SUMMARY
The expression of host genes can be altered during the process of viral infection. To investigate the viral infection‐induced up‐regulated gene expression changes of maize at different time intervals post‐inoculation with Sugarcane mosaic virus (SCMV), a suppression subtractive hybridization cDNA library was constructed. A total of 454 cDNA clones were identified to be viral infection‐induced up‐regulated genes. The influence of Rop1 on the infection of maize by SCMV was investigated. The results showed that transient silencing of the ZmRop1 gene through virus‐induced gene silencing enhanced the accumulation and systemic infection of SCMV and another potyvirus (Pennisetum mosaic virus) in maize plants, whereas transient over‐expression of ZmRop1 in maize protoplasts reduced SCMV accumulation. Furthermore, it was demonstrated that the heterologous expression of ZmRop1 impaired Potato virus X infection in Nicotiana benthamiana plants. These data suggest that ZmRop1 may play a role in plant defence responses to viral infection.
INTRODUCTION
Systemic viral infection in plants involves complicated molecular interactions between the invading virus and the host plant. In plant–virus interactions, one of the most striking effects of virus infection is the profound changes in host gene expression (Whitham et al., 2003). These alterations, which are related to disease development or immune responses, may have an impact on virus replication and movement (Havelda et al., 2008).
Maize dwarf mosaic disease is caused mainly by Sugarcane mosaic virus (SCMV) and a novel potyvirus, Pennisetum mosaic virus (PenMV), in China (Deng et al., 2008; Fan et al., 2003). In recent years, some differentially expressed genes associated with SCMV resistance in maize have been identified (Użarowska et al., 2009); however, the molecular mechanisms underlying the individual candidate genes and the development of SCMV infection in maize remain largely unclear. Suppression subtractive hybridization (SSH) is a powerful tool for obtaining gene expression profiles (Diatchenko et al., 1996). To investigate the gene expression profile in systemic leaves of maize infected by SCMV, an SSH library was constructed in this work, and one of the up‐regulated genes, identified as ZmRop1, which encodes ZmRop1 {a member of maize Rops [Rho‐related guanosine triphosphatases (GTPases) from plants]}, was selected for further study. Rops are versatile plant signalling regulators involved in different cellular processes (reviewed in Berken, 2006; Nibau et al., 2006), and are also involved in plant defence responses (Agrawal et al., 2003; Chen et al., 2010; Pathuri et al., 2009). There are nine Rops in maize (ZmRop1–ZmRop9), and ZmRop1, ZmRop4 and ZmRop9 have potential roles in plant–pathogen interactions and plant stress responses (Christensen et al., 2003). It has been reported that ZmRop1 (also known as ZmRacA), ZmRop2 (ZmRacB), ZmRop3 (ZmRacC) and ZmRop4 (ZmRacD) can induce the production of reactive oxygen species (ROS) in mammalian NIH 3T3 cells (Hassanain et al., 2000), and ZmRop2 has a specific role in male gametophyte function (Arthur et al., 2003). However, the functions of ZmRops are still unknown in most cases, in particular their roles in virus–plant interactions.
In the present study, our results showed that the ZmRop1 gene expression level was up‐regulated with the development of SCMV infection in maize leaves. Knock‐down of the ZmRop1 gene through virus‐induced gene silencing (VIGS) in maize plants enhanced the accumulation and systemic infection of SCMV and PenMV, whereas transient over‐expression of ZmRop1 in maize protoplasts impaired SCMV accumulation, and transient heterologous expression of ZmRop1 in Nicotiana benthamiana repressed Potato virus X (PVX) infection. These data suggest that ZmRop1 plays a role in maize defence responses against viral infection.
RESULTS
Differentially expressed maize genes after SCMV infection were acquired through SSH
To investigate the differentially expressed maize genes after SCMV infection, SSH cDNA libraries were constructed. Screening through the libraries, 454 mRNAs were identified as being up‐regulated with viral infection, 166 of which were cloned and sequenced. Sequence analyses indicated that 58 sequences were redundant (including one viral sequence) or not identified, whereas the other 108 sequences had potential coding capacities (Table S1, see Supporting Information). Among the 108 sequences, six showed relatively high homology to published maize gene sequences encoding protein kinase PK4, Rop1 small guanosine triphosphate (GTP)‐binding protein, ribulose‐1,5‐bisphosphate carboxylase/oxygenase (rubisco), ferredoxin‐thioredoxin‐reductase, glyceraldehyde‐3‐phosphate dehydrogenase/exohydrolase II and thioredoxin M. Thirty‐six per cent of the 108 sequences were either unclassified or hypothetical proteins, and the remaining sequences were involved in various processes, as shown in Fig. 1 and Table S1.
Figure 1.
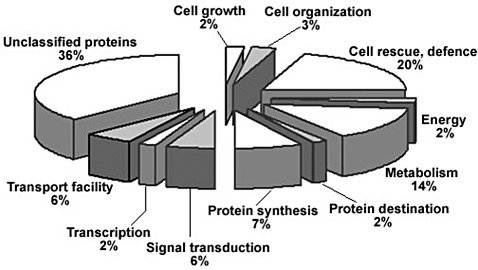
Distribution of partial up‐regulated gene transcripts in maize subtractive libraries post‐Sugarcane mosaic virus (SCMV) infection. A total of 108 gene transcripts with hits to the GenBank database are grouped into 11 functional categories. The percentage of transcripts in each category is listed.
ZmRop1 mRNA level was up‐regulated with the development of SCMV infection
To analyse the accumulation level of ZmRop1 mRNA during viral infection, total RNAs were extracted from maize Zong 31 and Va35 leaves collected at 2 days post‐inoculation (dpi), 5 dpi and 9 dpi. The results of quantitative real‐time reverse transcription‐polymerase chain reaction (QRT‐PCR) showed that the ZmRop1 mRNA levels in Zong 31 plants were similar in both the mock‐inoculated (with phosphate buffer) and noninoculated upper leaves of control maize plants. However, in the inoculated leaves of SCMV‐infected plants, the ZmRop1 mRNA level was approximately 44%, 10% and 20% higher than that of mock‐inoculated leaves at 2, 5 and 9 dpi, respectively. The ZmRop1 mRNA levels in SCMV‐infected maize systemic leaves were also increased. In the first upper systemic leaves of SCMV‐infected plants, the ZmRop1 mRNA level was approximately 52% and 212% higher than that in maize leaves of control plants at 5 and 9 dpi, respectively. For the second upper systemic leaves, the ZmRop1 mRNA level was approximately 122% higher than that in maize leaves of control plants at 9 dpi (Fig. 2). The profile of ZmRop1 mRNA levels in maize Va35 plants post‐SCMV infection was similar to that in Zong 31 plants (data not shown).
Figure 2.

The dynamic changes in ZmRop1 mRNA levels in maize Zong 31 leaves after Sugarcane mosaic virus (SCMV) infection. The analysis of the ZmRop1 mRNA level in maize (Zong 31) plants was assayed by quantitative reverse transcription‐polymerase chain reaction (QRT‐PCR) post‐viral infection. For each treatment, at least three samples were collected and subjected to testing. Each value is shown as the average of three independent experiments ± standard deviation (SD). IL, inoculated leaves; SL, systemic leaves. Different letters on the columns indicate significant differences by Student's t‐test (P < 0.05).
Transient silencing of ZmRop1 in maize plants facilitated viral infection
To investigate the potential role of ZmRop1 in SCMV infection in maize plants, we employed a C‐BMVA/G‐based silencing vector (BMV, Brome mosaic virus) (Ding et al., 2006) to transiently silence ZmRop1. The results showed that, at 10 dpi, mild white streaks were observed on the noninoculated upper leaves of maize (cv. Va35) inoculated with RNA transcripts representing C‐BMVA/G, whereas Va35 plants inoculated with pC‐BMVA/G/Rop1 RNA transcripts developed extensive light yellow and white streaking symptoms (Fig. 3A).
Figure 3.
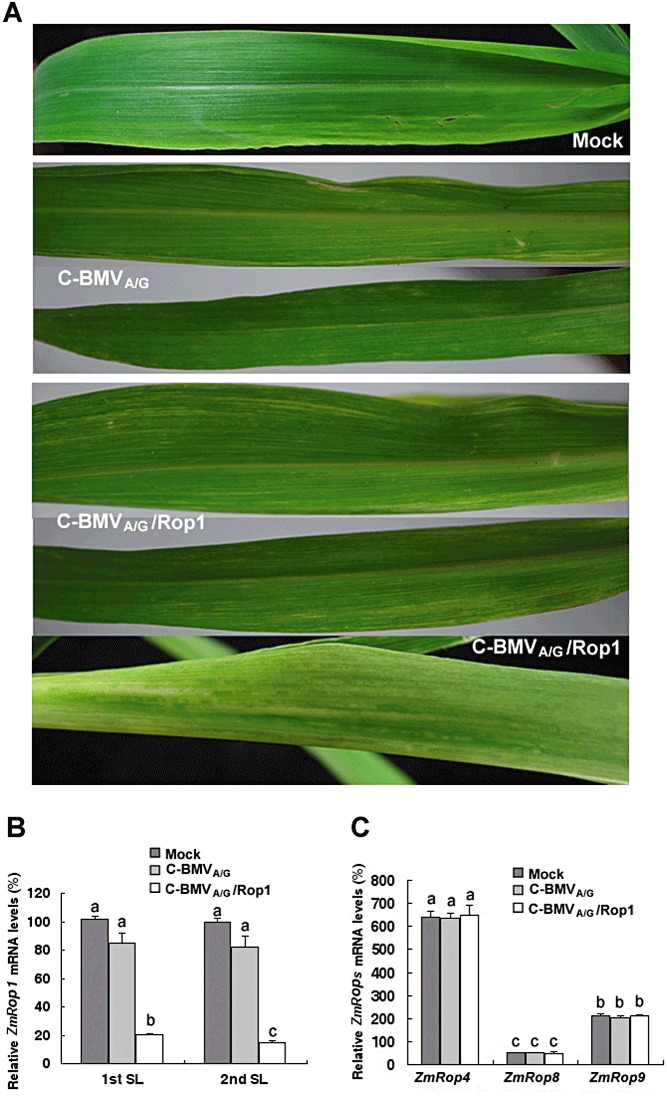
Transient silencing of ZmRop1 in maize plants. (A) Phenotypes of ZmRop1‐silenced maize (cv. Va35) plants. The first upper noninoculated leaves were photographed at 10 days post‐inoculation with FES buffer (mock inoculation) or RNA transcripts representing either the chimeric Brome mosaic virus (C‐BMVA/G) or C‐BMVA/G with a ZmRop1 insert (C‐BMVA/G/Rop1). Quantitative reverse transcription‐polymerase chain reaction (QRT‐PCR), detecting for ZmRop1 silencing efficiency at 14 days post‐inoculation (dpi) (B) and the ZmRop4, ZmRop8 and ZmRop9 transcript levels in ZmRop1‐silenced maize plants (C). Each value is shown as the average of three independent experiments ± standard deviation (SD). IL, inoculated leaves; SL, systemic leaves. Different letters on the columns indicate significant differences by Student's t‐test (P < 0.05).
To confirm that the phenotype difference was a result of ZmRop1 gene silencing through VIGS, the ZmRop1 mRNA levels of C‐BMVA/G‐, C‐BMVA/G/Rop1‐ and mock‐inoculated Va35 plants were detected by QRT‐PCR at different time points post‐inoculation. The results indicated that the ZmRop1 mRNA level in pC‐BMVA/G/Rop1 RNA transcript‐infected Va35 plants was approximately 18%–20% and 13%–15% of that in maize plants inoculated with pC‐BMVA/G RNA transcripts or FES buffer, respectively (Fig. 3B). In addition, to address the specificity of ZmRop1 silencing, we analysed the mRNA levels of other ZmRop genes (ZmRop4, ZmRop8 and ZmRop9) in ZmRop1‐silenced plants. The data showed that the expression of ZmRop4, ZmRop8 and ZmRop9 was not affected by ZmRop1 silencing (Fig. 3C). These results suggest that the symptom differences were probably caused by ZmRop1 gene silencing.
To elaborate the influence of ZmRop1 silencing on SCMV infection in maize plants, the second noninoculated upper leaves of C‐BMVA/G/Rop1‐infected, C‐BMVA/G‐infected and FES buffer mock‐inoculated plants were re‐inoculated with SCMV at 12 dpi. Systemic infection of SCMV in the first and second noninoculated leaves of ZmRop1‐silenced plants occurred at 7 and 11 dpi, respectively, in contrast with 10 and 14 dpi in the control plants, and RT‐PCR assay did not reveal any detectable level of SCMV RNA in the upper noninoculated leaves in control plants at 7 and 11 dpi, respectively (data not shown). In ZmRop1‐silenced plants, systemic leaves developed more severe mosaic symptoms than those infected with C‐BMVA/G or treated with FES buffer (Fig. 4A). QRT‐PCR and Western blotting were employed to analyse the SCMV RNA levels and viral accumulation in SCMV systemically infected leaves of ZmRop1‐silenced and control plants. The results of QRT‐PCR showed that the relative SCMV RNA levels in the systemic leaves of ZmRop1‐silenced plants were about 395% and 274% higher than that in control plants, respectively (Fig. 4B). Western blotting results also showed that SCMV accumulation in ZmRop1 gene‐silenced plants was about three times higher than that in control plants (Fig. 4C). In addition, the ZmRop1‐silenced and control plants were also inoculated with PenMV, and the results indicated that PenMV accumulated to a relatively higher level in ZmRop1‐silenced plants (Fig. 4C).
Figure 4.

Influence of transient silencing of ZmRop1 in maize (cv. Va35) plants on Sugarcane mosaic virus (SCMV) and Pennisetum mosaic virus (PenMV) systemic infection. (A) Phenotypes of ZmRop1‐silenced maize plants caused by SCMV infection. The second upper systemic leaves of maize seedlings above C‐BMVA/G‐ or C‐BMV A/G/Rop1‐inoculated leaves were challenge inoculated with SCMV. The first upper noninoculated leaves above SCMV‐inoculated leaves were photographed at 10 days post‐inoculation (dpi). (B) The first and second upper systemic leaves above SCMV‐inoculated leaves were harvested for quantitative reverse transcription‐polymerase chain reaction (QRT‐PCR) analysis to determine the SCMV RNA level. Each value is shown as the average of three independent experiments ± standard deviation (SD). IL, inoculated leaves; SL, systemic leaves. Different letters on the columns indicate significant differences by Student's t‐test (P < 0.05). (C) Accumulation of SCMV or PenMV in the first systemic leaves above SCMV (or PenMV)‐inoculated leaves was determined by Western blotting using SCMV/PenMV coat protein (CP) antibody, as indicated. The Coomassie brilliant blue‐stained 8% sodium dodecylsulphate‐polyacrylamide gel (bottom) for different samples was used as a loading control.
Transient over‐expression of ZmRop1 in maize protoplasts reduced virus accumulation
The large gene family and possible functional redundancy among family members present a potential problem for the determination of the function of Rops in plants. However, the unique regulatory feature of GTPases, i.e. cycling between guanosine diphosphate (GDP)‐ and GTP‐bound forms, allows the generation of gain‐of‐function point mutations at the conserved sites through all small GTPases. These mutations have been proven to be useful for functional studies of Rho and RAS GTPases in animals and yeast, and also for an understanding of the function of Rops in plants (Bloch et al., 2005). Thus, two opposite ZmRop1 mutants were generated: one constitutively active (CA) mutant (ZmRop1CA) substituting glycine‐17 with valine, and one dominant negative (DN) mutant (ZmRop1DN) substituting threonine‐22 with asparagine; CA and DN mutant proteins are expected to constitutively activate or block the ZmRop1‐dependent pathway, respectively. In addition, fusions between green fluorescent protein (GFP) and Rho GTPases were shown to localize and function in a similar manner to their cognate native proteins (Lavy et al., 2002). To further determine the role of ZmRop1 in virus infection, the effect of ZmRop1 on SCMV accumulation in maize protoplasts was evaluated. The coding sequences of ZmRop1, ZmRop1CA and ZmRop1DN were inserted into pGFP (a derivative of pUC19) to create pGFP‐ZmRop1, pGFP‐ZmRop1CA and pGFP‐ZmRop1DN, respectively (Fig. S1, see Supporting Information).
The mixtures of SCMV RNA and each GFP‐ZmRop1 fusion plasmid (pGFP‐ZmRop1, pGFP‐ZmRop1CA or pGFP‐ZmRop1DN) were simultaneously introduced into maize protoplasts, and the mixture of SCMV RNA and pGFP plasmid was used as a control. The maize protoplasts were harvested at 24 h after electroporation to determine the subcellular localization of GFP‐ZmRop1 fusion proteins by laser scanning confocal microscopy. The data showed that GFP‐ZmRop1, GFP‐ZmRop1CA and GFP‐ZmRop1DN were localized to the plasma membrane (PM) of maize protoplasts, whereas GFP alone was distributed throughout the cytoplasm (Fig. S3, see Supporting Information). The visualization data indicated that GFP‐ZmRop1 was expressed in maize protoplasts.
SCMV RNA and viral accumulation levels were investigated by QRT‐PCR and Western blotting. The results showed that the SCMV RNA levels in pGFP‐ZmRop1‐, pGFP‐ZmRop1CA‐ and pGFP‐ZmRop1DN‐transformed protoplasts were approximately 67%, 51% and 83%, respectively, of that in control protoplasts (electroporated with pGFP) at 24 h post‐electroporation (Fig. 5A). Western blotting analysis indicated that SCMV accumulated to a lower level in maize protoplasts transiently expressing GFP‐ZmRop1 fusion proteins than in those transiently expressing GFP alone (Fig. 5B). These data demonstrate that transient over‐expression of ZmRop1 or its mutants in maize protoplasts reduces SCMV accumulation.
Figure 5.
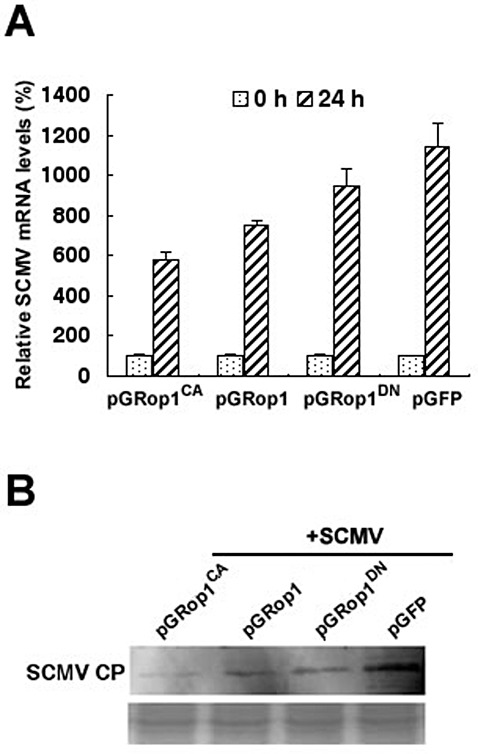
Effects of transient expression of ZmRop1 on Sugarcane mosaic virus (SCMV) multiplication. (A) SCMV RNA levels in electroporated maize protoplasts were assayed by real‐time reverse transcription‐polymerase chain reaction (RT‐PCR) at 0 and 24 h post‐transfection (hpt). Each value is shown as the average of three independent experiments ± standard deviation (SD). Different letters on the columns indicate significant differences by Student's t‐test (P < 0.05). (B) SCMV viral accumulation in transformed protoplasts was determined at 24 hpt by Western blotting uing SCMV coat protein (CP) antibody, as indicated. The Coomassie brilliant blue‐stained 8% sodium dodecylsulphate‐polyacrylamide gel (bottom) for different samples was used as a loading control. CP, coat protein.
Heterologous expression of ZmRop1 impaired viral infection in N. benthamiana
Nicotiana benthamiana often displays increased susceptibility to RNA viruses, probably as a result of its lack of an active salicylic acid (SA)‐ and virus‐inducible RNA‐dependent RNA polymerase (RdRP), which is required for defence against viruses (Yang et al., 2004). Nicotiana benthamiana is susceptible to PVX infection: even the N. benthamiana plants transformed with an SA‐inducible RdRP gene from Medicago truncatula did not show resistance to PVX (Yang et al., 2004). Agrobacterium cells harbouring p35S‐ZmRop1 were infiltrated into the entire lamina of fully expanded leaves of N. benthamiana, and Agrobacterium cells carrying the empty vector p35S‐GFP were used as a control. At 24 h after infiltration, in vitro PVX transcript was inoculated onto these leaves. The ZmRop1‐GFP‐induced green fluorescent foci were clearly visible under long‐wavelength ultraviolet light at 48 h post‐infiltration (data not shown).
At 6 days post‐PVX inoculation, N. benthamiana leaves were harvested to assay the PVX infection by RT‐PCR using the primer pairs P13/P14 and P17/P18 to detect the expression of ZmRop1 mRNA and PVX viral RNA, respectively. The RT‐PCR results showed that the target segments were amplified in these plants (data not shown), indicating that ZmRop1 and its derivative were expressed heterologously and PVX infection occurred in these plants.
At 12 dpi, the typical mosaic symptoms of PVX were observed in the upper noninoculated leaves of p35S‐GFP‐ and p35S‐ZmRop1DN‐infiltrated N. benthamiana plants, whereas the plants transiently expressing either ZmRop1 or ZmRop1CA developed mild mosaic symptoms. Intriguingly, the upper noninoculated leaves showed necrotic spots in N. benthamiana plants which heterologously expressed ZmRop1CA (Fig. 6A). Western blotting was employed to analyse PVX accumulation and the data showed that, at 12 dpi, the level of PVX coat protein (CP) in p35S‐GFP empty vector‐infiltrated plants was much higher than that in plants transiently expressing ZmRop1 and its CA derivative (Fig. 6B). These results suggest that the heterologous expression of ZmRop1 through agro‐infiltration can impair PVX infection in N. benthamiana.
Figure 6.

Influence of heterologous expression of ZmRop1 on Potato virus X (PVX) infection in Nicotiana benthamiana. (A) Symptoms caused by PVX infection in ZmRop1, ZmRop1CA and ZmRop1DN heterologous expression and control plants were photographed at 12 days post‐inoculation (dpi). CA, constitutively active, DN, dominant negative. (B) Analysis of PVX accumulation by Western blotting with PVX coat protein (CP) antibody, as indicated. The Coomassie brilliant blue‐stained 8% sodium dodecylsulphate‐polyacrylamide gel (bottom) for different samples was used as a loading control.
DISCUSSION
Viruses exploit various host factors to establish infection; however, some host factors are involved in defence against virus infection via the regulation of gene expression (Chen et al., 2008; Huang et al., 2010; Shi et al., 2011). Profiling the gene expression changes in the host may provide an insight into the molecular mechanisms that underlie host physiological and phenotypic responses to virus infection. To assess transcriptional changes in maize plants infected with SCMV‐BJ (BJ, Beijing isolate), maize leaves above the SCMV‐inoculated leaves were sampled at 15 dpi and subjected to SSH analysis. Among the 108 up‐regulated transcripts sequenced, 22 showed homology to stress‐ or pathogen defence‐related categories in maize and other species. ROS have been shown to be key mediators of local and systemic resistance responses in incompatible plant–pathogen reactions and to be involved in symptom development and pathogenesis in compatible plant–virus interactions (Sandermann, 2000). Rops have been suggested to be involved in host ROS production during plant–pathogen interactions (Agrawal et al., 2003). Previous work has demonstrated that ZmRop1 (ZmRacA) induces the activation of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, leading to superoxide production (Hassanain et al., 2000). Christensen et al. (2003) speculated that three Rops (ZmRop1, ZmRop4 and ZmRop9) are more likely to play an important role in pathogen and stress responses because of the maintenance of the expression levels of these three Rops in mature leaf and most vegetative tissues assayed. Therefore, as ZmRop1 is one of the genes up‐regulated post‐SCMV infection, it was selected for further research.
In this article, dynamic changes in ZmRop1 mRNA levels in SCMV‐inoculated and systemically infected maize leaves were investigated, and the resulting data showed that the ZmRop1 mRNA level was up‐regulated with the development of SCMV infection (Fig. 2). This phenomenon was unlikely to be caused by differences in leaf age, as both the previous report (Christensen et al., 2003) and our results showed that the ZmRop1 mRNA level was not related to leaf age (2, 3). Previous work has demonstrated that ZmRop4 and ZmRop9 may play potential roles in plant defence pathways and plant stress responses, and that ZmRop8 may have related functions to ZmRop1 in maize plants, as they share 96% amino acid sequence identity (Christensen et al., 2003). Our results showed that SCMV infection generally did not alter ZmRop4, ZmRop9 or ZmRop8 mRNA levels (Fig. S2, see Supporting Information). Therefore, ZmRop1, rather than other ZmRops, might play a specific role during SCMV infection. We observed that the ZmRop4 mRNA level in SCMV‐inoculated maize leaves at 2 dpi was approximately 65% higher than that in controls, whereas, at 5 and 9 dpi, the mRNA levels in both SCMV‐inoculated and systemic infected leaves were similar to that in the controls (Fig. S2). Whether or not ZmRop4 plays a role in the response to SCMV infection remains to be elucidated.
The above observation prompted us to further explore the role of ZmRop1 in SCMV infection. Post‐transcriptional gene silencing is a powerful method to study the role of a given gene (Burch‐Smith et al., 2004). A VIGS system based on a BMV infectious clone has been used to identify maize genes functionally involved in interactions with pathogens (van der Linde et al., 2011); thus, we used the BMV infectious clone to transiently silence the ZmRop1 gene. After inoculation with SCMV, systemic SCMV infection in the first and second upper leaves of ZmRop1‐silenced maize plants occurred earlier than that in control plants (data not shown). In ZmRop1‐silenced plants, more severe mosaic symptoms of SCMV were observed, and the viral accumulation of SCMV and PenMV in systemic leaves was much higher than that in control plants (Fig. 4), implying that silencing of the ZmRop1 gene facilitated viral accumulation in maize plants and led to more severe symptoms. This phenomenon is probably caused by ZmRop1 silencing, because the mRNA levels of other ZmRops (ZmRop4, ZmRop8 and ZmRop9) were almost unaffected by ZmRop1 gene silencing (Fig. 3C).
Complementary experiments were conducted by transient over‐expression of ZmRop1 in maize protoplasts or N. benthamiana to determine its effect on viral infection. Our results showed that both over‐expression of ZmRop1 or expression of its CA mutant apparently impaired SCMV and PVX viral accumulation, but the role of the DN mutant in this context was not obvious (5, 6). This result, combined with the observation that the transient silencing of ZmRop1 facilitated SCMV and PenMV infection, demonstrated that ZmRop1 could be a host defence factor during viral infection, and that the ZmRop1‐mediated viral defence response was probably not activated in maize protoplasts or N. benthamiana transiently expressing ZmRop1DN. In view of the limitations of the protoplast and heterologous system, ZmRop1 over‐expression and hairpin (RNAi) constructs have been created to be transformed into maize in order to obtain ZmRop1 transgenic plants for further investigations.
Under our experimental conditions, the first, second and third systemic leaves on SCMV‐inoculated maize plants developed mild systemic mosaic symptoms at 5, 9 and 14 dpi, respectively. RT‐PCR data showed that the SCMV accumulation level reached its maximum at 9 dpi for the first systemic leaves (data not shown). In this research, the most pronounced changes in ZmRop1 gene expression occurred when viral RNA accumulation reached its highest level (Fig. 2 and data not shown) and the leaf showed moderate systemic mosaic symptom development. In addition, the ZmRop1 mRNA profile in protoplast transfection was analysed, and no obvious alterations in the expression of ZmRop1 were detected at 12 and 24 h post‐transfection (data not shown). We speculated that the involvement of ZmRop1 in plant defence was not activated until the relatively late stages of the infection. The delayed host defence responses may be critical for SCMV to establish its systemic infection in maize plants.
Two loci, scmv1 and scmv2, have been shown to confer complete resistance to SCMV in maize (Dussle et al., 2000). Previous work has found that some of the differentially expressed genes are localized outside the scmv quantitative trait locus (QTL) region (Użarowska et al., 2009). ZmRop1 was mapped on maize chromosome 5L (Christensen et al., 2003), and localized outside the scmv QTL region. In combination with the delayed activation of ZmRop1 after SCMV infection, ZmRop1 might function further downstream in the signal transduction pathway mediated by genes localized in the scmv1 and/or scmv2 regions.
The important role of Rops in the plant defence response is well established. Plant Rops may function as positive (Kawasaki et al., 1999) or negative (Morel et al., 2004) regulators of ROS production in plants. Rapid production of ROS is often associated with plant defence against pathogens, and involved in host cell necrosis, cell wall strengthening and signal spreading (Király et al., 2008). For example, the expression of the GTP‐binding form of OsRac1 facilitates lignin accumulation through the enhancing activity of an enzyme involved in lignin biosynthesis and increased ROS production in rice (1999, 2006). Signalling of SA, a central component of plant resistance responses, such as the hypersensitive response, is mediated by ROS production, and the presence of ROS activates the Ca2+ signalling pathway (see review by Kawano, 2003). Recent work has indicated that OsRac1 interacts with multiple nucleotide‐binding site–leucine‐rich repeat (NBS‐LRR) proteins and is required for R protein‐mediated defence responses (Chen et al., 2010; Kawano et al., 2010). ZmRop1 has been reported to function as an activator of the oxidative burst in mammalian cells (Hassanain et al., 2000). In our study, PVX caused necrotic spots in plants transiently expressing ZmRop1CA (Fig. 6); therefore, it is possible that ZmRop1 might activate ROS production and thus be involved in a series of defence responses, such as the hypersensitive response or programmed cell death, to defend against viral infection. Further research is needed to test these possibilities. In this research, it was tested whether or not there were direct interactions between ZmRop1 and SCMV‐BJ‐encoded proteins. Each of the 10 SCMV‐encoded proteins (P1, HC‐Pro, P3, 6K1, CI, 6K2, VPg, NIa, NIb and CP; the 11th frame‐shift protein PIPO was not included in the test) was expressed in the pGADT7 vector individually, and ZmRop1 was inserted into the pGBKT7 vector. Co‐transformation results showed that there was no direct interaction between ZmRop1 and any of the tested SCMV proteins in yeast cells (data not shown).
The subcellular localization of Rops has been characterized in Arabidopsis (Lavy and Yalovsky, 2006) and rice (Chen et al., 2010). In this article, we revealed the subcellular localization of ZmRop1 in maize protoplasts. Consistent with previous reports that Rops (RACs) are located at the PM in their GTP‐ and GDP‐bound forms (Chen et al., 2010; Morel et al., 2004), our results showed that both forms of ZmRop1 were localized at the PM (Fig. S3). Rops can be subdivided into two major subgroups, type I and type II, according to differences in gene structure and the sequences of the C‐terminal hypervariable domains (Lavy and Yalovsky, 2006). ZmRop1 belongs to type II Rops based on its sequence homology with them. For another type II Rop, AtROP10, its polybasic domain and GC‐CG box in the C‐terminal hypervariable domain are essential for its association with the PM (Lavy and Yalovsky, 2006). Our results showed that the mutation of cysteine to serine in the GC‐CG box resulted in a change in ZmRop1 localization from PM to cytoplasm (Fig. S3); thus, the GC‐CG box is likely to be essential for ZmRop1 association with the PM.
In conclusion, our results suggest that ZmRop1 probably plays an important role in maize defence against viral infection. A better understanding of the specific roles of ZmRop1 in viral replication and systemic movement will be conducive to the elucidation of its function in virus–plant interactions.
EXPERIMENTAL PROCEDURES
Plant growth and virus sources
Maize (Zea mays L.) inbred line Zong 31 and cv. Va35 (both partially resistant to SCMV‐BJ and PenMV) plants were grown in growth chambers (22 °C day and 20 °C night, 16 h light and 8 h dark cycles) for virus inoculation and expression analysis. Maize (Zong 31) plants used for protoplast isolation were grown in the dark at 25 °C, according to the protocol of Sheen (1991). SCMV‐BJ (accession number AY042184) and PenMV isolate C (DQ977725) were isolated from diseased maize and Pennisetum centrasiaticum in the northern suburbs of Beijing and Shanxi Province (Deng et al., 2008; Fan et al., 2003), respectively, and maintained in maize (Zong 31) plants grown in an insect‐proof glasshouse in the above‐mentioned conditions. PVX was a gift from Dr Yiguo Hong (Warwick‐HRI, Warwick, UK).
SSH cDNA library construction
Maize leaves were collected for total RNA extraction using Trizol (Invitrogen/Life Technologies Inc., Carlsbad, CA, USA) at 15 days after SCMV or mock inoculation, and mRNA was isolated using an Oligotex™ mRNA isolation kit (Qiagen GmbH, Hilden, Germany). Procedures using the PCR‐Select™ cDNA subtraction kit (Clontech, Palo Alto, CA, USA) were conducted according to the manufacturer's protocol. Finally, the PCR products acquired after two rounds of SSH were cloned into the pGEM‐T Easy vector (Promega Inc., Madison, WI, USA). Ligated plasmids were transformed into DH5α cells. The library was amplified in Luria–Bertani (LB) medium containing 50 µg/mL ampicillin (growth medium). A portion of the library was plated, and colonies were picked into 96‐well microtitre plates containing growth medium. Copies of the library were stored in this format at −80 °C as a glycerol stock. Among them, 166 clones were subjected to DNA sequencing using the T7 and SP6 promoter sequence.
Plasmid construction
The sequences of the primers used in this section are listed in Table 1.
Table 1.
Primers used for polymerase chain reaction (PCR) amplification.
| Primer name | Sequence* | Restriction site | Usage |
|---|---|---|---|
| P1 | 5′‐ATGGCGTCCAGCGCCTCTCGGTTCAT‐3′ | Full‐length coding sequence | |
| P2 | 5′‐TCAGGACTTGAAGCATAGCATTTTTCTTCCACCG‐3′ | ||
| P3 | 5′‐TATGTCGACGTATGGCGTCCAGCGCCTCT‐3′ | SalI | pGFP‐ZmRop1 construction |
| P4 | 5′‐CTGGAGCTCTCAGGACTTGAAGCATAGCAT‐3′ | SacI | |
| P5 | 5′‐ ATGGATCCATGGCGTCCAGCGCCTCTCG‐3′ | BamHI | p35S‐ZmRop1 construction |
| P6 | 5′‐GTCGTCGACGGACTTGAAGCATAGCATTT‐3′ | SalI | |
| P7 | 5′‐CATAAGCTTGTCGACCCACGCGTCCGCCCAG‐3′ | HindIII | pC‐BMVA/G/Rop1 construction |
| P8 | 5′‐CATAAGCTTAGCAGCGGCGGATGAGCAGGGC‐3′ | HindIII | |
| P9 | 5′‐GGAAAAACCATAACCCTGGA‐3′ | Maize ubiquitin gene analysis | |
| P10 | 5′‐ATATGGAGAGAGGGCACCAG‐3′ | ||
| P11 | 5′‐TTTATGCGTGGCTTCTCG‐3′ | SCMV RNA analysis | |
| P12 | 5′‐TGTTGCTGGTGGCGTTGT‐3′ | ||
| P13 | 5′‐GAGCTCGGGTACCCGGGGATC‐3′ | ZmRop1‐GFP mRNA analysis | |
| P14 | 5′‐GAACTTCAGGGTCAGCTTGC‐3′ | ||
| P15 | 5′‐CCTCTGCAGTTGCCACC‐3′ | Tobacco RbcS RNA analysis | |
| P16 | 5′‐CCTGTGGGTATGCCTTCTTC‐3′ | ||
| p17 | 5′‐GAAAGATGTCAGCACCAGCTAGCAC‐3′ | PVX RNA analysis | |
| P18 | 5′‐TTATGGTGGTGGTAGAGTGACAACAG‐3′ | ||
| P19 | 5′‐AGGATGTTCTATGATGAACATCTTCGG‐3′ | ZmRop1 QPCR analysis | |
| P20 | 5′‐CTGCTTAACTCATAGAGCTAACCACAC‐3′ | ||
| P21 | 5′‐CCAATGATAACCATCGCCTCC‐3′ | ZmRop4 QPCR analysis | |
| P22 | 5′‐TGCTTACGCCTTACCATACCA‐3′ | ||
| P23 | 5′‐GGGATGTTCGATGGTGAACATCTTATC‐3′ | ZmRop8 QPCR analysis | |
| P24 | 5′‐CTAACTGATATAGCTAACCACGCCAG‐3′ | ||
| P25 | 5′‐AAAAGAAACAAGGGCGAAGG‐3′ | ZmRop9 QPCR analysis | |
| P26 | 5′‐TAATCACAAGGCGGGAAGGA‐3′ | ||
| P27 | 5′‐GTCGACGTATCGCGTCCAGCGC‐3′ | SalI | pZmRop1CA, pZmRop1DN and pZmRop1mS198 construction |
| P28 | 5′‐GAGCTCTCAGGACTTGAAGCATAG‐3′ | SacI | |
| P29† | 5′‐ACGGTCGGCGACGTTGCCGTGGGCAAGA‐3′ | ||
| P30† | 5′‐TGCCGTGGGCAAGAATTGTATGCTCATCTG‐3′ | ||
| P31† | 5′‐CACGCAAAGGATCTTCTATGATG‐3′ |
BMV, Brome mosaic virus; GFP, green fluorescent protein; PVX, Potato virus X; QPCR, quantitative polymerase chain reaction; SCMV, Sugarcane mosaic virus.
The restriction sites are shown in italic type.
The mutated nucleotides are shown in bold type.
The full‐length coding sequence of ZmRop1 was amplified by RT‐PCR from the total RNA extracted from the maize inbred line Zong 31 or cv. Va35 with the primer pair P1/P2 according to the ZmRop1 sequence deposited in GenBank (AF126052). The purified PCR product was inserted into pMD18‐T simple vector (TaKaRa Bio Inc., Otsu, Shiga, Japan) to create pZmRop1.
Glycine‐17, threonine‐22 and cysteine‐198 of ZmRop1 were mutated to valine, asparagine and serine by overlapping PCR from pZmRop1 with the primer pairs P27/P29 and P29/P28, P27/P30 and P30/P28, and P28/P31 and P31/P28, respectively, and the corresponding plasmids were named pZmRop1CA, pZmRop1DN and pZmRop1mS198, respectively.
The full‐length coding sequences of ZmRop1, ZmRop1CA, ZmRop1DN and ZmRop1mS198 were amplified by PCR using primers P3 and P4, doubly digested with SalI and SacI, and the fragments were cloned into the GFP expression vector pGFP, a derivative of pUC19 (TaKaRa Bio Inc.), to generate pGFP‐ZmRop1, pGFP‐ZmRop1CA, pGFP‐ZmRop1DN and pGFP‐ZmRop1mS198, respectively (see Fig. S1).
Vectors constructed from p35S‐GFP (provided by Professor Zejian Guo, China Agricultural University, Beijing, China) were used for the heterologous expression of ZmRop1 and its derivatives in N. benthamiana leaves. The full‐length coding sequences of ZmRop1, ZmRop1 CA and ZmRop1 DN were then amplified again using the primers P5 and P6, double digested with SacI and BamHI, and the fragments were inserted into p35S‐GFP. The constructs created were designated as p35S‐ZmRop1, p35S‐ZmRop1CA and p35S‐ZmRop1DN, respectively.
The BMV‐based vector pC‐BMVA/G was used in this study to silence the ZmRop1 gene in maize (cv. Va35). This pC‐BMVA/G vector consists of pF1‐11 for RNA1, pF2‐2 for RNA2 and pF3‐5/13′A/G for RNA3. A specific fragment from the 5′ terminal untranslated region (UTR) of the ZmRop1 gene was amplified by PCR using the primer pair P7/P8, and inserted into pF3‐5/13′A/G to create the plasmid pF3‐5/13′A/G/Rop1. The combination of pF3‐5/13′A/G/Rop1 and two other plasmids (pF1‐11 and pF2‐2) of pC‐BMVA/G was named pC‐BMVA/G/Rop1 (see Fig. S1).
Isolation and transfection of maize protoplasts
SCMV viral RNA was extracted from viral particles according to the established protocol (Dijkstra and de Jager, 1998). The isolation and transfection of maize protoplasts were performed according to the protocol of Sheen (1991) with modifications. A total of 2 × 105 maize protoplasts were transformed with 50 µg DNA each of the plasmids used in this research, or co‐transformed with 10 µg of purified SCMV viral RNA, via electroporation using the Gene Pulser Xcell system (Bio‐Rad Laboratories Inc., Hercules, CA, USA).
The viability of the electroporated protoplasts was determined using the fluorescein diacetate (FDA) (Sigma‐Aldrich Inc., St. Louis, MO, USA) staining method (Widholm, 1972); cell viability was observed using conventional fluorescence microscopy (Leica MZ FLIII Microsystem, Leica Microsystems Inc., Wetzlar, Germany) at 200× magnification, and determined by calculating the percentage of fluorescent cells from the total number of intact cells observed (Raghupathy et al., 2006).
The transfected maize protoplasts were suspended in culture medium [1000 mL containing 30 g sucrose, 4.43 g Murashige–Skoog (MS) salt (PhyoTechnology Laboratories Inc., Shawnee Mission, KS, USA), 2 mg glycine, 1 mg vitamin B1, 100 mg myoinositol, 0.5 mg 1‐naphthaleneacetic acid, 0.5 mg 6‐benzylaminopurine, 0.2 mg 2,4‐dichlorophenoxyacetic acid and 0.55 m mannitol, pH 5.7] and incubated in the dark at 25 °C. Maize protoplasts were harvested at the selected time points post‐electroporation and were used for further analyses.
GFP visualization
The transient expression of GFP was observed at different time points post‐electroporation or infiltration using a laser scanning confocal microscope (Carl Zeiss LSM510 META, Carl Zeiss Inc., Oberkochen, Germany) with a 40× (oil immersion) objective. For the excitation of GFP, an argon/krypton‐ion‐laser with a wavelength of 488 nm was used. Emissions were detected at 522 nm, and images were processed using the LSM confocal browser software package (Carl Zeiss Inc.).
Agrobacterium‐mediated transient expression
The recombinant plasmids p35S‐ZmRop1, p35S‐ZmRop1CA and p35S‐ZmRop1DN were introduced into Agrobacterium tumefaciens strain GV3101. The transformed A. tumefaciens cells were cultured, set to an optical density at 600 nm of 1.0 in MMA buffer [10 mm MgCl2, 10 mm 2‐(N‐morpholino)ethanesulphonic acid (MES), 100 µm acetosyringone] and used to infiltrate the leaves of 3‐week‐old N. benthamiana plants, essentially as described previously (Jia et al., 2003).
RNA transcripts and virus inoculation
Infectious RNAs from pC‐BMVA/G and pC‐BMVA/G/Rop1 were prepared by in vitro transcription using T3 DNA‐dependent RNA polymerase (Promega Inc.) as described previously (Ding et al., 2006). The second leaves of 10‐day‐old Va35 seedlings were inoculated with the purified infectious transcripts or FES buffer (0.1 m glycine, 0.06 m potassium phosphate, 1% sodium phosphate, 1% macaloid and 1% celite, pH 8.5–9.0). At 10–14 dpi with transcripts, the first upper noninoculated leaves of maize Va35 seedlings showing pronounced chlorotic streaks were re‐inoculated with crude sap from SCMV‐BJ‐ or PenMV‐C‐infected maize leaves. In later experiments using PVX (van Wezel et al., 2002), RNA transcripts were produced from plasmid PVX through in vitro transcription using T7 RNA polymerase (Promega Inc.). The RNA transcripts were then mechanically inoculated onto leaves of A. tumefaciens‐infiltrated N. benthamiana plants at 24 h post‐infiltration.
Semiquantitative and QRT‐PCR analysis
Total RNA was extracted from maize leaves or protoplasts and N. benthamiana leaves using TRIzol reagent (Invitrogen) and treated with 5 U of RNase‐free DNAase I (TaKaRa Bio Inc.) at 37 °C for 30 min. The DNase I‐treated total RNAs were recovered by ethanol precipitation. About 0.5 µg of total RNA was converted into cDNA (RT product) with SuperScript III reverse transcriptase (Invitrogen) using primers oligo (dT) and random 6‐mers (TaKaRa Bio Inc.) according to the manufacturer's instructions, yielding 20 µL of cDNA solutions. The RT products were individually diluted 10‐fold in Easy Dilution buffer (TaKaRa Bio Inc.) to be used as templates for the subsequent RT‐PCR analysis. The maize ubiquitin gene (U29159) was amplified with primers P9 and P10, and used as an internal control for both semiquantitative and QRT‐PCR analyses. Primers P11 and P12 were used for both semiquantitative and QRT‐PCR detection of SCMV infection. For SCMV detection, PCR amplification was conducted with 1 µL of RT product in a 25‐µL reaction with the following conditions: 94 °C for 2 min, followed by 30 cycles at 94 °C for 30 s, 60 °C for 30 s and 72 °C for 20 s, and then 72 °C for 10 min. The PCR products were visualized in a 1% agarose gel after staining with 1% (w/v) ethidium bromide, and analysed by an AlphaImager 2200 (Alpha Innotech Corp., San Leandro, CA, USA). For PVX infection analysis, the primer pairs P13/P14, P15/P16 and P17/P18 were used to detect the expression of ZmRop1s‐GFP fusion proteins, the accumulation of tobacco (N. benthamiana) NbRbcS mRNA (served as an internal control) and PVX genomic RNA, respectively. PCR amplification conditions were 94 °C for 2 min, followed by 30 cycles at 94 °C for 30 s, 58 °C for 30 s and 72 °C for 30 s, and a final 72 °C for 10 min.
QRT‐PCR was repeated three times for each sample and conducted on a DNA Engine Opticon™ 2 system (Bio‐Rad Laboratories Inc.) following the manufacturer's recommendations. The experiment was independently conducted at least three times with different RNA preparations for each treatment. The reactions contained 1 µL of 10‐fold‐diluted cDNA template, 1 µL of 10 mm deoxynucleoside triphosphates (dNTPs), 200 nm of each of the gene‐specific primer pairs (P19 and P20 for ZmRop1; P21 and P22 for ZmRop4; P23 and P24 for ZmRop8; P25 and P26 for ZmRop9; P11 and P12 for SCMV mRNA, Sangon Biotech, Shanghai, China), 0.4 µL ROX Dye II and 10 µL of 2 × SYBR Premix Ex Taq, in a total volume of mixture of 20 µL, as instructed by the manufacturer (TaKaRa Bio Inc.). Thermal cycling conditions consisted of 2 min at 50 °C and 10 min at 94 °C, followed by 40 cycles of 94 °C for 15 s, 60 °C for 15 s and 72 °C for 15 s, and 1 s at 80.5 °C for plate reading. After the cycling protocol, the final step was applied to all reactions by continuously monitoring the fluorescence through the dissociation temperature of the PCR product at a temperature transition rate of 0.1 °C/s to generate a melting curve. Quantification was conducted according to the 2−Δ Ct method (Pfaffl, 2001). The sequences of the primers used in the RT‐PCR experiments are listed in Table 1.
Western blotting assay
Maize proteins were isolated from maize protoplasts and leaves using established protocols (Gallagher et al., 1988) with some modifications. Proteins were extracted in 20 mm MES (pH 6.1), 0.25 m sucrose, 0.1 m ethyleneglycol‐bis(β‐aminoethylether)‐N,N′‐tetraacetic acid (EGTA), 1 mm dithiothreitol (DTT) and 0.2 mg/mL bovine serum albumin (BSA), and protease inhibitors [0.1 mM phenylmethylsulphonylfluoride (PMSF), 0.2 µg/mL aprotinin, 0.2 µg/mL pepstain A and 20 µg/mL benzamidine] were used when necessary. The proteins were separated into membrane‐associated and cytosolic fractions by 10 000 g centrifugation (Ivanchenko et al., 2000). Tobacco soluble proteins were extracted with buffer (220 mm Tris‐HCl, pH 7.4, 250 mm sucrose, 1 mm MgCl2, 50 mm KCl) containing β‐mercaptoethanol (10 mm) and PMSF (100 µm). The protein concentration was measured by the method of Bradford (1976). The yielded extracts were loaded and separated by 8% sodium dodecylsulphate‐polyacrylamide gel electrophoresis (SDS‐PAGE), and electroblotted onto nitrocellulose membranes; immunodetection was performed using antiserum against a bacterially expressed SCMV, PenMV and PVX CP or anti‐GFP polyclonal antibody [Tiangen Biotech (Beijing) Co., Ltd., Beijing China), followed by alkaline phosphatase‐conjugated protein A (Sigma‐Aldrich Inc.), and visualized by nitroblue tetrazolium/5‐bromo‐4‐chloroindol‐3‐yl phosphate (NBT/BCIP) (Roche Applied Sciences Inc., Indianapolis, IN, USA) staining.
Unless stated otherwise, all the experiments described in this work were repeated at least three times.
Supporting information
Fig. S1 Schematic diagram of pBMVA/G/Rop1 and pGFP‐ZmRop1s construction. (A) Schematic diagram of the C‐BMVA/G/Rop1 vector constructs, in which pF3‐5/13′A/G carries a specific 145‐bp PCR fragment of the ZmRop1 5′‐terminal untranslated region (UTR) (Rop1145). Black rectangles represent the viral sequence and grey rectangles represent Rop1145. The curly lines at both termini of each construct represent bacterial plasmid sequences. The clover‐like symbol represents the tRNA‐like structure at the 3′‐terminus of the viral RNA sequence. PshAI and SpeI are restriction sites for plasmid linearization prior to in vitro transcription, and HindIII in pF3‐5/13′A/G is used for Rop1145 insertion. T3 indicates the T3 promoter sequence (open rectangle) for in vitro transcription of viral sequences. (B) Schematic representation of four types of GFP‐ZmRop1 fusion construct. G, green fluorescent protein (GFP); CA, constitutively active; DN, dominant negative; 35 S, 35S promoter derived from Cauliflower mosaic virus; T, nopaline synthase (NOS) terminator derived from Agrobacterium tumefaciens.
Fig. S2 Quantitative reverse transcription‐polymerase chain reaction (QRT‐PCR) analysis of the effects of Sugarcane mosaic virus (SCMV) infection on the relative mRNA levels of ZmRop4 (A), ZmRop9 (B) and ZmRop8 (C) in maize leaves. Each value is shown as the average of three independent experiments ± standard deviation (SD). IL, inoculated leaves; SL, systemic leaves. Different letters on the columns indicate a significant difference by Student's t‐test (P < 0.05).
Fig. S3 The subcellular localization of ZmRop1. (A) Fluorescence micrographs of maize protoplasts transformed with four types of GFP‐ZmRop1 fusion construct using laser scanning confocal microscopy. Scale bar, 10 µm. pGRop1, pGFP‐ZmRop1; pGRop1CA, pGFP‐ZmRop1CA; pGRop1DN, pGFP‐ZmRop1DN; pGRop1mS198, pGFP‐ZmRop1mS198. CA, constitutively active, DN, dominant negative; C, cytoplasm; V, vacuole. (B) The expression levels of fusion proteins were detected by Western blotting using polyclonal antiserum against green fluorescent protein (GFP), as indicated. The Coomassie brilliant blue‐stained 8% sodium dodecylsulphate‐polyacrylamide gel (bottom) for different samples was used as a loading control.
Table S1 List of up‐regulated gene transcripts post‐Sugarcane mosaic virus (SCMV) infection with BLAST hits against the National Center for Biotechnology Information (NCBI) GenBank nonredundant (nr) database.
Supporting info item
Supporting info item
Supporting info item
Supporting info item
ACKNOWLEDGEMENTS
This work was supported by the National Basic Research Program of China (#2012CB114004), the Program for Changjiang Scholars and Innovative Research Team in the University (PCSIRT, No. IRT1042) and a grant from the Ministry of Science and Technology of China (2009ZX08003‐011B). We thank Dr Richard Nelson (Samuel Roberts Noble Foundation, Ardmore, OK, USA) for providing the BMV infectious clone C‐BMVA/G, Professors Jingrui Dai and Mingliang Xu (National Maize Development Center, Beijing, China) for their gifts of maize seeds of the inbred line Zong 31 and cv. Va35 and Professor Zejian Guo (China Agricultural University, Beijing, China) for the gift of the p35S‐GFP vector. We also thank Dr Xin‐Shun Ding (Samuel Roberts Noble Foundation) for his advice on some experiments, and Professor Jen Sheen (Department of Genetics, Harvard Medical School, Boston, MA, USA) for her valuable suggestions on maize protoplast isolation and transfection.
REFERENCES
- Agrawal, G.K. , Iwahashi, H. and Rakwal, R. (2003) Small GTPase ‘Rop’: molecular switch for plant defense responses. FEBS Lett. 546, 173–180. [DOI] [PubMed] [Google Scholar]
- Arthur, K.M. , Vejlupova, Z. , Meeley, R.B. and Fowler, J.E. (2003) Maize ROP2 GTPase provides a competitive advantage in male gametophyte. Genetics, 165, 2137–2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berken, A. (2006) ROPs in the spotlight of plant signal transduction. Cell. Mol. Life Sci. 63, 2446–2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloch, D. , Lavy, M. , Efrat, Y. , Efroni, I. , Bracha‐Drori, K. , Abu‐Abied, M. , Sadot, E. and Yalovsky, S. (2005) Ectopic expression of an activated RAC in Arabidopsis disrupts membrane cycling. Mol. Biol. Cell, 16, 1913–1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford, M.M. (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal. Biochem. 72, 248–254. [DOI] [PubMed] [Google Scholar]
- Burch‐Smith, T.M. , Anderson, J.C. , Martin, G.B. and Dinesh‐Kumar, S.P. (2004) Applications and advantages of virus‐induced gene silencing for gene function studies in plants. Plant J. 39, 734–746. [DOI] [PubMed] [Google Scholar]
- Chen, L. , Shiotani, K. , Togashi, T. , Miki, D. , Aoyama, M. , Wong, H.L. , Kawasaki, T. and Shimamoto, K. (2010) Analysis of the Rac/Rop small GTPase family in rice: expression, subcellular localization and role in disease resistance. Plant Cell Physiol. 51, 585–595. [DOI] [PubMed] [Google Scholar]
- Chen, Z.R. , Zhou, T. , Wu, X.H. , Hong, Y.G. , Fan, Z.F. and Li, H.F. (2008) Influence of cytoplasmic heat shock protein 70 on viral infection of Nicotiana benthamiana . Mol. Plant Pathol. 9, 809–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen, T.M. , Vejlupkova, Z. , Sharma, Y.K. , Arthur, K.M. , Spatafora, J.W. , Albright, C.A. , Meeley, R.B. , Duvick, J.P. , Quatrano, R.S. and Flower, J.E. (2003) Conserved subgroups and developmental regulation in the monocot rop gene family. Plant Physiol. 133, 1791–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng, C.L. , Wang, W.J. , Wang, Z.Y. , Jiang, X. , Cao, Y.Y. , Zhou, T. , Wang, F.R. , Li, H.F. and Fan, Z.F. (2008) The genomic sequence and biological properties of Pennisetum mosaic virus, a novel monocot‐infecting potyvirus. Arch. Virol. 153, 921–927. [DOI] [PubMed] [Google Scholar]
- Diatchenko, L. , Lau, Y.F. , Campell, A.P. , Chenchik, A. , Moqadam, F. , Huang, B. , Lukyanov, S. , Lukuanov, K. , Gurskaya, N. , Sverdlov, E.D. and Siebert, P.D. (1996) Suppression subtractive hybridization: a method for generating differentially regulated or tissue‐specific cDNA probes and libraries. Proc. Natl. Acad. Sci. USA, 93, 6025–6030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijkstra, J. and de Jager, C.P. (eds) (1998) Practical Plant Virology: Protocols and Exercises (Springer Lab Manual), pp. 308–311. Berlin, Heidelberg, New York: Springer Press. [Google Scholar]
- Ding, X.S. , Schneider, W.L. , Chaluvadi, S.R. , Mian, M.A. and Nelson, R.S. (2006) Characterization of a Brome mosaic virus strain and its use as a vector for gene silencing in monocotyledonous hosts. Mol. Plant–Microbe Interact. 19, 1229–1239. [DOI] [PubMed] [Google Scholar]
- Dussle, C.M. , Melchinger, A.E. , Kuntze, L. , Stork, A. and Lübberstedt, T. (2000) Molecular mapping and gene action of Scm1 and Scm2, two major QTL contributing to SCMV resistance in maize. Plant Breed. 119, 299–303. [Google Scholar]
- Fan, Z.F. , Chen, H.Y. , Liang, X.M. and Li, H.F. (2003) Complete sequence of the genomic RNA of the prevalent strain of a potyvirus infecting maize in China. Arch. Virol. 148, 773–782. [DOI] [PubMed] [Google Scholar]
- Gallagher, S. , Short, T.W. , Ray, P.M. , Pratt, L.H. and Briggs, W.R. (1988) Light‐mediated changes in two proteins found associated with plasma membrane fractions from pea stem sections. Proc. Natl. Acad. Sci. USA, 85, 8003–8007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassanain, H.H. , Sharma, Y.K. , Moldovan, L. , Khramtsova, V. , Berlinera, L.J. , Duvickb, J.P. and Goldschmidt‐Clermont, P.J. (2000) Plant Rac proteins induce superoxide production in mammalian cells. Biochem. Biophys. Res. Commun. 272, 783–788. [DOI] [PubMed] [Google Scholar]
- Havelda, Z. , Várallyay, E. , Válóczi, A. and Burgyán, J. (2008) Plant virus infection‐induced persistent host gene downregulation in systemically infected leaves. Plant J. 55, 278–288. [DOI] [PubMed] [Google Scholar]
- Huang, T. , Wei, T. , Laliberté, J. and Wang, A. (2010) A host RNA helicase‐like protein, AtRH8, interacts with the potyviral genome‐linked protein, VPg, associates with the virus accumulation complex, and is essential for infection. Plant Physiol. 152, 255–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanchenko, M. , Vejlupkova, Z. , Quatrano, R.S. and Fowler, J.E. (2000) Maize ROP7 GTPase contains a unique, CaaX box‐independent plasma membrane targeting signal. Plant J. 24, 79–90. [DOI] [PubMed] [Google Scholar]
- Jia, H.G. , Pang, Y.Q. and Fang, R.X. (2003) Agroinoculation as a simple way to deliver a Tobacco mosaic virus‐based expression vector. Acta Bot. Sin. 45, 770–773. [Google Scholar]
- Kawano, T. (2003) Roles of the reactive oxygen species‐generating peroxidase reactions in plant defense and growth induction. Plant Cell Rep. 21, 829–837. [DOI] [PubMed] [Google Scholar]
- Kawano, Y. , Akamatsu, A. , Hayashi, K. , Housen, Y. , Okuda, J. , Yao, A. , Nakashima, A. , Takahashi, H. , Yoshida, H. , Wong, H.L. , Kawasaki, T. and Shimamoto, K. (2010) Activation of a Rac GTPase by the NLR family disease resistance protein Pit plays a critical role in rice innate immunity. Cell Host Microbe, 7, 362–375. [DOI] [PubMed] [Google Scholar]
- Kawasaki, T. , Henmi, K. , Ono, E. , Hatakeyama, S. , Iwano, M. , Satoh, H. and Shimamoto, K. (1999) The small GTP‐binding protein Rac is a regulator of cell death in plants. Proc. Natl. Acad. Sci. USA, 96, 10 922–10 926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki, T. , Koita, H. , Nakatsubo, T. , Hasegawa, K. , Wakabayashi, K. , Takahashi, H. , Umemura, K. , Umezawa, T. and Shimamoto, K. (2006) Cinnamoyl‐CoA reductase, a key enzyme in lignin biosynthesis, is an effector of small GTPase Rac in defense signaling in rice. Proc. Natl. Acad. Sci. USA, 103, 230–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Király, L. , Hafez, Y.M. , Fodor, J. and Király, Z. (2008) Suppression of Tobacco mosaic virus‐induced hypersensitive‐type necrotization in tobacco at high temperature is associated with down regulation of NADPH oxidase and superoxide and stimulation of dehydroascorbate reductase. J. Gen. Virol. 89, 799–808. [DOI] [PubMed] [Google Scholar]
- Lavy, M. and Yalovsky, S. (2006) Association of Arabidopsis type‐II ROPs with the plasma membrane requires a conserved C‐terminal sequence motif and a proximal polybasic domain. Plant J. 46, 934–947. [DOI] [PubMed] [Google Scholar]
- Lavy, M. , Bracha‐Dori, K. , Sternberg, H. and Yalovsky, S. (2002) A cell‐specific, prenylation‐independent mechanism regulates targeting of type II RACs. Plant Cell, 14, 2431–2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Linde, K. , Kastner, C. , Kumlehn, J. , Kahmann, R. and Doehlemann, G. (2011) Systemic virus‐induced gene silencing allows functional characterization of maize genes during biotrophic interaction with Ustilago maydis . New Phytol. 189, 471–483. [DOI] [PubMed] [Google Scholar]
- Morel, J. , Fromentin, J. , Blein, J.P. , Simon‐Plas, F. and Elmayan, T. (2004) Rac regulation of NtrbohD, the oxidase responsible for the oxidative burst in elicited tobacco cells. Plant J. 37, 282–293. [DOI] [PubMed] [Google Scholar]
- Nibau, C. , Wu, H. and Cheung, A.Y. (2006) RAC/ROP GTPase: ‘hubs’ for signal integration and diversification in plants. Trends Plant Sci. 11, 1360–1385. [DOI] [PubMed] [Google Scholar]
- Pathuri, I.P. , Eichmann, R. and Hückelhoven, R. (2009) Plant small monomeric G‐proteins (RAC/ROPs) of barley are common elements of susceptibility to fungal leaf pathogens, cell expansion and stomata development. Plant Signal. Behav. 4, 1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffl, M.W. (2001) A new mathematical model for relative quantification in real‐time RT‐PCR. Nucleic Acids Res. 29, 2002–2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghupathy, M.B. , Griffiths, J.S. , Stobbs, L.W. , Brown, D.C.W. , Brandle, J.E. and Wang, A. (2006) Transfection of Arabidopsis protoplasts with a Plum pox virus (PPV) infectious clone for studying early molecular events associated with PPV infection. J. Virol. Methods, 136, 147–153. [DOI] [PubMed] [Google Scholar]
- Sandermann, H. (2000) Active oxygen species as mediators of plant immunity: three case studies. Biol. Chem. 381, 649–653. [DOI] [PubMed] [Google Scholar]
- Sheen, J. (1991) Molecular mechanisms underlying the differential expression of maize pyruvate, orthophosphate dikinase genes. Plant Cell, 3, 225–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi, Y. , Qin, Y. , Cao, Y. , Sun, H. , Zhou, T. , Hong, Y. and Fan, Z. (2011) Influence of an m‐type thioredoxin in maize on potyviral infection. Eur. J. Plant Pathol. 131, 317–326. [Google Scholar]
- Użarowska, A. , Dionisio, G. , Sarholz, B. , Piepho, H.P. , Xu, M. , Ingvardsen, C.R. , Wenzel, G. and Lübberstedt, T. (2009) Validation of candidate genes putatively associated with resistance to SCMV and MDMV in maize (Zea mays L.) by expression profiling. BMC Plant Biol. 9, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wezel, R. , Dong, X.L. , Blake, P. , Stanley, J. and Hong, Y.G. (2002) Differential roles of geminivirus Rep and AC4(C4) in the induction of necrosis in Nicotiana benthamiana . Mol. Plant–Microbe Interact. 3, 461–471. [DOI] [PubMed] [Google Scholar]
- Whitham, S.A. , Quan, S. , Chang, H.S. , Cooper, B. , Estes, B. , Zhu, T. , Wang, X. and Hou, Y.M. (2003) Diverse RNA viruses elicit the expression of common sets of genes in susceptible Arabidopsis thaliana plants. Plant J. 33, 271–283. [DOI] [PubMed] [Google Scholar]
- Widholm, J. (1972) The use of fluorescein diacetate and phenosafranine for determining viability of cultured plant cells. Stain Technol. 47, 189–194. [DOI] [PubMed] [Google Scholar]
- Yang, S.J. , Carter, S.A. , Cole, A.B. , Cheng, N.H. and Nelson, R.S. (2004) A natural variant of a host RNA‐dependent RNA polymerase is associated with increased susceptibility to viruses by Nicotiana benthamiana . Proc. Natl. Acad. Sci. USA, 101, 6297–6302. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 Schematic diagram of pBMVA/G/Rop1 and pGFP‐ZmRop1s construction. (A) Schematic diagram of the C‐BMVA/G/Rop1 vector constructs, in which pF3‐5/13′A/G carries a specific 145‐bp PCR fragment of the ZmRop1 5′‐terminal untranslated region (UTR) (Rop1145). Black rectangles represent the viral sequence and grey rectangles represent Rop1145. The curly lines at both termini of each construct represent bacterial plasmid sequences. The clover‐like symbol represents the tRNA‐like structure at the 3′‐terminus of the viral RNA sequence. PshAI and SpeI are restriction sites for plasmid linearization prior to in vitro transcription, and HindIII in pF3‐5/13′A/G is used for Rop1145 insertion. T3 indicates the T3 promoter sequence (open rectangle) for in vitro transcription of viral sequences. (B) Schematic representation of four types of GFP‐ZmRop1 fusion construct. G, green fluorescent protein (GFP); CA, constitutively active; DN, dominant negative; 35 S, 35S promoter derived from Cauliflower mosaic virus; T, nopaline synthase (NOS) terminator derived from Agrobacterium tumefaciens.
Fig. S2 Quantitative reverse transcription‐polymerase chain reaction (QRT‐PCR) analysis of the effects of Sugarcane mosaic virus (SCMV) infection on the relative mRNA levels of ZmRop4 (A), ZmRop9 (B) and ZmRop8 (C) in maize leaves. Each value is shown as the average of three independent experiments ± standard deviation (SD). IL, inoculated leaves; SL, systemic leaves. Different letters on the columns indicate a significant difference by Student's t‐test (P < 0.05).
Fig. S3 The subcellular localization of ZmRop1. (A) Fluorescence micrographs of maize protoplasts transformed with four types of GFP‐ZmRop1 fusion construct using laser scanning confocal microscopy. Scale bar, 10 µm. pGRop1, pGFP‐ZmRop1; pGRop1CA, pGFP‐ZmRop1CA; pGRop1DN, pGFP‐ZmRop1DN; pGRop1mS198, pGFP‐ZmRop1mS198. CA, constitutively active, DN, dominant negative; C, cytoplasm; V, vacuole. (B) The expression levels of fusion proteins were detected by Western blotting using polyclonal antiserum against green fluorescent protein (GFP), as indicated. The Coomassie brilliant blue‐stained 8% sodium dodecylsulphate‐polyacrylamide gel (bottom) for different samples was used as a loading control.
Table S1 List of up‐regulated gene transcripts post‐Sugarcane mosaic virus (SCMV) infection with BLAST hits against the National Center for Biotechnology Information (NCBI) GenBank nonredundant (nr) database.
Supporting info item
Supporting info item
Supporting info item
Supporting info item


