Abstract
The aim of the Complementary Nursing in Gynecologic Oncology study was to investigate the effects of a complex, nurse‐led, supportive care intervention using Complementary and Integrative Medicine (CIM) on patients’ quality of life (QoL) and associated patient‐reported outcomes. In this prospective, pragmatic, bicentric, randomized controlled trial, women with breast or gynecologic cancer undergoing a new regimen of chemotherapy (CHT) were randomly assigned to routine supportive care plus intervention (intervention group, IG) or routine care alone (control group, CG). The intervention consisted of CIM applications and counseling for symptom management, as well as CIM information material. The primary endpoint was global QoL measured with the EORTC‐QLQ‐C30 before and after CHT. Mixed linear models considering fixed and random factors were used to analyze the data. In total, 126 patients were randomly assigned into the IG and 125 patients into the CG (median age 51 years). The patients’ medical and socio‐demographic characteristics were homogenous at baseline and at follow‐up. No group effects on QoL were found upon completion of CHT (estimate −1.04 [−4.89; 2.81]; P = 0.596), but there was a significant group difference in favor of the IG 6 months later (estimate 6.643 [1.65; 11.64]; P = 0.010). IG patients did also experience significant better emotional functioning (P = 0.007) and less fatigue (P = 0.027). The tested supportive intervention did not improve patients’ QoL outcomes directly after CHT (T3), but was associated with significant QoL improvements when considering the change from baseline to the time point T4, which could be assessed 6 months after patients’ completion of CHT. This delayed effect may have resulted due to a strengthening of patients’ self‐management competencies.
Keywords: breast cancer, chemotherapy, complementary therapies, gynecologic cancer, health services research, nursing intervention, patient‐reported outcomes, quality of life, self‐care, supportive care
1. INTRODUCTION
The management of breast and gynecologic cancer patients’ care and treatment is complex and should recognize patient‐reported outcomes (PROs), which are endpoints that complement clinical outcomes, and can be directly experienced and self‐reported by the patients themselves. Patients report high levels of unmet needs and prefer to uptake self‐care methods from the spectrum of Complementary and Integrative Medicines (CIMs)1 to alleviate and self‐manage their symptoms often resulting of burdensome treatment phases like chemotherapy (CHT).2 The majority of cancer outpatients,3 and up to 80% of breast cancer survivors,4 complement their conventional cancer treatment with CIM. These numbers are startling, as a majority of patients do not talk about it in clinical consultations5 even though there is risk potential of some CIM therapies (eg, phytotherapeutics, supplements) due to interaction with standard therapies.6 Cancer patients often rely on healthcare advice from family and friends, or services from other non‐medical healthcare providers, which may be associated with further risks and high costs.7 Consequently, there is a need for safe, effective, and feasible cancer care deliveries including evidence‐based CIM applications and counseling to guarantee patients’ safety and address patients’ unmet complementary needs.
Integrating CIM methods in oncological care has resulted in positive effects on PROs8, 9 and is supported by international clinical guidelines.10, 11 Meta‐analyses indicate that, for instance, acupuncture and acupressure reduce nausea and pain,12 and aromatherapy has the potential to alleviate sleep and anxiety disorders.13 There is also recognized evidence that mind‐body‐methods like yoga and meditation increase patients’ quality of life (QoL), and reduce fatigue and distress.14 Patients often choose to communicate initially with their oncology nurses about their symptomatic burden and interest for CIM applications,15, 16 which is understandable as oncology nurses have closest and trustful relationships with the patients. Research indicates that oncology nurses have a positive and open attitude towards the integration of CIM methods into cancer care, but they are often struggling with structural and educational deficits.16, 17 Accordingly, there is a need to implement healthcare structures focusing on how nurses can respond to cancer patients’ complementary needs.
Research has highlighted that nursing interventions are effective in supporting patients during CHT,15 however, the effect of a nurse‐led supportive care packet including CIM tailored to patients’ needs during CHT, has not been shown so far. As oncology nurses represent one of the largest group of health professionals worldwide,18, 19 and the healthcare provision for oncology patients will continue to rise, integrated concepts of oncological care and treatment, especially for outpatients, will become more important.
In order to assess whether outpatients undergoing CHT benefit from a nurse‐led, supportive care intervention using CIM, the prospective, pragmatic, bicentric, randomized controlled study labeled CONGO (Complementary Nursing in Gynecologic Oncology) was conducted.20
2. METHODS
2.1. Patients
Details about the study design were published with the study protocol,20 designed and reported to meet CONSORT requirements.21 The study protocol was approved by the ethics committees of the University of Heidelberg (S‐008/2014) and the State Medical Council of Baden‐Wuerttemberg (B‐F‐2014‐037), Germany. Written informed consent was obtained for intervention participants and control participants prior to study entry.
From 31 July 2014 until 9 February 2016, all breast and gynecologic cancer patients who were scheduled for a new regimen of CHT were eligible to participate in this randomized controlled trial conducted at the National Center for Tumor Diseases (NCT) Heidelberg, and at the Community Hospital Karlsruhe (SKK), Germany. There was no age restriction for adults, and patients were allowed to participate in trials. Exclusion criteria were insufficient knowledge of the German language, cognitive disabilities and inability to give informed consent.
2.2. Randomization
Randomization was done with stratified block randomization, using the professional online randomization service randomizer.at. Patients were stratified at randomization by CHT treatment intention (curative, palliative) and participating center (NCT, SKK). The patients were randomly allocated (in a 1:1 ratio) to either routine care plus intervention (intervention group [IG]) or routine care (control group [CG]). Blinding was not possible, as the intervention could not be performed blindly.
2.3. Procedures
2.3.1. Intervention group
Patients in the IG received CHT with supportive therapy according to the clinics’ guidelines together with the CIM nurse‐led care. This means that based upon the patients’ symptomatic burden and preferences, patients were offered naturopathic applications from the supportive care package in addition to routine care. For example, if a patient suffered of pain, she was offered an Aconite or Solum oil application, or a Melissa oil abdominal rhythmical massage, or a Liver oil upper abdomen rhythmical massage in the outpatient clinic. Based upon their needs, patients also received standardized guidelines on the CIM interventions, so that the patients were able to follow these instructions at home between the CHT cycles, for managing their own symptoms. Patients were comprehensively and regularly counseled by the nurse, and patients were handed out a brochure and CD with more evidence‐based information on CIM.
Further details of the intervention, qualification and training of the nurses, and type and frequency of counseling were reported elsewhere.22 Due to possible longer palliative CHTs, the maximum time of the intervention was set to 24 weeks.
2.3.2. Control group
The CHT and supportive therapy plan according to the clinic's guidelines was not changed for the patients in the CG.23
2.4. Data collection
2.4.1. Recruitment and data collection
Patients’ medical and socio‐demographic characteristics were documented before the CHT (time point of randomization [T0]). PROs were collected at T1 (start of CHT, start of intervention), T2 (mid of CHT treatment, max. after 12 weeks), and T3 (end of intervention, max. after 24 weeks). Follow‐up assessment T4 was conducted 6 months after T3. Patients self‐reported the primary outcome on paper‐based questionnaires and weekly in the patient diary. Data was collected at the participating cancer centers and then transferred to the data management of the University Hospital Heidelberg.
2.5. Outcome measures
The primary endpoint was QoL at the end of CHT (T3). Secondary endpoints were QoL at T4 as well as the functional and symptom scales of the EORTC‐QLQ‐C30 at T3 and T4. All other PROs (eg, self‐efficacy, patient competence) were reported separately.
To assess QoL, the EORTC‐QLQ‐C30 was applied due to good psychometric properties.24, 25 It has been validated in German26 as well as with reference groups27 and consists of nine symptom scales, five functional scales, and the global QoL. According to the EORTC Scoring Manual,28 the latter is measured by two items on a 7‐point Likert scale ranging from 1 to 7, and then transformed linearly into a score between 0 and 100 points.
The five functional scales (physical functioning, role functioning, emotional functioning, cognitive functioning, social functioning) are measured with 4‐point Likert scales with higher scores representing higher functioning. In contrast, higher scores on the nine symptom scales (fatigue, nausea/vomiting, pain, dyspnea, insomnia, appetite loss, constipation, diarrhea, financial difficulties) measured with 3‐ranged Likert scales represent higher burden.
2.6. Statistical analysis
Statistical Analysis Software (version 9.4; SAS Institute, Cary, NC) was used for all analyses. Baseline group differences were assessed using descriptive measures combined with t‐tests or chi‐squared tests, as appropriate. The transformed score of the combination of the global QoL of the EORTC‐QLQ‐C30 was applied as the primary outcome and base for sample size calculation. To detect d = 0.4 with a power of 1 − β = 80% using a two‐sample t‐test at a two‐sided significance level α = 5%, a total of 200 patients for the randomized arm (100 per group) were required.20, 24
The intention‐to‐treat (ITT) population was used in the primary model where all patients were included in the group they were randomized to. The data were hierarchically arranged in two levels: observations (level 1) measured at different time points were nested in patients (level 2). Therefore, mixed linear models (MLMs) were applied to compare both groups on the primary and secondary outcomes. The treatment group, time of measurement, the interaction of treatment group and time, the QoL baseline score, and the stratification variables tumor stage and cancer center were included as fixed effects. Additionally, a random intercept (for the repeated measurements per patient) was included.
The secondary endpoints were analyzed using MLMs as well. All analyses were then repeated using the per protocol (PP) set excluding patients with major protocol violations. A P < 0.05 was considered significant. As predefined in our study protocol,20 only the results of the primary analysis are to be interpreted in a confirmatory manner; and when results of the secondary analyses are proven to be significant, these findings will be interpreted exploratory. Finally, for interpreting clinically relevant differences, the guidelines by Cocks et al.24 were applied.
3. RESULTS
3.1. Patients
Descriptive patient data and reasons for patients’ withdrawal is shown in the CONSORT diagram (see Figure 1). The response rate was 87% and 251 patients consented to be randomly allocated to intervention (IG) or CG. The medical and socio‐demographic characteristics of all randomized patients are reported in Table 1.
Figure 1.
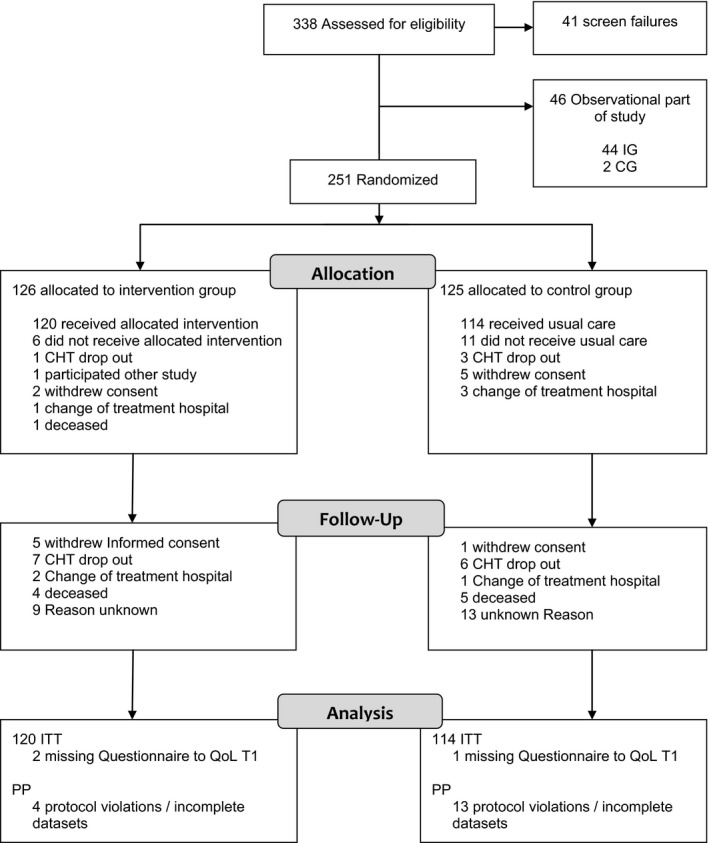
Study flow of the Complementary Nursing in Gynecologic Oncology‐study. CG, control group; CHT, chemotherapy; IG, intervention group; ITT, intention‐to‐treat; PP, per‐protocol; QoL, quality of life
Table 1.
Patient characteristics (medical and socio‐demographic)
| IG (n = 120) | CG (n = 114) | Total (N = 234) | P | |
|---|---|---|---|---|
| Age | ||||
| N | 120 | 114 | 234 | 0.631 |
| Mean ± SD | 52.6 ± 12.3 | 51.8 ± 11.4 | 52.2 ± 11.9 | |
| Median | 51.0 | 51.5 | 51.0 | |
| Cancer diagnosis | ||||
| Breast | 101 (84.2%) | 96 (84.2%) | 197 (84.2%) | 0.993 |
| Gynecologic (ovaries, uterus, cervix or other) | 19 (15.8%) | 18 (15.8%) | 37 (15.8%) | |
| Recurrent | ||||
| Yes | 11 (9.2%) | 11 (9.6%) | 22 (9.4%) | 0.899 |
| No | 109 (90.8%) | 103 (90.4%) | 212 (90.6%) | |
| Cancer center | ||||
| University hospital | 67 (55.8%) | 67 (58.8%) | 134 (57.3%) | 0.650 |
| Community hospital | 53 (44.2%) | 47 (41.2%) | 100 (42.7%) | |
| Intention of chemotherapy | ||||
| Curative | 102 (85.0%) | 99 (86.8%) | 201 (85.9%) | 0.686 |
| Palliative | 18 (15.0%) | 15 (13.2%) | 33 (14.1%) | |
| Postoperative chemotherapya | ||||
| Yes | 45 (37.5%) | 39 (34.2%) | 84 (35.9%) | 0.600 |
| No | 75 (62.5%) | 75 (65.8%) | 150 (64.1%) | |
| Preoperative chemotherapya | ||||
| Yes | 58 (48.3%) | 60 (52.6%) | 118 (50.4%) | 0.511 |
| No | 62 (51.7%) | 54 (47.4%) | 116 (49.6%) | |
| Radiochemotherapy | ||||
| Yes | 3 (2.5%) | 2 (1.8%) | 5 (2.1%) | 0.157 |
| No | 117 (97.5%) | 112 (98.2%) | 229 (97.9%) | |
| Immunotherapyb | ||||
| Yes | 21 (17.5%) | 33 (28.9%) | 54 (23.1%) | 0.038d |
| No | 99 (82.5%) | 81 (71.1%) | 180 (76.9%) | |
| Hormonal therapyc | ||||
| Yes | 1 (0.8%) | 4 (3.5%) | 5 (23.1%) | 0.157 |
| No | 119 (99.2%) | 110 (96.5%) | 229 (76.9%) | |
| Other treatments | ||||
| Yes | 2 (1.7%) | 2 (1.8) | 4 (1.7%) | 0.959 |
| No | 118 (98.3%) | 112 (98.2%) | 230 (98.3%) | |
| Place of residence | ||||
| Metropolitan city (>100 000 inhabitants) | 34 (28.3%) | 29 (27.6%) | 63 (28.0%) | 0.977 |
| Small town (20 000‐100 000 inhabitants) | 26 (21.7%) | 22 (21.0%) | 48 (21.3%) | |
| Countryside (<20 000 inhabitants) | 60 (50.0%) | 54 (51.4%) | 114 (50.7%) | |
| Missing | 0 | 9 | 9 | |
| Marital status | ||||
| Single | 12 (10.3%) | 13 (11.7%) | 25 (11.0%) | 0.033d |
| Married/living with a partner | 89 (76.0%) | 77 (69.4%) | 166 (72.8%) | |
| Divorced/separated | 5 (4.3%) | 16 (14.4%) | 21 (9.2%) | |
| Widowed | 11 (9.4%) | 5 (4.5%) | 16 (7.0%) | |
| Missing | 3 | 3 | 6 | |
| Place of birth | ||||
| Germany | 109 (91.6%) | 100 (87.7%) | 209 (89.7%) | 0.330 |
| In another country | 10 (8.4%) | 14 (12.3%) | 24 (10.3%) | |
| Missing | 1 | 0 | 1 | |
| Attitude towards CIM | ||||
| N | 104 | 100 | 204 | 0.636 |
| Mean ± SD | 8.1 ± 1.9 | 8.2 ± 1.8 | 8.1 ± 1.8 | |
| Experience with CIM | ||||
| Yes | 59 (50.4%) | 38 (34.2%) | 97 (42.5%) | 0.013d |
| No | 58 (49.6%) | 73 (65.8%) | 131 (57.5%) | |
| Missing | 3 | 3 | 6 | |
Abbreviations: CG, control group; CIM, Complementary and Integrative Medicine; IG, intervention group.
Administered chemotherapy treatments were: Carboplatin, Paclitaxel, Docetaxel, Epirubicin, Cyclophosphamid, Gemcitabin, Eribulin, Nab‐Paclitaxel, Doxorubicin, Topotecan, Cisplatin, Methotrexat, 5‐Fluorouracil, and other study regimes.
Administered immunotherapies were: Trastuzumab, Bevacizumab, Pertuzumab.
Administered hormonal therapies were: Tamoxifen.
Group difference, P < 0.05.
3.2. Primary outcome: global QoL measured with the EORTC‐QLQ‐C30
Overall development of patients’ global QoL is shown in Table 2 and Figure 2 for the ITT population (N = 231). At baseline, the global QoL levels did not differ between groups (P = 0.734). The QoL levels remained constant till T2, and decreased at T3 in both groups. Accordingly, no significant group effect was found for QoL at T3 defined as the main time point (effect estimate −1.04 [−4.89; 2.81]). At T4 (6 month follow‐up), the QoL levels had increased in both groups, but were more pronounced in the IG compared with the CG (P = 0.051). An estimated significant group effect of 6.643 [1.65; 11.64] (P = 0.010), indicating a difference between the two groups IG and CG in favor of the IG, was found in the secondary analysis for the global QoL levels considering the change from baseline (T1) to follow‐up (T4).
Table 2.
Course of the primary outcome Global Health Status (EORTC‐QLQ‐C30)
| Outcome (EORTC‐QLQ‐C30) | RA‐study arm | N = 231 | Mean (SD) T1 | N = 214 | Mean (SD) T2 | N = 199 | Mean (SD) T3 | N = 183 | Mean (SD) T4 |
|---|---|---|---|---|---|---|---|---|---|
| Global health status | IG | 118 | 59.9 ± 22.9 | 109 | 61.8 ± 19.0 | 98 | 54.5 ± 20.3 | 96 | 70.4 ± 19.8 |
| CG | 113 | 60.9 ± 23.0 | 105 | 62.0 ± 21.5 | 101 | 58.3 ± 19.7 | 87 | 64.5 ± 21.1a |
Abbreviations: CG, control group; IG, intervention group; RA, randomized study arm.
Group difference, P < 0.05.
Figure 2.
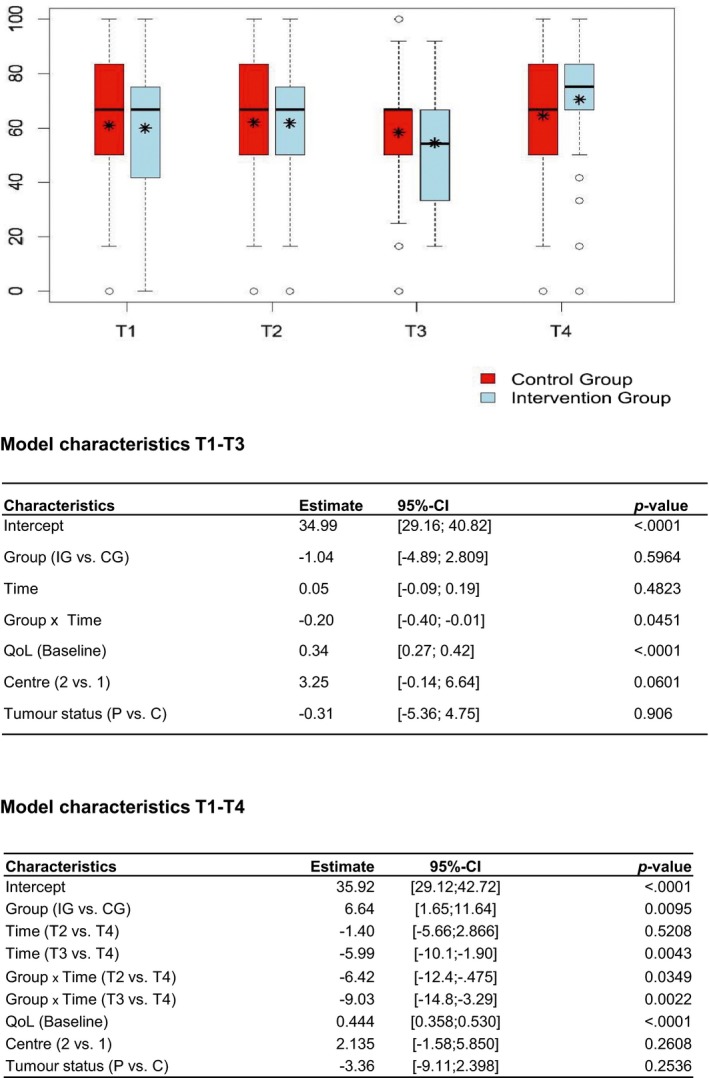
Process of the HRQoL (global domain of the EORTC‐QLQ‐C30), T1‐T4. The * represents the mean (value of the HRQoL).The _ represents the median
Sensitivity analyses were performed testing the robustness of the observed effects. Overall 214 patients could be included in the PP analysis, the other patients were excluded due to protocol violations (see Figure 1). The estimated group effects on the primary outcome in the PP analyses were similar compared to the effects observed in the ITT analyses: −2.11 [−6.10; 1.891] (P = 0.30) at T3, and 6.394 [1.142; 11.65] (P < 0.018) at T4. The sensitivity analyses where missing data were imputed led to very similar results, too.
3.3. Functional and symptom scales of the EORTC‐QLQ‐C30
In the univariate analyses, group differences were observed for other domains of the EORTC‐QLQ‐C30 reported in Table 3. At T4, significant group differences in favor of the IG were found for fatigue (P = 0.030), emotional functioning (P = 0.011), and dyspnea (P = 0.048). The first two findings were further confirmed by the multivariate analysis using the MLMs approach including the same fixed and random effects as in the primary analysis: patients from the IG experienced significant lower fatigue levels (effect estimate: −7.04 [−13.2; −.840], P = 0.027), and better emotional functioning (effect estimate: 8.196 [2.328; 14.07], P = 0.007). Only small group effects were measured with the EORTC‐QLQ‐C30 at the measurement time points T3 or T4. There was no group effect for dyspnea, presumably because the baseline values were not equal between the groups (P = 0.042).
Table 3.
Descriptive process of the secondary outcomes of the EORTC‐QLQ‐C30 domains
| Outcome (EORTC‐QLQ‐C30 domains) | RA‐study arm | N | Mean (SD) T1 | N | Mean (SD) T2 | N | Mean (SD) T3 | N | Mean (SD) T4 |
|---|---|---|---|---|---|---|---|---|---|
| Physical functioning | IG | 120 | 85.1 ± 19.3 | 107 | 75.3 ± 20.2 | 120 | 68.7 ± 23.5 | 96 | 80.9 ± 21.6 |
| CG | 113 | 82.6 ± 20.2 | 106 | 70.7 ± 21.7 | 114 | 67.3 ± 22.6 | 88 | 77.0 ± 21.4 | |
| Role functioning | IG | 120 | 64.3 ± 32.5 | 109 | 53.1 ± 30.4 | 98 | 44.4 ± 29.3 | 96 | 68.2 ± 26.5 |
| CG | 111 | 61.7 ± 34.1 | 106 | 54.7 ± 27.3 | 101 | 45.5 ± 30.0 | 88 | 61.7 ± 30.7 | |
| Emotional functioning | IG | 120 | 50.0 ± 26.0 | 108 | 62.8 ± 22.3 | 99 | 59.1 ± 25.4 | 96 | 65.9 ± 25.6a |
| CG | 114 | 50.7 ± 26.5 | 106 | 64.2 ± 22.4 | 101 | 56.5 ± 23.0 | 88 | 56.3 ± 24.7a | |
| Cognitive functioning | IG | 120 | 77.4 ± 24.8 | 109 | 72.2 ± 24.2 | 99 | 70.7 ± 26.6 | 96 | 73.4 ± 24.6 |
| CG | 114 | 77.2 ± 25.0 | 106 | 72.2 ± 23.5 | 101 | 70.3 ± 25.5 | 88 | 68.8 ± 26.7 | |
| Social functioning | IG | 120 | 59.3 ± 31.3 | 109 | 62.1 ± 29.4 | 99 | 54.5 ± 29.6 | 96 | 69.1 ± 27.9 |
| CG | 113 | 58.6 ± 29.6 | 106 | 59.7 ± 27.6 | 101 | 55.1 ± 29.5 | 88 | 63.6 ± 26.6 | |
| Fatigue | IG | 120 | 37.0 ± 29.1 | 109 | 50.2 ± 25.8 | 98 | 56.3 ± 26.9 | 96 | 36.2 ± 25.7a |
| CG | 113 | 41.2 ± 28.2 | 106 | 51.5 ± 24.6 | 101 | 59.4 ± 25.7 | 88 | 45.1 ± 29.1a | |
| Nausea/vomiting | IG | 120 | 8.9 ± 18.6 | 108 | 13.1 ± 20.1 | 98 | 13.1 ± 20.2 | 96 | 5.4 ± 14.6 |
| CG | 113 | 8.0 ± 17.6 | 106 | 13.5 ± 19.3 | 101 | 88 | 4.9 ± 13.6 | ||
| Pain | IG | 120 | 34.0 ± 32.7 | 109 | 26.8 ± 28.7 | 98 | 32.8 ± 33.7 | 96 | 27.8 ± 28.4 |
| CG | 113 | 35.5 ± 30.8 | 106 | 29.6 ± 28.0 | 101 | 35.1 ± 30.8 | 88 | 30.5 ± 29.8 | |
| Dyspnea | IG | 120 | 15.3 ± 25.2a | 109 | 33.3 ± 31.8a | 97 | 45.0 ± 32.3a | 95 | 23.2 ± 28.0a |
| CG | 112 | 22.3 ± 27.4a | 104 | 42.9 ± 30.0a | 100 | 49.3 ± 33.3a | 87 | 32.2 ± 33.1a | |
| Insomnia | IG | 119 | 44.8 ± 35.1 | 107 | 46.1 ± 34.5 | 98 | 50.0 ± 33.6 | 96 | 40.3 ± 31.7 |
| CG | 113 | 44.0 ± 33.7 | 106 | 41.8 ± 33.8 | 101 | 48.8 ± 35.5 | 88 | 48.1 ± 35.7 | |
| Appetite loss | IG | 119 | 21.8 ± 30.5 | 108 | 20.1 ± 26.5 | 97 | 20.3 ± 26.6 | 96 | 10.1 ± 21.1 |
| CG | 113 | 21.2 ± 30.9 | 105 | 20.0 ± 28.3 | 101 | 20.5 ± 30.9 | 88 | 13.6 ± 25.1 | |
| Constipation | IG | 119 | 15.1 ± 28.7 | 108 | 26.9 ± 32.0 | 98 | 24.5 ± 30.9 | 96 | 11.5 ± 24.1 |
| CG | 113 | 12.1 ± 25.2 | 104 | 22.1 ± 31.4 | 100 | 19.0 ± 29.7 | 88 | 9.8 ± 22.1 | |
| Diarrhea | IG | 119 | 12.0 ± 22.4 | 108 | 18.5 ± 31.4 | 97 | 20.3 ± 29.9 | 96 | 10.8 ± 21.9 |
| CG | 112 | 8.9 ± 20.0 | 105 | 24.8 ± 34.3 | 101 | 27.4 ± 37.8 | 85 | 7.8 ± 21.6 | |
| Financial problems | IG | 118 | 21.8 ± 31.5 | 109 | 21.1 ± 10328.6 | 99 | 24.9 ± 30.2 | 95 | 25.6 ± 29.4 |
| CG | 114 | 23.7 ± 31.3 | 103 | 22.7 ± 25.2 | 101 | 25.7 ± 29.4 | 88 | 27.3 ± 30.1 |
Abbreviations: CG, control group; IG, intervention group; RA, randomized study arm.
Group difference, P < 0.05.
4. DISCUSSION
Our results demonstrate that the administration of supportive care including nurse‐led CIM therapies, improves QoL compared to routine care in breast and gynecologic cancer patients 6 months after completion of CHT, but not directly after CHT as initially hypothesized.
The findings indicate that patients in both groups experienced an increase in their QoL in the timespan from completion of CHT (T3) until 6 months later (T4). During this follow‐up phase, an increase of QoL levels was measured in both groups, but more in the IG. Within the latter group, the improvement of 15.9 scores corresponded to a medium effect,24, 29 further demonstrating the health benefits of the tested intervention. Even though the significant QoL group difference between IG and CG measured at T4 is smaller than 10 points, and therefore does not reach the threshold to a medium effect,24, 30 the effect can be interpreted as clinically relevant though, as the supportive intervention was safe and addressed patients’ needs. Surprisingly, likewise as with the global QoL, the levels of fatigue and emotional functioning were stable in the IG and CG from the start of CHT until the midst of CHT, then a deterioration was assessed in both groups at the end of CHT (T3). At follow‐up (T4), 6 months after completion of CHT, both outcomes improved, but significantly more in the IG as shown by the univariate and multivariate analyses. Likewise as with the global QoL scores, the other two PROs improved, but compared with reference groups such as other chronic patients or people from the general population, the symptom burden was higher and functioning was more impaired.26, 27
Assessments of patients’ QoL experience have become widely accepted in clinical trials,31 as patients’ experience in QoL has been shown to be an important and prognostic factor for overall survival.32, 33 Consequently, healthcare interventions focusing on QoL that are feasible and can be translated back into clinical healthcare deliveries, also for outpatient care,29 are highly needed. To our knowledge, this study is the first randomized trial comparing the effect of a nurse‐led CIM intervention on QoL with routine care in outpatient cancer centers during CHT. To date, supportive interventions including CIM have been tested only within a few studies. These interventions, however, were not specifically integrated to supportive care during CHT,34, 35 considered exercise as the only intervention element,35 primarily considered other PROs like fatigue8, 35, 36, 37 or did not consider a nurse‐led approach.8, 9 The latter aspect, however, should be prioritized as nurses are often involved in patients’ discussions about CIM, but need more education and evidence‐based healthcare structures for conducting patient‐oriented care.17
Several aspects need to be considered as to why the integrated nurse‐led CIM approach reported here did not demonstrate QoL benefits in patients completing CHT. CHT is the most burdensome treatment phase for cancer patients,2 and oncological rehabilitation studies report that patients’ QoL needs almost a month to recover after primary treatment.38 This might be a possible explanation for not having found an effect shortly after the end of CHT. So, it can be hypothesized that the positive effect needs time to develop. This is supported by the finding that the delayed effect was also found for other scales of the EORTC‐QLQ‐C30 as shown for emotional functioning and fatigue. One other reason for the observed delayed effect of the CONGO‐study might lie in the fact that the intervention was administered during patients’ “teachable moments” and could therefore be effective over time. Previous research has indicated that psycho‐educative interventions are most successful for cancer patients and survivors if these are administered during or at the end of a strenuous treatment phase, so that their needs to strengthen their competencies, self‐efficacy, and health behaviors can be early addressed,39, 40 which is in line with the current findings. All CIM interventions had been standardized for the patient diary,22 so that patients could follow the CIM instructions at home and take care of their symptoms themselves.41 Preliminary results from the accompanying process evaluation42 confirm that patients were highly interested to continue applying the CIM interventions after positive experiences and wished to sustain them. Patients underlined how important it was for them to act autonomously and get back to normal as early as possible. The strong desire of patients to be independent and regain control is supported by many studies,43 and in our study we provided patients of the IG with self‐care strategies for the time during and after CHT.
The major strengths of our study are the large sample size and randomization, resulting in comparable groups of female cancer patients. Participating patients demonstrated high compliance and interest throughout the whole study, as well as high adherence with the study protocol. The findings of the study have high external validity as we used routine care as the CG. Thus, it was possible to focus on effectiveness (rather than efficacy) to best represent daily outpatient cancer care in the participating centers. Study quality was demonstrated by high data quality and an overall drop‐out rate of 21%, which can be regarded as normal and not affecting the robustness of the detected effects, as there was still a large sample size at T4 (n = 183).
The generalizability of our study may be limited to the studied population, focusing on adult German female outpatients treated for breast or gynecologic cancer in a University and community hospital. Other limitations may lie in the selection of indications used for the design and implementation of the CIM interventions as part of the study. Future implementation, supportive care and survivorship research might involve other patient groups and refine the symptom clusters, so that patients’ need can be addressed and met even more comprehensively. Further research is needed to investigate the optimal dose of the CIM counseling sessions, the interlinking of training modules for healthcare staff, and the best context conditions for the integration of such CIM supportive therapy interventions.
In conclusion, symptom management is highly relevant for cancer patients undergoing CHT, and the application of evidence‐based CIMs combined with regular counseling provide a safe and benign option, when administered by trained oncology nurses. Oncological centers that prioritize patient‐oriented care may consider integrating evidence‐based CIM healthcare services led by nurses. Our study does demonstrate a positive effect on PROs 6 months after patients completed their CHT, indicating further application and implementation of the tested nurse‐led CIM intervention.
CONFLICT OF INTEREST
None declared.
AUTHOR CONTRIBUTIONS
SJ, CH, CM, AS, AM, NK participated in the design and concept of the study. MB participated in the data collection and enrollment of patients. LU was involved in data analysis. NK was involved in literature research, data analysis, data interpretation, and writing of the report. SJ, CM, CH, MB, JS, NK participated in the interpretation of results. All authors have participated in drafting and finalizing the report.
ACKNOWLEDGMENTS
We thank all participating patients and their families. Special thanks go to the involved nurses Petra Neuberger, Susann Eismann, Claudia Koch, Ute Heyder, Sonja Goldmann and Christine Mandler, Markus Hoffmann from nursing management. Furthermore we want to thank our active members of the advisory committee: Dr. Markus Horneber, Prof. Dr. Klaus Linde, Prof. Dr. Hans‐Joachim Salize, Prof. Dr. Walter Haefeli, Gisela Blaser, Renate Schoenmakers, Inge Bördlein‐Wahl as well as Markus Qreini for the data management of the CONGO‐study.
Klafke N, Mahler C, von Hagens C, et al. The effects of an integrated supportive care intervention on quality of life outcomes in outpatients with breast and gynecologic cancer undergoing chemotherapy: Results from a randomized controlled trial. Cancer Med. 2019;8:3666–3676. 10.1002/cam4.2196
Funding information
This research was supported by a grant of the German Ministry of Education and Research (Bundesministerium für Bildung und Forschung, BMBF) under the research number 01GY1334 (Förderkennzeichen).
Trial registration: German Clinical Trials Register, DRKS00006056. Registered on 15 April 2014. Prospectively registered.
REFERENCES
- 1. Bonacchi A, Toccafondi A, Mambrini A, et al. Complementary needs behind complementary therapies in cancer patients. Psychooncology. 2015;24:1124‐1130. [DOI] [PubMed] [Google Scholar]
- 2. Groenvold M, Fayers PM, Petersen MA, Mouridsen HT. Chemotherapy versus ovarian ablation as adjuvant therapy for breast cancer: impact on health‐related quality of life in a randomized trial. Breast Cancer Res Treat. 2006;98:275‐284. [DOI] [PubMed] [Google Scholar]
- 3. Klafke N, Eliott JA, Wittert GA, Olver IN. Prevalence and predictors of complementary and alternative medicine (CAM) use by men in Australian cancer outpatient services. Ann Oncol. 2012;23:1571‐1578. [DOI] [PubMed] [Google Scholar]
- 4. Boon HS, Olatunde F, Zick SM. Trends in complementary/alternative medicine use by breast cancer survivors: comparing survey data from 1998 and 2005. BMC Womens Health. 2007;7:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Roter DL, Yost KJ, O'Byrne T, et al. Communication predictors and consequences of Complementary and Alternative Medicine (CAM) discussions in oncology visits. Patient Educ Couns. 2016;99:1519‐1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Posadzki P, Watson LK, Ernst E. Adverse effects of herbal medicines: an overview of systematic reviews. Clin Med (Lond). 2013;13:7‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Thanner M, Nagel E. Loss J [Cooperation between physicians and non‐medically trained practitioners: advantages and obstacles]. Forsch Komplementmed. 2013;20:23‐32. [DOI] [PubMed] [Google Scholar]
- 8. Witt CM, Ausserer O, Baier S, et al. Effectiveness of an additional individualized multi‐component complementary medicine treatment on health‐related quality of life in breast cancer patients: a pragmatic randomized trial. Breast Cancer Res Treat. 2015;149:449‐460. [DOI] [PubMed] [Google Scholar]
- 9. Ben‐Arye E, Samuels N, Schiff E, Raz OG, Sharabi IS, Lavie O. Quality‐of‐life outcomes in patients with gynecologic cancer referred to integrative oncology treatment during chemotherapy. Support Care Cancer. 2015;23:3411‐3419. [DOI] [PubMed] [Google Scholar]
- 10. Greenlee H, DuPont‐Reyes MJ, Balneaves LG, et al. Clinical practice guidelines on the evidence‐based use of integrative therapies during and after breast cancer treatment. CA Cancer J Clin. 2017;67:194‐232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Deng G, Frenkel M, Cohen L, et al. Evidence‐based clinical practice guidelines for integrative oncology: complementary therapies and botanicals. J Soc Integr Oncol. 2009;7:85‐120. [PubMed] [Google Scholar]
- 12. Towler P, Molassiotis A, Brearley SG. What is the evidence for the use of acupuncture as an intervention for symptom management in cancer supportive and palliative care: an integrative overview of reviews. Support Care Cancer. 2013;21:2913‐2923. [DOI] [PubMed] [Google Scholar]
- 13. Keyhanmehr A, Kolouri S, Heydarirad G, Mofid B, Mosavat S. Aromatherapy for the management of cancer complications: a narrative review. Complement Ther Clin Pract. 2018;31:175‐180. [DOI] [PubMed] [Google Scholar]
- 14. Cramer H, Lauche R, Klose P, Lange S, Langhorst J, Dobos GJ. Yoga for improving health‐related quality of life, mental health and cancer‐related symptoms in women diagnosed with breast cancer. Cochrane Database Syst Rev. 2017;1:CD010802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Coolbrandt A, Wildiers H, Aertgeerts B, et al. Characteristics and effectiveness of complex nursing interventions aimed at reducing symptom burden in adult patients treated with chemotherapy: a systematic review of randomized controlled trials. Int J Nurs Stud. 2014;51:495‐510. [DOI] [PubMed] [Google Scholar]
- 16. Chang H, Chang H. A review of nurses’ knowledge, attitudes, and ability to communicate the risks and benefits of complementary and alternative medicine. J Clin Nurs. 2015;24:1466‐1478. [DOI] [PubMed] [Google Scholar]
- 17. Spencer CN, Lopez G, Cohen L, et al. Nurse and patient characteristics predict communication about complementary and alternative medicine. Cancer. 2016;122:1552‐1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. World Health Organisation . Global strategic directions for strenghtening nursing and midwifery 2016‐2020. http://www.who.int/hrh/nursing_midwifery/global-strategic-midwifery2016-2020.pdf. Accessed June 13, 2018.
- 19. Bialous S. Vision of professional development of oncology nursing in the world. Asia Pac J Oncol Nurs. 2016;3:25‐27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Klafke N, Mahler C, von Hagens C, et al. A complex nursing care intervention on Complementary and Alternative Medicine (CAM) to increase quality of life in patients with breast and gynecologic cancer undegoing chemotherapy (CONGO study): study protocol for a partially randomized controlled trial. Trials. 2015;16, 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Calvert M, Blazeby J, Altman DG, Revicki DA, Moher D, Brundage MD. Reporting of patient‐reported outcomes in randomized trials: the CONSORT PRO extension. JAMA. 2013;309:814‐822. [DOI] [PubMed] [Google Scholar]
- 22. Klafke N, Mahler C, Von Hagens C, Blaser G, Bentner M, Joos S. Developing and implementing a complex Complementary and Alternative (CAM) nursing intervention for breast and gynecologic cancer patients undergoing chemotherapy—report from the CONGO (complementary nursing in gynecologic oncology) study. Support Care Cancer. 2016;24:2341‐2350. [DOI] [PubMed] [Google Scholar]
- 23. Kreienberg R, Albert U, Follmann M, et al. Interdisziplinäre S3‐Leitlinie für die Diagnostik, Therapie und Nachsorge des Mammakarzinoms. Senol Zeitsch Mammadiag Ther. 2013;10:164‐192. [Google Scholar]
- 24. Cocks K, King MT, Velikova G, Martyn St‐James M, Fayers PM, Brown JM. Evidence‐based guidelines for determination of sample size and interpretation of the European Organisation for the Research and Treatment of Cancer Quality of Life Questionnaire Core 30. J Clin Oncol. 2011;29:89‐96. [DOI] [PubMed] [Google Scholar]
- 25. Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ‐C30: a quality‐of‐life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365‐376. [DOI] [PubMed] [Google Scholar]
- 26. Schwarz R, Hinz A. Reference data for the quality of life questionnaire EORTC QLQ‐C30 in the general German population. Eur J Cancer. 2001;37:1345‐1351. [DOI] [PubMed] [Google Scholar]
- 27. Hjermstad MJ, Fayers PM, Bjordal K, Kaasa S. Using reference data on quality of life–the importance of adjusting for age and gender, exemplified by the EORTC QLQ‐C30 (+3). Eur J Cancer. 1998;34:1381‐1389. [DOI] [PubMed] [Google Scholar]
- 28. Fayers PM. Interpreting quality of life data: population‐based reference data for the EORTC QLQ‐C30. Eur J Cancer. 2001;37:1331‐1334. [DOI] [PubMed] [Google Scholar]
- 29. Hinz A, Weis J, Faller H, et al. Quality of life in cancer patients—a comparison of inpatient, outpatient and rehabilitation settings. Support Care Cancer. 2018;26:3533‐3541. [DOI] [PubMed] [Google Scholar]
- 30. Osoba D, Rodrigues G, Myles J, Zee B, Pater J. Interpreting the significance of changes in health‐related quality‐of‐life scores. J Clin Oncol. 1998;16:139‐144. [DOI] [PubMed] [Google Scholar]
- 31. Montazeri A. Quality of life data as prognostic indicators of survival in cancer patients: an overview of the literature from 1982 to 2008. Health Qual Life Outcomes. 2009;7:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kypriotakis G, Vidrine DJ, Francis LE, Rose JH. The longitudinal relationship between quality of life and survival in advanced stage cancer. Psychooncology. 2016;25:225‐231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ediebah DE, Quinten C, Coens C, et al. Quality of life as a prognostic indicator of survival: a pooled analysis of individual patient data from canadian cancer trials group clinical trials. Cancer. 2018;124:3409‐3416. [DOI] [PubMed] [Google Scholar]
- 34. Jefford M, Gough K, Drosdowsky A, et al. A randomized controlled trial of a nurse‐led supportive care package (survivorcare) for survivors of colorectal cancer. Oncologist. 2016;21:1014‐1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Steindorf K, Schmidt ME, Klassen O, et al. Randomized, controlled trial of resistance training in breast cancer patients receiving adjuvant radiotherapy: results on cancer‐related fatigue and quality of life. Ann Oncol. 2014;25:2237‐2243. [DOI] [PubMed] [Google Scholar]
- 36. de Raaf PJ, de Klerk C, Timman R, Busschbach JJ, Oldenmenger WH, van der Rijt CC. Systematic monitoring and treatment of physical symptoms to alleviate fatigue in patients with advanced cancer: a randomized controlled trial. J Clin Oncol. 2013;31:716‐723. [DOI] [PubMed] [Google Scholar]
- 37. Schmidt ME, Wiskemann J, Armbrust P, Schneeweiss A, Ulrich CM, Steindorf K. Effects of resistance exercise on fatigue and quality of life in breast cancer patients undergoing adjuvant chemotherapy: a randomized controlled trial. Int J Cancer. 2015;137:471‐480. [DOI] [PubMed] [Google Scholar]
- 38. Riedl D, Giesinger JM, Wintner LM, et al. Improvement of quality of life and psychological distress after inpatient cancer rehabilitation : results of a longitudinal observational study. Wien Klin Wochenschr. 2017;129:692‐701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Karvinen K, Bruner B, Truant T. The teachable moment after cancer diagnosis: perceptions from oncology nurses. Oncol Nurs Forum. 2015;42:602‐609. [DOI] [PubMed] [Google Scholar]
- 40. Park CL, Cho D, Salner AL, Dornelas E. A randomized controlled trial of two mail‐based lifestyle interventions for breast cancer survivors. Support Care Cancer. 2016;24:3037‐3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Coolbrandt A, Dierckx de Casterle B, Wildiers H, et al. Dealing with chemotherapy‐related symptoms at home: a qualitative study in adult patients with cancer. Eur J Cancer Care (Engl). 2016;25:79‐92. [DOI] [PubMed] [Google Scholar]
- 42. Klafke N, Mahler C, von Hagens C, et al. How the consolidated framework for implementation research can strengthen findings and improve translation of research into practice: a case study. Oncol Nurs Forum. 2017;44:e223‐e231. [DOI] [PubMed] [Google Scholar]
- 43. Weeks L, Balneaves LG, Paterson C, Verhoef M. Decision‐making about complementary and alternative medicine by cancer patients: integrative literature review. Open Med. 2014;8:e54‐e66. [PMC free article] [PubMed] [Google Scholar]


