Summary
Xanthomonas axonopodis pv. citri (Xac), the bacterium that causes citrus canker, contains a gene in the hrp [for hypersensitive response (HR) and pathogenicity] cluster that encodes a harpin protein called Hpa1. Hpa1 produced HR in the nonhost plants tobacco, pepper and Arabidopsis, whereas, in the host plant citrus, it elicited a weak defence response with no visible phenotype. Co‐infiltrations of Xac with or without the recombinant Hpa1 protein in citrus leaves produced a larger number of cankers in the presence of the protein. To characterize the effect of Hpa1 during the disease, an XacΔhpa1 mutant was constructed, and infiltration of this mutant caused a smaller number of cankers. In addition, the lack of Hpa1 hindered bacterial aggregation both in solution and in planta. Analysis of citrus leaves infiltrated with Hpa1 revealed alterations in mesophyll morphology caused by the presence of cavitations and crystal idioblasts, suggesting the binding of the harpin to plant membranes and the elicitation of signalling cascades. Overall, these results suggest that, even though Hpa1 elicits the defence response in nonhost plants and, to a lesser extent, in host plants, its main roles in citrus canker are to alter leaf mesophyll structure and to aggregate bacterial cells, and thus increase virulence and pathogen fitness. We expressed the N‐terminal and C‐terminal regions and found that, although both regions elicited HR in nonhost plants, only the N‐terminal region showed increased virulence and bacterial aggregation, supporting the role of this region of the protein as the main active domain.
Introduction
Plant‐pathogenic bacteria colonize their hosts through the secretion of virulence effector proteins which depends on the type III protein secretion system (TTSS). This system mediates the translocation of effector proteins across the bacterial membrane and the plant cell walls and plasma membranes. TTSS is encoded by the hrp [for hypersensitive response (HR) and pathogenicity] cluster, which is indispensable for pathogenicity in the host plant and for the induction of HR in nonhost plants. HR is a rapid, local, programmed cell death that is induced on recognition of the pathogen, and inhibits pathogen growth within the infected plants. The hrp cluster is composed of three different gene types: (i) hrp, only found in phytopathogens; (ii) hrc (hrp conserved in plant and animal bacterial pathogens), which constitutes the core of the translocon; and (iii) hpa (hrp associated), which contributes to pathogenicity and to HR induction in nonhost plants, but is not essential for bacterial pathogenic interactions with plants (Büttner and Bonas, 2002).
Among hpa genes are those that encode harpins, which are glycine‐rich, cysteine‐lacking, heat‐stable proteins. Harpins are secreted by the Hrp TTSS and trigger an HR‐like response when infiltrated into nonhost plants (He et al., 1993). The extensively studied harpin HrpZ of Pseudomonas syringae pv. phaseolicola was found to bind to lipid bilayers and to form ion‐conducting pores, which suggests that these proteins could exert their function from outside the plant cell (Lee et al., 2001b). Recently, it has been determined that the region of this protein that is essential for HR elicitor activity is also required for protein oligomerization and membrane pore formation (Haapalainen et al., 2011). Furthermore, this protein could elicit innate immune responses in plants in a mitogen‐activated protein kinase (MAPK)‐dependent signalling mechanism (Desikan et al., 2001; Lee et al., 2001a). The fact that harpins are secreted by the pathogen and exert their action from outside the plant cell (Büttner et al., 2004), and that they are able to bind to cell membranes and trigger the innate immune response, constitutes evidence that has led to a proposal that they resemble pathogen‐associated molecular patterns (PAMPs) (Engelhardt et al., 2009). In support of the idea that harpins participate in the induction of defence responses, it has been observed that transcripts of plant defence‐related genes are increased in plants which have been infiltrated with purified harpin proteins (Dong et al., 1999; Liu et al., 2006; Wang et al., 2008). Indeed, several harpin‐expressing plants have enhanced defence responses against different pathogens, and this suggests that harpins prime the defence response (Miao et al., 2010a; Peng et al., 2004; Shao et al., 2008; Sohn et al., 2007).
Another function attributed to the harpin HrpN of the phytopathogen Erwinia chrysanthemi is to favour cell aggregation as, when absent, the aggregative behaviour manifested as a pellicle at the air–liquid interface disappears. HrpN remains cell surface associated and it has been suggested that it might serve as an intercellular aggregative factor (Yap et al., 2006). However, the influence of the aggregative role of HrpN on bacterial virulence has not been studied (Yap et al., 2006).
In Xanthomonas, Hpa1 from X. oryzae pv. oryzae (Hpa1Xoo) and X. oryzae pv. oryzicola (Hpa1Xoc), HpaG from X. axonopodis pv. glycines (HpaGXag), XopA from X. axonopodis pv. vesicatoria (Xav) and HpaXm from X. citri ssp. malvacearum have been studied. These TTSS‐secreted proteins are delivered into the intercellular spaces of plant tissues and, when mutants in these genes are assayed in plant–pathogen interactions, they show reduced bacterial virulence (Kim et al., 2003; Noel et al., 2002; Zhu et al., 2000). Moreover, HpaGXag and Hpa1Xoo (Kim et al., 2003), Hpa1Xoc (Wang et al., 2008) and HpaXm (Miao et al., 2010b) elicit HR when they are infiltrated into leaves of nonhost plants, and recent reports have linked this elicitor activity to the fact that HpaGXag can oligomerize and form β‐sheet‐rich fibrils and, finally, amyloid‐like fibres, demonstrating that HR is dependent on this amyloid structure (Oh et al., 2007). Furthermore, minimum peptides in the N‐terminal region of the protein have been analysed (Kim et al., 2004; Wang et al., 2008), and it has been shown that the peptide involved in HR induction adopts a coiled‐coil conformation which, in turn, promotes oligomer formation (Ji et al., 2011; Miao et al., 2010b; Wang et al., 2008).
In this work, we analyse the role of Hpa1 from Xanthomonas axonopodis pv. citri (Xac) in citrus canker, and its capacity as an inducer of the plant basal immune response, and discuss the possible function of this harpin protein in the plant–pathogen interaction.
Results
Hpa1 induces HR in nonhost plants and a weak defence response in citrus
Hpa1 from Xac is highly similar to HpaGXag and Hpa1Xoo (92% and 69% identity, respectively), and these two previously studied harpins elicit an HR in tobacco leaves (Kim et al., 2003; Wang et al., 2008). Therefore, to validate whether Hpa1 from Xac could also elicit an HR, hpa1 was cloned, expressed in Escherichia coli and purified. The infiltration of Hpa1 pure protein into tobacco and pepper leaves showed typical HRs 16 h after injections, although no reaction was observed in cotton leaves (Fig. S1, see Supporting Information). The lack of HR in this plant may be explained by the fact that HR induction depends on the harpin–plant interaction; previous results have revealed that different harpins can induce diverse reactions in plant tissue. This is the case for HpaGXag, Hpa1Xoo, XopA and hrpN, which cause different reactions in tobacco leaves, ranging from no reaction in XopA infiltration to HR caused by HpaGXag, even at 50 nm (Kim et al., 2003).
We then analysed the response of different Arabidopsis thaliana cultivars to this harpin. Arabidopsis thaliana Col‐0 (Fig. 1A), Ler, Ws, Nossen and C24 leaves (Fig. S1) were infiltrated with 5 μm Hpa1 and, in all cases, an HR was visualized; however, at lower harpin concentrations, no response was observed (data not shown). We then tested whether Hpa1 could also elicit an HR in citrus leaves, as citrus is a Xac host plant. Therefore, Hpa1 was infiltrated into citrus leaves; no reaction was observed (Fig. 1A), even at 25 μm (data not shown), indicating that, in citrus, Hpa1 is not involved in triggering a cell death response. We then investigated its role as a basal defence response elicitor. We analysed whether Hpa1 was capable of triggering early events that occur during the basal immune response in A. thaliana and Citrus sinensis, such as the induction of MAPK cascades leading to the activation of WRKY‐type transcription factors and the expression of defence‐associated genes (Boller and Felix, 2009). First, leaves of host and nonhost plants were infiltrated with 5 μm Hpa1, and RNA from protein‐treated and mock infiltrations was extracted after 5 h for quantitative real‐time polymerase chain reaction (qRT‐PCR) analysis. The analysed genes were MAPK3 and MAPK kinase 4 (MKK4), which have been observed to be components of the kinase cascade of the plant basal response (Asai et al., 2002), and the WRKY30 transcription factor, which is induced by oxidative stress (Scarpeci et al., 2008) and by pathogen fungal elicitors. The results showed that MAPK3, MKK4 and WRKY30 expression was induced by the harpin (P < 0.01) in A. thaliana (Fig. 1B). However, citrus leaves showed no significant differences (P < 0.01) in the expression of MAPK3 and WRKY30 compared with mock infiltrations, and MKK4 retained the level of induction obtained for A. thaliana (Fig. 1B).
Figure 1.
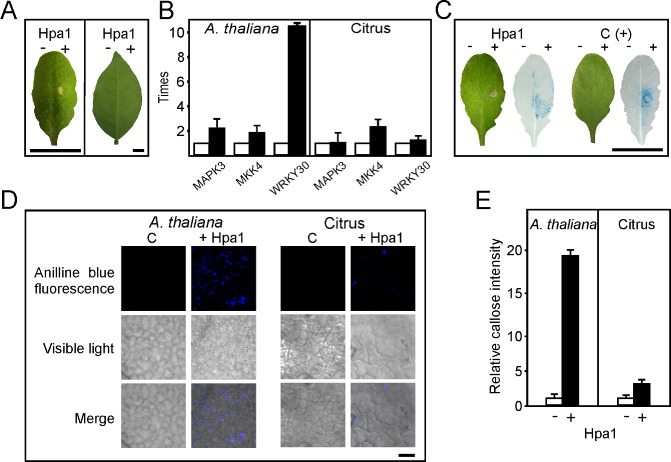
Analysis of Hpa1 elicitation of basal responses in Arabidopsis thaliana and citrus leaves. (A) Arabidopsis thaliana Col‐0 (left) and Citrus sinensis (right) leaves, 16 h after infiltration with 5 μm Hpa1 in 50 mm tris(hydroxymethyl)aminomethane (Tris, pH 8). As control, the same buffer was infiltrated on the left part of the leaves. (B) Quantitative real‐time polymerase chain reaction (qRT‐PCR) of genes involved in defence signalling. RNA extracted from leaves infiltrated with or without Hpa1 was analysed by RT‐PCR. Black bars indicate the expression levels of Hpa1‐treated leaves relative to mock infiltrations (white bars). Values represent the means of four independent experiments. Error bars indicate standard deviations. (C) Arabidopsis thaliana PrAtWRKY30::GUS was used to analyse WRKY30 expression after 5 h of treatment with Hpa1, or fungal elicitors [C(+)] as positive control or buffer as negative control. The hypersensitive response (HR) was followed in the left leaves 16 h after infiltration. (D) Aniline blue staining of callose deposition in A. thaliana and citrus leaves infiltrated with buffer and Hpa1, observed by light and fluorescence microscopy. (E) Relative callose intensities were quantified as described in Experimental procedures. − and + denote without and with Hpa1, respectively. Values represent means standardized to the mean callose intensity in buffer‐treated leaves. Error bars indicate standard deviations in the case of control treatment or propagated error in the case of Hpa1 treatment. The results are representative of three independent experiments. Bars represent 1 cm in (A) and (C) and 20 μm in (D).
To gain further insight into Hpa1‐triggered signalling, and as we observed a noticeable increase in WRKY30 expression, we performed experiments using an A. thaliana line bearing a β‐glucuronidase (GUS) reporter gene fused to the promoter region of WRKY30 (Scarpeci et al., 2008), which is turned on in A. thaliana leaves treated with fungal elicitors. Initially, we infiltrated A. thaliana PrAtWRKY30::GUS leaves with Hpa1 and, after 16 h, a clear HR was observed (Fig. 1C). Therefore, to detect the effect of Hpa1 on WRKY30 expression, GUS activity was assayed in A. thaliana leaves after 5 h of Hpa1 infiltration. The results showed that WRKY30 was induced by Hpa1 treatment in a similar manner to treatment with fungal elicitors (Fig. 1C).
As another marker for PAMP‐triggered immunity (Nguyen et al., 2010), we assayed callose deposition in A. thaliana and citrus leaves treated with 5 μm Hpa1. Differential stained leaves, unlike mock infiltrations, revealed that Hpa1 induced significantly greater callose deposition in citrus leaves than was induced by mock infiltration (P < 0.05), but less extensive than that induced in A. thaliana leaves (Fig. 1D, E), suggesting that Hpa1 is able to induce a weak basal immune response in the host plant. To test whether Hpa1 infiltration in citrus leaves could provide some immunity to subsequent bacterial inoculations, we infiltrated Hpa1 and, after 12 h, re‐infiltrated the same tissue with Xac wild‐type at 106 colony‐forming units (CFU)/mL, and quantified Xac growth in these leaves. As a control, we pre‐infiltrated the tissue with the TTSS mutant strain XachrpB– (Dunger et al., 2005), which reduces Xac growth when it is pre‐infiltrated in citrus leaves (Zimaro T. et al., IBR‐CONICET, Rosario, Argentina, unpublished results). As expected, we observed that Xac grew less well when the leaves had been treated previously with the nonpathogenic XachrpB– mutant that induced a response (Zimaro T. et al., IBR‐CONICET, Rosario, Argentina, unpublished results), whereas Hpa1 did not provide protection to subsequent Xac infections (Fig. S2, see Supporting information).
hpa1 is expressed in planta, is required for full virulence and co‐infiltrations of Xac and Hpa1 increase the number of cankers
To evaluate whether hpa1 is expressed during the pathogenic process, RNA was obtained from Xac recovered from C. sinensis‐infected leaves 0, 1, 2 and 3 days post‐inoculation. qRT‐PCRs showed that hpa1 expression increased on the first day post‐inoculation, reaching significantly different values (P < 0.05) each time it was analysed; it was highly induced at 3 days post‐inoculation (Fig. 2), suggesting a role of Hpa1 in the pathogenicity process.
Figure 2.
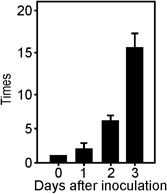
Analysis of hpa1 expression in planta. Quantitative real‐time polymerase chain reaction (qRT‐PCR) of hpa1 from Xanthomonas axonopodis pv. citri (Xac) infiltrated in citrus leaves. RNA extracted from Xac recovered from citrus leaves at the times stated was analysed by RT‐PCR. Bars indicate the expression levels of hpa1 relative to 0 days post‐inoculation. Values represent the means of three independent experiments. Error bars indicate standard deviations.
Next, we decided to investigate whether this protein plays a role in Xac virulence. To analyse the role of Hpa1 in citrus canker, we constructed an hpa1 deletion mutant strain, called XacΔhpa1, by marker exchange. We also complemented this mutant by conjugating a replicative vector that bears a copy of hpa1 (pBBR1Hpa1) into XacΔhpa1, generating XacΔhpa1c. In order to test the ability of these mutants to produce disease in citrus plants, XacΔhpa1, XacΔhpa1c and the wild‐type strains were infiltrated in citrus leaves at a concentration of 107 CFU/mL, and a reduction in disease symptoms in the mutant strain infiltration was observed (Fig. 3A). This virulence reduction was reversed in the infiltration performed with the complemented strain XacΔhpa1c (Fig. 3A). Afterwards, bacterial growth in planta was quantified in the three bacterial inoculations for 10 days. Xac wild‐type grew more rapidly than the mutant strain (almost one order of magnitude higher) each time it was analysed (P < 0.05), and the complemented strain showed intermediate growth (Fig. 3B). Moreover, infiltrations of serially diluted bacteria showed a significantly larger number of cankers (P < 0.05) per infiltrated area of wild‐type relative to XacΔhpa1 inoculations (Fig. 3C). At the highest initial CFU/mL infiltrations, five times more cankers were observed for the wild‐type, whereas, at 1 × 105 CFU/mL, cankers were observed only in wild‐type bacterial infiltrations (Fig. 3D). We then analysed whether the co‐infiltration of Xac at different concentrations (5 × 105 to 103 CFU/mL) and Hpa1 at the same time may provide clues about the participation of harpin in canker formation. Again, we observed that, in this case, the bacteria were significantly (P < 0.05) more virulent and more cankers were formed than in control infiltrations without the protein (Fig. 3E, F). As control, Xac was co‐infiltrated with 6His‐thioredoxin (Trx) and produced the same number of cankers as the wild‐type bacterium alone (data not shown).
Figure 3.
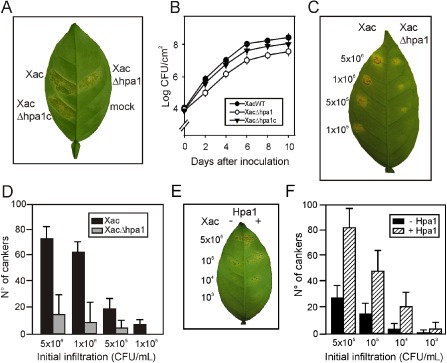
Hpa1 enhances Xanthomonas axonopodis pv. citri (Xac) virulence in citrus leaves. (A) Xac wild‐type, XacΔhpa1 and XacΔhpa1c strains were inoculated at 107 colony‐forming units (CFU)/mL, diluted in 10 mm MgCl2, into the intercellular spaces of fully expanded citrus leaves. The representative photograph was taken at 7 days post‐inoculation. (B) Bacterial growth of Xac, XacΔhpa1 and XacΔhpa1c in citrus leaves inoculated as described in (A). Values represent the means of three independent experiments. Error bars are standard deviations. (C) Serial dilutions of Xac and XacΔhpa1 were inoculated at the concentrations stated in citrus leaves. (D) Quantification of canker number in citrus leaves inoculated as described in (C). Bars are the means of 10 leaves assayed and error bars are standard deviations. The results are representative of three independent experiments. (E) Co‐infiltrations with Hpa1 enhance bacterial virulence. Xac wild‐type was co‐infiltrated with 5 μm Hpa1 in the right halves of citrus leaves at the bacterial concentrations indicated. (F) Quantification of canker number in leaves inoculated as described in (E).
Hpa1 aggregates bacterial cells in culture medium
We then evaluated whether Hpa1 may be an aggregative factor, and thus function as a recruiter of bacterial cells. As hpa1 is expressed in XVM2 (a minimal medium that mimics the conditions of the plant apoplast; Astua‐Monge et al., 2005) and not in rich medium (data not shown), we used this medium to analyse the ability of Xac, XacΔhpa1 and XacΔhpa1c to aggregate. Xac wild‐type showed bacterial aggregation when cultured in static liquid medium, whereas XacΔhpa1 was unable to associate, and the complemented strain aggregated in the same manner as Xac wild‐type (Fig. 4A); this suggests that Hpa1 is involved in cell–cell aggregation. Moreover, green fluorescent protein (GFP)‐expressing Xac wild‐type and XacΔhpa1 were cultured in static liquid XVM2 and visualized by confocal laser scanning microscopy after biofilm formation was observed. Notably, Xac wild‐type formed bacterial aggregates (Fig. 4B) that were absent in the hpa1 mutant strain culture (Fig. 4C). In order to further determine the role of Hpa1 as a bacterial aggregation protein, bacterial cell–cell aggregation in the presence and absence of Hpa1 recombinant protein was analysed. GFP‐expressing Xac previously cultured in XVM2 medium was incubated with (Fig. 4E) or without (Fig. 4D) 5 μm Hpa1 for 3 h and visualized by confocal microscopy. As an additional control, we incubated the bacterium with 6His‐Trx at the same concentration as Hpa1 and observed the same behaviour as with the Xac wild‐type alone (see below). Interestingly, the presence of the harpin protein caused a bacterial association resembling macrocolonies (Fig. 4E). We also analysed the effect of Hpa1 on XacΔhpa1 and observed that, in the presence of Hpa1, the mutant also formed macrocolonies (data not shown).
Figure 4.
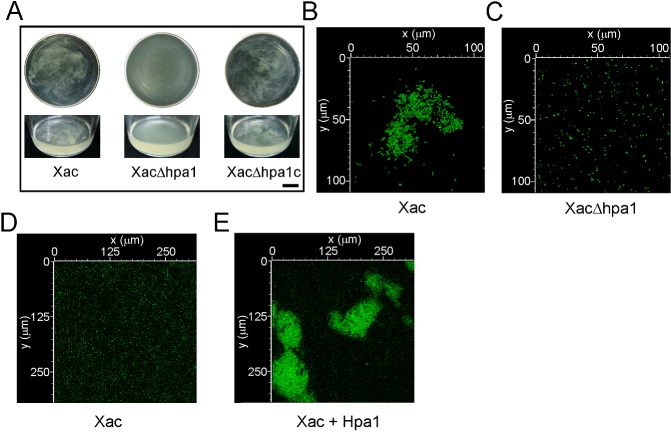
Hpa1 aggregates Xanthomonas axonopodis pv. citri (Xac) bacterial cells. (A) Representative photographs of Xac wild‐type, XacΔhpa1 and XacΔhpa1c strains grown statically in XVM2 medium in 100‐mL flasks. Top panel shows flask top views and bottom panel shows lateral views. Bar, 1 cm. (B) Photograph of a representative aggregate formed by green fluorescent protein (GFP)‐expressing Xac wild‐type previously grown statically in XVM2 medium. (C) Representative photograph of GFP‐expressing XacΔhpa1 grown as in (B). (D, E) Representative photographs of confocal laser scanning microscopy of the GFP‐expressing Xac wild‐type without (D) and with (E) 5 μm Hpa1.
Hpa1 is able to form amyloid fibres in vitro
As Hpa1 is very similar to HpaGXag, a harpin that has been shown previously to form amyloid fibrils (Oh et al., 2007), we decided to investigate the capacity of Hpa1 to form such a structure. To this end, we performed a binding assay with Congo red (CR). Like other harpins (Oh et al., 2007), Hpa1 fibrils bound to CR, and thus a red shift in the spectrum was observed, with a maximum difference in absorbance between CR alone and CR bound to Hpa1 fibrils at approximately 540 nm (Fig. 5A). Contrary to this, Trx used as a control did not bind to CR (Fig. 5B). This result suggests that the ability of Hpa1 to aggregate bacterial cells depends on its fibrillar structure.
Figure 5.
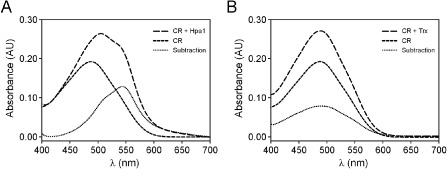
Hpa1 forms fibrils, revealed by Congo red (CR) binding. Absorbance spectra of the CR solution in the absence (short broken line) and presence (long broken line) of Hpa1 fibrils (A) and thioredoxin (Trx) (B), corrected for fibril scattering. The dotted lines indicate, for each protein analysed, the difference between CR with protein fibrils and CR alone. Each spectrum shown is the result of the accumulation of three runs.
Hpa1 is able to aggregate bacteria inside citrus tissue
Next, we determined whether this association effect extended to bacterial cells in citrus leaves. We infiltrated GFP‐expressing Xac and XacΔhpa1 in citrus leaves at 107 CFU/mL and visualized in vivo the development of bacterial aggregates during the infection process by confocal laser scanning microscopy. The bacterial cells were observed by the emission of GFP fluorescence and the plant tissue by the emission of chlorophyll autofluorescence. We took serial images every 0.5 μm, covering the entire infected leaf width. After 24 h of infection, wild‐type bacteria showed more bacterial aggregates than the mutant strain (Fig. 6A, B). Interestingly, the analysis of the 0.5‐μm stacks showed that the wild‐type strain formed larger bacterial cumuli, measuring 19.1 ± 1.12 μm in length, which were mainly observed near the stomata, whereas the mutant strain formed fewer and smaller bacterial aggregates of 8.1 ± 0.64 μm in length. Moreover, the observation of the 0.5‐μm stacks (z axis) revealed that Xac wild‐type developed bacterial colonies that extended to a greater depth from the abaxial surface (the surface with stomata) to the adaxial surface compared with the hpa1 mutant strain, suggesting that Xac wild‐type could advance further and become more embedded in the tissue (Fig. 6A, B). These results also suggest that, in planta, Hpa1 is able to agglomerate cells, and indicate that Hpa1 may contribute, at least in part, to increase virulence by aggregating bacterial cells in the plant tissue.
Figure 6.
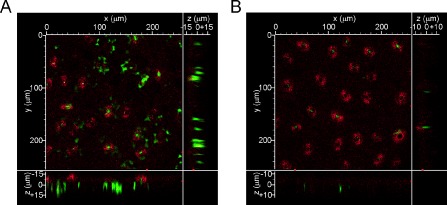
Hpa1 aggregates Xanthomonas axonopodis pv. citri (Xac) bacterial cells in citrus leaves. Representative photographs of confocal laser scanning microscopy of the green fluorescent protein (GFP)‐expressing bacteria 24 h post‐infiltration in citrus leaves. (A) Xac wild‐type. (B) XacΔhpa1.
Hpa1 alters citrus leaf morphology
Given that Hpa1 is able to induce HR in tobacco and A. thaliana plants, whereas no visible reaction is observed in citrus leaves, we questioned whether the protein could be exerting any other action in citrus leaf tissue morphology. Accordingly, we infiltrated Hpa1 at 5 μm in citrus leaves and, after 3 and 16 h, serial cross‐sections were analysed with a microscope equipped with an ocular micrometer which allows the observation of morphological or structural changes and quantitative measurements. Figure 7A shows that Hpa1 causes alterations in citrus leaves, including the disruption of the spongy mesophyll structure, revealed by the appearance of translucent cell‐lacking areas (top panels) and leaf contour distortion and widening (bottom panels). In addition, leaf thickness was increased after 16 h of treatment with Hpa1 (Fig. 7B). Normal citrus leaves present schizogenous cavities (Fig. 7A, bottom panel), and we observed that the area of these cavities was increased significantly (P < 0.05) after 3 or 16 h of Hpa1 treatment compared with buffer‐infiltrated leaves (Fig. 7C). After 16 h of Hpa1 treatment, we observed only half the number of cavities compared with 3 h of Hpa1 treatment or buffer treatment (data not shown), suggesting that this treatment with Hpa1 has a deleterious effect on cavity structure. Moreover, Hpa1 treatment increased significantly (P < 0.05) the number of idioblasts at the abaxial face of the leaves (Fig. 7D). These changes in leaf morphology were more noticeable in leaves at 16 h post‐infiltration than at 3 h post‐infiltration; this suggests a long‐term effect that might be attributed to the requirement for protein aggregation, which may occur later, although this requires further investigation.
Figure 7.
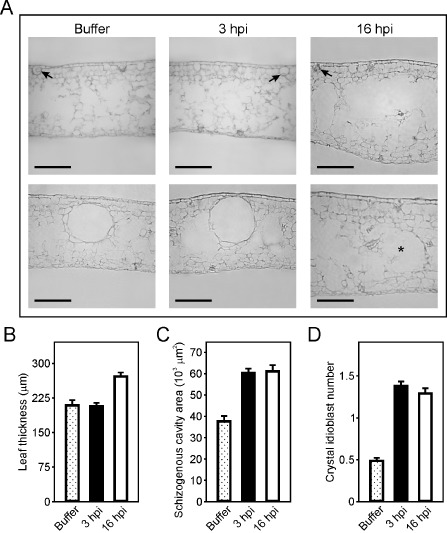
Hpa1 produces changes in the structure and morphology of citrus leaf tissue. (A) Representative transmission microscopy photographs of citrus leaf cross‐sections infiltrated with buffer or 5 μm Hpa1 protein for 3 or 16 h. Top panel shows the distortion and widening of the leaf contours after 16 h of Hpa1 treatment. Bottom panel shows the enhancement in cavity size after 3 h of Hpa1 treatment and a ghost of a pre‐existing cavity (denoted with an asterisk) after 16 h of Hpa1 treatment. Arrows indicate idioblast structures. Bars, 100 μm. These cross‐sections were used for microscopic quantitative measurements of leaf thickness (B), schizogenous cavity area (C) and idioblast number (D) in leaves infiltrated with buffer or 5 μm Hpa1 protein for 3 or 16 h. Thirty cross‐sections were analysed for each treatment from three independent experiments. Values represent the mean of these different measurements; error bars indicate standard deviations. hpi, hours post‐inoculation.
Contribution of N‐terminal and C‐terminal regions of Hpa1 to HR induction in nonhost plants, canker formation and bacterial aggregation
We then analysed whether Hpa1 N‐terminal and C‐terminal domains that bear two coiled‐coil regions, studied previously (Ji et al., 2011; Wang et al., 2008), are involved in HR induction, bacterial virulence and aggregation. We expressed and purified the two domains Hpa11–57 and Hpa158–137 fused to Trx, as their expression without the carrier protein could not be purified, probably because of their lability. Both protein regions induced HR in tobacco, pepper and A. thaliana Col‐0 (Fig. 8A), Ler, Ws, Nossen and C24 (Fig. S3, see Supporting Information). Both of the peptides were co‐infiltrated at 5 μm with Xac wild‐type at 5 × 106 and 105 CFU/mL, and a significantly (P < 0.05) larger number of cankers were observed in the presence of Hpa11–57, whereas no significant differences (P < 0.05) were observed when co‐infiltrations were performed with Hpa158–137 (Fig. 8B, C). At the lowest bacterial concentration used (5 × 103 CFU/mL), no significant differences (P < 0.05) were observed among treatments, probably because of the random and low quantity of cankers observed at this bacterial inoculums concentration (Fig. 8B, C). Finally, and in agreement with the previous results, Hpa11–57 caused bacterial cell–cell aggregation of GFP‐expressing Xac visualized by confocal microscopy, whereas the C‐terminal region produced only rare and small aggregates (Fig. 8D). Nevertheless, aggregates with the complete Hpa1 protein were larger (Fig. 4E) than those observed with the N‐terminal region alone, suggesting that the whole protein is required for full activity. As a control, we incubated the bacterium with 6His‐Trx and no aggregates were observed (Fig. 8D).
Figure 8.
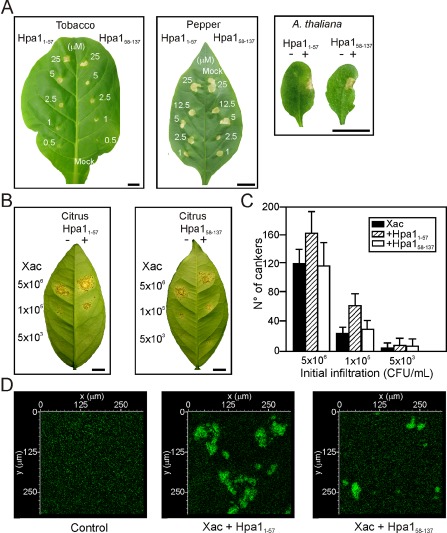
Contribution of Hpa1 N‐ and C‐terminal domains, Hpa11–57 and Hpa158–137, to the nonhost response, virulence and aggregation. (A) Hpa11–57 and Hpa158–137 elicit a hypersensitive response (HR) in tobacco, pepper and Arabidopsis thaliana Col‐0 at the concentrations stated. Bars, 1 cm. (B) Hpa11–57 enhances Xanthomonas axonopodis pv. citri (Xac) virulence in citrus leaves, whereas Hpa158–137 does not. The left halves of the leaves were co‐infiltrated with Xac and 6 His‐thioredoxin (Trx) and the right halves were co‐infiltrated with the fusion peptides at 5 μm. Bars, 1 cm. (C) Quantification of canker number in citrus leaves inoculated as described in (C). Bars are the means of six leaves assayed and error bars are the standard deviations. The results are representative of three independent experiments. (D) Representative photographs of confocal laser scanning microscopy of green fluorescent protein (GFP)‐expressing Xac with 6 His‐Trx (control), Hpa11–57 and Hpa158–137 at 5 μm.
Discussion
Several functions have been attributed to harpins from phytopathogens. One of the most characterized is their ability to bind to cell membranes and form ion‐conducting pores (Engelhardt et al., 2009; Haapalainen et al., 2011; Lee et al., 2001b). Although it has been determined that they can modulate ion channels (Lee et al., 2001b; Reboutier et al., 2007), the biochemical basis of plant cell membrane destabilization by harpins is unknown. In this work, we characterized the role of Hpa1 from Xac in its interaction with host and nonhost plants, and proposed a new function for this protein in promoting cell–cell aggregation and bacterial adhesion to the plant tissue.
It is noteworthy that the prominent feature of harpins is a high glycine content which plays an important role in the binding of hydrophobic membranes (Kourie and Henry, 2002). In addition, it has been shown that the glycine‐rich region of the amyloid β‐protein modulates the protein β‐sheet secondary structure, as well as the cytotoxic activity and aggregation (Pike et al., 1995). In this context, it has been demonstrated that HpaGXag, under plant apoplast‐like conditions, can form biologically active spherical oligomers, protofibrils and β‐sheet‐rich fibrils, and that the fibrillar form of HpaGXag is an amyloid (Oh et al., 2007). Other harpins, such as HrpN from Erwinia amylovora and HrpZ from P. syringae, can also form curvilinear protofibrils or fibrils, and it has been suggested that amyloidogenesis is a common feature of harpins (Oh et al., 2007). In addition, HpaGXag shares common biochemical characteristics with amyloid disease‐related proteins responsible for neuronal degeneration and cell death, suggesting similar action mechanisms in the induction of plant and animal cell death (Oh et al., 2007). Previously, it has been shown that the first 229 amino acids of HrpN from E. chrysanthemi, a region in which the glycine‐rich motif is located, promotes aggregation, and it has been suggested that this might possibly contribute to bacterial adhesion in the plant host (Yap et al., 2006). Taking into consideration the increase in virulence caused by the presence of Hpa1 in citrus canker, the ability of Hpa1 to form fibrils and promote bacterial aggregation and previous results (the fibrillar aggregation of HpaGXag, HrpZ from P. syringae and HrpN from E. amylovora in amyloid proteins, Oh et al., 2007; the assembly of HrpZ from P. syringae as protein oligomers, Haapalainen et al., 2011; and the promotion of cell aggregation by HrpN from E. chrysanthemi, Yap et al., 2006), we suggest that Hpa1 promotes cell–cell aggregation, which, in turn, enhances virulence. These results are in agreement with our previous work on Xac adhesins showing that cell–cell contact enhances Xac virulence (Gottig et al., 2009).
Several Hpa1 proteins from Xanthomonas have been dissected to characterize the minimum peptide required to elicit HR. By mutational and deletion analyses in HpaGXag, a 23‐amino‐acid peptide from N31 to Q53 was shown to be sufficient to elicit HR (Kim et al., 2004). Moreover, deletions of codons for 12 highly hydrophilic amino acids in this peptide in Hpa1Xoo caused a loss of HR in tobacco (Wang et al., 2008). In addition, two single missense mutations in Hpa1Xoo (L51P) and Hpa1Xoc (L53P), which destroy the integrity of a region predicted to form a coiled‐coil conformation, in turn inhibiting oligomer formation, eliminate HR elicitation activity in tobacco (Wang et al., 2008). Different techniques, such as size exclusion chromatography, electron microscopy, circular dichroism spectroscopy, CR staining and resistance to protease, have been used to show that HpaGXag can oligomerize first as a tetramer, then form β‐sheet‐rich fibrils and, finally, amyloid‐like fibres, and that HR is dependent on this amyloid structure (Oh et al., 2007). An HpaGXag mutant unable to form amyloid (L50P) also failed to elicit HR, indicating a positive correlation between fibrillogenesis and HR elicitor activity (Oh et al., 2007). In HpaXm, the Hpa1 from X. citri ssp. malvacearum, this activity was restricted to a peptide containing two heptads, LDQLLTQLIMALLQ, which were predicted to have a high probability of forming a coiled‐coil (Miao et al., 2010b). Recently, the structure–function of the two predicted Hpa1 coiled‐coil regions (the previously mentioned two‐heptad peptide in the N‐terminal region and another in the C‐terminal region of Hpa1) were analysed using biophysical and biochemical approaches, and it was determined that the coiled‐coil in the N‐terminal region is essential for the elicitation of HR. As generated peptides that favour or disrupt this coiled‐coil formation show no HR elicitation, it was proposed that specific oligomerization of the coiled‐coil regions of Hpa1 is required (Ji et al., 2011). We expressed the N‐terminal and C‐terminal regions of Hpa1 that contain the two predicted coiled‐coil regions, and observed that both induced HR, suggesting that, in the conditions assayed, both regions are able to cause a response. In agreement with the larger probability of coiled‐coil formation of the N‐terminal region, we observed a promotion of bacterial aggregation and increased virulence when this peptide was present, suggesting a major participation of this region in protein activity. However, the effects observed with the N‐terminal region were less than those with the complete protein, suggesting that the C‐terminal region contributes to the overall activity of the protein.
We also observed an increase in the number of schizogenous cavities in Hpa1‐treated citrus leaves, which leads to an increase in leaf thickness and resembles the differences in pepper leaf thickness caused by the induction of mesophyll cell hypertrophy by the type III effector protein AvrBs3 (Marois et al., 2002). AvrBs3 is a transcription activator‐like (TAL) effector which, once in the host cell nucleus, activates the transcription of a helix–loop–helix factor that regulates cell size and thus induces cell hypertrophy (Kay et al., 2007). PthA is the major factor of Xac pathogenicity required for canker elicitation (Brunings and Gabriel, 2003) and belongs to the same family of TAL effectors. PthA is sufficient to cause hypertrophy and hyperplasia in citrus canker lesions (Duan et al., 1999). As cell hypertrophy is one of the main features of citrus canker, we can speculate that Hpa1 may prepare the tissue for the action of the TAL effector that causes cell hypertrophy and hyperplasia by increasing the cavities and leaf thickness, and therefore promotes canker outcome. In this way, Hpa1 may enhance bacterial penetration in the leaf tissue, as observed in Xac wild‐type infection compared with the hpa1 mutant by confocal microscopy.
In summary, Hpa1 seems to have two functions in plant–pathogen interactions: first, the induction of the basal defence response, as observed in nonhost plants, and in agreement with previous results in transgenic cotton plants expressing Hpa1Xoo, where the induction of the basal defence response is sufficiently high to enhance resistance against Verticillium dahliae through a priming mechanism (Miao et al., 2010a); second, in increasing pathogen virulence, probably by causing changes in citrus leaf mesophyll structure and by aggregating bacterial cells, leading to greater tissue colonization. This dual role has been observed for the E. coli amyloid proteins known as ‘curli’. These highly aggregative surface fibre structures mediate interactions between individual bacteria, promoting biofilm formation, and between bacteria and host tissues, stimulating a host inflammatory response and contributing to bacterial persistence within the host (Epstein and Chapman, 2008). The fact that the presence of Hpa1 enhances pathogen virulence suggests that, once the bacterium has reached a threshold level of effector proteins, it can counteract the basal immune response (Chisholm et al., 2006), and thus inhibit the almost barely detectable Hpa1‐induced basal response. This aggregation activity is conceivable in a model in which Xac requires the harpin protein to recruit a larger number of pathogen cells to interact with the plant cell membrane and predispose the tissue to later effector action. Understanding the role of bacterial proteins involved in plant–pathogen interactions, such as Hpa1, is essential for the identification of new targets for disease control.
Experimental Procedures
Strains, culture conditions and media
Escherichia coli JM109 was used for DNA subcloning, and cells were cultivated at 37 °C in Luria–Bertani (LB) medium. Xac (Xcc99‐1330) and the derivative strains were grown at 28 °C in sucrose broth (SB), nutrient broth (NB) (Dunger et al., 2007) or XVM2 (Gottig et al., 2009). Antibiotics were used at the following final concentrations: ampicillin, 100 μg/mL for E. coli and 25 μg/mL for Xac; kanamycin, 40 μg/mL for both strains; gentamycin, 20 μg/mL for both strains; chloramphenicol, 30 μg/mL for E. coli.
Expression and purification of recombinant Hpa1, Hpa11–57 and Hpa158–137
The full‐length hpa1 gene was amplified by PCR from Xac genomic DNA using the oligonucleotides HPA1LNDE (5′‐TCTTAAGCATATGAATTCTTTGAACACACAG‐3′) and HPA1RHIND (5′‐CACGTAAGCTTTTACTGCATCGATCCGGTG‐3′), and cloned into pET28a vector (Novagen, Merck KGaA, Darmstadt, Germany) previously digested with the restriction enzymes NdeI and HindIII. Hpa11–57 was amplified with PNHpa1BamHI (5′‐TAAGGGATCCATGAATTCTTTGAACACACAG‐3′) and PNHpa1H (5′‐CACGTAAGCTTAGGCATTGTTGCTCTGCTGAAG‐3′), and Hpa158–137 with PCHpa1BamHI (5′‐TAAGGGATCCGAGCAGGGTCAGGGTCAA‐3′) and HPA1RHIND (5′‐CACGTAAGCTTTTACTGCATCGATCCGGTG‐3′). These fragments were cloned in pET32a vectors (Novagen) digested with BamHI and HindIII. The three constructs were transformed into E. coli strain BL21 (pLysS), and the synthesis of recombinant protein was induced by 0.5 mm isopropyl‐β‐d‐thiogalactopyranoside (IPTG) for 5 h at 18 °C. Hpa1, Hpa11–57 and Hpa158–137 were purified by affinity chromatography from the soluble fraction of the bacterial lysate using Ni2+‐nitrilotriacetate (Ni‐NTA) agarose (Qiagen, Hilden, Germany), and checked by sodium dodecylsulphate‐polyacrylamide gel electrophoresis (SDS‐PAGE).
Plant material, plant inoculations and GUS staining
Citrus sinensis cv. valencia was grown in a glasshouse at 26 ± 2 °C with a photoperiod of 16 h. Arabidopsis thaliana cultivars Col‐0, Ws, Nossen and C24 were used. Seeds were sown in soil in 10‐cm pots and grown in a controlled environment chamber at 23 ± 2 °C with a photoperiod of 16 h. Bacteria were grown in SB to an optical density of unity at 600 nm, harvested by centrifugation and resuspended in 10 mm MgCl2 at the required density. Infiltrations into leaves were performed with needleless syringes, and in planta assays were performed as described by Dunger et al. (2007). For GUS assay, the A. thaliana PrAtWRKY30::GUS line, bearing a 1.96‐kb 5′ genomic fragment upstream of the ATG initiation codon of the WRKY30 gene fused to the E. coli uidA reporter gene (Scarpeci et al., 2008), was used. PrAtWRKY30::GUS leaves were infiltrated using a needleless syringe with 5 μm Hpa1 or 1.3 mg/mL of autoclaved cellulase Onozuka R‐10 (Yakult Honsha, Tokyo, Japan) (Salinas‐Mondragon et al., 1999). After 5 h of treatment, PrAtWRKY30::GUS leaves were collected and rinsed in 50 mm sodium phosphate, pH 7.2, 10 mm ethylenediaminetetraacetic acid (EDTA) and 0.33 mg/mL potassium ferricyanide, and then transferred to the same solution containing, in addition, 0.5 mg/mL 5‐bromo‐4‐chloro‐3‐indolyl‐β‐d‐glucuronic acid (X‐Gluc; Gold Biotechnology, St. Louis, MO, USA). Leaves were vacuum infiltrated for 3 min, incubated at 37 °C for 24 h and destained by soaking in 70% (v/v) ethanol.
RNA preparation and RT‐PCR
Total RNA was isolated from citrus infiltrated leaves using TRIzol® reagent (Invitrogen, Grand Island, NY, USA) according to the manufacturer's instructions. At least 100 mg of frozen tissue were used for each total RNA extraction and samples were stored at −80 °C until use. RNA preparations of bacteria from inoculated leaves, at different post‐infection times, were performed as described previously (Gottig et al., 2008). For cDNA first‐strand synthesis 1 μg of total RNA was heated to 72 °C for 2 min and then cooled on ice. The reaction mix containing 200 U M‐MLV reverse transcriptase (Promega, Fitchburg, WI, USA), 0.25 μg primer dT22 (for plant RNA) or primer dN6 (for bacterial RNA) and 0.5 mm deoxynucleoside triphosphates (dNTPs) (reaction volume, 20 μL) was added and incubated for 1 h at 42 °C, and then for 10 min at 94 °C. qRT‐PCRs were performed with 1 μL of cDNA template, 0.5 U of Platinum Taq DNA Polymerase (Invitrogen), 1 × reaction buffer, 0.2 mm dNTPs and 20 pmol of each primer (reaction volume, 20 μL), and performed in a Mastercycler ep realplex Thermal Cycler (Eppendorf, Hamburg, Germany) using SYBR Green I (Roche, Basel, Switzerland) to monitor double‐stranded DNA (dsDNA) synthesis under the following conditions: 95 °C for 1 min, followed by 40 cycles of 95 °C for 15 s, 55 °C for 30 s and 72 °C for 40 s. The oligonucleotides used for A. thaliana were as follows: for MAPK3, 5′‐CCCAACTTCCCACGTCAGCCCTTAGC‐3′ and 5′‐TGGCTCATCATTCGGGTCGTGCAA‐3′; for MKK4, 5′‐GCTTGCAGAGAGAACCGGGGAAAAGGA‐3′ and 5′‐GGAAAAGAAGACGAGGACAGAGGACGA‐3′; for WRKY30, 5′‐CGCTGGACGATGGATTCAGTTGGAGA‐3′ and 5′‐TCGGTTCGAGGTTTTGTATCGGCATTG‐3′; for tubulin, used as control, 5′‐GAGAATGCTGATGAGTGCATGG‐3′ and 5′‐CAGGGAACCTCAGACAGCAAGT‐3′. For citrus, the following primers were used: for MAPK3, 5′‐TTACATGATGAAGCCGATGAAC‐3′ and 5′‐TGAGTGCTAATGCCTCCTGATA‐3′; for MKK4, 5′‐GCTTGCAGAGAGAACCGGGGAAAAGGA‐3′ and 5′‐GGAAAAGAAGACGAGGACAGAGGACGA‐3′; for WRKY30, 5′‐CGAAAGAAGCAAATGGGGTA‐3′ and 5′‐GGAAATAAACGTGGCGAAAA‐3′; for actin, used as control, 5′‐GAGCTGAAAGATTCCGTTGC‐3′ and 5′‐TTGATCTTCATGCTGCTTGG‐3′. The oligonucleotides used for Xac were as follows: for Hpa1, HPA1INTBF (5′‐ATCAGGATCCTGACCCCAGCCAGAACAC‐3′) and HPA1INTHR (5′‐ATCAAAGCTTCCGAAGCCACCACCAAAG‐3′); for 16S rRNA as reference gene, 16SUp (5′‐TGGTAGTCCACGCCCTAAACG‐3′) and 16SDown (5′‐CTGGAAAGTTCCGTGGATGTC‐3′). Values were normalized by the internal reference (Ctr) according to the equation ΔCt = Ct – Ctr, and quantified as 2–ΔCt. A second normalization by a control (mock treatment or 0 days post‐inoculation) (Ctc), ΔΔCt = Ct – Ctc, produces a relative quantification: 2–ΔΔCt (Livak and Schmittgen, 2001). The values are the mean of four independent experiments with n = 3. The results were analysed using one‐way analysis of variance (anova).
Callose staining
The detection of callose deposits was performed as described by Adam and Somerville (1996) with some modifications. This assay is based on the staining of callose with aniline blue and cytological observations at the sites of infiltration by UV fluorescence microscopy. Briefly, leaves were inoculated with Hpa1 protein and, after 5 h, were cleared in 96% ethanol in a Petri dish. Once the leaves had been destained completely, they were incubated in sodium phosphate buffer (0.07 m, pH 9) for 30 min. After this incubation, the buffer was discarded and an appropriate volume of aniline blue solution (0.05%) to cover the leaves was added to the dish. The tissue was immersed for 60 min and the aniline blue solution was discarded. The stained leaves were mounted in 50% glycerol and examined immediately by confocal laser scanning microscopy (Nikon Eclipse TE‐2000‐E2, Nikon, Tokyo, Japan). Callose was quantified as described by Luna et al. (2011). Briefly, the callose intensity was calculated from digital photographs by the number of blue pixels relative to the total number of pixels covering the plant material, using Photoshop CS3 software. The results shown are related to the control treatment with buffer in which the callose intensity was considered to be unity. Average callose measurements were based on at least 25 photographs from three independent experiments, and were analysed for statistical differences by one‐way anova (P < 0.05).
Mutant construction
For the construction of the hpa1 mutant (XacΔhpa1), hpa1 was isolated from Xac genomic DNA by PCR using the oligonucleotides HPA1INTBF (5′‐ATCAGGATCCTGACCCCAGCCAGAACAC‐3′) and HPA1INTHR (5′‐ATCAAAGCTTCCGAAGCCACCACCAAAG‐3′), digested with the restriction enzymes BamHI and HindIII, and cloned into the suicide vector pKMobGII, previously digested with the same enzymes. Escherichia coli S17‐1 cells transformed with this plasmid were used to perform the conjugation to Xac and, after selection for kanamycin resistance, XacΔhpa1 was verified by PCR. Complementation of XacΔhpa1 was performed by amplification of hpa1 with HPA1A (5′‐TACGCGGTTACAGCAATGAGAG‐3′) and HPA1B (5′‐CGCCCCACAACTGCCGTCGCTTAC‐3′) oligonucleotides, digested with EcoRI and PstI restriction enzymes present in the amplified product, and ligated to pBBR1MCS‐5 digested with the same enzymes. The resulting plasmid was transferred to XacΔhpa1, yielding XacΔhpa1c.
Bacterial aggregation assays
For bacterial aggregation assays in cultures, the different strains were grown overnight in SB medium, subcultured in XVM2 medium at a 1:50 dilution, incubated statically at 25 °C and visualized after 5 days. To analyse the aggregation capacity of Hpa1, Hpa11–57 and Hpa158–137, the bacterial strains were modified to express GFP. To this end, the coding sequence for EGFP from pEGFP‐1 (Clontech, Palo Alto, CA, USA) was digested with BamHI and XbaI, and ligated in frame with the LacZ‐α‐peptide of the broad‐host‐range vector pBBR1MCS‐5 (Kovach et al., 1995). The resulting plasmid was conjugated to Xac and XacΔhpa1 and selected for gentamycin resistance. GFP‐expressing strains were cultured overnight in XVM2, and 20 μL were incubated with or without 5 μm Hpa1, Hpa11–57 and Hpa158–137 for 3 h on glass slides in a humidity chamber. Bacterial aggregates were visualized by confocal laser scanning microscopy (Nikon Eclipse TE‐2000‐E2). Representative fields (n = 10) from three independent experiments were photographed.
In vivo imaging of infected leaves
For in vivo imaging of bacterial aggregation and localization in infected leaves, GFP‐expressing bacterial strains were grown overnight on SB medium with the appropriate antibiotics at 28 °C. These cultures were diluted in 10 mm MgCl2 at a bacterial concentration of 107 CFU/mL and syringe infiltrated into the abaxial face of fully expanded citrus leaves. After 24 h of infection, the leaves were sectioned, mounted with 50% glycerol and visualized by confocal laser scanning microscopy (Nikon Eclipse TE‐2000‐E2). Bacterial cumuli length was measured using the software EZ‐C1 Free Viewer version 3.90 (Nikon). Several fields from six independent experiments were observed, analysing the lengths of individual bacterial cumuli. The results are expressed as the means of these cumuli lengths with a confidence level of 95%.
Microscopic observations of citrus tissue
Citrus leaves were infiltrated with 5 μm Hpa1 and incubated for 3 or 16 h. Tissues were sectioned in a cryostat microtome, stained with Safranin fast green, and mounted and examined using a CH30 LB microscope (Olympus, Tokyo, Japan) equipped with an ocular micrometer that allows quantitative measurements. A total of 30 sections of tissue for each treatment were analysed to measure leaf tissue width, cavity width and length, and number of crystal idioblasts. Results were analysed using one‐way anova (P < 0.05). Representative sections (n = 30) of three independent experiments were photographed using a Axiolab microscope (Zeiss, Oberkochen, Germany) coupled with a digital camera.
CR binding
CR binding was carried out as described previously (Oh et al., 2007). Mixtures of 10 μm CR and 10 μm Hpa1 or Trx were incubated at room temperature for 30 min. Absorbance spectra in the region between 400 and 700 nm were recorded for the mixtures, and for CR and the proteins alone. Absorbance spectra were measured on a V‐630 spectrometer (Jasco, Easron, MD, USA) using cells with a 10‐mm path length at room temperature. A scanning rate of 100 nm/min, bandwidth of 1.5 nm and resolution of 0.1 nm were used.
Supporting information
Fig. S1 (A) Hpa1 develops a hypersensitive response (HR) in tobacco leaves and independently of the His tag. Tobacco leaf infiltrated with 6His‐Hpa1 at the concentrations stated in 50 mm tris(hydroxymethyl)aminomethane (Tris), pH 8. As controls, there are two infiltrated nonpathogenicity‐related proteins, 6His‐thioredoxin (Trx) and bovine serum albumin (BSA), in the right part of the leaf. (B) Hpa1 elicits HR in pepper at the concentrations stated. (C) Hpa1 does not cause HR in cotton leaves at the concentrations stated. Photographs were taken at 1 day post‐inoculation (dpi). (D) Characterization of the HR elicited by Hpa1 in tobacco leaves using 3,3′‐diaminobenzidine (DAB) and trypan blue staining assays. As positive control, Xanthomonas axonopodis pv. citri (Xac) wild‐type, at 108 colony‐forming units (CFU)/mL, was infiltrated. (E) Hpa1 (5 μm) induces HR in different Arabidopsis thaliana cultivars (right halves of the leaves). As control, the same buffer was infiltrated into the left halves of the leaves. Photographs were taken at 1 dpi. Bars, 1 cm.
Fig. S2 Bacterial populations of Xanthomonas axonopodis pv. citri (Xac) wild‐type in leaves that had been pre‐infiltrated with buffer [50 mm tris(hydroxymethyl)aminomethane (Tris), pH 8], 5 μm Hpa1 or XachrpB− [109 colony‐forming units (CFU)/mL] 12 h before inoculation with the pathogenic bacterium (106 CFU/mL). Values represent the mean of three samples; error bars indicate standard deviations.
Fig. S3 N‐terminal and C‐terminal regions of Hpa1 elicit a hypersensitive response (HR) in Arabidopsis thaliana. Ler, Ws, Nossen and C24 cultivars were infiltrated with 5 μm Hpa11‐57 or Hpa158‐137 in the right halves of the leaves. In the left halves of the leaves, thioredoxin (Trx) was infiltrated as control. Photographs were taken at 1 day post‐inoculation. Bar, 1 cm.
Acknowledgements
We thank Sonia Scarpeci and Rodrigo Vena for assistance with the confocal microscopy facility, Alejandra Martinez for the preparation of citrus leaf sections, Microquin for culture media and Rosana Wolochwianski for correcting the manuscript. This work was supported by grants from the Argentine Federal Government (PICT2006‐00678 and PICT2010‐0300 to JO) and CONICET (PIP2010‐2012 to JO and NG). GGS and FAF are Fellows and TES, EMV, EGO, NG and JO are staff members of the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET, Argentina).
References
- Adam, L. and Somerville, S.C. (1996) Genetic characterization of five powdery mildew disease resistance loci in Arabidopsis thaliana . Plant J. 9, 341–356. [DOI] [PubMed] [Google Scholar]
- Asai, T. , Tena, G. , Plotnikova, J. , Willmann, M.R. , Chiu, W.L. , Gomez‐Gomez, L. , Boller, T. , Ausubel, F.M. and Sheen, J. (2002) MAP kinase signalling cascade in Arabidopsis innate immunity. Nature, 415, 977–983. [DOI] [PubMed] [Google Scholar]
- Astua‐Monge, G. , Freitas‐Astua, J. , Bacocina, G. , Roncoletta, J. , Carvalho, S.A. and Machado, M.A. (2005) Expression profiling of virulence and pathogenicity genes of Xanthomonas axonopodis pv. citri . J. Bacteriol. 187, 1201–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boller, T. and Felix, G. (2009) A renaissance of elicitors: perception of microbe‐associated molecular patterns and danger signals by pattern‐recognition receptors. Annu. Rev. Plant Biol. 60, 379–406. [DOI] [PubMed] [Google Scholar]
- Brunings, A.M. and Gabriel, D.W. (2003) Xanthomonas citri: breaking the surface. Mol. Plant Pathol. 4, 141–157. [DOI] [PubMed] [Google Scholar]
- Büttner, D. and Bonas, U. (2002) Getting across—bacterial type III effector proteins on their way to the plant cell. EMBO J. 21, 5313–5322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Büttner, D. , Gurlebeck, D. , Noel, L.D. and Bonas, U. (2004) HpaB from Xanthomonas campestris pv. vesicatoria acts as an exit control protein in type III‐dependent protein secretion. Mol. Microbiol. 54, 755–768. [DOI] [PubMed] [Google Scholar]
- Chisholm, S.T. , Coaker, G. , Day, B. and Staskawicz, B.J. (2006) Host–microbe interactions: shaping the evolution of the plant immune response. Cell, 124, 803–814. [DOI] [PubMed] [Google Scholar]
- Desikan, R. , Hancock, J.T. , Ichimura, K. , Shinozaki, K. and Neill, S.J. (2001) Harpin induces activation of the Arabidopsis mitogen‐activated protein kinases AtMPK4 and AtMPK6. Plant Physiol. 126, 1579–1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong, H. , Delaney, T.P. , Bauer, D.W. and Beer, S.V. (1999) Harpin induces disease resistance in Arabidopsis through the systemic acquired resistance pathway mediated by salicylic acid and the NIM1 gene. Plant J. 20, 207–215. [DOI] [PubMed] [Google Scholar]
- Duan, Y.P. , Castañeda, A. , Zhao, G. , Erdos, G. and Gabriel, D.W. (1999) Expression of a single, host‐specific, bacterial pathogenicity gene in plant cells elicits division, enlargement, and cell death. Mol. Plant–Microbe Interact. 12, 556–560. [Google Scholar]
- Dunger, G. , Arabolaza, L.N. , Gottig, N. , Orellano, E.G. and Ottado, J. (2005) Participation of Xanthomonas axonopodis pv. citri hrp cluster in citrus canker and in non‐host plant responses. Plant Pathol. 54, 781–788. [Google Scholar]
- Dunger, G. , Relling, V.M. , Tondo, M.L. , Barreras, M. , Ielpi, L. , Orellano, E.G. and Ottado, J. (2007) Xanthan is not essential for pathogenicity in citrus canker but contributes to Xanthomonas epiphytic survival. Arch. Microbiol. 188, 127–135. [DOI] [PubMed] [Google Scholar]
- Engelhardt, S. , Lee, J. , Gabler, Y. , Kemmerling, B. , Haapalainen, M.L. , Li, C.M. , Wei, Z. , Keller, H. , Joosten, M. , Taira, S. and Nürnberger, T. (2009) Separable roles of the Pseudomonas syringae pv. phaseolicola accessory protein HrpZ1 in ion‐conducting pore formation and activation of plant immunity. Plant J. 57, 706–717. [DOI] [PubMed] [Google Scholar]
- Epstein, E.A. and Chapman, M.R. (2008) Polymerizing the fibre between bacteria and host cells: the biogenesis of functional amyloid fibres. Cell. Microbiol. 10, 1413–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottig, N. , Garavaglia, B.S. , Daurelio, L.D. , Valentine, A. , Gehring, C. , Orellano, E.G. and Ottado, J. (2008) Xanthomonas axonopodis pv. citri uses a plant natriuretic peptide‐like protein to modify host homeostasis. Proc. Natl. Acad. Sci. USA, 105, 18631–18636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottig, N. , Garavaglia, B.S. , Garofalo, C.G. , Orellano, E.G. and Ottado, J. (2009) A filamentous hemagglutinin‐like protein of Xanthomonas axonopodis pv. citri, the phytopathogen responsible for citrus canker, is involved in bacterial virulence. Plos ONE, 4, e4358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haapalainen, M. , Engelhardt, S. , Kufner, I. , Li, C.M. , Nürnberger, T. , Lee, J. , Romantschuk, M. and Taira, S. (2011) Functional mapping of harpin HrpZ of Pseudomonas syringae reveals the sites responsible for protein oligomerization, lipid interactions and plant defence induction. Mol. Plant Pathol. 12, 151–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He, S.Y. , Huang, H.C. and Collmer, A. (1993) Pseudomonas syringae pv. syringae harpinPss: a protein that is secreted via the Hrp pathway and elicits the hypersensitive response in plants. Cell, 73, 1255–1266. [DOI] [PubMed] [Google Scholar]
- Ji, Z. , Song, C. , Lu, X. and Wang, J. (2011) Two coiled‐coil regions of Xanthomonas oryzae pv. oryzae harpin differ in oligomerization and hypersensitive response induction. Amino Acids, 40, 381–392. [DOI] [PubMed] [Google Scholar]
- Kay, S. , Hahn, S. , Marois, E. , Hause, G. and Bonas, U. (2007) A bacterial effector acts as a plant transcription factor and induces a cell size regulator. Science, 318, 648–651. [DOI] [PubMed] [Google Scholar]
- Kim, J.G. , Park, B.K. , Yoo, C.H. , Jeon, E. , Oh, J. and Hwang, I. (2003) Characterization of the Xanthomonas axonopodis pv. glycines Hrp pathogenicity island. J. Bacteriol. 185, 3155–3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, J.G. , Jeon, E. , Oh, J. , Moon, J.S. and Hwang, I. (2004) Mutational analysis of Xanthomonas harpin HpaG identifies a key functional region that elicits the hypersensitive response in nonhost plants. J. Bacteriol. 186, 6239–6247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kourie, J.I. and Henry, C.L. (2002) Ion channel formation and membrane‐linked pathologies of misfolded hydrophobic proteins: the role of dangerous unchaperoned molecules. Clin. Exp. Pharmacol. Physiol. 29, 741–753. [DOI] [PubMed] [Google Scholar]
- Kovach, M.E. , Elzer, P.H. , Hill, D.S. , Robertson, G.T. , Farris, M.A. , Roop, R.M. and Peterson, K.M. (1995) Four new derivatives of the broad‐host‐range cloning vector pBBR1MCS, carrying different antibiotic‐resistance cassettes. Gene, 166, 175–176. [DOI] [PubMed] [Google Scholar]
- Lee, J. , Klessig, D.F. and Nürnberger, T. (2001a) A harpin binding site in tobacco plasma membranes mediates activation of the pathogenesis‐related gene HIN1 independent of extracellular calcium but dependent on mitogen‐activated protein kinase activity. Plant Cell, 13, 1079–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, J. , Klusener, B. , Tsiamis, G. , Stevens, C. , Neyt, C. , Tampakaki, A.P. , Panopoulos, N.J. , Noller, J. , Weiler, E.W. , Cornelis, G.R. , Mansfield, J.W. and Nürnberger, T. (2001b) HrpZ(Psph) from the plant pathogen Pseudomonas syringae pv. phaseolicola binds to lipid bilayers and forms an ion‐conducting pore in vitro. Proc. Natl. Acad. Sci. USA, 98, 289–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, F. , Liu, H. , Jia, Q. , Wu, X. , Guo, X. , Zhang, S. , Song, F. and Dong, H. (2006) The internal glycine‐rich motif and cysteine suppress several effects of the HpaG(Xooc) protein in plants. Phytopathology, 96, 1052–1059. [DOI] [PubMed] [Google Scholar]
- Livak, K.J. and Schmittgen, T.D. (2001) Analysis of relative gene expression data using real‐time quantitative PCR and the 2(–Delta Delta C(T)) method. Methods, 25, 402–408. [DOI] [PubMed] [Google Scholar]
- Luna, E. , Pastor, V. , Robert, J. , Flors, V. , Mauch‐Mani, B. and Ton, J. (2011) Callose deposition: a multifaceted plant defense response. Mol. Plant–Microbe Interact. 24, 183–193. [DOI] [PubMed] [Google Scholar]
- Marois, E. , Van den, A.G. and Bonas, U. (2002) The Xanthomonas type III effector protein AvrBs3 modulates plant gene expression and induces cell hypertrophy in the susceptible host. Mol. Plant–Microbe Interact. 15, 637–646. [DOI] [PubMed] [Google Scholar]
- Miao, W. , Wang, X. , Li, M. , Song, C. , Wang, Y. , Hu, D. and Wang, J. (2010a) Genetic transformation of cotton with a harpin‐encoding gene hpaXoo confers an enhanced defense response against different pathogens through a priming mechanism. BMC Plant Biol. 10, 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao, W.G. , Song, C.F. , Wang, Y. and Wang, J.S. (2010b) HpaXm from Xanthomonas citri subsp. malvacearum is a novel harpin with two heptads for hypersensitive response. J. Microbiol. Biotechnol. 20, 54–62. [PubMed] [Google Scholar]
- Nguyen, H.P. , Chakravarthy, S. , Velasquez, A.C. , McLane, H.L. , Zeng, L. , Nakayashiki, H. , Park, D.H. , Collmer, A. and Martin, G. (2010) Methods to study PAMP‐triggered immunity using tomato and Nicotiana benthamiana . Mol. Plant–Microbe Interact. 23, 991–999. [DOI] [PubMed] [Google Scholar]
- Noel, L. , Thieme, F. , Nennstiel, D. and Bonas, U. (2002) Two novel type III‐secreted proteins of Xanthomonas campestris pv. vesicatoria are encoded within the hrp pathogenicity island. J. Bacteriol. 184, 1340–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh, J. , Kim, J.G. , Jeon, E. , Yoo, C.H. , Moon, J.S. , Rhee, S. and Hwang, I. (2007) Amyloidogenesis of type III‐dependent harpins from plant pathogenic bacteria. J. Biol. Chem. 282, 13601–13609. [DOI] [PubMed] [Google Scholar]
- Peng, J.L. , Bao, Z.L. , Ren, H.Y. , Wang, J.S. and Dong, H.S. (2004) Expression of harpin(Xoo) in transgenic tobacco induces pathogen defense in the absence of hypersensitive cell death. Phytopathology, 94, 1048–1055. [DOI] [PubMed] [Google Scholar]
- Pike, C.J. , Walencewicz‐Wasserman, A.J. , Kosmoski, J. , Cribbs, D.H. , Glabe, C.G. and Cotman, C.W. (1995) Structure–activity analyses of beta‐amyloid peptides: contributions of the beta 25–35 region to aggregation and neurotoxicity. J. Neurochem. 64, 253–265. [DOI] [PubMed] [Google Scholar]
- Reboutier, D. , Frankart, C. , Briand, J. , Biligui, B. , Laroche, S. , Rona, J.P. , Barny, M.A. and Bouteau, F. (2007) The HrpN(ea) harpin from Erwinia amylovora triggers differential responses on the nonhost Arabidopsis thaliana cells and on the host apple cells. Mol. Plant–Microbe Interact. 20, 94–100. [DOI] [PubMed] [Google Scholar]
- Salinas‐Mondragon, R.E. , Garciduenas‐Pina, C. and Guzman, P. (1999) Early elicitor induction in members of a novel multigene family coding for highly related RING‐H2 proteins in Arabidopsis thaliana . Plant Mol. Biol. 40, 579–590. [DOI] [PubMed] [Google Scholar]
- Scarpeci, T.E. , Zanor, M.I. , Carrillo, N. , Mueller‐Roeber, B. and Valle, E.M. (2008) Generation of superoxide anion in chloroplasts of Arabidopsis thaliana during active photosynthesis: a focus on rapidly induced genes. Plant Mol. Biol. 66, 361–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao, M. , Wang, J. , Dean, R.A. , Lin, Y. , Gao, X. and Hu, S. (2008) Expression of a harpin‐encoding gene in rice confers durable nonspecific resistance to Magnaporthe grisea . Plant Biotechnol. J. 6, 73–81. [DOI] [PubMed] [Google Scholar]
- Sohn, S.I. , Kim, Y.H. , Kim, B.R. , Lee, S.Y. , Lim, C.K. , Hur, J.H. and Lee, J.Y. (2007) Transgenic tobacco expressing the hrpN(EP) gene from Erwinia pyrifoliae triggers defense responses against Botrytis cinerea . Mol. Cells, 24, 232–239. [PubMed] [Google Scholar]
- Wang, X.Y. , Song, C.F. , Miao, W.G. , Ji, Z.L. , Wang, X. , Zhang, Y. , Zhang, J.H. , Hu, J.S. , Borth, W. and Wang, J.S. (2008) Mutations in the N‐terminal coding region of the harpin protein Hpa1 from Xanthomonas oryzae cause loss of hypersensitive reaction induction in tobacco. Appl. Microbiol. Biotechnol. 81, 359–369. [DOI] [PubMed] [Google Scholar]
- Yap, M.N. , Rojas, C.M. , Yang, C.H. and Charkowski, A.O. (2006) Harpin mediates cell aggregation in Erwinia chrysanthemi 3937. J. Bacteriol. 188, 2280–2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, W. , MaGbanua, M.M. and White, F.F. (2000) Identification of two novel hrp‐associated genes in the hrp gene cluster of Xanthomonas oryzae pv. oryzae . J. Bacteriol. 182, 1844–1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 (A) Hpa1 develops a hypersensitive response (HR) in tobacco leaves and independently of the His tag. Tobacco leaf infiltrated with 6His‐Hpa1 at the concentrations stated in 50 mm tris(hydroxymethyl)aminomethane (Tris), pH 8. As controls, there are two infiltrated nonpathogenicity‐related proteins, 6His‐thioredoxin (Trx) and bovine serum albumin (BSA), in the right part of the leaf. (B) Hpa1 elicits HR in pepper at the concentrations stated. (C) Hpa1 does not cause HR in cotton leaves at the concentrations stated. Photographs were taken at 1 day post‐inoculation (dpi). (D) Characterization of the HR elicited by Hpa1 in tobacco leaves using 3,3′‐diaminobenzidine (DAB) and trypan blue staining assays. As positive control, Xanthomonas axonopodis pv. citri (Xac) wild‐type, at 108 colony‐forming units (CFU)/mL, was infiltrated. (E) Hpa1 (5 μm) induces HR in different Arabidopsis thaliana cultivars (right halves of the leaves). As control, the same buffer was infiltrated into the left halves of the leaves. Photographs were taken at 1 dpi. Bars, 1 cm.
Fig. S2 Bacterial populations of Xanthomonas axonopodis pv. citri (Xac) wild‐type in leaves that had been pre‐infiltrated with buffer [50 mm tris(hydroxymethyl)aminomethane (Tris), pH 8], 5 μm Hpa1 or XachrpB− [109 colony‐forming units (CFU)/mL] 12 h before inoculation with the pathogenic bacterium (106 CFU/mL). Values represent the mean of three samples; error bars indicate standard deviations.
Fig. S3 N‐terminal and C‐terminal regions of Hpa1 elicit a hypersensitive response (HR) in Arabidopsis thaliana. Ler, Ws, Nossen and C24 cultivars were infiltrated with 5 μm Hpa11‐57 or Hpa158‐137 in the right halves of the leaves. In the left halves of the leaves, thioredoxin (Trx) was infiltrated as control. Photographs were taken at 1 day post‐inoculation. Bar, 1 cm.


