Abstract
Objectives:
Intramuscular adipose tissue (IMAT) is recognized as a negative predictor of both muscle and mobility function in older adults, however the mechanism by which IMAT may negatively influence muscle and mobility function is currently unknown. The release of pro-inflammatory cytokines from IMAT provides a potential reason for these negative associations. To explore this hypothesis we compared IMAT and muscular inflammation in age-and BMI-matched older non-obese frail and non-frail adults. We also sought to examine the relationship between IMAT and inflammation, and muscle and mobility function in this group of older adults.
Design:
A case-control sampling was used for this study. Age-and BMI-matched non-obese frail and non-frail individuals (<65 years) were recruited.
Measurements:
MRI was used to quantify thigh IMAT and lean tissue. Unilateral muscle biopsies were used to quantify muscular inflammation as represented by interleukin-6 (IL-6) and tumor-necrosis factor alpha (TNF-α). Muscle and mobility function was also measured using a maximal voluntary isometric contraction, six-minute walk, and self-selected gait speed.
Participants:
26 older (80.7 +/− 5.4 years) individuals (8 frail and 18 non-frail) were enrolled.
Results:
The frail-group had increased IMAT (p<0.01) and decreased lean tissue (p<0.01), and elevated IL-6 muscle mRNA (p=0.02) and IL-6 protein content (p=0.02) compared to the non-frail group. IMAT was significantly associated with IL-6 mRNA (r=0.43, p=.04) and protein expression within the muscle (r=0.41, p= 0.045). IL-6 mRNA was significantly associated with six-minute walk (r=−0.63, p<0.01), and gait speed (r=−0.60, p <0.01) and IL-6 protein was significantly associated with muscle force (r=−0.54, p=0.01), six-minute walk (r=−0.66, p<0.01), and gait speed (r=−0.76, p<0.01). No significant relationships were found for any variables with TNF-a.
Conclusion:
Non-obese, older, frail individuals have increased IMAT and muscular inflammation when compared to their non-frail, age- and BMI-matched peers. A significant relationship exists between IMAT and muscle IL-6 expression as well as between IL-6 and muscle and mobility function of these older adults. This IMAT-inflammatory pathway provides a potential link between IMATs and decreased muscle and mobility function.
Keywords: Intramuscular fat, inflammation, frailty, mobility
Introduction
Intramuscular adipose tissue (IMAT), a small fat depot beneath the fascia of the thigh, has been identified as a potential contributor to mobility dysfunction in older adults (1, 2). IMAT does not solely reflect total body adiposity or locomotor muscle deterioration with aging (3–5) and may constitute a marker of muscle dysfunction linked to inactivity (4–6). IMAT may also play a more active inflammatory role influencing cytokines such as tumor necrosis factor alpha (TNF-α) and interleukin-6 (IL-6) within the muscle. (7, 8). Higher systemic levels of pro-inflammatory cytokines are associated with decreased muscle force and mobility (9–11). Increased circulating levels of pro-inflammatory cytokines such as TNF-α and IL-6 can result in cell apoptosis (12), decreased protein synthesis (13), and decreased muscle quality (14), all of which may contribute to decreased muscle force and mobility in older adults (15). The relationship of increased IMAT with decreased muscle and mobility function (1, 2, 5, 6) mirrors that of increased systemic inflammation and muscle and mobility function (9–11). IMAT is hypothesized to release pro-inflammatory cytokines directly within the muscle, resulting in decreased muscle and mobility function (4, 7, 16). To date, however, there has been little investigation into how increased levels of inflammation in locomotor muscle may be linked to increased IMAT in older adults and no study to date has examined the relationship of IMAT and muscular inflammation in older adults.
Therefore, the aim of this case-control sampling was to compare IMAT and muscular inflammation in non-obese, age- and BMI-matched older frail and non-frail adults. We hypothesized that sedentary, mobility-limited non-obese frail older adults would have higher IMAT and muscular inflammation levels than an age- and BMI-matched group of active, non-mobility limited, non-frail older adults. We also hypothesized a relationship would exist between IMAT and muscular inflammation, and between muscular inflammation and mobility function.
Methods
Participants
To maximize potential differences in IMAT, a convenience sample of two groups of older non-obese adults (a frail sedentary and a non-frail active group) were recruited. Recruitment occurred between 2011 and 2012 and took place at community health fairs, community senior centers, and via newspaper advertisement. Due to the difficulty in recruiting frail individuals we recruited in a 1:2 model. Individuals were matched for age plus or minus 5 years, and BMI plus or minus 2 kg/m2. Based on prior studies effects sizes of 1.36 to 2.39 for differences between active and sedentary adults in IMAT and inflammation we estimated that we would need between 3 and 6 frail and 5 and 12 non-frail individuals to achieve a power of 0.80 with a standard error of .05 to determine a significant difference in IMAT and muscular inflammation between the groups. We therefore conservatively sought to enroll a minimum of 6 frail and 12 non-frail individuals.
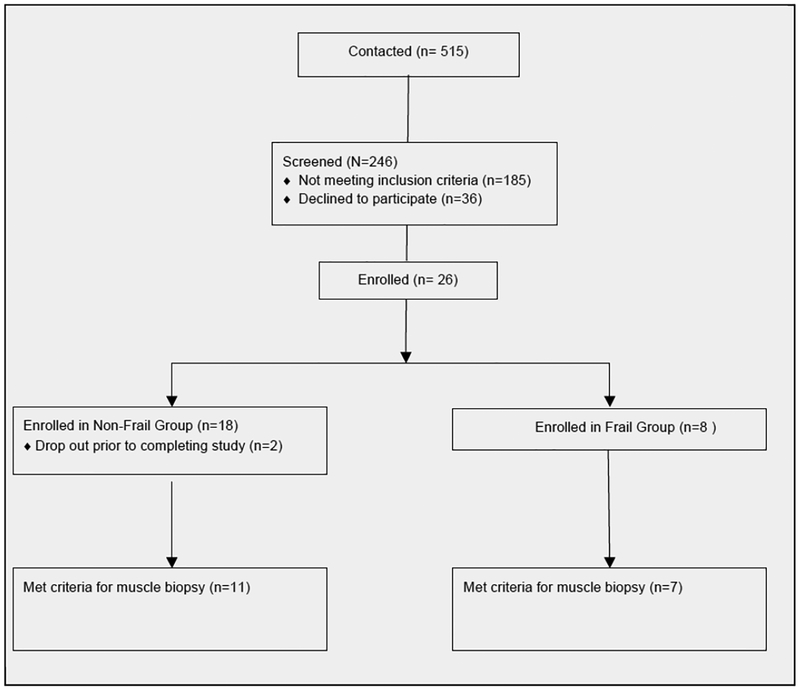
Both male and females were included if they were over the age of 65 years with a BMI of less than 30 kg/m2. Additionally the frail participants were required to be at least moderately frail as classified by a modified physical performance test score (MPPT) of < 25 (moderately frail), and reported little to no planned physical activity over the last year (17). The active non-frail group had MPPT scores of > 32 (not frail) and reported at least 90 minutes of moderate activity a week over the prior year (17). Exclusion criteria for both groups included diseases associated with increased IMAT and decreased functional mobility such as diabetes, COPD, chronic kidney disease, HIV, active cancer or a history of cancer in the last year, and any neurological diseases such as multiple sclerosis, Parkinson disease, or a history of a stroke. Additionally a subgroup of participants were given the option to participate in a percutaneous muscle biopsy if they were free from heart disease or any other condition known to increase systemic inflammatory levels and were not using any medication known to influence inflammation such as tobacco products, corticosteroids, non-steroidal anti-inflammatories, hormone replacements or anti-coagulants. Any individuals on statin medications were asked to hold all statins for at least 7 days prior to the muscle biopsy (Figure 1).
Figure 1.
Participant Recruitment
Study Design
Potential participants completed a telephone screen and an in-person screen to determine eligibility. Prior to all tests individuals provided written informed consent and signed an institution-approved consent form. During the in-person screen, the MPPT was performed and scored to ensure the participants met the requisite scores (17). Immediately after the in-person screening, demographic data were gathered and the first clinic-testing visit was scheduled within 2 weeks. During the first clinic testing session, individuals completed an activity questionnaire and underwent mobility and muscle force testing and an MRI to determine the cross sectional area of IMAT in the thigh. Within 1 week of this clinic-testing visit, individuals who met the inclusion criteria underwent a biopsy to determine inflammation within the muscle.
Activity, Mobility and Muscle force Testing
To quantify physical activity levels participants were asked to complete the Physical Activity Scale for the Elderly (PASE) (18). The PASE is a self-report measure of activity over the previous week that has been shown to be reliable and valid method for estimating levels of physical activity in older adults. The PASE includes not only planned physical activity but also considers the contribution of leisure time, volunteer, and housework activities (18–20). A tester blinded to group collected all muscle function and mobility measures. Mobility was determined using two tests: (1) distance covered in the six-minute walk test (6MW) and (2) self-selected gait-speed (GS) (21, 22). These performance tests were chosen to represent mobility function and have been shown to be both valid and reliable in this population (21–23). The 6MW test, a measure of the distance a subject walks in 6 minutes, was used to assess overall mobility (22). Self-selected gait-speed was measured over a 50-foot course. Individuals were instructed to walk at a comfortable pace starting at the word “go.” They were asked to walk out 25-feet and back. Timing took place from the command “go” until the starting line was crossed on the way back (17). Participants were allowed to use any walking aid they used on a daily basis.
Muscle force was determined by a maximum voluntary isometric contraction of the knee extensors (MVIC) on a KinCom dynamometer (Chattanooga Inc. TN) as follows: participants were stabilized by chest and thigh straps and seated with their knees fixed at 60 degrees of flexion with their arms folded across their chest. Prior to testing, participants practiced submaximal contractions at 50 and 75% of their perceived maximal effort prior to one practice maximal contraction trial. After a 2-minute rest period, three separate maximal contractions were performed. Each maximal contraction was held for 5 seconds with a 3-minute rest between trials. The outcome variable, muscle force, was calculated as the average force of three trials. The order of testing (right versus left) was randomized among subjects (5).
IMAT Determination
Magnetic resonance imaging (MRI) was used for determination of the cross-sectional area (CSA) of lean muscle mass and IMAT as has been done previously (5). Bilateral magnetic resonance imaging (MRI) scans of the thighs were obtained and subjects were placed supine in a 3.0 Tesla whole body MR imager (Siemens Trio, Siemens Medical, Erlangen, Germany). The legs were scanned in a coronal plane and the midpoint of the thigh was determined and defined as half way between the superior margin of the femoral head and the inferior margin of the femoral condyles. Axial imaging (5mm thick slices at 1 cm intervals) of the legs was then performed over 1/2 the length of the femur, centered at the midpoint of the thigh. Separate fat and water images were created with custom software using the three-point Dixon method (5, 24). A tissue model was then used to calculate estimates of total fat and nonfat volume fractions on a per-pixel basis, which were displayed in image form. Eleven images from the middle 1/3 of each thigh were used to determine average cross-sectional area (cm2) of IMAT and lean tissue. Manual tracing eliminated subcutaneous fat and bone and isolated the fascial border of the thigh to create a subfascial region of interest (ROI). Total IMAT and lean tissue were calculated by summing the value of percent fat fraction and percent lean tissue fraction over all pixels within the ROI using custom-written image analysis software (MATLAB; The MathWorks, Natick, Massachusetts). This sum was multiplied by the area of each pixel to give total fat and lean tissue CSAs within the ROI and the respective IMAT and lean tissue cross sectional areas were calculated after excluding subcutaneous adipose tissue and bone (24). The same investigator blinded to group performed measurements of individual participants. This technique has demonstrated high levels of intrarater reliability (25), test-retest reliability (26). and concurrent validity when compared to imaging of a cadaveric phantom limb (25). To normalize IMAT for thigh size, the percent of IMAT was calculated for each individual. This was done by dividing the area of IMAT (in cm2) by the overall area of the thigh (in cm2) excluding subcutaneous adipose tissue and bone.
Muscle Biopsy
All muscle biopsies were performed within one week of the first testing visit. The morning after a 12-hour fast a vastus lateralis percutaneous needle biopsy was performed on the dominant leg as defined by the participant (27). All individuals were asked to refrain from strenuous activity for 48 hours prior. The skin and fascia 12–15 cm above the lateral knee joint space was anesthetized with 5cc of 1% lidocaine, and a small incision was made. A Bergstrom biopsy needle was inserted 3–5 cm beyond the fascia into muscle and 3 passes made. The sample obtained was quickly dissected free from blood and visible fat, snap frozen in liquid nitrogen and stored at −80 C until analysis.
Pro-inflammatory Muscle Cytokines
IL-6 and TNF-α mRNA and protein were chosen as the pro-inflammatory cytokines of interest within the muscle (9–11). Muscle analysis was performed to determine the mRNA expression of IL-6 and TNF-α in the muscle tissue of the participants. Total RNA, cDNA synthesis and real-time quantitative PCR were conducted as previously reported.[28] Total RNA was extracted by homogenizing 15–20 mg muscle tissue with a hand-held homogenizing dispenser (PowerGen 125; Fisher Scientific) in a solution containing 0.75 ml Tri reagent (LS; Molecular Research Center, Cincinnati, OH) and 0.25 ml nuclease free water. The RNA was separated into an aqueous phase using 0.2 ml of chloroform and precipitated using 0.5 ml of isopropanol. Isolated RNA was washed with 1 ml of 75% ethanol, dried, and then suspended in a known amount of nuclease-free water (1.5 μl/mg tissue). RNA was DNase-treated using a commercially available kit (TURBO DNase-free, Life Technologies, Carlsbad, CA). RNA concentration was determined with a NanoDrop 2000 (ThermoFisher Scientific, Waltham, MA). Afterwards, 0.5 μg of total RNA was reverse transcribed into cDNA according to the manufacturer’s directions (iScript, BioRad, Hercules, CA). All isolated RNA and cDNA samples were stored at −80°C until analyzed. Real-time qPCR was carried out with an Applied Biosystems 7900HT fast sequence detection system. Taqman pre-designed primers (IL-6 and TNF-α) were purchased from Life Technologies. Values were normalized to beta 2-microglobulin then fold change values were calculated using the 2−ΔΔCt method (29).
Standard western blot procedures were used to determine protein expression of IL-6 and TNF-α in muscle homogenates(30). Frozen muscle tissue was homogenized in a buffer cocktail with protease inhibitors in a pre-chilled glass tube under ice. Muscle homogenates were centrifuged at 6000 rpm for 10min at 4°C, and the supernatant was subsequently collected and transferred to a new microcentrifuge tube. Total protein concentration for each sample was determined on a spectrophotometer using a colorimetric protein assay (Bio-Rad; Bradford) and an albumin standard curve. Whole muscle homogenates were diluted 1:1 in a 2X sample buffer. Homogenates were loaded at equal protein concentration on a Criterion Tris-HCL pre-cast polyacrylamide gel (Bio-Rad) and subjected to SDS-PAGE (150V) for 1h in running buffer. Each gel contained alternating frail and non-frail samples loaded in duplicate, and a molecular weight ladder. An internal control was loaded in duplicate on each gel for band normalization and comparisons across blots. Protein was transferred (50V; 1h) to a polyvinylidene diflouride membrane in ice cold transfer buffer then blocked for 1h at room temperature with 2% non-fat dry milk (NFDM) in Tris-buffered saline in 0.1% Tween-20 (TBST). Membranes were incubated overnight in primary antibody diluted in 2% NFDM in TBS. The next morning, blots were rinsed in TBST for 5 minutes, rocked in secondary antibody for 1h at room temperature in 2% NFDM in TBS then serially washed (15 min, 4 × 5 min) in TBST. Chemiluminescence reagent (ECL Plus, GE Healthcare) was applied to each blot for 5 min. Optical density measurements were obtained with a digital imager (Bio-Rad). Membranes were stripped (Restore PLUS, Thermo Scientific) of primary and secondary antibodies then re-probed for α-tubulin (1:50,000; Sigma Aldrich, St. Louis, MO). Densitometric analysis was performed using Quantity One software (Bio-Rad). After subtracting out background, all western blot data were normalized to the internal control and replicate samples were averaged. The following antibodies were used in this experiment: IL-6 (1:200; Santa Cruz Biotechnology, Santa Cruz, CA), TNF-α (1:500; Cell Signaling, Boston, MA), and Toll-like receptor 4 (1:500; Santa Cruz Biotechnology). Donkey anti-rabbit and goat anti-mouse IgG horseradish peroxidase-conjugated secondary antibodies (1:6000) were purchased from Santa Cruz Biotechnology.
Data Analysis
Data were analyzed with SPSS Statistics 20.0 (SPSS, Chicago, IL). Descriptive statistics were calculated for demographic variables and dependent measures. Due to the small sample size muscle and mobility function, IMAT and muscular inflammation in older frail and non-frail adults were compared using a Mann-Whitney U test. Spearman’s rho correlations were used to examine the relationship of IMAT and muscular inflammation and between inflammation and muscle force and mobility function. The level of significance was set at P<0.05.
Results
Participant Characteristics
A total of 26 individuals were enrolled, 8 frail and 18 non-frail older adults. Two individuals were eliminated for refusal to have an MRI and non-study related illness. All remaining individuals completed all testing resulting in data for 8 frail and 16 non-frail individuals. A subgroup of 7 frail and 11-non-frail individuals met the inclusion criteria for a muscle biopsy. The demographics for both groups of participants are summarized in Table 1. The frail and non-frail groups differed in all activity, mobility, and muscle force measures.
Table 1.
| Frail | Non-Frail | |
|---|---|---|
| n | 8 | 16 |
| Age (years) | 83.3 (79.5–87.0) | 78.1 (75.05–81.07) |
| BMI (kg/m2) | 25.0 (22.2–27.7) | 23.9 (22.5–25.3) |
| PASE | 58.6 (30.6–86.6) | 215.0 (185.0–245.0)* |
| MPPT | 16.3 (9.8–22.7) | 35.2 (34.5–35.8)* |
| % Lean | 81.9 (77.9–86.1) | 88.3 (87.3–89.4)* |
| % IMAT | 18.0 (13.9–22.1) | 11.7 (10.6–12.7)* |
| 6MW (meters) | 278.5 (155.1–401.9) | 540.8 (499.3–582.3)* |
| GS (m/sec) | 0.67 (0.49–0.85) | 1.2 (1.1–1.4)* |
| MVIC (N) | 161.9 (120.4–203.3) | 323.1(258.4–387.8)* |
Mean (95% confidence interval); BMI= Body Mass Index; PASE = Physical Activity; Scale for the Elderly; MPPT= Modified Physical Performance Test; % lean = percentage of thigh lean tissue average cross sectional area as measured with MRI excluding subcutaneous fat and bone; % IMAT = percentage thigh intramuscular adipose tissue average cross sectional area as measured with MRI excluding subcutaneous fat and bone; 6MW = distance in meters covered in six minute walk test; GS = self-selected gait speed in meters/second; MVIC = maximal voluntary isometric contraction of knee extensor muscles in Newtons;
significant difference between groups (p<.05)
Inflammation and IMAT
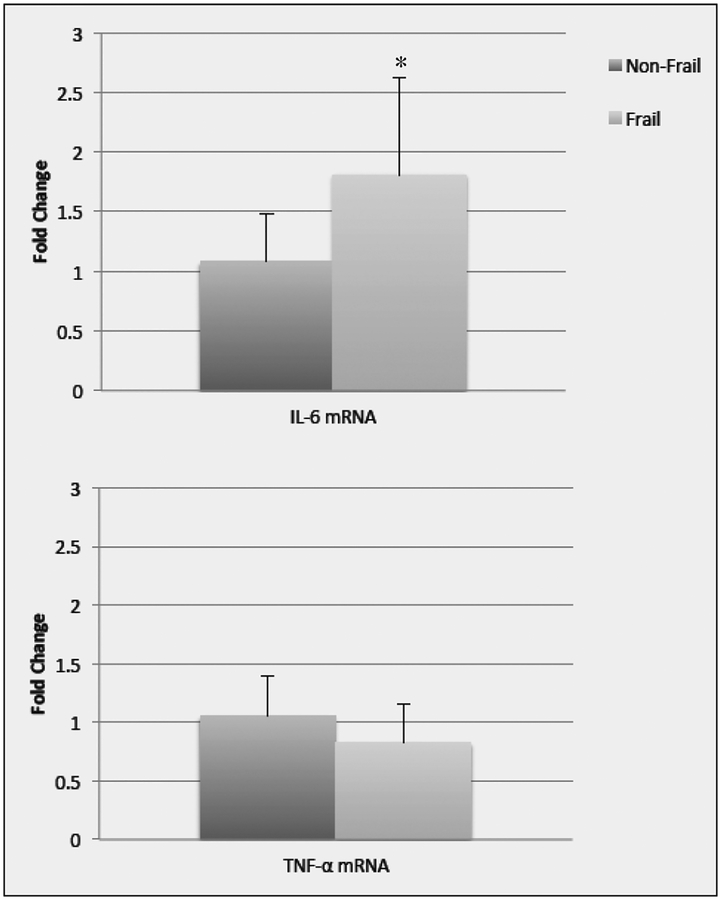
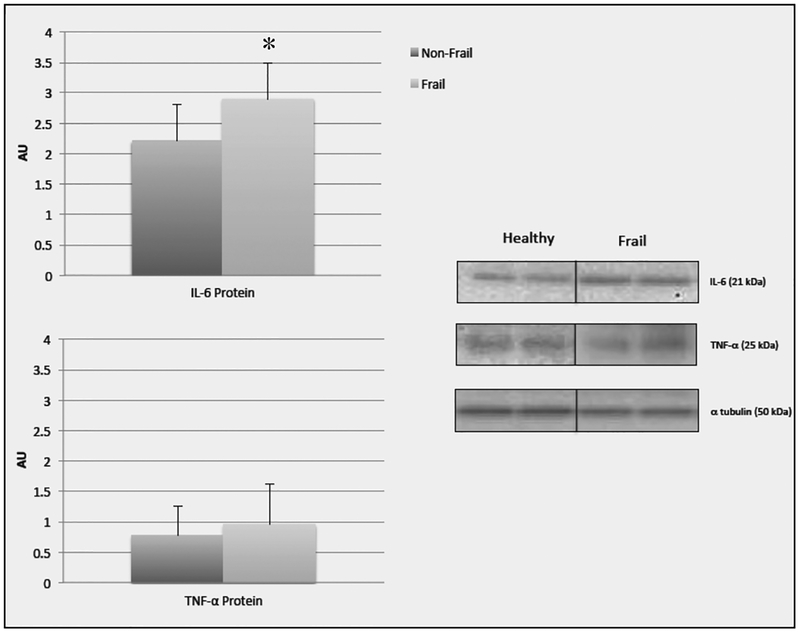
Despite having similar ages and BMIs, significant differences between the frail and non-frail groups were found for thigh IMAT with the frail group having more IMAT (p=0.002) and less lean tissue than the non-frail group (p=0.002) (Table 1). In the sub-group analysis, IL-6 mRNA (p=0.028) and protein expression (p= 0.019) were significantly higher in the frail group than the non-frail group. There was no significant between group differences in TNF-α mRNA (p=0.201) or protein expression (p= 0.248). (Figure 2a and 2b.)
Figure 2a.
Data (mean +/− SE) represents the fold change in mRNA for IL-6 and TNF-α in non-frail and frail older adults. *significant difference (p=0.02) between non-frail and frail older adults. Note: 11 non-frail and 7 frail older adults were included in the analysis except for IL-6 mRNA where one frail older adult was out of detectable range (>40 cycle threshold)
Figure 2b.
Data (mean +/− SE) represents the arbitrary units from Western blots for IL-6 and TNF-α protein in non-frail and frail older adults. Representative Western blot image and molecular weight for IL-6 and TNF-α from a non-frail and frail older adult (in replicate). Each gel contained alternating non-frail and frail samples loaded in replicate, and a molecular weight ladder. An internal control was loaded in duplicate on each gel for band normalization and comparisons across blots. Alpha tubulin was used to determine equal protein loading. *significant difference (p=0.03) between non-frail and frail older adults. Note: 11 non-frail and 7 frail older adults were included in all analysis
There was a moderate significant positive correlation between thigh IMAT and muscle expression of IL-6 mRNA (r=0.431 p=0.04) and IL-6 protein (r=0.411, p=.045). There was no significant correlation between TNF-α mRNA (r=0.240, p=0.177) or protein (r=0.032, p=.452) and thigh IMAT. Both IL-6 protein and mRNA in the muscle were strongly and significantly related to all mobility measures. TNF-α mRNA and protein were not significantly correlated (r values range from −0.27 to −0.005, p values range from 0.11 to 0.50) with any muscle force or mobility measures. IL-6 mRNA was strongly negatively correlated with GS (r=−0.61, p=0.005) and 6 MW distance (r=−0.63, p= 0.004) and muscle force was trending towards significance (r=−0.35, p= 0.08). IL-6 protein expression was strongly negatively correlated with GS (r=−0.76, p<0.001), 6 MW distance (r=−0.66, p=0.001), and muscle force (r=−0.54, p=0.01).
Discussion
This study is the first that we are aware of to examine the relationship of IMAT and inflammation in aging muscle tissue. We sought to examine the relationships and differences in IMAT, muscular inflammation, and muscle and mobility function in age- and BMI-matched, non-obese, frail and non-frail older adults. As hypothesized, we found that older frail adults had increased levels of IMAT and muscular inflammation when compared to their age- and BMI-matched non-frail peers. We also found significant positive relationships between IMAT and the expression of IL-6 mRNA and IL-6 protein within the muscle that were related to both muscle and mobility function in this group of older adults.
While a significant amount of work has been dedicated to the prevalence and impact of a loss of muscle mass (31, 32), surprisingly little research has examined the inflammatory state of skeletal muscle in older adults. While previous studies have compared muscle IL-6 mRNA and protein expression in healthy older and younger adults (33, 34), or changes in muscle inflammation pre-and-post chronic exercise (35, 36), this is the first study that we are aware of that compares pro-inflammatory cytokines in the muscle of otherwise healthy frail and non-frail older adults of similar ages and BMIs. Previous work has found little to no difference in IL-6 mRNA expression in the muscle of healthy young versus healthy older adults (33, 34). Our finding of increased IL-6 mRNA and protein within the muscle of frail older adults is clinically important as increased basal serum levels of pro-inflammatory cytokines such as IL-6 are one of the most important physiologic correlates of frailty (37,38). Older individuals with the highest levels of serum IL-6 experience decreased strength, impaired mobility, and an increased risk of death (10, 11, 39, 40). Our findings of a positive relationship between locomotor muscle IL-6 and impaired muscle and mobility function in frail older adults adds to the existing body of literature underscoring the potentially harmful nature of increased inflammatory levels in older adults.
Whether IMAT is merely a marker of muscle dysfunction or if it plays a more active role in muscle and mobility dysfunction remains unknown. IMAT is theorized to be a metabolically active component of muscle and a potential source of inflammatory regulation in older individuals (4, 5, 7, 16, 41). Our results, while only correlational, add to the speculation that the negative consequences of IMAT on mobility function may be from the release of pro-inflammatory cytokines that may act in a paracrine like manner on nearby muscle (4, 7, 16). An increase in pro-inflammatory cytokines from IMAT may be especially harmful to muscle. The close proximity of IMAT to muscle fibers would allow a direct release of pro-inflammatory cytokines, such as IL-6, onto the muscle fibers and may result in increased muscle and mobility dysfunction compared to systemic increases in inflammation. While this theory is currently only speculative, animal studies have suggested increased inflammation within the muscle leads to an immune response, catabolism, a loss of muscle mass, and decreased strength (12, 13, 42–45). The moderate relationships we found between IL-6 and muscle and mobility function suggest that muscle inflammation is related to muscle and mobility function in frail older adults and may be at least partially attributable to levels of IMAT within the locomotor muscles.
Higher levels of circulating IL-6 have been attributed to increased fat mass and obesity (46). Previous work demonstrates that up to 30% of circulating levels of IL-6 may be released from adipose tissue in obese subjects (47). Our novel data suggests that even in the absence of obesity, IL-6 mRNA and protein expression in the muscle are associated with IMAT. Our findings are also in agreement with Beasly et al., who reported a positive relationship between circulating serum levels of IL-6 with IMAT in older Caucasian women (7). We did not find any differences in the TNF-α expression between our groups. Findings have been equivocal on the effect of aging on TNF-α expression in muscle (33, 48). TNF-α is also known to have more variable expression than IL-6 (47). Subcutaneous adipose tissue releases large amounts of IL-6 but little TNF-α, however increases in visceral adipose tissue are associated with increased TNF-α release (47). It is possible that given the heterogeneity of adipose tissue, no direct relationship between IMAT and TNF-α expression exists.
The difference in activity levels between our groups also suggests that increased IMAT and IL-6 within the muscle of otherwise healthy frail older individuals may be at least partially due to sedentary behavior. Increases in both IMAT and serum levels of IL-6 have been associated with both increased age (16, 49–53) and decreased physical activity (4, 54–57). Our prospective experimental results provide additional detail to support large epidemiological studies suggesting that increased IMAT and intramuscular inflammation may be more a result of inactivity than aging. While some elevation of systemic inflammation may be expected with normal aging, a large part of this increase may be attributable to decreased physical activity or the existence of co-morbid conditions (51).
To date, the literature regarding aging and IMAT is equivocal, Delmonico et al. reported in a 5 year longitudinal study that IMAT levels increased even in individuals who lost weight (16). However, more recently Wroblewski et al., reported in a cross-sectional study of master athletes ages 40 to 70 plus years of age that IMAT levels did not increase significantly with age (57). Further support of the inactivity hypothesis was found by Manini et al. who demonstrated increased IMAT levels in the thigh and calf of young (19–28 years old) individuals after 30 days of single limb unloading. IMAT volume increased up to 20% in the lower extremity suggesting that even in young healthy individuals, increased IMAT is a consequence of decreased activity (4). Intramuscular inflammatory levels also differ significantly between sedentary and active adults, and increased physical activity may result in decreased inflammation in the muscle, even when no systemic changes in inflammation occur (35, 36, 58).
Taken together with our findings, increased IMAT and muscular inflammatory levels are more likely a product of disuse then age per se. While we did not have a younger group of individuals to compare, if increases in IMAT and muscular inflammation were solely an age and BMI related phenomenon we would expect to see similar levels of IMAT in our frail and non-frail age- and BMI-matched participants. Our results differ from those of Buford et al. (59) who found no difference in IMAT between non-frail older adults and frail older adults, but did find a difference in IMAT between young and older adults. Buford, however, studied sedentary adults with multiple co-morbid conditions. The participants in our study had markedly different activity levels and while we cannot attribute the between group IMAT differences in this case-control analysis to activity level alone, our findings do suggest that relative to IMAT deposition in the thigh muscles, all older adults are not created equal. This is an important finding as physical activity may be protective against locomotor IMAT deposition in older adults (4, 57). Goodpaster et al. identified that walking just two times per week nearly amerilorated an increase in IMAT in the midthigh of healthy mobile older adults age 70–89.
Limitations and Directions for Future Research
Our results are not without limitations. The small sample size and cross sectional nature of these results requires caution when interpreting the results. While we did control for multiple co-morbidities and illness in our age- and BMI-matched cohorts, it is possible that undiagnosed co-morbidities, or the small age discrepancy, could have influenced the differing levels of IMAT and inflammation. However, given that previous work has demonstrated no significant differences between IL-6 mRNA expression in young and old healthy individuals, this appears unlikely (33, 34). We also included both male and females in this analysis in an attempt to make our findings more generalizable. We do recognize that sex could have a potential influence on our results as previous work has found IMAT difference between males and females (7). However, even when our groups were analyzed by sex, the findings of differences between IMAT and inflammation in frail and non-frail older adults still remain (results not presented). Finally, because we controlled for both BMI and co-morbidities in both groups, our findings are likely conservative. The relationship of muscular inflammation and IMAT in older adults may be even more robust if we include individuals with obesity and diagnosed diseases typically associated with inflammation such as diabetes or heart disease.
This study is the first to examine the relationship of IMAT and inflammation in aging muscle tissue. A significant relationship was found between IMAT and IL-6 protein and mRNA expression. This IMAT-inflammatory pathway provides a potential mechanistic link underlying IMATs negative influence on muscle and mobility function and provides insight into the potential harmful effects of IMAT on skeletal muscle. Studies with larger samples are needed to more definitively delineate the relationship between IMAT and inflammation in older adults and to explore if exercise can be used to decrease both IMAT and inflammation in older adults.
Acknowledgements:
This work was supported by a grant from the University of Utah Research Foundation (10020526) and the project described was supported by the National Center for Research Resources and the National Center for advancing Translational Science, National Institutes of Health, through Grant 8UL1TR000105 (formerly UL1RR025764). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. Dr. Addison reports grants from NIH, during the conduct of the study. Dr. Drummond has nothing to disclose. Dr. LaStayo has nothing to disclose. Dr. Dibble has nothing to disclose. Dr. Wende has nothing to disclose. Dr. McClain has nothing to disclose. Dr. Marcus has nothing to disclose.
References
- 1.Goodpaster BH, et al. , Attenuation of skeletal muscle and strength in the elderly: The Health ABC Study. J Appl Physiol, 2001. 90(6): p. 2157–65. [DOI] [PubMed] [Google Scholar]
- 2.Visser M, et al. , Leg muscle mass and composition in relation to lower extremity performance in men and women aged 70 to 79: the health, aging and body composition study. J Am Geriatr Soc, 2002. 50(5): p. 897–904. [DOI] [PubMed] [Google Scholar]
- 3.Goodpaster BH, et al. , Association between regional adipose tissue distribution and both type 2 diabetes and impaired glucose tolerance in elderly men and women. Diabetes Care, 2003. 26(2): p. 372–9. [DOI] [PubMed] [Google Scholar]
- 4.Manini TM, et al. , Reduced physical activity increases intermuscular adipose tissue in healthy young adults. Am J Clin Nutr, 2007. 85(2): p. 377–84. [DOI] [PubMed] [Google Scholar]
- 5.Marcus RL, et al. , Intramuscular adipose tissue, sarcopenia, and mobility function in older individuals. J Aging Res, 2012. 2012: p. 629637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marcus R, Addison O, and LaStayo P, Intramuscular adipose tissue attenuates gains in muscle quality in older adults at high risk for falling. A brief report. J Nutr Health Aging, 2013: March; 17(3) p. 215–8. [DOI] [PubMed] [Google Scholar]
- 7.Beasley LE, et al. , Inflammation and race and gender differences in computerized tomography-measured adipose depots. Obesity, 2009. 17(5): p. 1062–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fontana L, et al. , Visceral fat adipokine secretion is associated with systemic inflammation in obese humans. Diabetes, 2007. 56(4): p. 1010–3. [DOI] [PubMed] [Google Scholar]
- 9.Schaap LA, et al. , Higher inflammatory marker levels in older persons: associations with 5-year change in muscle mass and muscle strength. J Gerontol A Biol Sci Med Sci, 2009. 64(11): p. 1183–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Visser M, et al. , Relationship of interleukin-6 and tumor necrosis factor-alpha with muscle mass and muscle strength in elderly men and women: the Health ABC Study. J Gerontol A Biol Sci Med Sci, 2002. 57(5): p. M326–32. [DOI] [PubMed] [Google Scholar]
- 11.Cesari M, et al. , Inflammatory markers and physical performance in older persons: the InCHIANTI study. J J Gerontol A Biol Sci Med Sci, 2004. 59(3): p. 242–8. [DOI] [PubMed] [Google Scholar]
- 12.Li YP, et al. , Skeletal muscle myocytes undergo protein loss and reactive oxygen-mediated NF-kappaB activation in response to tumor necrosis factor alpha. FASEB J, 1998. 12(10): p. 871–80. [DOI] [PubMed] [Google Scholar]
- 13.Guttridge DC, et al. , NF-kappaB-induced loss of MyoD messenger RNA: possible role in muscle decay and cachexia. Science, 2000. 289(5488): p. 2363–6. [DOI] [PubMed] [Google Scholar]
- 14.Hardin BJ, et al. , TNF-alpha acts via TNFR1 and muscle-derived oxidants to depress myofibrillar force in murine skeletal muscle. J Appl Physiol, 2008. 104(3): p. 694–9. [DOI] [PubMed] [Google Scholar]
- 15.Kidde J, et al. , Regional muscle and whole-body composition factors related to mobility in older individuals: a review. Physiother Can, 2009. 61(4): p. 197–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Delmonico MJ, et al. , Longitudinal study of muscle strength, quality, and adipose tissue infiltration. Am J Clin Nutr, 2009. 90(6): p. 1579–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brown M, et al. , Physical and performance measures for the identification of mild to moderate frailty. J Gerontol A Biol Sci Med Sci, 2000. 55(6): p. M350–5. [DOI] [PubMed] [Google Scholar]
- 18.Washburn RA, et al. , The Physical Activity Scale for the Elderly (PASE): development and evaluation. J Clin Epidemiol, 1993. 46(2): p. 153–62. [DOI] [PubMed] [Google Scholar]
- 19.Washburn RA, et al. , The Physical Activity Scale for the Elderly (PASE): Evidence for Validity. J Clin Epidemiol, 1999. 52(7): p. 643–651. [DOI] [PubMed] [Google Scholar]
- 20.Washburn RA and Ficker JL, Physical Activity Scale for the Elderly (PASE): the relationship with activity measured by a portable accelerometer. J Sports Med Phys Fitness, 1999. 39(4): p. 336–40. [PubMed] [Google Scholar]
- 21.Abellan van Kan G, et al. , Gait speed at usual pace as a predictor of adverse outcomes in community-dwelling older people an International Academy on Nutrition and Aging (IANA) Task Force. J Nutr Health Aging, 2009. 13(10): p. 881–9. [DOI] [PubMed] [Google Scholar]
- 22.Enright PL, et al. , The 6-min walk test: a quick measure of functional status in elderly adults. Chest, 2003. 123(2): p. 387–98. [DOI] [PubMed] [Google Scholar]
- 23.Kennedy DM, et al. , Assessing stability and change of four performance measures: a longitudinal study evaluating outcome following total hip and knee arthroplasty. BMC Musculoskelet Disord, 2005. 6: p. 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kovanlikaya A, et al. , Fat quantification using three-point dixon technique: in vitro validation. Acad Radiol, 2005. 12(5): p. 636–9. [DOI] [PubMed] [Google Scholar]
- 25.Dibble LE, et al. , High-intensity resistance training amplifies muscle hypertrophy and functional gains in persons with Parkinson’s disease. Mov Disord, 2006. 21(9): p. 1444–52. [DOI] [PubMed] [Google Scholar]
- 26.Elder CP, et al. , Intramuscular fat and glucose tolerance after spinal cord injury--a cross-sectional study. Spinal Cord, 2004. 42(12): p. 711–6. [DOI] [PubMed] [Google Scholar]
- 27.Marcus RL, et al. , Regional muscle glucose uptake remains elevated 1 week after cessation of resistance training independent of altered insulin sensitivity response in older adults with type 2 diabetes. Journal Endocrinol Invest, 2013. February; 36(2):111–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Drummond MJ, et al. , An increase in essential amino acid availability upregulates amino acid transporter expression in human skeletal muscle. Am J Physiol Endocrinol Metab, 2010. 298(5): p. E1011–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Livak KJ and Schmittgen TD, Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods, 2001. 25(4): p. 402–8. [DOI] [PubMed] [Google Scholar]
- 30.Drummond MJ, et al. , Skeletal muscle amino acid transporter expression is increased in young and older adults following resistance exercise. J Appl Physiol, 2011. 111(1): p. 135–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Masanes F, et al. , Prevalence of sarcopenia in healthy community-dwelling elderly in an urban area of Barcelona (Spain). J Nutr Health Aging, 2012. 16(2): p. 184–7. [DOI] [PubMed] [Google Scholar]
- 32.Vellas B, et al. , Designing pharmaceutical trials for sarcopenia in frail older adults: EU/US Task Force recommendations. J Nutr Health Aging, 2013. 17(7): p. 612–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Caldow MK, et al. , Inflammatory markers in skeletal muscle of older adults. Eur J Appl Physiol, 2012. [DOI] [PubMed] [Google Scholar]
- 34.Hamada K, et al. , Senescence of human skeletal muscle impairs the local inflammatory cytokine response to acute eccentric exercise. FASEB, 2005. 19(2): p. 264–6. [DOI] [PubMed] [Google Scholar]
- 35.Bruun JM, et al. , Diet and exercise reduce low-grade inflammation and macrophage infiltration in adipose tissue but not in skeletal muscle in severely obese subjects. Am J Physiol Endocrinol Metab, 2006. 290(5): p. E961–7. [DOI] [PubMed] [Google Scholar]
- 36.Lambert CP, et al. , Exercise but not diet-induced weight loss decreases skeletal muscle inflammatory gene expression in frail obese elderly persons. J Appl Physiol, 2008. 105(2):p. 473–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ershler WB and Keller ET, Age-associated increased interleukin-6 gene expression, late-life diseases, and frailty. Annu Rev Med, 2000. 51: p. 245–70. [DOI] [PubMed] [Google Scholar]
- 38.Kanapuru B and Ershler WB, Inflammation, coagulation, and the pathway to frailty. Am J Med, 2009. 122(7): p. 605–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Harris TB, et al. , Associations of elevated interleukin-6 and C-reactive protein levels with mortality in the elderly. Am J Med, 1999. 106(5): p. 506–12. [DOI] [PubMed] [Google Scholar]
- 40.Taaffe DR, et al. , Cross-sectional and prospective relationships of interleukin-6 and C-reactive protein with physical performance in elderly persons: MacArthur studies of successful aging. J Gerontol A Biol Sci Med Sci, 2000. 55(12): p. M709–15. [DOI] [PubMed] [Google Scholar]
- 41.Tuttle LJ, Sinacore DR, and Mueller MJ, Intermuscular adipose tissue is muscle specific and associated with poor functional performance. J Aging Res, 2012. 2012: p. 172957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Charters Y and Grimble RF, Effect of recombinant human tumour necrosis factor alpha on protein synthesis in liver, skeletal muscle and skin of rats. Biochem J, 1989. 258(2): p. 493–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Goodman MN, Tumor necrosis factor induces skeletal muscle protein breakdown in rats. Am J Physiol, 1991. 260(5 Pt 1): p. E727–30. [DOI] [PubMed] [Google Scholar]
- 44.Goodman MN, Interleukin-6 induces skeletal muscle protein breakdown in rats. Proc Soc Exp Biol Med, 1994. 205(2): p. 182–5. [DOI] [PubMed] [Google Scholar]
- 45.Wilcox PG, et al. , Tumor necrosis factor alpha decreases in vivo diaphragm contractility in dogs. Am J Respir Crit Care Med, 1994. 150(5 Pt 1): p. 1368–73. [DOI] [PubMed] [Google Scholar]
- 46.Cesari M, et al. , Sarcopenia, obesity, and inflammation--results from the Trial of Angiotensin Converting Enzyme Inhibition and Novel Cardiovascular Risk Factors study. Am J Clin Nutr, 2005. 82(2): p. 428–34. [DOI] [PubMed] [Google Scholar]
- 47.Mohamed-Ali V, et al. , Subcutaneous adipose tissue releases interleukin-6, but not tumor necrosis factor-alpha, in vivo.J Clin Endocrinol Metab, 1997. 82(12): p. 4196–200. [DOI] [PubMed] [Google Scholar]
- 48.Leger B, et al. , Human sarcopenia reveals an increase in SOCS-3 and myostatin and a reduced efficiency of Akt phosphorylation. Rejuvenation research, 2008. 11(1): p. 163–175B. [DOI] [PubMed] [Google Scholar]
- 49.Cartier A, et al. , Age-related differences in inflammatory markers in men: contribution of visceral adiposity. Metabolism, 2009. 58(10): p. 1452–8. [DOI] [PubMed] [Google Scholar]
- 50.Ershler WB, et al. , Interleukin-6 and aging: blood levels and mononuclear cell production increase with advancing age and in vitro production is modifiable by dietary restriction. Lymphokine Cytokine Res, 1993. 12(4): p. 225–30. [PubMed] [Google Scholar]
- 51.Ferrucci L, et al. , The origins of age-related proinflammatory state. Blood, 2005. 105(6):p. 2294–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hager K, et al. , Interleukin-6 and selected plasma proteins in healthy persons of different ages. Neurobiol Aging, 1994. 15(6): p. 771–2. [DOI] [PubMed] [Google Scholar]
- 53.Wei J, et al. , Increase of plasma IL-6 concentration with age in healthy subjects. Life Sci, 1992. 51(25): p. 1953–6. [DOI] [PubMed] [Google Scholar]
- 54.Autenrieth C, et al. , Association between different domains of physical activity and markers of inflammation. Med Sci Sports Exerc, 2009. 41(9): p. 1706–13. [DOI] [PubMed] [Google Scholar]
- 55.Fischer CP, et al. , Plasma levels of interleukin-6 and C-reactive protein are associated with physical inactivity independent of obesity. Scand J Med Sci Sports, 2007. 17(5): p. 580–7. [DOI] [PubMed] [Google Scholar]
- 56.Goodpaster BH, et al. , Effects of physical activity on strength and skeletal muscle fat infiltration in older adults: a randomized controlled trial. J Appl Physiol, 2008. 105(5): p. 1498–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wroblewski AP, et al. , Chronic exercise preserves lean muscle mass in masters athletes.Phys Sportsmed, 2011. 39(3): p. 172–8. [DOI] [PubMed] [Google Scholar]
- 58.Gielen S, et al. , Anti-inflammatory effects of exercise training in the skeletal muscle of patients with chronic heart failure. J Am Coll Cardiol, 2003. 42(5): p. 861–8. [DOI] [PubMed] [Google Scholar]
- 59.Buford TW, et al. , Age-related differences in lower extremity tissue compartments and associations with physical function in older adults. Experimental gerontology, 2012. 47(1):p. 38–44. [DOI] [PMC free article] [PubMed] [Google Scholar]