Abstract
Background/Aims:
Aminochrome, an endogenous compound formed during dopamine oxidation can induce neurotoxicity under certain aberrant conditions and induce Parkinson-like syndrome. Glutathione transferase M2 (GSTM2) activity of astrocytes by catalysing the conjugation of aminochrome with glutathione, can offer protection against aminochrome toxicity. Some medicinal toxicity through this plants may exert protective effect against aminochrome mechanism.
Methods:
In the present study, extracts from plants native to Cameroon, such as Alchornea laxiflora (leaves), Dacryodes edulis (barks), Annona muricata (seeds), Annona senegalensis (barks) were evaluated for their protection against aminochrome-induced toxicity in human glioblastoma/ astrocytoma U373MG wild type and U373MGsiGT6 cells in which GSTM2 expression was 74% silenced. The cells were pre-incubated with the plant extracts for 2 hr before addition of aminochrome (75 μM) and measurement of cell death/viability by flow cytometry after 24 hr incubation.
Results:
The extract of A. laxiflora (1 μg/ml), D. edulis (25 μg/ml), A. muricata (25 μg/ml) and A. senegalensis (25μg/ml) significantly decreased aminochrome-induced toxicity in U373siGST6 and U373MG cells. However, only A. laxiflora and A. muricata significantly increased the mitochondria membrane potential in U373siGST6 cells following aminochrome treatment.
Conclusion:
The results indicate that extracts of some Cameroon plants can provide protection against aminochrome-induced toxicity and mitochondria dysfunction in human glioblastoma/astrocytoma cells. Although further identification of active components of these extracts is needed, potential usefulness of these compounds in Parkinson’s disease may be suggested.
Keywords: Aminochrome, Medicinal Plants, U373MG cells, Neurotoxicity, Neuroprotection, Mitochondria Dysfunction.
Introduction
Parkinson’s disease (PD) is the second most common progressive neurodegenerative disorder after Alzheimer’s disease [1]. It is chara-cterized primarily by progressive degeneration of dopaminergic neurons containing neuromelanin in the substantia nigra pars compacta, which ultimately result in motors symptoms such as resting tremor, rigidity, bradykinesia and postural instability [2]. The most common drug used for PD is levodopa (L-dopa), a dopamine precursor, which increases dopamine levels in the brain and alleviates the symptoms. However, this drug has many side effects and loses its effectiveness in few years [3]. Hence, more novel therapies are needed.
Several cellular models of PD including exposure to 1-methyl-4-phenyl-1,2,3,6- tetrahydropyridine (MPTP), rotenone and the 6-hydroxydopamine (6-OHDA) are commonly used [4]. However, these drugs induce a rapid loss of dopaminergic cells in vitro [5], whereas the symptoms of Parkinsonism in humans may take up to years following nigral degeneration [6]. Moreover, in-vitro protection against these toxins does not necessarily translate into effective pharmacotherapy in PD [7, 8]. Dopamine oxidation to o-quinones appears to be a natural process as it accumulates with age [9]. However, under certain conditions, the o-quinones formed during dopamine oxidation, result in neurotoxicity through mitochondria dysfunction, formation and stabilization of neurotoxic protofibrils of alpha synuclein, protein degradation, dysfunction of both proteasomal and lysosomal systems, neuroinflammation, endoplasmic reticulum stress and oxidative stress [10, 11].
Astrocytes, the most abundant cells in the central nervous system (CNS), play important role in the maintenance of neuronal activity through release of neurotrophic factors, maintenance of ion gradients such as extracellular K+ and construction of the blood–brain barrier [12]. Studies suggest that neurotrophic mechanism has a powerful ability to protect degenerating dopamine neurons as well as promoting regeneration of the nigrostriatal dopamine system. Dopamine that is released during neurotransmission is removed from the synaptic cleft and back to the neuron by dopamine transporters. However, the synaptic terminals of dopaminergic neurons are surrounded by other neurons and astrocytes, which are also able to take up dopamine. In astrocytes, dopamine may be converted to aminochrome, but the astrocytes via their glutathione transferase M2 (GSTM2) are capable of catalysing the conjugation of aminochrome with glutathione and hence prevent aminochrome toxicity [13, 14, 15].
The search for novel therapeutic approaches targeting the presumed pathogenic mechanisms has been a major focus of research and it is expected that novel medications with disease-modifying properties will emerge from these efforts in the future. Medicinal plants were the first method of treating diseases in ancient world and are still an important part of various cultures. In Africa, despite widespread use of modern medicines, a high proportion of the rural people still use traditional medicines for health purposes and the plants used are potential source for drug development [16]. Cameroonian medicinal plants have been screened for a number of bioactive compounds including polyphenols, alkaloids, sterols, tannins, and triterpenes [17], which possess a wide spectrum of pharmacological activities. Moreover, some studies have shown that medicinal plants containing polyphenols have anti-oxidant and neuroprotective effects [18, 19] and may represent a potential source for drug development against PD.
In this perspective, an ethno pharmacological survey carried out in the Noun Division (western region of Cameroon) allowed identification of some medicinal plants where voucher specimens were deposited at the National Herbarium of Cameroon (Yaoundé). Preliminary experiments suggested that the methylene chloride-methanolic extracts of some of these plants contain considerable phenolic and flavonoids compounds with significant antioxidant and anti-inflammatory activities. Since oxidative stress and inflammation have been strongly implicated in the pathogenesis of PD, we hypothesized that some of these extracts will be capable of protecting the astrocytes against aminochrome toxicity.
Materials and Methods
Description of Cell Lines
A human glioblastoma astrocytoma (U373MG) is a cell line derived from a malignant tumour by explant technique. Two cell lines were used in this study: U373MG as the wild-type and U373MGsiGT6, in which GSTM2 expression is 74% silenced by siRNA.
Chemicals and Reagents
For viability/cytotoxicity analysis we used calcein AM (Invitrogen, Carlsbad, CA) and propidium iodide (Sigma-Aldrich, St. Louis, MO). Dopamine and tyrosinase were also purchased from Sigma- Aldrich. MitoProbe™ JC-1 Assay Kit for Flow Cytometry was purchased from Molecular Probes (Eugene, OR).
Plant collection and Preparation of Extracts
Different parts of plants (leaves, barks and seeds) were collected in the locality of Foumbot (Noun Division, West Region, Cameroon). These plants are used by traditional healers to improve memory loss or to treat some neurological/neuropsychiatric disorders such as epilepsy, headaches, depression, anxiety, migraine, convulsion and schizophrenia [See Table 1]. The different parts of plants collected were washed with distilled water and dried at room temperature for several weeks. The dried materials were powdered using a grinder. The powder obtained was kept at 4°C until the preparation of extracts. Hundred grams of powdered plant materials were soaked in 500 mL of solvent methylene chloride/methanol (1:1; v/v) for 48 hours. The final extracts were passed through Whatman N°1 filter paper and the filtrates obtained were concentrated under vacuum at low pressure on a rotary evaporator (RV10 Basic, IKA). The crude extracts obtained were stored at 4°C until further use.
Table 1:
List of the plants used in this study.
| Family | Scientific name Voucher Specimen Number |
Vernacular name in Bamoun* |
Part(s) used |
Mode of preparation |
Disease treated |
|---|---|---|---|---|---|
| Annonaceae | Annona muricata L. 32879/HNC | Chawa-chawa | Seeds | Decoction | Depression/Nervousness |
| Annona senegalensis Pers. 7783 YA | Kuopshe-kuopshe | Barks | Decoction | Convulsion/Memory loss | |
| Apocynaceae | Dacryodes edulis (G.Don) H.J.Lam 18258/HNC | Youom | Barks | Decoction | Depression/Epil epsy |
| Euphorbiaceae | Alchornea laxiflora (Benth.) Pax &K.Hoffm. 2093 YA | Meshé | Leaves | Decoction | Anxiety/Depression |
Evaluation of cytotoxicity of extracts plants
Cell culture and treatment with extracts
The different cells lines (U373MG and U373MGsiGST6) were cultured at 37°C under 5% CO2 in the petri dishes in RPMI-1640 medium (ATCC, Manassas, VA) containing 2 mM L-glutamine, 10 mM HEPES, 1 mM sodium pyruvate, 4.5g/l glucose, and 1500 mg/l sodium bicarbonate, supplemented with 10% fetal bovine serum (Hyclone Fetalclone III) (Thermo Scientific, Waltham, MA), 10 U /ml sodium penicillin, 10 U /ml streptomycin sulfate, and amphotericin. One mL of a cell suspension (5 × 106 cells) was seeded in 24 well plates and the cells were incubated overnight at 37°C with 5% CO2 to allow attachment of the cells to the bottom of the plate. Then, after removing the medium using a suction pump, fresh media was added, and the cells were incubated with different concentrations (12.5, 25, 50 and 100 μg/mL) of the extracts dissolved in DMSO and further diluted in the culture medium for 24 hours.
Cell death/viability assay by Flow Cytometry
Cell death/viability was measured by counting live and dead cells with flow cytometry apparatus (FACSCalibur, BD Biosciences, San Jose, CA), after staining with calcein AM and propidium iodide. These are fluorescent reagents that discriminate the population of live cells from the dead cells using 510-560 nm (excitation) and LP-590 nm (emission) for propidium iodide and 450-490 nm (excitation) and 515-565 nm (emission) for calcein AM. Calcein AM is a marker for live cells as these cells with intact membrane are distinguished by their ability to exclude propidium iodide (PI), which readily penetrates dead or damaged cells and intercalates into the DNA of these cells. Dual analysis was introduced using a quadrant dot plot, in which dead cells were identified as single PI-positive, live cells as calcein AM positive only, and cells in late apoptosis as double-positive for calcein AM and PI. Cells that stained negative for both calcein AM and PI were classified as live cells. Finally, the number of cells in each category was expressed as a percentage of the total number of stained cells.
Evaluation of the protective effect of plant extracts against aminochrome toxicity
Synthesis and Purification of Aminochrome
For synthesis of aminochrome, dopamine (7.5 mM) and 10 ng of tyrosinase were incubated in 25 mM potassium phosphate buffer pH 6 for 15-20 min at room temperature. For purification of aminochrome, the incubation solution was loaded on a CM-Sephadex C50-1000 (18 × 0.7 cm) column (Sigma-Aldrich) [20]. The red–orange solution corresponding to aminochrome was collected and detected spectrophotometrically by measuring the absorbance at 480 nm. Aminochrome concentration was determined by the molar extinction coefficient of 3058 M−1cm−1. Hence, concentration of aminochrome in micro molar = absorbance at 480 nm/molar extinction coefficient × 104 [21].
Cell culture and treatments
The different cell lines were cultured as described previously [22]. The cells were pre-incubated for 2 hours with the plant extracts dissolved in DMSO and further diluted in the culture medium before addition of aminochrome (final concentration: 75 μM) or aminochrome alone (negative control). Nicotine, a protector against aminochrome toxicity, served as a positive control [12]. The concentration of aminochrome (75 μM) was based on the previous study showing significant toxicity of this concentration after 24 h in the same cells lines [15]. After treatment, cell death/viability assay was measured as described previously.
Evaluation of the effect of plant extracts on aminochrome-induced mitochondrial membrane-potential impairment
The evaluation of mitochondria membrane potential was done according to the MitoProbe™ JC-1 Assay Kit. The cells lines were treated as described above for 24 h at 37°C before analysis with flow cytometry apparatus (FACSCalibur, BD Biosciences, USA). The ratio of red to green fluorescence was determined.
Statistical Analysis
All data are expressed as mean ± SD values. Statistical significance was assessed using analysis of variance (ANOVA) for multiple comparisons. Post hoc test was conducted using to distinguish which groups differed specifically P < 0.05 was set a priori as significant.
Results
An initial study was performed using various concentrations of the plant extracts to determine an optimal (non-toxic) concentration of each extract. The human astrocytoma cell line U373MG and U373MGsiGST6 (U373MG cells expressing a siRNA against GSTM2 with only 26% of GSTM2 expression) were used. The incubation of these cells with higher concentrations of the extracts (e.g. 50 μg/ml or 100 μg/ml of D. edulis, A. muricata, A. senegalensis) for 24 hours induced significant cell death. However, at 12.5 μg/ml or 25 μg/ml no toxicity compared to control (DMSO at 0.2 %) was observed (Data not shown). A. laxiflora, on the other hand had no toxicity at 0.1 and 1.0 μg/ml. Hence these lower concentrations were used in subsequent studies to determine potential protection against aminochrome-induced cell death and mitochondrial membrane potential impairments.
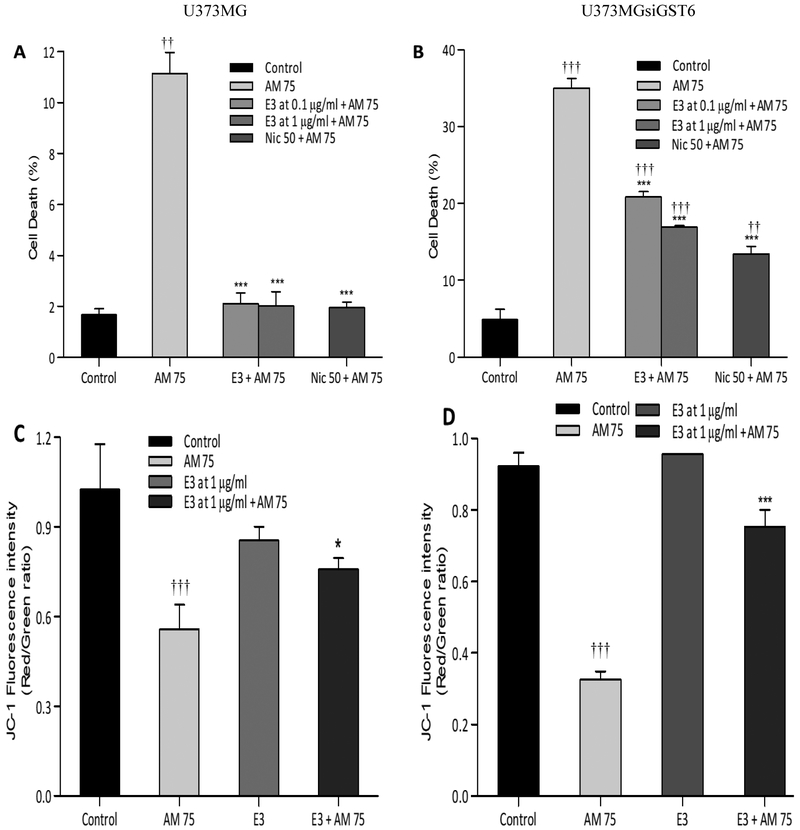
A. Laxiflora at both 0.1 and 1.0 μg/ml caused significant protection in both U373MG and U373siGST6 cells. Thus, the toxicity and U373siGST6, respectively) was reduced by A. Laxiflora to (2.1% and 20.8%, respectively with 0.1 μg/ml, P<0.001) and to (2.0% and 16.9%, respectively with 1.0 μg/ml, P 0.001) [Figure 1A and B]. We then measured the mitochondrial membrane potential using 1.0 μg/ ml A. laxiflora. Whereas 75 μM aminochrome caused significant reduction in membrane potential in both cell lines (0.55 and 0.32, respectively, P 0.001), A. laxiflora caused significant increase in membrane potential in both cell lines (0.75 and 0.78, respectively, P < 0.05 and P < 0.001). Hence A. laxiflora at concentration of 1.0 μg/ml protected against aminochrome induced impairments in mitochondrial membrane potential [Figure 1C and D].
Figure 1:
Effect of Alchornea laxiflora (E3) extracts on aminochrome-induced cell death (A: U373MG; B: U373MGsiGST6) and mitochondrial membrane- potential impairment (C: U373MG; D: U373MGsiGST6). The pre-incubation with 0.1 μg/ml and 1 μg/ml of A. laxiflora extracts for 2 hours significantly protected the cells against toxicity induced by aminochrome (AM 75 μM). The statistical significance was assessed using analysis of variance (ANOVA) for multiple comparisons (*P<0.05; ***P< 0.001) compared to AM 75 and (†P<0.05; †††P< 0.001) compared to control (DMSO at 0.2%). Nic 50 (Nicotine at 50 μM was used as a positive control).
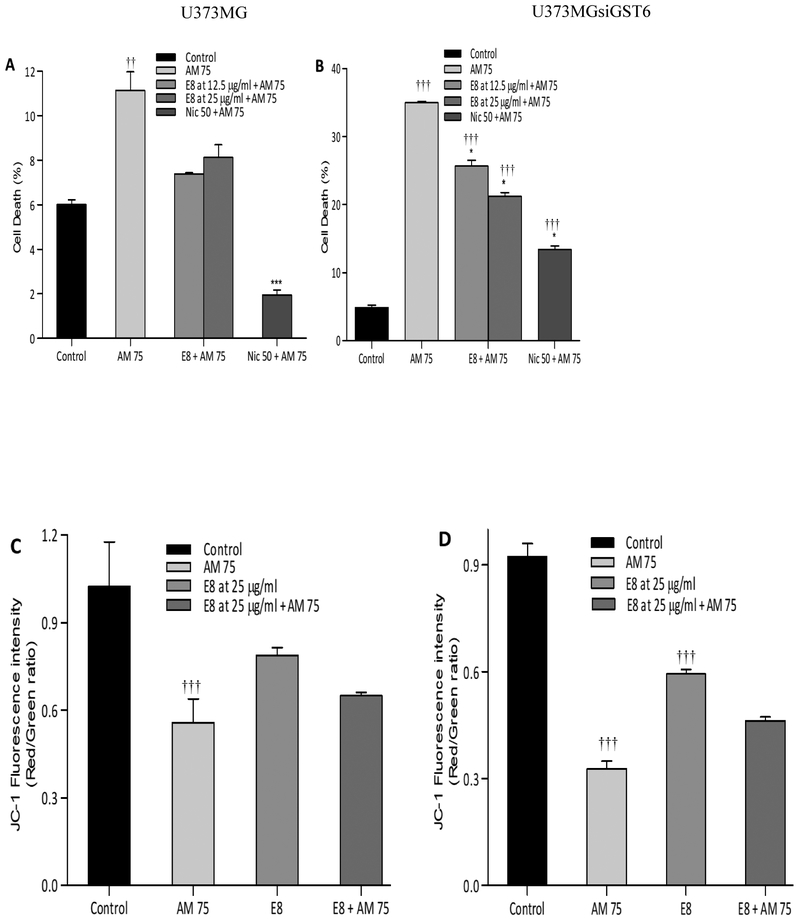
D. Edulis at both 12.5 and 25 μg/ml caused significant protection in both U373MG and U373siGST6 cells. Thus, the toxicity induced by 75 μM aminochrome was reduced by D. edulis to (7.8% and 25.6%, respectively with 12.5 μg/ml, P<0.05 - P<0.001) and to (8.3% and 16.9%, respectively with 25 μg/ml, P<0.05 - P 0.001) (Figure 2A and B). In this case, however, no significant increase in mitochondrial membrane potential was observed following either concentration of D. dulis [Figure 2C and D].
Figure 2:
Effect of Dacryodes edulis (E8) extracts on aminochrome-induced cell death (A: U373MG; B: U373MGsiGST6) and mitochondrial membrane-potential impairment (C: U373MG; D: U373MGsiGST6). The statistical significance was assessed using analysis of variance (ANOVA) for multiple comparisons (***P< 0.001) compared to AM 75 and (†††P< 0.001) compared to control (DMSO at 0.2%). Nic 50 (Nicotine at 50 μM was used as a positive control).
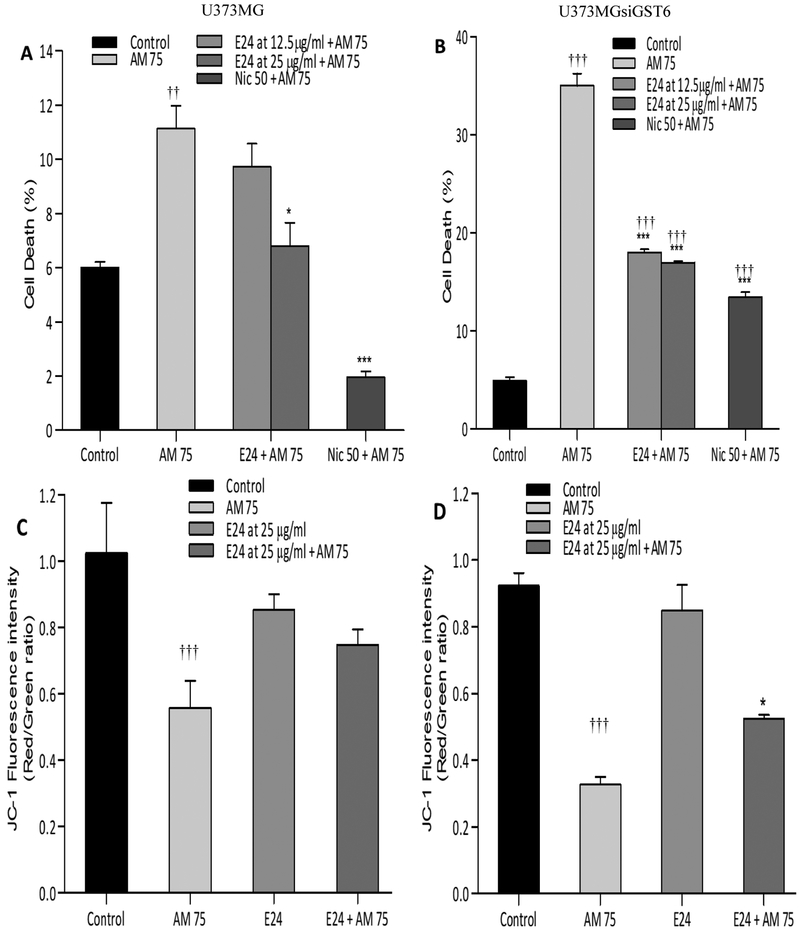
A. Muricata at both 12.5 and 25 μg/ml caused significant protection in U373MG and/or U373siGST6 cells. Thus, the toxicity induced by 75 μM aminochrome was reduced by A. muricata to (9.8% and 17.9%, respectively with 12.5 μg/ml, NS (non-significant) and P<0.001) and to (7.0% and 16.9%, respectively with 25 μg/ml, P<0.05 - P<0.001) [Figure 3A and B]. In this case, only the higher concentration of A. muricata caused a significant increase in mitochondrial membrane potential [Figure 3C and D].
Figure 3:
Effect of Knnona muricata (E24) extracts on aminochrome-induced cell death (A: U373MG; B: U373MGsiGST6) and mitochondrial membrane-potential impairment (C: U373MG; D: U373MGsiGST6). The statistical significance was assessed using analysis of variance (ANOVA) for multiple comparisons (*P 0.05; ***P< 0.001) compared to AM 75 and (††P 0.01; †††P 0.001) compared to control (DMSO at 0.2%). Nic 50 (Nicotine at 50 μM was used as a positive control).
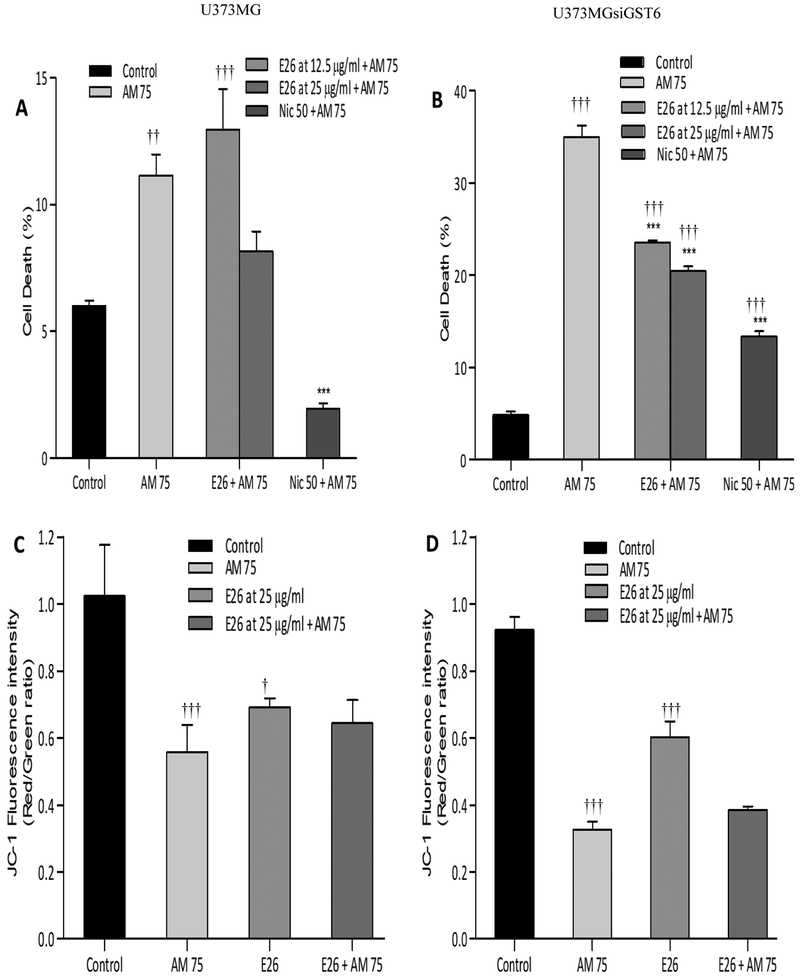
A. Senegalensis at 12.5 and 25 μg/ml caused significant protection in U373siGST6 cells only. Thus, the toxicity induced by 75 μM aminochrome was reduced by A.senegalensis to (23.5% and 20.4% P<0.001) [Figure 4A and B]. However, similar to D.edulis no effect on mitochondrial membrane potential was observed [Figure 4C and D].
Figure 4:
Effect of Annona senegalensis (E26) extracts on aminochrome-induced cell death (A: U373MG; B: U373MGsiGST6) and mitochondrial membrane-potential impairment (C: U373MG; D: U373MGsiGST6). The statistical significance was assessed using analysis of variance (ANOVA) for multiple comparisons (***p< 0.001) compared to AM 75 and (†P 0.05; ††P 0.01;†††P 0.001) compared to control (DMSO at 0.2%). Nic 50 (Nicotine at 50 μM was used as a positive control).
Discussion
The results of this study indicate that crude extracts of several native plants to Cameroon can protect against amiochrome-induced toxicity in intact human astrocytoma cells and astroyomas that were depleted up to 74% from glutathione transferase M2. Since GSTM2 as endogenous enzyme can protect against aminochrome induced toxicity by conjugating it with glutathione, the results strongly suggest induced by 75 μM aminochrome (11.1% and 35.0% in U373MG and that the crude extracts of the plants may at least partially exert their protective property through enhancement of this enzyme. Thus, extracts of the 4 plants: Alchornea laxiflora, Dacryodes edulis, Annona muricata and Annona senegalensis were all effective in reducing aminochrome toxicity and since aminochrome has been implicated in PD pathology, the results further suggest potential utility of these plant extracts in PD.
It is of relevance to note that dopamine itself by causing oxidative stress, may in certain circumstances contribute to selective degeneration of dopaminergic neurons of the substantia nigra [23]. Hence, when there is an excess amount of cytosolic DA it can undergo auto-oxidation to generate reactive oxygen species (ROS). ROS by altering mitochondrial respiration can induce changes in the permeability of brain mitochondria and hence cause damage to the neuron [24]. The auto-oxidation of DA may also produce quinones that can be oxidized to aminochrome, whose redox-cycling leads to the generation of the superoxide radicals and ultimate accumulation of neuromelanin, which may cause considerable toxicity to substantia nigra neurons [11, 23-27]. This toxicity may be mediated via PD-related proteins, such as α-synuclein and parkin, superoxide dismutase-2 (SOD2), inactivation of the DA transporter (DAT) and the rate limiting tyrosine hydroxylase (TH) enzyme as well as mitochondrial dysfunction [28].
As alluded to before, mitochondrial dysfunction is also closely related to increased ROS formation. Thus, complex I deficiencies of the respiratory chain in mitochondria account for the majority of neural apoptosis, which is considered a primary source of ROS in PD. Interestingly, enhanced production of ROS can inhibit complex I, resulting in a vicious cycle of positive feed forward mechanism. Moreover, oxidative phosphorylation, the main mechanism used by the mitochondria to form ATP can lead to formation of superoxide and hydrogen peroxide free radicals hence contributing to further disease progression [28, 29]. This scenario may account for the preferential cytotoxicity to the DA neurons by complex I inhibitors such as MPTP or rotenone [28]. In addition, ROS can interfere with elimination of damaged proteins leading to protein misfolding (e.g. generation of α-synuclein) which is believed to play a key role in the pathology of PD [30].
Although potential interaction of the examined extracts with protein misfolding is yet to be investigated, our results do suggest interaction of at least one of the extracts, i.e. Alchornea laxiflora and to some extent the higher concentration of Annona muricata with mitochondrial function. Thus, these specific extracts reduced the damage to mitochondrial membrane potential brought about by aminochrome exposure. However, the extent to which this interaction might contribute to their protective mechanism is also subject to further elucidation.
It is also noteworthy that the compounds tested in our experimental model, in majority of cases did not fully reverse the damage induced by aminochrome. Hence, further purification of the active components and examination of combination treatments of such extracts with other established or novel drugs (e.g. nicotine) would be of significant merit [31].
Conclusion
We conclude that crude extracts of several native plants to Cameroon such as Alchornea laxiflora, Dacryodes edulis, Annona muricata and Annona senegalensis can protect against amiochrome-induced toxicity in human astrocytoma cells. Moreover, two of these compounds (e.g. Alchornea laxiflora and Annona muricata) also prevented mitochondrial dysfunction induced by aminochrome. Further identification of the active components of these extracts and more detailed elucidation of their mechanism of action and potential utility in PD is warranted.
Acknowledgements
This work was supported by the International Center for Genetic Engineering and Biotechnology (ICGEB) [grant number S/CMR17-01](VNN); FONDECYT 1170033 (JSA) and NIH/NIAAA R03AA022479 (YT). The authors thank the Department of Molecular & Clinical Pharmacology, Faculty of Medicine, ICBM, University of Chile for providing the facilities to carry out this research.
References
- 1.Rudnick DA, Davidson NO. Functional relationships between lipid metabolism and liver regeneration. Int J Hepatol. 2012; 549241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wirdefeldt K, Adami H-O, Cole P, Trichopoulos D, Mandel J. Epidemiology and etiology of Parkinson’s disease: a review of the evidence. Eur J Epidemiol. 2011, 26:S1–58. [DOI] [PubMed] [Google Scholar]
- 3.Dexter DT, Jenner P. Parkinson disease: from pathology to molecular disease mechanisms. Free Radic Biol Med. 2013; 62: 132–144. [DOI] [PubMed] [Google Scholar]
- 4.Dorszewska J, Prendecki M, Lianeri M and Kozubski W. Molecular Effects of L-dopa Therapy in Parkinson’s Disease. Curr Genomics. 2014; 15:11–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Segura-Aguilar J. Neurotoxins as Preclinical Models for Parkinson's Disease. Neurotox Res. 2018; 34:870–877. [DOI] [PubMed] [Google Scholar]
- 6.Williams A. MPTP toxicity: clinical features. J Neural Transm Suppl. 1986; 20:5–9. [PubMed] [Google Scholar]
- 7.Braak H, Ghebremedhin E, Rüb U, Bratzke H, Tredici K. Stages in the development of Parkinson’s disease-related pathology. Cell Tissue Res. 2004; 318: 121–134. [DOI] [PubMed] [Google Scholar]
- 8.Segura-Aguilar J, Paris I, Muñoz P. The need of a new and more physiological preclinical model for Parkinson's disease. Cell Mol Life Sci. 2016; 73: 1381–1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Segura-Aguilar J. Can we conclude a potential therapeutic action for Parkinson's disease by using postmortem tissue and a preclinical model based on an exogenous neurotoxin? Cell Death Dis. 2018; 9:748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zecca L, Fariello R, Riederer P, Sulzer D, Gatti A, Tampellini D. The absolute concentration of nigral neuromelanin, assayed by a new sensitive method, increases throughout the life and is dramatically decreased in Parkinson's disease. FEES Letters 2002; 510:216–220. [DOI] [PubMed] [Google Scholar]
- 11.Segura-Aguilar J, Paris I. Mechanisms of Dopamine Oxidation and Parkinson’s Disease In: Handbook of Neurotoxicity. Edited by Kostrzewa RM. New York, NY: Springer New York; 2014: 865–883. [Google Scholar]
- 12.Segura-Aguilar J. On the role of endogenous neurotoxins and neuroprotection in Parkinson's disease. Neural Regen Res. 2017; 12:897–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ben Haim L, Carrillo-de Sauvage M-A, Ceyzériat Kand Escartin C. Elusive roles for reactive astrocytes in neurodegenerative diseases. Front Cell Neurosci. 2015; 9:278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baez S, Segura-Aguilar J, Widersten M, Johansson AS, Mannervik B. Glutathione transferases catalyse the detoxication of oxidized metabolites (o-quinones) of catecholamines and may serve as an antioxidant system preventing degenerative cellular processes. Biochem J. 1997; 324:25–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Segura-Aguilar J, Baez S, Widersten M, Welch CJ, Mannervik B. Human class Mu glutathione transferases, in particular isoenzyme M2-2, catalyze detoxication of the dopamine metabolite aminochrome. J Biol Chem. 1997; 272:5727–5731. [DOI] [PubMed] [Google Scholar]
- 16.Huenchuguala S, Muñoz P, Zavala P, Villa M, Cuevas C, Ahumada U, et al. Glutathione transferase mu 2 protects glioblastoma cells against aminochrome toxicity by preventing autophagy and lysosome dysfunction. Autophagy. 2014; 10:618–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elujoba AA, Odeleye OM, Ogunyemi CM. Traditional medicine development for medical and dental primary health care delivery system in Africa. African Journal of Traditional, Complementary Alternative Medicines. 2005; 2:46–61. [Google Scholar]
- 18.Kuete V, Efferth T. Cameroonian medicinal plants: pharmacology and derived natural products. Front Pharmacol. 2010; 1:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ebrahimi A, Schluesener H: Natural polyphenols against neurodegenerative disorders: Potentials and pitfalls. Ageing Res Rev. 2012; 11:329–345. [DOI] [PubMed] [Google Scholar]
- 20.Paris I, Perez-Pastene C, Cardenas S, Iturriaga-Vasquez P, Munoz P, Couve E, et al. Aminochrome induces disruption of actin, alpha- and beta-tubulin cytoskeleton networks in substantia-nigra derived cell line. Neurotox Res. 2010; 18: 82–92. [DOI] [PubMed] [Google Scholar]
- 21.Segura-Aguilar J, and Lind C. On the mechanism of the Mn3(þ)-induced neurotoxicity of dopamine: prevention of quinone-derived oxygen toxicity by DT diaphorase and superoxide dismutase. Chem Biol Interact. 1989; 72:309–324. [DOI] [PubMed] [Google Scholar]
- 22.Muñoz P, Huenchuguala S, Paris I, Cuevas C, Villa M, Caviedes P, et al. Protective Effects of Nicotine Against Aminochrome-Induced Toxicity in Substantia Nigra Derived Cells: Implications for Parkinson’s Disease. Neurotox Res. 2012; 22:177–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Segura-Aguilar J, Paris I, Muñoz P, Ferrari E, Zecca L and Zucca FA. Protective and toxic roles of dopamine in Parkinson’s disease. J Neurochem. 2014; 129: 898–915. [DOI] [PubMed] [Google Scholar]
- 24.Zucca FA, Basso E, Cupaioli FA, Ferrari E, Sulzer D, Casella L, et al. Neuromelanin of the human substantia nigra: an update. Neurotox Res. 2014; 25:13–23. [DOI] [PubMed] [Google Scholar]
- 25.Zucca FA, Segura-Aguilar J, Ferrari E, Muñoz P, Paris I, Sulzer D, et al. Interactions of iron, dopamine and neuromelanin pathways in brain aging and Parkinson's disease Prog Neurobiol. 2017; 155:96–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zucca FA, Vanna R, Cupaioli FA, Bellei C, De Palma A, Di Silvestre D, et al. Neuromelanin organelles are specialized autolysosomes that accumulate undegraded proteins and lipids in aging human brain and are likely involved in Parkinson’s disease. NPJ Parkinson’s Disease. 2018; 4:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sulzer D, Cassidy C, Horga G, Kang UJ, Fahn S, Casella L, et al. Neuromelanin detection by magnetic resonance imaging (MRI) and its promise as a biomarker for Parkinson's disease. NPJ Parkinson’s Disease. 2018; 4:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blesa J, Trigo-Damas I, Quiroga-Varela A, Jackson-Lewis VR. Oxidative stress and Parkinson’s disease. Front Neuroanat. 2015; 9:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Segura-Aguilar J. Aminochrome as preclinic model for Parkinson's disease. Oncotarget 2017, 8:45036–45037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schapira AHV, Cooper JM, Dexter D, Jenner P, Clark JB, and Marsden CD. Mitochondrial complex I deficiency in Parkinson’s disease. J Neurochem. 1990; 54: 823–827. [DOI] [PubMed] [Google Scholar]
- 31.Tizabi Y, Getachew B. Nicotinic Receptor Intervention in Parkinson's disease: Future Directions. Clin Pharmacol Transl Med. 2017; 1:14–19. [PMC free article] [PubMed] [Google Scholar]