Abstract
The ability of poly(anhydride-esters) composed of non-steroidal anti-inflammatory drugs that biodegrade to salicylic acid (SA) and adipic acid to prevent colonization by Pseudomonas aeruginosa and their effects on the foreign-body response were studied in vitro and in vivo, respectively. Soluble SA in bacterial medium at concentrations up to 300 mg/L did not affect the growth rate or viability of P. aeruginosa, indicating that SA does not exhibit a direct toxicity effect on the bacterium. Batch degradation rates of the salicylate-based polymer in the presence of an actively growing bacterial culture only marginally (14%) increased relative to polymer degradation rates in sterile medium. Short-term (3 h) bacterial adhesion studies in agitated batch systems indicated a 47% reduction in the rate of P. aeruginosa adhesion relative to a control polymer that does not release SA upon biodegradation. Long-term (3-day) biofilm accumulation studies indicated a dramatic reduction in biofilm formation on salicylate-based polymer versus controls. A recombinant P. aeruginosa pMHLAS, containing a fluorescent reporter gene prior to the las regulon, was employed to determine whether salicylate-based polymer prevents biofilm formation by the released SA inhibiting quorum sensing pathways. Long-term biofilm accumulation studies with P. aeruginosa pMHLAS insinuate that salicylate-based polymer prevents biofilm accumulation by inhibiting the las quorum sensing system. Furthermore, unlike control polymer, salicylate-based polymer implanted subcutaneously for a period of 4 weeks-resisted cell-mediated degradation and remained intact. Histological and immunohistochemical analysis indicated a reduction in overall encapsulation and paucity of macrophages in the area of the salicylate-based polymer implant.
Keywords: Biodegradable poly(anhydride-esters), Anti-biofilm control, Pseudomonas aeruginosa, Foreign-body response
1. Introduction
It is estimated that over 3 million artificial or prosthetic parts are implanted per annum in the United States alone [1,2]. Bacterial colonization and biofilm formation on any substratum proceeds by a series of complex physical and biological processes. The body reacts to prosthetic implants by first coating them with a film composed of various proteins (e.g., fibronectin, laminin, fibrin, collagen, immunoglobulins), some or all of which can serve as receptors for colonizing bacteria. Bacteria, transported to the substratum by either molecular diffusion or convective transport, can adhere by either a nonspecific adhesion mechanism governed by electrostatic forces acting between the cell and surface or by a specific adhesion binding reaction. Once attached to the substratum, bacteria can secrete copious amounts of insoluble extracellular polymers (e.g., polysaccharides, DNA), forming a tenacious three-dimensional matrix, termed a “biofilm” [3,4]. These bacterial polymers can mix with the extracellular polymers of other species, host cells, or blood platelets to form a mixed-cell line biofilm that is highly resistant to host immune defenses and rigorous antibiotic challenges [5,6].
To prevent biomaterial-related infections, current clinical strategy is to treat patients with antibiotics at high systemic concentrations. Since this approach is only marginally successful [7,8], the inevitable recourse is the surgical removal of the infected implant. A better approach is to provide a specific, localized anti-biofilm therapy, immediately upon implantation and directly at the site where it is most required—the material/body interface. Here, we address this significant scientific and social issue by designing implantable, self-healing biomaterials that biodegrade into bioactive molecules (i.e., non-steroidal anti-inflammatory drugs (NSAIDs)) that locally inhibit bacterial colonization and minimize inflammation. Specifically, poly(anhydride-esters) that biodegrade into salicylic acid (SA) [9,10], the hydrolysis product of aspirin [11], were evaluated for their ability to prevent bacteria adhesion and reduce foreign-body response. This series of polymers was originally designed to minimize pain and inflammation by locally releasing SA following implantation [12,13]. This manuscript describes how these polymers that biodegrade into SA—referred to as salicylate-based polymers—serendipitously prevent biofilm accumulation.
2. Materials and methods
2.1. Disk preparation
Synthesis of SA-derived poly(anhydride-ester) [14,15] and the inactive polyanhydride (control) [16] are detailed elsewhere [14-16]. These papers describe at length the synthesis of a poly(anhydride-ester) composed of alkyl chains linked by ester bonds to aromatic moieties of SA. The polymer, by design, undergoes hydrolytic degradation to release SA and adipic acid. Pellets of inactive (control) and active salicylate-based polymer (Fig. 1) were prepared by pressing ground polymers (~181 ± 23 mg) into 13 mm diameter × 1 mm thick disks for the in vitro studies and 6mm diameter × 3 mm thick disks (106±11 mg) for the in vivo studies in an IR pellet die (International Crystal Laboratories, Garfield, NJ) with a bench-top hydraulic press (Carver model M, Wabash, IN]. A pressure of 5000 psi was applied for 5 min at room temperature. No change in polymer color was observed upon applying pressure.
Fig. 1.
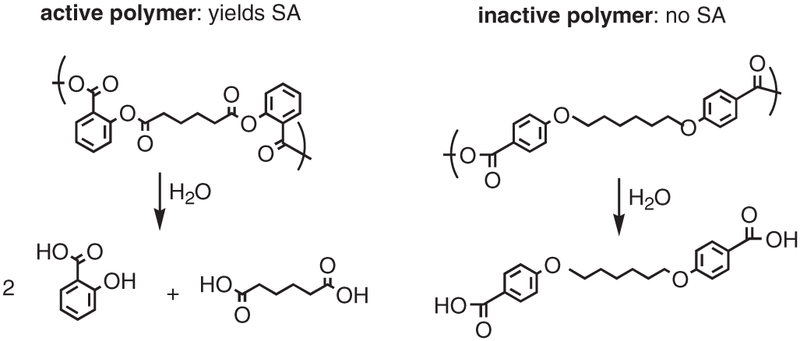
Biodegradation of active poly(anhydride-esters), referred to as salicylate-based polymers, releases salicylic acid (SA) and adipic acid compared with the biodegradation of inactive, control polyanhydride.
2.2. Bacterial strains and media
Wild-type Pseudomonas aeruginosa PAO1 (ATCC number BAA-47) was purchased from ATCC. A recombinant P. aeruginosa pMHLAS strain was obtained (courtesy of S. MØlin, Technical University Copenhagen, Denmark) and previously described [17]. The quorum sensing reporter system, pMHLAS, was constructed by a two-step cloning procedure. The PlasBgfp(ASV) translational fusion was made by amplifying a 348 bp PCR product starting 345 bp upstream of the lasB initiation codon, using the primers lasB fwd and lasB rev and chromosomal DNA of P. aeruginosa PAO1 as template. The PCR fragment was subsequently digested with XbaI and SphI and inserted into the corresponding site of pMH391 [17]. This gave rise to the plasmid pMHLB, which carries the translational PlasB-gfp(ASV) followed by translational stop codons in all three reading frames and two strong transcriptional terminators [18]. According to Henzter [17], the reporter cassette was inserted at random positions in the chromosomes of P. aeruginosa PAO1 by triparental mating. The selected transconjugants with random insertion of the mini-Tn5 elements showed no sign of phenotypic changes compared to the parental strains when cultivated in liquid medium or in biofilms. Should the las regulon be activated by homoserine lactones, then the activated cells would also express GFP and fluoresce.
Stock cultures of P. aeruginosa strains were kept at 4 °C on trypticase soy broth (TSB; Gibco, Co.) agar slants (for pMHLAS strains, agar contained 60 μg/mL gentamicin and 100 μg/mL ampicillin). Colonies were subcultured every 2–4 weeks to maintain culture viability. Before a bacterial challenge to the salicylate-based polymer samples, microorganisms were grown in pure culture overnight in 1 mL of trypticase soy broth (TSB, 10 g/L), started via loop inoculation from TSB plates. These cultures were used to subsequently inoculate a sterile 250 mL Erlenmeyer flask containing 50 mL of 10 g/L TSB that was then placed in a temperature-controlled rotary shake incubator at 37 °C. Typical growth curves for each bacterial strain were essentially undistinguishable and are available upon request.
Effects of soluble SA on suspended cultures of P. aeruginosa PAO1 were carried out as above but with culture media augmented with SA (Sigma, St. Louis, MO) with concentrations ranging from 10 to 300 mg/L (Fig. 2). Based upon the size and mass of the salicylate-based polymer disks, the active polymers contain 139.9 ± 2.1 mg of SA. Based on measured degradation rates, bacterial suspended cultures exposed to salicylate-based polymers did not experience ambient SA concentrations ⩾ 200 mg/L. Therefore, 300 mg/L is a concentration far in excess of any level attainable by the degradation of a single salicylate-based polymer disk in 10 mL fluid over the length of these experiments. Suspended cell concentrations were determined by direct cell count on samples withdrawn periodically from the suspended cultures. Samples were instantly filtered through 0.1 μm black polycarbonate membrane (Nuclepore, Whatman, Chicago, IL), stained using the Live/Dead Bac-Light™ assay (Molecular Probes, Salem OR). Live and dead cells were counted as per manufacturer’s instructions using Zeiss Axophot epi-fluoresecence microscope with a 100 × oil immersion objective. Live cells fluoresce green while any dead cells fluoresce red.
Fig. 2.
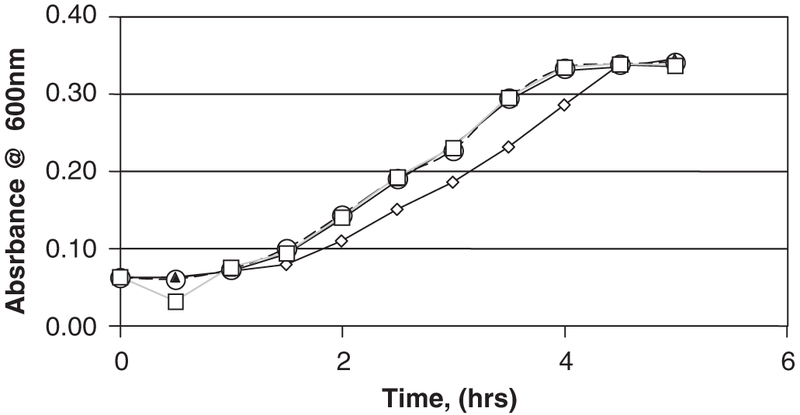
Suspended growth of P. aeruginosa wild-type in culture medium with various levels of soluble salicylic acid. ◇ = medium + 300 mg/L salicylic acid, ◯ = medium + 30.0 mg/L salicylic acid, ▲ = medium + 3.0 mg/L salicylic acid, and □ = medium alone.
To ascertain whether bacterial growth in the fluid phase would affect salicylate-based polymer degradation rates, release rates of SA from salicylate-based polymer were also quantified in the presence of actively growing suspended bacterial cultures of P. aeruginosa.
2.3. Bacterial challenges
2.3.1. Bacterial adhesion kinetics
For bacterial adhesion-only studies, salicylate-based polymer disks (1 mm thick, 13 mm diameter) in 10 mL scintillation vials were submerged with a suspension of P. aeruginosa PAO1 in buffered saline at a concentration of 1 × 106 cells/mL for time periods less than 3 h. At a designated time, contents of the vial were infinitely diluted with a stream of sterile buffered saline and polymer disks removed. Disks were placed into sterile buffer (5 mL) and sonicated for 30 s to suspend any attached bacterial cells. Resultant cell suspensions were vacuum-filtered through a 0.10 μm pore diameter black polycarbonate membrane and stained using the Live/Dead Bac-Light™ assay (Molecular Probes, Salem OR), and living and dead cells counted as per manufacturer’s instructions using Zeiss Axophot epi-fluoresecence microscope with a 100 × oil immersion objective. Live cells fluoresce green while any dead cells fluoresce red. All experiments were repeated at least in triplicate.
2.3.2. Biofilm formation
Salicylate-based polymer disks (1 mm thick × 13 mm diameter disc) were submerged in sterile medium and inoculated with P. aeruginosa PAO1. Every 8 h over a 3-day period, spent mdium was replaced with sterile fresh medium (5 mL), thus cultivating any adherent cultures in a semi-continuous fashion. The number of live/dead/total bacterial cells was measured daily as described above, in solution as well as directly on the salicylate-based polymer disk surfaces. The pH was periodically measured by micro-pH probe (Lazar Research Laboratories, Los Angeles, CA). Soluble SA concentration in the medium, released from the tablets, was also measured by collecting 0.5 mL samples for spectrophotometer readings at 235 nm. All experiments were repeated at least in triplicate.
2.3.3. Quorum sensing regulation
Increasing evidence indicates that cell-to-cell communication plays a crucial role for the maturation of biofilms, i.e., for the development of characteristic three-dimensional biofilm architecture. Although several signal molecule families have been identified in Gram-negative bacteria, the most intensively studied and best understood are those that belong to the N-acylhomoserine lactone (AHL) family. AHLs contain a homoserine lactone ring attached via an amide bond to an acyl side chain containing from 4 to 14 carbons. Two AHL-mediated quorum sensing circuits have been identified in P. aeruginosa. The las system consists of lasI, an AHL synthase gene responsible for the synthesis of OdDHL [N-(3-oxo-dodecanoyl)-l-homoserine lactone; 3-oxo-C12-HSL; PAI-1] [19], and lasR which encodes a LuxR-type transcriptional regulator protein [20,21]. The las system has been shown to regulate the expression of several virulence factors, such as extracellular enzymes (LasB elastase, LasA protease, alkaline protease), secondary metabolites (pyocyanin, hydrogen cyanide, pyoverdin), toxins (exotoxin A) and lasI itself. In the rhl system, the rhlI gene product directs the synthesis of BHL (N-butanoyl-l-homoserine lactone; C4-HSL; PAI-2), which, in conjunction with the rhlR gene product, activates transcription of the rhlAB rhamnolipid biosynthesis genes and the rhlI gene itself. The rhl system is also involved in modulating the expression of several of the virulence factors controlled by the las system [22,23]. Several studies using homoserine lactone-like analogs (furanones) have successfully negated biofilm formation [17].
The similarity of (a) the molecular structure of SA to certain furanones and (b) reported biofilm inhibition by SA without toxicity suggests that SA may inhibit biofilm formation by also interfering in homoserine lactone quorum sensing. Biofilm formation studies were repeated as described in Section 2.3.2 using the recombinant P. aeruginosa pMHLAS strain. Should SA inhibit the las regulon system, then SA will also affect the expression of the GFP reporter gene.
2.4. Foreign-body response studies
Two polymer systems were compared—the active polymer (i.e., salicylate-based polymer) and an inactive (inert) polyanhydride as our polymer control. For the following studies, a total of ten, 3–4 months of age, control mice (C57/Bl6 genetic background) were used for implantations. Each mouse received two implants (6 mm diameter, 3 mm thick) allowing for 10 implants per material. Polymer pellets (both active and inert) were sterilized under UV light and implanted subcutaneously in the dorsal region, through a midline incision. After 4 weeks, the animals were sacrificed and the polymers were retrieved and fixed in 10% zinc-buffered formalin for 24 h. Following processing and embedding, 6-μm sections were stained with Masson’s trichrome. Immunolocation of macrophages was performed with the Mac3 antibody as described previously [24]. All secondary peroxidase-conjugated antibodies were used at a dilution of 1:200. Controls included sections treated with pre-immune sera.
3. Results
3.1. Soluble salicylic acid effects on bacterial growth rates
Effects of soluble SA on suspended cultures of P. aeruginosa PAO1 were carried out with culture media augmented with SA at concentrations ranging from 10 to 300 mg/L. Representative results, summarized in Fig. 2, indicate that at concentrations below ~300 mg/L, SA does not exhibit any detrimental effects on the growth of P. aeruginosa. Note that 300 mg/L is a concentration far in excess of any level attainable by the degradation of a single salicylate-based polymer tablet in 10 mL fluid. Consequently, any retardation in P. aeruginosa colonization on salicylate-based polymer materials cannot be attributed to a growth-inhibitory or antibiotic effect of SA alone.
3.2. Polymer degradation rates
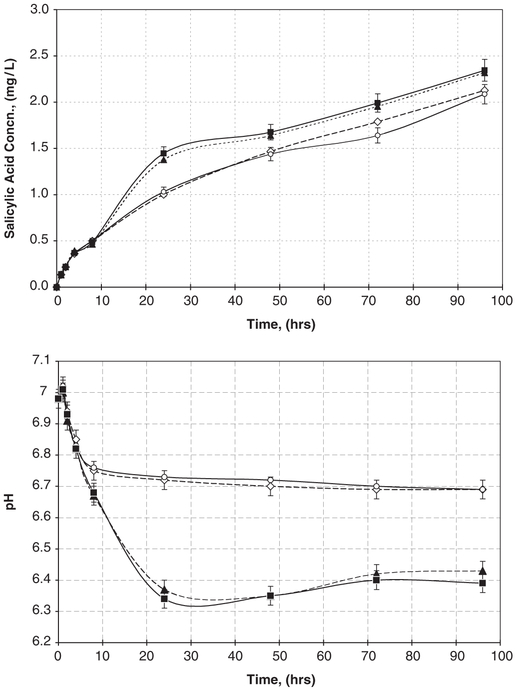
To ascertain whether bacterial growth in the fluid phase would affect salicylate-based polymer degradation rates, release rates of SA were also quantified in the presence of actively growing suspended bacterial cultures of P. aeruginosa. Samples of the liquid phase in which the salicylate-based polymer wafer was submerged were collected periodically, and SA concentrations, bacterial cell concentrations, and pH of the media were determined. Representative release rates of SA in both the absence and presence of actively growing bacterial suspensions are depicted in Fig. 3. Release rates of SA were only slightly higher in the presence of actively growing suspended bacteria cultures versus sterile medium. The corresponding difference in pH seen in these experiments may not be entirely due to the release of salicylic and adipic acids but rather due to active microbial growth producing CO2 in a weakly buffered growth medium.
Fig. 3.
Salicylic acid concentration (top graph) and pH changes (bottom graph) resulting from the hydrolytic degradation of active salicylate-based polymers in growing bacterial culture (closed symbols; two replicate experiments ■ and ▲) and in sterile medium (open symbols; two replicate experiments ◇ and ◯). Each data point is an average of three separate samples.
3.3. Bacterial adhesion on salicylate-based polymer disks
For bacterial adhesion-only studies, polymer disks (1 mm thick, 13 mm diameter) in 15 mL scintillation vials were submerged (10 mL total liquid volume) with a suspension of P. aeruginosa PAO1 in buffered saline at a concentration of 1 × 106 cells/mL for time periods less than 3 h. To minimize cell growth and replication, no carbon source was provided in the medium. Results of P. aeruginosa PAO1 adhesion, to both salicylate-based polymer releasing SA and the biodegradable inactive control are shown in Fig. 4.
Fig. 4.
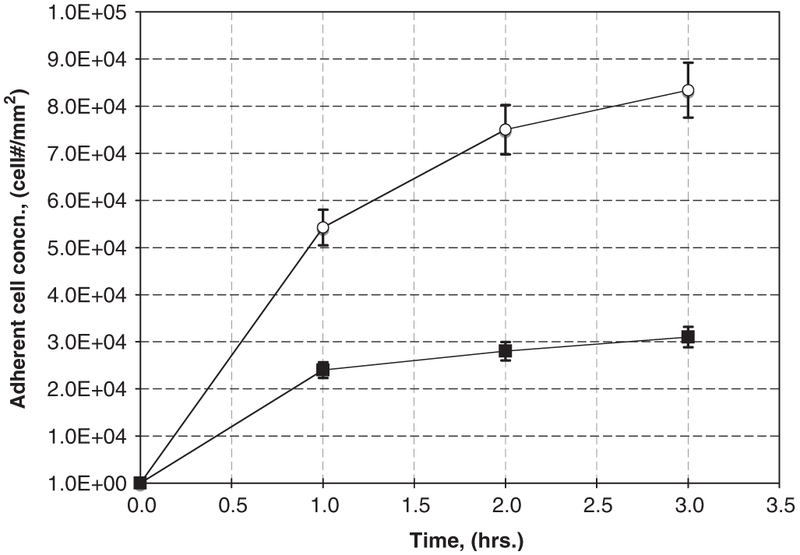
P. aeruginosa short-term adhesion-only studies with active salicylate-based polymer disks (■) relative to inactive control polymers that biodegrade but do not release SA (❍).
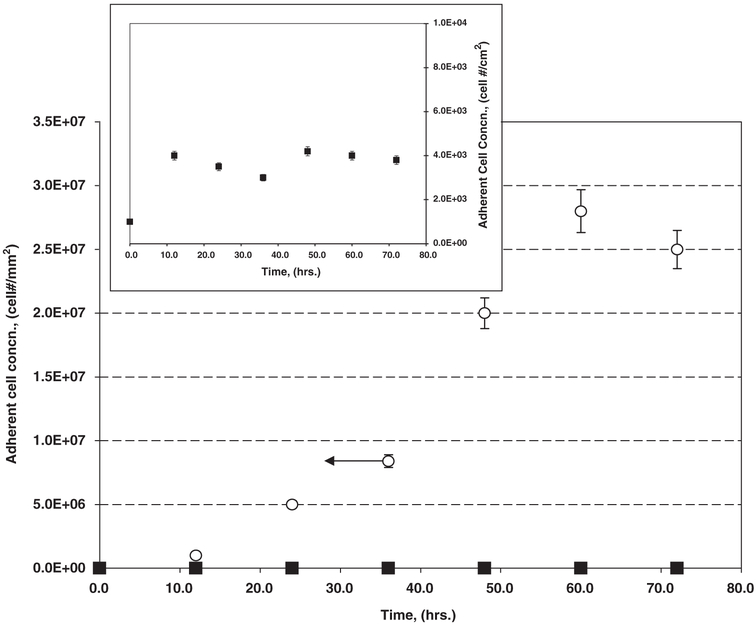
Fig. 4 illustrates adherent cell areal concentrations as a function of time for P. aeruginosa on either the biodegradable inactive controls or active salicylate-based polymer. Initial net accumulation rates (number of cells per time per area) were determined as per Poelstra et al. [25] and Busscher et al. [26], as the slope of the time course of adherent cell numbers per area at time = 0,
| (1) |
where Jo (cell Nr mm−2 s−1) is the initial rate of bacteria accumulation; X (t) (cell Nr mm−2) is the number of adherent cells per area at time t; and t (s) is the time. Ideally one would like to employ several early data points to estimate Jo. Here, we were able to use only 3–4 initial data points, thus introducing some error in the estimation of Jo. Consequently, these calculations can only be regarded as a qualitative comparison of initial accumulation. Initial net accumulation rates as per Eq. (1) were calculated as 14.7 and 6.9 cell/mm2/s, for control and salicylate-based polymer materials, respectively.
3.4. Biofilm formation on salicylate-based polymer disks
Salicylate-based polymer disks (1 mm thick × 13 mm diameter disc) were submerged in sterile medium and inoculated with P. aeruginosa PAO1. Experiments were carried out in triplicate. Every 8 h over a 3-day period, spent medium was replaced with sterile fresh medium, thus cultivating any adherent cultures in a semi-continuous fashion. The number of live/dead/total bacterial cells was measured, as described above, daily in solution as well as on the salicylate-based polymer disk surfaces. The pH and SA concentration in the medium were also measured.
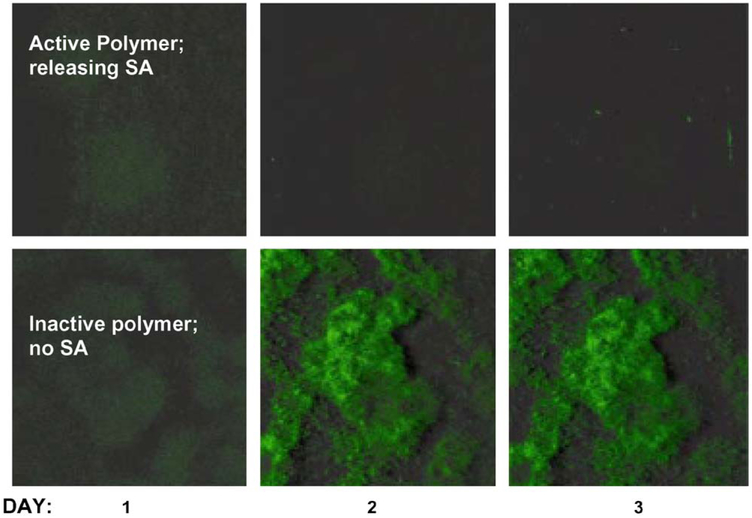
Fig. 5 summarizes a series of bacterial accumulation experiments on salicylate-based polymer and control biomaterials for P. aeruginosa PAO1 wild type. Photomicrographs of biofilm accumulation on salicylate-based polymer and control materials taken over the same 3-day period are shown in Fig. 6. Both results indicate a dramatic decrease in the overall amount of biofilm accumulation on the salicylate-based polymer versus the biodegradable, inactive control polymers.
Fig. 5.
Adherent cell accumulation by P. aeruginosa in long-term biofilm experiments performed in stirred batch systems comparing (major figure) active salicylate-based polymer disks (■) relative to inactive control polymers that biodegrade but do not release SA (❍). Inset provides the same adherent cell data for the salicylate-based polymers (■) on an expanded scale.
Fig. 6.
Time course photographs of biofilm formation on active salicylate-based polymers and inactive control polymer pellets.
3.5. Salicylate-based polymer effects on quorum sensing
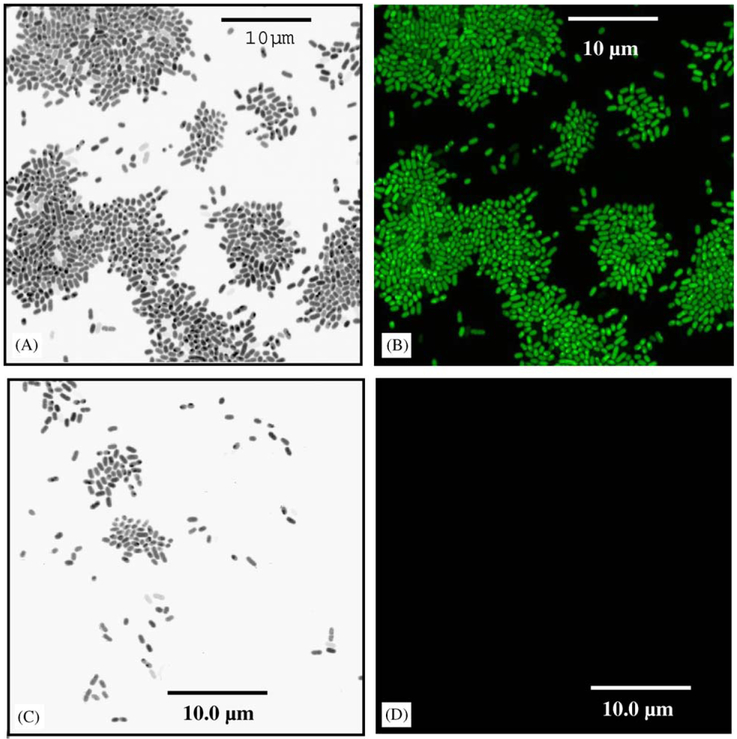
Studies using homoserine lactone-like analogs (furanones) have successfully negated biofilm formation [17]. Biofilm formation studies were repeated using the recombinant P. aeruginosa pMHLAS strain. Fig. 7 illustrates that P. aeruginosa pMHLAS accumulates to a much lesser degree on the SA-releasing polymers versus inactive, control polymers. Further, las regulon expression (as indicated by the GFP reporter gene expression) is clearly seen for the strain accumulating on the control biomaterials, while no such expression is seen in those few cells adsorbed to the salicylate-based polymer materials.
Fig. 7.
Influence of biodegrading polymers on las regulon activity in P. aeruginosa pMHLAS mutant: (A) Transmitted light micrograph of P. aeruginosa pMHLAS during the attachment phase of biofilm development on the inactive, control polymer; and (B) the same image under UV illumination indicating activation of the las regulon. (C) Transmitted light micrograph of P. aeruginosa pMHLAS during the attachment phase of biofilm development on active polymer, salicylate-based polymer that slowly releases salicylic acid upon biodegradation; and (D) the same image under UV illumination indicating inactivation of the las regulon.
3.6. In vivo foreign-body response
According to observation of Masson’s trichrome-stained histological sections (Fig. 8), salicylate-based polymers remained almost completely intact and allowed very low cell invasion (arrows in D). On the contrary, the inactive control polymer was extensively degraded and induced a large cellular influx (arrows in C). Semi-quantitative analysis indicated that approximately 80% of salicylate-based polymer was still intact, whereas only 20% of the control polymer remained after 4 weeks. In addition, the capsule surrounding salicylate-based polymer implants was loose and vascularized (Fig. 9B). Both observations suggest an alteration of the foreign-body response, an inflammation-driven process. Control implants displayed normal encapsulation with dense and largely avascular collagenous capsules (see Fig. 9A). Immunohistochemical analysis of sections with a macrophage-specific antibody revealed the presence of numerous macrophages in the capsule of control implants with many at the interface between the implantation site and the newly formed tissue (Fig. 9C). In contrast, macrophages were present at a significant distance from salicylate-based polymers, mostly at the outer edges of the foreign capsule (Fig. 9D).
Fig. 8.
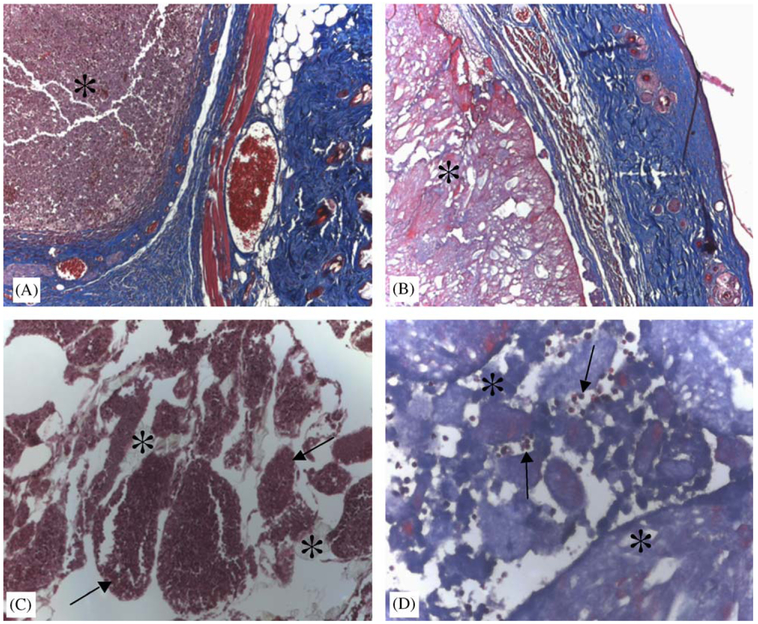
Salicylate-based polymer resists degradation and induces a limited foreign-body response. Area of initial polymer implantation is identified (*) in inactive control (A) and salicylate-based (B) polymer. Skin and capsule collagen stains blue and muscle tissue appears red. Higher-power images from the center of the implant indicate the degradation of the polymer (*) and invasion of cells (arrows) in inactive polymer (C) and salicylate-based polymer (D). Original magnification 100 × (A, B) and 400 × (C, D).
Fig. 9.
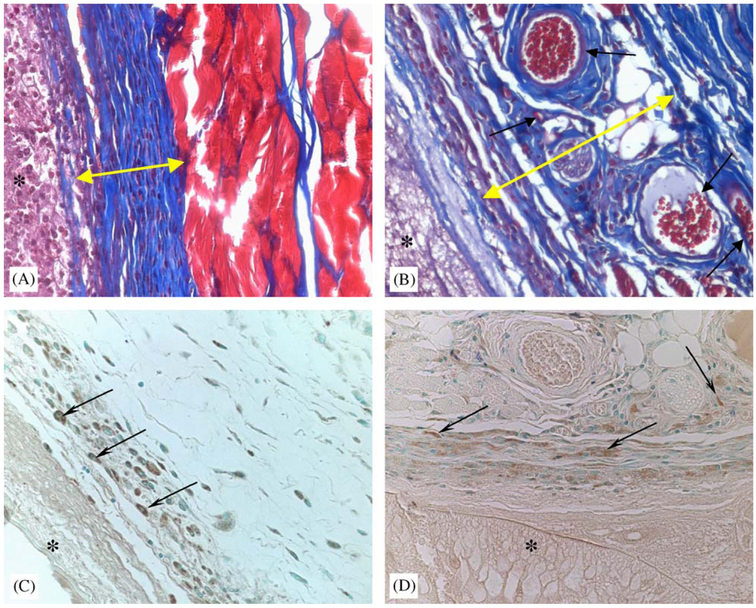
Salicylate-based polymer is encapsulated by vascularized capsules that are void of macrophages. Area of foreign-body capsule is identified by yellow bidirectional arrow (A, inactive control; B, salicylate-based polymers). The presence of blood vessels in the capsule surrounding Salicylate-based polymer is evident (arrows in B). Immunolocation of macrophages by the peroxidase reaction, brown color (arrows in C, D), indicate their presence in close proximity to the inactive control (*) and at a distance from the salicylate-based polymer.
4. Discussion
SA or salicylate is the major in vivo metabolite of acetylsalicylic acid, i.e., aspirin [11]. Farber et al. reports that SA inhibited adherence (55%), growth, and biofilm production of Staphylococcus epidermidis [27]. In a second study, Farber et al. further illustrated that NSAIDs, including sodium salicylate, inhibit bacterial biofilm production [28]. The authors studied the effect of soluble sodium salicylate on bacterial adherence and biofilm formation on contact lenses, lens cases, and commonly used medical polymers and observed that biofilm formation for S. epidermidis and P. aeruginosa was inhibited in a dose-related manner with sodium salicylate. Related studies also demonstrated that SA and other NSAIDs decreased bacterial colonization on contact lenses in a dose-dependent manner [29,30].
Stepanovic et al. explored the effect of aspirin on the biofilm-producing capacity of various Candida species [31]. Concentrations of soluble aspirin, which induced statistically significant decreases in yeast biofilm formation, ranged from 0.43 to 1.73 mM (59–239 mg/L SA) and depended on the tested yeast strain. Muller et al. reports that the inclusion of 5mM (691 mg/L) SA inhibited both growth and biofilm production by S. epidermidis by up to 55% [32].
Our results in Fig. 2 indicate that soluble SA below 300 mg/L had little effect on bacterial growth rate but concentrations ⩾ 300 mg/L decreased P. aeruginosa growth. Based upon the size and mass of the salicylate-based polymer disks used in this study, we estimate that the active polymers contain 139.9 ± 2.1 mg of SA. If this amount were instantly dispersed throughout in the 10 mL test volume, it would affect a concentration of ~14.0 gm/L, which—according to our batch growth curves- would be toxic to these bacterial cultures. However, based on the rates of polymer degradation, bacterial suspended cultures were never exposed to ambient SA concentrations ⩾ 100 mg/L, which were found not to be toxic. These results suggest that SA must prevent biofilms by a mechanism other than simply killing suspended bacterial cells.
Presence of enzymes secreted by growing bacteria and the acidic environment caused by bacterial generation of carbon dioxide could increase salicylate-based polymer degradation rates. Based upon the release of SA as shown in Fig. 3 (top), polymer degradation slightly increased when placed in the active, bacterial suspended culture. Degradation rates of polyanhydrides such as the salicylate-based polymer are typically slowed in acidic environments [9,25,26]. The combination of data shown in Fig. 3 is compelling: the presence of bacteria decreases the overall pH of the solution, which should slow the hydrolysis of the polymers, yet the polymers degraded slightly faster with bacteria present, suggesting that the bacteria may secrete enzymes (e.g., esterases) that are slightly enhancing polymer hydrolysis.
Polymer disks submerged in a P. aeruginosa suspension for up to 3 h also displayed differences in bacterial adhesion (Fig. 4). Both the inactive control and salicylate-releasing polymer disks displayed similar degradation profiles and generate degradation products with similar pKa values, thus the observed effects are due to the release of SA and not to dissimilar degradation rates or localized pH effects.
As shown in the long-term biofilm experiments (Figs. 5 and 6), the number of adherent cells accumulating on the inactive control polymer disks is five orders-of-magnitude more than on the salicylate-releasing polymers. Again, the slow release of SA resulting from salicylate-based polymer degradation significantly prevents biofilm formation, even relative to a polymer control undergoing an equivalent degradation rate. Local interfacial concentrations of SA either globally inhibit pathways leading to biofilm formation or have a more directed effect on a particular biofilm formation process.
Suspecting that SA may inhibit biofilm formation by interrupting quorum sensing control of polysaccharide secretion and subsequent biofilm formation, we repeated biofilm experiments employing a recombinant strain of P. aeruginosa. This strain carries a gene marker upstream of the las regulon quorum-sensing pathway. Should bacteria be activated by homo-serine lactone signals and begin polysaccharide secretions, the gfp gene would also be up-regulated and fluoresce. Results for the inactive control polymer disks (Fig. 7) indicate that was indeed the case. However, for recombinant cells adherent to salicylate-based polymers no GFP fluorescence was observed insinuating SA negates the las regulon system. This observation could mean that either SA acts as an analog competing with the homo-serine lactones or that SA merely inhibited some portion of the las pathway. Prithiviraj et al. [33] report that SA, at a concentration that did not inhibit P. aeruginosa growth was sufficient to significantly affect the ability of the bacteria to attach and form biofilm communities on abiotic surfaces. Furthermore, SA down regulated three known virulence factors of P. aeruginosa: pyocyanin, protease, and elastase. Using microarray technology to identify SA target genes, Prithiviraj et al. [33] reports that SA treatment affected the expression of 331 genes. In essence, that the las system appears influenced by SA in our work may not be a “quorum sensing” analog effect but rather a global effect on multiple metabolic pathways. A series of similar experiments were carried out with the bacterium, S. epidermidis that resulted in a similar reduction in adhesion and long-term biofilm formation (data available upon requested). The mode of interaction of released SA on S. epidermidis is unknown. Research has shown that Gram-positive bacteria do not employ homo-serine lactones as signal molecules; rather they use small peptides. Thus, since SA does exhibit similar effects on S. epidermidis, a species that does not employ the las pathway, it is most likely that SAs effects on P. aeruginosa could be far more global than simply inhibiting quorum signaling. More work is underway to determine SA’s role in the inhibition of biofilm formation.
5. Conclusion
Polymers of NSAIDs that degrade to produce salicylic acid and adipic acid were evaluated for their ability to reduce or eliminate bacterial biofilm accumulation. Results indicate that the wild-type P. aeruginosa exhibited slower adhesion rates and significantly less biofilm formation on salicylate-based polymer than on a degrading polymer that does not release salicylic acid.
Slow release of salicylic acid that occurs with the hydrolytic degradation of salicylate-based polymers has a significant impact on biofilm formation. In vitro, the salicylate-based polymers and inactive control polymers have similar degradation rates yet their in vivo degradation characteristics are significantly different in this animal model. After 4 weeks, the majority of salicylate-based polymers remained intact and exhibited significantly less infiltration of inflammation-associated cells such as macrophages and neutrophils. We have previously observed that a dampened inflammatory response, such as reduced foreign-body giant cell formation, can reduce the degradation rate of biodegradable implants [24]. Our current observations suggest that the released salicylic acid is modulating the inflammatory response and thus, leads to protection of the implant. The ability of salicylate-based polymers to modulate the macrophage response is also obvious by the lack of macrophages in the immediate proximity of the implantation site. Salicylic acid at therapeutic concentrations has been shown to suppress the expression of inflammatory molecules in macrophages such as cyclo-oxygenase (COX)-2, induced nitric oxide synthase (iNOS), and interleukin-4 [34]. At suprapharmacological concentrations it can also interfere with NF-kappaB binding. It is possible that in our implantation model, salicylate is influencing the expression of all these molecules and exerts a complex effect. Nevertheless, our in vivo studies demonstrate the ability of salicylate-based polymers implants to modulate the foreign-body response.
Acknowledgments
The authors gratefully acknowledge partial support from NIH (DE 13207) for KEU and AP and NIH (EB00987) for JDB.
References
- [1].Dankert J, Hogt A, Feijen J. Biomedical polymers: bacterial adhesion, colonization, and infection. CRC Crit Rev Biocompat 1986;2:219–29. [Google Scholar]
- [2].Stamm W Infection related to medical devices. Ann Intern Med 1978;89:764–85. [DOI] [PubMed] [Google Scholar]
- [3].Characklis W, Wilderer P, editors. Structure and function of biofilms. Wiley: Berlin; 1989. [Google Scholar]
- [4].Bryers JD, editor. Biofilms. 2nd ed. New York, NY: J. Wiley Interscience; 2000. [Google Scholar]
- [5].Brown M, Allison D, Gilbert P. Resistance of bacterial biofilms to antibiotics: a growth rate-related effect? Antimicrob. Chemother. 1988;22:777–80. [DOI] [PubMed] [Google Scholar]
- [6].Dunne W, Mason E, Kaplan S. Diffusion of rifampin and vancomycin through a Staphylococcus epidermidis biofilm. Antimicrob Agents Chemother 1993;37:2522–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Neut D, van Horn JR, van Kooten TG, van der Mei HC, Busscher HJ. Detection of biomaterial-associated infections in orthopaedic joint implants. Clin Orthopaed Rel Res 2003;413:261–8. [DOI] [PubMed] [Google Scholar]
- [8].Habash M, Reid G. Microbial biofilms: their development and significance for medical device-related infections. J Clin Pharmacol 1999;39:887–98. [DOI] [PubMed] [Google Scholar]
- [9].Erdmann L, Uhrich KE. Synthesis and degradation characteristics of salicylic acid-derived poly(anhydride-esters). Biomaterials 2000;21: 1941–6. [DOI] [PubMed] [Google Scholar]
- [10].Schmeltzer R, Schmalenberg K, Uhrich KE. Synthesis and cytotoxicity of salicylate-based poly(anhydride-esters). Biomacromolecules 2005;6:359–67. [DOI] [PubMed] [Google Scholar]
- [11].Budavari S The Merck index, 12th ed. Whitehouse Station: Merck Research Laboratories; 1996. [Google Scholar]
- [12].Erdmann L, Macedo B, Uhrich KE. Degradable poly(anhydride-ester) implants: effects of localized salicylic acid release on bone. Biomaterials 2000;21:2507–12. [DOI] [PubMed] [Google Scholar]
- [13].Harten RD, Svach DJ, Schmeltzer R, Uhrich KE. Salicylic acid-derived poly(anhydride-esters) inhibit bone formation in vivo. J Biomed Mater Res—A 2005;72A:354–62. [DOI] [PubMed] [Google Scholar]
- [14].Prudencio A, Schmeltzer RC, Uhrich KE. Effect of linker structure on salicylic acid-derived poly(anhydride-esters). Macromolecules 2005;38:6895–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Schmeltzer RC, Anastasiou TJ, Uhrich KE. Optimized synthesis of salicylate-based poly(anhydride-esters). Polym Bull 2003;49:441–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Campo CJ, Anastasiou T, Uhrich KE. Polyanhydrides: effects of ring substitution changes on polymer properties. Polym Bull 1999;42:61–8. [Google Scholar]
- [17].Hentzer M, Riedel K, Rasmussen TB, Heydorn A, Andersen JB, Parsek MR, et al. Inhibition of quorum sensing in Pseudomonas aeruginosa biofilm bacteria by a halogenated furanone compound. Microbiology 2002;148:87–102. [DOI] [PubMed] [Google Scholar]
- [18].Andersen JB, Sternberg C, Poulsen LK, Bjorn SP, Givskov M, Molin S. New unstable variants of green fluorescent protein for studies of transient gene expression in bacteria. Appl Environ Microbiol 1998; 64:2240–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Pearson JP, Gray KM, Passador L, Tucker KD, Eberhard A, Iglewski BH, et al. Structure of the autoinducer required for expression of Pseudomonas aeruginosa virulence genes. Proc Natl Acad Sci USA 1994;91:197–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Gambello MJ, Kaye S, Iglewski BH. LasR of Pseudomonas aeruginosa is a transcriptional activator of the alkaline protease gene (apr) and an enhancer of exotoxin A expression. Infect Immun 1993; 61:1180–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Passador L, Cook JM, Gambello MJ, Rust L, Iglewski BH. Expression of Pseudomonas aeruginosa virulence genes requires cell-to-cell communication. Science 1993;260:1127–30. [DOI] [PubMed] [Google Scholar]
- [22].Glessner A, Smith RS, Iglewski BH, Robinson JB. Roles of Pseudomonas aeruginosa las and rhl quorum-sensing systems in control of twitching motility. J Bacteriol 1999;181:1623–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Pearson JP, Passador L, Iglewski BH, Greenberg EP. A second N-acylhomoserine lactone signal produced by Pseudomonas aeruginosa. Proc Natl Acad Sci USA 1995;92:1490–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Kyriakides TR, Foster MJ, Keeney GE, Tsai A, Giachelli CM, Clark-Lewis I, et al. The CC chemokine ligand, CCL2/MCP1, participates in macrophage fusion and foreign body giant cell formation. Am J Pathol 2004;165:2157–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Poelstra KA, van der Mei HC, Gottenbos B, Grainger DW, van Horn JR, Busscher HJ. Pooled human immunoglobulins reduce adhesion of Pseudomonas aeruginosa in a parallel plate flow chamber. J Biomed Mater Res 2000;51(2):224–32. [DOI] [PubMed] [Google Scholar]
- [26].Busscher HJ, Van der Mei HC, Schakenraad JM. Analogies in the two-dimensional spatial arrangement of adsorbed proteins and adhering bacteria: bovine serum albumin and Streptococcus sanguis 12. J Biomater Sci Polym Edn 1991;3(1):85–94. [DOI] [PubMed] [Google Scholar]
- [27].Farber B, Wolff A. The use of nonsteroidal anti-inflammatory drugs to prevent adherence of Staphylococcus epidermidis to medical polymers. J Infect Dis 1992;166:861–5. [DOI] [PubMed] [Google Scholar]
- [28].Farber B, Hsieh H, Donnenfeld E, Perry H, Epstein A, Wolff A. A novel antibiofilm technology for contact lens solutions. Ophthalmology 1995;102:831–7. [DOI] [PubMed] [Google Scholar]
- [29].Bandara B, Sankaridurg P, Willcox M. Non-steroidal anti-inflammatory agents decrease bacterial colonisation of contact lenses and prevent adhesion to human corneal epithelial cells. Curr Eye Res 2004;29:245–9. [DOI] [PubMed] [Google Scholar]
- [30].Tomlinson A, Simmons P, Seal D, McFadyen A. Salicylate inhibition of Acanthamoeba attachment to contact lenses. Opthalmology 2000; 107:112–7. [DOI] [PubMed] [Google Scholar]
- [31].Stepanovic S, Vukovic D, Jesic M, Ranin L. Influence of acetylsalicylic acid (aspirin) on biofilm production by Candida species. J Chemother 2004;16:134–8. [DOI] [PubMed] [Google Scholar]
- [32].Muller E, Al-Attar J, Wolff A, Farber B. Mechanism of salicylate-mediated inhibition of biofilm in Staphylococcus epidermidis. J Infect Dis 1998;177:501–3. [DOI] [PubMed] [Google Scholar]
- [33].Prithiviraj B, Bais HP, Weir T, Suresh B, Najarro EH, Dayakar BV, et al. Down regulation of virulence factors of Pseudomonas aeruginosa by salicylic acid attenuates its virulence on Arabidopsis thaliana and Caenorhabditis elegans. Infect Immun 2005;73(9):5319–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Cieslik K, Zhu Y, Wu KK. Salicylate suppresses macrophage nitric-oxide synthase-2 and cyclo-oxygenase-2 expression by inhibiting CCAAT/enhancer-binding protein-beta binding via a common signaling pathway. J Biol Chem 2002;277:49304–10. [DOI] [PubMed] [Google Scholar]