Abstract
Background:
Sickle cell trait (SCT), sickle cell disease’s (SCD) carrier status, has been recently associated with worse cardiovascular and renal outcomes. An increased prevalence of atrial fibrillation (AF) is documented in SCD patients; however, studies in individuals with SCT are lacking.
Objectives:
To determine the association of SCT with AF
Methods:
Among African-American participants in the REasons for Geographic and Racial Differences in Stroke (REGARDS) Study we assessed the association of SCT (by ECG or medical history) with prevalent AF using logistic regression adjusting for age, sex, income, education, history of stroke, myocardial infarction, diabetes, hypertension, and chronic kidney disease. A second evaluation was performed a mean of 9.2 years later among available participants, and the same model was used to test the association of SCT with incident AF.
Results:
In 10,409 participants with baseline ECG data and genotyping, 778 (7.5%) had SCT and 811 (7.8%) had prevalent AF. After adjusting for age, sex, education and income, SCT was associated with AF, OR 1.32 (95% CI 1.03–1.70). The association with incident AF assessed at the second in-home visit with the same adjustments was similar; OR 1.25 (95% CI 0.77–2.03).
Conclusions:
SCT was associated with a higher prevalence of AF and a non-significantly higher incident AF over a 9.2 year period independent of AF risk factors. SCT remained associated with prevalent AF after adjusting for potential factors on the causal pathway such as hypertension and chronic kidney disease suggesting alternate mechanisms for the increased risk.
Keywords: Sickle Cell Trait, Atrial Fibrillation
Introduction
Sickle cell trait (SCT) is the heterozygous, purportedly asymptomatic carrier state for sickle cell disease (SCD) and is present in approximately 8% of African-Americans[1]. SCD profoundly affects individuals’ quality of life and leads to painful vaso-occlusive crises and progressive disability leading to early death. Once thought to be a benign condition[2], SCT is associated with an increased risk of chronic kidney disease (CKD)[3, 4], and other medical conditions[5–9]. Previous studies have shown no evidence to date that SCT leads to a lower life expectancy[10, 11], or stroke[12], and there are mixed data on the association with coronary artery disease[13–15]. Prior studies have not examined associations between arrhythmias such as atrial fibrillation (AF) and SCT.
AF has been documented in SCD patients in multiple studies[16–19]. In contrast to SCD, there are no similar studies documenting AF in SCT carriers. Cardiac problems in people with SCT have focused on athletes and sudden cardiac death[5, 20]. We evaluated the association with SCT and AF as AF is a common condition with an increasing prevalence[21] especially as the population ages and is a risk factor for ischemic stroke and heart failure[22].
Naik et al. demonstrated an association of SCT with an increased risk of CKD in several cohorts, including the REasons for Geographic and Racial Differences in Stroke (REGARDS) study [3, 4]. CKD and hypertension are known risk factors for AF[23], and SCT may be associated with AF by other poorly defined mechanisms beyond simply exacerbating other known risk factors Thus we hypothesized that SCT would be associated with an increased risk of prevalent and incident AF in African-Americans.
Methods
Study Cohort
REGARDS recruited 30,239 black and white men and women over 45 years old from 2003–2007, with the principal aim of identifying causes of racial and regional disparities in stroke incidence and mortality in the United States. The detailed study design has been published[24]. REGARDS recruited individuals from a commercially available list of U.S. residents. Participants were excluded if they could not speak English, had a self-reported race other than black or white, were on a waiting list for a nursing home, or had active cancer in the past year. Using a computer-assisted telephone interview, interviewers obtained demographic information and a cardiovascular medical history, including a physician diagnosis of AF. Consent was obtained initially on the telephone and subsequently in writing during an in-person evaluation 3–4 weeks later. During the visit, staff obtained blood and urine samples, medication history, and performed a resting ECG. ECGs were centrally interpreted for factors including prevalent AF. Participants were followed every 6 months by telephone for possible stroke and other outcomes[25]. A second in-home visit was performed an average of 9.2 years later among available REGARDS participants, where participants gave their health history and a second ECG was performed, and similarly interpreted for AF.
SCT status was determined by direct genotyping for rs334 using TaqMan®.[26, 27]
Genotyping for SCT was not performed on participants who self-reported as white. For this analysis, REGARDS participants were excluded if they did not self-identify as black, did not have stored DNA or consent for genetics research, or hemoglobin SS (SCD) or hemoglobin SC disease (a related sickling hemoglobinopathy). REGARDS was approved by the institutional review boards of all participating institutions and was conducted according to the provisions of the Declaration of Helsinki.
Outcomes
For the cross-sectional analysis, as previously reported[28], AF was defined at baseline as ECG evidence of AF at the time of the in-home physical exam or as a self-reported physician diagnosis of AF. For the incident AF analysis, Incident AF was defined as a self-reported physician diagnosis of AF or ECG evidence of AF in those free of AF by ECG or self-report at baseline.
Definitions
Age was specified as age at the time of entry into the REGARDS cohort. Income was categorized as <$20,000, $20,000–34,999, $35,000–74,999, or ≥$75,000 annually, and education as less than high- school, high-school graduate, some college, and college and above. Cardiovascular disease (CVD) was defined as a self-reported physician diagnosis of prior myocardial infarction or stroke. Hypertension was defined as the use of antihypertensive medications, or a resting in-home systolic blood pressure reading of ≥140 mmHg. Diabetes was defined as a prior self-reported physician diagnosis of diabetes, or the use of oral medications or insulin, excluding gestational diabetes. CKD was stratified into four groups (stage 0–1, 2, 3, 4+) according to the Kidney Disease: Improving Global Outcomes 2012 Clinical Practice Guidelines[29] incorporating estimated glomerular filtration rate from the CKD-EPI equation[30] and urine albumin concentration.
Statistical analysis
Baseline characteristics were compared by SCT status using chi-square analysis and t-tests for continuous variables. Staged logistic regression models were used to calculate odds ratios (ORs) of AF by presence of SCT. Covariates were added in three steps:
Model 1: adjusted for age, sex, income, and education
Model 2: Model 1 + body mass index, history of CVD, and diabetes
Model 3: Model 2 + CKD and hypertension
The purpose of Model 3 was to test for possible mediation of the association by CKD and hypertension, given the known association of SCT with CKD [3]. In addition to model 3, to control for population substructure, 10 principal components (PC) of genetic ancestry were generated in Eigenstrat[31] and added to Model 3. PC of genetic ancestry were measured in a case-control study and available in 67 percent of African-Americans. As these were not available in all individuals, we assessed the association of SCT with AF in those with PC of ancestry available. Additionally, a sensitivity analysis using electrocardiogram documented AF only was performed. Unadjusted baseline associations were also compared using Chi square analysis. All two-sided p values < 0.05 were considered significant.
The above analyses were repeated in those who had a 2nd home visit by REGARDS investigators who were free of AF at the initial visit to determine the OR of incident AF based on AF status at the second examination. Given that it is not possible to estimate the precise timing of developing AF based on ECG evidence seen on the 2nd home visit, cox proportional hazard models could not be used
All analysis was performed using STATA v 14.1 (StataCorp. 2015. Stata Statistical Software: Release 14. College Station, TX: StataCorp LP.)
Results
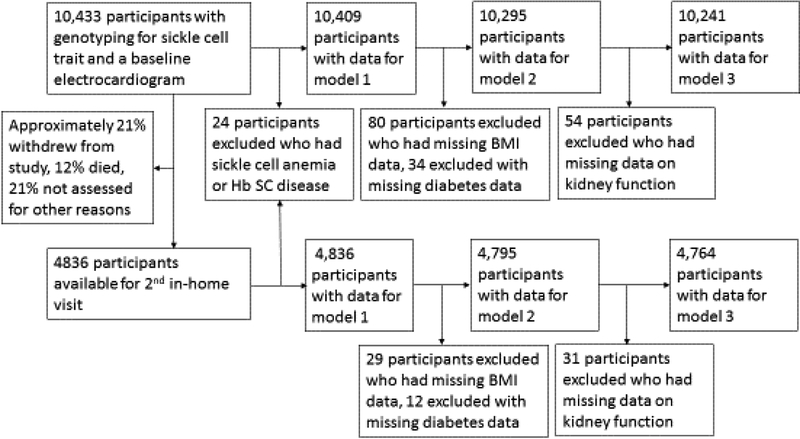
There were 10,433 African-American REGARDS participants who had genotyping for SCT as well as ECG and complete medical history (83% of the 12,514 African Americans in the study). Ten individuals homozygous for hemoglobin SS (sickle cell anemia) (0.09%) and 14 (0.13%) with Hemoglobin SC disease were excluded from the analysis. There were 778 participants with SCT (7.5%). Figure 1 outlines how many participants were excluded or had missing data at each stage of the analysis. Overall, 811 (7.8%) of these participants had either a medical history and/or ECG evidence of AF at baseline. Table 1 summarizes the baseline characteristics of participants with and without SCT. The mean baseline age was 64 years (range 45–96). Twice as many participants with SCT had stage 4 kidney disease by KDIGO criteria (2.9% vs 1.4% for people with and without SCT respectively, p=0.001).
Figure 1:
Summary of participant exclusions and missing data
Table 1:
Baseline characteristics of African American REGARDS participants by Sickle Cell Trait Status
| No Sickle Cell Trait | Sickle Cell Trait | P-value | |
|---|---|---|---|
| Mean Age (SD) | 64 (9) | 63.5 (9) | 0.14 |
| Female | 61% | 64% | 0.10 |
| Income < $20,000/year | 38.9% | 40.9% | 0.28 |
| Less than high school education | 19.7% | 19.2% | 0.84 |
| Mean BMI (SD) | 30.8 (6.7) | 30.7 (6.8) | 0.72 |
| History of cardiovascular disease | 13.7% | 13.5% | 0.85 |
| Diabetes | 30.3% | 32.2% | 0.25 |
| Stage 4 or higher chronic kidney disease | 1.4% | 2.9% | 0.001 |
| Hypertension | 66% | 63% | 0.07 |
| Mean Systolic Blood Pressure (SD) | 131 (17) | 132 (17.7) | 0.26 |
| Atrial Fibrillation | 7.3% | 9.5% | 0.02 |
SD= Standard Deviation
Table 2 outlines the main results of the cross sectional analysis. Overall, SCT was associated with an approximately 1.3-fold increased odds of AF in all models (lower limit of the 95% CI >1.0). In Model 1 (the demographic model) the OR (95% CI) was 1.32 (1.03–1.70). Further adjustment for AF risk factors (Model 2) or for CKD and hypertension (Model 3) did not attenuate or strengthen the association of SCT with AF.
Table 2:
Odds Ratios (ORs) and 95% Confidence Intervals (CIs) of Prevalent Atrial Fibrillation with Sickle Cell Trait
| N | N with prevalent AF | OR (95% CI) | |
|---|---|---|---|
| Model 1 | 10,409 | 811 | 1.32 (1.03, 1.70) |
| Model 2 | 10,295 | 797 | 1.33 (1.03, 1.71) |
| Model 3 | 10,241 | 793 | 1.32 (1.02, 1.70) |
Model 1: adjustment for age, sex, income and education. Model 2: Model 1+ adjustment for body mass index, cardiovascular disease history, diabetes. Model 3: Model 2 + adjustment for chronic kidney disease and hypertension
At the second in-home visit, an average 9.2 years after baseline, 4,836 participants with baseline known AF status and genotyping had an ECG. There was a similar association with incident AF and SCT as seen in prevalent AF with the OR in model 3 being 1.25 but the 95% confidence intervals were wider and crossed 1 (0.77, 2.03) (Table 3). In a sensitivity analysis classifying prevalent AF as AF present at either REGARDS exam, the association of SCT with AF was stronger than in analysis from the baseline visit, with an OR of 1.39 (1.01, 1.92) for model 3 (table 4). In this analysis, the total prevalence of ever having reported a history of AF or ever having ECG evidence of AF in the participants available for a 2nd in-home visit was slightly higher than the main cohort, at 10.1%.
Table 3:
Odds Ratios (ORs) and 95% Confidence Intervals (CIs) of Incident AF over 9.2 Years by Sickle Cell Trait
| N | N with incident AF | OR (95% CI) | |
|---|---|---|---|
| Model 1 | 4,836 | 208 | 1.25 (0.77, 2.03) |
| Model 2 | 4,795 | 206 | 1.27 (0.78, 2.07) |
| Model 3 | 4,764 | 206 | 1.28 (0.78, 2.08) |
Model 1: adjustment for age, sex, income and education. Model 2: Model 1+ adjustment for body mass index, cardiovascular disease history, diabetes. Model 3: Model 2 + adjustment for chronic kidney disease and hypertension
Table 4.
Odds Ratios (ORs) and 95% Confidence Intervals (CIs) of cross-sectional analysis of all prevalent AF noted at 2nd in-home visit by Sickle Cell Trait
| N | N with prevalent AF | OR (95% CI) | |
|---|---|---|---|
| Model 1 | 4,836 | 490 | 1.43 (1.05, 1.97) |
| Model 2 | 4,795 | 483 | 1.41 (1.03, 1.94) |
| Model 3 | 4,764 | 482 | 1.39 (1.01, 1.92) |
Model 1: adjustment for age, sex, income andeducation. Model 2: Model 1+ adjustment for body mass index, cardiovascular disease history, diabetes. Model 3: Model 2 + adjustment for chronic kidney disease and hypertension
In an additional sensitivity analysis of the odds of prevalence of ECG evidence of AF, the association of SCT with AF was greater, with ORs of 1.7–1.8 in all three models, while the association was weaker for AF defined by history alone (OR of 1.26 in all three models), but in both instances the 95% CIs included 1.0. When we restricted the analysis to individuals with PCs of genetic ancestry available, there was no association of SCT with AF in this subset (OR 1.01; 95% CI 0.71, 1.44), as such we did not further pursue adjusting for PC of genetic ancestry in additional models.
Discussion
The main findings of this study were a 32% higher odds of prevalent AF in African Americans with SCT after adjusting for age, gender, education, income, cardiovascular disease. Additional adjustment for CKD or hypertension did not attenuate this association. There was also a 26% higher odds of incident AF, though this finding was not statistically significant
To our knowledge, the association of SCT with AF has not been reported before. We had hypothesized that SCT could be associated with AF by acting through the intermediaries of CKD[23] which in turn may be associated with hypertension[32]. Arguing against CKD and hypertension mediating the association of SCT with AF is that after adjusting for CKD, hypertension, and known cardiovascular disease, the association of SCT with AF did not meaningfully change. These data are confusing but consistent with the surprising but robust recent epidemiological research demonstrating that SCT was not independently associated with risk of ischemic stroke among African Americans in the United States[12], even though ischemic stroke is associated with CKD and SCT is associated with risk of CKD.
While we have previously shown the substantial accuracy of self-reported AF[28], a strength of this study include the ability to capture AF data by both medical history and ECG. Medical history reported by REGARDS participants has also been validated by comparing it with Medicare claims data in other cardiovascular diseases[33] and it provides a better capture of paroxysmal or persistent AF, conditions which are still associated with thromboembolism and that require anticoagulation[34] as well as rhythm-controlled AF, and reported history of AF has been found to be a significant risk factor in developing ischemic stroke[28]. Furthermore, when a more strict definition of AF by ECG criteria alone was used, the direction of the association was similar with a greater magnitude. Additionally, the fact that there was a similar prevalent and incident association is helpful in that is provides an internal replication of results.
This study has several limitations. While data on prevalent AF at the time of entry into the study is robust, but the data on incident AF was less so: medical histories and a second in-home ECG were available from only about half of the participants, with approximately 12% dying between visits, 21% withdrawing from the study, and 21% not assessed for other reasons. The number of participants with incident AF was relatively small so our power was limited. Finally, when we looked at the subset of participants with PC of genetic ancestry available there was no association between SCT and AF. PC data was available in only a subset of the cohort, and as there were significantly more participants with AF in the subset that did not have PC data available, it appeared to be non-random, rather than a simple loss of power. In theory, SCT could be a marker of another nearby mutation and adjusting for population substructure may have attenuated this association. While possible, this would be unlikely due to the known pathologic implications of SCT and SCD as well as lack of attenuation of SCT with adverse outcomes after adjusting for PC of genetic ancestry for other diseases in other populations[3, 12]. Finally, as genotyping for SCT was limited to African American participants in REGARDS, these findings may not be generalizable to people of other races or living in other regions.
Conclusions
SCT was associated with AF independent of risk factors for AF. The mechanism does not appear to act through CKD and hypertension, known risk factors for AF and conclusions regarding a proposed mechanism cannot be drawn from the data. Given the association of HTN and CKD with AF, these data support the need to better understand the clinical consequences of SCT as well as other polymorphisms which may impact the association of SCT with cardiovascular diseases. These data add AF to the potential diseases associated with SCT and demonstrate the keen need to understand the health consequences of SCT as it seems less and less to be an asymptomatic carrier state of a common genetic disease.
Highlights.
Sickle cell trait was associated with a 1.32 increased odds of atrial fibrillation
This was independent of all measured risk factors.
Sickle cell trait may increase the risk of atrial fibrillation in African-Americans.
Acknowledgements:
We thank the staff and participants of REGARDS for their important contributions.
Funding:
This research project is supported by a cooperative agreement from the National Institute of Neurological Disorders and Stroke, National Institutes of Health, Department of Health and Human Service [U01 NS041588]. The authors thank the other investigators, the staff, and the participants of the REGARDS study for their valuable contributions. A full list of participating REGARDS investigators and institutions can be found at http://www.regardsstudy.org. Additional funding was provided by Lake Champlain Cancer Research Organization Inc. Additionally, this project has been funded in part with Federal funds from the Frederick National Laboratory for Cancer Research, National Institutes of Health, under contract [HHSN261200800001E]. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products or organizations imply endorsement by the US Government.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: no relevant disclosures
References
- 1.Ojodu J, Hulihan MM, Pope SN, Grant AM, Centers for Disease C, Prevention. Incidence of sickle cell trait--United States, 2010. MMWR Morbidity and mortality weekly report. 2014; 63: 1155–8. [PMC free article] [PubMed] [Google Scholar]
- 2.Motulsky AG. Frequency of sickling disorders in U.S. blacks. N Engl J Med. 1973; 288: 31–3. 10.1056/NEJM197301042880108. [DOI] [PubMed] [Google Scholar]
- 3.Naik RP, Derebail VK, Grams ME, Franceschini N, Auer PL, Peloso GM, Young BA, Lettre G, Peralta CA, Katz R, Hyacinth HI, Quarells RC, Grove ML, Bick AG, Fontanillas P, Rich SS, Smith JD, Boerwinkle E, Rosamond WD, Ito K, Lanzkron S, Coresh J, Correa A, Sarto GE, Key NS, Jacobs DR, Kathiresan S, Bibbins-Domingo K, Kshirsagar AV, Wilson JG, Reiner AP. Association of sickle cell trait with chronic kidney disease and albuminuria in African Americans. JAMA. 2014; 312: 2115–25. 10.1001/jama.2014.15063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Naik RP, Haywood C Jr. Sickle cell trait diagnosis: clinical and social implications. Hematology /the Education Program of the American Society of Hematology American Society of Hematology Education Program. 2015; 2015: 160–7. 10.1182/asheducation-2015.1.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsaras G, Owusu-Ansah A, Boateng FO, Amoateng-Adjepong Y. Complications associated with sickle cell trait: a brief narrative review. Am J Med. 2009; 122: 507–12. 10.1016/j.amjmed.2008.12.020. [DOI] [PubMed] [Google Scholar]
- 6.Kark JA, Posey DM, Schumacher HR, Ruehle CJ. Sickle-cell trait as a risk factor for sudden death in physical training. N Engl J Med. 1987; 317: 781–7. 10.1056/NEJM198709243171301. [DOI] [PubMed] [Google Scholar]
- 7.Harmon KG, Drezner JA, Klossner D, Asif IM. Sickle cell trait associated with a RR of death of 37 times in National Collegiate Athletic Association football athletes: a database with 2 million athlete-years as the denominator. British journal of sports medicine. 2012; 46: 325–30. 10.1136/bjsports-2011-090896. [DOI] [PubMed] [Google Scholar]
- 8.Davis CJ Jr., Mostofi FK, Sesterhenn IA. Renal medullary carcinoma. The seventh sickle cell nephropathy. The American journal of surgical pathology. 1995; 19: 1–11. [DOI] [PubMed] [Google Scholar]
- 9.Austin H, Key NS, Benson JM, Lally C, Dowling NF, Whitsett C, Hooper WC. Sickle cell trait and the risk of venous thromboembolism among blacks. Blood. 2007; 110: 908–12. 10.1182/blood-2006-11-057604. [DOI] [PubMed] [Google Scholar]
- 10.Stark AD, Janerich DT, Jereb SK. The incidence and causes of death in a follow-up study of individuals with haemoglobin AS and AA. International journal of epidemiology. 1980; 9: 325–8. [DOI] [PubMed] [Google Scholar]
- 11.Ashcroft MT, Desai P. Mortality and morbidity in Jamaican adults with sickle-cell trait and with normal haemoglobin followed up for twelve years. Lancet. 1976; 2: 784–6. [DOI] [PubMed] [Google Scholar]
- 12.Hyacinth HI, Carty CL, Seals SR, et al. Association of sickle cell trait with ischemic stroke among african americans: A meta-analysis. JAMA Neurology. 2018. 10.1001/jamaneurol.2018.0571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bucknor MD, Goo JS, Coppolino ML. The risk of potential thromboembolic, renal and cardiac complications of sickle cell trait. Hemoglobin. 2014; 38: 28–32. 10.3109/03630269.2013.832689. [DOI] [PubMed] [Google Scholar]
- 14.Nelson DA, Deuster PA, Carter R 3rd, Hill OT, Wolcott VL, Kurina LM. Sickle Cell Trait, Rhabdomyolysis, and Mortality among U.S. Army Soldiers. N Engl J Med. 2016; 375: 435–42. 10.1056/NEJMoa1516257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hyacinth HI, Cara CL, Seals SR, Irvin MR, Naik RP, Caughey M, Winkler C, Franceschini N, Burke GL, Zakai NA, Kopp JB, Judd SE, Adams RJ, Gee BE, Longstreth W, Egede LE, Lackland DT, Greenberg CS, Herman T, Manson JE, Key NS, Derebail VK, Kshirsagar AV, Folsom AR, Konety SH, Howard VJ, Allison M, Wilson JG, Correa A, Zhi D, Arnett D, Howard G, Cushman M, Reiner A, Safford MM. Association of Sickle Cell Trait with Risk of Coronary Heart Disease in African Americans. Blood. 2016; 128: 11-.27389540 [Google Scholar]
- 16.Holloman KL, Johnson CS, Haywood LJ. Electrocardiogram analysis in adult patients with sickle cell disease. Journal of the National Medical Association. 1987; 79: 809–14. [PMC free article] [PubMed] [Google Scholar]
- 17.Upadhya B, Ntim W, Brandon Stacey R, Henderson R, Leedy D, O’Brien FX, Knovich MA. Prolongation of QTc intervals and risk of death among patients with sickle cell disease. European journal of haematology. 2013; 91: 170–8. 10.1111/ejh.12127. [DOI] [PubMed] [Google Scholar]
- 18.Bode-Thomas F, Hyacinth HI, Ogunkunle O, Omotoso A. Myocardial ischaemia in sickle cell anaemia: evaluation using a new scoring system. Annals of tropical paediatrics. 2011; 31: 67–74. 10.1179/1465328110Y.0000000006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garadah T, Gabani S, Alawi MA, Abu-Taleb A. Prevalence and Predisposing Factors of Atrial Fibrillation in a Multi-Ethnic Society: The Impact of Racial Differences in Bahrain. Open journal of cardiovascular surgery. 2011; 4: 9–16. 10.4137/OJCS.S8032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Key NS, Connes P, Derebail VK. Negative health implications of sickle cell trait in high income countries: from the football field to the laboratory. Br J Haematol. 2015; 170: 5–14. 10.1111/bjh.13363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Piccini JP, Hammill BG, Sinner MF, Jensen PN, Hernandez AF, Heckbert SR, Benjamin EJ, Curtis LH. Incidence and prevalence of atrial fibrillation and associated mortality among Medicare beneficiaries, 1993–2007. Circ Cardiovasc Qual Outcomes. 2012; 5: 85–93. 10.1161/CIRCOUTCOMES.111.962688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zoni-Berisso M, Lercari F, Carazza T, Domenicucci S. Epidemiology of atrial fibrillation: European perspective. Clin Epidemiol. 2014; 6: 213–20. 10.2147/CLEP.S47385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Watanabe H, Watanabe T, Sasaki S, Nagai K, Roden DM, Aizawa Y. Close bidirectional relationship between chronic kidney disease and atrial fibrillation: the Niigata preventive medicine study. American heart journal. 2009; 158: 629–36. 10.1016/j.ahj.2009.06.031. [DOI] [PubMed] [Google Scholar]
- 24.Howard VJ, Cushman M, Pulley L, Gomez CR, Go RC, Prineas RJ, Graham A, Moy CS, Howard G. The reasons for geographic and racial differences in stroke study: objectives and design. Neuroepidemiology. 2005; 25: 135–43. 10.1159/000086678. [DOI] [PubMed] [Google Scholar]
- 25.Soliman EZ, Howard G, Cushman M, Kissela B, Kleindorfer D, Le A, Judd S, McClure LA, Howard VJ. Prolongation of QTc and risk of stroke: The REGARDS (REasons for Geographic and Racial Differences in Stroke) study. Journal of the American College of Cardiology. 2012; 59: 1460–7. 10.1016/j.jacc.2012.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hyacinth HI; Cara Carty L; Seals Samantha R; Irvin Marguerite R.; Naik Rakhi P.; Caughey Melissa; Winkler Cheryl; Franceschini Nora; Burke Gregory L; Zakai Neil A; Kopp Jeffrey B; Judd Suzanne E; Adams Robert J.; Gee Beatrice E.; Longstreth WT; Egede Leonard E; Lackland Daniel T; Greenberg Charles S.; Herman Taylor; Manson JoAnn E; Key Nigel S.; Derebail Vimal K.; Kshirsagar Abhijit V; Folsom Aaron R; Konety Suma H; Howard Virginia J; Allison Mattthew; Wilson James G.; Correa Adolfo; Zhi Degui; Arnett Donna; Howard George; Cushman Mary; Reiner Alexander; Safford Monika M. Association of Sickle Cell Trait with Risk of Coronary Heart Disease in African Americans American Society of Hematology 58th Annual Meeting & Exposition. San Diego, California, 2016. [Google Scholar]
- 27.Naik RP, Irvin MR, Judd S, Gutierrez OM, Zakai NA, Derebail VK, Peralta C, Lewis MR, Zhi D, Arnett D, McClellan W, Wilson JG, Reiner AP, Kopp JB, Winkler CA, Cushman M. Sickle Cell Trait and the Risk of ESRD in Blacks. Journal of the American Society of Nephrology : JASN. 2017; 28: 2180–7. 10.1681/asn.2016101086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Soliman EZ, Howard G, Meschia JF, Cushman M, Muntner P, Pullicino PM, McClure LA, Judd S, Howard VJ. Self-reported atrial fibrillation and risk of stroke in the Reasons for Geographic and Racial Differences in Stroke (REGARDS) study. Stroke. 2011; 42: 2950–3. 10.1161/STROKEAHA.111.621367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Levey AS, de Jong PE, Coresh J, El Nahas M, Astor BC, Matsushita K, Gansevoort RT, Kasiske BL, Eckardt KU. The definition, classification, and prognosis of chronic kidney disease: a KDIGO Controversies Conference report. Kidney Int. 2011; 80: 17–28. 10.1038/ki.2010.483. [DOI] [PubMed] [Google Scholar]
- 30.Levey AS, Stevens LA. Estimating GFR using the CKD Epidemiology Collaboration (CKD-EPI) creatinine equation: more accurate GFR estimates, lower CKD prevalence estimates, and better risk predictions. Am J Kidney Dis. 2010; 55: 622–7. 10.1053/j.ajkd.2010.02.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tran NT, Aslibekyan S, Tiwari HK, Zhi D, Sung YJ, Hunt SC, Rao DC, Broeckel U, Judd SE, Muntner P, Kent ST, Arnett DK, Irvin MR. PCSK9 variation and association with blood pressure in African Americans: preliminary findings from the HyperGEN and REGARDS studies. Front Genet. 2015; 6: 136 10.3389/fgene.2015.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kannel WB, Abbott RD, Savage DD, McNamara PM. Epidemiologic features of chronic atrial fibrillation: the Framingham study. N Engl J Med. 1982; 306: 1018–22. 10.1056/NEJM198204293061703. [DOI] [PubMed] [Google Scholar]
- 33.Colantonio LD, Levitan EB, Yun H, Kilgore ML, Howard G, Safford MM, Muntner P. Use of Medicare Claims Data for the Identification of Myocardial Infarction. The REasons for Geographic And Racial Differences in Stroke (REGARDS) Study CIRCULATION: LIPPINCOTT WILLIAMS & WILKINS TWO COMMERCE SQ, 2001 MARKET ST, PHILADELPHIA, PA: 19103 USA, 2017. [Google Scholar]
- 34.Ganesan AN, Chew DP, Hartshorne T, Selvanayagam JB, Aylward PE, Sanders P, McGavigan AD. The impact of atrial fibrillation type on the risk of thromboembolism, mortality, and bleeding: a systematic review and meta-analysis. Eur Heart J. 2016; 37: 1591–602. 10.1093/eurheartj/ehw007. [DOI] [PubMed] [Google Scholar]