Summary
The defence response of Zantedeschia aethiopica, a natural rhizomatous host of the soft rot bacterium Pectobacterium carotovorum, was studied following the activation of common induced resistance pathways—systemic acquired resistance and induced systemic resistance. Proteomic tools were used, together with in vitro quantification and in situ localization of selected oxidizing enzymes. In total, 527 proteins were analysed by label‐free mass spectrometry (MS) and annotated against the National Center for Biotechnology Information (NCBI) nonredundant (nr) protein database of rice (Oryza sativa). Of these, the fore most differentially expressed group comprised 215 proteins that were primed following application of methyl jasmonate (MJ) and subsequent infection with the pathogen. Sixty‐five proteins were down‐regulated following MJ treatments. The application of benzothiadiazole (BTH) increased the expression of 23 proteins; however, subsequent infection with the pathogen repressed their expression and did not induce priming. The sorting of primed proteins by Gene Ontology protein function category revealed that the primed proteins included nucleic acid‐binding proteins, cofactor‐binding proteins, ion‐binding proteins, transferases, hydrolases and oxidoreductases. In line with the highlighted involvement of oxidoreductases in the defence response, we determined their activities, priming pattern and localization in planta. Increased activities were confined to the area surrounding the pathogen penetration site, associating these enzymes with the induced systemic resistance afforded by the jasmonic acid signalling pathway. The results presented here demonstrate the concerted priming of protein expression following MJ treatment, making it a prominent part of the defence response of Z. aethiopica to P. carotovorum.
Introduction
Zantedeschia sp. (calla lilies) are herbaceous monocotyledonous ornamental geophytes that are highly sensitive to Pectobacterium carotovorum, the causal agent of soft rot disease. Currently, there is no effective way to control P. carotovorum in ornamental geophytes. The use of common bactericidal formulations to decrease P. carotovorum proliferation is relatively ineffective and these compounds are often phytotoxic (Gracia‐Garza et al., 2002; Snijder and van Tuyl, 2002). Currently, the only way to control P. carotovorum in Zantedeschia is through the use of strict sanitation measures and selection for more resistant cultivars (Luzzatto‐Knaan and Yedidia, 2009; Snijder et al., 2004a, b; Snijder and van Tuyl, 2002).
Evidence suggests that Zantedeschia aethiopica is capable of activating induced defence mechanisms to fight bacterial soft rot (Luzzatto et al., 2007a, b). The defence pathways and mechanisms involved have been found to depend on the plant's ability to recognize the pathogen and initiate defence signalling pathways, including a number of defence‐related genes. Similar to other plant systems, once recognition occurs, a large number of genes are activated and a systemic response is triggered (Rushton and Somssich, 1998; Walters and Heil, 2007). A similar systemic response has been shown recently for Z. aethiopica (Luzzatto‐Knaan et al., 2013). However, a defence response may be referred to as priming only if the plant displays a rapid and/or stronger activation of defence‐related genes when subsequently challenged by a pathogen (Conrath, 2011; Heil and Bostock, 2002; van Hulten et al., 2006; Luzzatto et al., 2007a; Sticher et al., 1997).
Induced systemic resistance (ISR) and systemic acquired resistance (SAR) are the two most recognized defence pathways in plants. These immune responses can be triggered by localized infection or by treatment with particular microbes or any of a diverse group of structurally unrelated organic and inorganic compounds (Heil and Bostock, 2002; Kúc, 2001; Pieterse et al., 1998, 2009, 2012; Proietti et al., 2013; Vallad and Goodman, 2004). SAR involves the salicylic acid (SA) signalling molecule and is characterized by elevated levels of pathogenesis‐related proteins and the hypersensitive response, whereas ISR is characterized by the jasmonic acid (JA)/ethylene signalling pathway and by increased levels of secondary metabolites and oxidizing enzymes (Constabel and Ryan, 1998; Glas et al., 2012; Kim et al., 2006; Li and Steffens, 2002; Luzzatto et al., 2007a; Pourcel et al., 2007).
Previously, we have shown that methyl jasmonate (MJ), acting via the JA signalling pathway, provides effective and long‐lasting resistance to P. carotovorum infection of Z. aethiopica, when compared with a control or the SA analogue benzothiadiazole (BTH). The MJ‐induced resistance was accompanied by the priming of polyphenolic compounds and increased antimicrobial activity, triggered by challenge inoculation with P. carotovorum (Luzzatto et al., 2007a, b). However, unlike in model plant systems, the activated expression of a large number of defence‐related genes and the roles of these genes in pathogen restriction have not been studied in Zantedeschia.
Microarray technology has become a powerful tool for the analysis of induced resistance and genome‐scale analyses of gene expression in model plants (Proietti et al., 2013; von Rad et al., 2005). However, the study of evolutionarily distant plants whose genomes have not been sequenced and for which few genomic data are available requires a different methodology. Proteomics is such an approach (Carpentier et al., 2008; Liska and Shevchenko, 2003). Proteomics allows the identification of the functional components of the gene (i.e. the protein), unravelling post‐translational modifications, even when a complete set of genomic data is not available (Carpentier et al., 2008; Deepak et al., 2008).
Plant proteomics lags behind other kingdoms, such as fungi (yeast), bacteria and animals (mammals), in terms of research and publications, mostly because of the relatively small amount of plant genome data available (Agrawal and Rakwal, 2006). The dicot Arabidopsis thaliana and the monocot Oryza sativa (rice) serve as model plants with sequenced genomes, as well as starting points for proteomic studies in other plant systems of interest (Lin et al., 2009). In some cases, the proteomic data analysis is performed against the National Center for Biotechnology Information (NCBI) nonredundant (nr) green plant database (Macarisin et al., 2009). With the benefits and pitfalls of this background, we have used proteomic tools to study the defence response of the ornamental monocot Z. aethiopica to the necrotrophic pathogen P. carotovorum. Based on previous studies, a label‐free quantification method for differential protein expression based on liquid chromatography‐mass spectrometry (LC‐MS) was utilized, and the MS data were analysed using the MaxQuant software and searching the O. sativa part of the NCBI nr database. Although this method has not been used very often in nonmodel plants, it has been employed to determine the relative abundance of proteins in different samples, such as poplar and wheat (Chen et al., 2012; Mak et al., 2006).
The plant responses were analysed following elicitation with two plant activators: BTH, which is associated with the SA‐dependent signalling pathway leading to SAR, and MJ, which is associated with the JA‐dependent signalling pathway leading to ISR (Pieterse et al., 2009, 2012; Vallad and Goodman, 2004). The overall objective of this study was to investigate protein expression following the elicitation of the two signalling pathways and following infection with the necrotrophic pathogen P. carotovorum in a nonmodel host Z. aethiopica. It is expected that the identification of expressed proteins according to their class and function might help us to better understand the mechanisms underlying the defence response of Z. aethiopica to P. carotovorum.
Results
Comparative quantitative proteomics
Extracts of three independent biological repeats were pooled in each treatment, and compared with a similar pool of another treatment. The proteins from each sample were trypsinized, resolved by reversed‐phase chromatography and analysed by tandem MS (MS/MS) analysis. The identification of proteins was performed by analysing the MS and MS/MS data against the closest related model organism for which a complete database was available, as the genome of Z. aethiopica was not available. Quantification was performed by label‐free analysis, calculating the peak volume of each identified peptide.
Six leaf treatments were compared: a distilled deionized water (ddw) control treatment; ddw followed by P. carotovorum infection; BTH treatment (5 μg/mL) with or without subsequent P. carotovorum infection; MJ treatment (10 mg/mL) with or without subsequent P. carotovorum infection. Analysis of all treatments was performed using MaxQuant software (Cox and Mann, 2008), resulting in 1371 peptide sequences that were identified and assigned to 527 proteins annotated against the Oryza part of the NCBI nr database. The threshold for differential expression was set at a two‐fold change (increase or decrease) in the relative intensities.
The expression of 134 of the 527 proteins was up‐regulated (two‐fold or greater) following P. carotovorum infection relative to each of the induction treatments (control, BTH or MJ), whereas 147 proteins were down‐regulated (two‐fold or greater) relative to the same induction treatments (Fig. 1). When the induction treatments with BTH or MJ were analysed relative to the control treatment, 54 and 93 up‐regulated (two‐fold or greater) proteins were observed, whereas 37 and 56 proteins were down‐regulated (two‐fold or greater), respectively. The same analysis (relative to control) following infection with P. carotovorum after either BTH or MJ treatment revealed similar numbers (58) of up‐regulated proteins (two‐fold or greater) for BTH + pathogen, whereas MJ + pathogen resulted in the up‐regulation of 120 proteins (two‐fold or greater) relative to the control. The largest group of up‐regulated proteins following infection with P. carotovorum was found among MJ‐treated plants (70 proteins). This group represented more than one‐half of all up‐regulated proteins following infection (Fig. 1). The smallest number of up‐regulated proteins following infection with P. carotovorum was observed for BTH‐induced plants. In plants treated only with the pathogen, 48 proteins were up‐regulated relative to the control. With the threshold used (two‐fold or greater), only a few proteins were common among the different treatments. The largest number of down‐regulated proteins was found in leaves that were only inoculated with the pathogen (83 proteins). The MJ + pathogen treatment repressed the expression of 51 proteins, relative to the treatment in which leaves were only induced with MJ, similar to the BTH + pathogen treatment with 52 repressed proteins relative to BTH alone (Fig. 1).
Figure 1.
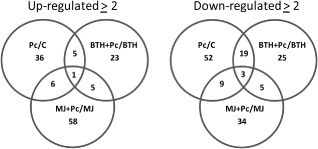
Venn diagrams showing the number of proteins whose expression was induced by the plant activators methyl jasmonate (MJ) and benzothiadiazole (BTH) and by Pectobacterium carotovorum infection. Zantedeschia aethiopica plants received the following treatments: C, distilled deionized water (ddw), BTH (5 μg/mL), MJ (10 mg/mL), followed by inoculation with P. carotovorum (Pc). Differences in expression between the inoculated and uninoculated treatments were calculated (threshold value, ≥2). Data are based on pooled protein samples from three independent experiments.
Protein classification
Gene Ontology (GO) categories were used to evaluate the potential functions of the differentially expressed proteins. The identified proteins were classified by molecular function, biological processes and cellular component, according to annotations in the Gene Ontology Annotation (UniProt‐GOA) Database (http://www.ebi.ac.uk/GOA/). The identified proteins corresponded to 13 functional groups and 18 biological processes associated with seven cellular components, covering a wide range of pathways and functions (Fig. 2). The cellular component analysis revealed a high percentage of genes corresponding to the cell, membrane, organelle, macromolecular complexes and extracellular region. For categories based on molecular function, the five most represented GO terms were binding, catalytic activity, structural molecule activity, antioxidant activity and electron carrier activity. For biological processes, the five most represented GO terms were metabolic progress, cellular progress, response to stimulus, biological regulation and cellular component organization or biogenesis (Fig. 2).
Figure 2.
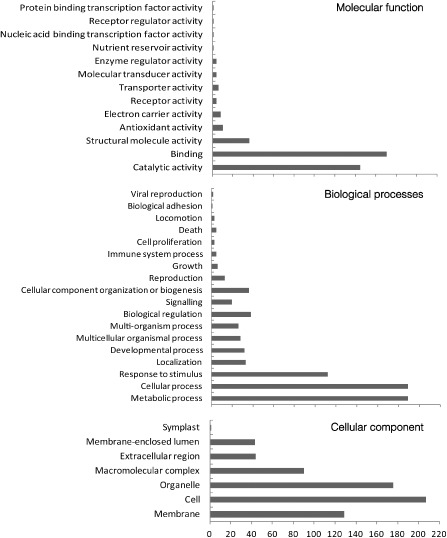
Level 2 Gene Ontology (GO) classification of all proteins. The identified proteins were classified on the basis of the molecular function term, biological processes term and cellular component term for each protein, from the annotations in the Gene Ontology Annotation (UniProt‐GOA) Database. The GO term distribution was extracted using blast2go.
Clustering of protein expression patterns based on expression intensities
The expression levels of proteins may vary between treatments, when the cell undergoes specific processes. The analysis of protein expression data requires the clustering of proteins into groups with similar expression patterns. We used the clustering algorithm called CLICK (CLuster Identification via Connectivity Kernels), which is applicable to gene and protein expression analyses (Sharan and Shamir, 2000). Our data yielded nine distinct expression patterns across all six treatments, four of which were selected according to their association with one of the defence pathways, and are presented here (Fig. 3).
Figure 3.
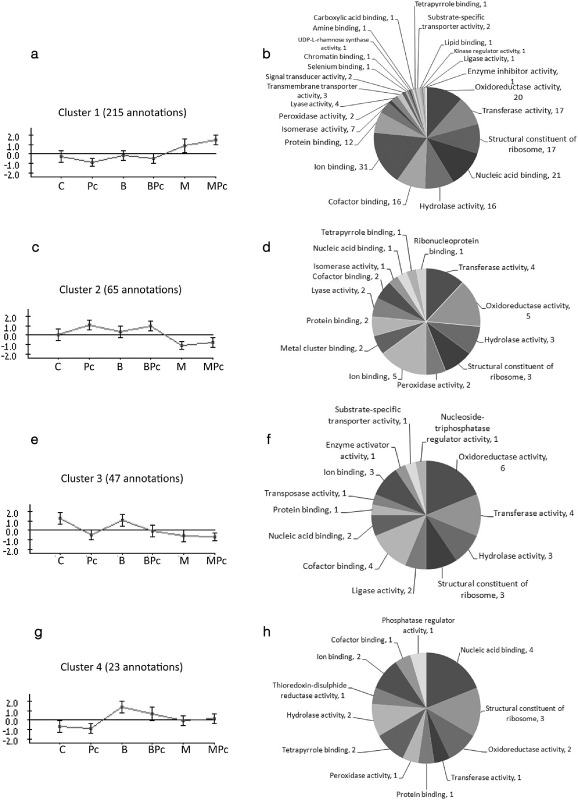
Clusters of leaf proteins expressed in the different treatments: C, distilled deionized water (ddw); B, benzothiadiazole (BTH; 5 μg/mL); M, methyl jasmonate (MJ; 10 mg/mL); Pc, Pectobacterium carotovorum; BPc, BTH + P. carotovorum; MPc, MJ + P. carotovorum. The pie charts on the right show the number of proteins in different functional classes [Gene Ontology (GO) level 3]. The CLICK (CLuster Identification via Connectivity Kernels) algorithm was used to cluster the annotated proteins on the basis of their expression levels. (a, b) Cluster 1. (c, d) Cluster 2. (e, f) Cluster 3. (g, h) Cluster 4.
Cluster 1 includes 215 proteins that were observed at elevated levels in the MJ treatment and whose expression was further increased following P. carotovorum infection, presenting a primed mode of action. These primed proteins were associated with 101 GO functions in 24 categories; 31 displayed ion binding activity, 21 nucleic acid binding activity, 20 oxidoreductase activity, 17 transferase activity, 17 were identified as structural constituents of the ribosome and 16 were associated with hydrolase activity.
Cluster 2 includes 65 proteins whose expression was repressed in the MJ treatments. These were associated with 20 GO functions in 14 categories; five were associated with ion binding activity, five with oxidoreductase activity, four with transferase activity, three with hydrolase activity and three with structural constituents of the ribosome.
Cluster 3 includes 47 proteins whose expression was repressed following P. carotovorum infection or MJ treatment. These were associated with 22 GO functions in 13 categories: six with oxidoreductase activity, four with transferase activity, four with cofactor binding, three with hydrolase activity and three with structural constituents of the ribosome.
Cluster 4 includes 23 proteins that were up‐regulated following BTH treatment and repressed following inoculation with P. carotovorum. These were associated with 11 GO functions in 12 categories: four with nucleic acid binding activity, three with structural constituents of the ribosome, two with oxidoreductase activity, two with tetrapyrrole activity and two with hydrolase activity.
Identification of differentially expressed proteins
Based on the LC‐MS data, of the 215 cluster 1 proteins whose expression levels increased following MJ treatment and increased further after pathogen infection, 88 differentially expressed proteins revealed a two‐fold or greater increase in expression (relative to the control). The protein data presented in Table 1 represent the first report of Z. aethiopica‐induced proteins. Table 2 shows the 30 proteins of cluster 2 whose expression was down‐regulated in response to MJ + P. carotovorum treatment. The expression levels of these 30 proteins were repressed by over 40%; the expression of nine was repressed by 40%–50%, the expression of nine others was repressed by over 50% and 12 were fully suppressed.
Table 1.
Proteins with increased differential expression (cluster 1) identified in Zantedeschia aethiopica leaves following treatment with methyl jasmonate and subsequent infection with P ectobacterium carotovorum (two‐fold or greater increase in expression relative to the control)
| Protein ID | Matched protein* | Access IDa | Fold changeb | Protein functionc | Process/cellular componentc |
|---|---|---|---|---|---|
| Two‐ to five‐fold change | |||||
| 14 | ATP‐dependent Clp protease proteolytic subunit | gi|108708097 | 2.81 | No assigned function | |
| 20 | Phosphoglycerate kinase | gi|114386666 | 2.02 | Transferase activity | |
| 38 | Putative 40S ribosomal protein | gi|115436514 | 2.19 | RNA binding | |
| 48 | Proteasome subunit α type | gi|12229919 | 2.28 | Hydrolase activity | |
| 56 | Ubiquitin‐fold modifier 1 | gi|20140685 | 2.96 | No assigned function | |
| 58 | Putative dnaK‐type molecular chaperone BiP | gi|41052596 | 2.82 | ATP binding | |
| 65 | Tubulin β‐5 chain | gi|1174600 | 2.05 | GTPase activity | |
| 67 | Putative elongation factor 1‐γ | gi|46806490 | 3.75 | Copper ion binding | |
| 83 | Putative aspartate transaminase | gi|47497041 | 2.06 | Transaminase activity | |
| 84 | Vacuolar H+‐pyrophosphatase | gi|1747294 | 2.49 | Hydrolase activity | |
| 99 | 40S ribosomal protein S3a | gi|1350986 | 2.00 | Structural constituent of ribosome | |
| 100 | Tubulin α‐2 chain, putative | gi|108706880 | 3.23 | Nucleotide binding | |
| 103 | Rhamnose biosynthetic enzyme 1, putative | gi|108707482 | 2.36 | UDP‐l‐rhamnose synthase activity | Flavonol biosynthetic process |
| 105 | Os03g0319300 | gi|115452695 | 4.99 | Calcium ion binding | Response to mechanical stimulus |
| 123 | Hypothetical protein OsI_13874 | gi|125546022 | 2.10 | Oxidoreductase activity | Oxidation–reduction process |
| 133 | 26S proteasome non‐ATPase regulatory subunit 6 | gi|20978545 | 2.70 | No assigned function | Proteasome complex |
| 150 | Putative TGF‐β receptor interacting protein | gi|54287644 | 3.23 | Receptor activity | Response to salt stress |
| 159 | Vacuolar ATPase B subunit | gi|14150751 | 2.98 | ATP binding | ATP metabolic process |
| 161 | Putative vacuolar protein‐ATPase | gi|52075907 | 2.02 | ATP binding | ATP metabolic process |
| 168 | Probable histone H2A.2 | gi|75294328 | 2.12 | No assigned function | Response to wounding |
| 173 | Elongation factor 1‐β | gi|90110019 | 2.32 | Translation elongation factor activity | Defence response to bacterium |
| 190 | Nuclear transport factor 2 | gi|15214179 | 3.50 | Ran GTPase binding | Transport |
| 196 | Senescence‐associated protein‐like protein | gi|29367553 | 2.58 | No assigned function | Membrane |
| 198 | 60S ribosomal protein | gi|109940148 | 3.52 | rRNA binding | Translation |
| 208 | Mitochondrial chaperonin‐60 | gi|22758324 | 2.11 | Copper ion binding | Response to heat |
| 212 | Pyruvate kinase family protein | gi|77548686 | 2.26 | Transferase activity | Response to cadmium ion |
| 225 | 5‐Methyltetrahydropteroyltriglutamate‐homocysteine methyltransferase | gi|77556632 | 4.99 | Methyltransferase activity | Methylation |
| 243 | Hypothetical protein OsI_02864 | gi|125526853 | 2.49 | No assigned function | |
| 249 | Hypothetical protein OsI_04319 | gi|125528274 | 2.87 | No assigned function | |
| 250 | Triose phosphate isomerase | gi|149390895 | 2.14 | No assigned function | |
| 253 | Hypothetical protein OsI_35570 | gi|125533843 | 3.15 | Structural constituent of ribosome | Wound healing |
| 256 | Hypothetical protein OsI_35999 | gi|125534271 | 2.18 | No assigned function | |
| 258 | Hypothetical protein OsI_37065 | gi|125535351 | 2.64 | No assigned function | |
| 261 | Hypothetical protein OsI_37384 | gi|125535694 | 2.37 | Copper ion binding | Response to salt stress |
| 281 | Hypothetical protein OsI_08139 | gi|125540364 | 2.36 | No assigned function | |
| 312 | Hypothetical protein OsI_19723 | gi|125552095 | 3.05 | No assigned function | |
| 323 | Hypothetical protein OsI_24355 | gi|125556650 | 2.78 | No assigned function | |
| 328 | Hypothetical protein OsI_25867 | gi|125558201 | 2.75 | No assigned function | |
| 332 | Isopentenyl pyrophosphate | gi|6856560 | 2.08 | No assigned function | |
| 338 | Hypothetical protein OsI_29415 | gi|125561721 | 4.70 | Oxidoreductase activity | Metabolic process |
| 360 | Oxygen‐evolving enhancer protein 1 | gi|149392519 | 3.70 | No assigned function | |
| 361 | Hypothetical protein OsI_17694 | gi|218195711 | 2.49 | No assigned function | |
| 374 | Hypothetical protein OsI_33537 | gi|218184502 | 2.17 | No assigned function | |
| 396 | Hypothetical protein OsI_01750 | gi|218188143 | 2.74 | No assigned function | |
| 402 | Hypothetical protein OsI_03305 | gi|218188864 | 2.26 | No assigned function | |
| 405 | Hypothetical protein OsI_04213 | gi|218189276 | 2.01 | No assigned function | |
| 428 | Hypothetical protein OsI_09120 | gi|218191664 | 4.21 | No assigned function | |
| 437 | Hypothetical protein OsI_10600 | gi|218192372 | 2.64 | No assigned function | |
| 446 | Hypothetical protein OsI_22416 | gi|218197916 | 2.98 | No assigned function | |
| 454 | Hypothetical protein OsI_17386 | gi|218195569 | 3.26 | No assigned function | |
| 484 | Hypothetical protein OsI_24541 | gi|218198925 | 2.99 | No assigned function | |
| 489 | Hypothetical protein OsI_26084 | gi|218199659 | 2.02 | No assigned function | |
| 494 | Hypothetical protein OsI_28098 | gi|218200594 | 2.38 | No assigned function | |
| 509 | Hypothetical protein OsI_31865 | gi|218202373 | 2.89 | No assigned function | |
| 516 | S‐Adenosylmethionine synthase 2 | gi|3024122 | 2.29 | Transferase activity | Lignin biosynthetic process |
| 523 | Putative superoxide dismutase [Cu–Zn] | gi|42408425 | 2.61 | No assigned function | |
| ≥Five‐fold change | |||||
| 12 | 30S ribosomal protein S1, chloroplast precursor | gi|108707824 | 5.09 | No assigned function | |
| 134 | Hypothetical protein OsI_07937 | gi|218191112 | 5.20 | Oxidoreductase activity | Oxidation–reduction process |
| 259 | Hypothetical protein OsI_37152 | gi|125535486 | 25.54 | No assigned function | |
| 439 | Hypothetical protein OsI_10996 | gi|218192543 | 12.38 | No assigned function | |
| 458 | Hypothetical protein OsI_18129 | gi|218195939 | 6.09 | No assigned function | |
| Detected following treatment | |||||
| 27 | Ribosomal protein L16 | gi|114812068 | d | Structural constituent of ribosome | Translation |
| 36 | ATP‐citrate synthase β chain protein | gi|75249275 | d | Transferase activity | Metabolic process |
| 116 | α‐Tubulin | gi|10441016 | d | Transferase activity | Purine‐nucleoside phosphorylase activity |
| 126 | Probable methylenetetrahydrofolate reductase | gi|75294984 | d | Oxidoreductase activity | Oxidation–reduction process |
| 192 | Putative poly(A)‐binding protein | gi|46389987 | d | Nucleotide binding | |
| 204 | Putative myo‐inositol‐1‐phosphate synthase | gi|20043019 | d | Inositol‐3‐phosphate synthase activity | Defence response to bacterium |
| 205 | Hypothetical protein OsI_29960 | gi|218201483 | d | Kinase binding | Protein transport |
| 219 | ATP‐AMP transphosphorylase A | gi|585337 | d | Transferase activity | Response to cadmium ion |
| 224 | 5‐Methyltetrahydropteroyltriglutamate‐homocysteine methyltransferase | gi|77556631 | d | Methyltransferase activity | Methylation |
| 265 | Hypothetical protein OsI_38175 | gi|125536468 | d | No assigned function | |
| 274 | Hypothetical protein OsI_06046 | gi|125538279 | d | No assigned function | |
| 285 | Hypothetical protein OsI_09265 | gi|125541449 | d | No assigned function | |
| 289 | Hypothetical protein OsI_10220 | gi|125542606 | d | No assigned function | |
| 292 | Hypothetical protein OsI_10803 | gi|125543162 | d | No assigned function | |
| 322 | Hypothetical protein OsI_23958 | gi|125556326 | d | No assigned function | |
| 336 | Hypothetical protein OsI_29091 | gi|125561408 | d | No assigned function | |
| 388 | Hypothetical protein OsI_38794 | gi|218187100 | d | No assigned function | |
| 398 | Hypothetical protein OsI_02106 | gi|218188296 | d | No assigned function | |
| 399 | Hypothetical protein OsI_02961 | gi|218188712 | d | No assigned function | |
| 403 | Hypothetical protein OsI_04088 | gi|218189221 | d | No assigned function | |
| 442 | Hypothetical protein OsI_12134 | gi|218193097 | d | No assigned function | |
| 452 | Hypothetical protein OsI_16908 | gi|218195338 | d | No assigned function | |
| 467 | Hypothetical protein OsI_20905 | gi|218197235 | d | No assigned function | |
| 468 | Hypothetical protein OsI_21033 | gi|218197290 | d | No assigned function | |
| 481 | Hypothetical protein OsI_23984 | gi|218198689 | d | No assigned function | |
| 505 | Hypothetical protein OsI_30663 | gi|218201808 | d | No assigned function | |
| 515 | Thioredoxin M5 | gi|11135471 | d | No assigned function |
*Matched proteins were identified using ion‐trap liquid chromatography‐tandem mass spectrometry (LC‐MS/MS) (Orbitrap, Thermo Fisher Scientific).
Access ID is the access identification of the protein determined via comparison with the Oryza part of the National Center for Biotechnology Information (NCBI) nonredundant (nr) database.
Fold change according to quantification and analysis carried out using MaxQuant 1.2.2.5 software.
Protein function/process/cellular component based on Gene Ontology Annotation (UniProt‐GOA) Database [Gene Ontology (GO) level 3].
Fold could not be calculated; control intensities were below detection level.
Table 2.
Proteins with repressed differential expression (cluster 2) identified in Z antedeschia aethiopica leaves following treatment with methyl jasmonate and subsequent infection with P ectobacterium carotovorum (≥40% reduction relative to the control)
| Protein ID | Matched protein* | Access IDa | Fold changeb | Protein functionc | Process/cellular componentc |
|---|---|---|---|---|---|
| 40%–50% change | |||||
| 155 | Hypothetical protein OsI_17265 | gi|218195501 | 0.60 | No assigned function | Plastid organization |
| 251 | Hypothetical protein OsI_05369 | gi|125529268 | 0.56 | No assigned function | |
| 214 | Fructose‐bisphosphate aldolase | gi|78099750 | 0.56 | Lyase activity | Response to abscisic acid stimulus |
| 218 | Monothiol glutaredoxin‐S12 | gi|122063510 | 0.55 | Oxidoreductase activity | Cell redox homeostasis |
| 304 | Hypothetical protein OsI_16800 | gi|125549171 | 0.54 | No assigned function | |
| 89 | Catalase‐1 | gi|108706015 | 0.54 | Peroxidase activity | Response to oxidative stress |
| 492 | Hypothetical protein OsI_27178 | gi|218200179 | 0.54 | No assigned function | |
| 163 | Putative alanine aminotransferase | gi|50510015 | 0.52 | Transaminase activity | Biosynthetic process |
| 66 | 26S proteasome ATPase subunit Rpt | gi|11991116 | 0.51 | ATP binding | Protein catabolic process |
| 50%–95% change | |||||
| 455 | Hypothetical protein OsI_17466 | gi|218195608 | 0.47 | No assigned function | |
| 391 | Hypothetical protein OsI_00149 | gi|218187401 | 0.45 | No assigned function | |
| 276 | Hypothetical protein OsI_06200 | gi|125538439 | 0.44 | No assigned function | |
| 267 | Hypothetical protein OsI_38775 | gi|125537077 | 0.43 | No assigned function | |
| 233 | Hypothetical protein OsI_16423 | gi|218195106 | 0.39 | No assigned function | |
| 128 | Putative galactose kinase | gi|31249736 | 0.30 | Kinase activity | Metabolic process |
| 95 | Pentose‐5‐phosphate 3‐epimerase | gi|109940150 | 0.29 | Isomerase activity | Metabolic process |
| 244 | Hypothetical protein OsI_03093 | gi|125527088 | 0.24 | No assigned function | |
| 518 | Malate dehydrogenase, glyoxysomal | gi|3183079 | 0.14 | Structural constituent of ribosome | |
| ≥95% change | |||||
| 75 | Small GTP‐binding protein | gi|642121 | 0.00 | GTP binding | Signal transduction |
| 239 | Hypothetical protein OsI_00615 | gi|125524634 | 0.00 | No assigned function | |
| 252 | Hypothetical protein OsI_34765 | gi|125533056 | 0.00 | No assigned function | |
| 309 | Hypothetical protein OsI_19495 | gi|125551858 | 0.00 | No assigned function | |
| 327 | Hypothetical protein OsI_11322 | gi|218192705 | 0.00 | No assigned function | |
| 346 | Hypothetical protein OsI_32141 | gi|125564469 | 0.00 | No assigned function | |
| 414 | Hypothetical protein OsI_06478 | gi|218190367 | 0.00 | No assigned function | |
| 424 | Hypothetical protein OsI_08447 | gi|218191345 | 0.00 | No assigned function | |
| 432 | Hypothetical protein OsI_09642 | gi|218191917 | 0.00 | No assigned function | |
| 469 | Hypothetical protein OsI_21076 | gi|218197310 | 0.00 | No assigned function | |
| 500 | Hypothetical protein OsI_30076 | gi|218201535 | 0.00 | No assigned function | |
| 511 | Zinc finger protein ZFP252 | gi|28849865 | 0.00 | No assigned function |
*Matched proteins were identified using ion‐trap liquid chromatography‐tandem mass spectrometry (LC‐MS/MS) (Orbitrap, Thermo Fisher Scientific).
Access ID is the access identification of the protein determined via comparison with the Oryza part of the National Center for Biotechnology Information (NCBI) nonredundant (nr) database.
Fold change according to quantification and analysis carried out using MaxQuant 1.2.2.5 software.
Protein function/process/cellular component based on Gene Ontology Annotation (UniProt‐GOA) Database [Gene Ontology (GO) level 3].
Table 3 lists the 20 of the 47 annotated proteins whose expression levels decreased by ≥40% relative to the control following P. carotovorum infection (cluster 3). Table 4 lists the five of the 23 annotated proteins whose expression levels increased by two‐fold or greater relative to the control after BTH treatment and decreased following infection with the pathogen (cluster 4).
Table 3.
Proteins with repressed differential expression (cluster 3) identified in Z antedeschia aethiopica leaves following P ectobacterium carotovorum infection (≥40% reduction relative to the control)
| Protein ID | Matched protein* | Access IDa | Fold changeb | Protein functionc | Process/cellular componentc |
|---|---|---|---|---|---|
| 40%–50% change | |||||
| 456 | Hypothetical protein OsI_17671 | gi|218195699 | 0.59 | No assigned function | |
| 357 | Hypothetical protein OsI_09033 | gi|218191618 | 0.55 | No assigned function | |
| 145 | Glutamine synthetase | gi|121343 | 0.54 | Ligase activity | Glutamine biosynthetic process |
| 379 | Hypothetical protein OsI_35121 | gi|218185255 | 0.53 | No assigned function | |
| 320 | Hypothetical protein OsI_22460 | gi|125554832 | 0.52 | No assigned function | |
| 298 | Hypothetical protein OsI_13894 | gi|125546040 | 0.51 | No assigned function | |
| 34 | Os01g0253300 | gi|115435706 | 0.50 | Binding | Interspecies interaction |
| 50%–95% change | |||||
| 122 | Glycolate oxidase | gi|122246745 | 0.48 | Oxidoreductase activity | Interspecies interaction |
| 172 | Os07g0626400 | gi|115473475 | 0.45 | Heat shock protein binding | Protein folding |
| 362 | Catalase isozyme | gi|152013381 | 0.41 | ||
| 120 | Os03g0773800 | gi|115455637 | 0.39 | Oxidoreductase activity | Oxidation–reduction process |
| 140 | Glycolate oxidase | gi|75326731 | 0.36 | Oxidoreductase activity | Interspecies interaction |
| 169 | Putative 40S ribosomal protein | gi|24417185 | 0.31 | Structural constituent of ribosome | Translation |
| 189 | Putative aminotransferase | gi|42407771 | 0.23 | Transferase activity | Metabolic process |
| 329 | Hypothetical protein OsI_25927 | gi|125558262 | 0.22 | No assigned function | |
| 230 | Hypothetical protein OsI_16326 | gi|125548728 | 0.21 | No assigned function | |
| ≥95% change | |||||
| 44 | Ubiquitin‐conjugating enzyme | gi|226492411 | 0.00 | Ligase activity | Protein ubiquitination |
| 275 | Hypothetical protein OsI_06150 | gi|125538387 | 0.00 | No assigned function | |
| 351 | Hypothetical protein OsI_32438 | gi|125564750 | 0.00 | No assigned function | |
| 400 | Hypothetical protein OsI_03090 | gi|218188764 | 0.00 | No assigned function |
*Matched proteins were identified using ion‐trap liquid chromatography‐tandem mass spectrometry (LC‐MS/MS) (Orbitrap, Thermo Fisher Scientific).
Access ID is the access identification of the protein determined via comparison with the Oryza part of the National Center for Biotechnology Information (NCBI) nonredundant (nr) database.
Fold change according to quantification and analysis carried out using MaxQuant 1.2.2.5 software.
Protein function/process/cellular component based on Gene Ontology Annotation (UniProt‐GOA) Database [Gene Ontology (GO) level 3].
Table 4.
Proteins with increased differential expression (cluster 4) identified in Z antedeschia aethiopica leaves following treatment with benzothiadiazole (BTH) and subsequent infection with P ectobacterium carotovorum (two‐fold or greater change relative to the control)
| Protein ID | Matched protein* | Access IDa | Fold changeb | Protein functionc | Process/cellular componentc |
|---|---|---|---|---|---|
| Two‐ to five‐fold change | |||||
| 15 | Photosystem II 47‐kDa protein | gi|109156612 | 2.41 | Chlorophyll binding | Electron transport chain |
| 72 | 40S ribosomal protein | gi|109940154| | 2.02 | No assigned function | Ribosome |
| 504 | Hypothetical protein OsI_30475 | gi|218201704 | 2.96 | No assigned function | |
| Detected following treatment | |||||
| 59 | Putative EF‐hand Ca2+ binding protein CCD1 | gi|41053013 | d | Calcium ion binding | |
| 506 | Hypothetical protein OsI_30699 | gi|218201824 | d | No assigned function |
*Matched proteins were identified using ion‐trap liquid chromatography‐tandem mass spectrometry (LC‐MS/MS) (Orbitrap, Thermo Fisher Scientific).
Access ID is the access identification of the protein determined via comparison with the Oryza part of the National Center for Biotechnology Information (NCBI) nonredundant (nr) database.
Fold change according to quantification and analysis carried out using MaxQuant 1.2.2.5 software.
Protein function/process/cellular component based on Gene Ontology Annotation (UniProt‐GOA) Database [Gene Ontology (GO) level 3].
Fold could not be calculated; control intensities were below detection level.
In vitro analysis of peroxidase (POD) and polyphenol oxidase (PPO) activity
Cluster 1 represents the priming of Z. aethiopica‐induced proteins with intense levels of expression following induction with MJ, and even higher expression levels following inoculation with the pathogen. Of the 215 primed proteins, 101 were assigned to GO functional groups; 20 of these primed proteins were associated with oxidoreductase activity (Fig. 3a,b). Oxidoreductases include oxidases and dehydrogenases, namely PODs, phenoloxidases, hydroxylases, oxygenases and reductases (Baker et al., 1997). The high proportion of oxidoreductases among the cluster 1 proteins led to the investigation of these activities in vitro and in situ by examining POD (EC 1.11.1.7) and PPO (EC 1.14.18.1) activity using colorimetric assays and semi‐denaturing polyacrylamide gels.
The results revealed priming of PPO activity following MJ treatment. Pectobacterium carotovorum infection of these plants was associated with a more than two‐fold increase in PPO activity relative to the uninfected MJ‐treated plants and a 12‐fold increase relative to the noninduced, infected plants. PPO activity was detected only in the MJ treatments (with or without the pathogen), with an evident priming response following challenge with the pathogen (Fig. 4a). The activity gel revealed different isoenzymes in the MJ treatments with two common bands (∼75 and 45 kDa) and an additional band (∼20 kDa) observed solely in the priming treatment (MJ + pathogen treatment) (Fig. 4c).
Figure 4.
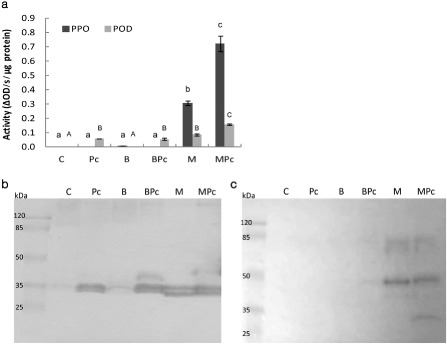
In vitro peroxidase (POD) and polyphenol oxidase (PPO) activity of the total soluble protein extracted from Zantedeschia aethiopica leaves. Leaves were treated with distilled deionized water (ddw) (control, C), 5 μg/mL benzothiadiazole (B) or 10 mg/mL methyl jasmonate (M) and, 24 h later, inoculated with Pectobacterium carotovorum (Pc). Proteins were extracted 24 h after inoculation (10 mm sodium acetate, pH 5.6). (a) In vitro enzymatic activity was quantified spectrophotometrically (475 nm) using 3,3′,5,5′‐tetramethylbenzidine (TMB) in the presence of H2O2 as the POD substrate and dopa as the substrate for PPO. Bars represent mean POD and PPO activity, based on four biological replicates for each treatment ± SE. Treatments followed by the same letter are not significantly different according to the Tukey–Kramer multiple range test at P < 0.01. (b, c) Sodium dodecylsulphate‐polyacrylamide gel electrophoresis (SDS‐PAGE) (10%) activity gels for PODs using TMB in the presence of H2O2 as the substrate (b) and for PPO using dopa as the substrate (c). Each lane contained 30 μg of protein. In all cases, extracted protein was pooled from the leaves of three plants that received the same treatment. Molecular weight (MW) markers are shown at the left of each gel.
A similar priming trend was observed for POD activity. We observed a 1.5‐fold increase in POD activity in plants in the MJ + P. carotovorum treatment, relative to the plants that were treated with MJ, but not inoculated. POD activity was also induced following treatment with P. carotovorum alone (Fig. 4a). The POD activity gel demonstrates this activation (Fig. 4b). It also shows that MJ activates the same POD as the pathogen, and does so just as strongly. Gel analysis revealed that different isoenzymes were expressed in each treatment, implying the existence of common POD isoenzymes (∼35 kDa) and a specific isoenzyme for the MJ treatments (∼30 kDa). An additional isoenzyme (∼45 kDa) was expressed in the treatments that included both induction and infection (BTH + pathogen and MJ + pathogen; Fig. 4). Notably, the pathogen‐free BTH treatment was not associated with any POD activity in vitro (Fig. 4b).
In situ localization of POD and PPO activity
Oxidizing enzymes play an active part in defending the plant against pathogens. Diaminobenzidine (DAB) is a common synthetic substrate that polymerizes instantly through the action of PODs and on contact with hydrogen peroxide (H2O2) to develop a dark colour (Cohen et al., 2011). In a similar way, following oxidation by PPO, dopa is oxidized to yield dopachrome, which then further polymerizes to form an aggregate of dark melanin (Constabel and Ryan, 1998).
Staining with DAB and dopa was used to localize POD and PPO activity in Z. aethiopica leaf tissue. Briefly, following induction and P. carotovorum inoculation, leaf discs were incubated in 0.1% DAB or 25 mm dopa. The leaf discs were bleached in boiling ethanol and observed under a light microscope (Fig. 5). The results show that, in the control and BTH treatments, no polymerized product accumulated in the leaf tissue at the wound or the P. carotovorum infection site. Under the same conditions, POD and PPO activities were observed around the wounds in the leaves that had been treated with MJ, with augmented levels observed at and around the penetration area following infection with P. carotovorum. This observation implies that POD and PPO activity are involved in the plant's induced defence response to the pathogen (Fig. 5).
Figure 5.
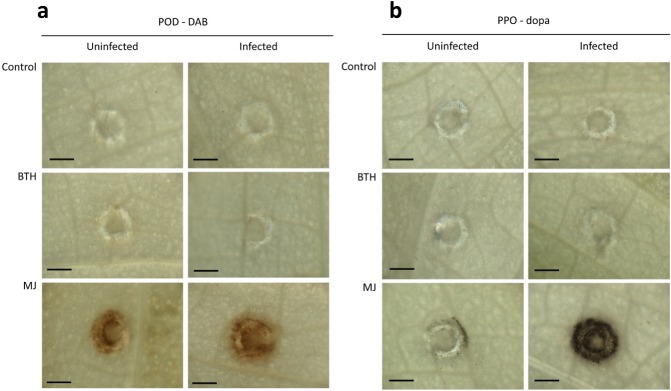
Light microscopy images of peroxidase (POD) and polyphenol oxidase (PPO) activity in leaf discs of Zantedeschia aethiopica treated with plant activators: distilled deionized water (ddw) (control), 5 μg/mL benzothiadiazole (BTH) or 10 mg/mL methyl jasmonate (MJ). The discs were inoculated with Pectobacterium carotovorum 24 h later. Enzymatic activity was recorded 24 h after inoculation following incubation with diaminobenzidine (DAB) for the localization of POD activity (a) and dopa for the localization of PPO activity (b). POD or PPO activity was visualized via the accumulation of a polymerized product, as shown by the brown/black ring around the wounded or infected site (bar, 500 μm).
Discussion
The use of label‐free MS analysis, in combination with bioinformatics tools, facilitates proteomic research in organisms for which the available genomic data are limited at best (Carpentier et al., 2008). We have focused on Z. aethiopica, a natural rhizomatous host of the soft rot pathogen P. carotovorum, to unravel the proteome underlying the defence response of a less frequently studied monocot to the soft rot bacterium. The differential expression of proteins following the induction of common plant defence pathways and subsequent inoculation with P. carotovorum was analysed using the most similar monocot database available, that of O. sativa. The role of the JA/ethylene signalling pathway in the defence response of Z. aethiopica to P. carotovorum has been documented previously in work based on the use of physiological and biochemical tools (Luzzatto et al., 2007a, b). However, this is the first attempt to investigate the molecular basis of this response using a proteomic approach. A total of 527 proteins were annotated in Z. aethiopica leaves in response to the following six treatments: a ddw control, BTH and MJ induction treatments and the same induction treatments followed by challenge inoculation with the pathogen.
The first level of organization of the Z. aethiopica proteome was based on Venn diagrams depicting the number of up‐regulated and down‐regulated proteins in the P. carotovorum infected/uninfected state. Our analysis of infected/uninfected treatments revealed 134 up‐regulated proteins (with a threshold of two‐fold or greater change), the largest number of which were distinct proteins observed in the MJ + pathogen treatment. One hundred and forty‐seven proteins were down‐regulated and the largest number of distinct down‐regulated proteins was associated with P. carotovorum infection. Overlapping proteins were more likely to be among those down‐regulated, suggesting a significant role for bacterial suppression of gene expression.
To obtain a better understanding of how proteins are associated with various molecular cell functions, GO terms were used to categorize the proteome. The different categories allowed us to identify proteins associated with several cellular components that are related to the membrane and the extracellular region. These could be related to a defensive state established following the activation of a resistance response. In a similar manner, cellular components, such as signalling, response to stimuli, multi‐organism processes and immune system processes, could be congruent with a defensive mode. The next category, molecular function, revealed potential defence responses associated with molecular transducer activity, binding, catalytic activities, transporter activities and antioxidant activity.
Clustering of expression patterns of differential proteins based on the algorithm CLICK, according to the intensity of expression, enabled the visualization of specific proteins with similar behaviours across different manipulations. Each of the four expression patterns presented here demonstrates a defence‐associated phenomenon, of which the first and by far the largest cluster may represent the unique physiological state known as priming, with 215 proteins displaying a similar expression trend line (cluster 1). Primed plants show rapid and/or strong activation of defence responses when subsequently challenged by a pathogen (Conrath, 2011; Conrath et al., 2002, 2006; Heil and Bostock, 2002; Sticher et al., 1997).
The results presented here show that induction with MJ, but not BTH, was further augmented following infection with P. carotovorum. This suggests that the JA/ethylene signalling pathway plays a role in protein priming involved in the plant defence response to this necrotroph. Priming has been reported previously in the same pathosystem, based on the augmented accumulation of total and specific polyphenolic compounds in Z. aethiopica leaves following MJ treatment and challenge with P. carotovorum (Luzzatto et al., 2007a). However, the quantitative outline of priming following MJ induction is shown here for the first time, revealing 88 proteins whose expression levels increased by more than two‐fold relative to the control. Of the up‐regulated proteins, the most abundant were 21 identified as nucleic acid binding proteins, implying the involvement of transcription factors, and 31 identified as ion binding proteins, with an additional 16 identified as cofactor binding proteins. These results are congruent with the high proportion of potentially de novo synthesized proteins (27 of 88) among those expressed in the primed state, suggesting an important role for transcriptional regulation activities in the priming process.
A recent study discussing the molecular aspects of priming assigned a key role for mitogen‐activated protein kinases (MAPKs), also known as serine/threonine‐specific protein kinases, in plant defence. These enzymes were found to function downstream of receptors and to transmit extracellular stimuli into intracellular responses, whilst amplifying transduction signals (Beckers et al., 2009; Conrath, 2011). A protein from this group with 100% identity to several protein kinases from O. sativa and other monocots was identified in this study (protein ID 243, Table 1), and its level of expression was 2.5‐fold higher in the primed state than in the control. MAPK was also reported to play a role in the ISR response of cucumber which is triggered by the beneficial fungus Trichoderma during the activation of the JA/ethylene signalling pathway (Shoresh et al., 2006).
Another protein family with a well‐established relationship to defence signalling is the transferases, specifically the methyl transferases. This family of proteins catalyses the formation of small‐molecule to volatile methyl esters, which are involved in the attraction of pollinators, as well as in defence against herbivores and pathogens (Kollner et al., 2010).
We identified 17 primed transferases, including a methyl transferase (225) whose expression level increased nearly five‐fold and another protein (224) that was expressed only in the treatment and below detection level in the control. Methyl esters act as interplant signals that are produced by infected plants and subsequently induce defence responses in their uninfected neighbours (Kollner et al., 2010; Zubieta et al., 2003).
Other notable induced proteins include: a 60‐kDa chaperonin (208), which is essential for correct protein folding and sustained resistance to biotic stress; S‐adenosylmethionine synthase (516), which is specifically regulated by fungal and bacterial elicitors and is involved in the plant's response to wounding; ribosomal proteins (38, 99, 198, 12, 27) required for protein translation; these might be associated with plant innate immunity, specifically the mass production of antimicrobial proteins; thioredoxin (515), which is involved in intracellular signalling and resistance to oxidative stress, as reported previously in the context of disease resistance of tomato, and which is significantly up‐regulated in response to JA treatments (Afroz et al., 2010; Mysore et al., 2003); and, finally, oxidoreductases (123, 338, 134, 126). The expression levels of certain oxidoreductases increased more than two‐fold (123, 338), whereas another increased more than five‐fold (134) and one was defined as a de novo protein (126). Two of these proteins, 123 and 134, have been mentioned in relation to primary metabolism pathways involved in plant defence (Bolton, 2009).
The second noteworthy defence‐related phenomenon involves the protein expression trends presented in clusters 2 and 3. These trends correspond to a pattern of repressed protein expression that may be seen as a trade‐off effect. The priming of defence‐related genes is often accompanied by the down‐regulation of cellular processes or, in some cases, antagonistic pathways (Glazebrook, 2005; Pieterse et al., 2001, 2009). The repression may be the result of the allocation of plant resources to the production of defence compounds or the detoxification of defence products during the induced resistance response, which carry costs for plant fitness and growth (Walters and Heil, 2007). Trade‐offs observed between the rate of plant growth and disease resistance support the hypothesis that plant growth and defence are regulated by a network of interconnecting signalling pathways (Pieterse et al., 2009). The consequence is expressed in the attenuation of functions, such as growth and reproduction (van Hulten et al., 2006). Quantitative analysis of cluster 2 repressed proteins was based on a comparison of the expression levels in plants treated with MJ and then inoculated with P. carotovorum with the expression levels observed in the control. The threshold was set to ≥40% reduced expression and up to 100% reduction. This analysis revealed that a total of 65 proteins were down‐regulated following the MJ + pathogen treatment. Cluster 3 analysis (pathogen/control) under the same conditions revealed 45 proteins whose expression was down‐regulated following inoculation with the pathogen. An examination of the cluster 3 trend line showed that some of the same proteins were down‐regulated in response to MJ treatment and following P. carotovorum infection, suggesting that MJ and the pathogen affect similar signalling pathways.
Several studies have reported trade‐offs related to antagonism between the JA/ethylene and SA signalling pathways (Glazebrook, 2005; Pieterse et al., 2001, 2009; Proietti et al., 2013), and such trade‐offs are also evident in the down‐regulation of proteins observed in this study. Both clusters 2 and 3 present trade‐offs associated with the down‐regulation of catalase isoenzymes (89 in Table 2 and 362 in Table 3, EC 1.11.1.6) in response to the activation of the JA signalling pathway by MJ or the pathogen. Catalase protects the cell from the toxic effects of H2O2 generated as a by‐product of cell metabolism by catalysing its decomposition. There have been several recent reports of reduced catalase activity following the application of MJ to Ricinus communis leaves and Arabidopsis thaliana roots (Loyola‐Vargas et al., 2012; Soares et al., 2010).
The protein expression pattern of cluster 4 demonstrates a cross‐talk between the two most recognized defence pathways, SAR and ISR (Heil and Bostock, 2002; Pieterse et al., 2009; Proietti et al., 2013; Thaler et al., 2012). It clearly reveals that proteins that are elevated following the application of BTH reveal low levels of expression following treatment with MJ. In addition, subsequent inoculation with P. carotovorum (which activates the JA signalling pathway) represses BTH protein expression, revealing an antagonistic interaction between the SA signalling pathway (activated by BTH) and the JA signalling pathway (activated by the pathogen). Antagonism between the two signalling pathways has been widely described and is often accompanied by trade‐offs in gene expression (Glazebrook, 2005; Pieterse et al., 2001, 2009; Proietti et al., 2013). This antagonism has been documented for P. carotovorum, which was found to suppress the SA‐mediated SAR response of A. thaliana during an early stage of that plant–pathogen interaction (Vidal et al., 1997). Twenty‐three proteins were expressed at higher levels following treatment with BTH. This increased expression was slightly repressed following inoculation with P. carotovorum (BTH + pathogen); only five of these proteins displayed more than two‐fold increased expression, two of which were detected in the treatment, but were below the detection level in the control.
A consideration of the proteins involved in the priming of defence processes in Z. aethiopica suggests an important role for oxidoreductases, which account for 20 of the GO function classifications supported by protein annotations. As these enzymes are related to biotic and abiotic stress, further effort was devoted to the study of the activities and localization of defence‐related members of the group. Oxidoreductase activities have been shown earlier to increase following JA treatment and in correlation with the accumulation of secondary metabolites (Constabel and Ryan, 1998; Thipyapong and Steffens, 1997). Moreover, the involvement of PODs and PPOs in the JA‐dependent signalling observed in response to pathogens and herbivores has led, in some cases, to the use of these enzymes as indicators for these responses (Baker et al., 1997; Cosio and Dunand, 2009; Halfeld‐Vieira et al., 2006; Li and Steffens, 2002). PODs, which have been localized to a variety of cellular locations, catalyse the oxidation of phenolic compounds and other substrates through the associated reduction of H2O2 (Cosio and Dunand, 2009; Pourcel et al., 2007). PPOs are nuclear‐encoded proteins that catalyse the oxygen‐dependent oxidation of polyphenols to quinones and are thought to be involved in plant defence activities (Koussevitzky et al., 2004; Li and Steffens, 2002; Mayer, 2006; Pourcel et al., 2007).
The in vitro activity observed in this study clearly demonstrates a role for oxidoreductases, such as PODs and PPOs, in the priming response of Z. aethiopica following treatment with MJ, but not BTH. POD activity in Z. aethiopica leaves was activated following P. carotovorum infection, as well as following MJ treatment. No POD or PPO activity was observed in the control treatment. According to the priming model, these activities were further augmented following challenge with P. carotovorum. POD activity gels showed parallel induced bands for the P. carotovorum‐only treatment, the MJ‐only treatment (but not BTH treatment) and the MJ + pathogen treatment, again supporting the hypothesis of a common signalling pathway for MJ and P. carotovorum.
Afroz et al. (2010) have reported recently that, in a proteomic analysis of tomato cultivars, PPO was significantly up‐regulated in plants treated with JA, but not SA. Over‐expression of PPO in transgenic tomato was found to enhance bacterial disease resistance, and the down‐regulation of constitutive and induced expression of PPO resulted in the hypersusceptibility to pathogens (Li and Steffens, 2002; Thipyapong and Steffens, 1997).
In order to localize in situ the POD and PPO activities that were observed in vitro, these activities were visualized in leaf tissues. Our results show that MJ treatment increased POD and PPO activity following infection with P. carotovorum, implying the direct involvement of these enzymes in plant induced‐defence responses and priming. No polymerized dark product accumulated around the penetration site in the control or BTH treatment. Treatment with BTH, which acts through the SA signalling pathway, did not appear to increase the expression of these oxidizing enzymes, whereas subsequent infection with P. carotovorum apparently had an antagonistic effect.
In summary, a series of induced resistance treatments was applied to Z. aethiopica leaves, which were further inoculated with the soft rot bacterium P. carotovorum, and proteomic techniques were used to elucidate the defence processes involved in this pathosystem. This approach shed light on the nature of the response and the pathways involved. This is the first time that priming has been clearly demonstrated to play a key role in the defence response of a monocot geophyte to the soft rot bacterium P. carotovorum.
Experimental Procedures
Chemicals
All of the chemicals used in this study were purchased from Sigma‐Aldrich (St. Louis, MO, USA) unless indicated otherwise. Chemicals for electrophoresis were purchased from Bio‐Rad (Hercules, CA, USA). BTH (Bion; 50% active ingredient) was kindly provided by Syngenta (Basel, Switzerland). Chemicals used to prepare samples and all solvents used for high‐performance liquid chromatography (HPLC) and LC‐MS/MS were HPLC‐ and LC‐MS/MS‐grade materials purchased from BDH (Poole, Dorset, UK).
Plant material
Zantedeschia aethiopica plants were grown in pots in the glasshouse for two seasons (25/10 °C maximum/minimum, natural daylight). The youngest fully spread leaf was cut at the base of the petiole and suspended (25 °C) in water (control) or BTH (5 μg/mL) in water or sprayed with MJ (10 mg/mL). After 24 h, leaves were treated with ddw or P. carotovorum [104 colony‐forming units (cfu)/mL], applied to four spots on each leaf (challenge inoculation), and incubated for an additional 24 h. There were three biological replicates of each treatment.
Extraction of proteins
Plant material was harvested 24 h after the induction treatment and frozen in liquid nitrogen. Each sample was then placed in a 2‐mL tube and ground in a bead beater (Retsch, Haan, Germany) with two 3‐mm tungsten beads at 23 Hz/s for 2 min. Leaf tissue was homogenized with 10 mm sodium acetate buffer (pH 5.6) and centrifuged at 10 000 g for 15 min at 4 °C. The supernatant was used as a crude extract. Protein concentration was determined according to the Bradford assay with bovine serum albumin used as a standard.
Proteolysis
The proteins were mixed with 8 m urea and 100 mm ammonium bicarbonate, and reduced with 2.8 mm dithiothreitol (DTT) (60 °C for 30 min), modified with 8.8 mm iodoacetamide in 100 mm ammonium bicarbonate (in the dark at room temperature for 30 min) and then digested overnight at 37 °C with modified trypsin (Promega, Madison, WI, USA) in a solution of 2 m urea and 25 mm ammonium bicarbonate at a 1 : 50 enzyme to substrate ratio. A second digestion took 4 h.
MS analysis
The peptides were desalted using C18 tips (Harvard, Holliston, MA, USA), dried and resuspended in 0.1% formic acid. The peptides were then resolved by reversed‐phase chromatography on 0.075 × 200‐mm2 fused silica capillaries (J&W) packed with Reprosil reversed‐phase material (Dr Maisch HPLC GmbH, Ammerbuch‐Entringen, Germany). The peptides were eluted with linear 214‐min gradients of 7%–40% for 8 min in 95% acetonitrile with a 0.1% aqueous formic acid solution at a flow rate of 0.25 μL/min. MS was performed using an ion‐trap mass spectrometer (Orbitrap, Thermo Fisher Scientific, Waltham, MA, USA) in a positive mode, with repetitively full MS scans, followed by collision‐induced dissociation (CID) of the seven most dominant ions selected from the first MS scan.
Five wash runs were made between the samples. For the blank, 0.1% formic acid was injected using a 34‐min gradient of 7%–40%, followed by 9 min of 95% acetonitrile with an aqueous 0.1% formic acid solution. The MS data were analysed using MaxQuant 1.2.2.5 software (M. Mann's group, Max Planck Institute) searching against the Oryza part of the NCBI nr database with 1% false discovery rate (FDR). The data were quantified by label‐free analysis using the same software by calculating the elution peak volume of the identified peptides. The protein intensities were normalized by comparing the summed intensities of each sample with that of the control. Ratios were calculated from the normalized intensities of the proteins and were loaded to Perseus software (Mattias Mann's laboratory), log2 transformed and the significance A and B values (which are the P values for the detection of significant outlier ratios) were calculated as described by Cox and Mann (2008).
Functional and clustering analysis
GenInfo identifier (GI) numbers were converted to UniProt accessions using the DAVID gene ID conversion tool (http://david.abcc.ncifcrf.gov/conversion.jsp; da Huang et al., 2008). The Gene Ontology Annotation (UniProt‐GOA) Database (http://www.ebi.ac.uk/GOA/) was used to classify each UniProt‐identified item by GO term. The GO term distribution was extracted using blast2go (http://www.blast2go.com/b2ghome). GO enrichment analysis was performed using the Singular Enrichment Analysis (SEA) tool in agriGO, and statistical significance was determined using the hypergeometric test (P < 0.05) (http://bioinfo.cau.edu.cn/agriGO/index.php; Du et al., 2010). Proteins were sorted into clusters based on their expression intensities, using the CLICK algorithm and Expander 6 software (Ulitsky et al., 2010).
Spectrophotometric assay of POD and PPO activity in vitro and in situ
POD activity was assayed using 3,3′,5,5′‐tetramethylbenzidine (TMB) in the presence of H2O2 as the POD substrate. PPO activity was assayed using dopa as a substrate. The reaction was performed at 25 °C in 10 mm sodium acetate buffer (pH 5.6) with 5 mm dopa in a total volume of 200 μL using 96‐well microtitre plates. Enzyme activity was monitored at 475 nm with a spectrophotometer plate reader. All experiments were carried out with at least three biological replications. Statistical comparisons were made using one‐way analysis of variance (ANOVA) by PRISM 5.00 software (GraphPad, San Diego, CA, USA). Where ANOVA yielded significance (P < 0.01), post hoc analysis was performed using the Tukey–Kramer multiple comparisons test. Data presented are the means ± standard error (SE).
Protein gel electrophoresis and in‐gel activity
Proteins were separated using 10% sodium dodecylsulphate‐polyacrylamide gel electrophoresis (SDS‐PAGE). The protein samples were mixed with sample buffer consisting of 0.5 m Tris‐HCl (pH 6.8), 10% glycerol and bromophenol blue as the tracking dye. Proteins were separated using SDS‐PAGE (10% running gel, pH 8.8 and 5% stacking gel, pH 6.8) at room temperature in Tris‐glycine buffer (pH 10.8). Electrophoretic separation was performed at 100 V for stacking and 130 V for separation. A prestained protein molecular weight marker (#SM0441; Fermentas, Pittsburgh, PA, USA) was used as a standard. After electrophoresis, the gels were treated with reaction buffer (10 mm sodium acetate, pH 5.6) for 10 min and then rinsed with ddw for 10 min. The gel was incubated for 10 min with 25 mL of reaction buffer containing 0.1 mg/mL TMB, and then 3 mm H2O2 was added to assay POD activity. For the analysis of PPO activity, the gel was incubated with 25 mm dopa in 25 mL of reaction buffer for 30 min, and then photographed.
POD and PPO activity in situ
Following induction treatments, discs (20 mm in diameter) were excised from Z. aethiopica leaves and inoculated with 10 μL of P. carotovorum (106 cfu/mL) using a 10‐μL tip, as described previously (Luzzatto et al., 2007b). The leaf discs were then transferred to 24‐well microtitre plates, in which they were immersed in 1 mL DAB (1 mg/mL) or 1 mL dopa (25 mm) in reaction buffer. The plates were then incubated on a shaker at room temperature for 10 h. To visualize the in situ accumulation of the enzymatic reaction product, the leaf discs were bleached with boiling ethanol (96%) and viewed under a Leica MZFLIII stereomicroscope (Solms, Germany) equipped with a DS‐Fi1 camera (Nikon, Melville, NY, USA) and NIS‐Elements (Nikon) software (ver. 3.06).
Acknowledgements
We thank the Smoler Proteomics Center at Technion, Israel for the MS proteomic analysis, and Syngenta for kindly providing the BTH used in this study. This work was supported by a grant from the Chief Scientist of the Israel Ministry of Agriculture (No. 2560898).
References
- Afroz, A. , Khan, M.R. and Komatsu, S. (2010) Determination of proteins induced in response to jasmonic acid and salicylic acid in resistant and susceptible cultivars of tomato. Protein Pept. Lett. 17, 836–846. [DOI] [PubMed] [Google Scholar]
- Agrawal, G.K. and Rakwal, R. (2006) Rice proteomics: a cornerstone for cereal food crop proteomes. Mass Spectrom. Rev. 25, 1–53. [DOI] [PubMed] [Google Scholar]
- Baker, P.J. , Waugh, M.L. , Wang, X.G. , Stillman, T.J. , Turnbull, A.P. and Engel, P.C. (1997) Determinants of substrate specificity in the superfamily of amino acid dehydrogenases. Biochemistry, 36, 16 109–16 115. [DOI] [PubMed] [Google Scholar]
- Beckers, G.J.M. , Jaskiewicz, M. , Liu, Y.D. , Underwood, W.R. , He, S.Y. and Zhang, S.Q. (2009) Mitogen‐activated protein kinases 3 and 6 are required for full priming of stress responses in Arabidopsis thaliana . Plant Cell, 21, 944–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton, M.D. (2009) Primary metabolism and plant defense—fuel for the fire. Mol. Plant–Microbe Interact. 22, 487–497. [DOI] [PubMed] [Google Scholar]
- Carpentier, S.C. , Panis, B. , Vertommen, A. , Swennen, R. , Sergeant, K. , Renaut, J. , Laukens, K. , Witters, E. , Samyn, B. and Devreese, B. (2008) Proteome analysis of non‐model plants: a challenging but powerful approach. Mass Spectrom. Rev. 27, 354–377. [DOI] [PubMed] [Google Scholar]
- Chen, S. , Yuan, H.M. , Liu, G.F. , Li, H.Y. and Jiang, J. (2012) A label‐free differential quantitative proteomics analysis of a TaLEA‐introduced transgenic Populus simonii × Populus nigra dwarf mutant. Mol. Biol. Rep. 39, 7657–7664. [DOI] [PubMed] [Google Scholar]
- Cohen, Y. , Rubin, A.E. and Vaknin, M. (2011) Post‐infection application of DL‐3‐amino‐butyric acid (BABA) induces multiple forms of resistance against Bremialactucae in lettuce. Eur. J. Plant Pathol. 130, 13–27. [Google Scholar]
- Conrath, U. (2011) Molecular aspects of defence priming. Trends Plant Sci. 16, 524–531. [DOI] [PubMed] [Google Scholar]
- Conrath, U. , Pieterse, C.M. and Mauch‐Mani, B. (2002) Priming in plant–pathogen interactions. Trends Plant Sci. 7, 210–216. [DOI] [PubMed] [Google Scholar]
- Conrath, U. , Beckers, G.J. , Flors, V. , Garcia‐Agustin, P. , Jakab, G. and Mauch, F. (2006) Priming: getting ready for battle. Mol. Plant–Microbe Interact. 19, 1062–1071. [DOI] [PubMed] [Google Scholar]
- Constabel, C.P. and Ryan, C.A. (1998) A survey of wound‐ and methyl jasmonate‐induced leaf polyphenol oxidase in crop plants. Phytochemistry, 47, 507–511. [Google Scholar]
- Cosio, C. and Dunand, C. (2009) Specific functions of individual class III peroxidase genes. J. Exp. Bot. 60, 391–408. [DOI] [PubMed] [Google Scholar]
- Cox, J. and Mann, M. (2008) MaxQuant enables high peptide identification rates, individualized p.p.b.‐range mass accuracies and proteome‐wide protein quantification. Nat. Biotechnol. 26, 1367–1372. [DOI] [PubMed] [Google Scholar]
- Deepak, S.A. , Shibato, J. , Ishii, H. , Ogawa, Y. , Yoshida, Y. and Iwahashi, H. (2008) Proteomics approach for investigating the disease resistance using cucumber as a model plant. Am. J. Biochem. Biotechnol. 4, 231–238. [Google Scholar]
- Du, Z. , Zhou, X. , Ling, Y. , Zhang, Z. and Su, Z. (2010) agriGO: a GO analysis toolkit for the agricultural community. Nucleic Acids Res. 38, 64–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glas, J.J. , Schimmel, B.C.J. , Alba, J.M. , Escobar‐Bravo, R. , Schuurink, R.C. and Kant, M.R. (2012) Plant glandular trichomes as targets for breeding or engineering of resistance to herbivores. Int. J. Mol. Sci. 13, 17 077–17 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glazebrook, J. (2005) Contrasting mechanisms of defense against biotrophic and necrotrophic pathogens. Annu. Rev. Phytopathol. 43, 205–227. [DOI] [PubMed] [Google Scholar]
- Gracia‐Garza, J.A. , Blom, T.J. , Brown, W. and Allen, W. (2002) Pre‐ and post‐plant applications of copper‐based compounds to control Erwinia soft rot of calla lilies. Can. J. Plant Pathol. 24, 274–280. [Google Scholar]
- Halfeld‐Vieira, B.A. , Vieira, J.R. , Romeiro, R.D. , Silva, H.S.A. and Baracat‐Pereira, M.C. (2006) Induction of systemic resistance in tomato by the autochthonous phylloplane resident Bacillus cereus . Pesqui. Agropecu. Bras. 41, 1247–1252. [Google Scholar]
- Heil, M. and Bostock, R.M. (2002) Induced systemic resistance (ISR) against pathogens in the context of induced plant defences. Ann. Bot. 89, 503–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Huang , W. , Sherman, B.T. , Stephens, R. , Baseler, M.W. , Lane, H.C. and Lempicki, R.A. (2008) DAVID gene ID conversion tool. Bioinformation, 2, 428–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hulten, M. , Pelser, M. , van Loon, L.C. , Pieterse, C.M.J. and Ton, J. (2006) Costs and benefits of priming for defense in Arabidopsis. Proc. Natl. Acad. Sci. USA, 103, 5602–5607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, H.J. , Chen, F. , Wang, X. and Rajapakse, N.C. (2006) Effect of methyl jasmonate on secondary metabolites of sweet basil (Ocimum basilicum L.). J. Agric. Food Chem. 54, 2327–2332. [DOI] [PubMed] [Google Scholar]
- Kollner, T.G. , Lenk, C. , Zhao, N. , Seidl‐Adams, I. , Gershenzon, J. and Chen, F. (2010) Herbivore‐induced SABATH methyltransferases of maize that methylate anthranilic acid using S‐adenosyl‐L‐methionine. Plant Physiol. 153, 1795–1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koussevitzky, S. , Ne'eman, E. and Harel, E. (2004) Import of polyphenol oxidase by chloroplasts is enhanced by methyl jasmonate. Planta, 219, 412–419. [DOI] [PubMed] [Google Scholar]
- Kúc, J. (2001) Concepts and direction of induced systemic resistance in plants and its application. Eur. J. Plant Pathol. 107, 7–12. [Google Scholar]
- Li, L. and Steffens, J.C. (2002) Overexpression of polyphenol oxidase in transgenic tomato plants results in enhanced bacterial disease resistance. Planta, 215, 239–247. [DOI] [PubMed] [Google Scholar]
- Lin, M.K. , Lee, Y.J. , Lough, T.J. , Phinney, B.S. and Lucas, W.J. (2009) Analysis of the pumpkin phloem proteome provides insights into angiosperm sieve tube function. Mol. Cell Proteomics, 8, 343–356. [DOI] [PubMed] [Google Scholar]
- Liska, A.J. and Shevchenko, A. (2003) Expanding the organismal scope of proteomics: cross‐species protein identification by mass spectrometry and its implications. Proteomics, 3, 19–28. [DOI] [PubMed] [Google Scholar]
- Loyola‐Vargas, V. , Ruiz‐May, E. , Galaz‐Avalos, R. and De‐la‐Pena, C. (2012) The role of jasmonic acid in root mitochondria disruption. Plant Signal Behav. 7, 611–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luzzatto, T. , Golan, A. , Yishay, M. , Bilkis, I. , Ben‐Ari, J. and Yedidia, I. (2007a) Priming of antimicrobial phenolics during induced resistance response towards Pectobacterium carotovorum in the ornamental monocot calla lily. J. Agric. Food Chem. 55, 10 315–10 322. [DOI] [PubMed] [Google Scholar]
- Luzzatto, T. , Yishay, M. , Lipsky, A. , Ion, A. , Belausov, E. and Yedidia, I. (2007b) Efficient, long‐lasting resistance against the soft rot bacterium Pectobacterium carotovorum in calla lily provided by the plant activator methyl jasmonate. Plant Pathol. 56, 692–701. [Google Scholar]
- Luzzatto‐Knaan, T. and Yedidia, I. (2009) Induction of disease resistance in ornamental geophytes. Isr. J. Plant Sci. 57, 401–410. [Google Scholar]
- Luzzatto‐Knaan, T. , Kerem, Z. , Lipsky, A. and Yedidia, I. (2013) A systemic response of geophytes is demonstrated by patterns of protein expression and the accumulation of signal molecules in Zantedeschia aethiopica . Plant Physiol. Biochem. 71, 218–225. [DOI] [PubMed] [Google Scholar]
- Macarisin, D. , Wisniewski, M.E. , Bassett, C. and Thannhauser, T.W. (2009) Proteomic analysis of beta‐aminobutyric acid priming and abscisic acid—induction of drought resistance in crabapple (Malus pumila): effect on general metabolism, the phenylpropanoid pathway and cell wall enzymes. Plant Cell Environ. 32, 1612–1631. [Google Scholar]
- Mak, Y.X. , Willows, R.D. , Roberts, T.H. , Wrigley, C.W. , Sharp, P.J. and Copeland, L.E.S. (2006) Black Point is associated with reduced levels of stress, disease‐ and defence‐related proteins in wheat grain. Mol. Plant Pathol. 7, 177–189. [DOI] [PubMed] [Google Scholar]
- Mayer, A.M. (2006) Polyphenol oxidases in plants and fungi: going places? A review. Phytochemistry, 67, 2318–2331. [DOI] [PubMed] [Google Scholar]
- Mysore, K.S. , D'Ascenzo, M.D. , He, X.H. and Martin, G.B. (2003) Overexpression of the disease resistance gene Pto in tomato induces gene expression changes similar to immune responses in human and fruitfly. Plant Physiol. 132, 1901–1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieterse, C.M. , Van Wees, S.C. , Van Pelt, J.A. , Knoester, M. , Laan, R. and Gerrits, H. (1998) A novel signaling pathway controlling induced systemic resistance in Arabidopsis . Plant Cell, 10, 1571–1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieterse, C.M.J. , Van Pelt, J.A. , Van Wees, S.C.M. , Ton, J. , Leon‐Kloosterziel, K.M. and Keurentjes, J.J.B. (2001) Rhizobacteria‐mediated induced systemic resistance: triggering, signalling and expression. Eur. J. Plant Pathol. 107, 51–61. [Google Scholar]
- Pieterse, C.M.J. , Leon‐Reyes, A. , Van der Ent, S. and Van Wees, S.C.M. (2009) Networking by small‐molecule hormones in plant immunity. Nat. Chem. Biol. 5, 308–316. [DOI] [PubMed] [Google Scholar]
- Pieterse, C.M.J. , Van der Does, D. , Zamioudis, C. , Leon‐Reyes, A. and Van Wees, S.C.M. (2012) Hormonal modulation of plant immunity. Annu. Rev. Cell Dev. Biol. 28, 489–521. [DOI] [PubMed] [Google Scholar]
- Pourcel, L. , Routaboul, J.M. , Cheynier, V. , Lepiniec, L. and Debeaujon, I. (2007) Flavonoid oxidation in plants: from biochemical properties to physiological functions. Trends Plant Sci. 12, 29–36. [DOI] [PubMed] [Google Scholar]
- Proietti, S. , Bertini, L. , Timperio, A.M. , Zolla, L. , Caporale, C. and Caruso, C. (2013) Crosstalk between salicylic acid and jasmonate in Arabidopsis investigated by an integrated proteomic and transcriptomic approach. Mol. Biosyst. 9, 1169–1187. [DOI] [PubMed] [Google Scholar]
- von Rad, U. , Mueller, M.J. and Durner, J. (2005) Evaluation of natural and synthetic stimulants of plant immunity by microarray technology. New Phytol. 165, 191–202. [DOI] [PubMed] [Google Scholar]
- Rushton, P.J. and Somssich, I.E. (1998) Transcriptional control of plant genes responsive to pathogens. Curr. Opin. Plant Biol. 1, 311–315. [DOI] [PubMed] [Google Scholar]
- Sharan, R. and Shamir, R. (2000) CLICK: a clustering algorithm with applications to gene expression analysis. Proc. Int. Conf. Intell. Syst. Mol. Biol. 8, 307–316. [PubMed] [Google Scholar]
- Shoresh, M. , Gal‐On, A. , Leibman, D. and Chet, I. (2006) Characterization of a mitogen‐activated protein kinase gene from cucumber required for Trichoderma‐conferred plant resistance. Plant Physiol. 142, 1169–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snijder, R.C. and van Tuyl, J.M. (2002) Evaluation of tests to determine resistance of Zantedeschia spp. Araceae to soft rot caused by Erwinia carotovora subspecies carotovora . Eur. J. Plant Pathol. 108, 565–571. [Google Scholar]
- Snijder, R.C. , Cho, H.R. , Hendriks, M.M.W.B. , Lindhout, P. and van Tuyl, J.M. (2004a) Genetic variation in Zantedeschia spp. (Araceae) for resistance to soft rot caused by Erwinia carotovora subsp. carotovora . Euphytica, 135, 119–128. [Google Scholar]
- Snijder, R.C. , Lindhout, P. and van Tuyl, J.M. (2004b) Genetic control of resistance to soft rot caused by Erwinia carotovora subsp. carotovora in Zantedeschia spp. (Araceae), section Aestivae . Euphytica, 136, 319–325. [Google Scholar]
- Soares, A.M.D.S. , Souza, T.F.D. , Jacinto, T.N. and Machado, O.L.T. (2010) Effect of methyl jasmonate on antioxidative enzyme activities and on the contents of ROS and H2O2 in Ricinus communis leaves. Braz. J. Plant Physiol. 22, 151–158. [Google Scholar]
- Sticher, L. , Mauch‐Mani, B. and Metraux, J.P. (1997) Systemic acquired resistance. Annu. Rev. Phytopathol. 35, 235–270. [DOI] [PubMed] [Google Scholar]
- Thaler, J.S. , Humphrey, P.T. and Whiteman, N.K. (2012) Evolution of jasmonate and salicylate signal crosstalk. Trends Plant Sci. 17, 260–270. [DOI] [PubMed] [Google Scholar]
- Thipyapong, P. and Steffens, J.C. (1997) Tomato polyphenol oxidase—differential response of the polyphenol oxidase F promoter to injuries and wound signals. Plant Physiol. 115, 409–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulitsky, I. , Maron‐Katz, A. , Shavit, S. , Sagir, D. , Linhart, C. and Elkon, R. (2010) Expander: from expression microarrays to networks and functions. Nat. Protoc. 5, 303–322. [DOI] [PubMed] [Google Scholar]
- Vallad, E.G. and Goodman, R.M. (2004) Systemic acquired resistance and induced systemic resistance in conventional agriculture. Crop Sci. 44, 1920–1934. [Google Scholar]
- Vidal, S. , deLeon, I.P. , Denecke, J. and Palva, E.T. (1997) Salicylic acid and the plant pathogen Erwinia carotovora induce defense genes via antagonistic pathways. Plant J. 11, 115–123. [Google Scholar]
- Walters, D. and Heil, M. (2007) Costs and trade‐offs associated with induced resistance. Physiol. Mol. Plant Pathol. 71, 3–17. [Google Scholar]
- Zubieta, C. , Ross, J.R. , Koscheski, P. , Yang, Y. , Pichersky, E. and Noel, J.P. (2003) Structural basis for substrate recognition in the salicylic acid carboxyl methyltransferase family. Plant Cell, 15, 1704–1716. [DOI] [PMC free article] [PubMed] [Google Scholar]


