–116. [DOI] [PubMed] [Google Scholar]
- Avenot, H. , Simoneau, P. , Iacomi‐Vasilescu, B. and Bataille‐Simoneau, N. (2005) Characterization of mutations in the two‐component histidine kinase gene AbNIK1 from Alternaria brassicicola that confer high dicarboximide and phenylpyrrole resistance. Curr. Genet. 47, 234–243. [DOI] [PubMed] [Google Scholar]
- Bahn, Y. (2008) Master and commander in fungal pathogens: the two‐component system and the HOG signal pathway. Eukaryot. Cell, 7, 2017–2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boland, G.J. and Hall, R. (1994) An index of host plant hosts susceptible to Sclerotinia sclerotiorum . Can. J. Plant Pathol. 16, 93–108. [Google Scholar]
- Catlett, N.L. , Yoder, O.C. and Turgeon, B.G. (2003) Whole‐genome analysis of two‐component signal transduction genes in fungal pathogens. Eukaryot. Cell, 2, 1151–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Y. , Zhang, A.F. , Gao, T.C. , Zhang, Y. , Wang, W.X. , Ding, K.J. , Chen, L. , Sun, Z. , Fang, X.Z. and Zhou, M.G. (2012) Integrated use of pyraclostrobin and epoxiconazole for the control of Fusarium head blight of wheat in Anhui Province of China. Plant Dis. 96, 1495–1500. [DOI] [PubMed] [Google Scholar]
- Cho, Y. , Kim, K.H. , La, R.M. , Scott, D. , Santopietro, G. , Callihan, M. , Mitchell, T.K. and Lawrence, C.B. (2009) Identification of novel virulence factors associated with signal transduction pathways in Alternaria brassicicola . Mol. Microbiol. 72, 1316–1333. [DOI] [PubMed] [Google Scholar]
- Cui, W. , Beever, R.E. , Parkes, S.L. , Weeds, P.L. and Templeton, M.D. (2002) An osmosensing histidine kinase mediates dicarboximide fungicide resistance in Botryotinia fuckeliana (Botrytis cinerea). Fungal Genet. Biol. 36, 187–198. [DOI] [PubMed] [Google Scholar]
- Duan, Y.B. , Liu, S.M. , Ge, C.Y. , Feng, X.J. , Chen, C.J. and Zhou, M.G. (2012) In vitro inhibition of Sclerotinia sclerotiorum by mixtures of azoxystrobin, SHAM, and thiram. Pestic. Biochem. Physiol. 103, 101–107. [Google Scholar]
- Duan, Y.B. , Ge, C.Y. , Liu, S.M. , Chen, C.J. and Zhou, M.G. (2013) Effect of phenylpyrrole fungicide fludioxonil on mycelial morphology and biological characterization of Sclerotinia sclerotiorum . Pestic. Biochem. Phys. 106, 61–67. [Google Scholar]
- Fujimura, M. , Ochiai, N. , Ichiishi, A. , Usami, R. , Horikoshi, K. and Yamaguchi, I. (2000) Sensitivity to phenylpyrrole fungicides and abnormal glycerol accumulation in os and cut mutant strains of Neurospora crassa . J. Pestic. Sci. 25, 31–36. [Google Scholar]
- Fujimura, M. , Ochiai, N. , Oshima, M. , Motoyama, T. , Ichiishi, A. , Usami, R. , Horikoshi, K. and Yamaguchi, I. (2003) Putative homologs of SSK22 MAPKK kinase and PBS2 MAPK kinase of Saccharomyces cerevisiae encoded by os‐4 and os‐5 genes for osmotic sensitivity and fungicide resistance in Neurospora crassa . Biosci. Biotechnol. Biochem. 67, 186–191. [DOI] [PubMed] [Google Scholar]
- Hagiwara, D. , Matsubayashi, Y. , Marui, J. , Furukawa, K. , Yamashino, T. , Kanamaru, K. , Kato, M. , Abe, K. , Kobayashi, T. and Mizuno, T. (2007) Characterization of the NikA histidine kinase implicated in the phosphorelay signal transduction of Aspergillus nidulans, with special reference to fungicide responses. Biosci. Biotechnol. Biochem. 71, 844–847. [DOI] [PubMed] [Google Scholar]
- Harel, A. , Bercovich, S. and Yarden, O. (2006) Calcineurin is required for sclerotial development and pathogenicity of Sclerotinia sclerotiorum in an oxalic acid‐independent manner. Mol. Plant–Microbe Interact. 19, 682–693. [DOI] [PubMed] [Google Scholar]
- Henkel, E. and Stoltz, M. (1982) A newly drafted colour test for the determination of triglycerides convenient for manual and mechanized analysis (glycerolphosphate‐oxidase‐PAP method). Fresenius' J. Anal. Chem. 311, 451–452. [Google Scholar]
- Huang, H.C. and Kokko, E.G. (1992) Pod rot of dry peas due to infection by ascospores from Sclerotinia sclerotiorum . Plant Dis. 76, 597–600. [Google Scholar]
- Kuang, J. , Hou, Y.P. , Wang, J.X. and Zhou, M.G. (2011) Sensitivity of Sclerotinia sclerotiorum to fludioxonil: in vitro determination of baseline sensitivity and resistance risk. Crop Prot. 30, 876–882. [Google Scholar]
- Leberer, E. , Dignard, D. , Harcus, D. , Thomas, D.Y. and Whiteway, M. (1992) The protein kinase homologue Ste20p is required to link the yeast pheromone response G‐protein βγ subunits to downstream signaling components. EMBO J. 11, 4815–4824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lew, R.R. (2010) Turgor and net ion flux responses to activation of the osmotic MAP kinase cascade by fludioxonil in the filamentous fungus Neurospora crassa . Fungal Genet. Biol. 47, 721–726. [DOI] [PubMed] [Google Scholar]
- Liu, H. , Styles, C.A. and Fink, G.R. (1993) Elements of the yeast pheromone response pathway required for filamentous growth of diploids. Science, 262, 1741–1744. [DOI] [PubMed] [Google Scholar]
- Liu, W. , Leroux, P. and Fillinger, S. (2008) The HOG1‐like MAP kinase Sak1 of Botrytis cinerea is negatively regulated by the upstream histidine kinase Bos1 and is not involved in dicarboximide‐ and phenylpyrrole‐resistance. Fungal Genet. Biol. 45, 1062–1074. [DOI] [PubMed] [Google Scholar]
- Lumsden, R.D. and Dow, R.L. (1973) Histopathology of Sclerotinia sclerotiorum infection of bean. Phytopathology, 63, 708–715. [Google Scholar]
- Ma, H.X. , Feng, X.J. , Chen, Y. , Chen, C.J. and Zhou, M.G. (2009) Occurrence and characterization of dimethachlon insensitivity in Sclerotinia sclerotiorum in Jiangsu Province of China. Plant Dis. 93, 36–42. [DOI] [PubMed] [Google Scholar]
- Ma, Z. , Luo, Y. and Michailides, T.J. (2006) Molecular characterization of the two‐component histidine kinase gene from Monilinia fructicola . Pest Manag. Sci. 62, 991–998. [DOI] [PubMed] [Google Scholar]
- Maier, F.J. , Malz, S. , Losch, A.P. , Lacour, T. and Schafer, W. (2005) Development of a highly efficient gene targeting system for Fusarium graminearum using the disruption of a polyketide synthase gene as a visible marker. FEMS Yeast Res. 5, 653–662. [DOI] [PubMed] [Google Scholar]
- Motoyama, T. , Kadokura, K. , Ohira, T. , Ichiishi, A. , Fujimura, M. , Yamaguchi, I. and Kudo, T.A. (2005a) Two‐component histidine kinase of the rice blast fungus is involved in osmotic stress response and fungicide action. Fungal Genet. Biol. 42, 200–212. [DOI] [PubMed] [Google Scholar]
- Motoyama, T. , Ohira, T. , Kadokura, K. , Ichiishi, A. , Fujimura, M. , Yamaguchi, I. and Kudo, T. (2005b) An Os‐1 family histidine kinase from a filamentous fungus confers fungicide‐sensitivity to yeast. Curr. Genet. 47, 298–306. [DOI] [PubMed] [Google Scholar]
- Mullins, E.D. , Chen, X. , Romaine, P. , Raina, R. , Geiser, D.M. and Kang, S. (2001) Agrobacterium‐mediated transformation of Fusarium oxysporum: an efficient tool for insertional mutagenesis and gene transfer. Phytopathology, 91, 173–180. [DOI] [PubMed] [Google Scholar]
- Nemecek, J.C. , Wuthrich, M. and Klein, B.S. (2006) Global control of dimorphism and virulence in fungi. Science, 312, 583–588. [DOI] [PubMed] [Google Scholar]
- O'Rourke, S.M. and Herskowitz, I. (1998) The Hog1 MAPK prevents cross talk between the HOG and pheromone response MAPK pathways in Saccharomyces cerevisiae . Gene Dev. 2, 2874–2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochiai, N. , Fujimura, M. , Motoyama, T. , Ichiishi, A. , Usami, R. , Horikoshi, K. and Yamaguchi, I. (2001) Characterization of mutations in the two‐component histidine kinase gene that confer fludioxonil resistance and osmotic sensitivity in the os‐1 mutants of Neurospora crassa . Pest Manag. Sci. 57, 437–442. [DOI] [PubMed] [Google Scholar]
- Ochiai, N. , Tokai, T. , Nishiuchi, T. , Takahashi‐Ando, N. , Fujimura, M. and Kimura, M. (2007) Involvement of the osmosensor histidine kinase and osmotic stress‐activated protein kinases in the regulation of secondary metabolism in Fusarium graminearum . Biochem. Biophys. Res. Commun. 363, 639–644. [DOI] [PubMed] [Google Scholar]
- Oshima, M. , Fujimura, M. , Banno, S. , Hashimoto, C. , Motoyama, T. , Ichiishi, A. and Yamaguchi, I. (2002) A point mutation in the two‐component histidine kinase BcOS‐1 gene confers dicarboximide resistance in field isolates of Botrytis cinerea . Phytopathology, 92, 75–80. [DOI] [PubMed] [Google Scholar]
- Posas, F. , Wurgler‐Murphy, S.M. , Maeda, T. , Witten, E.A. , Thai, T.C. and Saito, H. (1996) Yeast HOG1 MAP kinase cascade is regulated by a multistep phosphorelay mechanism in the SLN1‐YPD1‐SSK1 two‐component osmosensor. Cell, 86, 865–875. [DOI] [PubMed] [Google Scholar]
- Purdy, L.H. (1979) Sclerotinia sclerotiorum: history, diseases and symptomatology, host range, geographic distribution and impact. Phytopathology, 69, 875–880. [Google Scholar]
- Raitt, D.C. , Posas, F. and Saito, H. (2000) Yeast Cdc42 GTPase and Ste20 PAK‐like kinase regulate Sho1‐dependent activation of the Hog1 MAPK pathway. EMBO J. 19, 4623–4631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramer, S.W. and Davis, R.W. (1993) A dominant truncation allele identifies a gene, STE20 that encodes a putative protein kinase necessary for mating in Saccharomyces cerevisiae . Proc. Natl. Acad. Sci. USA, 90, 452–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rispail, N. and Pietro, D.A. (2010) The two‐component histidine kinase Fhk1 controls stress adaptation and virulence of Fusarium oxysporum . Mol. Plant Pathol. 11, 395–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts, R.L. and Fink, G.R. (1994) Elements of a single MAP kinase cascade in Saccharomyces cerevisiae mediate two developmental programs in the same cell type: mating and invasive growth. Gene Dev. 8, 2974–2985. [DOI] [PubMed] [Google Scholar]
- Saito, H. and Tatebayashi, K. (2004) Regulation of the osmoregulatory HOG MAPK cascade in yeast. J. Biochem. 136, 267–272. [DOI] [PubMed] [Google Scholar]
- San‐Jose, C. , Monge, R.A. , Perez‐Diaz, R. , Pla, J. and Nombela, C. (1996) The mitogen activated protein kinase homolog HOG1 gene controls glycerol accumulation in the pathogenic fungus Candida albicans . J. Bacteriol. 178, 5850–5852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher, M. , Enderlin, C. and Selitrennikoff, C. (1997) The osmotic‐1 locus of Neurospora crassa encodes a putative histidine kinase similar to osmosensors of bacteria and yeast. Curr. Microbiol. 34, 340–347. [DOI] [PubMed] [Google Scholar]
- Segmüller, N. , Ellendorf, U. , Tudzynski, B. and Tudzynski, P. (2007) BcSAK1, a stress‐activated mitogen‐activated protein kinase, is involved in vegetative differentiation and pathogenicity in Botrytis cinerea . Eukaryot. Cell, 6, 211–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi, Z.Q. , Zhou, M.G. and Ye, Z.Y. (2000) Resistance of Sclerotinia sclerotiorum to carbendazim and dimethachlon. Chin. J. Oil Crop Sci. 22, 54–57. [Google Scholar]
- Steadman, J.R. (1979) Control of plant diseases caused by Sclerotinia species. Phytopathology, 69, 904–907. [Google Scholar]
- Tanaka, C. and Izumitsu, K. (2010) Two‐component signaling system in filamentous fungi and the mode of action of dicarboximide and phenylpyrrole fungicides In: Fungicides (Carisse O., ed.), pp. 523–538. Rijeka: InTech. [Google Scholar]
- Tu, J.C. (1997) An integrated control of white mold (Sclerotinia sclerotiorum) of beans, with emphasis on recent advances in biological control. Bot. Bull. Acad. Sin. 38, 73–76. [Google Scholar]
- Viaud, M. , Fillinger, S. , Liu, W. , Polepalli, J.S. , Pecheur, L.P. , Kunduru, A.R. , Leroux, P. and Legendre, L. (2006) A class III histidine kinase acts as a novel virulence factor in Botrytis cinerea . Mol. Plant–Microbe Interact. 19, 1042–1050. [DOI] [PubMed] [Google Scholar]
- West, A.H. and Stock, A.M. (2001) Histidine kinases and response regulator proteins in two‐component signaling systems. Trends Biochem. Sci. 26, 369–376. [DOI] [PubMed] [Google Scholar]
- Wojda, I. , Alonso‐Monge, R. , Bebelman, J.P. , Mager, W.H. and Siderius, M. (2003) Response to high osmotic conditions and elevated temperature in Saccharomyces cerevisiae is controlled by intracellular glycerol and involves coordinate activity of MAP kinase pathways. Microbiology, 149, 1193–1204. [DOI] [PubMed] [Google Scholar]
- Yamada‐Okabe, T. , Mio, T. , Ono, N. , Kashima, Y. , Matsui, M. , Arisawa, M. and Yamada‐Okabe, H. (1999) Roles of three histidine kinase genes in hyphal development and virulence of the pathogenic fungus Candida albicans . J. Bacteriol. 181, 7243–7247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan, L. , Yang, Q. , Sundin, G.W. , Li, H. and Ma, Z. (2010) The mitogen‐activated protein kinase kinase BOS5 is involved in regulating vegetative differentiation and virulence in Botrytis cinerea . Fungal Genet. Biol. 47, 753–760. [DOI] [PubMed] [Google Scholar]
- Yan, L. , Yang, Q. , Jiang, J. , Michailides, T.J. and Ma, Z. (2011) Involvement of a putative response regulator Brrg‐1 in the regulation of sporulation, sensitivity to fungicides, and osmotic stress in Botrytis cinerea . Appl. Microbiol. Biotechnol. 90, 215–226. [DOI] [PubMed] [Google Scholar]
- Yang, Q. , Yan, L. , Gu, Q. and Ma, Z. (2012) The mitogen‐activated protein kinase kinase kinase BcOs4 is required for vegetative differentiation and pathogenicity in Botrytis cinerea . Appl. Microbiol. Biotechnol. 96, 481–492. [DOI] [PubMed] [Google Scholar]
- Yoshimi, A. , Tsuda, M. and Tanaka, C. (2004) Cloning and characterization of the histidine kinase gene Dic1 from Cochliobolus heterostrophus that confers dicarboximide resistance and osmotic adaptation. Mol. Genet. Genomics, 271, 228–236. [DOI] [PubMed] [Google Scholar]
- Yu, J.H. , Hamari, Z. , Han, K.H. , Seo, J.A. , Reyes‐Domínguez, Y. and Scazzocchio, C. (2004) Double‐joint PCR: a PCR‐based molecular tool for gene manipulations in filamentous fungi. Fungal Genet. Biol. 41, 973–981. [DOI] [PubMed] [Google Scholar]
- Zhang, Y. , Lamm, R. , Pillonel, C. , Lam, S. and Xu, J.R. (2002) Osmoregulation and fungicide resistance: the Neurospora crassa os‐2 gene encodes a HOG1 mitogen‐activated protein kinase homologue. Appl. Environ. Microbiol. 68, 532–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng, D. , Zhang, S. , Zhou, X. , Wang, C. , Xiang, P. , Zheng, Q. and Xu, J.R. (2012) The Fghog1 pathway regulates hyphal growth, stress responses, and plant infection in Fusarium graminearum . PLoS ONE, 7, e49495. [DOI] [PMC free article] [PubMed] [Google Scholar]


