SUMMARY
With the aim of identifying novel regulators of host and nonhost resistance to fungi in rice, we carried out a systematic mutant screen of mutagenized lines. Two mutant wrky22 knockout lines revealed clear‐cut enhanced susceptibility to both virulent and avirulent Magnaporthe oryzae strains and altered cellular responses to nonhost Magnaporthe grisea and Blumeria graminis fungi. In addition, the analysis of the pathogen responses of 24 overexpressor OsWRKY22 lines revealed enhanced resistance phenotypes on infection with virulent M. oryzae strain, confirming that OsWRKY22 is involved in rice resistance to blast. Bioinformatic analyses determined that the OsWRKY22 gene belongs to a well‐defined cluster of monocot‐specific WRKYs. The co‐regulatory analysis revealed no significant co‐regulation of OsWRKY22 with a representative panel of OsWRKYs, supporting its unique role in a series of transcriptional responses. In contrast, inquiring a subset of biotic stress‐related Affymetrix data, a large number of resistance and defence‐related genes were found to be putatively co‐expressed with OsWRKY22. Taken together, all gathered experimental evidence places the monocot‐specific OsWRKY22 gene at the convergence point of signal transduction circuits in response to both host and nonhost fungi encountering rice plants.
INTRODUCTION
WRKY proteins are characterized by the highly conserved WRKYGQK domain and a zinc‐finger motif (Eulgem et al., 2000). Arabidopsis and rice WRKY genes have been classified into three groups depending on the number and type of WRKY domains and zinc‐finger motif (Wu et al., 2005; Zhang and Wang, 2005).
In plants, the WRKYs encode large gene families of transcription factors which are implicated in diverse developmental and physiological functions. They have been shown to be involved in response to wounding (Hara et al., 2000), cold (Marèet al., 2004) and drought (Pnueli et al., 2002). In Arabidopsis, they also play a crucial role in response to infection with Blumeria graminis (Lippok et al., 2007), Erwinia carotovora (Li et al., 2004), Erysiphe cichoracearum (Li et al., 2006), Hyaloperonospora parasitica (Knoth et al., 2007), Botrytis cinerea (Abuqamar et al., 2006; Mao et al., 2011), Alternaria brassicicola (Zheng et al., 2006), Ralstonia solanacearum (Hu et al., 2008) and Pseudomonas syringae (Journot‐Catalino et al., 2006; Murray et al., 2007; Xu et al., 2006; Zheng et al., 2007).
A systematic expression analysis of rice WRKYs revealed that 15 of the 45 genes tested were induced on infection with Xanthomonas oryzae pv. oryzae (Xoo) and Magnaporthe oryzae, the causal agents of bacterial blight and fungal blast, respectively (Ryu et al., 2006). Ramamoorthy et al. (2008) analysed the rice WRKY gene family and showed that several OsWRKYs are co‐regulated under abiotic stress and on hormone treatments. Custom OsWRKY array analysis has recently shown that 24 members of the rice WRKY family are differentially regulated in response to biotic or abiotic stress conditions, and some are significantly co‐expressed (Berri et al., 2009).
Several studies have also suggested the importance of specific OsWRKYs (mainly belonging to group 2) in the transcriptional regulation of defence‐related genes in rice response to pathogens. More precisely, OsWRKY71 (group 2A) may function as a transcriptional regulator upstream of OsNH1 and OsPR1b in the defence signalling pathway (Liu et al., 2007), while OsWRKY13 (group 2E) acts as an activator of the salicylic acid (SA)‐dependent pathway and a suppressor of the jasmonic acid (JA)‐dependent pathway (Qiu et al., 2007). OsWRKY6 (group 2D) has been shown to play a role in the pathogen‐ or SA‐inducible expression of OsPR1a; indeed, OsWRKY6 is induced by Xoo and SA, and activates OsPR1 promoter in rice (Hwang et al., 2011). Shimono et al. (2007) identified a group 3 gene, OsWRKY45, which is transcriptionally up‐regulated by SA and benzothiadiazole, a functional analogue of SA. Zhang et al. (2008) isolated another gene belonging to group 3, OsWRKY55 (OsWRKY31 according to the nomenclature of Wu et al., 2005), which is induced by the rice blast fungus and auxin. OsWRKY89 (group 1) was strongly induced by treatments with methyl jasmonate and UV‐B radiation (Wang et al., 2007). All the WRKY genes mentioned above have been defined as positive regulators of resistance as their over‐expression has been associated with enhanced resistance to Xoo and/or M. oryzae. In contrast, Peng et al. (2008) identified OsWRKY62 (group 2A) as a negative regulator of rice defence. Indeed, transgenic plants over‐expressing this gene showed compromised Xoo resistance. Later, Peng et al. (2010) reported that, more generally, OsWRKY group 2A members (including OsWRKY62) play both positive and negative regulatory roles in tuning rice innate immunity. In most cases, when mutant lines that knocked down the expression of the above‐mentioned genes were screened for a phenotype to infection with blast or Xoo, clear‐cut phenotypes were lacking. For example, OsWRKY55 over‐expressing lines were more resistant to M. oryzae, but the RNA‐interference knock‐down plants did not show the expected specific hypersusceptible phenotypes (Zhang et al., 2008). This probably occurs because of the functional redundancy of phylogenetically related and unrelated WRKY genes (Berri et al., 2009). To decipher the function of a specific gene, it is therefore relevant to ascertain the relation with its closest paralogues and to verify whether it might be part of a co‐regulatory network.
In the present study, we screened 35 insertion lines in OsWRKYs belonging to different phylogenetic groups for their infection phenotype against two M. oryzae isolates. Only one line, corresponding to OsWRKY22, exhibited a greater number of enlarged lesions in response to both strains and was analysed further. We showed that OsWRKY22 is a relevant regulator of rice resistance to M. oryzae and is also involved in the rice response to the nonhost barley powdery mildew. To better describe OsWRKY22, we analysed the co‐regulation and expression profiles after blast infection of this gene together with its closest paralogues belonging to the same subgroup, which is poorly described so far. We highlighted that this OsWRKY subgroup within group 3 has closest homologues only in monocot plants. By depicting the genes co‐expressed with OsWRKY22, we also reported that this gene might play different roles during the rice response to host and nonhost fungi.
RESULTS
wrky22 mutants show increased susceptibility to M. oryzae
Seedlings from T2 generations carrying mutations in 23 WRKY genes (corresponding to 35 mutant lines) were tested for altered susceptibility or resistance to a M. oryzae strain FR13 which is virulent on the corresponding wild‐type rice cultivar. A clear‐cut phenotype was observed only for two allelic lines in the gene OsWRKY22 (Os01g60490). The wrky22 mutants were also challenged with the avirulent M. oryzae strain CL3.6.7. When homozygous wrky22‐1 and wrky22‐2 mutants were inoculated with FR13 or CL3.6.7 at 4 and 2 weeks after sowing, respectively, plants reproducibly displayed enhanced susceptibility (Fig. 1a,b) with a significantly increased number of enlarged lesions (Fig. 1c). Reverse transcription‐polymerase chain reaction (RT‐PCR) analysis using primers located in the second and third exons of WRKY22 confirmed the highly reduced transcription levels of this gene in both wrky22‐1 and wrky22‐2 homozygous lines (Fig. 1d). The wrky22 mutants grew at the same rate and flowered at the same time as wild‐type plants, and showed normal plant architecture (data not shown). Homozygous wrky22‐1 and wrky22‐2 mutants were also inoculated with barley powdery mildew (Blumeria graminis f. sp. hordei, Bgh) to assess an involvement in nonhost interactions. However, no macroscopic phenotype was observed.
Figure 1.
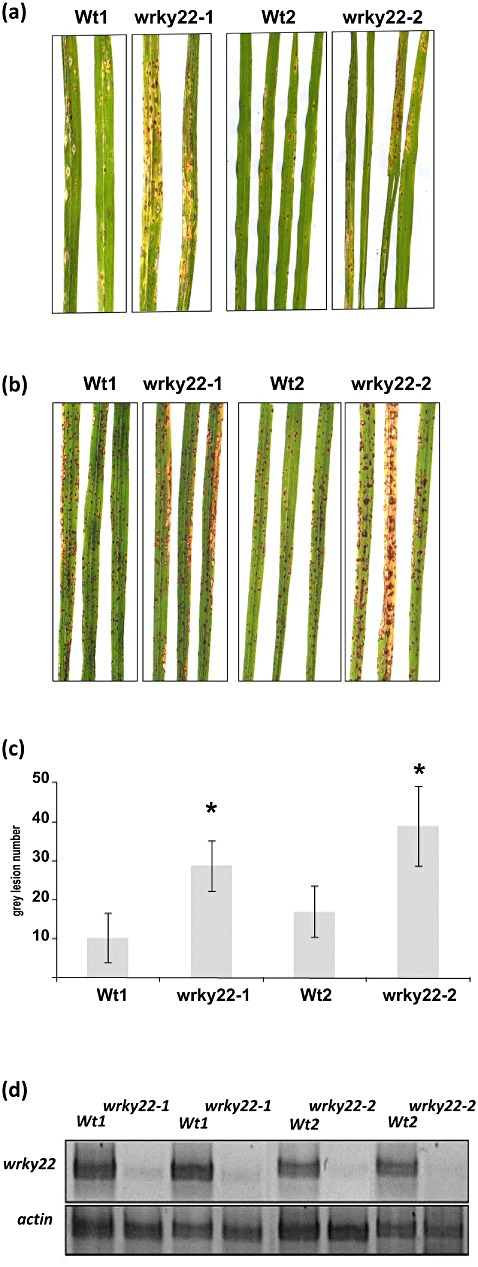
Phenotypes of the two wrky22 T‐DNA insertion lines and corresponding nullizygous (wild‐type, Wt) plants after Magnaporthe infection. (a) Symptoms of 4‐week‐old wrky22 mutant lines upon M. oryzae virulent FR13 infection at 7 days post‐inoculation. (b) Symptoms of 2‐week‐old wrky22 mutant lines upon M. oryzae avirulent CL3.6.7 infection. (c) Number of grey lesions surrounded by brown zones in wrky22 mutants and corresponding nullizygous (Wt) plants at 7 days post‐inoculation with the avirulent M. oryzae strain CL3.6.7. (d) Nested reverse transcription‐polymerase chain reaction (RT‐PCR) of OsWRKY22 and house‐keeping actin expression in wrky22 mutants and Wt plants before inoculation.
Both allelic mutant lines were further investigated at the cellular level by analysing the formation of H2O2 or callose deposition at 24 and 48 h post‐inoculation (hpi) (Fig. 2) with M. oryzae FR13 (virulent), CL3.6.7 (avirulent) and BR32 (nonadapted). In addition, the two nonhost species M. grisea BR29 and barley powdery mildew (Bgh) were included in these cytological analyses to learn more about the putative involvement of OsWRKY22 during nonhost interactions. Figure 2 shows the results obtained with wrky22‐1, but the same cellular events occurred in both OsWRKY22 allelic lines.
Figure 2.
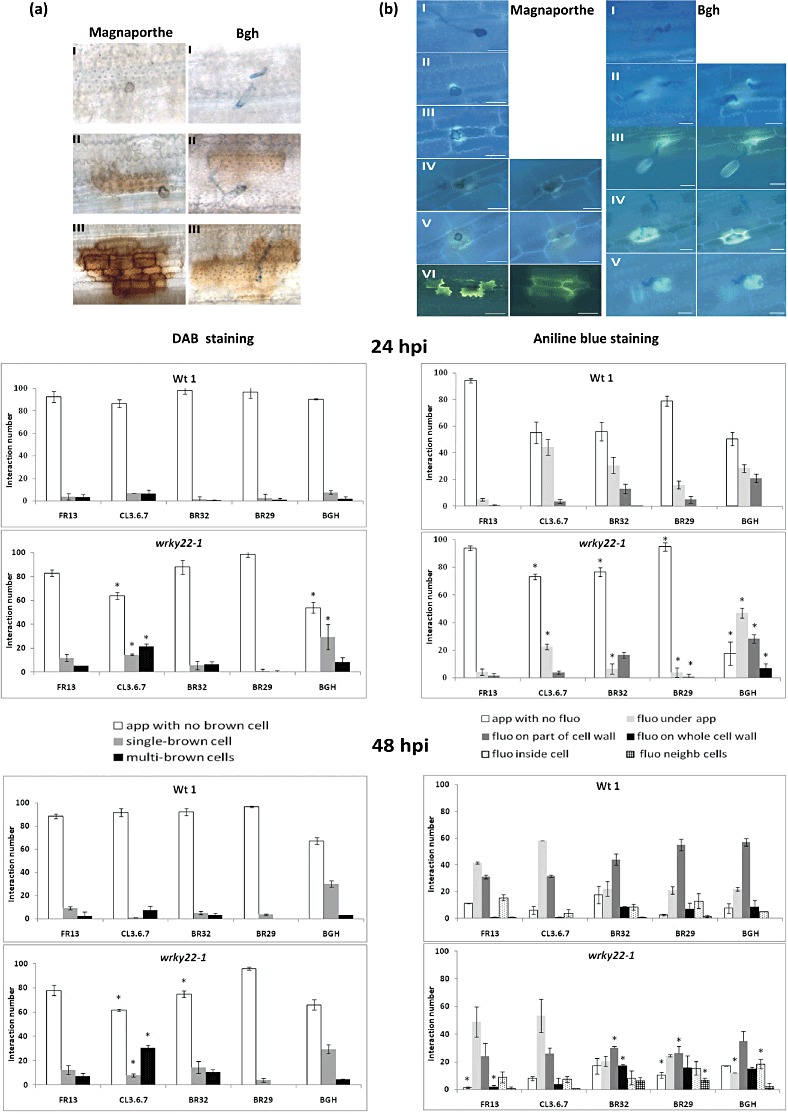
Magnaporthe oryzae, M. grisea and Blumeria graminis f. sp. hordei (Bgh) leaf infection process at the cellular level in wrky22 mutants and corresponding nullizygous wild‐type (Wt) plants at 24 and 48 h post‐inoculation (hpi). (a) Classification of cytological interaction types revealed after 3,3′‐diaminobenzidine (DAB) staining (H2O2 production): I, appressorium with no brown cell; II, single brown cell; III, multiple brown cells. (b) Classification of cytological interaction types revealed after aniline blue staining (callose deposition): I, appressorium with no fluorescence; II, fluorescence under appressorium; III, fluorescence on part of the cell wall; IV, fluorescence on the whole cell wall; V, fluorescence inside the cell; VI, fluorescence occurring in the neighbouring cells. Left panels show Magnaporthe and right panels Bgh infection process. The histograms represent the frequency of DAB‐associated cellular phenotypes or callose deposition at 24 and 48 hpi with Magnaporthe FR13 (virulent), BR32 and CL3.6.7 (avirulent) isolates, the nonhost M. grisea BR29 and Bgh strains. The mean and standard deviation of three replicates (constituting 100 interactions) are shown (*P < 0.05). Bars, 20 µm.
The frequency of multicellular H2O2 production was significantly higher at both 24 and 48 hpi in the mutant plants after inoculation with CL3.6.7 (Fig. 2a). However, 3,3′‐diaminobenzidine (DAB) staining did not differ significantly between wild‐type and mutants in response to FR13, BR32 and BR29, respectively. In contrast, at 24 hpi, the occurrence of fluorescence corresponding to callose deposition was higher in wild‐type plants than in wrky22 mutants during interactions with Magnaporthe CL3.6.7/BR32/BR29 (Fig. 2b). When plants were inoculated with Bgh, the occurrence of fluorescence was higher at 24 hpi in wrky22 mutants.
In conclusion, wrky22 mutants showed different cellular responses relative to the wild‐type with regard to both H2O2 formation and callose deposition. This is likely to correlate with the enhanced disease susceptibility phenotype following infection with CL3.6.7 strain as observed at 7 days post‐inoculation (dpi) (Fig. 1a–c).
Over‐expression of WRKY22 results in enhanced disease resistance to M. oryzae
Transgenic Nipponbare rice lines over‐expressing the OsWRKY22 cDNA were regenerated. None of the over‐expressing transformed plants exhibited morphological defects at the vegetative and flowering stages compared with control plants transformed with empty vector (data not shown). The transcription levels of OsWRKY22 were measured by quantitative RT‐PCR (Fig. 3a). In over‐expressing (OE) plants, the WRKY22 transcript level was found to be 600–3000 fold higher than in controls. We tested 24 T0 transformation events for resistance to the compatible strain of blast fungus FR13 in comparison with control plants containing empty vector. Twenty‐one (91%) transgenic lines over‐expressing WRKY22 showed significantly enhanced disease resistance (Fig. 3b). Three independent transgenic lines from T0 seeds (bearing only one T‐DNA insertion and denoted by #1, #2 and #3 in Fig. 3b) were then multiplied to obtain T1 progeny plants segregating for the introduced T‐DNA. Thirteen, 26 and 27 plants for each of these T1 lines were challenged with FR13 and the phenotype of the OE lines was confirmed as resistant (Fig. 4a). Indeed, 75% of the OE analysed plants were totally or partially resistant (Fig. 4b). In addition, this enhanced resistance to FR13 correlated strongly with higher basal WRKY transcript levels measured before inoculation, as shown by the quantitative RT‐PCR data in Fig. 4c.
Figure 3.
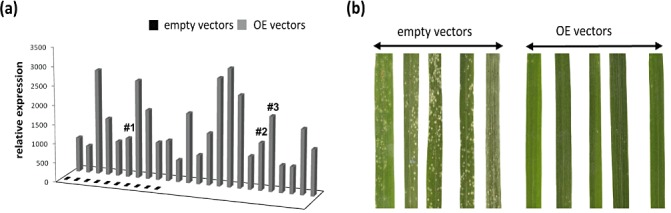
Over‐expressing (OE) OsWRKY22 rice cv. Nipponbare T0 plants. (a) OsWRKY22 expression levels in OE transgenic lines and in empty vector control plants before inoculation. #1, #2 and #3 indicate the three selected T0 lines to obtain T1 progeny plants segregating for the introduced T‐DNA. (b) Enhanced disease resistance phenotype of the OE lines and phenotype of the empty vector plants upon M. oryzae FR13 virulent infection. A representative sample of leaves from OE and empty vector control plants at 7 days post‐inoculation is shown.
Figure 4.
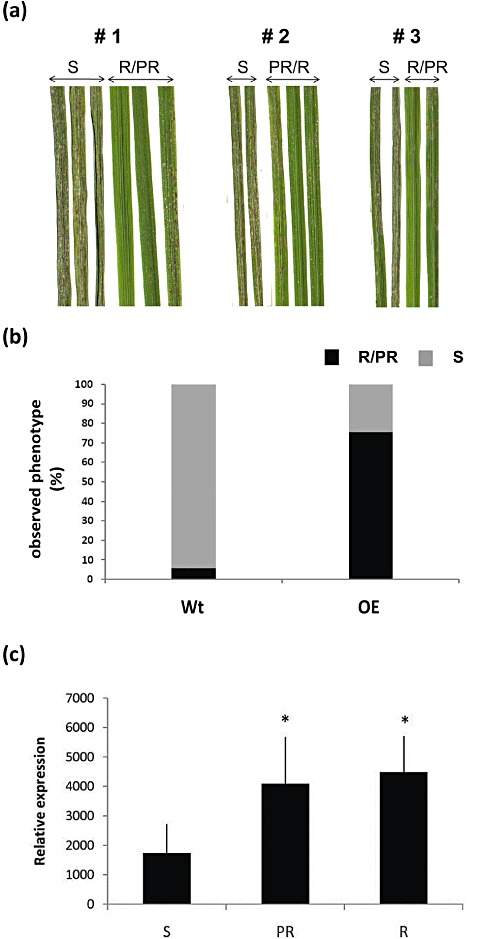
Over‐expressing (OE) OsWRKY22 rice cv. Nipponbare T1 plants. Three independent transgenic lines (#1, #2 and #3, all carrying only one T‐DNA) with 13, 26 and 27 plants for each line were analysed for phenotyping and molecular analysis. (a) Symptoms of three OE transgenic lines on Magnaporthe oryzae FR13 virulent infection: S, susceptible; R, resistant; PR, partially resistant. A representative sample of leaves from OE plants is shown. (b) Frequency of observed and expected S/R/PR phenotypes of OE transgenic lines and nullizygous (wild‐type, Wt) plants upon infection with FR13. (c) Basal OsWRKY22 expression levels in OE transgenic lines and nullizygous Wt plants before inoculation. The mean and standard deviation of three replicates are shown (*P < 0.05).
OsWRKY22 is repressed by virulent and induced by avirulent M. oryzae isolates
As wrky22 mutant plants and over‐expressing lines showed increased susceptibility and resistance to M. oryzae, respectively, the differential regulation of WRKY22 in rice in response to blast infection was investigated. Gene expression was measured in a time course experiment at early infection stages (between 0 and 24 hpi) using 2‐week‐old Nipponbare leaves challenged with virulent and avirulent isolates. The inoculation with the virulent isolate FR13 strongly repressed OsWRKY22 transcription at 8 hpi, whereas transcript levels between infected and mock plants were not statistically significantly different at the later time points. With regard to inoculation with the avirulent CL3.6.7 strain, OsWRKY22 was found to be statistically up‐regulated (compared with the mock plants) only at 12 hpi (Fig. 5).
Figure 5.
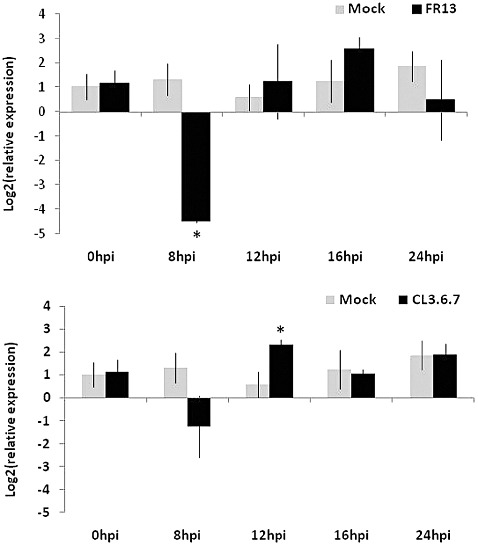
OsWRKY22 gene expression following Magnaporthe or mock inoculation by quantitative reverse transcription‐polymerase chain reaction (qRT‐PCR). The strains used were Magnaporthe FR13 (virulent) and CL3.6.7 (avirulent) isolates. Values represent the means of log2(relative expression) and standard deviation for four biological replicates. Statistical differences between mock and infected samples were assessed by t‐test analysis (*P < 0.05). All calculations for relative quantification were performed as described in Pfaffl (2001) and the reference gene used was actin (Os03g50885).
OsWRKY22 belongs to a monocot subgroup within group 3
To better describe OsWRKY22 in relation to its closest OsWRKY paralogues, the following in silico analyses were carried out: (i) hierarchical clustering analyses of rice WRKYs using full protein sequences; and (ii) neighbour‐joining (NJ) analysis of all‐against‐all blastp best results of the rice group 3 WRKY.
Using a novel modularity‐based clustering method, based on the full protein sequences and tuning to the median of the E‐value similarities in the entire dataset, the relationships between WRKY family members were obtained (Fig. 6a). The branches in the tree describe the hierarchical structure of the OsWRKY family divided into the three main groups (1, 2, 3) and several subgroups, supported by high modularity values at each node. This hierarchical clustering tree highlights, for the first time, the division of group 3 into three consistent subgroups (I, II and III).
Figure 6.
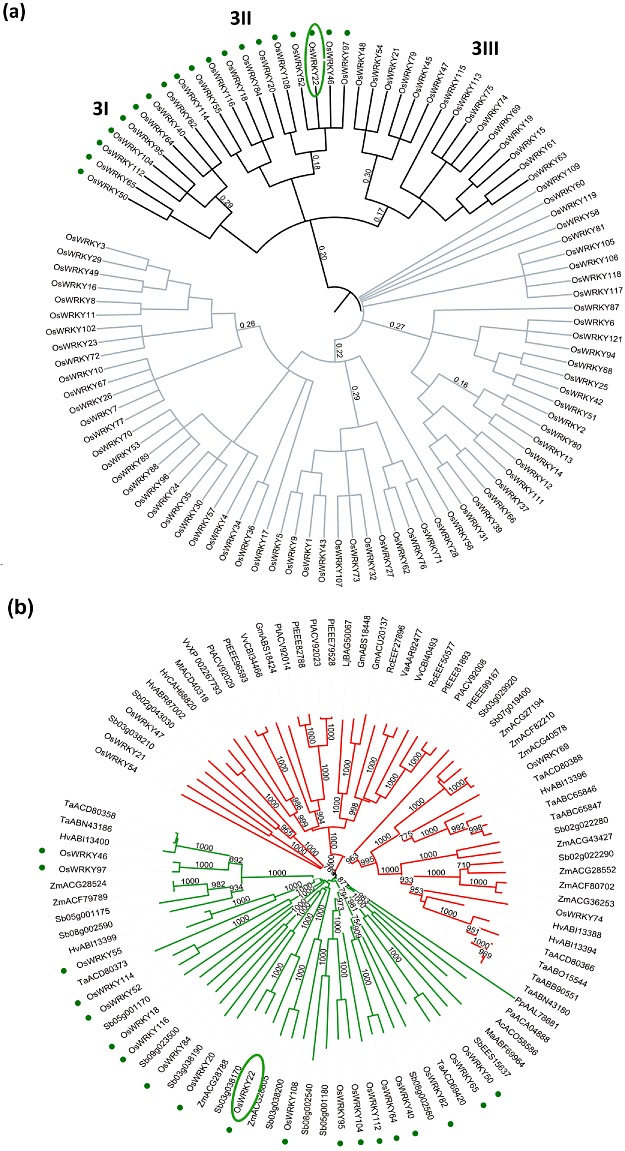
In silico analysis of the monocot‐specific OsWRKY proteins. (a) Hierarchical clustering tree of the OsWRKYs obtained using the full protein sequences. Subgroups 3I, 3II and 3III are the three subclusters within group 3 identified in this study. (b) Neighbour‐joining tree based on all‐against‐all blastp best hits (E‐values > 1e−30) of all group 3 OsWRKYs. The 19 WRKYs belonging to subgroups 3I and 3II (green dots) are identifiable within the monocot‐specific group (in green) bearing only sequences from cereals. Gm, Glycine max; Hv, Hordeum vulgare; Lj, Lotus japonica; Mt, Medicago truncatula; Pt, Populus trichocarpa; Rc, Ricinus communis; Sb, Sorghum bicolor; Ta, Triticum aestivum; Vv, Vitis vinifera; Zm, Zea mays. Outgroups: Ac, Areca catechu; Ma, Musa acuminate; Pa, Picea abies; Pp, Physcomitrella patens.
To assess which rice WRKYs had the closest homologues in monocot/dicot plants, an all‐against‐all blastp analysis of the whole OsWRKY family was carried out against the nonredundant protein databases. All OsWRKYs have dicotyledonous closest homologues, except for a small set within group 3. Subsequently, an NJ tree was built using the best hits (E‐values > 10−30) of OsWRKYs belonging to group 3 (Fig. 6b). Two divergent groups were identified: the first contained rice, sorghum, corn, wheat and barley proteins, and the second group included cereal WRKYs as well as proteins from various dicotyledonous plants (Arabidopsis, Vitis vinifera, Lotus japonica, Glycine max, Ricinus communis, Populus trichocarpa, Medicago truncatula). Nineteen OsWRKY proteins, including OsWRKY22, were closest homologues only to other cereal WRKYs and were split into two subgroups, in agreement with our hierarchical clustering analysis. We concluded that these 19 genes within group 3 can be referred to as monocot/cereal‐specific (MCS) OsWRKYs.
OsWRKY22 is poorly co‐regulated with MCS and OsWRKY members
To test whether OsWRKY22 is strongly co‐regulated with the other MCS OsWRKY members, we gathered a large set of Affymetrix expression data in a matrix [Rice Expression Matrix (REM)] and performed co‐regulation analysis using RI‐REG, a software tool specifically developed to calculate the Pearson coefficient. A stringent cut‐off (0.8) was applied during linear and logarithmic co‐regulation analysis to include only the genes best co‐regulated with the MCS WRKYs. Therefore, 37 genes (from 94 in the Affymetrix dataset) are present in the networks (Fig. 7), including the 14 MCS WRKY genes occurring in the Affymetrix chip.
Figure 7.
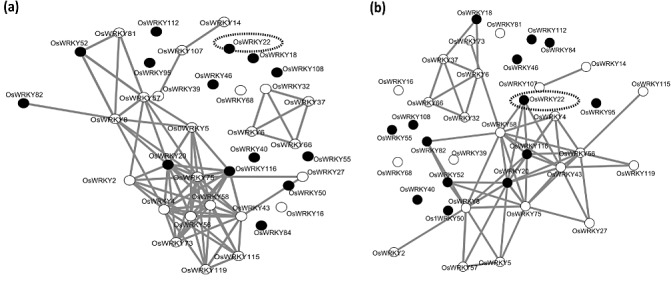
Co‐regulatory networks of the monocot/cereal‐specific (MCS) OsWRKY genes and other members of the OsWRKY family. (a) Linear Pearson analysis. (b) Logarithmic Pearson analysis. The 14 MCS OsWRKY genes (black nodes) and their best co‐regulated OsWRKYs (white nodes) are included in the graphical representation (lines connecting nodes represent Pearson coefficient threshold > 0.8).
Eighteen and four WRKY genes formed one major and one small network, respectively, with very similar structures in both P‐lin and P‐log analyses, but the number of connections decreased considerably in the P‐log network. The remaining 15 genes were outside these two main networks. In the P‐lin analysis, four MCS WRKYs (OsWRKY116, OsWRKY20, OsWRKY82, OsWRKY52) fell only into the major network (Fig. 7a). Most of the genes belonging to the major network were linked by multiple connections, including the four MCS WRKYs. Surprisingly, the MCS WRKY genes were not directly connected, except for OsWRKY116 and OsWRKY20. In the P‐log network (Fig. 7b), OsWRKY22 belonged to the major and OsWRKY18 to the small network, suggesting that these genes are co‐regulated with other rice WRKYs in specific conditions. OsWRKY22 was connected only with two MCS (OsWRKY116, OsWRKY20) and two other members of the rice WRKY family (OsWRKY58 and OsWRKY43). Increasing the cut‐off to 0.9, only OsWRKY116 and OsWRKY20 were connected in P‐lin, whereas no connections among MCS OsWRKY genes were found in the P‐log network (data not shown).
In conclusion, MCS WRKYs are poorly co‐regulated and especially only in specific conditions. OsWRKY116 and OsWRKY20 are the only two MCS WRKY genes strongly co‐regulated.
WRKY22 and MCS OsWRKY involvement in the blast response
To learn more about the potential involvement of OsWRKY22 and MCS WRKYs in response to different Magnaporthe isolates, the gene expression of 10 members (OsWRKY22, OsWRKY20, OsWRKY108, OsWRKY55, OsWRKY84, OsWRKY112, OsWRKY52, OsWRKY46, OsWRKY50, OsWRKY95) was measured by quantitative RT‐PCR. Nipponbare leaves challenged with M. oryzae virulent FR13, avirulent CL3.6.7, nonadapted BR32 and M. grisea nonhost isolate BR29 were collected at 12 hpi (before fungal penetration of leaf epidermal cells), 24 hpi (beginning of fungus penetration) and 48 hpi (multicellular propagation for host strains) after infection.
The OsWRKY22 transcript level was not differentially regulated on BR32 and BR29 infection at the considered time points. With regard to the other MCS WRKYs, eight of 10 genes were significantly induced or repressed following plant inoculation with virulent, avirulent and nonhost Magnaporthe strains (Fig. S1, see Supporting Information). In particular, some of them were involved in the rice response to several isolates at specific time points. OsWRKY112 and OsWRKY20 expression profiles did not show differences between infected and mock plants at the tested time points (data not shown).
Genes co‐expressed with OsWRKY22 putatively involved in the rice defence response
OsWRKY22 function was investigated, identifying genes co‐expressed with WRKY22 during the rice defence response. For this purpose, a subset of the Rice Expression Matrix, including only the experiments in biotic stress conditions (286 of 707), was inquired. The resulting 147 genes co‐regulated with OsWRKY22 (41% of those annotated) were classified into eight major classes in relation to their potential defence function in rice (Fig. 8 and Table S1, see Supporting Information). Disease resistance genes were the most abundant (20%), including mainly leucine‐rich repeat (LRR) genes. Mildew resistance locus a (Mla1 and Mla6) were also present. The pathogenesis‐related (PR)/PR‐like genes represented 9%, including mainly lipid transfer protein‐like (LTP), defensins and subtilisins. The defence‐related genes co‐expressed with OsWRKY22 (11%) included genes involved in the synthesis of antimicrobial molecules or pathogen recognition (flavonoids, extensin, terpenes, ankyrin, harpins, lectins). The cell wall‐related class represented only 2%. Genes related to primary/secondary metabolism constituted a high proportion of the co‐expressed genes (18%) and most were related to protein folding and degradation processes (bric‐a‐brac tramtrack broad complex, ubiquitin complex, proteases, speckle genes). Genes involved in signalling (13%) during pathogen detection, including kinase receptors and lipase/lipase‐like genes, were also co‐expressed with OsWRKY22. The hormone transduction class included only two genes related to ethylene and jasmonate synthesis. Among regulators (7%), the most abundant were MYB and helix–loop–helix (HLH) transcription factors. Genes indirectly involved in the pathogen response, such as the F‐box family, SNARE domain, flavin monooxygenase and reticuline oxidase‐like protein, were also present in the list of genes co‐expressed with OsWRKY22 (‘others’ in Fig. 8).
Figure 8.
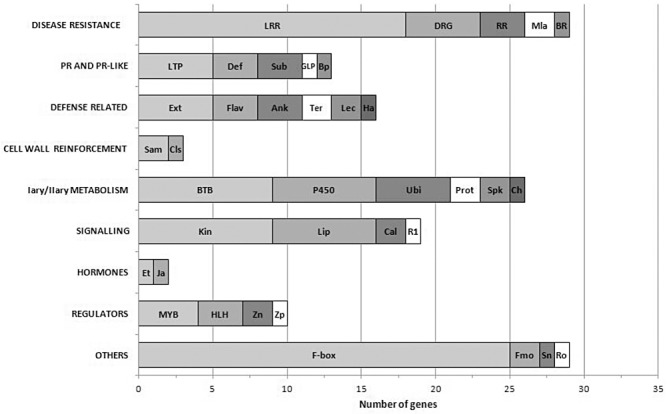
Bar charts of the genes co‐expressed with OsWRKY22 putatively involved in the rice defence response (147 genes) according to their known or predicted function. The number of genes is shown on the x‐axis and the main categories on the y‐axis. LRR: different categories of leucine‐rich repeat (LRR) resistance genes; DRG: defence‐related genes; RR: rust resistance; Mla: MLA protein; BR: blight resistance. LTP: LTP family; Def: defensins; Sub: subtilisins; GLP: germin‐like proteins; Bp: BURP domain. Ext: extensin family; Flav: flavonoids‐related genes; Ank: ankyrin repeat family; Ter: terpene synthases putative; Lec: lectin domain; Ha: harpin‐induced protein. Sam: SAM‐dependent carboxyl methyltransferase; Cls: cellulose synthase‐like family. BTB: bric‐a‐brac tramtrack broad complex; P450: cytochrome P450; Ubi: several proteins belonging to the ubiquitin complex; Prot: proteases; Spk: speckle‐type POZ proteins; Ch: chorismate mutase. Kin: different categories of receptor kinase; Lip: GDSL‐like lipases; Cal: calmodulin‐binding proteins; R1: RGA‐1. Et: 1‐aminocyclopropane‐1‐carboxylate oxidase; Ja: jasmonate O‐methyltransferase. MYB: MYB transcription factor; HLH: helix–loop–helix DNA‐binding domain; Zn: zinc finger C3HC4‐type domain; Zp: bZIP transcription factor domain. F‐box: F‐box domain; Fmo: flavin mono‐oxygenase; Sn: SNARE domain; Ro: reticuline oxidase‐like protein.
No OsWRKY genes were present among regulators. Interestingly, the two MCS OsWRKY116 and OsWRKY20, shown to be co‐regulated with OsWRKY22 in the P‐log network of this study, were not among the genes co‐expressed with OsWRKY22, when only the subset of biotic stress‐related experiments was analysed. This finding confirms that OsWRKY22 acts independently from other rice WRKYs as the master regulator of specific signal transduction pathways leading to defence‐related rice‐specific responses to fungal pathogens.
DISCUSSION
We have screened several knock‐down insertion lines corresponding to 23 WRKY genes from different phylogenetic groups to identify novel OsWRKY genes involved in plant innate immunity. Altered susceptibility to both virulent and avirulent M. oryzae strains was associated with typical blast lesions for the gene OsWRKY22.
At the cellular level, the wrky22 mutant plants were impaired in callose deposition in response to the avirulent M. oryzae and the nonhost M. grisea strains at 24 hpi, whereas higher callose deposition occurred in wrky22 mutants in response to Bgh. In plants, the first layer of defence encountered by pathogens is the cell wall (Thordal‐Christensen, 2003). When attacked, plants physically reinforce cell walls by lignification (Göhre and Robatzek, 2008) and the deposition of callose (Kudlicka and Brown, 1997). The OsWRKY22 protein does not seem to play the same role at early time points in response to penetration attempts of host and nonhost fungi, which may rely on a different elicitor repertoire of M. oryzae or Bgh. These differences most probably reflect the different lifestyles of the two pathogens, i.e. facultative versus obligate biotrophy. Ambivalent responses against these pathogens have been reported previously for barley (Jarosch et al., 1999; Zellerhoff et al., 2008). In addition, a significantly higher H2O2 production following inoculation with the avirulent M. oryzae strain, as well as with the nonhost Bgh isolate, was observed. This higher H2O2 accumulation was maintained only in the avirulent interaction at 48 hpi. Reactive oxygen species (ROS), including H2O2, are involved in rapid programmed cell death to block pathogen progression (Thordal‐Christensen et al., 1997), and are usually associated with plant resistance mechanisms (Shetty et al., 2008). However, ROS accumulation alone is often insufficient to ensure disease resistance (Heath, 2000; Hückelhoven, 2007). Despite the high level of H2O2 production, the wrky22 mutants also exhibited an increased number of blast lesions compared with wild‐type plants. This observation supports the hypothesis that, at late stages of infection, M. oryzae benefits from ROS‐induced cell death, because the pathogen switches to necrotrophy. A similar scenario has been described for the mlo mutant plants inoculated with M. oryzae strains (Jarosch et al., 1999). However, this enhanced H2O2 production boosts the notion that the avirulent blast pathogen and the nonhost Bgh fungus are able to penetrate more easily into wrky22 mutant epidermal cells, which then respond, activating a second layer of defence responses, i.e. hypersensitive response reflected by whole‐cell or multi‐cell DAB staining, as also reported for the Arabidopsis pen mutants (Collins et al., 2003; Lipka et al., 2005; Stein et al., 2006).
WRKY genes can function as either repressors or activators of different biological processes (Hu et al., 2008; 2004, 2006; Qiu et al., 2007; Wang et al., 2006) and respond to different pathogens (Tao et al., 2009). Our results revealed that OsWRKY22 is likely to play a central role not only in the regulation of upstream defence responses to virulent and avirulent rice Magnaporthe strains, but also in the rice response to the nonhost powdery mildew isolate.
We also showed that rice transgenic plants over‐expressing OsWRKY22 displayed an enhanced resistance to the virulent host M. oryzae strain. Several OsWRKYs were found to be involved in the rice response to Xanthomonas oryzae (Liu et al., 2007; Peng et al., 2008), Pseudomonas syringae DC3000 (Qiu and Yu, 2009) and M. oryzae (Chujo et al., 2007; Zhang et al., 2008). However, most of the enhanced resistance phenotypes mentioned above were observed in ectopic over‐expressing lines, as claimed by Pandey and Somssich (2009). In contrast, in the data presented here, rice plants showed opposite phenotypes to Magnaporthe virulent infection, depending on whether OsWRKY22 was knocked‐down or over‐expressed. The only other two WRKY genes previously presenting a different phenotype in mutant and over‐expressing lines were OsWRKY45 (Shimono et al., 2007) and OsWRKY89 (Wang et al., 2007).
In our previous phylogenetic analysis using Arabidopsis and rice WRKY domains, OsWRKY22 clustered in group 3 within a subgroup called 3C (Berri et al., 2009). Further analyses were performed in the present study to describe OsWRKY22 in relation to its subgroup. A novel clustering method was used to better define the hierarchical structure of group 3, as there is no consistency with regard to the number of its subclusters and genes so far (Berri et al., 2009; Wu et al., 2005; Xie et al., 2005; Zhang and Wang, 2005). The hierarchical OsWRKY tree was in good agreement with previous phylogenetic classifications (Berri et al., 2009; Ross et al., 2007; Wu et al., 2005). Nevertheless, it consistently established the partition of group 3 into three subgroups (3I, 3II and 3III), taking into account also single conserved residues/motifs. This approach is relevant when analysing poorly conserved proteins, such as WRKYs. The NJ tree of the all‐against‐all blastp showed that the 19 OsWRKYs belonging to subgroups 3I–3II had closest homologues only in monocot/cereal plants. Several authors have suggested a monocot/rice‐specific group of WRKY proteins (Berri et al., 2009; Mangelsen et al., 2008; Wu et al., 2005; Yang et al., 2009; Zhang and Wang, 2005); however, no detailed analyses demonstrating the split of this clade from the rest of the gene family had been carried out before that presented here.
We observed that 74% of MCS WRKYs are positioned in segmentally duplicated blocks (data not shown), whereas OsWRKY22, OsWRKY116, OsWRKY20 and OsWRKY108 are reported to be tandemly duplicated genes (Ramamoorthy et al., 2008; Ross et al., 2007; Xie et al., 2005). Although extensively duplicated, only a small portion of MCS OsWRKY genes were co‐expressed and only at specific conditions in our co‐regulation analysis. Nevertheless, the same genes were also connected to OsWRKY members of other phylogenetic groups, in agreement with the analysis for the Arabidopsis WRKY family (Berri et al., 2009). Although WRKY22 is involved in the blast response, as well as other monocot‐specific WRKYs (as suggested by the quantitative RT‐PCR data), this gene appears to play a unique and independent role in the rice defence response, as supported by the co‐regulatory network analysis and the list of genes co‐expressed with OsWRKY22 putatively involved in the response to biotic stress.
To understand the role of a gene, it is crucial to identify the genes associated with a specific regulatory network. Given the phenotype of wrky22 mutants following blast infection, we explored a subset of the Rice Expression Matrix involved in the pathogen response and identified a list of genes co‐expressed with OsWRKY22, which are likely to be putative targets, regulators or partners in its network. Indeed, similar correlation analyses have been shown previously to represent a powerful tool to investigate the gene function and to identify new candidate genes involved in specific cellular processes (Gigolashvili et al., 2009; Hirai et al., 2007; Murgia et al., 2011; Vandepoele et al., 2009). On the basis of the thorough analysis of the co‐expressed genes, we propose a putative defence signalling model involving OsWRKY22 (Fig. S2, see Supporting Information). Three interaction types are shown: virulent M. oryzae, avirulent M. oryzae and nonhost Bgh. Cell wall components and pathogen‐associated molecular patterns (PAMPs) are recognized by specific receptors that activate or repress OsWRKY22 transcription. These events stimulate or repress the induction of specific sets of defence‐related responses involved in the oxidative burst, cell wall reinforcement, secretion of antimicrobial molecules, protein degradation and activation of different transcription factors.
Further validation and investigations of the candidate genes described in the defence signalling model will be an intriguing research perspective. To this aim, high‐throughput technologies for expression (RNA‐seq) and interactions (ChIP‐Seq), modelling networks from sequences, and integrated expression and genetics approaches will facilitate the discovery of the biological functions of OsWRKY22 and other MCS WRKYs implicated in rice–blast (Pandey and Somssich, 2009; Roccaro and Somssich, 2011) as well as rice–powdery mildew interactions.
EXPERIMENTAL PROCEDURES
Identification of OsWRKY insertion mutants
Thirty‐five WRKY insertion mutants from the collections of the Génoplante Oryza Tag Line (http://oryzatagline.cirad.fr/) (Larmande et al., 2008; Sallaud et al., 2004) and National Institute of Agrobiological Sciences (http://tos.nias.affrc.go.jp/) (Miyao et al., 2007), corresponding to 23 different genes belonging to different phylogenetic groups (Table S2, see Supporting Information), were screened in our laboratory for rice–M. oryzae interaction. All mutant homozygous lines were derived by self‐pollination. The genotype of the progeny was determined by PCR analysis using the primers listed in Table S3 (see Supporting Information). Gene‐specific primers for screening wild‐type plants and T‐DNA/Tos17 border primer combined with the gene‐specific primer for screening homozygous lines were used. The number of T‐DNA/Tos17 inserts in the mutant lines was determined by Southern blot analysis (see Appendix S1).
Over‐expression of OsWRKY22
The open reading frame (ORF) of OsWRKY22 (Wa32) was amplified by hemi‐nested PCR using the following primer combinations: Wa32cloF1/R1 and Wa32cloF2/R1 (Table S3). Appropriately sized DNA fragments were gel purified (Machery Nagel, Düren, Germany) and then subcloned between the two BamHI sites of the pCR® 4 Blunt TOPO® Vector (Life Technologies Ltd., Paisley, UK). The binary vector of the OsWRKY22 ORF was obtained by ligation into the corresponding restriction sites of the multiple‐cloning site of pC2300 Ubi Tnos, a pCAMBIA2300 (CAMBIA, Canberra, Australia) derivative [J. C. Breitler, Centre de Coopération Internationale en Recherche Agronomique pour le Développement (CIRAD), Montpellier, France, unpublished data]. Positive clones were selected on Luria–Bertani (LB)–kanamycin plates after incubation at 37 °C overnight, and screened for inserts by colony PCR. The OsWRKY22 over‐expressing construct was transferred into the rice variety Nipponbare by Agrobacterium‐mediated transformation following the protocol of Toki et al. (2006). The copy number of each construct in transgenic plants was determined by Southern blot analysis.
DAB and aniline blue staining
The production of H2O2 and ROS was detected using the ‘DAB‐uptake method’ described by Thordal‐Christensen et al. (1997). The inoculated leaf samples of the wrky22 mutant and wild‐type were examined by light microscopy. Callose was stained by the method reported in Zellerhoff et al. (2008). Leaves were analysed by epifluorescence microscopy using a Leica (Leica Microsystems GmbH, Wetzlar, Germany) DMRBE microscope and a long‐pass filter (excitation filter, 340–380 nm; dichromatic mirror, 400 nm; suppression filter LP, 425 nm). A total of 100 infection sites was inspected per leaf and scored independently for both stains. Three leaves were analysed for each data point and the mean with standard deviation was calculated. Statistically significantly different comparisons between wild‐type and mutants were assessed by t‐test analysis.
Plant material and infection assays
All rice plants used in this study had the Nipponbare genetic background. Rice plants were grown according to Faivre‐Rampant et al. (2008). The isolates BR29, BR32, CL3.6.7 and FR13 were chosen from the Magnaporthe strain collection (CIRAD). Inoculation assays were carried out as described previously (Faivre‐Rampant et al., 2008). Macroscopic phenotypes (necrosis, lesions) on rice leaves were observed at 7 dpi.
Bgh was maintained on the compatible barley (Hordeum vulgare) line Golden Promise by weekly transfer. Inoculation assay on secondary leaves of 2‐week‐old rice plants was carried out in analogy with barley plants, as described previously by Zellerhoff et al. (2010). Rice leaves were examined for macroscopic lesions at 7 dpi.
Leaf samples were harvested at 24 and 48 hpi with Magnaporthe and Blumeria strains for microscopy, and at 24 hpi for transcriptome analysis.
Computational analysis of the group 3 WRKY protein sequences
A novel modularity‐based clustering was developed and applied to the 101 rice WRKY full protein sequences. The procedure consisted of several steps, including the use of blast E values as a proxy for similarity, the re‐scaling of E values into similarities using a sigmoid function centred around the median of the data and the repeated re‐clustering of clusters with a different median E value. Full details are presented in Appendix S1.
An all‐against‐all blastp search was conducted using the complete set of OsWRKY sequences in the query of the nonredundant protein databases of GenBank. Alignment of the 19 monocot‐specific OsWRKY proteins and their closest homologues (corresponding to E values < 10−30) was performed using clustalx 2. Bootstrap values were obtained using a random number generator seed of 111 and number of bootstrap trials of 1000. A tree image showing relationships among clusters was obtained using iTOL (http://itol.embl.de/index.shtml).
Transcriptome analysis
Three biological replicates for each interaction type and the corresponding mock were extracted and analysed independently by GeneChip® Rice Genome Array. Total RNA was isolated using TRIzol reagent (Invitrogen) following the manufacturer's instructions and purified using Qiagen RNeasy columns. RNA was quantified using a NanoDrop ND‐1000 spectrophotometer and quality was tested using a 2100 Bioanalyser (Agilent Technologies, Inc., Santa Clara, CA, USA). For Affymetrix GeneChip® analysis, 8 µg of total RNA were used to make biotin‐labelled cRNA targets. All the processes for cDNA and cRNA synthesis, cRNA fragmentation, hybridization, washing, staining and scanning were conducted according to the GeneChip® standard protocol (Eukaryotic Target Preparation, Affymetrix, Affymetrix Inc., Santa Clara, CA, USA). CEL files were included in the Rice Expression Matrix (see below) and analysed together with other raw expression data. Information on the GeneChip® Rice Genome Array can be found in the Affymetrix website http://www.affymetrix.com/estore/index.jsp.
Rice Expression Matrix and clustering analysis
As of October 2010, the Rice Expression Matrix consisted of 728 hybridizations and was constructed by gathering the publicly available Affymetrix experiments from platform GPL2025 in Gene Expression Omnibus (GEO; http://www.ncbi.nlm.nih.gov/geo/) and the 21 hybridizations carried out in this study. The raw microarray data were normalized using the RMA (Robust Multichip Average) Express program, as reported by Bolstad et al. (2003). Further details are given in Appendix S1.
Correlation analysis software (RI‐REG) and co‐regulatory networks
The Visual C++ program (RI‐REG), developed by Berri et al. (2009), was applied to analyse gene co‐regulation. The Rice Expression Matrix was used without further modification, scaling, array normalization or processing of replicates. The Pearson correlation coefficient for each gene pair was calculated using the linear values (linear analysis) or following log transformation (Menges et al., 2008). Graphical representations of WRKY gene networks were produced using igraph (http://igraph.sourceforge.net; Csárdi and Nepusz, 2006). More details are given in Appendix S1.
Real‐time quantitative RT‐PCR analysis
RNA (15 µg) was treated with DNase I (Fermentas, Burlington, Ontario, Canada), and 5 µg of RNA were denatured for 5 min at 65 °C in water with oligo(dT)18 (3.5 µm) and deoxynucleoside triphosphate (dNTP) (1.5 µm). Reverse transcription was carried out for 60 min at 37 °C with 200 U of reverse transcriptase M‐MLV (Promega, Madison, WI, USA). Two microlitres of cDNA (dilution 1:10) were then used for quantitative RT‐PCR. Forward and reverse primers are given in Table S3. All calculations for relative quantification were performed as described in Pfaffl (2001) using a mathematical model to determine the relative quantification of the target gene compared with the reference gene (actin) from inoculated leaves versus controls (mock). The mean and standard deviation of four biological replicates are reported. A t‐test was performed to establish whether infected plants were significantly different from mock plants (*P < 0.05).
Supporting information
Fig. S1 Monocot‐specific OsWRKY gene expression by quantitative reverse transcription‐polymerase chain reaction (qRT‐PCR) of 2‐week‐old Nipponbare leaves following Magnaporthe or gelatin inoculation at 12, 24 and 48 h post‐inoculation (hpi). The strains used were Magnaporthe FR13 (virulent), CL3.6.7 (avirulent), BR32 (nonadapted) and nonhost M. grisea BR29. Values represent the means of log2(relative expression) and standard deviation for four biological replicates. Statistical differences between mock and infected samples were assessed by t‐test analysis (*P < 0.05). All calculations for relative quantification were performed as described in Pfaffl (2001) and the reference gene used was actin (Os03g50885).
Fig. S2 Putative schematic overview of OsWRKY22 involvement in rice responses to blast and barley powdery mildew.
Table S1 List of genes co‐expressed with OsWRKY22 putatively involved in the rice defence response.
Table S2 List of insertion mutant lines analysed in this study.
Table S3 List of primers used in this study.
Appendix S1 Supporting methods.
Supporting info item
Supporting info item
Supporting info item
Supporting info item
Supporting info item
ACKNOWLEDGEMENTS
We acknowledge the Cariplo Foundation (Italy) for the funding of Riceimmunity (2005‐1866) and Dryrice (2008‐3179) projects. T.N. acknowledges the support of the Newton Fellowship Scheme of the Royal Society (grant number: NF080750). A.P. was supported by the Biotechnology and Biological Sciences Research Council (BBSRC) grant BB/F00964X/1. Part of this work was carried out on the RicE Functional GEnomics (REFUGE) platform funded by the Agropolis Foundation. We are grateful to Dr John Williams for critical reading of the manuscript. WRKY genes in the present study are named according to the nomenclature elaborated by the rice WRKY working group. During the revision process of this manuscript, a paper describing this new nomenclature has been published online in the journal RICE (Rice WRKY working group et al., 2012). We hope that this nomenclature will become generally acceptable to the scientific community.
REFERENCES
- Abuqamar, S. , Chen, X. , Dhawan, R. , Bluhm, B. , Salmeron, J. , Lam, S. , Dietrich, R.A. and Mengiste, T. (2006) Expression profiling and mutant analysis reveals complex regulatory networks involved in Arabidopsis response to Botrytis infection. Plant J. 48, 28–44. [DOI] [PubMed] [Google Scholar]
- Berri, S. , Abbruscato, P. , Faivre‐Rampant, O. , Brasileiro, A.C.M. , Fumasoni, I. , Satoh, K. , Kikuchi, S. , Mizzi, L. , Morandini, P. , Pè, M.E. and Piffanelli, P. (2009) Characterization of WRKY co‐regulatory networks in rice and Arabidopsis. BMC Plant Biol. 9, 120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolstad, B. , Irizarry, R. , Strand, M. and Speed, T. (2003) A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics, 19, 185–193. [DOI] [PubMed] [Google Scholar]
- Chujo, T. , Takai, R. , Akimoto‐Tomiyama, C. , Ando, S. , Minami, E. , Nagamura, Y. , Kaku, H. , Shibuya, N. , Yasuda, M. , Nakashita, H. , Umemura, K. , Okada, A. , Okada, K. , Nojiri, H. and Yamane, H. (2007) Involvement of the elicitor‐induced gene OsWRKY53 in the expression of defense related genes in rice. Biochim. Biophys. Acta, 1769, 497–505. [DOI] [PubMed] [Google Scholar]
- Collins, N.C. , Thordal‐Christensen, H. , Lipka, V. , Bau, S. , Kombrink, E. , Qiu, J.L. , Hückelhoven, R. , Stein, M. , Freialdenhoven, A. , Somerville, S.C. and Schulze‐Lefert, P. (2003) SNARE‐protein‐mediated disease resistance at the plant cell wall. Nature, 425, 973–977. [DOI] [PubMed] [Google Scholar]
- Csárdi, G. and Nepusz, T. (2006) The Igraph software package for complex network research. Int. J. Compl. Syst. 1695. [Google Scholar]
- Eulgem, T. , Rushton, P. , Robatzek, S. and Somssich, I. (2000) The WRKY superfamily of plant transcription factors. Trends Plant Sci. 5, 199–206. [DOI] [PubMed] [Google Scholar]
- Faivre‐Rampant, O. , Thomas, J. , Allègre, M. , Morel, J.‐B. , Tharreau, D. , Nottéghem, J.‐L. , Lebrun, M.‐H. , Schaffrath, U. and Piffanelli, P. (2008) Characterisation of the model system rice–Magnaporthe for the study of nonhost resistance in cereals. New Phytol. 180, 899–910. [DOI] [PubMed] [Google Scholar]
- Gigolashvili, T. , Yatusevich, R. , Rollwitz, I. , Humphry, M. , Gershenzon, J. and Flügge, U.I. (2009) The plastidic bile acid transporter 5 is required for the biosynthesis of methionine‐derived glucosinolates in Arabidopsis thaliana . Plant Cell, 21, 1813–1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Göhre, V. and Robatzek, S. (2008) Breaking the barriers: microbial effector molecules subvert plant immunity. Annu. Rev. Phytopathol. 46, 189–215. [DOI] [PubMed] [Google Scholar]
- Hara, K. , Yagi, M. , Kusano, T. and Sano, H. (2000) Rapid systemic accumulation of transcripts encoding a tobacco WRKY transcription factor upon wounding. Mol. Gen. Genet. 263, 30–37. [DOI] [PubMed] [Google Scholar]
- Heath, M.C. (2000) Nonhost resistance and non specific plant defenses. Curr. Opin. Plant Biol. 3, 315–319. [DOI] [PubMed] [Google Scholar]
- Hirai, M.Y. , Sugiyama, K. , Sawada, Y. , Tohge, T. , Obayashi, T. , Suzuki, A. , Araki, R. , Sakurai, N. , Suzuki, H. , Aoki, K. , Goda, H. , Nishizawa, O.I. , Shibata, D. and Saito, K. (2007) Omics‐based identification of Arabidopsis Myb transcription factors regulating aliphatic glucosinolate biosynthesis. Proc. Natl. Acad. Sci. USA, 104, 6478–6483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu, J. , Barlet, X. , Deslandes, L. , Hirsch, J. , Feng, D.X. , Somssich, I. and Marco, Y. (2008) Transcriptional responses of Arabidopsis thaliana during wilt disease caused by the soil‐borne phytopathogenic bacterium Ralstonia solanacearum . PLoS ONE, 3, e2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hückelhoven, R. (2007) Cell wall‐associated mechanisms of disease resistance and susceptibility. Annu. Rev. Phytopathol. 45, 101–127. [DOI] [PubMed] [Google Scholar]
- Hwang, S.H. , Won Yie, S. and Hwang, D.J. (2011) Heterologous expression of OsWRKY6 gene in Arabidopsis activates the expression of defense related genes and enhances resistance to pathogens. Plant Sci. 181, 316–323. [DOI] [PubMed] [Google Scholar]
- Jarosch, B. , Kogel, K.H. and Schaffrath, U. (1999) The ambivalence of the barley Mlo locus: mutations conferring resistance against powdery mildew (Blumeria graminis f. sp. hordei) enhance susceptibility to the rice blast fungus Magnaporthe grisea . Mol. Plant–Microbe Interact. 12, 508–514. [Google Scholar]
- Journot‐Catalino, N. , Somssich, I.E. , Roby, D. and Kroj, T. (2006) The transcription factors WRKY11 and WRKY17 act as negative regulators of basal resistance in Arabidopsis thaliana . Plant Cell, 18, 3289–3302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoth, C. , Ringler, J. , Dangl, J. and Eulgem, T. (2007) Arabidopsis WRKY70 is required for full RPP4‐mediated disease resistance and basal defense against Hyaloperonospora parasitica . Mol. Plant–Microbe Interact. 20, 120–128. [DOI] [PubMed] [Google Scholar]
- Kudlicka, K. and Brown Jr, R.M. (1997) Cellulose and callose biosynthesis in higher plants I. Solubilization and separation of (1‐>3)‐ and (1‐>4)‐[beta]‐glucan synthase activities from mung bean. Plant Physiol. 115, 643–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larmande, P. , Gay, C. , Lorieux, M. , Perin, C. , Bouniol, M. , Droc, G. , Sallaud, C. , Perez, P. , Barnola, I. , Biderre‐Petit, C. , Martin, J. , Morel, J.B. , Johnson, A.A.T. , Bourgis, F. , Ghesquiere, A. , Ruiz, M. , Courtois, B. and Guiderdoni, E. (2008) Oryza Tag Line, a phenotypic mutant database for the Genoplante rice insertion line library. Nucleic Acids Res. 36, D1022–D1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, J. , Brader, G. and Palva, E.T. (2004) The WRKY70 transcription factor: a node of convergence for jasmonate‐mediated and salicylate‐mediated signals in plant defense. Plant Cell, 16, 319–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, J. , Brader, G. , Kariola, T. and Palva, E.T. (2006) WRKY70 modulates the selection of signaling pathways in plant defense. Plant J. 46, 477–491. [DOI] [PubMed] [Google Scholar]
- Lipka, V. , Dittgen, J. , Bednarek, P. , Bhat, R. , Wiermer, M. , Stein, M. , Landtag, J. , Brandt, W. , Rosahl, S. , Scheel, D. , Llorente, F. , Molina, A. , Parker, J. , Somerville, S. and Schulze‐Lefert, P. (2005) Pre‐ and postinvasion defenses both contribute to nonhost resistance in Arabidopsis. Science, 310, 1180–1183. [DOI] [PubMed] [Google Scholar]
- Lippok, E. , Birkenbihl, R.P. , Rivory, G. , Brümmer, J. , Schmelzer, E. , Logemann, E. and Somssich, I.E. (2007) Expression of AtWRKY33 encoding a pathogen or PAMP‐responsive WRKY transcription factor is regulated by a composite DNA motif containing W box elements. Mol. Plant–Microbe Interact. 20, 420–429. [DOI] [PubMed] [Google Scholar]
- Liu, X. , Bai, X. , Wang, X. and Chu, C. (2007) OsWRKY71, a rice transcription factor, is involved in rice defense response. J. Plant Physiol. 164, 969–979. [DOI] [PubMed] [Google Scholar]
- Mangelsen, E. , Kilian, J. , Berendzen, K.W. , Kolukisaoglu, U.H. , Harter, K. , Jansson, C. and Wanke, D. (2008) Phylogenetic and comparative gene expression analysis of barley (Hordeum vulgare) WRKY transcription factor family reveals putatively retained functions between monocots and dicots. BMC Genomics, 9, 194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao, G. , Meng, X. , Liu, Y. , Zheng, Z. , Chen, Z. and Zhang, S. (2011) Phosphorylation of a WRKY transcription factor by two pathogen‐responsive MAPKs drives phytoalexin biosynthesis in Arabidopsis. Plant Cell, 23, 1639–1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marè, C. , Mazzucotelli, E. , Crosatti, C. , Francia, E. , Stanca, A. and Cattivelli, L. (2004) Hv‐WRKY38: a new transcription factor involved in cold and drought‐response in barley. Plant Mol. Biol. 55, 399–416. [DOI] [PubMed] [Google Scholar]
- Menges, M. , Dóczi, R. , Ökrész, L. , Morandini, P. , Mizzi, L. , Soloviev, M. , Murray, J.A.H. and Bögre, L. (2008) Comprehensive gene expression atlas for the Arabidopsis MAP kinase signalling pathways. New Phytol. 179, 643–662. [DOI] [PubMed] [Google Scholar]
- Miyao, A. , Iwasaki, Y. , Kitano, H. , Itoh, J.I. , Maekawa, M. , Murata, K. , Yatou, O. , Nagato, Y. and Hirochika, H. (2007) A large‐scale collection of phenotypic data describing an insertional mutant population to facilitate functional analysis of rice genes. Plant Mol. Biol. 63, 625–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murgia, I. , Tarantino, D. , Soave, C. and Morandini, P. (2011) Arabidopsis CYP82C4 expression is dependent on Fe availability and circadian rhythm, and correlates with genes involved in the early Fe deficiency response. J. Plant Physiol. 168, 894–902. [DOI] [PubMed] [Google Scholar]
- Murray, S.L. , Ingle, R.A. , Petersen, L.N. and Denby, K.J. (2007) Basal resistance against Pseudomonas syringae in Arabidopsis involves WRKY53 and a protein with homology to a nematode resistance protein. Mol. Plant–Microbe Interact. 20, 1431–1438. [DOI] [PubMed] [Google Scholar]
- Pandey, S.P. and Somssich, I.E. (2009) The role of WRKY transcription factors in plant immunity. Plant Physiol. 150, 1648–1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng, Y. , Bartley, L.E. , Chen, X. , Dardick, C. , Chern, M. , Ruan, R. , Canlasa, P.E. and Ronald, P.C. (2008) OsWRKY62 is a negative regulator of basal and Xa21‐mediated defense against Xanthomonas oryzae pv. oryzae in rice. Mol. Plant, 1, 446–458. [DOI] [PubMed] [Google Scholar]
- Peng, Y. , Bartley, L.E. , Canlasa, P.E. and Ronald, P.C. (2010) OsWRKY IIa transcription factors modulate rice innate immunity. Rice 3, 36–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffl, M. (2001) A new mathematical model for relative quantification in real‐time RT‐PCR. Nucleic Acids Res. 29, 2002–2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pnueli, L. , Hallak‐Herr, E. , Rozenberg, M. , Cohen, M. , Goloubinoff, P. , Kaplan, A. and Mittler, R. (2002) Molecular and biochemical mechanisms associated with dormancy and drought tolerance in the desert legume Retama raetam . Plant J. 31, 319–330. [DOI] [PubMed] [Google Scholar]
- Qiu, D. , Xiao, J. , Ding, X. , Xiong, M. , Cai, M. , Cao, Y. , Li, X. , Xu, C. and Wang, S. (2007) OsWRKY13 mediates rice disease resistance by regulating defense‐related genes in salicylate‐ and jasmonate‐dependent signaling. Mol. Plant–Microbe Interact. 20, 492–499. [DOI] [PubMed] [Google Scholar]
- Qiu, Y. and Yu, D. (2009) Over‐expression of the stress‐induced OsWRKY45 enhances disease resistance and drought tolerance in Arabidopsis. Environ. Exp. Bot. 65, 35–47. [Google Scholar]
- Ramamoorthy, R. , Jiang, S.‐Y. , Kumar, N. , Venkatesh, P.N. and Ramachandran, S. (2008) A comprehensive transcriptional profiling of the WRKY gene family in rice under various abiotic and phytohormone treatments. Plant Cell Physiol. 49, 865–879. [DOI] [PubMed] [Google Scholar]
- Rice WRKY Working Group, Shen , Shen, Q.J. , Yu, D. , Jeon, J.S. , Piffanelli, P. , Abbruscato, P. , Guo, Z.J. , Zhang, Y. , Itoh, T. , Lee, S.S. , Buell, R. , Nagato, Y. , McCouch S., Yano, M. , Wang, G.L. , Jena, K.K. , Xiong, L. , Meyers, B. , Jaiswal, P. and Yamazaki, Y. (2012) Nomenclature report on rice WRKY's. Conflict regarding gene names and its solution. Rice, 5, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roccaro, M. and Somssich, I.E. (2011) Chromatin immunoprecipitation to identify global targets of WRKY transcription factor family members involved in plant immunity. Methods Mol. Biol. 712, 45–58. [DOI] [PubMed] [Google Scholar]
- Ross, C.A. , Liu, Y. and Shen, Q.J. (2007) The WRKY gene family in rice (Oryza sativa). J. Integr. Plant Biol. 49, 827–842. [Google Scholar]
- Ryu, H. , Han, M. , Lee, S. , Cho, J. , Ryoo, N. , Heu, S. , Lee, Y. , Bhoo, S. , Wang, G. , Hahn, T. and Jeon, J. (2006) A comprehensive expression analysis of the WRKY gene superfamily in rice plants during defense response. Plant Cell Rep. 25, 836–847. [DOI] [PubMed] [Google Scholar]
- Sallaud, C. , Gay, C. , Larmande, P. , Bès, M. , Piffanelli, P. , Piégu, B. , Droc, G. , Regad, F. , Bourgeois, E. , Meynard, D. , Périn, C. , Sabau, X. , Ghesquière, A. , Glaszmann, J.C. , Delseny, M. and Guiderdoni, E. (2004) High throughput T‐DNA insertion mutagenesis in rice: a first step towards in silico reverse genetics. Plant J. 39, 450–464. [DOI] [PubMed] [Google Scholar]
- Shetty, N.P. , Jørgensen, H.J.L. , Jensen, J.D. , Collinge, D.B. and Shetty, H.S. (2008) Roles of reactive oxygen species in interactions between plants and pathogens. Eur. J. Plant Pathol. 121, 267–280. [Google Scholar]
- Shimono, M. , Sugano, S. , Nakayama, A. , Jiang, C.‐J. , Ono, K. , Toki, S. and Takatsuji, H. (2007) Rice WRKY45 plays a crucial role in benzothiadiazole inducible blast resistance. Plant Cell, 19, 2064–2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein, M. , Dittgen, M. , Sanchez‐Rodriguez, C. , Hou, B.‐H. , Molina, A. , Schulze‐Lefert, P. , Lipka, V. and Somerville, S. (2006) Arabidopsis PEN3/PDR8, an ATP binding cassette transporter, contributes to nonhost resistance to inappropriate pathogens that enter by direct penetration. Plant Cell, 18, 731–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao, Z. , Liu, H. , Qiu, D. , Zhou, Y. , Li, X. , Xu, C. and Wang, S. (2009) A pair of allelic WRKY genes play opposite roles in rice–bacteria interactions. Plant Physiol. 151, 936–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thordal‐Christensen, H. (2003) Fresh insights into processes of nonhost resistance. Curr. Opin. Plant Biol. 6, 351–357. [DOI] [PubMed] [Google Scholar]
- Thordal‐Christensen, H. , Zhang, Z. , Wei, Y. and Collinge, D.B. (1997) Subcellular localization of H2O2 in plants. H2O2 accumulation in papillae and hypersensitive response during the barley–powdery mildew interaction. Plant J. 11, 1187–1194. [Google Scholar]
- Toki, H. , Hara, N. , Ono, K. , Onodera, H. , Tagiri, A. , Oka, S. and Tanaka, H. (2006) Early infection of scutellum tissue with Agrobacterium allows high‐speed transformation of rice. Plant J. 47, 969–976. [DOI] [PubMed] [Google Scholar]
- Vandepoele, K. , Quimbaya, M. , Casneuf, T. , De Veylder, L. and Van de Peer, Y. (2009) Unraveling transcriptional control in Arabidopsis using cis‐regulatory elements and coexpression networks. Plant Physiol. 150, 535–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, D. , Amornsiripanitch, N. and Dong, X. (2006) A genomic approach to identify regulatory nodes in the transcriptional network of systemic acquired resistance in plants. PLoS Pathog. 2, e123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, H. , Hao, J. , Chen, X. , Hao, Z. , Wang, X. , Lou, Y. , Peng, Y. and Guo, Z. (2007) Over‐expression of rice WRKY89 enhances ultraviolet B tolerance and disease resistance in rice plants. Plant Mol. Biol. 65, 799–815. [DOI] [PubMed] [Google Scholar]
- Wu, K. , Guo, Z. , Wang, H. and Li, J. (2005) The WRKY family of transcription factors in rice and Arabidopsis and their origins. DNA Res. 12, 9–26. [DOI] [PubMed] [Google Scholar]
- Xie, Z. , Zhang, Z. , Zou, X. , Huang, J. , Ruas, P. , Thompson, D. and Shen, Q. (2005) Annotations and functional analyses of the rice WRKY gene superfamily reveal positive and negative regulators of abscisic acid signaling in aleurone cells. Plant Physiol. 137, 176–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, X. , Chen, C. , Fan, B. and Chen, Z. (2006) Physical and functional interactions between pathogen‐induced Arabidopsis WRKY18, WRKY40, and WRKY60 transcription factors. Plant Cell, 18, 1310–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, B. , Jiang, Y. , Rahman, M.H. , Deyholos, M.K. and Kav, N.N. (2009) Identification and expression analysis of WRKY transcription factor genes in canola (Brassica napus L.) in response to fungal pathogens and hormone treatments. BMC Plant Biol. 9, 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zellerhoff, N. , Jansen, M. and Schaffrath, U. (2008) Barley Rom1 antagonizes Rar1 function in Magnaporthe oryzae infected leaves by enhancing epidermal and diminishing mesophyll defence. New Phytol. 180, 702–710. [DOI] [PubMed] [Google Scholar]
- Zellerhoff, N. , Himmelbach, A. , Dong, W. , Bieri, S. , Schaffrath, U. and Schweizer, P. (2010) Nonhost resistance of barley to different fungal pathogens is associated with largely distinct, quantitative transcriptional responses. Plant Physiol. 152, 2053–2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, J. , Peng, Y. and Guo, Z. (2008) Constitutive expression of pathogen inducible OsWRKY31 enhances disease resistance and affects root growth and auxin response in transgenic rice plants. Cell Res. 18, 508. [DOI] [PubMed] [Google Scholar]
- Zhang, Y. and Wang, L. (2005) The WRKY transcription factor superfamily: its origin in eukaryotes and expansion in plants. BMC Evol. Biol. 5, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng, Z. , Qamar, S. , Chen, Z. and Mengiste, T. (2006) Arabidopsis WRKY33 transcription factor is required for resistance to necrotrophic fungal pathogens. Plant J. 48, 592–605. [DOI] [PubMed] [Google Scholar]
- Zheng, Z. , Mosher, S.L. , Fan, B. , Klessig, D.F. and Chen, Z. (2007) Functional analysis of Arabidopsis WRKY25 transcription factor in plant defense against Pseudomonas syringae . BMC Plant Biol. 7, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 Monocot‐specific OsWRKY gene expression by quantitative reverse transcription‐polymerase chain reaction (qRT‐PCR) of 2‐week‐old Nipponbare leaves following Magnaporthe or gelatin inoculation at 12, 24 and 48 h post‐inoculation (hpi). The strains used were Magnaporthe FR13 (virulent), CL3.6.7 (avirulent), BR32 (nonadapted) and nonhost M. grisea BR29. Values represent the means of log2(relative expression) and standard deviation for four biological replicates. Statistical differences between mock and infected samples were assessed by t‐test analysis (*P < 0.05). All calculations for relative quantification were performed as described in Pfaffl (2001) and the reference gene used was actin (Os03g50885).
Fig. S2 Putative schematic overview of OsWRKY22 involvement in rice responses to blast and barley powdery mildew.
Table S1 List of genes co‐expressed with OsWRKY22 putatively involved in the rice defence response.
Table S2 List of insertion mutant lines analysed in this study.
Table S3 List of primers used in this study.
Appendix S1 Supporting methods.
Supporting info item
Supporting info item
Supporting info item
Supporting info item
Supporting info item


