Summary
Two mutants (tri6Δ and noxABΔ) of the fungal pathogen Fusarium graminearum were assessed for their ability to prime immune responses in wheat (cv. Roblin) against challenge with pathogenic F. graminearum. Priming treatments generated Fusarium head blight (FHB)‐resistant wheat phenotypes and reduced the accumulation of fungal mycotoxins in infected tissues. Microarray analysis identified 260 transcripts that were differentially expressed during the priming period. Expression changes were observed in genes associated with immune surveillance systems, signalling cascades, antimicrobial compound production, oxidative burst, secondary metabolism, and detoxification and transport. Specifically, genes related to jasmonate, gibberellin and ethylene biosynthesis exhibited differential expression during priming. In addition, the induction of the phenylpropanoid pathways that lead to flavonoid, coumarin and hydroxycinnamic acid amide accumulation was also observed. This study highlights the utility of nonpathogenic mutants to both elicit and delineate stages of defence responses in wheat.
Keywords: Fusarium, noxAB, plant immunity, priming, resistance, tri6, wheat
Fusarium head blight (FHB), caused by the fungal pathogen Fusarium graminearum, affects a number of economically important cereal crops. In addition to yield reductions, FHB causes the accumulation of trichothecene mycotoxins that present a health hazard to both animals and humans. Microbial biological control agents (BCAs) have been shown previously to reduce the severity of FHB and the accumulation of fungal mycotoxins in Fusarium‐infected cereal heads (Khan and Doohan, 2009; Xue et al., 2009). Nonpathogenic Fusarium strains have been used effectively as BCAs, acting through various mechanisms, including nutrient and niche competition, and induced resistance in the host (reviewed by Alabouvette et al., 2009). Induced resistance, which occurs following a particular stimulus, leads to heightened resistance against subsequent pathogen attacks. This heightened physiological state, in which plants are able to more rapidly and/or better activate immune responses, is known as the ‘primed state’. Nonpathogenic strains of Fusarium have been used to induce disease resistance in asparagus and tomato (Aimé et al., 2013; He et al., 2002), and priming has been shown to provide disease protection without incurring heavy fitness costs (Van Hulten et al., 2006). Previously, Petti et al. (2010) investigated the localized transcriptome associated with bacterial‐induced priming in barley, and Aimé et al. (2013) investigated the expression of six defence response‐related genes in various tissues during avirulent Fusarium‐induced priming in tomato. To date, no study has described priming‐induced resistance (PIR) against FHB in wheat. Here, we report two F. graminearum deletion mutants (tri6Δ and noxABΔ) that activate PIR against FHB in wheat (cv. Roblin), and the associated global transcriptional changes that accompany this phenotype.
The F. graminearum tri6Δ mutant strain contains a knockout in the Tri6 gene which encodes a global transcriptional regulator found in the trichothecene gene cluster (Nasmith et al., 2011). This strain is unable to produce the mycotoxin deoxynivalenol (DON) in culture and is nonpathogenic (Nasmith et al., 2011; Scherm et al., 2011; Seong et al., 2009). The F. graminearum noxABΔ mutant strain has two genes related to the catalytic unit of NADH oxidase deleted (Wang et al., 2014). Unlike the tri6Δ mutant strain, high‐performance liquid chromatography (HPLC) analysis showed that the noxABΔ mutant strain has the capacity to produce 15‐acetyldeoxynivalenol (15‐ADON) in culture (Wang et al., 2014). In pathogenicity assays, the tri6Δ strain is able to infect, but not spread beyond, the inoculated wheat spikelets, whereas, in wheat inoculated with the noxABΔ strain, the fungus managed a limited spread into wheat spikelets adjacent to those inoculated (Fig. 1a). With mutant strains, less DON/15ADON accumulated compared with wheat heads infected with pathogenic F. graminearum (Fig. 1a). To investigate the potential of the tri6Δ and noxABΔ strains to prime resistance in wheat and offer protection against pathogenic F. graminearum, wheat spikelets were point inoculated with either tri6Δ or noxABΔ strain (spikelet 0) and subsequently challenged on an adjacent spikelet (spikelet 2) with pathogenic F. graminearum (Fig. 2a). Priming treatment with the tri6Δ strain consistently exhibited a more resistant phenotype than with the noxABΔ strain, as indicated by the level of infection and accumulation of DON in the wheat heads (Fig. 1b). This suggests that priming by the Δtri6 mutant induces an early response by the host, whereas the ΔnoxAB mutant evokes a delayed response leading to more disease spread. Yet, disease symptoms and DON levels after both priming treatments were greatly reduced when compared with those in wheat heads infected only with pathogenic F. graminearum (Fig. 1b).
Figure 1.
Priming‐induced resistance to Fusarium head blight in wheat (cv. Roblin). (a) Disease symptoms caused by pathogenic Fusarium graminearum (Fg; DAOM 233423; Canadian Collection of Fungal Cultures, Agriculture and Agri‐Food Canada, Ottawa, ON, Canada) and the F. graminearum mutant strains tri6Δ and noxABΔ (tri6Δ, DAOM 237993; noxABΔ, DAOM 239571) were compared by point inoculation of 1000 spores to spikelet 0 (Fig. 2a). (b) The priming potential of tri6Δ and noxABΔ was evaluated by comparing mock treatments (dH2O) with point inoculation treatments of 1000 spores of either tri6Δ or noxABΔ to spikelet 0, followed by point inoculation pathogen challenge treatment consisting of 1000 spores of pathogenic F. graminearum to spikelet 2 (Fig. 2a), 24 h later (tri6Δ + Fg; noxABΔ + Fg). Images were taken 21 days after application of initial treatments. The mean percentage of spikelet infection per head (% infected) is indicated ± standard deviation. Combined deoxynivalenol (DON) and 15‐acetyldeoxynivalenol (15‐ADON) levels in infected tissues were measured in an enzyme‐linked immunosorbent assay (ELISA) and are presented as parts per million (ppm) ± standard deviation. Asterisks indicate a statistically significant difference between the two priming treatments in three independent experiments, as determined by Students's t‐test (P = 0.05).
Figure 2.
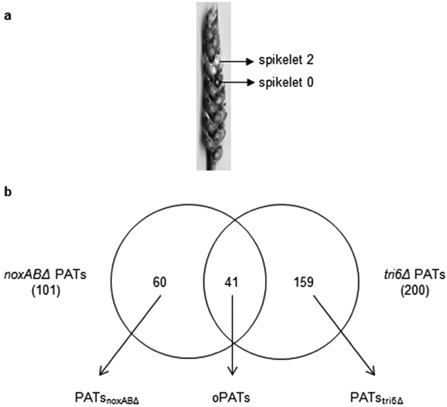
Analysis of wheat spikelets primed with Fusarium graminearum mutant strains. (a) Site of point inoculation for priming experiments on wheat (cv. Roblin) heads. Priming or mock priming treatments were applied to spikelet 0 and pathogen challenge or mock challenge treatments were applied to spikelet 2. RNA samples were isolated from spikelet 2 for microarray analysis, as described previously (Nasmith et al., 2011; Wang et al., 2010). Microarray analysis was performed with three biological replicates and, for each experiment, RNA was extracted from eight spikelets. (b) Venn diagram of the number of transcripts showing two‐fold or greater change in abundance at either 8 h or 24 h post‐pathogen challenge, relative to primed but not challenged as well as challenged but not primed controls. The dataset of priming‐associated transcripts (PATs) differentially expressed during both noxABΔ‐induced priming (noxABΔ PATs) and tri6Δ‐induced priming (tri6Δ PATs) is defined as overlapping PATs (oPATs), and datasets with nonoverlapping PATs specific to either noxABΔ‐ or tri6Δ‐induced priming are defined as PATsnoxABΔ and PATstri6Δ, respectively.
To characterize the genetic mechanisms underlying PIR against FHB in wheat, DNA microarray analysis was conducted using the Affymetrix Wheat GeneChip Array on RNA extracted from spikelet 2 at 8 h and 24 h post‐challenge (Fig. 2a). All raw expression data can be accessed through http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE55653. In our analyses, probesets were considered to be differentially expressed if: (i) the fold change was at least two; (ii) the P value (Baldi and Long, 2001) was ≤0.001; and (iii) the false discovery rate (Benjamini and Hochberg, 1995) was ≤0.05. In cases in which multiple probesets quantified expression of the same transcript, we manually removed redundant probesets from the datasets. The priming phenotype only occurs when both the priming agent and the pathogen are present (Van Der Ent et al., 2009); therefore, we identified priming‐associated transcripts (PATs) in primed and challenged (P&C) plant tissues showing differential expression relative to wheat spikelets that were (i) primed but not challenged (P&C vs. P) or (ii) challenged but not primed (P&C vs. C) at either of the 8 h or 24 h post‐pathogen challenge time points (Table S1, see Supporting Information). This analysis identified 101 differentially expressed PATs during noxABΔ‐induced priming (noxABΔ PATs), and 200 differentially expressed PATs during tri6Δ‐induced priming (tri6Δ PATs; Fig. 2). Furthermore, we compiled a dataset of 41 overlapping PATs (oPATs) composed of differentially expressed transcripts found in both the noxABΔ and tri6Δ PATs (Fig. 2). As the tri6Δ and noxABΔ strains differ in their relative virulence, but both generate priming phenotypes, the transcripts in the oPATs are more likely to highlight the essential transcriptional changes associated with priming. We also defined datasets containing PATs that were either uniquely primed by noxABΔ (PATsnoxABΔ) or tri6Δ (PATstri6Δ) mutant stains (Fig. 2). We identified 60 PATsnoxABΔ and 159 PATstri6Δ (Fig. 2b; Table S1). Quantitative reverse transcription‐polymerase chain reaction (qRT‐PCR) analysis of selected PATs was used to support our analysis (Table S2, see Supporting Information).
The three datasets were analysed with respect to what is currently known about host responses during F. graminearum infection, general plant–microbe interactions and the observed priming phenotype. Transcripts were assigned to functional categories based on annotations provided by HarvEST Wheat1 chip version 1.59 (http://harvest.ucr.edu). A large proportion (41%) of the noxABΔ PATs overlapped with the tri6Δ PATs, whereas only 21% of the tri6Δ PATs overlapped with the noxABΔ PATs (Fig. 2). The majority of the PATs were up‐regulated: 90% in oPATs, 92% in PATstri6Δ and 65% in PATsnoxABΔ (Table S1).
Transcripts found in the oPATs, PATsnoxABΔ and PATstri6Δ are associated with a wide range of roles in plant immunity (Tables 2, 3, 4, and complete datasets in Table S1). Pathogen recognition plays a critical role in the activation of plant immune responses (reviewed in Dodds and Rathjen, 2010). Recognition events occurring at the plant cell surface are based on the perception of various molecular stimuli, known as pathogen‐, microbe‐ or damage‐associated molecular patterns (PAMPs, MAMPs or DAMPs, respectively) by membrane‐bound pattern recognition receptors (PRRs). Many PRRs are receptor‐like kinases (RLKs) responsible for the recognition of fungal PAMPs, such as chitin and xylanase, and PAMP recognition activates an immune response known as PAMP‐triggered immunity (PTI) (reviewed in Deslandes and Rivas, 2012; Zipfel, 2009). There are several transcripts annotated as membrane‐bound RLKs in the PATstri6Δ (Table 4). However, membrane‐bound RLK transcripts are not found in the oPATs or PATsnoxABΔ. Indeed, PATstri6Δ contains the largest proportion of transcripts associated with signal transduction (5%; Table 1), suggesting an important role for these processes in tri6Δ‐induced priming. Consistent with this interpretation, signalling modules known to function downstream of PTI activation are exclusive to PATstri6Δ. For example, mitogen‐activated protein kinase (MAPK) and calcium/calmodulin‐dependent protein kinase (CAMK) signalling systems are both associated with PAMP recognition and PTI activation (Bhardwaj et al., 2011; Gao et al., 2013), and transcripts with annotations related to both of these signalling systems are exclusive to PATstri6Δ (Table 4). Furthermore, MAPK cascades are hypothesized to direct the expression of WRKY transcription factors, which are important mediators of plant defence responses, and are also exclusively in PATstri6Δ (Table 4; Eulgem, 2006; Ross et al., 2007). Similarly, transcription factors belonging to the Myb family, which are known to be involved in defence responses to fungal pathogens, are exclusive to PATstri6Δ (Table 4; Liu et al., 2013). These results suggest that mutants varying in pathogenicity and inducing the priming response differentially (Fig. 1) may be used to tease out stages of plant defence responses.
Table 2.
Selected overlapping priming‐associated transcripts (oPATs) with predicted functions related to recognition and signalling, defence response or detoxification and transport
| Probeset | Predicted function | Fold changeb | |||||||
|---|---|---|---|---|---|---|---|---|---|
| tri6Δ | noxABΔ | ||||||||
| PC vs. C | PC vs. P | PC vs. C | PC vs. P | ||||||
| 8 h | 24 h | 8 h | 24 h | 8 h | 24 h | 8 h | 24 h | ||
| Recognition and signalling | |||||||||
| Ta.9507.2.S1_ata | Jasmonate‐ZIM‐domain protein 1 (JAZ1) | 2.58 | 0.44 | 8.80 | 2.67 | 3.00 | 2.35 | ||
| Ta.27345.1.S1_ata | Mlo3 | 2.08 | 10.16 | 2.28 | 5.29 | ||||
| Defence response | |||||||||
| Ta.26340.1.A1_at | Chitinase | 2.06 | 8.82 | 2.11 | 3.79 | ||||
| Ta.18497.1.S1_at | Peroxidase precursor | 3.49 | 8.92 | 3.39 | 5.44 | ||||
| Ta.8483.1.S1_at | Blue copper protein | 2.10 | 4.19 | 2.02 | 7.38 | ||||
| TaAffx.29050.1.S1_s_at | Agmatine coumaroyltransferase | 3.08 | 5.96 | 2.80 | 3.03 | ||||
| Ta.21061.3.S1_at | trans‐Cinnamate 4‐monooxygenase (C4H) | 3.09 | 7.28 | 3.64 | 3.65 | ||||
| TaAffx.28302.2.S1_at | Dirigent‐like protein | 2.36 | 3.96 | 2.10 | 6.49 | ||||
| Ta.7022.2.S1_at | Phenylalanine ammonia‐lyase | 2.09 | 4.92 | 2.10 | 2.38 | 3.42 | |||
| Ta.8559.1.S1_at | Polyphenol oxidase | 0.36 | 0.45 | 0.36 | 0.35 | ||||
| Ta.5610.1.S1_at | Nonsymbiotic haemoglobin | 3.28 | 2.26 | 2.51 | 13.10 | ||||
| Detoxification and transport | |||||||||
| Ta.23833.3.S1_x_ata | Plasma membrane intrinsic protein 1;3 (PIP1;3) | 0.46 | 0.49 | 0.38 | 0.47 | ||||
| Ta.28728.2.S1_x_at | Plasma membrane intrinsic protein 2;4 (PIP2;4) | 0.41 | 0.48 | 0.30 | 0.46 | ||||
| TaAffx.29128.1.S1_at | ABC transporter‐like | 2.18 | 2.19 | 2.18 | 5.25 | ||||
| TaAffx.113701.1.S1_s_at | Pleiotropic drug resistance protein | 2.29 | 3.91 | 2.75 | 2.02 | ||||
Quantitative reverse transcription‐polymerase chain reaction (qRT‐PCR) data available (Table S2).
Data shown for probesets exhibiting two‐fold greater or two‐fold lower (values less than unity) change in expression at either 8 h or 24 h post‐pathogen challenge, relative to primed but not challenged (PC vs. P) as well as challenged but not primed (PC vs. C) controls.
Predicted function shows the best significant blastx database hit from HarvEST.
Table 3.
Selected PATsnoxABΔ with predicted functions related to recognition and signalling, defence response or detoxification and transport
| Probeset | Predicted function | Fold changeb | |||
|---|---|---|---|---|---|
| noxABΔ | |||||
| PC vs. C | PC vs. P | ||||
| 8 h | 24 h | 8 h | 24 h | ||
| Recognition and signalling | |||||
| TaAffx.31548.1.S1_ata | Gibberellin receptor GID1L2 | 2.51 | 3.34 | ||
| Ta.1207.1.S1_at | 12‐Oxophytodienoate reductase (OPR1) | 2.13 | 2.28 | ||
| Ta.22602.1.S1_a_at | Alpha‐dioxygenase (α‐DOX2) | 2.26 | 4.28 | ||
| Defence response | |||||
| Ta.9336.2.S1_x_at | Blue copper‐binding protein | 2.19 | 3.37 | ||
| TaAffx.45277.1.S1_x_at | Phenylalanine ammonia‐lyase | 2.22 | 2.32 | 4.36 | |
| Ta.14545.1.S1_at | O‐Methyltransferase ZRP4 | 2.50 | 8.26 | ||
| Ta.82.1.S1_at | Peroxidase precursor | 0.46 | 3.28 | ||
| Detoxification and transport | |||||
| Ta.21281.1.S1_at | Pleiotropic drug resistance (PDR)‐type ABC transporter | 2.15 | 2.25 | ||
| Ta.30922.1.S1_at | Glutathione S‐transferase | 2.29 | 0.49 | 4.49 | |
Quantitative reverse transcription‐polymerase chain reaction (qRT‐PCR) data available (Table S2).
Data shown for probesets exhibiting two‐fold greater or two‐fold lower (values less than unity) change in expression at either 8 h or 24 h post‐pathogen challenge, relative to primed but not challenged (PC vs. P) as well as challenged but not primed (PC vs. C) controls.
Predicted function shows the best significant blastx database hit from HarvEST.
Table 4.
Selected PATstri6Δ with predicted functions related to recognition and signalling, defence response or detoxification and transport
| Probeset | Predicted function | Fold changeb | |||
|---|---|---|---|---|---|
| tri6Δ | |||||
| PC vs. C | PC vs. P | ||||
| 8 h | 24 h | 8 h | 24 h | ||
| Recognition and signalling | |||||
| TaAffx.103209.1.S1_at | Resistant to Pseudomonas syringae 2 (RPS2) | 2.04 | 3.39 | ||
| TaAffx.53376.1.S1_ata | Receptor‐like protein kinase 2 | 2.36 | 2.23 | 2.74 | |
| TaAffx.84014.1.S1_ata | Cysteine‐rich receptor‐like protein kinase 6 precursor (CRK6) | 2.02 | 2.58 | ||
| Ta.25487.1.S1_at | Cysteine‐rich receptor‐like protein kinase 10 (CRK10) | 2.12 | 3.03 | ||
| Ta.8585.1.S1_at | Gibberellin receptor GID1B | 2.40 | 10.76 | ||
| Ta.5671.1.S1_at | Scarecrow‐like transcription factor 5 (SCL5) | 2.27 | 2.03 | ||
| TaAffx.128684.1.S1_x_at | 12‐Oxophytodienoic acid reductase (OPR1) | 2.46 | 9.11 | ||
| Ta.18630.1.A1_x_at | Allene oxide synthase (AOS) | 2.10 | 3.32 | ||
| TaAffx.71241.1.A1_ata | 1‐Amino‐cyclopropane‐1‐carboxylic acid synthase (ACC synthase, ACS) | 2.58 | 3.14 | ||
| Ta.236.1.S1_at | Mitogen‐activated protein kinase 5 (MPK5) | 2.01 | 2.59 | ||
| Ta.16181.1.S1_at | Calcium/calmodulin‐dependent protein kinase (CAMK) | 2.05 | 3.79 | ||
| Ta.4725.1.S1_at | WRKY14 | 2.24 | 3.60 | ||
| Ta.5405.1.S1_x_at | MYB family transcription factor | 2.23 | 5.71 | ||
| Ta.11849.1.S1_at | Myb transcription factor | 0.24 | 0.42 | ||
| Defence response | |||||
| Ta.4328.1.S1_at | Pathogenesis related protein 10 (PR‐10) | 2.08 | 4.58 | ||
| Ta.24544.1.S1_at | Hs1 (HsPro homologue) | 2.16 | 2.65 | ||
| Ta.21556.1.S1_at | Wheat‐induced resistance 1B (WIR1B) | 2.09 | 4.48 | ||
| Ta.10133.1.S1_x_at | Protease inhibitor/seed storage/lipid transfer protein precursor | 2.06 | 0.43 | ||
| Ta.9220.1.S1_a_at | Putative phenylalanine ammonia‐lyase (PAL) | 2.27 | 6.73 | ||
| Ta.25703.2.A1_s_at | Flavonol synthase/flavanone 3‐hydroxylase | 6.48 | 3.29 | ||
| Detoxification and transport | |||||
| Ta.6990.1.S1_at | Pleiotropic drug resistance protein | 2.26 | 4.12 | ||
| Ta.2793.1.S1_at | Multidrug resistance protein 1 | 2.40 | 41.50 | ||
| TaAffx.120297.1.S1_at | ABC transporter family protein | 2.13 | 5.29 | ||
| Ta.12715.1.S1_s_at | Heavy metal transport/detoxification protein | 2.24 | 2.32 | ||
| TaAffx.110629.1.S1_at | Glutathione S‐transferase | 2.73 | 2.97 | ||
| TaAffx.111585.1.S1_ata | UDP‐glycosyltransferase/sinapate 1‐glucosyltransferase | 2.72 | 31.74 | ||
| Ta.12887.1.S1_at | UDP‐glucuronosyl/UDP‐glucosyl transferase | 2.89 | 2.13 | 28.11 | |
Quantitative reverse transcription‐polymerase chain reaction (qRT‐PCR) data available (Table S2).
Data shown for probesets exhibiting two‐fold greater or two‐fold lower (values less than unity) change in expression at either 8 h or 24 h post‐pathogen challenge, relative to primed but not challenged (PC vs. P) as well as challenged but not primed (PC vs. C) controls.
Predicted function shows the best significant blastx database hit from HarvEST.
Table 1.
Functional categorization of the 60 PATsnoxABΔ, 41 overlapping priming‐associated transcripts (oPATs) and 159 PATstri6Δ from Fig. 2. The percentage of transcripts allocated to each category is indicated
| Functional category | PATsnoxABΔ | oPATs | PATstri6Δ |
|---|---|---|---|
| Cellular component organization, biogenesis and development | 5.0% | 2.4% | 3.8% |
| Energy | 1.7% | 0.0% | 1.9% |
| General metabolism | 1.7% | 14.6% | 6.9% |
| Hormone metabolism | 3.3% | 2.4% | 6.9% |
| Other | 6.7% | 7.3% | 6.3% |
| Photosynthesis | 0.0% | 0.0% | 0.0% |
| Protein degradation | 1.7% | 2.4% | 0.0% |
| Protein synthesis | 1.7% | 0.0% | 1.9% |
| Redox homeostasis, detoxification and secondary metabolism | 8.3% | 12.2% | 4.4% |
| Signal transduction | 1.7% | 0.0% | 5.0% |
| Stress | 8.3% | 14.6% | 7.5% |
| Transcription | 6.7% | 0.0% | 8.8% |
| Transport | 3.3% | 9.8% | 7.5% |
| Unclassified | 50.0% | 34.1% | 39.0% |
Functional categories were based on MAPMAN (Thimm et al., 2004) classifications.
The colonization of host plants requires pathogens to overcome PTI. To this end, many pathogens secrete effector proteins that interfere with PTI responses. Accordingly, plants have evolved to recognize these effector proteins and activate a second branch of immune responses, known as effector‐triggered immunity (ETI; reviewed in Dodds and Rathjen, 2010). Effector recognition is mediated either directly or indirectly by the disease resistance (R) proteins. Indirect interaction with R proteins requires a deployment of proteins that guard the actual targets of the effectors (reviewed in Dodds and Rathjen, 2010). For example, in A. thaliana, RPS2 is an R protein that activates ETI through indirect interaction with a bacterial effector protein (reviewed in Xin and He, 2013). In the Pseudomonas–Arabidopsis interaction, RIN4 (an important regulator of ETI) is targeted for degradation by the bacterial effector AvrRpt2 (Liu et al., 2009). Degradation of RIN4 leads to the activation of RPS2, and the onset of ETI (Mackey et al., 2003). A transcript annotated as RPS2 is found in PATstri6Δ (Table 4). This finding is surprising, as no effector proteins, nor associated race‐specific resistance ETI phenotypes, have been identified in any F. graminearum pathosystem, although both of these phenomena have been identified in multiple F. oxysporum pathosystems (Thatcher et al., 2012). It is possible that the wheat transcript annotated as RPS2 represents a cognate receptor for a yet‐to‐be identified F. graminearum effector protein, or may guard other wheat proteins targeted by these putative effector proteins. Interestingly, a transcript annotated as Mlo3, a guard protein targeted by effectors, is found in the oPATs; (Table 2; Panstruga, 2005). In barley, the transmembrane protein Mlo3 interacts with the Ca2+ sensor calmodulin and negatively regulates cell death during powdery mildew infection (Miklis et al., 2007). Importantly, loss‐of‐function Mlo alleles in barley confer complete resistance to biotrophic powdery mildews, whilst conferring enhanced susceptibility to necrotrophic F. graminearum (Jansen et al., 2005). Overall, the discovery of genes encoding guard proteins in oPATs suggests that effector recognition and the activation of PTI/ETI are important in PIR against FHB in wheat.
Many PATs were associated with hormone metabolism (Tables 2, 3 and S1), including jasmonic acid (JA), gibberellic acid (GA) and ethylene (ET). PATstri6Δ contained the largest proportion of transcripts associated with this functional category (6.9%), compared with either PATsnoxABΔ (3.3%) or oPATs (2.4%; Table 1). JA and its derivatives are important systemic signalling molecules that regulate plant immunity, and are produced in response to abiotic cues, such as wounding, as well as to biotic cues, such as pathogen challenge, symbiosis and priming (Aimé et al., 2013; De Geyter et al., 2012; Kazan and Manners, 2008; Memelink, 2009; Petti et al., 2010). Transcripts associated with JA biosynthesis are found in all three PATs datasets, including 12‐oxo‐phytodienoic acid reductase 1 (OPR1), alpha‐dioxygenase (α‐DOX2), allene oxide synthase (AOS) and jasmonate‐ZIM‐domain protein 1 (JAZ1) (Tables 2, 3, 4). There are two major recognized branches of the JA signalling pathway, one of which is controlled by MYC‐type transcription factors that are negatively regulated by JAZ repressor proteins (Van der Does et al., 2013). A transcript annotated as JAZ1 is an up‐regulated oPAT (Table 2); JAZ transcripts themselves are positively regulated in a MYC2‐dependent manner (Chini et al., 2007; Thines et al., 2007); therefore, the up‐regulation of JAZ transcripts during PIR against FHB in wheat may be indicative that MYC2‐dependent JA signalling is important to this phenomenon.
The second major branch of the JA signalling pathway requires both JA and ET signalling (Van der Does et al., 2013). All ET‐regulated responses begin with ET biosynthesis, and 1‐amino‐cyclopropane‐1‐carboxylic acid synthase (ACC synthase; ACS) is an essential enzyme in this process. A transcript annotated as ACS is an up‐regulated PATstri6Δ (Table 4).
GAs are plant hormones that regulate plant growth as well as immune responses (Kazan and Manners, 2012). Various nodes in GA signalling are up‐regulated PATsnoxABΔ and PATstri6Δ, including scarecrow‐like (SCL) transcription factors 5 and 9, as well as GID1 (GA insensitive dwarf1) (Tables 3 and 4). In A. thaliana, SCL transcription factors belong to a family of proteins that positively regulate GA signalling (Zhang et al., 2011), and GID1 is a gibberellin receptor that activates GA signalling (Sun, 2010). It is of particular interest that both JA and GA signalling pathways are represented in the PATs, as antagonistic and synergistic interactions are known to occur between these pathways (Kazan and Manners, 2012). For example, low GA levels can prevent JAZ repression of MYC2, leaving JA signalling unencumbered (Hou et al., 2010). However, when GA levels reach a certain threshold, JAZ proteins actively repress MYC2, leading to suppression of MYC2‐dependent JA responses. Conversely, another mechanism allows high GA levels to directly de‐repress MYC2‐dependent JA responses (Hong et al., 2012). It has been proposed that underlying physiological conditions determine the outcome of JA–GA interactions, resulting in local and systemic changes in gene expression co‐ordinated specifically to changing environmental circumstances (Hong et al., 2012). The simultaneous up‐regulation of JA and GA signalling pathways during PIR against FHB in wheat implies a synergistic rather than antagonistic interplay between these two hormone pathways, and it is tempting to speculate that the JA, ET and GA pathways contribute to the systemic activation of the priming phenotype in wheat.
In addition to recognition and signalling, PIR against FHB in wheat is associated with the differential expression of transcripts with roles in defence responses. For example, chitinases, which directly attack fungal structures, are up‐regulated oPATs (Table 2). The expression of other defence‐related transcripts is idiosyncratic to tri6Δ‐induced priming. For example, the WIR1 family of proteins (Wheat‐induced resistance 1), lipid transfer proteins (LTPs) and PR‐10 (pathogenesis‐related class 10) are all exclusive to PATstri6Δ (Table 4). WIR1 proteins are hypothesized to be membrane‐spanning proteins that enhance the adhesion of the plasma membrane to the cell wall during pathogen attack (Bull et al., 1992); they are induced in wheat and barley in response to a number of microbial pathogens (Tufan et al., 2012). Similarly, some LTPs are speculated to play a role in reinforcing the cuticle through the incorporation of fatty acids, and have been reported previously to be key factors in priming in barley and FHB resistance in wheat (Petti et al., 2010; Schweiger et al., 2013). PR‐10 has functions related to secondary metabolism and antimicrobial activity, and is positively correlated with resistance to F. graminearum in cereals (Bernardo et al., 2007; Makandar et al., 2006; Mohammadi et al., 2011).
We observed several PATs with annotations related to reactive oxygen species (ROS), known to play key roles in plant immunity (Nanda et al., 2010). For example, superoxides and hydroxyl radicals may damage fungal structures directly (Hemetsberger et al., 2012). ROS produced by peroxidases during the synthesis of lignin, suberin and during pathogen‐induced cell wall modifications have been hypothesized to slow the influx of fungal toxins and efflux of plant nutrients (Kang and Buchenauer, 2000), and several oPATs and PATsnoxABΔ are annotated as peroxidases (Tables 2 and 3). In addition, several transcripts found in the oPATs and PATsnoxABΔ datasets are annotated as blue‐copper proteins, which function as electron transporters during redox processes, are induced during stress and wounding, and have previously been associated with disease resistance in wheat (Tables 2 and 3; Coram et al., 2010; Li et al., 2012).
Secondary metabolism also plays a major role in the development of the priming phenotype in wheat; multiple oPATs, PATsnoxABΔ and PATstri6Δ are annotated as part of the general phenylpropanoid pathway, including phenylalanine ammonia‐lyase, polyphenol oxidase and trans‐cinnamate 4‐monooxygenase (Tables 2, 3, 4). In addition, one oPAT was annotated as agmatine coumaroyltransferase (ACT), an enzyme that catalyses the biosynthesis of hydroxycinnamic acid amides (HCAAs), and one PATtri6Δ was annotated as flavonol synthase (Tables 2 and 3; Held et al., 1993). Flavonoids, coumarins and HCAAs can function as phytoalexins, but can also be deposited in plant cell walls as structural reinforcements (reviewed in Vogt, 2010). Recently, resistance to FHB associated with the wheat Fhb1 locus has been shown to be associated with the up‐regulation of flavonoid and HCAA biosynthesis, and the specific deposition of these compounds into the plant cell wall (Gunnaiah et al., 2012). Consistent with our previous observations, many secondary metabolic pathways are known to be regulated in a JA‐ and GA‐dependent manner (De Geyter et al., 2012; Hong et al., 2012).
During infection, F. graminearum produces a number of toxic secondary metabolites that disrupt host cell functions. Accordingly, the ability of the host to mitigate the effects of these toxins, either through detoxification or export, is critical for disease resistance. For example, glycosyltransferases (GTs) are known to play important roles in general detoxification, and some UDP‐glycosyltransferases (UGTs) are specifically associated with DON detoxification (Lulin et al., 2010; Poppenberger et al., 2003; Schweiger et al., 2013). There are multiple transcripts annotated as UGTs in PATstri6Δ (Table 4). Additional genes with predicted function in transport and stress tolerance were also found in the three PAT datasets (Tables 2, 3, 4).
We have successfully demonstrated the utility of nonpathogenic strains of F. graminearum to induce resistance in wheat heads and have profiled the global transcriptome during this phenomenon. The early arrest of the pathogen induced by the treatment with the tri6Δ mutant indicated that genes involved in the early defence response might be responsible for this phenotype. The expression of RLKs and MAPKs only in the PATstri6Δ dataset supports this hypothesis.
The wide array of transcripts associated with plant immunity during PIR against FHB probably reflects the importance of each of the multiple battlegrounds on which the F. graminearum–wheat interaction takes place. PIR in wheat is associated with the active transcription of genes that are involved in immune surveillance, signalling, reinforcement of the plant cell wall, and detoxification and/or export of fungal toxins. JA, ET and GA pathways are all suggested to play roles in the development of this phenotype, and we have highlighted transcripts encoding the phenylpropanoid pathway, detoxification/transport systems as well as PRR proteins that warrant further study regarding the genetic improvement of wheat through either traditional or advanced molecular means. Furthermore, our results support the use of nonpathogenic F. graminearum mutant strains as a management strategy for FHB in wheat.
Supporting information
Table S1 Priming‐associated transcripts (PATs) differentially expressed during priming‐induced resistance to Fusarium head blight in wheat.
Table S2 Quantitative reverse transcription‐polymerase chain reaction (qRT‐PCR) analysis of selected priming‐associated transcripts (PATs). Relative levels of expression were measured as described previously (Wang et al., 2010).
Acknowledgements
We would like to thank Dr Barbara Blackwell and Sally Buffam for DON analyses. The financial support from Agriculture and Agri‐Food Canada's Canadian Crops Genomics Initiative and Grain Farmers of Ontario is gratefully acknowledged.
Reproduced with the permission of the Minister of Agriculture And Agri‐Food Canada.
References
- Aimé, S. , Alabouvette, C. , Steinberg, C. and Olivain, C. (2013) The endophytic strain Fusarium oxysporum Fo47: a good candidate for priming the defence responses in tomato roots. Mol. Plant–Microbe Interact. 26, 918–926. [DOI] [PubMed] [Google Scholar]
- Alabouvette, C. , Olivain, C. , Migheli, Q. and Steinberg, C. (2009) Microbiological control of soil‐borne phytopathogenic fungi with special emphasis on wilt‐inducing Fusarium oxysporum . New Phytol. 184, 529–544. [DOI] [PubMed] [Google Scholar]
- Baldi, P. and Long, A.D. (2001) A Bayesian framework for the analysis of microarray expression data: regularized t‐test and statistical inferences of gene changes. Bioinformatics, 17, 509–519. [DOI] [PubMed] [Google Scholar]
- Benjamini, Y. and Hochberg, Y. (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. B, 57, 289–300. [Google Scholar]
- Bernardo, A. , Bai, G. , Guo, P. , Xiao, K. , Guenzi, A.C. and Ayoubi, P. (2007) Fusarium graminearum‐induced changes in gene expression between Fusarium head blight‐resistant and susceptible wheat cultivars. Funct. Integr. Genomics, 7, 69–77. [DOI] [PubMed] [Google Scholar]
- Bhardwaj, V. , Meier, S. , Petersen, L.N. , Ingle, R.A. and Roden, L.C. (2011) Defence responses of Arabidopsis thaliana to infection by Pseudomonas syringae are regulated by the circadian clock. PLoS ONE, 6, e268968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bull, J. , Mauch, F. , Hertig, C. , Rebmann, G. and Dudler, R. (1992) Sequence and expression of a wheat gene that encodes a novel protein associated with pathogen defence. Mol. Plant–Microbe Interact. 5, 516–519. [DOI] [PubMed] [Google Scholar]
- Chini, A. , Fonseca, S. , Fernández, G. , Adie, B. , Chico, J.M. , Lorenzo, O. , García‐Casado, G. , López‐Vidriero, I. , Lozano, F.M. , Ponce, M.R. , Micol, J.L. and Solano, R. (2007) The JAZ family of repressors is the missing link in jasmonate signalling. Nature, 448, 666–671. [DOI] [PubMed] [Google Scholar]
- Coram, T.E. , Huang, X. , Zhan, G. , Settles, M.L. and Chen, X. (2010) Meta‐analysis of transcripts associated with race‐specific resistance to stripe rust in wheat demonstrates common induction of blue copper‐binding protein, heat‐stress transcription factor, pathogen‐induced WIR1A protein, and ent‐kaurene synthase transcripts. Funct. Integr. Genomics, 10, 383–392. [DOI] [PubMed] [Google Scholar]
- De Geyter, N. , Gholami, A. , Goormachtig, S. and Goossens, A. (2012) Transcriptional machineries in jasmonate‐elicited plant secondary metabolism. Trends Plant Sci. 17, 349–359. [DOI] [PubMed] [Google Scholar]
- Deslandes, L. and Rivas, S. (2012) Catch me if you can: bacterial effectors and plant targets. Trends Plant Sci. 17, 644–655. [DOI] [PubMed] [Google Scholar]
- Dodds, P.N. and Rathjen, J.P. (2010) Plant immunity: towards an integrated view of plant–pathogen interactions. Nat. Rev. Genet. 11, 539–548. [DOI] [PubMed] [Google Scholar]
- Eulgem, T. (2006) Dissecting the WRKY web of plant defence regulators. PLoS Pathog. 2, e126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao, X. , Chen, X. , Lin, W. , Chen, S. , Lu, D. , Niu, Y. , Li, L. , Cheng, C. , McCormack, M. , Sheen, J. and Ping, H. (2013) Bifurcation of Arabidopsis NLR immune signaling via Ca2+‐dependent protein kinases. Plos Pathog. 9, e1003127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnaiah, R. , Kushalappa, A.C. , Duggavathi, R. , Fox, S. and Somers, D.J. (2012) Integrated metabolo‐proteomic approach to decipher the mechanisms by which wheat qtl (Fhb1) contributes to resistance against Fusarium graminearum . Plos ONE, 7, e40695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He, C.Y. , Hsiang, T. and Wolyn, D.J. (2002) Induction of systemic disease resistance and pathogen defence responses in Asparagus officinalis inoculated with nonpathogenic strains of Fusarium oxysporum . Plant Pathol. 51, 225–230. [Google Scholar]
- Held, B.M. , Wang, H. , John, I. , Wurtele, E.S. and Colbert, J.T. (1993) An mRNA putatively coding for an O‐methyltransferase accumulates preferentially in maize roots and is located predominantly in the region of the endodermis. Plant Physiol. 102, 1001–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemetsberger, C. , Herrberger, C. , Zechmann, B. , Hillmer, M. and Doehlemann, G. (2012) The Ustilago maydis effector Pep1 suppresses plant immunity by inhibition of host peroxidase activity. Plos Pathog. 8, e1002684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong, G.J. , Xue, X.Y. , Mao, Y.B. , Wang, L.J. and Chen, X.Y. (2012) Arabidopsis MYC2 interacts with DELLA proteins in regulating sesquiterpene synthase gene expression. Plant Cell, 24, 2635–2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou, X. , Lee, L.Y.C. , Xia, K. , Yan, Y. and Yu, H. (2010) DELLAs modulate jasmonate signaling via competitive binding to JAZs. Dev. Cell, 19, 884–894. [DOI] [PubMed] [Google Scholar]
- Jansen, C. , von Wettstein, D. , Schäfer, W. , Kogel, K.‐H. , Felk, A. and Maier, F.J. (2005) Infection patterns in barley and wheat spikes inoculated with wild‐type and trichodiene synthase gene disrupted Fusarium graminearum . Proc. Natl. Acad. Sci. USA, 102, 16 892–16 897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang, Z. and Buchenauer, H. (2000) Ultrastructural and immunocytochemical investigation of pathogen development and host responses in resistant and susceptible wheat spikes infected by Fusarium culmorum . Physiol. Mol. Plant Pathol. 57, 255–268. [Google Scholar]
- Kazan, K. and Manners, J.M. (2008) Jasmonate signaling: toward an integrated view. Plant Physiol. 146, 1459–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazan, K. and Manners, J.M. (2012) JAZ repressors and the orchestration of phytohormone crosstalk. Trends Plant Sci. 17, 22–31. [DOI] [PubMed] [Google Scholar]
- Khan, M.R. and Doohan, F.M. (2009) Bacterium‐mediated control of Fusarium head blight disease of wheat and barley and associated mycotoxin contamination of grain. Biol. Control, 48, 42–47. [Google Scholar]
- Li, Y.C. , Meng, F.R. , Zhang, C.Y. , Zhang, N. , Sun, M.S. , Ren, J.P. , Niu, H.B. , Wang, X. and Yin, J. (2012) Comparative analysis of water stress‐responsive transcriptomes in drought‐susceptible and ‐tolerant wheat (Triticum aestivum L.). J. Plant Biol. 55, 349–360. [Google Scholar]
- Liu, J. , Elmore, J.M. and Coaker, G. (2009) Investigating the functions of the RIN4 protein complex during plant innate immune responses. Plant Signal. Behav. 4, 1107–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, X. , Yang, L. , Zhou, X. , Zhou, M. , Lu, Y. , Ma, L. , Ma, H. and Zhang, Z. (2013) Transgenic wheat expressing Thinopyrum intermedium MYB transcription factor TiMYB2R. J. Exp. Bot. 64, 2243–2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lulin, M. , Yi, S. , Aizhong, C. , Zengjun, Q. , Liping, X. , Peidu, C. , Dajun, L. and Xiu‐E, W. (2010) Molecular cloning and characterization of an up‐regulated UDP‐glucosyltransferase gene induced by DON from Triticum aestivum L. cv. Wangshuibai. Mol. Biol. Rep. 37, 785–795. [DOI] [PubMed] [Google Scholar]
- Mackey, D. , Belkhadir, Y. , Alonso, J.M. , Ecker, J.R. and Dangl, J.L. (2003) Arabidopsis RIN4 is a target of the type III virulence effector AvrRpt2 and modulates RPS2‐mediated resistance. Cell, 112, 379–389. [DOI] [PubMed] [Google Scholar]
- Makandar, R. , Essig, J.S. , Schapaugh, M.A. , Trick, H.N. and Shah, J. (2006) Genetically engineered resistance to Fusarium head blight in wheat by expression of Arabidopsis NPR1. Mol. Plant–Microbe Interact. 19, 123–129. [DOI] [PubMed] [Google Scholar]
- Memelink, J. (2009) Regulation of gene expression by jasmonate hormones. Phytochemistry, 70, 1560–1570. [DOI] [PubMed] [Google Scholar]
- Miklis, M. , Consonni, C. , Bhat, R.A. , Lipka, V. , Schulze‐Lefert, P. and Panstruga, R. (2007) Barley MLO modulates actin‐dependent and actin‐independent antifungal defence pathways at the cell periphery. Plant Physiol. 144, 1132–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammadi, M. , Anoop, V. , Gleddie, S. and Harris, L.J. (2011) Proteomic profiling of two maize inbreds during early gibberella ear rot infection. Proteomics, 11, 3675–3684. [DOI] [PubMed] [Google Scholar]
- Nanda, A.K. , Andrio, E. , Marino, D. , Pauly, N. and Dunand, C. (2010) Reactive oxygen species during plant–microorganism early interactions. J. Integr. Plant Biol. 52, 195–204. [DOI] [PubMed] [Google Scholar]
- Nasmith, C.G. , Walkowiak, S. , Wang, L. , Leung, W.W.Y. , Gong, Y. , Johnston, A. , Harris, L.J. , Guttman, D.S. and Subramaniam, R. (2011) Tri6 is a global transcription regulator in the phytopathogen Fusarium graminearum . PLoS Pathog. 7, e1002266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panstruga, R. (2005) Serpentine plant MLO proteins as entry portals for powdery mildew fungi. Biochem. Soc. Trans. 33, 389–392. [DOI] [PubMed] [Google Scholar]
- Petti, C. , Khan, M. and Doohan, F. (2010) Lipid transfer proteins and protease inhibitors as key factors in the priming of barley responses to Fusarium head blight disease by a biocontrol strain of Pseudomonas fluorescens . Funct. Integr. Genomics, 10, 619–627. [DOI] [PubMed] [Google Scholar]
- Poppenberger, B. , Berthiller, F. , Lucyshyn, D. , Sieberer, T. , Schuhmacher, R. , Krska, R. , Kuchler, K. , Glössl, J. , Luschnig, C. and Adam, G. (2003) Detoxification of the Fusarium mycotoxin deoxynivalenol by a UDP‐glucosyltransferase from Arabidopsis thaliana . J. Biol. Chem. 278, 47 905–47 914. [DOI] [PubMed] [Google Scholar]
- Ross, C.A. , Liu, Y. and Shen, Q.J. (2007) The WRKY gene family in rice (Oryza sativa). J. Integr. Plant Biol. 49, 827–842. [Google Scholar]
- Scherm, B. , Orrù, M. , Balmas, V. , Spanu, F. , Azara, E. , Delogu, G. , Hammond, T.M. , Keller, N.P. and Migheli, Q. (2011) Altered trichothecene biosynthesis in TRI6‐silenced transformants of Fusarium culmorum influences the severity of crown and foot rot on durum wheat seedlings. Mol. Plant Pathol. 12, 759–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweiger, W. , Steiner, B. , Ametz, C. , Siegwart, G. , Wiesenberger, G. , Berthiller, F. , Lemmens, M. , Jia, H. , Adam, G. , Muehlbauer, G.J. , Kreil, D.P. and Buerstmayr, H. (2013) Transcriptomic characterization of two major Fusarium resistance quantitative trait loci (QTLs), Fhb1 and Qfhs.ifa‐5A, identifies novel candidate genes. Mol. Plant Pathol. 14, 772–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seong, K.Y. , Pasquali, M. , Zhou, X. , Song, J. , Hilburn, K. , McCormick, S. , Dong, Y. , Xu, J.R. and Kistler, H.C. (2009) Global gene regulation by Fusarium transcription factors Tri6 and Tri10 reveals adaptations for toxin biosynthesis. Mol. Microbiol. 72, 354–367. [DOI] [PubMed] [Google Scholar]
- Sun, T. (2010) Gibberellin‐GID1‐DELLA: a pivotal regulatory module for plant growth and development. Plant Physiol. 154, 567–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thatcher, L.F. , Gardiner, D.M. , Kazan, K. and Manners, J.M. (2012) A highly conserved effector in Fusarium oxysporum is required for full virulence on Arabidopsis. Mol. Plant–Microbe Interact. 25, 180–190. [DOI] [PubMed] [Google Scholar]
- Thimm, O. , Bläsing, O. , Gibon, Y. , Nagel, A. , Meyer, S. , Krüger, P. , Selbig, J. , Müller, L.A. , Rhee, S.Y. and Stitt, M. (2004) MAPMAN: a user‐driven tool to display genomics data sets onto diagrams of metabolic pathways and other biological processes. Plant J. 37, 914–939. [DOI] [PubMed] [Google Scholar]
- Thines, B. , Katsir, L. , Melotto, M. , Niu, Y. , Mandaokar, A. , Liu, G. , Nomura, K. , He, S.Y. , Howe, G.A. and Browse, J. (2007) JAZ repressor proteins are targets of the SCFCOI1 complex during jasmonate signalling. Nature, 448, 661–665. [DOI] [PubMed] [Google Scholar]
- Tufan, H.A. , McGrann, G.R.D. , Maccormack, R. and Boyd, L.A. (2012) TaWIR1 contributes to post‐penetration resistance to Magnaporthe oryzae, but not Blumeria graminis f. sp. tritici, in wheat. Mol. Plant Pathol. 13, 653–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Does, D. , Leon‐Reyes, A. , Koornneef, A. , Van Verk, M.C. , Rodenburg, N. , Pauwels, L. , Goossens, A. , Körbes, A.P. , Memelink, J. , Ritsema, T. , Van Wees, S.C. and Pieterse, C.M. (2013) Salicylic acid suppresses jasmonic acid signaling downstream of SCFCOI1‐JAZ by targeting GCC promoter motifs via transcription factor ORA59. Plant Cell, 25, 744–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Der Ent, S. , Van Hulten, M. , Pozo, M.J. , Czechowski, T. , Udvardi, M.K. , Pieterse, C.M.J. and Ton, J. (2009) Priming of plant innate immunity by rhizobacteria and β‐aminobutyric acid: differences and similarities in regulation. New Phytol. 183, 419–431. [DOI] [PubMed] [Google Scholar]
- Van Hulten, M. , Pelser, M. , Van Loon, L.C. , Pieterse, C.M.J. and Ton, J. (2006) Costs and benefits of priming for defence in Arabidopsis. Proc. Natl. Acad. Sci. USA, 103, 5602–5607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt, T. (2010) Phenylpropanoid biosynthesis. Mol. Plant, 3, 2–20. [DOI] [PubMed] [Google Scholar]
- Wang, J.R. , Wang, L. , Gulden, S. , Rocheleau, H. , Balcerzak, M. , Hattori, J. , Cao, W. , Han, F. , Zheng, Y.L. , Fedak, G. and Ouellet, T. (2010) RNA profiling of Fusarium head light‐resistant wheat addition lines containing the Thinopyrum elongatum chromosome 7E. Can. J. Plant Pathol. 32, 188–214. [Google Scholar]
- Wang, L. , Mogg, C. , Walkowiak, S. , Joshi, M. and Subramaniam, R. (2014) Characterization of NADPH oxidase genes NoxA and NoxB in Fusarium graminearum . Can. J. Plant Pathol. 36, 12–21. [Google Scholar]
- Xin, X.F. and He, S.Y. (2013) Pseudomonas syringae pv. tomato DC3000: a model pathogen for probing disease susceptibility and hormone signaling in plants. Annu. Rev. Phytopathol. 51, 473–498. [DOI] [PubMed] [Google Scholar]
- Xue, A.G. , Voldeng, H.D. , Savard, M.E. and Fedak, G. (2009) Biological management of Fusarium head blight and mycotoxin contamination in wheat. World Mycotoxin J. 2, 193–201. [Google Scholar]
- Zhang, Z.L. , Ogawa, M. , Fleet, C.M. , Zentella, R. , Hu, J. , Heo, J.O. , Lim, J. , Kamiya, Y. , Yamaguchi, S. and Sun, T.P. (2011) SCARECROW‐LIKE 3 promotes gibberellin signaling by antagonizing master growth repressor DELLA in Arabidopsis. Proc. Natl. Acad. Sci. USA, 108, 2160–2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zipfel, C. (2009) Early molecular events in PAMP‐triggered immunity. Curr. Opin. Plant Biol. 12, 414–420. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Priming‐associated transcripts (PATs) differentially expressed during priming‐induced resistance to Fusarium head blight in wheat.
Table S2 Quantitative reverse transcription‐polymerase chain reaction (qRT‐PCR) analysis of selected priming‐associated transcripts (PATs). Relative levels of expression were measured as described previously (Wang et al., 2010).


