Summary
Xanthomonas oryzae pv. oryzicola (Xoc) is the causal agent of bacterial leaf streak, a devastating disease in rice. Xoc uses a type III secretion (T3S) system, which is encoded by the hrp–hrc–hpa (hypersensitive response and pathogenicity, hrp‐conserved and hrp‐associated) genes, to inject repertoires of T3S effectors (T3Es) into plant cells. Many of the hrp–hrc–hpa genes have roles in pathogenesis, but the role of hrpE3, which shows homology to hpaE in X. campestris pv. vesicatoria (Xcv), is poorly understood. In this study, hrpE3 was shown to be transcribed independent of the hrpD operon, and its expression was dependent on a promoter within hpaB. The expression of hrpE3 was positively regulated by HrpG and HrpX, a finding probably caused by an imperfect plant‐inducible promoter (PIP) box (TTCGT‐N16‐TTCGA) in the hrpE3 promoter. The secretion of HrpE3 was dependent on T3S, and subcellular localization of HrpE3 was cytoplasmic and nuclear in plant cells. A mutation in hrpE3 reduced the virulence of Xoc by decreasing disease lesion length and bacterial growth in planta. Full virulence was restored to the mutant when Xoc hrpE3, but not Xcv hpaE, was expressed in trans. The differences in transcription, secretion via the T3S system and bacterial virulence in plants were attributed to N‐terminal amino acid differences between Xoc HrpE3 and Xcv HpaE. Collectively, the results demonstrate that hrpE3 encodes a T3E protein which is delivered into the plant cell through the T3S system, localizes to the cytoplasm and nucleus, and is required for full virulence in rice.
Introduction
In Gram‐negative phytopathogenic bacteria, the hypersensitive response and pathogenicity (hrp) genes are normally required for the activation of defence, e.g. the hypersensitive response (HR), in nonhost plants, and for parasitic growth in susceptible host plants. The hrp genes encode a type III secretion (T3S) system, which enables a bacterium to deliver T3S effector proteins (T3Es) into plant cells (Alfano and Collmer, 1997; Cunnac et al., 2009; Gürlebeck et al., 2006; He, 1998; Li et al., 2011b). The hrp genes in Xanthomonas species have been classified as hrp group II, which differs from hrp group I of Erwinia amylovora and Pseudomonas syringae (Alfano and Collmer, 1997; Tang et al., 2006). The elucidation of the hrp–hrc–hpa cluster in Xanthomonas oryzae pv. oryzicola (Xoc) strain RS105 (Li et al., 2011a; Zou et al., 2006) and the completed genome sequence of Xoc BLS256 (Bogdanove et al., 2011) have revealed that the core hrp cluster contains 10 hrp, nine hrc (hrp‐conserved) and eight hpa (hrp‐associated) genes. The organization of the Xoc hrp cluster is similar to that of X. oryzae pv. oryzae (Xoo) (Cho et al., 2008; Lee et al., 2005; Ochiai et al., 2005; Salzberg et al., 2008), X. campestris pv. vesicatoria (Xcv) (Bonas et al., 2000), X. campestris pv. campestris (Xcc) and X. axonopodis pv. citri (Xac) (da Silva et al., 2002). Although there is shared synteny in hrp genes of closely related Xanthomonas spp., only Xoc contains hrpE3, which is homologous to hpaE in Xcv (Büttner et al., 2007; Weber et al., 2007). In Xcv, hpaE is not shown to be secreted via the T3S system, but its expression is dependent on HrpG and HrpX (Büttner et al., 2007). In Xoc, hrpE3 is flanked by hpaB and hpa4 in the hrp–hrc–hpa cluster (Li et al., 2011a; Zou et al., 2006). It is unclear whether hrpE3 is independently transcribed or co‐transcribed as part of an operon.
The expression of the hrp–hrc–hpa genes is generally suppressed in rich media, but highly induced in planta and in hrp‐inducing media (Brito et al., 1999; Schulte and Bonas, 1992; Wei et al., 2000; Xiao et al., 1992, 2007). Typically, the expression of hrp–hrc–hpa genes is controlled by two key regulatory genes: hrpG and hrpX (Büttner and Bonas, 2006; Kim et al., 2003; Tang et al., 2006; Zou et al., 2006). HrpG is related to OmpR, a response regulatory protein in the highly conserved, two‐component signal transduction paradigm. Presumably, HrpG perceives an environmental signal via a histidine protein kinase (HPK), but the cognate HPK for HrpG has not been identified (Wengelnik et al., 1996, 1999). HrpX is an AraC‐type transcriptional activator (Wengelnik and Bonas, 1996), which forms a homodimer and contains a helix–turn–helix (HTH) motif. The HTH motif interacts with the plant‐inducible promoter (PIP) box (TTCGC‐N15‐TTCGC) in hrp transcripts by binding and activating transcription (Furutani et al., 2006; Koebnik et al., 2006). The PIP box was later described as TTCGB‐N15‐TTCGB, where ‘B’ refers to any base except adenine (Cunnac et al., 2004; Mole et al., 2007; Tsuge et al., 2005).
In Xanthomonas, HrpG and HrpX form a regulatory cascade in which HrpG regulates the expression of the hrpA operon and hrpX. HrpX subsequently activates the expression of PIP box‐containing hrp transcripts (hrpB to hrpF) (Wengelnik and Bonas, 1996; Wengelnik et al., 1996, 1999) and other HrpX‐regulated genes (Furutani et al., 2009; Jiang et al., 2009b; Li et al., 2012). Another cis element resembling the −10 binding sequence (YANNRT: Y, C/T; N, A/T/G/C; R, A/G) of RNA polymerase σ70 factor is present 30–32 bp downstream of the PIP box in Xoo (Furutani et al., 2006). However, the intergenic region between hpaB and hrpE3 in Xoc is only 38 nucleotides and does not contain the PIP or −10 box (Li et al., 2011a; Zou et al., 2006). It is unclear whether a PIP box and/or σ70‐binding sequence is present further upstream of hrpE3.
In addition to the PIP box, some T3Es regulated by HrpX have the following characteristics: (i) their expression is co‐regulated with hrp genes (Tang et al., 2006); and (ii) the first 50 N‐terminal amino acids of the translated genes contain T3S signals [containing 20% serine (Ser) or proline (Pro) residues] (Furutani et al., 2009; Schechter et al., 2004). T3Es in Xoo, Ralstonia solanacearum and P. syringae are enriched in Ser and Pro residues in the first 50 N‐terminal amino acids (Alfano and Collmer, 2004; Cunnac et al., 2004; Furutani et al., 2009; Schechter et al., 2006; Tampakaki et al., 2004). The transcription and translocation of T3Es have been examined using several reporter systems, including the calmodulin‐dependent adenylate cyclase (Cya) from Bordetella pertussis (Casper‐Lindley et al., 2002; Cunnac et al., 2004; Schechter et al., 2006), the glucuronidase protein encoded by gusA (Mukaihara et al., 2010) and avirulence proteins lacking T3S signal sequences, e.g. AvrBs1 (Jiang et al., 2009b; Roden et al., 2004) and AvrXa10 (Li et al., 2011b). It remains unclear whether HrpE3 is secreted via the T3S system in a manner consistent with other T3Es.
Although hpa gene products contribute to virulence, hpa mutations generally have a mild effect on disease severity compared with other hrp–hrc mutations (Cho et al., 2008; Huguet et al., 1998; Kim et al., 2003; Li et al., 2011a; Zou et al., 2006). However, some Hpa proteins are indispensable for pathogenicity, including HpaB and HpaC, which work as exit control proteins for the secretion of some T3Es into plant cells (Büttner et al., 2004; Gürlebeck et al., 2006; Li et al., 2011b). Generally, T3Es in Xanthomonas spp. are classified into two types: TALEs (transcriptional activator‐like effectors) and NTALEs (non‐TAL effectors) (Jiang et al., 2009b; Song and Yang, 2010; Yang and White, 2004). tale genes, such as avrXa10, trigger HR in an HpaB‐dependent manner in rice cultivars containing the corresponding resistance (R) genes (Cunnac et al., 2009; Gürlebeck et al., 2006; Sugio et al., 2005). This avirulence (avr)–R gene‐mediated response has been used previously to explore the secretion of NTALEs by fusing the first 50 amino acids of NTALES with an N‐terminally‐truncated AvrXa10 (Li et al., 2011b).
In this study, we investigated the transcription, regulation and secretion of HrpE3. The contribution of hrpE3 to virulence was investigated using a genetic approach, and the gene was shown to be required for a full level of pathogenicity in the Xoc–rice interaction.
Results
hrpE3 is required for full virulence in rice and cannot be replaced by hpaE from Xcv
Sequence analysis of the hpaB–hpa4 intergenic region in Xoc RS105 led to the identification of hrpE3 (Zou et al., 2006), which is homologous to hpaE, a virulence factor in Xcv (Büttner et al., 2007). To determine whether hrpE3 is required for virulence in Xoc, we constructed the hrpE3 deletion mutant RΔhrpE3 (Table 1; Fig. S1, see Supporting Information). The wild‐type strain RS105 and mutant RΔhrpE3 were inoculated to seedlings and adult plants of the susceptible rice line IR24. Strain RΔhrpE3 displayed significantly (t‐test, P = 0.01) smaller lesions (Fig. 1A,B) and reduced bacterial growth (Fig. 1C) relative to the wild‐type. When hrpE3 (under the control of a 397‐bp promoter region, see construct pP1HrpE3‐c‐Myc in Table 1) was introduced into the deletion mutant, virulence was restored (see strain designated CRΔhrpE3, Fig. 1).
Table 1.
Strains and plasmids used in this study
| Strains or plasmids | Relevant characteristics | Reference or source |
|---|---|---|
| Xanthomonas oryzae pv. oryzae | ||
| PXO99A | Wild‐type, Philippine race 6 | This laboratory |
| PΔhrcU | hrcU deletion mutant of strain PXO99A | Li et al. (2011b) |
| Xanthomonas oryzae pv. oryzicola | ||
| RS105 | Wild‐type, Chinese race 2; Rifr | Zou et al. (2006) |
| RΔhrpE3 | hrpE3 deletion mutant of strain RS105; Rifr | This work |
| CRhrpE3 | RΔhrpE3 containing pP2HrpE3‐c‐Myc; Rifr Kmr | This work |
| CRhrpE3(201hpaE) | RΔhrpE3 containing pP2HpaE‐c‐Myc; Rifr Kmr | This work |
| RΔhrcC | hrcC deletion mutant of strain RS105; Rifr | Li et al. (2011a) |
| RΔhrpG | hrpG deletion mutant of strain RS105, Rifr | Jiang et al. (2009a) |
| RΔhrpX | hrpX deletion mutant of strain RS105; Rifr | Jiang et al. (2009a) |
| RΔhpaB | hpaB deletion mutant of strain RS105, Rifr | Li et al. (2011b) |
| RΔhrcV | hrcV deletion mutant of strain RS105; Rifr | This laboratory |
| RΔhrpD5 | hrpD5 deletion mutant of strain RS105, Rifr | Li et al. (2011a) |
| RΔhrpD6 | hrpD6 deletion mutant of strain RS105, Rifr | Li et al. (2011a) |
| RΔhrpE | hrpE deletion mutant of strain RS105, Rifr | Li et al. (2011a) |
| Xanthomonas campestris pv. vesicatoria | ||
| 23‐1 | Wild‐type, Chinese race | Laboratory collection |
| Escherichia coli | ||
| DH5α |
F− Φ80dlacZ ΔM15Δ (lacZYA‐argF)U169 endA1 deoR recA1 hsdR17(rK
− mK+) phoA supE44
λ− thi‐l gyrA96 relA1 |
Clontech |
| Agrobacterium tumefaciens | ||
| EH105 | Rifr | This laboratory |
| Plasmids | ||
| pMD18‐T | pUC ori, cloning vector; Apr | TaKaRa |
| pUFR034 | IncW, Mob(p), Mob −, LacZa +, PK2 replicon, cosmid; Kmr | De Feyter et al., 1990 |
| pKMS1 | 6.4‐kb derivative from pK18mobGII, sacB +; Kmr | Zou et al., 2011 |
| pKΔhrpE3 | 1250‐bp fusion ligated in pKMS1 with a 285‐bp deletion in hrpE3; Kmr | This work |
| pP1HrpE3‐c‐Myc | pUFR034 expressing hrpE3 under the control of its own 397‐bp promoter with a c‐Myc tag; Kmr | This work |
| pP2HrpE3‐c‐Myc | pUFR034 expressing hrpE3 under the control of its own 201‐bp promoter with a c‐Myc tag; Kmr | This work |
| pP3HrpE3‐c‐Myc | pUFR034 expressing hrpE3 under the control of its own 101‐bp promoter with a c‐Myc tag; Kmr | This work |
| p987HpaE‐c‐myc | pUFR034 expressing hpaE under the control of its own 987‐bp promoter with a c‐Myc tag; Kmr | This work |
| pP1HpaE‐c‐myc | pUFR034 expressing hpaE under the control of hrpE3 398‐bp promoter with a c‐Myc tag; Kmr | This work |
| pP2HpaE‐c‐myc | pUFR034 expressing hpaE under the control of hrpE3 201‐bp promoter with a c‐Myc tag; Kmr | This work |
| pP2nE3‐cE | pUFR034 expressing the fusion encoding the 50 N‐terminal amino acids of HrpE3 and the last 44 C‐terminal amino acids of HpaE under a 201‐bp hrpE3 promoter; Kmr | This work |
| pP1GUS | pUFR034 expressing GUS under the 397‐bp promoter of hrpE3; Kmr | This work |
| pP2GUS | pUFR034 expressing GUS under the 201‐bp promoter of hrpE3; Kmr | This work |
| pP3GUS | pUFR034 expressing GUS under the 101‐bp promoter of hrpE3; Kmr | This work |
| p987GUS | pUFR034 expressing GUS under the 987‐bp promoter of hpaE; Kmr | This work |
| p397GUS | pUFR034 expressing GUS under the 397‐bp promoter of hpaE; Kmr | This work |
| p201GUS | pUFR034 expressing GUS under the 201‐bp promoter of hpaE; Kmr | This work |
| p101GUS | pUFR034 expressing GUS under the 101‐bp promoter of hpaE; Kmr | This work |
| pavrXa10 | pUFR034 expressing AvrX10, Kmr | Li et al. (2011b) |
| pavrXa10Δ | pUFR034 expressing AvrXa10Δ, Kmr | Li et al. (2011b) |
| pP2avrXa10Δ | avrXa10Δ expression from the 205‐bp promoter of hrpE3 in pUFR034; Kmr | This work |
| pP1E3avrXa10Δ | The first 50 amino acids of HrpE3 fused with AvrXa10Δ under a 397‐bp promoter region of hrpE3 in pUFR034; Kmr | This work |
| pP2E3avrXa10Δ | The first 50 amino acids of HrpE3 fused with AvrXa10Δ under a 201‐bp promoter region of hrpE3 in pUFR034; Kmr | This work |
| pP3E3avrXa10Δ | The first 50 amino acids of HrpE3 fused with AvrXa10Δ under a 101‐bp promoter region of hrpE3 in pUFR034; Kmr | This work |
| pP2EavrXa10Δ | The first 50 amino acids of HpaE fused with AvrXa10Δ under a 201‐bp promoter region of hrpE3 in pUFR034; Kmr | This work |
| pEGAD | lacZa+, contains GFP; Kmr | This laboratory |
| pHrpE3‐GFP | A 285‐bp hrpE3 was fused in frame with gfp in vector pEGAD; Kmr | This work |
| pHpaE‐GFP | A 300‐bp hpaE was fused in frame with gfp in vector pEGAD; Kmr | This work |
| pXopR‐GFP | A 1314‐bp XopR was fused in frame with gfp gene in vector pEGAD; Kmr | Guo et al. (2012) |
Apr, ampicillin resistance; GFP, green fluorescent protein; GUS, β‐glucuronidase; Kmr, kanamycin resistance; Rifr, rifampicin resistance.
Figure 1.
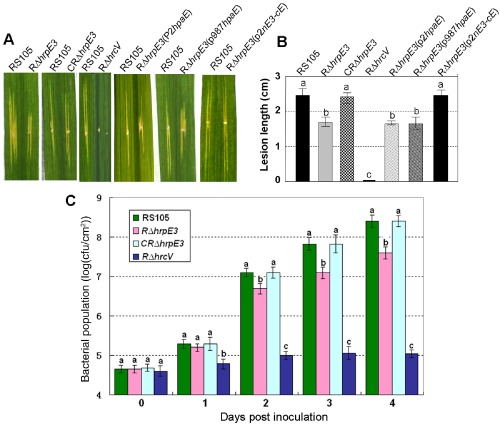
hrpE3 is required for the virulence and growth of Xanthomonas oryzae pv. oryzicola (Xoc) in rice. (A) Bacterial leaf streak (BLS) symptoms caused by Xoc strains RS105 (wild‐type), RΔhrpE3 (hrpE3 mutant), CRΔhrpE3 (complemented hrpE3 mutant with pP1hrpE3‐c‐Myc), RΔhrpE3(pP2hpaE‐c‐Myc), RΔhrpE3(p987hpaE), RΔhrpE3(p2nE3‐cE) and RΔhrcV (hrcV mutant; nonpathogenic control). Xoc strains [1 × 108 colony‐forming units (cfu)/mL] were inoculated to rice cultivar IR24 (2 months old) by leaf needling, and lesions were measured at 14 days post‐inoculation (dpi). The left half of each leaf was inoculated with the wild‐type RS105, and the right half was inoculated with Xoc derivatives. (B) BLS lesion lengths formed in rice (2 months old) inoculated with Xoc strains and assessed at 14 dpi. (C) Bacterial population in rice leaves inoculated with Xoc and derivatives. Leaf discs (0.5 cm in diameter) were excised from the inoculated areas, homogenized in sterile water, diluted and plated on nutrient agar (NA) plates. Data points represent the means ± standard deviation (SD) from three replicates.
We then investigated whether hpaE from Xcv could restore the virulence defect in the Xoc hrpE3 mutant. Two constructs were used for this objective: hpaE under the control of the hrpE3 promoter (201‐bp promoter region upstream of the hrpE3 predicted start codon; construct pP2HpaE‐c‐Myc, Table 1) and hpaE under the control of its native promoter (987‐bp region upstream of the hpaE predicted start codon; construct p987hpaE, Table 1). Regardless of the promoter used, hpaE failed to complement the virulence deficiency in mutant RΔhrpE3 (Fig. 1A,B).
The predicted protein sequences of HrpE3 (ABH07404) and HpaE (YP_362146) differ primarily at their N‐termini. Thus, we sought to determine whether a chimeric gene containing the N‐terminus of HrpE3 fused to the C‐terminus of HpaE could restore virulence to RΔhrpE3. Plasmid p2nE3‐cE was designed to investigate this possibility; this chimeric construct contains 351 nucleotides from Xoc RS105 (spanning 201 bp upstream of hrpE3 and extending 150 bp into the coding region) fused to a 132‐bp region containing the C‐terminus of hpaE from Xcv 23‐1. Notably, the impaired virulence of the hrpE3 mutant was restored to wild‐type levels when p2nE3‐cE was introduced into RΔhrpE3 (Fig. 1A–C). These results indicate that the C‐terminal functions of HrpE3 and HpaE are functionally interchangeable in Xoc, but that the N‐terminal regions are not.
hrpE3 is transcribed by a promoter located within hpaB
In Xcv, the hrpE, hpaB and hpaE genes are in the hrpE operon, and hpaB and hpaE are co‐transcribed, but they are not in the same operon with hrpE (Büttner et al., 2007; Rossier et al., 2000; Weber et al., 2007). However, the transcriptional organization of hrpE3 and the upstream regions in Xoc remain unclear. A 38‐bp intergenic region is present between hpaB and hrpE3 in Xoc (Fig. S1), which prompted us to determine the location of the hrpE3 promoter. We first determined the transcriptional linkages between the hrpD5 and hrpE3 regions in the wild‐type RS105 and deletion mutants RΔhrpD5, RΔhrpD6, RΔhrpE, RΔhpaB and RΔhrpE3 (Table 1, Fig. 2). Xoc RS105 and mutant strains were incubated in XOM3, an hrp‐inducing medium, for 16 h, and reverse transcription‐polymerase chain reaction (RT‐PCR) was performed to assess the expression of the intergenic junctions using primer pairs flanking each open reading frame (ORF) (Table S1, see Supporting Information). If the entire hrpD5 to hrpE3 region was co‐transcribed, a 3302‐bp PCR product would be predicted; however, this was not detected in the wild‐type RS105 or the five mutant strains (Fig. 2). However, when primers encompassing hrpD5 to hpaB were used in RT‐PCR, a 2295‐bp PCR product detected in RS105 was larger than those in the hrpD6, hrpE, hpaB and hrpE3 mutants (Fig. 2B, see lane designated D5‐aB). These results suggest that hrpD5, hrpD6, hrpE and hpaB are co‐transcribed as polycistronic mRNA. This hypothesis was supported by the PCR products observed when smaller regions were amplified (e.g. 349‐bp PCR product when primers extended from hrpD6 to hrpE; 729‐bp product using primers spanning hrpE to hpaB; see products amplified from strain RS105, lanes D6‐E and E‐aB, Fig. 2B). The predicted 804‐bp PCR product spanning hpaB–hrpE3 (lane designated aB‐E3) was not obtained in the wild‐type RS105 or mutants, suggesting that hpaB and hrpE3 are independently transcribed (Fig. 2B, lane ab‐E3). However, a 285‐bp PCR product was amplified from RS105, RΔhrpD5, RΔhrpD6 and RΔhrpE when primers specific to hrpE3 were used in RT‐PCR (Fig. 2B, lane E3). The absence of the 285‐bp PCR product in the hpaB and hrpE3 mutants suggests that transcription of hrpE3 begins in the coding region of hpaB. Thus, hrpE3 is transcribed independently from a promoter located within hpaB, an organization that is clearly different from hpaE in Xcv, where hpaE and hpaB are co‐transcribed (Büttner et al., 2007).
Figure 2.
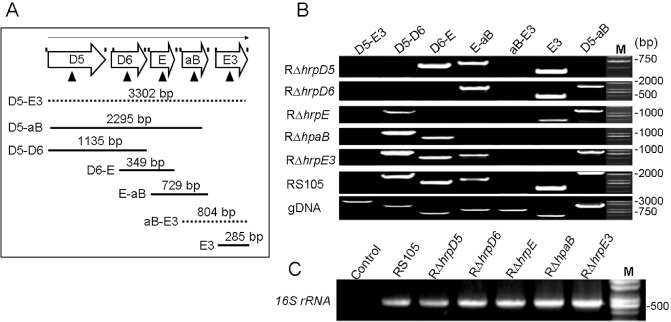
Reverse transcription‐polymerase chain reaction (RT‐PCR) analysis of transcriptional units in the hrpD5–hrpE3 region of Xanthomonas oryzae pv. oryzicola (Xoc). (A) Schematic representation of the hrpD5–hrpE3 region; RT‐PCR primers are indicated by vertical lines above the open arrows and were designed to span the intergenic junctions. Triangular arrows present the open reading frame (ORF)‐deleted mutations in the region from hrpD5 to hrpE3. The open arrows represent hrpD5 (D5), hrpD6 (D6), hrpE (E), hpaB (aB) and hrpE3 (E3). The full lines indicate the physical map location of PCR products amplified in this experiment; the dotted lines represent the deletions in PCR products D5‐E3 and aB‐E3. D5‐E3 denotes PCRs with the primer pair hrpD5F‐hrpE3R, D5‐aB with hrpD5F‐hpaBR, D5‐D6 with hrpD5F‐hrpD6R, D6‐E with hrpD6F‐hrpER, E‐aB with hrpEF‐hpaBR, aB‐E3 with hpaBF‐hrpE3R and E3 with hrpE3F‐hrpE3R. (B) Transcripts detected by RT‐PCR using cDNA isolated from RS105, RΔhrpD5, RΔhrpD6, RΔhrpE, RΔhpaB and RΔhrpE3; bacterial strains were incubated in the hrp (hypersensitive response and pathogenicity)‐inducing medium XOM3 for 16 h. (C) The 16S rRNA gene was used to verify the absence of significant variation in cDNA levels. Lane M represents a molecular weight marker (TaKaRa). All experiments were repeated three times, and similar results were obtained each time.
5′‐Rapid amplification of cDNA ends (5′‐RACE) was used to further define the promoter region for hrpE3. When RACE outer and inner primers (O‐F and I‐F) (Table S1) were used in nested PCR with the anchor‐specific hrpE3 outer and inner primers (hrpE3O‐R and hrpE3I‐R, Table S1), a 201‐bp fragment was amplified (Fig. 3A). Sequence analysis indicated that this 201‐bp region was located in the coding region of hpaB. Included within this 201‐bp region were an imperfect PIP box (TTCGTT‐N16‐TTCGA) sequence (as in Xcv) and a −10 box (GAGCCT), 32 bp downstream of the PIP box, that is changed to GCGCCT in the corresponding region upstream of hpaE (Fig. 3A). In comparing the predicted translational start codons of hpaE and hrpE3, we note that hrpE3 contains an additional 14 nucleotides of leader sequence, including a predicted Shine Dalgarno (SD) site, which are not present in hpaE (Fig. 3A). The differences above may help to explain the divergent transcriptional organization of the two genes.
Figure 3.
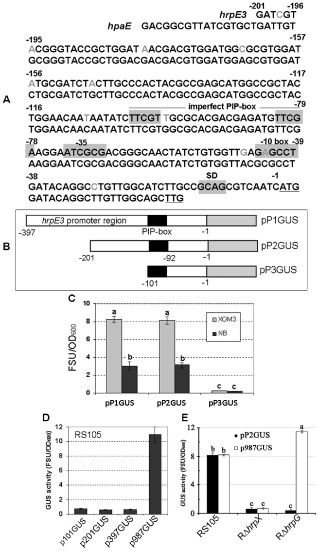
Analysis of the hrpE3 promoter region. (A) The comparison of the nucleotide sequences 201 bp upstream of the Xanthomonas oryzae pv. oryzicola (Xoc) hrpE3 start codon (ATG) and the X. campestris pv. vesicatoria (Xcv) hpaE start codon (TTG). The letters underlined indicate the predicted start codon of hrpE3 or hpaE. A predicted Shine Dalgarno (SD) sequence is shaded in grey upstream of the hrpE3 start codon and is absent in hpaE. A putative imperfect plant‐inducible promoter (PIP) box is shaded in grey and a −10 box 32 bp downstream of the PIP box is marked in grey, but some important nuclear acids are altered correspondingly in the upstream of hpaE. The grey letters in the hrpE3 promoter region display the different nucleotides from those in the upstream of hpaE. (B) Schematic map of the hrpE3 promoter region containing the PIP box (in black) fused to a promoterless gusA (GUS). Three different sized fragments upstream of hrpE3 in Xoc were subcloned as three promoter regions containing 397 (pP1), 201 (pP2) or 101 (pP3) nucleotides, to form pP1GUS, pP2GUS and pP3GUS, respectively. (C) β‐Glucuronidase (GUS) activity of the pP1GUS, pP2GUS and pP3GUS constructs in the wild‐type Xoc RS105. GUS activity was measured after 16 h of growth in the hrp (hypersensitive response and pathogenicity)‐inducing medium (XOM3) and the nutrient‐rich medium (NB). (D) Detection of GUS activity in Xoc RS105 strain containing transcriptional fusions to fragments upstream of the hpaE start codon of Xcv. Upstream fragments included 987 (p987), 397 (p987), 201 (p201) and 101 (p101) nucleotides fused to a promoterless gusA to generate p987GUS, p397GUS, p201GUS and p101GUS, respectively. (E) The comparison of GUS activity in Xoc RS105, RΔhrpX and RΔhrpG containing either pP2GUS or p987GUS. Bacterial strains were cultured in XOM3 medium for 16 h, and GUS activity was assayed as described in Experimental procedures. All experiments were repeated three times with similar results. Statistical differences in (C) and (D) are indicated by different letters.
The information presented above (see Figs 2 and 3A) suggests that hrpE3 is transcribed under its own promoter. To test this hypothesis, three transcriptional fusions were constructed between the hrpE3 promoter region and a promoterless gusA gene. The constructs included pP1GUS, pP2GUS and pP3GUS (Table 1). The gusA gene was fused to the 397‐, 201‐ and 101‐bp regions upstream of the hrpE3 translational start codon shown in Fig. 3B. These constructs were then introduced into the wild‐type Xoc strain RS105. β‐Glucuronidase (GUS) activity was measured after 16 h of growth in hrp‐inducing medium (XOM3) and a nutrient‐rich medium (NB). GUS activity of Xoc containing either pP1GUS or pP2GUS was significantly higher than Xoc (pP3GUS), regardless of the growth medium (t‐test, P = 0.01). This result suggests that the 101‐bp DNA region in pP3GUS does not contain the promoter region necessary for hrpE3 transcription. Furthermore, GUS activity was higher when Xoc RS105 containing pP1GUS or pP2GUS was cultured in XOM3 relative to NB (Fig. 3C). These results suggest that the hrpE3 promoter is plant inducible, and that the promoter region is located in the 201‐bp region upstream of the hrpE3 translational start site.
For comparative purposes, four transcriptional fusions were constructed between putative hpaE promoter regions and the promoterless gusA. These included gusA fused to a 987‐bp fragment (containing a 263‐bp region upstream of hrpE and the intact hrpE and hpaB genes) and the 397‐, 201‐ and 101‐bp regions upstream of the hpaE predicted start codon; these plasmids were designated p987GUS, p397GUS, p201GUS and p101GUS, respectively (Table 1). These constructs were transferred into the wild‐type RS105, and GUS activity was measured in XOM3 medium. GUS activity of Xoc containing p987GUS was significantly higher (t‐test, P = 0.01) than Xoc harbouring p397GUS, p201GUS or p101GUS, with no significant differences among the last three constructs (Fig. 3D). The data established that hpaE is transcribed as part of an hrpE–hpaB–hpaE operon, an organization that is clearly different from hrpE3 in Xoc.
hrpE3 expression is positively regulated by HrpG and HrpX
In Xcv, the expression of hpaE, which is homologous to hrpE3 in Xoc, is regulated by HrpG and HrpX (Büttner et al., 2007). To investigate whether hrpE3 is regulated similarly in Xoc, we transferred pP2GUS, which contains the 201‐bp region upstream of hrpE3 fused to a promoterless gusA, into the hrpG and hrpX mutants, RΔhrpG and RΔhrpX, respectively. The bacterial strains were cultured in XOM3 medium for 16 h, and GUS activity was assayed. GUS expression in RΔhrpG and RΔhrpX containing pP2GUS was approximately sevenfold lower (P = 0.01, t‐test) than in RS105 (pP2GUS) (Fig. 3E). For comparative purposes, we transferred the transcriptional fusion p987GUS into Xoc RS105, RΔhrpG and RΔhrpX to investigate promoter activity in the 987‐bp region upstream of hpaE. In XOM3 medium, GUS expression in RΔhrpX (pP987GUS) was significantly lower (P = 0.01, t‐test) than in RS105 and RΔhrpG containing pP987GUS (Fig. 3E). In summary, these data indicate that the hrpE3 promoter is positively regulated by both HrpG and HrpX, whereas the transcript containing hpaE is positively regulated by HrpX, but not HrpG.
The expression of hrpE3 is induced in planta
Rice seedlings of cultivar IR24 were infiltrated with Xoc RS105, and the expression of hrpE3 was monitored by RT‐PCR using the hrpE3‐specific primers hrpE3‐F/hrpE3‐R (Table S1) at 0, 3, 6, 12 and 24 h post‐inoculation (hpi). The expression of hrpE3 was elevated at 0, 3, 6 and 12 hpi, with maximal expression detected at 12 hpi (Fig. 4A), indicating that expression of hrpE3 is induced in planta. We also investigated the in planta expression of hrpE3 in mutants RΔhrcC, RΔhrpX, RΔhrpG and RΔhpaB, which are defective in hrcC, hrpX, hrpG and hpaB, respectively. Although hrpE3 was highly expressed in RS105 and mutant RΔhrcC, there was no obvious expression of hrpE3 detected in RΔhrpG, RΔhrpX and RΔhpaB strains (Fig. 4B).
Figure 4.
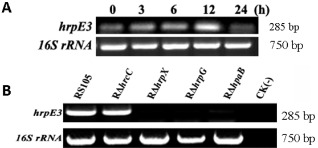
Detection of hrpE3 expression in planta by semi‐quantitative reverse transcription‐polymerase chain reaction (RT‐PCR). (A) Time point detection of hrpE3 expression in rice. Seedlings of rice cultivar IR24 (2 weeks old) were inoculated with Xanthomonas oryzae pv. oryzicola (Xoc) using a needleless syringe and assayed by semi‐quantitative RT‐PCR at 0, 3, 6, 12 and 24 h post‐inoculation (hpi). PCR products were electrophoretically separated on a 1.2% agarose gel. 16S rRNA was amplified as a constitutive control. (B) Detection of hrpE3 expression in Xoc RS105 and hrcC, hrpG, hrpX and hpaB mutants. Strains were infiltrated into rice seedlings (as described above), incubated for 12 h and total RNA was extracted for RT‐PCR. Water [CK(‐)] was used as a negative control. PCR products were electrophoretically separated as described above, and 16S rRNA was amplified as a control. The experiments were repeated three times with similar results, and one experiment is displayed.
HrpE3 is secreted through the T3S system, but HpaE is not
In many Gram‐negative phytopathogenic bacteria, T3Es contain a secretion signal within the N‐terminal 50 amino acids that is highly enriched in Ser and Pro residues (Alfano and Collmer, 2004; Cunnac et al., 2004; Furutani et al., 2009; Schechter et al., 2006; Tampakaki et al., 2004). For example, Furutani et al. (2009) showed that Xanthomonas effectors contained up to 20% Ser/Pro residues in their N‐terminal sequences. One strategy for the investigation of secretion via the T3S system is to fuse the N‐terminus of a candidate effector with an Avr protein that lacks a secretion signal, and investigate whether the fused effector can be translocated into plant cells (Jiang et al., 2009b; Li et al., 2011a). Sequence analysis of the predicted translational product of HrpE3 indicated the presence of three Ser and six Pro residues (18% Ser/Pro) in the first 50 amino acids (Fig. 5A). In comparison, HpaE from Xcv contains six Ser and seven Pro residues in the N‐terminal 50 amino acids (Fig. 5A), suggesting that HpaE may also be a T3E. However, it is important to note that the N‐terminal 50 amino acids of HrpE3 and HpaE are very different from each other (Fig. 5A). To investigate whether these proteins are secreted by the T3S system, the N‐terminal 50 amino acids of HrpE3 and HpaE were fused with a truncated AvrXa10 that lacked 28 amino acid residues at the N‐terminus (avrXa10Δ). The chimeric protein E3avrXa10Δ was expressed from the three putative hrpE3 promoters described in Fig. 3B, resulting in pP1E3avrXa10Δ, pP2E3avrXa10Δ and pP3E3avrXa10Δ (Fig. 5B). The fusion protein EavrXa10Δ, consisting of the first 50 amino acids of HpaE fused to AvrXa10Δ, was expressed under the control of the 201‐bp promoter of hrpE3 in plasmid pP2EavrXa10Δ (Table 1). It is important to mention that the wild‐type RS105 harbouring avrXa10 does not trigger an HR in rice line IRBB10, which carries the cognate R gene Xa10 (Li et al., 2011b). Therefore, we used Xoo strain PXO99A to investigate whether the fused protein is translocated into IRBB10. The T3S‐deficient mutant PΔhrcU was used as a negative control.
Figure 5.
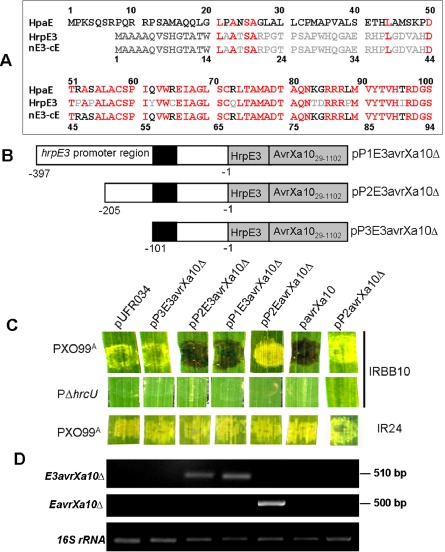
Detection of HrpE3 secretion via the type III secretion (T3S) system. (A) Amino acid sequence alignment of Xanthomonas oryzae pv. oryzicola (Xoc) HrpE (ABH07404), X. campestris pv. vesicatoria (Xcv) HpaE (YP_362146) and the fused chimeric protein nE3‐cE; the different amino acids among HrpE3, HpaE and nE3‐cE are shown in grey and black letters, and the same in red letters. (B) Schematic maps showing HrpE3 constructs fused to AvrXa10. The 50 amino acids at the N‐terminus of HrpE3 contain a higher percentage of serine (S) and proline (P) residues, and may comprise a T3S signal. The 50 N‐terminal amino acids of HrpE3 were fused to a truncated version of AvrXa10Δ in which the first 28 amino acids were deleted, generating a fusion designated E3avrXa10Δ. The transcription of this fusion was driven by three fragments containing 397, 201 and 101 bp of hrpE3 upstream DNA; these were designated pP1E3avrXa10Δ, pP2E3avrXa10Δ and pP3E3avrXa10Δ, respectively. The black rectangle indicates the location of the putative plant‐inducible promoter (PIP) box in the promoter region of hrpE3. (C) The X. oryzae pv. oryzae (Xoo)–rice pathosystem was used to evaluate secretion and translocation of the HrpE3‐AvrXa10 chimeric proteins. For comparative purposes, an HpaE‐AvrXa10 chimeric protein was included. This construct was designated pP2EavrXa10Δ and contained the 50 N‐terminal amino acids from HpaE fused to AvrXa10Δ; the promoter driving transcription was the 201‐bp region upstream of hrpE3. Xoo strains containing the fusions were inoculated into rice cultivar IRBB10 or IR24 (see Experimental procedures). The native avrXa10 was used as a positive control (construct pavrXa10) and the N‐terminal truncated avrXa10Δ under control of the 201‐bp hrpE3 promoter region (pP2avrXa10Δ) served as a negative control. PXO99A and PΔhrcU containing the empty vector pURF034 were also included. Phenotypes were photographed 3 days after inoculation in three independent experiments. (D) Detection of the fused genes E3avrXa10Δ and EavrXa10Δ in rice IRBB10 leaves infiltrated with Xoo strains and analysed by reverse transcription‐polymerase chain reaction (RT‐PCR) at 12 h post‐inoculation (hpi) (detailed in Experimental procedures). The 16S rRNA gene of Xoo was used as an internal control.
PXO99A containing pP1E3avrXa10Δ or pP2E3avrXa10Δ produced an HR in IRBB10, as did PXO99A (pavrXa10) (Fig. 5C). However, an HR was not elicited by PXO99A containing pP3E3avrXa10Δ, pP2EavrXa10Δ or pP2avrXa10Δ (Fig. 5C); the latter construct contains the 201‐bp hrpE3 promoter fused to avrXa10 with the N‐terminal deletion. As predicted, the T3S‐deficient strain PΔhrcU did not elicit bacterial blight symptoms in rice cultivar IRBB10. Xoo strain PXO99A containing the constructs mentioned above induced water‐soaked lesions in rice cultivar IR24, which does not contain the Xa10 gene (Fig. 5C).
We then used semi‐quantitative RT‐PCR to investigate whether or not the translational fusions present in pP2E3avrXa10Δ and pP2EavrXa10Δ were expressed in the infiltrated leaves. The E3::avrXa10Δ fusion was amplified in rice leaves inoculated with PXO99A containing either pP1E3avrXa10Δ or pP2E3avrXa10Δ, and the E::avrXa10 fusion was detected in rice leaves inoculated with PXO99A (pP2EavrXa10Δ) (Fig. 5D). To summarize, the hrpE3 promoter and the first 50 N‐terminal amino acids of HrpE3, but not HpaE, enable the N‐terminal truncated AvrXa10 to be secreted through the T3S system and translocated into rice cells for HR induction.
To confirm secretion via the T3S system, HrpE3 was expressed as a C‐terminal Myc epitope‐tagged derivative in plasmid pP2HrpE3‐c‐Myc (Table 1), which was introduced into Xoc strains RS105, RΔhrcC, RΔhrpG, RΔhrpX and RΔhpaB. Bacterial cells were incubated in XOM3 medium, and total protein extracts (TE) and culture supernatants (SN) were analysed for the presence of HrpE3‐c‐Myc using an epitope‐specific antibody. The HrpE3 protein was detected in the TE and SN of the wild‐type RS105 (Fig. 6A). However, HrpE3 was only detected in the TE of hrp deletion mutants (RΔhrcC, RΔhrpG, RΔhrpX and RΔhpaB) (Fig. 6A); this indicates that the secretion of HrpE3 did not occur in the mutants and was hrp dependent.
Figure 6.
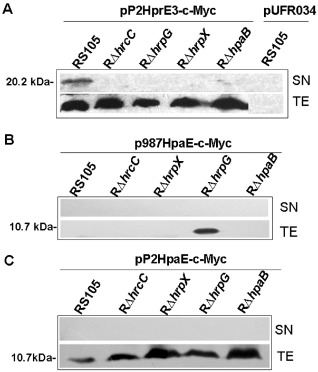
Comparison of HrpE3 and HpaE secretion by immunoblotting. (A) Detection of HrpE3 secretion in different genetic background strains RS105, RΔhrcC, RΔhrpG, RΔhrpX and RΔhpaB harbouring pP2HrpE3‐c‐Myc (hrpE3 under the control of the 201‐bp hrpE3 promoter). (B) Immunodetection of HpaE in Xanthomonas oryzae pv. oryzicola (Xoc) strains RS105, RΔhrcC, RΔhrpG, RΔhrpX and RΔhpaB harbouring p987HpaE‐c‐Myc [hpaE under the control of the X. campestris pv. vesicatoria (Xcv)‐derived 987‐bp upstream promoter]. (C) Immunodetection of HpaE in Xoc strains RS105, RΔhrcC, RΔhrpG, RΔhrpX and RΔhpaB harbouring pP2HpaE‐c‐Myc (hpaE under the control of the 201‐bp hrpE3 promoter). After incubation in XOM3 medium for 16 h, the total protein extracts (TE) and culture supernatants (SN) of each tested strain were analysed by immunoblotting using anti‐c‐Myc antibodies (Genescript). The experiments were repeated three times, and representative results are shown.
Using a similar strategy, the secretion of HpaE was evaluated in Xoc RS105 and the four hrp deletion mutants. The HpaE‐c‐Myc protein was detected in the TE of the hrpG mutant when expressed under the control of the 987‐bp hpaE promoter region (p987HpaE‐c‐Myc) (Fig. 6B); however, it was not detectable in TE or SN from other strains. When expressed from the 201‐bp hrpE3 promoter, the HpaE‐c‐Myc fusion was detected in TE of all strains, but was undetectable in SN (see pP2HpaE‐c‐Myc) (Fig. 6C). The undetectable HpaE in the SN fractions of the wild‐type and mutant strains implies that HpaE is not secreted via the T3S system.
HrpE3 localizes to the cytoplasm of host cells
The localization of HrpE3 in plant cells was studied by fusing the hrpE3 coding region as a translational fusion to gfp (encoding green fluorescent protein), whereas HpaE was used for comparison. For comparative purposes, a known T3E, XopR (Akimoto‐Tomiyama et al., 2012), was also fused with GFP and used as a positive control. To confirm whether the fused proteins were synthesized, we first evaluated whether they could be detected in tobacco leaves inoculated with Agrobacterium tumefaciens EH105 containing pEGAD, pHrpE3‐GFP, pHpaE‐GFP or pXopR‐GFP (Table 1). After transient expression for 36 h, tobacco leaves were collected, and total proteins were extracted, precipitated and analysed for expression of GFP by immunoblotting (see Experimental procedures). As predicted, a 35.9‐kDa HrpE3‐GFP, a 36.9‐kDa HpaE‐GFP and a 75.01‐kDa XopR‐GFP were expressed as GFP fusions and could be detected using an anti‐GFP antibody (Fig. 7A).
Figure 7.
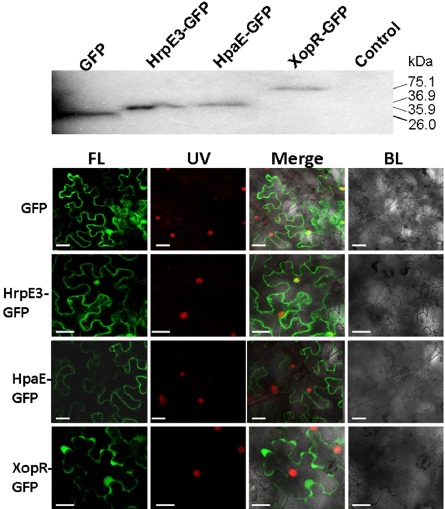
Subcellular localization of HrpE3 in plant cells. (A) Detection of the fusion protein HrpE3‐GFP in tobacco (Nicotiana benthamiana). Total proteins were extracted from tobacco leaves infiltrated with Agrobacterium tumefaciens containing pEGAD, pHrpE3‐GFP, pHpaE‐GFP and pXopR‐GFP, and analysed by immunoblotting with anti‐GFP (Genescript). Tobacco leaves infiltrated with A. tumefaciens EH105 containing empty vector were used as a control. The numbers represent the size of proteins in kilodaltons (kDa). (B) Detection of green fluorescent protein (GFP) fused proteins in tobacco cells. Tobacco epidermal cells expressing HrpE3‐GFP, HpaE‐GFP and XopR‐GFP fusions or GFP alone are shown. The XopR‐GFP fusion was used as a positive control. Expression was driven by the 35S cauliflower mosaic virus (CaMV) promoter. For confocal laser scanning microscopy, tobacco samples were taken 36 h after Agrobacterium‐mediated delivery of GFP, HrpE3‐GFP, HpaE‐GFP and XopR‐GFP. Images were acquired by fluorescence (FL) at 503 nm, exposure to ultraviolet light (UV) at 395 nm and using black light (BL). White bars represent 10 nm, and experiments were repeated three times with similar results.
Fluorescence microscopy was used to examine the localization of the GFP fusions described above. Although HrpE3‐GFP was localized to the cytoplasm and nucleus, HpaE‐GFP was only observed in the cytoplasm (Fig. 7B). The localization of XopR‐GFP was observed at the plasma membrane and in the cytoplasm, which is consistent with previous results (Akimoto‐Tomiyama et al., 2012). In general, our results suggest a cytoplasmic location for HrpE3‐GFP, although nuclear localization cannot be completely excluded.
Discussion
Previously, we have reported that the hrpD operon in Xoc contains eight co‐transcribed genes (hrcQ to hpaB; Li et al., 2011a), which is similar to the organization of the hrpD operon in the closely related pathogen Xoo (Cho et al., 2008). In the tomato/pepper pathogen Xcv, this region is subdivided into several transcriptional units. For example, the hrpD operon contains four co‐transcribed genes, the hrpE operon contains hrpD5–hrpD6–hrpE and a third transcript contains hpaB–hpaE (Büttner et al., 2007; Weber et al., 2007). The results presented in this study show that hrpE3, the hpaE homologue in Xcv, is transcribed independently under a promoter located within hpaB of the hrpD operon. Thus, the transcriptional organization of hrpE3 is clearly different from hpaE in Xcv, where hpaE and hpaB are co‐transcribed (Büttner et al., 2007). It is important to note that these genes are part of the nonconserved region of the hrp pathogenicity island (Büttner et al., 2007), and divergent transcription in this region may provide clues regarding host specificity: Xoc HrpE3 plays a role in virulence in rice, whereas Xcv HpaE plays a role in pepper.
The expression of hrpE3 was evaluated in various genetic backgrounds to further explore its regulation in Xoc. hrpE3 was expressed in the hrcC deletion mutant (RΔhrcC), which was not surprising as hrcC encodes a membrane component of the T3S system in Xanthomonas spp. (Büttner and Bonas, 2002a, b) and was not expected to have an impact on the regulation of hrpE3 (Fig. 6A). The absence of hrpE3 transcription in the RΔhrpX and RΔhrpG mutants is consistent with a role for hrpX and hrpG at the apex of the Hrp regulon (Guo et al., 2012; Li et al., 2011a; Wengelnik et al., 1996, 1999). The absence of hrpE3 expression in the hpaB deletion mutant (Figs 2B and 4B) is probably a result of the deletion of the hrpE3 promoter region, which mapped within the hpaB coding region (Fig. 3A, Fig. S1).
In this study, we have demonstrated that hrpE3 is required for full virulence of Xoc in rice (Fig. 1). Büttner et al. (2007) have shown previously that the hrpE3 homologue, hpaE, contributes to the virulence of Xcv in pepper and is required for HR induction in resistant host plants. HrpE3 and HpaE differ in their N‐terminal 50 amino acids, but share a high degree of identity in their C‐terminal residues (Fig. 5A). Despite their similarities, we found that hpaE could not restore full virulence to the hrpE3 mutant (Fig. 1), and did not interfere with Xoc virulence in rice when the wild‐type RS105 was overexpressed with hpaE in trans (data not shown), regardless of the promoter used to drive expression. To explore whether the C‐terminal regions of hrpE3 and hpaE were functionally interchangeable, we designed plasmid p2nE3‐cE. This chimeric construct contains the hrpE3 promoter, 150 bp of the 5′ coding region of hrpE3, and 132 bp from hpaE; the latter segment encodes the 45 amino acid residues at the C‐terminus of hpaE. Notably, we found that p2nE3‐cE complemented the hrpE3 mutant for virulence in rice (Fig. 1), suggesting that functions at the C‐terminal regions of hrpE3 and hpaE are conserved.
In vitro and in vivo secretion assays demonstrated that HrpE3 is secreted by the T3S system of Xoc (Figs 5 and 6) and translocated into the plant cytoplasm (Fig. 7). This is in sharp contrast with HpaE, which is not secreted by the T3S system (Büttner et al., 2007), which is consistent with our findings. As our results suggest that the C‐terminal regions of HrpE3 and HpaE are functionally interchangeable, it is probable that the differences in secretion via the T3S system and the delivery into plant cells between HrpE3 and HpaE are attributed to their N‐termini. As HpaE is not secreted via the T3S system, it probably functions inside the bacterial cell to influence effector secretion, possibly as a helper for Xcv T3E translocation (Büttner et al., 2007). The subcellular localization of HrpE3 is cytoplasmic–nuclear in plant cells (Fig. 7B), suggesting that HrpE3 functions as a genuine T3E. Thus, the major functional polymorphisms of HrpE3 and HpaE in bacterial virulence in plants, T3S‐dependent secretion and subcellular localizations in plant cells are possibly determined by the polymorphisms in the N‐terminal differences between HrpE3 and HpaE. However, the host cell function targeted by HrpE3 remains unknown. Our results do not exclude the possibility that HrpE3 may also function to facilitate the translocation of T3Es in Xoc. These possibilities are not mutually exclusive, because individual T3Es often exhibit multiple functions in host–pathogen interactions (Cunnac et al., 2009). Thus, whether or not the mutation in hrpE3 diminishes or compromises the translocation of other T3Es is a question that warrants further investigation.
Experimental Procedures
Bacterial strains, plasmids and growth conditions
The bacterial strains and plasmids used in this study are listed in Table 1. Xanthomonas strains and other derivatives were grown in nutrient agar (NA; 0.1% yeast extract, 0.3% beef extract, 0.5% peptone, 1% sucrose and 1.5% agar) or NB (NA without agar) (Li et al., 2011a) at 28 °C. The hrp‐inducing medium for X. oryzae strains was XOM3 (Li et al., 2011a; Xiao et al., 2007). To obtain the hrpE3 deletion mutant, NA without sucrose (NAN) was used. Agrobacterium tumefaciens strain EH105 and related constructs were grown in Luria–Bertani medium (LB; Miller, 1972) at 28 °C. Escherichia coli was grown in LB at 37 °C. Antibiotics were used at the following concentrations (μg/mL) when required: rifampicin (Rif), 50; kanamycin (Km), 25; ampicillin (Ap), 50.
DNA manipulation and plasmid construction
DNA isolation, restriction enzyme digestion, cloning, electroporation, PCR, Southern blots and immunoblotting were performed according to standard procedures (Sambrook et al., 1989). The primers used for PCR are listed in Table S1. PCR products were first cloned into pMD18‐T (TaKaRa, Dalian, China), verified by sequencing, and analysed using VECTOR NTI software (http://www.invitrogen.com). The promoter analysis of hrpE3 was conducted using tools available at http://www.fruitfly.org/seq_tools/promoter.html.
Construction of an hrpE3 deletion mutant
In Xoc strain RS105, hrpE3 (nucleotides 18 351–18 635) is located 38 bp downstream of hpaB and 454 bp upstream of hpa4 (accession no. AY875714) (Zou et al., 2006) (Fig. S1). To generate a nonpolar mutation in hrpE3, fragments located 500 bp upstream and 750 bp downstream of hrpE3 (Fig. S1) were amplified from RS105 genomic DNA using the primers E3UP‐F/E3UP‐R and E3II‐F/E3II‐R (Table S1), respectively. PCR products were cloned into pMD18‐T and verified by DNA sequencing. The constructs were then digested with SmaI/BamHI and BamHI/HindIII, respectively, to release the cloned fragments, which were then subcloned into the suicide vector pKMS1 (Zou et al., 2011) at SmaI and HindIII sites, resulting in pKΔhrpE3 (Table 1). This construct was then introduced into Xoc RS105. A mutant lacking hrpE3 was initially obtained on NAN medium and then on NA following the procedure described by Jiang et al. (2009a). The mutant was verified by PCR amplification with the primers E3UP‐F/E3II‐R (Table S1) and by Southern blot analysis using the hrpE3 gene as a probe (Fig. S1).
Complementation of the hrpE3 mutant
For the complementation of the hrpE3 mutant (RΔhrpE3), a 682‐bp DNA fragment containing a 397‐bp upstream region and a 285‐bp coding region of hrpE3 was amplified from RS105 genomic DNA using the primers E3P1‐F/E3Pm‐R (Table S1). After confirmation by sequencing, the amplified DNA fragment was cloned into pUFR034 at EcoRI and BamHI sites, resulting in plasmid pP1HrpE3‐c‐Myc (Table 1).
For comparative studies, the entire ORF of hpaE with a c‐Myc tag was amplified from genomic DNA of Xcv 23‐1 (Table 1) using the primers hpaE‐F1/aEPm‐R (Table S1). This amplified DNA was fused to a 201‐bp promoter region of hrpE3, which was amplified from the genome of Xoc RS105 with the primers E3P2‐F/E3P‐R (Table S1). This chimeric construct was then cloned into pUFR034 at EcoRI and BamHI sites, generating pP2HpaE‐c‐Myc (Table 1).
To determine whether hpaE from Xcv could complement the Xoc hrpE3 mutant for virulence, a 1290‐bp fragment, containing hpaE (303 bp) and 987 bp of upstream DNA (Büttner et al., 2007), was amplified from genomic DNA of Xcv 23‐1 with the primers aEP987‐F and aEPm‐R (Table S1). After sequencing, this DNA fragment was cloned into pUFR034 at EcoRI sites, creating p987hpaE (Table 1).
A construct was also designed to determine whether a chimeric fusion between the N‐terminal and C‐terminal regions of HrpE3 and HpaE could restore virulence to the hrpE3 mutant. For these experiments, a genetic fusion named p2nE3‐cE was constructed; this contains the first 50 amino acids of HrpE3 and the C‐terminal region of HpaE (44 amino acids). To generate this fusion, primer pair E3P2‐F/nE3‐R (Table S1) was used to amplify 351 nucleotides from Xoc RS105 spanning 201 bp upstream of hrpE3 and extending 150 bp into the coding region. Next, a 132‐bp region containing the C‐terminus of HpaE was amplified from Xcv 23‐1 genomic DNA using the primers cE‐F/aEPm‐R (Table S1). After BamHI digestion, the two fragments were ligated and used as a template to amplify a 483‐bp fusion with the primers E3P2‐F/aEPm‐R. After confirmation by sequence analysis, this fusion was cloned in pUFR034 at the EcoRI site, creating pP2nE3‐cE (Table 1).
The constructs described above, pP1HrpE3‐c‐Myc, pP2hpaE, p987hpaE and pP2nE3‐cE, were then transferred into the hrpE3 mutant, RΔhrpE3. One representative transformant was used in subsequent studies after verification by PCR.
Pathogenicity and HR assays
Oryza sativa ssp. indica rice cultivars IR24 and IRBB10 (susceptible to Xoc infection) were used to assay pathogenicity. IR24 and IRBB10 plants were inoculated with a needleless syringe as seedlings (2 weeks old) and by leaf needling as adults (2 months old). The bacterial suspensions used in pathogenicity assays were adjusted to 1 × 108 colony‐forming units (cfu)/mL. Rice cultivar IRBB10, which contains the Xa10 gene, is resistant to Xoo strains with avrXa10, and this interaction results in an HR (Li et al., 2011b; Yang and White, 2004). The ability of Xoc strains to induce an HR was evaluated on tobacco (Nicotiana benthamiana) by infiltration of a bacterial suspension at 1 × 108 cfu/mL into fully expanded leaves with a needleless syringe. Plants were observed at 24 hpi for HR in tobacco, at 3 days post‐inoculation (dpi) for water soaking or HR induction in rice seedlings, and at 14 dpi for lesion length in adult rice plants. Plants were maintained in a glasshouse, and all experiments were repeated at least three times.
Measurement of bacterial growth in rice
Suspensions of Xoc RS105 and derivatives were adjusted to 1 × 108 cfu/mL and infiltrated into newly expanded leaves of rice IR24 (2 weeks old) with a needleless syringe at three locations per leaf. Three leaf discs (0.8 cm in diameter) were excised with a cork borer from each infiltrated area. After sterilization in 70% ethanol and 30% hypochlorite, the discs were macerated using a sterile mortar and pestle in 1 mL of distilled water, diluted and plated to determine cfu/cm2. Serial dilutions were spotted in triplicate on NA plates with appropriate antibiotics. The plates were incubated at 28 °C for 3–4 days until single colonies could be counted. The bacterial population (cfu/cm2 of leaf area) was then estimated, and the standard deviation was calculated using colony counts from three triplicate spots from each of three samples obtained at each time point. Experiments were repeated at least three times.
Semi‐quantitative RT‐PCR
To evaluate the expression of selected genes in the pathogen, single colonies of Xoc RS105 and deletion mutants (Table 1) were cultured, and total bacterial RNAs were extracted as described previously (Li et al., 2011a) using the Trizol method (Invitrogen, Shanghai, China). The extracted RNAs were treated with DNase I (TaKaRa) to remove any contaminating genomic DNA, purified and then used as templates for PCR amplification of hrpD5, hrpD6, hrpE, hpaB and hrpE3 with the primers listed in Table S1. cDNA synthesis and semi‐quantitative RT‐PCR were performed as described by Li et al. (2011a). The RT‐PCR products were sequenced to confirm the specificity of the primers for the five hrp–hrc–hpa genes. The resulting amplified products were analysed on 1.2% agarose gels. The Xoc 16S rRNA gene was used as an internal control to verify the absence of significant variation at the cDNA level.
To investigate whether hrpE3 and hrpE3 fused genes were expressed in planta, total RNAs were extracted from plant leaves inoculated with Xoc strains (1 × 108 cfu/mL). The procedure outlined above was followed for RNA extraction and cDNA synthesis. Semi‐quantitative RT‐PCR was performed with primer pairs specific for each gene (Table S1), and the rice 16S rRNA gene was used as an internal control. Similar results were obtained from three independent experiments.
Promoter analysis of hrpE3 by 5′‐RACE and GUS assays
5′‐RACE was used to determine the transcriptional start site of hrpE3. Xoc RS105 (10 mL) was incubated in XOM3 medium at 28 °C for 16 h, and total RNA was extracted using an RNeasy Midi kit (TaKaRa). The isolated RNA was treated with RNase‐free DNase I (TaKaRa) at 37 °C for 2.5 h, followed by a second purification using an RNeasy column. cDNA fragments were obtained using a 5′‐RACE kit (TaKaRa), and an anchor sequence was added to the 5′ end of the cDNA using terminal deoxynucleotide transferase. The tailed cDNA was then amplified using the nested gene‐specific primers hrpE3O‐R and hrpE3I‐R (Table S1) and RACE outer primer O‐F and inner primer I‐F (Table S1), as recommended in the 5′‐Full RACE Kit (TakaRa). PCR products were then cloned into pMD18‐T and sequenced.
To construct transcriptional fusions between the hrpE3 promoter and gusA, the putative promoter‐containing regions of hrpE3 were fused to a promoterless gusA (Mitsuhara et al., 1996) with its ribosome binding site. Regions upstream of the start codon of hrpE3 were PCR amplified using total DNA of Xoc RS105 as the template. The primer pairs E3P1‐F/E3P‐R, E3P2‐F/E3P‐R and E3P3‐F/E3P‐R (Table S1) were used to amplify the hrpE3 upstream regions, and these were fused to gusA, which was amplified with the primer pair gusF/gusR (Table S1). Transcriptional fusions were cloned into pUFR034, resulting in pP1GUS, pP2GUS and pP3GUS (Table 1). For comparative analysis of hpaE and hrpE3 promoter activity, 987‐, 397‐, 201‐ and 101‐bp fragments upstream of the hpaE coding region were amplified with the primer pairs aEP987‐F/aEP‐R, aEP397‐F/aEP‐R, aEP201‐F/aEP‐R and aEP101‐F/aEP‐R (Table S1), respectively, using genomic DNA of Xcv 23‐1 as a template (Table 1). After sequence verification, these fragments were fused with gusA at the HindIII site and cloned into pUFR034 at EcoRI and XhoI sites, resulting in p987GUS, p397GUS, p201GUS and p101GUS (Table 1). Constructs were then transferred into RS105, RΔhrpX and RΔhrpG by electroporation and verified by colony‐PCR.
For GUS assays, Xoc strains were cultured in XOM3 or NB to an optical density at 600 nm (OD600) of 0.5. Bacterial cells were diluted and disrupted in sonication buffer [20 mm Tris‐HCl, pH 7.0; 10 mm 2‐mercaptoethanol, 5 mm ethylenediaminetetraacetic acid (EDTA) and 1% Triton X‐100]. GUS activities were determined at 15‐min intervals up to 4 h by evaluating OD415 using 4‐methylumbelliferone‐d‐glucuronide as the substrate (Jefferson et al., 1987). One unit (U) was defined as 1 nmol of 4‐methylumbelliferone produced per min per OD600 value of the bacterium.
T3S assays for HrpE3 and HpaE
Two strategies were used to detect the secretion of HrpE3 and HpaE through the T3S system. In one approach, a c‐Myc epitope‐encoding sequence was fused to the C‐terminus of HrpE3 or HpaE prior to the stop codon. To generate HrpE3‐c‐Myc expression constructs, hrpE3 with three promoter regions (−397 to −1, −201 to −1 and −101 to −1 bp upstream of the hrpE3 translational start site) were amplified from genomic DNA of Xoc strain RS105 with the primers listed in Table S1. The PCR products were cloned in frame into pURF034 with a c‐Myc epitope‐encoding sequence, resulting in pP1HrpE3‐c‐Myc, pP2HrpE3‐c‐Myc and pP3HrpE3‐c‐Myc (Table 1). These constructs were then transformed into Xoc RS105, RΔhrcC, RΔhpaB, RΔhrpG and RΔhrpX (Table 1) for protein secretion investigation. For comparative purposes, constructs p987HpaE‐c‐Myc and pP2HpaE‐c‐Myc (Table 1) were transferred into RS105, RΔhrcC, RΔhrpX, RΔhrpG and RΔhpaB strains for detection of HpaE‐mediated secretion.
In the second strategy, we fused a variant of AvrXa10 lacking 28 amino acids at the N‐terminus to the first 50 amino acid residues located at the N‐terminus of HrpE3 or HpaE. The truncated form of AvrXa10 had been cloned previously into pUFR034 and was designated as pΔ28AvrX10 (Li et al., 2011b). To generate HrpE3‐AvrXa10 expression constructs, the N‐terminal coding sequence of hrpE3 (1 to 150 bp) plus three putative promoter regions (−397 to −1, −201 to −1 and −101 to −1 bp upstream of the hrpE3 translational start site) were amplified from genomic DNA of Xoc RS105 using the primers listed in Table S1. The PCR products were cloned into the EcoRI and XhoI sites of pURF034 in frame with the truncated AvrXal10 fragment in pΔ28AvrX10; this resulted in pP1E3avrXa10Δ, pP2E3avrXa10Δ and pP3E3avrXa10Δ (Table 1). For comparative purposes, nucleotides encoding the N‐terminal 50 amino acids of HpaE were amplified from genomic DNA of Xcv strain 23‐1 with the primers hpaE‐F1/aEP‐R1 (Table S1). This amplified DNA was fused with the 201‐bp promoter (P2) of hrpE3, which was amplified from Xoc RS105 with the primers E3P2‐F/E3P‐R1 (Table S1). The fused fragment was cloned in frame with pavrXa10Δ at the EcoRI/XhoI sites, resulting in pP2EavrXa10Δ (Table 1). These constructs were transformed into the wild‐type Xoo strain PXO99A and the Xoo hrcU mutant PΔhrcU (Table 1). Transformed strains were assayed for HR induction in rice IRBB10.
For Western blot analysis, Xoc strains were pre‐incubated in NB medium, resuspended in sterile distilled water at OD600 = 2.0 and washed twice. The bacterial suspension (40 μL) was transferred into 1 mL of the hrp‐inducing medium XOM3 (pH 6.5) and incubated at 28 °C for 16 h. Bacterial cells and supernatants were separated by centrifugation, and proteins in the supernatant fraction were precipitated with 12.5% trichloroacetic acid (Laemmli, 1970). Proteins were separated on 10% sodium dodecylsulphate‐polyacrylamide gel electrophoresis (SDS‐PAGE) gels and transferred to membranes for immunoblotting using anti‐c‐Myc (Genescript, Nanjing, China) as the primary antibody. Primary antibodies were recognized using a secondary anti‐rabbit antibody (Genescript) and visualized on autoradiographs by chemiluminescence (Transgene, Shanghai, China).
Subcellular localization of HrpE3 in planta
GFP was used as a reporter to investigate the subcellular localization of HrpE3 in planta. The coding region of hrpE3 was amplified from genomic DNA of Xoc RS105 with the primers hrpE3‐F1/hrpE3‐R (Table S1). After sequence verification, the intact hrpE3 ORF without a stop codon was cloned in frame with gfp at EcoRI and HindIII sites in vector pEGAD (Guo et al., 2012), generating pHrpE3‐GFP (Table 1). Using the same strategy, pHpaE‐GFP was also constructed (Table 1). Expression of the hrpE3‐gfp fusion was driven by duplicate cauliflower mosaic virus (CaMV) 35S promoters. As a positive control, xopR (PXO_03819), encoding a 239‐amino‐acid membrane‐binding protein (Akimoto‐Tomiyama et al., 2012), was amplified from genomic DNA of Xoo PXO99A using the primers xopR‐F/xopR‐R (Table S1); the product was subcloned into pEGAD at EcoRI and HindIII sites, creating pXopR‐GFP (Table 1). The constructs described above were individually introduced into A. tumefaciens strain EH105 and infiltrated into N. benthamiana leaves for transient expression in epidermal cells. At 36 hpi, tobacco leaves were collected and total proteins were extracted as described previously (Akimoto‐Tomiyama et al., 2012). Proteins in the supernatant fraction were precipitated with 12.5% trichloroacetic acid (Laemmli, 1970), separated on 10% SDS‐PAGE gels, and transferred to membranes for immunoblotting using the primary antibody anti‐GFP (Genescript). Primary antibodies were recognized using a secondary anti‐rabbit antibody (Genescript) and visualized on autoradiographs by chemiluminescence (Transgene).
For observation by fluorescence microscopy, epidermal cells were used to investigate the localization of fusion proteins at 36 hpi with A. tumefaciens. Three independently infiltrated leaves expressing GFP under duplicate CaMV 35S promoters were placed in an enzyme solution (2% cellulase, 1% pectinase, 0.2 mol/L CaCl2, 50 mmol/L 2‐(N‐morpholino)ethanesulphonic acid (MES) and 0.6 mol/L mannitol) containing 1 μg/mL 4′,6‐diamidino‐2‐phenylindole (DAPI). After incubation for 3 h, the treated leaves were observed using confocal laser scanning microscopy (Leica Microsystems, Solms, Germany). Fluorescence images were acquired after exposure to fluorescent (FL, 509 nm), ultraviolet (UV, 395 nm) and black light (BL) irradiation. The assays were repeated three times with similar results, and the findings of one representative experiment are presented here.
Supporting information
Fig. S1 (A) Physical and functional map of hrpE3 and flanking genes (hpaB and hpa4) in the hrp (hypersensitive response and pathogenicity) cluster (AY875714) of Xanthomonas oryzae pv. oryzicola (Xoc) strain RS105. Open arrows indicate length, location and orientation of hpaB, hrpE3 and hpa4. (B) Strategy used for the deletion of hrpE3 by removal of a 285‐bp coding region (white) of hrpE3 using the E3UP (amplifies approximately 500 bp, grey) and E3II (amplifies 750 bp, black) primers. The fused PCR fragments were digested at SmaI and HindIII, and ligated in pKMS1. (C) Confirmation of deletion in mutant RΔhrpE3 by polymerase chain reaction (PCR) amplification with the primer pair (upF and downR) and by Southern hybridization using the left fragment as the probe, confirming that the PCR product and hybridized band in RΔhrpE3 is 285 bp shorter than that in the wild‐type RS105. Genomic DNAs from Xoc RS105 and RΔhrpE3 were digested with MscI.
Table S1 List of oligonucleotide primers used in this study.
Acknowledgements
We are grateful to Dr Carol Bender (Oklahoma State University, Stillwater, OK, USA) for reviewing the manuscript prior to submission. This work was supported by the Natural Science Foundation of China (31230059 and 31071656), the Special Fund for Agro‐Scientific Research in the Public Interest of China (201303015) and the Key Basic Research Project of the Shanghai Committee of Science and Technology (11JC1406300).
References
- Akimoto‐Tomiyama, C. , Furutani, A. , Tsuge, S. , Washington, E.J. , Nishizawa, Y. , Minami, E. and Ochiai, H. (2012) XopR, a type III effector secreted by Xanthomonas oryzae pv. oryzae, suppresses microbe‐associated molecular pattern‐triggered immunity in Arabidopsis thaliana . Mol. Plant–Microbe Interact. 25, 505–514. [DOI] [PubMed] [Google Scholar]
- Alfano, J.R. and Collmer, A. (1997) The type III (Hrp) secretion pathway of plant pathogenic bacteria: trafficking harpins, Avr proteins, and death. J. Bacteriol. 179, 5655–5662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfano, J.R. and Collmer, A. (2004) Type III secretion system effector proteins: double agents in bacterial disease and plant defense. Annu. Rev. Phytopathol. 42, 385–414. [DOI] [PubMed] [Google Scholar]
- Bogdanove, A.J. , Koebnik, R. , Lu, H. , Furutani, A. , Angiuoli, S.V. , Patil, P.B. , Van Sluys, M.A. , Ryan, R.P. , Meyer, D.F. , Han, S.W. , Aparna, G. , Rajaram, M. , Delcher, A.L. , Phillippy, A.M. , Puiu, D. , Schatz, M.C. , Shumway, M. , Sommer, D.D. , Trapnell, C. , Benahmed, F. , Dimitrov, G. , Madupu, R. , Radune, D. , Sullivan, S. , Jha, G. , Ishihara, H. , Lee, S.W. , Pandey, A. , Sharma, V. , Sriariyanun, M. , Szurek, B. , Vera‐Cruz, C.M. , Dorman, K.S. , Ronald, P.C. , Verdier, V. , Dow, J.M. , Sonti, R.V. , Tsuge, S. , Brendel, V.P. , Rabinowicz, P.D. , Leach, J.E. , White, F.F. and Salzberg, S.L. (2011) Two new complete genome sequences offer insight into host and tissue specificity of plant pathogenic Xanthomonas spp. J. Bacteriol. 193, 5450–5464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonas, U. , Van den Ackerveken, G. , Büttner, D. , Hahn, K. , Marois, E. , Nennstiel, D. , Noel, L. , Rossier, O. and Szurek, B. (2000) How the bacterial plant pathogen Xanthomonas campestris pv. vesicatoria conquers the host. Mol. Plant–Microbe Interact. 1, 73–76. [DOI] [PubMed] [Google Scholar]
- Brito, B. , Marenda, M. , Barberis, P. , Boucher, C. and Genin, S. (1999) prhJ and hrpG, two new components of the plant signal‐dependent regulatory cascade controlled by PrhA in Ralstonia solanacearum . Mol. Microbiol. 31, 237–251. [DOI] [PubMed] [Google Scholar]
- Büttner, D. and Bonas, U. (2002a) Getting across—bacterial type III effector proteins on their way to the plant cell. Eur. Mol. Biol. Organ. J. 21, 5313–5322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Büttner, D. and Bonas, U. (2002b) Port of entry—the type III secretion translocon. Trends Microbiol. 10, 186–192. [DOI] [PubMed] [Google Scholar]
- Büttner, D. and Bonas, U. (2006) Who comes first? How plant pathogenic bacteria orchestrate type III secretion. Curr. Opin. Microbiol. 9, 193–200. [DOI] [PubMed] [Google Scholar]
- Büttner, D. , Gürlebeck, D. , Noel, L.D. and Bonas, U. (2004) HpaB from Xanthomonas campestris pv. vesicatoria acts as an exit control protein in type III‐dependent protein secretion. Mol. Microbiol. 54, 755–768. [DOI] [PubMed] [Google Scholar]
- Büttner, D. , Noel, L. , Stuttmann, J. and Bonas, U. (2007) Characterization of the nonconserved hpaB‐hrpF region in the hrp pathogenicity island from Xanthomonas campestris pv. vesicatoria . Mol. Plant–Microbe Interact. 20, 1063–1074. [DOI] [PubMed] [Google Scholar]
- Casper‐Lindley, C. , Dahlbeck, D. , Clark, E.T. and Staskawicz, B.J. (2002) Direct biochemical evidence for type III secretion‐dependent translocation of the AvrBs2 effector protein into plant cells. Proc. Natl. Acad. Sci. USA, 99, 8336–8341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho, H.J. , Park, Y.J. , Noh, T.H. , Kim, Y.T. , Kim, J.G. , Song, E.S. , Lee, D.H. and Lee, B.M. (2008) Molecular analysis of the hrp gene cluster in Xanthomonas oryzae pathovar oryzae KACC10859. Microb. Pathog. 44, 473–483. [DOI] [PubMed] [Google Scholar]
- Cunnac, S. , Boucher, C. and Genin, S. (2004) Characterization of the cis‐acting regulatory element controlling HrpB‐mediated activation of the type III secretion system and effector genes in Ralstonia solanacearum . J. Bacteriol. 186, 2309–2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunnac, S. , Lindeberg, M. and Collmer, A. (2009) Pseudomonas syringae type III secretion system effectors: repertoires in search of functions. Curr. Opin. Microbiol. 12, 53–60. [DOI] [PubMed] [Google Scholar]
- De Feyter, R. , Kado, C.I. and Gabriel, D.W. (1990) Small, stable shuttle vectors for use in Xanthomonas . Gene 88, 65–72. [DOI] [PubMed] [Google Scholar]
- Furutani, A. , Nakayama, T. , Ochiai, H. , Kaku, H. , Kubo, Y. and Tsuqe, S. (2006) Identification of novel HrpXo regulons preceded by two cis‐acting elements, a plant‐inducible promoter box and a –10 box‐like sequence, from the genome database of Xanthomonas oryzae pv. oryzae . FEMS Microbiol. Lett. 259, 133–141. [DOI] [PubMed] [Google Scholar]
- Furutani, A. , Takaoka, M. , Sanada, H. , Noquchi, Y. , Oku, T. , Tsuno, K. , Ochiai, H. and Tsuqe, S. (2009) Identification of novel type III secretion effectors in Xanthomonas oryzae pv. oryzae . Mol. Plant–Microbe Interact. 22, 96–106. [DOI] [PubMed] [Google Scholar]
- Guo, W. , Cai, L.L. , Zou, H.S. , Ma, W.X. , Liu, X.L. , Zou, L.F. , Li, Y.R. , Chen, X.B. and Chen, G.Y. (2012) The ketoglutarate transport protein KgtP is secreted through the type III secretion system and contributes to virulence in Xanthomonas oryzae pv. oryzae . Appl. Environ. Microbiol. 78, 5672–5681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gürlebeck, D. , Thieme, F. and Bonas, U. (2006) Type III effector proteins from the plant pathogen Xanthomonas and their role in the interaction with the host plant. J. Plant Physiol. 163, 233–255. [DOI] [PubMed] [Google Scholar]
- He, S.Y. (1998) Type III protein secretion system in plant and animal pathogenic bacteria. Annu. Rev. Phytopathol. 36, 363–392. [DOI] [PubMed] [Google Scholar]
- Huguet, E. , Hahn, K. , Wengelnik, K. and Bonas, U. (1998) hpaA mutants of Xanthomonas campestris pv. vesicatoria are affected in pathogenicity but retain the ability to induce host‐specific hypersensitive reaction. Mol. Microbiol. 29, 1379–1390. [DOI] [PubMed] [Google Scholar]
- Jefferson, R.A. , Kavanagh, T.A. and Bevan, M.W. (1987) GUS fusions: β‐glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J. 6, 3901–3907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, J. , Zou, H.S. , Li, Y.R. and Chen, G.Y. (2009a) Expression of the hrcC, hrpE and hpa3 genes is not regulated by the hrpG and hrpX genes in a rice pathogen Xanthomonas oryzae pv. oryzicola . Wei Sheng Wu Xue Bao 49, 1018–1025. [PubMed] [Google Scholar]
- Jiang, W. , Jiang, B.L. , Xu, R.Q. , Huang, J.D. , Wei, H.Y. , Jiang, G.F. , Cen, W.J. , Liu, J. , Ge, Y.Y. , Li, G.H. , Su, L.L. , Hang, X.H. , Tang, D.J. , Lu, G.T. , Feng, J.X. , He, Y.Q. and Tang, J.L. (2009b) Identification of six type III effector genes with the PIP box in Xanthomonas campestris pv. campestris and five of them contribute individually to full pathogenicity. Mol. Plant–Microbe Interact. 22, 1401–1411. [DOI] [PubMed] [Google Scholar]
- Kim, J.G. , Park, B.K. , Yoo, C.H. , Jeon, E. , Oh, J. and Hwang, I. (2003) Characterization of the Xanthomonas axonopodis pv. glycines Hrp pathogenicity island. J. Bacteriol. 185, 3155–3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koebnik, R. , Kruger, A. , Thieme, F. , Urban, A. and Bonas, U. (2006) Specific binding of the Xanthomonas campestris pv. vesicatoria AraC‐type transcriptional activator HrpX to plant‐inducible promoter boxes. J. Bacteriol. 188, 7652–7660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli, U.K. (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227, 680–685. [DOI] [PubMed] [Google Scholar]
- Lee, B.M. , Park, Y.J. , Park, D.S. , Kang, H.W. , Kim, J.G. , Song, E.S. , Park, I.C. , Yoon, U.H. , Hahn, J.H. , Koo, B.S. , Lee, G.B. , Kim, H. , Park, H.S. , Yoon, K.O. , Kim, J.H. , Jung, C.H. , Koh, N.H. , Seo, J.S. and Go, S.J. (2005) The genome sequence of Xanthomonas oryzae pathovar oryzae KACC10331, the bacterial blight pathogen of rice. Nucleic Acids Res. 33, 577–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Y. , Xiao, Y. , Zou, L. , Zou, H. and Chen, G. (2012) Identification of HrpX regulon genes in Xanthomonas oryzae pv. oryzicola using a GFP visualization technique. Arch. Microbiol. 194, 281–291. [DOI] [PubMed] [Google Scholar]
- Li, Y.R. , Zou, H.S. , Che, Y.Z. , Cui, Y.P. , Guo, W. , Zou, L.F. , Chatterjee, S. , Biddle, E.M. , Yang, C.H. and Chen, G.Y. (2011a) A novel regulatory role of HrpD6 in regulating hrp‐hrc‐hpa genes in Xanthomonas oryzae pv. oryzicola . Mol. Plant–Microbe Interact. 24, 1086–1101. [DOI] [PubMed] [Google Scholar]
- Li, Y.R. , Che, Y.Z. , Zou, H.S. , Cui, Y.P. , Guo, W. , Zou, L.F. , Biddle, E.M. , Yang, C.H. and Chen, G.Y. (2011b) Hpa2 is required by HrpF to translocate Xanthomonas oryzae TAL effectors into rice for pathogenicity. Appl. Environ. Microbiol. 77, 3809–3818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller, J.H. (1972) Experiments in Molecular Genetics. New York: Cold Spring Harbor Laboratory Press. [Google Scholar]
- Mitsuhara, I. , Uqaki, M. , Hirochika, H. , Ohshima, M. , Murakami, T. , Gotoh, Y. , Katayose, Y. , Nakamura, R. , Nishimiya, S. , Ueno, K. , Mochizuki, A. , Tanimoto, H. , Tsugawa, H. , Otsuki, Y. and Ohashi, Y. (1996) Efficient promoter cassettes for enhanced expression of foreign genes in dicotyledonous and monocotyledonous plants. Plant Cell Physiol. 37, 49–59. [DOI] [PubMed] [Google Scholar]
- Mole, B.M. , Baltrus, D.A. , Dangl, J.L. and Grant, S.R. (2007) Global virulence regulation networks in phytopathogenic bacteria. Trends Microbiol. 15, 363–371. [DOI] [PubMed] [Google Scholar]
- Mukaihara, T. , Tamura, N. and Iwabuchi, M. (2010) Genome‐wide identification of a large repertoire of Ralstonia solanacearum type III effector proteins by a new functional screen. Mol. Plant–Microbe Interact. 23, 251–262. [DOI] [PubMed] [Google Scholar]
- Ochiai, H. , Inoue, Y. , Takeya, M. , Sasaki, A. and Kaku, H. (2005) Genome sequence of Xanthomonas oryzae pv. oryzae suggests contribution of large numbers of effector genes and insertion sequences to its race diversity. Jpn. Agric. R. Q. 39, 275–287. [Google Scholar]
- Roden, J.A. , Belt, B. , Ross, J.B. , Tachibana, T. , Varqas, J. and Mudgett, M.B. (2004) A genetic screen to isolate type III effectors translocated into pepper cells during Xanthomonas infection. Proc. Natl. Acad. Sci. USA 101, 16 624–16 629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossier, O. , Van den Ackerveken, G. and Bonas, U. (2000) HrpB2 and HrpF from Xanthomonas are type III‐secreted proteins and essential for pathogenicity and recognition by the host plant. Mol. Microbiol. 38, 828–838. [DOI] [PubMed] [Google Scholar]
- Salzberg, S.L. , Sommer, D.D. , Schatz, M.C. , Phillippy, A.M. , Rabinowicz, P.D. , Tsuge, S. , Furutani, A. , Ochiai, H. , Delcher, A.L. , Kelley, D. , Madupu, R. , Puiu, D. , Radune, D. , Shumway, M. , Trapnell, C. , Aparna, G. , Jha, G. , Pandey, A. , Patil, P.B. , Ishihara, H. , Meyer, D.F. , Szurek, B. , Verdier, V. , Koebnik, R. , Dow, J.M. , Ryan, R.P. , Hirata, H. , Tsuyumu, S. , Won Lee, S. , Seo, Y.S. , Sriariyanum, M. , Ronald, P.C. , Sonti, R.V. , Van Sluys, M.A. , Leach, J.E. , White, F.F. and Bogdanove, A.J. (2008) Genome sequence and rapid evolution of the rice pathogen Xanthomonas oryzae pv. oryzae PXO99. BMC Genomics 9, 204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook, J. , Fritsch, E.F. and Maniatis, T. (1989) Molecular Cloning: A Laboratory Manual, 2nd edn New York: Cold Spring Harbor Laboratory Press. [Google Scholar]
- Schechter, L.M. , Roberts, K.A. , Jamir, Y. , Alfano, J.R. and Collmer, A. (2004) Pseudomonas syringae type III secretion system targeting signals and novel effectors studied with a Cya translocation reporter. J. Bacteriol. 186, 543–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schechter, L.M. , Vencato, M. , Jordan, K.L. , Schneider, S.E. , Schneider, D.J. and Collmer, A. (2006) Multiple approaches to a complete inventory of Pseudomonas syringae pv. tomato DC3000 type III secretion system effector proteins. Mol. Plant–Microbe Interact. 19, 1180–1192. [DOI] [PubMed] [Google Scholar]
- Schulte, R. and Bonas, U. (1992) Expression of the Xanthomonas campestris pv. vesicatoria hrp gene cluster, which determines pathogenicity and hypersensitivity on pepper and tomato, is plant inducible. J. Bacteriol. 174, 815–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva, A.C. , Ferro, J.A. , Reinach, F.C. , Farah, C.S. , Furlan, L.R. , Quaggio, R.B. , Monteiro‐Vitorello, C.B. , Van Sluys, M.A. , Almeida, N.F. , Alves, L.M. , do Amaral, A.M. , Bertolini, M.C. , Camargo, L.E. , Camarotte, G. , Cannavan, F. , Cardozo, J. , Chambergo, F. , Ciapina, L.P. , Cicarelli, R.M. , Coutinho, L.L. , Cursino‐Santos, J.R. , El‐Dorry, H. , Faria, J.B. , Ferreira, A.J. , Ferreira, R.C. , Ferro, M.I. , Formighieri, E.F. , Franco, M.C. , Greggio, C.C. , Gruber, A. , Katsuyama, A.M. , Kishi, L.T. , Leite, R.P. , Lemos, E.G. , Lemos, M.V. , Locali, E.C. , Machado, M.A. , Madeira, A.M. , Martinez‐Rossi, N.M. , Martins, E.C. , Meidanis, J. , Menck, C.F. , Miyaki, C.Y. , Moon, D.H. , Moreira, L.M. , Novo, M.T. , Okura, V.K. , Oliveira, M.C. , Oliveira, V.R. , Pereira, H.A. , Rossi, A. , Sena, J.A. , Silva, C. , de Souza, R.F. , Spinola, L.A. , Takita, M.A. , Tamura, R.E. , Teixeira, E.C. , Tezza, R.I. , Trindade dos Santos, M. , Truffi, D. , Tsai, S.M. , White, F.F. , Setubal, J.C. and Kitajima, J.P. (2002) Comparison of the genomes of two Xanthomonas pathogens with differing host specificities. Nature 417, 459–463. [DOI] [PubMed] [Google Scholar]
- Song, C. and Yang, B. (2010) Mutagenesis of 18 type III effectors reveals virulence function of XopZ(PXO99) in Xanthomonas oryzae pv. oryzae . Mol. Plant–Microbe Interact. 23, 893–902. [DOI] [PubMed] [Google Scholar]
- Sugio, A. , Yang, B. and White, F.F. (2005) Characterization of the hrpF pathogenicity peninsula of Xanthomonas oryzae pv. oryzae . Mol. Plant–Microbe Interact. 18, 546–554. [DOI] [PubMed] [Google Scholar]
- Tampakaki, A.P. , Fadouloqlou, V.E. , Gazi, A.D. , Panopoulos, N.J. and Kokkinidis, M. (2004) Conserved features of type III secretion. Cell. Microbiol. 6, 805–816. [DOI] [PubMed] [Google Scholar]
- Tang, X. , Xiao, Y. and Zhou, J.M. (2006) Regulation of the type III secretion system in phytopathogenic bacteria. Mol. Plant–Microbe Interact. 19, 1159–1166. [DOI] [PubMed] [Google Scholar]
- Tsuge, S. , Terashinma, S. , Furutani, A. , Ochiai, H. , Oku, T. , Tsuno, K. , Kaku, H. and Kubo, Y. (2005) Effects on promoter activity of base substitutions in the cis‐acting regulatory element of HrpXo regulons in Xanthomonas oryzae pv. oryzae . J. Bacteriol. 187, 2308–2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber, E. , Berger, C. , Bonas, U. and Koebnik, R. (2007) Refinement of the Xanthomonas campestris pv. vesicatoria hrpD and hrpE operon structure. Mol. Plant–Microbe Interact. 20, 559–567. [DOI] [PubMed] [Google Scholar]
- Wei, Z. , Kim, J.F. and Beer, S.V. (2000) Regulation of hrp genes and type III protein secretion in Erwinia amylovora by HrpX/HrpY, a novel two component system, and HrpS. Mol. Plant–Microbe Interact. 13, 1251–1262. [DOI] [PubMed] [Google Scholar]
- Wengelnik, K. and Bonas, U. (1996) HrpXv, an AraC‐type regulator, activates expression of five of the six loci in the hrp cluster of Xanthomonas campestris pv. vesicatoria . J. Bacteriol. 178, 3462–3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wengelnik, K. , Van den Ackerveken, G. and Bonas, U. (1996) HrpG, a key hrp regulatory protein of Xanthomonas campestris pv. vesicatoria is homologous to two‐component response regulators. Mol. Plant–Microbe Interact. 9, 704–712. [DOI] [PubMed] [Google Scholar]
- Wengelnik, K. , Rossier, O. and Bonas, U. (1999) Mutations in the regulatory gene hrpG of Xanthomonas campestris pv. vesicatoria result in constitutive expression of all hrp genes. J. Bacteriol. 181, 6828–6831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao, Y. , Lu, Y. , Heu, S. and Hutcheson, S.W. (1992) Organization and environmental regulation of the Pseudomonas syringae pv. syringae 61 hrp cluster. J. Bacteriol. 174, 1734–1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao, Y.L. , Li, Y.R. , Liu, Z.Y. , Xiang, Y. and Chen, G.Y. (2007) Establishment of the hrp‐inducing systems for the expression of the hrp genes of Xanthomonas oryzae pv. oryzicola . Wei Sheng Wu Xue Bao 47, 396–401. [PubMed] [Google Scholar]
- Yang, B. and White, F.F. (2004) Diverse members of the AvrBs3/PthA family of type III effectors are major virulence determinants in bacterial blight disease of rice. Mol. Plant–Microbe Interact. 17, 1192–1200. [DOI] [PubMed] [Google Scholar]
- Zou, L.F. , Wang, X.P. , Xiang, Y. , Zhang, B. , Li, Y.R. , Xiao, Y.L. , Wang, J.S. , Walmsley, A.R. and Chen, G.Y. (2006) Elucidation of the hrp clusters of Xanthomonas oryzae pv. oryzicola that control the hypersensitive response in nonhost tobacco and pathogenicity in susceptible host rice. Appl. Environ. Microbiol. 72, 6212–6224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou, L.F. , Li, Y.R. and Chen, G.Y. (2011) A non‐marker mutagenesis strategy to generate poly‐hrp gene mutants in the rice pathogen Xanthomonas oryzae pv. oryzicola . Agric. Sci. China 10, 1139–1150. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 (A) Physical and functional map of hrpE3 and flanking genes (hpaB and hpa4) in the hrp (hypersensitive response and pathogenicity) cluster (AY875714) of Xanthomonas oryzae pv. oryzicola (Xoc) strain RS105. Open arrows indicate length, location and orientation of hpaB, hrpE3 and hpa4. (B) Strategy used for the deletion of hrpE3 by removal of a 285‐bp coding region (white) of hrpE3 using the E3UP (amplifies approximately 500 bp, grey) and E3II (amplifies 750 bp, black) primers. The fused PCR fragments were digested at SmaI and HindIII, and ligated in pKMS1. (C) Confirmation of deletion in mutant RΔhrpE3 by polymerase chain reaction (PCR) amplification with the primer pair (upF and downR) and by Southern hybridization using the left fragment as the probe, confirming that the PCR product and hybridized band in RΔhrpE3 is 285 bp shorter than that in the wild‐type RS105. Genomic DNAs from Xoc RS105 and RΔhrpE3 were digested with MscI.
Table S1 List of oligonucleotide primers used in this study.


