Summary
d‐Galacturonic acid is the most abundant monosaccharide component of pectic polysaccharides that comprise a significant part of most plant cell walls. Therefore, it is potentially an important nutritional factor for Botrytis cinerea when it grows in and through plant cell walls. The d‐galacturonic acid catabolic pathway in B. cinerea consists of three catalytic steps converting d‐galacturonic acid to pyruvate and l‐glyceraldehyde, involving two nonhomologous galacturonate reductase genes (Bcgar1 and Bcgar2), a galactonate dehydratase gene (Bclgd1) and a 2‐keto‐3‐deoxy‐l‐galactonate aldolase gene (Bclga1). Knockout mutants in each step of the pathway (ΔBcgar1/ΔBcgar2, ΔBclgd1 and ΔBclga1) showed reduced virulence on Nicotiana benthamiana and Arabidopsis thaliana leaves, but not on Solanum lycopersicum leaves. The cell walls of N. benthamiana and A. thaliana leaves were shown to have a higher d‐galacturonic acid content relative to those of S. lycopersicum. The observation that mutants displayed a reduction in virulence, especially on plants with a high d‐galacturonic acid content in the cell walls, suggests that, in these hosts, d‐galacturonic acid has an important role as a carbon nutrient for B. cinerea. However, additional in vitro growth assays with the knockout mutants revealed that B. cinerea growth is reduced when d‐galacturonic acid catabolic intermediates cannot proceed through the entire pathway, even when fructose is present as the major, alternative carbon source. These data suggest that the reduced virulence of d‐galacturonic acid catabolism‐deficient mutants on N. benthamiana and A. thaliana is not only a result of the inability of the mutants to utilize an abundant carbon source as nutrient, but also a result of the growth inhibition by catabolic intermediates.
Introduction
Botrytis cinerea is a necrotrophic fungal plant pathogen infecting over 200 host plants and causing significant economic damage (pre‐ and post‐harvest) to crops worldwide. The wide variety of symptoms on different tissues and plants suggests that B. cinerea possesses a large arsenal of weapons to invade its hosts (Choquer et al., 2007). The infection process includes the penetration of the host tissue, killing of the host cells and lesion expansion, followed by tissue maceration and sporulation (van Kan, 2006). Botrytis cinerea produces a variety of compounds capable of killing plant cells, such as phytotoxic metabolites and proteins, oxalic acid and hydrogen peroxide (van Kan, 2006). However, the ultimate purpose of a necrotroph is not to kill its host, but to decompose the plant tissue and utilize the host‐derived nutrients for its own growth. Botrytis cinerea secretes multiple cell wall‐degrading enzymes (including pectinases, cellulases and hemicellulases) to facilitate plant tissue colonization and to release carbohydrates for consumption; several of these enzymes have been demonstrated to be required for full virulence (Choquer et al., 2007).
Botrytis cinerea often penetrates host leaf tissue at the anticlinal cell wall and subsequently grows into and through the middle lamella, which consists mostly of low‐methyl‐esterified pectin (ten Have et al., 2002). The importance of pectin degradation for the virulence of B. cinerea was demonstrated by targeted mutagenesis in endo‐polygalacturonase (endo‐PG) genes. Strains with mutations in endo‐PG genes have a reduced capacity for pectin decomposition and, consequently, release reduced amounts of nutrients for the fungus to potentially catabolize. Strains with a mutation in the Bcpg1 gene were reduced in virulence by 25% (ten Have et al., 1998), whereas mutants in the Bcpg2 gene were reduced in virulence by >50% (Kars et al., 2005). These experiments, however, could not distinguish whether pectin decomposition during infection occurs for the purpose of plant tissue colonization or for the release of monosaccharides that serve as nutrients for fungal growth, or both. In order to distinguish between the relevance of pectin decomposition for plant tissue colonization or for nutrient acquisition by the fungus, it is imperative to obtain B. cinerea mutants that produce the full spectrum of plant cell wall‐decomposing enzymes, but cannot catabolize the monosaccharides that are released from the cell wall polymers.
Current knowledge of monosaccharide utilization and catabolism by B. cinerea during plant infection is limited. NMR spectroscopy has suggested that, during colonization of sunflower cotyledons, B. cinerea converts glucose and fructose present in the host plant into mannitol via pathways involving the enzymes mannitol‐1‐phosphate dehydrogenase (BcMPD), mannitol‐1‐phosphate phosphatase and mannitol‐2‐dehydrogenase (BcMTDH) (Dulermo et al., 2009, 2010). Transcript levels of Bcmpd and Bcmtdh, as well as enzyme activities of BcMPD and BcMTDH, increased during the progress of infection (Dulermo et al., 2009). Analysis of single‐ and double‐knockout mutants in the Bcmpd and Bcmtdh genes, however, revealed that deletion of these genes did not abolish mannitol metabolism and did not affect virulence on sunflower (Dulermo et al., 2010). A different study showed that hexokinase is required for B. cinerea development and for virulence on apple and tomato fruit. The extent of reduction in virulence of a hexokinase‐deficient mutant was correlated with the content of sugars in the fruit, in particular fructose (Rui and Hahn, 2007).
The monosaccharide d‐galacturonic acid is the most abundant component of pectin polysaccharides (Caffall and Mohnen, 2009; Mohnen, 2008), and might constitute an important part of the nutrition of B. cinerea when it grows in and through plant cell walls. Recently, we have characterized the d‐galacturonic acid catabolic pathway in B. cinerea, which consists of three catalytic steps converting d‐galacturonic acid to pyruvate and l‐glyceraldehyde. The pathway involves two nonhomologous galacturonate reductase genes (Bcgar1 and Bcgar2), a galactonate dehydratase gene (Bclgd1) and a 2‐keto‐3‐deoxy‐L‐galactonate aldolase gene (Bclga1) (Zhang et al., 2011). Their transcript levels were induced substantially when the fungus was cultured in media containing d‐galacturonic acid, pectate or pectin as the sole carbon source. BcGAR1 and BcGAR2 jointly contribute to the conversion of d‐galacturonic acid to l‐galactonate, albeit to a different extent. BcLGD1 converts l‐galactonate to 2‐keto‐3‐deoxy‐l‐galactonate, which is subsequently catalysed by BcLGA1 to pyruvate and l‐glyceraldehyde. Targeted gene replacement of the four genes in B. cinerea, either separately or in combination, yielded mutants that were affected in growth on d‐galacturonic acid or pectic substrates (pectate, apple pectin, citrus pectin) as the sole carbon source. The extent of growth reduction of the mutants on pectic substrates (as sole carbon source) was correlated with the proportion of d‐galacturonic acid in the substrate. The growth of the mutants on apple pectin (containing only 61% d‐galacturonic acid) was reduced by ∼50%, whereas growth on citrus pectin (containing 78% d‐galacturonic acid) was reduced by ∼75%, and growth on sodium pectate (containing >99% d‐galacturonic acid) was negligible (Zhang et al., 2011).
In this study, we analysed the d‐galacturonic acid content in leaf cell wall extracts of three plant species (Solanum lycopersicum, Nicotiana benthamiana and Arabidopsis thaliana) and analysed the expression profiles of B. cinerea d‐galacturonic acid catabolic genes in planta, as well as the virulence of B. cinerea mutants in these genes.
Results
The d‐galacturonic acid content in cell walls differs among plant species
The alcohol‐insoluble residue (AIR) fraction, mainly consisting of cell wall polysaccharides, was extracted from leaves of S. lycopersicum, N. benthamiana and A. thaliana. For all three plant species, AIR makes up ∼70% of the leaf dry weight (not shown). The polysaccharides were hydrolysed and the monosaccharide composition and contents were quantified by chemical analysis (Fig. 1). The contents of neutral sugars in the hydrolysed AIR fraction differed among the three plant species. The content of glucose (the most abundant neutral sugar) in the AIR polysaccharides of S. lycopersicum was significantly higher than that in N. benthamiana and A. thaliana. The content of uronic acids (>95% of which is d‐galacturonic acid) in the AIR polysaccharides of S. lycopersicum was 60–70% of the levels in N. benthamiana and A. thaliana (Fig. 1).
Figure 1.
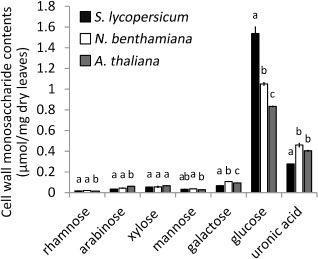
Cell wall monosaccharide contents of leaves of Solanum lycopersicum, Nicotiana benthamiana and Arabidopsis thaliana. The monosaccharides analysed are given underneath the columns. Contents are given in μmol/mg dry weight. Bars indicate means ± standard deviation. For each monosaccharide, the letters above the bars indicate statistical significance; bars not sharing letters represent significant mean differences at P < 0.05 by Student's t‐test.
The contents of glucose, fructose and sucrose were measured in water‐soluble leaf extracts of the three plant species. The contents of glucose and sucrose were higher in N. benthamiana than in S. lycopersicum and A. thaliana, whereas the content of fructose was higher in S. lycopersicum and N. benthamiana than in A. thaliana (Fig. S1, see Supporting Information). The overall contents of free sugars in plant leaves were negligible relative to the sugars assimilated in the AIR extract from plant cell walls (Fig. 1).
Transcript levels of d‐galacturonic acid catabolic genes during infection
The expression profiles of the d‐galacturonic acid catabolic genes Bcgar1, Bcgar2, Bclgd1 and Bclga1 were quantified during infection of wild‐type B. cinerea on leaves of S. lycopersicum, N. benthamiana and A. thaliana at two time points (Fig. 2). All four genes were expressed in each host plant and there were only marginal differences in transcript levels between 2 and 3 days post‐inoculation (dpi) (relative to an internal standard transcript). The transcript levels of the four genes were all higher in N. benthamiana and A. thaliana than in S. lycopersicum, except for Bcgar1 in N. benthamiana at 2 dpi. The relative transcript levels of Bclgd1 were more than two‐fold higher in N. benthamiana and A. thaliana than in S. lycopersicum. The transcript levels observed in planta (higher in N. benthamiana and A. thaliana than in S. lycopersicum; Fig. 2) correlate with the contents of d‐galacturonic acid in the AIR polysaccharides, which were higher in N. benthamiana and A. thaliana than in S. lycopersicum (Fig. 1).
Figure 2.
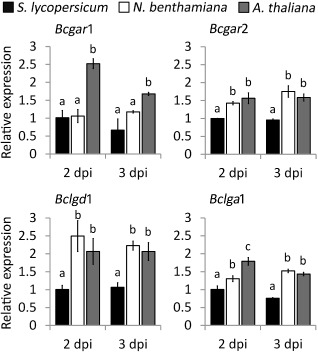
Relative transcript levels of d‐galacturonic acid catabolic genes during infection on Solanum lycopersicum, Nicotiana benthamiana and Arabidopsis thaliana. Infected plants were sampled at 2 and 3 days post‐inoculation (dpi) for RNA extraction. mRNA levels of d‐galacturonic acid catabolic genes were normalized to the levels of the constitutive reference gene Bcrpl5 and calibrated to the levels on S. lycopersicum at time point 2 dpi (set as 1), according to the 2–ΔΔCt method. Data are represented as means ± standard deviation from one biological repeat. Three technical replicates of each repeat were analysed and three independent biological repeats were performed, all with similar results. For each time point, letters above the bars indicate statistical significance; bars not sharing letters represent significant mean differences at P < 0.05 by Student's t‐test.
Virulence of d‐galacturonic acid catabolism‐deficient mutants on S. lycopersicum, N. benthamiana and A. thaliana leaves
To investigate to what extent d‐galacturonic acid serves as an important carbon source for B. cinerea growth during infection, the virulence of ΔBcgar1/ΔBcgar2, ΔBclgd1 and ΔBclga1 mutants was tested on leaves of the three plant species. For each step in the d‐galacturonic acid catabolic pathway, two independent mutants were tested and yielded essentially identical results.
On S. lycopersicum leaves, each mutant generated lesion sizes similar to that of the wild‐type strain (Fig. S2A, see Supporting Information). The fungal biomass on S. lycopersicum leaves was monitored at 2 and 3 dpi, and each mutant showed a similar fungal biomass to that of the wild‐type strain (Fig. S2B).
On N. benthamiana leaves, mutants in all three steps of the catabolic pathway produced smaller lesions relative to those on the wild‐type strain (Fig. 3). ΔBcgar1/ΔBcgar2 and ΔBclga1 mutants displayed ∼25% reduction in lesion size at 3 dpi, whereas ΔBclgd1 mutants displayed ∼45% reduction (Fig. 3A,C). Immunological quantification indicated that the biomass of each mutant was similar to that of the wild‐type strain at 2 dpi (not shown). At 3 dpi, however, the biomass of ΔBcgar1/ΔBcgar2 and ΔBclga1 mutants on N. benthamiana was ∼71% of that of the wild‐type strain, and the biomass of ΔBclgd1 mutants was ∼42% of that of the wild‐type strain (Fig. 3B). This suggests that the catabolism of d‐galacturonic acid released from pectin in N. benthamiana leaves is important for the expansion of lesions, but not for the initial colonization. In addition to N. benthamiana, all mutants produced smaller lesions on N. tabacum leaves relative to those of the wild‐type strain. ΔBclgd1 mutants displayed a greater decrease in lesion size than did ΔBcgar1/ΔBcgar2 and ΔBclga1 mutants (not shown).
Figure 3.
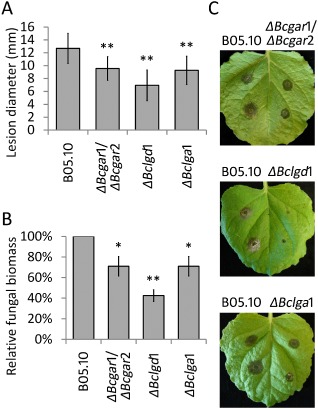
Virulence of d‐galacturonic acid catabolism‐deficient mutants on Nicotiana benthamiana. (A) Lesion development of Botrytis cinerea on N. benthamiana leaves was evaluated at 3 days post‐inoculation (dpi) by determining the average lesion diameter on four leaves from eight plants each. Data represent means ± standard deviation (n ≥ 50 independent lesions). (B) Botrytis cinerea biomass by immunological detection at 3 dpi on N. benthamiana. Six discs (30 mm in diameter, containing the whole lesions in the centre) from three leaves of three plants were sampled as a pool for quantification. The fungal biomass of the mutants was normalized to that of the wild‐type strain. Experiments were repeated at least twice with similar results. *P < 0.05, **P < 0.01 by Student's t‐test. (C) Disease symptoms of B. cinerea on N. benthamiana leaves at 3 dpi.
Finally, the virulence of ΔBcgar1/ΔBcgar2, ΔBclgd1 and ΔBclga1 mutants was investigated on leaves of A. thaliana ecotype Columbia (Col‐0). The A. thaliana leaf is much smaller than that of tomato and tobacco; thus, only one droplet of a suspension of conidia of wild‐type or mutants was inoculated onto each detached leaf. Wild‐type strain B05.10 caused extensive necrosis on Col‐0, whereas the mutants showed a significant reduction in the extent of necrosis at 3 dpi (Fig. 4A). As the shapes of expanding lesions on A. thaliana were not circular, it was not possible to determine the lesion diameter. The fungal biomass was quantified by immunodetection using pools of eight leaves sampled at different time points. Fungal biomass on Col‐0 did not differ between the mutants and the wild‐type strain at 2 dpi (data not shown). However, at 3 dpi, the biomasses of ΔBcgar1/ΔBcgar2, ΔBclgd1 and ΔBclga1 mutants on Col‐0 were ∼62%, ∼50% and ∼70% of that of the wild‐type strain, respectively (Fig. 4B). These data are similar to the results on N. benthamiana (Fig. 3B).
Figure 4.
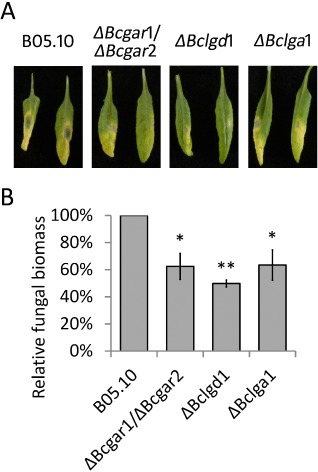
Virulence of d‐galacturonic acid catabolism‐deficient mutants on Arabidopsis thaliana. (A) Disease symptoms of Botrytis cinerea on A. thaliana leaves at 3 days post‐inoculation (dpi). Two representative leaves are shown for each plant/strain combination. (B) Botrytis cinerea biomass accumulation by immunological detection at 3 dpi. Eight detached leaves were sampled as a pool for quantification. The fungal biomass of the mutants was normalized to that of the wild‐type strain. Data represent means ± standard deviation from two independent biological repeats. *P < 0.05, **P < 0.01 by Student's t‐test.
Transcript levels of endo‐PG genes in d‐galacturonic acid catabolism‐deficient mutants
In plant tissues that are infected by B. cinerea, pectin is mainly depolymerized by secreted endo‐PGs and exo‐PGs (Kars et al., 2005; Williamson et al., 2007; Wubben et al., 1999), releasing d‐galacturonic acid that potentially serves as a nutrient for fungal growth. We considered the possibility that the reduced virulence in d‐galacturonic acid catabolism‐deficient mutants might be an indirect consequence of the altered regulation of endo‐PG genes. To assess whether the expression profiles of endo‐PG genes were affected in d‐galacturonic acid catabolism‐deficient mutants, the transcript levels of Bcpg1–6 were investigated in the wild‐type strain and in mutants during infection on A. thaliana at 2 and 3 dpi (Fig. 5). Bcpg1 showed similar transcript levels in the d‐galacturonic acid catabolism‐deficient mutants as in the wild‐type strain at 2 and 3 dpi. Transcripts of Bcpg2, Bcpg3 and Bcpg5 were not detected under these experimental conditions. The transcript levels of Bcpg4 and Bcpg6 were higher in all the mutants relative to those in the wild‐type strain at 2 and 3 dpi. These results are in agreement with reports indicating that transcript levels of Bcpg4 and Bcpg6 (but not Bcpg1, Bcpg2, Bcpg3 and Bcpg5) are induced in cultures containing d‐galacturonic acid as sole carbon source (Wubben et al., 2000). The expression profiles of Bcpg4 and Bcpg6 suggest that d‐galacturonic acid (or catabolic pathway intermediates) accumulates in mutants during infection to a concentration sufficient to hyperinduce Bcpg4 and Bcpg6 transcripts.
Figure 5.
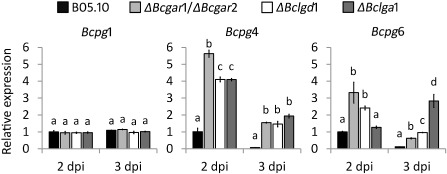
Relative transcript levels of Bcpg1, Bcpg4 and Bcpg6 in wild‐type Botrytis cinerea and d‐galacturonic acid catabolism‐deficient mutants during infection on Arabidopsis thaliana leaves. Infected plants were sampled at 2 and 3 days post‐inoculation (dpi) for RNA extraction. mRNA levels of Bcpg1, Bcpg4 and Bcpg6 genes were normalized to the levels of the constitutive reference gene Bcrpl5 and calibrated to wild‐type strain B05.10 levels at time point 2 dpi (set as 1), according to the 2–ΔΔCt method. Data are represented as means ± standard deviation from one biological repeat. Three technical replicates of each repeat were analysed and three independent biological repeats were performed, which showed similar results. For each time point, letters above the bars indicate statistical significance; bars not sharing letters represent significant mean differences at P < 0.01 by Student's t‐test.
During infection on N. benthamiana, higher transcript levels of Bcpg4 and Bcpg6 were also observed in the d‐galacturonic acid catabolism‐deficient mutants in comparison with those in the wild‐type strain, especially at 3 dpi (Fig. S3, see Supporting Information). By contrast, on S. lycopersicum, Bcpg4 and Bcpg6 exhibited similar transcript levels in the d‐galacturonic acid catabolism‐deficient mutants and in the wild‐type, and these transcript levels were negligible compared with those on N. benthamiana and A. thaliana (Fig. S3). These results indicate that the deficiency of d‐galacturonic acid catabolism in B. cinerea does not impair the expression of endo‐PG genes. Therefore, the misregulation of endo‐PG genes is not the cause of the reduced virulence of the mutants.
Defence responses in A. thaliana
In order to test whether the reduced virulence of the B. cinerea mutants in A. thaliana could be explained by altered defence responses, the expression was monitored of several A. thaliana genes that are involved in basal resistance to pathogens, including pad3, required for the production of camalexin (Böttcher et al., 2009; Zhou et al., 1999), PR1, a marker gene for salicylic acid‐dependent defence (Cao et al., 1994; Delaney et al., 1994; Penninckx et al., 1996), and PDF1.2, a marker for jasmonic acid/ethylene‐dependent defence (Penninckx et al., 1998; Thomma et al., 1998). Moreover, the expression was monitored of genes which contribute to partial resistance against B. cinerea, including AtPGIP1, AtPME3 and AtrbohD (van Baarlen et al., 2007; Ferrari et al., 2003, 2006; Raiola et al., 2011; Torres et al., 2005). Transcript levels of these genes were determined following inoculation of A. thaliana with B. cinerea wild‐type and d‐galacturonic acid catabolism‐deficient mutants. Most defence‐related genes tested were induced to a similar extent by the wild‐type and mutant B. cinerea strains; in a few cases, the defence genes showed lower expression levels in response to the mutants (Fig. S4, see Supporting Information).
Growth inhibition by intermediates in the d‐galacturonic acid catabolic pathway
It was striking that the extent of virulence reduction of ΔBclgd1 mutants on N. benthamiana and A. thaliana was higher than that of Bcgar1/ΔBcgar2 and ΔBclga1 mutants (Figs 3 and 4). If the reduction in virulence was merely caused by the inability of mutants to utilize d‐galacturonic acid as carbon source for growth, one would expect that mutants in all three steps would show the same extent of reduction in virulence, which would reflect the contribution of d‐galacturonic acid as a carbon source to the fungus. As the virulence of the ΔBclgd1 mutants was reduced even more than that of Bcgar1/ΔBcgar2 and ΔBclga1 mutants, we hypothesized that the accumulation of the intermediate l‐galactonate (substrate of the BcLGD1 protein) might result in growth inhibition to B. cinerea. To test this, the growth of d‐galacturonic acid catabolism‐deficient mutants was monitored on agar containing fructose as the most abundant carbon source, with or without a small amount of d‐galacturonic acid. Unexpectedly, all three d‐galacturonic acid catabolism‐deficient mutants showed dose‐dependent growth reduction when compared with the wild‐type strain (Fig. 6A). The growth of all three mutants was similar to that of the wild‐type strain on medium with 0.05 mm d‐galacturonic acid; however, growth was clearly reduced with 0.3 mm d‐galacturonic acid between days 3 and 5 of incubation and severely reduced with 1 mm d‐galacturonic acid within the first 3 days. Colony diameters of Bcgar1/ΔBcgar2, ΔBclgd1 and ΔBclga1 mutants were 32%, 64% and 33% smaller on medium containing fructose + 0.3 mm d‐galacturonic acid and 49%, 73% and 54% smaller on medium containing fructose + 1 mm d‐galacturonic acid, respectively, when compared with the diameters on medium with fructose only. The wild‐type strain did not show any growth reduction on medium with or without d‐galacturonic acid. Transcripts of Bcgar1, Bcgar2, Bclgd1 and Bclga1 were repressed in the presence of glucose (Zhang et al., 2011). Therefore, we tested the growth of Bcgar1/ΔBcgar2, ΔBclgd1 and ΔBclga1 mutants on agar plates containing glucose as the major carbon source with or without 1 mm d‐galacturonic acid. The growth of all three mutants was similar on agar plates containing 50 mm glucose with d‐galacturonic acid when compared with growth without d‐galacturonic acid. On agar plates containing 10 mm glucose with d‐galacturonic acid, however, the growth of all three mutants was reduced slightly when compared with growth without d‐galacturonic acid (Fig. 6B).
Figure 6.
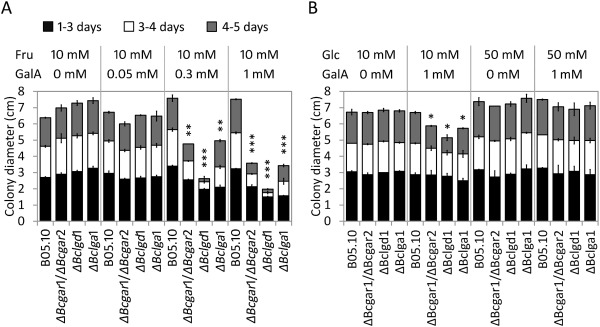
Radial growth of d‐galacturonic acid catabolism‐deficient mutants on agar medium containing fructose (A, Fru) or glucose (B, Glc) as the major carbon source, with different concentrations of d‐galacturonic acid (GalA) as indicated at the top. Colony diameter was measured at 3, 4 and 5 days after incubation at 20 °C. Data presented are the means ± standard deviation from two biological repeats, with three technical replicates of each repeat. Asterisks indicate a significant difference from the wild‐type strain based on Student's t‐test (*P < 0.05, **P < 0.01, ***P < 0.001).
Discussion
The fundamental question at the onset of this study was whether the important role of endo‐PGs in the virulence of B. cinerea (ten Have et al., 1998; Kars et al., 2005) relates to a function in tissue disintegration and colonization, and/or to a function in the release of an abundant source of monosaccharide nutrients from pectic polymers. These functions might possibly be distinguished by analysing mutants with a normal set of pectinases, and thus fully able to decompose pectin, but unable to catabolize the d‐galacturonic acid released by the action of these enzymes. If pectin degradation by B. cinerea is merely for the purpose of host tissue invasion and colonization, but does not contribute significantly to the release of nutrients for fungal growth, one would anticipate that the virulence of mutants in d‐galacturonic acid catabolic genes would be unaltered when compared with that of the wild‐type. If, however, d‐galacturonic acid makes up a substantial proportion of the nutrition for the fungus, one would anticipate that the virulence of such mutants in d‐galacturonic acid catabolic genes would be reduced. Furthermore, it might be expected that the extent of virulence reduction would be proportional to the d‐galacturonic acid content in the host cell wall.
We analysed the d‐galacturonic acid content in the cell wall extracts of leaves from three plant species that are commonly used for B. cinerea infection assays: S. lycopersicum, N. benthamiana and A. thaliana. Although substantial data are available on the leaf cell wall composition of A. thaliana (Harholt et al., 2006; Zablackis et al., 1995; Zandleven et al., 2007), only limited information is available on the composition of pectins and cell walls of leaves from S. lycopersicum (Curvers et al., 2010) and none on N. benthamiana. Although the last two species are both Solanaceae and might be anticipated to have similar cell wall architecture, the composition of monosaccharides released from AIR in N. benthamiana was remarkably similar to that in A. thaliana and distinct from the composition in S. lycopersicum. The content of d‐galacturonic acid in S. lycopersicum was only 60%–70% of that in the other two species, whereas the content of glucose was higher in S. lycopersicum leaves when compared with that in the other two plants. The amount of free monosaccharides in all three plants (on a mol/dry weight basis) was extremely low when compared with the monosaccharides assimilated in cell wall polysaccharides. The differences in d‐galacturonic acid content between the three hosts might affect the nutrients available for B. cinerea growth when it colonizes the leaves of the respective hosts.
We have previously described a set of B. cinerea d‐galacturonic acid catabolism‐deficient mutants, which are blocked in one of three enzymatic steps in the catabolic pathway (Zhang et al., 2011). These mutants were unable to grow on polygalacturonic acid as sole carbon source, and showed severely reduced growth on apple pectin and citrus pectin, substrates that, in addition to d‐galacturonic acid, contain neutral sugars. The extent of residual growth of the mutants correlated with the proportion of neutral sugars in these pectic substrates (Zhang et al., 2011). The B. cinerea d‐galacturonic acid catabolism‐deficient mutants showed a significant reduction in virulence on N. benthamiana and A. thaliana leaves when compared with the wild‐type, but a similar virulence on S. lycopersicum leaves. The differential virulence was correlated with the content of d‐galacturonic acid in the cell walls of the plants tested. The initial interpretation of the data was that, in N. benthamiana and A. thaliana, d‐galacturonic acid makes up an important part of the nutrition for B. cinerea. The decomposition of pectin would release d‐galacturonic acid monomers that cannot be utilized by the mutants for growth, thereby leading to significantly reduced lesion outgrowth. The observation that the virulence of the mutants on S. lycopersicum leaves was similar to that of the wild‐type was tentatively explained by the low d‐galacturonic acid content in this host, and the use of alternative carbon sources by the mutants.
The transcript levels of d‐galacturonic acid catabolic genes during infection by wild‐type B. cinerea correlated with the level of d‐galacturonic acid in the leaf cell walls of the species tested, with relatively high transcript levels in N. benthamiana and A. thaliana, as opposed to low transcript levels in S. lycopersicum. In individual mutant strains, the transcript levels of the other pathway genes were not altered during leaf infections, when compared with the levels in the wild‐type. Specifically, there was no significant change in the transcript levels of Bclgd1 and Bclga1 in the ΔBcgar1/ΔBcgar2 mutant, of Bcgar1, Bcgar2 and Bclga1 in the ΔBclgd1 mutant, or of Bcgar1, Bcgar2 and Bclgd1 in the ΔBclga1 mutant (not shown). These data suggest that d‐galacturonic acid itself is responsible for the induction of the catabolic genes, and the catabolic intermediates derived from d‐galacturonic acid are not required for induction of transcription. This finding is in agreement with the observation that, in an Aspergillus niger knockout mutant in the gaaA gene (orthologue of Bcgar2), transcript levels of genes downstream in the pathway were elevated in the presence of d‐galacturonic acid, similar to that in the wild‐type (Mojzita et al., 2010).
We tested whether a nonfunctional d‐galacturonic acid catabolic pathway might affect the in planta expression of B. cinerea endo‐PG genes, which could lead indirectly to a reduction in virulence. Transcript levels of the Bcpg1 gene, which is important for the virulence of B. cinerea (ten Have et al., 1998), were unaltered in the d‐galacturonic acid catabolism‐deficient mutants. By contrast, the expression of the Bcpg4 and Bcpg6 genes, which can be induced by the culture of B. cinerea in the presence of d‐galacturonic acid (Wubben et al., 2000), was elevated in the mutants relative to the wild‐type strain. This suggests that the mutants sense elevated levels of d‐galacturonic acid, when compared with the wild‐type, presumably because the compound is catabolized in the wild‐type strain, leading to lower intra‐ and/or extracellular d‐galacturonic acid concentrations. The inability to catabolize d‐galacturonic acid in the mutants leads to prolonged elevated levels of Bcpg4 and Bcpg6 transcripts. Expression analyses corroborated that the reduced virulence of d‐galacturonic acid catabolism‐deficient mutants was not a result of an indirect impact on the expression of B. cinerea endo‐PG genes.
Furthermore, we tested whether the reduced virulence of B. cinerea mutants on A. thaliana could be explained by an elevation in defence responses. All A. thaliana defence‐related genes tested were induced to a similar extent by the wild‐type and mutant B. cinerea strains; some defence genes even showed lower transcript levels in response to the mutants. The conclusion that the reduced virulence of d‐galacturonic acid catabolism‐deficient mutants was not caused by an altered regulation of B. cinerea endo‐PG genes, or by a modulation of defence responses in the host, strengthened the initial hypothesis that d‐galacturonic acid catabolism influences virulence directly, and therefore d‐galacturonic acid may serve as an important nutritional component for the fungus.
This hypothesis was challenged by the striking observation that the mutants did not all show the same reduction in virulence. The ΔBclgd1 mutant (blocked in the second step in the pathway) showed markedly stronger reduction in virulence than the mutants blocked in the first and third steps. This observation was indicative of growth inhibitory effects exerted by catabolic intermediates, which would partly or largely explain the differences between the ΔBclgd1 mutant and the other mutants. As the catabolic pathway intermediates (l‐galactonate and 2‐keto‐3‐deoxy‐l‐galactonate, accumulating in the ΔBclgd1 and ΔBclga1 mutants, respectively) are not commercially available, it is not feasible to evaluate directly the growth‐inhibiting effects of these compounds in B. cinerea. Therefore, experiments were performed in which B. cinerea wild‐type and mutants were grown on agar, containing fructose as the most abundant carbon source, supplemented with different amounts of d‐galacturonic acid. The growth of all three mutants was reduced on medium containing fructose supplemented with d‐galacturonic acid when compared with growth on fructose alone. The growth reduction of the mutants in the presence of d‐galacturonic acid occurred in a dose‐dependent manner (Fig. 6A). This suggests that the accumulation of all three intermediates in the d‐galacturonic acid catabolic pathway is inhibitory to B. cinerea, with l‐galactonate (product of the first step in the pathway, accumulating in the ΔBclgd1 mutant) having the most severe growth‐reducing effect. The mutants grew at a similar rate to the wild‐type in the first 3 days, but then showed a severe decline in radial growth between days 3–4 and days 4–5. The ΔBclgd1 mutant showed nearly complete growth cessation in the presence of 0.3 and 1 mm d‐galacturonic acid. These observations provide support that the intermediates first need to be generated and accumulate before exerting their growth‐reducing effect.
Growth reduction was not observed when glucose (50 mm) was provided as the major carbon source, because the expression of d‐galacturonic acid catabolic genes is repressed by glucose (Zhang et al., 2011). In addition, the expression of putative d‐galacturonic acid transporter genes is repressed by glucose (Zhang and van Kan, unpublished results). With 10 mm glucose and 1 mm d‐galacturonic acid in the medium, the growth of mutants was reduced slightly between days 4 and 5 of incubation. This observation can be explained by the glucose being depleted after 3–4 days of incubation, leading to the release of repression of the d‐galacturonic catabolic genes and putative d‐galacturonic acid transporter genes. This enables the fungus to switch to d‐galacturonic acid transport and consumption, resulting in the accumulation of pathway intermediates to levels sufficient to cause growth reduction. Future studies with knockout mutants of putative d‐galacturonic acid transporter genes in the background of a ΔBclgd1 mutant could confirm the growth‐reducing effect of catabolic pathway intermediates both in planta and in vitro. Failure to import d‐galacturonic acid would prevent the accumulation of the inhibitory intermediates and thereby alleviate the growth reduction. Such experiments were, however, beyond the scope of the present study.
The d‐galacturonic acid catabolic pathway has been characterized in Hypocrea jecorina and Aspergillus niger (Martens‐Uzunova and Schaap, 2008; Richard and Hilditch, 2009). In these fungi, there is no evidence of growth‐reducing effects of catabolic pathway intermediates. Intracellular accumulation of 2‐keto‐3‐deoxy‐l‐galactonate was observed in the H. jecorina lga1 mutant and the Aspergillus niger gaaC mutant (ΔBclga1 mutant in B. cinerea), but did not appear to affect hyphal viability or sporulation (Hilditch et al., 2007; Wiebe et al., 2010). In addition, the degradation of 2‐keto‐3‐deoxy‐l‐galactonate was observed in H. jecorina and Aspergillus niger mutant cultures (Wiebe et al., 2010), suggesting that alternative mechanisms exist in these fungi, which prevent the accumulation of inhibitory compounds. Such alternative pathways are either less effective or missing in B. cinerea.
The observed growth‐reducing effects of catabolic pathway intermediates forced us to reconsider the interpretation of the virulence assays. It was particularly striking that the extent of reduction in virulence of the mutants in different steps of the catabolic pathway was strongly correlated with the extent of growth reduction in vitro (on fructose plus d‐galacturonic acid). Furthermore, the reduced virulence was especially observed in host species with larger contents of d‐galacturonic acid in their cell walls. Solanum lycopersicum has 30%–40% smaller amounts of d‐galacturonic acid in its cell wall when compared with N. benthamiana and A. thaliana. This leads to less pronounced induction of the expression of d‐galacturonic acid catabolic genes in S. lycopersicum than in the other two hosts, as corroborated by the observed expression profiles (Fig. 2). The levels of glucose and sucrose in all three plants tested are negligible when compared with the monosaccharides deposited in cell walls. The concentrations of free monosaccharides in leaves are insufficient to cause catabolite repression of B. cinerea d‐galacturonic acid catabolic genes (Fig. S2). The consequence is that the growth‐reducing effects of d‐galacturonic acid catabolic intermediates during infection on S. lycopersicum leaves are negligible and lesions of the mutants are as large as those of the wild‐type. By contrast, the higher d‐galacturonic acid levels in N. benthamiana and A. thaliana leaves cause greater expression of d‐galacturonic acid catabolic genes, and more rapid and greater accumulation of catabolic intermediates, leading to slower lesion outgrowth.
Ideally, instead of comparing host species with distinct pectin composition, one might prefer to compare the virulence of B. cinerea on a single wild‐type host species and on an isogenic mutant with an altered content of d‐galacturonic acid in its cell wall. The A. thaliana qua1 mutant is deficient in pectin synthesis and contains 25% less d‐galacturonic acid in the cell wall (Bouton et al., 2002). However, this mutant is severely dwarfed and displays numerous features that would influence the interaction with B. cinerea in an unpredictable manner. Pectin is of such crucial relevance for plant cell architecture (Mohnen, 2008) that it is not feasible to compare the virulence of pathogens on genotypes from the same plant species with markedly different d‐galacturonic acid contents.
The question at the onset of this study was whether the important role of endo‐PGs in the virulence of B. cinerea relates to a function in tissue disintegration and colonization, and/or to a function in the release of an abundant source of monosaccharide nutrients from pectic polymers. The data presented here suggest that the reduced virulence of d‐galacturonic acid catabolism‐deficient B. cinerea mutants on N. benthamiana and A. thaliana is only partly caused by the inability of mutants to utilize pectic monosaccharides that serve as an important nutrient source. The growth inhibitory effect of the d‐galacturonic acid catabolic pathway intermediates might make a more significant contribution to the reduced virulence phenotype of the mutants.
Experimental Procedures
Fungal strain and growth conditions
Botrytis cinerea wild‐type strain B05.10 and the mutant strains ΔBcgar1/ΔBcgar2, ΔBclgd1 and ΔBclga1 used in this study were routinely grown on Malt Extract Agar (Oxoid, Basingstoke, UK; 50 g/L) in the dark at 20 °C for 3–4 days. The plates were placed for one night under near‐UV light (350–400 nm) to promote sporulation, and were subsequently returned to darkness. Spores were harvested 4–7 days later in 10–20 mL of water, and the suspension was filtered over glasswool to remove mycelium fragments. The spore suspension was centrifuged at 120 g for 5 min. The supernatant was discarded and the spores in the pellet were resuspended at the desired density. For radial growth assays, conidia of the strains were inoculated on Gamborg's B5 (Duchefa, Haarlem, the Netherlands) agarose medium supplemented with 10 mm (NH4)H2PO4, either fructose (10 mm) or glucose (10 or 50 mm) as carbon source and d‐galacturonic acid (0.05, 0.3 or 1 mm). Cultures were grown at 20 °C and the colony diameter was measured after 3, 4 and 5 days of incubation.
Plant material and growth conditions
Solanum lycopersicum (Moneymaker) and N. benthamiana plants were grown in a glasshouse at 20 °C. Arabidopsis thaliana wild‐type ecotype Columbia (Col‐0) plants were grown in a growth chamber at 20 °C and 70% relative humidity under a 12‐h light/dark cycle.
Plant infection
Leaves of 5–6‐week‐old S. lycopersicum, N. benthamiana and A. thaliana plants were inoculated with B. cinerea. Droplets of a suspension of conidia of wild‐type and mutants (2 μL, 106 conidia/mL in potato dextrose broth, 1.2 g/L) were inoculated on opposite sides of the central vein (for S. lycopersicum, three to four droplets per leaf half; for N. benthamiana, one to two droplets per leaf half). Each comparison of wild‐type and mutant was performed on four leaflets of one composite tomato leaf, on two composite leaves per plant and for two plants per experiment, or on three to four leaves per N. benthamiana plant and six plants per experiment, leading to a total of at least 50 lesions per experiment. Lesion diameters were measured with a digital calliper at 3 dpi. Six discs containing the infection lesions in the centre (30 mm in diameter) from three leaves of three plants were sampled at 2 and 3 dpi as a pool for RNA isolation and biomass quantification.
For A. thaliana infection, one droplet of a suspension of conidia of wild‐type or mutants was inoculated on one side of each detached leaf. Eight leaves of each inoculation were sampled at 1, 2 and 3 dpi as a pool for RNA isolation and biomass quantification.
Each mutant was tested in at least two independent experiments. Lesion sizes and fungal biomass were analysed statistically by Student's t‐test using a two‐tailed distribution and two‐sample unequal variance.
RNA extraction and quantitative reverse transcription‐polymerase chain reaction (qRT‐PCR) analysis
The infected plant material was freeze dried and partially used for RNA extraction. Total RNA was isolated using a Nucleospin® RNA plant kit (Macherey‐Nagel, Düren, Germany), according to the manufacturer's instructions. First‐strand cDNA was synthesized from 1 μg of total RNA with SuperScript® III Reverse Transcriptase (Invitrogen, Carlsbad, CA, USA), according to the manufacturer's instructions.
qRT‐PCR was performed using an ABI7300 PCR machine (Applied Biosystems, Foster City, CA, USA) in combination with the qPCR SensiMix kit (BioLine, London, UK). The primers to detect the transcripts of B. cinerea and A. thaliana genes are listed in Tables S1 and S2 (see Supporting Information), respectively. Real‐time PCR conditions were as follows: an initial 95 °C denaturation step for 10 min, followed by denaturation for 15 s at 95 °C and annealing/extension for 45 s at 60 °C for 40 cycles. The data were analysed on 7300 System SDS software (Applied Biosystems). The transcript levels of target genes were normalized to the transcript levels of the constitutively expressed gene Bcrpl5 (for B. cinerea genes) and Atactin (for A. thaliana genes), according to the 2–ΔΔCt method.
Measurement of B. cinerea biomass
The freeze‐dried infected plant material was incubated in the extraction buffer (35 mg/mL) provided in the QuickStix™ kit for B. cinerea (Enviro‐Logix, Portland, ME, USA). After 10 min of incubation, the supernatants were used for fungal biomass quantification with a lateral flow device (Dewey et al., 2008), which quantifies a stable water‐soluble, extracellular epitope (Dewey and Meyer, 2000). The signal intensity of the monoclonal antibody reaction was determined with an optical reader (Enviro‐Logix) and converted into fungal biomass (μg/mg plant tissue), which was calculated on the basis of the standard curves generated by known amounts of dry mycelium diluted in extraction buffer. The fungal biomass of mutants was normalized to that of the wild‐type strain.
AIR preparation and sugar composition analysis
Leaves of 5–6‐week‐old plants were freeze dried and milled. AIR was extracted with 70% ethanol at 50 °C as described by Hilz et al. (2005). The obtained AIR was dissolved and prehydrolysed with 72% w/w sulphuric acid at 30 °C for 1 h, followed by hydrolysis with additional double‐distilled H2O for 3 h at 100 °C. The uronic acid content of the hydrolysed samples was determined by m‐hydroxydiphenyl assay (Blumenkrantz and Asboe‐Hansen, 1973; Kintner and Van Buren, 1982), using an auto‐analyser (Skalar Analytical BV, Breda, the Netherlands). Galacturonic acid was used as a standard for quantification. After hydrolysis, the neutral sugars were converted into alditol acetates and determined by gas chromatography (Englyst and Cummings, 1984), using inositol as the internal standard.
Sugar extraction of plant leaves and sucrose, d‐fructose and d‐glucose determination
Leaves of 5–6‐week‐old plants were freeze dried and milled. Powered materials were extracted with 70% ethanol. The supernatant was filtered with a 0.2‐μm filter and evaporated to remove ethanol. The sugar extracts obtained were dissolved in water and purified with chloroform. The water phase was transferred to a fresh tube and incubated at 65 °C for 10 min to inactivate the enzymes in the samples. The sucrose, d‐fructose and d‐glucose contents were measured with a sucrose, d‐fructose and d‐glucose assay kit (Megazyme, Bray, Ireland), according to the manufacturer's instructions.
Supporting information
Fig. S1 Sugar contents in leaves of Solanum lycopersicum, Nicotiana benthamiana and Arabidopsis thaliana. Bars indicate means ± standard deviation. Letters above the bars indicate statistical significance; bars not sharing letters represent significant mean differences at P < 0.05 by Student's t‐test.
Fig. S2 Virulence of d‐galacturonic acid catabolism‐deficient mutants on Solanum lycopersicum leaves. (A) Lesion sizes of Botrytis cinerea wild‐type and mutants were evaluated at 3 days post‐inoculation (dpi) by determining the average lesion diameter on two composite leaves from two plants each. Data represent means ± standard deviation (n ≥ 50 independent lesions). (B) Botrytis cinerea biomass accumulation by immunological detection at 2 and 3 dpi on S. lycopersicum. Six lesion discs (30 mm in diameter) from three leaves of two plants were sampled as a pool for quantification. Data represent means ± standard deviation from two independent biological repeats. Letters above the bars indicate statistical significance; bars not sharing letters represent significant mean differences at P < 0.05 by Student's t‐test.
Fig. S3 Relative transcript levels of Bcpg1, Bcpg4 and Bcpg6 in wild‐type Botrytis cinerea and d‐galacturonic acid catabolism‐deficient mutants during infection on Nicotiana benthamiana and Solanum lycopersicum leaves. Infected plants were sampled at 2 and 3 days post‐inoculation (dpi) for RNA extraction. mRNA levels of Bcpg1, Bcpg4 and Bcpg6 genes were normalized to the levels of the constitutive reference gene Bcrpl5 and calibrated to wild‐type strain B05.10 levels at time point 2 dpi on N. benthamiana leaves (set as 1), according to the 2–ΔΔCt method. Data are represented as means ± standard deviation from one biological repeat. Three technical replicates of each repeat were analysed and three independent biological repeats were performed, which showed similar results. For each time point on each plant leaf, letters above the bars indicate statistical significance; bars not sharing letters represent significant mean differences at P < 0.05 by Student's t‐test.
Fig. S4 Relative transcript levels of Arabidopsis thaliana defence‐related genes during infection with Botrytis cinerea wild‐type strain and d‐galacturonic acid catabolism‐deficient mutants. Infected leaves were sampled at 1, 2 and 3 days post‐inoculation (dpi) for RNA extraction. mRNA levels of A. thaliana genes were normalized to the levels of the constitutive reference gene Atactin and calibrated to the levels with B. cinerea wild‐type at time point 1 dpi (set as 1), according to the 2–ΔΔCt method. Data are represented as means ± standard deviation from one biological repeat. Three technical replicates of each repeat were analysed and three independent biological repeats were performed, which all showed similar results.
Table S1 Quantitative reverse transcription‐polymerase chain reaction (qRT‐PCR) primers of Botrytis cinerea genes used in this study.
Table S2 Quantitative reverse transcription‐polymerase chain reaction (qRT‐PCR) primers of Arabidopsis thaliana genes used in this study.
Acknowledgements
The authors are grateful to Yvonne Westphal and Henk Schols (Wageningen University, Laboratory of Food Chemistry, Wageningen, the Netherlands) for providing facilities, advice and assistance for the determination of plant cell wall composition. The authors are also grateful to Barbara Blanco‐Ulate and Ann Powell (Plant Sciences Department, University of California, Davis, CA, USA), Guido van den Ackerveken (Utrecht University, Utrecht, the Netherlands) and Pierre de Wit (Wageningen University, Laboratory of Phytopathology, Wageningen, the Netherlands) for comments on a draft version of the manuscript and for fruitful discussions. The authors acknowledge funding by the Foundation Technological Top Institute Green Genetics (Project 2CC035RP) and the Netherlands Graduate School Experimental Plant Sciences.
References
- van Baarlen, P. , Woltering, E.J. , van Staats, M. and Kan, J.A.L. (2007) Histochemical and genetic analysis of host and non‐host interactions of Arabidopsis with three Botrytis species: an important role for cell death control. Mol. Plant. Pathol. 8, 41–54. [DOI] [PubMed] [Google Scholar]
- Blumenkrantz, N. and Asboe‐Hansen, G. (1973) New method for quantitative determination of uronic acids. Anal. Biochem. 54, 484–489. [DOI] [PubMed] [Google Scholar]
- Böttcher, C. , Westphal, L. , Schmotz, C. , Prade, E. , Scheel, D. and Glawischnig, E. (2009) The multifunctional enzyme CYP71B15 (PHYTOALEXIN DEFICIENT3) converts cysteine‐indole‐3‐acetonitrile to camalexin in the indole‐3‐acetonitrile metabolic network of Arabidopsis thaliana . Plant Cell, 21, 1830–1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouton, S. , Leboeuf, E. , Mouille, G. , Leydecker, M.T. , Talbotec, J. , Granier, F. , Lahaye, M. , Höfte, H. and Truong, H.N. (2002) Quasimodo 1 encodes a putative membrane‐bound glycosyltransferase required for normal pectin synthesis and cell adhesion in Arabidopsis . Plant Cell, 14, 2577–2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caffall, K.H. and Mohnen, D.B. (2009) The structure, function, and biosynthesis of plant cell wall pectic polysaccharides. Carbohydr. Res. 344, 1879–1900. [DOI] [PubMed] [Google Scholar]
- Cao, H. , Bowling, S.A. , Gordon, A.S. and Dong, X. (1994) Characterization of an Arabidopsis mutant that is nonresponsive to inducers of systemic acquired resistance. Plant Cell, 6, 1583–1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choquer, M. , Fournier, E. , Kunz, C. , Levis, C. , Pradier, J.M. , Simon, A. and Viaud, M. (2007) Botrytis cinerea virulence factors: new insights into a necrotrophic and polyphageous pathogen. FEMS Microbiol. Lett. 277, 1–10. [DOI] [PubMed] [Google Scholar]
- Curvers, K. , Seifi, H. , de Mouille, G., Rycke, R. , van Asselbergh, B., Hecke, A. , Vanderschaeghe, D. , Höfte, H. , van Callewaert, N., Breusegem, F. and Höfte, M. (2010) Abscisic acid deficiency causes changes in cuticle permeability and pectin composition that influence tomato resistance to Botrytis cinerea . Plant Physiol. 154, 847–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaney, T.P. , Uknes, S. , Vernooij, B. , Friedrich, L. , Weymann, K. , Negrotto, D. , Gaffney, T. , Gut‐Rella, M. , Kessmann, H. , Ward, E. and Ryals, J. (1994) A central role of salicylic acid in plant disease resistance. Science, 266, 1247–1250. [DOI] [PubMed] [Google Scholar]
- Dewey, F.M. , Hill, M. and DeScenzo, R. (2008) Quantification of Botrytis and laccase in winegrapes. Am. J. Enol. Vitic. 59, 47–54. [Google Scholar]
- Dewey, F.M. and Meyer, U.M. (2000) Efficacy of different immunogens for raising monoclonal antibodies to Botrytis cinerea . Mycol. Res. 104, 979–987. [Google Scholar]
- Dulermo, T. , Rascle, C. , Chinnici, G. , Gout, E. , Bligny, R. and Cotton, P. (2009) Dynamic carbon transfer during pathogenesis of sunflower by the necrotrophic fungus Botrytis cinerea: from plant hexoses to mannitol. New Phytol. 183, 1149–1162. [DOI] [PubMed] [Google Scholar]
- Dulermo, T. , Rascle, C. , Billon‐Grand, G. , Gout, E. , Bligny, R. and Cotton, P. (2010) Novel insights into mannitol metabolism in the fungal plant pathogen Botrytis cinerea . Biochem. J. 427, 323–332. [DOI] [PubMed] [Google Scholar]
- Englyst, H.N. and Cummings, J.H. (1984) Simplified method for the measurement of total non‐starch polysaccharides by gas–liquid chromatography of constituent sugars as alditol acetates. Analyst, 109, 937–942. [DOI] [PubMed] [Google Scholar]
- Ferrari, S. , Plotnikova, J.M. , De Lorenzo, G. and Ausubel, F.M. (2003) Arabidopsis local resistance to Botrytis cinerea involves salicylic acid and camalexin and requires EDS4 and PAD2, but not SID2, EDS5 or PAD4. Plant J. 35, 193–205. [DOI] [PubMed] [Google Scholar]
- Ferrari, S. , Galletti, R. , Vairo, D. , Cervone, F. and De Lorenzo, G. (2006) Antisense expression of the Arabidopsis thaliana AtPGIP1 gene reduces polygalacturonase‐inhibiting protein accumulation and enhances susceptibility to Botrytis cinerea . Mol. Plant–Microbe Interact. 19, 931–936. [DOI] [PubMed] [Google Scholar]
- Harholt, J. , Jensen, J.K. , Sørensen, S.O. , Orfila, C. , Pauly, M. and Scheller, H.V. (2006) ARABINAN DEFICIENT 1 is a putative arabinoslytransferase involved in biosynthesis of pectic arabinan in Arabidopsis . Plant Physiol. 140, 49–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ten Have, A. , Mulder, W. , van Visser, J. and Kan, J.A.L. (1998) The endopolygalacturonase gene Bcpg1 is required for full virulence of Botrytis cinerea . Mol. Plant–Microbe Interact. 11, 1009–1016. [DOI] [PubMed] [Google Scholar]
- ten Have, A. , Tenberge, K.B. , Benen, J.A.E. , van Tudzynski, P.J.V. and Kan, J.A.L. (2002) The contribution of the cell wall degrading enzymes to pathogenesis of fungal plant pathogens In: The Mycota XI, Agricultural Applications (Kempken F., ed.), pp. 341–358. Berlin: Springer‐Verlag. [Google Scholar]
- Hilditch, S. , Berghall, S. , Kalkkinen, N. , Penttila, M. and Richard, P. (2007) The missing link in the fungal d‐galacturonate pathway: identification of the ‐threo‐3‐deoxy‐hexulosonate aldolase. J. Biol. Chem. 282, 26 195–26 201. [DOI] [PubMed] [Google Scholar]
- Hilz, H. , Bakx, E.J. , Schols, H.A. and Voragen, A.G.J. (2005) Cell wall polysaccharides in black currants and bilberries—characterisation in berries, juice, and press cake. Carbohydr. Polym. 59, 477–488. [Google Scholar]
- van Kan, J.A.L. (2006) Licensed to kill: the lifestyle of a necrotrophic plant pathogen. Trends Plant Sci. 11, 247–253. [DOI] [PubMed] [Google Scholar]
- Kars, I. , Krooshof, G.H. , Wagemakers, L. , Joosten, R. , van Benen, J.A. and Kan, J.A.L. (2005) Necrotizing activity of five Botrytis cinerea endopolygalacturonases produced in Pichia pastoris . Plant J. 43, 213–225. [DOI] [PubMed] [Google Scholar]
- Kintner, P.K. and Van Buren, J.P. (1982) Carbohydrate interference and its correction in pectin analysis using the m‐hydroxydiphenyl method. J. Food Sci. 47, 756–759. [Google Scholar]
- Martens‐Uzunova, E.S. and Schaap, P.J. (2008) An evolutionary conserved d‐galacturonic acid metabolic pathway operates across filamentous fungi capable of pectin degradation. Fungal Genet. Biol. 45, 1449–1457. [DOI] [PubMed] [Google Scholar]
- Mohnen, D. (2008) Pectin structure and biosynthesis. Curr. Opin. Plant Biol. 11, 226–277. [DOI] [PubMed] [Google Scholar]
- Mojzita, D. , Wiebe, M. , Hilditch, S. , Boer, H. , Penttilä, M. and Richard, P. (2010) Metabolic engineering of fungal strains for conversion of d‐galacturonate to meso‐galactarate. Appl. Environ. Microbiol. 76, 169–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penninckx, I.A. , Eggermont, K. , Terras, F.R. , Thomma, B.P. , De Samblanx, G.W. , Buchala, A. , Metraux, J.P. , Manners, J.M. and Broekaert, W.F. (1996) Pathogen‐induced systemic activation of a plant defensin gene in Arabidopsis follows a salicylic acid‐independent pathway. Plant Cell, 8, 2309–2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penninckx, I.A. , Thomma, B.P. , Buchala, A. , Metraux, J.P. and Broekaert, W.F. (1998) Concomitant activation of jasmonate and ethylene response pathways is required for induction of a plant defensin gene in Arabidopsis . Plant Cell, 10, 2103–2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raiola, A. , Lionetti, V. , Elmaghraby, I. , Immerzeel, P. , Mellerowicz, E.J. , Salvi, G. , Cervone, F. and Bellincampi, D. (2011) Pectin methylesterase is induced in Arabidopsis upon infection and is necessary for a successful colonization by necrotrophic pathogens. Mol. Plant–Microbe Interact. 24, 432–440. [DOI] [PubMed] [Google Scholar]
- Richard, P. and Hilditch, S. (2009) d‐galacturonic acid catabolism in microorganisms and its biotechnological relevance. Appl. Microbiol. Biotechnol. 82, 597–604. [DOI] [PubMed] [Google Scholar]
- Rui, O. and Hahn, M. (2007) The Botrytis cinerea hexokinase, Hxk1, but not the glucokinase, Glk1, is required for normal growth and sugar metabolism, and for pathogenicity on fruits. Microbiology, 153, 2791–2802. [DOI] [PubMed] [Google Scholar]
- Thomma, B.P. , Eggermont, K. , Penninckx, I.A. , Mauch‐Mani, B. , Vogelsang, R. , Cammue, B.P. and Broekaert, W.F. (1998) Separate jasmonate‐dependent and salicylate‐dependent defense‐response pathways in Arabidopsis are essential for resistance to distinct microbial pathogens. Proc. Natl. Acad. Sci. USA, 95, 15 107–15 111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres, M.A. , Jones, J.D. and Dangl, J.L. (2005) Pathogen‐induced, NADPH oxidase‐derived reactive oxygen intermediates suppress spread of cell death in Arabidopsis thaliana . Nat. Genet. 37, 1130–1134. [DOI] [PubMed] [Google Scholar]
- Wiebe, M.G. , Mojzita, D. , Hilditch, S. , Ruohonen, L. and Penttilä, M. (2010) Bioconversion of d‐galacturonate to keto‐deoxy‐l‐galactonate (3‐deoxy‐l‐threo‐hex‐2‐ulosonate) using filamentous fungi. BMC Biotechnol. 10, 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson, B. , Tudzynski, B. , van Tudzynski, P. and Kan, J.A.L. (2007) Botrytis cinerea: the cause of grey mould disease. Mol. Plant. Pathol. 8, 561–580. [DOI] [PubMed] [Google Scholar]
- Wubben, J.P. , ten Mulder, W., van Have, A., Kan, J.A.L. and Visser, J. (1999) Cloning and partial characterization of endopolygalacturonase genes from Botrytis cinerea . Appl. Environ. Microbiol. 65, 1596–1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wubben, J.P. , ten Have, A. , van Kan, J.A.L. and Visser, J. (2000) Regulation of endopolygalacturonase gene expression in Botrytis cinerea by galacturonic acid, ambient pH and carbon catabolite repression. Curr. Genet. 37, 152–157. [DOI] [PubMed] [Google Scholar]
- Zablackis, E. , Huang, J. , Müller, B. , Darvill, A.G. and Albersheim, P. (1995) Characterization of the cell‐wall polysaccharides of Arabidopsis thaliana leaves. Plant Physiol. 107, 1129–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zandleven, J. , Sørensen, S.O. , Harholt, J. , Beldman, G. , Schols, H.A. , Scheller, H.V. and Voragen, A.J. (2007) Xylogalacturonan exists in cell walls from various tissues of Arabidopsis thaliana . Phytochemistry, 68, 1219–1226. [DOI] [PubMed] [Google Scholar]
- Zhang, L. , van Thiewes, H. and Kan, J.A.L. (2011) The d‐galacturonic acid catabolic pathway in Botrytis cinerea . Fungal Genet. Biol. 48, 990–997. [DOI] [PubMed] [Google Scholar]
- Zhou, N. , Tootle, T.L. and Glazebrook, J. (1999) Arabidopsis PAD3, a gene required for camalexin biosynthesis, encodes a putative cytochrome P450 monooxygenase. Plant Cell, 11, 2419–2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 Sugar contents in leaves of Solanum lycopersicum, Nicotiana benthamiana and Arabidopsis thaliana. Bars indicate means ± standard deviation. Letters above the bars indicate statistical significance; bars not sharing letters represent significant mean differences at P < 0.05 by Student's t‐test.
Fig. S2 Virulence of d‐galacturonic acid catabolism‐deficient mutants on Solanum lycopersicum leaves. (A) Lesion sizes of Botrytis cinerea wild‐type and mutants were evaluated at 3 days post‐inoculation (dpi) by determining the average lesion diameter on two composite leaves from two plants each. Data represent means ± standard deviation (n ≥ 50 independent lesions). (B) Botrytis cinerea biomass accumulation by immunological detection at 2 and 3 dpi on S. lycopersicum. Six lesion discs (30 mm in diameter) from three leaves of two plants were sampled as a pool for quantification. Data represent means ± standard deviation from two independent biological repeats. Letters above the bars indicate statistical significance; bars not sharing letters represent significant mean differences at P < 0.05 by Student's t‐test.
Fig. S3 Relative transcript levels of Bcpg1, Bcpg4 and Bcpg6 in wild‐type Botrytis cinerea and d‐galacturonic acid catabolism‐deficient mutants during infection on Nicotiana benthamiana and Solanum lycopersicum leaves. Infected plants were sampled at 2 and 3 days post‐inoculation (dpi) for RNA extraction. mRNA levels of Bcpg1, Bcpg4 and Bcpg6 genes were normalized to the levels of the constitutive reference gene Bcrpl5 and calibrated to wild‐type strain B05.10 levels at time point 2 dpi on N. benthamiana leaves (set as 1), according to the 2–ΔΔCt method. Data are represented as means ± standard deviation from one biological repeat. Three technical replicates of each repeat were analysed and three independent biological repeats were performed, which showed similar results. For each time point on each plant leaf, letters above the bars indicate statistical significance; bars not sharing letters represent significant mean differences at P < 0.05 by Student's t‐test.
Fig. S4 Relative transcript levels of Arabidopsis thaliana defence‐related genes during infection with Botrytis cinerea wild‐type strain and d‐galacturonic acid catabolism‐deficient mutants. Infected leaves were sampled at 1, 2 and 3 days post‐inoculation (dpi) for RNA extraction. mRNA levels of A. thaliana genes were normalized to the levels of the constitutive reference gene Atactin and calibrated to the levels with B. cinerea wild‐type at time point 1 dpi (set as 1), according to the 2–ΔΔCt method. Data are represented as means ± standard deviation from one biological repeat. Three technical replicates of each repeat were analysed and three independent biological repeats were performed, which all showed similar results.
Table S1 Quantitative reverse transcription‐polymerase chain reaction (qRT‐PCR) primers of Botrytis cinerea genes used in this study.
Table S2 Quantitative reverse transcription‐polymerase chain reaction (qRT‐PCR) primers of Arabidopsis thaliana genes used in this study.


