Abstract
Tumor necrosis factor-α (TNF-α) is initially synthesized as a transmembrane protein that is cleaved by TNF-α converting enzyme (TACE) to release soluble TNF-α. The elevated level of TNF-α in the brain and circulation in heart failure (HF) suggests an increase in the TACE-mediated ectodomain shedding process. The present study sought to determine whether TACE is upregulated in cardiovascular/autonomic brain regions like subfornical organ (SFO) and hypothalamic paraventricular nucleus (PVN) in rats with ischemia-induced HF and whether TACE plays a role in TNF-α-driven sympathetic excitation. We found that TACE was expressed throughout the SFO and PVN, with significantly higher levels in HF than in sham-operated (Sham) rats. Intracerebroventricular (ICV) injection of recombinant TACE induced a mild increase in blood pressure (BP), heart rate (HR) and renal sympathetic nerve activity (RSNA) that peaked at 15-20 min in both Sham and HF rats. HF rats had a secondary prolonged increase in these variables that was prevented by the TNF-α inhibitor SPD304. ICV administration of the TACE inhibitor TAPI-1 decreased BP, HR and RSNA in Sham and HF rats, with an exaggerated reduction in HR and RSNA in the HF rats. Direct microinjection of TACE or TAPI-1 into PVN or SFO of Sham and HF rats elicited BP, HR and RSNA responses similar to ICV TACE or TAPI-1. ICV infusion of angiotensin II and interleukin-1β increased TACE expression in SFO and PVN of normal rats. These data suggest that a TACE-mediated increase in soluble TNF-α in the brain contributes to sympathetic excitation in HF.
Keywords: transmembrane TNF-α, subfornical organ, cytokines, hypothalamic paraventricular nucleus
Graphical Abstract
INTRODUCTION
Increased peripheral and central nervous system levels of the pro-inflammatory cytokine tumor necrosis factor-α (TNF-α) have been implicated in the pathophysiology of a variety of cardiac, cerebrovascular and metabolic diseases, including systolic heart failure (HF), hypertension, atherosclerosis, stroke, obesity and diabetes mellitus.1–6 TNF-α is initially synthesized as a membrane bound protein, transmembrane TNF-α (tmTNF-α).7 TNF-α converting enzyme (TACE), a sheddase that is also identified as a disintegrin and metalloprotease 17 (ADAM17),8 proteolytically cleaves the extracellular domain of tmTNF-α to free the fully functional form of soluble TNF-α (sTNF-α), a process called ectodomain shedding.9–12 sTNF-α is responsible for most of the adverse actions of TNF-α.
An abnormal increase in TACE expression occurs in disease states associated with TNF-α-related inflammation in the periphery. The production of sTNF-α in the tissues and circulation can be greatly reduced by TACE inhibitors. Treatment with a TACE inhibitor provided a robust protection against LPS-induced endotoxin shock in vivo in mice.13 Knockdown of TACE prevented DOCA-salt-elicited hypertension in mice14 and thwarted the development of cardiac hypertrophy in spontaneously hypertensive rats.15 Inhibition of TACE activity improved insulin sensitivity in insulin-resistant hypertensive rats16 and effectively prevented focal ischemia-induced brain injury in the rat.17 These observations indicate that TACE plays an important role in variety of pathological conditions.
TACE is also associated with heart diseases in humans. TACE expression was significantly greater in the myocytes of patients with myocarditis or dilated cardiomyopathy, compared with control subjects.18, 19 The level of TACE expression increases with the severity of cardiac compromise and correlates with increased LV volume and decreased LV systolic function. In patients with acute myocardial infarction, the TACE level in the circulation and in myocytes is increased and is associated with elevated myocardial TNF-α.20, 21 These findings suggest that TACE may be an important contributor to cardiac dysfunction.
While TACE clearly has peripheral effects in cardiovascular disease states, the role of brain TACE in the regulation of sympathetic nerve activity has not yet been investigated. TNF-α is highly expressed in cardiovascular/autonomic regions of the brain in HF and contributes significantly to neurohumoral activation in the development of the disease.22, 23 The augmented level of the TNF-α in the brain and circulation in HF suggests a rise in TACE-mediated shedding process.
The present study sought to determine whether TACE expression is upregulated in cardiovascular and autonomic brain regions such as the subfornical organ (SFO) and hypothalamic paraventricular nucleus (PVN), in rats with ischemia-induced HF, and whether increased TACE activity in the brain, particularly in the PVN and SFO, contributes to TNF-α-induced sympathetic excitation in HF. We further evaluated factors that might upregulate TACE expression in SFO and PVN in HF.
METHODS
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Animals
Experiments were carried out on adult male Sprague-Dawley rats (225-275g), purchased from Envigo/Harlan Sprague Dawley (Indianapolis, IN). Animals were housed in temperature (23 ± 2°C) and light-controlled (12:12-h light-dark cycle) animal care facility at the University of Iowa. Standard rat chow and tap water were provided ad libitum. The experimental protocols performed in this study were approved by the University of Iowa Institutional Animal Care and Use Committee. The experimental procedures were conducted in accordance with the National Institutes of Health “Guide for the Care and Use of Laboratory Animals.”
HF and sham-operated (Sham) rats were produced by coronary artery ligation or sham surgery, as previously described 22, 24. The experimental protocols below were conducted approximately 4-5 weeks following induction of HF or sham operation.
Drugs and Drug Administration
Drugs were administered into the left lateral ventricle of the brain or microinjected into the PVN and SFO. Recombinant TACE was purchased from ACRO Biosystem (Newark, NJ). TAPI-1, a selective TACE/ADAM17 inhibitor, SPD-304, a small molecule TNF-α inhibitor,25 and angiotensin II (ANG II) were purchased from Sigma Aldrich (St. Louis, MO). IL-1β was purchased from EMD Millipore (Billerica, MA).
The doses of TAPI-1 (1μg) for ICV injection or PVN and SFO microinjection were derived from a study26 indicating that infusion of TAPI-1 into the hippocampus of anesthetized rats at a dose of 0.75 μg significantly inhibited TACE/ADAM17 activity. The dose of SPD304 (5μg) was based on a dose (2.5μg) used for 4th cerebroventricular injection to effectively inhibit the effect of TNF-α in a previously published report.27
Experimental protocols
TACE expression and activity in SFO and PVN in Sham and HF rats
-
1)
Sham (n=6) and HF (n=6) rats were anesthetized with urethane (1.5g/kg) and transcardially perfused with heparinized saline followed by 4% paraformaldehyde in 0.01M PBS. The collected brains were cut into 16μm coronal sections for immunofluorescent studies to examine the expression of TACE in PVN and SFO, and its expression in neurons and astrocytes.
-
2)
Sham (n=12) and HF (n=12) rats were anesthetized with urethane and decapitated immediately. The brain tissues were rapidly removed and frozen in liquid nitrogen. The PVN (including some surrounding tissue) and the SFO were harvested to measure mRNA or protein level of TACE by real time PCR and Western blot, respectively.
-
3)
Sham (n=12) and HF (n=12) rats were anesthetized with urethane and treated with ICV injection of vehicle (VEH) or TAPI-1. At 90 mins after injection, these rats were decapitated, and the brain tissues were rapidly removed and frozen in liquid nitrogen. The PVN (including some surrounding tissue) and the SFO were harvested to measure TACE activity using TACE Activity Assay Kit (Anaspec, Inc), Fremont, CA).
TACE effects on sympathetic nerve activity and hemodynamics in Sham and HF rats
Sham and HF rats were anesthetized with urethane and underwent electrophysiological recordings of renal sympathetic nerve activity (RSNA), arterial blood pressure (BP), and heart rate (HR) in the following treatment groups:
-
1)
Sham (n=6) and HF (n=6) rats were treated with ICV injection of TACE (300 ng in 3μl aCSF over 1 min). Artificial cerebrospinal fluid (aCSF) served as vehicle (VEH) control.
-
2)
Sham (n=6) and HF (n=6) rats were treated with ICV injection of TACE (300 ng in 3μl aCSF over 1 min) immediately following ICV pretreatment with the TNF-α inhibitor SPD 304 (5 μg in 10μl 5% DMSO in aCSF over 5min). 5% DMSO in aCSF served as VEH control.
-
3)
Sham (n=6) and HF (n=6) rats were treated with ICV injection of the TACE inhibitor TAPI-1 (1 μg in 4 μl 5% DMSO in aCSF over 1 min). 5% DMSO in aCSF served as vehicle control.
-
4)
Sham (n=12) and HF (n=12) rats were treated with bilateral PVN microinjection of TACE (50 ng/side) and TAPI-1 (0.25 μg/side). 5% DMSO in aCSF served as vehicle control.
-
5)
Sham (n=8) and HF (n=8) rats were treated with SFO microinjection of TACE (50 ng) and TAPI-1 (0.25 μg). 5% DMSO in aCSF served as vehicle control.
Effect of ICV ANG II and IL-1β on TACE expression in SFO and PVN in normal rats
Urethane-anesthetized normal rats received an ICV infusion of vehicle (a CSF, 20μl, n=15), ANG II (400 ng in 20μl, n=15) or IL-1β (400 ng in 20μl, n=15) over 4 hours.
-
1)
Six hours after initiating the injection, rats were decapitated, and the brain tissues were rapidly removed and frozen in liquid nitrogen. The PVN (including some surrounding tissue) and the SFO were harvested to measure mRNA (n=6) and protein (n=6) levels of TACE.
-
2)
Some rats (n=3) from each group were transcardially perfused with 4% paraformaldehyde and the brains were collected for immunofluorescent studies to examine TACE expression in the PVN and SFO.
Specific Materials and Methods
Please see the online data supplement (http://hyper.ahajournals.org).
Statistical Analysis
Electrophysiological recording data of sympathetic nerve activity and hemodynamics were analyzed with CED Spike2 software (Cambridge Electronic Design). Responses of mean arterial pressure (MAP, mmHg), HR in beats per minute (bpm), windowed RSNA (spikes/s) and integrated RSNA (mV) sampled over 5 min intervals after ICV injection or PVN microinjection were compared with baseline values averaged over 5 min intervals immediately preceding each intervention. Integrated voltage (mV) was used for statistical analysis of RSNA, and was reported as a percentage change from baseline control. The level of TACE protein in Western analysis was expressed as a ratio normalized to β-actin and the level of TACE mRNA was stated as fold change compared to Sham or VEH control. The TACE immunofluorescent intensity was reported as arbitrary units (AU) and TACE activity was reported as relative fluorescence units (RFU) per mg of protein. All values are expressed as the mean ± SEM. The significance of differences among groups was analyzed by one-way or two-way repeated-measure ANOVA followed by post hoc Fisher’s test. For other unpaired data, a Student’s t-test was used for comparison between groups. p< 0.05 was considered to indicate statistical significance.
RESULTS
Echocardiographic assessment of heart failure
HF was echocardiographically defined by left ventricular (LV) ejection fraction (LVEF) and ischemic zone as a percent of the LV circumference (%IZ). Animals with % IZ >35 and LVEF < 40 % were assigned to the HF treatment groups. %IZ and LVEF did not differ among the HF treatment groups. LV end-diastolic volume (LVEDV) was significantly increased and LVEF was greatly reduced in HF compared with Sham rats (Table S1).
TACE expression in PVN and SFO in Sham and HF rats
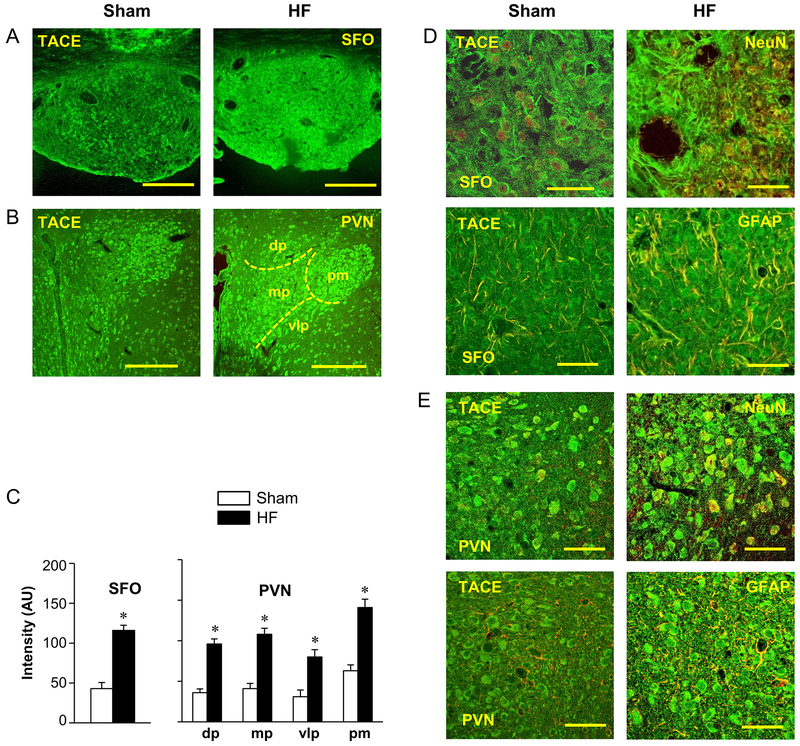
Immunofluorescent studies revealed substantial expression of TACE-like immunoreactivity in the SFO and PVN (Figure 1) in both Sham and HF rats. In SFO, TACE was evenly expressed throughout the nucleus, but with an upregulated expression of TACE-like immunoreactivity (fluorescent units) in HF compared with Sham rats (Figure 1.A and 1.C). In PVN, TACE was distributed in all four commonly recognized subdivisions - the dorsal parvocellular (PVN-dp), the ventrolateral parvocellular (PVN-vlp), the medial parvocellular (PVN-mp) and the posterior magnocellular (PVN-pm) - in both Sham and HF rats. The PVN-pm had higher densities of TACE-like immunoreactivity than PVN-dp, PVN-mp and PVN-vlp in Sham rats. In HF rats, compared to the Sham, TACE-like immunoreactivity was more intense in all four subdivisions (HF vs Sham, * p<0.05), especially in PVN-vlp and PVN-mp subdivisions (Figure 1.B and 1.C).
Figure 1.
Laser confocal images showing expression of TNF-α converting enzyme (TACE, green) in (A) subfornical organ (SFO) and (B) hypothalamic paraventricular nucleus (PVN) of sham-operated (Sham) and heart failure (HF) rats. Rough boundaries of the four subdivisions of the PVN are indicated. dp: dorsal parvocellular subdivision of PVN; mp: medial parvocellular subdivision of PVN; vlp: ventrolateral parvocellular subdivision of PVN; pm: posterior magnocellular subdivision of PVN. Scale bar: 200 μm. (C) Quantification of TACE immunoreactivity in SFO and PVN in Sham and HF rats. Values in arbitrary units (AU) are expressed as mean ± SEM. * p<0.05, compared to Sham rats (n=6 in each group). (D, E) High power images showing the co-localized expression (merged: yellow) of TACE (green) with NeuN (left, red) and the astrocyte marker GFAP (right, red) in SFO (D) and PVN (E) in a HF rat. Scale bar: 50 μm.
Confocal immunofluorescent images in Sham and HF rats also revealed that TACE colocalized with the neuronal marker NeuN and the astrocyte marker GFAP, indicating that TACE was expressed by and may be synthesized in both neurons and astrocytes in the SFO (Figure 1.D) and PVN (Figure 1.E).
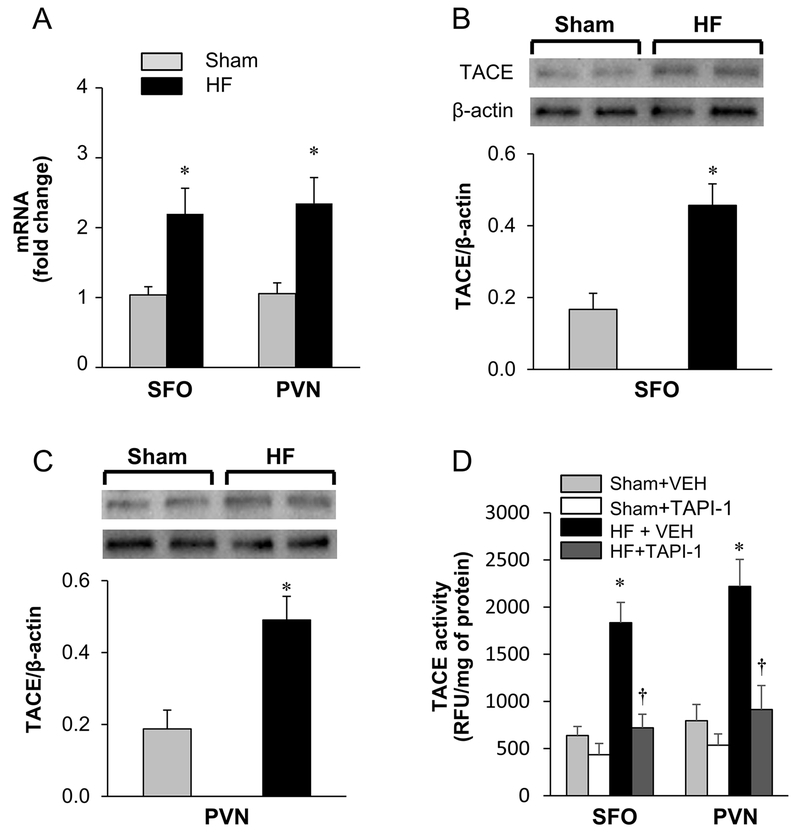
Molecular studies confirmed the HF-induced increase in TACE expression in SFO and PVN (Figure 2). RT-PCR analysis revealed that the mRNA levels of TACE were elevated (p<0.05*) in the SFO (2.2 fold*) and PVN (2.5 fold*) of HF rats (n=6) compared with Sham rats (Figure 2.A). Western blot analysis confirmed that the protein level of TACE was also significantly higher in the SFO (Figure 2.B) and PVN (Figure 2.C) of HF than Sham control rats.
Figure 2.
(A) Real-time PCR indicating the fold change of TACE mRNA in subfornical organ (SFO) and hypothalamic paraventricular nucleus (PVN) in sham-operated (Sham) and heart failure (HF) rats. (B and C) Western blot analysis showing the expression of TACE protein in SFO and PVN in Sham and HF rats. (D) TACE activity in the SFO and PVN in Sham and HF rats treated with ICV vehicle or TAPI-1. Values are expressed as mean ± SEM. Western data is normalized to β-actin. * p<0.05, compared to Sham (n=6 in each group). † p<0.05, compared with HF + vehicle (VEH).
TACE activity in SFO and PVN in Sham and HF rats
In HF rats treated with ICV VEH, TACE activity in the SFO (HF vs. Sham: 1834 ± 215 vs. 638 ± 96 RFU) and PVN (HF vs. Sham: 2220 ± 285 vs. 796 ± 172 RFU) was higher than that in Sham rats treated with ICV VEH. After treatment with TACE inhibitor TAPI-1, TACE activity in SFO (720 ± 144 RFU) and PVN (913 ± 255 RFU) of HF is significantly reduced. Treatment with ICV TAPI-1 didn’t significantly alter TACE activity in SFO (435 ± 119 RFU) and PVN (536 ± 120 RFU) in Sham rats (Figure 2.D).
Hemodynamic and Sympathetic effects of TACE in Sham and HF rats
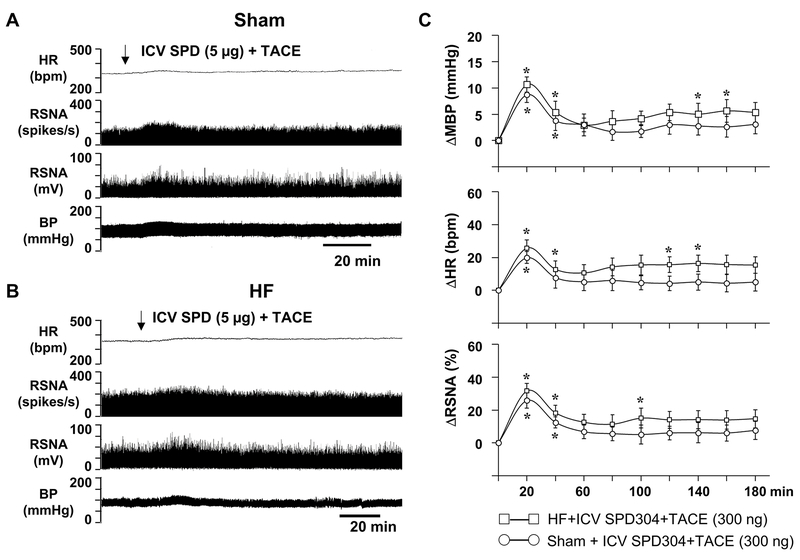
In Sham rats (n=6), ICV administration of TACE (300 ng) induced a rapid and mild increase in MAP (8.9±1.2 mmHg*), HR (17.6±3.2*) and RSNA (25.5±2.7*) that peaked at 15-20 min and returned to baseline within 40 min after the injection (Figure 3.A and 3.C). In HF rats (n=6), ICV injection of the same dose of TACE (300 ng) elicited a two-phase response - an early small response similar to that induced by TACE in Sham rats, and a later long-lasting response with the peak increase in MAP (12.6± 1.7* mmHg), HR (35.2 ± 4.5* bpm) and RSNA (41.5±5.2* % change) occurring 2-3 hours after the injection (Figure 3.B and 3.C). The late excitatory response in MBP, HR and RSNA was sustained at a higher than baseline level for the rest of 4-5 hour recording period, closely resembling the pattern and time course of ICV TNF-α-induced increases in these variables in normal animals. However, the baseline levels of MBP (75.4 ± 3.2 mmHg), HR (338 ± 8 bpm) and integrated RSNA (10.9 ±1.7 mV) in HF rats were different from the baseline levels of MBP (86.5 ± 3.1 mmHg), HR (312 ± 9 bpm) and integrated RSNA (8.0 ± 1.1 mV) in Sham rats. ICV injection of an equal volume of vehicle (aCSF) did not change MAP, HR and RSNA in Sham or HF rats (data not shown).
Figure 3.
Representative tracings showing the effects of intracerebroventricular (ICV) TACE on blood pressure (BP), heart rate (HR) and renal sympathetic nerve activity (RSNA) in (A) sham-operated (Sham) and (B) heart failure (HF) rats. BP, mmHg; HR, beats/min (bpm): RSNA, windowed (spikes/s) and integrated (mV) activity. (C) Grouped data showing the change (Δ) from baseline in mean blood pressure (MBP, mmHg), HR (bpm) and RSNA elicited by ICV TACE in Sham and HF rats. Integrated RSNA was used for data analysis. Values are expressed as mean ± SEM (n=6 in each group). * p<0.05, compared with baseline. † p<0.05, compared with Sham.
Effects of TNF-α inhibitor on TACE-induced changes in MAP, HR and RSNA in Sham and HF rats
In Sham rats (n=6), ICV pretreatment with the SPD304, a small molecule inhibitor that prevents the binding of TNF-α to its receptors, had no effect on ICV TACE-induced early excitatory responses in MAP, HR and RSNA (Figure 4.A, compared with Figure 3A). In HF rats, ICV pretreatment with SPD304 (5 μg) blocked the TACE-induced late prolonged excitatory responses in MAP, HR and RSNA, but the early excitatory responses in MAP, HR and RSNA persisted (Figure 4.B, compared with Figure 3B). The baseline levels of MBP, HR and RSNA in Sham or HF rats in this group treated with SPD304 and TACE were not different from those of Sham or HF animals treated with TACE only in Figure 3. ICV pretreatment with vehicle for SPD304 (aCSF with 5% DMSO) did not affect the TACE-induced increases in MAP, HR and RSNA in Sham and HF rats (data not shown).
Figure 4.
Representative tracings showing the effects of intracerebroventricular (ICV) TACE on blood pressure (BP), heart rate (HR) and renal sympathetic nerve activity (RSNA) in (A) sham-operated (Sham) and (B) heart failure (HF) rats pretreated with ICV TNF-α inhibitor SPD 304. BP, mmHg; HR, beats/min (bpm): RSNA, windowed (spikes/s) and integrated (mV) activity. (C), Grouped data showing the change (Δ) from baseline in mean blood pressure (MBP, mmHg), HR (bpm) and RSNA (%) elicited by ICV TACE in Sham and HF rats pretreated with SPD 304. Integrated RSNA was used for data analysis. Values are expressed as mean ± SEM (n=6 in each group). * p<0.05, compared with baseline. † p<0.05, compared with Sham.
Hemodynamic and sympathetic effects of TACE inhibitor TAPI-1 in Sham and HF rats
ICV administration of the TACE inhibitor TAPI-1 (1 μg) induced decreases in MAP (9.1± 1.4* mmHg), HR (18.6 ± 2.5* bpm) and RSNA (19.4 ±3.9* % change) in sham animals (n=6) (Figure 5.A and 5.C). In HF rats, ICV TAPI-1 caused a substantially larger (*p<0.05) reduction in HR (28.5 ± 3.0* bpm) and RSNA (33.5 ± 4.2* % change) but a similar drop in MAP (6.8 ± 0.8 mmHg) (Figure 5.B and 5.C). ICV injection of an equal volume of vehicle for TAPI-1 (aCSF with 5% DMSO) had no noticeable effect on MBP, HR and RSNA in Sham and HF rats (data not shown).
Figure 5.
Representative tracings showing the effects of intracerebroventricular (ICV) TACE inhibitor TAPI-1 on blood pressure (BP), heart rate (HR) and renal sympathetic nerve activity (RSNA) in (A) sham-operated (Sham) and (B) heart failure (HF) rats. BP, mmHg; HR, beats/min (bpm): RSNA, windowed (spikes/s) and integrated (mV) activity. (C), Grouped data showing the change (Δ) from baseline in mean blood pressure (MBP, mmHg), HR (bpm) and RSNA (%) elicited by ICV TAPI-1 in Sham and HF rats. Integrated RSNA was used for data analysis. Values are expressed as means ± SEM (n=6 in each group). * p<0.05, compared with baseline; † p<0.05 compared with Sham.
Effects of microinjection of TACE or TAPI-1 into PVN and SFO on MAP, HR and RSNA in Sham and HF rats
Like ICV injection of TACE, bilateral PVN microinjection of TACE (50 ng/side) in either Sham or HF rats induced mild excitatory response in MAP, HR and RSNA that peaked at 10-15 mins after injection. Like ICV administration, bilateral PVN microinjection of TACE elicited both an initial and a late long-lasting excitatory response on MAP, HR and RSNA that peaked at 1.5-2 hours in HF rats (Figure 6.A, 6.B and 6.E). Bilateral PVN microinjection of TACE inhibitor TAPI-1 caused decreases in MBP (90.7 ± 2.8 to 82.5 ± 2.1 mmHg), HR (306 ± 7 to 291 ± 6 bpm ) and integrated RSNA (−16.3 ± 2.2 % change from a baseline of 10.7 ± 2.0 mV) in Sham rats, and even greater reductions in MBP (from 81.5 ± 2.3 to 68.1±1.7 mmHg), HR (328 ± 8 to 304 ± 6 bpm) and integrated RSNA (−28.1±3.3 % change from a baseline of 15.0 ± 2.4 mV) in HF rats, measured at 30-45 min (Figure 6C, 6D and 6E). In the HF rats, the responses to TAPI-1 began within 5-10 minutes after PVN microinjection and were sustained for 1-2 hours.
Figure 6.
Representative tracings showing the effects of bilateral PVN microinjection of TACE (A and B) and its inhibitor TAPI-1 (C and D) on blood pressure (BP), heart rate (HR) and renal sympathetic nerve activity (RSNA) in sham-operated (Sham) and heart failure (HF) rats. BP, mmHg; HR, beats/min (bpm): RSNA, windowed (spikes/s) and integrated (mV) activity. (E), Grouped data showing the change (Δ) from baseline in mean blood pressure (MBP, mmHg), HR (bpm) and integrated RSNA (%) elicited by PVN microinjection of TACE and TAPI-1 in Sham and HF rats. Values are expressed as means ± SEM (n=6 in each group). * p<0.05, compared with baseline; † p<0.05 compared with corresponding Sham. Bar scale: 15 min.
SFO microinjection of TACE or TAPI-1 induced similar responses in BP, HR and RSNA with bilateral PVN microinjection of these two reagents in Sham and HF, respectively (Figure S1 and S2). PVN and SFO microinjection of an equal volume of vehicle (5% DMSO in aCSF) had no effect on BP, HR and RSNA in either Sham or HF rats.
Effect of ICV ANG II and IL-1β on TACE expression in SFO and PVN in normal rats
In normal rats, ICV injection of ANG II or IL-1β significantly (*p<0.05 vs. aCSF) increased TACE mRNA level in SFO (2.06 ± 0.21* and 1.85 ± 0.18*, respectively) and PVN (2.13 ± 0.20* and 1.95 ± 0.21*, respectively) compared with ICV aCSF (Figure S3.A). ICV ANG II and IL-1β did not affect TACE mRNA in the cerebral cortex. Western blot analysis also revealed a significant (*p<0.05) increase in TACE protein in SFO (0.44±0.06* and 0.47±0.05* respectively) and PVN (0.51 ± 0.06*, 0.47 ± 0.07*, respectively) of ANG II or IL-1β-infused rats (Figure S3.B), compared with VEH-treated rats (SFO, 0.06 ± 0.04; PVN, 0.08 ± 0.05). Similarly, confocal images exhibited that the expression of TACE-like immunoreactivity was substantially higher throughout all subdivisions of PVN and the SFO in the rats treated with ICV ANG II or IL-1β, compared with aCSF-infused rats (Figure S3.C).
DISCUSSION
We previously reported that both central and peripheral administration of TNF-α increases sympathetic outflow28, 29 and upregulates expression of TNF-α and its TNFR1 receptor in cardiovascular/autonomic regions of the brain in HF.22, 23 In the present study, we demonstrated an important role for TACE in mediating TNF-α-driven sympathetic excitation in the brain in ischemia-induced HF, likely via an ectodomain shedding mechanism. The major novel findings are: 1) Expression of TACE by neurons and astrocytes is upregulated in the SFO and PVN of rats with HF and TACE activity is also increased in the brain in HF; 2) ICV TACE induces an early excitatory response in hemodynamics and sympathetic nerve activity in both Sham and HF animals, but a long-lasting pressor response in HF rats that can be blocked by pretreatment with a TNF-α inhibitor; 3) ICV administration of a TACE inhibitor decreases TACE activity in PVN and SFO and reduces MAP, HR and RSNA in Sham and HF animals, but the reduction in HR and RSNA is exaggerated in rats with HF; 4) Direct microinjection of TACE and its inhibitor TAPI-1 into PVN or SFO caused MAP, HR and RSNA responses similar to ICV administration, and 5) ICV administration of ANG II and IL-1β increases TACE activity in SFO and PVN in normal rats. These findings suggest that TACE activity is upregulated in cardiovascular regions of the brain in HF and that TACE-mediated ectodomain shedding is an important inflammatory mechanism contributing to sympathetic excitation. TACE is a potential target for therapeutic intervention in chronic inflammatory states like HF and hypertension.
TACE is found in peripheral tissues and the brain.30, 31 In the present study, we found that TACE is abundantly expressed in cardiovascular and autonomic brain regions such as PVN and SFO and can be found in both neurons and glial cells. The expression of TACE in the astrocytes and neurons implies that TACE may be produced by either cell type in the brain. We also observed some other unidentified cells in SFO and PVN expressing TACE. Those cellular elements may contain microglia, endothelial cells and perivascular macrophages in the brain vessels. We further discovered that TACE mRNA and protein as well as TACE activity are markedly upregulated in SFO and PVN in HF rats, suggesting that upregulation of TACE activity in these regions may contribute to the central inflammation in HF. A similar upregulation of TACE/ADAM17 expression in hypothalamus has been reported in the DOCA-salt hypertensive mice.14 Chronic treatment with TACE/ADAM17 siRNA reversed its expression and increased ACE2 activity in the brain to counter the effect of RAS activation on hypertension in this model. We predict that the higher level of TACE in cardiovascular/autonomic regions of the brain could cause a prolonged inflammation and have a major impact on hemodynamics and renal sympathetic nerve activity in the development of HF and hypertension. Notably, TACE is also expressed in brain regions other than the SFO and PVN and may have similar or diverse functions in central nervous system.31–33
An intriguing finding of the present study is that central administration of TACE induced a prolonged pressor response in the HF rats, not observed in the Sham animals. This delayed excitatory response, which closely resembles the time course and response pattern induced by ICV TNF-α in normal animals, was blocked by pretreatment with a TNF-α inhibitor SPD304 that had no effect on the early TACE-induced response in Sham or HF rats. These observations suggest that the later long-lasting excitatory response to ICV TACE in HF is TNF-α driven and is probably caused by newly produced sTNF-α in the brain. We hypothesize that levels of TACE and tmTNF-α increase in the brain in HF, eventually reaching a steady state that promotes the excessive production of sTNF-α and augmented sympathetic nerve activity. ICV administration of exogenous TACE under these conditions likely induces the production of still more sTNF-α to further promote sympathetic excitation, while inhibition of TACE activity reduces the level of soluble TNF-α and thus attenuates the sympathetic activation in HF. In contrast, in Sham rats exogenous TACE, acting upon the relatively low ambient levels of tmTNF-α under normal conditions, is unable to produce excessive sTNF-α and the prolonged excitatory responses observed in the HF rats. This interpretation is consistent with the more pronounced decrease in HR and RSNA in HF than Sham rats induced by the TACE inhibitor TAPI-1. Comparable BP, HR and RSNA responses were observed with direct microinjection of TACE and its inhibitor TAPI-1 into the PVN or SFO, indicating that TACE activity in these two major autonomic regions contributes to the central inflammatory effect on sympathetic excitation in HF.
Though its major function as a TNF-α sheddase is to cleave transmembrane TNF-α to release sTNF-α, TACE is also responsible for shedding of a number of other membrane-anchored proteins including transforming growth factor-α, epidermal growth factor receptor, amyloid precursor proteins, and chemokine fractalkine/CX3CL1.34–36 Additionally, TACE potentiates RAS activity in activated conditions by mediating ANG II–induced suppression of ACE2 activity.14, 37 Whereas our data suggests that the late long-lasting response to TACE in HF is most likely due to its shedding effect on sTNF-α production in the brain, the early pressor responses to ICV TACE in both Sham and HF rats that were not blocked by the TNF-α inhibitor SPD304 may be elicited by TACE-mediated cleavage of other substrates. Identifying the active molecules that contribute to the early excitatory response, which is nearly identical in Sham and HF animals, is beyond the scope of this study.
TACE is upregulated in the brain in HF by mechanisms that are not yet fully understood. In the present study, we examined two potential mechanisms for upregulation of TACE activity in the HF brain. Brain renin-angiotensin system activity is increased in HF, and our data demonstrate that ICV ANG II increases TACE expression in the brain. This observation is consistent with a previous report showing that ANG II–induced cardiac hypertrophy and fibrosis is effectively prevented by knockdown of TACE expression with its small-interfering RNA.15 Seemingly, TACE has biological function associated with the downstream effects of ANG II. It is well-documented that renin-angiotensin system is activated in the brain38, 39 and contributes largely for the activation of a number of downstream events in HF, including ROS production40 and inflammation.41 Thus, an increase in locally produced ANG II or upregulation of its receptor in the brain may be responsible for upregulated TACE activity in HF setting. Inflammation in cardiovascular regions of the brain is another characteristic of HF.23, 42 We found that ICV injection of IL-1β also increases TACE expression in the PVN and SFO, suggesting that the high inflammatory condition in the brain has a potential to increase TACE activity by neurons or glial cells. In the context of HF, upregulated expression of IL-1β42 and/or other cytokines like IL-643 in the hypothalamus could be drivers of TACE activity in the brain. This result also raises a possibility of the positive feedback loop by TNF-α to promote TACE activity in conditions like HF and hypertension.
How ANG II or IL-1β prompts TACE activity in the brain remains a mystery. MAPK and oxidative stress have been shown to promote the expression of TACE/ADAM17 or its activity.44–46 A recent in vitro study from Lazartigues group,47 using cultured primary hypothalamic neurons, demonstrated that ANG II-increased TACE/ADAM17 activity was entirely prevented by the inhibitors for MAPK and NADPH oxidase, suggesting ANG II-enhanced TACE activity is mediated by MAPK signaling pathways and oxidative stress. Both ANG II and cytokines excite their G protein-coupled receptors to drive oxidative stress by increasing reactive oxygen species and activate mitogen-activated protein kinase (MAPK).48 Therefore, TACE activity could be elevated in the brain by exposure to mediators that have a potential to activate MAPK or cause oxidative stress. We have previously demonstrated that three major effector kinases of MAPK family were highly phosphorylated in PVN and SFO of HF rats.48
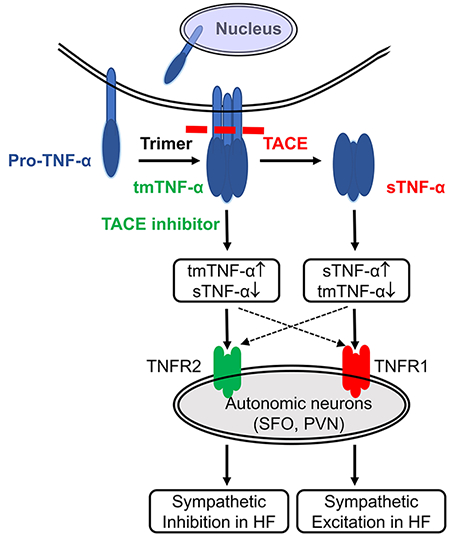
Perspectives
The role of TNF-α in the regulation of cardiovascular function and sympathetic outflow in HF has been well-documented in our studies and others. Those studies have focused on the adverse inflammatory effects of the soluble form of TNF-α. sTNF-α and tmTNF-α both exhibit biological functions, but they may have opposing actions. sTNF-α elicits pro-inflammatory and toxic responses by binding predominantly to TNF-α receptor 1,49 but tmTNF-α may have anti-inflammatory and protective effects by binding preferentially to TNF receptor 2.50, 51 TACE acts by cleaving sTNF-α from tmTNF-α via an ectodomain shedding process. Thus, interventions that lessen brain TACE activity have the potential to reduce the generation of the inflammatory sTNF-α and raise the level of the anti-inflammatory tmTNF-α. This approach holds promise for the development of novel therapeutic agents to ameliorate sympathetic over-activation in HF.
The present study has revealed an important role of TACE in the regulation of sympathetic drive and suggests that ectodomain shedding by TACE is a critical mechanism for TNF-α-caused sympathetic activation in HF. This study provides new insights into the central inflammatory mechanisms in HF, with important implications for other cardiovascular and metabolic diseases like hypertension and obesity that share the similar neurohumoral mechanisms.
Supplementary Material
Novelty and Significance.
-
1)
What Is New?
TNF-α converting enzyme (TACE/ADAM17) is richly expressed in neurons and astrocytes in the PVN and SFO and is upregulated in HF rats.
Central administration of TACE induces a two-phase response in BP, HR and RSNA in HF rats. The second prolonged excitatory response on these variables is blocked by a TNF-α inhibitor.
Central administration of ANG II or the inflammatory cytokine IL-1β increases TACE expression in the brain.
-
2)
What Is Relevant?
This study reveals a previously unrecognized role for brain TACE in the regulation cardiovascular function and sympathetic outflow in heart failure.
TACE in the brain, particularly in the PVN and SFO, may play an important role in TNF-α–driven central inflammation in pathophysiological conditions like hypertension and heart failure.
-
3)
Summary
The data suggest that elevated TACE activity in autonomic regions of the brain contributes to the augmented sympathetic activity in HF. Overactivity of the renin-angiotensin system and elevated inflammatory mediators play a role in promoting TACE activity in the brain in HF.
ACKNOWLEDGEMENTS
We thank Kathy Zimmerman, RDMS, RDCS, FASE, for diligent and expert assistance in the performance of the echocardiograms to assess cardiac function.
SOURCES OF FUNDING
This study was supported by research grants from National Institutes of Health grants R01 HL-139521 (to S-G. Wei), RO1 HL-073986 (to R.B. Felder) and S10 OD-019941 (to R.M. Weiss). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
DISCLOSURES
None
REFERENCES
- 1.Levine B, Kalman J, Mayer L, Fillit HM and Packer M. Elevated circulating levels of tumor necrosis factor in severe chronic heart failure. N Engl J Med. 1990;323:236–41. [DOI] [PubMed] [Google Scholar]
- 2.Mehaffey E and Majid DSA. Tumor necrosis factor-alpha, kidney function, and hypertension. Am J Physiol Renal Physiol. 2017;313:F1005–F1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hotamisligil GS and Spiegelman BM. Tumor necrosis factor alpha: a key component of the obesity-diabetes link. Diabetes. 1994;43:1271–8. [DOI] [PubMed] [Google Scholar]
- 4.Zinman B, Hanley AJ, Harris SB, Kwan J and Fantus IG. Circulating tumor necrosis factor-alpha concentrations in a native Canadian population with high rates of type 2 diabetes mellitus. J Clin Endocrinol Metab. 1999;84:272–8. [DOI] [PubMed] [Google Scholar]
- 5.Clausen BH, Lambertsen KL, Babcock AA, Holm TH, Dagnaes-Hansen F and Finsen B. Interleukin-1beta and tumor necrosis factor-alpha are expressed by different subsets of microglia and macrophages after ischemic stroke in mice. J Neuroinflammation. 2008;5:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feldman AM, Combes A, Wagner D, Kadakomi T, Kubota T, Li YY and McTiernan C. The role of tumor necrosis factor in the pathophysiology of heart failure. J Am Coll Cardiol. 2000;35:537–44. [DOI] [PubMed] [Google Scholar]
- 7.Pennica D, Nedwin GE, Hayflick JS, Seeburg PH, Derynck R, Palladino MA, Kohr WJ, Aggarwal BB and Goeddel DV. Human tumour necrosis factor: precursor structure, expression and homology to lymphotoxin. Nature. 1984;312:724–9. [DOI] [PubMed] [Google Scholar]
- 8.Gearing AJ, Beckett P, Christodoulou M, Churchill M, Clements J, Davidson AH, Drummond AH, Galloway WA, Gilbert R, Gordon JL and et al. Processing of tumour necrosis factor-alpha precursor by metalloproteinases. Nature. 1994;370:555–7. [DOI] [PubMed] [Google Scholar]
- 9.Kriegler M, Perez C, DeFay K, Albert I and Lu SD. A novel form of TNF/cachectin is a cell surface cytotoxic transmembrane protein: ramifications for the complex physiology of TNF. Cell. 1988;53:45–53. [DOI] [PubMed] [Google Scholar]
- 10.Horiuchi K A brief history of tumor necrosis factor alpha--converting enzyme: an overview of ectodomain shedding. Keio J Med. 2013;62:29–36. [DOI] [PubMed] [Google Scholar]
- 11.Black RA, Rauch CT, Kozlosky CJ, Peschon JJ, Slack JL, Wolfson MF, Castner BJ, Stocking KL, Reddy P, Srinivasan S, Nelson N, Boiani N, Schooley KA, Gerhart M, Davis R, Fitzner JN, Johnson RS, Paxton RJ, March CJ and Cerretti DP. A metalloproteinase disintegrin that releases tumour-necrosis factor-alpha from cells. Nature. 1997;385:729–33. [DOI] [PubMed] [Google Scholar]
- 12.Moss ML, Jin SL, Milla ME, Bickett DM, Burkhart W, Carter HL, Chen WJ, Clay WC, Didsbury JR, Hassler D, Hoffman CR, Kost TA, Lambert MH, Leesnitzer MA, McCauley P, McGeehan G, Mitchell J, Moyer M, Pahel G, Rocque W, Overton LK, Schoenen F, Seaton T, Su JL, Becherer JD and et al. Cloning of a disintegrin metalloproteinase that processes precursor tumour-necrosis factor-alpha. Nature. 1997;385:733–6. [DOI] [PubMed] [Google Scholar]
- 13.Mohler KM, Sleath PR, Fitzner JN, Cerretti DP, Alderson M, Kerwar SS, Torrance DS, Otten-Evans C, Greenstreet T, Weerawarna K and et al. Protection against a lethal dose of endotoxin by an inhibitor of tumour necrosis factor processing. Nature. 1994;370:218–20. [DOI] [PubMed] [Google Scholar]
- 14.Xia H, Sriramula S, Chhabra KH and Lazartigues E. Brain angiotensin-converting enzyme type 2 shedding contributes to the development of neurogenic hypertension. Circ Res. 2013;113:1087–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang X, Oka T, Chow FL, Cooper SB, Odenbach J, Lopaschuk GD, Kassiri Z and Fernandez-Patron C. Tumor necrosis factor-alpha-converting enzyme is a key regulator of agonist-induced cardiac hypertrophy and fibrosis. Hypertension. 2009;54:575–82. [DOI] [PubMed] [Google Scholar]
- 16.Togashi N, Ura N, Higashiura K, Murakami H and Shimamoto K. Effect of TNF-alpha--converting enzyme inhibitor on insulin resistance in fructose-fed rats. Hypertension. 2002;39:578–80. [DOI] [PubMed] [Google Scholar]
- 17.Wang X, Feuerstein GZ, Xu L, Wang H, Schumacher WA, Ogletree ML, Taub R, Duan JJ, Decicco CP and Liu RQ. Inhibition of tumor necrosis factor-alpha-converting enzyme by a selective antagonist protects brain from focal ischemic injury in rats. Mol Pharmacol. 2004;65:890–6. [DOI] [PubMed] [Google Scholar]
- 18.Satoh M, Nakamura M, Saitoh H, Satoh H, Maesawa C, Segawa I, Tashiro A and Hiramori K. Tumor necrosis factor-alpha-converting enzyme and tumor necrosis factor-alpha in human dilated cardiomyopathy. Circulation. 1999;99:3260–5. [DOI] [PubMed] [Google Scholar]
- 19.Satoh M, Nakamura M, Satoh H, Saitoh H, Segawa I and Hiramori K. Expression of tumor necrosis factor-alpha--converting enzyme and tumor necrosis factor-alpha in human myocarditis. J Am Coll Cardiol. 2000;36:1288–94. [DOI] [PubMed] [Google Scholar]
- 20.Akatsu T, Nakamura M, Satoh M and Hiramori K. Increased mRNA expression of tumour necrosis factor-alpha and its converting enzyme in circulating leucocytes of patients with acute myocardial infarction. Clin Sci (Lond). 2003;105:39–44. [DOI] [PubMed] [Google Scholar]
- 21.Shimoda Y, Satoh M, Nakamura M, Akatsu T and Hiramori K. Activated tumour necrosis factor-alpha shedding process is associated with in-hospital complication in patients with acute myocardial infarction. Clin Sci (Lond). 2005;108:339–47. [DOI] [PubMed] [Google Scholar]
- 22.Wei SG, Yu Y, Weiss RM and Felder RB. Endoplasmic reticulum stress increases brain MAPK signaling, inflammation and renin-angiotensin system activity and sympathetic nerve activity in heart failure. Am J Physiol Heart Circ Physiol. 2016;311:H871–H880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu Y, Wei SG, Weiss RM and Felder RB. TNF-alpha receptor 1 knockdown in the subfornical organ ameliorates sympathetic excitation and cardiac hemodynamics in heart failure rats. Am J Physiol Heart Circ Physiol. 2017;313:H744–H756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Francis J, Weiss RM, Wei SG, Johnson AK and Felder RB. Progression of heart failure after myocardial infarction in the rat. Am J Physiol Regul Integr Comp Physiol. 2001;281:R1734–45. [DOI] [PubMed] [Google Scholar]
- 25.He MM, Smith AS, Oslob JD, Flanagan WM, Braisted AC, Whitty A, Cancilla MT, Wang J, Lugovskoy AA, Yoburn JC, Fung AD, Farrington G, Eldredge JK, Day ES, Cruz LA, Cachero TG, Miller SK, Friedman JE, Choong IC and Cunningham BC. Small-molecule inhibition of TNF-alpha. Science. 2005;310:1022–5. [DOI] [PubMed] [Google Scholar]
- 26.Taylor CJ, Ireland DR, Ballagh I, Bourne K, Marechal NM, Turner PR, Bilkey DK, Tate WP and Abraham WC. Endogenous secreted amyloid precursor protein-alpha regulates hippocampal NMDA receptor function, long-term potentiation and spatial memory. Neurobiol Dis. 2008;31:250–60. [DOI] [PubMed] [Google Scholar]
- 27.Fritze D, Zhang W, Li JY, Chai B and Mulholland MW. TNFalpha causes thrombin-dependent vagal neuron apoptosis in inflammatory bowel disease. J Gastrointest Surg. 2014;18:1632–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wei SG, Yu Y, Zhang ZH and Felder RB. Proinflammatory cytokines upregulate sympathoexcitatory mechanisms in the subfornical organ of the rat. Hypertension. 2015;65:1126–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wei SG, Zhang ZH, Beltz TG, Yu Y, Johnson AK and Felder RB. Subfornical organ mediates sympathetic and hemodynamic responses to blood-borne proinflammatory cytokines. Hypertension. 2013;62:118–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lomniczi A, Cornea A, Costa ME and Ojeda SR. Hypothalamic tumor necrosis factor-alpha converting enzyme mediates excitatory amino acid-dependent neuron-to-glia signaling in the neuroendocrine brain. J Neurosci. 2006;26:51–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Skovronsky DM, Fath S, Lee VM and Milla ME. Neuronal localization of the TNFalpha converting enzyme (TACE) in brain tissue and its correlation to amyloid plaques. J Neurobiol. 2001;49:40–6. [DOI] [PubMed] [Google Scholar]
- 32.Hurtado O, Cardenas A, Lizasoain I, Bosca L, Leza JC, Lorenzo P and Moro MA. Up-regulation of TNF-alpha convertase (TACE/ADAM17) after oxygen-glucose deprivation in rat forebrain slices. Neuropharmacology. 2001;40:1094–102. [DOI] [PubMed] [Google Scholar]
- 33.Palazuelos J, Crawford HC, Klingener M, Sun B, Karelis J, Raines EW and Aguirre A. TACE/ADAM17 is essential for oligodendrocyte development and CNS myelination. J Neurosci. 2014;34:11884–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hinkle CL, Sunnarborg SW, Loiselle D, Parker CE, Stevenson M, Russell WE and Lee DC. Selective roles for tumor necrosis factor alpha-converting enzyme/ADAM17 in the shedding of the epidermal growth factor receptor ligand family: the juxtamembrane stalk determines cleavage efficiency. J Biol Chem. 2004;279:24179–88. [DOI] [PubMed] [Google Scholar]
- 35.Jones BA, Riegsecker S, Rahman A, Beamer M, Aboualaiwi W, Khuder SA and Ahmed S. Role of ADAM-17, p38 MAPK, cathepsins, and the proteasome pathway in the synthesis and shedding of fractalkine/CX(3) CL1 in rheumatoid arthritis. Arthritis Rheum. 2013;65:2814–25. [DOI] [PubMed] [Google Scholar]
- 36.Allinson TM, Parkin ET, Condon TP, Schwager SL, Sturrock ED, Turner AJ and Hooper NM. The role of ADAM10 and ADAM17 in the ectodomain shedding of angiotensin converting enzyme and the amyloid precursor protein. Eur J Biochem. 2004;271:2539–47. [DOI] [PubMed] [Google Scholar]
- 37.Patel VB, Clarke N, Wang Z, Fan D, Parajuli N, Basu R, Putko B, Kassiri Z, Turner AJ and Oudit GY. Angiotensin II induced proteolytic cleavage of myocardial ACE2 is mediated by TACE/ADAM-17: a positive feedback mechanism in the RAS. J Mol Cell Cardiol. 2014;66:167–76. [DOI] [PubMed] [Google Scholar]
- 38.Zucker IH, Wang W, Pliquett RU, Liu JL and Patel KP. The regulation of sympathetic outflow in heart failure. The roles of angiotensin II, nitric oxide, and exercise training. Ann N Y Acad Sci. 2001;940:431–43. [PubMed] [Google Scholar]
- 39.Zhang W, Huang BS and Leenen FH. Brain renin-angiotensin system and sympathetic hyperactivity in rats after myocardial infarction. Am J Physiol Heart Circ Physiol. 1999;276:H1608–15. [DOI] [PubMed] [Google Scholar]
- 40.Gao L, Wang W, Li YL, Schultz HD, Liu D, Cornish KG and Zucker IH. Superoxide mediates sympathoexcitation in heart failure: roles of angiotensin II and NAD(P)H oxidase. Circ Res. 2004;95:937–44. [DOI] [PubMed] [Google Scholar]
- 41.Yu Y, Wei SG, Weiss RM and Felder RB. Angiotensin II Type 1a Receptors in the Subfornical Organ Modulate Neuroinflammation in the Hypothalamic Paraventricular Nucleus in Heart Failure Rats. Neuroscience. 2018;381:46–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wei SG, Yu Y, Weiss RM and Felder RB. Inhibition of Brain Mitogen-Activated Protein Kinase Signaling Reduces Central Endoplasmic Reticulum Stress and Inflammation and Sympathetic Nerve Activity in Heart Failure Rats. Hypertension. 2016;67:229–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Helwig BG, Musch TI, Craig RA and Kenney MJ. Increased interleukin-6 receptor expression in the paraventricular nucleus of rats with heart failure. Am J Physiol Regul Integr Comp Physiol. 2007;292:R1165–73. [DOI] [PubMed] [Google Scholar]
- 44.de Queiroz TM, Xia H, Filipeanu CM, Braga VA and Lazartigues E. alpha-Lipoic acid reduces neurogenic hypertension by blunting oxidative stress-mediated increase in ADAM17. Am J Physiol Heart Circ Physiol. 2015;309:H926–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xu P and Derynck R. Direct activation of TACE-mediated ectodomain shedding by p38 MAP kinase regulates EGF receptor-dependent cell proliferation. Mol Cell. 2010;37:551–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Soond SM, Everson B, Riches DW and Murphy G. ERK-mediated phosphorylation of Thr735 in TNFalpha-converting enzyme and its potential role in TACE protein trafficking. J Cell Sci. 2005;118:2371–80. [DOI] [PubMed] [Google Scholar]
- 47.Xu J, Sriramula S, Xia H, Moreno-Walton L, Culicchia F, Domenig O, Poglitsch M and Lazartigues E. Clinical Relevance and Role of Neuronal AT1 Receptors in ADAM17-Mediated ACE2 Shedding in Neurogenic Hypertension. Circ Res. 2017;121:43–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wei SG, Yu Y, Zhang ZH, Weiss RM and Felder RB. Angiotensin II-triggered p44/42 mitogen-activated protein kinase mediates sympathetic excitation in heart failure rats. Hypertension. 2008;52:342–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Grell M, Wajant H, Zimmermann G and Scheurich P. The type 1 receptor (CD120a) is the high-affinity receptor for soluble tumor necrosis factor. Proc Natl Acad Sci U S A. 1998;95:570–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Grell M, Douni E, Wajant H, Lohden M, Clauss M, Maxeiner B, Georgopoulos S, Lesslauer W, Kollias G, Pfizenmaier K and Scheurich P. The transmembrane form of tumor necrosis factor is the prime activating ligand of the 80 kDa tumor necrosis factor receptor. Cell. 1995;83:793–802. [DOI] [PubMed] [Google Scholar]
- 51.Canault M, Peiretti F, Mueller C, Kopp F, Morange P, Rihs S, Portugal H, Juhan-Vague I and Nalbone G. Exclusive expression of transmembrane TNF-alpha in mice reduces the inflammatory response in early lipid lesions of aortic sinus. Atherosclerosis. 2004;172:211–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.