SUMMARY
Plant parasitic nematodes impose a severe constraint on plantain and banana productivity; however, the sterile nature of many cultivars precludes conventional breeding for resistance. Transgenic plantain cv. Gonja manjaya (Musa AAB) plants, expressing a maize cystatin that inhibits nematode digestive cysteine proteinases and a synthetic peptide that disrupts nematode chemoreception, were assessed for their ability to resist nematode infection. Lines were generated that expressed each gene singly or both together in a stacked defence. Nematode challenge with a single species or a mixed population identified 10 lines with significant resistance. The best level of resistance achieved against the major pest species Radopholus similis was 84% ± 8% for the cystatin, 66% ± 14% for the peptide and 70% ± 6% for the dual defence. In the mixed population, trial resistance was also demonstrated to Helicotylenchus multicinctus. A fluorescently labelled form of the chemodisruptive peptide underwent retrograde transport along certain sensory dendrites of R. similis as required to disrupt chemoreception. The peptide was degraded after 30 min in simulated intestinal fluid or boiling water and after 1 h in nonsterile soil. In silico sequence analysis suggests that the peptide is not a mammalian antigen. This work establishes the mode of action of a novel nematode defence, develops the evidence for its safe and effective deployment against multiple nematode species and identifies transgenic plantain lines with a high level of resistance for a proposed field trial.
INTRODUCTION
Several nematodes are major pathogens of banana and plantain crops (Atkinson, 2003; Brentu et al., 2004; Gowen and Quénéhervé, 1990) with the migratory endoparasite, Radopholus similis, often the most damaging species (Bridge et al., 1995; Haegeman et al., 2010; Price, 2006). It causes lesions in roots that encourage secondary fungal and bacterial infections (Duncan and Moens, 2006). Parasitism leads to yield loss from stunted plant growth and plant toppling in strong winds as a result of reduced anchorage from damaged root systems. Helicotylenchus multicinctus is a second root parasite (Bridge et al., 1995; McSorley and Parrado, 1983), whose importance is often more evident when R. similis is absent (Brentu et al., 2004; Price, 2006). Experimental application of nematicides has indicated that damage caused by nematodes in a range of African countries results in a 71% ± 16% reduction in yield over 3 years (Atkinson, 2003). Nematicides are widely applied to soils in intensive banana plantations, but they are environmentally toxic and a risk to human health (Atkinson, 2003). In sub‐Saharan Africa, plantains are predominantly cultivated on small plots and nematicides are inappropriate on the basis of both cost and grower safety. Banana and plantain together (Musa spp.) represent the fourth most important staple food of sub‐Saharan Africa, where about 73% of the world's plantains are grown (FAOSTAT, 2009; http://bit.ly/faostat). They provide more than 25% of the carbohydrates consumed by approximately 60 million people in West Africa (Swennen and Vuylsteke, 2001).
Conventional breeding has not produced nematode‐resistant hybrid plantains, and resistance that has been achieved for other bananas is only effective against single species and not against concurrent infections by several nematodes (Lorenzen et al., 2010; Pinochet, 1988). A key difficulty is that the cultivated plantain and banana varieties are sterile with a triploid genome that has arisen by somatic mutations or somaclonal variation (Lorenzen et al., 2010). These characteristics hamper improvement by traditional cross‐pollination techniques. Attempts to increase the genetic diversity of plantain cultivars by induced mutation, molecular genetics and other approaches (Lorenzen et al., 2010) have provided limited success (Escalant and Jain, 2004). They rely on plantain germplasm possessing genes of value for the required crop improvement. Transgenic introduction of one or more genes to plantain overcomes both this constraint and ploidy issues. Agrobacterium‐mediated transformation of embryogenic cell suspensions and meristematic tissues has been developed to improve bananas and plantains (Ganapathi et al., 2001; Khanna et al., 2004; May et al., 1995; 2005, 2008). The first improvements sought for bananas and plantains have been resistance to fungal and bacterial diseases (Paul et al., 2011; Tripathi et al., 2010) and biofortification (Mayer et al., 2008). This work extends this range to nematode control.
Several transgenes confer plant resistance to both tropical and temperate plant parasitic nematodes (Atkinson et al., 2003). Cystatins inhibit nematode digestive cysteine proteinase activity, suppressing the growth and multiplication of these pests (Urwin et al., 1997). They have provided resistance to a range of plant parasitic nematodes in different crops (Chan et al., 2010; Gao et al., 2011; 1997, 2000; Vain et al., 1998) and have proven efficacy under field conditions (2001, 2003). The control of R. similis has been achieved in a containment trial with transgenic dessert banana expressing a cystatin (Atkinson et al., 2004a). A second defence is based on the secretion from plant roots of synthetic peptides that disrupt nematode chemoreception and interfere with the perception of the location of host plants (Liu et al., 2005; Winter et al., 2002). One peptide, which inhibits acetylcholinesterase, provided 94.9% ± 0.8% resistance to the potato cyst nematode Globodera pallida when expressed in transgenic potato plants under control of a root cap‐specific promoter (Lilley et al., 2011). The synthetic peptide used in the current work is a constrained 7‐mer termed nAChRbp (Wang et al., 2011) with the sequence CTTMHPRLC. It binds to nematode nicotinic acetylcholine receptors (nAChRs) and inhibits the chemoreception of G. pallida at 1 µm (Winter et al., 2002). Transgenic potato plants expressing this peptide displayed up to 77% ± 4% resistance against G. pallida in a field trial and had no impact on the community of nontarget soil nematodes (Green et al., 2012). A fluorescently tagged version of nAChRbp is taken up by the infective stage of the cyst nematodes Heterodera schachtii and G. pallida via certain chemosensory sensillae in the amphidial pouches. The peptide undergoes retrograde transport along sensory dendrites to neuronal cell bodies, resulting in a loss of orientation by infective stage G. pallida towards potato root exudate (Wang et al., 2011). The transgenic plantains described in the current work secrete the peptide from their roots as a result of an N‐terminal cleaved extracellular export signal (Liu et al., 2005). Uptake and inhibition, therefore, presumably occur in soil at or near the rhizoplane prior to invasion, in contrast with the cystatin that only has an effect following invasion after the commencement of feeding. This work also develops a prima facie basis for the biosafety of the peptide used to control nematodes to accompany that already provided for a cystatin (Atkinson et al., 2004b).
We have transformed the plantain cv. Gonja manjaya (Musa spp. AAB) to express both a cystatin and a peptide to provide either single or dual defence against nematodes. The challenge of these transgenic plantains demonstrates that both defences provide a level of resistance to nematodes in a contained screenhouse trial that justifies a future field trial in Africa.
RESULTS
Uptake of fluorescent peptide by R. similis
The nAChRbp peptide conjugated to the fluorophore Alexa Fluor 488 accumulated in the two amphidial pouches of adult female R. similis after 16 h of incubation. The amphids enclose chemosensory sensillae and open via a pore to the anterior surface of the nematode (Fig. 1). Fluorescence was not evident along the pharyngeal lumen, and so ingestion of the peptide had not occurred. Strong fluorescent emissions were evident in several neuronal cell bodies surrounding the pharynx behind its median bulb. The nerve ring around the pharynx anterior to these cells was also observed as emitting fluorescence (Fig. 1a) and the course of the sensory dendrites to their cell bodies could be determined (Fig. 1b).
Figure 1.
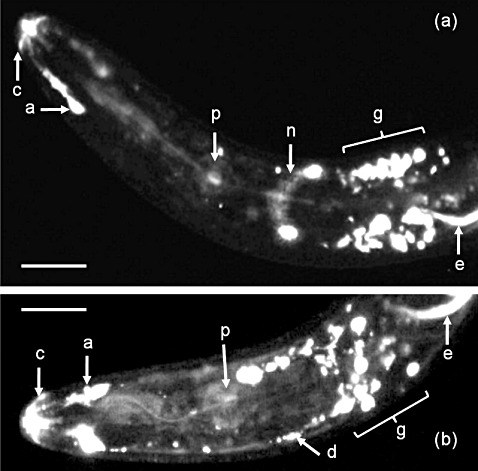
Neuronal uptake of Alexa Fluor 488‐labelled nAChRbp peptide by adult female Radopholus similis. The peptide was detected in the nematode by epifluorescent illumination. (a) Ventral view of the pharyngeal region of the nematode; (b) right lateral view of the same region. a, amphidial pouch; c, cephalic framework; d, tract of amphidial dendrites; e, excretory canal; g, region of the lateral and ventral neuronal ganglia; n, nerve ring; p, pharyngeal bulb. The excretory canal, which is open to the lateral surface, also takes up the labelled peptide and the cephalic framework shows autofluorescence. Scale bar, 10 µm.
Stability and allergenicity of the peptide
The peptide at 1 mg/mL was totally degraded after 1 h of exposure to nonsterile soil (Fig. 2a). It was digested within 30 min in simulated intestinal fluid containing 20 mg/mL pancreatin from porcine pancreas with amylase, trypsin, lipase, ribonuclease and protease enzyme activities (Fig. 2b), and was destroyed by boiling in water for 30 min as measured by alkaline phosphatase (ALP) inhibition activity. In silico analysis of the peptide sequence using the allergenicity prediction tools Allergenonline (http://www.allergenonline.com) and Allermatch (http://allermatch.org; Fiers et al., 2004) suggests that the peptide is not an allergen.
Figure 2.
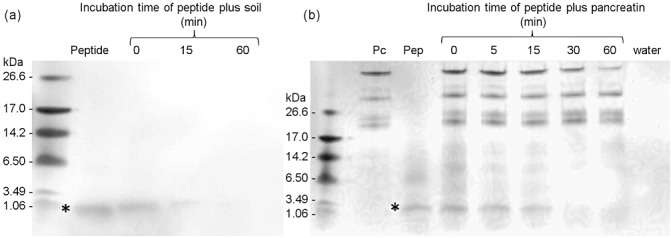
Degradation of the nAChRbp peptide. Tris‐tricine sodium dodecylsulphate‐polyacrylamide gel electrophoresis (SDS‐PAGE) showing degradation of the nAChRbp peptide by soil water (a) and the digestion of the peptide (Pep) when incubated in pancreatin (Pc) (b). The M r of the peptide is indicated (*).
Generation of transgenic plantains
Plantain cv. Gonja manjaya (Musa spp. AAB) was transformed with constructs designed to express a maize kernel cystatin (CCII), the nAChRbp peptide or both genes in combination (Fig. 3a). Agrobacterium‐infected cells multiplied and proliferated on kanamycin selective medium in contrast with control untransformed cells (Fig. 3b). The regenerated transgenic shoots were proliferated and transferred to rooting medium (Fig. 3c). All the shoots developed roots within 2–3 weeks. Transgenic shoots were also obtained from Agrobacterium‐infected meristematic explants. Assay by polymerase chain reaction (PCR) confirmed that a total of 245 independent transformed lines of plantain cv. Gonja manjaya were generated with cystatin, peptide or double constructs. The rooted plantlets were transferred to soil in pots in a containment facility. There were no apparent phenotypic alterations observed during the vegetative growth of plants (Fig. 3d,e).
Figure 3.
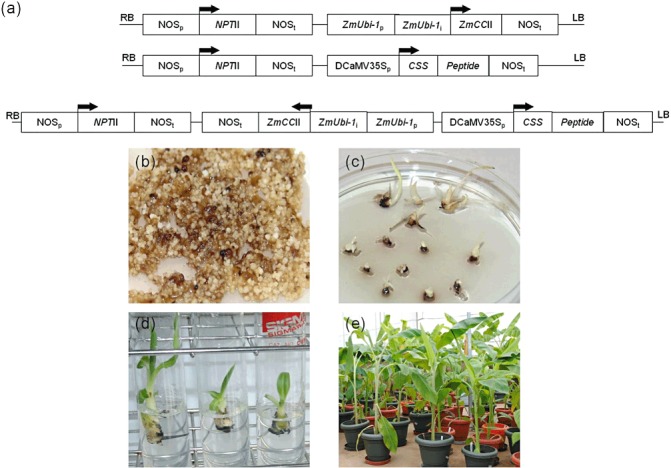
Generation of transgenic lines of plantain cultivar Gonja manjaya. (a) The T‐DNAs used for transformation. The maize ubiquitin promoter (ZmUbi‐1 p), intron (ZmUbi‐1 i) and the nopaline synthase terminator (NOSt) were used for the expression of the maize cystatin (CCII). The enhanced cauliflower mosaic virus (CaMV) 35S promoter and NOSt were used for the expression of the export signal (CSS) and synthetic peptide. The kanamycin resistance gene NEOMYCIN PHOSPHOTRANSFERASE II (NPTII) was used as a selectable marker and is shown between the left (LB) and right (RB) borders of the T‐DNA. Translation start sites and the direction of transcription are shown by arrows. (b) Proliferation of Agrobacterium‐infected cells on kanamycin selective medium. (c) Germinating transgenic seedlings from mature embryos on selective medium. (d) Transgenic plantlets on proliferation medium. (e) Transgenic plants in pots in the glasshouse.
Screening for transgene expression
Transformation generated 15 independent lines containing the cystatin construct (C series lines). All 15 lines were confirmed to express the maize cystatin by western blot analysis. One hundred and thirty independent lines were generated containing the peptide construct (P series lines). Of these lines, 48 of the 52 screened were confirmed to express the peptide by immuno‐dot‐blot and ALP inhibition assays. Of the 100 independent lines generated containing the dual construct (D series lines), 48 were confirmed to express both the cystatin and peptide by western blot, immuno‐dot‐blot and ALP inhibition assays. A subset of transgenic lines, based on the highest levels of transgene expression, was selected for challenge in containment with plant parasitic nematodes. A range of cystatin expression levels, at or above 0.01% total soluble protein, were observed (Fig. 4). Similar cystatin levels have been found previously to confer resistance to R. similis in banana (Atkinson et al., 2004a). No correlation was identified between the level of cystatin expression and the subsequent nematode resistance achieved. This was also true for peptide‐expressing lines.
Figure 4.
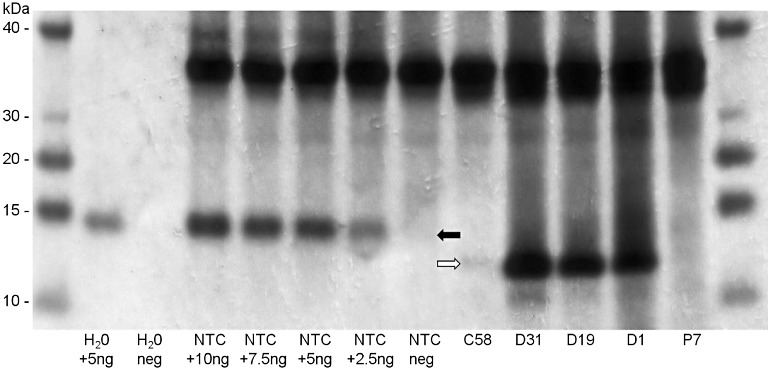
Detection of cystatin expression in transgenic plantain lines. Total soluble protein extracted from roots of transgenic plantain lines was analysed by western blotting. The M
r of the transgenic maize cystatin is indicated (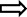 ). Nontransformed Gonja manjaya control (NTC) protein extract was spiked with the indicated quantity of 6 × His‐tagged maize cystatin for positive controls (
). Nontransformed Gonja manjaya control (NTC) protein extract was spiked with the indicated quantity of 6 × His‐tagged maize cystatin for positive controls ( ); the increase in the size of the band in the positive controls corresponds to the size of the 6 × His tag.
); the increase in the size of the band in the positive controls corresponds to the size of the 6 × His tag.
Challenge of transgenic plantain lines
The first trial evaluated one cystatin‐expressing line, two peptide‐expressing lines and five lines transformed with the dual cystatin and peptide construct. Plants were inoculated with banana root material that provided approximately 1000 nematodes with a population mix of 68% R. similis and 28% H. multicinctus as the two dominant plant parasitic species, as well as 4% Meloidogyne spp. Such a population mix was considered to be similar to the challenge that the plants would face in the field.
At the end of the trial, the number of nematodes per unit weight of roots was calculated for each line. The mean number of nematodes recovered from nontransgenic control plants was 736 ± 171 per 25 g root fresh weight at harvest, which corresponds to 1228 ± 584 nematodes per root system. Analysis by one‐way analysis of variance (ANOVA) established that all transgenic lines trialled, except D18, provided significant resistance to R. similis. The data are presented as the percentage resistance relative to the nematode densities on nontransgenic roots in Fig. 5. Five lines also provided significant levels of resistance of 84% ± 8% (C58; P < 0.01), 66% ± 14% (P11; P < 0.05), 64% ± 11% (P22; P < 0.05), 69% ± 6% (D31; P < 0.05) and 57% ± 10% (D63; P < 0.05) to total nematodes. Therefore, lines of each of the three constructs showed resistance to both R. similis and total nematodes. The numbers of H. multicinctus in roots were low, but, even so, those recovered from plants of lines P22 and D46 showed sufficiently low variance that significant resistance to this nematode was also detected.
Figure 5.
Resistance of transgenic plantain lines challenged with a mixed population of nematodes. Percentage resistance against total plant parasitic nematodes ( ), Radopholus similis (
), Radopholus similis ( ) and Helicotylenchus multicinctus (
) and Helicotylenchus multicinctus ( ) for lines in the first trial expressing the cystatin (C58), the nAChRbp peptide (P11 and P22) or both anti‐nematode genes (D63, D1, D46, D31 and D18). Values are mean nematode numbers ± standard error of the mean per unit weight of roots expressed relative to that for nontransformed Gonja manjaya controls (NTC). One‐way analysis of variance (ANOVA) with a priori contrasts identified those means that differed from the corresponding value for NTC plants (**P < 0.01; *P < 0.05).
) for lines in the first trial expressing the cystatin (C58), the nAChRbp peptide (P11 and P22) or both anti‐nematode genes (D63, D1, D46, D31 and D18). Values are mean nematode numbers ± standard error of the mean per unit weight of roots expressed relative to that for nontransformed Gonja manjaya controls (NTC). One‐way analysis of variance (ANOVA) with a priori contrasts identified those means that differed from the corresponding value for NTC plants (**P < 0.01; *P < 0.05).
A second screenhouse trial infected with R. similis only was carried out with a further 10 dual defence lines to identify additional lines of interest. Line C58, expressing the maize cystatin, was retrialled to confirm its efficacy and to determine if challenge by R. similis from sterile culture on carrot discs provided similar results to those achieved by adding infected roots at planting. The data were analysed by transgenic line with six to eight sibling plants, each using the same approaches as in the first trial. The total of 300 R. similis added to the soil increased to 1398 ± 422 per 25 g fresh roots at harvest on nontransgenic control plants after 12 weeks, which corresponds to 3052 ± 889 R. similis per root system. Line C58 provided a resistance of 78% ± 8%, which is similar to that of 85% ± 8% against R. similis in the first trial (Fig. 6). Three double construct lines provided significant resistance to R. similis in this trial of 67% ± 10% (D30, P < 0.01), 56% ± 14% (D66, P < 0.05) and 51% ± 11% (D12, P < 0.05). The highest resistance provided to R. similis was comparable with that of 70% ± 6% for line D31 in the first trial. The two screenhouse trials therefore identified a subset of four lines among those showing resistance that spanned the three constructs (C58, P11, D30 and D31) and displayed >67% nematode resistance. They have been selected for future contained field trials.
Figure 6.
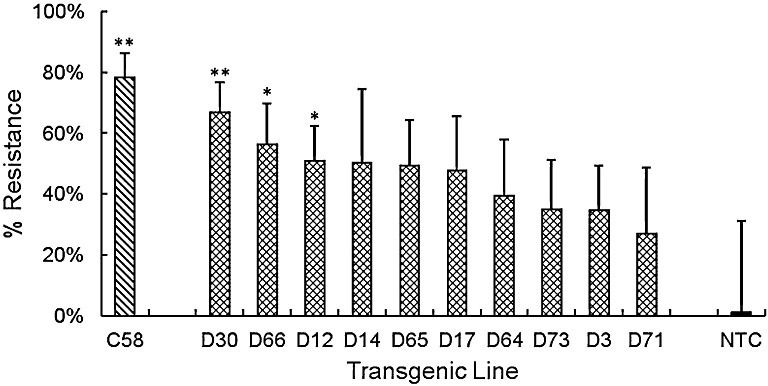
Resistance of transgenic plantain lines challenged with Radopholus similis. The percentage resistance is presented for lines in the second trial expressing the cystatin (C58) or both anti‐nematode genes. Values are mean nematode numbers ± standard error of the mean per unit weight of roots expressed relative to that for nontransformed Gonja manjaya controls (NTC). One‐way analysis of variance (ANOVA) with a priori contrasts identified those means that differed from the corresponding value for NTC plants (**P < 0.01; *P < 0.05).
DISCUSSION
Two hundred and forty‐five independent transgenic plantain lines were generated for the assessment of nematode resistance conferred by two distinct approaches. Of the 18 lines trialled, 11 showed resistance to nematodes associated with the expression of a maize cystatin, a secreted, synthetic peptide or both transgenes. The peptide's mode of action was shown to involve retrograde transport along certain sensory dendrites of R. similis, and prima facie evidence is provided that it represents no food safety risk. The plantain lines were developed using a highly efficient protocol for the establishment of embryogenic cell suspensions, and Agrobacterium‐mediated transformation of plantain cv. Gonja manjaya was developed. About 50–60 transgenic plants/0.5 mL settled cell volume were regenerated for plantain cv. Gonja manjaya in 5–6 months after agro‐infection of embryogenic cell suspensions. Similar transformation efficiency has been reported for other cultivars of banana and plantain (Ganapathi et al., 2001; Ghosh et al., 2009; Khanna et al., 2004).
Two challenges with nematodes were carried out against a subset of highly expressing lines to the capacity of the screenhouse. After the first trial of 12 weeks, the recovered population mix on nontransgenic control plantains was 91% ± 13.5% R. similis and 5.9% ± 1.3% H. multicinctus. These proportions are consistent with the rapid multiplication of R. similis on plantain (Fogain, 2000). Likewise, R. similis contributed the majority of the reduced number of nematodes from the transgenic plants, with H. multicinctus providing only 6.1%–15.2% of the recovered individuals. The similar proportions at the start and end of the trial suggested that suppression of nematode multiplication was common to the two nematodes. The reliability of the challenges was also shown by the similar value of resistance to R. similis for line C58 in the two trials, which was somewhat higher than that recorded previously (70% ± 10%) for Cavendish banana expressing the modified rice cystatin OcIΔD86 (Atkinson et al., 2004a). Four lines, C58, P11, D30 and D31, provided an estimated resistance to R. similis of >67%, and low variance of H. multicinctus on lines P22 and D46 allowed significant resistance levels of 74% ± 11% and 75% ± 9% to be obtained. None of the dual defence lines screened to date provided a cumulative level of resistance that improved on the level achieved by the single defence lines. They may prove to be of value in preventing the selection of virulence as nematodes must overcome two distinct defence mechanisms.
Fewer R. similis were recovered from line C58 in the second trial than were added to each pot, but the actual multiplication or failure to increase could not be established for any line. This uncertainty arose because uncounted nematodes are present in the soil and some of these within plants may have survived the duration of the experiment to contribute to the final counts. At the end of each trial, nontransgenic control plants provided the equivalent of 2944 ± 684 total nematodes/100 g roots in the first trial and 5592 ± 1688 R. similis/100 g roots in the second trial, which were similar to corresponding values in previous work (Atkinson et al., 2004a). The duration of the trial was limited to ensure that nematode multiplication on control plants was not checked by root damage, which would have resulted in an underestimate of resistance for the effective transgenic lines. The densities of nematodes required to damage bananas vary with cultivar in Uganda (Speijer and Ssango, 1999) and, presumably, with agronomy and the virulence of populations. The economic threshold determined from root necrosis has been suggested to be 16–149 R. similis/100 g root in Uganda (Pattison et al., 2002), whereas, for dessert bananas in Costa Rica, where populations can reach >30 000 R. similis/100 g roots (Moens et al., 2001), damage thresholds occur at about 2000 R. similis/100 g roots (Gowen and Quénéhervé, 1990). In the Cameroon, an approximately exponential increase occurred on plantains from <2000 R. similis/100 g to up to 140 000 R. similis/100 g in just 4–5 months. This phase of increase was followed by a rapid decline post‐harvest when the plant was cut back. The cycle was repeated as the following ratoon plant developed from the parental corm (Fogain, 2000). Regular nematicide treatment kept R. similis below 80 000 nematodes/100 g roots and prevented toppling of plantain as a result of root rots that otherwise caused the main yield loss at the field site (Fogain, 2000). This suggests that >67% reduction in nematode population increase over 12 weeks in the current work would be sufficient to prevent a damaging population buildup over 4–5 months.
The safety of a cystatin‐based transgenic defence has been well established for a rice cystatin (Atkinson et al., 2004b). The maize kernel cystatin selected for this work lacks novelty as a dietary protein. It is consumed as part of the staple diet throughout Africa and a similar protein is present in human saliva (Veerman et al., 1996). It is also unlikely to differ in its toxicology from structurally similar proteins (Abraham et al., 2006), such as the rice cystatin, which is also not an allergen in mammals (Meredith and Atkinson, 2000). Effort in this work centred, therefore, on developing a prima facie case for food safety of the nAChRbp peptide. The preliminary safety assessments for the synthetic peptide described here establish that it is destroyed by the high temperature required for cooking plantain and by simulated intestinal fluid. In addition, the peptide sequence is not recognized as a potential allergen by Allergenonline (http://www.allergenonline.com) or Allermatch (http://allermatch.org; Fiers et al., 2004), two tools that meet Food and Agriculture Organization/World Health Organization (FAO/WHO) Codex alimentarius guidelines for allergenicity assessment (http://bit.ly/CodexAlimentarius). The lack of allergenicity of the 1.16‐kDa peptide is also consistent with the observation that proteins of less than 3 kDa do not normally elicit an allergic response in mammals (Van Beresteijn et al., 1994).
Expression of OcIΔD86 cystatin by potato plants does not pose a substantial environmental risk to aerial invertebrate associates of a transgenic potato crop (Cowgill and Atkinson, 2003; 2002b, 2004), or perturb soil organism communities in the field (Cowgill et al., 2002a). Field work is required to confirm whether this is also the case for the maize cystatin when expressed in plantain. The peptide is rapidly degraded in the soil, presumably being utilized by soil microorganisms. It does not kill nontarget invertebrates at levels above those produced by transgenic plants (Wang, 2009) and its release from transgenic potato does not perturb nontarget soil nematode communities in the field (Green et al., 2012). Defining the uptake of the peptide along the sensory neurones, as described here for the migratory nematode R. similis and previously for infective juveniles of cyst nematodes (Wang et al., 2011), helps to support a common mode of action and to determine if nontarget species are likely to be affected by this nonlethal defence. Its biosafety can be enhanced further by the expression from the promoter of a root cap‐specific gene, which also raised the resistance level provided by an acetylcholinesterase‐inhibiting peptide above that achieved using the cauliflower mosaic virus (CaMV) 35S promoter (Lilley et al., 2011). A study of any environmental impact of nematode‐resistant plantains expressing the cystatin and/or the peptide is an important activity for the intended field trial.
This is the first study to develop transgenic nematode resistance in a widely grown tropical subsistence crop. Previous field trials using cystatin‐expressing potato plants (2001, 2003) have suggested that resistance levels recorded in containment provide a reliable indicator of resistance in field conditions, and work by Marin et al. (2000) indicates that glasshouse‐based assays of nematode resistance are indicative of field responses to R. similis by a range of banana cultivars. This work has identified highly promising plantain lines for a planned field trial to confirm this assumption with the future aim of enhancing food security for those Africans that depend on this crop.
EXPERIMENTAL PROCEDURES
Peptide degradation by soil
The nAChRbp peptide (CTTMHPRLC; synthesised by GL Biochem, Shanghai, China; Winter et al., 2002), at 1 mg/mL in 80 µL of aqueous solution, was added to 80‐mg aliquots of soil collected from the University of Leeds field station, Headley Hall Farm, Tadcaster, West Yorkshire, UK. The soil suspension was incubated at 20 °C and aliquots were removed at 0, 15 and 60 min, 18, 48 and 96 h for analysis by tris‐tricine sodium dodecylsulphate‐polyacrylamide gel electrophoresis (SDS‐PAGE).
Peptide stability in simulated intestinal fluid
The peptide, at 10 mg/mL, was added to simulated intestinal fluid, prepared as described by Fu et al. (2002), to achieve a pancreatin to test protein ratio of 18 : 1 (w/w). The samples were incubated at 37 °C and aliquots were removed at 0, 5, 15, 30 and 60 min for analysis by tris‐tricine SDS‐PAGE.
Neuronal uptake of fluorescent peptide
Mixed stages of R. similis were soaked in a 183 µm aqueous solution of the Alexa Fluor 488‐labelled peptide (Cambridge Research Biochemicals, Billingham, Cleveland, UK) at room temperature in the dark for 16 h. Fluorescence was observed as described by Wang et al. (2011).
Cloning of nematode resistance constructs
For the creation of the maize cystatin construct, the coding region of the maize kernel cystatin gene CCII (Abe et al., 1995) was amplified from cDNA using the forward primer 5′‐GCATATGCATCATGGCCGACAACACCGG‐3′ and reverse primer 5′‐ACCCCCGGGTTAGGCGCTAGCACCCTCGTCAAC‐3′. The cystatin sequence was cloned as an NsiI–SmaI fragment to replace the β‐glucuronidase (GUS) gene of plasmid pWRG2920 (Christou and Ford, 1995) that had been removed by digestion with PstI and SmaI. A 2901‐bp PvuII fragment of this construct containing, in 5′ to 3′ order, the maize ubiquitin promoter and first intron sequence (Toki et al., 1992), the CCII gene and a nopaline synthase polyadenylation site (NOSt; Bevan et al., 1983), was ligated into the SmaI site of the binary vector pBI101 (Jefferson et al., 1987).
For creation of the peptide construct, a 407‐bp fragment containing, in 5′ to 3′ order, the calreticulin extracellular transport sequence (Borisjuk et al., 1998), the peptide coding region (Winter et al., 2002) and a NOSt site, was amplified from the pBI:MDK‐Pep plasmid (Green et al., 2012; Lilley et al., 2011; Liu et al., 2005). The primers used for amplification were Peptide forward 5′‐AGGTCAACTAGTAAGGAGATATATAACAATGGCTACTCA‐3′ and NOSt reverse 5′‐GTACTAGGTACCCGGCCAGTGAATTCCCGATCTA‐3′. The PCR product was ligated into the pCR‐Blunt II‐TOPO plasmid (Invitrogen, Carlsbad, CA, USA). An XbaI–SacI fragment of this plasmid, containing the PCR product insert, was then ligated into the corresponding sites of pBI121 (Chen et al., 2003; Jefferson et al., 1987) to replace the GUS gene and create pBI:SigPep‐NOSt. A 786‐bp CaMV 35S‐derived promoter sequence with enhanced expression in monocots (D35S; Omirulleh et al., 1993) was amplified from the pCAMBIA1303 plasmid using the primers D35S forward 5′‐ACTGTAAAGCTTATGGTGGAGCACGACACTC‐3′ and D353 reverse 5′‐AGGTACTCTAGATCGAGAGAGATAGATTTGTAGAGAGA‐3′. Digestion of the PCR product produced an HindIII–XbaI fragment that was ligated into the corresponding sites of the pBI121 SigPep‐NOSt plasmid, replacing the standard CaMV 35S promoter fragment already present in the pBI121 plasmid (Chen et al., 2003; Jefferson et al., 1987), to create pBI:D35S‐SigPep‐NOSt.
The stacked gene construct was created by ligating the PvuII fragment containing CCII into the klenow‐filled HindIII restriction site of the pBI:D35S‐SigPep‐NOSt plasmid. All clones were verified by sequencing before transformation into Agrobacterium tumefaciens strain EHA105 (Hood et al., 1993) by the freeze–thaw method (Holsters et al., 1978).
Plant material, transformation and regeneration
Plantain cv. Gonja manjaya (Musa AAB) was transformed by one of two methods. Embryogenic cell suspensions generated from tiny multiple buds (Tripathi et al., 2011) were transformed as described by Tripathi et al. (2010). Fine cross‐sections (thickness, 0.4–0.6 mm) of intercalary meristematic tissues excised from the corm of in vitro shoots were transformed as described by Tripathi et al. (2008). The Agrobacterium‐infected embryogenic cell suspensions or meristematic explants were washed and regenerated on selective medium supplemented with timentin (200 mg/L) and kanamycin (100 mg/L). Transgenic shoots were maintained and multiplied on proliferation medium consisting of Murashige and Skoog (MS) medium (Murashige and Skoog, 1962) supplemented with 5 mg/L benzylaminopurine (BAP). Growth was at 26 ± 2 °C with 16 h of 94 µmol/m2/s light in every 24 h. Individual shoots were transferred to rooting medium [MS medium supplemented with 1 mg/L indole‐3‐butyric acid (IBA)]. Molecular analysis was used to select rooted plantlets for further micropropagation to achieve sufficient siblings for nematode challenge and maintenance of the line in vitro. Following multiplication, plants for nematode challenge were transferred to soil in a screenhouse containment facility.
PCR detection of transgenes
Genomic DNA for PCR detection of the transgene sequence was extracted using a DNeasy kit (Qiagen, Hilden, Germany) from tissue of in vitro plants ground with liquid nitrogen. The primers used for the detection of CCII were UbiEnd forward 5′‐TTGATGTGGGTTTTACTGATGC‐3′ and ZmCys reverse 5′‐TTAGGCGCTAGCACCCTCGTCAAC‐3′, which amplified a 572‐bp region from the 3′ end of the ubiquitin intron sequence to the 3′ end of the maize cystatin sequence. The primers for the detection of the peptide were D35SEnd forward 5′‐TGGATTGATGTGATATCTCCACT‐3′ and Peptide reverse 5′‐ACGATCCCCGCTGCACCC‐3′, which amplified a 279‐bp region from the 3′ end of the D35S promoter to the 3′ end of the peptide coding sequence. PCR amplification was carried out in a total volume of 20 µL containing 1 µL of 150 ng/µL genomic DNA, 0.5 µL of each 50 pmol/µL primer, 0.5 µL of 10 mm deoxynucleoside triphosphates (dNTPs), 1 × Taq polymerase reaction buffer and 1 U Taq polymerase (NEB, Ipswich, MA, USA). Thermocycling began with denaturation at 94 °C for 1 min, followed by 35 cycles of 94 °C for 30 s, 55 °C for 30 s and 72 °C for 1 min, and a final extension of 72 °C for 10 min.
Detection of CCII
Whole‐protein extract from roots of fresh in vitro plants was collected in 100 µL of CelLytic P lysis buffer (Sigma, Munich, Germany) containing a protease inhibitor cocktail (Roche, Basel, Switzerland) according to the manufacturer's instructions. The concentration of total soluble protein was measured using the DC Protein Assay (Biorad, Hercules, CA, USA). Sample preparation was in a total volume of 20 µL containing 2 µg total protein extract, 5 µL of 4 × NuPAGE LDS Sample Buffer and 2 µL of 10 × NuPAGE Sample Reducing Agent (Invitrogen). Samples were incubated at 96 °C for 10 min prior to separation on NuPAGE 10% Bis Tris gels according to Invitrogen instructions. The proteins were blotted onto Immobilon‐P membrane (Millipore, Billerica, MA, USA), using standard equipment and procedures (Novex XCell II, Invitrogen). Expressed CCII protein was detected following incubation with a 1:500 dilution of CCII‐specific primary rabbit polyclonal antibody (Yorkshire Biosciences, York, UK), followed by a 1:24 000 dilution of anti‐rabbit IgG ALP conjugate (Sigma). Secondary antibody detection was by 5‐bromo‐4‐chloro‐3′‐indolyphosphate/nitroblue tetrazolium (BCIP/NBT) substrate (Sigma).
Detection of the peptide
In vitro‐grown transgenic plantlets were transferred to tubes of sterile tap water and maintained for 14 days at 25 °C with 16 h of 94 µmol/m2/s light in every 24 h. Root exudate of each line was applied to an Immobilon‐P membrane (Millipore) held in a 96‐well dot‐blot module (Scie‐Plas, Cambridge, UK) in two 200‐µL aliquots. Peptide detection was with a 1 : 500 dilution of nAChRbp‐specific primary rabbit polyclonal antibody (Yorkshire Biosciences), and secondary antibody and detection were as for western blot detection of CCII. The active peptide content of the pooled exudates that were positive by dot‐blot was quantified by colorimetric assay of ALP inhibition, as described by Liu et al. (2005). ALP inhibition was carried out in a total volume of 31 µL containing 20 µL of root exudate, 10 µL of distilled water and 1 µL of 50 µg/mL ALP, incubated in the dark for 30 min. To this reaction, 400 µL of Sigma Fast p‐nitrophenyl phosphate reaction buffer (Sigma) was added, and each sample was split between four wells, each containing 100 µL, on a 96‐well microreader plate. The progression of the ALP reaction was measured for 1 h with 5‐min reading intervals at 405 nm by an ELx800 plate reader (Biotek Instruments, Winooski, VT, USA). The quantification of the expression level of the peptide for each line was calculated from a standard curve of control samples spiked with known quantities of synthesized peptide (Yorkshire Biosciences). Each line was measured twice in independent experiments.
Collection and maintenance of nematode inocula
Roots were collected from infected bananas in the field at Namulonge, Uganda, and nematode counts were made on subsamples of mixed roots to determine the level of inoculum required for the first trial. The population mix of plant parasitic nematodes in the first trial inoculum was 68% R. similis and 28% H. multicinctus. Monoxenic cultures of R. similis, maintained on sterile carrot discs according to the modified methods of Southey (1986), were used as inoculum for the second trial. The carrot disc cultures were maintained at 25 °C in the dark and subcultured every 8 weeks. Nematodes were recovered for subculture or inocula by washing carrot discs in sterile tap water. For subculture, 100 surface‐sterilized nematodes were used to establish each new carrot disc culture. The number and genus of nematodes present were checked by microscope prior to subculture or infection for both roots and carrot disc inocula.
Screenhouse trials of nematode resistance
Both nematode challenge trials were carried out in a screenhouse at the National Agricultural Research Laboratories at Kawanda, Uganda. Eighteen transgenic lines were challenged with nematodes in a primary bioassay with 8–10 plants per line. Established plants, grown for 10 weeks in an onsite glasshouse before transfer to the screenhouse, were inoculated with either chopped roots estimated to contain a mixed population of 1000 nematodes for the first trial or 300 R. similis watered onto GF/A filter paper placed onto exposed roots for the second trial. The trials continued for 12 weeks in the screenhouse at ambient environmental conditions, with a mean daily temperature minimum of 15.3 °C and maximum of 27.3 °C, and 76% relative humidity. Manual watering was carried out daily. At trial end, the fresh weights of green and root tissue were measured before the roots were cut into c. 5‐cm pieces and 25‐g aliquots were used for nematode collection. Root samples were placed in polypropylene bags and submerged in 100 mL of 1% H2O2 solution (Southey, 1986). After 7 days of incubation at room temperature in the dark, nematodes were collected before identification to genus, in the case of the first trial, and counting using a stereobinocular microscope.
ACKNOWLEDGEMENTS
The authors thank the Biotechnology and Biological Sciences Research Council (BBSRC) and Department for International Development (DFID) for financial support [Sustainable Agriculture Research for International Development (SARID) initiative], the National Agriculture Research Laboratories, Uganda for providing laboratory and glasshouse facilities, Jayne Green for technical assistance, James Kawuma, Douglas Mwesigwa and Elvis Mbiru for technical support, Danny Coyne for supplying nematodes, and Catherine Lilley for critical reading of the manuscript.
REFERENCES
- Abe, M. , Abe, K. , Domoto, C. and Arai, S. (1995) Two distinct species of corn cystatin in corn kernels. Biosci. Biotechnol. Biochem. 59, 756–758. [DOI] [PubMed] [Google Scholar]
- Abraham, Z. , Martinez, M. , Carbonero, P. and Diaz, I. (2006) Structural and functional diversity within the cystatin gene family of Hordeum vulgare . J. Exp. Bot. 57, 4245–4255. [DOI] [PubMed] [Google Scholar]
- Atkinson, H.J. (2003) Strategies for resistance to nematodes in Musa spp In: Genetic Transformation Strategies to Address the Major Constraints to Banana and Plantain Production in Africa (Atkinson H.J., Dale J., Harding R., Kiggundu A., Kunert K., Muchwezi J.M., Sagi L. and Viljoen A., eds), pp. 74–107. Montpellier: INIBAP. [Google Scholar]
- Atkinson, H.J. , Urwin, P.E. and McPherson, M.J. (2003) Engineering plants for nematode resistance. Annu. Rev. Phytopathol. 41, 615–639. [DOI] [PubMed] [Google Scholar]
- Atkinson, H.J. , Grimwood, S. , Johnston, K. and Green, J. (2004a) Prototype demonstration of transgenic resistance to the nematode Radopholus similis conferred on banana by a cystatin. Transgenic Res. 13, 135–142. [DOI] [PubMed] [Google Scholar]
- Atkinson, H.J. , Johnston, K.A. and Robbins, M. (2004b) Prima facie evidence that a phytocystatin for transgenic plant resistance to nematodes is not a toxic risk in the human diet. J. Nutr. 134, 431–434. [DOI] [PubMed] [Google Scholar]
- Bevan, M. , Barnes, W.M. and Chilton, M.‐D. (1983) Structure and transcription of the nopaline synthase region of T‐DNA. Nucleic Acids Res. 11, 369–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borisjuk, N. , Sitailo, L. , Adler, K. , Malysheva, L. , Tewes, A. , Borisjuk, L. and Manteuffel, R. (1998) Calreticulin expression in plant cells: developmental regulation, tissue specificity and intracellular distribution. Planta, 206, 504–514. [DOI] [PubMed] [Google Scholar]
- Brentu, C.F. , Speijer, P.R. , Green, K. , Hemeng, B. , De Waele, D. and Coyne, D.L. (2004) Micro‐plot evaluation of the yield reduction potential of Pratylenchus coffeae, Helicotylenchus multicinctus and Meloidogyne javanica on plantain cv. Apantu‐pa (Musa spp., AAB‐group) in Ghana. Nematology, 6, 455–462. [Google Scholar]
- Bridge, J. , Price, N.S. and Kofi, P. (1995) Plant parasitic nematodes of plantain and other crops in Cameroon, West Africa. Fundam. Appl. Nematol. 18, 251–260. [Google Scholar]
- Chan, Y.L. , Yang, A.H. , Chen, J.T. , Yeh, K.W. and Chan, M.T. (2010) Heterologous expression of taro cystatin protects transgenic tomato against Meloidogyne incognita infection by means of interfering sex determination and suppressing gall formation. Plant Cell Rep. 29, 231–238. [DOI] [PubMed] [Google Scholar]
- Chen, P.‐Y. , Wang, C.‐K. , Soong, S.‐C. and To, K.‐Y. (2003) Complete sequence of the binary vector pBI121 and its application in cloning T‐DNA insertion from transgenic plants. Mol. Breed. 11, 287–293. [Google Scholar]
- Christou, P. and Ford, T.L. (1995) The impact of selection parameters on the phenotype and genotype of transgenic rice callus and plants. Transgenic Res. 4, 44–51. [Google Scholar]
- Cowgill, S.E. and Atkinson, H.J. (2003) A sequential approach to risk assessment of transgenic plants expressing protease inhibitors: effects on non‐target herbivorous insects. Transgenic Res. 12, 439–449. [DOI] [PubMed] [Google Scholar]
- Cowgill, S.E. , Bardgett, R.D. , Kiezebrink, D.T. and Atkinson, H.J. (2002a) The effect of transgenic nematode resistance on non‐target organisms in the potato rhizosphere. J. Appl. Ecol. 39, 915–923. [Google Scholar]
- Cowgill, S.E. , Wright, C. and Atkinson, H.J. (2002b) Transgenic potatoes with enhanced levels of nematode resistance do not have altered susceptibility to nontarget aphids. Mol. Ecol. 11, 821–827. [DOI] [PubMed] [Google Scholar]
- Cowgill, S.E. , Danks, C. and Atkinson, H.J. (2004) Multitrophic interactions involving genetically modified potatoes, nontarget aphids, natural enemies and hyperparasitoids. Mol. Ecol. 13, 639–647. [DOI] [PubMed] [Google Scholar]
- Duncan, L. and Moens, M. (2006) Migratory endoparasitic nematodes In: Plant Nematology (Perry R.N. and Moens M., eds), pp. 123–152. Wallingford, Oxfordshire: CABI Publishing. [Google Scholar]
- Escalant, J.V. and Jain, S.M. (2004) Banana improvement: future perspectives In: Banana Improvement: Cellular, Molecular Biology, and Induced Mutations (Jain S.M. and Swennen R., eds), pp. 359–367. Enfield, NH: Science Publishers Inc. [Google Scholar]
- Fiers, M.W. , Kleter, G.A. , Nijland, H. , Peijnenburg, A.A. , Nap, J.P. and van Ham, R.C. (2004) Allermatch, a web tool for the prediction of potential allergenicity according to current FAO/WHO Codex alimentarius guidelines. BMC Bioinformatics, 5, 133–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogain, R. (2000) Effect of Radopholus similis on plant growth and yield of plantains (Musa, AAB). Nematology, 2, 129–133. [Google Scholar]
- Fu, T.‐J. , Abbott, U.R. and Hatzos, C. (2002) Digestibility of food allergens and nonallergenic proteins in simulated gastric fluid and simulated intestinal fluid: a comparative study. J. Agric. Food Chem. 50, 7154–7160. [DOI] [PubMed] [Google Scholar]
- Ganapathi, T.R. , Higgs, N.S. , Balint‐Kurti, P.J. , Arntzen, C.J. , May, G.D. and Van Eck, J.M. (2001) Agrobacterium‐mediated transformation of embryogenic cell suspensions of the banana cultivar Rasthali (AAB). Plant Cell Rep. 20, 157–162. [DOI] [PubMed] [Google Scholar]
- Gao, S. , Yu, B. , Zhai, H. , He, S.‐Z. and Liu, Q.‐C. (2011) Enhanced stem nematode resistance of transgenic sweetpotato plants expressing oryzacystatin‐I gene. Agric. Sci. China, 10, 519–525. [Google Scholar]
- Ghosh, A. , Ganapathi, T. , Nath, P. and Bapat, V. (2009) Establishment of embryogenic cell suspension cultures and Agrobacterium‐mediated transformation in an important Cavendish banana cv. Robusta (AAA). Plant Cell Tissue Organ Cult. 97, 131–139. [Google Scholar]
- Gowen, S. and Quénéhervé, P. (1990) Nematode parasites of bananas, plantains and abaca In: Plant Parasitic Nematodes in Subtropical and Tropical Agriculture (Luc M., Sikora R.A. and Bridge J., eds), pp. 431–460. Wallingford, Oxfordshire: CABI Publishing. [Google Scholar]
- Green, J. , Wang, D. , Lilley, C.J. , Urwin, P.E. and Atkinson, H.J. (2012) Transgenic potatoes for potato cyst nematode control can replace pesticide use without impact on soil quality. PLoS ONE, 7, e30973 DOI:10.1371/journal.pone.0030973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haegeman, A. , Elsen, A. , De Waele, D. and Gheysen, G. (2010) Emerging molecular knowledge on Radopholus similis, an important nematode pest of banana. Mol. Plant Pathol. 11, 315–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holsters, M. , De Waele, D. , Depicker, A. , Messens, E. , Van Montagu, M. and Schell, J. (1978) Transfection and transformation of Agrobacterium tumefaciens . Mol. Gen. Genet. 163, 181–187. [DOI] [PubMed] [Google Scholar]
- Hood, E.E. , Gelvin, S.B. , Melchers, L.S. and Hoekema, A. (1993) New Agrobacterium helper plasmids for gene transfer to plants. Transgenic Res. 2, 208–218. [Google Scholar]
- Jefferson, R.A. , Kavanagh, T.A. and Bevan, M.W. (1987) GUS fusions: β‐glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J. 16, 3901–3907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanna, H. , Becker, D. , Kleidon, J. and Dale, J. (2004) Centrifugation assisted Agrobacterium tumefaciens‐mediated transformation (CAAT) of embryogenic cell suspensions of banana (Musa spp. Cavendish AAA and Lady finger AAB). Mol. Breed. 14, 239–252. [Google Scholar]
- Lilley, C.J. , Wang, D. , Atkinson, H.J. and Urwin, P.E. (2011) Effective delivery of a nematode‐repellent peptide using a root‐cap‐specific promoter. Plant Biotechnol. J. 9, 151–161. [DOI] [PubMed] [Google Scholar]
- Liu, B. , Hibbard, J.K. , Urwin, P.E. and Atkinson, H.J. (2005) The production of synthetic chemodisruptive peptides in planta disrupts the establishment of cyst nematodes. Plant Biotechnol. J. 3, 487–496. [DOI] [PubMed] [Google Scholar]
- Lorenzen, J. , Tenkouano, A. , Bandyopadhyay, R. , Vroh, B. , Coyne, D. and Tripathi, L. (2010) Overview of banana and plantain (Musa spp.) improvement in Africa: past and future. Acta Hortic. 879, 595–603. [Google Scholar]
- Marin, D.H. , Barker, K.R. , Kaplan, D.T. , Sutton, T.B. and Opperman, C.H. (2000) Development and evaluation of a standard method for screening for resistance to Radopholus similis in bananas. Plant Dis. 84, 689–693. [DOI] [PubMed] [Google Scholar]
- May, G.D. , Afza, R. , Mason, H.S. , Wiecko, A. , Novak, F.J. and Arntzen, C.J. (1995) Generation of transgenic banana (Musa acuminata) plants via Agrobacterium‐mediated transformation. Nat. Biotechnol. 13, 486–492. [Google Scholar]
- Mayer, J.E. , Pfeiffer, W.H. and Beyer, P. (2008) Biofortified crops to alleviate micronutrient malnutrition. Curr. Opin. Plant Biol. 11, 166–170. [DOI] [PubMed] [Google Scholar]
- McSorley, R. and Parrado, J.L. (1983) The spiral nematode, Helicotylenchus multicinctus, on bananas in Florida and its control. Proc. Fla. State Hort. Soc. 96, 201–207. [Google Scholar]
- Meredith, C. and Atkinson, H.J. (2000) Development of methods to predict the allergenic potential of genetically modified foods and new protein products. Toxicology, 148, 41–42. [Google Scholar]
- Moens, T. , Araya, M. and De Waele, D. (2001) Correlation between nematode numbers and damage to banana (Musa AAA) roots under commercial conditions. Nematropica, 31, 55–65. [Google Scholar]
- Murashige, T. and Skoog, F. (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plantarum, 15, 473–497. [Google Scholar]
- Omirulleh, S. , Ábrahám, M. , Golovkin, M. , Stefanov, I. , Karabev, M.K. , Mustárdy, L. , Mórocz, S. and Dudits, D. (1993) Activity of a chimeric promoter with the doubled CaMV 35S enhancer element in protoplast‐derived cells and transgenic plants in maize. Plant Mol. Biol. 21, 415–428. [DOI] [PubMed] [Google Scholar]
- Pattison, A.B. , Stanton, J.M. , Cobon, J.A. and Doogan, V.J. (2002) Population dynamics and economic threshold of the nematodes Radopholus similis and Pratylenchus goodeyi on banana in Australia. Int. J. Pest Manage. 48, 107–111. [Google Scholar]
- Paul, J.‐Y. , Becker, D.K. , Dickman, M.B. , Harding, R.M. , Khanna, H.K. and Dale, J.L. (2011) Apoptosis‐related genes confer resistance to Fusarium wilt in transgenic ‘Lady Finger’ bananas. Plant Biotechnol. J. 9, 1141–1148. [DOI] [PubMed] [Google Scholar]
- Pinochet, J. (1988) Comments on the difficulty in breeding bananas and plantains for resistance to nematodes. Rev. Nématol. 11, 3–5. [Google Scholar]
- Price, N.S. (2006) The banana burrowing nematode, Radopholus similis (Cobb) Thorne, in the Lake Victoria region of East Africa: its introduction, spread and impact. Nematology, 8, 801–817. [Google Scholar]
- Southey, J. (1986) Laboratory Methods for Work with Plant and Soil Nematodes. Reference Book 402. London: Her Majesty's Stationery Office. [Google Scholar]
- Speijer, P.R. and Ssango, F. (1999) Evaluation of musa host plant response using nematode densities and damage indices. Nematropica, 29, 185–192. [Google Scholar]
- Swennen, R. and Vuylsteke, D. (2001) Banana (Musa L.) In: Crop Production in Tropical Africa (Raemaekers R.H., ed.), pp. 530–552. Brussels: Directorate General for International Co‐operation. [Google Scholar]
- Toki, S. , Takamatsu, S. , Nojiri, C. , Ooba, S. , Anzai, H. , Iwata, M. , Christensen, A.H. , Quail, P.H. and Uchimiya, H. (1992) Expression of a maize Ubiquitin gene promoter‐bar chimeric gene in transgenic rice plants. Plant Physiol. 100, 1503–1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathi, J.N. , Muwonge, A. and Tripathi, L. (2011) Highly efficient regeneration and transformation protocol for plantain cv. ‘Gonja Manjaya’ (Musa spp. AAB) using embryogenic cell suspension. In Vitro Cell. Dev. Biol., Plant. DOI:10.1007/s11627‐011‐9422‐z Online from 11 Jan 2012. [Google Scholar]
- Tripathi, L. , Tripathi, J.N. and Hughes, J.d.A. (2005) Agrobacterium‐mediated transformation of plantain (Musa spp.) cultivar Agbagba. Afr. J. Biotechnol. 4, 1378–1383. [Google Scholar]
- Tripathi, L. , Tripathi, J.N. and Tushemereirwe, W.K. (2008) Rapid and efficient production of transgenic East African Highland Banana (Musa spp.) using intercalary meristematic tissues. Afr. J. Biotechnol. 7, 1438–1445. [Google Scholar]
- Tripathi, L. , Mwaka, H. , Tripathi, J.N. and Tushemereirwe, W.K. (2010) Expression of sweet pepper Hrap gene in banana enhances resistance to Xanthomonas campestris pv. musacearum . Mol. Plant Pathol. 11, 721–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urwin, P.E. , Lilley, C.J. , McPherson, M.J. and Atkinson, H.J. (1997) Resistance to both cyst and root‐knot nematodes conferred by transgenic Arabidopsis expressing a modified plant cystatin. Plant J. 12, 455–461. [DOI] [PubMed] [Google Scholar]
- Urwin, P.E. , Levesley, A. , McPherson, M.J. and Atkinson, H.J. (2000) Transgenic resistance to the nematode Rotylenchulus reniformis conferred by Arabidopsis thaliana plants expressing proteinase inhibitors. Mol. Breed. 6, 257–264. [Google Scholar]
- Urwin, P.E. , Troth, K.M. , Zubko, E.I. and Atkinson, H.J. (2001) Effective transgenic resistance to Globodera pallida in potato field trials. Mol. Breed. 8, 95–101. [Google Scholar]
- Urwin, P.E. , Green, J. and Atkinson, H.J. (2003) Expression of a plant cystatin confers partial resistance to Globodera, full resistance is achieved by pyramiding a cystatin with natural resistance. Mol. Breed. 12, 263–269. [Google Scholar]
- Vain, P. , Worland, B. , Clarke, M.C. , Richard, G. , Beavis, M. , Liu, H. , Kohli, A. , Leech, M. , Snape, J. , Christou, P. and Atkinson, H.J. (1998) Expression of an engineered cysteine proteinase inhibitor (Oryzacystatin‐IΔD86) for nematode resistance in transgenic rice plants. Theor. Appl. Genet. 96, 266–271. [Google Scholar]
- Van Beresteijn, E.C.H. , Peeters, R.A. , Kaper, J. , Meijer, R.J.G.M. , Robben, A.J.P.M. and Schmidt, D.G. (1994) Molecular mass distribution, immunological properties and nutritive value of whey protein hydrolysates. J. Food Prot. 57, 619–625. [DOI] [PubMed] [Google Scholar]
- Veerman, E.C. , van den Keybus, P.A. , Vissink, A. and Nieuw Amerongen, A.V. (1996) Human glandular salivas: their separate collection and analysis. Eur. J. Oral Sci. 104, 346–352. [DOI] [PubMed] [Google Scholar]
- Wang, D. (2009) Reducing the environmental risks and hazards of crop production by biosafe use of transgenic crops. PhD Thesis, Faculty of Biological Sciences, University of Leeds, Leeds.
- Wang, D. , Jones, L.M. , Urwin, P.E. and Atkinson, H.J. (2011) A synthetic peptide shows retro‐ and anterograde neuronal transport before disrupting the chemosensation of plant‐pathogenic nematodes. PLoS ONE, 6, e17475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter, M.D. , McPherson, M.J. and Atkinson, H.J. (2002) Neuronal uptake of pesticides disrupts chemosensory cells of nematodes. Parasitology, 125, 561–565. [PubMed] [Google Scholar]


