Summary
The biological function(s) of the cpkk1, cpkk2 and cpkk3 genes, encoding the three mitogen‐activated protein kinase kinases (MAP2Ks) of Cryphonectria parasitica, the causal agent of chestnut blight, were examined through knockout strains. Cpkk1, the Mkk1 orthologue, acts in a phosphorylation cascade essential for cell integrity; Cpkk2 is the Ste7 orthologue involved in the pheromone response pathway; Cpkk3 is the Pbs2 orthologue, the MAP2K activated during the high‐osmolarity response. Our analysis confirmed the role of each MAP2K in its respective signalling cascade with some peculiarities: abnormal hyphae with a reduced number of septa and thinner cell walls were observed in Δcpkk1 mutants, and a strong growth defect on solid media was evident in Δcpkk2 mutants, when compared with the controls. Virulence on chestnut was affected in both the Δcpkk1 and Δcpkk2 strains, which were also unable to complete the developmental steps essential for mating. No alterations were reported in Δcpkk3, except under hyperosmotic conditions and in the presence of fludioxonil. Δcpkk2 mutants, however, showed higher sensitivity during growth in medium containing the antibiotic G418 (Geneticin).
Keywords: chestnut blight, Cryphonectria parasitica, growth, MAP kinase, mating, pathogenesis
Introduction
The filamentous ascomycete Cryphonectria parasitica is the causal agent of chestnut blight, which has devastated the chestnut forestry of North America since the early 20th century (Dawe and Nuss, 2013). Infection of the fungus with specific hypoviruses (Hypoviridae family) is known to cause hypovirulence, resulting in biological control (Milgroom and Cortesi, 2004). Strains containing Cryphonectria hypovirus 1 (CHV1), the best‐characterized hypovirus type member, display characteristic symptoms, such as reduced or altered sporulation, laccase production, pigmentation, oxalate accumulation, virulence expression and interference with the secretory pathway (Dawe and Nuss, 2013; Turina and Rostagno, 2007). As these phenotypic changes are pleiotropic and take place in a coordinated and specific manner, it has been suggested that hypoviruses perturb one or more regulatory pathways (Nuss, 2005). Preliminary studies have suggested that signal transduction components may be targets for CHV1 in inducing hypovirulence. Previous reports have elucidated the roles of elements belonging to the heterotrimeric G‐protein and mitogen‐activated protein kinase (MAPK) families (Nuss, 2010).
In microbial and higher eukaryotes, MAPK pathways play pivotal roles in intracellular and intercellular signalling. Development, growth and adaptation to the environment are regulated physiological processes orchestrated by MAPKs, which are composed of three functionally interlinked and successive protein kinases: MAP3K (MAP kinase kinase kinase), MAP2K (MAP kinase kinase) and MAPK. MAPK pathways are the basic modules used to transduce signals through a stepwise phosphorylation relay (Imajo et al., 2006), and the presence of MAPK phosphatases modulates MAPK activity by dephosphorylation (Bhalla et al., 2002). In filamentous fungi, MAPK pathways have established functions in three main processes: (i) the mating pheromone response, characterized by the key function of the Fus3 homologue regulating the expression of genes involved in sporulation, mating and the sexual cycle; (ii) the maintenance of cell wall integrity, centred on the activity of the Slt2 homologue, a regulator of expression of genes related to cell wall biogenesis and structure, adaptation to stress and toxic compounds; and (iii) the response to changes in osmolarity and nutrient sensing, in which the MAPK Hog1 plays a fundamental role in the activation of defence genes in response to oxidative stress, osmotic shock and threats to water homeostasis (Hamel et al., 2012; Rispail et al., 2009). In pathogenic fungi, MAPKs are also involved in virulence and correlated phenomena, such as appressoria formation, host penetration and tissue colonization (Zhao et al., 2007).
The aim of the present study was to perform a functional characterization of the three MAP2Ks encoded by the C. parasitica genome: Cpkk1, Cpkk2 and Cpkk3. Recent proposed nomenclature that takes into account the yeast orthologue would denominate them Cpa‐MKK1, Cpa‐Ste7 and Cpa‐Pbs2, respectively (Hamel et al., 2012). Detailed phenotypic analyses of MAP2K mutants allowed us to assess the commonalities and specificities of the role of each MAP2K in comparison with those already studied in other filamentous fungi and phylogenetically related ascomycete species.
Results
Construction of MAP2K mutants
To analyse the functions of the cpkk1, cpkk2 and cpkk3 genes in C. parasitica, mutants were generated by site‐directed double homologous recombination during integrative transformation. To this end, a different linearized plasmid for each transformation (see Experimental procedures), containing a gene cassette conferring hygromycin resistance (hph) and flanked by MAP2K‐specific sequences, was introduced into strain Δcpku80, a C. parasitica isolate highly efficient at homologous recombination (Lan et al., 2008) (Fig. S1, see Supporting Information). Polymerase chain reaction (PCR) screening of transformants with hph and locus‐specific primers (data not shown) and Southern blot analyses confirmed the gene replacements of the wild‐type allele with the disrupted allele containing the hph fragment (Fig. S1). Western blot experiments using previously prepared specific Cpkk1 and Cpkk2 polyclonal antibodies (Turina et al., 2006) detected no protein products in the respective null mutants at 24 h post‐inoculation (Fig. 1). In order to avoid the possibility of random mutations occurring ectopically in the genome, two distinct knockout strains originating from different independent transformation events were analysed for each MAP2K, and all experiments aimed at verifying phenotypic changes were performed on at least two single‐spore isolates from each of the two independently obtained knockout strains. We documented and discussed as phenotypic changes caused by specific gene knockout only those common to both independently obtained strains.
Figure 1.
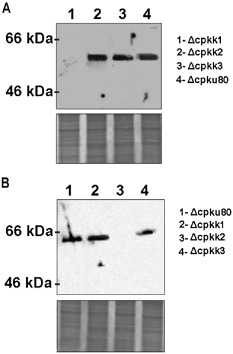
Western blot analyses on mitogen‐activated protein kinase kinase (MAP2K)‐null mutants using Cpkk1 (A) and Cpkk2 (B) polyclonal antibodies. The developed films show the absence of signal in the Δcpkk1 and Δcpkk2 strains using the respective anti‐MAP2K polyclonal antibody. Equal loading of protein samples was confirmed in a parallel gel that was stained only with Coomassie blue (bottom panels in A and B).
Phenotypic characterization of MAP2K knockout strains
Different growth alterations were observed in MAP2K‐null mutants when compared with the wild‐type strain (Δcpku80 was used as the control). Under standard growth conditions on PDAmb medium [potato dextrose agar (PDA) containing l‐methionine (100 mg/L) and biotin (1 mg/L), 25 °C, 12 h/12 h light/dark], both Δcpkk1 and Δcpkk2 strains resulted in impaired growth with the absence of aerial hyphae (Fig. 2). Moreover, the growth of Δcpkk2 always ceased before the colony reached the edge of the plate. On the contrary, in Endothia parasitica (EP) complete liquid medium, only the Δcpkk1 strain exhibited a slower growth rate than the Δcpku80 isolate, resulting in a smaller amount of mycelium (Table 1). The growth of Δcpkk3 was unaffected in either solid or liquid medium.
Figure 2.
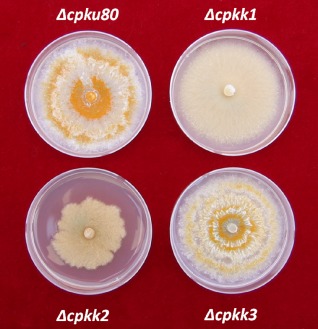
Fungal colony morphology on PDAmb [potato dextrose agar (PDA) containing l‐methionine (100 mg/L) and biotin (1 mg/L)] after 6 days. Knockout strains Δcpkk1, Δcpkk2, Δcpkk3 and wild‐type Δcpku80 are shown. Representative photographs from one of three experiments are shown.
Table 1.
Comparison of mitogen‐activated protein kinase kinase (MAP2K)‐null mutants, complemented strains and wild‐type isolate (Δcpku80). Reported values are means ± standard deviation. Data followed by the same letter are not significantly different according to Tukey's test (P < 0.05). Colony diameter was taken after 7 days of growth
| Characteristic | Δcpkk1 | Δcpkk2 | Δcpkk3 | Δcpku80 | Δcpkk1‐compl | Δcpkk2‐compl |
|---|---|---|---|---|---|---|
| Growth on PDAmb (cm) | 2.69 ± 0.23a | 1.95 ± 0,30b | 7.23 ± 0.41c | 7.42 ± 0.60c | 7.23 ± 0.35c | 7.59 ± 0.31c |
| Biomass dry weight (g) from liquid culture | 0.07 ± 0.01a | 0.27 ± 0.03b | 0.28 ± 0.01b | 0.28 ± 0.02b | 0.29 ± 0.04b | 0.27 ± 0.01b |
| Growth on PDAmb + 2 m sorbitol (cm) | 1.64 ± 0.14a | 1.21 ± 0.18b | No | 5.95 ± 0.30c | 5.77 ± 0.12c | 5.86 ± 0.16c |
| Growth on PDAmb + 4% NaCl (cm) | 0.75 ± 0.41a | 1.05 ± 0.11b | No | 2.00 ± 0.10c | 1.88 ± 0.14c | 1.97 ± 0.11c |
| Growth on PDAmb + 0.125 mm menadione (cm) | 0.76 ± 0.09a | 0.89 ± 0.13a | 2.33 ± 0.15b | 2.41 ± 0.10b | 2.18 ± 0.17b | 2.27 ± 0.20b |
| Growth on PDAmb + 0.005% SDS (cm) | No | 0.68 ± 0.10a | 2.86 ± 0.33b | 4.68 ± 0.34c | 4.24 ± 0.54c | 4.61 ± 0.27c |
| Growth on PDAmb + 100 mm H2O2 (cm) | No | 0.86 ± 0.12a | 5.19 ± 0.32b | 5.02 ± 0.23b | 4.95 ± 0.31b | 5.05 ± 0.27b |
| Asexual sporulation (conidiospores × 107/mL) | No | No | 2.91 ± 0.15a | 2.72 ± 0.25a | 2.34 ± 0.18a | 2.47 ± 0.11a |
| Stromal pustule production | No | No | Yes | Yes | Yes | Yes |
| Canker area on chestnut at 21 days post‐inoculation (cm2) | 0.27 ± 0.14a | 0.21 ± 0.06a | 2.91 ± 0.69b | 3.12 ± 0.58b | 2.98 ± 0.58b | 2.96 ± 0.54b |
PDAmb, potato dextrose agar (PDA) containing l‐methionine (100 mg/L) and biotin (1 mg/L); SDS, sodium dodecylsulphate.
The cpkk2‐null mutant strain also displayed a strong reduction in vegetative growth on malt agar substrate compared with growth on PDAmb. On the contrary, the other strains, including the control, were unaffected by malt agar (Fig. S2, see Supporting Information).
Changes in colour pigmentation were observed as a result of the loss of either cpkk1 or cpkk2 function. In comparison with Δcpku80, the Δcpkk1 strain appeared completely white, with extremely hyaline hyphae on the edge of the colony. In contrast, the cpkk2‐null mutant showed light yellowish mycelium. No colour alterations were observed in the Δcpkk3 mutant (Fig. 2).
Growth defects in the Δcpkk3 knockout strain were only noted under acute hyperosmotic conditions. Specifically, on PDAmb supplemented with 2 m sorbitol or 4% NaCl, the Δcpkk3 strain was completely unable to grow, whereas the other mutants were compromised to a lesser extent in their growth capabilities (Table 1, Figs S3 and S4, see Supporting Information).
Laccase‐type phenol oxidase activity was estimated by the colour of the reaction assay on plates containing tannic acid. A strong dark‐brown halo was detected around Δcpkk2 colonies, suggesting an increase in phenol oxidase activity (Fig. 3), but such variation was not confirmed in liquid culture containing tannic acids (not shown), linking a possible phenol oxidase up‐regulation only to growth on solid medium. No specific growth inhibition caused by tannic acid was observed, and no differences were detected between the wild‐type laccase phenotype and the other two MAP2K‐null mutants (data not shown).
Figure 3.
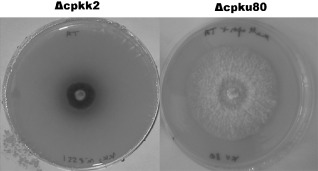
Polyphenol oxidase activity on Bavendamm's medium at 5 days post‐inoculation. The level of brown coloration is correlated with the phenol oxidase activity of the strain degrading tannic acid contained in the medium. A very strong dark‐brown halo was observed around the Δcpkk2 colony, whereas, in these specific experimental conditions, the colour intensity of the halo was much lower for the wild‐type strain Δcpku80.
In order to evaluate a putative involvement of the three MAP2Ks in temperature sensitivity, the diameter of each fungal colony was measured after 5 days of growth on PDAmb plates at 8, 12, 30 and 37 °C. The absence of growth was noted at the two extreme temperatures tested (8 and 37 °C) for all isolates, including the control, whereas significant differences were observed at 12 °C. More specifically, although all the isolates grew more slowly than normal, the Δcpkk1 mutant failed to grow at all (Fig. S5, see Supporting Information). At 30 °C, all strains were equally delayed in growth (data not shown).
Experiments to evaluate the responses to oxidative and detergent/surfactant stresses were also carried out. The MAP2K‐null mutants and the wild‐type isolate were grown on PDAmb supplemented with 0.125 mm menadione, 100 mm hydrogen peroxide (H2O2) or 0.005% sodium dodecylsulphate (SDS). Sensitivity to menadione was observed in the Δcpkk1 and Δcpkk2 strains, whereas absence of growth was detected for Δcpkk1 in the presence of SDS, which also partially influenced the growth of the other strains. Hydrogen peroxide proved to be extremely toxic for the cpkk1‐null mutant, as no growth was recorded; under the same conditions, growth of the Δcpkk2 strain was also strongly reduced (Table 1, Figs S6–S8, see Supporting Information). Sensitivity to growth on solid media containing calcofluor white (CFW) and Congo red was evaluated: the results (Fig. S9, see Supporting Information) showed a statistically significant specific inhibition of Δcpkk1 in both media, whereas specific inhibition of Δcpkk2 was only present in CFW‐containing medium.
Four different antifungal compounds commonly used for selection in C. parasitica were also tested at different concentrations in PDAmb medium: no difference among the various mutants and wild‐type was observed for benomyl and hygromycin B at the high inhibitory concentration tested (data not shown). Surprisingly, Δcpkk2 strains exhibited specific higher sensitivity to G418 (Fig. S10, see Supporting Information): the minimal concentration to completely inhibit growth of the wild‐type and Δcpkk1 and Δcpkk3 strains was 50 μg/mL, whereas a concentration of 3.75 μg/mL was sufficient to completely inhibit growth of Δcpkk2 strains; on the contrary, Δcpkk3 strains were fully resistant to inhibitory working concentrations of fludioxonil (Fig. S11, see Supporting Information).
Cell wall digestion assay
Resistance to cell wall‐degrading enzymes was tested on each of the C. parasitica MAP2K‐null mutants and the wild‐type isolate. Starting with the same amounts of young mycelia grown in liquid medium for 16 h in the dark, the number of spheroplasts produced after 2 h of digestion was measured. Only in the case of the Δcpkk1 strain was the number of spheroplasts significantly higher than for the other strains, indicating a strong sensitivity to digestion and a low level of cell wall resistance (Fig. 4A).
Figure 4.
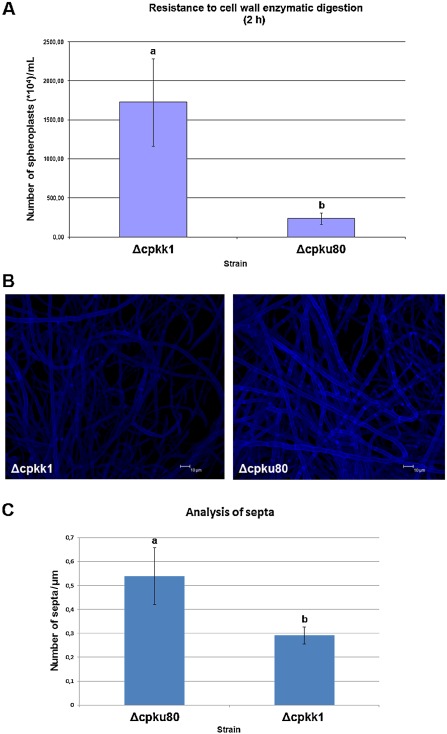
Characterization of the cpkk1‐null mutant strain. (A) Resistance to cell wall enzymatic digestion. The Δcpkk1 strain showed an extreme sensitivity to enzymatic digestion, resulting in a larger number of spheroplasts per millilitre, when compared with the Δcpku80 strain (1.7 × 107 ± 0.56 × 107 versus 0.24 × 107 ± 0.07 × 107). (B) Confocal micrographs of Δcpkk1 and Δcpku80 mycelia after calcofluor white staining. Hyphae of the cpkk1‐null mutant are thinner and less septated than those of the wild‐type. (C) Graphical representation of the number of septa per micrometre observed in the Δcpkk1 and Δcpku80 strains (0.29 ± 0.04 versus 0.54 ± 0.12). Data were analysed with one‐way analysis of variance (ANOVA), Tukey's test, P < 0.05.
Confocal microscopic observations of CFW‐stained hyphae and hyphal fusion during colony growth
After CFW staining, mycelia of the MAP2K‐null mutants were microscopically inspected and compared with those of Δcpku80. The Δcpkk1 strain showed thinner hyphae than the control (Fig. S12A, see Supporting Information), and the frequency of septa per length unit of mycelium was lower (Fig. 4B,C). The observed fluorescence was also lower in the Δcpkk1 strain (Fig. S12B). No differences were noted in the other MAP2K mutants (data not shown).
We also observed hyphal fusion events in the subperipheral region of a growing colony: fusions among different hyphae were commonly found within Δcpkk1, Δcpkk3 and Δcpku80 strains, but we failed to detect any fusion in Δcpkk2 strains (Fig. S13, see Supporting Information).
Mating experiments
MAP2K‐null mutants (derived from Δcpku80, a mating type Mat‐2 strain) were compared with the wild‐type strain (Δcpku80) with regard to the production of conidia (also serving as spermatia in mating experiments) and the ability to serve as the female parent in sexual crosses with the opposite mating type strain EP67 (Mat‐1). Asexual sporulation was evaluated after 1 month of growth on PDAmb (Table 1). Conidiation was completely absent for the Δcpkk1 and Δcpkk2 strains; thus, spermatization of strain EP67 was not possible. When inoculated on agar substrate adjacent to chestnut twigs as female parent, strains Δcpkk1 and Δcpkk2 were unable to colonize the bark tissue and erupt to produce stroma and stromal pustules; no mating products were observed after the addition of a conidial suspension from strain EP67 (Fig. 5). Taken together, these findings show that the developmental impairments in both the cpkk1 and cpkk2 knockout strains are such that it was impossible to evaluate their competence for mating, either as female or male. On the contrary, mature perithecia with protruding ostiolar necks were present in abundance after 3 months in the control crosses EP67 (♀ or ♂) × Δcpku80 (♂ or ♀) and for Δcpkk3 (♂ or ♀) (Fig. 5).
Figure 5.
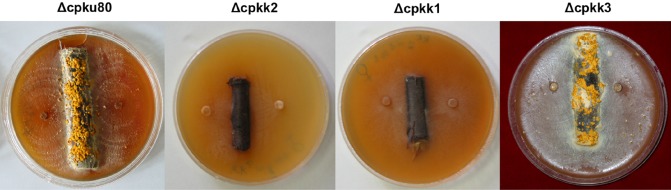
Stromal pustule formation on chestnut twigs. The Δcpkk1 and Δcpkk2 strains are unable to erupt from bark to produce stromal pustules. Photographs were taken 3 months after inoculation with each respective strain.
Deletion of cpkk1 and cpkk2 reduces C. parasitica virulence
Pathogenicity assays were performed to evaluate whether disruption of the MAP2K genes affected virulence. Wild‐type and MAP2K C. parasitica mutants were inoculated onto dormant European chestnut stems. At 21 days post‐inoculation, the canker diameter area was measured: deletion of cpkk1 or cpkk2 resulted in a dramatic reduction in virulence, as the mutants were unable to form any cankers even 8 weeks after inoculation. The pathogenic behaviour was not affected in the cpkk3 knockout strains, suggesting that Cpkk3 is dispensable for virulence (Table 1 and Fig. 6).
Figure 6.
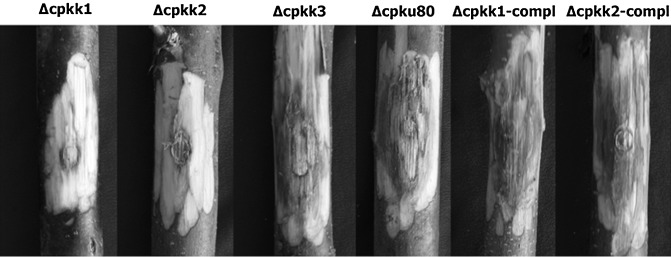
Virulence assay on chestnut stems inferred from the area of the cankers induced by each tested strain. Photographs were taken at 21 days post‐inoculation.
Complementation experiments
Considering the intriguing phenotypic changes in the cpkk1‐ and cpkk2‐null mutants of C. parasitica, complementation experiments were carried out. Vectors expressing each specific MAP2K wild‐type allele, and conferring resistance to G418, were assembled and used to transform the respective mutants. G418‐resistant transformants were then analysed for their morphology, growth on different media, asexual sporulation and virulence. PCR analyses confirmed that the complemented transformants contained an additional wild‐type allele of the specific MAP2K gene (Fig. S14, see Supporting Information). All altered functions were restored and the complemented strains were closely similar to the wild‐type Δcpku80, confirming unequivocally that the differential phenotypic characters of the null mutants were caused by their gene disruptions (Table 1 and Fig. 6).
Discussion
Three conserved MAPK pathways involved in the transduction of a variety of extracellular signals and the regulation of different developmental processes have been described in fungal pathogens (Hamel et al., 2012; Rispail et al., 2009; Zhao et al., 2007). The most studied and best characterized is the Fus3/Kss1 pathway, also known as the pheromone response pathway, because of its activation on binding of pheromone to its membrane receptor. This MAPK cascade regulates various phenomena of fungal biology, ranging from conidiation, gamete maturation, haploid fruiting (morphological switch from a budding yeast to hyphal growth), hyphal differentiation and, in a number of cases, virulence (Müller et al., 2003; Tscharke et al., 2003; Zhao et al., 2007).
The Pkc1‐Slt2 signalling pathway is a phosphorylation cascade regulating cell integrity and promoting cell wall biosynthesis. Impairment of this cascade results in perturbed growth, defects in cell wall integrity and the accumulation of secretory vesicles (Irie et al., 1993; Loewith et al., 2000; Mazzoni et al., 1993). In yeast, this MAPK pathway is also involved in responses to low osmolarity, high temperature, alkaline pH and nutrient starvation (Heinisch, 2005). Considering that the ability to maintain cell wall integrity and resistance is critical for the establishment of disease in a host, the Pkc1‐Slt2 MAPK cascade has also been found to play an important role in virulence, as demonstrated for several plant‐pathogenic fungi, including Claviceps purpurea, Fusarium graminearum, Botrytis cinerea and Colletotrichum lagenarium (Hou et al., 2002; Kojima et al., 2002; Mey et al., 2002; Rui and Hahn, 2007).
The third MAPK cascade, known as the high‐osmolarity glycerol (Hog) response pathway, is primarily required for growth under hyperosmotic conditions, but recent studies in yeast have also related it to resistance to non‐osmotic stresses, such as arsenite, low temperature and acid pH (Mollapour and Piper, 2006; Panadero et al., 2006; Thorsen et al., 2006). Moreover, it is involved in fungicide resistance in Magnaporthe oryzae and Neurospora crassa (Motoyama et al., 2008; Zhang et al., 2002), and in the virulence of several filamentous fungi (Hamel et al., 2012).
Several MAPKs and their downstream effectors have been characterized already in C. parasitica. In the pheromone response pathway, Fus3 and Ste11 homologues (encoded by cpmk2 and cpst11), the kinases activated by and activating MAP2K Cpkk2, respectively, have been analysed and addressed for possible role(s) in fungal development, pathogenesis and hypovirus infection (Choi et al., 2005; Park et al., 2012). Transcription factors, such as Ste12 and Pro1, activated by Cpmk2, and downstream target genes, such as Mf2/1 and Mf2/2, have also been studied to better define the molecular mechanisms triggered by or regulating the pheromone response (Deng et al., 2006; Sun et al., 2009; Turina et al., 2003; Zhang et al., 1993).
Concerning the high‐osmolarity growth cascade, only CpMK1, the Hog1 homologue activated by MAP2K Cpkk3, has been analysed (Park et al., 2004). In addition to osmosensitivity, disruption of the cpmk1 gene resulted in several pleiotropic hypovirulence‐associated changes, such as reduced pigmentation, conidiation, laccase production and virulence expression. Moreover, this MAPK seems to be regulated by hypovirus infection after hypertonic stress (Park et al., 2004).
In contrast, no elements of the cell integrity pathway have been investigated so far in C. parasitica, except Cpkk1, whose function was recently revealed in our laboratory using silencing techniques (Rostagno et al., 2010). Experiments demonstrated that Cpkk1 plays an important role in conidiation, growth on minimal medium and virulence. A previous study has shown that hypovirus infection does not regulate mRNA accumulation of the three MAP2Ks (Rostagno et al., 2009), but is able to induce hyperphosphorylation of Cpkk1, observed during active mycelial growth and juvenile status of the fungus (Turina et al., 2006). Apart from these studies, knowledge of this MAPK pathway in C. parasitica, especially during pathogen–plant interactions, is still limited.
In the present study, we have characterized the three MAP2K genes, cpkk1, cpkk2 and cpkk3, identified in the C. parasitica genome, which appear to be functional homologues of the corresponding MAP2K genes in yeast and filamentous fungi. Our functional analysis generally confirmed the assignment of each MAP2K to the expected signalling cascade described above—specifically, the cell integrity pathway for Cpkk1, the high‐osmolarity growth cascade for Cpkk3 and the pheromone response pathway for Cpkk2—although we could not demonstrate a direct role in mating for Cpkk2 because of major impairments in developmental processes not strictly related to mating, but necessary for mating to occur.
Cpkk1 and Cpkk2 are necessary for growth, sporulation, pathogenesis and developmental steps important for mating
Phenotypic analysis of the Δcpkk1 and Δcpkk2 strains revealed common vegetative growth defects.
The importance of MAPK cascades in fungal growth is universally recognized; therefore, it is evident that, in the absence of a correct signalling system, severe growth defects may result because of the fact that environmental conditions or internal stimuli are not well perceived and/or transduced (Lew, 2011). Nevertheless, in different fungi, the roles of MAPK homologues in vegetative growth may differ greatly, and the same MAPK deletion can give rise to diverse morphofunctional behaviour. For instance, in the basidiomycete Ustilago maydis, contrary to the situation in C. parasitica, no influence on cell morphology or growth was observed as a result of deletion of mkk1 (Carbó and Pérez‐Martín, 2010). In M. oryzae, mutants deleted for Mmk2 were defective in cell wall integrity, conidiation and virulence, but not in growth rate (Mehrabi et al., 2009). The PaMkk1 mutant of the model saprophytic filamentous fungus Podospora anserina showed no pigmentation, differentiation of aerial hyphae or fruiting body development, but growth was not affected (Kicka et al., 2006). In addition, no influence on growth was observed in the Mkk2 mutant of the human pathogen Cryptococcus neoformans (Gerik et al., 2005).
Concerning the pheromone pathway, the Fuz7 (a Cpkk2 homologue) gene of U. maydis, similar to the situation in C. parasitica, has been shown to be fundamental for growth and spore formation (Kahmann and Kamper, 2004). By contrast, Ste7 homologues are dispensable for vegetative growth in M. oryzae and F. graminearum (Ramamoorthy et al., 2007; Zhao et al., 2005).
The impossibility of performing complete mating experiments because of the absence of stromal pustule formation on chestnut wood and a lack of sporulation suggests that both Cpkk1 and Cpkk2 are required for essential developmental prerequisites to mating, which are not mating dependent. Thus, we cannot provide direct evidence for the likely role of Cpkk2 in mating.
Considering that C. parasitica is a phytopathogenic fungus, effects on virulence were of major interest. Neither the cpkk1‐ nor cpkk2‐null mutant was able to produce canker areas after artificial inoculation on chestnut stems. Among other possibilities, we envision three different scenarios to explain such behaviour: (i) the two serine/threonine kinases may influence directly the expression of genes involved in pathogenesis (thus, the absence of virulence would be a result of a direct impairment in the production of pathogenic effectors); (ii) the deletion of the MAP2K genes results in such strong destabilizing events for metabolism that even virulence is affected secondarily (therefore the fungus is avirulent, just as it was unable to mate, in response to the many defects that perturb its survival); (iii) Cpkk1 and Cpkk2 are involved, independently, in the recognition of the host plant or, in other words, in the transduction of a signal activated after the interaction with the plant; therefore, in the absence of either, the plant would not be perceived and the activation of the virulence machinery would not occur.
As reported above for filamentous growth, virulence may also be influenced in different ways by orthologous MAPKs in different fungal pathogens. Similar effects to those observed in C. parasitica were reported in U. maydis, in which the Fuz7‐null mutant is non‐pathogenic (Kahmann and Kamper, 2004). Ste7 orthologues also play a fundamental role in the virulence of M. oryzae and F. graminearum, (Ramamoorthy et al., 2007; Zhao et al., 2005).
Considering the only MAPK pathway completely characterized in C. parasitica—the mating response pathway—Δcpmk2, Δcpkk2 and Δcpste11 share some commonalities in their phenotype, but have three different vegetative and pathogenic behaviours. The Δcpste11 isolate showed no significant morphological changes, except for enhanced sporulation, and is still pathogenic, even if less than the wild‐type (Park et al., 2012). On the contrary, the Δcpmk2 strain is unable to sporulate, like the Δcpkk2 mutant, and displays reduced growth on both solid and liquid media, but is partially virulent on chestnut (Choi et al., 2005). As the Δcpkk2 strain is totally apathogenic, it is likely that Cpkk2 has additional downstream targets in addition to Cpmk2 that contribute to virulence. The knockout of one of the transcription factors (CpST12) controlled by the pheromone response cascade did not lead to impaired growth on PDA medium, but produced abundant conidia (both features strongly impaired in our Δcpkk2 mutant); however, like the latter, there was impairment of canker production on chestnut stems, but the production of stromal pustules on autoclaved chestnut twigs was normal (Deng et al., 2006).
Cpkk1 is essential for correct hyphal development and cell wall structure
The Δcpkk1 strain displayed no virulence and lacked aerial mycelium; such features may be a direct consequence of the morphological problems detected through evaluation of the mycelia by confocal microscopy. More specifically, thinner hyphae, reduced frequency of septa per length unit of mycelium, thinner cell walls and hypersensitivity to enzymatic digestion were all recorded in comparison with the control Δcpku80. Moreover, reduced fluorescence after CFW staining was observed. Considering that CFW is a strong non‐specific fluorochrome that binds to cellulose and chitin, a fainter signal may imply a lower or altered biosynthesis of cell wall components. Growth itself in CFW‐ and Congo red‐containing media was impaired. These data confirmed that Cpkk1, as part of the cell integrity pathway, plays a fundamental role in the regulation of cell wall properties, such as mechanical resistance, composition, structure and susceptibility to chemical compounds, such as surfactant agents or oxidants. The observed hypersensitivity to SDS, which disrupts plasma membranes and lyses cells with cell wall defects, and to menadione or H2O2, which have been used extensively to study cell integrity phenotypes in fungi (Carbó and Pérez‐Martín, 2010; Nikolaou et al., 2009; Rui and Hahn, 2007), may be a consequence of the abnormal cell wall composition and thickness. Mutants in the cell integrity MAPK cascade in M. oryzae, Claviceps purpurea, Colletotrichum lagenarium, B. cinerea and F. graminearum all show similar effects to those observed in the C. parasitica Δcpkk1 strain (Hou et al., 2002; Kojima et al., 2002; Mey et al., 2002; Rui and Hahn, 2007; Xu et al., 1998), but septal frequency is correlated to a functional Cpkk1 for the first time.
Cpkk1 is required for growth at low temperature
Interestingly, after a temperature growth assay, growth of the cpkk1‐null mutant was completely abolished at 12 °C, in comparison with other strains in which growth was only partially reduced. This result suggests that the cell integrity MAPK pathway may transduce signals derived from the perception of low temperature. Currently, we do not know exactly how the stimulus is perceived, but it is likely that low‐temperature sensing receptors may be involved in the activation of the cascade. A similar outcome has been reported for the Hog response pathway in yeast (Panadero et al., 2006), whereas the Pkc1‐Slt2 pathway seems to play a role in the response to high temperature (Heinisch, 2005). These differences clearly illustrate that, although the components of the MAPK cascade are homologous in different organisms, the biological functions controlled by them may be quite dissimilar.
Hard surface perception, medium composition sensing and hyphal fusions are regulated by Cpkk2
Loss of function of Cpkk2 was responsible for the reduced growth of the Δcpkk2 mutant on solid substrates. Nevertheless, the vegetative growth rate in liquid medium was not affected. This finding suggests that Cpkk2 (but not Cpkk1) may be involved in the sensing of hard surfaces. A similar phenotype has already been reported for the Δcpmk2 mutant (Choi et al., 2005). Ste‐7 homologues are also involved in surface sensing in M. oryzae, F. oxysporum and U. maydis (Lanver et al., 2010; Liu et al., 2011; Pérez‐Nadales and Di Pietro, 2011). How the pheromone response MAPK cascade is activated by the hard surface stimulus is still unknown. It has been proposed that the transmembrane protein Msb2 may play a ‘sensor’ role in surface perception upstream of the MAPK pathway.
Cpkk2 seems to be involved in some way in the growth response to substrate composition. On malt agar plates, growth of the cpkk2‐null mutant was strongly affected in comparison with growth on PDAmb and with the effects produced in the other two knockout strains. This finding indicates that nutrients are perceived in a different manner by Δcpkk2 and the other two MAP2K mutants. Among the possible explanations, we can envision either a direct toxic effect of the maltose medium or a lack of ability to use maltose as a carbon source specific to the Δcpkk2 strain. To our knowledge, this is the first evidence of direct involvement of a pheromone response cascade element in nutrient substrate sensing in filamentous fungi.
A further characterization of the Δcpkk2 mutants revealed increased sensitivity towards the antibiotic G418 sulphate (Geneticin) when compared with the wild‐type and other MAP2K knockout strains. Such a phenotype may be related to decreased detoxification in the mutant or a higher specific sensitivity during protein translation in the specific steps affected by G418. Regulation of translation was shown to be dependent on a MAPK cascade orthologous to Cpkk2 in mammals: MNK1—a MAPK‐activated protein kinase—phosphorylates directly the protein eIF4E (Cargnello and Roux, 2011), but the biological significance of such a phosphorylation event is still debated; the MNK1 catalytic domain belongs to the CAMK family of kinases and orthologous sequences are present in the C. parasitica genome, but, so far, they remain uncharacterized.
An additional feature of cpkk2‐null mutants has been noted by microscopic analysis: hyphae are not able to carry out vegetative self‐fusion. This result has been reported previously in filamentous fungi, in which elements of the pheromone response MAPK cascade or homologues have been deleted (Pandey et al., 2004; Prados Rosales and Di Pietro, 2008). Vegetative hyphal fusion is a ubiquitous phenomenon during the growth of filamentous fungi: a model system for studying its genetic regulation is N. crassa (Fleissner et al., 2009); the biological role of self‐fusion seems to be related to general homeostasis, translocation of nutrients and water, developmental processes and non‐meiotic gene transfer within the fungal colony (Read et al., 2009). The pheromone signaling pathway has been suggested to play a role in the transduction of all of these intrahyphal communication events (Fleissner et al., 2009).
Tannin degradation may be controlled by Cpkk2 on solid media
Cpkk2 may be involved in the control of the phenol oxidase activity of C. parasitica. Despite the slight growth inhibition on malt agar plates containing tannic acid, a strong dark brown colour was detected around colonies of the cpkk2‐null mutant. Browning of the medium is the result of the breakdown of tannic acid by the phenol oxidase activities of extracellular fungal enzymes. Tannic acid is a toxic compound that may resemble either a breakdown product of lignin or an antimicrobial agent secreted by the host plant. As part of their defence barrier, tannic acid is abundant in plants; thus, for phytopathogenic fungi, extracellular phenol oxidase activity most probably represents a response to eliminate this compound. The strong brown halo observed around Δcpkk2 colonies may be caused by an alteration in laccase pattern expression: possibly, the absence of the pheromone response MAP2K leads to higher phenol oxidase activity.
Roles of Cpkk3 in C. parasitica
Cpkk3 is the putative homologue of S. cerevisiae Pbs2. In the present study, we observed phenotypic growth defects of the cpkk3‐null mutant only under hyperosmotic conditions, confirming that it belongs functionally to the high‐osmolarity growth response MAPK cascade. Furthermore, as cpkk3‐null mutants showed high resistance to the phenylpyrrole compound fludioxonil, it is probable that, as observed in N. crassa and M. oryzae (Motoyama et al., 2008; Noguchi et al., 2007), Cpkk3 may be positioned downstream of a histidine kinase involved in sensitivity to fludioxonil.
Unlike the observations in Cpkk1 and Cpkk2, Cpkk3 does not affect vegetative growth and is dispensable for conidiation, mating, resistance to oxidative stress or heat shock, eruption from bark, dead wood colonization and virulence. The deletion of cpmk1, which encodes the Hog1 homologue, putatively acting downstream of Cpkk3, produced different alterations from those observed in the cpkk3‐null mutant, such as reduced pigmentation, conidiation, laccase production and virulence (Park et al., 2004). These data suggest that Cpmk1 can either control several functions or take part in different pathways (resulting in the activation of different sets of genes) which are not influenced by the upstream MAP2K Cpkk3; alternatively, in the absence of Cpkk3, other kinases can partially complement its function. Therefore, we might speculate that Cpmk1 can be activated by various signals through different kinases and, in the absence of Cpkk3, only the ‘transduction pathway’ responding to high osmolarity is affected.
The opposite situation from C. parasitica was reported in B. cinerea and Colletotrichum lagenarium, in which the Pbs2 homologue is very important, not only for the osmolarity response, but also in pathogenesis, as these mutants failed to cause disease in host plants (Kojima et al., 2002; Yan et al., 2010), whereas Hog1 deletion strains were fully virulent (Kojima et al., 2006; Segmuller et al., 2007). In these cases, the MAP2K Pbs2 can control functions in which the downstream MAPK is not involved. This implies that, in such fungi, additional stimuli (for example, the presence of the host plant which induces the pathogenic response) can activate Pbs2.
A further case was reported for the human pathogens Candida albicans and Cryptococcus neoformans, in which both the Hog1 and Pbs2 homologues are necessary for virulence (Alonso‐Monge et al., 2006; Kojima et al., 2006).
All of these studies suggest that osmolarity‐responsive MAPKs display different involvements and levels of importance in different fungal species.
The results presented here highlight portions of the complex cellular networks occurring in C. parasitica in response to environmental stimuli; additional studies specifically aimed at elucidating the activation of each MAPK module or the involvement of MAPK proteins in hypovirus interaction are still required to better characterize the complex interplay at the base of virulence and virus‐induced hypovirulence phenomena.
Experimental Procedures
Fungal strains and growth conditions
All chemicals and solvents were purchased from Sigma‐Aldrich (St. Louis, MO, USA), unless otherwise stated.
Cryphonectria parasitica strains used in the present study were maintained on PDAmb and kept at 6 °C. For long storage, strains were conserved under 15% glycerol at −80 °C. Evaluation of vegetative growth was performed in Petri plates on PDAmb at 25 °C under a constant level of light (Kim et al., 1995). Growth inhibition and resistance to stresses were tested on PDAmb supplemented with SDS, sorbitol, NaCl, H2O2, menadione, G418 sulphate or fludioxonil (see Figs S3, S4, S6, S7, S8, S10 and S11 for the concentrations used). Radial growth was assessed by measuring the diameters of colonies after 7 days. Liquid mycelial cultures were grown in 500 mL of EP complete medium for 3 days with shaking at 200 rpm (Puhalla and Anagnostakis, 1971). The primary inoculum was prepared as described previously (Kim et al., 1995). To determine biomass, 100 mL of liquid cultures were filtered through Miracloth (Calbiochem‐Merk4biosciences, Darmstad, Germany), washed with sterile water and lyophilized for 24 h to measure the dry weight. Each experiment was repeated three times, and three biological replicates were included in each experiment. All statistical analyses were performed using one‐way analysis of variance (ANOVA) with Systat software (Cranes Software International Ltd, Bangalore, Karnataka, India). The significance of the differences was assessed with Tukey's test (P < 0.05).
Microscopy
Confocal observations of hyphae were made on mycelia grown in liquid medium, washed once with 0.6 m MgSO4 and stained with CFW. Photographs were taken with a Leica TCS‐SP2 microscope (Leica Microsystems, Wetzlar, Germany). Calcofluor fluorescence was excited with the 405‐nm line of a mercury lamp and recorded at 420–440 nm. For light microscopy, mycelium was grown on PDAmb for 2 days and observed using a bright field.
Construction of knockout strains
A hygromycin B resistance cassette (hph) was used to replace the cpkk1, cpkk2 and cpkk3 genes in C. parasitica. The hph cassette was obtained from the plasmid pCB1004 (Carroll et al., 1994) after digestion with HpaI, and introduced into a fragment of the coding sequence of each MAP2K amplified by PCR. Primers were designed on the basis of known MAP2K sequences (Rostagno et al., 2010; Turina et al., 2006) (Table S1, see Supporting Information). Briefly, MAP2K amplified fragments were cloned into the TOPO TA Vector (Invitrogen Life Technology, Carlsbad, CA, USA), digested with a specific single‐site‐cutting enzyme (NruI for cpkk1, SnaBI for cpkk2 and BsaBI for cpkk3) and fused with the 1.4‐kb hph cassette. The resulting plasmids were linearized in a unique site flanking the MAP2K gene fragment prior to transformation (BamHI was employed for pΔCpkk1, SmaI for pΔCpkk2 and EcoRV for pΔCpkk3), and used to transform the Δcpku80 strain (Lan et al., 2008). Spheroplast preparation and transformation were performed as described previously (Churchill et al., 1990). Transformants were selected from agar plates supplemented with 150 mg/L hygromycin B (Duchefa, Haarlem, The Netherlands). Initial screens were carried out by PCR with specific hph inner primers and MAP2K primers designed outside the region used for the knockout vector. After obtaining nuclear homogeneity and stability through single‐spore isolation, Southern blot analysis was carried out as described previously (Turina et al., 2003).
Complementation of cpkk1‐ and cpkk2‐null mutant strains
As the C. parasitica genome has been released and can be accessed at http://genome.jgi‐psf.org/Crypa2/Crypa2.home.html, DNA copies of the full‐length cpkk1 and cpkk2 genes (the entire protein coding sequence plus approximately 800 and 300 bp upstream and downstream of the mRNA of the gene) were amplified by PCR using the primers cpkk1‐complF/cpkk1‐complR and cpkk2‐complF/cpkk2‐complR (Table S1). Fragments were cloned into pGEMT vector (Promega, Madison, WI, USA) to obtain pCpkk1 and pCpkk2. A Geneticin (G418) resistance cassette of 1.2 kb, consisting of the Escherichia coli neomycin phosphotransferase gene (NptII), under the control of the Aspergillus nidulans tryptophan synthase gene promoter (TrpC), was inserted into each vector (in the EcoRV site in pCpkk1 and the EcoRI site in pCpkk2). Briefly, the cassette was constructed as follows: NptII and TrpC were amplified from the plasmids TOPO TA Vector and pCB1004, respectively, using the primers NptIIF/NptIIR and TrpCF/TrpCR (Table S1). Subsequently, the TrpC promoter was cloned in the appropriate orientation at the 5′ end of the NptII gene in a SmaI site of a pBSK plasmid (Stratagene, La Jolla, CA, USA). The pCpkk1 and pCpkk2 plasmids were then used to transform Δcpkk1 and Δcpkk2 mutant spheroplasts after linearization with PstI (pCpkk1) or EcoRV (pCpkk2). The selected transformants were subjected to single‐spore purification using G418 (20 μg/mL) as marker and further verified by PCR analyses to confirm both the presence of the respective wild‐type allele and the original deleted allele, in order to exclude external contamination.
DNA manipulations
Genomic DNA from C. parasitica strains was extracted using the method described by Turina et al. (2003). Southern blot analyses were performed according to standard protocols (Sambrook et al., 1989). One microgram of DNA was digested with appropriate restriction enzymes (SacI for cpkk1, BamHI for cpkk2 and SmaI in case of cpkk3), blotted onto a Hybond N+ nylon membrane (GE Healthcare, Uppsala, Sweden) after separation and hybridized with radioactively labelled specific probes synthesized as described previously (Rastgou et al., 2009) using the T7 RNA polymerase.
Protein analysis and laccase activity assay
Total proteins of the selected strains were extracted in buffer A after 24 h as described by Turina et al. (2006). Western blot analyses were carried out as detailed previously using polyclonal anti‐cpkk1 and anti‐cpkk2 primary antibodies (Turina et al., 2006).
Laccase activity was gauged by growing fungal strains on Bavendamm's medium (0.05% tannic acid, 1.5% malt extract, 2.0% agar) and assessing the resultant darkening of the medium after 5 days (Rigling et al., 1989). Furthermore, the same test was carried out in liquid medium containing 1.5% malt extract or EP minimal medium (Puhalla and Anagnostakis, 1971) containing 0.05% tannic acid.
Spheroplast cell wall resistance evaluation
To test the susceptibility of the Δcpkk1, Δcpkk2 and Δcpkk3 strains to cell wall‐degrading enzymes, fungal mycelium was cultured in 200 mL of EP complete liquid medium in the total absence of light at 120 rpm for 23 h. One milligram of harvested mycelium was washed once with 0.6 m MgSO4, transferred into a flask with 30 mL of osmotically stabilized protoplasting solution containing β‐glucuronidase and lytic enzyme from Trichoderma harzianum, and incubated at 26 °C under slow shaking (Churchill et al., 1990). After 2 h, the concentration of spheroplasts was determined with a haemocytometer. The Δcpku80 strain was used as a control.
Analysis of septa
In order to evaluate the frequency of septa per micrometre of mycelium, five photographs taken by confocal microscopy from different positions in the fungal colonies were analysed. Three hyphae of different lengths for each photograph were considered for the septal count. Frequency data were obtained by dividing the number of septa by the hyphal length. For each MAP2K mutant and the Δcpku80 strain, three different colonies from different experimental plates were subjected to analysis.
Male and female fertility analysis
To test the sexual fertility of MAP2K‐null mutants, mating experiments were carried out. Mating was performed on autoclaved twigs of chestnut tree embedded in 2% water agar as described previously (Anagnostakis, 1979). Mutant and complemented (mating type Mat‐2) strains were tested as both male and female in crosses with strain EP67 (mating type Mat‐1). Reciprocal Δcpku80 × EP67 crosses served as controls. After spermatization with conidia from the male parent, plates were incubated at 25 °C until perithecia could be observed in the stroma.
Conidiation was evaluated after 1 month of growth on PDAmb plates under standard conditions at 25 °C. Conidia from each plate were harvested with 10 mL of sterile water and their concentrations were determined using a haemocytometer.
Virulence assay
Virulence assays were performed on stems of European chestnut tree (Castanea sativa) with four replicates per fungal strain as described previously (Rostagno et al., 2010). Inoculated stems were kept at 24 °C under normal light covered with plastic bags to maintain moisture. Three independent experiments were carried out.
Supporting information
Fig. S1 Restriction maps and Southern blot analyses of mitogen‐activated protein kinase kinase (MAP2K)‐null mutants and Δcpku80 strain (used as wild‐type). (A) Gene cpkk1. (B) Gene cpkk2. (C) Gene cpkk3. Gene disruption was generated for each MAP2K through the insertion of a 1.4‐kb hygromycin cassette (Hyg) in the coding sequence. Total fungal DNA from the Δcpkk1, Δcpkk2 and Δcpkk3 mutants was digested with SacI, BamHI and SmaI, respectively. The digested fragments were separated on a 1% agarose gel by electrophoresis, and hybridized with 32P‐labelled specific probes indicated in the restriction maps: each probe detected a digested fragment that differed in size compared with the wild‐type, indicating that transformants underwent the desired replacement at the MAP2K site through double homologous recombination.
Fig. S2 Comparison of mitogen‐activated protein kinase kinase (MAP2K)‐null mutant colony morphology after 7 days of growth on PDAmb [potato dextrose agar (PDA) containing l‐methionine (100 mg/L) and biotin (1 mg/L)] (left) versus malt agar plates (right). (A) Strain Δcpkk1. (B) Strain Δcpkk2. (C) Strain Δcpkk3. (D) Strain Δcpku80. Medium composition significantly affected the growth of the cpkk2‐null mutant.
Fig. S3 Colony morphology of mitogen‐activated protein kinase kinase (MAP2K)‐null mutants grown for 7 days on PDAmb [potato dextrose agar (PDA) containing l‐methionine (100 mg/L) and biotin (1 mg/L)] supplemented with 2 m sorbitol: no growth was observed in the cpkk3‐null mutant and a strong reduction in growth was reported for the other strains.
Fig. S4 Growth of mitogen‐activated protein kinase kinase (MAP2K)‐null mutants after 7 days on PDAmb [potato dextrose agar (PDA) containing l‐methionine (100 mg/L) and biotin (1 mg/L)] supplemented with different concentrations of NaCl. The cpkk3‐null mutant displayed the strongest impairment in growth with increasing NaCl concentration in the medium.
Fig. S5 Growth experiment at different temperatures. (A) One‐way analysis of variance (ANOVA) of mitogen‐activated protein kinase kinase (MAP2K)‐null mutant growth compared with the wild‐type strain at 12 °C (Tukey's test, P < 0.05). Fungal strains were grown on PDAmb [potato dextrose agar (PDA) containing l‐methionine (100 mg/L) and biotin (1 mg/L)] for 5 days. The Δcpkk1 strain could not grow at 12 °C. Results from three different experiments are shown: Δcpkk1, 0 cm; Δcpkk2, 0.60 ± 0 cm; Δcpkk3, 1.87 ± 0.17 cm; Δcpku80, 1.95 ± 0.12 cm. (B) Growth at 25 °C (Tukey's test, P < 0.05). Fungal strains were grown on PDAmb for 5 days. Results from three different experiments are shown: Δcpkk1, 2.08 ± 0.16 cm; Δcpkk2, 1.38 ± 0.13 cm; Δcpkk3, 6.32 ± 0.22 cm; Δcpku80, 6.80 ± 0.41 cm.
Fig. S6 Colony morphology of wild‐type (Δcpku80) and mitogen‐activated protein kinase kinase (MAP2K)‐null mutants grown for 7 days on PDAmb [potato dextrose agar (PDA) containing l‐methionine (100 mg/L) and biotin (1 mg/L)] supplemented with 0.125 mm menadione. cpkk1‐ and cpkk2‐null mutants displayed a strong sensitivity to the oxidative stress induced by menadione.
Fig. S7 Colony morphology of wild‐type (Δcpku80) and mitogen‐activated protein kinase kinase (MAP2K)‐null mutants grown for 7 days on PDAmb [potato dextrose agar (PDA) containing l‐methionine (100 mg/L) and biotin (1 mg/L)] supplemented with 0.005% sodium dodecylsulphate (SDS). Reduction of growth was reported for all strains, except the Δcpkk1 isolate, in which the absence of growth was observed.
Fig. S8 Colony morphology of wild‐type (Δcpku80) and mitogen‐activated protein kinase kinase (MAP2K)‐null mutants grown for 7 days on PDAmb [potato dextrose agar (PDA) containing l‐methionine (100 mg/L) and biotin (1 mg/L)] supplemented with 100 mm H2O2. All strains, including the wild‐type, were affected, but only the cpkk1‐null mutant displayed absence of growth under the same conditions.
Fig. S9 Wild‐type (Δcpku80) and mitogen‐activated protein kinase kinase (MAP2K)‐null mutants growth in PDAmb [potato dextrose agar (PDA) containing l‐methionine (100 mg/L) and biotin (1 mg/L)] containing Congo red (1 mg/mL) and calcofluor white (0.5 mg/mL). The y axis represents the average of the difference between the diameter on PDAmb and the diameter on Congo red‐ and calcofluor white‐containing media. Error bars represent the standard deviation. Red type: t‐test values for two‐sample comparisons (each knockout strain compared with the wild‐type). Asterisks represent a statistically significant difference when compared with the wild‐type strain.
Fig. S10 Wild‐type (Δcpku80) and mitogen‐activated protein kinase kinase (MAP2K)‐null mutants growth in PDAmb [potato dextrose agar (PDA) containing l‐methionine (100 mg/L) and biotin (1 mg/L)] containing different concentrations of G418 (Geneticin) antibiotic. Four representative concentrations are shown to illustrate the sensitivity of Δcpkk2 to the presence of Geneticin in the medium.
Fig. S11 Wild‐type (Δcpku80) and mitogen‐activated protein kinase kinase (MAP2K)‐null mutants growth on PDAmb [potato dextrose agar (PDA) containing l‐methionine (100 mg/L) and biotin (1 mg/L)] supplemented with fludioxonil. Concentrations of 100, 50, 25, 12.5 and 6.25 mg/L were tested: at 100 mg/L, only the Δcpkk3 strain displayed normal growth, whereas, at the same concentration, the growth of all the other strains was fully inhibited.
Fig. S12 cpkk1‐null mutants show lower diameter hyphae and lower fluorescence on calcofluor staining of cell walls compared with Δcpku80. Quantification of the cell diameter and calcofluor fluorescence intensity was performed using ImageJ software (National Institutes of Health, Bethesda, MD, USA). In both cases, the numbers are relative to measurements taken for strain Δcpku80, arbitrarily given the value of 100. For each strain, the diameters of 100 hyphae were measured on five different slides.
Fig. S13 Representative photographs of Δcpkk2 and Δcpku80 hyphal fusions. Fungal strains were inoculated on PDAmb [potato dextrose agar (PDA) containing l‐methionine (100 mg/L) and biotin (1 mg/L)] and observed after 2 days by light microscopy. Several fusion events were observed in Δcpku80 mycelium (white arrowheads), but no hyphal fusion was detected in Δcpkk2 colonies. Lack of hyphal fusion was evident at the hyphal contact points (asterisks). Bars, 20 μm.
Fig. S14 Agar gel electrophoresis of polymerase chain reaction (PCR) products obtained using DNA templates from knockout and complemented strains. Oligonucleotides were designed immediately upstream and downstream of the hygromycin resistance gene insertion point in each of the two genes. Oligonucleotides were: for Δcpkk1, Cpkk1‐screenF (5′‐GCCGAGGGTGTCCTGCAGGG‐3′) and Cpkk1‐screenRev (5′‐GCACAACAGGATATTCGAGG‐3′); for Δcpkk2, Cpkk2‐korealF (5′‐GGAGGATATGATTCAGAAGTGTCTCTT‐3′) and Cpkk2‐korealRev (5′‐ACGCTGGCCTGTATCTGGTG‐3′). The lower band in both gels represents the c. 100‐bp fragment amplified from the locus without insertion, whereas the higher band running with the 1.5‐kbp fragment represents the locus with the hygromycin resistance gene insertion.
Table S1 Oligonucleotide primers used in this study.
Acknowledgements
The first author was supported by a postdoctoral fellowship partially co‐financed by Regione Piemonte.
References
- Alonso‐Monge, R. , Roman, E. , Nombela, C. and Pla, J. (2006) The MAP kinase signal transduction network in Candida albicans . Microbiology, 152, 905–912. [DOI] [PubMed] [Google Scholar]
- Anagnostakis, S.L. (1979) Sexual reproduction of Endothia parasitica in the laboratory. Mycologia, 71, 213–215. [Google Scholar]
- Bhalla, U.S. , Ram, P.T. and Iyengar, R. (2002) MAP kinase phosphatase as a locus of flexibility in a mitogen‐activated protein kinase signaling network. Science, 297, 1018–1023. [DOI] [PubMed] [Google Scholar]
- Carbó, N. and Pérez‐Martín, J. (2010) Activation of the cell wall integrity pathway promotes escape from G2 in the fungus Ustilago maydis . PLoS Genet. 6, e1001009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cargnello, M. and Roux, P.P. (2011) Activation and function of the MAPKs and their substrates, the MAPK‐activated protein kinases. Microbiol. Mol. Biol. Rev. 75, 50–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll, A. , Sweigard, J. and Valent, B. (1994) Improved vectors for selecting resistance to hygromycin. Fungal Genet. Newsl. 41, 22. [Google Scholar]
- Choi, E.S. , Chung, H.J. , Kim, M.J. , Park, S.M. , Cha, B.J. , Yang, M.S. and Kim, D.H. (2005) Characterization of the ERK homologue CpMK2 from the chestnut blight fungus Cryphonectria parasitica . Microbiology, 151, 1349–1358. [DOI] [PubMed] [Google Scholar]
- Churchill, A.C.L. , Ciuffetti, L.M. , Hansen, D.R. , Van Etten, H.D. and Van Alfen, N.K. (1990) Transformation of the fungal pathogen Cryphonectria parasitica with a variety of heterologous plasmids. Curr. Genet. 17, 25–31. [Google Scholar]
- Dawe, A.L. and Nuss, D.L. (2013) Hypovirus molecular biology: from Koch's postulates to host self‐recognition genes that restrict virus transmission. Adv. Virus Res. 86, 109–147. [DOI] [PubMed] [Google Scholar]
- Deng, F. , Allen, T.D. and Nuss, D.L. (2006) Ste12 transcription factor homologue CpST12 is down‐regulated by hypovirus infection and required for virulence and female fertility of the chestnut blight fungus Cryphonectria parasitica . Eukaryot. Cell, 6, 235–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleissner, A. , Leeder, A.C. , Roca, M.G. , Read, N.D. and Glass, N.L. (2009) Oscillatory recruitment of signaling proteins to cell tips promotes coordinated behavior during cell fusion. Proc. Natl. Acad. Sci. USA, 106, 19 393–19 398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerik, K.J. , Donlin, M.J. , Soto, C.E. , Banks, A.M. , Banks, I.R. , Maligie, M.A. , Selitrennikoff, C.P. and Lodge, J.K. (2005) Cell wall integrity is dependent on the PKC1 signal transduction pathway in Cryptococcus neoformans . Mol. Microbiol. 58, 393–408. [DOI] [PubMed] [Google Scholar]
- Hamel, L.P. , Nicole, M.C. , Duplessis, S. and Ellis, B.E. (2012) Mitogen‐activated protein kinase signaling in plant‐interacting fungi: distinct messages from conserved messengers. Plant Cell, 24, 1327–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinisch, J.J. (2005) Baker's yeast as a tool for the development of antifungal kinase inhibitors targeting protein kinase C and the cell integrity pathway. Biochim. Biophys. Acta, 1754, 171–182. [DOI] [PubMed] [Google Scholar]
- Hou, Z. , Xue, C. , Peng, Y. , Katan, T. , Kistler, H.C. and Xu, J.R. (2002) A mitogen‐activated protein kinase gene (MGV1) in Fusarium graminearum is required for female fertility, heterokaryon formation, and plant infection. Mol. Plant–Microbe Interact. 15, 1119–1127. [DOI] [PubMed] [Google Scholar]
- Imajo, M. , Tsuchiya, Y. and Nishida, E. (2006) Regulatory mechanisms and functions of MAP kinase signaling pathways. IUBMB Life, 58, 312–317. [DOI] [PubMed] [Google Scholar]
- Irie, K. , Takase, M. , Lee, K.S. , Levin, D.E. , Araki, H. , Matsumoto, K. and Oshima, Y. (1993) MKK1 and MKK2, which encode Saccharomyces cerevisiae mitogen‐activated protein kinase‐kinase homologs, function in the pathway mediated by protein kinase C. Mol. Cell. Biol. 13, 3076–3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahmann, R. and Kamper, J. (2004) Ustilago maydis: how its biology relates to pathogenic development. New Phytol. 164, 31–42. [DOI] [PubMed] [Google Scholar]
- Kicka, S. , Bonnet, C. , Sobering, A.K. , Ganesan, L.P. and Silar, P. (2006) A mitotically inheritable unit containing a MAP kinase module. Proc. Natl. Acad. Sci. USA, 103, 13 445–13 450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, D.H. , Rigling, D. , Zhang, L. and Van Alfen, N.K. (1995) A new extracellular laccase of Cryphonectria parasitica is revealed by deletion of Lac1. Mol. Plant–Microbe Interact. 8, 259–266. [Google Scholar]
- Kojima, K. , Kikuchi, T. , Takano, Y. , Oshiro, E. and Okuno, T. (2002) The mitogen‐activated protein kinase gene MAF1 is essential for the early differentiation phase of appressorium formation in Colletotrichum lagenarium . Mol. Plant–Microbe Interact. 15, 1268–1276. [DOI] [PubMed] [Google Scholar]
- Kojima, K. , Bahn, Y.S. and Heitman, J. (2006) Calcineurin, Mpk1 and Hog1 MAPK pathways independently control fludioxonil antifungal sensitivity in Cryptococcus neoformans . Microbiology, 152, 591–604. [DOI] [PubMed] [Google Scholar]
- Lan, X. , Yao, Z. , Zhou, Y. , Shang, J. , Lin, H. , Nuss, D.L. and Chen, B. (2008) Deletion of the cpku80 gene in the chestnut blight fungus, Cryphonectria parasitica, enhances gene disruption efficiency. Curr. Genet. 53, 59–66. [DOI] [PubMed] [Google Scholar]
- Lanver, D. , Mendoza‐Mendoza, A. , Brachmann, A. and Kahmann, R. (2010) Sho1 and Msb2‐related proteins regulate appressorium development in the smut fungus Ustilago maydis . Plant Cell, 22, 2085–2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lew, R.R. (2011) How does a hypha grow? The biophysics of pressurized growth in fungi. Nat. Rev. Microbiol. 9, 509–518. [DOI] [PubMed] [Google Scholar]
- Liu, W. , Zhou, X. , Li, G. , Li, L. , Kong, L. , Wang, C. , Zhang, H. and Xu, J.R. (2011) Multiple plant surface signals are sensed by different mechanisms in the rice blast fungus for appressorium formation. PLoS Pathog. 7, e1001261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewith, R. , Hubberstey, A. and Young, D. (2000) Skh1, the MEK component of the mkh1 signaling pathway in Schizosaccharomyces pombe . J. Cell Sci. 113, 153–160. [DOI] [PubMed] [Google Scholar]
- Mazzoni, C. , Zarzov, P. , Rambourg, A. and Mann, C. (1993) The SLT2 (MPK1) MAP kinase homolog is involved in polarized cell growth in Saccharomyces cerevisiae . J. Cell Biol. 123, 1821–1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehrabi, R. , Zhao, X. , Kim, Y. and Xu, J.R. (2009) The cAMP signaling and MAP kinase pathways in plant pathogenic fungi In: The Mycota, Vol. 5 Part 2 (Esser K., ed.), pp. 157–172. Berlin, Heidelberg: Springer‐Verlag. [Google Scholar]
- Mey, G. , Held, K. , Scheffer, J. , Tenberge, K.B. and Tudzynski, P. (2002) CPMK2, an SLT2‐homologous mitogen‐activated protein (MAP) kinase, is essential for pathogenesis of Claviceps purpurea on rye: evidence for a second conserved pathogenesis‐related MAP kinase cascade in phytopathogenic fungi. Mol. Microbiol. 46, 305–318. [DOI] [PubMed] [Google Scholar]
- Milgroom, M.G. and Cortesi, P. (2004) Biological control of chestnut blight with hypovirulence: a critical analysis. Annu. Rev. Phytopathol. 42, 311–338. [DOI] [PubMed] [Google Scholar]
- Mollapour, M. and Piper, P.W. (2006) Hog1p mitogen‐activated protein kinase determines acetic acid resistance in Saccharomyces cerevisiae . FEMS Yeast Res. 6, 1274–1280. [DOI] [PubMed] [Google Scholar]
- Motoyama, T. , Ochiai, N. , Morita, M. , Iida, Y. , Usami, R. and Kudo, T. (2008) Involvement of putative response regulator genes of the rice blast fungus Magnaporthe oryzae in osmotic stress response, fungicide action, and pathogenicity. Curr. Genet. 54, 185–195. [DOI] [PubMed] [Google Scholar]
- Müller, P. , Weinzierl, G. , Brachmann, A. , Feldbrügge, M. and Kahmann, R. (2003) Mating and pathogenic development of the smut fungus Ustilago maydis are regulated by one mitogen‐activated protein kinase cascade. Eukaryot. Cell, 2, 1187–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolaou, E. , Agrafioti, I. , Stumpf, M. , Quinn, J. , Stansfield, I. and Brown, A.J.P. (2009) Phylogenetic diversity of stress signalling pathways in fungi. BMC Evol. Biol. 9, 44. doi: 10.1186/1471-2148-9-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi, R. , Banno, S. , Ichikawa, R. , Fukumori, F. , Ichiishi, A. , Kimura, M. , Yamaguchi, I. and Fujimura, M. (2007) Identification of OS‐2 MAP kinase‐dependent genes induced in response to osmotic stress, antifungal agent fludioxonil, and heat shock in Neurospora crassa . Fungal Genet. Biol. 44, 208–218. [DOI] [PubMed] [Google Scholar]
- Nuss, D.L. (2005) Hypovirulence: mycoviruses at the fungal–plant interface. Nat. Rev. Microbiol. 3, 632–642. [DOI] [PubMed] [Google Scholar]
- Nuss, D.L. (2010) Mycoviruses In: Cellular and Molecular Biology of Filamentous Fungi (Borkovich K.A. and Ebbole D.J., eds), pp. 145–152. Washington, DC: ASM Press. [Google Scholar]
- Panadero, J. , Pallotti, C. , Rodriguez‐Vargas, S. , Randez‐Gil, F. and Prieto, J.A. (2006) A downshift in temperature activates the high osmolarity glycerol (HOG) pathway, which determines freeze tolerance in Saccharomyces cerevisiae . J. Biol. Chem. 281, 4638–4645. [DOI] [PubMed] [Google Scholar]
- Pandey, A. , Roca, M.G. , Read, N.D. and Glass, N.L. (2004) Role of a mitogen‐activated protein kinase pathway during conidial germination and hyphal fusion in Neurospora crassa . Eukaryot. Cell, 3, 348–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, J.A. , Kim, J.M. , Park, S.M. and Kim, D.H. (2012) Characterization of CpSte11, a MAPKKK gene of Cryphonectria parasitica, and initial evidence of its involvement in the pheromone response pathway. Mol. Plant Pathol. 13, 240–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, S.M. , Choi, E.S. , Kim, M.J. , Cha, B.J. , Yang, M.S. and Kim, D.H. (2004) Characterization of HOG1 homologue, CpMK1, from Cryphonectria parasitica and evidence for hypovirus‐mediated perturbation of its phosphorylation in response to hypertonic stress. Mol. Microbiol. 51, 1267–1277. [DOI] [PubMed] [Google Scholar]
- Pérez‐Nadales, E. and Di Pietro, A. (2011) The membrane mucin Msb2 regulates invasive growth and plant infection in Fusarium oxysporum . Plant Cell, 23, 1171–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prados Rosales, R.C. and Di Pietro, A. (2008) Vegetative hyphal fusion is not essential for plant infection by Fusarium oxysporum . Eukaryot. Cell, 7, 162–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puhalla, J.E. and Anagnostakis, S.L. (1971) Genetic and nutritional requirements of Endothia parasitica . Phytopathology, 61, 169–173. [Google Scholar]
- Ramamoorthy, V. , Zhao, X. , Snyder, A.K. , Xu, J.R. and Shah, D.M. (2007) Two mitogen‐activated protein kinase signalling cascades mediate basal resistance to antifungal plant defensins in Fusarium graminearum . Cell. Microbiol. 9, 1491–1506. [DOI] [PubMed] [Google Scholar]
- Rastgou, M. , Koohi Habibi, M. , Izadpanah, K. , Luisoni, E. , Masenga, V. , Milne, R.G. , Wolf, Y.I. , Koonin, E.V. and Turina, M. (2009) Molecular characterization of the plant virus genus Ourmiavirus and evidence of inter‐kingdom reassortment of viral genome segments as its possible route of origin. J. Gen. Virol. 90, 2525–2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read, N.D. , Lichius, A. , Shoji, J.Y. and Goryachev, A.B. (2009) Self‐signalling and self‐fusion in filamentous fungi. Curr. Opin. Microbiol. 12, 608–615. [DOI] [PubMed] [Google Scholar]
- Rigling, D. , Heiniger, U. and Hohl, H.R. (1989) Reduction of laccase activity in dsRNA‐containing hypovirulent strains of Cryphonectria (Endothia) parasitica . Phytopathology, 79, 219–223. [Google Scholar]
- Rispail, N. , Soanes, D.M. , Ant, C. , Czajkowski, R. , Grünler, A. , Huguet, R. , Perez‐Nadales, E. , Poli, A. , Sartorel, E. , Valiante, V. , Yang, M. , Beffa, R. , Brakhage, A.A. , Gow, N.A. , Kahmann, R. , Lebrun, M.H. , Lenasi, H. , Perez‐Martin, J. , Talbot, N.J. , Wendland, J. and Di Pietro, A. (2009) Comparative genomics of MAP kinase and calcium‐calcineurin signalling components in plant and human pathogenic fungi. Fungal Genet. Biol. 46, 287–298. [DOI] [PubMed] [Google Scholar]
- Rostagno, L. , Crivelli, G. and Turina, M. (2009) Study of mRNA expression by real time PCR of Cpkk1, Cpkk2 and Cpkk3, three MEKs of Cryphonectria parasitica, in virus‐free and virus‐infected isogenic isolates. J. Phytopathol. 158, 409–416. [Google Scholar]
- Rostagno, L. , Prodi, A. and Turina, M. (2010) Cpkk1, MAPKK of Cryphonectria parasitica, is necessary for virulence on chestnut. Phytopathology, 100, 1100–1110. [DOI] [PubMed] [Google Scholar]
- Rui, O. and Hahn, M. (2007) The Slt2‐type MAP kinase Bmp3 of Botrytis cinerea is required for normal saprotrophic growth, conidiation, plant surface sensing and host tissue colonization. Mol. Plant Pathol. 8, 173–184. [DOI] [PubMed] [Google Scholar]
- Sambrook, J. , Fritsch, E.F. and Maniatis, T. (1989) Molecular Cloning: A Laboratory Manual, 2nd edn Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press. [Google Scholar]
- Segmuller, N. , Ellendorf, U. , Tudzynski, B. and Tudzynski, P. (2007) BcSak1, a stress‐activated mitogen‐activated protein kinase, is involved in vegetative differentiation and pathogenicity in Botrytis cinerea . Eukaryot. Cell, 6, 211–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, Q. , Choi, G.H. and Nuss, D.L. (2009) Hypovirus‐responsive transcription factor gene pro1 of the chestnut blight fungus Cryphonectria parasitica is required for female fertility, asexual spore development, and stable maintenance of hypovirus infection. Eukaryot. Cell, 8, 262–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorsen, M. , Di, Y.J. , Tangemo, C. , Morillas, M. , Ahmadpour, D. , Van der Does, C. , Wagner, A. , Johansson, E. , Boman, J. , Posas, F. , Wysocki, R. and Tamás, M.J. (2006) The MAPK Hog1p modulates Fps1p‐dependent arsenite uptake and tolerance in yeast. Mol. Biol. Cell 17, 4400–4410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tscharke, R.L. , Lazera, M. , Chang, Y.C. , Wickes, B.L. and Kwon‐Chung, K.J. (2003) Haploid fruiting in Cryptococcus neoformans is not mating type alpha‐specific. Fungal Genet. Biol. 39, 230–237. [DOI] [PubMed] [Google Scholar]
- Turina, M. and Rostagno, L. (2007) Virus‐induced hypovirulence in Cryphonectria parasitica: still an unresolved conundrum. J. Plant Pathol. 89, 165–178. [Google Scholar]
- Turina, M. , Prodi, A. and Van Alfen, N.K. (2003) Role of the Mf1‐1 pheromone precursor gene of the filamentous ascomycete Cryphonectria parasitica . Fungal Genet. Biol. 40, 242–251. [DOI] [PubMed] [Google Scholar]
- Turina, M. , Zhang, L. and Van Alfen, N.K. (2006) Effect of Cryphonectria hypovirus 1 (CHV1) infection on Cpkk1, a mitogen‐activated protein kinase kinase of the filamentous fungus Cryphonectria parasitica . Fungal Genet. Biol. 43, 764–774. [DOI] [PubMed] [Google Scholar]
- Xu, J.R. , Staiger, C.J. and Hamer, J.E. (1998) Inactivation of the mitogen‐activated protein kinase Mps1 from the rice blast fungus prevents penetration of host cells but allows activation of plant defense responses. Proc. Natl. Acad. Sci. USA, 95, 12 713–12 718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan, L. , Yang, Q. , Sundin, G.W. , Li, H. and Ma, Z. (2010) The mitogen‐activated protein kinase kinase BOS5 is involved in regulating vegetative differentiation and virulence in Botrytis cinerea . Fungal Genet. Biol. 47, 753–760. [DOI] [PubMed] [Google Scholar]
- Zhang, L. , Churchill, A.C. , Kazmierczak, P. , Kim, D.H. and Van Alfen, N.K. (1993) Hypovirulence‐associated traits induced by a mycovirus of Cryphonectria parasitica are mimicked by targeted inactivation of a host gene. Mol. Cell. Biol. 13, 7782–7792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Y. , Lamm, R. , Pillonel, C. , Lam, S. and Xu, J.R. (2002) Osmoregulation and fungicide resistance: the Neurospora crassa os‐2 gene encodes a HOG1 mitogen‐activated protein kinase homologue. Appl. Environ. Microbiol. 68, 532–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, X. , Kim, Y. , Park, G. and Xu, J.R. (2005) A mitogen‐activated protein kinase cascade regulating infection‐related morphogenesis in Magnaporthe grisea . Plant Cell, 17, 1317–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, X. , Mehrabi, R. and Xu, J.R. (2007) Mitogen‐activated protein kinase pathways and fungal pathogenesis. Eukaryot. Cell, 6, 1701–1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 Restriction maps and Southern blot analyses of mitogen‐activated protein kinase kinase (MAP2K)‐null mutants and Δcpku80 strain (used as wild‐type). (A) Gene cpkk1. (B) Gene cpkk2. (C) Gene cpkk3. Gene disruption was generated for each MAP2K through the insertion of a 1.4‐kb hygromycin cassette (Hyg) in the coding sequence. Total fungal DNA from the Δcpkk1, Δcpkk2 and Δcpkk3 mutants was digested with SacI, BamHI and SmaI, respectively. The digested fragments were separated on a 1% agarose gel by electrophoresis, and hybridized with 32P‐labelled specific probes indicated in the restriction maps: each probe detected a digested fragment that differed in size compared with the wild‐type, indicating that transformants underwent the desired replacement at the MAP2K site through double homologous recombination.
Fig. S2 Comparison of mitogen‐activated protein kinase kinase (MAP2K)‐null mutant colony morphology after 7 days of growth on PDAmb [potato dextrose agar (PDA) containing l‐methionine (100 mg/L) and biotin (1 mg/L)] (left) versus malt agar plates (right). (A) Strain Δcpkk1. (B) Strain Δcpkk2. (C) Strain Δcpkk3. (D) Strain Δcpku80. Medium composition significantly affected the growth of the cpkk2‐null mutant.
Fig. S3 Colony morphology of mitogen‐activated protein kinase kinase (MAP2K)‐null mutants grown for 7 days on PDAmb [potato dextrose agar (PDA) containing l‐methionine (100 mg/L) and biotin (1 mg/L)] supplemented with 2 m sorbitol: no growth was observed in the cpkk3‐null mutant and a strong reduction in growth was reported for the other strains.
Fig. S4 Growth of mitogen‐activated protein kinase kinase (MAP2K)‐null mutants after 7 days on PDAmb [potato dextrose agar (PDA) containing l‐methionine (100 mg/L) and biotin (1 mg/L)] supplemented with different concentrations of NaCl. The cpkk3‐null mutant displayed the strongest impairment in growth with increasing NaCl concentration in the medium.
Fig. S5 Growth experiment at different temperatures. (A) One‐way analysis of variance (ANOVA) of mitogen‐activated protein kinase kinase (MAP2K)‐null mutant growth compared with the wild‐type strain at 12 °C (Tukey's test, P < 0.05). Fungal strains were grown on PDAmb [potato dextrose agar (PDA) containing l‐methionine (100 mg/L) and biotin (1 mg/L)] for 5 days. The Δcpkk1 strain could not grow at 12 °C. Results from three different experiments are shown: Δcpkk1, 0 cm; Δcpkk2, 0.60 ± 0 cm; Δcpkk3, 1.87 ± 0.17 cm; Δcpku80, 1.95 ± 0.12 cm. (B) Growth at 25 °C (Tukey's test, P < 0.05). Fungal strains were grown on PDAmb for 5 days. Results from three different experiments are shown: Δcpkk1, 2.08 ± 0.16 cm; Δcpkk2, 1.38 ± 0.13 cm; Δcpkk3, 6.32 ± 0.22 cm; Δcpku80, 6.80 ± 0.41 cm.
Fig. S6 Colony morphology of wild‐type (Δcpku80) and mitogen‐activated protein kinase kinase (MAP2K)‐null mutants grown for 7 days on PDAmb [potato dextrose agar (PDA) containing l‐methionine (100 mg/L) and biotin (1 mg/L)] supplemented with 0.125 mm menadione. cpkk1‐ and cpkk2‐null mutants displayed a strong sensitivity to the oxidative stress induced by menadione.
Fig. S7 Colony morphology of wild‐type (Δcpku80) and mitogen‐activated protein kinase kinase (MAP2K)‐null mutants grown for 7 days on PDAmb [potato dextrose agar (PDA) containing l‐methionine (100 mg/L) and biotin (1 mg/L)] supplemented with 0.005% sodium dodecylsulphate (SDS). Reduction of growth was reported for all strains, except the Δcpkk1 isolate, in which the absence of growth was observed.
Fig. S8 Colony morphology of wild‐type (Δcpku80) and mitogen‐activated protein kinase kinase (MAP2K)‐null mutants grown for 7 days on PDAmb [potato dextrose agar (PDA) containing l‐methionine (100 mg/L) and biotin (1 mg/L)] supplemented with 100 mm H2O2. All strains, including the wild‐type, were affected, but only the cpkk1‐null mutant displayed absence of growth under the same conditions.
Fig. S9 Wild‐type (Δcpku80) and mitogen‐activated protein kinase kinase (MAP2K)‐null mutants growth in PDAmb [potato dextrose agar (PDA) containing l‐methionine (100 mg/L) and biotin (1 mg/L)] containing Congo red (1 mg/mL) and calcofluor white (0.5 mg/mL). The y axis represents the average of the difference between the diameter on PDAmb and the diameter on Congo red‐ and calcofluor white‐containing media. Error bars represent the standard deviation. Red type: t‐test values for two‐sample comparisons (each knockout strain compared with the wild‐type). Asterisks represent a statistically significant difference when compared with the wild‐type strain.
Fig. S10 Wild‐type (Δcpku80) and mitogen‐activated protein kinase kinase (MAP2K)‐null mutants growth in PDAmb [potato dextrose agar (PDA) containing l‐methionine (100 mg/L) and biotin (1 mg/L)] containing different concentrations of G418 (Geneticin) antibiotic. Four representative concentrations are shown to illustrate the sensitivity of Δcpkk2 to the presence of Geneticin in the medium.
Fig. S11 Wild‐type (Δcpku80) and mitogen‐activated protein kinase kinase (MAP2K)‐null mutants growth on PDAmb [potato dextrose agar (PDA) containing l‐methionine (100 mg/L) and biotin (1 mg/L)] supplemented with fludioxonil. Concentrations of 100, 50, 25, 12.5 and 6.25 mg/L were tested: at 100 mg/L, only the Δcpkk3 strain displayed normal growth, whereas, at the same concentration, the growth of all the other strains was fully inhibited.
Fig. S12 cpkk1‐null mutants show lower diameter hyphae and lower fluorescence on calcofluor staining of cell walls compared with Δcpku80. Quantification of the cell diameter and calcofluor fluorescence intensity was performed using ImageJ software (National Institutes of Health, Bethesda, MD, USA). In both cases, the numbers are relative to measurements taken for strain Δcpku80, arbitrarily given the value of 100. For each strain, the diameters of 100 hyphae were measured on five different slides.
Fig. S13 Representative photographs of Δcpkk2 and Δcpku80 hyphal fusions. Fungal strains were inoculated on PDAmb [potato dextrose agar (PDA) containing l‐methionine (100 mg/L) and biotin (1 mg/L)] and observed after 2 days by light microscopy. Several fusion events were observed in Δcpku80 mycelium (white arrowheads), but no hyphal fusion was detected in Δcpkk2 colonies. Lack of hyphal fusion was evident at the hyphal contact points (asterisks). Bars, 20 μm.
Fig. S14 Agar gel electrophoresis of polymerase chain reaction (PCR) products obtained using DNA templates from knockout and complemented strains. Oligonucleotides were designed immediately upstream and downstream of the hygromycin resistance gene insertion point in each of the two genes. Oligonucleotides were: for Δcpkk1, Cpkk1‐screenF (5′‐GCCGAGGGTGTCCTGCAGGG‐3′) and Cpkk1‐screenRev (5′‐GCACAACAGGATATTCGAGG‐3′); for Δcpkk2, Cpkk2‐korealF (5′‐GGAGGATATGATTCAGAAGTGTCTCTT‐3′) and Cpkk2‐korealRev (5′‐ACGCTGGCCTGTATCTGGTG‐3′). The lower band in both gels represents the c. 100‐bp fragment amplified from the locus without insertion, whereas the higher band running with the 1.5‐kbp fragment represents the locus with the hygromycin resistance gene insertion.
Table S1 Oligonucleotide primers used in this study.


