Abstract
Objective:
To evaluate the RE-AIM framework’s effect on retention of participants and implementation outcomes of a 5-year cancer research education program on psychosocial distress screening in cancer centers across the United States.
Methods:
A one-group pre-/post-test design was used to evaluate the program on participant retention and implementation outcomes at 6-, 12-, and 24-months after enrolling in the program (baseline) and analyzed using descriptive statistics.
Results:
Seventy-two cancer centers participated in 4 cohorts. Participant retention was 100%. At baseline and 24 months, respectively, 52 (72%) and 64 (88%) of the cancer centers had formulated a psychosocial distress screening policy; 51 (71%) and 70 (98%) had started screening in more than one clinic/population; 15 (21%) and 45 (63%) had started auditing health records for documentation of screening. Each outcome rate improved at the cancer-center level over the 24 months.
Conclusion:
RE-AIM can be used as a framework for cancer research education programs. Future research is needed on the use of a randomized adaptive design to test the optimal support for implementation of quality care standards according to cancer centers’ needs.
Keywords: RE-AIM, dissemination frameworks, implementation science, cancer, distress, psychosocial, quality care standards
Background
In the context of cancer, psychosocial distress is unpleasant emotional experience that arise from psychological, social, or spiritual concerns. It may interfere with patients’ abilities to cope with a diagnosis of, and treatment for, cancer (NCCN, 2019). In large-sample studies, the prevalence of psychosocial distress has ranged between 33% (Wang et al, 2017) and 46% (Carlson et al, 2018). However, when distressed patients are identified through routine screening and referred to appropriate health care resources, they report lower psychosocial distress levels over time (Carlson et al., 2010).
Based on the strength of the evidence, the United States’ National Academy of Medicine (NAM) established psychosocial distress screening as a clinical practice guideline for identifying and addressing psychosocial concerns, to integrate biomedical and psychosocial cancer care (National Academy of Medicine/Institute of Medicine [NAM/IOM]), 2008). Building upon on the NAM guideline recommendation, the American College of Surgeons Commission on Cancer (CoC) adopted psychosocial distress screening as a program accreditation standard as of 2015 (Commission on Cancer (CoC), 2016; IOM/NAM, 2008). The CoC standard mandates that the cancer committee, which insures that the cancer care institution complies with all CoC accreditation standards, addresses patients’ psychosocial concerns by having a policy for using a standardized, validated instrument to screen patients for psychosocial distress, at least once during a pivotal medical visit, and for referring distressed patients to appropriate psychosocial health care services (Commission on Cancer, 2016). We have described comprehensive psychosocial distress screening as a 5-step process: (1) screening patients using a standardized, validated instrument; (2) evaluating patients who screen positive for sources of distress; (3) referring patients, when needed, to psychosocial health care services; (4) following-up with patients and the primary oncology team, to integrate biomedical and psychosocial cancer care; and (5) documenting and auditing steps 1–4 for quality assurance and improvement (Lazenby, Tan, Pasacreta, Ercolano, & McCorkle, 2015).
The core values of dissemination and implementation science—rigor and relevance, efficiency and speed, collaboration, improved capacity, and cumulative knowledge—have been applied to biomedical and public health interventions, such as behavioral interventions meant to prevent disease (Russell E. Glasgow et al., 2012; Centers for Disease Control and Prevention, 2018). Cancer care professional societies have used these core values for rapid dissemination and implementation of biomedical evidence-based clinical practice guidelines (American Society of Clinical Oncology, 2018). However, these core values have not been used for rapid dissemination and implementation of psychosocial evidence-based clinical practice guidelines. One reason may be that psychosocial health care services inherently involve programmatic complexities, such as staffing, training, and resources (Ercolano et al., 2018; Knies et al., 2018). involved in disseminating and implementing psychosocial evidence-based guideline recommendations, such as psychosocial distress screening. These programmatic complexities not only involve fine-tuning the clinical steps of psychosocial distress screening in real-world practice settings. This complexity necessitates a dissemination and implementation framework for full-scale broad-based implementation. To our knowledge, a dissemination and implementation framework has not been used for supporting implementation of guideline recommendations in psychosocial oncology.
One such framework is the RE-AIM, which stands for Reach, Effectiveness, Adoption, Implementation, and Maintenance (Glasgow, Vogt, & Boles, 1999). Originally developed as a framework for reporting research and literature review results, the RE-AIM framework is widely used to translate research into practice and to help plan programs such that they have greater chances of working in real-world practice settings (Gaglio, Shoup, & Glasgow, 2013).
We used RE-AIM as a framework for disseminating and implementing psychosocial distress screening at the cancer center-level, through a 5-year National Cancer Institute-funded cancer research education grant, the Screening for Psychosocial Distress Program (SPDP). Table 1 describes the elements of RE-AIM for programmatic implementation as they pertain to SPDP’s outcomes. The purposes of this article are (1) to evaluate the fit of the RE-AIM framework for disseminating and implementing psychosocial distress screening at the program-level, and (2) to evaluate the SPDP’s outcomes by each RE-AIM element.
Table 1.
Definitions of the RE-AIM Framework’s Elements as They Pertain to the Screening for Psychosocial Distress Program (SPDP)
| RE-AIM Framework Element | Definition of RE-AIM Element as It Pertains to the SPDP (adapted from RE-AIM.ORG)(RE-AIM.ORG, 2018) |
|---|---|
| Reach | The absolute number of participants willing to participate in the SPDP. |
| Effectiveness | The impact of the SPDP on Adoption, Implementation, and Maintenance Outcomes. |
| Adoption | The absolute number of settings who are willing to initiate all components of the CoC’s standard and of the 5 steps of comprehensive psychosocial distress screening. Measured at 6 months. |
| Implementation | At the setting level, implementation refers to the intervention agents’ fidelity over time to the components of the CoC standard and the 5 steps of comprehensive distress screening. Measured at 12 months. |
| Maintenance | The extent to which the components of the CoC standard and the 5 steps of comprehensive psychosocial distress screening become institutionalized as part of the routine organizational policies. Measured at 24 months. |
Methods
Design
The SPDP was a dissemination and implementation initiative for cancer care professionals that used a one-group pre-/post-test design. It was delivered by expert faculty to each cohort over a 2-year period and consisted of 2 annual workshops, with printed reference materials for the introductory (Year 1) and advanced (Year 2) workshops. The introductory workshop focused on the evidence for psychosocial distress screening, an overview of the RE-AIM framework, and adoption aspects of Steps 1–3 of comprehensive psychosocial distress screening. The advanced workshop focused on adoption of Steps 4–5 of comprehensive distress screening and on implementation and maintenance of psychosocial distress screening programs. These workshops were supplemented with 10 themed 1-hour conference calls, 6 in Year 1 and 4 in Year 2. During the calls, an expert faculty member presented on a topic for 15 minutes and then problem-solved with participants the remaining time. Figure 1 illustrates the program structure. We have described in detail the design, dose, and content (syllabus) of the education intervention in more detail elsewhere (Lazenby et al., 2018; Lazenby et al., 2015).
Figure 1:
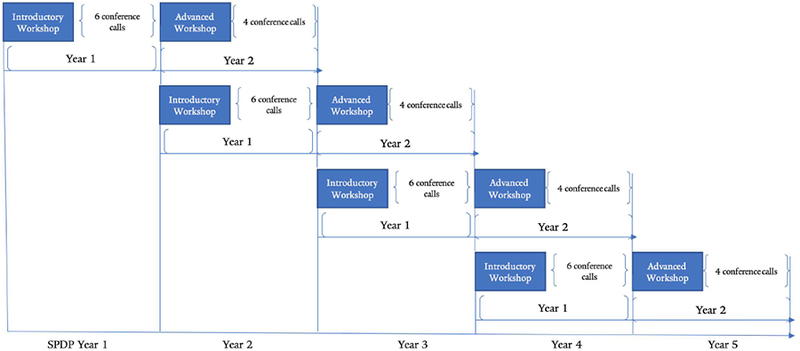
SPDP Structure
Participants, Recruitment, and Setting
Participants were dyads, 2 cancer care professionals who were involved in managerial decision-making or the delivery of psychosocial care services, from cancer care institutions in the United States (US) who had the support of their supervisors for implementing psychosocial distress screening, as evidenced in letters of support. We recruited on the American Psychosocial Oncology Society (APOS) website and listserv, in the CoC’s newsletter, and in other trade periodicals. Each cohort consisted of 18 dyads.
The 2 workshops were delivered in person by expert faculty a day before the APOS annual scientific meeting. Participants were given free access to all scientific sessions at the meeting and given stipends to defray the cost of travel and lodging. The conference calls were conducted on the Yale University conferencing system. Participation in the educational workshop was free.
Data Collection and Analysis
We used investigator-developed Internet-based forms to collect self-report data from dyads at 4 timepoints, baseline and 6, 12, and 24 months after attending the first workshop.
REACH data were collected at baseline, at the time of recruitment and included institutional characteristics: type of cancer center, and whether it had a cancer committee that governs cancer care, a psychosocial oncology committee, and whether the institution screened for psychosocial distress in at least 1 clinic or patient population. It also included the participants’ individual characteristics of sex, race, and professional discipline.
ADOPTION data that were collected at the 6-month timepoint and included whether the institution from which the participants came (1) had a cancer committee, (2) a psychosocial oncology committee (which is not a CoC accreditation standard or a step in comprehensive distress screening), (3) a psychosocial distress screening policy, (4) had initiated screening in more than 1 clinic or patient population with a standardized, validated instrument, (5) had built referral networks internal and external to the institution, and (6) were auditing health records for documentation that patients had been screened and referred if necessary. If the institution was screening for psychosocial distress, we asked which screening instrument they had adopted. We asked this question only at the 6-month data collection timepoint.
Data for IMPLEMENTAITON and MAINTENANCE, respectively were collected using ADOPTION variables at 12 months and at 24 months. We collected the numbers of providers in internal and external networks at 6, 12, and 24 months, which we have reported elsewhere (Lazenby, et al., 2018). We collected number of providers only at ADOPTION (6 months) by category of number (0, 1–3, 4–6, 7–9, 10+) of psychosocial providers in each network.
Note that hereafter, when we speak of Reach, Effectiveness, Adoption, Implementation, and Maintenance outcomes, we capitalize the words. When we speak of the acts of adoption, implementation, or maintenance, we do not capitalize the words.
Not every dyad answered all the questions on questionnaires at every data collection timepoint, and when they did not, we left a blank in their response. Sometimes dyadic members changed over the 2 years, and when they did, their answers to questions sometimes changed between data collection periods. These changes are often reflected by lower frequencies in subsequent data collection periods.
Measures
We measured fit of the RE-AIM framework for the dissemination and implementation of psychosocial distress screening using the rate of retention over the 24 months of participation in the cancer research education program.
We measured Effectiveness—that is, impact of the SPDP on Adoption, Implementation, and Maintenance—using the change in rates of Adoption, Implementation, and Maintenance outcomes over time at the cancer-center level.
Analysis
The data were imported and stored in a secure Microsoft Access® database and exported to Microsoft Excel® for analysis of frequencies and percentages. We did not test for statistical significance of the change in observed frequencies over time, it was expected that the frequencies would increase over time greater than the probability of chance.
The Yale University Institutional Review Board exempted this cancer research education project from review.
Results
Reach
One-hundred forty-four cancer care professionals from 72 cancer centers located in 37 different United States’ states and the District of Columbia participated in the SPDP over 4 cohorts. They were largely (n=53, 75%) community or ambulatory cancer centers. A majority of them (n=51, 71%) had already started screening patients for psychosocial distress in at least 1 clinic or patient population. Seventy percent of the cancer care professionals who participated were social workers (n=59, 41%) or nurses (n=41, 28%). Table 2 describes participants’ institutional and individual characteristics.
Table 2.
Institutional and Individual Characteristics of Participants Cohorts 1 through 4 at Baseline
| Institutional Characteristics | All Cohorts N=72 |
Cohort 1 N=18 |
Cohort 2 N=18 |
Cohort 3 N=18 |
Cohort 4 N=18 |
|
|---|---|---|---|---|---|---|
| n (%) | n (%) | n (%) | n (%) | n (%) | ||
| Type of cancer center | NCI Designated | 15 (21) | 2 (11) | 3 (17) | 8 (44.4) | 2 (11) |
| Community or Ambulatory | 54 (75) | 15 (83) | 15 (83) | 9 (50.0) | 14 (89) | |
| Advocacy | 1 (1) | 1 (5.6) | 0 | 0 | 2 (11) | |
| Veterans Affairs | 1 (1) | 0 | 0 | 1 (5.6) | 0 | |
| All Cohorts N=144 |
Cohort 1 N=36 |
Cohort 2 N=36 |
Cohort 3 N=36 |
Cohort 4 N=36 |
||
| n (%) | n (%) | n (%) | n (%) | n (%) | ||
| Sex | Female | 131 (91) | 31 (86) | 35 (97) | 32 (89) | 33 (92) |
| Race | White | 122 (85) | 27 (75) | 34 (94) | 32 (89) | 29 (81) |
| Black or African American | 7 (5) | 2 (5.6) | 1 (2.8) | 1 (2.8) | 3 (8.3) | |
| Asian | 3 (2) | 0 | 0 | 2 (5.6) | 1 (2.8) | |
| Hawaiian or Other Pacific Islander | 2 (1) | 1 (2.8) | 0 | 0 | 1 (2.8) | |
| American Indian or Alaskan Native | 1 (.06) | 1 (2.8) | 0 | 0 | 0 | |
| Other | 6 (4) | 2 (5.6) | 1 (2.8) | 1 (2.8) | 2 (5.6) | |
| Not reported | 3 (2) | 3 (8.3) | 0 | 0 | 0 | |
| Ethnicity | Hispanic | 8 (5.5) | 3 (8.3) | 3 (8.3) | 0 | 2 (5.6) |
| Discipline (select all that apply) | Social Work | 59 (41) | 21 (58) | 14 (39) | 14 (39) | 10 (28) |
| Nursing | 41 (28) | 7 (19) | 11 (31) | 12 (33) | 11 (31) | |
| Psychiatry/Psychology | 31 (22) | 6 (17) | 8 (22) | 7 (19) | 10 (28) | |
| Oncology | 3 (2) | 2 (5.6) | 1 (2.8) | 0 | 0 | |
| Chaplaincy | 2 (1.5) | 0 | 0 | 1 (2.8) | 1 (2.8) | |
| Other | 8 (5.5) | 0 | 2 (5.6) | 2 (5.6) | 4 (11.1) |
Adoption
Rates of adoption of CoC accreditation standards and the 5 steps of comprehensive psychosocial distress screening ranged from a low of 42% of participant cancer centers that were auditing health records for documentation of psychosocial distress screening to a high of 89% of participant cancer centers who had a cancer committee for overseeing compliance with psychosocial distress screening accreditation standards (Table 3). Table 4 describes rates of adoption of a standardized, validated instrument by which to measure psychosocial distress, which ranged from 91% for Cohort 4 to 100% for Cohort 1. The most commonly adopted instrument was the NCCN Distress Thermometer with NCCN Problem List.
Table 3.
Adoption, Implementation, and Maintenance (12- and 24-Months Data Timepoint) of Components of CoC Accreditation Standard and Steps of Comprehensive Psychosocial Distress Screening
| CoC Accreditation Standard Component or Step in Comprehensive Psychosocial Distress Screening | Cohorts | Baseline | Adoption 6 Months | Implementation 12 Months | Maintenance 24 Months |
|---|---|---|---|---|---|
| All N=72; each cohort N=18 | All N=72; each cohort N=18 | All N=72; each cohort N=18 | |||
| n (%) | n (%) | n (%) | n (%) | ||
| The cancer center has a cancer committee for overseeing CoC compliance | All | 63 (88) | 64 (89) | 65 (90) | 67 (93) |
| 1 | 15(83) | 15 (83) | 15 (83) | 16 (89) | |
| 2 | 15 (83) | 17 (94) | 17 (94) | 18 (100) | |
| 3 | 18 (100) | 18 (100) | 18 (100) | 18 (100) | |
| 4 | 15 (83) | 14 (78) | 15 (83) | 15 (83) | |
| The cancer center has formulated a psychosocial distress policya | All | NA | 52 (72) | 60 (83) | 64 (88) |
| 1 | NA | 12 (67) | 12 (67) | 15 (83) | |
| 2 | NA | 16 (84) | 18 (100) | 18 (100) | |
| 3 | NA | 15 (83) | 17 (94) | 16 (89) | |
| 4 | NA | 13 (93) | 13 (72) | 15 (83) | |
| The cancer center has a psychosocial oncology committee | All | 30 (42) | 35 (49) | 40 (56) | 38 (53) |
| 1 | 11 (61) | 11 (61) | 12 (67) | 10 (56) | |
| 2 | 7 (39) | 7 (39) | 10 (56) | 11 (61) | |
| 3 | 5 (28) | 9 (50) | 8 (44) | 9 (50) | |
| 4 | 7 (39) | 8 (44) | 10 (56) | 8 (44) | |
| The cancer center has started psychosocial distress screeningb | All | 51 (71) | 55 (76) | 67 (93) | 70 (98) |
| 1 | 8 (44) | 12 (67) | 17 (94) | 17 (94) | |
| 2 | 15 (83) | 18 (100) | 18 (100) | 18 (100) | |
| 3 | 16 (89) | 13 (72) | 16 (89) | 18 (100) | |
| 4 | 12 (67) | 12 (67) | 16 (89) | 17 (94) | |
| The cancer center has an internal referral network of psychosocial providers | All | NA | 57 (79) | 67 (93) | 69 (96) |
| 1 | NA | 15 (83) | 17 (94) | 18 (100) | |
| 2 | NA | 18 (100) | 17 (94) | 18 (100) | |
| 3 | NA | 13 (72) | 16 (89) | 18 (100) | |
| 4 | NA | 11 (61) | 17 (94) | 15 (83) | |
| The cancer center has an external referral network of psychosocial providers | All | NA | 45 (63) | 56 (78) | 62 (86) |
| 1 | NA | 11 (61) | 15 (83) | 17 (94) | |
| 2 | NA | 17 (94) | 14 (78) | 14 (78) | |
| 3 | NA | 9 (50) | 13 (72) | 16 (89) | |
| 4 | NA | 8 (44) | 14 (78) | 15 (83) | |
| The cancer center audits health records for documentation of psychosocial distress screening | All | 15(21) | 30 (42) | 39 (54) | 45 (63) |
| 1 | 4 (22) | 4 (22) | 9 (50) | 10 (56) | |
| 2 | 6(33) | 8 (44) | 12 (67) | 10 (56) | |
| 3 | 0 | 11 (85) | 8 (44) | 15 (83) | |
| 4 | 5 (27) | 7 (58) | 10 (56) | 10 (56) |
Not a CoC accreditation standard or a step in comprehensive psychosocial distress screening
At baseline, the questionnaire asked participants if they had started screening in at least 1 clinic or patient population, but at Adoption, Maintenance, and Implementation, the questionnaire asked participants if they screened in more than 1 clinic or patient population.
Table 4.
Standardized, Validated Screening Instruments Adopted by the 6-Month Data Collection Timepoint
| Instrument Adoption | The Cancer Centers that had Started Screening at 6 Months | |||||
|---|---|---|---|---|---|---|
| All Cohorts N=55 |
Cohort 1 N=12 |
Cohort 2 N=18 |
Cohort 3 N=13 |
Cohort 4 N=12 |
||
| n (%) | n (%) | n (%) | n (%) | n (%) | ||
| Cancer centers that adopted a standardized, validated screening instrument | 52 (95) | 12 (100) | 17 (94) | 12 (92) | 11 (91) | |
| n | n | n | n | n | ||
| Instrument adopted | NCCN Distress Thermometer | 6 | 0 | 2 | 2 | 2 |
| (Select all that apply) | NCCN Distress Thermometer with NCCN Problem List | 24 | 6 | 8 | 4 | 6 |
| NCCN Distress Thermometer with Adapted Problem List | 17 | 4 | 5 | 5 | 3 | |
| PHQ-2 | 13 | 4 | 4 | 3 | 2 | |
| PHQ-9 | 11 | 0 | 3 | 5 | 3 | |
| Edmonton Symptom Assessment Scale | 5 | 1 | 1 | 2 | 1 | |
| Other Instrument | 18 | 1 | 8 | 5 | 3 | |
The adoption of a referral network by the number of psychosocial providers internal and external to the cancer center to whom patients who endorse distress are sent are displayed in Table 5. The rates of adoption ranged from 56% of Cohort 3 participants having no psychosocial providers to whom they referred internally to 11% of Cohort 4 participants; at the same time, 28% of Cohort 4 participants had 10+ internal psychosocial providers. By contrast, 61% of Cohort 1 participants had at least 1 psychosocial provider to whom they referred patients external to the cancer center; and yet, 66% of Cohort 2 participants and 94% of Cohort 3 and 4 participants had no external providers in their referral networks.
Table 5.
Adoption (Measured at 6 Months) of a Referral Network by Numbers of Psychosocial Providers Internal and External to the Cancer Center
| Number of psychosocial providers in referral network | Cohort | |||
|---|---|---|---|---|
|
1
(N=18) |
2
(N=18 |
3
(N=18) |
4
(N=18) |
|
| n (%) | n (%) | n (%) | n (%) | |
| Internal | ||||
| 0 | 3 (17) | 6 (33) | 10 (56) | 2 (11) |
| 1–3 | 9 (50) | 3 (17) | 1 (6) | 2 (11) |
| 4–6 | 5 (28) | 3 (17) | 3 (17) | 3 (17) |
| 7–9 | 1 (6) | 1 (6) | 7 (39) | |
| 10+ | 1 (6) | 5 (28) | 3 (17) | 4 (22) |
| External | ||||
| 0 | 7 (39) | 12 (66) | 17 (94) | 17 (94) |
| 1–3 | 5 (28) | 1 (6) | 0 | 1 (6) |
| 4–6 | 4 (22) | 0 | 1 (6) | 0 |
| 7–9 | 0 | 0 | 0 | 0 |
| 10+ | 2 (11) | 5 (28) | 0 | 0 |
Implementation and Maintenance
Implementation (12-month) rates ranged from 54% of participant cancer centers that were auditing health records for documentation of psychosocial distress screening to 93% that were screening patients for psychosocial distress and that had an internal referral network (Table 3). Maintenance (24-month) rates ranged from 63% of participant cancer centers that were auditing health records for documentation of psychosocial distress screening to 98% that were screening patients for psychosocial distress (Table 3).
Effectiveness
Each rate of achievement of the components of the CoC accreditation standard and steps of comprehensive psychosocial distress screening increased from Adoption to Maintenance with the exception of cancer centers having a psychosocial oncology committee, which went from 49% of all participant cancer centers at Adoption, to 56% and 53% at Implementation and Maintenance, respectively.
Fit of the RE-AIM Framework with Dissemination and Implementation of Psychosocial Distress Screening
The retention rate for all 4 cohorts (a total of 72 cancer centers) over the 24 months of participation in the SPDP, the primary measure of fit between the RE-AIM framework for dissemination and implementation of psychosocial distress screening, was 100%.
Discussion
Professional scientific and practice bodies put forth evidenced-based clinical practice guidelines as a strategy for improving standard practice. However, there is a gap between these guidelines and dissemination and implementation of them in real-world settings. To fill this gap, we sought to evaluate the fit of the RE-AIM framework for disseminating and implementing comprehensive psychosocial distress screening, a NAM and CoC evidenced-based clinical practice guideline, through a 5-year NCI-funded cancer research education grant. We found that the RE-AIM framework fit the dissemination and implementation of the CoC accreditation components and the practical steps of comprehensive distress screening in real-world settings. The primary measure of fit was the retention rate of participant cancer centers in the 2-year-long training, the SPDP; that rate was 100%.
Another way to evaluate the fit of the RE-AIM framework with disseminating and implementing evidence-based clinical practice guidelines in psychosocial oncology is the Effectiveness of the SPDP, as measured by Adoption, Implementation, and Maintenance outcomes. Participant cancer centers improved their rates of Adoption, Implementation, and Maintenance on all CoC accreditation components and all 5 steps of comprehensive psychosocial distress screening. The 3% dip in the rate of participant cancer centers who had instituted a psychosocial committee to oversee adoption, implementation, and maintenance of psychosocial distress screening represented a loss of the committee by 2 cancer center in each of Cohorts 1 and 4 but a gain of 1 in each of Cohorts 2 and 3 at the Maintenance (24-month) timepoint. So while some cancer centers experienced a lack of maintenance on this one measure, others experienced a gain. It should be noted, however, that having a psychosocial committee is neither a CoC accreditation standard nor a step in comprehensive distress screening. We devised this outcome as a strategy for creating key stakeholder buy-in.
Key stakeholder engagement is as essential to implementation of a quality care standard as the evidence that backs it up (Glasgow, Green, Taylor, & Stange, 2012). The make-up of stakeholders, and their varying levels of authority to add resources to implementation projects, change from cancer center to cancer center. We found that using a stakeholder-engagement strategy like a psychosocial oncology committee early in adoption improved Implementation and Maintenance.
One of the greatest barriers to implementation is administration’s commitment of staffing resources (Knies et al., 2018). Using the distress screening clinical practice guideline as justification for the staffing resources necessary to move from adoption to implementation and maintenance does not always convince administration. Rather, stakeholder engagement during adoption process seems to be protective of staffing resources necessary for implementation and maintenance. For example, by adopting a psychosocial oncology committee early, the cancer centers in Cohorts 1 and 4 protected themselves from a slowing down of implementation and maintenance when the committee disbanded after the adoption phase. A dissemination and implementation framework forces interaction between the evidence-based standard and the stakeholders who hold the power to implement and maintain the standard. This adaptivity of the RE-AIM to real-world settings, where the make-up of stakeholders and their commitment of resources vary, is a good fit for disseminating and implementing psychosocial distress screening at the program level.
Our second purpose in this article was to describe the SPDP’s outcomes by RE-AIM element. The rates of adoption of a CoC-mandated cancer committee reflect that not all participants in the SPDP were CoC-accredited institutions or were seeking CoC accreditation. The same is true of having a psychosocial distress screening policy. Having a psychosocial oncology committee is not a CoC accreditation standard. The rates of employing a psychosocial oncology committee at adoption, implementation, and maintenance were the lowest of the outcome measures, despite its importance in stakeholder engagement.
The lowest rate of adoption, implementation, and maintenance of the other outcome measures—screening patients for psychosocial distress, having referral networks both internal and external to the cancer center, and auditing health records for documentation of psychosocial distress screening—was for auditing. Health care providers in the US were required to demonstrate “meaningful use” of electronic health records by 2014, which was when the SPDP started, and a 2016 law mandated the electronic exchange of health care information between providers (Office of the National Coordinator for Health Information Technology, 2019). Implementing these 2 mandates competed with the auditing outcome. The timing, however, was auspicious in that it allowed participants to work with their information technology departments to include, and audit the psychosocial distress screening components within the electronic health record platform for some cancer centers.
Limitations/Strengths
The one-group pre/post-test design was a limitation of the dissemination and implementation support program. However, we have shown the effectiveness of such a program that is guided by the RE-AIM framework. Our sample was varied by type and size of cancer center and by geography; this was a strength. However, their needs varied; this was a limitation for a one-group design. Future research can use a design by which participants are randomized to an intervention dose and type (e.g., guided by RE-AIM framework or by a systems approach framework) according to their response, to test for optimal adaptive support (Almirall, Nahum-Shani, Sherwood, & Murphy, 2014; Murphy, 2005). Another limitation was that the data were self-report. However, it was a strength that the SPDP data collection was focused around CoC accreditation standards and the 5 steps of comprehensive psychosocial distress screening, which gave participants outcomes by which they could measure their progress toward adoption, implementation, and maintenance.
Another strength of this program was the collaboration with APOS. Participants had access to the latest science in psychosocial oncology by attending the APOS annual scientific meetings. Psychosocial oncology professional societies such as APOS can take up the dissemination and implementation mantle by building networks of experts and using a framework such as the RE-AIM for training and supporting cancer centers in the real-world implementation of quality care standards.
Conclusion
Using the RE-AIM framework as a model for this cancer research education program on psychosocial distress screening aligned it with the core values of dissemination and implementation science. It provided rigor in outcome measures; relevance to the institutions who needed training; efficiently and speedily getting psychosocial distress screening programs in diverse cancer centers up to full maintenance; collaboration between the program, participants, and a professional society; improved capacity among participants; and knowledge on dissemination and implementation of quality care standards in psychosocial oncology in real-world practice settings. Other cancer research education programs in psychosocial oncology can use the RE-AIM framework to the same ends. In addition, using an adaptive design in future research would allow for testing optimal support for implementation of quality care standards by cancer centers.
Funding:
This study was funded by a grant (R25 CA177553–04; principal investigator: McCorkle, R.) from the National Cancer Institute at the National Institutes of Health.
Footnotes
Conflict of Interest statement: We have no conflicts of interest to declare.
Disclosure: The views expressed are those of the authors and do not necessarily represent those of the National Cancer Institute.
Ethics approval: The Yale University Institutional Review Board exempted this cancer research education project from review.
References
- Almirall D, Nahum-Shani I, Sherwood NE, & Murphy SA (2014). Introduction to SMART designs for the development of adaptive interventions: with application to weight loss research. Translational behavioral medicine, 4(3), 260–274. doi: 10.1007/s13142-014-0265-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Society of Clinical Oncology. (2018, November 4, 2018). ASCO iPhone and iPad Apps. Retrieved from https://itunes.apple.com/us/developer/asco/id387427538
- Carlson LE, Zelinski EL, Toivonen KI, Sundstrom L, Jobin CT, Demaskos P, & Zebrack B (2018, December 28). Prevalence of psychosocial distress in cancer patients across 55 North American Cancer Centers. Journal of Psychosocial Distress. [Epub ahead of print]. doi: 10.1080/07347332.2018.1521490 [DOI] [PubMed] [Google Scholar]
- Carlson LE, Groff SL, Maciejewski O, & Bultz BD (2010). Screening for distress in lung and breast cancer outpatients: A randomized controlled trial. Journal of Clinical Oncology, 28(33), 4884–91. doi: 10.1200/JCO.2009.27.3698. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. (2018). Effective Interventions: HIV Prevention that Works. Retrieved November 4, 2018, from https://effectiveinterventions.cdc.gov/
- Commission on Cancer. (2016). Cancer Program Standards: Ensuring Patient-Centered Care (2016 ed.). Chicago, IL: American College of Surgeons. [Google Scholar]
- Ercolano E, Hoffman E, Tan H, Pasacreta N, Lazenby M, & McCorkle R (2018). Managing Psychosocial Distress: Lessons Learned in Optimizing Screening Program Implementation. Oncology (Williston Park), 32(10), 488–490, 492–483. [PMC free article] [PubMed] [Google Scholar]
- Gaglio B, Shoup JA, & Glasgow RE (2013). The RE-AIM framework: a systematic review of use over time. Am J Public Health, 103(6), e38–46. doi: 10.2105/ajph.2013.301299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasgow RE, Green LW, Taylor MV, & Stange KC (2012). An evidence integration triangle for aligning science with policy and practice. Am J Prev Med, 42(6), 646–654. doi: 10.1016/j.amepre.2012.02.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasgow RE, Vinson C, Chambers D, Khoury MJ, Kaplan RM, & Hunter C (2012). National Institutes of Health Approaches to Dissemination and Implementation Science: Current and Future Directions. American Journal of Public Health, 102(7), 1274–1281. doi: 10.2105/ajph.2012.300755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasgow RE, Vogt TM, & Boles SM (1999). Evaluating the public health impact of health promotion interventions: the RE-AIM framework. Am J Public Health, 89(9), 1322–1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knies AK, Jutagir DR, Ercolano E, Pasacreta N, Lazenby M, & McCorkle R (2018). Barriers and facilitators to implementing the commission on cancer’s distress screening program standard. Palliat Support Care, 1–9. doi: 10.1017/s1478951518000378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazenby M, Ercolano E, Knies A, Pasacreta N, Grant M, Holland JC, … McCorkle R (2018). Psychosocial Distress Screening: An Educational Program’s Impact on Participants’ Goals for Screening Implementation in Routine Cancer Care. Clin J Oncol Nurs, 22(3), E85–e91. doi: 10.1188/18.Cjon.E85-e91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazenby M, Tan H, Pasacreta N, Ercolano E, & McCorkle R (2015). The five steps of comprehensive psychosocial distress screening. Curr Oncol Rep, 17(5), 447. doi: 10.1007/s11912-015-0447-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy SA (2005). An experimental design for the development of adaptive treatment strategies. Statistics in Medicine, 24(10), 1455–1481. doi: doi: 10.1002/sim.2022 [DOI] [PubMed] [Google Scholar]
- National Academy of Medicine/Institute of Medicine (NAM/IOM). (2008). Cancer Care for the Whole Patient: Meeting Psychosocial Health Needs. Washington, DC: The National Academies Press. [PubMed] [Google Scholar]
- National Comprehensive Cancer Network (NCCN). (2019, February 5). Clinical Practice Guidelines in Oncology Distress Management Version 1.2019. Retrieved February 20, 2019, from https://www.nccn.org/professionals/physician_gls/pdf/distress.pdf. [DOI] [PMC free article] [PubMed]
- Office of the National Coordinator for Health Information Technology. (2019). Health IT Legislation. Retrieved December 10, 2018, from https://www.healthit.gov/topic/laws-regulation-and-policy/health-it-legislation [Google Scholar]
- RE-AIM.ORG. (2018). Retrieved November 5, 2018, from http://www.re-aim.org/about/frequently-asked-questions/#define.
- Wang GL, Cheng CT, Feng AC, Hsu SH, Hou YC, & Chiu CY (2017). Prevalence, risk factors, and the desire for help of newly diagnosed cancer patients: A large-sample study. Palliative and Supportive Care, 15(3), 295–304. doi: 10.1017/S1478951516000717. [DOI] [PubMed] [Google Scholar]


