Abstract
BCAR1 (also known as p130Cas/BCAR1) is an adaptor protein that belongs to the CAS family of scaffold proteins. In the last years, increasing evidence has demonstrated the ability of p130Cas/BCAR1 to activate signaling originating from mechanical stimuli, cell-extracellular matrix (ECM) adhesion and growth factor stimulation cascades during normal development and disease in various biological models. In this review we will specifically discuss the more recent data on the contribution of p130Cas/BCAR1 in the regulation of tissue homeostasis and its potential implications in pathological conditions.
Keywords: p130Cas/BCAR1, intracellular signalling pathways, development, cancer, adaptor protein, cell motility
Introduction
p130Cas/BCAR1 (p130 Crk-associated substrate; also known as Breast Cancer Anti-Estrogen Resistance 1 [BCAR1]), is part of the Cas (Crk-associated substrate) family of adaptor proteins. The other family members are Nedd9 (Neural precursor cell expressed, developmentally downregulated 9); Human enhancer of filamentation-1 (HEF-1 or CAs-L), EFS (Embryonal Fyn-associated substrate) and CASS4 (Cas scaffolding protein family member 4). These proteins share structure similarity characterized by the presence of multiple protein interaction domains and several tyrosine and serine phosphorylation motifs (Cabodi et al., 2010a; Barrett et al., 2013; Nikonova et al., 2014) (Figure 1). p130Cas/BCAR1 protein is characterized by an amino (N)-terminal Src-homology 3 (SH3) domain, an adjacent large substrate-binding domain containing 15 repetitions of the YxxP motif, a main site of tyrosine phosphorylation on the p130Cas/BCAR1 molecule that generates SH2-binding sites, a proline and serine rich region, and a highly conserved four-helix bundle (focal adhesion targeting [FAT] domain) (Bouton et al., 2001; Defilippi et al., 2006; Tikhmyanova et al., 2010a; Barrett et al., 2013).
Figure 1. Cartoon illustrating p130Cas/BCAR1 domains.
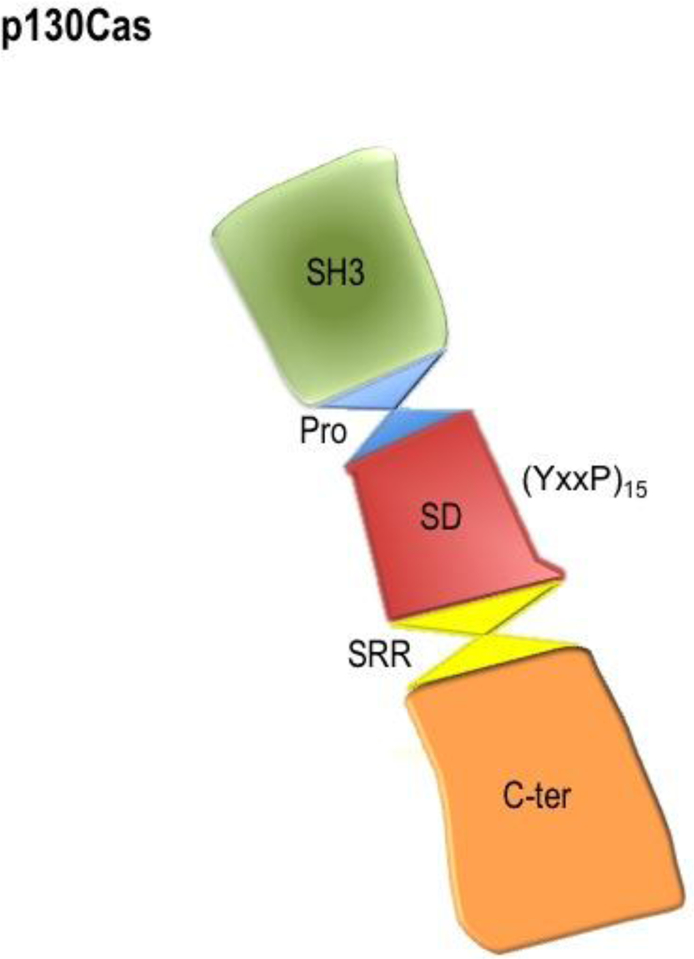
The structural domains of p130Cas/BCAR1 proteins (SH3, substrate domain SD, serine rich region SRR and C-terminus) are shown. The binding proteins of these domains have been extensively described in several recent reviews (Cabodi et al., 2010a; Tikhmyanova et al., 2010a; Barrett et al., 2013).
p130Cas/BCAR1 phosphorylation and mechano-transducer properties
Although p130Cas/BCAR1 is devoid of any enzymatic or transcriptional activity, the presence of multiple tyrosine residues in the substrate domain allows extensive changes in phosphorylation that drive the formation of multi-protein signaling complexes. This results in the induction and/or maintenance of signaling pathways with pleiotropic effects on cell motility, cell adhesion, cytoskeleton remodeling, invasion, survival and proliferation (Defilippi et al., 2006). Tyrosine phosphorylation occurs upon integrin-mediated adhesion, receptor tyrosine kinase (RTK) or chemokine receptor activation, and other upstream signals such as hypoxia. Phosphorylated p130Cas/BCAR1 tyrosines can associate with signaling effectors such as the non-receptor focal adhesion kinases (FAK), SRC family kinases (SFKs), ABL, as well as with phosphatases, assembling a variety of signaling molecules in a membrane proximal molecular hub capable of tuning specific cell functions in several physiological and pathological contexts (Cabodi et al., 2010a).
More recently, mechanical stretch, which enables cells to sense and respond to mechanical forces, has been shown to increase tyrosine phosphorylation of p130Cas/BCAR1 and its association with signaling molecules such as the Crk/C3G complex that leads to the activation of Rap1 GTPase, ERK and other signaling pathways (Tamada et al., 2004; Sawada et al., 2006). These observations demonstrate that mechanical stress by unfolding and extending p130Cas/BCAR1 substrate domain, unmasks effector binding sites and phosphorylation motifs, enabling the conversion of external force into intracellular biochemical signals (Sawada et al., 2006). Besides the mechanical stress-mediated consequences on p130Cas/BCAR1 substrate domain phosphorylation, it has been recently proposed that the direct binding of the SH3 domain of p130Cas/BCAR1 with FAK or vinculin is required for placing p130Cas/BCAR1 in the correct positions into focal adhesion in order to function as a proper mechanosensor. Thus, the capability of p130Cas/BCAR1 to act as a mechanosensor is not only due to the phosphorylation status of its substrate domain but also to the association of its SH3 domain to focal adhesion proteins (Janostiak et al., 2011; Janostiak et al., 2014). Moreover, phosphorylation of tyrosine 12 is capable of antagonizing the mechanical activation of CAS by disrupting its SH3 domain binding capacity (Janostiak et al., 2011). These data demonstrate that the phosphorylation status of distinct tyrosines can control the p130Cas/BCAR1 ability to act as a proper mechanosensor into adherent cells. Altogether these findings emphasize the crucial role of p130Cas/BCAR1 in the control of mechanosensing in cell physiology and possibly during disease. One tissue that is strongly exposed to mechanical stress is the skeletal muscle. Indeed in muscles exposures to mechanical stresses induced p130Ca/BCAR1 tyrosine phosphorylation (Akimoto et al., 2013). However, the muscle specific deletion of p130Cas/BCAR1 did not alter the skeletal muscle adaptation induced by stretching or running, suggesting that in vivo, the absence of p130Cas/BCAR1 can be compensated by other mechanical stress-induced pathways or by the involvement of other p130Cas/BCAR1 family members (Akimoto et al., 2013).
p130Cas/BCAR1 in early embryonic development in mice and flies
Although studies of the p130Cas/BCAR1 protein highlight its important roles in cancer and other pathogenic conditions, defining its precise function in early and late tissue and organ development still requires additional investigations. Indeed, p130Cas/BCAR1 is a ubiquitous protein expressed at early stages during development. The germ-line knockout (KO) p130Cas/BCAR1 mouse model previously generated by and Honda and co-workers (Honda et al., 1998), showed that early p130Cas/BCAR1 deficiency is embryonic lethal, with p130Cas/BCAR1-null embryos dying in utero at 12.5 dpc. Death results from systemic congestion and growth retardation, due in particular to massive heart and blood vessels defects. These results underline the unique role of p130Cas/BCAR1 in early mouse development, implying that at these stages, the four paralogous in the Cas family, although with overlapping expression profiles, cannot compensate for p130Cas depletion. Thus, early lethal defects in p130Cas/BCAR1 knock-out mice have prevented to establish further specific functions for p130Cas/BCAR1 in late or adult tissue development till the generation of tissue-specific deletions (see below).
The role of p130Cas/BCAR1 has been recently studied in a Drosophila genetic mutant. The Drosophila Cas gene is highly expressed in the embryonic nervous system as well as in the ventral ectoderm at earlier developmental stages (Huang et al., 2007; Tomancak et al., 2007). A partial deletion of the single Cas gene caused lethality only in 10% of embryos (Tikhmyanova et al., 2010b). In spite of this technical issue, it was revealed that Dcas is an important regulator of integrin pathway genes, including integrins and their effector kinases Fak56D and Src42A. Indeed a synthetic lethal phenotype was observed in double mutants of Dcas and Src or FAK56D. Moreover, these mutants showed defective expression and localization of Shg/E-cadherin to cell junctions, resulting in alterations of cell polarity, thus indicating the requirement of concomitant expression of Dcas and FAK56D genes for the accurate E-cadherin localization. These data suggest the existence of a dynamic equilibrium among DCas, Fak56D, and Shg/E-cadherin proteins, regulating the Dcas-dependent Shg/E-cadherin turn-over. These data raise the possibility that p130Cas/BCAR1 levels by affecting cell junction stability may control cell polarity, both in physiological and pathological conditions.
p130Cas/BCAR1 and tissue homeostasis
Several evidence highlighting the role for p130Cas/BCAR1 in regulating various mechanisms implicated during development have been recently reviewed (Barrett et al., 2013; Nikonova et al., 2014). To date, only few mouse models with tissue specific deletion of p130Cas/BCAR1 have been generated (Akimoto et al., 2013; Nagai et al., 2013; Riccomagno et al., 2014), thus limiting further conclusions regarding its precise in vivo function.
In the current review we will discuss the most recent findings regarding the role of p130Cas/BCAR1 in tissue homeostasis (Figure 2).
Figure 2: Functional effects of p130Cas/BCAR1 expression on organ development.
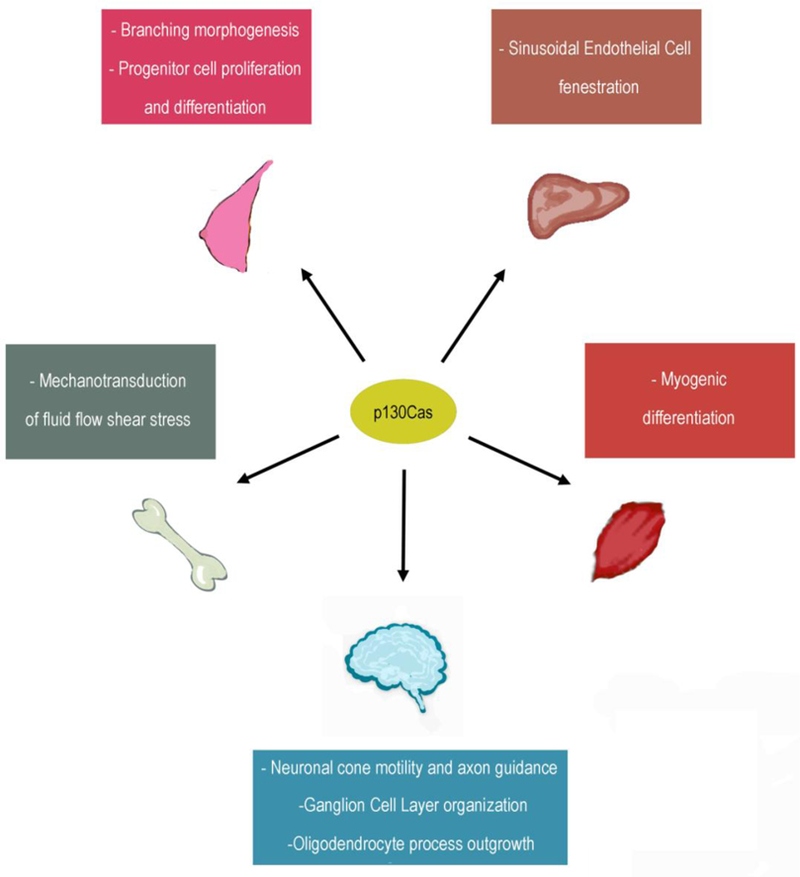
The main functional consequences of p130Cas/BCAR1 expression in selected organs are highlighted.
p130Cas/BCAR1 in the mammary gland
In normal human breast tissue the expression of p130Cas/BCAR1 is mainly detected in the epithelial compartment (Tornillo et al., 2013). In mouse mammary gland epithelium, p130Cas/BCAR1 expression is regulated during mammary gland development is highly enriched in the basal cell population (Tornillo et al., 2013). It was recently reported that alteration of p130Cas/BCAR1 expression level can affect morphogenesis and homeostasis of the mammary gland. Indeed, it was shown that over-expression of p130Cas/BCAR1 promotes mammary branching morphogenesis in vivo during puberty as well as in mammary organoids cultivated ex vivo upon EGF or FGF stimulation (Cabodi et al., 2006; Camacho Leal Mdel et al., 2012). Consistent with p130Cas/BCAR1 ability to exert a positive effect in mammary cell growth, its over expression in vivo leads to mammary hyperplasia and delayed involution by increasing the proliferation and survival signaling in mouse mammary cells (Cabodi et al., 2006). The hyper proliferation observed can be explained by the fact that p130Cas/BCAR1 over expression leads to the expansion of luminal progenitors cells, altering their differentiation potential and changing their commitment to basal cell fate (Tornillo et al., 2013). These functional alterations result from p130Cas/BCAR1-dependent aberrant activation of the tyrosine kinase c-Kit. These findings suggest that p130Cas/BCAR1 expression levels must be finely tuned in the mammary gland in order to prevent alteration in proliferation, survival and cell fate commitment.
p130Cas/BCAR1 in muscle development
The link between modulation of p130Cas/BCAR1 expression and myogenic differentiation was initially demonstrates in C2C12 myoblasts. In this cell model, the adhesion-dependent p130Cas/BCAR1 phosphorylation, by inducing actin remodeling and favoring nuclear localization of the MAL/SRF transcription factor, promotes myogenic differentiation (Kawauchi et al., 2012). In line with these findings, the Lmo7 transcription factor that has been implicated in the expression of muscle relevant genes, co-localizes in focal adhesion with p130Cas/BCAR1 (Wozniak et al., 2013). This interaction negatively regulates Lmo7 transcription activity, thus suggesting that p130Cas/BCAR1-dependent recruitment of this transcription factor might be a mechanism through which myoblasts regulate their differentiation (Wozniak et al., 2013). Recently, Jeong et al. reported that the caspase-dependent cleavage of p130Cas/BCAR1 antagonizes myogenic differentiation. Indeed, it was demonstrated that the over expression of the p130Cas/BCAR1 cleavage product leads to alteration of muscle specific gene transcription such as MyoD thus impairing the muscle differentiation program (Jeong da et al., 2014).
These data indicate that during myogenic differentiation, a stable and phosphorylable p130Cas/BCAR1 protein may finely tune myoblast differentiation. However, as mentioned above, the muscle specific deletion of p130Cas/BCAR1 protein does not produce significantly functional alteration in skeletal muscles (Akimoto et al., 2013), indicating that further investigation is required in order to better understand the role of p130Cas/BCAR1 in muscle development and differentiation in vivo and in pathological conditions.
p130Cas/BCAR1 in the nervous system
To date, the function of p130Cas/BCAR1 in the nervous system is still largely unclear, but very recently, the analysis of the transcriptome of developing cerebellar granule cells have identified p130Cas/BCAR1 as one of the genes involved in cellular mechanisms regulating different stages of cerebellar development (Furuichi et al., 2011).
Several pieces of evidence have suggested that phosphorylation of p130Cas/BCAR1 may mediate integrin signaling during neural development. In particular, Drosophila Dcas was shown to be essential for integrin-mediated motor axon guidance and fasciculation (Huang et al., 2007). Additional reports supporting a role for p130Cas/BCAR1 protein downstream of integrins during vertebrate neural development were generated by in vitro studies (Bargon et al., 2005; Bourgin et al., 2007).
Netrins are evolutionary conserved secreted proteins that control neuron axon outgrowth and guidance. A role for p130Cas/BCAR1 in netrin-1 dependent axon guidance was described. Indeed, it was reported that p130Cas/BCAR1 is expressed in axons and growth cones and co-localize with netrin-1 receptor. Moreover, in primary cultured neurons, netrin-1 can induce the tyrosine phosphorylation of p130Cas/BCAR1 and the binding of p130Cas/BCAR1 with Fyn and FAK kinases. The netrin-1-dependent tyrosine phosphorylation of p130Cas/BCAR1 is required to activate Rac1 and Cdc42 that are crucial regulators of cone motility and axon guidance. In line with these results, the silencing of p130Cas/BCAR1 inhibits netrin-1 dependent neurite outgrowth and axon attraction, indicating that p130Cas/BCAR1 is required for netrin-1-dependent signaling in commissural axon guidance (Liu et al., 2007).
More recently, Riccomagno and co-workers showed that p130Cas/BCAR1 is highly expressed in the inner neuroblastic layer of the mouse retina, and together with beta1 integrin is required for proper ganglion cell layer (GCL) organization. Specifically, a dynamic localization of the phosphorylated form of p130Cas/BCAR1 during the stages of retinal development was observed. Whilst specific conditional deletion of p130Cas/BCAR1 in mouse retinal neurons did not show severe retina developmental defects, a combined triple conditional knockout of p130Cas/BCAR1, NEDD and SIN leads to a dramatic disruption of the GCL structure, phenocopying the loss of the beta1 integrin. Altogether these findings suggest that Cas family is essential for the beta1 integrin dependent vertebrate retina development (Riccomagno et al., 2014).
In an oligodendrocyte cells, p130Cas/BCAR1 has been shown to be a target of the Src family kinase Fyn and its long-term silencing leads to primary oligodendrocytes cell death by enhanced apoptosis. Moreover, p130Cas/BCAR1 is required for oligodendrocyte process outgrowth and cell migration, implying a potential role for p130Cas in myelination processes (Gonsior et al., 2014).
In summary, these findings points out p130Cas/BCAR1 as a key modulator of different aspects of development of the nervous system.
p130Cas/BCAR1 in the bone
The mechanosensing function of p130Cas/BCAR1 has been shown to play an important role in bone tissue homeostasis. Within the bone tissue pericellular space, fluid flows exert a mechanical stress that can be sensed by osteoblast cells. Kaneko et al. recently reported that the absence of alphav integrin in osteoblasts impairs Src-dependent phosphorylation of p130Cas/BCAR1 and JNK, as well as the inhibition of YAP/TAZ transcriptional activity. The inhibition of Src/p130Cas/BCAR1/Jnk/YAP/TAZ axis impairs the capacity to respond to mechanical forces, suggesting that active signaling downstream to alphav integrin, involving also p130Cas/BCAR1, is required for transducing mechanical stress by fluid shear stress in primary osteoblast cells (Kaneko et al., 2014). Further evidence in osteoclast cells highlights the role of p130Cas/BCAR1 for the maintenance of a correct bone homeostasis. It was previously reported that Src-dependent p130Cas/BCAR1 phosphorylation is involved in the adhesion-induced actin ring formation that is considered a marker for osteoclast activation (Nakamura et al., 1998). Consistently, the conditional deletion of p130Cas/BCAR1 in osteoclasts leads to an increase in bone volume due to a reduction of osteoclast functionality and to inhibition of actin ring formation (Vives et al., 2011; Nagai et al., 2013). These data strengthen the significance of p130Cas/BCAR1 adaptor in bone homeostasis, and suggest that the understanding of the precise molecular mechanisms that are regulated by p130Cas/BCAR1 can be instrumental for the development of new therapeutic approaches to treat bone diseases.
p130Cas/BCAR1 in the liver
In the liver, p130Cas/BCAR1 is expressed in the sinusoidal endothelial cells (SECs), but not in the hepatocytes. Transgenic mice carrying a hypomorphic p130Cas/BCAR1 allele lacking exon 2, that encodes for the SH3 domain, are embryonic lethal, similar to p130Cas/BCAR1 total KO mice, and display a progressive liver degeneration accompanied by hepatocyte apoptotic cell death during embryonic life (Tazaki et al., 2010). Further in vitro experiments performed over-expressing the mutant ΔSH3 p130Cas/BCAR1 in the NP31 SEC cell line, have demonstrated a dominant negative function that results in reduced p130Cas/BCAR1 tyrosine phosphorylation, defective p130Cas/BCAR1-CrkII interaction, actin stress fiber formation, and loss of cell fenestration that serves for oxygen and nutrients uptake to hepatocytes. Altogether these findings underline an important role of p130Cas/BCAR1 protein in regulating liver endothelial cells (Tazaki et al., 2010).
Pathophysiology of p130Cas/BCAR1
At the molecular level, the importance of p130Cas/BCAR1 for the regulation of signaling pathways controlling cell proliferation, survival actin cytoskeleton organization and extracellular matrix degradation in many pathological conditions has been well documented. Indeed, in the past years, p130Cas/BCAR1 expression has been shown to be fundamental not only for cell transformation and cancer progression, but also in several other diseases.
p130Cas/BCAR1 and Cancer
The relevance of p130Cas/BCAR1 adaptor protein in cancer has been extensively supported by demonstrating the contribution of de-regulated expression levels of p130Cas/BCAR1 to cellular transformation and malignancy. Indeed, altered expression of p130Cas/BCAR1 has been identified in several human tumours. At the same time, current knowledge implicates p130Cas/BCAR1 in acquired resistance to cancer treatment (Tornillo et al., 2014) Altered levels of p130Cas/BCAR1 expression in cancers can result from gene amplification, transcription up regulation, or changes in protein stability, although the exact mechanisms have not been identified yet. Over expression of p130Cas/BCAR1 has been detected in human breast, prostate, ovarian, lung, colorectal, pancreatic and hepatocellular carcinoma, as well as in glioma, melanoma, anaplastic large cell lymphoma and chronic myelogenous leukemia (Tikhmyanova et al., 2010a). Conversely, lowering the amount of p130Cas/BCAR1 expression in breast, prostate and ovarian cancer is sufficient to block tumor growth and progression of cancer cells (Cabodi et al., 2010b; Dai et al., 2011; Nick et al., 2011).
In the past years, several observations suggest that an aberrant activation of the p130Cas/BCAR1 signaling network signature in diverse type of tumors leads to up-regulation of key regulatory signaling pathways promoting cell transformation.
Specifically, in ErbB2-positive breast cancer, p130Cas/BCAR1 is necessary to induce invasion in three-dimensional culture cells by supporting and amplifying ErbB2 downstream signals, triggering MMP9 secretion and modulating several coding and non-coding genes (Cabodi et al., 2010b; Tornillo et al., 2011; Pincini et al., 2013). Moreover, in breast and lung cancer, p130Cas/BCAR1 expression has been correlated to the acquirement of mesenchymal traits, thus supporting its role in driving malignancy and metastasis dissemination (Tikhmyanova and Golemis, 2011; Bisaro et al., 2012; Deng et al., 2014).
Several reports indicate the key role of p130Cas/BCAR1 in prostate cancer. In vitro studies have shown that increasing p130Cas/BCAR1 protein levels is sufficient to restore cell motility in Du145 prostate cancer cell line expressing the metastasis suppressor KAI1/CD82 (Zhang et al., 2003). In human prostate cancer, high levels of p130Cas/BCAR1 correlate with high EGFR and KAI1/CD82 expression and with disease progression in hormone refractory prostate cancers (Fromont et al., 2007; Celhay et al., 2010). Consistently, p130Cas/BCAR1 has been recently proposed as a diagnostic marker to predict recurrence in low risk patients undergoing radical prostatectomy (Fromont et al., 2012). Taken together these data point out the relevance of p130Cas/BCAR1 expression as a prognostic marker for prostate cancer progression thus implying that p130Cas/BCAR1 can be used potentially as a new target for therapeutic interventions in prostate cancer.
In hepatocellular carcinoma (HCC), p130Cas/BCAR1 expression has been found to correlate with low E-cadherin and beta-catenin expression, worse patho-histological grades and prognosis (Guo et al., 2008). Specifically, p130Cas/BCAR1 positive expression correlates with abnormal expression of beta-catenin and reduced expression of E-cadherin. HCC patients with positive expression of p130Cas/BCAR1 are associated with higher risk of developing lymph-node metastasis. On the basis of these results it is intriguing to speculate on the possibility that in HCC the over expression of p130Cas/BCAR1 by altering the stability of the cadherin/catenin complex impairs cell-cell adhesion and promotes more efficient cell invasion.
More recent studies have implicated p130Cas/BCAR1 in malignant brain tumors. Specifically, p130Cas/BCAR1 tyrosine phosphorylation induced by Platelet derived growth factor ligand (PDGF)-BB was a key event for the establishment of a migration phenotype in U87 glioma cells and in vascular smooth muscle cells (Evans et al., 2011; Pellet-Many et al., 2011). Further investigations have refined the molecular mechanisms involved in glioma cell migration and invasion. Indeed it was recently reported that PDGF-BB-dependent migration and invasion requires the scaffold protein Downstream Of Kinase 1 (DOK1). PDGF-BB leads to DOK1 tyrosine phosphorylation that in turn triggers the tyrosine phosphorylation of p130Cas/BCAR1 and Rap1 activity that are necessary for glioma cell migration and 3D invasion. Thus these results delineate the existence of a DOK1/p130Cas/BCAR1/Rap1 signaling pathway that is fundamental for glioma cell motility (Barrett et al., 2014).
In line with these findings, in pancreatic cancer cells activation of the small GTPase Rap1 downstream of EGFR has been reported to be strictly dependent on p130Cas/BCAR1 tyrosine phosphorylation and subsequent formation of p130Cas/BCAR1-Nck1 complex. Though, activation of the p130Cas/BCAR1/Rap1 signaling pathway seems to be determinant for EGFR-induced metastasis rather than primary tumor growth (Huang et al., 2012). Interestingly, p130Cas/BCAR1 gene locus was recently identified in a genome wide screening of 7,683 pancreatic cancer patients as a new gene for pancreatic cancer susceptibility, opening new perspectives for the study of p130Cas/BCAR1 in pancreatic cancer (Wolpin et al., 2014).
It has been reported that p130Cas/BCAR1 tyrosine phosphorylation may also be regulated by lisophosphatidic acid (LPA) that induces migration and invasion of ovarian cancer cells by activating the Gαi2 G protein subunit (Ward et al., 2013). LPA-dependent activation of Gαi2 promotes p130Cas/BCAR1 translocation into the invadopodia whereby it mediates the formation of a macromolecular complex together with Src, beta-Pix, Rac1. The formation of this signaling complex drives the p130Cas/BCAR1-mediated activation of Rac1 that in turn induces the invasive migration of ovarian cancer cells (Ward et al., 2015). Interestingly, the high levels of p130Cas/BCAR1 expression in human ovarian cancer specimens correlate with poor clinical outcome, further underlining the relevance of p130Cas/BCAR1 in this type of cancer. In addition, silencing of p130Cas/BCAR1 in tumor specimens was sufficient to inhibit tumor and induce cell death through apoptosis and autophagy (Nick et al., 2011).
Overall these findings indicate that p130Cas/BCAR1 plays a key role in the growth and invasion of several types of cancer cells and suggest that down-regulation of p130Cas/BCAR1 might be a valid therapeutic strategy to cure these deadly diseases.
p130Cas/BCAR1 in vascular remodeling and diseases
It has been demonstrated that p130Cas/BCAR1 is a crucial regulator of Vasculature Smooth Muscle Cells (VSMC) contractility by promoting actin cytoskeleton remodeling (Tang, 2009).
It was recently proposed that Cystein-rich Protein 2 (CRP2), previously demonstrated as a crucial player of VSMC migration, by sequestering p130Cas/BCAR1 at focal adhesion, alters lamellipodia formation and consequently reduces VSMCs motility. Lacking of CRP2 and p130Cas/BCAR1 phosphorylation can promote neointima formation following arterial injury, suggesting the intriguing possibility that the sequestration of p130Cas/BCAR1 by CRP2 can prevent neointima formation, and vascular remodeling that ultimately cause atherosclerosis. In line with these results, the BCAR1 gene was identified as one of the genes that can predispose to carotid intima-media thickness (cIMT) and atherosclerosis (Gertow et al., 2012).
Pulmonary arterial hypertension (PAH) is a disease characterized by a dramatic increase in pulmonary artery pressure due to vasculature remodeling that ultimately causes the right ventricular failure and death. A role for p130Cas/BCAR1 in PAH has been recently described. Indeed, p130Cas/BCAR1 over expression was detected both in serum and in walls of distal pulmonary arteries. Modulation of p130Cas/BCAR1 expression and RTK-dependent phosphorylation was sufficient to alter migration and proliferation of endothelial and smooth muscle vascular cells derived from PAH patients. Consistently, increased expression and phosphorylation of p130Cas/BCAR1 were observed in animal models of pulmonary hypertension (PH). Interestingly, the attenuation of p130Cas/BCAR1 tyrosine phosphorylation by using RTK inhibitors was sufficient to partially rescue PH in these animal models. These results support the hypothesis that high levels of p130Cas/BCAR1 and its tyrosine phosphorylation upon RTK activation can promote PAH by amplifying intracellular signaling (Tu et al., 2012).
p130Cas/BCAR1 in microbial pathogenesis
The importance of p130Cas/BCAR1 as a mediator of bacterial infections has been documented in several studies (Hamid et al., 1999; Weidow et al., 2000; Sun and Barbieri, 2003; Deng et al., 2005) and recently reviewed (Barrett et al., 2013).
Importantly, more recent data has identified p130Cas/BCAR1 as a platform to promote Kaposi’s sarcoma-associated herpesvirus (KSHV) trafficking. Specifically, in this study in human microvascular dermal endothelial cells, it was demonstrated that p130Cas/BCAR1 serves as a platform to support KSHV trafficking. Early during KSHV de novo infection, p130Cas is found in lipid rafts associated to CIB1, a EphrinA2 (EphA2)-associated adaptor protein, allowing successful nuclear entry of the KSHV genome. Thus interfering with EphA2 and p130Cas/BCAR1 can impair KSHV-induced entry signal complex and the downstream trafficking signalosome, eventually blocking KSHV infection and associated malignancies (Bandyopadhyay et al., 2014).
Overall, these studies demonstrate that prokaryotes exploit signaling network involving p130Cas/BCAR1 to sustain their infection.
Conclusions and perspectives
Accumulating evidence indicates that p130Cas/BCAR1 is profoundly involved in the regulation of basic signaling mechanisms both in developmental/physiological processes and in diseases. p130Cas/BCAR1 over-expression is detrimental in several diseases. Down-regulation of p130Cas/BCAR1 as well as interference with p130Cas/BCAR1 association with effector downstream signaling molecules might be a promising therapeutic strategy in many diseases. Moreover, understanding the mechanisms through which p130Cas/BCAR1 expression is up-regulated might provide new perspectives to identify pharmacological targets for the treatment of a wide range of pathologies.
Table1:
p130Cas/BCAR1 genetic modified models
| Genetic model | Phenotype | Refs |
|---|---|---|
| MMTV-p130Cas/BCAR1 Tg mouse | Mammary gland hyperplasia during pregnancy and delayed involution | (Cabodi et al., 2006) |
| MMTV-p130Cas/BCAR1, NeuT Tg mouse | Accelerated onset of focal mammary tumors, due to increased cell survival and proliferation | (Cabodi et al., 2006) |
| p130Cas total KO mice | Embryonic lethal (12.5 dpc) characterized by massive heart and blood vessels defects. | (Honda et al., 1998) |
| DCas/Fak56D Drosophila KO | Embryonic lethal, defective expression and localization of shg/E-cadherin to cell junctions resulting in cell polarity defects. | (Tikhmyanova and Golemis, 2011) |
| Retina-specific triple KO p130Cas/BCAR1/NEDD9/Sin | Severe disorganization of the ganglion cell layer | (Riccomagno et al., 2014) |
| Osteoclasts-specific p130Cas/BCAR1 KO mice | Increased bone mass due to reduced osteoclasts functions and actin ring formation. | (Nagai et al., 2013) |
| Muscle-specific p130Cas/BCAR1 KO mice | No gross defects in skeletal muscle | (Akimoto et al., 2013) |
MMTV, mouse mammary tumour virus; Dcas, Drosophila p130Cas/BCAR1 gene; Fak, Focal adhesion kinase.
Table 2:
Molecular signaling associated with p130Cas/BCAR1 expression in human malignancies.
| Type of malignancy | Molecular signaling | Refs |
|---|---|---|
| Breast cancer | Amplification of ErbB2 downstream signals, increased migration and invasion Tamoxifen resistance and poor prognosis p130Cas/BCAR1/Cox2 axis and EMT |
Cabodi, Tinirello, Pincini, (Dorssers et al., 2004) |
| Lung cancer | TGF-beta1/p130Cas/BCAR1 and EMT | (Deng et al., 2014) |
| Prostate tumors | High EGFR and KAI1/CD82 expression and disease progression | (Zhang et al., 2003; Fromont et al., 2012) |
| Hepatocellular carcinoma | Abnormal expression of Beta-catenin, reduced expression of E-cadherin and cell invasion. | (Guo et al., 2008) |
| Brain Tumors | DOK1/p130Cas/BCAR1/Rap1axis and increased cell motility | (Barrett et al., 2014) |
| Pancreatic Cancer | p130Cas/BCAR1/Rap1 and metastatization | (Huang et al., 2012) |
| Ovarian Cancer | Src/beta-Pix/p130Cas/BCAR1/Rac1complex and cell invasion | (Ward et al., 2015) |
| Vascular remodeling | p130Cas/BCAR1mediated VSMC contractility and actin cytoskeleton remodeling | (Tang, 2009; Tu et al., 2012) |
| Microbial pathogenesis | p130Cas/BCAR1 and KSHV trafficking | (Bandyopadhyay et al., 2014) |
ER, oestrogen receptor; EMT, epithelial mesenchymal transition; TGF-beta1, Transforming growth factor-beta; EGFR, Epidermal Growth Factor Receptor; RAP1, Ras-related protein 1; DOK1, Docking protein-1; VSMC,Vasculature Smooth Muscle Cells; KSHV, Kaposi’s sarcoma-associated herpesvirus.
Acknowledgements
This work was supported by AIRC (Associazione Italiana Ricerca Cancro) to SC (IG11346) and PD (IG-11896); MIUR (FIRB giovani 2008 RBFR08F2FS to SC), MIUR (Ministero Università Ricerca, PRIN 2010/2011) to PD and Compagnia San Paolo, Torino; Progetto d’Ateneo, Università di Torino 2011 to PD. Giusy Tornillo is supported by Breast Cancer Campaign UK (2012MayPR076).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interests
The authors declare that they have no conflict of interests.
References
- Akimoto T, Okuhira K, Aizawa K, Wada S, Honda H, Fukubayashi T and Ushida T Skeletal muscle adaptation in response to mechanical stress in p130cas−/− mice. Am J Physiol Cell Physiol 304 (2013), pp. C541–7. [DOI] [PubMed] [Google Scholar]
- Bandyopadhyay C, Veettil MV, Dutta S and Chandran B p130Cas Scaffolds the Signalosome To Direct Adaptor-Effector Cross Talk during Kaposi’s Sarcoma-Associated Herpesvirus Trafficking in Human Microvascular Dermal Endothelial Cells. J Virol 88 (2014), pp. 13858–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bargon SD, Gunning PW and O’Neill GM The Cas family docking protein, HEF1, promotes the formation of neurite-like membrane extensions. Biochim Biophys Acta 1746 (2005), pp. 143–54. [DOI] [PubMed] [Google Scholar]
- Barrett A, Evans IM, Frolov A, Britton G, Pellet-Many C, Yamaji M, Mehta V, Bandopadhyay R, Li N, Brandner S, Zachary IC and Frankel P A crucial role for DOK1 in PDGF-BB-stimulated glioma cell invasion through p130Cas and Rap1 signalling. J Cell Sci 127 (2014), pp. 2647–58. [DOI] [PubMed] [Google Scholar]
- Barrett A, Pellet-Many C, Zachary IC, Evans IM and Frankel P p130Cas: a key signalling node in health and disease. Cell Signal 25 (2013), pp. 766–77. [DOI] [PubMed] [Google Scholar]
- Bisaro B, Montani M, Konstantinidou G, Marchini C, Pietrella L, Iezzi M, Galie M, Orso F, Camporeale A, Colombo SM, Di Stefano P, Tornillo G, Camacho-Leal MP, Turco E, Taverna D, Cabodi S, Amici A and Defilippi P p130Cas/Cyclooxygenase-2 axis in the control of mesenchymal plasticity of breast cancer cells. Breast Cancer Res 14 (2012), p. R137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourgin C, Murai KK, Richter M and Pasquale EB The EphA4 receptor regulates dendritic spine remodeling by affecting beta1-integrin signaling pathways. J Cell Biol 178 (2007), pp. 1295–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouton AH, Riggins RB and Bruce-Staskal PJ Functions of the adapter protein Cas: signal convergence and the determination of cellular responses. Oncogene 20 (2001), pp. 6448–58. [DOI] [PubMed] [Google Scholar]
- Cabodi S, del Pilar Camacho-Leal M, Di Stefano P and Defilippi P Integrin signalling adaptors: not only figurants in the cancer story. Nat Rev Cancer 10 (2010a), pp. 858–70. [DOI] [PubMed] [Google Scholar]
- Cabodi S, Tinnirello A, Bisaro B, Tornillo G, del Pilar Camacho-Leal M, Forni G, Cojoca R, Iezzi M, Amici A, Montani M, Eva A, Di Stefano P, Muthuswamy SK, Tarone G, Turco E and Defilippi P p130Cas is an essential transducer element in ErbB2 transformation. Faseb j 24 (2010b), pp. 3796–808. [DOI] [PubMed] [Google Scholar]
- Cabodi S, Tinnirello A, Di Stefano P, Bisaro B, Ambrosino E, Castellano I, Sapino A, Arisio R, Cavallo F, Forni G, Glukhova M, Silengo L, Altruda F, Turco E, Tarone G and Defilippi P p130Cas as a new regulator of mammary epithelial cell proliferation, survival, and HER2-neu oncogene-dependent breast tumorigenesis. Cancer Res 66 (2006), pp. 4672–80. [DOI] [PubMed] [Google Scholar]
- Camacho Leal Mdel P, Pincini A, Tornillo G, Fiorito E, Bisaro B, Di Luca E, Turco E, Defilippi P and Cabodi S p130Cas over-expression impairs mammary branching morphogenesis in response to estrogen and EGF. PLoS One 7 (2012), p. e49817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celhay O, Yacoub M, Irani J, Dore B, Cussenot O and Fromont G Expression of estrogen related proteins in hormone refractory prostate cancer: association with tumor progression. J Urol 184 (2010), pp. 2172–8. [DOI] [PubMed] [Google Scholar]
- Dai Y, Qi L, Zhang X, Li Y, Chen M and Zu X CrkI and p130(Cas) complex regulates the migration and invasion of prostate cancer cells. Cell Biochem Funct 29 (2011), pp. 625–9. [DOI] [PubMed] [Google Scholar]
- Defilippi P, Di Stefano P and Cabodi S p130Cas: a versatile scaffold in signaling networks. Trends Cell Biol 16 (2006), pp. 257–63. [DOI] [PubMed] [Google Scholar]
- Deng B, Tan QY, Wang RW, Jiang YG, Zhou JH and Huang W P130cas is required for TGF-beta1-mediated epithelial-mesenchymal transition in lung cancer. Oncol Lett 8 (2014), pp. 454–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Q, Sun J and Barbieri JT Uncoupling Crk signal transduction by Pseudomonas exoenzyme T. J Biol Chem 280 (2005), pp. 35953–60. [DOI] [PubMed] [Google Scholar]
- Dorssers LC, Grebenchtchikov N, Brinkman A, Look MP, van Broekhoven SP, de Jong D, Peters HA, Portengen H, Meijer-van Gelder ME, Klijn JG, van Tienoven DT, Geurts-Moespot A, Span PN, Foekens JA and Sweep FC The prognostic value of BCAR1 in patients with primary breast cancer. Clin Cancer Res 10 (2004), pp. 6194–202. [DOI] [PubMed] [Google Scholar]
- Evans IM, Yamaji M, Britton G, Pellet-Many C, Lockie C, Zachary IC and Frankel P Neuropilin-1 signaling through p130Cas tyrosine phosphorylation is essential for growth factor-dependent migration of glioma and endothelial cells. Mol Cell Biol 31 (2011), pp. 1174–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromont G, Rozet F, Cathelineau X, Ouzzane A, Doucet L, Fournier G and Cussenot O BCAR1 expression improves prediction of biochemical reccurence after radical prostatectomy. Prostate 72 (2012), pp. 1359–65. [DOI] [PubMed] [Google Scholar]
- Fromont G, Vallancien G, Validire P, Levillain P and Cussenot O BCAR1 expression in prostate cancer: association with 16q23 LOH status, tumor progression and EGFR/KAI1 staining. Prostate 67 (2007), pp. 268–73. [DOI] [PubMed] [Google Scholar]
- Furuichi T, Shiraishi-Yamaguchi Y, Sato A, Sadakata T, Huang J, Shinoda Y, Hayashi K, Mishima Y, Tomomura M, Nishibe H and Yoshikawa F Systematizing and cloning of genes involved in the cerebellar cortex circuit development. Neurochem Res 36 (2011), pp. 1241–52. [DOI] [PubMed] [Google Scholar]
- Gertow K, Sennblad B, Strawbridge RJ, Ohrvik J, Zabaneh D, Shah S, Veglia F, Fava C, Kavousi M, McLachlan S, Kivimaki M, Bolton JL, Folkersen L, Gigante B, Leander K, Vikstrom M, Larsson M, Silveira A, Deanfield J, Voight BF, Fontanillas P, Sabater-Lleal M, Colombo GI, Kumari M, Langenberg C, Wareham NJ, Uitterlinden AG, Gabrielsen A, Hedin U, Franco-Cereceda A, Nyyssonen K, Rauramaa R, Tuomainen TP, Savonen K, Smit AJ, Giral P, Mannarino E, Robertson CM, Talmud PJ, Hedblad B, Hofman A, Erdmann J, Reilly MP, O’Donnell CJ, Farrall M, Clarke R, Franzosi MG, Seedorf U, Syvanen AC, Hansson GK, Eriksson P, Samani NJ, Watkins H, Price JF, Hingorani AD, Melander O, Witteman JC, Baldassarre D, Tremoli E, de Faire U, Humphries SE and Hamsten A Identification of the BCAR1-CFDP1-TMEM170A locus as a determinant of carotid intima-media thickness and coronary artery disease risk. Circ Cardiovasc Genet 5 (2012), pp. 656–65. [DOI] [PubMed] [Google Scholar]
- Gonsior C, Biname F, Fruhbeis C, Bauer NM, Hoch-Kraft P, Luhmann HJ, Trotter J and White R Oligodendroglial p130Cas is a target of Fyn kinase involved in process formation, cell migration and survival. PLoS One 9 (2014), p. e89423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo C, Liu QG, Yang W, Zhang ZL and Yao YM Relation among p130Cas, E-cadherin and beta-catenin expression, clinicopathologic significance and prognosis in human hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int 7 (2008), pp. 490–6. [PubMed] [Google Scholar]
- Hamid N, Gustavsson A, Andersson K, McGee K, Persson C, Rudd CE and Fallman M YopH dephosphorylates Cas and Fyn-binding protein in macrophages. Microb Pathog 27 (1999), pp. 231–42. [DOI] [PubMed] [Google Scholar]
- Honda H, Oda H, Nakamoto T, Honda Z, Sakai R, Suzuki T, Saito T, Nakamura K, Nakao K, Ishikawa T, Katsuki M, Yazaki Y and Hirai H Cardiovascular anomaly, impaired actin bundling and resistance to Src-induced transformation in mice lacking p130Cas. Nat Genet 19 (1998), pp. 361–5. [DOI] [PubMed] [Google Scholar]
- Huang M, Anand S, Murphy EA, Desgrosellier JS, Stupack DG, Shattil SJ, Schlaepfer DD and Cheresh DA EGFR-dependent pancreatic carcinoma cell metastasis through Rap1 activation. Oncogene 31 (2012), pp. 2783–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Z, Yazdani U, Thompson-Peer KL, Kolodkin AL and Terman JR Crk-associated substrate (Cas) signaling protein functions with integrins to specify axon guidance during development. Development 134 (2007), pp. 2337–47. [DOI] [PubMed] [Google Scholar]
- Janostiak R, Brabek J, Auernheimer V, Tatarova Z, Lautscham LA, Dey T, Gemperle J, Merkel R, Goldmann WH, Fabry B and Rosel D CAS directly interacts with vinculin to control mechanosensing and focal adhesion dynamics. Cell Mol Life Sci 71 (2014), pp. 727–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janostiak R, Tolde O, Bruhova Z, Novotny M, Hanks SK, Rosel D and Brabek J Tyrosine phosphorylation within the SH3 domain regulates CAS subcellular localization, cell migration, and invasiveness. Mol Biol Cell 22 (2011), pp. 4256–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong da E, Lee EK, Song WK and Kim W The 31-kDa caspase-generated cleavage product of p130Cas antagonizes the action of MyoD during myogenesis. Biochem Biophys Res Commun 444 (2014), pp. 509–13. [DOI] [PubMed] [Google Scholar]
- Kaneko K, Ito M, Naoe Y, Lacy-Hulbert A and Ikeda K Integrin alphav in the mechanical response of osteoblast lineage cells. Biochem Biophys Res Commun 447 (2014), pp. 352–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawauchi K, Tan WW, Araki K, Abu Bakar FB, Kim M, Fujita H, Hirata H and Sawada Y p130Cas-dependent actin remodelling regulates myogenic differentiation. Biochem J 445 (2012), pp. 323–32. [DOI] [PubMed] [Google Scholar]
- Liu G, Li W, Gao X, Li X, Jurgensen C, Park HT, Shin NY, Yu J, He ML, Hanks SK, Wu JY, Guan KL and Rao Y p130CAS is required for netrin signaling and commissural axon guidance. J Neurosci 27 (2007), pp. 957–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai Y, Osawa K, Fukushima H, Tamura Y, Aoki K, Ohya K, Yasuda H, Hikiji H, Takahashi M, Seta Y, Seo S, Kurokawa M, Kato S, Honda H, Nakamura I, Maki K and Jimi E p130Cas, Crk-associated substrate, plays important roles in osteoclastic bone resorption. J Bone Miner Res 28 (2013), pp. 2449–62. [DOI] [PubMed] [Google Scholar]
- Nakamura I, Jimi E, Duong LT, Sasaki T, Takahashi N, Rodan GA and Suda T Tyrosine phosphorylation of p130Cas is involved in actin organization in osteoclasts. J Biol Chem 273 (1998), pp. 11144–9. [DOI] [PubMed] [Google Scholar]
- Nick AM, Stone RL, Armaiz-Pena G, Ozpolat B, Tekedereli I, Graybill WS, Landen CN, Villares G, Vivas-Mejia P, Bottsford-Miller J, Kim HS, Lee JS, Kim SM, Baggerly KA, Ram PT, Deavers MT, Coleman RL, Lopez-Berestein G and Sood AK Silencing of p130cas in ovarian carcinoma: a novel mechanism for tumor cell death. J Natl Cancer Inst 103 (2011), pp. 1596–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikonova AS, Gaponova AV, Kudinov AE and Golemis EA CAS proteins in health and disease: an update. IUBMB Life 66 (2014), pp. 387–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellet-Many C, Frankel P, Evans IM, Herzog B, Junemann-Ramirez M and Zachary IC Neuropilin-1 mediates PDGF stimulation of vascular smooth muscle cell migration and signalling via p130Cas. Biochem J 435 (2011), pp. 609–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pincini A, Tornillo G, Orso F, Sciortino M, Bisaro B, Leal Mdel P, Lembo A, Brizzi MF, Turco E, De Pitta C, Provero P, Medico E, Defilippi P, Taverna D and Cabodi S Identification of p130Cas/ErbB2-dependent invasive signatures in transformed mammary epithelial cells. Cell Cycle 12 (2013), pp. 2409–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riccomagno MM, Sun LO, Brady CM, Alexandropoulos K, Seo S, Kurokawa M and Kolodkin AL Cas adaptor proteins organize the retinal ganglion cell layer downstream of integrin signaling. Neuron 81 (2014), pp. 779–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawada Y, Tamada M, Dubin-Thaler BJ, Cherniavskaya O, Sakai R, Tanaka S and Sheetz MP Force sensing by mechanical extension of the Src family kinase substrate p130Cas. Cell 127 (2006), pp. 1015–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J and Barbieri JT Pseudomonas aeruginosa ExoT ADP-ribosylates CT10 regulator of kinase (Crk) proteins. J Biol Chem 278 (2003), pp. 32794–800. [DOI] [PubMed] [Google Scholar]
- Tamada M, Sheetz MP and Sawada Y Activation of a signaling cascade by cytoskeleton stretch. Dev Cell 7 (2004), pp. 709–18. [DOI] [PubMed] [Google Scholar]
- Tang DD p130 Crk-associated substrate (CAS) in vascular smooth muscle. J Cardiovasc Pharmacol Ther 14 (2009), pp. 89–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tazaki T, Sasaki T, Uto K, Yamasaki N, Tashiro S, Sakai R, Tanaka M, Oda H, Honda Z and Honda H p130Cas, Crk-associated substrate plays essential roles in liver development by regulating sinusoidal endothelial cell fenestration. Hepatology 52 (2010), pp. 1089–99. [DOI] [PubMed] [Google Scholar]
- Tikhmyanova N and Golemis EA NEDD9 and BCAR1 negatively regulate E-cadherin membrane localization, and promote E-cadherin degradation. PLoS One 6 (2011), p. e22102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tikhmyanova N, Little JL and Golemis EA CAS proteins in normal and pathological cell growth control. Cell Mol Life Sci 67 (2010a), pp. 1025–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tikhmyanova N, Tulin AV, Roegiers F and Golemis EA Dcas supports cell polarization and cell-cell adhesion complexes in development. PLoS One 5 (2010b), p. e12369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomancak P, Berman BP, Beaton A, Weiszmann R, Kwan E, Hartenstein V, Celniker SE and Rubin GM Global analysis of patterns of gene expression during Drosophila embryogenesis. Genome Biol 8 (2007), p. R145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tornillo G, Bisaro B, Camacho-Leal Mdel P, Galie M, Provero P, Di Stefano P, Turco E, Defilippi P and Cabodi S p130Cas promotes invasiveness of three-dimensional ErbB2-transformed mammary acinar structures by enhanced activation of mTOR/p70S6K and Rac1. Eur J Cell Biol 90 (2011), pp. 237–48. [DOI] [PubMed] [Google Scholar]
- Tornillo G, Defilippi P and Cabodi S Cas proteins: dodgy scaffolding in breast cancer. Breast Cancer Research 16 (2014), p. 443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tornillo G, Elia AR, Castellano I, Spadaro M, Bernabei P, Bisaro B, Camacho-Leal Mdel P, Pincini A, Provero P, Sapino A, Turco E, Defilippi P and Cabodi S p130Cas alters the differentiation potential of mammary luminal progenitors by deregulating c-Kit activity. Stem Cells 31 (2013), pp. 1422–33. [DOI] [PubMed] [Google Scholar]
- Tu L, De Man FS, Girerd B, Huertas A, Chaumais MC, Lecerf F, Francois C, Perros F, Dorfmuller P, Fadel E, Montani D, Eddahibi S, Humbert M and Guignabert C A critical role for p130Cas in the progression of pulmonary hypertension in humans and rodents. Am J Respir Crit Care Med 186 (2012), pp. 666–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vives V, Laurin M, Cres G, Larrousse P, Morichaud Z, Noel D, Cote JF and Blangy A The Rac1 exchange factor Dock5 is essential for bone resorption by osteoclasts. J Bone Miner Res 26 (2011), pp. 1099–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward JD, Ha JH, Jayaraman M and Dhanasekaran DN LPA-mediated migration of ovarian cancer cells involves translocalization of Galphai2 to invadopodia and association with Src and beta-pix. Cancer Lett 356 (2015), pp. 382–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward Y, Lake R, Martin PL, Killian K, Salerno P, Wang T, Meltzer P, Merino M, Cheng SY, Santoro M, Garcia-Rostan G and Kelly K CD97 amplifies LPA receptor signaling and promotes thyroid cancer progression in a mouse model. Oncogene 32 (2013), pp. 2726–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidow CL, Black DS, Bliska JB and Bouton AH CAS/Crk signalling mediates uptake of Yersinia into human epithelial cells. Cell Microbiol 2 (2000), pp. 549–60. [DOI] [PubMed] [Google Scholar]
- Wolpin BM, Rizzato C, Kraft P, Kooperberg C, Petersen GM, Wang Z, Arslan AA, Beane-Freeman L, Bracci PM, Buring J, Canzian F, Duell EJ, Gallinger S, Giles GG, Goodman GE, Goodman PJ, Jacobs EJ, Kamineni A, Klein AP, Kolonel LN, Kulke MH, Li D, Malats N, Olson SH, Risch HA, Sesso HD, Visvanathan K, White E, Zheng W, Abnet CC, Albanes D, Andreotti G, Austin MA, Barfield R, Basso D, Berndt SI, Boutron-Ruault MC, Brotzman M, Buchler MW, Bueno-de-Mesquita HB, Bugert P, Burdette L, Campa D, Caporaso NE, Capurso G, Chung C, Cotterchio M, Costello E, Elena J, Funel N, Gaziano JM, Giese NA, Giovannucci EL, Goggins M, Gorman MJ, Gross M, Haiman CA, Hassan M, Helzlsouer KJ, Henderson BE, Holly EA, Hu N, Hunter DJ, Innocenti F, Jenab M, Kaaks R, Key TJ, Khaw KT, Klein EA, Kogevinas M, Krogh V, Kupcinskas J, Kurtz RC, LaCroix A, Landi MT, Landi S, Le Marchand L, Mambrini A, Mannisto S, Milne RL, Nakamura Y, Oberg AL, Owzar K, Patel AV, Peeters PH, Peters U, Pezzilli R, Piepoli A, Porta M, Real FX, Riboli E, Rothman N, Scarpa A, Shu XO, Silverman DT, Soucek P, Sund M, Talar-Wojnarowska R, Taylor PR, Theodoropoulos GE, et al. Genome-wide association study identifies multiple susceptibility loci for pancreatic cancer. Nat Genet 46 (2014), pp. 994–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wozniak MA, Baker BM, Chen CS and Wilson KL The emerin-binding transcription factor Lmo7 is regulated by association with p130Cas at focal adhesions. PeerJ 1 (2013), p. e134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XA, He B, Zhou B and Liu L Requirement of the p130CAS-Crk coupling for metastasis suppressor KAI1/CD82-mediated inhibition of cell migration. J Biol Chem 278 (2003), pp. 27319–28. [DOI] [PubMed] [Google Scholar]


