Summary
Ralstonia solanacearum is a Gram‐negative soil‐borne bacterium that causes bacterial wilt disease in more than 200 plant species, including economically important Solanaceae species. In R. solanacearum, the hypersensitive response and pathogenicity (Hrp) type III secretion system is required for both the ability to induce the hypersensitive response (HR) in nonhost plants and pathogenicity in host plants. Recently, 72 effector genes, called rip (Ralstonia protein injected into plant cells), have been identified in R. solanacearum RS1000. RS1002, a spontaneous nalixidic acid‐resistant derivative of RS1000, induced strong HR in the nonhost wild eggplant Solanum torvum in an Hrp‐dependent manner. An Agrobacterium‐mediated transient expression system revealed that Rip36, a putative Zn‐dependent protease effector of R. solanacearum, induced HR in S. torvum. A mutation in the putative Zn‐binding motif (E149A) completely abolished the ability to induce HR. In agreement with this result, the RS1002‐derived Δrip36 and rip36E149A mutants lost the ability to induce HR in S. torvum. An E149A mutation had no effect on the translocation of Rip36 into plant cells. These results indicate that Rip36 is an avirulent factor that induces HR in S. torvum and that a putative Zn‐dependent protease motif is essential for this activity.
Ralstonia solanacearum is a Gram‐negative soil‐borne bacterium that causes bacterial wilt disease in more than 200 plant species in over 50 plant families, including many economically important crops, such as potato, tomato, tobacco, banana and eggplant, worldwide (Hayward, 1991; Lebeau et al., 2010). The pathogen enters host plants through natural openings or wounds in the root system, penetrates the xylem vessels, colonizes the root cortex and vascular parenchyma, proliferates and spreads throughout the vascular system, and produces a large amount of exopolysaccharide (EPS) which blocks water transport, resulting in the wilting and death of infected plants (Genin and Denny, 2012; Schell, 2000). In addition, R. solanacearum secretes plant cell wall‐degrading enzymes, such as endopolygalacturonase and endoglucanase, which break down pectins and cellulosic glucans in the cell wall (Gonzalez and Allen, 2003; Liu et al., 2005).
Numerous Gram‐negative plant pathogenic bacteria use the Hrp type III secretion system (T3SS) encoded by a cluster of approximately 20 hypersensitive response (HR) and pathogenicity (hrp) genes (Alfano and Collmer, 1997; Galan and Wolf‐Watz, 2006; Tang et al., 2006; Van Gijsegem et al., 2000). Basically, Hrp T3SS acts as a specialized system for the injection of virulence factors, so‐called effector proteins, from pathogens into host plant cytoplasm. Generally, effector proteins modulate host cellular functions to establish infection and promote propagation in plants. Recent studies have unveiled biochemical activities of several T3SS effectors, including proteases, phosphatases, ubiquitin ligases, ribosyltransferases, transcriptional activators and phosphothreonine lyases (Dean, 2011; Deslandes and Rivas, 2012; Grant et al., 2006; Hann et al., 2010; Staskawicz et al., 2001; Zhou and Chai, 2008). Like many other plant pathogenic bacteria, Hrp T3SS is essential for the growth of R. solanacearum in host plants, as Hrp T3SS‐deficient mutants completely lose their pathogenicity, and effector proteins collectively contribute to virulence, as the mutation of a single effector gene produces little or no effect in most cases (Boucher et al., 1987; Cunnac et al., 2004; Kanda et al., 2003; Mukaihara et al., 2004; Zolobowska and Van Gijsegem, 2006). In R. solanacearum strain GMI1000, 74 T3SS effector proteins have been identified by in silico analysis of the whole genome sequence and functional genomic approaches (Cunnac et al., 2004; Poueymiro and Genin, 2009). To date, three effector proteins, namely AvrA, PopP1 and PopP2, have been shown to act as avirulence factors in nonhost plants. For example, AvrA is responsible for HR elicitation in Nicotiana tabacum and N. benthamiana (Carney and Denny, 1990; Poueymiro et al., 2009; Robertson et al., 2004). PopP1, a member of the YopJ/AvrRxv (C55) family of cysteine proteases, is the major HR elicitor in Petunia St40 (Lavie et al., 2002) and N. glutinosa (Poueymiro et al., 2009). PopP2, another member of the YopJ‐like family, confers RRS1‐R‐mediated resistance on Arabidopsis (Deslandes et al., 2003). Mutation of the conserved cysteine residue in the catalytic triad of PopP2 abolishes its ability to induce RRS1‐R‐mediated resistance in Arabidopsis (Tasset et al., 2010). Some of the above‐mentioned effector proteins have been shown to contribute to virulence in host plants. For example, AvrA is required for the early stage of root infection of the legume Medicago truncatula (Turner et al., 2009) and bacterial fitness on tomato leaves (Macho et al., 2010). Macho et al. (2010) have also shown that PopP2 is required for bacterial fitness on eggplant or bean. Thus, effector proteins play important roles in the interaction between R. solanacearum and its host/nonhost plants.
Plants have evolved the ability to recognize potential pathogens by detecting conserved microbial molecules, such as bacterial flagellin, elongation factor and peptidoglycans, called pathogen/microbe‐associated molecular patterns (PAMPs/MAMPs), leading to the activation of the basal defence response, called PAMP‐triggered immunity (PTI) (Monaghan and Zipfel, 2012). Subsequently, pathogens developed effector proteins to suppress PTI for successful infection. Furthermore, plants developed a system for the recognition of bacterial effectors through the sensing of their virulence by the product of the resistance (R) gene. Therefore, effectors are virulence factors which promote disease in compatible interactions, but function as avirulence factors, which induce resistance responses, in incompatible interactions. Understanding the mechanism by which an Avr protein is recognized by the corresponding R protein may lead to the identification of the virulence function of the effector protein and the detection system in plants, and contribute to the engineering of more durable resistance in crops.
In R. solanacearum strain RS1000, 72 T3SS effector proteins, the so‐called Rip (Ralstonia protein injected into plant cells) proteins, have been identified using a calmodulin‐dependent adenylate cyclase (Cya) reporter system, and their translocations into plant cells have been demonstrated (Mukaihara et al., 2010). It has been shown that the wild eggplant Solanum torvum is highly tolerant to most R. solanacearum strains (Clain et al., 2004; Gousset et al., 2005). Hence, this plant species is widely used as a rootstock for eggplant or tomato cultivation. Infiltration of R. solanacearum RS1002, a spontaneous nalixidic acid‐resistant derivative of RS1000, into the leaves of S. torvum Sw. cv. Torubamubiga induced HR, a rapid cell death in the infiltrated area (Fig. 1A). To identify the RS1000 effector protein eliciting HR, we cloned the entire coding region of each rip gene into a binary vector pEl2Ω‐MCS (Fig. 2; Ohtsubo et al., 1999) under the control of the high expression promoter 35S‐Ω. Agrobacterium tumefaciens strain GV3101 was transformed by the resultant plasmids, and then each Rip was transiently expressed in the leaves of S. torvum and tobacco (N. tabacum L. cv. Xanthi NC) via agroinfiltration. We tested 64 RS1000 effectors and found that Rip36 specifically induced HR in S. torvum leaves, but not in tobacco leaves (Fig. 2B–D). Transient expression of AvrA, an avirulence protein in tobacco leaves (Carney and Denny, 1990; Poueymiro et al., 2009; Robertson et al., 2004), specifically induced HR only in tobacco leaves, indicating that the system worked effectively.
Figure 1.
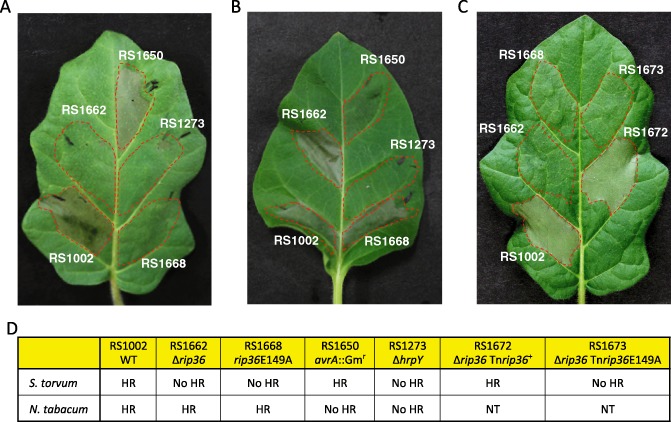
Hypersensitive responses (HRs) induced by inoculation of Ralstonia solanacearum wild‐type (WT) RS1002 and its derivatives in Solanum torvum Torubamubiga (A, C) and tobacco (B) leaves. Leaves were infiltrated with bacterial suspensions (OD 600 of 0.3) of R. solanacearum RS1002 (WT), RS1662 (Δrip36), RS1668 (rip36E149A), RS1650 (avrA::Gmr) or RS1273 (ΔhrpY), or the complemented strains RS1672 (Δrip36 Tnrip36 +) or RS1673 (Δrip36 Tnrip36E149A), and incubated under a 16 h light/8 h dark cycle at 28 °C. Photographs show representative results of S. torvum at 1 day post‐inoculation (dpi) (A, C) or tobacco at 2 dpi (B) from three independent experiments, which gave similar results. The broken lines represent the infiltrated area. (D) Summary of the plant responses caused by various strains in S. torvum and tobacco leaves. NT, not tested.
Figure 2.
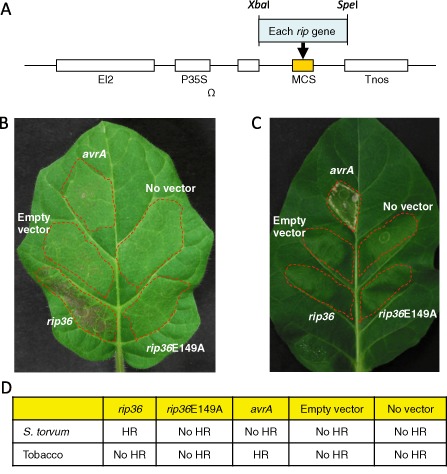
Effects of Agrobacterium‐mediated transient expression of Rip36, Rip36E149A and AvrA in Solanum torvum Torubamubiga and tobacco leaves. (A) Construction of a series of pEl2Ω‐MCS plasmids expressing the Rip effector. Leaves were infiltrated with Agrobacterium tumefaciens GV3101 harbouring no plasmid, an empty plasmid, a plasmid expressing Rip36, Rip36E149A or AvrA at OD 600 of 0.5, and incubated under a 16 h light/8 h dark cycle at 28 °C. Photographs show representative results of S. torvum at 2 days post‐inoculation (dpi) (B) or tobacco at 4 dpi (C) from three independent experiments, which gave similar results. The broken lines represent the infiltrated area. (D). Summary of plant responses. HR, hypersensitive response.
Rip36 is a Zn‐dependent protease‐like T3SS effector with a putative Zn‐binding motif (HExxH). Therefore, we next investigated the molecular mechanism of Rip36‐induced HR in S. torvum. To examine whether the HExxH motif of Rip36 is required to induce HR, we changed the essential glutamic acid of position 149 to alanine (HELIH to HALIH) using a QuickChange XL site‐directed mutagenesis kit (Stratagene, La Jolla, CA, USA). Experimental details for DNA construction are described in Supporting Information Text S1. Wild‐type (WT) rip36 and rip36E149A genes were cloned into a binary vector pEl2Ω‐MCS (Ohtsubo et al., 1999) and transiently expressed in leaves of S. torvum and tobacco (N. tabacum L. cv. Xanthi NC) via a transient Agrobacterium‐mediated expression system. We found that Rip36E149A no longer induced HR in S. torvum (Fig. 2). This result indicates that the putative Zn‐dependent protease motif of Rip36 is required for HR elicitation in S. torvum.
To confirm the results obtained from the transient expression analysis in R. solanacearum, we constructed rip36 and avrA mutant derivatives of strain RS1002, as described in Text S1. The R. solanacearum mutants and plasmids used in this study are listed in Table 1.
Table 1.
Bacterial strains and plasmids
| Strain | Description | Reference |
|---|---|---|
| Ralstonia solanacearum | ||
| RS1002 | RS1000 Nalr | Mukaihara et al. (2004) |
| RS1273 | RS1002 ΔhrpY | Mukaihara et al. (2004) |
| RS1650 | RS1002 avrA::Gmr | This study |
| RS1662 | RS1002 Δrip36 | This study |
| RS1668 | RS1002 rip36E149A | This study |
| RS1672 | RS1662 chr::mini‐Tn5rip36 + | This study |
| RS1673 | RS1662 chr::mini‐Tn5rip36E149A | This study |
| Agrobacterium tumefaciens | ||
| GV3101 | Gmr, Rifr | Lamblin et al. (2001) |
| Escherichia coli | ||
| DH5α | F‐ λ‐ ø80dlacZΔM15 Δ(lacZYA‐argF)U169 recA1 endA1 hsdR17(rK ‐mK +) supE44 thi‐1 gyrA relA1 | Takara, Kyoto, Japan |
| S17‐1 | thi pro hsdR‐ hsdM+ recA [chr::RP4‐2‐Tc::Mu‐Km::Tn7] | Schäfer et al. (1994) |
| S17‐1 λpir | λ pir lysogen of S17‐1 | Simon et al. (1983) |
| Plasmid | ||
| pEl2Ω‐MCS | Binary vector that can replicate in both E. coli and A. tumefaciens, used for transient expression analysis | Ohtsubo et al. (1999) |
| pEl2Ω‐MCS_avrA | pEl2Ω‐MCS carrying rip5 at XbaI and SpeI sites | This study |
| pEl2Ω‐MCS_rip36 | pEl2Ω‐MCS carrying rip36 at XbaI and SpeI sites | This study |
| pEl2Ω‐MCS_rip36E149A | pEl2Ω‐MCS carrying rip36E149A at XbaI and SpeI sites | This study |
| pARO‐HA‐'Cya | CyaA reporter plasmid | Murata et al. (2006) |
| pARO‐rip36‐HA‐'Cya | pARO‐HA‐'Cya carrying rip36 at XbaI and XhoI sites | Mukaihara et al. (2010) |
| pK18mobsacB | Small mobilizable vector, Kmr, sucrose sensitive (sacB) | Schäfer et al. (1994) |
| pK18‐Δrip36 | pK18mobsacB‐derived plasmid that contains a 1.6‐kb chimeric PCR product deleting rip36, Kmr | This study |
| pK18‐rip36E149A | pK18mobsacB‐derived plasmid that contains a 657‐bp rip36E149A, Kmr | This study |
| pK18‐avrA | pK18mobsacB‐derived plasmid that contains a 2.8‐kb PCR product containing avrA, Kmr | This study |
| pK18‐avrA::Gmr | pK18mobsacB‐derived plasmid that contains disrupted avrA by the insertion of a Gmr cassette into pK18‐avrA, Gmr, Kmr | This study |
| pBSL118 | Mini‐Tn5‐derived plasmid vector for insertion mutagenesis, Ampr, Kmr | Alexeyev et al. (1995) |
| pBSL‐rip36 | pBSL118‐derived plasmid that contains a 2.09‐kb rip36, Ampr, Kmr | This study |
| pBSL‐rip36E149A | pBSL118‐derived plasmid that contains a 2.09‐kb rip36E149A, Ampr, Kmr | This study |
In S. torvum, HR was induced by inoculation of strains RS1002 (parental WT) and RS1650 (avrA::Gmr), but not strains RS1662 (Δrip36) and RS1668 (rip36E149A) (Fig. 1A). HR in tobacco leaves was induced by inoculation of the WT, Δrip36 and rip36E149A strains, but not by the avrA::Gmr strain (Fig. 1B). This result clearly indicates that Rip36 and AvrA of RS1002 are required for HR in S. torvum and tobacco, respectively. Furthermore, Rip36 abolished the ability to induce HR in S. torvum when the Zn‐binding motif HELIH was changed to HALIH, indicating that the putative Zn‐dependent protease motif is required for HR elicitation in S. torvum. We also confirmed that these HRs were dependent on hrpY, a gene encoding an essential component of the Hrp T3SS, supporting the hypothesis that these T3SS effectors determine HR.
To confirm that Rip36 is a crucial avirulence factor in the induction of HR in S. torvum, we introduced the WT rip36 and rip36E149A genes into a Δrip36 mutant strain, and generated two types of complemented strain, as described in Text S1. The leaves of S. torvum were infiltrated with the resultant complemented strains, RS1672 (Δrip36 Tnrip36 +) and RS1673 (Δrip36 Tnrip36E149A). As expected, HR in S. torvum leaves was restored by the introduction of Tnrip36 +, but not Tnrip36E149A (Fig. 1C). This result further confirmed that Rip36 is an avirulence factor that induces HR in S. torvum and that the Zn‐protease motif is essential for this HR.
To exclude the possibility that the E149A mutation of Rip36 affects the ability to translocate into plant cells via the Hrp T3SS, we constructed R. solanacearum strains expressing the Rip36‐HA‐'Cya and Rip36E149A‐HA‐'Cya fusion proteins, as described previously (Murata et al., 2006; Text S1). An in planta adenylate cyclase assay was carried out as described previously (Murata et al., 2006). RS1273 (ΔhrpY) was used as a negative control because this strain lacks the ability to translocate effectors into plant cells (Mukaihara and Tamura, 2009). The cyclic adenosine monophosphate (cAMP) in S. torvum leaves inoculated with the WT strain expressing Rip36E149A‐HA‐'Cya was increased to a level similar to that in leaves inoculated with the strain expressing Rip36‐HA‐'Cya (Fig. 3A). As predicted, the increase in the cAMP level was completely abolished by the ΔhrpY mutation (Fig. 3A). This result indicates that the E149A mutation in the putative Zn‐binding motif has no effect on the translocation of Rip36 into plant cells.
Figure 3.
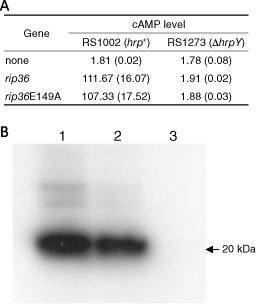
Translocation and stability of Rip36 and Rip36E149A. (A) The cyclic adenosine monophosphate (cAMP) levels of Solanum torvum leaves inoculated with Ralstonia solanacearum RS1002 (hrp +) or RS1273 (ΔhrpY) strains expressing the calmodulin‐dependent adenylate cyclase (Cya) fusion with Rip36 or Rip36E149A. The cAMP level is shown as an average of three replications with standard deviations in parentheses. (B) The stability of Rip36 and Rip36E149A in S. torvum leaves after infiltration. Leaves of S. torvum were infiltrated with RS1002 (lane 1) and the rip36E149A (lane 2) and Δrip36 (lane 3) mutants, and total proteins were prepared from the inoculated leaves at 15 h post‐inoculation. The Rip36 and Rip36E149A proteins were detected by an anti‐Rip36 peptide antibody after sodium dodecylsulphate‐polyacrylamide gel electrophoresis (SDS‐PAGE). Photograph shows a representative result of Western blotting from two independent experiments. An arrow indicates the position of the 20‐kDa marker protein.
Next, we tested the stability of the Rip36E149A protein by immunoblot analysis. Briefly, R. solanacearum strains RS1002 (WT), RS1662 (Δrip36) and RS1668 (rip36E149A) were grown overnight in BG medium (Bacto‐Agar 15 g, glucose 5 g/L) at 28 °C, pelleted, washed with distilled water and then resuspended at an optical density at 600 nm (OD600) of 0.3. The leaves of 5–6‐week‐old S. torvum were infiltrated with the bacterial suspensions and incubated at 28 °C in a growth chamber. After 15 h, the infiltrated parts of the leaves were collected, frozen with liquid nitrogen and ground with a mortar and pestle. Then, 1.5 mL of 50 mm Tris‐HCl buffer (pH 7.5) was added per 0.5 mg of leaf tissue. Cell debris was removed by centrifugation twice for 10 min at 10 000 × g, and the supernatant was collected in new tubes. Proteins were then precipitated from the supernatant by adding 5% trichloroacetic acid and incubating for 1 h at 4 °C. Precipitated proteins were pelleted by centrifugation and washed with 70% ethanol, dried, resuspended in 50 μL PBST buffer (137 mm NaCl, 8.1 mm Na2HPO4, 2.68 mm KCl, 1.47 mm KH2PO4 and Tween‐20, pH 7.4) and investigated by Western blot analysis after sodium dodecylsulphate‐polyacrylamide gel electrophoresis (SDS‐PAGE), as described previously (Nguyen et al., 2012). A rabbit polyclonal anti‐Rip36‐specific peptide antibody was generated using the synthetic peptide (peptide sequence: QGLQARSKYKPRNA, 205–218 amino acids) by Operon Biotechnologies (Tokyo, Japan) by the same method as described previously (Nguyen et al., 2012). Bands corresponding to the expected size of the Rip36 protein were detected in WT and rip36E149A mutant strains, whereas no band was detected in the Δrip36 strain (Fig. 3B). On the basis of these results, it is clear that the point mutation protein Rip36E149A is as stable as WT Rip36, and that the E149A mutation in the Zn‐protease motif has no effect on the translocation and stability of Rip36 in plant cells.
To examine whether Rip36 contributes to the virulence of R. solanacearum on host eggplant (Solanum melongena cv. Senryo‐nigou), RS1002 (WT) and the Δrip36 and rip36E149A mutants [5 × 104 colony‐forming units (cfu)/mL] were infiltrated into eggplant leaves and bacterial growth was measured at 2 and 5 days post‐inoculation (dpi). This quantitative assay is reported to be more sensitive than the disease scoring method (Macho et al., 2010). However, no significant difference in bacterial multiplication was observed between RS1002, Δrip36 and rip36E149A mutant strains at either 2 or 5 dpi (Fig. 4A). This result suggests that Rip36 has no or little effect on the virulence of R. solanacearum in host eggplant, probably because of the functional redundancy of a large effector repertoire, as reported by Mukaihara et al. (2010).
Figure 4.
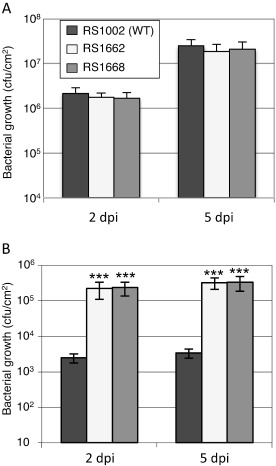
Effect of rip36 mutation on the growth of Ralstonia solanacearum RS1002 on host eggplant (A) and nonhost Solanum torvum (B). Leaves were infiltrated with wild‐type (WT) RS1002 or Δrip36 and rip36E149A mutants at 5 × 104 colony‐forming units (cfu)/mL. Bacterial growth was measured 2 and 5 days after inoculation. Error bars indicate the standard deviation measured from six biological replicates. Means of three independent experiments are presented. Asterisks indicate a significant difference from the growth of the RS1002 WT strain in a t‐test (***P < 0.001).
We further measured the bacterial growth on nonhost S. torvum. The leaves of S. torvum were infiltrated with RS1002 (WT) and the Δrip36 and rip36E149A mutants (5 × 104 cfu/mL), and bacterial growth was measured at 2 and 5 dpi. The Δrip36 and rip36E149A mutants increased significantly in S. torvum leaves when compared with the WT RS1002 at both 2 and 5 dpi (Fig. 4B). This indicates that Rip36 is the major avirulence factor in RS1002 that elicits resistance and restricts bacterial growth in S. torvum leaves.
In this study, we have shown that the R. solanacearum T3SS effector Rip36 functions as an avirulence protein to induce HR in S. torvum. We have also shown that the putative Zn‐dependent protease motif of Rip36 is essential for its avirulence function. In the fungal pathogen Magnaporthe grisea, the causal agent of rice blast disease, an AVR‐Pita avirulence protein is known to contain a Zn‐dependent protease motif. The protease motif is essential for the avirulence function of AVR‐Pita because a point mutation in the conserved motif completely abolishes the ability to induce rice blast resistance mediated by the corresponding disease resistance (R) protein Pi‐ta in rice (Orbach et al., 2000). It is also known that AVR‐Pita interacts directly with Pi‐ta, and that a mutation in the protease motif results in the loss of avirulence and disrupts the physical interaction between them. This direct interaction raises the possibility that the Pi‐ta protein may sense the cleavage by AVR‐Pita (Jia et al., 2000). Our results also indicate that the Zn‐protease activity of Rip36, like Avr‐Pita, is required for the induction of HR, although the enzymatic activity has not yet been tested. It has been reported that the Rip36 homologue NleD from enteropathogenic Escherichia coli cleaves c‐JUN N‐terminal kinase by its Zn‐dependent protease activity and inhibits the inflammatory reaction, an animal innate immunity response (Baruch et al., 2011). In R. solanacearum, Rip36 might cleave a particular S. torvum protein to exhibit its virulence function, and the resultant peptide fragment might be specifically recognized by a resistance protein, leading to HR. The Rip36 homologue HopH1 from Pseudomonas syringae pv. tomato DC3000 may contribute to virulence on host plants because the deletion of both hopH1 and hopC1 from this pathogen reduced both lesion formation and growth in Arabidopsis and tomato (Wei et al., 2007). It is interesting that the putative protease motif of HopH1 is required for its virulence function. The mechanism of HR induction by Rip36 might be similar to that of the cysteine protease effectors AvrRpt2 from P. syringae pv. tomato DC3000 (Axtell and Staskawicz, 2003) and AvrPphB from P. syringae pv. phaseolicola (Shao et al., 2002). AvrRpt2 and AvrPphB effectors cleave Arabidopsis RIN4 and PBS1 proteins, respectively, whose cleavage is monitored by the corresponding R proteins RPS2 and RPS5, respectively (Mackey et al., 2003; Shao et al., 2003). To reveal the mechanism of Rip36‐induced HR in S. torvum, the identification of the target protein is now under investigation.
Supporting information
Text S1 Plasmid constructions and generation of Ralstonia solanacearum strains.
Acknowledgements
This work was supported in part by the Grants‐in‐Aid for Scientific Research (No. 24658042) from the Ministry of Education, Culture, Sports, Science and Technology of Japan and Joint Research Project by Okayama Prefecture.
References
- Alexeyev, M.F. , Shokolenko, I.N. and Croughan, T.P. (1995) New mini‐Tn5 derivatives for insertion mutagenesis and genetic engineering in gram‐negative bacteria. Can. J. Microbiol. 41, 1053–1055. [DOI] [PubMed] [Google Scholar]
- Alfano, J.R. and Collmer, A. (1997) The type III (Hrp) secretion pathway of plant pathogenic bacteria: trafficking harpins, AvrA proteins and death. J. Bacteriol. 179, 5655–5662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axtell, M.J. and Staskawicz, B.J. (2003) Initiation of RPS2‐specified disease resistance in Arabidopsis is coupled to the AvrRpt2‐directed elimination of RIN4. Cell, 112, 369–377. [DOI] [PubMed] [Google Scholar]
- Baruch, K. , Gur‐Arie, L. , Nadler, C. , Koby, S. , Yerushalmi, G. , Ben‐Neriah, Y. , Yogev, O. , Shaulian, E. , Guttman, C. , Zarivach, R. and Rosenshine, I. (2011) Metalloprotease type III effectors that specifically cleave JNK and NF‐κB. EMBO J. 30, 221–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucher, C.A. , Van Gijsegemm, F. , Barberis, P.A. , Arlat, M. and Zischek, C. (1987) Pseudomonas solanacearum genes controlling both pathogenicity on tomato and hypersensitivity on tobacco are clustered. J. Bacteriol. 169, 5626–5632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carney, B.F. and Denny, T.P. (1990) A cloned avirulence gene from Pseudomonas solanacearum determines incompatibility on Nicotiana tabacum at the host species level. J. Bacteriol. 172, 4836–4843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clain, C. , Da Silva, D. , Fock, I. , Vaniet, S. , Carmeille, A. , Gousset, C. , Sihachakr, D. , Luisetti, J. , Kodja, H. and Besse, P. (2004) RAPD genetic homogeneity and high levels of bacterial wilt tolerance in Solanum torvum Sw. (Solanaceae) accessions from Reunion Island. Plant Sci. 166, 1533–1540. [Google Scholar]
- Cunnac, S. , Occhialini, A. , Barberis, P. , Boucher, C. and Gennin, S. (2004) Inventory and functional analysis of the large Hrp regulon in Ralstonia solanacearum: identification of novel effector proteins translocated to plant host cells through the type III secretion system. Mol. Microbiol. 23, 115–128. [DOI] [PubMed] [Google Scholar]
- Dean, P. (2011) Functional domains and motifs of bacterial type III effector proteins and their roles in infection. FEMS Microbiol. 35, 1100–1125. [DOI] [PubMed] [Google Scholar]
- Deslandes, L. and Rivas, S. (2012) Catch me if you can: bacterial effectors and plant targets. Trends Plant Sci. 17, 644–655. [DOI] [PubMed] [Google Scholar]
- Deslandes, L. , Olivier, J. , Peeters, N. , Feng, D. , Khounlotham, M. , Boucher, C. , Somssich, I. , Genin, S. and Marco, Y. (2003) Physical interaction between RRS1‐R, a protein conferring resistance to bacterial wilt, and PopP2, a type III effector targeted to the plant nucleus. Proc. Natl. Acad. Sci. USA, 100, 8024–8029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galan, J.E. and Wolf‐Watz, H. (2006) Protein delivery into eukaryotic cells by type III secretion machines. Nature, 444, 567–573. [DOI] [PubMed] [Google Scholar]
- Genin, S. and Denny, T.P. (2012) Pathogenomics of the Ralstonia solanacearum species complex. Annu. Rev. Phytopathol. 50, 67–89. [DOI] [PubMed] [Google Scholar]
- Gonzalez, E.T. and Allen, C. (2003) Characterization of a Ralstonia solanacearum operon required for polygalacturonate degradation and uptake of galacturonic acid. Mol. Plant–Microbe Interact. 16, 536–544. [DOI] [PubMed] [Google Scholar]
- Gousset, C. , Collonnier, C. , Mulya, K. , Mariska, I. , Rotino, G.L. , Besse, P. , Servaes, A. and Sihachakr, D. (2005) Solanum torvum, as a useful source of resistance against bacterial and fungal diseases for improvement of eggplant (S. melongena L.). Plant Sci. 170, 319–327. [Google Scholar]
- Grant, S.R. , Fisher, E.J. , Chang, J.H. , Mole, B.M. and Dangl, J.L. (2006) Subterfuge and manipulation: type III effector proteins of phytopathogenic bacteria. Annu. Rev. Microbiol. 60, 425–449. [DOI] [PubMed] [Google Scholar]
- Hann, D.R. , Gimenez‐Ibanez, S. and Rathjen, J.P. (2010) Bacterial virulence effectors and their activities. Curr. Opin. Plant Biol. 13, 388–393. [DOI] [PubMed] [Google Scholar]
- Hayward, A.C. (1991) Biology and epidemiology of bacterial wilt caused by Pseudomonas solanacearum . Annu. Rev. Phytopathol. 29, 65–87. [DOI] [PubMed] [Google Scholar]
- Jia, Y. , McAdams, S.A. , Bryan, G.T. , Hershey, H.P. and Valent, B. (2000) Direct interaction of resistance gene and avirulence gene products confers rice blast resistance. EMBO J. 19, 4004–4014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanda, A. , Yasukohchi, M. , Ohnishi, K. , Kiba, A. , Okuno, T. and Hikichi, Y. (2003) Ectopic expression of Ralstonia solanacearum effector protein PopA early in invasion results in loss of virulence. Mol. Plant–Microbe Interact. 16, 447–455. [DOI] [PubMed] [Google Scholar]
- Lamblin, F. , Saladin, G. , Dehorter, B. , Cronier, D. , Grenier, E. , Lacoux, J. , Bruyant, P. , Lainé, E. , Chabbert, B. , Girault, F. , Monties, B. , Morvan, C. , David, H. and David, A. (2001) Overexpression of a heterologous sam gene encoding S‐adenosylmethionine synthetase in flax (Linum usitatissimum) cells: consequences on methylation of lignin precursors and pectins. Physiol. Plant. 112, 223–232. [DOI] [PubMed] [Google Scholar]
- Lavie, M. , Shillington, E. , Eguiluz, C. , Grimsley, N. and Boucher, C. (2002) PopP1, a new member of YopJ/AvrRxv family of type III effector proteins, acts as a host‐specificity factor and modulates aggressiveness of Ralstonia solanacearum . Mol. Plant–Microbe Interact. 15, 1058–1068. [DOI] [PubMed] [Google Scholar]
- Lebeau, A. , Daunay, M.C. , Frary, A. , Palloix, A. , Wang, J.F. , Dintinger, J. , Chiroleu, F. , Wicker, E. and Prior, P. (2010) Bacterial wilt resistance in tomato, pepper, and eggplant: genetic resources respond to diverse strains in the Ralstonia solanacearum species complex. Phytopathology, 101, 154–165. [DOI] [PubMed] [Google Scholar]
- Liu, H. , Zhang, S. , Schell, M.A. and Denny, T.P. (2005) Pyramiding unmarked deletions in Ralstonia solanacearum shows that secreted proteins in addition to plant cell‐wall‐degrading enzymes contribute to virulence. Mol. Plant–Microbe Interact. 18, 1296–1305. [DOI] [PubMed] [Google Scholar]
- Macho, A.P. , Guidot, A. , Barberis, P. , Beuzon, C.R. and Genin, S. (2010) A competitive index assay identifies several Ralstonia solanacearum type III effector mutant strains with reduced fitness in host plants. Mol. Plant–Microbe Interact. 23, 1197–1205. [DOI] [PubMed] [Google Scholar]
- Mackey, D. , Belkhadir, Y. , Alonso, J.M. , Ecker, J.R. and Dangl, J.L. (2003) Arabidopsis RIN4 is a target of the type III virulence effector AvrRpt2 and modulates RPS‐2 mediated resistance. Cell, 112, 379–389. [DOI] [PubMed] [Google Scholar]
- Monaghan, J. and Zipfel, C. (2012) Plant pattern recognition receptor complexes at the plasma membrane. Curr. Opin. Plant Biol. 15, 349–357. [DOI] [PubMed] [Google Scholar]
- Mukaihara, T. and Tamura, N. (2009) Identification of novel Ralstonia solanacearum type III effector proteins through translocation analysis of hrpB‐regulated gene products. Microbiology, 155, 2235–2244. [DOI] [PubMed] [Google Scholar]
- Mukaihara, T. , Tamura, N. , Murata, Y. and Iwabuchi, M. (2004) Genetic screening of Hrp type III‐related pathogenicity genes controlled by the HrpB transcriptional activator in Ralstonia solanacearum . Mol. Microbiol. 54, 863–875. [DOI] [PubMed] [Google Scholar]
- Mukaihara, T. , Tamura, N. and Iwabuchi, M. (2010) Genome‐wide identification of a large repertoire of Ralstonia solanacearum type III effector proteins by a new functional screen. Mol. Plant–Microbe Interact. 23, 251–262. [DOI] [PubMed] [Google Scholar]
- Murata, Y. , Tamura, N. , Nakaho, K. and Mukaihara, T. (2006) Mutations in the lrpE gene of Ralstonia solanacearum affects Hrp pili production and virulence. Mol. Plant–Microbe Interact. 19, 884–895. [DOI] [PubMed] [Google Scholar]
- Nguyen, L.C. , Taguchi, F. , Tran, Q.M. , Naito, K. , Yamamoto, M. , Ohnishi‐Kameyama, M. , Ono, H. , Yoshida, M. , Chiku, K. , Ishii, T. , Inagaki, Y. , Toyoda, K. , Shiraishi, T. and Ichinose, Y. (2012) Type IV pilin is glycosylated in Pseudomonas syringae pv. tabaci 6605 and is required for surface motility and virulence. Mol. Plant Pathol. 13, 764–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtsubo, N. , Mitsuhara, I. , Koga, M. , Seo, S. and Ohashi, U. (1999) Ethylene promotes the necrotic lesion formation and basic PR gene expression in TMV‐infected tobacco. Plant Cell Physiol. 40, 808–817. [Google Scholar]
- Orbach, M.J. , Farrall, L. , Sweigard, J.A. , Chumley, F.G. and Valent, B. (2000) A telomeric avirulence gene determines efficacy for the rice blast resistance gene Pi‐ta . Plant Cell, 12, 2019–2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poueymiro, M. and Genin, S. (2009) Secreted proteins from Ralstonia solanacearum: a hundred tricks to kill a plant. Curr. Opin. Microbiol. 12, 44–52. [DOI] [PubMed] [Google Scholar]
- Poueymiro, M. , Cunnac, S. , Barberis, P. , Deslandes, L. , Peeters, N. , Cazale‐Noel, A.C. , Boucher, C. and Genin, S. (2009) Two type III secretion system effectors from Ralstonia solanacearum GMI1000 determine host‐range specificity on tobacco. Mol. Plant–Microbe Interact. 22, 538–550. [DOI] [PubMed] [Google Scholar]
- Robertson, A.E. , Wechter, W.P. , Denny, T.P. , Fortnum, B.A. and Kluepfel, D.A. (2004) Relationship between avirulence gene (avrA) diversity in Ralstonia solanacearum and bacterial wilt incidence. Mol. Plant–Microbe Interact. 17, 1376–1384. [DOI] [PubMed] [Google Scholar]
- Schäfer, A. , Tauch, A. , Jager, W. , Kalinowski, J. , Thierbach, G. and Puhler, A. (1994) Small mobilizable multi‐purpose cloning vectors derived from the Escherichia coli plasmids pK18 and pK19: selection of defined deletions in the chromosome of Corynebacterium glutamicum . Gene, 145, 69–73. [DOI] [PubMed] [Google Scholar]
- Schell, M.A. (2000) Control of virulence and pathogenicity genes of Ralstonia solanacearum by an elaborate sensory network. Annu. Rev. Phytopathol. 38, 263–292. [DOI] [PubMed] [Google Scholar]
- Shao, F. , Merritt, P.M. , Bao, Z. , Innes, R.W. and Dixon, J.E. (2002) A Yersinia effector and a Pseudomonas avirulence protein define a family of cysteine proteases functioning in bacterial pathogenesis. Cell, 109, 575–588. [DOI] [PubMed] [Google Scholar]
- Shao, F. , Golstein, C. , Ade, J. , Stoutemyer, M. , Dixon, J.E. and Innes, R.W. (2003) Cleavage of Arabidopsis PBS1 by a bacterial type III effector. Science, 301, 1230–1233. [DOI] [PubMed] [Google Scholar]
- Simon, R. , Priefer, U. and Pühler, A. (1983) A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in Gram‐negative bacteria. Nature Biotechnol. 1, 784–791. [Google Scholar]
- Staskawicz, B.J. , Mudget, M.B. , Dangle, J.L. and Galán, J.E. (2001) Common and contrasting themes of plant and animal diseases. Science, 292, 2285–2289. [DOI] [PubMed] [Google Scholar]
- Tang, X. , Xiao, Y. and Zhou, J.M. (2006) Regulation of the type III secretion system in phytopathogenic bacteria. Mol. Plant–Microbe Interact. 19, 1159–1166. [DOI] [PubMed] [Google Scholar]
- Tasset, C. , Bernoux, M. , Jauneau, A. , Pouzet, C. , Briere, C. , Kieffer‐Jacquinod, S. , Rivas, S. , Marco, Y. and Deslandes, L. (2010) Autoacetylation of the Ralstonia solanacearum effector PopP2 targets a lysine residue essential for RRS1‐R‐mediated immunity in Arabidopsis. PLoS Pathog. 6, e1001202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner, M. , Jauneau, A. , Genin, S. , Tavella, M.J. , Vailleau, F. , Gentzbittel, L. and Jardinaud, M.F. (2009) Dissection of bacterial wilt on Medicago truncatula revealed two type III secretion system effectors acting on root infection process and disease development. Plant Physiol. 150, 1713–1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Gijsegem, F. , Vasse, J. , Camus, J.C. , Marenda, M. and Boucher, C. (2000) Ralstonia solanacearum produces Hrp‐dependent pili that are required for popA secretion but not for attachment of bacteria to plant cells. Mol. Microbiol. 36, 259–260. [DOI] [PubMed] [Google Scholar]
- Wei, C.F. , Kvitko, B.H. , Shimizu, R. , Crabill, E. , Alfano, J.R. , Lin, N.C. , Martin, G.B. , Huang, H.C. and Collmer, A. (2007) A Pseudomonas syringae pv. tomato DC3000 mutant lacking the type III effector HopQ1‐1 is able to cause disease in the model plant Nicotiana benthamiana . Plant J. 51, 32–46. [DOI] [PubMed] [Google Scholar]
- Zhou, J.M. and Chai, J. (2008) Plant pathogenic bacterial type III effectors subdue host responses. Curr. Opin. Microbiol. 11, 179–185. [DOI] [PubMed] [Google Scholar]
- Zolobowska, L. and Van Gijsegem, F. (2006) Induction of lateral root structure formation on petunia roots: a novel effect of GMI1000 Ralstonia solanacearum infection impaired in hrp mutants. Mol. Plant–Microbe Interact. 19, 597–606. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Text S1 Plasmid constructions and generation of Ralstonia solanacearum strains.


