SUMMARY
The robust and reliable identification of fungi underpins virtually every element of plant pathology, from disease diagnosis to studies of biology, management/control, quarantine and, even more recently, comparative genomics. Most plant diseases are caused by fungi, typically pleomorphic organisms, for which the taxonomy and, in particular, a dual nomenclature system have frustrated and confused practitioners of plant pathology. The emergence of DNA sequencing has revealed cryptic taxa and revolutionized our understanding of relationships in the fungi. The impacts on plant pathology at every level are already immense and will continue to grow rapidly as new DNA sequencing technologies continue to emerge. DNA sequence comparisons, used to resolve a dual nomenclature problem for the first time only 19 years ago, have made it possible to approach a natural classification for the fungi and to abandon the confusing dual nomenclature system. The journey to a one fungus, one name taxonomic reality has been long and arduous, but its time has come. This will inevitably have a positive impact on plant pathology, plant pathologists and future students of this hugely important discipline on which the world depends for food security and plant health in general. This contemporary review highlights the problems of a dual nomenclature, especially its impact on plant pathogenic fungi, and charts the road to a one fungus, one name system that is rapidly drawing near.
INTRODUCTION
The responsible and accurate diagnosis of plant diseases is fundamental to the implementation of effective management strategies. This process includes the isolation of a putative pathogen, accurate identification and, subsequently, proof of pathogenicity via the testing of Koch's postulates. Although these steps are essentially relatively simple, they are also fraught with complications. The effective isolation of putative pathogens is often difficult and sometimes requires long periods of trial and error before success is achieved. The testing of Koch's postulates, although on the surface appears to be simple, can be extremely difficult. Sometimes it is not possible, at least in a manner that appropriately reflects natural ecological situations. Although the above two steps in disease diagnosis harbour problems, it is the identification of putative pathogens that can be most complicated. For the fungi, this is a particularly pertinent issue and one that is currently the subject of substantial debate.
It is an interesting fact that most plant diseases are caused by ascomycete fungi. The taxonomy of these organisms has been problematic ever since they were first recognized. One of the most difficult elements of fungal taxonomy with which plant pathologists have had to contend is pleomorphism in fungal pathogens. This pleomorphism arises from the fact that many ascomycete fungi occur in either their sexual (teleomorph) or asexual (anamorph) states alone, or in combination. To complicate this situation further, some fungi have more than one asexual morph (synanamorph) and these are often linked to unique ecological niches. This unusual situation has complicated the taxonomy of ascomycetes since the mid‐19th century; it has also confused plant pathologists and confounded plant disease diagnosis.
DUAL NOMENCLATURE
Pleomorphism is encountered in many of the most important ascomycete plant pathogens. The most obvious manifestation is found when fungi have both sexual (teleomorph) and asexual (anamorph) states. Common examples (Fig. 1) include species of Calonectria with Cylindrocladium asexual states, Gibberella with Fusarium asexual states, Ceratocystis with Thielaviopsis asexual states, Grosmannia with Leptographium asexual states, and Botryosphaeriaceae, Mycosphaerellaceae and Teratosphaeriaceae with large numbers of very distinct asexual states, to name just a few. For most of these fungi, the asexual states are the most commonly found and typically represent the morphs that produce repeating cycles of infective spores that give rise to plant disease epidemics. Sexual states for many pleomorphic ascomycete plant pathogens are very seldom encountered and represent the more durable overwintering forms that often initiate new infections in new rotations (e.g. apple scab caused by Venturia inaequalis, anamorph Fusicladium pomi, synanamorph Spilocaea pomi) (Schubert et al., 2003).
Figure 1.

Symptoms of diseases caused by fungi belonging to the groups illustrated in the phylogenetic trees (Fig. 2) and where a single genus name (alternatives in parentheses) will simplify many aspects of dealing with them. (A) Diaporthe rhusicola (Phomopsis) leaf spot on Rhus pendulina. (B) Leaf disease caused by Teratosphaeria (Kirramyces) cryptica on Eucalyptus sp. (C) Ramularia lamii (Mycosphaerella) leaf spot on Leonotus leonurus. (D) Black pod rot on peanut caused by Calonectria illicicola (Cylindrocladium). (E) Streaked discoloration of Platanus wood caused by Ceratocystis platani (Thielaviopsis). (F) Neofusicoccum protearum (Botryosphaeria) canker on Protea sp.
The existence of very different morphological forms of many commonly encountered ascomycete plant pathogens has presented a long‐standing and complicated challenge for fungal taxonomists. It has commonly confused and often irritated plant pathologists. A solution to this problem was presented by promoting a dual system of fungal nomenclature, promulgated by Saccardo (1904), who recommended both sexual (Fungi Perfecti) and asexual (Fungi Imperfecti) names for fungi. (Correction added after online publication 6 Dec 2011: In the preceding sentence, the terms ‘sexual’ and ‘asexual’ were corrected.) This approach was considered by the International Botanical Congress (IBC) in Vienna, Austria (Briquet, 1905), and captured in Article 49 (precursor of the current Article 59) of the International Code of Botanical Nomenclature (ICBN) (Briquet, 1912; Hennebert, 2003; Taylor, 2011; Weresub and Pirozynski, 1979), allowing for different names to be applied to different morphs of the same fungus. These morphs are often discovered separately, commonly several years apart, and this also means that the same fungus may have not only different generic names, but also different species' names. Thus, Cylindrocladium scoparium is the anamorph of Calonectria morganii (not Calonectria scoparia, which is a different species; Crous et al., 1993a; 2000a, 2000b), Botryosphaeria rhodina is the teleomorph of Lasiodiplodia theobromae (Alves et al., 2008), and Thielaviopsis ungeri is the anamorph of Ceratocystis coerulescens (Paulin‐Mahady et al., 2002). To add stability to this dual nomenclature, where a sexual state is clearly shown to be connected to an asexual morph, the ICBN has dictated that priority should accrue to the sexual morph (McNeill et al., 2006). Unfortunately, since the onset of DNA sequencing in fungi in the early 1990s, many published anamorph–teleomorph connections have been refuted. This has led to either the anamorph or teleomorph gaining yet another name, or the anamorph/teleomorph genus being split into several cryptic genera (Crous and Groenewald, 2005).
Long‐standing and active debate has underpinned problems relating to pleomorphism in fungi. Perhaps the most active period of discussion was in the late 1970s, when the famous Kananaskis conferences were held. The first of these considered the taxonomy of the conidial fungi (Kendrick, 1971). The focus here was on the discovery of many previously unrecognized morphological characteristics that could be used to identify conidial fungi, the asexual forms of mostly ascomycetes. The recognition of these characters, and patterns sometimes linking them to sexual morphs (Hughes, 1953), arose largely from the fact that electron microscopy became readily available to mycologists (for example, Cole and Samson, 1979). However, the many limitations of focusing particularly on asexual forms of fungi to promote more accurate taxonomy were clear and this gave rise to the second Kananaskis conference, in which a focus on the ‘Whole Fungus’ (Kendrick, 1979) was promoted. In an attempt to accommodate this bizarrely artificial system, a new terminology was introduced, which is still used amongst mycologists and plant pathologists today. Here, sexual states are referred to as teleomorphs, asexual states as anamorphs and the whole fungus (encompassing all known morphs) as the holomorph (Weresub and Pirozynski, 1979).
Subsequent to the second Kananaskis conference, plant pathologists have attempted to accommodate the dual nomenclature system for ascomycete plant pathogens. This has led to many name changes for economically important plant pathogens, consistent with the warning by Hawksworth and Sutton (1974) that this would occur. For instance, Cylindrocladium parasiticum, the causal agent of Cylindrocladium pod rot of peanut, was linked to Calonectria ilicicola (Crous et al., 1993b), whereas Cylindrocladium ilicicola was shown to be the anamorph of Calonectria lauri (Lechat et al., 2010). The accommodation of these changes has not always been easy. Many practitioners of plant pathology have been confused and frustrated by having to deal with two names for a single plant pathogen. This confusion has also frustrated important quarantine regulations linked to import and export requirements, where some countries list the anamorph name for an organism, whereas others list the teleomorph. Although genetically identical, these are frequently perceived as different taxa by quarantine officers who are not well versed with the constantly changing anamorph–teleomorph taxonomy. Even in the Basidiomycota, dual nomenclature has taken its toll. A striking example is the recent identification of an invasive new rust on Myrtaceae as myrtle rust (Uredo rangelii) in Australia (Carnegie et al., 2010). This raised confusion as to whether or not the much feared Eucalyptus rust (Puccinia psidii), a serious quarantine organism, and listed on quarantine lists in countries in which eucalypts are cultivated (Coutinho et al., 1998; Glen et al., 2007), had been introduced into Australia. Genetically, these names represent the same fungus or, at least, very closely related fungi causing the same disease, which suggests that they should be treated in a similar fashion related to quarantine. However, they have not been treated equally and this has caused very substantial complications relating to the treatment of the new P. psidii sensu lato invasion in Australia (Carnegie and Cooper, 2011).
For a period of time, it became relatively commonplace to provide names for fungi having more than one asexual state, so‐called synanamorphs. Because this is a relatively common occurrence in ascomycetes, the practice had the potential to result in a huge proliferation of fungal names. It clearly would have added further confusion to practitioners of mycology, including plant pathologists. The naming of synanamorphs was consequently discouraged (Gams, 1995), although it was relevant not to lose valuable information regarding asexual morphs that often had important ecological value (Malloch and Cain, 1972). For example, a single fungus might have an asexual morph with wet sticky spores adapted to insect dispersal and dry wind‐borne conidia (e.g. the Pesotum and Sporothrix asexual states of some Ophiostoma spp., respectively). With a dual nomenclature system, the decision as to which of these states would justify having a name was generally arbitrary and often the source of conflict.
In order to further reduce confusion arising from a dual name for pleomorphic ascomycete plant pathogens, mycologists generally agreed in 2005 not to assign asexual state names to fungi that were known in their sexual form (McNeill et al., 2006). Yet, this remains only a recommendation and, although it has been taken up as part of the editorial policy by leading mycological journals (Hawksworth, 2007a), it is still not strictly adhered to (see Põldmaa, 2011). Following the whole fungus approach and the rules of the ICBN (McNeill et al., 2006), priority was always given to the sexual state, perceived to be the more important morph. This is despite the fact that this morph is often less commonly observed in the case of many plant pathogens, and, as a consequence, mycologists and plant pathologists have used the names associated with the forms that they have observed in the field or the laboratory.
THE IMPACT OF MOLECULAR BIOLOGY
Despite the complications and confusion presented by the dual nomenclature for fungi to practitioners and, in particular, plant disease diagnosticians, this has been the only system approved. To be fair, taxonomic mycologists have debated this problem actively since the mid‐1970s. Such debates (often heated) and discussions have been hosted at all International Mycological Congresses (IMCs), including and subsequent to the second IMC in Tampa, FL, USA in 1977.
In the early 1990s, molecular methods and, in particular, DNA sequences, providing opportunities for phylogenetic inference, began to have a significant impact on the taxonomy of fungi. For example, Berbee and Taylor (1992) provided the first example of the linking of an asexual fungus, in this case the important human pathogen Sporothrix schenkii, to a teleomorph genus (Ophiostoma) on the basis of DNA sequences and phylogenetic inference. This and many subsequent studies, including increasingly robust phylogenetic information, began to substantially question the need to perpetuate a dual nomenclature for fungi (Reynolds and Taylor, 1992; and others).
DNA sequence data for the fungi have accumulated exponentially subsequent to the initial study of Berbee and Taylor (1992). Amongst the most important discoveries has been that many fungal names, including important plant pathogens, previously treated as one organism, in many cases represent large numbers of separate taxa (Bensch et al., 2010; Crous and Groenewald, 2005). In this regard, the implications for plant pathology are immense. Many species previously thought to cause diseases have been shown to be different from those that are actually involved. Just as an example, diseases of woody plants caused by Botryosphaeria spp. were, for many years, attributed to a small number of taxa, notably B. dothidea and B. ribis (Slippers et al., 2004). Yet, various phylogenetic studies have shown that the fungi associated with these diseases represent numerous different taxa. In most cases, phylogenetic inference has made it possible to recognize differences, but the taxa have also been shown to be different on the basis of morphological, ecological and other characteristics. Some other important examples of plant pathogens that have been affected in this way include species of Fusarium (2004, 2009), Calonectria (2010a, 2010b, 2010c), Ceratocystis (van Wyk et al., 2009), Ophiostoma, Grosmannia (Zipfel et al., 2006) and numerous taxa in the Teratosphaeriaceae and Mycosphaerellaceae (2007, 2009a, 2009b, 2009c).
ONE FUNGUS, ONE NAME
DNA sequence comparisons have made it possible to reliably connect asexual states of fungi to their sexual states. Some examples for important plant pathogenic genera are illustrated in Fig. 2. Perhaps more importantly, it has become possible to connect asexual states of fungi to teleomorph‐typified generic names, without ever having seen the sexual morphs (see, for example, species in the Botryosphaeriaceae and Teratosphaeriaceae, Fig. 2).
Figure 2.
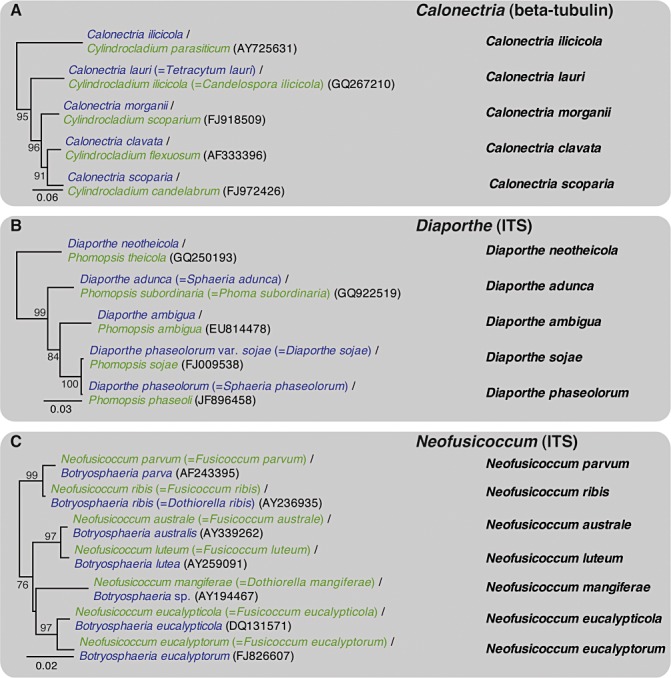
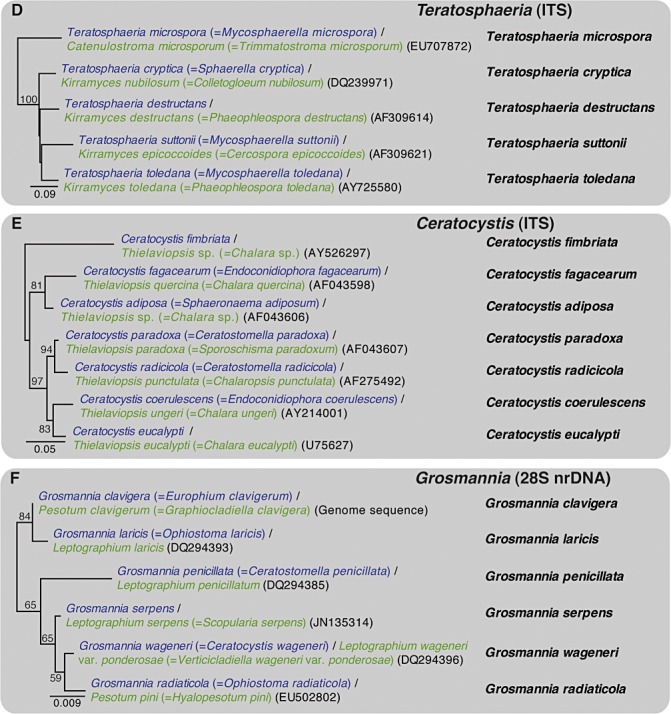
Phylogenetic trees for selected species of the plant pathogenic genera (following strict priority): (A) Calonectria; (B) Diaporthe; (C) Neofusicoccum; (D) Teratosphaeria; (E) Ceratocystis; (F) Grossmania. Trees were constructed using neighbour‐joining analysis with HKY85 as substitution model in PAUP version 4.0b10. Teleomorph names in the trees are shown in blue and anamorph names in green. Basionyms are presented in either blue or green in parentheses, and GenBank accession numbers are in black in parentheses. The single name highlighted in the right‐hand column for each species presents the name that will probably be used for the fungi following the ‘one fungus, one name’ approach. Bootstrap support values are based on 1000 replicates and the scale bar indicates the number of substitutions per site. ITS, internal transcribed spacer.
In a recent treatment of diverse genera of Ascomycota with pleomorphic life cycles, Rossman and Seifert (2011) presented five options that might be followed when deciding on a single name for a fungus. These included:
-
•
strict priority irrespective of names originally typified by anamorphic or teleomorphic elements with strict application of priority of both generic names and species' epithets (Gräfenhan et al., 2011; Schroers et al., 2011; Summerbell et al., 2011);
-
•
teleomorph priority with anamorphic species' epithets (Chaverri et al., 2011);
-
•
teleomorph priority with earlier anamorph species' epithets not considered (Hirooka et al., 2011; Mejía et al., 2011; Sultan et al., 2011);
-
•
teleotypification (Réblová and Seifert, 2011); or
-
•
single species' names but allowing two genera per clade (Põldmaa, 2011).
Of these various options, the easiest to implement would be strict priority with relation to genus and species' epithet (Table S1, see Supporting Information). Given this situation, Crous et al. (2006) took the unprecedented step of describing new genera in the Botryosphaeriaceae linked to obvious phylogenetic lineages in the family, using the oldest available name for the lineage (strict priority), irrespective of whether this was an asexual or sexual state. Thus, a single name was given to each genus and this accommodated all known morphs of the fungus. The genus Neofusicoccum is used for the clade with unnamed Botryosphaeria‐like teleomorphs (Crous et al., 2006). Likewise, Damm et al. (2008) used this approach to name an asexual Phialophora‐like fungus in the teleomorph genus Jattaea, Lombard et al. (2009, 2010a, b, c) described Cylindrocladium species using the older generic name Calonectria, and Crous et al. (2007, 2009a, b, c) described asexual morphs in Teratosphaeria. Similarly, this approach was used to describe sexual Davidiella species in the older generic name Cladosporium (Crous et al., 2011), and Phomopsis species in the older, sexual genus Diaporthe (Crous et al., 2011). Gräfenhan et al. (2011) and Schroers et al. (2011) used the same approach in their revisions of parts of Fusarium, Aveskamp et al. (2010) in their treatment of Phoma, and Summerbell et al. (2011) followed this route with Acremonium.
Although the application of a single name for a fungus as illustrated above was contrary to the intention of Article 59, it did not contradict the rules of the ICBN. The reason was that Article 59 was a later addition that only became operative when both the sexual and asexual states were known. Thus, these names are legal under the ICBN, although some mycologists have been openly antagonistic towards their application (e.g. Gams and Jaklitsch, 2011). The decision to apply a single name for a fungus was, in itself, fuelled by a desperate desire for meaningful names for genetic entities that were associated with important plant diseases. Some of these could have up to four different morphs, but for which it was felt that one name, linked to a DNA signature or DNA barcode (or genome), would suffice. The approach of one fungus having one name is also essential for the rapidly proliferating whole genome sequencing projects, where plant pathologists must compare species representing single entities with their closest neighbours. For instance, comparing Mycosphaerella tritici (now Zymoseptoria; see Quaedvlieg et al., 2011) with Mycosphaerella fijiensis (now Pseudocercospora; see Crous et al., 2009d), is not very informative, as these are simply two genera in the same family, not two species of the same genus.
The debate regarding dual nomenclature has increased concurrently with the growing mass of sequence data for fungi. Indeed, there has been increasing support from mycologists to abandon dual nomenclature and to allow only one name for each fungus (Hawksworth, 2007b). Already, in 2005, at the 17th IBC in Vienna, Austria, a Special Committee on the Nomenclature of Fungi with a Pleomorphic Life Cycle was appointed with the mandate to provide guidance on a proposal to prohibit dual nomenclature and to review the need for Article 59. However, the committee failed to reach a consensus and, as a body, could not make a recommendation for acceptance or rejection of any particular proposal (Redhead, 2010). Thus, the topic was again open for discussion at one of the nomenclature sessions at the 9th IMC in Edinburgh, UK, in 2010, leading to an extensive debate of the matter. The results of a questionnaire circulated among delegates confirmed that the majority favoured a progressive move towards adopting one name for each fungal taxon. There was also considerable support to delete Article 59, with provision that existing names were not retroactively invalidated (Norvell et al., 2010). The immediate problem that arose was the fact that fungal nomenclature continues to be governed by the ICBN and that any changes to the code would need to be made at the 18th IBC in Melbourne, Australia, in 2011. The next opportunity for change would have been at the IBC in Beijing, China, in 2017.
The dissent and confusion related to the dual nomenclature system for fungi is a rising tide. This has been strongly linked to the fact that available data regarding the identity of fungi, emerging through the availability of DNA sequence data, have made the current rules largely redundant, further fuelling confusion. The speed at which changes to the rules can be made is inconsistent with the available knowledge. Ignoring this fact makes no sense, and also leads to erroneous diagnoses of important plant diseases. It is against this backdrop that a symposium was arranged under the auspices of the International Commission on the Taxonomy of Fungi following discussions at the IMC in Edinburgh, UK, to debate this issue further. This symposium, ‘One Fungus: One Name’, was held at the Royal Netherlands Academy of Arts and Sciences offices in Amsterdam, the Netherlands, during 20–21 April 2011. The meeting included a number of presentations outlining the current problems, and presenting potential solutions, concluding with the ‘Amsterdam Declaration on Fungal Nomenclature’, published recently (Hawksworth et al., 2011). The latter declaration was co‐authored by numerous prominent mycologists and plant pathologists, and endorsed by a variety of other bodies, including the governing bodies of the International Mycological Association and the European Mycological Association, as well as the Nomenclature Committee for Fungi appointed by the Vienna IBC. However, it was not unanimously supported (Gams and Jaklitsch, 2011).
Effectively, the Amsterdam Declaration recognized the desire of mycologists to adopt one name for each fungal species, and captures the view that a unified BioCode (Greuter et al., 2011) or an independent MycoCode needs to be considered for the fungi. The Declaration included various other recommendations that facilitate a one fungus, one name taxonomy and also one that will reduce an undue proliferation of fungus names. It did not contain specific proposals, but rather a road‐map giving the direction that should be taken, and serving as a guide to mycologists attending the IBC in Melbourne, Australia.
ONE FUNGUS WHICH NAME?
Although not unanimous, there has been broad support amongst mycologists, especially those using DNA sequence data to identify fungi, to move away from the system of dual nomenclature (confusion illustrated in Fig. 2). This is especially so as DNA sequence data and the genomes of fungi become increasingly available to mycologists and plant pathologists who deal with fungal names. Clearly this support, also prompted by the ‘Amsterdam Declaration’, led to a momentous decision at the 18th IBC in Melbourne, Australia, to implement several radical changes to the ICBN (Hawksworth, 2011; McNeill et al., 2011; Norvell, 2011). The latter, which was renamed the International Code of Nomenclature for Algae, Fungi and Plants (ICN), proposed that, from 1 January 2013, all nomenclatural details of fungal novelties should be registered in a database, such as MycoBank (Crous et al., 2004), although the repository of choice has yet to be approved by the Nomenclature Committee for Fungi. Furthermore, as from 1 January 2012, the electronic publication of new names will be permissible (see Knapp et al., 2011 for details), and either English or Latin would be acceptable for the validation of new fungal descriptions. Perhaps, surprisingly to many, the more than 200 registered delegates of the entire Nomenclature Section of the Congress voted overwhelmingly to abandon the dual nomenclature system (from 1 January 2013), thus paving the way for a new era in the taxonomy of fungi where one name will be applied to every fungal taxon (Hawksworth, 2011; McNeill et al., 2011; Norvell, 2011). The International Committee for the Taxonomy of Fungi will hold a follow‐up meeting to the One Fungus: One Name symposium held in Amsterdam in 2011 in order to set up a series of subcommittees and guidelines to streamline the integration of names into a single nomenclature for fungi. The choice of these names is crucially important and will impact strongly on plant pathology and plant pathologists.
Although the force towards the application of only one name for a fungus became so overwhelming that it eventually became a reality, the issue of which name to use for these fungi is somewhat more complex. Based on the accepted recommendations, all legitimate fungal names are now treated equally for the purposes of establishing priority, essentially meaning that anamorphic genera compete with teleomorph genera based on priority, i.e. precedence by date [thus Trichoderma (1794) not Hypocrea (1825), Alternaria (1817) not Lewia (1986), Cladosporium (1816) not Davidiella (2003), Fusarium (1809) not Gibberella (1877), Sphaceloma (1874) not Elsinoë (1900), Diaporthe (1870) not Phomopsis (1905), Phyllosticta (1818) not Guignardia (1892), etc.]. Exceptions (younger, more commonly used genera) for conservation would, however, be considered by the Committee, and a support structure and database must now be established to manage this process.
CONCLUSIONS
The dual system of fungal nomenclature has served plant pathologists relatively well in the past, although it has often been noted as the source of substantial confusion. Plant pathology students are well known to have been confused (and bemused) at the fact that a single plant pathogen can have both different and correct genus and species' names. However, more importantly, as DNA sequence data have become available and an increasing instability of names has emerged, the credibility of mycologists has come into question by practitioners of mycology, importantly including plant pathologists.
As DNA sequence data become available for increasing numbers of fungi, including plant pathogens, previously unrecognized relationships between these fungi will emerge. Indeed, this is already happening increasingly regularly, and fungal pathogens are increasingly being found to bear generic names that are inconsistent with their phylogenetic relationships. Thus, pathogens believed to be related on the basis of their names are increasingly being found to be unrelated or only distantly related (Ceratocystis and Ophiostoma are good examples). As robust identities and relationships emerge from increasingly informative phylogenetic studies, the justification for a single name applied to a single fungal taxon will become overwhelmingly evident. Indeed, the pressure in this direction is already substantial and, as pointed out by John Taylor, one of the keynote speakers at the One Fungus: One Name symposium, ‘the horse has already bolted’ (Taylor, 2011). Put another way, DNA sequence data, as they apply to fungal names, might be seen as a metaphorical earthquake; what we are now dealing with is the tsunami in terms of the application of available knowledge.
We believe that the debate regarding the application of single names for fungi has largely passed and the outcome of the recent meeting of the 18th IBC in Melbourne, Australia, has reaffirmed this fact. Yet much needs to be done regarding practical issues. Clearly, a reliable and stable system of fungal nomenclature is required, but, for fungi, it must also be one that is able to respond to the realities of the data available to mycologists and plant pathologists. The fact that the ICBN has always included the fungi is the result of a long history of poorly understood relationships between fungi and other organisms. This dates back as far as Linnaeus's (1753) Species Plantarum. Today, we clearly understand that fungi are not plants; they are only very distantly related to plants and, indeed, are more closely related to animals (James et al., 2006), or to use a quote from the eminent Fusarium taxonomist, W. F. O. Marasas, ‘Fungi are more closely related to the mycologists that study them than to the plants on which they occur’. The rapidly increasing number of fungal genomes available for study will more deeply elucidate these relationships. Furthermore, they will increasingly demand a more responsive taxonomic system for the fungi. Our view is that a taxonomic code that is tailored to the needs of mycologists is inevitable. This might already be offered by the newly accepted ICN, or it could emerge as an adaptation of the proposed universal BioCode (Greuter et al., 2011); alternatively, it may be a newly developed MycoCode. Whichever end point is finally reached, the needs and support of practitioners and, in particular, plant pathologists will be crucially important.
A commonly accepted benchmark is that there are at least 1.5 million species of fungi on earth (Hawksworth, 1991), which we are describing at an average of 1200 per year, suggesting that it will take more than 1170 years to describe the number expected to exist (Hibbett et al., 2011). However, of the species currently being described, Hawksworth (2004) reported that only 20% were being deposited in culture collections (Biological Resource Centres). It is obvious that a system is needed that can address these issues more realistically. In other words, a system that is forward looking, and not locked into a code of naming fungi solely on the basis of a botanical methodology. To address the vast fungal biodiversity that exists, we need to re‐evaluate the manner in which we are recording it. Similar to research grants and funding agencies that require taxonomists to publish in open access journals, the question might be raised as to whether mycologists funded on such grants should be permitted to name fungi that lack a DNA barcode voucher. Surely this would be a more progressive approach in an environment in which several mycologists and plant pathologists are already calling for genomes, not mere genes, to be sequenced.
Biologists have dreamed of having a natural classification for all living organisms for decades. In this regard, it is interesting to look back to the words of Charles Darwin in a letter to Thomas Huxley in 1857 (http://www.darwinproject.ac.uk/entry‐2143): ‘In regard to Classification, and all the endless disputes about the Natural System which no two authors define in the same way, I believe it ought, in accordance to my heterodox notions, to be simply genealogical. But as we have no written pedigrees, you will, perhaps, say this will not help much; but I think it ultimately will, whenever heterodoxy becomes orthodoxy, for it will clear away an immense amount of rubbish about the value of characters and will make the difference between analogy and homology, clear. The time will come I believe, though I shall not live to see it, when we shall have very fairly true genealogical trees of each great kingdom of nature’. The relatively limited morphological characteristics of fungi and the complications arising from pleomorphism have made this dream especially relevant to mycologists. With the powerful molecular tools available, we have reached a point at which a natural classification for fungi is possible and this could include those that cannot be cultured, perhaps that we will never be able to see. However, we are trapped in history and face the difficulty of applying current knowledge, in the face of long‐standing and traditional rules that define how we name fungi. This situation must change much more rapidly than the current code allows, if we are to maintain our credibility and serve plant pathologists appropriately.
Supporting information
Table S1 Selected examples of plant pathogenic ascomycete genera, with their various morphs listed as synonyms.
Supporting info item
ACKNOWLEDGEMENTS
The idea for this paper emerged from the One Fungus: One Name symposium held at Trippenhuis, the home of the Royal Dutch Academy of Sciences (KNAW), in April 2011, organized by the Centraalbureau voor Schimmelcultures, and at which the first author presented one of the introductory lectures. We thank many colleagues and friends for useful, entertaining and sometimes energetic discussions, and especially those that have shared our desire to reach a more effective taxonomic scheme for the fungi. The manuscript was also substantially improved by suggestions from an anonymous reviewer, for which we are grateful. We acknowledge funding from the DST/NRF Centre of Excellence in Tree Health Biotechnology, the National Research Foundation (South Africa) and KNAW, the Netherlands, which funds the CBS‐KNAW Fungal Biodiversity Centre based in Utrecht.
REFERENCES
- Alves, A. , Crous, P.W. , Correia, A. and Phillips, A.J.L. (2008) Morphological and molecular data reveal cryptic speciation in Lasiodiplodia theobromae . Fungal Divers. 28, 1–13. [Google Scholar]
- Aveskamp, M.M. , Gruyter, H. , Woudenberg, J. , Verkley, G. and Crous, P.W. (2010) Highlights of the Didymellaceae: a polyphasic approach to characterise Phoma and related pleosporalean genera. Stud. Mycol. 65, 1–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bensch, K. , Groenewald, J.Z. , Dijksterhuis, J. , Starink‐Willemse, M. , Andersen, B. , Summerell, B.A. , Shin, H.‐D. , Dugan, F.M. , Schroers, H.‐J. , Braun, U. and Crous, P. (2010) Species and ecological diversity within the Cladosporium cladosporioides complex (Davidiellaceae, Capnodiales). Stud. Mycol. 67, 1–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berbee, M.L. and Taylor, J.W. (1992) 18S ribosomal RNA gene sequence characters place the pathogen Sporothrix schenckii in the genus Ophiostoma . Exp. Mycol. 16, 87–91. [Google Scholar]
- Briquet, J. (1905) Texte synoptique des documents destinés à servir de base aux débats du Congrès International de Nomenclature Botanique de Vienne 1905. Commission Internationale de Nomenclature Botanique. Berlin: Friedländer. [Google Scholar]
- Briquet, J. (1912) International Rules of Botanical Nomenclature Adopted by the International Botanical Congresses of Vienna, 1905 and Brussels, 1910. Jena: G. Fischer. [Google Scholar]
- Carnegie, A.J. and Cooper, K. (2011) Emergency response to the incursion of an exotic myrtaceous rust in Australia. Australas. Plant Pathol. 40, 346–359. [Google Scholar]
- Carnegie, A.J. , Lidbetter, J.R. , Walker, J. , Horwood, M.A. , Tesoriero, L. , Glen, M. and Priest, M.J. (2010) Uredo rangelii, a taxon in the guava rust complex, newly recorded on Myrtaceae in Australia. Australas. Plant Pathol. 39, 463–466. [Google Scholar]
- Chaverri, P. , Salgado, C. , Hirooka, Y. , Rossman, A.Y. and Samuels, G.S. (2011) Delimitation of Neonectria and Cylindrocarpon (Nectriaceae, Hypocreales, Ascomycota) and related genera with Cylindrocarpon‐like anamorphs. Stud. Mycol. 68, 57–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole, G.T. and Samson, R.A. (1979) Patterns of Development in Conidial Fungi. London: Pitman Publishing Limited. [Google Scholar]
- Coutinho, T.A. , Wingfield, M.J. , Alfenas, A.C. and Crous, P.W. (1998) Eucalyptus rust: a disease with the potential for serious international implications. Plant Dis. 82, 819–825. [DOI] [PubMed] [Google Scholar]
- Crous, P.W. and Groenewald, J.Z. (2005) Hosts, species and genotypes: opinions versus data. Australas. Plant Pathol. 34, 463–470. [Google Scholar]
- Crous, P.W. , Alfenas, A.C. and Wingfield, M.J. (1993a) Calonectria scoparia and Calonectria morganii sp. nov., and variation among isolates of their Cylindrocladium anamorphs. Mycol. Res. 97, 701–708. [Google Scholar]
- Crous, P.W. , Wingfield, M.J. and Alfenas, A. (1993b) Cylindrocladium parasiticum sp. nov., a new name for C. crotalariae . Mycol. Res. 97, 889–896. [Google Scholar]
- Crous, P.W. , Gams, W. , Stalpers, J.A. , Robert, V. and Stegehuis, G. (2004) MycoBank: an online initiative to launch mycology into the 21st century. Stud. Mycol. 50, 19–22. [Google Scholar]
- Crous, P.W. , Slippers, B. , Wingfield, M.J. , Rheeder, J. , Marasas, W.F.O. , Philips, A.J.L. , Alves, A. , Burgess, T.I. , Barber, P. and Groenewald, J. (2006) Phylogenetic lineages in the Botryosphaeriaceae. Stud. Mycol. 55, 235–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous, P.W. , Braun, U. and Groenewald, J.Z. (2007) Mycosphaerella is polyphyletic. Stud. Mycol. 58, 1–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous, P.W. , Groenewald, J.Z. , Summerell, B.A. , Wingfield, B.D. and Wingfield, M.J. (2009a) Co‐occurring species of Teratosphaeria on Eucalyptus . Persoonia, 22, 38–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous, P.W. , Schoch, C.L. , Hyde, K.D. , Wood, A.R. , Gueidan, C. , de Hoog, G.S. and Groenewald, J.Z. (2009b) Phylogenetic lineages in the Capnodiales. Stud. Mycol. 64, 17–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous, P.W. , Summerell, B.A. , Carnegie, A.J. , Wingfield, M.J. and Groenewald, J.Z. (2009c) Novel species of Mycosphaerellaceae and Teratosphaeriaceae. Persoonia, 23, 119–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous, P.W. , Summerell, B.A. , Carnegie, A.J. , Wingfield, M.J. , Hunter, G.C. , Burgess, T.I. , Andjic, V. , Barber, P.A. and Groenewald, J.Z. (2009d) Unravelling Mycosphaerella: do you believe in genera? Persoonia, 23, 99–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous, P.W. , Groenewald, J.Z. , Shivas, R.G. , Edwards, J. , Seifert, K.A. , Alfenas, A.C. , Alfenas, R.F. , Burgess, T.I. , Carnegie, A.J. , Hardy, G.E.S.J. , Hiscock, N. , Hüberli, D. , Jung, T. , Louis‐Seize, G. , Okada, G. , Pereira, O.L. , Stukely, M.J.C. , Wang, W. , White, G.P. , Young, A.J. , McTaggart, A.R. , Pascoe, I.G. , Porter, I.J. and Quaedvlieg, W. (2011) Fungal planet description sheets: 69–91. Persoonia, 26, 108–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damm, U. , Crous, P.W. and Fourie, P.H. (2008) A fissitunicate ascus mechanism in the Calosphaeriaceae, and novel species of Jattaea and Calosphaeria on Prunus wood. Persoonia, 20, 39–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gams, W. (1995) How natural should anamorph genera be? Can. J. Bot. 73 (Suppl 1), S747–S753. [Google Scholar]
- Gams, W. and Jaklitsch, W. (2011) A critical response to the ‘Amsterdam Declaration’. Mycotaxon, 116, 501–512. [Google Scholar]
- Glen, M. , Alfenas, A.C. , Zauza, E.A.V. , Wingfield, M.J. and Mohammed, C. (2007) Puccinia psidii: a threat to the Australian environment and economy—a review. Australas. Plant Pathol. 36, 1–16. [Google Scholar]
- Gräfenhan, T. , Schroers, H.‐J. , Nirenberg, H.I. and Seifert, K.A. (2011) An overview of the taxonomy, phylogeny, and typification of nectriaceous fungi in Cosmospora, Acremonium, Fusarium, Stilbella, and Volutella . Stud. Mycol. 68, 79–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greuter, W. , Garrity, G. , Hawksworth, D.L. , Jahn, R. , Kirk, P.M. , Knapp, S. , McNeill, J. , Michel, E. , Patterson, D.J. , Pyle, R. and Tindall, B.J. (2011) Draft BioCode (2011): principles and rules regulating the naming of organisms. Bionomina, 3, 26–44; Taxon, 60, 201–212; Bull. Zool. Nomen. 68, 10–28. [Google Scholar]
- Hawksworth, D.H. (1991) The fungal dimension of biodiversity: magnitude, significance and conservation. Mycol. Res. 95, 641–655. [Google Scholar]
- Hawksworth, D.L. (2004) Fungal diversity and its implications for genetic resource collections. Stud. Mycol. 50, 9–18. [Google Scholar]
- Hawksworth, D.L. (2007a) Mycological research: instructions and guidelines for authors. Mycol. Res. 111, 117–126. [DOI] [PubMed] [Google Scholar]
- Hawksworth, D.L. (2007b) Mycologists speak on nomenclatural issues. Mycol. Res. 111, 1363–1364. [Google Scholar]
- Hawksworth, D.L. (2011) A new dawn for the naming of fungi: impacts of decisions made in Melbourne in July 2011 on the future publication and regulation of fungal names. MycoKeys, 1, 7–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawksworth, D.L. and Sutton, B.C. (1974) Comments on Weresub, Malloch and Pirozynski's proposal for Article 59. Taxon, 23, 659–661. [Google Scholar]
- Hawksworth, D.L. , Crous, P.W. , Redhead, S.A. , Reynolds, D.R. , Samson, R.A. , Seifert, K.A. , Taylor, J.W. , Wingfield, M.J. , Abaci, Ö. , Aime, C. , Asan, A. , Bai, F.‐Y. , de Beer, Z.W. , Begerow, D. , Berikten, D. , Boekhout, T. , Buchanan, P.K. , Burgess, T. , Buzina, W. , Cai, L. , Cannon, P.F. , Crane, J.L. , Damm, U. , Daniel, H.‐M. , van Diepeningen, A.D. , Druzhinina, I. , Dyer, P.S. , Eberhardt, U. , Fell, J.W. , Frisvad, J.C. , Geiser, D.M. , Geml, J. , Glienke, C. , Gräfenhan, T. , Groenewald, J.Z. , Groenewald, M. , de Gruyter, J. , Guého‐Kellermann, E. , Guo, L.‐D. , Hibbett, D.S. , Hong, S.‐B. , de Hoog, G.S. , Houbraken, J. , Huhndorf, S.M. , Hyde, K.D. , Ismail, A. , Johnston, P.R. , Kadaifciler, D.G. , Kirk, P.M. , Kõljalg, U. , Kurtzman, C.P. , Lagneau, P.‐E. , Lévesque, C.A. , Liu, X. , Lombard, L. , Meyer, W. , Miller, A. , Minter, D.W. , Najafzadeh, M.J. , Norvell, L. , Ozerskaya, S.M. , Öziç, R. , Pennycook, S.R. , Peterson, S.W. , Pettersson, O.V. , Quaedvlieg, W. , Robert, V.A. , Ruibal, C. , Schnürer, J. , Schroers, H.‐J. , Shivas, R. , Slippers, B. , Spierenburg, H. , Takashima, M. , Taşkın, E. , Thines, M. , Thrane, U. , Uztan, A.H. , van Raak, M. , Varga, J. , Vasco, A. , Verkley, G. , Videira, S.I.R. , de Vries, R.P. , Weir, B.S. , Yilmaz, N. , Yurkov, A. and Zhang, N. (2011) The Amsterdam Declaration on fungal nomenclature. IMA Fungus, 2, 105–112; Mycotaxon, 116, 491–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennebert, G.L. (2003) Fundamentals for suppression of dual nomenclature in pleomorphic fungi and integration of anamorphic fungi (deuteromycetes) into the Ascomycota and Basidiomycota. Mycotaxon, 88, 509–514. [Google Scholar]
- Hibbett, D.S. , Ohman, A. , Glotzer, D. , Nuhn, M. , Kirk, P. and Nilsson, R.H. (2011) Progress in molecular and morphological taxon discovery in Fungi and options for formal classification of environmental sequences. Fungal. Biol. Rev. 25, 38–47. [Google Scholar]
- Hirooka, Y. , Rossman, A.Y. and Chaverri, P. (2011) A morphological and phylogenetic revision of the Nectria cinnabarina species complex. Stud. Mycol. 68, 35–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes, S.J. (1953) Conidiophores, conidia and classification. Can. J. Bot. 31, 557–659. [Google Scholar]
- James, T.Y. , Kauff, F. , Schoch, C.L. , Matheny, P.B. , Hofstetter, V. , Cox, C.J. , Celio, G. , Gueidan, C. , Fraker, E. , Miadlikowska, J. , Lumbsch, H.T. , Rauhut, A. , Reeb, V. , Arnold, A.E. , Amtoft, A. , Stajich, J.E. , Hosaka, K. , Sung, G.‐H. , Johnson, D. , O'Rourke, B. , Binder, M. , Curtis, J.M. , Slot, J.C. , Wang, Z. , Wilson, A.W. , Schüßler, A. , Longcore, J.E. , O'Donnell, K. , Mozley‐Standridge, S. , Porter, D. , Letcher, P.M. , Powell, M.J. , Taylor, J.W. , White, M.M. , Griffith, G.W. , Davies, D.R. , Sugiyama, J. , Rossman, A.Y. , Rogers, J.D. , Pfister, D.H. , Hewitt, D. , Hansen, K. , Hambleton, S. , Shoemaker, R.A. , Kohlmeyer, J. , Volkmann‐Kohlmeyer, B. , Spotts, R.A. , Serdani, M. , Crous, P.W. , Hughes, K.W. , Matsuura, K. , Langer, E. , Langer, G. , Untereiner, W.A. , Lücking, R. , Büdel, B. , Geiser, D.M. , Aptroot, A. , Diederich, P. , Schmitt, I. , Schultz, M. , Yahr, R. , Hibbett, D.S. , Lutzoni, F. , McLaughlin, D.J. , Spatafora, J.W. and Vilgalys, R. (2006) Reconstructing the early evolution of the fungi using a six gene phylogeny. Nature, 443, 818–822. [DOI] [PubMed] [Google Scholar]
- Kendrick, W.B. (1971) Taxonomy of Fungi Imperfecti. Toronto, ON: University of Toronto Press. [Google Scholar]
- Kendrick, W.B. (1979) The Whole Fungus. Ottawa, ON: National Museum of Natural Sciences. [Google Scholar]
- Knapp, S. , McNeill, J. and Turland, N.J. (2011) Changes to publication requirements made at the XVIII International Botanical Congress in Melbourne—what does e‐publication mean for you? Mycotaxon, 117, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechat, C. , Crous, P.W. and Groenewald, J.Z. (2010) The enigma of Calonectria species occurring on leaves of Ilex aquifolium in Europe. IMA Fungus, 1, 101–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linnaeus, C. (1753) Species Plantarum. Stockholm: Salvius. [Google Scholar]
- Lombard, L. , Rodas, C.A. , Crous, P.W. , Wingfield, B.D. and Wingfield, M.J. (2009) Calonectria (Cylindrocladium) species associated with dying Pinus cuttings. Persoonia, 23, 41–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombard, L. , Crous, P.W. , Wingfield, B.D. and Wingfield, M.J. (2010a) Multigene phylogeny and mating tests reveal three cryptic species related to Calonectria pauciramosa . Stud. Mycol. 66, 15–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombard, L. , Crous, P.W. , Wingfield, B.D. and Wingfield, M.J. (2010b) Phylogeny and systematics of the genus Calonectria . Stud. Mycol. 66, 31–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombard, L. , Crous, P.W. , Wingfield, B.D. and Wingfield, M.J. (2010c) Species concepts in Calonectria (Cylindrocladium). Stud. Mycol. 66, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malloch, D. and Cain, R. (1972) The Trichocomataceae: ascomycetes, with Aspergillus, Paecilomyces, and Penicillium imperfect states. Can. J. Bot. 50, 2613–2638. [Google Scholar]
- McNeill, J. , Barrie, F.R. , Burdet, H.M. , Demoulin, V. , Hawksworth, D.L. , Marhold, K. , Nicolson, D.H. , Prado, J. , Silva, P.C. , Skog, J.E. , Wiersema, J.H. and Turland, N.J. (2006) International Code of Botanical Nomenclature (Vienna Code) Adopted by the Seventeenth International Botanical Congress, Vienna, Austria, July 2005 (Electronic Ed.). Vienna: International Association for Plant Taxonomy; Available at http://ibot.sav.sk/icbn/main.htm. [Google Scholar]
- McNeill, J. , Turland, N.J. , Monro, A.M. and Lepsci, B.J. (2011) XVIII International Botanical Congress: preliminary mail vote and report of Congress action on nomenclature proposals. Taxon, 60, 1507–1520. [Google Scholar]
- Mejía, L.C. , Castlebury, L.A. , Rossman, A.Y. , Sogonov, M.V. and White, J.F. Jr (2011) A systematic account of the genus Plagiostoma (Gnomoniaceae, Diaporthales) based on morphology, host‐associations, and a four‐gene phylogeny. Stud. Mycol. 68, 211–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norvell, L.L. (2011) Fungal nomenclature. 1. Melbourne approves a new Code. Mycotaxon, 116, 481–490. [Google Scholar]
- Norvell, L.L. , Hawksworth, D.L. , Petersen, R.H. and Redhead, S.A. (2010) IMC9 Edinburgh nomenclature sessions. Mycotaxon, 113, 503–511; IMA Fungus, 1, 143–147; Taxon, 59, 1867–1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell, K. , Ward, T.J. , Geiser, D.M. , Kistler, H.C. and Aoki, T. (2004) Genealogical concordance between the mating type locus and seven other nuclear genes supports formal recognition of nine phylogenetically distinct species within the Fusarium graminearum clade. Fungal Genet. Biol. 41, 600–623. [DOI] [PubMed] [Google Scholar]
- O'Donnell, K. , Gueidan, C. , Sink, S. , Johnston, P.R. , Crous, P.W. , Glenn, A. , Riley, R. , Zitomer, N.C. , Colyer, P. , Waalwijk, C. , van der Lee, T. , Moretti, A. , Kang, S. , Kim, H.‐S. , Geiser, D.M. , Juba, J.H. , Baayen, R.P. , Cromey, M.G. , Bithel, S. , Sutton, D.A. , Skovgaard, K. , Ploetz, R. , Kistler, H.C. , Elliott, M. , Davis, M. and Sarver, B.A.J. (2009) A two‐locus DNA sequence database for typing plant and human pathogens within the Fusarium oxysporum species complex. Fungal Genet. Biol. 46, 936–948. [DOI] [PubMed] [Google Scholar]
- Paulin‐Mahady, A.E. , Harrington, T.C. and McNew, D. (2002) Phylogenetic and taxonomic evaluation of Chalara, Chalaropsis, and Thielaviopsis anamorphs associated with Ceratocystis . Mycologia, 94, 62–72. [PubMed] [Google Scholar]
- Põldmaa, K. (2011) Tropical species of Cladobotryum and Hypomyces producing red pigments. Stud. Mycol. 68, 1–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quaedvlieg, W. , Kema, G.H.J. , Groenewald, J.Z. , Verkley, G.J.M. , Seifbarghi, S. , Razavi, M. , Mirzadi Gohari, A. , Mehrabi, R. and Crous, P.W. (2011) Zymoseptoria gen. nov.: a new genus to accommodate Septoria‐like species occurring on graminicolous hosts. Persoonia, 26, 57–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Réblová, M. and Seifert, K.A. (2011) Discovery of the teleomorph of the hyphomycete, Sterigmatobotrys macrocarpa, and epitypification of the genus to holomorphic status. Stud. Mycol. 68, 193–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redhead, S.A. (2010) Report on the Special Committee on the nomenclature of fungi with a pleomorphic life cycle. Taxon, 59, 1863–1866. [Google Scholar]
- Reynolds, D.R. and Taylor, J.W. (1992) Article 59: reinterpretation or revision? Taxon, 41, 91–98. [Google Scholar]
- Rossman, A.Y. and Seifert, K.A. (2011) Phylogenetic revision of taxonomic concepts in the Hypocreales and other Ascomycota—a tribute to Gary J. Samuels. Stud. Mycol. 68, 1–256. 21523187 [Google Scholar]
- Saccardo, P.A. (1904) De Diagnostica et nomenclatura mycologica, Admonita quaedam. Ann. Mycol. 2, 195–198. [English translation by Clements, F.E. (1904) J. Mycol. 10, 109–112.]. [Google Scholar]
- Schoch, C.L. , Crous, P.W. , Wingfield, M.J. and Wingfield, B.D. (2000a) Phylogeny of Calonectria and selected hypocrealean genera with cylindrical macroconidia. Stud. Mycol. 45, 45–62. [Google Scholar]
- Schoch, C.L. , Crous, P.W. , Witthuhn, R.C. , Cronright, G. , El‐Gholl, N.E. and Wingfield, B.D. (2000b) Recombination in Calonectria morganii and phylogeny with other heterothallic small‐spored Calonectria species. Mycologia, 92, 665–673. [Google Scholar]
- Schroers, H.J. , Gräfenhan, T. , Nirenberg, H.I. and Seifert, K.A. (2011) A revision of Cyanonectria and Geejayessia gen. nov., and related species with Fusarium‐like anamorphs. Stud. Mycol. 68, 115–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert, K. , Ritschel, A. and Braun, U. (2003) A monograph of Fusicladium s. lat. (Hyphomycetes). Schlechtendalia, 9, 1–132. [Google Scholar]
- Slippers, B. , Crous, P.W. , Denman, S. , Coutinho, T.A. , Wingfield, B.D. and Wingfield, M.J. (2004) Combined multiple gene genealogies and phenotypic characters differentiate several species previously identified as Botryosphaeria dothidea . Mycologia, 96, 83–101. [PubMed] [Google Scholar]
- Sultan, A. , Johnston, P.R. , Park, D. and Robertson, A.W. (2011) Two new pathogenic ascomycetes in Guignardia and Rosenscheldiella on New Zealand's pygmy mistletoes (Korthalsella: Viscaceae). Stud. Mycol. 68, 237–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summerbell, R.C. , Gueidan, C. , Schroers, H.‐J. , de Hoog, G.S. , Starink, M. , Arocha Rosete, Y. , Guarro, J. and Scott, J.A. (2011) Acremonium phylogenetic overview and revision of Gliomastix, Sarocladium, and Trichothecium . Stud. Mycol. 68, 139–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor, J.E. (2011) One fungus = one name: DNA and fungal nomenclature twenty years after PCR. IMA Fungus, 2, 113–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weresub, L.K. and Pirozynski, K.A. (1979) Pleomorphism of fungi as treated in the history of mycology and nomenclature In: The Whole Fungus 1 (Kendrick B., ed.), pp. 17–25. Ottawa, ON: National Museums of Canada. [Google Scholar]
- van Wyk, M. , Wingfield, B.D. , Clegg, P.A. and Wingfield, M.J. (2009) Ceratocystis larium sp. nov., a new species from Styrax benzoin wounds associated with incense harvesting in Indonesia. Persoonia, 22, 75–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zipfel, R.D. , de Beer, Z.W. , Jacobs, K. , Wingfield, B.D. and Wingfield, M.J. (2006) Multi‐gene phylogenies define Ceratocystiopsis and Grosmannia distinct from Ophiostoma . Stud. Mycol. 55, 75–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Selected examples of plant pathogenic ascomycete genera, with their various morphs listed as synonyms.
Supporting info item


