Abstract
Background
Long-term growth in HIV-infected infants treated early in resource-limited settings is poorly documented. Incidence of growth retardation, instantaneous risk of death related to malnutrition and growth parameters evolution during the first five years of life of uninfected and early treated HIV-infected children were compared and associated factors with growth retardation were identified.
Methods
Weight-for-age (WAZ), weight-for-length (WLZ), and length-for-age (LAZ) Z-scores were calculated. The ANRS-PEDIACAM cohort includes four groups of infants with three enrolled during the first week of life: HIV-infected (HI, n = 69), HIV-exposed uninfected (HEU, n = 205) and HIV-unexposed uninfected (HUU, n = 196). The last group included HIV-infected infants diagnosed before 7 months of age (HIL, n = 141). The multi-state Markov model was used to describe the incidence of growth retardation and identified associated factors.
Results
During the first 5 years, 27.5% of children experienced underweight (WAZ<-2), 60.4% stunting (LAZ<-2) and 41.1% wasting (WLZ<-2) at least once. The instantaneous risk of death observed from underweight state (35.3 [14.1–88.2], 84.0 [25.5–276.3], and 6.0 [1.5–24.1] per 1000 person-months for 0–6 months, 6–12 months, and 12–60 months respectively) was higher than from non-underweight state (9.6 [5.7–16.1], 20.1 [10.3–39.4] and 0.3 [0.1–0.9] per 1000 person-months). Compared to HEU, HIL and HI children were most at risk of wasting (adjusted HR (aHR) = 4.3 (95%CI: 1.9–9.8), P<0.001 and aHR = 3.3 (95%CI: 1.4–7.9), P = 0.01 respectively) and stunting for HIL (aHR = 8.4 (95%CI: 2.4–29.7). The risk of underweight was higher in HEU compared to HUU children (aHR = 5.0 (CI: 1.4–10.0), P = 0.001). Others associated factors to growth retardation were chronic pathologies, small size at birth, diarrhea and CD4< 25%.
Conclusions
HIV-infected children remained at high risk of wasting and stunting within the first 5 years period of follow-up. There is a need of identifying suitable nutritional support and best ways to integrate it with cART in pediatric HIV infection global care.
Introduction
Growth retardation is a major public health problem, mainly in children aged less than five year of age, with an estimated 3.1 million deaths annually, accounting for 45% of all child deaths [1, 2]. In the last few years, about half of global under-five deaths occurred in sub-Saharan Africa (SSA), with more than 50% attributable to malnutrition [3, 4]. Moreover, SSA is also impacted by HIV infection as more than 90% of the 1.8 million children (< 15 years) living with HIV globally currently reside at the end of 2017, with only 52% of them on antiretroviral therapy (ART). During that period, there were 110,000 AIDS-related deaths among children below the age of 15 [5, 6]. In Cameroon, the estimated number of new pediatric HIV infections was 4500 in 2017 and vertical transmission rate at 13% after breastfeeding ends[5, 7]. Prevalence of stunting, underweight, and wasting among children under 5 years in 2014 was 31.7%, 14.8%, and 5.2% respectively [8].
Malnutrition is a common comorbidity among HIV-infected children and HIV infection is also diagnosed frequently in children with severe malnutrition [9]. These two pathologic conditions interact and can each impede physical growth, cognitive, and social development of the child [4, 10]. ART initiation can help to resolve malnutrition as shown by many authors [11–14]. But, growth improvement seems to be greater for children initiated early on ART compared with those initiated at older ages [11–13]. Also, some authors indicated that nutritional status of a certain number of HIV-infected children on ART will not be completely restored even with significant gain on weight and/or height [12, 15, 16]. Long-term growth in HIV-infected infants initiated early on cART in resource-limited settings is poorly documented [13]. Following this observation and having an ongoing cohort constituted of early treated HIV-infected and HIV-uninfected children with longer follow-up periods, we found it necessary to conduct this study assuming a long-term direct benefit of early ART on growth catch-up.
We compared incidence of growth retardation, instantaneous risk of death related to growth retardation, and growth evolution over the first 5 years of life of early treated HIV-infected and HIV-uninfected children born to HIV positive or negative mothers and identified factors associated with growth retardation in Cameroon.
Methods
Ethics statement
The ANRS-PEDIACAM study was granted ethical approval in Cameroon by the National Ethics Committee and in France by the Biomedical Research Committee of the Pasteur Institute of Paris. The Cameroon Ministry of Public Health gave administrative authorization to start the study. Written informed consent was obtained from parents or guardians prior to inclusion of infants into the research project
Data source
Data used in this analysis were obtained from the ANRS-PEDIACAM, an ongoing prospective observational study based in three referral hospitals in Cameroon (The Maternity of the Central hospital/Mother and Child Center of the Chantal Biya Foundation (MCH/MCC-CBF) in Yaoundé, the Essos Hospital Center in Yaoundé (EHC) and the Laquintinie Hospital in Douala (LH)) under the coordination of the Centre Pasteur of Cameroon. The ANRS-PEDIACAM cohort study was designed to assess the feasibility of early HIV diagnosis and access of HIV-infected infants to antiretroviral multi-therapy and evaluate infant response to vaccines of the Expanded Program on Immunisation (EPI). A synthesis of the study protocol was described elsewhere [17]. Briefly, infant inclusion into the ANRS-PEDIACAM study was conducted in two consecutive phases. The first phase, planned from first week of life to 14 weeks, included all infants born live to HIV-infected mothers and an equivalent number of infants born to HIV uninfected mothers matched individually on gender and recruitment site. The newborn pairs were followed, tested for HIV (where needed) at the first follow-up visit planned at 6 weeks of age and given routine vaccinations. The samples were tested for HIV RNA by a real-time polymerase chain reaction (RT PCR) (Biocentric HIV Charge Virale) using the TaqMan technology in an in-house protocol validated by the French National Agency for Research on AIDS and Viral Hepatitis [18] or by Biocentric HIV DNA cell kit to test for HIV proviral DNA. In case of positive or indeterminate test result, a second test on a different sample was planned to confirm HIV infection [17]. For infants who tested HIV negative, a second was performed if the child became symptomatic or six week after weaning for breastfed infants. All identified HIV-infected infants and selected controls of uninfected infants followed from birth, born to HIV-infected mothers (HEU) or to HIV-uninfected mothers (HUU) were included in the second phase for prolonged follow up planned from 14 weeks. Inclusion into the second phase was also offered until October 2011 to HIV-infected children not identified during the first week of life but diagnosed before 7 months of age (HIL). After inclusion in the second phase, all HIV-infected infants were offered systematic cART according to Cameroonian guidelines. First line treatment was zidovudine (or stavudine for infant with anemia) and lamivudine associated with lopinavir/ritonavir if nevirapine (NVP) had been used for PMTCT, or with NVP otherwise. Follow-up was subsequently planned every 3 months for HIV-infected infants and every 6 months for HIV-uninfected infants, until the first 2 years. From 2 years, children were followed every 6 months until the last included child had 5 years [17]. Incentives, including free medical support for consultation, biological analysis, additional vaccines and reimbursement of transport costs, were provided to parents/caregivers by the project during follow up visits. The project provided during its first phase free milk as required to women who choose formula feeding.
Participants and data collection
A case report form (CRF) was used to collect at the baseline and at different visits. This included data on the family environment (socio-demographic and economic characteristics of the family, information on parents), monitoring of pregnancy and childbirth (anthropometric parameters at birth, data concerning the PMTCT, clinical features of the infant) information on the anthropometric parameters of the children (weight, height, cranial and brachial perimeter). Weight was measure with the child supine and the crown of the head touching a vertical headboard using scale and flexible measuring tape respectively. For those aged 2 to 5 years, they step onto scale with one foot on each side. For the length, the child stands against the stadiometer on the board facing the practitioner Clinical status (clinical events since the last protocol visit), family environment, data related to ARV treatments received since the last visit (including adherence to treatment) was collected longitudinally. Biological data (blood count, lipid balance, viral load assessment (for HIV-infected children) and CD4 count were also collected.
Main outcome definition and covariables
The main outcome was growth retardation including underweight, stunting or wasting defined at each visit using anthropometric indices as weight-for-age (WAZ) <-2, weight-for-length (WLZ) <-2, and length-for-age (LAZ) <-2 Z-scores respectively (7, 14). Z-score was calculated using the 2006 WHO growth standard [19].
Prior to the data analysis, a number of variables were recoded. Thus, the mother’s marital status was recoded in married, cohabitation and single/divorced/widow; the level of household income in <100,000 CFA francs and ≥100,000; the level of CD4 in <25% and ≥ 25%. The hemoglobin level was recoded according to the 2011 WHO criteria [20] as follows: not anemia (≥ 11 g / dl in children aged 0–24 months and ≥ 10 g / dl in in children aged 24–60 months), mild or moderate anemia (7 to 10.9 g / dl in children aged 0–24 months and 7–9.9 g / dl in children aged 24–60 months), and severe anemia (<7 g / dl). Sign of systemic involvement was defined at each visit by the presence of one or more of the following signs: fever, jaundice, hepatomegaly, adenopathy or parotitis. Chronic pathologies refers to a persistent pathologic condition observed or notified since the last visit (tuberculosis, sickle cell disease, malformations, chronic skin disease, etc.). Small for gestational age and gender (SGAG) defined as previously [21] as a birth weight Z-score adjusted for gestational age at delivery and gender that is more than two standard deviations below the mean (-2SD) and small birth size by any height Zscore < -2SD, in line with international recommendations [22].
Statistical analysis
The Markov multi-state models were used to assess the incidence of growth retardation and identify associated factors. This choice was motivated by several constraints, including the presence of: (a) interval censorship with the moments and the rhythm of visits fixed a priori, (b) the informative censoring due to competing risk of malnutrition with death among children aged 0–5 years [3, 4, 9, 23–25], (c) the recurrence between healthy and malnourished states that need to be taken into account in the modeling process, (d) the episodic absences and loss to follow-up (LTFU) in the ANRS-PEDIACAM study, and (e) the presence of time-dependent variables.
We considered three states in our analysis: "healthy" (without malnutrition), "Malnourished" (when children presented growth retardation at the visit) and Death (for deceased children). Healthy and malnourished states were transient while the Death state was absorbent. Possible transitions between states were "Healthy—malnourished," "Healthy to Death," "Malnourished to Healthy" and "Malnourished—death". Such model is well described by Jackson [26] and implemented in the R package "msm". In order to combine multi-state models with multiple imputation, we implemented the algorithm for performing likelihood ratio tests with data from multiple imputation proposed by Xiao-Li [27, 28]. This model allowed us to investigate the factors associated with the instantaneous risk of underweight, stunting and wasting.
Growth patterns up to the age of 5 on the basis of WAZ, LAZ and WLZ was studied using the two-stage Heckman approach, whose generalization to repeated data has been proposed by Shelton et al. [29]. This method was chosen because its capacity to account for biases generated by missing data on the growth indices (dependent variables). Due to the participants’ behavior concerning LTFU, we hypothesize that the mechanism of the missing data was informative, placing us in the context of Missing Not At Random according to the missing data typology proposed by Rubin et al. [30].
In the first stage, a GEE probit model was used to identify factors associated with missing data at any visit of the scheduled follow-up, taking into account both the variables observed at baseline and time-dependent variables. The residuals of this model were used to compute the inverse mills ratio (IMR), representing the effect of all unobserved variables that can influence the missingness process. In the second stage, a GEE identity was used to model the marginal expectation of anthropometric indices. At this stage the IMR was added as a covariate to adjust for the bias due to the missing data in both univariable and multivariable analyses. In order to obtain valid hypothesis tests of the model parameters (p-values, standard errors and confidence intervals), we performed 500 bootstrap replications of each of the models adjusted with the IMR. Indeed, since the IMR has been estimated and not observed, the classical standard errors estimated in the model do not take into account the Heteroscedasticity induced by the selection of the sample [31], which invalidates the estimated standard errors.
In order to measure the influence of the biases due to the missing data on the conclusions made when they are not taken into account, we conducted an analysis using a standard GEE without taking into account the IMR. The results obtained with this sensitivity analysis were compared with those resulting from a procedure incorporating the bias correction.
The likelihood ratio test on multiple imputation data was carried out for the choice of the multi-state model variables, the Wald test for the GEE model and the choice based on a bootstrap procedure for the second step in the Heckman’s method. In order to take into account the potential confounding factors in the model, all variables with a p-value <0.25 were retained for the multivariable analysis. We followed a backward procedure for the selection of the variables to be maintained in the model. Before each variable was removed an investigation was done to take into account interaction terms. A variable was considered statistically significant if it had a p-value less than 0.05 or when the confidence interval of the associated estimate did not contain the value 0.
Due to the moderate proportion of missing around 10% accounting for all variables, we first imputed values using multiple imputations by conditional approach under the Missing At Random hypothesis. All variables (except anthropometric parameters) with missing data were included in the imputation model. This strategy for handling missing data was chosen because it allows both problems of bias and precision that could induce an inappropriate method to be resolved, especially when the imputation is done correctly [32]. We considered 10 multiple imputations with 10 iterations.
All analyzes were carried out using WHO Anthro (for the estimation of Z-scrores during follow-up) and R software, with the packages mice and mitools for multiple imputation, msm for multi-state model, geepack and BSagri for GEE.
Results
Study population description
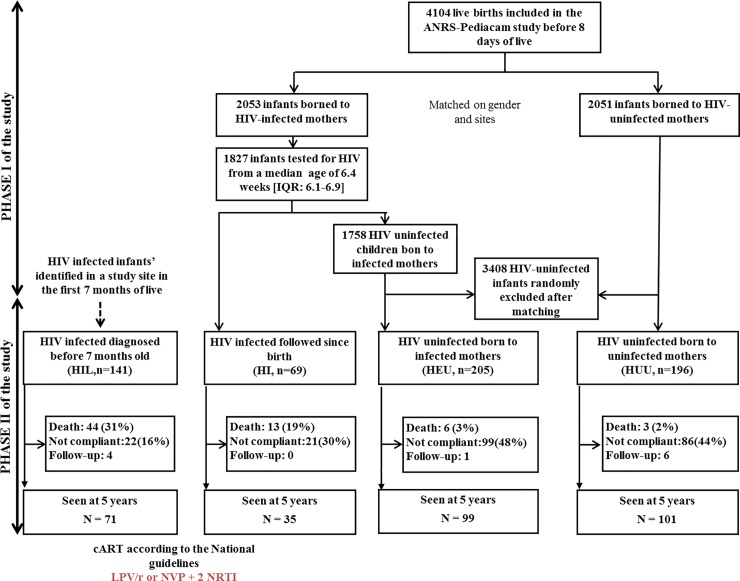
Initially, a total of 4104 mother-child pairs were enrolled in the first phase of the ANRS-PEDIACAM study between November 2007 and October 2010, including 2051 HIV-exposed infants and 2053 HIV-unexposed infants. Among them, 1827 infants were tested for HIV at 6 weeks. For this analysis, 611 infants selected and included in the second phase of the ANRS-PEDIACAM study were enrolled for long-term follow-up, including 69 (11.3%) HI, 205 (33.5%) HEU, 196 (32.1%) HUU and 141 (23.1%) HIL, see Fig 1. Among the 210 children identify, 193 started cART at a median age of 4.1 months. A total of 124 were on LPV/r-based regimen while 69 were of NVP-based regimen. The median age at enrolment in the second phase of the study was 3.4 months (Interquartile range (IQR): 2.3–3.7) for HI, 4.3 months (IQR: 3.6–5.0) for HEU, 4.0 months (IQR: 3.6–4.9) for HUU, and 4.3 months (IQR: 3.2–5.6) for HIL. About 52.9% of children were girls. Nearly half of the children (48.9%) came from the Maternity of the Central hospital/Mother and Child Center of the Chantal Biya Foundation (MCH/MCC-CBF), 22.9% at the Laquintinie Hospital (LH) and 28.2% at the Essos Hospital Center (EHC). Concerning children's home environment, 56.6% and 54.5% of parents reported having water supply and a functional refrigerator at home, respectively. At birth, the proportion of small for gestational age and gender in HIV-infected infants was significantly higher than in HIV-uninfected infants (9.5% vs 5.2% p<10–2). Globally, the two groups of children differed significantly (Table 1).
Fig 1. Brief synthesis of children included and followed until the age of 5, ANRS-PEDIACAM study, Cameroun, Nov. 2007- Dec.2015.
IQR: interquartile range; Not compliant: failed to return for study visit over a period of one year; Follow-up: children regularly monitored, but under the age of 5 at the last visit; PHASE I: first study period planned the first week of life until 14 weeks; PHASE II: second study period for prolonged follow-up (three-monthly till 2 years and six-monthly till 5 years for HIV infected, six monthly till 5 years for HIV-uninfected).
Table 1. Comparison of the characteristics of mothers and children at the inclusion into the phase 2 of the ANRS-PEDIACAM study according to infant HIV status, Cameroun, Nov. 2007- Dec.2015.
| Inclusion group | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| HIV infected children | HIV uninfected children | Total | P | ||||||||
| HI | HIL | HEU | HUU | ||||||||
| N | % | N | % | N | % | N | % | N | % | ||
| Mothers characteristics | 69 | 11.3 | 141 | 23.1 | 205 | 33.6 | 196 | 32.1 | 611 | 100 | |
| Clinical site | |||||||||||
| MCH/MCC-CBF * | 30 | 43.5 | 76 | 53.9 | 98 | 47.8 | 95 | 48.5 | 299 | 48.9 | 0.40 |
| LH † | 19 | 27.5 | 25 | 17.7 | 55 | 26.8 | 41 | 20.9 | 140 | 22.9 | |
| EHC ‡ | 20 | 29.0 | 40 | 28.4 | 52 | 25.4 | 60 | 30.6 | 172 | 28.2 | |
| Mother’s vital status | |||||||||||
| Deceased | 0 | 0.0 | 15 | 10.6 | 0 | 0.0 | 0 | 0.0 | 15 | 2.5 | < .001 |
| Mother’s marital status | |||||||||||
| Married | 44 | 63.8 | 85 | 60.3 | 135 | 65.9 | 133 | 67.9 | 397 | 65.0 | 0.87 |
| Cohabitation | 2 | 2.9 | 4 | 2.8 | 5 | 2.4 | 3 | 1.5 | 14 | 2.3 | |
| Single/divorced/widow | 23 | 33.3 | 50 | 35.5 | 65 | 31.7 | 60 | 30.6 | 198 | 32.4 | |
| Mother’s level of education | |||||||||||
| Higher education | 8 | 11.6 | 6 | 4.3 | 43 | 21.0 | 84 | 42.9 | 141 | 23.1 | < .001 |
| Secondary | 48 | 69.6 | 80 | 56.7 | 123 | 60.0 | 103 | 52.6 | 354 | 57.9 | |
| Primary | 12 | 17.4 | 53 | 37.6 | 37 | 18.0 | 8 | 4.1 | 110 | 18.0 | |
| Electricity at home | |||||||||||
| Yes | 65 | 94.2 | 111 | 78.7 | 196 | 95.6 | 194 | 99.0 | 566 | 92.6 | < .001 |
| No | 2 | 2.9 | 24 | 17.0 | 5 | 2.4 | 0 | 0.0 | 31 | 5.1 | |
| Water supply at home | |||||||||||
| Yes | 36 | 52.2 | 63 | 44.7 | 106 | 51.7 | 141 | 71.9 | 346 | 56.6 | < .001 |
| No | 31 | 44.9 | 72 | 51.1 | 95 | 46.3 | 51 | 26.0 | 249 | 40.8 | |
| Functional fridge at home | |||||||||||
| Yes | 34 | 49.3 | 53 | 37.6 | 108 | 52.7 | 138 | 70.4 | 333 | 54.5 | < .001 |
| No | 32 | 46.4 | 80 | 56.7 | 92 | 44.9 | 53 | 27.0 | 257 | 42.1 | |
| Children characteristics | |||||||||||
| Sex | |||||||||||
| Female | 42 | 60.9 | 75 | 53.2 | 108 | 52.7 | 98 | 50.0 | 323 | 52.9 | 0.49 |
| SGAG at birth $ | |||||||||||
| Yes | 13 | 18.8 | 7 | 5.0 | 17 | 8.3 | 4 | 2.0 | 41 | 6.7 | < .001 |
| No | 56 | 81.2 | 101 | 71.6 | 188 | 91.7 | 191 | 97.4 | 536 | 87.7 | |
| Small size at birth | |||||||||||
| Yes | 4 | 5.8 | 3 | 2.1 | 13 | 6.3 | 1 | 0.5 | 21 | 3.4 | < .001 |
| No | 57 | 82.6 | 36 | 25.5 | 189 | 92.2 | 191 | 97.4 | 473 | 77.4 | |
| Hospitalisation | |||||||||||
| Yes | 14 | 20.3 | 78 | 55.3 | 10 | 4.9 | 4 | 2.0 | 106 | 17.3 | < .001 |
| No | 52 | 75.4 | 63 | 44.7 | 195 | 95.1 | 192 | 98.0 | 502 | 82.2 | |
| Chronic pathologies | |||||||||||
| Yes | 0 | 0.0 | 12 | 8.5 | 0 | 0.0 | 2 | 1.0 | 14 | 2.3 | < .001 |
| No | 65 | 94.2 | 106 | 75.2 | 205 | 100.0 | 194 | 99.0 | 570 | 93.3 | |
| Diarrhea | |||||||||||
| Yes | 9 | 13.0 | 55 | 39.0 | 8 | 3.9 | 8 | 4.1 | 80 | 13.1 | < .001 |
| No | 55 | 79.7 | 83 | 58.9 | 197 | 96.1 | 188 | 95.9 | 523 | 85.6 | |
| CD4 (in %) | |||||||||||
| < 25 | 30 | 43.5 | 95 | 67.4 | 15 | 7.3 | 28 | 14.3 | 168 | 27.5 | < .001 |
| ≥ 25 | 34 | 49.3 | 43 | 30.5 | 185 | 90.2 | 156 | 79.6 | 418 | 68.4 | |
| Anemia | |||||||||||
| Mild | 18 | 26.1 | 24 | 17.0 | 18 | 8.8 | 25 | 12.8 | 85 | 13.9 | < .001 |
| Moderate / Severe | 16 | 23.2 | 58 | 41.1 | 8 | 3.9 | 10 | 5.1 | 92 | 15.1 | |
| No | 31 | 44.9 | 56 | 39.7 | 177 | 86.3 | 149 | 76.0 | 413 | 67.6 | |
| Status | |||||||||||
| Compliant | 35 | 50.7 | 75 | 53.2 | 100 | 48.8 | 107 | 54.6 | 317 | 51.9 | < .001 |
| Deceased | 13 | 18.8 | 44 | 31.2 | 6 | 2.9 | 3 | 1.5 | 66 | 10.8 | |
| Not compliant | 21 | 30.4 | 22 | 15.6 | 99 | 48.3 | 86 | 43.9 | 228 | 37.3 | |
HI: HIV infected followed since birth; HIL: HIV infected diagnosed before 7 months old; HEU: HIV uninfected born to infected mothers; HUU: HIV uninfected born to uninfected mothers
*MCH/MCC-CBF = Maternity of the Central hospital/Mother and Child Center of the Chantal Biya Foundation
† LH = Laquintinie Hospital
‡ EHC = Essos Hospital Center
$ SGAG = Small for Gestational Age and Gender; Compliant: children regularly monitored; Chronic pathologies: included chronic diseases or malformations observed and notified by the clinician since the last visit; Anemia: obtained from the level of hemoglobin, according to the 2011 WHO criteria; Not compliant: lost-to-follow-up for over a year before 5 years.
From enrolment until 60 months, 48% of infants were lost-to-follow up (not compliant) in HEU, 44% in HUU, 30% in HI, and 16% in HIL. In total, 10.8% of infants (n = 66) died during the same period including 18.8% (n = 13), 2.9% (n = 6), 1.5% (n = 3) and 31.2% (n = 44) respectively from HI, HEU, HUU, and HIL children groups. The median age at death was not statistically different between groups (6.7 months (IQR = 3.2–12.2) among HI, 6.2 (IQR = 4.0–9.2) among HIL, 16.6 (IQR = 6.6–41.8) among HEU, and 30.6 (IQR = 17.9–47.5) among HUU; p = 0.14). Also, median follow-up time at the last visit was 60 months (IQR = 41.0–60.2) among HI, 59.8 months (IQR = 11.7–60.2) among HIL, 59.6 months (IQR = 33.8–60.3) among HEU, and 60.0 months (IQR = 42.6–60.4) among HUU. Among alive children, 2.0% (11/545) were less than 5 years at the last visit, with a median age of 54.6 months (range 54.3–55.4) (Fig 1). Overall, 61%, 42.3%, and 27.7% of children experienced wasting, stunting, and underweight at least once respectively with significantly high proportions in HIL group. Regardless of the indicator of malnutrition used, there was a high proportion of malnutrition among children from HIL group, followed respectively by children from HI, HEU and HUU groups (Table 2). Among children who died, 63.3% were stunted (38/60), 51.6% (32/62) were underweight, and 26.7% (16/60) wasted.
Table 2. Distribution of children according to whether they have undergone at least one or not malnutrition during follow-up in the ANRS-PEDIACAM study, Cameroun, Nov. 2007- Dec.2015.
| Inclusion group | Total | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| HI | HIL | HEU | HUU | P | |||||||
| n | % | n | % | n | % | n | % | N | % | ||
| WAZ < -2SD (n = 607) | |||||||||||
| Yes | 24 | 36.9 | 88 | 62.4 | 41 | 20.0 | 15 | 7.7 | 168 | 27.7 | 0.004 |
| LAZ < -2SD (n = 605) | |||||||||||
| Yes | 47 | 72.3 | 115 | 82.7 | 124 | 60.5 | 83 | 42.3 | 369 | 61.0 | 0.071 |
| WLZ < -2SD (n = 605) | |||||||||||
| Yes | 32 | 49.2 | 68 | 48.9 | 91 | 44.4 | 65 | 33.2 | 256 | 42.3 | 0.403 |
HI: HIV infected followed since birth; HIL: HIV infected diagnosed before 7 months old; HEU: HIV uninfected born to infected mothers; HUU: HIV uninfected born to uninfected mothers; WAZ: Weight-for-age Zscore; LAZ: Length-for-age Zscroe; WLZ: Weight-for-length Zscore; SD: standard deviation.
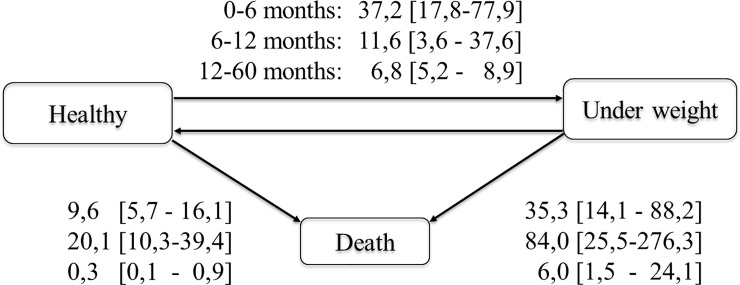
The transitions intensity between healthy status to underweight estimated from the multi-state model were 37.2 per 1000 person-months (95% Confidence interval (95%CI): 17.8–77.9) between 0–6 months, 11.6 per 1000 person-months (95%CI: 3.6–37.6) between 6–12 months, and 6.8 per 1000 person-months (95%CI: 5.2–8.9) between 12–60 months. Between healthy to death, it was 9.6 (95%CI: 5.7–16.1), 20.1 (95%CI: 10.3–39.4), and 0.3 (95%CI: 0.1–0.9) per 1000 person-months between 0–6 months, 6–12 months and 12–60 months respectively. Between underweight to death status, it was 35.3 (95%CI: 14.1–88.2), 84.0 (95%CI: 25.5–276.3) and 6.0 (95%CI: 1.5–24.1) per 1000 person-months between 0–6 months, 6–12 months and 12–60 months respectively. Globally, the incidence of growth retardation was higher in HIV-infected children (HI and HIL) than in uninfected children (HEU and HUU) regardless of the indicator of malnutrition considered (see Fig 2).
Fig 2. Transitions intensities per 1000 person-months between healthy malnutrition and death status for underweight, ANRS-PEDIACAM study, Cameroun, Nov. 2007- Dec.2015.
Factors associated with growth retardation
In multivariable multistate analysis, children’s group, children’s age, and occurrence of chronic pathologies since last visited were independently associated with wasting (see Table 3). At any particular time during follow-up, HI children experienced wasting more frequently compared to HEU (adjusted HR (aHR): 3.3 (95%CI: 1.4–7.9) regarding the HI children and aHR: 4.3 (95%CI: 1.9–9.8) regarding the HIL children. HEU and HUU groups were comparable (aHR: 0.7 (95%IC = 0.3–1.5)). The most at risk age group was 0–6 months compared to 6–12 months with an aHR of 8.8 (CI = 3.4–23.2). Children with chronic pathologies since the last visit were 6.7 times more likely to be wasted (CI: 3.7–12.3). HIL and HI children died frequently from healthy status compared to HEU children (aHR = 14.8[4.1–53.0] 5.8[1.5–22.3] respectively); compared to children aged 6–12 months, the aHR of death was 2.2[1.1–4.7] and 0.0[0.0–0.2] in children aged 0–6 months and 12–60 months resp. Recovery after wasting was explained by children’s group (high odds in HI compared to HEU), young age (0–6 months compared to 6–12 months), male gender, non-hospitalization before inclusion and the absence of general signs at the visit (Table 3).
Table 3. Multivariable multistate models and factors associated with the risk of wasting (WLZ <-2SD) and underweight (WAZ <- 2SD) in the ANRS-PEDIACAM study, Cameroun, Nov. 2007- Dec.2015.
| Transitions | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Healthy- malnourished | Healthy-death | Malnourished-Healthy | ||||||||||
| aHR | L | U | P | aHR | L | U | P | aHR | L | U | P | |
| Factors associated with wasting (WLZ < - 2SD) | ||||||||||||
| Children’s groupe (ref = HEU) | ||||||||||||
| HI | 3.3 | 1.4 | 7.9 | 0.01 | 5.8 | 1.5 | 22.3 | 0.01 | 3.3 | 1.4 | 7.8 | 0.01 |
| HIL | 4.3 | 1.9 | 9.8 | < .001 | 14.8 | 4.1 | 53.0 | < .001 | 4.4 | 2.0 | 9.5 | < .001 |
| HUU | 0.7 | 0.3 | 1.5 | 0.31 | 0.2 | 0.0 | 4.8 | 0.35 | 1.0 | 0.5 | 1.9 | 0.96 |
| Children’s age (months, ref = (6–12]) | ||||||||||||
| (0–6] | 8.8 | 3.4 | 23.2 | < .001 | 2.2 | 1.1 | 4.7 | 0.03 | 5.3 | 2.4 | 12.0 | < .001 |
| (12–60] | 0.8 | 0.4 | 1.7 | 0.60 | 0.0 | 0.0 | 0.2 | < .001 | 0.7 | 0.4 | 1.3 | 0.22 |
| Gender: Female | 0.7 | 0.4 | 1.2 | 0.26 | 1.4 | 0.8 | 2.7 | 0.26 | 0.6 | 0.3 | 0.9 | 0.02 |
| Hospitalisation before inclusion | 1.1 | 0.6 | 2.1 | 0.83 | 1.9 | 0.9 | 3.7 | 0.07 | 0.3 | 0.2 | 0.6 | < .001 |
| General signs during the visit | 0.5 | 0.3 | 0.9 | 0.03 | ||||||||
| Chronic pathologies | 6.7 | 3.7 | 12.3 | < .001 | ||||||||
| Factors associated with underweight(WAZ < - 2SD) | ||||||||||||
| Children’s groupe (ref = HI) | ||||||||||||
| HEU | 1.4* | 0.6 | 3.2 | 0.43 | 0.2 | 0.0 | 0.8 | 0.03 | 1.9 | 1.0 | 3.5 | 0.04 |
| HIL | 2.2* | 1.0 | 4.9 | 0.05 | 1.4 | 0.5 | 4.4 | 0.53 | 1.6 | 0.9 | 2.8 | 0.12 |
| HUU | 0.3* | 0.1 | 1.0 | 0.04 | 0.1 | 0.0 | 0.6 | 0.01 | 1.8 | 0.8 | 4.0 | 0.15 |
| HEU: CD4 <25% | 0.2 | 0.0 | 2.6 | 0.24 | ||||||||
| HIL : CD4 <25% | 1.2 | 0.2 | 6.9 | 0.87 | ||||||||
| HUU: CD4 <25% | 0.0 | 0.0 | Inf | 0.53 | ||||||||
| CD4 (ref = ≥ 25%) | ||||||||||||
| < 25% | 3.0† | 1.3 | 7.3 | 0.01 | 0.7 | 0.5 | 1.1 | 0.14 | ||||
| < 25%: HEU | 0.5 | 0.0 | 6.1 | 0.58 | ||||||||
| < 25%: HIL | 1.6 | 0.3 | 10.3 | 0.61 | ||||||||
| < 25%: HUU | 0.0 | 0.0 | Inf | 0.68 | ||||||||
| Children’s age (months, ref = (6–12]) | ||||||||||||
| (0–6] | 2.7 | 1.4 | 5.2 | 0.00 | 1.3 | 0.3 | 4.9 | 0.74 | 1.8 | 1.0 | 3.1 | 0.04 |
| (12–60] | 0.3 | 0.2 | 0.5 | < .001 | 0.2 | 0.1 | 0.8 | 0.02 | 0.6 | 0.4 | 1.0 | 0.07 |
| Sex: Female | 0.5 | 0.4 | 0.8 | 0.00 | 2.4 | 0.8 | 6.8 | 0.11 | 0.6 | 0.4 | 0.9 | 0.01 |
| Professional activity of the mother (ref = paid activity) | ||||||||||||
| Training/student | 0.6 | 0.3 | 1.2 | 0.14 | 0.4 | 0.2 | 0.8 | 0.01 | ||||
| Housewife/unemployed | 0.9 | 0.6 | 1.5 | 0.73 | 0.6 | 0.4 | 0.9 | 0.01 | ||||
| Water supply at home | 0.7 | 0.5 | 1.1 | 0.17 | 1.5 | 1.0 | 2.2 | 0.04 | ||||
| Hospitalisation before inclusion | 1.9 | 1.2 | 2.9 | 0.01 | 0.8 | 0.3 | 2.4 | 0.74 | 0.6 | 0.4 | 0.9 | 0.02 |
| Chronic pathologies | 2.2 | 0.9 | 5.5 | 0.09 | 0.3 | 0.1 | 0.8 | 0.02 | ||||
*In children’ with more than 25% of CD4
†in HIV-infected infants; HI: HIV infected followed since birth; HIL: HIV infected diagnosed before 7 months old; HEU: HIV uninfected born to infected mothers; HUU: HIV uninfected born to uninfected mothers; WAZ: Weight-for-age Zscore; WLZ: Weight-for-length Zscore; SD: standard deviation; aHR: adjusted hazard risk; L: Lower bound of the confidence interval; U: Upper bound of the confidence interval; P: Pvalue; Inf: Value greater than 100.
Concerning factors associated with underweight (see Table 3), we identified children’s group, mainly in those with more than 25% of CD4, low CD4 count, children’s age, gender, and hospitalization before inclusion. In children with more than 25% CD4 at the last visit, compared to HI children, the aHR was 2.2 (95%CI: 1.0–4.9) among HIL children and 0.3 (95%CI: 0.1–1.0) in HUU ones. Among HI children, those with less than 25% CD4 were more likely to develop underweight than those with CD4 count > 25% (aHR: 3.0, 95%CI: 1.3–7.3). Compared to the 6–12 months age group, children aged 0–6 months were 2.7 more likely to develop underweight (95%CI: 1.4–5.2) while children aged 12–60 months were protected (aHR: 0.3, 95%CI: 0.2–0.5), female gender were protected (aHR: 0.5, 95%CI: 0.4–0.8). Hospitalized prior to inclusion presented a risk of 1.9 (95%CI: 1.2–2.9). Although, not statistical significant, children with chronic pathologies since the last visit had a risk of 2.2 (95%CI: 0.9–5.5). “Healthy-death” transition was associated with children group (HEU and HUU protected compared to HI children) and age (children aged 12–60 months protected compared to those aged 6–12 months). Factors independently associated with the odds of recover after underweight were children’s age, the male gender, mothers’ professional activity (more observed in mothers with paid activity), presence of running water at home, absence of hospitalization before inclusion, and absence of chronic pathology since the last visit (see Table 3).
Associated factors with stunting, as show in Table 4 were children’s group, clinical site, age (children aged 0–6 months were at risk and children aged 12–60 months protected compared to those aged 6–12 months), small size at birth (aHR: 2.0, 95%CI: 1.1–3.6), diarrhea (aHR: 1.8, 95%CI: 1.2–2.8), the low level of CD4 count (aHR: 1.4, 95%CI: 1.1–1.8). In the MCH/MCC-CBF, compared to HEU children, the adjusted hazard risk of stunting was 8.4 (95%CI: 2.4–19.7) in HIL children, 1.5 (95%CI: 0.9–2, 5) in HI children and 0.8 (95%CI: 0.5–1.2) in HUU. Compared to HEU children, the risks were comparable as well in LH as in EHC with HI, HIL and HUU children. On the other hand, compared to children included in the MCH/MCC-CBF, the aHR of stunting was respectively 2.4 (95%CI: 1.5–3.8), 2.8 (95%CI: 0.8–9.6), 0.1 (95%CI: 0.0–0.7) and 3.8 (95%CI: 1.3–11.1) in the LH among HEU, HI, HIL, and HUU children. Although not statistical significant, the presence of water supply at home was a protective factor of stunting (aHR: 0.8, 95%CI: 0.6–1.0). The presence of systemic clinical signs at the visit was associated with death from healthy state (aHR = 11.0, CI = 1.4–88.0). Recovery after stunting was associated with children group (HUU compared to HEU), clinical site (LH compared to MCH/MCC-CBF), age of the child (0–6 months compared to 6–12 months) and the absence of chronic pathologies since the last visit.
Table 4. Multivariable multistate models and factors associated with the risk of stunting (LAZ <-2SD) in the ANRS-PEDIACAM study, Cameroun, Nov. 2007- Dec.2015.
| Transitions | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Healthy- malnourished | Healthy-death | Malnourished-Healthy | ||||||||||
| aHR | L | U | P | aHR | L | U | P | aHR | L | U | P | |
| Children’s group (ref = HEU) | ||||||||||||
| HI | 1.5* | 0.9 | 2.5 | 0.12 | 0.9 | 0.6 | 1.3 | 0.64 | ||||
| HIL | 8.4* | 2.4 | 29.7 | 0.00 | 0.8 | 0.5 | 1.3 | 0.32 | ||||
| HUU | 0.8* | 0.5 | 1.2 | 0.27 | 1.5 | 1.0 | 2.2 | 0.03 | ||||
| HI: LH | 1.8 | 0.5 | 6.3 | 0.38 | ||||||||
| HIL: LH | 0.4 | 0.0 | 5.7 | 0.53 | ||||||||
| HUU: LH | 1.2 | 0.4 | 3.6 | 0.79 | ||||||||
| HI: EHC | 1.9 | 0.5 | 6.9 | 0.31 | ||||||||
| HIL: EHC | 1.5 | 0.1 | 19.6 | 0.77 | ||||||||
| HUU: EHC | 0.9 | 0.3 | 3.1 | 0.89 | ||||||||
| Clinical site (ref = MCH/MCC-CBF) | ||||||||||||
| LH | 2.4† | 1.5 | 3.8 | < .001 | 0.5 | 0.1 | 5.3 | 0.59 | 0.6 | 0.4 | 0.9 | 0.01 |
| EHC | 1.0† | 0.6 | 1.6 | 0.86 | 0.4 | 0.0 | 122.1 | 0.75 | 1.2 | 0.8 | 1.7 | 0.36 |
| LH: HI | 2.8 | 0.8 | 9.6 | 0.09 | ||||||||
| EHC: HI | 1.2 | 0.3 | 4.3 | 0.76 | ||||||||
| LH: HIL | 0.1 | 0.0 | 0.7 | 0.02 | ||||||||
| EHC: HIL | 0.2 | 0.0 | 1.0 | 0.06 | ||||||||
| LH: HUU | 3.8 | 1.3 | 11.1 | 0.02 | ||||||||
| EHC: HUU | 1.2 | 0.3 | 4.0 | 0.83 | ||||||||
| Children’s age (months, ref = (6–12]) | ||||||||||||
| (0–6] | 1.6 | 1.0 | 2.5 | 0.06 | 2.5 | 1.5 | 4.4 | 0.00 | ||||
| (12–60] | 0.2 | 0.1 | 0.3 | < .001 | 0.9 | 0.6 | 1.5 | 0.81 | ||||
| Water supply at home | 0.8 | 0.6 | 1.0 | 0.07 | 0.2 | 0.0 | 2.4 | 0.23 | 1.6 | 0.9 | 3.0 | 0.14 |
| Small size at birth | 2.0 | 1.1 | 3.6 | 0.03 | 1.5 | 0.9 | 2.5 | 0.13 | ||||
| General signs during the visit | 1.2 | 0.8 | 1.7 | 0.41 | 11.0 | 1.4 | 88.0 | 0.02 | ||||
| Diarrhea since the latest visit | 1.8 | 1.2 | 2.8 | 0.01 | ||||||||
| Chronic pathologies | 0.4 | 0.1 | 0.9 | 0.03 | ||||||||
| CD4 < 25% | 1.4 | 1.1 | 1.8 | 0.02 | ||||||||
*In children’ from the Maternity of the Central hospital/Mother and Child Center of the Chantal Biya Foundation
†in HIV-exposed uninfected children; HI: HIV infected followed since birth; HIL: HIV infected diagnosed before 7 months old; HEU: HIV uninfected born to infected mothers; HUU: HIV uninfected born to uninfected mothers; MCH/MCC-CBF: Maternity of the Central hospital/Mother and Child Center of the Chantal Biya Foundation; LH: Laquintinie Hospital; EHC: Essos Hospital Center; LAZ: Length-for-age Zscore; SD: standard deviation; aHR: adjusted hazard risk; L: Lower bound of the confidence interval; U: Upper bound of the confidence interval; P: Pvalue.
Factors associated with growth patterns up to the age of 5
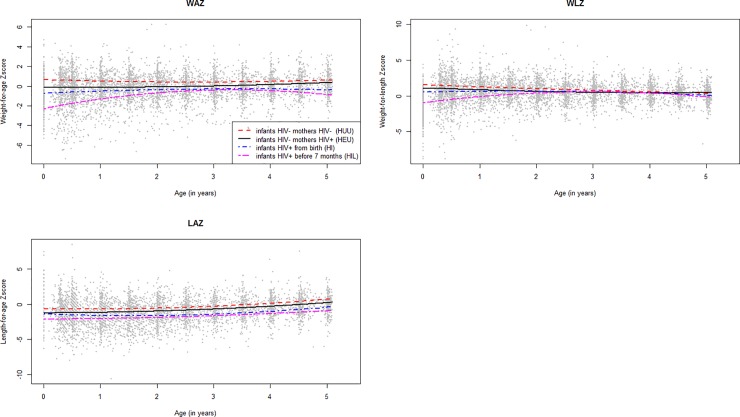
Fig 3 presents the average evolution of the anthropometric indices of children followed in the ANRS-PEDIACAM study according to the different groups. For weight-for-age Z-score (WAZ), mean growth of HIL children was delayed compared to children in other groups, especially in the first two years of life. For the weight-for-length Z-score (WLZ), from 24 months of age, we observed an identical mean evolution in all groups. We will not present results from univariable analyze and Heckman’s first-stage models explaining the non-response process.
Fig 3. Mean growth of children according to anthropometric index and infants’ group at inclusion, from a second order polynomial regression model including 2775 observations for WAZ, 2763 observations for LAZ, and 2758 observations for WLZ, ANRS-PEDIACAM study, Cameroun, Nov. 2007- Dec.2015.
In Multivariable analysis of the WLZ evolution, two significant interactions were observed between children’s group with the onset of an infectious pathology, and with anemia Table 5. A mean reduction of WLZ in HIL children in the presence of anemia and infectious pathology compared to HEU children. Moreover, the onset of infectious pathologies in HEU children reduced the WLZ by nearly 14%. Other variables such as female gender, multiple birth, reported developmental delay, presence of systemic clinical signs, chronic pathologies since last visit, low birth weight (SGAG), and mother’s primary level of education were independently associated to the reduction of the mean WLZ evolution relative to the reference class (coefficient <0 and P <0.05, see Table 5). By cons, changes of residence, breastfeeding, and the presence of electricity were independently associated to an increase of the average WLZ evolution compared to the reference class.
Table 5. Multivariable model, GEE identity with exchangeable correlation structure, describing the factors associated with the evolution of WLZ in the ANRS-PEDIACAM cohort (with and without taking into account biases due to missing data on the WLZ), Cameroun, Nov. 2007- Dec.2015.
| Multivariable analysis without IMR N = 4919 visits by 605 children |
Multivariable analysis with IMR: SE, L, U et P obtained by bootstrap* | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Coef | SE | L | U | P | Coef | SE | L | U | P | ||
| IMR | -0.69 | 0.239 | -1.13 | -0.18 | 0.002 | ||||||
| Clinical site (ref = MCH/MCC-CBF) | |||||||||||
| LH | 0.90 | 0.106 | 0.69 | 1.11 | < .001 | 0.94 | 0.078 | 0.79 | 1.09 | < .001 | |
| EHC | 0.10 | 0.088 | -0.07 | 0.27 | 0.257 | 0.04 | 0.071 | -0.09 | 0.19 | 0.546 | |
| Sex: Female | -0.21 | 0.077 | -0.36 | -0.06 | 0.007 | -0.16 | 0.061 | -0.28 | -0.04 | 0.010 | |
| Age | 0.84 | 0.056 | 0.73 | 0.95 | < .001 | 0.96 | 0.063 | 0.83 | 1.08 | < .001 | |
| Age2 | -0.16 | 0.010 | -0.18 | -0.14 | < .001 | -0.17 | 0.008 | -0.18 | -0.15 | < .001 | |
| Multiple birth | -0.39 | 0.223 | -0.83 | 0.04 | 0.077 | -0.40 | 0.181 | -0.78 | -0.09 | 0.030 | |
| Children’s groupe (ref = HEU) | |||||||||||
| HI | 0.08 | 0.137 | -0.19 | 0.35 | 0.564 | -0.08 | 0.118 | -0.30 | 0.15 | 0.524 | |
| HIL | 0.05 | 0.135 | -0.21 | 0.32 | 0.701 | -0.17 | 0.135 | -0.41 | 0.11 | 0.216 | |
| HUU | 0.27 | 0.115 | 0.04 | 0.49 | 0.020 | 0.17 | 0.094 | -0.01 | 0.35 | 0.082 | |
| Infectious patologies (ref = No) | -0.14 | 0.066 | -0.27 | -0.01 | 0.038 | -0.14 | 0.048 | -0.23 | -0.05 | 0.002 | |
| Anemia (ref = No) | 0.06 | 0.088 | -0.11 | 0.24 | 0.469 | 0.07 | 0.063 | -0.06 | 0.18 | 0.302 | |
| HI: Infectious patologies | -0.17 | 0.126 | -0.42 | 0.07 | 0.167 | -0.17 | 0.101 | -0.37 | 0.05 | 0.090 | |
| HIL: Infectious patologies | -0.29 | 0.105 | -0.49 | -0.08 | 0.006 | -0.28 | 0.084 | -0.44 | -0.10 | < .001 | |
| HUU: Infectious patologies | 0.02 | 0.094 | -0.16 | 0.21 | 0.807 | 0.03 | 0.073 | -0.12 | 0.17 | 0.680 | |
| HI: Anemia | -0.02 | 0.172 | -0.36 | 0.32 | 0.899 | -0.07 | 0.128 | -0.34 | 0.17 | 0.586 | |
| HIL: Anemia | -0.47 | 0.128 | -0.72 | -0.21 | < .001 | -0.38 | 0.105 | -0.59 | -0.17 | < .001 | |
| HUU: Anemia | -0.15 | 0.130 | -0.40 | 0.11 | 0.258 | -0.12 | 0.101 | -0.32 | 0.07 | 0.238 | |
| CD4 ≥_25% | 0.13 | 0.069 | -0.01 | 0.26 | 0.063 | 0.04 | 0.064 | -0.08 | 0.17 | 0.528 | |
| Home change | 0.17 | 0.072 | 0.03 | 0.32 | 0.016 | 0.20 | 0.050 | 0.10 | 0.30 | < .001 | |
| Developmental delay | -0.68 | 0.131 | -0.93 | -0.42 | < .001 | -0.67 | 0.102 | -0.87 | -0.48 | < .001 | |
| General signs during the visit | -0.14 | 0.087 | -0.31 | 0.03 | 0.107 | -0.14 | 0.067 | -0.27 | -0.01 | 0.032 | |
| Breastfeeding | 0.06 | 0.072 | -0.08 | 0.20 | 0.391 | 0.21 | 0.081 | 0.04 | 0.35 | 0.016 | |
| Diarrhea | -0.27 | 0.105 | -0.48 | -0.07 | 0.010 | -0.15 | 0.091 | -0.35 | 0.02 | 0.096 | |
| Chronic pathologies | -0.63 | 0.175 | -0.97 | -0.29 | < .001 | -0.63 | 0.138 | -0.87 | -0.32 | < .001 | |
| SGAG (ref = No) | -0.46 | 0.169 | -0.79 | -0.13 | 0.006 | -0.51 | 0.125 | -0.77 | -0.27 | < .001 | |
| Mother’s level of education (ref = higher) | |||||||||||
| Secondary | -0.01 | 0.091 | -0.19 | 0.17 | 0.882 | 0.02 | 0.069 | -0.11 | 0.15 | 0.808 | |
| Primary | -0.24 | 0.136 | -0.51 | 0.03 | 0.080 | -0.28 | 0.111 | -0.47 | -0.04 | 0.016 | |
| Electricity supply at home | 0.34 | 0.238 | -0.13 | 0.81 | 0.152 | 0.35 | 0.183 | 0.02 | 0.74 | 0.052 | |
*standard errors (SE) and confidence intervalle (L,U) obtained based on 500 replications bootstrap, due to the introduction of the IMR in the model; IMR: Inverse Mills ratio obtained from the residuals of the first stage model; Coef: coefficients; L: Lower bound of the confidence interval; U: Upper bound of the confidence interval; P: Pvalue; HI: HIV infected followed since birth; HIL: HIV infected diagnosed before 7 months old; HEU: HIV uninfected born to infected mothers; HUU: HIV uninfected born to uninfected mothers; MCH/MCC-CBF: Maternity of the Central hospital/Mother and Child Center of the Chantal Biya Foundation; LH: Laquintinie Hospital; EHC: Essos Hospital Center; SGAG: small-for-gestational age and gender; WLZ: Weight-for-length.
Concerning the mean evolution of WAZ, HIV-infected children contributed negatively while the HUU children's contribution was positive compared to HEU children (P <0.001, see S1 Table). In addition, the following mother’s and child’s characteristics impacted positively (breastfeeding, the presence of electricity and water at home) or negatively (multiple births, chronic pathologies, anemia, CD4 count < 25%, unemployed mother, developmental delay, presence of systemic clinical signs during the visit, SGAG, diarrhea, secondary and primary education level of the mother) WAZ evolution.
Similar observation was made at with LAZ (see S2 Table). We also observed a significant interaction with positive coefficients between the children group and anemia, showing that the negative effects of HI and HIL children (respectively positive effects of HUU children) compared to HEU children on LAZ were attenuated (respectively accentuated) in the presence of anemia. Furthermore, in children, the presence of anemia contributes negatively to the evolution of WAZ. It was also observed that children with small birth size contribute negatively to the LAZ evolution.
A mean quadratic effect of age was observed on WLZ, WAZ or LAZ profiles. Taking into consideration the selection bias of missing data, the estimated IMR effect was significantly less than 0 with WLZ and WAZ profiles and some coefficients of the standard GEE models have changed their significance (see Table 5 and S1 Table). No significant effect of IMR was observed on the LAZ evolution (see S2 Table).
Discussion
The main focus of this analysis was to compare incidence of growth retardation, instantaneous risk of death related to growth retardation, and growth evolution over the first five years of life between early treated HIV-infected, HEU and HUU children in Cameroon. Other studies [13, 14] also addressed the issue of growth among children in the context of early cART in sub-Saharan Africa. But, the distinctiveness of our study was its statistical approach, which considered deaths as a competitive risk with malnutrition, and the recurrence of malnutrition during follow-up.
Overall, our results showed that, regardless of the anthropometric index considered, early treated HIV-infected children (particularly those from HIL group) had growth retardation and average growth rate lower than that of HIV-uninfected children indicating no catching up of growth indices over the first five years of life. It is known that administration of early ART helps to suppress viral load and improve the immune response leading drastic reduction of morbidity, mortality [4]. These observations can be the consequences of the differences in characteristics of early ART treated HIV-infected children and HIV-uninfected children. As presented in Table 1, early HIV-infected children environment is socioeconomically disadvantage (low education level of mothers, low access to electricity and water etc.) compared to HIV-uninfected children. Furthermore, a significant number of early treated HIV-infected children will fail to achieve or to maintain virological suppression. Thus, several factors can influence the response to antiretroviral therapy, and therefore the nutritional response. Even with a significant gain in weight and height in presence of cART, the nutritional status could not be completely restored for a significant number of children who remained malnourished, despite the treatment [33]. ART alone is not sufficient and specific interventions are needed to improve the nutritional care of HIV-infected children.
In the HIL group, a high proportion of deaths occurred during the first year of life corroborating the fact that without cART, one third of HIV-infected children died before the age of one year [34]. This was probably due to advanced stage of the disease or CMV coinfection as recently described at time of enrolment [35]. Many authors have also reported that malnourished children died in average 2 to 3 times in the first month of cART treatment compared to non-malnourished children [9, 23–25].
Some authors have reported the negative effect of HIV infection on growth in different settings [36, 37]. Bailey et al. in Congo found in a prospective cohort of children that, the risk of underweight, stunting and wasting was higher in HIV-infected children than in HIV-uninfected children during the first two years of life [36]. In Rwanda, in a cohort of children aged 0–48 months, HIV-infected children were more likely to be underweight and stunting, but not wasting compared to HIV-uninfected children. In that cohort, comparable anthropometric indices were observed between HEU and HUU [37]. HIV-infected children suffer from opportunistic infections, such as diarrhea and persistent malabsorption, with inflammatory effects that can cause intestinal dysfunction and affect height and weight [38].
HUU children had a risk of wasting and stunting comparable to that of HEU. Furthermore, in children with CD4 levels greater than 25%, those in HUU group were protected against underweight compared to those in HI group. However, regardless of the anthropometric index considered, the average growth evolution was negatively impacted by HIV infection and in-utero HIV exposition. This requires special attention to be given to children born to HIV-infected mothers to ensure growth comparable to that of HUU. Nutritional supplements during the first years of life observed by Rebacca et al. as in many other studies indicates that such action in this period would improve the growth of HIV-exposed children [39].
Many other factors, such as male gender, small birth size, multiple birth, mother's primary or secondary education, SGAG, lack of running water or electricity at home, unemployment of the mother have been identified as potentially and independently compromising the growth of children in this study. In addition, others factors related to the child's health status such as the occurrence of chronic pathologies, reported episodes of diarrhea, anemia, and low CD4 levels affecting the growth of children have also been identified in other contexts [40].
In the modeling process, we found in some models, mainly for the variable children’s group fairly broad confidence intervals. This is due to the low number of events observed in HUU children during follow-up. The interaction was significant between the groups of children and the clinical site, reflecting the heterogeneity in the management of the children. The study sites were located in referral hospital, pioneers and pole of excellence in pediatric HIV care in Cameroon, managing the largest number of HIV-infected and HIV exposed children.
Heckman's method allowed us to avoid falsely concluding on the effect (or not) of different variables on the evolution of anthropometric indices as reported by Protopopescu et al [31]. This analysis approach is necessary in our context where it is not obvious to involve HIV-uninfected children’s parents (infected or not) to research studies involving HIV-infected children and with no direct benefit.
The multi-state models of the Markovian type used allowed us to study the dynamics evolution of the growth retardation taking into account the censorship interval observed on the occurrence of the growth retardation. Available studies are mostly based on Cox proportional hazard models that consider right censorship of deaths and first occurrence of growth retardation. Another interesting aspect of this study was the missing data consideration using the most well-known methods for minimizing induced biases based on the hypothesis of the non-response process: multiple imputation and Heckman methods.
Three common growth indicators encountered during the first five years of live in the literature were used: weight-for-age, weight for length, and length-for-age Z-score. One of the perspectives of this work would be to define, using these 3 indices, a single indicator for the growth retardation. We also plan to propose a user friendly R package, which summarizes all the statistical methods used in this manuscript, for wide use by the scientific community. A comparative analysis between the Markov multi-state model and the joint frailty model for recurrent events and death [41] should be considered to find the most appropriate method for studying the incidence of growth retardation and the instantaneous risk of death. The advantage of the latter method (Rondeau et al) is to take into account the potential selection biases due to unmeasured covariates, using a shared frailty term.
Conclusions
Growth retardation in the ANRS-PEDIACAM study was identified as a real health problem affecting at least once more than 60% of children followed during the first 5 years of life in three referral hospitals based in urban setting in Cameroon. HIV-infected children diagnosed before the age of 7 months were most affected, especially during the first two years of life, where their mean growth evolution diverged drastically from that of other groups. Uninfected infants born to HIV-infected mothers were comparable to un-exposed children in terms of the instantaneous risk of growth retardation. Overall, mother’s or child HIV infection affects the child's growth during the first years of life, regardless of the availability of antiretroviral therapy. However, it is imperative to strengthen the care of HIV-infected children and to follow international guidelines with regard to the early detection and initiation of ARVs. Special attention should be given to the statistical methodology envisaged for the study of the growth of children in order to take into account the potential biases due to missing data and other constraints related to the epidemiological problem.
Supporting information
(DOCX)
(DOCX)
Acknowledgments
We would like to thank all those who contributed to this study, especially the parents who agreed the inclusion of their children in this study. We thank the coordination team and all the staff involved in the ANRS-PEDIACAM study. We gratefully acknowledge very helpful and constructive comments and suggestions from the academic editor and the anonymous referee, which lead to significant improvements of this manuscript.
The ANRS-Pediacam Study Team is as follows:
Primary investigators: Prof Albert Faye (Hôpital Robert Debré / Univ. Paris 7, France) and Dr Mathurin Cyrille Tejiokem (Centre Pasteur du Cameroun, Yaoundé).
Co-investigators: Prof Françoise Barré-Sinoussi and Dr Daniel Scott (Unité de Régulations des Infections Rétrovirales, Institut Pasteur de Paris, France), Dr Frédéric Tangy (Unité de Génomique virale et Vaccinantion, Institut Pasteur de Paris, France), Dr Josiane Warszawski (Equipe 4 (VIH et IST)—INSERM U1018 (CESP)/Univ. Paris Sud 11, France), Prof Stéphane Blanche (Service d’Immunologie et Hématologie Pédiatrique, Hôpital Necker Enfants Malades, Paris, France), Dr Catherine Dollfus (Hôpital Trousseau, Paris, France), Dr Laurence Baril (GSK Bio, Risenxart, Belgium), Dr Anfumbom Kfutwah (Centre Pasteur du Cameroun), Dr Ida Penda (Hôpital Laquintinie, Douala, Cameroun), Dr Georgette Guemkam and Dr Ateba Ndongo Francis (Centre Mère et Enfant de la Fondation Chantal Biya, Yaoundé, Cameroun), Dr Suzie Tetang Ndiang (Centre Hospitalier d’Essos, Yaoundé, Cameroun).
Other members of the ANRS-Pediacam team: (by site and alphabetic order).
Centre Pasteur du Cameroun: Epouner Denise, Etienne NOMEGNE, Françis Yuya, Mbanzouen William, Ngoupo Paul Alain, Owona Félicité, Sofeu Casimir Ledoux, Dr Tchendjou Patrice.
Center Hospital Maternity/Mother and Child care Center in Yaoundé: Bossolo Juste, Ehongo Jean Marie, Dr Evouna Armel, Mbida Patricia, Dr Ndongo Jean Audrey, Dr Nguefack Félicité, Prof Mbu Robinson, and Prof Koki Paul.
Essos Hospital Center in Yaoundé: Bekono Ernestine, Belinga Marie Louise, Evoundou Dieudonné, Derboise GWEHA, Dr Nga Annie, Nguen Suzanne, Dr Njom Nlend Anne, Onono Yvette, Dr Wamba Guillaume, Dr Zeudja.
Laquintinie Hospital in Douala: Alibien Michelle, Dr Dissongo Jean II, Djene Julie, Ewané Valery, Dr Makwet Nicaise, Dr Mbangué Madeleine, Ngo Sohna Aurore, Dr Ngwa, Obedat Shiro.
The lead author of the ANRS-PEDIACAM study group is Mathurin Cyrille Tejiokem (email: tejiokem@pasteur-yaounde.org, mathurin.tejiokem@gmail.com)
Data Availability
The Cameroon Ministry of Public Health (Division of operational Research and the Centre Pasteur du Cameroun) and the ANRS own the data underlying the results of this study. Thus, data are available upon request due to ethical and legal restrictions. To get access to the ANRS 12225 PEDIACAM study data, a researcher should submit a request detailing the planned analysis on the requested data. Requests should be sent to the ANRS (ped@anrs.fr or laure-amelie.monteynard@anrs.fr), or to the ANRS 12225 PEDIACAM study coordinators (tejiokem@pasteur-yaounde.org or albert.faye@aphp.fr). All requests will be reviewed by the Scientific Committee of the study, if still active, and if not, by the principal/coordinating team in accordance with the ANRS and the Cameroon Ministry of Public Health. The authors did not receive any special access privileges to the data. Interested researchers will be able to replicate the results of this study by following the protocol outlined in the Methods section of the paper.
Funding Statement
This study is funded by the French National Agency for Research on HIV/AIDS and viral hepatitis (ANRS, France). The ANRS is informed at regular intervals about the progress of the study. The ANRS had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript. All the co-authors have contributed collectively to this study and have approved the final version of this manuscript and its submission to PLoS ONE.
References
- 1.WHO. Alimentation du nourrisson et du jeune enfant. Aide-mémoire N° 342Juillet 2015. 02/06/2016. Available from: http://www.who.int/mediacentre/factsheets/fs342/fr/. [Google Scholar]
- 2.Black RE, Victora CG, Walker SP, Bhutta ZA, Christian P, de Onis M, et al. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet. 2013;382(9890):427–51. 10.1016/S0140-6736(13)60937-X [DOI] [PubMed] [Google Scholar]
- 3.UNICEF. Committing to Child Survival: A Promise Renewed. Progress Report 2014. 3 United Nations Plaza, New York, NY 10017, USA: UNICEF; Septembre 2014. [Google Scholar]
- 4.Jesson J, Leroy V. Challenges of malnutrition care among HIV-infected children on antiretroviral treatment in Africa. Med Mal Infect. 2015;45(5):149–56. 10.1016/j.medmal.2015.03.002 [DOI] [PubMed] [Google Scholar]
- 5.UNAIDS. UNAIDS gap report 2014.2014:[26 p.]. Available from: http://www.unaids.org/fr/resources/campaigns/2014/2014gapreport/gapreport.
- 6.UNAIDS/WHO. Fact sheet—World AIDS day 2018. 2017 Global HIV statistics.
- 7.Global aids update http://www.unaids.org/sites/default/files/media_asset/global-AIDS-update-2016_en.pdf.
- 8.INS. Enquête par grappes à indicateurs multiples (MICS5), 2014, Rapport Final2015. Available from: http://slmp-550-104.slc.westdc.net/~stat54/downloads/2016/MICS5_CMR2014_RAPPORT_FINAL.pdf.
- 9.Fergusson P, Tomkins A. HIV prevalence and mortality among children undergoing treatment for severe acute malnutrition in sub-Saharan Africa: a systematic review and meta-analysis. Trans R Soc Trop Med Hyg. 2009;103(6):541–8. 10.1016/j.trstmh.2008.10.029 [DOI] [PubMed] [Google Scholar]
- 10.Debeaudrap P, Bodeau-Livinec F, Pasquier E, Germanaud D, Ndiang ST, Nlend AN, et al. Neurodevelopmental outcomes in HIV-infected and uninfected African children. AIDS. 2018;32(18):2749–57. 10.1097/QAD.0000000000002023 [DOI] [PubMed] [Google Scholar]
- 11.Parchure RS, Kulkarni VV, Darak TS, Mhaskar R, Miladinovic B, Emmanuel PJ. Growth Patterns of HIV Infected Indian Children in Response to ART: A Clinic Based Cohort Study. Indian J Pediatr. 2015;82(6):519–24. 10.1007/s12098-014-1659-1 [DOI] [PubMed] [Google Scholar]
- 12.Jesson J, Koumakpaï S, Diagne NR, Amorissani-Folquet M, Kouéta F, Aka A, et al. Effect of Age at Antiretroviral Therapy Initiation on Catch-up Growth Within the First 24 Months Among HIV-infected Children in the IeDEA West African Pediatric Cohort. Pediatr Infect Dis J. 2015;34(7):e159–68. 10.1097/INF.0000000000000734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shiau S, Arpadi S, Strehlau R, Martens L, Patel F, Coovadia A, et al. Initiation of antiretroviral therapy before 6 months of age is associated with faster growth recovery in South African children perinatally infected with human immunodeficiency virus. J Pediatr. 2013;162(6):1138–45, 45.e1-2. 10.1016/j.jpeds.2012.11.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jesson J, Dahourou DL, Amorissani Folquet M, Malateste K, Yonaba C, N'Gbeche MS, et al. Malnutrition, Growth Response and Metabolic Changes Within the First 24 Months After ART Initiation in HIV-infected Children Treated Before the Age of 2 Years in West Africa. Pediatr Infect Dis J. 2018;37(8):781–7. 10.1097/INF.0000000000001932 [DOI] [PubMed] [Google Scholar]
- 15.Prendergast A, Bwakura-Dangarembizi MF, Cook AD, Bakeera-Kitaka S, Natukunda E, Nahirya Ntege P, et al. Hospitalization for severe malnutrition among HIV-infected children starting antiretroviral therapy. AIDS. 2011;25(7):951–6. 10.1097/QAD.0b013e328345e56b [DOI] [PubMed] [Google Scholar]
- 16.Mwiru RS, Spiegelman D, Duggan C, Seage GR, Semu H, Chalamilla G, et al. Growth among HIV-infected children receiving antiretroviral therapy in Dar es Salaam, Tanzania. J Trop Pediatr. 2014;60(3):179–88. 10.1093/tropej/fmt104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tejiokem MC, Faye A, Penda IC, Guemkam G, Ateba Ndongo F, Chewa G, et al. Feasibility of early infant diagnosis of HIV in resource-limited settings: the ANRS 12140-PEDIACAM study in Cameroon. PLoS One. 2011;6(7):e21840 10.1371/journal.pone.0021840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rouet F, Ekouevi DK, Chaix ML, Burgard M, Inwoley A, Tony TD, et al. Transfer and evaluation of an automated, low-cost real-time reverse transcription-PCR test for diagnosis and monitoring of human immunodeficiency virus type 1 infection in a West African resource-limited setting. J Clin Microbiol. 2005;43(6):2709–17. 10.1128/JCM.43.6.2709-2717.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.WHO. Multicentre Growth Reference Study Group. WHO Child Growth Standards: length/height-for- age, weight-for- age, weight-for- length, weight-for- height and body mass index-for- age: methods and development. Geneva: World Health Organization; 2006. Available from: http://www.who.int/childgrowth/standards/technical_report/en/. [Google Scholar]
- 20.OMS. Concentrations en hémoglobine permettant de diagnostiquer l’anémie et d’en évaluer la sévérité. Système d’informations nutritionnelles sur les vitamines et les minéraux. Genève; 2011. [Google Scholar]
- 21.Sofeu CL, Warszawski J, Ateba Ndongo F, Penda IC, Tetang Ndiang S, Guemkam G, et al. Low birth weight in perinatally HIV-exposed uninfected infants: observations in urban settings in Cameroon. PLoS One. 2014;9(4):e93554 10.1371/journal.pone.0093554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee PA, Chernausek SD, Hokken-Koelega AC, Czernichow P, Board ISfGAA. International Small for Gestational Age Advisory Board consensus development conference statement: management of short children born small for gestational age, April 24-October 1, 2001. Pediatrics. 2003;111(6 Pt 1):1253–61. 10.1542/peds.111.6.1253 [DOI] [PubMed] [Google Scholar]
- 23.Mwiru RS, Spiegelman D, Duggan C, Seage GR, Semu H, Chalamilla G, et al. Nutritional Status and Other Baseline Predictors of Mortality among HIV-Infected Children Initiating Antiretroviral Therapy in Tanzania. J Int Assoc Provid AIDS Care. 2015;14(2):172–9. 10.1177/2325957413500852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brian CZ, Thuli P, Holly MZ, Holly F, Margaret EF. Risk factors associated with increased mortality among HIV infected children initiating antiretroviral therapy (ART) in South Africa. PLoS One. 2011;6(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anaky MF, Duvignac J, Wemin L, Kouakoussui A, Karcher S, Touré S, et al. Scaling up antiretroviral therapy for HIV-infected children in Côte d'Ivoire: determinants of survival and loss to programme. Bull World Health Organ. 2010;88(7):490–9. 10.2471/BLT.09.068015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jackson CH. Multi-State Models for Panel Data: The msm Package for R. Journal of statistical software; January 2011. [Google Scholar]
- 27.Meng X, Rubin DB. Performing likelihood ratio tests with multiply-imputed data sets. Great Britain 1992. p. 103–11. [Google Scholar]
- 28.Mistler SA. A SAS Macro for Computing Pooled Likelihood Ratio Tests with Multiply Imputed Data. Arizona State University 2013. [Google Scholar]
- 29.Shelton BJ, Gilbert GH, Lu Z, Bradshaw P, Chavers LS, Howard G. Comparing longitudinal binary outcomes in an observational oral health study. Stat Med. 2003;22(12):2057–70. 10.1002/sim.1469 [DOI] [PubMed] [Google Scholar]
- 30.Chavance M, Manfredi R. [Modeling incomplete observations]. Rev Epidemiol Sante Publique. 2000;48(4):389–400. [PubMed] [Google Scholar]
- 31.Protopopescu C, Raffi F, Roux P, Reynes J, Dellamonica P, Spire B, et al. Factors associated with non-adherence to long-term highly active antiretroviral therapy: a 10 year follow-up analysis with correction for the bias induced by missing data. J Antimicrob Chemother. 2009;64(3):599–606. 10.1093/jac/dkp232 [DOI] [PubMed] [Google Scholar]
- 32.White IR, Royston P, Wood AM. Multiple imputation using chained equations: Issues and guidance for practice. Stat Med. 2011;30(4):377–99. 10.1002/sim.4067 [DOI] [PubMed] [Google Scholar]
- 33.Sunguya BF, Poudel KC, Otsuka K, Yasuoka J, Mlunde LB, Urassa DP, et al. Undernutrition among HIV-positive children in Dar es Salaam, Tanzania: antiretroviral therapy alone is not enough. BMC Public Health. 2011;11(1):869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Newell ML, Coovadia H, Cortina-Borja M, Rollins N, Gaillard P, Dabis F, et al. Mortality of infected and uninfected infants born to HIV-infected mothers in Africa: a pooled analysis. Lancet. 2004;364(9441):1236–43. 10.1016/S0140-6736(04)17140-7 [DOI] [PubMed] [Google Scholar]
- 35.Kfutwah AK, Ngoupo PA, Sofeu CL, Ndongo FA, Guemkam G, Ndiang ST, et al. Cytomegalovirus infection in HIV-infected versus non-infected infants and HIV disease progression in Cytomegalovirus infected versus non infected infants early treated with cART in the ANRS 12140-Pediacam study in Cameroon. BMC Infect Dis. 2017;17(1):224 10.1186/s12879-017-2308-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bailey RC, Kamenga MC, Nsuami MJ, Nieburg P, St Louis ME. Growth of children according to maternal and child HIV, immunological and disease characteristics: a prospective cohort study in Kinshasa, Democratic Republic of Congo. Int J Epidemiol. 1999;28(3):532–40. 10.1093/ije/28.3.532 [DOI] [PubMed] [Google Scholar]
- 37.Lepage P, Msellati P, Hitimana DG, Bazubagira A, Van Goethem C, Simonon A, et al. Growth of human immunodeficiency type 1-infected and uninfected children: a prospective cohort study in Kigali, Rwanda, 1988 to 1993. Pediatr Infect Dis J. 1996;15(6):479–85. [DOI] [PubMed] [Google Scholar]
- 38.Miller TL, Agostoni C, Duggan C, Guarino A, Manary M, Velasco CA, et al. Gastrointestinal and nutritional complications of human immunodeficiency virus infection. J Pediatr Gastroenterol Nutr. 2008;47(2):247–53. 10.1097/MPG.0b013e318181b254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Heidkamp RA, Stoltzfus RJ, Fitzgerald DW, Pape JW. Growth in late infancy among HIV-exposed children in urban Haiti is associated with participation in a clinic-based infant feeding support intervention. J Nutr. 2012;142(4):774–80. 10.3945/jn.111.155275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Teklemariam Z, Mitiku H, Mesfin F. Prevalence of anemia and nutritional status among HIV-positive children receiving antiretroviral therapy in Harar, eastern Ethiopa. HIV AIDS (Auckl). 2015;7:191–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rondeau V, Mathoulin-Pelissier S, Jacqmin-Gadda H, Brouste V, Soubeyran P. Joint frailty models for recurring events and death using maximum penalized likelihood estimation: application on cancer events. Biostatistics. 2007;8(4):708–21. 10.1093/biostatistics/kxl043 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
Data Availability Statement
The Cameroon Ministry of Public Health (Division of operational Research and the Centre Pasteur du Cameroun) and the ANRS own the data underlying the results of this study. Thus, data are available upon request due to ethical and legal restrictions. To get access to the ANRS 12225 PEDIACAM study data, a researcher should submit a request detailing the planned analysis on the requested data. Requests should be sent to the ANRS (ped@anrs.fr or laure-amelie.monteynard@anrs.fr), or to the ANRS 12225 PEDIACAM study coordinators (tejiokem@pasteur-yaounde.org or albert.faye@aphp.fr). All requests will be reviewed by the Scientific Committee of the study, if still active, and if not, by the principal/coordinating team in accordance with the ANRS and the Cameroon Ministry of Public Health. The authors did not receive any special access privileges to the data. Interested researchers will be able to replicate the results of this study by following the protocol outlined in the Methods section of the paper.