SUMMARY
Iron is an essential element for most living organisms, and pathogens are likely to compete with their hosts for the acquisition of this element. The bacterial plant pathogen Dickeya dadantii has been shown to require its siderophore‐mediated iron uptake system for systemic disease progression on several host plants, including Arabidopsis thaliana. In this study, we investigated the effect of the iron status of Arabidopsis on the severity of disease caused by D. dadantii. We showed that symptom severity, bacterial fitness and the expression of bacterial pectate lyase‐encoding genes were reduced in iron‐deficient plants. Reduced symptoms correlated with enhanced expression of the salicylic acid defence plant marker gene PR1. However, levels of the ferritin coding transcript AtFER1, callose deposition and production of reactive oxygen species were reduced in iron‐deficient infected plants, ruling out the involvement of these defences in the limitation of disease caused by D. dadantii. Disease reduction in iron‐starved plants was also observed with the necrotrophic fungus Botrytis cinerea. Our data demonstrate that the plant nutritional iron status can control the outcome of an infection by acting on both the pathogen's virulence and the host's defence. In addition, iron nutrition strongly affects the disease caused by two soft rot‐causing plant pathogens with a large host range. Thus, it may be of interest to take into account the plant iron status when there is a need to control disease without compromising crop quality and yield in economically important plant species.
INTRODUCTION
Acting as a catalyst in many metabolic processes, such as respiration and photosynthesis, iron is essential for the growth of almost all organisms. Despite its high abundance in the Earth's crust, its availability in aerobic and alkaline soils is poor because it is generally present in the form of insoluble ferric hydroxides (Lindsay and Schwab, 1982). About 30% of croplands are too alkaline for optimal crop growth (Marschner, 1995). In the presence of oxygen, the more soluble ferrous form can generate noxious reactive oxygen species (ROS) through the Fenton reaction (Pierre and Fontecave, 1999). Therefore, cellular iron acquisition, utilization and storage are subject to different levels of homeostatic regulation.
Plants use two strategies to acquire iron from the soil (Briat et al., 2007a; Romheld and Marschner, 1986) The so‐called ‘strategy I’ described in nongraminaceous species involves a reduction mechanism. The plant copes with iron deficiency by increasing root H+‐ATPase activity and secreting organic acids that lower the rhizospheric pH and increase iron solubility. Soluble ferric ions are reduced at the plasma membrane through the activity of an iron deficiency‐inducible root ferric‐chelate reductase. In A. thaliana, FRO2 was identified as the major root iron‐chelate reductase (Robinson et al., 1999), and the reduced iron is taken up by the root iron‐regulated transporter IRT1 (Eide et al., 1996). The metal is internalized in the root epidermis and cortex, and then mobilized to the aerial parts through the vascular system. Strategy II, based on iron chelation, is used by Poaceae which secrete phytosiderophores that bind ferric iron in the root medium. The iron–phytosiderophore complexes are specifically recognized by a high‐affinity transporter (Kobayashi et al., 2010) belonging to the YS1 family, and iron is internalized into the root. Inside the plant, iron is transported essentially as iron–citrate and iron–nicotianamine complexes (Briat et al., 2007b; Morrissey and Guerinot, 2009). Storage and buffering in dedicated compartments, including apoplast and organelles (vacuole, plastids), protect the cell from iron toxicity (Briat et al., 2007b; Morrissey and Guerinot, 2009). In plastids, ferritins represent the major iron‐containing proteins. In A. thaliana, the ferritins AtFER1–4 are mainly involved in buffering iron and protecting the plant cells against oxidative stress (Ravet et al., 2009). Loading of iron in vacuolar stores is mediated by VIT1 (Kim et al., 2006), and iron mobilization from the vacuole to the cytosol is mediated by the divalent metal transporters AtNRAMP3 and AtNRAMP4 during seedling development (Lanquar et al., 2005).
In addition to the physical properties of soils, biotic factors have an impact on iron availability to plants (Lemanceau et al., 2009). Plants interact with a variety of microorganisms which, like grasses, produce siderophores which are secreted in response to iron deficiency (Andrews et al., 2003; Winkelmann, 2007). Iron–siderophore complexes are specifically recognized and transported across the microbial cell envelope, providing the microorganism with iron. Microbial siderophores can exert a beneficial effect on plant growth because they increase significantly the solubility of iron in the soil. However, most of them display a higher affinity than phytosiderophores and other plant iron carriers for this metal and, consequently, iron can be a stake in competitive relationships between plants and microorganisms. In plant–pathogen interactions, the production of siderophores by the pathogen is an efficient mechanism to acquire iron in the host and to promote infection (Expert, 1999; Haas et al., 2008).
Dickeya dadantii is an enterobacterium which causes soft rot on a large range of host plant species. It causes economically important damage on different crops, including potatoes, chicory and ornamentals such as Saintpaulia ionantha (Perombelon, 2002; Toth et al., 2003). Bacterial cells invade the intercellular spaces of parenchymatous tissues and secrete large quantities of plant cell wall‐degrading enzymes, leading to tissue disorganization (Fagard et al., 2007; Murdoch et al., 1999). Under iron deficiency, D. dadantii releases two siderophores: the hydroxycarboxylate achromobactin, which is produced when iron becomes limiting (Münzinger et al., 2000), and the catecholate chrysobactin (Persmark et al., 1989), which prevails under severe iron deficiency. Chrysobactin and achromobactin production are required for the systemic progression of maceration symptoms on the hosts (Dellagi et al., 2005; Enard et al., 1988; Franza et al., 2005). In A. thaliana, the genes encoding the iron storage protein ferritin AtFER1, and the vacuolar metal transporters AtNRAMP3 and AtNRAMP4, are involved in basal resistance to D. dadantii, indicating that changes in plant iron trafficking occur during infection (Dellagi et al., 2005; Segond et al., 2009). In addition, following D. dadantii inoculation, both plant iron deficiency markers IRT1 and FRO2 are up‐regulated, indicating that enhanced iron acquisition by the plant occurs upon infection (Segond et al., 2009).
In this work, we assessed whether the plant iron status was likely to influence the development of the disease caused by this pathogen on Arabidopsis plants. We showed that, in plants grown under low‐iron conditions, this metal is the essential factor limiting the development of D. dadantii. The reduced progression of disease symptoms observed on iron‐starved plants correlates with reduced expression of major bacterial virulence genes. We analysed the defence reactions known to be activated in A. thaliana in response to D. dadantii infection in relation to the plant iron status. We concluded that the decreased susceptibility of iron‐starved plants to disease is a result of the low plant iron content, rather than to a general amplification of defence responses to the pathogen. We found that iron deficiency in A. thaliana also decreases the incidence of grey mold disease caused by the fungus Botrytis cinerea. These data highlight the existence of a link between plant iron status and susceptibility/resistance to microbial disease.
RESULTS
Effect of iron starvation in Arabidopsis on the disease caused by D. dadantii
In order to investigate the effect of plant iron status on the disease severity caused by the bacterial plant pathogen D. dadantii, we compared the severity of symptoms after inoculation of +Fe and −Fe plants (see Experimental procedures, Fig. 1). Symptom severity was scored over 6 days using the scale presented in Fig. 2B. We observed a delay in the development of symptoms in −Fe relative to +Fe plants (Fig. 2C), visible after 2 days post‐inoculation (dpi). Accordingly, we found reduced bacterial growth in −Fe plants (Fig. 2D), whereas almost no growth occurred during the first 24 h post‐inoculation (hpi). Thereafter, the number of bacterial counts increased, but always remained lower than that observed in +Fe plants. These data led us to consider that the pathogenicity of D. dadantii was affected on iron‐starved plants.
Figure 1.
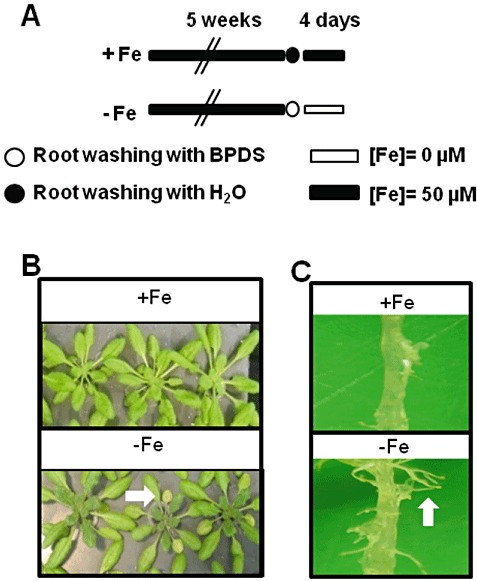
Iron deficiency‐triggered phenotypic modifications in Arabidopsis plants. (A) Growth conditions for +Fe and −Fe plants. Arabidopsis plants were grown for 5 weeks in a 50 µm Fe‐ethylenediaminetetraacetate (Fe‐EDTA)‐containing nutritional solution; the roots were then washed with distilled water or bathophenanthroline disulphonic acid (BPDS) as indicated. Next, plants were transferred to fresh nutritional solution with (+Fe) or without (–Fe) 50 µm Fe‐EDTA for 4 days. (B) Photographs of +Fe and −Fe Arabidopsis plants at the same scale. Chlorotic leaves are indicated (white arrow). (C) Photographs of roots from +Fe and −Fe plants. Secondary roots are indicated in −Fe plants (white arrow).
Figure 2.
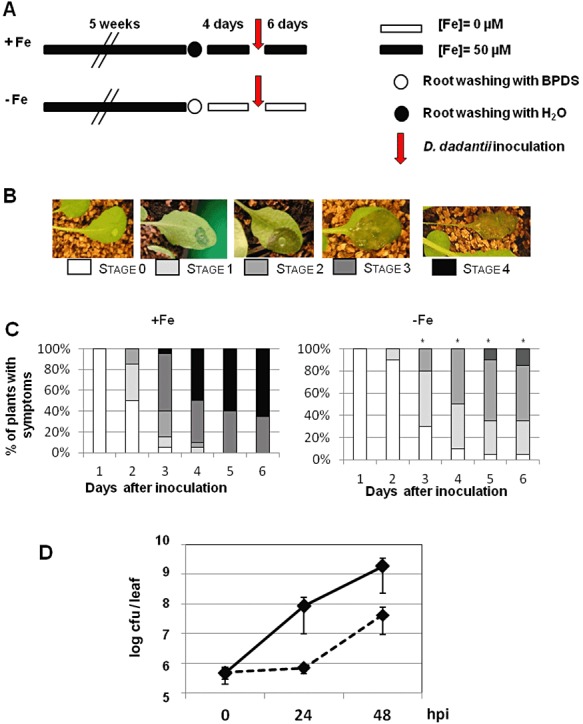
Effect of iron deficiency on disease development and Dickeya dadantii growth in Arabidopsis plants. (A) Experimental design for +Fe and −Fe plants (see Fig. 1). Leaves were inoculated with a D. dadantii bacterial suspension [107 colony‐forming units (cfu)/mL] and symptoms were scored for 6 days. (B) Symptom severity scale: stage 0, no symptoms; stage 1, maceration at the site of inoculation; stage 2, maceration covering about one‐half of the leaf; stage 3, maceration covering the whole leaf; stage 4, maceration has spread to the rest of the plant. (C) Disease severity on +Fe and −Fe plants scored at the indicated times after inoculation. Data are representative of four independent experiments with 24–30 plants in each experiment. Asterisks indicate significant difference in symptom severity calculated using Fisher's exact test (P < 0.05). (D) Bacterial populations of D. dadantii at the indicated times after inoculation in +Fe plants (full line) and −Fe plants (broken line). Bars, standard error. BPDS, bathophenanthroline disulphonic acid; hpi, h post‐inoculation.
Iron re‐supply to iron‐starved plants restores D. dadantii pathogenicity
To determine whether iron availability was the main factor limiting the infection process, we re‐supplied −Fe plants with iron after bacterial inoculation. Symptom evolution on these plants was compared with that observed on −Fe and +Fe plants. For this purpose, +Fe and −Fe plants were inoculated as shown in Fig. 2. Twenty‐four hours after inoculation, −Fe plants were transferred to the iron‐containing medium. These plants were referred to as ‘–Fe/+Fe’. To rule out a possible mechanical stress effect during the transfer, control experiments consisted of +Fe and −Fe plants inoculated and transferred at 24 hpi to the same +Fe and −Fe media, respectively. The data (Fig. 3) indicate that, in +Fe plants, the disease intensity increased over time. Symptoms of stage 4 were apparent in approximately 75% of inoculated plants at 6 dpi. In −Fe plants, the intensity of the symptoms was reduced significantly, but iron re‐supply reversed this effect: a complete rescue was observed at 6 dpi. Thus, iron availability in iron‐starved plants is a limiting factor for the development of the infection process.
Figure 3.
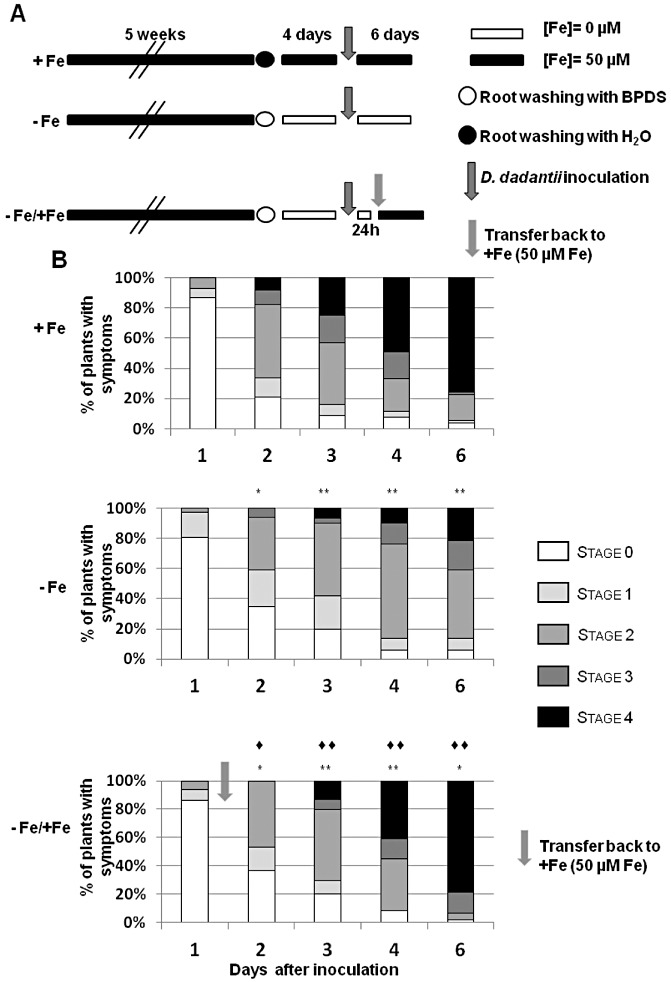
Effect of iron re‐supply on Dickeya dadantii disease development in iron‐starved Arabidopsis plants. (A) Experimental design for +Fe, −Fe and −Fe/+Fe plants. Leaves were inoculated with a D. dadantii bacterial suspension [107 colony‐forming units (cfu)/mL]. To investigate the effect of iron on disease progression, −Fe plants were re‐supplied with 50 µm Fe‐ethylenediaminetetraacetate (Fe‐EDTA), 24 h after bacterial inoculation (designated −Fe/+Fe) by transfer to iron‐sufficient medium (light grey arrow). (B) Disease severity on +Fe, −Fe and −Fe/+Fe plants scored at the indicated times after inoculation. Asterisks indicate significant difference in symptom severity compared with +Fe plants at the same time point calculated using Fisher's exact test (*P < 0.05; **P < 0.01). Diamonds indicate significant difference in symptom severity compared with −Fe plants at the same time point calculated using Fisher's exact test (◆, P < 0.05; ◆◆, P < 0.01). Data are representative of three independent experiments with 24–30 plants in each experiment. BPDS, bathophenanthroline disulphonic acid.
Effect of plant iron status on the expression of D. dadantii pectate lyase‐encoding genes
In D. dadantii, pectate lyase isoenzymes PelA–PelD are major determinants of symptom production on host plants. In planta detection of bacterial pel gene expression by reverse transcription‐polymerase chain reaction (RT‐PCR) has been described previously on soil‐grown seedlings during the first 24 hpi (Kraepiel et al., 2011; Lebeau et al., 2008). As the symptom severity was reduced in −Fe plants, we hypothesized that this could be a result of changes in the transcriptional activity of the corresponding pectate lyase‐encoding genes, pelA–pelD. To test this possibility, plants were grown and infected as indicated in Fig. 2. Leaves from +Fe and −Fe plants were infiltrated with a bacterial suspension [107 colony‐forming units (cfu)/mL] and were harvested at 3, 7, 10 and 24 hpi. The relative expression of pelA, pelB, pelC and pelD genes was monitored by quantitative RT‐PCR and normalized against the reference gene rpoB. The fold induction of pel gene expression in +Fe relative to −Fe plants was calculated using the 2–ΔΔCt method. The expression of pelA, pelB, pelC and pelD genes was similar in +Fe and −Fe plants, until 7 hpi (Fig. 4). After 10 h, there was an increase in the expression levels of pelB, pelC and pelD genes in +Fe plants only. This increase was transient and, after 24 h, their expression levels were similar in both +Fe and −Fe plants. The expression of the pelA gene remained unchanged during the experiment. Thus, there is a direct correlation between the expression levels of pel genes, the amount of symptoms and bacterial growth, indicating a reduction in bacterial virulence when plants are iron starved.
Figure 4.

Effect of Arabidopsis iron status on the expression of Dickeya dadantii pectinase genes in planta. Plants were grown under −Fe or +Fe conditions and inoculated with a D. dadantii bacterial suspension [107 colony‐forming units (cfu)/mL] as indicated in Fig. 2. Leaves were harvested at the indicated times after inoculation. The relative expression of the indicated pectate lyase genes was monitored by quantitative reverse transcription‐polymerase chain reaction (RT‐PCR), and is expressed as the fold induction in +Fe inoculated plants relative to −Fe inoculated plants. Data are expressed as 2–ΔΔCt with the endogenous reference gene rpoB. Bars, standard error; n= 3 independent biological experiments. hpi, h post‐inoculation.
Effect of plant iron status on the expression of Arabidopsis defence genes
The decreased virulence of D. dadantii observed on iron‐starved plants could result from enhanced plant resistance. To investigate this possibility, we analysed the expression of the defence genes PR1, CHIB and AtFER1, known to be up‐regulated in A. thaliana during the first 24 h following D. dadantii infection (Dellagi et al., 2005; Fagard et al., 2007). PR1 and CHIB genes are representative of the salicylic acid (SA) and ethylene/jasmonic acid (Et/JA)‐dependent signalling pathways, respectively, which mediate plant defence (Glazebrook, 2005). The AtFER1 gene encodes a plastidial ferritin involved in basal resistance to D. dadantii (Dellagi et al., 2005). Leaves from +Fe and −Fe plants were infiltrated with a bacterial suspension (107 cfu/mL) or with 10 mm MgSO4 as a control. RNAs were extracted from infected and control leaves at 3, 10 and 24 hpi, and the expression of defence genes was monitored by quantitative RT‐PCR. In +Fe as well as −Fe plants, we observed the up‐regulation of the three genes at 24 hpi. However in −Fe plants, the transcript level of PR1 was two‐fold higher and that of AtFER1 was four‐fold lower than that measured in +Fe plants (Fig. 5). Thus, these data indicate that there is no strict correlation between the expression levels of these defence genes and the reduced disease severity observed in −Fe plants.
Figure 5.
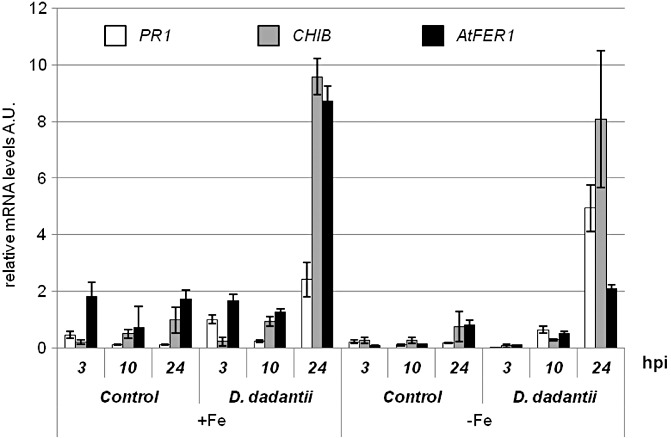
Effect of Arabidopsis iron status on the expression of defence genes in response to Dickeya dadantii. Plants were grown under −Fe or +Fe conditions as indicated in Fig. 2. The relative transcript levels of the indicated Arabidopsis defence genes were monitored in control plants inoculated with 10 mm MgSO4 and in plants inoculated with a D. dadantii bacterial suspension [107 colony‐forming units (cfu)/mL]. Leaves were harvested at the indicated times after inoculation and gene expression was assayed by quantitative reverse transcription‐polymerase chain reaction (RT‐PCR) relative to the internal control EF1α transcript level. Bars, standard error; n= 3 technical repeats. Data are representative of two independent experiments. hpi, h post‐inoculation.
Effect of plant iron status on callose deposition following infection
Callose deposition is a plant immunity marker used to study the plant response to pathogen‐associated molecular patterns (PAMPs) or to virulence‐promoting pathogen effectors (Luna et al., 2011). Inoculation of D. dadantii in Arabidopsis leaves triggers strong callose deposition, mainly around the leaf vascular system (Fagard et al., 2007). Leaves from +Fe and −Fe plants were infiltrated with a bacterial suspension (107 cfu/mL) or with 10 mm MgSO4 as a control. Callose deposition was monitored by aniline blue staining, 8 h following infection. Figure 6A and 6B shows that callose deposits accumulated to a significantly higher level in D. dadantii‐infected leaves of +Fe plants relative to −Fe plants. Interestingly, in −Fe plants, bacterial infection did not trigger callose deposition. In addition, the background level of callose deposition observed on MgSO4‐treated leaves seemed to be reduced in leaves from −Fe Arabidopsis plants. These data suggest that iron limitation results in reduced callose deposition in healthy and infected Arabidopsis plants. Thus, although −Fe plants displayed reduced susceptibility to D. dadantii relative to +Fe plants, this cannot be linked to increased callose deposition.
Figure 6.

Impact of iron status on callose deposition and oxidative stress in response to Dickeya dadantii. Control leaves were infiltrated with 10 mm MgSO4 and D. dadantii inoculated leaves were infiltrated with a bacterial suspension [107 colony‐forming units (cfu)/mL]. (A) Photograph of callose deposition detected with aniline blue staining in leaves 8 h after the indicated treatments. Bar, 200 µm. (B) Numbers of callose deposits per unit of leaf surface were quantified using ImageJ software; n= 18 leaves. (C) Photograph of hydrogen peroxide staining in Arabidopsis leaves with the fluorescent dye 2′,7′‐dichlorofluorescein‐diacetate (DCFH‐DA), 16 h after the indicated treatments. (D) Quantification of H2O2‐mediated fluorescence as the index of grey pixels/leaf. Bars, standard error; n= 18 leaves. Asterisks indicate a statistically significant difference between +Fe and −Fe in response to D. dadantii (Mann–Whitney, P < 0.01). Data are representative of three independent experiments.
Effect of plant iron status on ROS accumulation following infection
A strong oxidative burst is generated in A. thaliana during the first 24 h following infection by D. dadantii, and this reaction constitutes an effective defence towards the bacterium (Fagard et al., 2007). This burst has been shown to result mainly from the activity of the NADPH oxidase AtRbohD. As iron can also generate ROS in aerobic environments (Pierre and Fontecave, 1999), we investigated the level of ROS production following bacterial infection in +Fe and −Fe plants. The fluorescent dye 2′,7′‐dichlorofluorescein‐diacetate (DCFH‐DA) was used to detect intracellular hydrogen peroxide as an indicator of ROS production. Leaves from +Fe and −Fe plants were infiltrated with a bacterial suspension (107 cfu/mL) or with 10 mm MgSO4 as a control. The fluorescence was monitored at the 16 hpi time point (Fig. 6C,D). A significantly higher fluorescence was observed on leaves infiltrated with the bacterial suspension in +Fe relative to −Fe plants. No increase in fluorescence was observed in −Fe leaves following bacterial treatment compared with the control. These results indicate that plant iron availability may contribute to the production of ROS in response to D. dadantii. Thus, although −Fe plants displayed reduced susceptibility to D. dadantii relative to +Fe plants, this cannot be linked to increased accumulation of ROS.
Effect of iron deficiency in Arabidopsis on the disease caused by B. cinerea
We were interested to determine whether reduced symptom severity caused by iron deprivation was specifically occurring against the bacterial pathogen D. dadantii. To address this point, we chose another microbial plant pathogen, the ascomycete B. cinerea, which shares several characteristics with D. dadantii: (i) it causes maceration symptoms owing to a large amount of plant cell wall‐degrading enzymes; (ii) it infects a large host range of plant species. We then investigated the effect of plant iron status on the disease caused by this fungus. We grew +Fe and −Fe plants, and inoculated excised leaves with B. cinerea mycelium plugs. Lesion surfaces were scored on each leaf over 4 days. Figure 7B shows that the macerated surfaces increased over time in leaves from +Fe and −Fe plants. However, from 72 hpi onwards, leaves from −Fe plants displayed significantly smaller lesions. This indicates that, in the same way as for D. dadantii, the severity of the disease caused by B. cinerea is reduced in iron‐starved plants. We further investigated whether the growth of B. cinerea strain BO 5.10 was affected under iron limitation by analysing the growth of the mycelium in a minimal medium amended or not with iron. Figure 8A shows that iron starvation resulted in a three‐fold decline in growth after 5 days of culture. Figure 8B shows that iron limitation resulted in the production of three‐fold higher levels of siderophores as detected by the chrome azurol S (CAS) assay. These data indicate that B. cinerea strain BO 5.10 responds to low iron with reduced growth and increased siderophore production. Thus, reduced B. cinerea aggressiveness on −Fe plants could result from a decrease in pathogen fitness.
Figure 7.
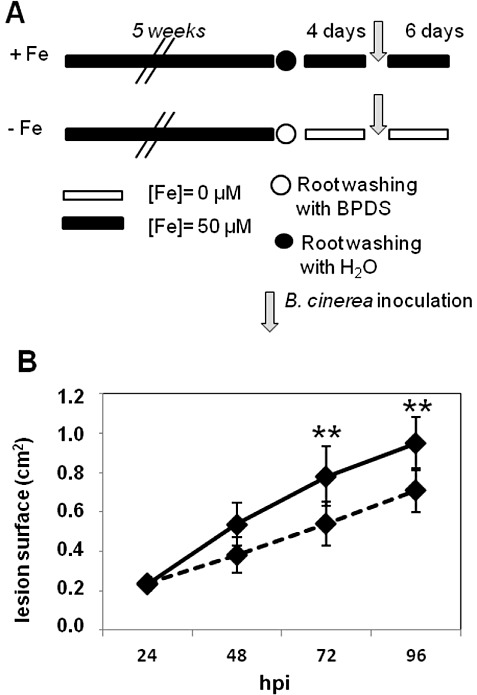
Effect of iron deficiency on Botrytis cinerea symptom development in Arabidopsis leaves. (A) Experimental design for +Fe and −Fe plants. Detached leaves were inoculated with B. cinerea mycelium plugs. (B) Surfaces of macerated lesions were monitored at the indicated times after inoculation in +Fe plants (full line) and iron‐starved plants (broken line) Bars, standard error; n= 20–24 leaves. Asterisks indicate statistically significant differences between iron‐starved and nonstarved plants: P < 0.001 (Mann–Whitney). Data are representative of three independent experiments. BPDS, bathophenanthroline disulphonic acid; hpi, h post‐inoculation.
Figure 8.
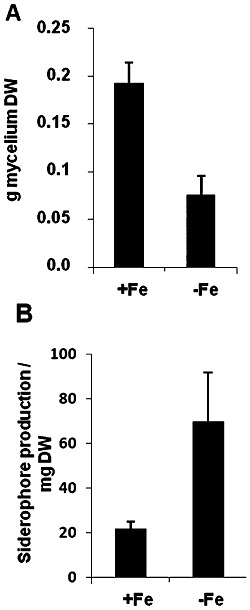
Impact of iron deficiency on Botrytis cinerea growth and siderophore production. Botrytis cinerea liquid cultures were grown for 5 days in Czapek medium with (+Fe) or without (–Fe) iron. (A) Botrytis cinerea mycelium dry weight. (B) Siderophore production expressed as the relative absorbance at 630 nm monitored by chrome azurol S (CAS) assay/mg of mycelium dry weight (DW).
DISCUSSION
In plants, as in humans and other animals, the availability of iron is one of the factors which may limit the growth of pathogenic microorganisms within the host. Although the role of high‐affinity iron uptake systems in the virulence of plant pathogens is beginning to be well documented, the role of the plant iron status per se on the susceptibility to disease has only been investigated in a few cases (Anderson and Guerra, 1985; Macur et al., 1991). In this work, we explored this question on the model plant A. thaliana. As discussed below, we found that the plant iron status influences the microbial fitness, as well as reactions of the plant basal immunity, in a complex manner. Using the pathogens D. dadantii and B. cinerea, we show that conditions favourable for plant growth are also greatly beneficial to the pathogen.
To analyse the effects exerted by the plant iron status on the susceptibility to pathogens, we devised a protocol on A. thaliana plants displaying similar sizes, allowing relevant comparisons to be made between +Fe and −Fe conditions. This enabled us to show that plants exposed to iron deficiency are less susceptible to disease caused by D. dadantii and B. cinerea. To explain this effect, we focused our analysis on the D. dadantii–A. thaliana pathosystem and considered three explanations, which are not mutually exclusive: (i) in planta bacterial growth is limited by low iron availability; (ii) bacterial virulence is reduced in iron‐starved plants; and (iii) plant defence is increased in iron‐starved plants.
Indeed, in iron‐starved plants, symptom spread and bacterial growth were greatly reduced within the first 24 h of infection by D. dadantii, suggesting that the reduced mobilization of nutritional iron from the root to the aerial parts of the plant results in reduced bacterial fitness because of decreased iron availability in the leaf. This view is in agreement with the fact that high‐affinity iron acquisition systems, such as the production of siderophores, are required by D. dadantii to cause systemic infections on its hosts (Dellagi et al., 2005; Enard et al., 1988; Franza et al., 2005). In addition, in iron‐starved plants, we found a reduced expression of the genes encoding pectate lyases, which are the major virulence factors of D. dadantii. The reduced expression of these genes must result in a lower production of the corresponding enzymes, and thus in reduced bacterial growth and invasiveness. Moreover, the reduced expression of these genes could be the result of a physiological trade‐off in bacterial cells which encounter stress conditions in iron‐starved plants. Interestingly, by re‐supplying iron‐starved plants with iron, we were able to restore the symptom severity to a level almost similar to that observed on nonstarved plants, thus indicating that iron in the root medium acts as a limiting factor for the infection process. Iron re‐supply must result in an uptake of this metal by the plant, thus rescuing bacterial growth and the expression of virulence genes.
We showed that, in A. thaliana, the iron status has some impact on defence reactions activated in response to D. dadantii. It is noteworthy that, in iron‐starved plants, the defences known to be effective against this bacterium (Fagard et al., 2007) are unaffected, as is the case for the Et/JA‐mediated response, or strongly reduced, as is the case for callose and ROS production. It seems clear that these defences do not contribute to the increased resistance against D. dadantii in iron‐starved plants. The reduced production of ROS in such plants may result from the fact that iron is required for the formation of ROS through the Fenton reaction (Pierre and Fontecave, 1999). In addition, iron is needed for the activity of several ROS‐generating enzymes, which are haem dependent, such as NADPH‐oxidases (AtRbohD and AtRbohF) and peroxidases (Mittler et al., 2011). Thus, it is very probable that the production of ROS following infection is hindered because the iron levels are reduced. The reduced callose deposition following D. dadantii infection in iron‐starved plants may be the result of the involvement of iron in this process. It may also be related to low ROS production. This assumption is supported by the fact that callose deposition in response to the bacterial plant defence elicitors, flagellin‐derived peptide Flg22 (Luna et al., 2011) and oligogalacturonides (Galletti et al., 2008), is dependent on ROS production. As anticipated, the up‐regulation of the AtFER1 gene in response to D. dadantii infection was also affected in iron‐starved plants. Very probably, this effect results from the low levels of ROS and iron, as these two factors are known to control the transcriptional expression of the AtFER1 gene (Gaymard et al., 1996; Petit et al., 2001).
The only defence that appeared to be enhanced in iron‐starved plants was the expression of the SA pathway marker gene PR1. Would this pathway be involved in the enhanced resistance to D. dadantii in iron‐starved plants? Previous data, based on the study of the Arabidopsis SA‐deficient mutant sid2, indicating that defences mediated by SA are not effective against D. dadantii infection (Fagard et al., 2007), do not support this hypothesis. However, the mode of action of SA, which is supposed to interfere with the intracellular redox status, has still not been clarified. We can assume that some SA‐mediated effects depend on the plant physiological status. Iron‐starved plants are metabolically different from nonstarved plants (Yang et al., 2010) and SA is a powerful iron‐scavenging molecule (Nurchi et al., 2009). This compound might be used by the plant to mobilize iron under deficiency and to withhold this metal from pathogens.
Our data are reminiscent of the observations reported by Luna et al. (2011). These authors demonstrated that plant nutrition conditions have an important impact on the establishment of defences. They showed that, in A. thaliana, modification of the level of saccharose or vitamins in the growth medium affects the intensity of callose deposition and of hydrogen peroxide accumulation in response to Flg22 and chitosan. It is therefore of primary importance to consider the effects of nutrition on plant defences, especially in the context of agronomically important species.
We tested the possible effect of iron on the infection process of a fungal pathogen, B. cinerea. Interestingly, we found that iron‐starved Arabidopsis plants display enhanced resistance against this pathogen. Whether this effect is related to changes in the expression of fungal virulence and/or of the plant defence system requires further investigations. We found that iron starvation reduces B. cinerea growth and stimulates the accumulation of siderophores, suggesting that iron‐starved plants could affect the fitness of the fungus. Konetschny‐Rapp et al. (1988) chemically identified the siderophore produced by a rose‐derived strain of B. cinerea as being the ferrirhodin. Interestingly, high‐affinity iron uptake systems have been identified as virulence determinants in several phytopathogenic fungi (Eichhorn et al., 2006; Greenshields et al., 2007; Haas et al., 2008; Oide et al., 2006). Thus, further investigation of the role of B. cinerea iron uptake mechanisms in disease progress could provide valuable data to improve crop protection.
Iron‐starved plants displayed reduced susceptibility to two pathogens with similar lifestyles (necrotrophic, large host range and soft rot causing). It would be worth investigating whether −Fe plants display a similar behaviour when infected with biotrophic or hemibiotrophic pathogens. We should mention that studies conducted on other pathosystems showed different results from ours. For instance, Anderson and Guerra (1985) observed an increase in severity of Fusarium solani‐initiated disease on iron‐restricted bean seedlings. Similarly, infection levels by Verticillium dahliae of tomato resistant genotypes increased when the plants were grown under iron‐deficient conditions (Macur et al., 1991). In these reports, the authors studied plant pathogens that invaded the root vascular system. Thus, the influence of plant iron status on host–pathogen relationships may differ according to the pathogen considered. In addition, it would be worth studying the effect of long‐term iron starvation, such as that occurring in calcareous soils, on plant diseases. This is reminiscent of what has been reported for nitrogen fertilization, which can either promote or limit infections depending on the pathogen, the host plant and the chemical form of nitrogen supplied (Huber and Watson, 1974; Snoeijers et al., 2000). In the same way, crop iron fertilization could influence the intensity of disease caused by pathogens. Similarly, depending on the level of starvation, opposite effects of iron deficiency have been described on immunity in mammals (Weiss, 2002).
Therefore, no generalization should be made concerning the role of plant mineral nutrition on microbial disease development. Such investigations are of primary importance in an agronomical context, where reductions in mineral fertilization and pesticide crop treatment are becoming compulsory. Together, these reports provide valuable data for the development of crop genotypes with efficient mineral use and uptake properties without an increase in susceptibility to plant pathogens and/or decrease in yield or crop quality.
EXPERIMENTAL PROCEDURES
Plant material and growth conditions
Arabidopsis thaliana seeds from the Col‐0 ecotype were obtained from the INRA Versailles collection (Versailles, France). For hydroponic cultures, seeds were first stratified for 2 days at 4 °C in a nutrient solution (described below) containing 0.1% agar. Seeds were then individually sown in Eppendorf tubes cut at the bottom and filled with 0.75% agar. They were placed in PVC holders floating on the nutrient solution. Plants were allowed to grow for 5–6 weeks. The nutrient solution contained 0.25 mm Ca(NO3)2.4H2O, 1 mm KH2PO4, 0.5 mm KNO3, 1 mm MgSO4.7H2O, 50 µm H3BO3, 19 µm MnCl2.4H2O, 10 µm ZnCl2, 1 µm CuSO4.5H2O, 0.02 µm Na2MoO4.2H2O and 50 µm FeNa‐ethylenediaminetetraacetate (FeNa‐EDTA). Plants were subjected to an 8‐h light/16‐h dark cycle, at 19 °C, with 70% relative humidity.
To study the effect of plant iron status on microbial infection, we used the hydroponic system described previously (Segond et al., 2009). In order to obtain plants with reduced iron content, without affecting plant size, we first grew plants under iron‐replete conditions (50 µm Fe‐EDTA, Fig. 1A) for 5 weeks. Then, iron deficiency was achieved as follows. Roots were washed for 5 min with a medium containing the reductant sodium dithionite (5.7 mm) and the chelator bathophenanthroline disulphonic acid (BPDS, 0.3 mm), both from Sigma (St. Louis, OH, USA). Roots were then washed with distilled water and transferred to iron‐depleted medium. Four days later, the plants displayed typical iron deficiency symptoms: leaf chlorosis and the presence of a larger number of secondary roots (Fig. 1B,C). These plants were referred to as ‘–Fe plants’. Control plants were grown for 6 weeks in the presence of 50 µm Fe‐EDTA. They were referred to as ‘+Fe plants’. The iron content was found to be reduced by 30% in −Fe leaves [61 ± 1.41 µg/g dry weight (DW)] relative to +Fe leaves (90 ± 8.3 µg/g DW). Under these conditions, leaves of +Fe and −Fe plants have almost the same size (Fig. 1).
Bacterial and fungal strains and culture conditions
The wild‐type strain, Dickeya dadantii 3937 (previously named Erwinia chrysanthemi 3937 our collection), was isolated from Saintpaulia ionantha H. Wendl. (African violet). The species D. dadantii consists of strains previously belonging to the Erwinia chrysanthemi species, sharing genotypic and phenotypic characteristics which led to their transfer to a novel taxon (Samson et al., 2005). Growth conditions were as described in Dellagi et al. (2005). The B. cinerea wild‐type strain BO5.10 was maintained on malt agar medium for virulence tests and cultivated on potato dextrose agar for conidia production. For the determination of fungal DW and siderophore production, the fungus was cultivated in liquid Czapek medium, as described by Reignault et al. (2000), with 3% glucose as carbon source. Liquid medium was supplemented or not with 36 µm FeSO4.7H2O. Cultures of 50 mL were inoculated with a final concentration of 3 × 105 conidia/mL and grown for 5 days.
Determination of fungal biomass and siderophores in fungal culture filtrates
Liquid cultures of B. cinerea were filtered on tissue with a pore size of 100 µm and the mycelium DW was determined by weighing the fungal biomass after 2 days of drying at 80 °C. Siderophores in the culture filtrates were determined spectrophotometrically using a CAS–iron(III) assay, according to Schwyn and Neilands (1987). CAS–iron absorbs at 630 nm and, when a strong chelator removes the iron from the dye, its colour turns from blue to orange. The results correspond to the relative absorbance per milligram of fungal DW. The relative absorbance was calculated as follows: [A 630(reference) −A 630(culture filtrate)/A 630(reference)]× 100%. Uninoculated culture medium was used as reference.
Plant inoculations and determination of bacterial number
For the scoring of symptoms and the determination of bacterial populations in planta, a small hole was made with a needle within the leaf and 5 µL of a bacterial suspension at a density of 107 cfu/mL, made up in 50 mm potassium phosphate buffer (pH 7), were spotted on the hole. One leaf per plant was inoculated and 24–30 plants were used in each experiment. Symptom severity was scaled (Fig. 2B) as follows: 0, no symptoms; 1, maceration at the site of inoculation; 2, maceration spreading to about one‐half of the leaf; 3, maceration spreading to the whole leaf; 4, maceration starting to spread to the rest of the plant. Fisher's exact test was used to compare symptom distributions (Simple Interactive Statistical Binomial website, http://www.quantitativeskills.com/sisa/). For the determination of bacterial number, leaves were harvested in 0.9% NaCl and ground using a pestle and sterile sand. The resulting suspensions were used for serial dilutions, followed by plating on M9 minimal medium (Sambrook et al., 1989).
For RNA extractions, callose or H2O2 staining, we used a syringe without a needle to infiltrate the entire leaf or a portion of the leaf with the bacterial suspension at 107 cfu/mL in 10 mm MgSO4 (half a leaf was infiltrated for callose and H2O2 staining). For RNA analysis, callose and H2O2 staining, three leaves were inoculated on each plant, and six plants were used in each experiment.
Detached A. thaliana leaves were inoculated with B. cinerea mycelium plugs and lesion spreading was scored as described in Arbelet et al. (2010).
RNA extraction and quantitative RT‐PCR
Leaves were harvested at the indicated time points after treatment and then frozen in liquid nitrogen. Total RNAs were purified with TRIzol reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's instructions. The total RNA concentration was determined using a NanoDrop ND‐1000 spectrophotometer (NanoDropTechnologies Inc., Wilmington, DE, USA). RNA samples were treated with Turbo DNaseI (Ambion, Saint‐Aubin, France) RNase‐free to remove any DNA contamination. A total of 1 µg of DNase‐treated RNA was reverse transcribed using the High Capacity cDNA Reverse Transcription Kit and 50 ng of random hexamers following the supplier's instructions. One microlitre of the 1:10 diluted cDNA was subjected to real‐time quantitative PCR using SYBR Green PCR Mastermix (Applied Biosystems, Foster City, CA,USA) and gene‐specific primers designed to amplify 100–150‐bp fragments from each gene of interest and the reference genes EF1α and rpoB used for A. thaliana and D. dadantii, respectively. Primer sequences are indicated in Table S1 (see Supporting Information). Real‐time quantitative PCR analysis was performed using a 7300 system (Applied Biosystems). For the fold expression of D. dadantii pectinase genes in planta, data were expressed as 2–ΔΔCt. For example, for pelB, the fold expression at a given time point is: 2–{[Ct pelB (+Fe) − Ct rpoB(+Fe)]−[Ct pelB (–Fe) − Ct rpoB(–Fe)]}.
Callose deposition analysis by aniline blue staining and quantification
Leaves were stained with aniline blue and then examined by epifluorescence microscopy, as described previously (Fagard et al., 2007). For each leaf, a representative image was obtained with an exposure time of 835 ms. Image J software was used for image analysis (National Institutes of Health, Bethesda, MD, USA). The colour image was transformed into an eight‐bit greyscale picture and the callose spots were counted in a field of 0.6 mm2. Experiments were performed three times with similar results.
Hydrogen peroxide staining and quantification
The H2O2 detection method was adapted from Zhang et al. (2004). At different time points following leaf infiltration with the bacterial strain or the control 10 mm MgSO4, leaves were excised and then stained by vacuum infiltration in a 300 µm DCFH‐DA solution in the dark at room temperature. Observations were performed 15 min later. Whole‐leaf images were taken using an Olympus SZX12 binocular magnifier and pictures were captured after 30 s of excitation at 470 nm. RGB split was performed on whole‐leaf pictures using ImageJ software (National Institutes of Health), green was converted into grey and mean grey values were calculated. Experiments were performed three times with similar results.
Quantification of plant iron content
Leaves were harvested from healthy −Fe and +Fe plants. The iron content was determined using the protocol described in Lanquar et al. (2010).
Supporting information
Table S1 Sequence of primers used in this study.
Supporting info item
ACKNOWLEDGEMENTS
This work was supported by grants from the Institut National de la Recherche Agronomique (INRA). N.P.K. was granted from the government of Vietnam. A.A. and D.S. were funded by the Ministère de l’Enseignement Supérieur et de la Recherche. We thank O. Patrit for technical help, and S. Thomine and N. Chen for iron quantifications.
REFERENCES
- Anderson, A.J. and Guerra, D. (1985) Responses of bean to root colonization with Pseudomonas putida in a hydroponic system. Phytopathology, 75, 992–995. [Google Scholar]
- Andrews, S.C. , Robinson, A.K. and Rodriguez‐Quinones, F. (2003) Bacterial iron homeostasis. FEMS Microbiol. Rev. 27, 215–237. [DOI] [PubMed] [Google Scholar]
- Arbelet, D. , Malfatti, P. , Simond‐Cote, E. , Fontaine, T. , Desquilbet, L. , Expert, D. , Kunz, C. and Soulie, M.C. (2010) Disruption of the bcchs3a chitin synthase gene in Botrytis cinerea is responsible for altered adhesion and overstimulation of host plant immunity. Mol. Plant–Microbe Interact. 23, 1324–1334. [DOI] [PubMed] [Google Scholar]
- Briat, J.‐F. , Curie, C. and Gaymard, F. (2007a) Iron utilization and metabolism in plants. Curr. Opin. Plant Biol. 10, 276–282. [DOI] [PubMed] [Google Scholar]
- Briat, J.F. , Arnaud, N. , Cellier, F. , Curie, C. , Gaymard, F. , Ravel, K. , Seguela, M. and Vert, G. (2007b) Iron uptake and storage in plants. Am. J. Hematol. 82, 506–507. [Google Scholar]
- Dellagi, A. , Rigault, M. , Segond, D. , Roux, C. , Kraepiel, Y. , Cellier, F. , Briat, J.F. , Gaymard, F. and Expert, D. (2005) Siderophore‐mediated upregulation of Arabidopsis ferritin expression in response to Erwinia chrysanthemi infection. Plant J. 43, 262–272. [DOI] [PubMed] [Google Scholar]
- Eichhorn, H. , Lessing, F. , Winterberg, B. , Schirawski, J. , Kaemper, J. , Mueller, P. and Kahmann, R. (2006) A ferroxidation/permeation iron uptake system is required for virulence in Ustilago maydis . Plant Cell, 18, 3332–3345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eide, D. , Broderius, M. , Fett, J. and Guerinot, M.L. (1996) A novel iron‐regulated metal transporter from plants identified by functional expression in yeast. Proc. Natl. Acad. Sci. USA, 93, 5624–5628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enard, C. , Diolez, A. and Expert, D. (1988) Systemic virulence of Erwinia‐chrysanthemi 3937 requires a functional iron assimilation system. J. Bacteriol. 170, 2419–2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Expert, D. (1999) Withholding and exchanging iron: interactions between Erwinia spp. and their plant hosts. Annu. Rev. Phytopathol. 37, 307–334. [DOI] [PubMed] [Google Scholar]
- Fagard, M. , Dellagi, A. , Roux, C. , Perino, C. , Rigault, M. , Boucher, V. , Shevchik, V.E. and Expert, D. (2007) Arabidopsis thaliana expresses multiple lines of defense to counterattack Erwinia chrysanthemi . Mol. Plant–Microbe Interact. 20, 794–805. [DOI] [PubMed] [Google Scholar]
- Franza, T. , Mahe, B. and Expert, D. (2005) Erwinia chrysanthemi requires a second iron transport route dependent on the siderophore achromobactin for extracellular growth and plant infection. Mol. Microbiol. 55, 261–275. [DOI] [PubMed] [Google Scholar]
- Galletti, R. , Denoux, C. , Gambetta, S. , Dewdney, J. , Ausubel, F.M. , De Lorenzo, G. and Ferrari, S. (2008) The AtrbohD‐mediated oxidative burst elicited by oligogalacturonides in Arabidopsis is dispensable for the activation of defense responses effective against Botrytis cinerea . Plant Physiol. 148, 1695–1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaymard, F. , Boucherez, J. and Briat, J.F. (1996) Characterization of a ferritin mRNA from Arabidopsis thaliana accumulated in response to iron through an oxidative pathway independent of abscisic acid. Biochem. J. 318, 67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glazebrook, J. (2005) Contrasting mechanisms of defense against biotrophic and necrotrophic pathogens. Annu. Rev. Phytopathol. 43, 205–227. [DOI] [PubMed] [Google Scholar]
- Greenshields, D.L. , Liu, G.S. , Feng, J. , Selvaraj, G. and Wei, Y.D. (2007) The siderophore biosynthetic gene SID1, but not the ferroxidase gene FET3, is required for full Fusarium graminearum virulence. Mol. Plant Pathol. 8, 411–421. [DOI] [PubMed] [Google Scholar]
- Haas, H. , Eisendle, M. and Turgeon, B.G. (2008) Siderophores in fungal physiology and virulence. Annu. Rev. Phytopathol. 46, 149–187. [DOI] [PubMed] [Google Scholar]
- Huber, D.M. and Watson, R.D. (1974) Nitrogen form and plant disease. Annu. Rev. Phytopathol. 12, 139–165. [DOI] [PubMed] [Google Scholar]
- Kim, S.A. , Punshon, T. , Lanzirotti, A. , Li, L. , Alonso, J.M. , Ecker, J.R. , Kaplan, J. and Guerinot, M.L. (2006) Localization of iron in Arabidopsis seed requires the vacuolar membrane transporter VIT1. Science, 314, 1295–1298. [DOI] [PubMed] [Google Scholar]
- Kobayashi, T. , Nakanishi, H. and Nishizawa, N.K. (2010) Recent insights into iron homeostasis and their application in graminaceous crops. Proc. Jpn. Acad., Ser. B, Phys. Biol. Sci. 86, 900–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konetschny‐Rapp, S. , Jung, G. , Huschka, H.‐G. and Winkelmann, G. (1988) Isolation and identification of the principal siderophore of the plant pathogenic fungus Botrytis cinerea . Biol. Metals, 1, 90–98. [Google Scholar]
- Kraepiel, Y. , Pedron, J. , Patrit, O. , Simond‐Cote, E. , Hermand, V. and Van Gijsegem, F. (2011) Analysis of the plant bos1 mutant highlights necrosis as an efficient defence mechanism during D. dadantii/Arabidospis thaliana interaction. PLoS ONE 6, e18991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanquar, V. , Lelievre, F. , Bolte, S. , Hames, C. , Alcon, C. , Neumann, D. , Vansuyt, G. , Curie, C. , Schroder, A. , Kramer, U. , Barbier‐Brygoo, H. and Thomine, S. (2005) Mobilization of vacuolar iron by AtNRAMP3 and AtNRAMP4 is essential for seed germination on low iron. EMBO J. 24, 4041–4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanquar, V. , Ramos, M.S. , Lelievre, F. , Barbier‐Brygoo, H. , Krieger‐Liszkay, A. , Kraemer, U. and Thomine, S. (2010) Export of vacuolar manganese by AtNRAMP3 and AtNRAMP4 is required for optimal photosynthesis and growth under manganese deficiency. Plant Physiol. 152, 1986–1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebeau, A. , Reverchon, S. , Gaubert, S. , Kraepiel, Y. , Simond‐Cote, E. , Nasser, W. and Van Gijsegem, F. (2008) The GacA global regulator is required for the appropriate expression of Erwinia chrysanthemi 3937 pathogenicity genes during plant infection. Environ. Microbiol. 10, 545–559. [DOI] [PubMed] [Google Scholar]
- Lemanceau, P. , Expert, D. , Gaymard, F. , Bakker, P. and Briat, J.F. (2009) Role of iron in plant–microbe interactions. Adv. Bot. Res. 51, 491–549. [Google Scholar]
- Lindsay, W.L. and Schwab, A.P. (1982) The chemistry of iron in soils and its availability to plants. J. Plant Nutr. 5, 821–840. [Google Scholar]
- Luna, E. , Pastor, V. , Robert, J. , Flors, V. , Mauch‐Mani, B. and Ton, J. (2011) Callose deposition: a multifaceted plant defense response. Mol. Plant–Microbe Interact. 24, 183–193. [DOI] [PubMed] [Google Scholar]
- Macur, R.E. , Mathre, D.E. and Olsen, R.A. (1991) Interactions between iron nutrition and verticillium wilt resistance in tomato. Plant Soil, 134, 281–286. [Google Scholar]
- Marschner, H. (1995) Mineral Nutrition of Higher Plants, New York: Academic Press. [Google Scholar]
- Mittler, R. , Vanderauwera, S. , Suzuki, N. , Miller, G. , Tognetti, V.B. , Vandepoele, K. , Gollery, M. , Shulaev, V. and Van Breusegem, F. (2011) ROS signaling: the new wave? Trends Plant Sci. 16, 300–309. [DOI] [PubMed] [Google Scholar]
- Morrissey, J. and Guerinot, M.L. (2009) Iron uptake and transport in plants: the good, the bad, and the ionome. Chem. Rev. 109, 4553–4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Münzinger, M. , Budzikiewicz, H. , Expert, D. , Enard, C. and Meyer, J.M. (2000) Achromobactin, a new citrate siderophore of Erwinia chrysanthemi . Z. Naturforsch. C, 55, 328–332. [DOI] [PubMed] [Google Scholar]
- Murdoch, L. , Corbel, J.C. , Reis, D. , Bertheau, Y. and Vian, B. (1999) Differential cell wall degradation by Erwinia chrysanthemi in petiole of Saintpaulia ionantha . Protoplasma, 210, 59–74. [Google Scholar]
- Nurchi, V.M. , Pivetta, T. , Lachowicz, J.I. and Crisponi, G. (2009) Effect of substituents on complex stability aimed at designing new iron(III) and aluminum(III) chelators. J. Inorg. Biochem. 103, 227–236. [DOI] [PubMed] [Google Scholar]
- Oide, S. , Moeder, W. , Krasnoff, S. , Gibson, D. , Haas, H. , Yoshioka, K. and Turgeon, B.G. (2006) NPS6, encoding a nonribosomal peptide synthetase involved in siderophore‐mediated iron metabolism, is a conserved virulence determinant of plant pathogenic ascomycetes. Plant Cell, 18, 2836–2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perombelon, M.C.M. (2002) Potato diseases caused by soft rot erwinias: an overview of pathogenesis. Plant Pathol. 51, 1–12. [Google Scholar]
- Persmark, M. , Expert, D. and Neilands, J.B. (1989) Isolation, characterization, and synthesis of chrysobactin, a compound with siderophore activity from Erwinia‐chrysanthemi . J. Biol. Chem. 264, 3187–3193. [PubMed] [Google Scholar]
- Petit, J.M. , Briat, J.F. and Lobreaux, S. (2001) Structure and differential expression of the four members of the Arabidopsis thaliana ferritin gene family. Biochem. J. 359, 575–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierre, J.L. and Fontecave, M. (1999) Iron and activated oxygen species in biology: the basic chemistry. Biometals, 12, 195–199. [DOI] [PubMed] [Google Scholar]
- Ravet, K. , Touraine, B. , Kim, S.A. , Cellier, F. , Thomine, S. , Guerinot, M.L. , Briat, J.F. and Gaymard, F. (2009) Post‐translational regulation of atfer2 ferritin in response to intracellular iron trafficking during fruit development in Arabidopsis. Mol. Plant, 2, 1095–1106. [DOI] [PubMed] [Google Scholar]
- Reignault, P. , Kunz, C. , Delage, N. , Moreau, E. , Vedel, R. , Hamada, W. , Bompeix, G. and Boccara, M. (2000) Host‐ and symptom‐specific pectinase isozymes produced by Botrytis cinerea . Mycol. Res. 104, 421–428. [Google Scholar]
- Robinson, N.J. , Procter, C.M. , Connolly, E.L. and Guerinot, M.L. (1999) A ferric‐chelate reductase for iron uptake from soils. Nature, 397, 694–697. [DOI] [PubMed] [Google Scholar]
- Romheld, V. and Marschner, H. (1986) Evidence for a specific uptake system for iron phytosiderophores in roots of grasses. Plant Physiol. 80, 175–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook, J. , Fritsch, E.F. and Maniatis, T. (1989) Molecular Cloning: A Laboratory Manual. 2nd edn. Cold Spring Harbor, NY: Cold Spring Harbor Press. [Google Scholar]
- Samson, R. , Legendre, J.B. , Christen, R. , Fischer‐Le Saux, M. , Achouak, W. and Gardan, L. (2005) Transfer of Pectobacterium chrysanthemi (Burkholder et al. 1953) Brenner et al. 1973 and Brenneria paradisiaca to the genus Dickeya gen. nov as Dickeya chrysanthemi comb. nov and Dickeya paradisiaca comb. nov and delineation of four novel species, Dickeya dadantii sp nov., Dickeya dianthicola sp nov., Dickeya dieffenbachiae sp nov and Dickeya zeae sp nov. Int. J. Syst. Evol. Microbiol. 55, 1415–1427. [DOI] [PubMed] [Google Scholar]
- Schwyn, B. and Neilands, J.B. (1987) Universal chemical assay for the detection and determination of siderophores. Anal. Biochem. 160, 47–56. [DOI] [PubMed] [Google Scholar]
- Segond, D. , Dellagi, A. , Lanquar, V. , Rigault, M. , Patrit, O. , Thomine, S. and Expert, D. (2009) NRAMP genes function in Arabidopsis thaliana resistance to Erwinia chrysanthemi infection. Plant J. 58, 195–207. [DOI] [PubMed] [Google Scholar]
- Snoeijers, S.S. , Perez‐Garcia, A. , Joosten, M. and De Wit, P. (2000) The effect of nitrogen on disease development and gene expression in bacterial and fungal plant pathogens. Eur. J. Plant Pathol. 106, 493–506. [Google Scholar]
- Toth, I.K. , Bell, K.S. , Holeva, M.C. and Birch, P.R.J. (2003) Soft rot erwiniae: from genes to genomes. Mol. Plant Pathol. 4, 17–30. [DOI] [PubMed] [Google Scholar]
- Weiss, G. (2002) Iron and immunity: a double‐edged sword. Eur. J. Clin. Invest. 32, 70–78. [DOI] [PubMed] [Google Scholar]
- Winkelmann, G. (2007) Ecology of siderophores with special reference to the fungi. Biometals, 20, 379–392. [DOI] [PubMed] [Google Scholar]
- Yang, T.J.W. , Lin, W.‐D. and Schmidt, W. (2010) Transcriptional profiling of the Arabidopsis iron deficiency response reveals conserved transition metal homeostasis networks. Plant Physiol. 152, 2130–2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, C. , Gutsche, A.T. and Shapiro, A.D. (2004) Feedback control of the Arabidopsis hypersensitive response. Mol. Plant–Microbe Interact. 17, 357–365. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Sequence of primers used in this study.
Supporting info item


