Abstract
The adverse consequences of herbicide drift towards sensitive crops have been extensively reported in the literature. However, little to no information is available on the consequences of herbicide drift onto weed species inhabiting boundaries of agricultural fields. Exposure to herbicide drift could be detrimental to long-term weed management as several weed species have evolved herbicide-resistance after recurrent selection with sublethal herbicide rates This study investigated the deposition of glyphosate, 2,4-D, and dicamba spray particle drift from applications with two different nozzles in a low speed wind tunnel, and their impact on growth and development of Amaranthus spp. Herbicide drift resulted in biomass reduction or complete plant mortality. Inflection points (distance to 50% biomass reduction) for Amaranthus tuberculatus were 7.7, 4.0, and 4.1 m downwind distance for glyphosate, 2,4-D, and dicamba applications with the flat-fan nozzle, respectively, whereas these values corresponded to 2.8, 2.5, and 1.9 m for applications with the air-inclusion nozzle. Inflection points for Amaranthus palmeri biomass reduction were 16.3, 10.9, and 11.5 m for glyphosate, 2,4-D, and dicamba applications with the flat-fan nozzle, respectively, whereas these values corresponded to 7.6, 5.4, and 5.4 m for applications with the air-inclusion nozzle. Plants were more sensitive to glyphosate at higher exposure rates than other herbicides, whereas plants were more sensitive to 2,4-D and dicamba at lower exposure rates compared to glyphosate. Applications with the flat-fan nozzle resulted in 32.3 and 11.5% drift of the applied rate at 1.0 and 3.0 m downwind, respectively, whereas the air-inclusion nozzle decreased the dose exposure in the same distances (11.4 and 2.7%, respectively). Herbicide drift towards field boundaries was influenced by nozzle design and exposed weeds to herbicide rates previously reported to select for herbicide-resistant biotypes.
Introduction
Spray drift is defined as the part of the application (particles or vapors) that is deflected away from the target during or following applications [1]. Many environmental and application technique factors influence spray particle drift, such as wind speed and direction, sprayer boom height, and spray droplet size [2–4]. Spray droplet size which is directly influenced by nozzle design, nozzle orifice size, operating pressure, and physicochemical properties of the solution, is often the focal point of particle drift mitigation efforts [5–7].
Risk assessment of herbicide drift includes the surrounding vegetation characterization, as non-target sensitive vegetation coexist with agricultural fields [8,9]. The adverse consequences of herbicide drift towards sensitive crops have been extensively reported in the literature [10–13]. However, little to no information is available on the consequences of herbicide drift on agricultural weed species. Weed species including horseweed (Erigeron canadensis L.), waterhemp [Amaranthus tuberculatus (Moq.) J. D. Sauer], Palmer amaranth (Amaranthus palmeri S. Wats.), velvetleaf (Abutilon theophrasti Medik), giant ragweed (Ambrosia trifida L. AMBTR), and others are often abundant in field boundaries and ditches surrounding agricultural lands in the US Midwest [14–17] (Fig 1).
Fig 1. Waterhemp (Amaranthus tuberculatus) population located on field border in eastern Nebraska.
Exposure to herbicide drift could be detrimental to long-term weed management as several weed species have evolved resistance after recurrent selection with sublethal herbicide rates [18–27]. Previous research reported that recurrent selection with low rates of herbicides progressively selected for herbicide metabolism alleles present within the standing genetic variation of the population, additively leading to herbicide resistance [28–30]. In most recurrent selection studies, weed populations selected with sublethal rates of a given herbicide also evolved resistance to other herbicide sites of action [18,22,23,26]. This highlights the nature of non-target site resistance (NTSR) and influence of metabolic alleles selected in weed populations upon recurrent selection with low herbicides rates [29,31,32]. It has been suggested that recurrent selection with sublethal doses of herbicides not only select polygenic alleles within the standing genetic variation of the population, but also could induce new stress-related mutations within surviving individuals [33]. Furthermore, it has been suggested that sublethal herbicide rates could act as stress agents inducing DNA mutations, epigenetic alterations, transcriptional remodeling, protein modifications, and other events that could ultimately confer levels of herbicide resistance [34]. Stress-induced epigenetic changes (DNA methylation, histone modifications, and others) are normally reverted soon after stress exposure, although in specific cases they can be carried over for multiple generations [35]. The reproductive system of weed species influences herbicide resistance evolution. For instance, when plants are recurrently selected with sublethal rates of herbicides, recombination and accumulation of minor resistance genes can occur at a faster rate in cross-pollinated species such as waterhemp and Palmer amaranth [20,36].
Despite the potential adverse implications towards resistance evolution from sublethal rate exposure via herbicide drift, near-field weed populations are often ignored and not managed in agricultural landscapes [14,15,17,37]. Therefore, the objectives of this study were to investigate the near-field deposition of glyphosate, 2,4-D, and dicamba spray particle drift from applications with two different nozzles (different droplet spectrum resulting in low and high drift potentials) in a low speed wind tunnel, and their impact on waterhemp and Palmer amaranth growth and development under controlled environment.
Material and methods
Plant material
A waterhemp population collected from a corn field (Zea mays L.) in northeastern Nebraska (Cuming County) in the fall of 2014, and a Palmer amaranth population collected from a sorghum (Sorghum bicolor L.) field in southwestern Nebraska (Hayes County) in the fall of 2015 were used in this study. No specific permissions were required for field seed collections, and field collections did not involve endangered or protected species. Both waterhemp and Palmer amaranth populations were previously confirmed susceptible to glyphosate, 2,4-D, and dicamba with dose-response bioassays (unpublished data). Waterhemp and Palmer amaranth seeds were sown into plastic tubes (1 L) containing commercial potting mix (Berger BM7 Bark Mix, Saint Modeste, QC, Canada) and maintained under greenhouse conditions (30/20 C [day/night] with a 16 h photoperiod) at the Pesticide Application Technology Laboratory (University of Nebraska-Lincoln, West Central Research and Extension Center, North Platte, NE). LED growth lights (520 μmol s−1, Philips Lighting, Somerset, NJ, USA) provided supplemental lighting to ensure a 16-h photoperiod. Plants were supplied with water including fertilizer solution (0.2% v/v) as needed (UNL 5-1-4, Wilbur-Ellis Agribusiness, Aurora, CO, USA).
Droplet size study
A droplet size study was conducted in the low speed wind tunnel at the Pesticide Application Technology Laboratory. Droplet size distribution data were collected using a Sympatec Helos/Vario KR laser diffraction system (Sympatec Inc., Clausthal, Germany) measuring at a distance of 0.3 m from the nozzle tip. The diffraction system was equipped with a R7 lens which detects droplets ranging from 9 to 3700 μm in diameter. Nozzles were attached to an actuator and traversed vertically at constant speed (0.2 m s-1) to ensure the entire spray plume crossed the laser diffraction system [38]. Applications were performed with two even (banding) nozzles; a conventional flat-fan nozzle (TP95015EVS) and an air-inclusion (AI) nozzle (AI95015EVS) (TeeJet Technologies Spraying Systems Co., Glendale Heights, IL, USA); and three herbicide solutions: glyphosate, 2,4-D, and dicamba (Table 1). The glyphosate treatment had the addition of ammonium sulfate solution at 5% v/v to overcome antagonistic effects of cationic salts in hard water (Bronc®, Wilbur-Ellis Agribusiness, Aurora, CO, USA). Solutions were prepared at 140 L ha-1 carrier volume. Applications were performed at 230 kPa with constant wind speed of 6.71 m s-1. The DV0.1, DV0.5, and DV0.9 (droplet diameters which 10, 50, and 90% of the spray volume are contained in droplets of smaller diameter, respectively), and the percentage of the spray volume in droplets smaller than 150 μm (driftable fines) were recorded. The relative span (RS), a dimensionless parameter that estimates the distribution spread and its homogeneity was calculated [39] (Eq 1):
| [1] |
Table 1. Herbicide solutions, rates, and product manufacturers for solutions tested in the droplet size and spray particle drift studiesa.
| Herbicide | Active ingredient | Product manufacturer | Rate |
|---|---|---|---|
| Clarity® | Dicamba diglycolamine salt | BASF Corporation, Research, Triangle Park, NC, USA | 280 g ae ha-1 |
| Roundup PowerMax® | Glyphosate potassium salt | Bayer CropScience, Research, Triangle Park, NC, USA | 867 g ae ha-1 |
| Weedar® 64 | 2,4-D dimethylamine salt | Nufarm Inc, Alsip, IL, USA | 532 g ae ha-1 |
aGlyphosate solution had the addition of ammonium sulfate solution at 5% v/v (Bronc®, Wilbur-Ellis Agribusiness, Aurora, CO, USA).
The treatment design was a factorial arrangement with herbicide solution and nozzle as factors in a complete randomized experimental design with three replications and repeated. Droplet size data were subjected to analysis of variance in SAS (SAS v9.4, SAS Institute Inc., Cary, NC, USA) and comparisons among treatments were performed using Fisher’s Protected LSD test (P ≤ 0.05).
Wind tunnel particle drift study
A spray particle drift deposition study was conducted in the low speed wind tunnel at the Pesticide Application Technology Laboratory. Glyphosate, 2,4-D, and dicamba solutions were prepared as previously described (Table 1) with the addition of 1,3,6,8-pyrene tetra sulfonic acid tetra sodium salt (PTSA) as a fluorescent tracer (Spectra Colors Corporation, Kearny, NJ, USA) at 1000 ppm concentration [40]. Herbicide solutions were sprayed at 140 L ha-1 using two different even nozzles (banding) at 230 kPa (AI95015EVS and TP95015EVS) under a 4.47 m s-1 wind speed. The average air temperature and relative humidity during this study were 25 C and 45%, respectively. Mylar cards (100 mm x 100 mm) (Grafix Plastics, Cleveland, OH) were used to collect particle drift deposition at different downwind distances: 1.0, 1.5, 2.0, 2.5, 3.0, 4.0, 5.0, 7.0, and 12.0 m from the nozzle. Simultaneously, waterhemp and Palmer amaranth plants (15–20 cm-tall) were also positioned at the same downwind distances (Fig 2). Applications were performed at 51 cm height in relation to Mylar cards and plants.
Fig 2. Herbicide particle drift study conducted in the low speed wind tunnel with waterhemp (Amaranthus tuberculatus), Palmer amaranth (Amaranthus palmeri), and drift collectors (Mylar cards) positioned at different downwind distances from the nozzle.
After applications, Mylar cards were collected and placed into pre-labeled plastic zip-top bags and were immediately transferred to a dark container to avoid PTSA photodegradation. Spray particle drift deposition was determined for each Mylar card by fluorometric analysis at the Pesticide Application Technology Laboratory. Mylar cards were washed using 40 ml of a 9:1 solution of distilled water and 91% isopropyl alcohol. With the tracer completely suspended, a 1.5 ml aliquot was transferred to glass cuvette and analyzed using a Trilogy® fluorimeter with a PTSA module (Turner Designs, Sunnyvale, CA, USA). Relative fluorescence units (RFU) data were converted into mg L-1 using a calibration curve for the tracer, and posteriorly to deposition percentage as compared to the theoretical application rate of 140 L ha-1. The deposition data for each nozzle by herbicide solution combination (nozzle*herbicide) was estimated with a four-parameter symmetric log-logistic model using the drc package in R software (R Foundation for Statistical Computing, Vienna, Austria) (Eq 2):
| [2] |
where y represents deposition (% from applied rate), b is the slope at the inflection point, c is the lower limit of the model (fixed to 0%), d is the upper limit (applied rate fixed to 100%), and e is the inflection point (distance to 50% spray drift deposition) [41]. The distance to 5% application rate deposition (D5) was estimated for each nozzle*herbicide combination.
After applications, waterhemp and Palmer Amaranth plants were maintained under greenhouse conditions as previously described. Above ground plant biomass was harvested 28 days after treatment (DAT) and oven dried at 65°C to constant weight. The biomass data were converted into percentage of biomass reduction as compared to the untreated control. The symmetric four-parameter log-logistic model was used to describe biomass reduction using the drc package in R statistical software (Eq 2), where y represents biomass reduction (%), b is the slope at the inflection point, c is the lower limit of the model (fixed to 0%), d is the upper limit, and e is the inflection point (distance to 50% biomass reduction).
In swath (0 m distance) plant biomass reduction for each nozzle*herbicide treatment was estimated with herbicide applications using a research spray chamber calibrated to deliver 140 L ha-1 with the same nozzles, herbicide solutions, and spraying parameters used in the wind tunnel study.
Results and discussion
Droplet size
A significant interaction between nozzle design and herbicide solution was detected for the DV0.1 (p = 0.0002), DV0.5 (p < 0.0001), DV0.9 (p < 0.0001), RS (p < 0.0001), and driftable fines (p < 0.0001). Nozzle design had the greatest influence on droplet size, whereas herbicide solution had minor impact as previously reported [5,42,43] (Table 2). The preorifice component of the AI nozzle is designed to reduce the solution pressure as it exits the nozzle, thereby increasing the droplet size of the spray [5,42].
Table 2. Droplet size distribution and spray classification for the two nozzles and three herbicide solutions tested in the droplet size and spray particle drift study at 230 kPaa.
| Nozzleb | Herbicidec | Droplet size characteristicsd | Spray classificatione |
||||
|---|---|---|---|---|---|---|---|
| DV0.1 | DV0.5 | DV0.9 | RS | Driftable fines | |||
| ______________ μm ______________ | % | ||||||
| TP95015EVS | Glyphosate | 89 D | 201 D | 348 E | 1.29 A | 30.7 A | F |
| 2,4-D | 98 C | 212 C | 360 D | 1.23 B | 26.2 C | F | |
| Dicamba | 96 C | 209 C | 355 DE | 1.24 B | 26.9 B | F | |
| AI95015EVS | Glyphosate | 392 B | 805 A | 1212 B | 1.02 C | 0.6 D | UC |
| 2,4-D | 408 A | 801 A | 1223 A | 1.02 C | 0.4 D | UC | |
| Dicamba | 411 A | 789 B | 1166 C | 0.96 D | 0.4 D | UC | |
aMeans within a column followed by the same letter are not significantly different based on the LSD test (P ≤ 0.05).
bTeeJet Technologies, Spraying Systems Co., Glendale Heights, IL, USA.
cGlyphosate solution had the addition of ammonium sulfate solution at 5% v/v (Bronc®, Wilbur-Ellis Agribusiness, Aurora, CO, USA).
dAbbreviations: DV0.1, DV0.5, and DV0.9: Parameters which represent the droplet size such that 10, 50, and 90% of the spray volume is contained in droplets of lesser values, respectively
Driftable fines: Percent of spray volume that contains droplets less than 150 μm diameter
RS: Relative span, a dimensionless parameter that estimates the spread of a distribution.
eThe spray classifications for this study were based on reference curves created from reference nozzle data at the Pesticide Application Technology Laboratory as described by ASABE S572.1 where F = Fine, and UC = Ultra Coarse.
Wind tunnel particle drift deposition
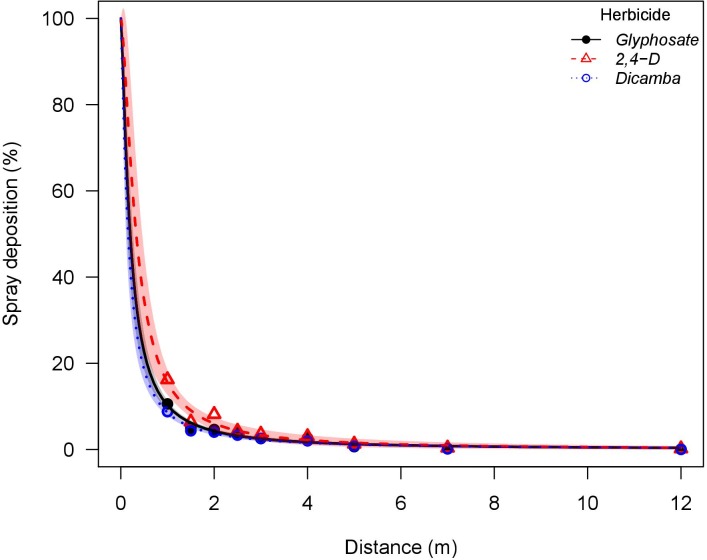
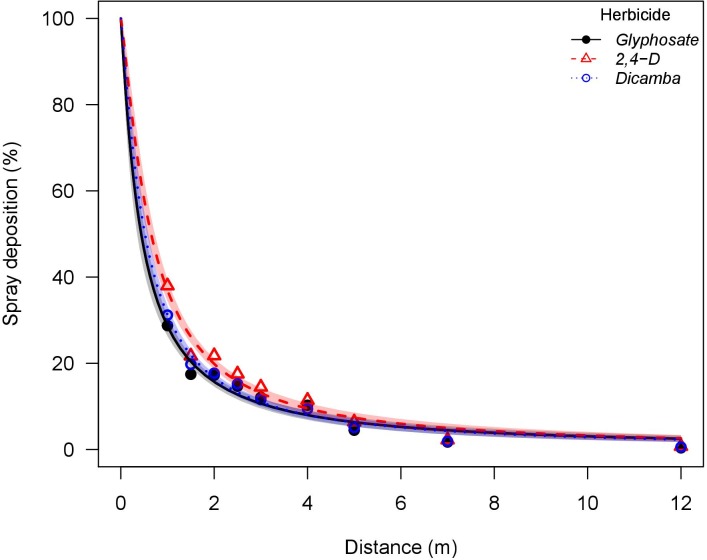
The nozzle treatments selected herein created two scenarios: a low drift potential (AI nozzle producing Ultra Coarse droplets with less than 1% of driftable fines) and a high drift potential (flat-fan nozzle producing Fine droplets with more than 25% of driftable fines). The estimated particle drift potential of treatments included in this wind tunnel study are consistent with previously reported field scale particle drift potential, where similar nozzle designs, droplet size classifications, and study methods were used. A study reported that 5% of applications of water with PTSA solution (93.5 L ha-1) using an AI nozzle at an average wind speed of 5.7 m s-1 deposited at 2.3 m downwind, whereas this distance corresponded to 4.5 m for applications with a flat-fan nozzle [43]. In this wind tunnel study, applications with the AI nozzle had 5% of the applied rate being deposited at 1.9 m downwind when herbicides were pooled, whereas this distance corresponded to 6.5 m for applications with the flat-fan nozzle. This indicates that the wind tunnel drift simulation method reproduced near-field spray drift conditions (Figs 3 and 4). Herbicide applications with the AI nozzle had smaller e parameter (distance to 50% spray drift deposition), ranging from 0.16 to 0.33 m across herbicides, when compared to applications with the flat-fan nozzle (0.44 to 0.65 m) (Table 3). The same trend was observed in the D5 parameter, where applications with the AI nozzle had 5% of the total applied rate being deposited from 1.57 to 2.27 m across herbicides, whereas these distances are increased to 6.11 and 6.97 m with the flat-fan nozzle. These results indicate the greater spray particle drift potential of the flat-fan nozzle. The greater b parameter (slope at the inflection point) of applications with the AI nozzle (ranging from 1.28 to 1.52 across herbicides) when compared to the flat-fan nozzle (1.10 to 1.24) indicates a faster decay rate of spray deposits resulting in less spray deposition at further downwind distances.
Fig 3. Glyphosate, 2,4-D, and dicamba particle drift study using an air-inclusion nozzle (AI95015EVS) conducted in a low speed wind tunnel.
Shaded area indicates the 95% confidence limits. Glyphosate solution had the addition of ammonium sulfate solution at 5% v/v (Bronc®, Wilbur-Ellis Agribusiness, Aurora, CO, USA).
Fig 4. Glyphosate, 2,4-D, and dicamba particle drift study using a flat-fan nozzle (TP95015EVS) conducted at a low speed wind tunnel.
Shaded area indicates the 95% confidence limits. Glyphosate solution had the addition of ammonium sulfate solution at 5% v/v (Bronc®, Wilbur-Ellis Agribusiness, Aurora, CO, USA).
Table 3. Log-logistic model parameters estimates, standard errors, and distance to 5% application rate deposition (D5) as influenced by downwind distance for each nozzle*herbicide treatment combination tested in the spray particle drift studya.
| Nozzleb | Herbicide | Log-logistic model parametersc | ||
|---|---|---|---|---|
| b | e | D5 | ||
| _______________ m _______________ | ||||
| TP95015EVS | Glyphosate | 1.10 ± 0.07 | 0.44 ± 0.04 | 6.28 ± 0.60 |
| 2,4-D | 1.24 ± 0.07 | 0.65 ± 0.04 | 6.97 ± 0.56 | |
| Dicamba | 1.19 ± 0.07 | 0.52 ± 0.04 | 6.11 ± 0.53 | |
| AI95015EVS | Glyphosate | 1.36 ± 0.19 | 0.20 ± 0.05 | 1.77 ± 0.12 |
| 2,4-D | 1.52 ± 0.15 | 0.33 ± 0.05 | 2.27 ± 0.14 | |
| Dicamba | 1.28 ± 0.21 | 0.16 ± 0.06 | 1.57 ± 0.11 | |
aGlyphosate solution had the addition of ammonium sulfate solution at 5% v/v (Bronc®, Wilbur-Ellis Agribusiness, Aurora, CO, USA).
bTeeJet Technologies, Spraying Systems Co., Glendale Heights, IL.
cb parameter corresponds to the slope at the inflection point; e parameter corresponds to the distance to 50% application deposition; c parameter (lower limit) fixed to 0%; d parameter (upper limit) fixed to 100%; D5 corresponds to the distance to 5% application rate deposition.
These findings corroborate the results from a field study investigating spray particle drift [44], where applications (water plus fluorescent tracer) with AI nozzles resulted in less particle drift compared to applications with conventional flat-fan nozzles. It has been reported that the distance where sorghum plants were lethally injured by glyphosate drift decreased 34% for applications with AI nozzles compared to conventional flat-fan nozzles [45]. Similar wind tunnel study results were reported, where applications of dicamba alone and in tank mixtures with glyphosate using AI nozzles resulted in less herbicide particle drift compared to conventional flat-fan nozzles [46,47].
Plants response to herbicide drift
Herbicide drift exposure subjected waterhemp and Palmer amaranth plants to either physiological stress (biomass reduction) or mortality (Table 4). The parameter estimates for the log-logistic biomass reduction model for waterhemp and Palmer amaranth are presented in Tables 5 and 6, respectively. The estimated d parameters (in-swath biomass reduction or upper limit) were greater than 84% biomass reduction for all nozzle*herbicide treatments, confirming that waterhemp and Palmer amaranth populations used in this study were susceptible to glyphosate, 2,4-D, and dicamba. Plants had greater biomass reduction when exposed to herbicide drift from applications with the flat-fan nozzle (greater drift potential).
Table 4. Waterhemp and Palmer amaranth mortality and estimations of biomass reduction using a log-logistic model as influenced by downwind distances for each nozzle*herbicide combination tested in the spray particle drift studyab.
| Nozzlec | Distance | Waterhemp mortality (biomass reduction) |
Palmer amaranth mortality (biomass reduction) | ||||
|---|---|---|---|---|---|---|---|
| TP95015EVS | Glyphosate | 2,4-D | Dicamba | Glyphosate | 2,4-D | Dicamba | |
| m | _________________________________________ % ____________________________________ | ||||||
| 1.0 | 100 (89) | 83 (83) | 83 (74) | 100 (93) | 0 (75) | 67 (86) | |
| 1.5 | 83 (87) | 83 (75) | 50 (67) | 100 (93) | 0 (72) | 83 (83) | |
| 2.0 | 17 (85) | 0 (69) | 17 (62) | 100 (93) | 0 (69) | 17 (79) | |
| 2.5 | 17 (82) | 17 (63) | 0 (57) | 83 (92) | 0 (66) | 17 (77) | |
| 3.0 | 0 (78) | 0 (57) | 0 (53) | 83 (92) | 0 (64) | 0 (74) | |
| 4.0 | 17 (71) | 0 (49) | 0 (46) | 83 (91) | 0 (60) | 0 (69) | |
| 5.0 | 0 (63) | 0 (42) | 0 (40) | 67 (89) | 0 (56) | 0 (65) | |
| 7.0 | 0 (50) | 0 (32) | 0 (33) | 33 (83) | 0 (50) | 0 (58) | |
| 12.0 | 0 (27) | 0 (19) | 0 (22) | 0 (64) | 0 (41) | 0 (47) | |
| AI95015EVS | 1.0 | 100 (91) | 0 (60) | 17 (54) | 100 (94) | 0 (59) | 0 (59) |
| 1.5 | 67 (80) | 17 (53) | 0 (49) | 83 (91) | 0 (55) | 0 (56) | |
| 2.0 | 0 (67) | 0 (48) | 0 (45) | 100 (87) | 0 (53) | 0 (54) | |
| 2.5 | 0 (55) | 0 (44) | 0 (42) | 33 (83) | 0 (50) | 0 (52) | |
| 3.0 | 0 (44) | 0 (41) | 0 (39) | 33 (79) | 0 (49) | 0 (50) | |
| 4.0 | 0 (28) | 0 (35) | 0 (35) | 17 (71) | 0 (46) | 0 (48) | |
| 5.0 | 0 (19) | 0 (32) | 0 (33) | 0 (64) | 0 (44) | 0 (46) | |
| 7.0 | 0 (9) | 0 (26) | 0 (28) | 0 (52) | 0 (40) | 0 (43) | |
| 12.0 | 0 (3) | 0 (19) | 0 (22) | 0 (32) | 0 (35) | 0 (39) | |
aGlyphosate solution had the addition of ammonium sulfate solution at 5% v/v (Bronc®, Wilbur-Ellis Agribusiness, Aurora, CO, USA).
bBiomass reduction as compared to the untreated control.
cTeeJet Technologies, Spraying Systems Co., Glendale Heights, IL.
Table 5. Log-logistic model parameters estimates and standard errors for waterhemp biomass reduction as influenced by downwind distance for each nozzle*herbicide combinations tested in the spray particle drift studyab.
| Nozzlec | Herbicide | Log-logistic model parametersd | ||
|---|---|---|---|---|
| b | d (%) | e (m) | ||
| TP95015EVS | Glyphosate | 1.92 ± 0.38 | 91.04 ± 4.00 | 7.71 ± 0.69 |
| 2,4-D | 1.28 ± 0.17 | 96.79 ± 4.58 | 4.04 ± 0.45 | |
| Dicamba | 1.07 ± 0.16 | 90.32 ± 4.87 | 4.10 ± 0.59 | |
| AI95015EVS | Glyphosate | 2.44 ± 0.33 | 98.18 ± 4.24 | 2.75 ± 0.16 |
| 2,4-D | 0.83 ± 0.14 | 87.96 ± 4.93 | 2.48 ± 0.43 | |
| Dicamba | 0.61 ± 0.12 | 90.80 ± 5.04 | 1.91 ± 0.46 | |
aGlyphosate solution had the addition of ammonium sulfate solution at 5% v/v (Bronc®, Wilbur-Ellis Agribusiness, Aurora, CO, USA).
bBiomass reduction as compared to the untreated control.
cTeeJet Technologies, Spraying Systems Co., Glendale Heights, IL.
dc parameter (lower limit) fixed to 0%; b parameter corresponds to the slope at the inflection point; d parameter corresponds to the upper limit, e parameter corresponds to the distance to 50% biomass reduction.
Table 6. Log-logistic model parameters estimates and standard errors for Palmer amaranth biomass reduction as influenced by downwind distance for each nozzle*herbicide combinations tested in the spray particle drift studyab.
| Nozzlec | Herbicide | Log-logistic model parametersd | ||
|---|---|---|---|---|
| b | d (%) | e (m) | ||
| TP95015EVS | Glyphosate | 2.55 ± 0.83 | 93.13 ± 1.92 | 16.31 ± 2.24 |
| 2,4-D | 0.86 ± 0.14 | 84.76 ± 3.55 | 10.91 ± 1.98 | |
| Dicamba | 0.91 ± 0.14 | 95.59 ± 3.75 | 11.50 ± 1.82 | |
| AI95015EVS | Glyphosate | 1.53 ± 0.19 | 98.28 ± 3.09 | 7.55 ± 0.63 |
| 2,4-D | 0.46 ± 0.10 | 85.85 ± 4.27 | 5.38 ± 1.53 | |
| Dicamba | 0.37 ± 0.09 | 90.98 ± 4.28 | 5.37 ± 1.80 | |
aGlyphosate solution had the addition of ammonium sulfate solution at 5% v/v (Bronc®, Wilbur-Ellis Agribusiness, Aurora, CO, USA).
bBiomass reduction as compared to the untreated control.
cTeeJet Technologies, Spraying Systems Co., Glendale Heights, IL.
dc parameter (lower limit) fixed to 0%; b parameter corresponds to the slope at the inflection point; d parameter corresponds to the upper limit, e parameter corresponds to the distance to 50% biomass reduction; D5 corresponds to the distance with 5% application rate deposition.
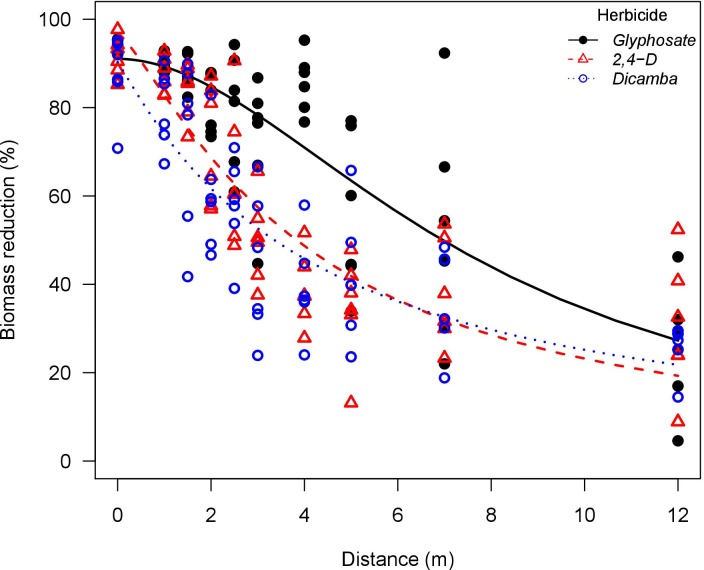
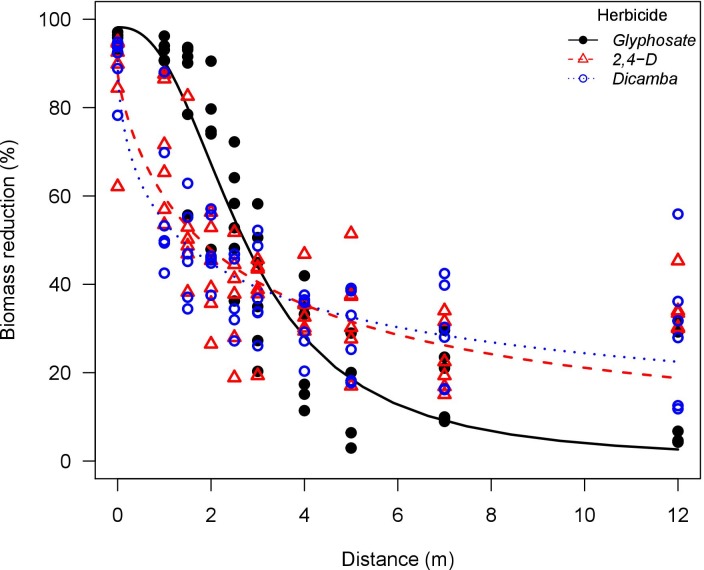
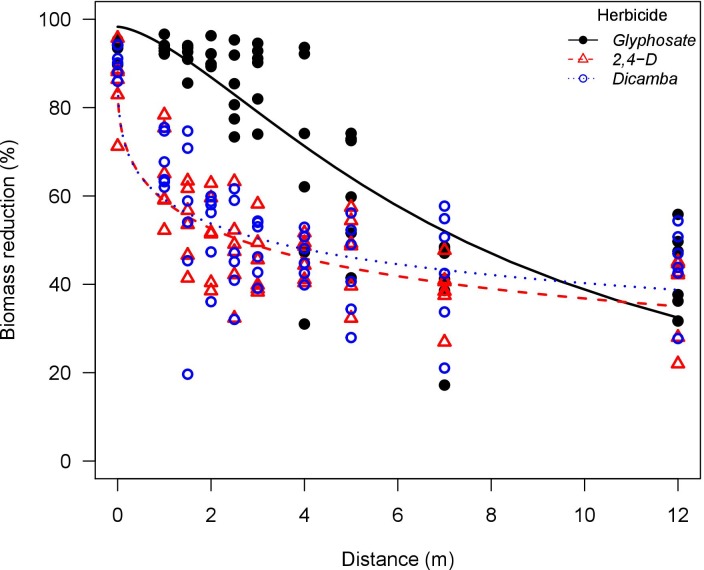
Across the herbicides tested, Palmer amaranth had higher biomass reduction compared to waterhemp. The susceptibility differences between waterhemp and Palmer amaranth were more evident with glyphosate, corroborating a previous report [17]. Palmer amaranth was extremely susceptible to glyphosate drift from both nozzles, in which the biomass reduction curve as influenced by downwind distances did not even reach the e parameter (distance to 50% biomass reduction) for applications with the flat-fan nozzle (Figs 5 and 6). In scenarios where the weed biotypes are extremely susceptible to a given herbicide, selection pressure will take place in extended downwind distances from the sprayed area as further distance is required to plants reach the no observable effect level (NOEL).
Fig 5. Waterhemp (Amaranthus tuberculatus) biomass reduction as influenced by glyphosate, 2,4-D, and dicamba particle drift using a flat-fan nozzle (TP95015EVS) in a low speed wind tunnel.
Glyphosate solution had the addition of ammonium sulfate solution at 5% v/v (Bronc®, Wilbur-Ellis Agribusiness, Aurora, CO, USA).
Fig 6. Palmer amaranth (Amaranthus palmeri) biomass reduction as influenced by glyphosate, 2,4-D, and dicamba particle drift using a flat-fan nozzle (TP95015EVS) in a low speed wind tunnel.
Glyphosate solution had the addition of ammonium sulfate solution at 5% v/v (Bronc®, Wilbur-Ellis Agribusiness, Aurora, CO, USA).
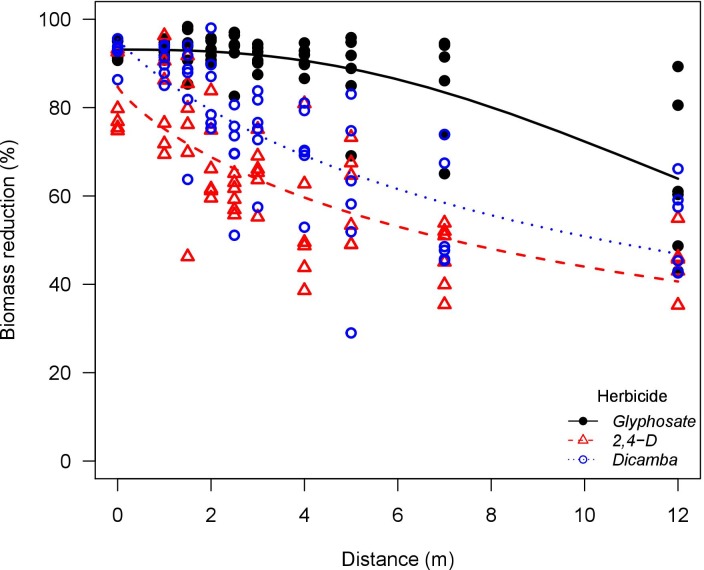
Glyphosate was more active at higher exposure rates compared to 2,4-D and dicamba. The e parameters (distance to 50% biomass reduction) also support this observation. Glyphosate applications had greater e parameter when compared to 2,4-D and dicamba, especially in applications with the flat-fan nozzle where plants are exposed to higher herbicide rates. Conversely, 2,4-D and dicamba were more active than glyphosate under lower exposure rates. This is more evident in the biomass reduction curves for waterhemp and Palmer amaranth exposed to herbicide drift from the AI nozzle (Figs 7 and 8). In fact, glyphosate applications had greater b parameter (slope at the inflection point) in general, indicating that biomass reduction curves had faster decay rate as the downwind distance was increased when compared to 2,4-D and dicamba. This indicates that glyphosate would reach no observable effect level at shorter downwind distances when compared to 2,4-D and dicamba. This corroborates previous reports relating low rates of 2,4-D and dicamba to high crop injury potential on soybean (Glycine max (L.) Merr.), cotton (Gossypium hirsutum L.), tomato (Solanum lycopersicum L.), and other broadleaf species [48–50].
Fig 7. Waterhemp (Amaranthus tuberculatus) biomass reduction as influenced by glyphosate, 2,4-D, and dicamba particle drift using an air-inclusion nozzle (AI95015EVS) in a low speed wind tunnel.
Glyphosate solution had the addition of ammonium sulfate solution at 5% v/v (Bronc®, Wilbur-Ellis Agribusiness, Aurora, CO, USA).
Fig 8. Palmer amaranth (Amaranthus palmeri) biomass reduction as influenced by glyphosate, 2,4-D, and dicamba particle drift using an air-inclusion nozzle (AI95015EVS) in a low speed wind tunnel.
Glyphosate solution had the addition of ammonium sulfate solution at 5% v/v (Bronc®, Wilbur-Ellis Agribusiness, Aurora, CO, USA).
Herbicide drift and plant exposure to sublethal rates
Estimations of spray drift deposition as influenced by downwind distance and nozzle type (pooled across herbicides) are provided in Table 7. Applications with the flat-fan nozzle resulted in near-field spray drift ranging from 32.3 (1.0 m) to 11.5% (3.0 m) of the applied rate. The use of the AI nozzle decreased the dose exposure in the same distance range, with drift deposition estimations ranging from 11.4 (1.0 m) to 2.7% (3 m) of the applied rate.
Table 7. Spray drift deposition estimations with 95% confidence intervals (CI 95%) as influenced by downwind distance and nozzle type (pooled herbicides) using a log-logistic non-linear regression model in the spray particle drift study.
| Nozzlea | Distance | Spray depositionb | CI 95% |
|---|---|---|---|
| m | _________________________ % _________________________ | ||
| TP95015EVS | 1.0 | 32.3 | (31.1–33.5) |
| 1.5 | 22.8 | (22.1–23.5) | |
| 2.0 | 17.4 | (16.8–18.0) | |
| 2.5 | 13.9 | (13.3–14.5) | |
| 3.0 | 11.5 | (10.9–12.1) | |
| 4.0 | 8.5 | (7.9–9.1) | |
| 5.0 | 6.7 | (6.1–7.2) | |
| 7.0 | 4.6 | (4.1–5.1) | |
| 12.0 | 2.5 | (2.1–2.8) | |
| AI95015EVS | 1.0 | 11.4 | (10.1–12.8) |
| 1.5 | 6.8 | (6.1–7.5) | |
| 2.0 | 4.7 | (4.0–5.3) | |
| 2.5 | 3.4 | (2.8–4.1) | |
| 3.0 | 2.7 | (2.1–3.3) | |
| 4.0 | 1.8 | (1.3–2.4) | |
| 5.0 | 1.3 | (0.9–1.8) | |
| 7.0 | 0.8 | (0.5–1.2) | |
| 12.0 | 0.4 | (0.2–0.6) | |
aTeeJet Technologies, Spraying Systems Co., Glendale Heights, IL.
bSpray drift deposition (%) in relation to the applied rate of 140.3 L ha-1.
It has been reported that progenies of an initially susceptible population of annual ryegrass (Lolium rigidum Gaudin) shifted towards glyphosate resistance (up to 2.1-fold in the LD50) after being recurrently selected with sublethal rates of glyphosate [21]. These authors exposed three generations of Lolium rigidum plants to sublethal rates of glyphosate ranging from 150 g ae ha-1 to 350 g ae ha-1 (17 to 40% of the 867 g ae ha-1 commonly adopted field rate in glyphosate tolerant crops). In a similar study, it was reported that a glyphosate-susceptible Palmer amaranth population evolved glyphosate resistance (2.2-fold in the LD50) after being recurrently selected under sublethal rates of glyphosate for four generations [25]. The author reported that glyphosate doses of 105, 126, 210, and 420 g ae ha-1 (12, 15, 24, and 48% of the 867 g ae ha-1 commonly adopted field rate in glyphosate tolerant crops, respectively) were used as generations progressed during the recurrent selection study. In a Raphanus raphanistrum L. population, the plants evolved 2,4-D resistance (8.6-fold in the LD50) after being recurrently selected during four generations [18]. The authors exposed plants to 125, 250, and 750 g 2,4-D ae ha-1 (12, 24, and 73% of the 1065 g ae ha-1 recommended rate for 2,4-D-tolerant soybean) as generations progressed. Another study reported that a 2,4-D and dicamba-susceptible Palmer amaranth population had its susceptibility reduced to both herbicides (2.8 and 2.0-fold in the LD50 for dicamba and 2,4-D, respectively) after recurrent selection with sublethal rates of dicamba for three selection generations [26]. The authors exposed plants to 140, 280, and 420 g dicamba ae ha-1 (25, 50, and 75% of the 560 g ae ha-1 recommended rate for dicamba-tolerant soybean) during the selection generations. Recurrent selection studies with sublethal rates of pyroxasulfone and diclofop-methyl were also associated with resistance evolution in weeds in previous studies [19,20,22,23,27].
Despite similar dose ranges, herbicide drift exposure differs from previously reported sublethal rate studies in terms of spray deposition pattern on plants and herbicide concentration within spray droplets. Unlike an intentional sublethal rate application with a constant carrier volume (usually ranging from 94 to 188 L ha-1), spray drift deposition is not consistent across field edges, which could influence plant response to the herbicide exposure. The higher herbicide concentration of spray drift droplets at lower carrier volumes could also influence plant response to herbicide exposure. Previous research indicated that glyphosate was more active at lower carrier volumes (more concentrated droplets) on oat (Avena sativa L.), wheat (Triticum aestivum L.), and several annual grass weed species such as Echinochloa crusgalli L., Panicum dichotomiflorum Michx., Setaria viridis (L.) Beauv., Setaria pumila (Poir.) Roem. et Schult, and Digitaria sanguinalis (L.) Scop., especially when lower glyphosate rates were compared [51,52]. Another study reported that carrier volume also influenced glyphosate activity on corn, whereas soybean was not affected [53,54]. It has also been reported that carrier volume influenced low rates of 2,4-D activity on cotton plants with lower carrier volumes (more concentrated droplets) resulting in more herbicide injury [53]. Similarly, lower rates of 2,4-D and dicamba were more active on cotton when lower carrier volumes with more concentrated droplets were used [50]. A study highlighted that the active ingredient concentration within droplets could influence the diffusion process of herbicide foliar uptake [55]. However, the authors mentioned that glyphosate foliar uptake has been investigated more than other herbicides. Additionally, it has been suggested that carrier volume could influence glyphosate activity because of water hardness, surfactant concentration, and spray droplet dynamics [52]. Herbicides tested in this study (glyphosate, 2,4-D, and dicamba) have systemic activity and can still be effective at lower carrier volumes and coverage, whereas contact herbicides usually require higher carrier volumes and adequate coverage [56–58]. Therefore, spray drift and injury potential from contact herbicides needs to be further investigated.
The results of this study indicate that herbicide drift towards field edges expose weeds to a range of herbicide rates reported to select for herbicide resistance. A previous study reported that only 3% of a total of 215 Palmer amaranth populations collected from roadsides, ditches, and field borders in eastern Arkansas were completely susceptible to glyphosate [14]. Glyphosate resistance was also confirmed in waterhemp and Palmer amaranth populations located on field borders and ditches in Nebraska [17]. Similarly, the presence of herbicide-resistant giant ragweed (Ambrosia trifida L.) in crop fields throughout the U.S. Corn Belt and Ontario (Canada) was strongly correlated to the species presence on crop field edges such as railroad sidings, ditch banks, and fencerows [15].
This study confirmed that nozzle selection influenced spray drift and consequent herbicide dose exposure on field edges, although spray drift could also be influenced by other parameters not tested, such as wind speed and boom height. The distance range with herbicide exposure and selection pressure is further increased for applications with the flat-fan nozzle (higher drift potential). It has been suggested that plants exposed to low doses of herbicides experience physiological stress, whereas plants exposed to even lower rates (hormetic doses) could also be subjected to stress [34]. Therefore, further studies are necessary to investigate if weeds could evolve herbicide resistance after recurrent selection with different exposure ranges of herbicide drift.
Despite the herbicide drift exposure and its potential implications on resistance evolution and weed management, near-field weed populations are often neglected and not properly managed in agricultural landscapes [14,15,17,37]. It has been reported that unmanaged field margins with resistant-prone weeds can exacerbate the risk of resistance, especially when outcrossing occurs with resistant populations near field [37]. Having plants under selection pressure for herbicide resistance on field borders could be detrimental for in-field weed management as pollen-mediated gene flow plays an important role in dispersing herbicide resistance alleles in cross-pollinated species such as waterhemp and Palmer amaranth [59–61]. Preventing resistance-prone weeds on field margins is an important best management practice (BMP) to delay herbicide resistance, although the additional management costs and time constraints pose a challenge for growers [18,62]. Growers should consider additional strategies to mitigate near-field spray drift [43,63], and implement appropriate control strategies to manage weed populations on field borders, such as mowing, using boomless nozzles for weed control in areas of difficult access (fencerows, electrical lines), or planting and maintaining field borders to a less-weedy and easier to manage species [37].
Supporting information
(ZIP)
Acknowledgments
The authors would like to thank all undergraduate students, graduate students, and professional staff at the University of Nebraska-Lincoln who assisted with data collection and analysis.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
The authors would like to thank CAPES (Brazilian Government Foundation) for the financial support to the graduate student BCV (proc 013041/2013-04). This project was partially supported by the Nebraska Agricultural Experiment Station with funding from the Hatch Multistate Research capacity funding program from the USDA National Institute of Food and Agriculture. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Matthews G, Bateman R, Miller P. Pesticide Application Methods 4th edition Hoboken, NJ: Wiley-Blackwell; 2014. [Google Scholar]
- 2.Hewitt AJ. Spray drift: impact of requirements to protect the environment. Crop Prot. 2000;19: 623–627. 10.1016/S0261-2194(00)00082-X [DOI] [Google Scholar]
- 3.Nordby A, Skuterud R. The effects of boom height, working pressure and wind speed on spray drift. Weed Res. 1974;14: 385–395. 10.1111/j.1365-3180.1974.tb01080.x [DOI] [Google Scholar]
- 4.Zhu H, Reichard DL, Fox RD, Brazee RD, Ozkan HE. Simulation of Drift of Discrete Sizes of Water Droplets from Field Sprayers. Trans ASAE. 1994;37: 1401–1407. 10.13031/2013.28220 [DOI] [Google Scholar]
- 5.Creech CF, Henry RS, Fritz BK, Kruger GR. Influence of Herbicide Active Ingredient, Nozzle Type, Orifice Size, Spray Pressure, and Carrier Volume Rate on Spray Droplet Size Characteristics. Weed Technol. 2015;29: 298–310. 10.1614/WT-D-14-00049.1 [DOI] [Google Scholar]
- 6.Hilz E, Vermeer AWP. Spray drift review: The extent to which a formulation can contribute to spray drift reduction. Crop Prot. 2013;44: 75–83. 10.1016/j.cropro.2012.10.020 [DOI] [Google Scholar]
- 7.Schampheleire MD, Nuyttens D, Baetens K, Cornelis W, Gabriels D, Spanoghe P. Effects on pesticide spray drift of the physicochemical properties of the spray liquid. Precis Agric. 2008;10: 409–420. 10.1007/s11119-008-9089-6 [DOI] [Google Scholar]
- 8.Egan JF, Bohnenblust E, Goslee S, Mortensen D, Tooker J. Herbicide drift can affect plant and arthropod communities. Agric Ecosyst Environ. 2014;185: 77–87. 10.1016/j.agee.2013.12.017 [DOI] [Google Scholar]
- 9.de Snoo GR, van der Poll RJ. Effect of herbicide drift on adjacent boundary vegetation. Agric Ecosyst Environ. 1999;73: 1–6. 10.1016/S0167-8809(99)00008-0 [DOI] [Google Scholar]
- 10.Ding W, Reddy KN, Krutz LJ, Thomson SJ, Huang Y, Zablotowicz RM. Biological Response of Soybean and Cotton to Aerial Glyphosate Drift. J Crop Improv. 2011;25: 291–302. 10.1080/15427528.2011.559633 [DOI] [Google Scholar]
- 11.Jones GT, Norsworthy JK, Barber T, Gbur E, Kruger GR. Off-target Movement of DGA and BAPMA Dicamba to Sensitive Soybean. Weed Technol. 2019;33: 51–65. 10.1017/wet.2018.121 [DOI] [Google Scholar]
- 12.Kalsing A, Rossi CVS, Lucio FR, Zobiole LHS, Cunha LCV da, Minozzi GB. Effect of Formulations and Spray Nozzles on 2,4-D Spray Drift under Field Conditions. Weed Technol. 2018;32: 379–384. 10.1017/wet.2018.18 [DOI] [Google Scholar]
- 13.Reddy KN, Ding W, Zablotowicz RM, Thomson SJ, Huang Y, Krutz LJ. Biological responses to glyphosate drift from aerial application in non-glyphosate-resistant corn. Pest Manag Sci. 2010;66: 1148–1154. 10.1002/ps.1996 [DOI] [PubMed] [Google Scholar]
- 14.Bagavathiannan MV, Norsworthy JK. Multiple-Herbicide Resistance Is Widespread in Roadside Palmer Amaranth Populations. PLOS ONE. 2016;11: e0148748 10.1371/journal.pone.0148748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Regnier EE, Harrison SK, Loux MM, Holloman C, Venkatesh R, Diekmann F, et al. Certified Crop Advisors’ Perceptions of Giant Ragweed (Ambrosia trifida) Distribution, Herbicide Resistance, and Management in the Corn Belt. Weed Sci. 2016;64: 361–377. 10.1614/WS-D-15-00116.1 [DOI] [Google Scholar]
- 16.Samuelson S. Response of Problematic Weed Populations in Nebraska to Glyphosate [Internet]. M.Sc. Thesis, University of Nebraska-Lincoln. 2017. Available: http://digitalcommons.unl.edu/agronhortdiss/122
- 17.Vieira BC, Samuelson SL, Alves GS, Gaines TA, Werle R, Kruger GR. Distribution of glyphosate-resistant Amaranthus spp. in Nebraska. Pest Manag Sci. 2018;74: 2316–2324. 10.1002/ps.4781 [DOI] [PubMed] [Google Scholar]
- 18.Ashworth MB, Walsh MJ, Flower KC, Powles SB. Recurrent selection with reduced 2,4-D amine doses results in the rapid evolution of 2,4-D herbicide resistance in wild radish (Raphanus raphanistrum L.). Pest Manag Sci. 2016;72: 2091–2098. 10.1002/ps.4364 [DOI] [PubMed] [Google Scholar]
- 19.Busi R, Gaines TA, Walsh MJ, Powles SB. Understanding the potential for resistance evolution to the new herbicide pyroxasulfone: field selection at high doses versus recurrent selection at low doses. Weed Res. 2012;52: 489–499. 10.1111/j.1365-3180.2012.00948.x [DOI] [Google Scholar]
- 20.Busi R, Girotto M, Powles SB. Response to low-dose herbicide selection in self-pollinated Avena fatua. Pest Manag Sci. 2016;72: 603–608. 10.1002/ps.4032 [DOI] [PubMed] [Google Scholar]
- 21.Busi R, Powles SB. Evolution of glyphosate resistance in a Lolium rigidum population by glyphosate selection at sublethal doses. Heredity. 2009;103: 318–325. 10.1038/hdy.2009.64 [DOI] [PubMed] [Google Scholar]
- 22.Manalil S, Busi R, Renton M, Powles SB. Rapid Evolution of Herbicide Resistance by Low Herbicide Dosages. Weed Sci. 2011;59: 210–217. 10.1614/WS-D-10-00111.1 [DOI] [Google Scholar]
- 23.Neve P, Powles S. Recurrent selection with reduced herbicide rates results in the rapid evolution of herbicide resistance in Lolium rigidum. Theor Appl Genet. 2005;110: 1154–1166. 10.1007/s00122-005-1947-2 [DOI] [PubMed] [Google Scholar]
- 24.Neve P, Powles S. High survival frequencies at low herbicide use rates in populations of Lolium rigidum result in rapid evolution of herbicide resistance. Heredity. 2005;95: 485–492. 10.1038/sj.hdy.6800751 [DOI] [PubMed] [Google Scholar]
- 25.Norsworthy JK. Repeated Sublethal Rates of Glyphosate Lead to Decreased Sensitivity in Palmer Amaranth. Crop Manag. 2012; 10.1094/CM-2012-0403-01-RS [DOI] [Google Scholar]
- 26.Tehranchian P, Norsworthy JK, Powles S, Bararpour MT, Bagavathiannan MV, Barber T, et al. Recurrent Sublethal-Dose Selection for Reduced Susceptibility of Palmer Amaranth (Amaranthus palmeri) to Dicamba. Weed Sci. 2017;65: 206–212. 10.1017/wsc.2016.27 [DOI] [Google Scholar]
- 27.Vila-Aiub MM, Ghersa CM. Building up resistance by recurrently exposing target plants to sublethal doses of herbicide. Eur J Agron. 2005;22: 195–207. 10.1016/j.eja.2004.01.004 [DOI] [Google Scholar]
- 28.Busi R, Neve P, Powles S. Evolved polygenic herbicide resistance in Lolium rigidum by low-dose herbicide selection within standing genetic variation. Evol Appl. 2013;6: 231–242. 10.1111/j.1752-4571.2012.00282.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gaines TA, Lorentz L, Figge A, Herrmann J, Maiwald F, Ott M-C, et al. RNA-Seq transcriptome analysis to identify genes involved in metabolism-based diclofop resistance in Lolium rigidum. Plant J. 2014;78: 865–876. 10.1111/tpj.12514 [DOI] [PubMed] [Google Scholar]
- 30.Neve P, Busi R, Renton M, Vila-Aiub MM. Expanding the eco-evolutionary context of herbicide resistance research. Pest Manag Sci. 2014;70: 1385–1393. 10.1002/ps.3757 [DOI] [PubMed] [Google Scholar]
- 31.Yu Q, Han H, Cawthray GR, Wang SF, Powles SB. Enhanced rates of herbicide metabolism in low herbicide-dose selected resistant Lolium rigidum. Plant Cell Environ. 2013;36: 818–827. 10.1111/pce.12017 [DOI] [PubMed] [Google Scholar]
- 32.Yuan JS, Tranel PJ, Stewart CN. Non-target-site herbicide resistance: a family business. Trends Plant Sci. 2007;12: 6–13. 10.1016/j.tplants.2006.11.001 [DOI] [PubMed] [Google Scholar]
- 33.Gressel J. Low pesticide rates may hasten the evolution of resistance by increasing mutation frequencies. Pest Manag Sci. 2010;67: 253–257. 10.1002/ps.2071 [DOI] [PubMed] [Google Scholar]
- 34.Dyer WE. Stress-induced evolution of herbicide resistance and related pleiotropic effects. Pest Manag Sci. 2018;74: 1759–1768. 10.1002/ps.5043 [DOI] [PubMed] [Google Scholar]
- 35.Markus C, Pecinka A, Karan R, Barney JN, Merotto A. Epigenetic regulation–contribution to herbicide resistance in weeds? Pest Manag Sci. 2018;74: 275–281. 10.1002/ps.4727 [DOI] [PubMed] [Google Scholar]
- 36.Yu Q, Powles S. Metabolism-Based Herbicide Resistance and Cross-Resistance in Crop Weeds: A Threat to Herbicide Sustainability and Global Crop Production. Plant Physiol. 2014;166: 1106–1118. 10.1104/pp.114.242750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Norsworthy JK, Ward SM, Shaw DR, Llewellyn RS, Nichols RL, Webster TM, et al. Reducing the Risks of Herbicide Resistance: Best Management Practices and Recommendations. Weed Sci. 2012;60: 31–62. 10.1614/WS-D-11-00155.1 [DOI] [Google Scholar]
- 38.Butts TR, Butts LE, Luck JD, Fritz BK, Hoffmann WC, Kruger GR. Droplet size and nozzle tip pressure from a pulse-width modulation sprayer. Biosyst Eng. 2019;178: 52–69. 10.1016/j.biosystemseng.2018.11.004 [DOI] [Google Scholar]
- 39.Hewitt AJ. Spray optimization through application and liquid physical property variables–I. The Environmentalist. 2008;28: 25–30. 10.1007/s10669-007-9044-5 [DOI] [Google Scholar]
- 40.Hoffmann WC, Fritz BK, Ledebuhr MA. Evaluation of 1, 3, 6, 8-Pyrene Tetra Sulfonic Acid Tetra Sodium Salt (PTSA) as an Agricultural Spray Tracer Dye. Appl Eng Agric. 2014; 25–28. 10.13031/aea.30.10313 [DOI] [Google Scholar]
- 41.Knezevic SZ, Streibig JC, Ritz C. Utilizing R Software Package for Dose-Response Studies: The Concept and Data Analysis. Weed Technol. 2007;21: 840–848. 10.1614/WT-06-161.1 [DOI] [Google Scholar]
- 42.Dorr GJ, Hewitt AJ, Adkins SW, Hanan J, Zhang H, Noller B. A comparison of initial spray characteristics produced by agricultural nozzles. Crop Prot. 2013;53: 109–117. 10.1016/j.cropro.2013.06.017 [DOI] [Google Scholar]
- 43.Vieira BC, Butts TR, Rodrigues AO, Golus JA, Schroeder K, Kruger GR. Spray particle drift mitigation using field corn (Zea mays L.) as a drift barrier. Pest Manag Sci. 2018;74: 2038–2046. 10.1002/ps.5041 [DOI] [PubMed] [Google Scholar]
- 44.Bueno MR, da Cunha JPAR, de Santana DG. Assessment of spray drift from pesticide applications in soybean crops. Biosyst Eng. 2017;154: 35–45. [Google Scholar]
- 45.Johnson AK, Roeth FW, Martin AR, Klein RN. Glyphosate Spray Drift Management with Drift-Reducing Nozzles and Adjuvants. Weed Technol. 2006;20: 893–897. 10.1614/WT-05-162.1 [DOI] [Google Scholar]
- 46.Alves GS, Kruger GR, Cunha JPAR da, Vieira BC, Henry RS, Obradovic A, et al. Spray Drift from Dicamba and Glyphosate Applications in a Wind Tunnel. Weed Technol. 2017;31: 387–395. 10.1017/wet.2017.15 [DOI] [Google Scholar]
- 47.Alves GS, Kruger GR, Cunha JPAR da, Santana DG de, Pinto LAT, Guimarães F, et al. Dicamba Spray Drift as Influenced by Wind Speed and Nozzle Type. Weed Technol. 2017;31: 724–731. 10.1017/wet.2017.61 [DOI] [Google Scholar]
- 48.Egan JF, Barlow KM, Mortensen DA. A Meta-Analysis on the Effects of 2,4-D and Dicamba Drift on Soybean and Cotton. Weed Sci. 2014;62: 193–206. 10.1614/WS-D-13-00025.1 [DOI] [Google Scholar]
- 49.Kruger GR, Johnson WG, Doohan DJ, Weller SC. Dose Response of Glyphosate and Dicamba on Tomato (Lycopersicon esculentum) Injury. Weed Technol. 2012;26: 256–260. 10.1614/WT-D-11-00073.1 [DOI] [Google Scholar]
- 50.Smith HC, Ferrell JA, Webster TM, Fernandez JV. Cotton Response to Simulated Auxin Herbicide Drift Using Standard and Ultra-low Carrier Volumes. Weed Technol. 2017;31: 1–9. 10.1614/WT-D-16-00101.1 [DOI] [Google Scholar]
- 51.Buhler DD, Burnside OC. Effect of Spray Components on Glyphosate Toxicity to Annual Grasses. Weed Sci. 1983;31: 124–130. 10.1017/S0043174500068673 [DOI] [Google Scholar]
- 52.Roider CA, Griffin JL, Harrison SA, Jones CA. Carrier Volume Affects Wheat Response to Simulated Glyphosate Drift. Weed Technol. 2008;22: 453–458. 10.1614/WT-07-111.1 [DOI] [Google Scholar]
- 53.Banks PA, Schroeder J. Carrier Volume Affects Herbicide Activity in Simulated Spray Drift Studies1. Weed Technol. 2002;16: 833–838. 10.1614/0890-037X(2002)016[0833:CVAHAI]2.0.CO;2 [DOI] [Google Scholar]
- 54.Ellis JM, Griffin JL, Jones CA. Effect of Carrier Volume on Corn (Zea mays) and Soybean (Glycine max) Response to Simulated Drift of Glyphosate and Glufosinate. Weed Technol. 2002;16: 587–592. 10.1614/0890-037X(2002)016[0587:EOCVOC]2.0.CO;2 [DOI] [Google Scholar]
- 55.Wang CJ, Liu ZQ. Foliar uptake of pesticides—Present status and future challenge. Pestic Biochem Physiol. 2007;87: 1–8. 10.1016/j.pestbp.2006.04.004 [DOI] [Google Scholar]
- 56.Butts TR, Samples CA, Franca LX, Dodds DM, Reynolds DB, Adams JW, et al. Spray droplet size and carrier volume effect on dicamba and glufosinate efficacy. Pest Manag Sci. 2018;74: 2020–2029. 10.1002/ps.4913 [DOI] [PubMed] [Google Scholar]
- 57.Creech CF, Henry RS, Werle R, Sandell LD, Hewitt AJ, Kruger GR. Performance of Postemergence Herbicides Applied at Different Carrier Volume Rates. Weed Technol. 2015;29: 611–624. 10.1614/WT-D-14-00101.1 [DOI] [Google Scholar]
- 58.Knoche M. Effect of droplet size and carrier volume on performance of foliage-applied herbicides. Crop Prot. 1994;13: 163–178. 10.1016/0261-2194(94)90075-2 [DOI] [Google Scholar]
- 59.Gaines TA, Ward SM, Bukun B, Preston C, Leach JE, Westra P. Interspecific hybridization transfers a previously unknown glyphosate resistance mechanism in Amaranthus species. Evol Appl. 2012;5: 29–38. 10.1111/j.1752-4571.2011.00204.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Oliveira MC, Gaines TA, Patterson EL, Jhala AJ, Irmak S, Amundsen K, et al. Interspecific and intraspecific transference of metabolism-based mesotrione resistance in dioecious weedy Amaranthus. Plant J. 2018;96: 1051–1063. 10.1111/tpj.14089 [DOI] [PubMed] [Google Scholar]
- 61.Sarangi D, Tyre AJ, Patterson EL, Gaines TA, Irmak S, Knezevic SZ, et al. Pollen-mediated gene flow from glyphosate-resistant common waterhemp (Amaranthus rudis Sauer): consequences for the dispersal of resistance genes. Sci Rep. 2017;7: srep44913 10.1038/srep44913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Riar DS, Norsworthy JK, Steckel LE, Stephenson DO, Eubank TW, Bond J, et al. Adoption of Best Management Practices for Herbicide-Resistant Weeds in Midsouthern United States Cotton, Rice, and Soybean. Weed Technol. 2013;27: 788–797. [Google Scholar]
- 63.Foster HC, Sperry BP, Reynolds DB, Kruger GR, Claussen S. Reducing Herbicide Particle Drift: Effect of Hooded Sprayer and Spray Quality. Weed Technol. 2018;32: 714–721. 10.1017/wet.2018.84 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(ZIP)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.