Abstract
BACKGROUND
Ambiguous histopathologic diagnoses represent a challenge for clinicians because of a lack of definitive diagnosis and related uncertainty about management.
OBJECTIVE
To review the literature on atypical melanocytic proliferations and detail synonymous terms, epidemiology, diagnostic work-up, histopathology, treatment, and prognosis.
METHODS
Databases from PubMed and Web of Science were searched for articles related to atypical melanocytic proliferations.
RESULTS
Intraepidermal melanocytic proliferations with features worrisome for possible melanoma in situ (MIS) are generally excised as for MIS. Reported rates of upstaging of such cases to invasive melanoma on review of the excision are very low. Because invasion, lymph node spread, and metastasis can occur in atypical melanocytic lesions with a thick intradermal component, these are often treated as for malignant melanoma.
CONCLUSION
Because the diagnosis dictates treatment, it is incumbent to establish a diagnosis as definitive as possible, obtaining second or third opinions and using ancillary studies when appropriate. When the diagnosis remains uncertain, it is difficult to provide guidelines for treatment. Clinical care decisions for patients with an uncertain diagnosis are best done on a case-by-case basis weighing probabilities of adverse outcomes against potential benefits and risks from various treatment options.
Early detection of melanoma is key from a patient prognosis standpoint, and as such the authors rely on an accurate histopathological assessment. Most histological diagnoses involving melanocytic lesions can be made with a high level of certainty, reproducibility, and agreement among dermatopathologists; however, there exists a subset of melanocytic neoplasms that can be difficult to classify as benign or malignant based on conventional microscopic analysis.1–10 These lesions are often referred to as atypical melanocytic proliferations.
When evaluating an atypical melanocytic proliferation, pathologists may not be able to establish a definitive diagnosis and/or disagree with each other on the nature of the tumor. A lack of standardized diagnostic terminology makes it challenging to gauge clinical behavior and guide treatment.11 Atypical melanocytic proliferations often involve expert consultation and require management based on a review of the clinical situation and favored opinions by pathologists. There is a paucity of literature on these lesions and their management. There is risk of over- and undertreating patients with ambiguous melanocytic tumors because it is implicitly not known which lesion is benign and which is malignant. Here, the authors present a review of the literature on atypical melanocytic proliferations, including the current consensus on nomenclature, diagnosis, biologic potential, and treatment. Atypical melanocytic proliferations may also be seen in the nail unit and represent a separate diagnostic and therapeutic dilemma.
Nomenclature
Key Points
Multiple descriptive terms representing histologically ambiguous melanocytic proliferations are used to refer to lesions that do not clearly fit into a “benign” or “malignant” category (Table 1).
Inconsistent nomenclature that varies by institution may lead to patient/provider confusion and a lack of consensus on how to manage these lesions.
Imprecise terminology and evasion of a specific diagnosis should be avoided because there is risk of misunderstanding by clinicians, who may then opt for the wrong treatment option.
TABLE 1.
Diagnostic Terms Used to Describe Atypical Melanocytic Lesions
| Diagnostic Term | Abbreviation | Definition |
|---|---|---|
| Atypical intraepidermal melanocytic proliferation12–16 | AIMP | High-grade intraepidermal melanocytic dysplasia insufficient for a diagnosis of melanoma in situ |
| Intraepidermal borderline melanocytic tumor17–23 | Intraepidermal BMT | High-grade intraepidermal melanocytic dysplasia insufficient for a diagnosis of melanoma in situ |
| Dermal borderline melanocytic tumor17–23 | Dermal BMT | Atypical melanocytic lesion with a thick dermal component, prognostically indeterminate |
| De novo intraepidermal melanocytic dysplasia24,25 | DNIEMD | A purely intraepidermal BMT; possible precursor lesion to melanoma in situ |
| Atypical junctional melanocytic hyperplasia26–29 | AJMH | A proliferation of uniformly atypical melanocytes that are more than dysplastic nevi but less than those of melanoma in situ |
| Pagetoid melanocytic proliferation26,30 | PMP | Atypical melanocytes singly or in nests disbursed through the full thickness of the epidermis, including the granular layer, with no involvement in the dermis |
| Minimal deviation melanoma31–42 | MDM | Resembles a benign acquired or Spitz’s nevi, but with an expansive, aberrant vertical growth phase; has less cellular atypia than melanoma; and is at the border between benign and malignant |
| Superficial atypical melanocytic proliferations of uncertain significance43–47 | SAMPUS | High-grade intraepidermal melanocytic dysplasia insufficient for a diagnosis of melanoma in situ |
| Melanocytic tumors of uncertain malignant potential43–47 | MELTUMP | Atypical melanocytic lesion with a thick dermal component, prognostically indeterminate |
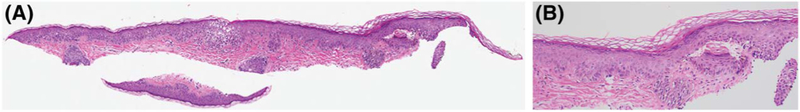
There is no established nomenclature for how to refer to lesions whose histological features do not fit into a “benign” or “malignant” category. In the literature, a variety of terms have been used, such as “atypical intraepidermal melanocytic proliferation” (AIMP),12–16 “borderline melanocytic tumor” (BMT) with intraepidermal and dermal variants,17–23 “de novo intraepidermal melanocytic dysplasia” (DNIEMD),24,25 “atypical junctional melanocytic hyperplasia” (AJMH),26–29 “pagetoid melanocytic proliferation” (PMP),26,30 and “minimal deviation melanoma” (MDM).31–42 Of these terms, one of the most commonly used is AIMP. It is used as a descriptive term for a diagnostic problem when unable to exclude melanoma in situ (MIS).43,44 The term AIMP may be used because the available material or clinical information is inadequate (small partial biopsy with no knowledge of clinical size and appearance of a lesion) (Figure 1A). Throughout this article, atypical melanocytic lesions are referred to by the terminology used in the original studies. Table 1 provides a quick reference for the terms and their definitions.
Figure 1.
(A) Small partial shave biopsy (hematoxylin and eosin, original magnification ×2) diagnosed as atypical intraepidermal melanocytic proliferation. (B) Examination at higher power (hematoxylin and eosin, original magnification ×20) demonstrates subtle focal findings of atypical melanocytes confined to the epidermis; although lentigo maligna could not be ruled out, criteria for melanoma in situ were not met.
In 2004, Elder and Xu proposed to classify atypical melanocytic lesions into 2 broad categories based on the risk assessment: “superficial atypical melanocytic proliferations of uncertain significance” (SAMPUS), and “melanocytic tumors of uncertain malignant potential” (MELTUMP) (Table 1). With superficial tumors the risk of adverse outcome is low, whereas a thick tumor has a greater chance for harm to the patient, if the tumor is indeed a melanoma. Melanocytic tumors of uncertain malignant potential is a term that is used by pathologists for melanocytic tumors with an intradermal component if, for whatever reasons, they cannot render a definitive diagnosis and cannot exclude invasive melanoma. Atypical melanocytic lesions with a thick dermal component have also been referred to as dermal BMT. An alternate analogous approach would be to simply report tumor thickness and/or other prognostic features. These proposals essentially bypass the need for a diagnosis and address the prognosis of the worst-case scenario, that is, if the lesion was a melanoma. Terms such as SAMPUS or MELTUMP are associated with the risk of overuse by pathologists to avoid establishing a firm diagnosis for various reasons.
The presence of multiple descriptive terms that represent the same entity (a histologically ambiguous melanocytic proliferation) contributes to the confusion on the side of clinicians and patients and related uncertainty for how to manage these lesions. Advocates for standardizing ambiguous terminology have proposed to expand the classification scheme of melanocytic neoplasms, including the World Health Organization classification of melanocytic tumors of the skin, to include a third category called melanocytic lesions of intermediate malignant potential,45 or the SAMPUS and MELTUMP categories as put forth by Elder and Xu.43,46,47 In 2014, the Melanocytic Pathology Assessment Tool and Hierarchy for Diagnosis (MPATH-Dx) reporting schema was published to standardize reporting and simplify treatments. This tool categorizes histologic diagnoses into a hierarchy of 7 categories based on consensus regarding management. Classes 2 to 4 comprise the “Variable Classification” group or the gray-zone of melanocytic lesions of uncertain malignant potential. Atypical intraepidermal melanocytic proliferation was described as Variable Classification, which most frequently mapped to Class 2 or Class 3, with suggestive management of “narrow but complete re-excision (<5 mm)” (Class 2 lesions) or “repeat excision with at least 5 mm (but <1 cm) margins” (Class 3 lesions).48 Studies evaluating this schema have found interrater agreements between MPATH-Dx categorization and treatment suggestions of 0.70 (95% confidence interval 0.68–0.71) and 0.72 (95% confidence interval 0.71–0.73),49 and consensus diagnosis among experienced dermatopathologists of 64% for Class 2 and 84% for Class 3 lesions.50 This proposal is well-intended, but it remains doubtful to what extent the unknown can rationally be subclassified. Furthermore, evidence-based data on the utility of this schema for patients are lacking. Atypical melanocytic lesions (Variable Classification) are broadly mapped to a range from Class 2 to Class 5, which highlights the uncertainty in optimal management of these lesions. In addition, this schema may lead to unnecessary reexcision of Spitz nevi without atypia and blue nevus without atypia, and wider excision of special site nevi (acral, genital, flexural, etc.), which are mapped from Class 1 to Class 2.
Epidemiology
The incidence of atypical melanocytic proliferations is unknown because of the absence of a histopathological diagnosis code and the variable nomenclature among institutions; however, these lesions are not uncommon in clinical practice.51 A retrospective review of 400 cases of intradermal nevi found that 25 cases (6.2%) had features of AJMH.51 Studies have described a high percentage of these lesions on the lower extremities of women.52–54 In a retrospective study of 263 skin biopsies diagnosed as DNIEMD, 82% of lesions were found in women and 71% of all lesions were on lower extremities.25 The frequency of using ambiguous terms is, of course, highly dependent on the pathologist(s) involved in such series. Some dermatopathologists strive to render precise diagnoses on melanocytic lesions in nearly all of their reports, whereas others have a tendency to avoid specific diagnoses and/or frequently prefer reporting a diagnostic problem instead of making a diagnostic decision.
Diagnosis
Key Points
Hematoxylin and eosin sectioning remains the gold standard in evaluating melanocytic lesions.
Even experienced dermatopathologists may at times be unable to establish a definitive diagnosis with light microscopy alone.
Immunohistochemistry, cytogenetic studies, and gene expression assays have emerged as potential adjuncts to improve diagnostic accuracy.
The histological descriptions of AIMP and SAMPUS refer to melanocytic proliferations confined to the epidermis or epidermis and superficial dermis, respectively. Histologically, it is not immediately clear whether a lesion is a subtle MIS, a junctional nevus with atypical features, or benign melanocyte hyperplasia. A few pagetoid melanocytes, for example, may be seen above the dermal–epidermal junction. There may also be some inflammation or subtle features of irritation, making the pathologist wonder whether the pagetoid melanocytes reflect MIS or a pseudo-melanomatous change of a nevus secondary to inflammation or external trauma. If the pathologist cannot reach a final conclusion, the default option may be a descriptive report of “AIMP” (Figure 1B). Another feature that may lead to an uncertain diagnosis is when a nevus is associated with a more complex growth pattern and stromal fibrosis.
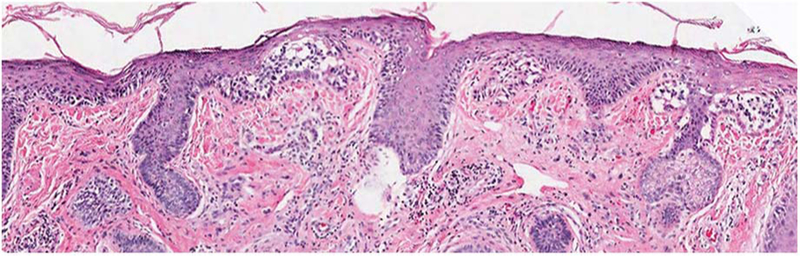
Morphologic evaluation on hematoxylin and eosin sectioning is paramount and remains the gold standard in the evaluation of melanocytic lesions.51,55 Although reliable parameters exist for the distinction of the majority of melanomas from most nevi, even experienced dermatopathologists may at times not be able to establish a definitive diagnosis based on light microscopic parameters alone. Limits to histologic evaluation include the growing trend toward increasingly smaller biopsies (sampling error). Although less cosmetically pleasing, a larger biopsy may be necessary to yield a definitive diagnosis (Figure 2), as evidenced by high rates (12%–16%) of MIS upstaged to invasive melanoma in final excision specimens.56,57 There are several noninvasive methods to facilitate diagnosis, such as the use of a Woods lamp, dermoscopy, or confocal microscopy. These methods are valuable, but limited to user expertise and device availability, and histologic examination remains the criterion standard.58–62
Figure 2.
Subsequent larger tissue sample of specimen in Figure 1 (hematoxylin and eosin, original magnification ×10) confirms the diagnosis of melanoma in situ.
Additional boundaries to histologic diagnosis include a lack of specificity and sensitivity of some of the morphologic features used for the diagnosis of melanoma, leading to interobserver variability. Sometimes, what one pathologist may call atypical, another may call benign or malignant.63–66 At the XXIX Symposium of the International Society of Dermatopathology in 2008, international experts in dermatopathology were asked to evaluate 57 cases previously deemed MELTUMP in an effort to obtain histopathologic criteria that could reliably classify atypical melanocytic tumors as benign or malignant. There was no good consensus. Diagnostic agreement was reached in only 30% of cases. In addition, pathologists incorrectly classified 53% of cases who had a favorable outcome as malignant and 27% of cases who had an unfavorable outcome (local recurrence, metastasis, or death) as benign. None of the traditional histological criteria used to differentiate between melanoma and benign nevi were found statistically significant in differentiating between favorable and unfavorable MELTUMPs.67
Given the acknowledged limitations in light microscopic analysis, miscellaneous ancillary methods have been explored to improve diagnostic accuracy, including immunohistochemistry and molecular technologies, such as cytogenetic studies (comparative genomic hybridization [CGH], fluorescent in situ hybridization [FISH]), and gene expression arrays. Immunohistochemical (IHC) studies for melanocyte differentiation antigens can facilitate recognition of melanocytes and are particularly useful when it is difficult to see lesional melanocytes, such as in the setting of dense inflammation.51,68,69 However, differentiation markers do not distinguish benign from malignant cells. Biomarkers, such as Ki-67, p16, or R21, have low sensitivity and specificity, which is why currently available IHC methods have limited value for distinguishing atypical melanocytic nevi from melanomas.
Cytogenetic studies hold greater promise. Comparative genomic hybridization identifies genome-wide chromosomal alterations in cancerous lesions. Array-based CGH, first introduced in 1998,70 has largely replaced whole-genome CGH. It is more sensitive, technically easier to perform, and allows for detection of smaller or more focal alterations, providing a much higher resolution.71 Bastian and colleagues pioneered the application of this technique to melanoma; the most common chromosomal aberrations found in melanoma and distinct from melanocytic nevi were loss of 9q and 10, and gains in 7.72–74 Further studies have also found marked differences in the genetic makeup of melanomas based on anatomical location and sun-exposure pattern.73,75 The estimated sensitivity and specificity of this technique for melanoma are 80% to 90%.71 False-negative results may occur because of failure of CGH to detect genomic aberrations in small populations of tumoral cells.76
The 4-probe FISH assay has also shown promise as an ancillary diagnostic tool.77,78 As most melanomas have copy-number increases of 11q and 6p, to differentiate from common nevi, the initial assay combined 4 probes targeting genes on 6p25, 6q23, 11q13, and centromere 6. The initial assay had 87% sensitivity and 95% specificity for melanoma and identified 6 of 6 cases as melanomas that were histologically ambiguous and subsequently metastasized.79 Additional validation studies found an estimated sensitivity of 80% to 100% and specificity of 95% for melanoma.71 For histologically ambiguous melanocytic tumors, combining the histopathologic diagnosis with FISH results in optimized diagnosis, by increasing sensitivity compared with FISH alone (90% vs 43% for FISH alone) and improving specificity (76% vs 52% for histopathologic diagnosis alone).80 A potential pitfall for false-positive results with FISH analysis is lesions with polyploidy.81 A prospective analysis of 140 lesions by Zembowicz and colleagues82 found that of all abnormal FISH results, 27% were false-positive results secondary to tetraploidy. Probes for chromosome 9p21 (CDKN2A) have been included to overcome limitations imposed by tetraploidy, as homozygous 9p21 deletion has shown a high discriminatory value.83 Melanomas (especially spitzoid) frequently show homozygous 9p21 deletion.84 Benign nevi may harbor single deletions of 9p21; however, homozygous deletions are rare. New probe sets are being developed to enhance sensitivity and specificity; an expanded FISH panel (addition of 4 new probes, including 9p21, to the initial 4 sets of probes) improved sensitivity for ambiguous melanocytic tumors.85 Compared with CGH, FISH is less expensive, requires less technical expertise to perform, can be used on limited amounts of tissue,86 and is more widely available. However, FISH is limited to the detection of chromosomal alterations targeted by specific probes. This can serve advantageous in lesions with minor subpopulations of genomic aberrations that are at risk of false-negative results with CGH.76
Another potential diagnostic adjunct is a gene expression signature that aims to differentiate benign and malignant melanocytic neoplasms. In a cohort of 437 samples that included a broad spectrum of melanocytic lesions, some of which were ambiguous, the 23-gene probe had a sensitivity of 90% and a specificity of 91% for differentiating benign nevi from primary malignant melanoma.87 A comparison study of the diagnostic utility of CGH versus the 23-gene expression probe for classification of melanocytic lesions demonstrated concordant results from both tools for benign and malignant lesions, but a higher percentage of discordance for ambiguous lesions.88
Recent advances in melanoma genomics have prompted the discovery of numerous molecular pathways and genes that may be used as diagnostic aids. For example, characterization of the microRNA (miRNA) transcriptome in melanoma is ongoing, but robust preclinical evidence suggests involvement in oncogenesis of miR-21, miR-125b, miR-150, miR-155, miR-205, and miR-211.89 Studies have found miR-211 to be one of the most differentially expressed miRNAs when comparing melanoma with normal epidermal melanocytes, with significantly decreased expression of miR-211 in melanomas when compared with nevi (p < .0001).90,91 Studies have suggested that miR-211 is a potent tumor suppressor and influences gene pathways involved in cell invasion.92–96 These findings support miR-211 as a leading miRNA candidate to aid in melanoma diagnosis. Examination of 109 melanocytic lesions found that miRNA in situ hybridization for fluorescent detection of miR-211 was accurate in discrimination between melanoma and nevi with 90% sensitivity and 86.2% specificity.97
Another biomarker currently under evaluation to aid in the diagnosis of melanoma is BRCA1-associated protein 1 (BAP-1), a tumor suppressor gene. Both somatic and germline mutations in BAP-1 have been described in various tumors, including cutaneous and uveal melanomas, atypical cutaneous melanocytic tumors, mesothelioma, renal cell carcinoma, and lung adenocarcinoma.98 Germline mutations in BAP-1, known as BAP-1 tumor syndrome, confer inherited susceptibility to the aforementioned cutaneous and internal malignancies.99 Loss of BAP-1 expression can be identified in melanocytic lesions with IHC analysis100 and may prove most useful in evaluation of atypical spitzoid melanocytic proliferations.101–103 BAP-1 expression is also being evaluated as a potential prognostic marker for melanoma, especially uveal melanoma.104–106
These molecular approaches have substantial potential to impact the management of melanocytic lesions of uncertain malignant potential, especially if histopathologic features are less than definitive. Combining multiple diagnostic modalities, such as dermatoscopy, histology, and molecular tools, has shown promise in enhancing early detection of melanomas.107,108 However, all of these applications require further validation studies, with long-term follow-up. A comparison of the molecular approaches is presented in Table 2.
TABLE 2.
Advantages and Disadvantages of Molecular Studies for the Diagnosis of Atypical Melanocytic Proliferations
| Molecular Tool | Diagnoses Studied In | Advantages | Disadvantages |
|---|---|---|---|
| IHC59,68,69 | MIS; actinic keratoses; solar lentigines; and AIMP | Facilitate identification of melanocytes when compared with H&E alone, especially MITF | Melan-A overestimates epidermal melanocytes, suggesting potential overdiagnosis of MIS |
| Useful for when tissue is limited | S100 underestimates epidermal melanocytes, suggesting potential underdiagnosis of MIS | ||
| CGH70–76 | Benign melanocytic nevi and malignant melanoma | Sensitivity and specificity of 80%–90% for diagnosing melanoma | Novel technique with no long-term clinical follow-up |
| Detects aberrations of the entire genome in a single assay | Available only at select laboratories | ||
| Some subtypes of melanoma have distinct genomic patterns | Variable insurance coverage | ||
| May increase understanding of the development of melanoma and aid in design of therapeutic strategies | Requires a substantial amount of tissue | ||
| May predict melanoma prognosis | Failure to detect chromosomal abnormalities in a minor subpopulation of tumor cell results in false-negative results | ||
| 4-probe FISH assay71,77–82,86,107,108 | Atypical melanocytic nevi; melanoma; and ambiguous melanocytic proliferations | Sensitivity of 80%–100% and specificity of 95% for diagnosing melanoma | Limited number of probes |
| Validated in numerous difficult diagnostic situations | Targets only specific chromosomal aberrations | ||
| May enhance detection of early melanoma when combined with dermatoscopy and histology | Dependent on individual experience | ||
| Increased sensitivity for spitzoid melanomas seen with inclusions of an additional probe | False-positive tests secondary to polyploidy | ||
| Requires less technical expertise than CGH | False-negative tests due to sampling error | ||
| Often covered by insurance | Limited long-term follow-up | ||
| Limited utility for prognostication of melanoma | |||
| Gene expression signature87,88 | Benign melanocytic nevi and malignant melanoma | Classifies melanocytic lesions as benign or malignant with a sensitivity of 90% and a specificity of 91% | Further validation needed, especially in large cohorts of difficult atypical melanocytic proliferations and melanoma subtypes |
| Analysis by qRT-PCR can detect changes in gene expression that may not be an effect of gains or losses of DNA | Currently available studies excluded metastatic lesions, therefore only useful for distinguishing between benign nevi and primary melanomas | ||
| Estimates of sensitivity and specificity may have been biased by use of archived formalin-fixed paraffinembedded tissue sections, which were more likely to be degraded |
AIMP, atypical intraepidermal melanocytic proliferation; CGH, comparative genomic hybridization; FISH, fluorescent in situ hybridization; H&E, hematoxylin and eosin; IHC, immunohistochemistry; Melan-A, melanoma antigen recognized by T cells 1; MIS, melanoma in situ; MITF, microphthalmia-associated transcription factor; qRT-PCR, quantitative reverse transcription polymerase chain reaction.
Progression to Invasive Malignancy
Key Points
Few studies have evaluated the biologic potential of atypical melanocytic proliferations.
The rate of upstaging from AIMP or DNIEMD to melanoma is estimated at 4.2% to 9.8%.
Lesions with a largely dermal component (MELTUMP and dermal BMT) have higher risk of metastatic spread.
Few studies have investigated the biologic potential of atypical melanocytic proliferations. It is possible that most lesions currently reported as atypical melanocytic proliferation (AIMP or SAMPUS) are in fact benign, and that their significance lies in the potential pitfall for overdiagnosis of malignant melanoma.26,54 This hypothesis is based on the fact that the majority of the lesion lies intraepithelial with a minor dermal component; thus, the chance of distal metastasis is low.44 However, a retrospective analysis of 306 AIMPs treated by conventional excision found a change in final diagnosis from AIMP to melanoma in 4.2% (13/306) after complete histopathological evaluation of the excisional specimen,13 suggesting that a subset of these lesions are melanomas when completely sampled and reviewed. Of these melanomas, 85% were in situ and 15% were invasive. Specific risk factors associated with diagnostic change included location on the head and neck or acral areas, extension of AIMP to the base of the biopsy specimen, biopsy performed through punch technique, and an initial histopathologic differential diagnosis of melanoma. The authors recommend that for AIMPs with these features, clinicians should counsel patients on their increased risk of upstaging to melanoma before excision and consider treating similar to MIS.13
One small study investigated the prognostic significance of AJMH by reviewing biopsies between 2003 and 2004 from a private dermatopathology laboratory. There were 27 cases that fit into the strict criteria of AJMH; of these cases, 19 were available for follow-up. Sixteen patients had been treated with re-excision of their lesions with 5-mm margin, and 3 patients had a re-excision with a 1-mm margin. No upstaging was described for any of these patients. All 19 patients were followed for a period of 2 to 6 years, and no recurrences occurred.53
A retrospective analysis of 82 skin biopsies diagnosed as DNIEMD found that 8 of the lesions (9.8%) occurred in the context of fully evolved melanoma.24 Overall, the authors determined that DNIEMDs are a distinct entity, associated with the atypical mole phenotype and a personal and/or family history of melanoma. Similar results were found in a larger retrospective review of 263 skin biopsies diagnosed as DNIEMD.25 This study described an increased association of DNIEMD with malignant melanoma, dysplastic nevi, and nonmelanoma skin cancer. The authors proposed that these lesions might represent a transitional or evolutionary step to MIS, and/or a de novo precursor lesion to melanoma.24 In addition, the positive association between these lesions, dysplastic nevi, and melanoma suggests that they may serve as a marker for increased risk of developing melanoma.25
The risk of metastatic spread is higher for lesions with a largely dermal component (those categorized as MELTUMP or dermal BMT). One prospective study followed 32 patients with BMTs whom underwent wide local excision and sentinel lymph node biopsy (SLNB) with a mean follow-up of <5 years, and the study concluded that the dermal variant of these lesions can have lymph node spread and progression of disease if not adequately treated.17 Retrospective reviews of MELTUMP tumors have reported that the risk of developing regional metastases or death from metastasized disease ranges from 1.0% to 2.4%.52,109 One retrospective study described lymphatic invasion in 25% of patients and a statistically significant association between MELTUMP lymphatic invasion and melanoma metastases or melanoma-related death.11 Although a number of MELTUMPs have been reported to be associated with melanocyte deposits in lymph nodes,67 it is important to recognize that regional lymph node involvement does not constitute proof of malignancy, as there are reports of benign melanocytic nevi spreading to lymph nodes and/or cutaneous lymphatics.44 Many MELTUMPs with positive lymph nodes are associated with a subsequent indolent clinical course.
An overview of the published data evaluating atypical melanocytic proliferations is presented in Table 3.
TABLE 3.
Available Literature Evaluating Outcomes of Atypical Melanocytic Lesions
| Study | Study Design | Diagnosis | No. of Patients | Median Follow-Up (mo) | Outcome |
|---|---|---|---|---|---|
| Abraham and colleagues11 | Retrospective cross-sectional study | MELTUMP | 32 | 111 | 2 patients died of melanoma-related disease; 25% of patients had lymphatic invasion |
| Berk and colleagues126 | Retrospective cross-sectional study | Melanoma, MELTUMP | 13 (melanoma) 7 (MELTUMP) | 27 (melanoma) 22 (MELTUMP) | Positive SLN in 20% of patients with melanoma and 33% of patients with MELTUMP; emphasis on the need for close follow-up in patients with MELTUMP |
| Cerroni and colleagues67 | Tutorial held at XXIX Symposium of the International Society of Dermatopathology | MELTUMP | 57 | NR | MELTUMPs as a group exist and may be biologically different from conventional melanoma and benign melanocytic nevi; terminology is highly controversial; there is uncertainty in classification and interpretation |
| Cunningham and colleagues116 | Retrospective cross-sectional study | MELTUMP | 27 | 22.8 | 44% of lesions were graded as melanoma Stage 1A or 1B; 4% of these lesions were upstaged to Stage 1B after excision |
| El Tal and colleagues53 | Retrospective cross-sectional study | AJMH | 27 | 24–72 | Most common location was lower extremity; no recurrences observed |
| Green and colleagues109 | Retrospective cross-sectional study | MELTUMP | 42 | 23 | 1 patient developed locally recurrent disease; 1 patient developed regional metastases; and 1 patient died of metastatic disease |
| Jessup and Cohen25 | Retrospective cross-sectional study | DNIEMD | 263 | NR | 71% of lesions were found on the lower extremities; 24% of patients had a personal history of melanoma |
| Kaltoft and colleagues52 | Retrospective cross-sectional study | MELTUMP | 67 | NR | 6% of patients had regional nodal dissemination at the time of diagnosis; 1 patient developed regional spread at follow-up; and 1 patient died of distant metastases 1 yr after diagnosis |
| Magro and colleagues17 | Prospective cohort study | Dermal BMT | 32 | 50 | 34% of patients had positive SLN; 3% of patients died of metastatic disease |
| Meyers and colleagues125 | Retrospective cross-sectional study | MELTUMP | 31 | 16 | 16% of patients had positive SLN; younger age and greater Breslow depth were associated with a positive SLN |
| Mills and colleagues7 | Retrospective cross-sectional study | Atypical melanocytic proliferation | 24 | 49 | 29% of patients had positive SLN; no recurrences at median follow-up |
| Okamura and colleagues26 | Retrospective cross-sectional study | AJMH | 400 | 6–24 | 6.2% of cases exhibited benign AJMH; 40% of these lesions were located on the face; no patients developed melanoma |
| Phillips and colleagues39 | Retrospective cross-sectional study | MDM | 21 | 57 | 2 patients died of widespread metastatic disease |
| Pusiol and colleagues46 | Retrospective cross-sectional study | MELTUMP | 14 | >36 | 1 patient with positive SLN; all patients free of disease at follow-up |
| Sachdeva and colleagues24 | Retrospective cross-sectional study | DNIEMD | 82 | NR | 55% of patients had atypical mole phenotype; 27% of patients had a previous or subsequent diagnosis of melanoma |
| Zhang and colleagues12 | Retrospective cross-sectional study | AIMP | 413 | NR | 2.9% of lesions had positive or equivocal margins after initial excision; increased risk of incomplete excision associated with location on the head and neck or a preoperative biopsy that includes MIS in the differential diagnosis |
| Zhang and colleagues13 | Retrospective cross-sectional study | AIMP | 306 | NR | 4.2% of lesions were upstaged to melanoma on review of excision specimen; risk factors for upstaging included location on head and neck or acral areas, lesion extension to base of biopsy specimen, use of punch biopsy technique, and melanoma in initial histopathologic differential diagnosis |
AIMP, atypical intraepidermal melanocytic proliferation; AJMH, atypical junctional melanocytic hyperplasia; BMT, borderline melanocytic tumor; DNIEMD, de novo intraepidermal melanocytic dysplasia; MDM, minimal deviation melanoma; MELTUMP, melanocytic tumors of uncertain malignant potential; MIS, melanoma in situ; NR, not recorded; SLN, sentinel lymph node.
Management
Key Points
The malignant potential of atypical melanocytic proliferations is unknown; there are no evidence-based surgical guidelines.
If possible, it is recommended that the entire lesion be sampled for histopathologic review to direct comprehensive management.
For intraepidermal lesions, treatment should aim for complete re-excision with clear margins and close follow-up.
Atypical lesions with largely dermal component (e.g., MELTUMP) should be treated as malignant melanoma because of the risk of invasion and distant metastasis.
Nonsurgical alternatives, such as imiquimod cream, may be considered for poor surgical candidates or used as an adjuvant when residual melanocytic proliferation is seen at the peripheral margin after excision.
Medicolegal disputes in dermatopathology are often related to delayed diagnosis or misdiagnosis of melanoma.110 As a result, there is an increasing push to diagnose melanoma early. Although less catastrophic than misdiagnosing a melanoma, there are still consequences to overdiagnosis, including increased health care expenditures, patient distress, and increased morbidity associated with unnecessary treatment. Invasive treatments, such as large surgical excision, SLNB, lymphadenectomy, and systemic chemotherapy, have all been described for the management of atypical melanocytic lesions of uncertain malignant potential.111,112
As the malignant potential is unknown, there are no evidence-based surgical guidelines. Most lesions may be initially sampled with shave or incisional biopsies; however, these techniques may not capture the entire breadth of the clinical lesion.113 To achieve the most accurate diagnosis, it is recommended that, if possible, the entire lesion be sampled for histopathologic review. This of course may be limited because of cosmetic or anatomical considerations. For lesions on the trunk, saucerization technique may be used to facilitate pathologic assessment without impairing further appropriate management such as staging. When faced with a large pigmented lesion on the face suspicious for lentigo maligna (LM), broad superficial shave biopsy or multiple small biopsies of morphologically distinct regions are preferred. These techniques maintain cosmesis while providing broad areas for histologic assessment and rarely affect future management including staged excision.114 However, if the diagnosis is equivocal, a further biopsy may aid in the diagnosis.
The pathology report should be as precise as possible. If one pathologist cannot reach a diagnosis, it is best to consult with a more experienced colleague or several colleagues. If consensus cannot be reached or if there is agreement that the morphologic features are too ambiguous, ancillary studies may be helpful for an accurate diagnosis. If, despite ancillary studies, a definitive diagnosis cannot be established, it is preferable for the pathologist to at least favor whether or not the lesion is more likely benign or indolent versus malignant and capable of harming the patient. In the latter case, one may report thickness and other parameters as if the lesion was a melanoma.
The lesion should be managed with sufficient therapy for the most clinically significant entity in the differential diagnosis,115 and the uncertainty and difficulty in categorizing the lesion with presently available means should be shared with the patient.43 An open dialog should be established, with emphasis on the uncertain malignant potential of these lesions and the inherent challenge in predicting their clinical behavior.11,55 The histologic description should be taken into consideration along with the clinical course of the lesion and patient factors, such as age and health status. Depending on anatomic location or cosmetic concerns, treatment should aim for complete re-excision with clear margins and close follow-up, as there is potential for upstaging to melanoma as seen in a substantial proportion of melanomas.24,43,54,67,116–118 Treatment for MIS or malignant melanoma often recommends larger surgical margins than that for AIMP.119
Surgical management of atypical melanocytic proliferations and MIS is often challenging. With a background of actinic melanocytic hyperplasia, the boundaries of the proliferation are often poorly defined with a potential extensive subclinical disease.29,120,121 Before surgery, improved margin delineation may be enhanced with use of a Woods lamp, dermoscopy, or confocal microscopy; however, these techniques depend largely on clinician skill and experience and have not been extensively studied.59,122,123 The use of topical 5% imiquimod cream has been investigated as potential adjuvant treatment for LM after surgical resection, in which histologic analysis showed persistent involvement of LM at the peripheral margin. To date, no randomized, prospective trial has been performed to determine the efficacy of topical imiquimod cream as an adjunct to surgical resection of LM. One retrospective cohort study found that 94.4% of patients who used imiquimod as adjuvant therapy after surgery demonstrated clearance of LM after a mean follow-up of 43.1 months.120 This cohort of patients included cases of AIMP in which “early or evolving LM” was included in the diagnosis. These findings support the consideration of adjuvant therapy in cases where atypical melanocytic proliferations are seen at peripheral margins on patients with a background of sun-damaged skin, or in cases where patients are poor surgical candidates.
In cosmetically concerning areas, the use of Mohs micrographic surgery or staged excision with circumferential margin assessment may warrant consideration. In a retrospective analysis of 413 AIMPs treated with conventional excision, Zhang and colleagues12 found that AIMPs on the head/neck, especially those with a preoperative histologic diagnosis of MIS on the differential, were associated with a higher frequency (12.5%, 4/32) of incomplete removal after conventional excision. The authors hypothesized that a preoperative diagnosis of AIMP may have been due to inadequate sampling of MIS, or that there is similar clinical behavior between AIMP and MIS. There was no significant difference in the frequency of positive or equivocal margins based on size of surgical margins for lesions on the head/neck compared with those on the trunk/extremities. An increased rate of incomplete excision was also seen for AIMPs on acral surfaces; however, this result was not statistically significant.12
Clinicians may consider treating AIMPs at higher risk of diagnostic change to melanoma (those on the head/neck and/or those with preoperative diagnosis of MIS on the differential) similar to an MIS in the same anatomic location. Upstaging of AIMP to melanoma after conventional excision is important, as it will increase the risk of subsequent melanoma and alter follow-up recommendations.124 The general consensus for atypical melanocytic lesions with a largely dermal component, e.g., MELTUMPs, is treatment as malignant melanoma because of the aforementioned potential for lymph node involvement, invasion of cutaneous lymphatic vessels, and distant metastases.7,43,44,52,116,125–127 Close consideration should be given to lesion characteristics, sufficient sampling, patient characteristics, and ancillary diagnostic techniques, such as CGH and FISH before invasive adjuncts, such as SLNB.11,128
Conclusion
The authors’ review of the literature of atypical melanocytic proliferations highlights the overall ambiguity of these lesions and the potential pitfalls that they present, including, but not limited to, diagnostic disagreement, institutional variations in nomenclature, uncertain biologic potential, and lack of histologic criteria, and management recommendations. To improve diagnostic specificity, stronger efforts are needed to improve ancillary methods for when light microscopy fails.
The various terms and abbreviations for atypical melanocytic lesions (Table 1) are not always well defined or easily used in clinical parlance, and improved characterization of these lesions represents a major gap in the literature. The inconsistent nomenclature highlights 2 points: (1) imprecise terminology should be avoided because it is critical to establish a diagnosis as definitive as possible by obtaining second or third opinions and using ancillary studies when appropriate, and (2) irrespective of the descriptive terminology used, these terms suggest an uncertain prognosis, warranting complete sampling of the clinical lesion when possible. Communication between the pathologist and treating clinician is essential for clinicopathologic correlation.
In addition, the authors’ review highlights the importance of having an open discussion with patients in regards to the management of these lesions. The decision to re-biopsy, excise, or monitor may vary depending on the aforementioned factors. Optimal margins may differ in the size based on anatomic location. Although reported rates of upstaging to frank melanoma are low, there is a paucity of published data in this area, and invasion, lymph node spread, and metastasis can occur. Additional studies showing long-term outcomes, recurrence, and progression to melanoma are needed to determine the biologic potential of these lesions and to further guide clinical management.
Acknowledgments
This research was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748. The authors have indicated no significant interest with commercial supporters.
References
- 1.Braun RP, Gutkowicz-Krusin D, Rabinovitz H, Cognetta A, et al. Agreement of dermatopathologists in the evaluation of clinically difficult melanocytic lesions: how golden is the “gold standard”? Dermatology 2012;224:51–8. [DOI] [PubMed] [Google Scholar]
- 2.Okun MR, Edelstein LM, Kasznica J, Kirkham N, et al. What criteria reliably distinguish melanoma from benign melanocytic lesions? Histopathology 2000;37:464–72. [DOI] [PubMed] [Google Scholar]
- 3.Cook MG, Clarke TJ, Humphreys S, Fletcher A, et al. The evaluation of diagnostic and prognostic criteria and the terminology of thin cutaneous malignant melanoma by the CRC melanoma pathology panel. Histopathology 1996;28:497–512. [DOI] [PubMed] [Google Scholar]
- 4.Kim JC, Murphy GF. Dysplastic melanocytic nevi and prognostically indeterminate nevomelanomatoid proliferations. Clin Lab Med 2000; 20:691–712. [PubMed] [Google Scholar]
- 5.Briggs JC. Melanoma precursor lesions and borderline melanomas. Histopathology 1985;9:1251–62. [DOI] [PubMed] [Google Scholar]
- 6.Cheung WL, Smoller BR. Dermatopathology updates on melanocytic lesions. Dermatol Clin 2012;30:617–22. [DOI] [PubMed] [Google Scholar]
- 7.Mills OL, Marzban S, Zager JS, Sondak VK, et al. Sentinel node biopsy in atypical melanocytic neoplasms in childhood: a single institution experience in 24 patients. J Cutan Pathol 2012;39: 331–6. [DOI] [PubMed] [Google Scholar]
- 8.Wick MR, Patterson JW. Cutaneous melanocytic lesions: selected problem areas. Am J Clin Pathol 2005;124(Suppl):S52–83. [DOI] [PubMed] [Google Scholar]
- 9.Rywlin AM. Intraepithelial melanocytic neoplasia (IMN) versus intraepithelial atypical melanocytic proliferation (IAMP). Am J Dermatopathol 1988;10:92–3. [PubMed] [Google Scholar]
- 10.Piérard GE, Piérard-Franchimont C, Delvenne P. Simulants of malignant melanoma. Oncol Rev 2015;9:278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abraham RM, Karakousis G, Acs G, Ziober AF, et al. Lymphatic invasion predicts aggressive behavior in melanocytic tumors of uncertain malignant potential (MELTUMP). Am J Surg Pathol 2013; 37:669–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang J, Miller CJ, Sobanko JF, Shin TM, et al. Frequency of and factors associated with positive or equivocal margins in conventional excision of atypical intraepidermal melanocytic proliferations (AIMP): a single academic institution cross-sectional study. J Am Acad Dermatol 2016;75:688–95. [DOI] [PubMed] [Google Scholar]
- 13.Zhang J, Miller CJ, Sobanko JF, Shin TM, et al. Diagnostic change from atypical intraepidermal melanocytic proliferation to melanoma after conventional excision—a single academic institution cross-sectional study. Dermatol Surg 2016;42:1147–54. [DOI] [PubMed] [Google Scholar]
- 14.Chisholm C, Greene JF. Progression from atypical/dysplastic intraepidermal proliferations and carcinoma in situ to invasive tumors: a pathway based on current knowledge. Am J Dermatopathol 2011; 33:803–10. [DOI] [PubMed] [Google Scholar]
- 15.Urso C, Giannini A, Bartolini M, Bondi R. Histological analysis of intraepidermal proliferations of atypical melanocytes. Am J Dermatopathol 1990;12:150–5. [PubMed] [Google Scholar]
- 16.Rywlin AM. Malignant melanoma in situ, precancerous melanosis, or atypical intraepidermal melanocytic proliferation. Am J Dermatopathol 1984;6(Suppl):97–9. [PubMed] [Google Scholar]
- 17.Magro CM, Crowson AN, Mihm MC, Gupta K, et al. The dermal-based borderline melanocytic tumor: a categorical approach. J Am Acad Dermatol 2010;62:469–79. [DOI] [PubMed] [Google Scholar]
- 18.Magro CM, Abraham RM, Guo R, Li S, et al. Deep penetrating nevus-like borderline tumors: a unique subset of ambiguous melanocytic tumors with malignant potential and normal cytogenetics. Eur J Dermatol 2014;24:594–602. [DOI] [PubMed] [Google Scholar]
- 19.Zembowicz A, Scolyer RA. Nevus/Melanocytoma/Melanoma: an emerging paradigm for classification of melanocytic neoplasms? Arch Pathol Lab Med 2011;135:300–6. [DOI] [PubMed] [Google Scholar]
- 20.Scolyer RA, Murali R, McCarthy SW, Thompson JF. Histologically ambiguous (“borderline”) primary cutaneous melanocytic tumors: approaches to patient management including the roles of molecular testing and sentinel lymph node biopsy. Arch Pathol Lab Med 2010; 134:1770–7. [DOI] [PubMed] [Google Scholar]
- 21.Margo CE, Roper DL, Hidayat AA. Borderline melanocytic tumor of the conjunctiva: diagnostic and therapeutic considerations. J Pediatr Ophthalmol Strabismus 1991;28:268–70. [DOI] [PubMed] [Google Scholar]
- 22.Weedon D Borderline melanocytic tumors. J Cutan Pathol 1985;12: 266–70. [DOI] [PubMed] [Google Scholar]
- 23.Brodell RT, Santa Cruz DJ. Borderline and atypical melanocytic lesions. Semin Diagn Pathol 1985;2:63–86. [PubMed] [Google Scholar]
- 24.Sachdeva M, Frambach GE, Crowson AN, Deng AC, et al. De novo intraepidermal epithelioid melanocytic dysplasia as a marker of the atypical mole phenotype—a clinical and pathological study of 75 patients. J Cutan Pathol 2005;32:622–8. [DOI] [PubMed] [Google Scholar]
- 25.Jessup CJ, Cohen LM. De novo intraepidermal epithelioid melanocytic dysplasia: a review of 263 cases. J Cutan Pathol 2010; 37:852–9. [DOI] [PubMed] [Google Scholar]
- 26.Okamura JM, Barr RJ, Cantos KA. Benign atypical junctional melanocytic hyperplasia associated with intradermal nevi: a common finding that may be confused with melanoma in situ. Mod Pathol 2000;13:857–60. [DOI] [PubMed] [Google Scholar]
- 27.Mutasim DF. What is atypical junctional melanocytic Hyperplasia? Junctional melanocytic hyperplasia. In: Practical Skin Pathology Basel, Switzerland: Springer International Publishing; 2015; pp. 3–6. [Google Scholar]
- 28.Dalton SR, Fillman EP, Altman CE, Gardner TL, et al. Atypical junctional melanocytic proliferations in benign lichenoid keratosis. Hum Pathol 2003;34:706–9. [DOI] [PubMed] [Google Scholar]
- 29.Johnson TM, Headington JT, Baker SR, Lowe L. Usefulness of the staged excision for lentigo maligna and lentigo maligna melanoma: the “square” procedure. J Am Acad Dermatol 1997;37(5 Pt 1):758–64. [DOI] [PubMed] [Google Scholar]
- 30.Ackerman AB, Borghi S. “Pagetoid melanocytic proliferation” is the latest evasion from a diagnosis of “melanoma in situ”. Am J Dermatopathol 1991;13:583–604. [DOI] [PubMed] [Google Scholar]
- 31.Reed RJ. Minimal deviation melanoma. Hum Pathol 1990;21: 1206–11. [DOI] [PubMed] [Google Scholar]
- 32.Reed RJ. Dimensionalities: borderline and intermediate melanocytic neoplasia. Hum Pathol 1999;30:521–4. [DOI] [PubMed] [Google Scholar]
- 33.Reed RJ. Minimal deviation melanoma. Borderline and intermediate melanocytic neoplasia. Clin Lab Med 2000;20:745–58. [PubMed] [Google Scholar]
- 34.Stas M, van den Oord JJ, Garmyn M, Degreef H, et al. Minimal deviation and/or naevoid melanoma: is recognition worthwhile? A clinicopathological study of nine cases. Melanoma Res 2000;10: 371–80. [DOI] [PubMed] [Google Scholar]
- 35.Muhlbauer JE, Margolis RJ, Mihm MC, Reed RJ. Minimal deviation melanoma: a histologic variant of cutaneous malignant melanoma in its vertical growth phase. J Invest Dermatol 1983;80(1 Suppl):63–65s. [DOI] [PubMed] [Google Scholar]
- 36.Sagebiel RW. Histopathology of borderline and early malignant melanomas. Am J Surg Pathol 1979;3:543–52. [DOI] [PubMed] [Google Scholar]
- 37.Podnos YD, Jimenez JC, Zainabadi K, Jakowatz JG, et al. Minimal deviation melanoma. Cancer Treat Rev 2002;28:219–21. [DOI] [PubMed] [Google Scholar]
- 38.Reed RJ, Webb SV, Clark WH. Minimal deviation melanoma (halo nevus variant). Am J Surg Pathol 1990;14:53–68. [DOI] [PubMed] [Google Scholar]
- 39.Phillips ME, Margolis RJ, Merot Y, Sober AJ, et al. The spectrum of minimal deviation melanoma: a clinicopathologic study of 21 cases. Hum Pathol 1986;17:796–806. [DOI] [PubMed] [Google Scholar]
- 40.Reed RJ. Minimal deviation malignant melanoma arising in a congenital nevus. Am J Surg Pathol 1978;2:215–20. [PubMed] [Google Scholar]
- 41.Becker DW, Miller CJ, Keller HB. Metastatic minimal-deviation melanoma. Ann Plast Surg 1980;4:230–7. [PubMed] [Google Scholar]
- 42.Warner TF, Seo IS, Bennett JE. Minimal deviation melanoma with epidermotropic metastases arising in a congenital nevus. Am J Surg Pathol 1980;4:175–83. [DOI] [PubMed] [Google Scholar]
- 43.Elder DE, Xu X. The approach to the patient with a difficult melanocytic lesion. Pathology 2004;36:428–34. [DOI] [PubMed] [Google Scholar]
- 44.Mooi WJ. “Lentiginous melanoma”: full-fledged melanoma or melanoma precursor? Adv Anat Pathol 2014;21:181–7. [DOI] [PubMed] [Google Scholar]
- 45.Zembowicz A A new perspective for the classification of melanocytic lesions. In: 4th Melanoma Pathology Symposium of the International Melanoma Pathology Working Group Tampa, FL: John Wiley & Sons; 2011; pp. 1012. [Google Scholar]
- 46.Pusiol T, Piscioli F, Speziali L, Zorzi MG, et al. Clinical features, dermoscopic patterns, and histological diagnostic model for melanocytic tumors of uncertain malignant potential (MELTUMP). Acta Dermatovenerol Croat 2015;23:185–94. [PubMed] [Google Scholar]
- 47.Pusiol T, Morichetti D, Piscioli F, Zorzi MG. Theory and practical application of superficial atypical melanocytic proliferations of uncertain significance (SAMPUS) and melanocytic tumours of uncertain malignant potential (MELTUMP) terminology: experience with second opinion consultation. Pathologica 2012;104:70–7. [PubMed] [Google Scholar]
- 48.Piepkorn MW, Barnhill RL, Elder DE, Knezevich SR, et al. The MPATH-Dx reporting schema for melanocytic proliferations and melanoma. J Am Acad Dermatol 2014;70:131–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Carney PA, Reisch LM, Piepkorn MW, Barnhill RL, et al. Achieving consensus for the histopathologic diagnosis of melanocytic lesions: use of the modified Delphi method. J Cutan Pathol 2016;43:830–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lott JP, Elmore JG, Zhao GA, Knezevich SR, et al. Evaluation of the melanocytic pathology assessment tool and hierarchy for diagnosis (MPATH-Dx) classification scheme for diagnosis of cutaneous melanocytic neoplasms: results from the international melanoma pathology study group. J Am Acad Dermatol 2016;75:356–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nybakken GE, Sargen M, Abraham R, Zhang PJ, et al. MITF accurately highlights epidermal melanocytes in atypical intraepidermal melanocytic proliferations. Am J Dermatopathol 2013;35:25–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kaltoft B, Hainau B, Lock-Andersen J. Melanocytic tumour with unknown malignant potential–a Danish study of 67 patients. Melanoma Res 2015;25:64–7. [DOI] [PubMed] [Google Scholar]
- 53.El Tal AK, Mehregan D, Malick F, Atanasovski M. Atypical junctional melanocytic hyperplasia: a study of its prognostic significance. J Am Acad Dermatol 2012;66:AB141. [Google Scholar]
- 54.Buonaccorsi JN, Lynott J, Plaza JA. Atypical melanocytic lesions of the thigh with spitzoid and dysplastic features: a clinicopathologic study of 29 cases. Ann Diagn Pathol 2013;17:265–9. [DOI] [PubMed] [Google Scholar]
- 55.Abraham RM, Ming ME, Elder DE, Xu X. An atypical melanocytic lesion without genomic abnormalities shows locoregional metastasis. J Cutan Pathol 2012;39:21–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Möller MG, Pappas-Politis E, Zager JS, Santiago LA, et al. Surgical management of melanoma-in-situ using a staged marginal and central excision technique. Ann Surg Oncol 2009;16:1526–36. [DOI] [PubMed] [Google Scholar]
- 57.Hazan C, Dusza SW, Delgado R, Busam KJ, et al. Staged excision for lentigo maligna and lentigo maligna melanoma: a retrospective analysis of 117 cases. J Am Acad Dermatol 2008;58:142–8. [DOI] [PubMed] [Google Scholar]
- 58.Paraskevas L-R, Halpern AC, Marghoob AA. Utility of the Wood’s light: five cases from a pigmented lesion clinic. Br J Dermatol 2005; 152:1039–44. [DOI] [PubMed] [Google Scholar]
- 59.Robinson JK. Use of digital epiluminescence microscopy to help define the edge of lentigo maligna. Arch Dermatol 2004;140:1095–100. [DOI] [PubMed] [Google Scholar]
- 60.Menzies SW, Emery J, Staples M, Davies S, et al. Impact of dermoscopy and short-term sequential digital dermoscopy imaging for the management of pigmented lesions in primary care: a sequential intervention trial. Br J Dermatol 2009;161:1270–7. [DOI] [PubMed] [Google Scholar]
- 61.He J, Wang N, Tsurui H, Kato M, et al. Noninvasive, label-free, three-dimensional imaging of melanoma with confocal photothermal microscopy: differentiate malignant melanoma from benign tumor tissue. Sci Rep 2016;6:30209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Song E, Grant-Kels JM, Swede H, D’Antonio JL, et al. Paired comparison of the sensitivity and specificity of multispectral digital skin lesion analysis and reflectance confocal microscopy in the detection of melanoma in vivo: a cross-sectional study. J Am Acad Dermatol 2016;75:1187–92.e2. [DOI] [PubMed] [Google Scholar]
- 63.Brochez L, Verhaeghe E, Grosshans E, Haneke E, et al. Inter-observer variation in the histopathological diagnosis of clinically suspicious pigmented skin lesions. J Pathol 2002;196:459–66. [DOI] [PubMed] [Google Scholar]
- 64.Farmer ER, Gonin R, Hanna MP. Discordance in the histopathologic diagnosis of melanoma and melanocytic nevi between expert pathologists. Hum Pathol 1996;27:528–31. [DOI] [PubMed] [Google Scholar]
- 65.Bush JW, Hunt EL, Fraga GR. BAM! Utilizing the frequency of benign, atypical and malignant diagnoses for quality improvement in the histopathologic diagnosis of melanocytic neoplasms. J Cutan Pathol 2015;42:712–6. [DOI] [PubMed] [Google Scholar]
- 66.Barnhill RL, Cerroni L, Cook M, Elder DE, et al. State of the art, nomenclature, and points of consensus and controversy concerning benign melanocytic lesions: outcome of an international workshop. Adv Anat Pathol 2010;17:73–90. [DOI] [PubMed] [Google Scholar]
- 67.Cerroni L, Barnhill R, Elder D, Gottlieb G, et al. Melanocytic tumors of uncertain malignant potential: results of a tutorial held at the XXIX Symposium of the International Society of Dermatopathology in Graz, October 2008. Am J Surg Pathol 2010;34:314–26. [DOI] [PubMed] [Google Scholar]
- 68.Buonaccorsi JN, Prieto VG, Torres-Cabala C, Suster S, et al. Diagnostic utility and comparative immunohistochemical analysis of MITF-1 and SOX10 to distinguish melanoma in situ and actinic keratosis: a clinicopathological and immunohistochemical study of 70 cases. Am J Dermatopathol 2014;36:124–30. [DOI] [PubMed] [Google Scholar]
- 69.Kim J, Taube J, McCalmont T, Glusac E. Quantitative comparison of MiTF, Melan-A, HMB-45 and Mel-5 in solar lentigines and melanoma in situ. J Cutan Pathol 2011;38:775–9. [DOI] [PubMed] [Google Scholar]
- 70.Pinkel D, Segraves R, Sudar D, Clark S, et al. High resolution analysis of DNA copy number variation using comparative genomic hybridization to microarrays. Nat Genet 1998;20:207–11. [DOI] [PubMed] [Google Scholar]
- 71.March J, Hand M, Truong A, Grossman D. Practical application of new technologies for melanoma diagnosis: part II. Molecular approaches. J Am Acad Dermatol 2015;72:943–58. [DOI] [PubMed] [Google Scholar]
- 72.Bastian BC, LeBoit PE, Hamm H, Bröcker EB, et al. Chromosomal gains and losses in primary cutaneous melanomas detected by comparative genomic hybridization. Cancer Res 1998;58:2170–5. [PubMed] [Google Scholar]
- 73.Bastian BC, Kashani-Sabet M, Hamm H, Godfrey T, et al. Gene amplifications characterize acral melanoma and permit the detection of occult tumor cells in the surrounding skin. Cancer Res 2000;60: 1968–73. [PubMed] [Google Scholar]
- 74.Bastian BC, Olshen AB, LeBoit PE, Pinkel D. Classifying melanocytic tumors based on DNA copy number changes. Am J Pathol 2003;163: 1765–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Curtin JA, Fridlyand J, Kageshita T, Patel HN, et al. Distinct sets of genetic alterations in melanoma. N Engl J Med 2005;353:2135–47. [DOI] [PubMed] [Google Scholar]
- 76.Wang L, Rao M, Fang Y, Hameed M, et al. A genome-wide high-resolution array-CGH analysis of cutaneous melanoma and comparison of array-CGH to FISH in diagnostic evaluation. J Mol Diagn 2013;15:581–91. [DOI] [PubMed] [Google Scholar]
- 77.Gerami P, Barnhill RL, Beilfuss BA, LeBoit P, et al. Superficial melanocytic neoplasms with pagetoid melanocytosis. Am J Surg Pathol 2010;34:816–21. [DOI] [PubMed] [Google Scholar]
- 78.DeMarchis EH, Swetter SM, Jennings CD, Kim J. Fluorescence in situ hybridization analysis of atypical melanocytic proliferations and melanoma in young patients. Pediatr Dermatol 2014;31:561–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gerami P, Jewell SS, Morrison LE, Blondin B, et al. Fluorescence in situ hybridization (FISH) as an ancillary diagnostic tool in the diagnosis of melanoma. Am J Surg Pathol 2009;33:1146–56. [DOI] [PubMed] [Google Scholar]
- 80.Vergier B, Prochazkova-Carlotti M, de la Fouchardière A, Cerroni L, et al. Fluorescence in situ hybridization, a diagnostic aid in ambiguous melanocytic tumors: European study of 113 cases. Mod Pathol 2011; 24:613–23. [DOI] [PubMed] [Google Scholar]
- 81.Fang Y, Dusza S, Jhanwar S, Busam KJ. Fluorescence in situ hybridization (FISH) analysis of melanocytic nevi and melanomas: sensitivity, specificity, and lack of association with sentinel node status. Int J Surg Pathol 2012;20:434–40. [DOI] [PubMed] [Google Scholar]
- 82.Zembowicz A, Yang S-E, Kafanas A, Lyle SR. Correlation between histologic assessment and fluorescence in situ hybridization using MelanoSITE in evaluation of histologically ambiguous melanocytic lesions. Arch Pathol Lab Med 2012;136:1571–9. [DOI] [PubMed] [Google Scholar]
- 83.Gerami P, Li G, Pouryazdanparast P, Blondin B, et al. A highly specific and discriminatory FISH assay for distinguishing between benign and malignant melanocytic neoplasms. Am J Surg Pathol 2012;36:808–17. [DOI] [PubMed] [Google Scholar]
- 84.Gammon B, Beilfuss B, Guitart J, Gerami P. Enhanced detection of spitzoid melanomas using fluorescence in situ hybridization with 9p21 as an adjunctive probe. Am J Surg Pathol 2012;36:81–8. [DOI] [PubMed] [Google Scholar]
- 85.Guo R, Shah K, Erickson LA, Flotte T, et al. The expanded eight-probe melanoma fluorescence in situ hybridization (FISH) assay as an ancillary tool in diagnosing ambiguous melanocytic lesions: an updated clinical, pathological and cytogenetic review of 416 cases. In: United States and Canadian Academy of Pathology Annual Meeting Seattle, WA: 2016; pp. 128A–129A. [Google Scholar]
- 86.Busam KJ. Molecular pathology of melanocytic tumors. Semin Diagn Pathol 2013;30:362–74. [DOI] [PubMed] [Google Scholar]
- 87.Clarke LE, Warf MB, Flake DD, Hartman AR, et al. Clinical validation of a gene expression signature that differentiates benign nevi from malignant melanoma. J Cutan Pathol 2015;42:244–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang G, Wang M, Alomari A, Andea A. Comparison of genomic abnormalities and gene expression analysis in atypical, ambiguous and malignant melanocytic lesions. In: United States and Canadian Academy of Pathology Annual Meeting Seattle, WA: 2016; pp. 137A. [Google Scholar]
- 89.Latchana N, Ganju A, Howard JH, Carson WE. MicroRNA dysregulation in melanoma. Surg Oncol 2016;25:184–9. [DOI] [PubMed] [Google Scholar]
- 90.Kozubek J, Ma Z, Fleming E, Duggan T, et al. In-depth characterization of microRNA transcriptome in melanoma. PLoS One 2013;8:e72699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Boyle GM, Woods SL, Bonazzi VF, Stark MS, et al. Melanoma cell invasiveness is regulated by miR-211 suppression of the BRN2 transcription factor. Pigment Cel Melanoma Res 2011;24:525–37. [DOI] [PubMed] [Google Scholar]
- 92.Levy C, Khaled M, Iliopoulos D, Janas MM, et al. Intronic miR-211 assumes the tumor suppressive function of its host gene in melanoma. Mol Cel 2010;40:841–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mazar J, DeYoung K, Khaitan D, Meister E, et al. The regulation of miRNA-211 expression and its role in melanoma cell invasiveness. PLoS One 2010;5:e13779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Babapoor S, Fleming E, Wu R, Dadras SS. A novel miR-451a isomiR, associated with amelanotypic phenotype, acts as a tumor suppressor in melanoma by retarding cell migration and invasion. PLoS One 2014;9: e107502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Xu Y, Brenn T, Brown ERS, Doherty V, et al. Differential expression of microRNAs during melanoma progression: miR-200c, miR-205 and miR-211 are downregulated in melanoma and act as tumour suppressors. Br J Cancer 2012;106:553–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Duncan LM, Deeds J, Hunter J, Shao J, et al. Down-regulation of the novel gene melastatin correlates with potential for melanoma metastasis. Cancer Res 1998;58:1515–20. [PubMed] [Google Scholar]
- 97.Babapoor S, Horwich M, Wu R, Levinson S, et al. microRNA in situ hybridization for miR-211 detection as an ancillary test in melanoma diagnosis. Mod Pathol 2016;29:461–75. [DOI] [PubMed] [Google Scholar]
- 98.Matatall KA, Agapova OA, Onken MD, Worley LA, et al. BAP1 deficiency causes loss of melanocytic cell identity in uveal melanoma. BMC Cancer 2013;13:371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Soura E, Eliades PJ, Shannon K, Stratigos AJ, et al. Hereditary melanoma: update on syndromes and management. J Am Acad Dermatol 2016;74:411–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tetzlaff MT, Torres-Cabala CA, Pattanaprichakul P, Rapini RP, et al. Emerging clinical applications of selected biomarkers in melanoma. Clin Cosmet Investig Dermatol 2015;8:35–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Busam KJ, Sung J, Wiesner T, von Deimling A, et al. Combined BRAFV600E-positive melanocytic lesions with large epithelioid cells lacking BAP1 expression and conventional Nevomelanocytes. Am J Surg Pathol 2013;37:193–9. [DOI] [PubMed] [Google Scholar]
- 102.Wiesner T, Murali R, Fried I, Cerroni L, et al. A distinct subset of atypical spitz tumors is characterized by BRAF mutation and loss of BAP1 expression. Am J Surg Pathol 2012;36:818–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Piris A, Mihm MC, Hoang MP. BAP1 and BRAFV600E expression in benign and malignant melanocytic proliferations. Hum Pathol 2015; 46:239–45. [DOI] [PubMed] [Google Scholar]
- 104.Kalirai H, Dodson A, Faqir S, Damato BE, et al. Lack of BAP1 protein expression in uveal melanoma is associated with increased metastatic risk and has utility in routine prognostic testing. Br J Cancer 2014; 111:1373–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.van de Nes JAP, Nelles J, Kreis S, Metz CHD, et al. Comparing the prognostic value of BAP1 mutation pattern, chromosome 3 status, and BAP1 immunohistochemistry in uveal melanoma. Am J Surg Pathol 2016;40:796–805. [DOI] [PubMed] [Google Scholar]
- 106.van Essen TH, van Pelt SI, Versluis M, Bronkhorst IH, et al. Prognostic parameters in uveal melanoma and their association with BAP1 expression. Br J Ophthalmol 2014;98:1738–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Nardone B, Martini M, Busam K, Marghoob A, et al. Integrating clinical/dermatoscopic findings and fluorescence in situ hybridization in diagnosing melanocytic neoplasms with less than definitive histopathologic features. J Am Acad Dermatol 2012;66: 917–22. [DOI] [PubMed] [Google Scholar]
- 108.Ponti G, Ruini C, Massi D, Pellacani G, et al. Fluorescence in-situ hybridization and dermoscopy in the assessment of controversial melanocytic tumors. Melanoma Res 2013;23:474–80. [DOI] [PubMed] [Google Scholar]
- 109.Green RJ, Taghizadeh R, Lewis CJ, Lawrence C, et al. Melanocytic tumours of uncertain malignant potential (MELTUMPs)—a diagnostic and management dilemma. Eur J Plast Surg 2015;38: 13–6. [Google Scholar]
- 110.Marsch A, High WA. Medicolegal issues with regard to melanoma and pigmented lesions in dermatopathology. Dermatol Clin 2012;30: 593–615. [DOI] [PubMed] [Google Scholar]
- 111.Massi G Melanocytic nevi simulant of melanoma with medicolegal relevance. Virchows Arch 2007;451:623–47. [DOI] [PubMed] [Google Scholar]
- 112.LeBoit PE. What sentinel node biopsy in patients with melanoma (or patients whose doctors worry that they could have melanoma) might and might not do. Clin Dermatol 2009;27:588–93. [DOI] [PubMed] [Google Scholar]
- 113.Witheiler DD, Cockerell CJ. Sensitivity of diagnosis of malignant melanoma: a clinicopathologic study with a critical assessment of biopsy techniques. Exp Dermatol 1992;1:170–5. [DOI] [PubMed] [Google Scholar]
- 114.Dalton SR, Gardner TL, Libow LF, Elston DM. Contiguous lesions in lentigo maligna. J Am Acad Dermatol 2005;52:859–62. [DOI] [PubMed] [Google Scholar]
- 115.Sagebiel RW. Diagnosis and management of premalignant melanocytic proliferations. Pathology 1985;17:285–90. [DOI] [PubMed] [Google Scholar]
- 116.Cunningham C, Divekar P, Hohle R, Jenkins R, et al. Management of melanocytic tumour of uncertain malignant potential over 5 years 2009–2014. Br J Dermatol 2015;173(Suppl S1):21–76. [Google Scholar]
- 117.Etzkorn JR, Sobanko JF, Elenitsas R, Newman JG, et al. Low recurrence rates for in situ and invasive melanomas using Mohs micrographic surgery with melanoma antigen recognized by T cells 1 (MART-1) immunostaining: tissue processing methodology to optimize pathologic staging and margin assessment. J Am Acad Dermatol 2015;72:840–50. [DOI] [PubMed] [Google Scholar]
- 118.Iorizzo LJ, Chocron I, Lumbang W, Stasko T. Importance of vertical pathology of debulking specimens during Mohs micrographic surgery for lentigo maligna and melanoma in situ. Dermatol Surg 2013;39: 365–71. [DOI] [PubMed] [Google Scholar]
- 119.Kunishige JH, Brodland DG, Zitelli JA. Surgical margins for melanoma in situ. J Am Acad Dermatol 2012;66:438–44. [DOI] [PubMed] [Google Scholar]
- 120.Swetter SM, Chen FW, Kim DD, Egbert BM. Imiquimod 5% cream as primary or adjuvant therapy for melanoma in situ, lentigo maligna type. J Am Acad Dermatol 2015;72:1047–53. [DOI] [PubMed] [Google Scholar]
- 121.Breuninger H, Schlagenhauff B, Stroebel W, Schaumburg-Lever G, et al. Patterns of local horizontal spread of melanomas: consequences for surgery and histopathologic investigation. Am J Surg Pathol 1999; 23:1493–8. [DOI] [PubMed] [Google Scholar]
- 122.Tannous ZS, Mihm MC, Flotte TJ, González S. In vivo examination of lentigo maligna and malignant melanoma in situ, lentigo maligna type by near-infrared reflectance confocal microscopy: comparison of in vivo confocal images with histologic sections. J Am Acad Dermatol 2002;46:260–3. [DOI] [PubMed] [Google Scholar]
- 123.Langley RGB, Burton E, Walsh N, Propperova I, et al. In vivo confocal scanning laser microscopy of benign lentigines: comparison to conventional histology and in vivo characteristics of lentigo maligna. J Am Acad Dermatol 2006;55:88–97. [DOI] [PubMed] [Google Scholar]
- 124.van der Leest RJT, Flohil SC, Arends LR, de Vries E, et al. Risk of subsequent cutaneous malignancy in patients with prior melanoma: a systematic review and meta-analysis. J Eur Acad Dermatol Venereol 2015;29:1053–62. [DOI] [PubMed] [Google Scholar]
- 125.Meyers MO, Yeh JJ, Deal AM, Byerly FL, et al. Age and Breslow depth are associated with a positive sentinel lymph node in patients with cutaneous melanocytic tumors of uncertain malignant potential. J Am Coll Surg 2010;211:744–8. [DOI] [PubMed] [Google Scholar]
- 126.Berk DR, LaBuz E, Dadras SS, Johnson DL, et al. Melanoma and melanocytic tumors of uncertain malignant potential in children, adolescents and young adults-the stanford experience 1995–2008. Pediatr Dermatol 2010;27:244–54. [DOI] [PubMed] [Google Scholar]
- 127.McArthur GJ, Banwell ME, Cook MG, Powell BW. The role of sentinel node biopsy in the management of melanocytic lesions of uncertain malignant potential (MUMP). J Plast Reconstr Aesthet Surg 2007;60:952–4. [DOI] [PubMed] [Google Scholar]
- 128.Busam K FISH—applications and limitations for melanocytic lesions. Pigment Cel Melanoma Res 2010;23:993–4. [Google Scholar]