Abstract
This paper describes a new microfluidic biosensor with capabilities of studying single cell biophysical properties. The chip contains four parallel sensing channels, where each channel includes two constriction regions separated by a relaxation region. All channels share a pair of electrodes to record the electrical impedance. Single cell impedance magnitudes and phases at different frequencies were obtained. The deformation and transition time information of cells passing through two sequential constriction regions were gained from the time points on impedance magnitude variations. Constriction channels separated by relaxation regions have been proven to improve the sensitivity of distinguishing single cells. The relaxation region between two sequential constriction channels provides extra time stamps that can be identified in the impedance plots. The new chip allows simultaneous measurement of the biophysical attributes of multiple cells in different channels, thereby increasing the overall throughput of the chip. Using the biomechanical parameters represented by the time stamps in the impedance results, breast cancer cells (MDA-MB-231) and the normal epithelial cells (MCF-10A) could be distinguished by 85%. The prediction accuracy at the single-cell level reached 97% when both biomechanical and bioelectrical parameters were utilized. While the new label-free assay has been tested to distinguish between normal and cancer cells, its application can be extended to include cell–drug interactions and circulating tumor cell detection in blood.
I. INTRODUCTION
Microfluidic technologies have enabled better understanding of cancer pathobiology by facilitating acquisition of novel biosignatures that are based on bioelectrical and biomechanical properties of cancer cells.1–4 These biosignatures prove useful as biomarkers of metastatic potential and therapeutic responsiveness. The biomechanical characteristics of cancer cells can be derived from velocity measurements as cells enter and then transit through constriction channels designed to impose mechanical deformation stress.5–9 Bioelectrical properties are revealed through the use of impedance spectroscopy at the single-cell level either as cells are held in constriction channels or as cells pass through flow cytometry microchannels.10–12
Multiple biophysical characteristics of cancer cells can be extracted from a microfluidic environment. Impedance spectroscopy measures the impedance amplitude ratio and phase shift that occurs over a wide frequency range, and these data provide information on the specific membrane capacitance and cytoplasmic conductivity of single cells.13 Most of the studies on the mechanical properties of cell lines at the single-cell level have been carried out in a single constriction microfluidic channel, either with or without additional electrodes for impedance measurement.2,14–16 The impedance measurements obtained from cells within constriction channels can better identify the intrinsic biophysical properties of cancer cells1,10,11 and can be used to distinguish cancer cell lines from normal cell lines at a single-cell level with an accuracy of >70%–95% at the population level.10,17 Collection of biomechanical properties involves measuring transit times through video/image processing. Consequently, high-throughput analysis of single cells makes postprocessing time consuming.6 The parameters extracted from the images of the cells passing through the constriction channels are entry time, cell elongation, passing time, and cell sizes. The elongation of the cells is highly depending on the dimensions of the microfabricated constriction channels. For example, Lim's group found that the elongation of the deformed breast cancer cells in a 10 μm by 10 μm constriction channel can vary from 20 μm to 35 μm.18 The entry time, passing time, elongation of the cells, and cell sizes can differentiate 95% of the breast cancer cells (MCF-7) and normal cells (MCF-10A).18 However, the direct measurement of cell elongation from the optical microscope image can introduce measurement errors. Fabry's group used the image intensity to identify the different geometries of the cells undergoing deformation in a short constriction channel.19 However, the intensity measurement requires staining processes of the nucleus and the cytoskeleton, which increases the sample preparation time. One way to increase the predication rate of cancer cells from normal cells through transit-time-based microfluidics is to perform cyclic deformation separated by relaxation regions. Our group has demonstrated that microchannels with cyclic deformation and relaxation regions can distinguish human breast cancer cell lines MDA-MB-231, HCC-1806, and MCF-7 from normal breast cells MCF-10A with 81%–85% confidence rate.20 Video/image processing is commonly used to image cell movements in these constriction channels, which necessitates extensive image-processing time to obtain biomechanical information from the cell transit times.8,15,21
Using the biomechanical and bioelectrical parameters directly measured from impedance spectroscopy can distinguish the cells with different dielectric properties. Sun's group used a single constriction channel with two electrodes to detect different cells.1 The parameters they selected were amplitude ratio, phase shift, and overall passing time of single cells. Directly using these three parameters, they were only able to distinguish the cells with significant different dielectric properties such as adult red blood cells without the nucleus and early stage red blood cells with the nucleus.17 Distinguishing between two cell lines with comparable specific membrane capacitance and cytoplasm conductivity has proven to be challenging via impedance spectroscopy. Scientists tried different ways to add biomechanical characteristics in the impedance measurement results to increase the prediction rate. Chen's group found that the impedance information at 10 kHz and 100 kHz on two different microconstriction channels with cross sections of 6 μm by 6 μm and 8 μm by 8 μm reached 59.6% prediction accuracy on breast cancer cell line EMT6.10 The prediction rate can increase to 70.2% with additional transition time of the cells through a single-constriction channel.10 The throughput of measuring the bioelectrical properties of single cells in a single constriction channel was limited. In addition, the possibility of measuring multiple cells simultaneously without interference between different impedance results of cells is a hindrance on the performance of a single constriction channel.
Here, we present a less time-consuming method using impedance spectroscopy as an alternative to image processing to monitor cell transit times through microchannels. By modifying the microfluidic constriction channel configurations, we are able to create time stamps on time-domain impedance results that can be used to track cell velocity in the microchannel and to simultaneously evaluate the bioelectrical properties of cells. Compared to single constriction microfluidic channels for cell bioelectrical and biomechanical analysis, multiparallel channels with relaxation regions have higher throughputs and provide additional information for cell sensing. This device was used to analyze breast cell line MDA-MB-231 and normal epithelial cell line MCF-10A.
II. MATERIALS AND METHODS
A. Cell culture and sample preparation
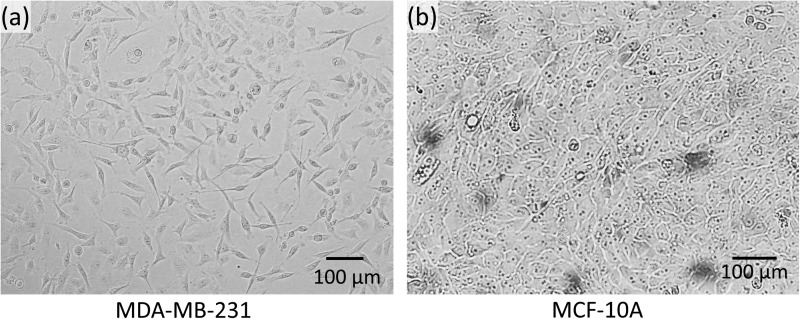
To evaluate the performance of the microfluidic device with parallel constriction channels, a highly metastatic breast cancer cell line MDA-MB-231 [passage 29, American Type Culture Collection (ATCC), Manassas, VA] and a normal breast epithelial cell line MCF-10A (passage 17, ATCC, Manassas, VA) were used via impedance spectroscopy. MDA-MB-231 cells were grown in F12:DMEM (Lonza, Basel, Switzerland) with 10% fetal bovine serum (FBS), 4 mM glutamine, and penicillin-streptomycin (100 units/ml). MCF-10A cells were grown in F12:DMEM with penicillin-streptomycin (100 units/ml), 2.5 mM l-glutamine, 20 ng/ml epidermal growth factor (EGF), 0.1 μg/ml cholera toxin, 10 μg/ml insulin, 0.5 μg/ml hydrocortisone, and 5% horse serum. The cells were grown in T-25 cm2 culture flasks at 37 °C in a 5% CO2 in air atmosphere until cells were ready for subculture. Figure 1 shows the optical microscope images of the cultured MDA-MB-231 and MCF-10A before trypsinization. After the cells were fully confluent, the MDA-MB-231 and MCF-10A cells were trypsinized (trypsin-EDTA, 1×) for 2 min and 10 min, respectively. Then, the cells were diluted in the culture medium to reach a final concentration of ∼4 × 104 cells/ml.
FIG. 1.
Micrographs depicting the morphology of (a) breast cancer cell line MDA-MB-231 and (b) normal epithelial cell line MCF-10A.
B. Device fabrication
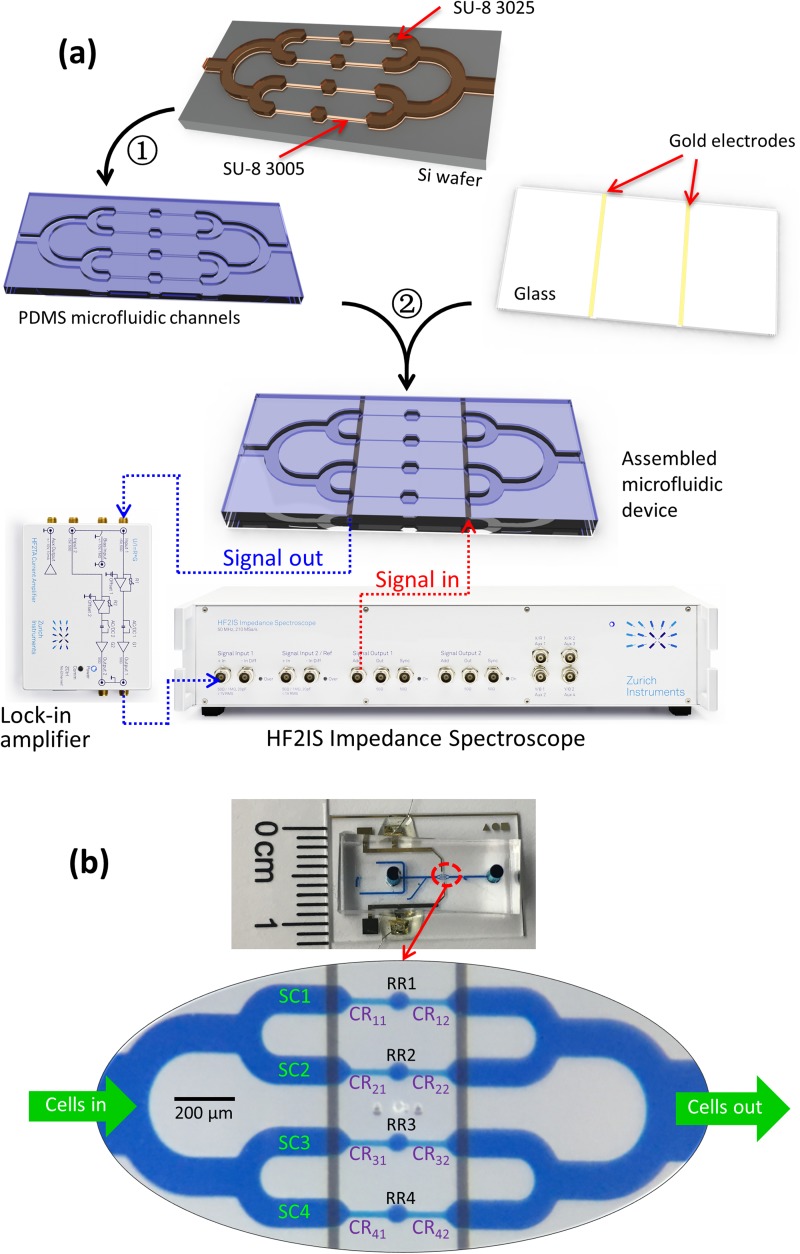
We fabricated the microfluidic channels by polydimethylsiloxane (PDMS) soft-lithography, followed by PDMS-glass bonding after the plasma treatment. As illustrated in Fig. 2(a), four microchannels with constriction (cross section: 8 × 8 μm2; length: 120 μm) and relaxation regions (cross section: 20 × 30 μm2; length: 40 μm) were connected in parallel. One electrode pair is shared across four channels. The microfluidic channel devices were fabricated on a silicon wafer with two layers of SU-8 (SU-8 3005 and SU-8 3025, MicroChem, Newton, MA) photolithography. Tridecafluoro-1,1,2,2-tetrahydrooctyl-1-trichlorosilane (TFOCS, Fisher Scientific) was coated on the surface of the molds for the easy release of PDMS. The detailed fabrication procedures are available in the supplementary material. The gold electrodes were deposited on glass by E-beam evaporation and lift-off. After the plasma treatment, the PDMS microchannel was aligned and bonded to the glass electrode under a microscope without adding methanol. Wires were soldered on the gold electrode pads after bonding [Fig. 2(b)] using the solder paste.22
FIG. 2.
(a) Device fabrication processes and experimental setup: ① PDMS replica molding, ② PDMS to glass bonding after the plasma treatment; (b) illustration of the channel configurations; SC: sensing channel; CR: constriction region; RR: relaxation region; CRxy, x: row number (x = 1, 2, 3, 4), y: column number (y = 1, 2).
C. Experimental
The microfluidic device was mounted on an inverted microscope (Zeiss Axio Observer, LSM-510, Thornwood, NY). The cell suspension solution was injected into the inlet at a constant flow rate of 10 μl/min controlled by a syringe pump. The wires are connected to an impedance analyzer (HF2IS impedance spectroscope, Zurich Instruments, Zurich, Switzerland) with the affiliated lock-in amplifier. An AC signal with 1 V in amplitude, and four frequencies at 1 kHz, 10 kHz, 100 kHz, and 1 MHz was applied on the electrodes for impedance measurement. The impedance data were recorded through LabView® and processed by Matlab®. Simultaneously, videos of the cells traveling through the parallel microfluidic channel were recorded at 240 frames per second (fps). The movement of the cells was aligned with impedance measurement results to validate our assumption that impedance spectroscopy can recognize the location of the cells passing through the constriction channels and relaxation regions. According to our previous research and that of others on bioelectrical characteristics at single-cell level analysis, the amplitude ratio and phase shift are important biomarkers to distinguish different cell types.11
As illustrated in Fig. 2(b), four parallel sensing channels (SC1, SC2, SC3, and SC4) share one pair of electrodes. In each sensing channel (SC), two sequential constriction regions (CR) are separated by one relaxation region (RR). Each constriction channel is named by the row number and column number. The first and second sequential constriction regions are labeled as CRx1 and CRx2, where x is the SC sequential number 1–4. The relaxation regions in each SC are named as RR1, RR2, RR3, and RR4.
III. RESULTS
A. Data collection and parameter definition
The shared pair of electrodes measure the overall impedance value of four channels in parallel. The impedance drops when the cell is in the RR because the impedance of the culture medium is lower than the cell. The time stamps generated by the RR helped identify the impedance shift of each cell. More importantly, the parallel SC allows more cells to pass through simultaneously. If one cell is deforming in one channel, other cells are still able to pass through other channels and be detected by the impedance variations. The impedance measurements were repeated on multiple devices with multiple samples (6 devices for MDA-MB-231 with 6 samples; and 5 devices for MCF-10A with 5 samples).
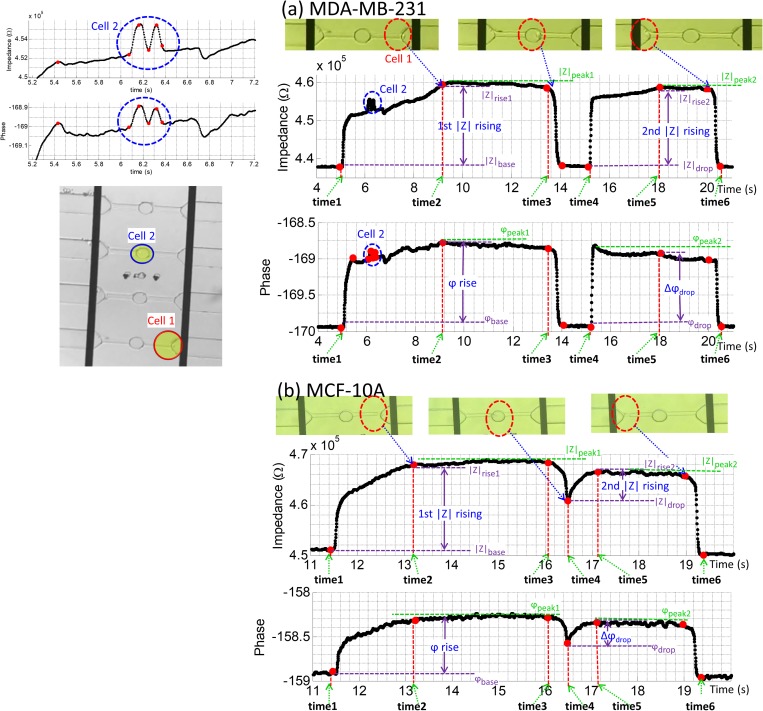
The location of a larger MDA-MB-231 (1, red) and a smaller MDA-MB-231 cancer cell (2, blue) in the microchannel with respect to time is plotted in Fig. 3(a). The impedance profile of the larger cancer cell 1 is well-resolved. As seen in Fig. 3, six time stamps were collected from a single cell passing through the channel SC4. By comparing the time stamps of the cell location and the impedance values, it can be seen that due to the deformation channel design, the impedance profile of this cell exhibits two peaks of roughly similar magnitude separated by a return to a baseline impedance value [Fig. 3(a)]. The impedance maxima at t = 9–13 s and t = 16–20 s correlated with the location of cell 1 in the CR41 and CR42, and the impedance dropped sharply as cell 1 entered the RR4 (t = 14–15 s); here the impedance value returned to the original baseline typical of the medium solution. A smaller cancer cell 2 entered another channel SC2 as cell 1 was passing through the microchannel, which was detected by the electrode pair at t ≈ 6 s (video “vv2.avi” in the supplementary material).
FIG. 3.
The impedance plot of an example cell through the device: (a) MDA-MB-231 (two cells); (b) MCF-10A (one cell).
The relaxation region in the middle of constriction channel causes a sharp drop in the impedance amplitude which can be correlated to the position of the cell in the relaxation region. Once a cell enters the relaxation region, the medium starts to flow around the deformed cell and reduces the measured impedance value. This allows us to label time stamps to obtain velocity profiles of each cell. If the cell recovers to its original spherical shape, as illustrated in Fig. 3(a) (14–15 s), the impedance will reduce to its baseline as no cells traveling in SC. If the cell still keeps the deformed rod shape and travels to CRx2 without full recovery, as illustrated in Fig. 3(b) (∼16.5 s), the impedance will show a drop in the amplitude and continue to rise again at the entrance of CRx2.
Both the bioelectrical properties and biomechanical properties can be obtained from the impedance measurement results. The amplitude ratio, phase shift, and travel time of single cells can be obtained directly from these impedance measurements as plotted in Fig. 3. The variables that represent the velocity profiles of a single cell can be defined as follows: the rise time CRx1 channel ; the rise time in CRx2 channel ; and the overall travel time of the cell in the whole channel . The amplitude rise in CRx1 is defined as ; the amplitude in CRx2 channel is defined as , where and are the rising impedance when the cell deforms in CRx1 and CRx2, respectively. is the baseline of the impedance value before the cell enters CRx1 channel. is the impedance value when the cell enters the RR and causes an impedance drop. is defined as the maximum value of impedance when the cell is traveling in either CRx1 or CRx2 channel. Similarly, the phase (φ) peak and baseline can also be collected at the same time points (videos “vv1.avi,” “vv2.avi,” “vv3.avi” in the supplementary material). Additionally, more combinations that reveal the bioelectrical and biomechanical properties of the cells can be obtained from the impedance plots in Fig. 3. Comparing the deformation differences can be used as another parameter. The rise time ratio is defined as the ratio of the 1st rising time and the 2nd rising time . Another parameter we included is the impedance rise slope, which is defined as and for the impedance rise slope in CRx1 channel and CRx2 channel, respectively. In some cases, as illustrated in Fig. 3(a), the impedance can drop back to the baseline if the cell relaxes in the RR and recovers back to its original shape. In other cases, as illustrated in Fig. 3(b), the cell enters CRx2 channel while maintaining its rod shape, which does not allow the impedance to reach the baseline. Therefore, we can define the impedance drop ratio , as the ratio of the magnitude of impedance drop and the maximum impedance rise. Similar to the impedance drop, as shown in Fig. 3, the phase will also drop when the cell reaches the RR1–4. Similar to the impedance drop ratio, the phase drop ratio is defined as . If the impedance magnitude drop of a cell reaches the baseline, the phase drop will also reach the baseline. If cells (whether similar size or not) are present at different channels at the same time and they cause blockage of the current (they deform and attach to walls), the baseline will be shifting to a new value. This will not affect the identification of rising, falling, and dropping of impedance values.
B. Data analysis
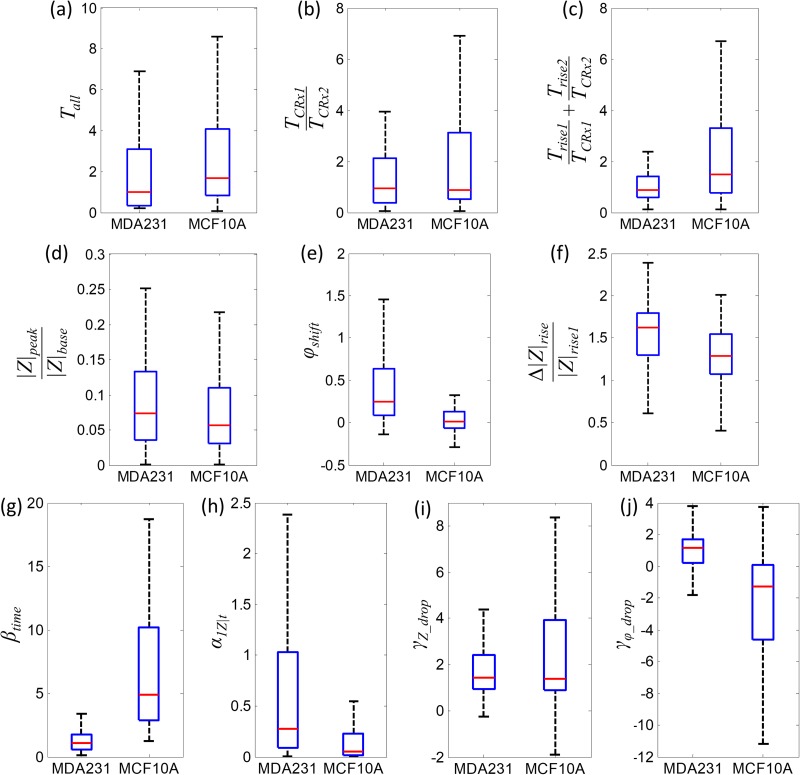
Many bioelectrical and biomechanical parameters can be extracted from the impedance measurement results. The total transit time includes the travel time of cells through CRx1, RR, and CRx2. is broadly used in single constriction channels to characterize cancer cells.11,23 However, this chip with four parallel channels with RRs shows significant overlapping of cancer cells (CA) and normal cells (NR) in the total transition time [Fig. 4(a)]. The travel time ratio in CRx1 and CRx2 also shows overlap with a similar median value [Fig. 4(b)]. If we select the rise time as a parameter, the ratio of rise time and travel time in each CR can better distinguish CA and NR cells at the population level [Fig. 4(c)]. Therefore, can be a good parameter to represent the biomechanical properties of CA and NR cells. Researchers found that impedance spectroscopy is a useful tool in differentiating tumor cells from their normal counterparts based on the amplitude ratios and phase shifts.24,25 The bioelectrical parameters include impedance amplitudes and phase shifts. The commonly used amplitude ratio is defined as the ratio of the relative impedance amplitude peak to impedance baseline, when no cell is traveling in the constriction channel.1,10 The phospholipid abundance differs in human cancer cells (MDA-MB-231) and normal breast epithelial cells (MCF-10A) and leads to the impedance and phase shift differences.26 The amplitude ratio shows a low differential rate between CA and NR cells [Fig. 4(d)]. The phase shifts of CA and NR show a better separation at the population level [Fig. 4(e)]; however, the Q75 line of MCF-10A is still higher than the Q25 line of MDA-MB-231, which means more than 25% of the cells cannot be distinguished by phase shifts only. A prediction accuracy of lower than 75% is not sufficient to distinguish CA and NR. Another bioelectrical parameter is the ratio of the impedance rise difference in CRx1 and CRx2 to the impedance rise in CRx1, which is defined as [Fig. 4(f)]. In the configuration of two sequential CR separated by one RR, the first deformation and second deformation can be compared and used as a biophysical marker for CA and NR cells. The impedance rise in CRx2 is directly affected by the biophysical status of the cells in RR. If the cell recovers to its original spherical shape, the secondary deformation and impedance rise in CRx2 will be more similar to the deformation and impedance rise in CRx1. The different cell membrane stiffnesses and cytoskeleton strength of MDA-MB-231 and MCF-10A will behave differently in the RR and result in different impedance rise in CRx2. The impedance drop ratio and phase drop ratio is used as a parameter to describe bioelectrical properties of cells in RR. The useful biomechanical and bioelectrical parameters for distinguishing CA and NR cells become , , , and . The rise time ratio shows a clear separation between the Q75 of CA cells and the Q25 value of NR cells [Fig. 4(g)]. The impedance rise slope in CRx1 shows a wider distribution of CA cells than NR cells [Fig. 4(h)]. The impedance drop ratio shows a significant overlap between CA and NR cells [Fig. 4(i)]; however, the phase drop ratio has separated Q25 line of CA and Q75 line of NR, which may contribute more in prediction accuracy between the CA and NR cells.
FIG. 4.
CA (cancer cells MDA-MB-231, n = 101) and NR (normal cells MCF-10A, n = 103) distinguished at the population level with the use of selected parameters: (a) total transit time; (b) transit time ratio of the passing time in CRx1 and CRx2; (c) the sum of rise time to passing time ratio in CRx1 and CRx2; (d) amplitude ratio: the ratio of relative impedance peak and impedance baseline; (e) phase shifts; (f) the ratio of the difference of impedance rise in CRx1 and CRx2 to the impedance rise in CRx1; (g) rise time ratio; (h) impedance rise slope in CRx1; (i) impedance drop ratio; (j) phase drop ratio. The blue box plot depicts the quantile numbers: maxima (upper dash whisker), Q75, Q50 (median, red bar), Q25, and minima (lower dashed whisker).
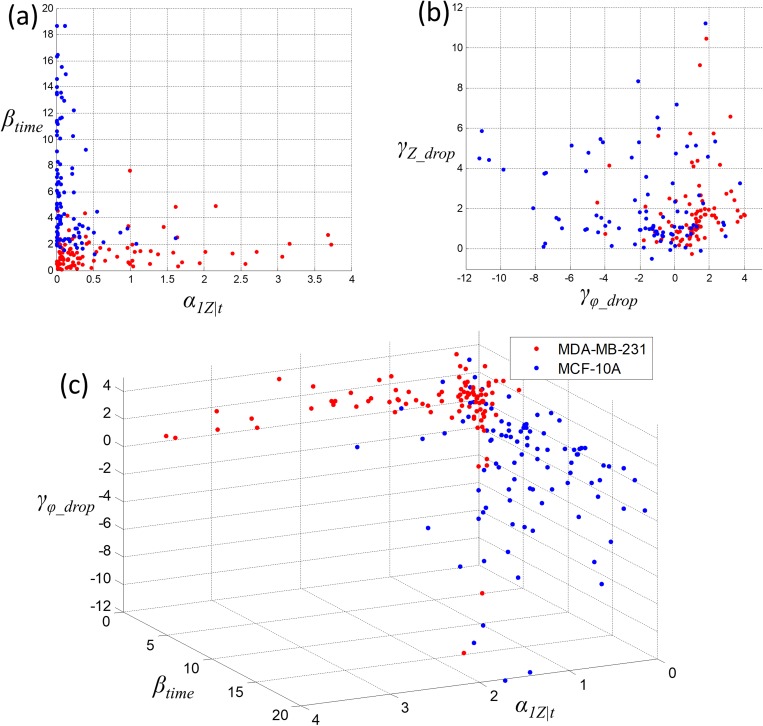
Figure 5 shows the scatter plot at the single-cell level by using 3 parameters, , , and to distinguish cancer cells (CA) and normal cells (NR). The vertical axis in Fig. 5(a) is the rising time ratio , which represents the biomechanical properties of the cells because it is a representation of how the cells behave in the relaxation region. In Fig. 5(a), using the vertical axis parameter, rising time ratio, a boundary between CA (red dots) and NR (blue dots) can be found to distinguish CA and NR at the single-cell level. This can be identified as the distinguish boundary between CA and NR using only the biomechanical properties. In Fig. 5(b), both the impedance drop ratio and phase drop ratio can also be used to establish a boundary to distinguish CA and NR. The parameter α1Z|t combines both the biomechanical and bioelectrical properties of the cells. The overall impedance includes the effect of both membrane capacitance and the cytoplasm resistance. We are not able to specify each parameter to a specific biological/biochemical property of a cell. The cancer cells have higher nuclear-cytoplasmic ratio (N/C ratio), which increased the membrane impedance of the cells. According to the literature,26 the membrane of cancer cells shows a higher impedance. In Fig. 5(c), together with three parameters selected, , , and , we can further improve the prediction accuracy between CA and NR at the single-cell level.
FIG. 5.
Scatter plot distinguishes at single-cell level cancer cells (MDA-MB-231, n = 101) and normal cells (MCF-10A, n = 103): (a) biomechanical parameters of rise time ratio; (b) bioelectrical parameters of impedance drop and phase drop ratios; and (c) combined biomechanical and bioelectrical properties.
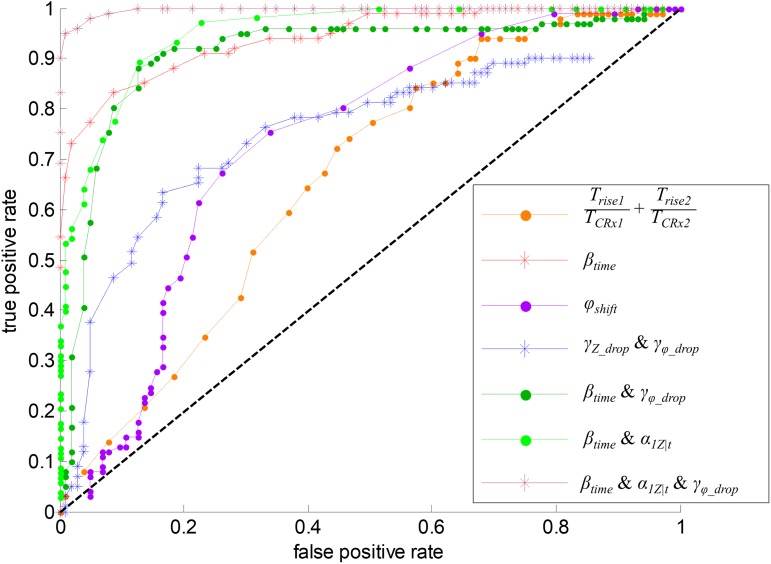
Figure 6 indicates the receiver operating characteristic (ROC) curve for the best separation between MDA-MB-231 and MCF-10A at the single-cell level. The area under curve (AUC) value can show the ability to differentiate cancer cells (MDA-MB-231) and normal cells (MCF-10A). The black line represents the boundary that CA and NR cannot be distinguished. The biomechanical parameter involving cells traveling time during impedance rise (orange curve in Fig. 6) shows a separation rate of 63% between CA and NR cells. (red curve in Fig. 6) shows that the separation ratio between CA and NR can reach 85%. The phase shift only (purple curve in Fig. 6) has a separation ratio of 63%; however, the false positive rate using cannot be decreased below 16% unless the true positive rate is also lower than 41%. The rate of differentiation between CA and NR with and has only 62% (blue line in Fig. 6). Combining the biomechanical parameter and a bioelectrical parameter with higher prediction accuracy shown in Fig. 4(j), , the differentiation rate between CA and NR can reach 85% (dark green curve in Fig. 6). If considering the combination of and , a parameter with both of the bioelectrical impedance rise and the biomechanical travel time during the impedance rise, the differentiating rate between CA and NR can reach 92% (light green curve in Fig. 6). When considering three parameters , , and , the maroon curve in Fig. 6 shows a low false positive rate and a high true positive rate. The rate at which between CA and NR can be identified reaches 97%.
FIG. 6.
ROC curve depicting the ability to discriminate CA (MDA-MB-231) and NR (MCF-10A) using different biophysical parameters. (ROC curve of other parameters is available in the supplementary material.)
IV. DISCUSSION
Cancer cell characteristics studied by label-free methods are focusing on the biophysical attributes, such as the abnormal nuclei, cell cytoskeleton strength, membrane stiffness and adhesion properties.18,27–30 The biomechanical properties of cancer cells can be measured and distinguished from normal cells by atomic force microscopy (AFM).27,31–34 The main issue with AFM measurement on cells is the extensive experimental procedures for both sample preparation and data acquisition. The biomechanical response time of cells to the cantilever stimulation is about 1 ms, while the mapping time for AFM on a cell can reach several minutes.35,36 Maintaining a survivable environment during the AFM measurement to keep the viability of the cells in the culture medium is challenging. The uncertainty of the consistency of the cells during the AFM mapping can introduce errors during experiments. Even with improved AFM stimulation on single cells, the data collection of cells at a large population by AFM can reach over 2 h for hundreds of cells,32 while our microfluidic chip presented in this paper can harvest the bioelectrical and biomechanical profile data within 20 s for a single cell. Meanwhile, probing for cells in aqueous environment by AFM cantilever tip with a spherical glass bead needs additional processes for fabrication and calibration.31,37,38 Based on the principle of AFM, Bagnall's group have developed a microfabricated cantilever structure on chip can also be used to collect biomechanical data from cultured cells; however, using this method, the data acquisition requires extensive video/image processing.39–41 To overcome the issues of AFM measurements for biomechanical properties of the cells, the microfluidic constriction channels become a superior tool for high throughput analysis of single cells. The time of data collection for both bioelectrical and biomechanical properties is also significantly reduced. The purpose of AFM and our microfluidic chip involves collecting biophysical properties from a large population of cells by studying single cells through a label-free technique. The CRs in our chip force the cells to deform and RRs allow the cells to recover, which has the similar function with the indentations by AFM cantilevers.
The use of parallel constriction channels for higher throughput cell analysis has been studied.42,43 However, capturing the biomechanical characteristics of cells directly from video analysis is difficult without proper time stamps from the constriction channel. Our device differs from existing devices by the addition of a RR within each constriction channel. This RR adds another crucial dimension to characterizing the biomechanical property of cells and also provides the means to assign time point labels for identifying the location of cells in the impedance measurement results. The six time points include the initial transit of CRx1 channel, followed by the transit through RR, and finally the transit through CRx2 channel. The CRx1 channel probed the deformation of the cell at a constriction channel, which is similar with other approaches of single cell biomechanical characterization with a single constriction channel. New to this device, the RR removes the mechanical stress which enables the cell to return to its original spherical shape. The CRx2 then reimposes mechanical stress thus probing the cells' biomechanical properties during the secondary deformation. The CA and NR cells are differentiated by the secondary deformation process, specifically defined here as the impedance rise slope at the entrance of CRx2 channel. The entry time at CRx1 and CRx2 is related to the cytoskeleton strength and the cell membrane stiffness. The deformability is one of the differences in biomechanical properties between cancer and normal cells. The impedance rise slope includes the bioelectrical impedance properties and the rise time, which is one of the biomechanical parameters. The phase drop ratio is the bioelectrical parameter related to the capacitance of the cells, including the specific membrane capacitance and the capacitance from cytoskeleton and nucleus.
The impedance properties of normal and cancer cells are less well known. Here, we incorporated electrodes to serve a dual role of providing time stamps on biomechanical changes in single cells and analyzing impedance changes in response to mechanical stress. There are more ways to label time stamps on constriction channels. For example, a more direct way is to embed more electrodes on the constriction channels with individual or differential impedimetric measurement.44,45 Zhou, et al. added two sets of electrodes at the entrance and the exit of a single constriction channel to detect the passing time of MCF-7.45 The separation between MCF-7 and drug treated MCF-7 was distinguished by the impedance of cells entering and exiting the channel, as well as the passing time with p < 0.001 at the population level.45 The time variables represent the velocity information and are indicative of the biomechanical properties of each cell. In our work, the presence of the RR amplified the difference of biomechanical behaviors between cancer and normal cells; the value of the RR here was predictable based upon on earlier findings of repetitive nanoindentation of cancer and normal cells.32 We also showed in previous work that using the two sequential constriction channels with one RR can distinguish cancer and normal cells at a ratio of ∼83% using selected biomechanical parameters.8 Both of these studies confirmed that cancer cells are more deformable than normal cells, and that cancer cells recover their original spherical shape faster than the normal cells. Therefore, the cancer cells face the secondary deformation process at CRx2 channel in a different biomechanical condition than normal cells. Normal cells, less deformable than cancer cells, result in a smaller secondary entrance time compared to the . The timing parameter shows the difference in biomechanical properties of the two cell lines. As a result, the of the breast cancer cell line MDA-MB-231 is smaller than the of the normal breast cell line MCF-10A. In the ROC curve, we showed similar conclusions to our prior work;8,15 we can reach a prediction ratio of 85.2% to identify cancer and normal cells if we rely only on the biomechanical properties.
The amplitude ratios and phase shifts represent the bioelectrical properties of each cell. Both the specific membrane capacitance and cytoplasm conductivity contribute to the bioelectrical data. Previous work on the bioelectrical characteristics of MDA-MB-231 and MCF-10A in single constriction channel, reported the membrane capacitance and cytoplasm conductivity of MDA-MB-231 are 1.63 ± 0.17 μF/cm2 and 24.9 ± 1.12 MΩ;13 and the membrane capacitance and cytoplasm conductivity of MCF-10A are 1.94 ± 0.14 μF/cm2 and 24.8 ± 1.05 MΩ.13 The dielectric parameters of MDA-MB-231 and MCF-10A cells are measured in constriction channels when the cells are deformed into rod shape. The key to improving discrimination between cancer and normal cells here is that both of the biomechanical and bioelectrical properties become the biosignatures of the cells. The phase drop ratio improves the prediction rate between CA and NR. MCF-10A is less deformable than MDA-MB-231, which causes a slower rod-to-spherical shape recovery in the RR. The impedance and phase drop will not reach the baseline if the cell retains the rod shape during the passing through RR. Based on the AUC, the ROC curve prediction rate between MDA-MB-231 and MCF-10A reaches 97% by combining both the biomechanical and bioelectrical properties.
We show that the shared pair of electrodes across four parallel channels can be used to measure bioelectrical parameters to increase the rate of cell identification. Even though multiple channels in parallel are added while still using a single electrode pair, the device is still sensitive enough to identify single cells or multiple cells passing through the parallel microchannels, which yields a 97% prediction rate between cancer and normal cell lines.
V. CONCLUSIONS
We developed a device with parallel constriction channels accompanied by a single sensor to improve the throughput and data collection of deformability assays. The two constriction regions and one relaxation region in each channel served to establish time stamps for collecting the biomechanical variables of the cells so that the biomechanical properties of the cells can be extracted from the time stamps in the impedance measurement. From the impedance measurement data, both biomechanical and bioelectrical properties were collected for data analysis. The potential for this device to effectively diagnose cancerous and normal breast cells is demonstrated. In the future, further refinements can be made. For example, embedding additional widths and lengths of the constriction channels and relaxation regions can potentially improve sensitivity to resolve more heterogeneous tissue samples. In addition, the acquisition of data and analysis of the increased variables such design features provide can also help understand the dynamic changes of cell biomechanical behaviors. We should add that this is the first time we report this new bioassay. The throughput of our device is scalable allowing tens or hundreds of microchannels in the array, which can process more than 2 ml of the liquid sample within a few minutes. Doing so may require multiplexing between different electrode pairs or integrating more impedance analyzers with the system (electronics can be custom made). In this paper, we used one pair of electrodes for 4 channels. The impedance data can be collected from multi-electrodes at the same time, which can increase the throughput significantly. More work needs to be done to study the influence of the microfluidic chip parameters including the length of the constriction regions, the length and width of the relaxation region and the ratio of the two on the predication rate between normal and cancer cells. Also, the question remains to be answered if the more comprehensive biophysical attributes proposed here can be extended to other cancer cells and if they can be used to even distinguish between different cancer cells with different degrees of invasiveness.
SUPPLEMENTARY MATERIAL
See the supplementary material for the detailed fabrication procedures and protocols of the microfluidic device; three videos to illustrate the image of cells and the impedance measurement results (vv1.avi, vv2.avi, vv3.avi); an illustration of the shift of impedance baseline when multiple cells passing through different channels; and the ROC curve of all parameters as the supplementary results of Fig. 6.
ACKNOWLEDGMENTS
The work was primarily funded through National Institutes of Health (NIH) National Cancer Institute (Award No. R21CA210216). The chips were fabricated in the Micro & Nano Fabrication Laboratory at Virginia Tech.
REFERENCES
- 1.Chen J., Li J., and Sun Y., Lab Chip 12(10), 1753–1767 (2012). 10.1039/c2lc21273k [DOI] [PubMed] [Google Scholar]
- 2.Chen J., Xue C., Zhao Y., Chen D., Wu M.-H., and Wang J., Int. J. Mol. Sci. 16(5), 9804–9830 (2015). 10.3390/ijms16059804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.TruongVo T., Kennedy R., Chen H., Chen A., Berndt A., Agarwal M., Zhu L., Nakshatri H., Wallace J., and Na S., J. Micromech. Microeng. 27(3), 035017 (2017). 10.1088/1361-6439/aa5bbb [DOI] [Google Scholar]
- 4.Zheng Y., Nguyen J., Wei Y., and Sun Y., Lab Chip 13(13), 2464–2483 (2013). 10.1039/c3lc50355k [DOI] [PubMed] [Google Scholar]
- 5.Zheng Y., Shojaei-Baghini E., Wang C., and Sun Y., Biosens. Bioelectron. 42, 496–502 (2013). 10.1016/j.bios.2012.10.081 [DOI] [PubMed] [Google Scholar]
- 6.Adamo A., Sharei A., Adamo L., Lee B., Mao S., and Jensen K. F., Anal. Chem. 84(15), 6438–6443 (2012). 10.1021/ac300264v [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guan G., Chen P. C., Peng W. K., Bhagat A. A., Ong C. J., and Han J., J. Micromech. Microeng. 22(10), 105037 (2012). 10.1088/0960-1317/22/10/105037 [DOI] [Google Scholar]
- 8.Ren X., Ghassemi P., Babahosseini H., Strobl J., and Agah M., ACS Sens. 2(2), 290–299 (2017). 10.1021/acssensors.6b00823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hu S. and Lam R. H., Microfluid. Nanofluid. 21(4), 68 (2017). 10.1007/s10404-017-1903-x [DOI] [Google Scholar]
- 10.Chen J., Zheng Y., Tan Q., Shojaei-Baghini E., Zhang Y. L., Li J., Prasad P., You L., Wu X. Y., and Sun Y., Lab Chip 11(18), 3174–3181 (2011). 10.1039/c1lc20473d [DOI] [PubMed] [Google Scholar]
- 11.Babahosseini H., Srinivasaraghavan V., Zhao Z., Gillam F., Childress E., Strobl J. S., Santos W. L., Zhang C., and Agah M., Lab Chip 16(1), 188–198 (2016). 10.1039/C5LC01201E [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Song H., Rosano J. M., Wang Y., Garson C. J., Prabhakarpandian B., Pant K., Klarmann G. J., Perantoni A., Alvarez L. M., and Lai E., Anal. Methods 8(41), 7437–7444 (2016). 10.1039/C6AY01377E [DOI] [Google Scholar]
- 13.Han A., Yang L., and Frazier A. B., Clin. Cancer Res. 13(1), 139–143 (2007). 10.1158/1078-0432.CCR-06-1346 [DOI] [PubMed] [Google Scholar]
- 14.Xue C., Wang J., Zhao Y., Chen D., Yue W., and Chen J., Micromachines 6(11), 1794–1804 (2015). 10.3390/mi6111457 [DOI] [Google Scholar]
- 15.Babahosseini H., Strobl J. S., and Agah M., Anal. Methods 9(5), 847–855 (2017). 10.1039/C6AY03342C [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luo Y., Chen D., Zhao Y., Wei C., Zhao X., Yue W., Long R., Wang J., and Chen J., Sens. Actuators B 202, 1183–1189 (2014). 10.1016/j.snb.2014.05.028 [DOI] [Google Scholar]
- 17.Zheng Y., Shojaei-Baghini E., Azad A., Wang C., and Sun Y., Lab Chip 12(14), 2560–2567 (2012). 10.1039/c2lc21210b [DOI] [PubMed] [Google Scholar]
- 18.Hou H. W., Li Q., Lee G., Kumar A., Ong C., and Lim C. T., Biomed. Microdevices 11(3), 557–564 (2009). 10.1007/s10544-008-9262-8 [DOI] [PubMed] [Google Scholar]
- 19.Lange J. R., Steinwachs J., Kolb T., Lautscham L. A., Harder I., Whyte G., and Fabry B., Biophys. J. 109(1), 26–34 (2015). 10.1016/j.bpj.2015.05.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ren X., Ghassemi P., Kanaan Y. M., Naab T., Copeland R. L., Dewitty R. L., Kim I., Strobl J. S., and Agah M., ACS Sens. 3(8), 1510–1521 (2018). 10.1021/acssensors.8b00301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nyberg K. D., Hu K. H., Kleinman S. H., Khismatullin D. B., Butte M. J., and Rowat A. C., Biophys. J. 113(7), 1574–1584 (2017). 10.1016/j.bpj.2017.06.073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ren X., Kumbur E. C., Zhou J. G., Noh M., and Chong P. L.-G., J. Membr. Sci. 540, 27–34 (2017). 10.1016/j.memsci.2017.06.041 [DOI] [Google Scholar]
- 23.Apichitsopa N., Jaffe A., and Voldman J., Lab Chip 18(10), 1430–1439 (2018). 10.1039/C8LC00240A [DOI] [PubMed] [Google Scholar]
- 24.Mulhall H., Labeed F., Kazmi B., Costea D., Hughes M., and Lewis M., Anal. Bioanal. Chem. 401(8), 2455–2463 (2011). 10.1007/s00216-011-5337-0 [DOI] [PubMed] [Google Scholar]
- 25.Liang X., Graham K., Johannessen A., Costea D., and Labeed F., Integr. Biol. 6(5), 545–554 (2014). 10.1039/C3IB40255J [DOI] [PubMed] [Google Scholar]
- 26.Dória M. L., Cotrim C. Z., Simões C., Macedo B., Domingues P., Domingues M. R., and Helguero L. A., J. Cell. Physiol. 228(2), 457–468 (2013). 10.1002/jcp.24152 [DOI] [PubMed] [Google Scholar]
- 27.Cross S. E., Jin Y.-S., Rao J., and Gimzewski J. K., Nat. Nanotechnol. 2(12), 780–783 (2007). 10.1038/nnano.2007.388 [DOI] [PubMed] [Google Scholar]
- 28.Xu W., Mezencev R., Kim B., Wang L., McDonald J., and Sulchek T., PloS One 7(10), e46609 (2012). 10.1371/journal.pone.0046609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Swaminathan V., Mythreye K., O'Brien E. T., Berchuck A., Blobe G. C., and Superfine R., Cancer Res. 71(15), 5075–5080 (2011). 10.1158/0008-5472.CAN-11-0247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Agus D. B., Alexander J. F., Arap W., Ashili S., Aslan J. E., Austin R. H., Backman V., Bethel K. J., Bonneau R., and Chen W.-C., Sci. Rep. 3, 1449 (2013). 10.1038/srep01449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Babahosseini H., Carmichael B., Strobl J., Mahmoodi S., and Agah M., Biochem. Biophys. Res. Commun. 463(4), 587–592 (2015). 10.1016/j.bbrc.2015.05.100 [DOI] [PubMed] [Google Scholar]
- 32.Babahosseini H., Strobl J. S., and Agah M., Nanotechnology 26(35), 354004 (2015). 10.1088/0957-4484/26/35/354004 [DOI] [PubMed] [Google Scholar]
- 33.Lekka M., Laidler P., Gil D., Lekki J., Stachura Z., and Hrynkiewicz A., Eur. Biophys. J. 28(4), 312–316 (1999). 10.1007/s002490050213 [DOI] [PubMed] [Google Scholar]
- 34.Rosenbluth M. J., Lam W. A., and Fletcher D. A., Biophys. J. 90(8), 2994–3003 (2006). 10.1529/biophysj.105.067496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heu C., Berquand A., Elie-Caille C., and Nicod L., J. Struct. Biol. 178(1), 1–7 (2012). 10.1016/j.jsb.2012.02.007 [DOI] [PubMed] [Google Scholar]
- 36.Deng X., Xiong F., Li X., Xiang B., Li Z., Wu X., Guo C., Li X., Li Y., and Li G., J. Nanobiotechnol. 16(1), 102 (2018). 10.1186/s12951-018-0428-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Babahosseini H., Strobl J. S., and Agah M., Nanomedicine 10(17), 2635–2638 (2015). 10.2217/nnm.15.104 [DOI] [PubMed] [Google Scholar]
- 38.Nikkhah M., Strobl J. S., De Vita R., and Agah M., Biomaterials 31(16), 4552–4561 (2010). 10.1016/j.biomaterials.2010.02.034 [DOI] [PubMed] [Google Scholar]
- 39.Arntz Y., Seelig J. D., Lang H., Zhang J., Hunziker P., Ramseyer J., Meyer E., Hegner M., and Gerber C., Nanotechnology 14(1), 86 (2002). 10.1088/0957-4484/14/1/319 [DOI] [Google Scholar]
- 40.Bagnall J. S., Byun S., Begum S., Miyamoto D. T., Hecht V. C., Maheswaran S., Stott S. L., Toner M., Hynes R. O., and Manalis S. R., Sci. Rep. 5, 18542 (2015). 10.1038/srep18542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bagnall J. S., Byun S., Miyamoto D. T., Kang J. H., Maheswaran S., Stott S. L., Toner M., and Manalis S. R., Integr. Biol. 8(5), 654–664 (2016). 10.1039/c5ib00284b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huang S., Undisz A., Diez-Silva M., Bow H., Dao M., and Han J., Integr. Biol. 5(2), 414–422 (2013). 10.1039/C2IB20161E [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rosenbluth M. J., Lam W. A., and Fletcher D. A., Lab Chip 8(7), 1062–1070 (2008). 10.1039/b802931h [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reale R., De Ninno A., Businaro L., Bisegna P., and Caselli F., Microfluid. Nanofluid. 22(4), 41 (2018). 10.1007/s10404-018-2055-3 [DOI] [Google Scholar]
- 45.Zhou Y., Yang D., Zhou Y., Khoo B. L., Han J., and Ai Y., Anal. Chem. 90(1), 912–919 (2017). 10.1021/acs.analchem.7b03859 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
See the supplementary material for the detailed fabrication procedures and protocols of the microfluidic device; three videos to illustrate the image of cells and the impedance measurement results (vv1.avi, vv2.avi, vv3.avi); an illustration of the shift of impedance baseline when multiple cells passing through different channels; and the ROC curve of all parameters as the supplementary results of Fig. 6.