Abstract
Relative to neutral memories, negative and positive memories both exhibit an increase in memory longevity, subjective memory re-experiencing, and amygdala activation. These memory enhancements are often attributed to shared influences of arousal on memory. Yet prior work suggests the intriguing possibility that arousal affects memory networks in valence-specific ways. Psychophysics work has shown that arousal-related heart rate deceleration (HRD) responses are related to enhanced amygdala-visual functional connectivity (AVFC) and visual perception of negative stimuli. However, in the memory realm, it is not known if the effect of AVFC influences subsequent negative memory outcomes as a function of the magnitude of physiological arousal during encoding. Using psycho-autonomic interaction (PAI) analyses and trial-level measures of heart rate deceleration (HRD) as an objective measure of arousal during encoding of emotional stimuli, our findings suggest the magnitude of the HRD modulates the effect of AVFC on subsequent negative memory vividness. Specifically, AVFC effects in early visual regions predicted negative memory vividness, not neutral or positive vividness, but only in the presence of heightened physiological arousal. This novel approach was grounded in a replication of prior working showing enhanced HRD effects in the insula for negative stimuli. These findings demonstrate that the effect of arousal on emotional memory networks depends on valence and provide further evidence that negative valence may enhance the incorporation of visuo-sensory regions into emotional memory networks.
Keywords: Amygdala, arousal, emotional memory, encoding, functional connectivity, heart rate deceleration, negative, physiology, V1, vividness
Introduction
William James made a prescient observation when he wrote; “An impression may be so exciting emotionally as almost to leave a scar upon the cerebral tissues” [1]. Negative memories can differ from positive or neutral both in brain and in behavior [2], with functional magnetic resonance imaging (fMRI) studies linking negative valence with enhanced visual processing during successful encoding [3], post-encoding rest [4], and retrieval [2,4,5] of vivid memories [6]. Almost all levels of the ventral visual system receive feedback projections from the amygdala, including early visual cortex [7], yet the impact of amygdala-related arousal on memory may depend on valence: A study capitalizing on between-subject variability in regional activity levels suggested encoding of high- vs. low-arousal negative stimuli was linked with increased amygdala connectivity with visual areas, while encoding of high-arousal positive stimuli decreased the strength of those connections [8].
This study directly examines the effects of physiological arousal and amygdala-cortical connectivity on subsequent memory strength by acquiring an objective, trial-level metric of arousal during encoding: Heart rate deceleration (HRD). HRD is a common metric of parasympathetic response associated with stimulus attention and orienting, with an exaggerated decelerative response to emotional stimuli [9]. In fearful situations, the freezing response is accompanied by HRD, which results from vagal nerve activation initiated by cholinergic activation of amygdala projections to the brainstem. Together, the HRD response and freezing response are thought to facilitate an organism’s sensory processing of its surroundings to assess threats [10]. Memory research has provided evidence that HRD responses can predict subsequent memory for negative stimuli [11] and recent work suggests enhanced arousal and behavioral inhibition might enhance memory selectivity [12], however, the neural mechanisms of these effects have not been formally tested.
Recent psychophysics work has shown concurrent HRD responses and AVFC enhance visual sensitivity [13], raising the possibility that arousal enhances perceptual encoding of negative stimuli into memory. Prior work has shown that HRD magnitudes are correlated with activation of the amygdala [14], visual cortex, and ventral visual regions [15,16]—regions also associated with emotionally-enhanced memory [2]. If negative valence is associated with perceptual enhancements related to HRD-related increases in arousal during the initial experience of negative stimulus [13], this could lead to long-term consequences on memory vividness specifically for negative (not positive) stimuli, consistent with our recent model proposing enhanced perceptual recapitulation of negative memories [2]. Alternatively, HRD responses could relate to AVFC and memory vividness for all arousing stimuli (positive and negative, consistent with an arousal account) or even for neutral stimuli (consistent with an attention-based account of HRD).
Here, we asked: Does the magnitude of physiological arousal during encoding facilitate the “scarring” of negative experiences into long-term memory? We examined the effect of valence on AVFC profiles associated with an interaction between trial-level HRD responses and later memory vividness 24-hours later by conducting psycho-autonomic interaction (PAI) analyses [17]. We focused on amygdala connectivity because of its known role in emotional memory and in the HRD response. In humans, direct stimulation of the amygdala leads to dose-dependent HRD responses [14] and enhances declarative memory [18]. We predicted that heightened HRD response magnitudes and greater AVFC would specifically predict vividness for negative, but not positive, memories, with effects for neutral falling intermediately.
Methods
All procedures were approved by the Boston College Institutional Review Board and written informed consent was obtained from all participants. Full explanations of the study stimuli, memory task, and procedures, including fMRI acquisition parameters, pre-preprocessing, and thresholding, have been previously reported [4]. We outline the key methods for the current analyses.
Participants.
Thirty-three participants were recruited as a part of a larger study examining the effects of stress and sleep on emotional memory. The participants included in the present analysis did not undergo the stress condition. Data from seven participants were excluded from present analyses: One due to a structural anomaly (female, 23), one due to chance-level memory performance (d’<0; male, 25), one from due to psychophysiology data loss (male, 24), and three due to poor HR signal recordings (2 females). One additional participant (male, 25) was removed from the whole-brain analysis due to fMRI parameter estimates of Negative PAI beyond 4SD of the group mean upon follow-up testing. Exclusion of this data did not qualitatively alter the pattern of valence-specific results. The final analyzed sample included twenty-six participants ages 18–29 years (M=22.0, SD=2.8, 12 females).
Recognition Memory Task.
Participants incidentally encoded negative, neutral, and positive scenes while undergoing concurrent fMRI and psychophysiological recording. Each scene was presented for 3s and was preceded by a 1.5s presentation of its line-drawing sketch. A jittered fixation between trials (6–12s) allowed the physiological response to return to baseline. The next day, participants completed a surprise recognition task of old and new line-drawings. For each line-drawing, participants used a 0–4 scale to make an Old-New memory and vividness rating (0=“New”, 1= “Old, Not Vivid”, 2=“Old, Somewhat Vivid, 3=“Old, Vivid”, 4=“Old, Extremely Vivid”).
Heart rate data acquisition, pre-processing, and event analysis.
HR data were sampled at 1000 Hz during encoding using an MRI-compatible fiber-optic oximetry sensor (Model 7500FO Fiber-Optic Pulse Oximeter, Nonin Medical, Inc) attached to the left index finger in conjunction with the BIOPAC System MP150 module and AcqKnowledge software (BIOPAC Systems Inc., Goleta, CA). HR data recording was time-locked to onset of the MRI scanner.
HR data for each encoding run was analyzed using custom MATLAB scripts. First, the HR data were adjusted for a ~4s time delay between the stimulus presentation and the change in HR [19]. To reduce high-frequency fMRI noise, the HR data were then smoothed (moving median window=1.5s), detrended, z-scored, and averaged in 0.5s time-bins.
Event-related HRD was calculated by identifying the minimum HR value that occurred within 1–7s after the onset of the scene image, compared to a 1s pre-trial baseline. The sign of the HRD values were then inverted such that a more positive value corresponded to a stronger deceleration response. The HRD traces were inspected for artifacts (0–2% of trials), which were removed from the remaining analyses. As our neural hypotheses were specifically about HRDs, only trials with an HRD were included as effects of interest in the fMRI analyses.
fMRI analysis.
fMRI analyses were carried out in SPM8 (Wellcome Department of Cognitive Neurology, London, United Kingdom) implemented in MATLAB R2014a. We applied a similar PAI approach using parametric modulation analyses as Farrow and colleagues [17]. For each participant, three fixed-effects models were created with the following effects of interest: (1) a Subsequent Vividness (SubViv) model containing subsequently remembered items (hits) separately by valence with trial-level SubViv ratings as parametric modulators and one column containing all of the missed trials; (2) an HRD model containing all HRD events by stimulus valence (hits and misses collapsed within valence) with the trial-level HRD values as the parametric modulator; and (3) a PAI model containing all hits by valence with SubViv and HRD as the first and second parametric modulators (to control for the main effects) followed by the trial-level PAI term (SubViv*HRD) and one column containing all of the missed trials. The PAI model allowed us to examine AFC patterns above and beyond those patterns separately associated with SubViv or HRD. Each of the fixed-effects models also contained a regressor of trials that showed a negative HRD value (~20% of trials) or a HR artifact (~1% of trials) and a matrix of regressors was added to the end of each fixed-effects models to control for item-level objective salience of the scene images [4], 7 head-motion parameters, and drift. All event-related encoding trials were modelled using 6s box-car functions.
Functional connectivity analyses were conducted using left and right amygdala seed regions drawn from a probability atlas of the human brain [20]. Statistical maps of parametric functional connectivity of the amygdala were generated using the Generalized PPI (gPPI) Toolbox [21]. For each amygdala seed region, the gPPI toolbox was used to 1) generate task/psychological regressors, 2) estimate the BOLD signal in the amygdala seed regions to create the physiological variable, and 3) calculate the psychophysiological interaction terms by convolving the timecourse vectors with the corresponding parametric modulator vectors. For all participants, six whole-brain functional parametric t images were saved from each of the three models to examine the main effects of SubViv, HRD, and the PAI effect for each valence and amygdala seed. These t contrasts were entered into three separate group-level full factorial models to separately assess the main effects of 1) subsequent vividness, 2) HRD, and 3) the PAI effect as a function of valence.
Results
HRD results.
Analysis of the HRD trials entered into the fMRI analysis showed that normalized HRD levels did not statistically differ between negative and positive hits (Mneg=0.69, Mpos=0.65: t(25)=−1.07, p=0.30). As expected, negative hits elicited stronger HRD responses than neutral hits (Mneu=0.62: t(25)=2.16, p=0.04), but positive and neutral hit HRD magnitudes did not statistically differ (t(25)=0.66, p=0.51).
fMRI results.
There were no whole-brain hemisphere-by-valence interactions; thus, we focus our findings on AFC collapsed across hemisphere.
Main effects of vividness.
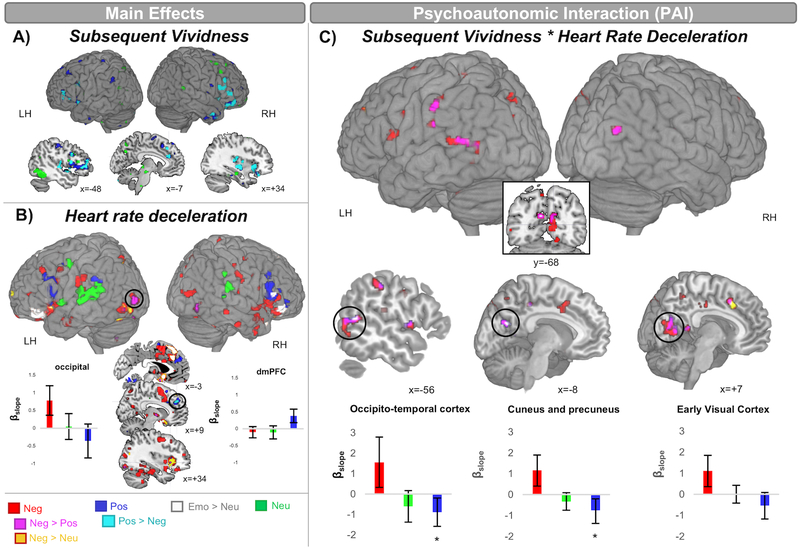
Valence-specific positive memory vividness effects were observed in the inferior frontal gyrus (BA44/45/47), middle frontal gyrus, insula, and putamen (depicted in Fig. 1A in cyan and peak coordinates in Supplementary Table, Supplemental digital content 1). Neutral item vividness was associated with AVFC in the left inferior temporal gyrus (Fig. 1A, green). AFC was not modulated by the main effects of negative item vividness.
Fig. 1.
Statistical maps of amygdala parametric functional connectivity with subsequent vividness (1A), heart rate deceleration (1B), and their interactions (1C). Results are collapsed across the right and left amygdala. Error bars represent 95% within-subject confidence intervals. βslope corresponds to the parameter estimate of the slopes. LH=Left hemisphere, RH=Right hemisphere. Statistical maps thresholded at p<0.005, k=10. *Denotes p=0.02 (one-sample t-test).
Main effects of heart rate deceleration.
Compared to neutral stimuli, emotional stimuli showed greater AFC with ventrolateral prefrontal cortex (BA45/47), dorsomedial prefrontal cortex (BA6), orbital frontal cortex (BA11), and the subgenual area (BA25). Consistent with prior research [13,15], AFC increased as a function of negative arousal in the insula and the ventral visual stream, (BA18/19/20/37; Fig. 1B, red). Valence-specific effects of negative arousal were observed in left inferior and middle occipital gyri (Fig. 1B, magenta). Negative HRD effects were greater than neutral HRD effects in the left inferior occipital gyrus (BA19; Fig. 1B, yellow and magenta). AFC increased with the inferior parietal lobule/superior temporal gyrus (i.e., ventral attention network) and frontal areas for neutral and positive stimuli, respectively (Fig. 1B, green and blue). See peak coordinates of HRD effects in Supplementary Table, Supplemental digital content 2.
Psycho-autonomic interaction.
Valence-specific PAI effects for negative stimuli were observed in a large swath of the cuneus and lingual gyrus (BA17/18/19; including V1), the cuneus and dorsal posterior cingulate (BA18/31), a portion of left occipito-temporal cortex (BA19/37/39), and the right superior occipital cortex (BA21/22/37) (Fig. 1C, magenta). Several of these regions also showed whole-brain Negative PAI effects greater than Neutral PAI (Fig 1C, white). Consistent with a valence pattern, the neutral PAI effect estimates fell numerically between those of negative and positive and follow-up one-sample t-tests confirmed that two of these visual processing clusters showed below zero PAI effects for positive stimuli (Fig. 1C bar plots). There were no suprathreshold PAI effects of neutral and positive stimuli. See peak coordinates of PAI effects in Supplementary Table, Supplemental digital content 3. Control analyses confirmed that the valence-specific PAI effects in visual cortex were also robust to controlling for TR-wise skin conductance level and respiration in a subset of n=21 participants (at a threshold of p<0.05), suggesting these effects were not driven by other physiological signals.
Although we had strong motivation for seeding the amygdala, results of additional medial temporal lobe seed analyses suggest that some PAI effects with visual areas might not always be specific to the amygdala (see Supplementary Figures and Table, Supplemental digital content 4 for results of control location comparisons). However, some specificity is reported compared to other medial temporal lobe areas (i.e., entorhinal cortex and parahippocampal cortex) and a ventral attention seed region.
Discussion
Using PAI analyses to examine interactions between trial-level metrics of arousal and subsequent memory vividness, we provide evidence that arousal increases AVFC during encoding in a way that corresponds specifically to the memory vividness of negative, not positive, memories. These results provide empirical support for William James’ conjecture that the arousal of a negative event is what sears it into memory. In fact, we found that the link between AVFC and subsequent vividness for negative memories was contingent upon the consideration of HRD: There was no main effect of negative memory vividness on AVFC; the relation to AVFC only emerged when the interaction between with HRD was considered. Although we did not predict this contingency a priori, it is intriguingly consistent with affective “tagging” theories of negative memories: Arousal tags negative memory traces during encoding, which are then prioritized and selectively consolidated [22]. By utilizing trial-level changes in HRD, we were further able to show that these “tagging” effects are sensitive to the magnitude of the physiological arousal response, going beyond prior work using subjective arousal ratings or arousal categories (high vs. low). We grounded these novel PAI findings in a replication of prior work demonstrating HRD main effects for negative stimuli in ventral visual regions [13] and the insula [15]. Together, these results underscore that not only does HRD relate to AFC in valence-specific ways, the implications for memory vividness are also valence-specific.
These findings provide foundational work for further investigations into valence-specific effects of arousal. First, although the effects in visual cortex survived controlling for overall skin conductance level, analysis of event-related sympathetic responses could definitively confirm if this effect is specific to parasympathetic HRD, or if it is broadly related to other markers of arousal. Second, further work is needed to understand how the Negative PAI effect with visual regions could propagate along the medial temporal lobe as a function of arousal to enhance negative memory vividness. Third, we can only infer based on prior work that valence is somehow gating visual cortex inputs, possibly altering perceptual encoding (e.g., field of view [23], perceptual vividness [24], visual sensitivity [13], or signal-to-noise ratios) and influencing the resolution of negative information entering memory stores [25]. Although the predicted effects in visual regions for negative stimuli might reflect enhanced memory-related visual processing, the main effects of vividness and HRD of amygdala-prefrontal connectivity observed for positive stimuli could reflect self-referential processing, relational encoding, semantic processing, or possibly promote broader, more or associative perceptual encoding [12,23]. Future work is needed to understand how amygdala engagement and arousal determines the content and scope of the information that is enhanced in vivid negative, compared to positive, long-term memories.
Supplementary Material
Acknowledgements
We thank Tala Berro, Ryan Daley, and Stephanie Sherman for help with data collection and Stephanie McMains, Tammy Moran, and Ross Mair from the Harvard Center for Brain Science. This research was supported by The National Science Foundation [1539361 to EAK and Jessica D. Payne, DGE1258923 to SK], The National Institute of Health [F31MH113304–01 to SK, S10OD020039 to Harvard CBS], Sigmi Xi [to SK]. Portions of this work were included in the dissertation of SK.
Additional data are available (https://osf.io/4uvx9/).
References
- 1.James W Principles of Psychology. New York, NY: Holt; 1890, pg. 670. [Google Scholar]
- 2.Bowen HJ, Kark SM, Kensinger EA. NEVER forget: negative emotional valence enhances recapitulation. Psychonomic bulletin & review 2018; 25 (3):870–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mickley Steinmetz KR, Kensinger EA. The effects of valence and arousal on the neural activity leading to subsequent memory. Psychophysiology 2009; 46 (6):1190–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kark SM, Kensinger EA. Post-encoding Amygdala-Visuosensory Coupling Is Associated with Negative Memory Bias in Healthy Young Adults. Journal of Neuroscience 2019; 39 (16):3130–3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kark SM, Kensinger EA. Effect of emotional valence on retrieval-related recapitulation of encoding activity in the ventral visual stream. Neuropsychologia 2015; 78:221–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mickley KR, Kensinger EA. Emotional valence influences the neural correlates associated with remembering and knowing. Cognitive, affective & behavioral neuroscience 2008; 8 (2):143–152. [DOI] [PubMed] [Google Scholar]
- 7.Amaral DG, Behniea H, Kelly JL. Topographic organization of projections from the amygdala to the visual cortex in the macaque monkey. Neuroscience 2003; 118 (4):1099–1120. [DOI] [PubMed] [Google Scholar]
- 8.Mickley Steinmetz KR, Addis DR, Kensinger EA. The effect of arousal on the emotional memory network depends on valence. NeuroImage 2010; 53 (1):318–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lang PJ, Greenwald MK, Bradley MM, Hamm AO. Looking at pictures: affective, facial, visceral, and behavioral reactions. Psychophysiology 1993; 30 (3):261–273. [DOI] [PubMed] [Google Scholar]
- 10.Lacey J, Lacey B. Some autonomic central nervous system interrelationships In: Black P, editor. Physiological correlates of emotion. New York:: Academic Press; 1970. pp. 205–227. [Google Scholar]
- 11.Cunningham TJ, Crowell CR, Alger SE, Kensinger EA, Villano MA, Mattingly SM, et al. Psychophysiological arousal at encoding leads to reduced reactivity but enhanced emotional memory following sleep. Neurobiology of learning and memory 2014; 114:155–164. [DOI] [PubMed] [Google Scholar]
- 12.Clewett D, Murty VP. Echoes of Emotions Past: How Neuromodulators Determine What We Recollect. eNeuro 2019; 6 (2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lojowska M, Ling S, Roelofs K, Hermans EJ. Visuocortical changes during a freezing-like state in humans. NeuroImage 2018; 179:313–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Inman CS, Bijanki KR, Bass DI, Gross RE, Hamann S, Willie JT. Human amygdala stimulation effects on emotion physiology and emotional experience. Neuropsychologia 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Critchley HD, Rotshtein P, Nagai Y, O’Doherty J, Mathias CJ, Dolan RJ. Activity in the human brain predicting differential heart rate responses to emotional facial expressions. NeuroImage 2005; 24 (3):751–762. [DOI] [PubMed] [Google Scholar]
- 16.Hermans EJ, Henckens MJ, Roelofs K, Fernandez G. Fear bradycardia and activation of the human periaqueductal grey. NeuroImage 2013; 66:278–287. [DOI] [PubMed] [Google Scholar]
- 17.Farrow TF, Johnson NK, Hunter MD, Barker AT, Wilkinson ID, Woodruff PW. Neural correlates of the behavioral-autonomic interaction response to potentially threatening stimuli. Frontiers in human neuroscience 2012; 6:349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Inman CS, Manns JR, Bijanki KR, Bass DI, Hamann S, Drane DL, et al. Direct electrical stimulation of the amygdala enhances declarative memory in humans. Proceedings of the National Academy of Sciences of the United States of America 2018; 115 (1):98–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shermohammed M, Mehta PH, Zhang J, Brandes CM, Chang LJ, Somerville LH. Does Psychosocial Stress Impact Cognitive Reappraisal? Behavioral and Neural Evidence. Journal of cognitive neuroscience 2017; 29 (11):1803–1816. [DOI] [PubMed] [Google Scholar]
- 20.Hammers A, Allom R, Koepp M, Free S, Myers R, Lemieux L, et al. Three-dimensional maximum probability atlas of the human brain, with particular reference to the temporal lobe. Human brain mapping 2003; 19 (4):224–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McLaren DG, Ries ML, Xu G, Johnson SC. A generalized form of context-dependent psychophysiological interactions (gPPI): a comparison to standard approaches. NeuroImage 2012; 61 (4):1277–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bennion KA, Payne JD, Kensinger EA. Selective effects of sleep on emotional memory: What mechanisms are responsible? Translational Issues in Psychological Science 2015; 1 (1):79–88. [Google Scholar]
- 23.Schmitz TW, De Rosa E, Anderson AK. Opposing influences of affective state valence on visual cortical encoding. The Journal of neuroscience : the official journal of the Society for Neuroscience 2009; 29 (22):7199–7207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Todd RM, Schmitz TW, Susskind J, Anderson AK. Shared neural substrates of emotionally enhanced perceptual and mnemonic vividness. Frontiers in behavioral neuroscience 2013; 7:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xie W, Zhang W. Negative emotion enhances mnemonic precision and subjective feelings of remembering in visual long-term memory. Cognition 2017; 166:73–83. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.