Abstract
Japan introduced a Helicobacter pylori eradication therapy strategy in 2013, with the aim of decreasing the number of gastric cancer‐related death, the number of new cases of gastric cancer, and associated medical costs. Five years have passed since then, but no reduction in the annual number of gastric cancer has been observed. In addition, it was suggested that the number of deaths due to gastric cancer could be reduced to 30,000 a year by 2020, but the annual death toll in 2017 remained at more than 45,000. Based on the above evidence, it was examined whether it was possible to reach the target value based on the data from the last 5 years. The number of deaths per year in 2020 is predicted to be more than 40,000, which is clearly different from the target value. Logically, the effect of the strategy might appear by 2023. However, there is a possibility that the risk of gastric cancer may increase in some populations due to the influence of proton pump inhibitors and dysbiosis in the gastric microbiome. To solve these problems, combined therapy with PPIs and aspirin for patients after H pylori eradication should be considered.
Keywords: atrophic gastritis, dysbiosis, gastric cancer, gastric intestinal metaplasia, Helicobacter pylori eradication, latent gastric cancer, proton pump inhibitors
1. INTRODUCTION
Gastric cancer is clearly associated with infection by Helicobacter pylori, and in populations without H pylori infection, the gastric cancer incidence rates are very low.1 This fact led to the development of a strategy to prevent gastric cancer via the administration of H pylori eradication therapy. In Japan, the provision of eradication therapy for chronic gastritis due to H pylori infection by health insurance was started in February 2013, regardless of the presence of symptoms.2 The main purpose of this strategy was to reduce the number of gastric cancer‐related death and the number of new cases of gastric cancers. This strategy was believed to be economically beneficial because it would drastically reduce the cost of treating patients with gastric cancer within 5 years.3, 4 However, according to report published by the Ministry of Health, Labor and Welfare in January 2019, the number of new cases of gastric cancer in 2016 was 134 650, which was the largest number to date (Data S1).5 There was a 0.6% decrease in the number of deaths due to gastric cancer in 2016 compared to the previous year.6 One reason for the discrepancy between these results and the predictions may be that an insufficient number of people achieved eradication of H pylori. It is also possible that the strategy is ineffective. The purpose of this review was to provide evidence that it is impossible to attain the goal of the strategy and to explain the reasons for this failure.
2. A REALITY FAR FROM THE PREDICTED RESULTS
2.1. Theory underlying the strategy and the predicted number of gastric cancer‐related deaths
In Japan, owing to the baby boom that occurred between 1947 and 1949, the largest age group in the population is near 70 years. However, in Japan, the population naturally decreases due to various diseases after the age of 75 years. With the decrease in the prevalence of H pylori infection, the severity of atrophic gastritis and gastric intestinal metaplasia in the Japanese population has drastically decreased since 2010.7, 8 Furthermore, not only the rapid decline in H pylori infection rates after the baby boom but also the entire population is decreasing, and the total number of H pylori‐positive persons will also decrease after 2025 (Data S2). For that reason, it is clear that the number of deaths due to gastric cancer might naturally decline after 2025 even without the strategy to eliminate gastric cancer. Nonetheless, there is an urgent need for the eradication of H pylori to prevent gastric cancer in the elderly, including in baby boomers, who have a high risk of gastric cancer.2, 3, 4 This strategy was implemented to achieve a short‐term rather than a long‐term goal.
The specific predicted values were as follows.
2.1.1. Predicted the number of new cases of gastric cancer
The basis for this prediction was the incidence of gastric intestinal metaplasia in Japan,9 and the prevention rate of metachronous gastric cancer (MGC) by H pylori eradication therapy.10 In Japan, the prevalence of gastric intestinal metaplasia in patients with H pylori was as follows: 51‐60 years, 47.5%; 61‐70 years, 57.6%; older than 71 years, 57.1%.9 In a study of gastric cancer reduction effect by H pylori eradication therapy on MGC in Japan, the incidence of secondary gastric cancer was reduced by approximately one‐third over 3 years.10 For subjects in this study, the prevalence of gastric intestinal metaplasia was approximately 50%.10 From the above, it was predicted that approximately 30% of gastric cancer would be suppressed by the eradication of H pylori in the group of over 50 years of age.3, 4 Asaka noted that the number of new cases of gastric cancer is 107,000 per year in Japan, therefore, 535,000 people develop gastric cancer in 5 years. However, if H pylori eradication therapy is performed for all people aged 50 years or older, the number of annual gastric cancers will be 32,000 (107,000 × 0.3). Therefore, within 5 years, the occurrence of 375,000 (535,000‐32,000 × 5) gastric cancers can be eliminated.4
2.1.2. Predicted number of deaths
Because this strategy is intended to prevent the development of new gastric cancer, the number of patients dying from gastric cancer should decrease. Asaka and Kato described that if the gastric cancer elimination project is successful and about 50% of people with H pylori infection receive eradication therapy, the number of deaths from gastric cancer will be about 30,000 in 2020.2 Asaka described that this strategy could prevent approximately 150,000 deaths from gastric cancer over 5 years.3, 4 In fact, the calculation of the 2020 forecast value was made from the decrease in expected value in 5 years. Asaka explained that this strategy could prevent approximately 150,000 deaths from gastric cancer within 5 years. The number 150,000 was obtained assuming that 40% of people who developed gastric cancer would die (375,000 × 0.4).4 The hypothesis that the number of annual deaths would be 30,000 after 5 years was, first, assuming that the number of deaths in 5 years would be 70,000 if the strategy was not implemented.3, 4 Therefore, with a baseline of 50,000, the total reduction in 5 years is 100,000 (150,000−20,000 × 5 × 2−1). If half of the subjects receive eradication therapy, reduced numbers are 20,000 at 5 years later, and the annual number of deaths is 30,000 (50,000−20,000).
In Japan, the eradication success rates of first‐line treatment were about 90%, and most unsuccessful patients receive second‐line and third‐line treatment and, endoscopy is mandated before the first‐line treatment. However, the evaluation of the cost‐effectiveness of the strategy did not take into the cost of confirming that patients were H pylori‐negative after undergoing eradication therapy, the cost of endoscopies, or costs incurred by treatment failure. The cost‐effectiveness was evaluated based on the cost of the first test for H pylori and the cost of the first‐line treatment.4 Specifically, the eradication rate used in designing this strategy was based on the number of people undergoing first‐line treatment only.4 The number of subjects (42.266 million people) was based on 78% of the 54 million people over the age of 50 years being infected with H pylori. However, this infection rate is derived from data from 1990,11 and it is clearly different from the actual infection rate immediately prior to the implementation of the strategy. The use of this overly high H pylori morbidity rate may be one of the causes of the exaggeration of the predicted effectiveness of the strategy.
2.2. Results after implementing the strategy
The Japanese government approved the H pylori eradication therapy strategy for individuals with peptic ulcers in 2000 and further extended this therapy to every H pylori‐positive person in 2013.2 The adaptation of this eradication therapy covered all H pylori‐infected individuals, regardless of endoscopic/pathological findings, and became an indiscriminate eradication strategy. As a result, in Japan, 600,000 people annually from 2001 to 2013 and 1.4‐1.6 million people annually since 2013 have been administered H pylori eradication therapy,12, 13 and in total, 13.6 million people who were H pylori‐positive were treated between 2001 and 2016.14 Assuming that on average, 1.5 million people per year have received H pylori eradication therapy since the implementation of the expanded strategy in 2013, the total number of patients treated from 2013 to 2016 was 7.5 million, which is only 17.5% of the original target of 42.26 million people. However, by the time of the implementation of this strategy in 2013, the H pylori infection rate had decreased, and the number of H pylori‐positive people aged 50‐79 years was estimated to be 18.6 million people 15 (Data S3). Therefore, by 2016, approximately 40% of H pylori‐positive people in the 50‐79 year old age group may have already received H pylori eradication therapy. However, in general, gastric cancer screening in Japan has been expanded to include all individuals older than 40 years. Therefore, there are 22.2 million subjects undergoing gastric cancer screening, half (11.1 million people) of whom might receive H pylori eradication therapy prior to 2020. In the 5 years since the strategy was implemented, the number of deaths from gastric cancer has tended to decrease slightly (Data S4). However, according to the regression line (R 2 = 0.9737), the value predicted in 2020 is greater than 40,000, and the possibility of achieving that expected value is extremely low (Figure 1).
Figure 1.
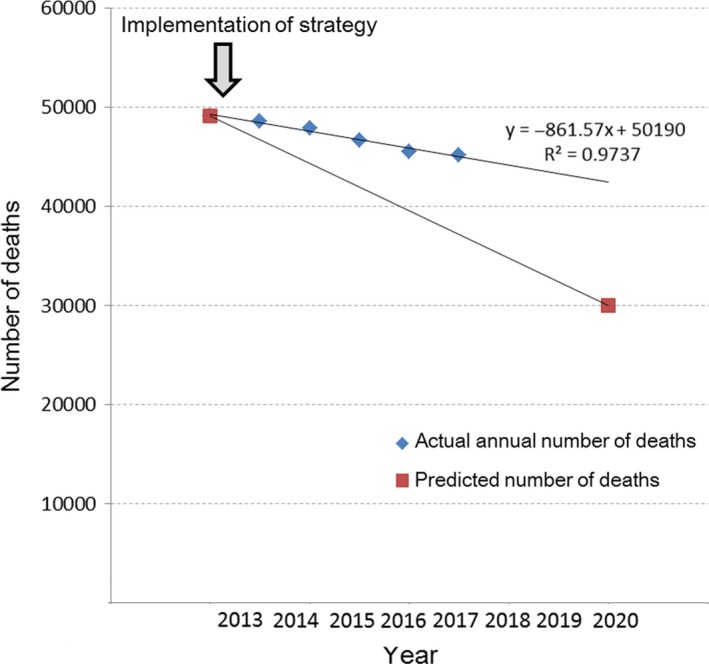
Predictive number before strategy and actual number after strategy. The number of deaths per year showed a very strong linear relationship (R 2 = 0.9737). According to extension of that straight line, even in 2020, the number of deaths from gastric cancer would over 40,000
The reason for the difference between the predicted and actual deaths due to gastric cancer could be that gastric cancer incidence rate did not decrease as much as predicted. Clearly, the number of gastric cancer‐related deaths has decreased, although only slightly. However, the decrease in the number of deaths over 5 years has been approximately 10,000 people out of the 7.5 million who have received H pylori therapy, which is only 0.13% (Data S4). Although the number of gastric cancer sufferers is increasing, age‐adjusted morbidity rates from 2003 to 2016 have been declining gradually (Figure 2).5, 16 However, the rates after 2013 are also on the regression line (R 2 = 0.6892). These results suggest that H pylori eradication therapy could not have the effect of prevention of gastric cancer within 5 years. The increase in the number of affected individuals reflects Japan's population structure, and a gradual decline in age‐adjusted morbidity rates may reflect a natural decline in H pylori infection rates (Data S2).
Figure 2.
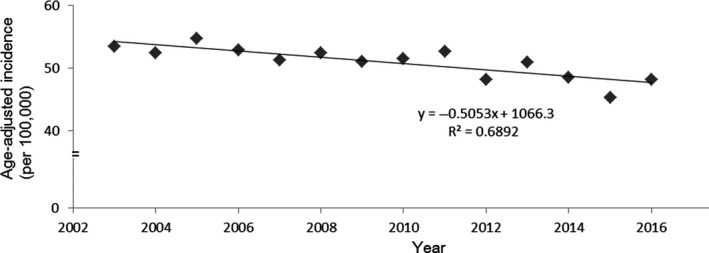
Age‐adjusted morbidity of gastric cancer from 2003 to 2016 in Japan. All data are on the regression line (y = −0.5053x + 1066.3, R 2 = 0.6892). Changes after 2013 cannot be confirmed
3. CAN H PYLORI TREATMENT REDUCE THE INCIDENCE OF GASTRIC CANCER?
3.1. Latent gastric cancer after H pylori eradication
In this strategy, emphasis was placed on the reduction in the incidence of MGC to one‐third of the original rate in only 3 years.2, 3, 4, 10 This result led to the hypothesis that the occurrence of gastric cancer is suppressed immediately upon the eradication of H pylori and that the number of deaths due to gastric cancer could be drastically reduced within several years.3, 4 However, other research groups in Japan have reported that the eradication of H pylori does not prevent the occurrence of MGC.17, 18 In those studies, the incidence of MGC increased 4 to 5 years after H pylori eradication. In 2017, Maehata et al investigated why gastric cancer became more prevalent during the second half of the 11‐year study period.19 They observed that the gastric cancer that occurred within 5 years of eradication was morphologically characterized by small depressions covered with nonneoplastic mucosa. Because this finding differs from the early‐stage gastric cancer (EGC), which occurs in patients with conventional atrophic gastritis, it may be overlooked and classified as latent gastric cancer if the endoscopist is unable to recognize it (Figure 3).20 It has also been reported that the incidence of primary EGC increases 5 years after H pylori has been eradicated.21 Moreover, this new form has been observed not only in MGC but also in primary EGC after H pylori eradication (Figure 4).22, 23, 24, 25
Figure 3.
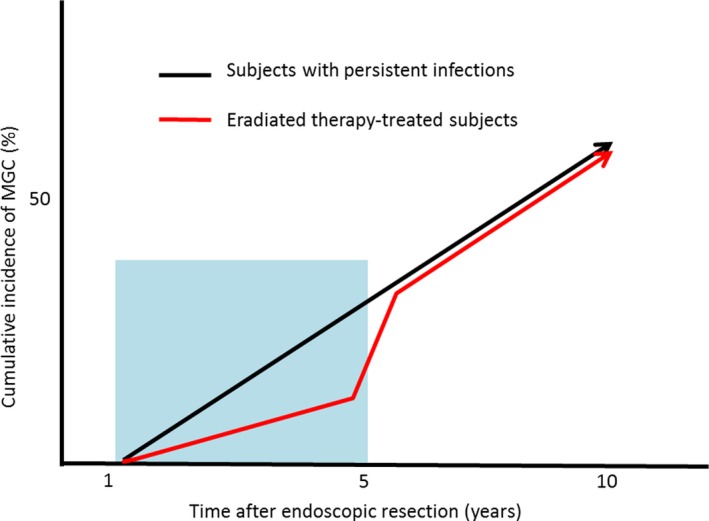
The effect of gastric cancer detection rate by latent gastric cancer. A short period of research within 5 years leads to erroneous results that H pylori eradication can prevent gastric cancer development
Figure 4.
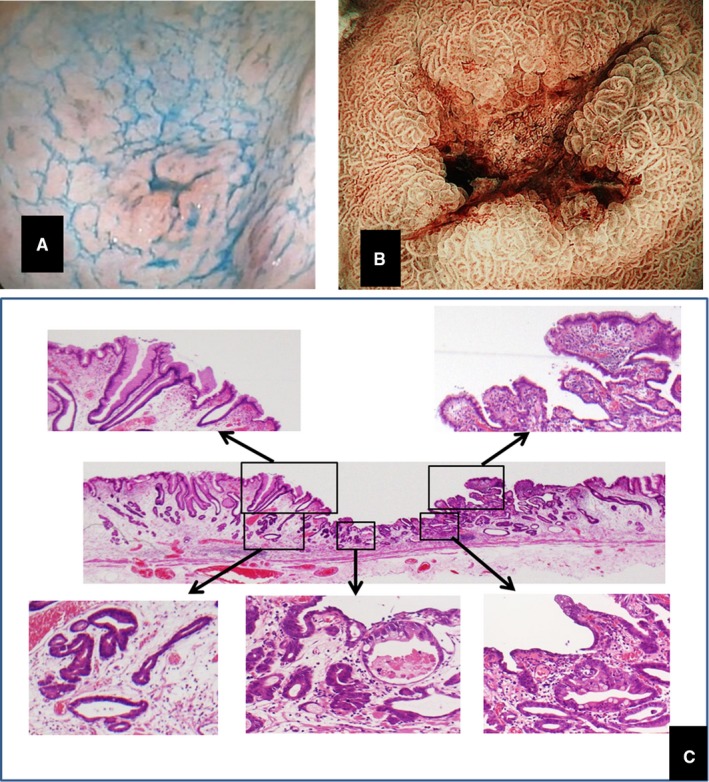
Typical early‐stage gastric cancer after H pylori eradication. A, Primary early‐stage gastric cancer found 3 years after H pylori eradication. B, The lesion removed by endoscopic submucosal dissection. The diameter of the depressed part is 3 mm. C, Carcinoma tissue is seen to be covered by nonneoplastic tissue
Because this form of EGC is different from that of gastric cancer that occurs in the conventional H pylori‐infected stomach, there is a possibility that this form of cancer may be misdiagnosed or diagnosed after it has progressed to a later stage. In addition, the belief among endoscopists that gastric cancer is less likely to develop after H pylori eradication may lead to a detection bias. Various gastric mucosal changes (eg, mottled patchy erythema) occur after the eradication of H pylori.26 However, because there is no such change in the gastric mucosa without H pylori treatment, changes due to carcinogenesis can be easily detected by endoscopy. These factors may lead to differences in cancer detection rates.
3.2. Logical lead time from the eradication of H pylori to a reduction in the mortality from gastric cancer
At a certain point, level of intestinal metaplasia in the Correa pathway reaches the “point of no return”, which might limit of the efficacy of H pylori eradication for preventing gastric cancer.27, 28 Residual DNA methylation in the gastric mucosa after H pylori eradication plays a role in gastric carcinogenesis. This “point of no return” is closely related to the residual DNA methylation level.29 In a meta‐analysis of 10 studies performed in 2016, a reduction in the risk of gastric cancer due to H pylori eradication was not observed when considering the stages of intestinal metaplasia and dysplasia.30 In 2017, it was found that it takes 5‐10 years after H pylori eradication for atrophic gastritis to histologically improve to level found in uninfected subjects.31 Further, in another report from 2018, according to a 5‐year follow‐up survey of 1,755 cases after H pylori eradication, gastric cancer occurred in 5% of OLGA stage III and IV cases. Furthermore, 53% of stage II cases did not change, 19% progressed to stage III, and only 28% returned to stage 0 or I.32 These recent reports show that even in patients with gastritis in treatment‐responsive stages, the recovery time is long, and there are certainly cases that still progress to gastric cancer even after H pylori eradication.
When discussing the incidence of gastric cancer, it is necessary to consider the pathological criteria for diagnosing EGC. The mismatch in the pathological diagnoses of gastric cancer between Japan and the West has become a problem; lesions diagnosed as high‐grade dysplasia in the West are frequently classified as carcinoma in Japan.33, 34 However, lesions diagnosed as EGC based on Japanese diagnostic criteria have been demonstrated to progress to advanced cancer. In a Japanese study that followed EGC discovered in 1980,35 the median of Kaplan‐Meier until the early cancer progresses to advanced cancer was 44 months (interquartile range [IQR]: 30‐80), cumulative 5‐year risk for progression to the advanced stage was estimated as 63.0% (95% confidence interval 48.1‐77.9%). Furthermore, the median until the death of delayed operation cases was 125 months (IQR = 45‐195). Almost all those EGCs may have originated from H pylori infections because the rate of H pylori positivity in the 1980s was estimated to be 70%‐80% in Japan.7 Considering the lead time from the development of atrophic gastritis or intestinal metaplasia to progression to EGC (A) and the lead time from the development of advanced cancer to death (B), in theory, it would take more than 3.7+A+B years to observe the effect of eradicating H pylori on gastric cancer‐related deaths (Figure 5). Based on the above evidence, an observable effect of H pylori eradication on gastric cancer‐related death cannot be expected within 5 years of the implementation of the strategy, although it might be possible to observe an effect within 10 years. However, even 10 years after the eradication of H pylori, the incidence rate of gastric cancer remains equivalent to the rate between 5 and 9 years after the administration of therapy, and permanent follow‐up is necessary.21 Leung et al divided the subjects who received H pylori eradication therapy into three groups of less than 40 years, 40‐59 years, and 60 years or older, and investigated the incidence rates of gastric cancer, and the incidence rates were compared in three periods (less than 5 years, 5‐9 years, and 10 years or longer) for the three groups.36 As for the results, although the incidences of gastric cancer in the H pylori‐treated cohort were comparable to the age‐matched general population in the first 9 years after eradication, after 10 or more years of H pylori treatment, the risk of gastric cancer among the older (≥60 years) and middle (40‐59 years) age groups became significantly lower than that of the matched general population. This evidence indicates that gastric cancer incidence and mortality cannot be reduced less than 9 years after the initiation of eradication therapy but may be reduced after 10 years.
Figure 5.
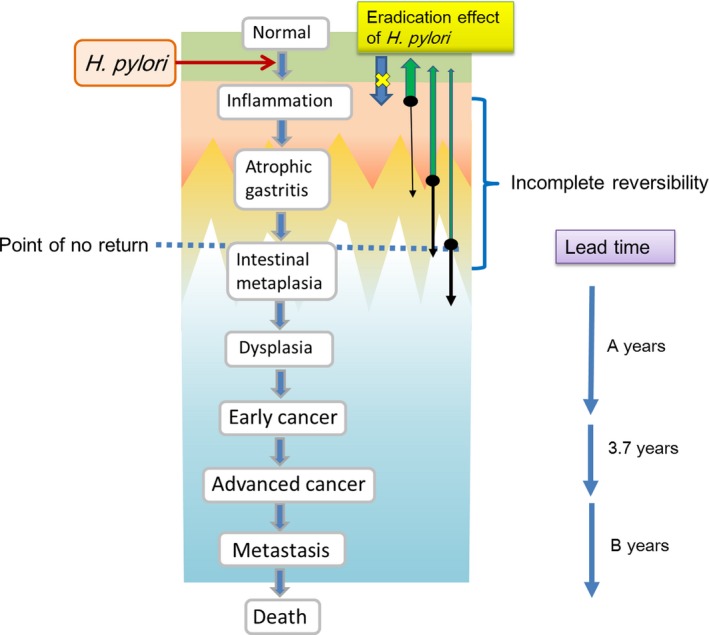
Lead time to the reduction in gastric cancer deaths from eradicating H pylori. After decreasing of gastric cancer which occurs from over stage of the "point of no return", the effect of H pylori eradication might be observed
3.2.1. Influence of proton pump inhibitors (PPIs)
It has been suggested that the incidence of reflux esophagitis may increase after H pylori eradication in Asians, who demonstrate marked increases in gastric acid secretion after H pylori eradication.37 In a large‐scale cross‐sectional study of Japanese subjects, the prevalence of reflex esophagitis in subjects with chronic H pylori infection and successful H pylori eradication were significantly different (2.3% and 8.8%; P < 0.0001).38 A report from Taiwan stated that eradication of H pylori increased the reflux esophagitis morbidity rate from 13.7% to 27.3%.39 A researcher from South Korea reported that compared with the prevalence of reflux esophagitis in the persistent infection group, the prevalence of reflux esophagitis increased after successful H pylori eradication (OR 2.34).40 In addition, there is a possibility that PPIs will be used for the treatment of reflux esophagitis. However, the use of PPIs has been reported to increase the risk of gastric cancer.41, 42 Regarding causality between PPIs and gastric cancer, sample size, observation period, and sampling bias when gastric cancer is not confirmed before administration of PPIs, the involvement of potential drugs involved in gastric cancer development, such as aspirin, affects the research results.43 However, if gastric cancer increases in patients using PPI after H pylori eradication, the direct relationship between H pylori and PPIs might be denied. Furthermore, if the endoscopy is performed before H pylori eradication, sampling bias may be eliminated. In a cohort study in Hong Kong with 63,397 subjects, it was observed that 153 patients (0.24%) developed gastric cancer during a median follow‐up period of 7.6 years, and PPIs use was associated with an increased gastric cancer risk (HR 2.44).44 Long‐term use of PPIs was still associated with an increased gastric cancer risk in subjects even after the administration of H pylori eradication therapy. From the above evidence, it can be concluded that the prevalence of gastric cancer due to the use of PPIs may increase in certain populations after the eradication of H pylori. However, the risk of PPIs for gastric cancer might be avoided using a combination with aspirin. From the results of the cohort study, Cheung et al suggested that aspirin may be effective in preventing gastric cancer after H pylori eradication.45, 46 Interestingly, they found that gastric cancer risk reduction with aspirin in H pylori‐eradicated subjects (HR 0.30 [95% CI: 0.15‐0.61]) was greater than that reported in the meta‐analysis (pooled odds ratio [OR] 0.78 [95% CI: 0.69‐0.87]) including studies on both H pylori‐infected and H pylori‐negative subjects.45 The prevention effect on gastric cancer was more prominent in those who used aspirin daily or for 5 or more years. Furthermore, it has been reported that PPIs and aspirin combination therapy may prevent esophageal adenocarcinoma in Barrett's eosophagus.47 Concerning the relationship between aspirin and events of gastrointestinal bleeding, Garcia Rodriguez et al reported that concomitant use of PPIs and aspirin decreased upper gastrointestinal bleeding risks relative to aspirin monotherapy.48 From the above, it is expected to perform a randomized prospective study on whether combined therapy with PPIs and aspirin for patients after H pylori eradication can prevent gastric cancer and/or esophageal adenocarcinoma.
3.3. Gastric cancer and stomach microbiota
As atrophic gastritis progresses, H pylori itself spontaneously disappears from the stomach.49 However, this condition leads to the highest risk of developing gastric cancer.50 , 51 According to the gastric microbiota profile generated by 16S rRNA analysis, many other bacteria exist in the stomach besides H pylori.52 Chinese researchers confirmed that H pylori was less common in the tumoral microhabitat than nontumoral microhabitats by conducting a gastric microbiota analysis targeting the 16S rRNA gene in 230 normal, 247 peritumoral and 229 tumoral tissues.53 H pylori colonization alone is not sufficient to induce gastric carcinogenesis, and H pylori‐induced gastric cancer is promoted by the presence of a complex microbiota.53 Furthermore, dysbiosis of the microbiome in the stomach, which is involved in the development of gastric cancer, occurs when the relative abundance of H pylori decreases.54 Research from Portugal suggested that the gastric microbiome in patients with gastric carcinoma exhibits a dysbiotic microbial community (eg, nitrosating bacteria) with genotoxic potential.55 (Figure 6) Researchers from South Korea reported that the gastric microbiome in the successful H pylori eradication group resembled that in the H pylori‐negative intestinal metaplasia group, which is regarded as a high‐risk group.56 Studies of the gastric microbiome, such as these, may provide new strategies to prevent gastric cancer in the future.
Figure 6.
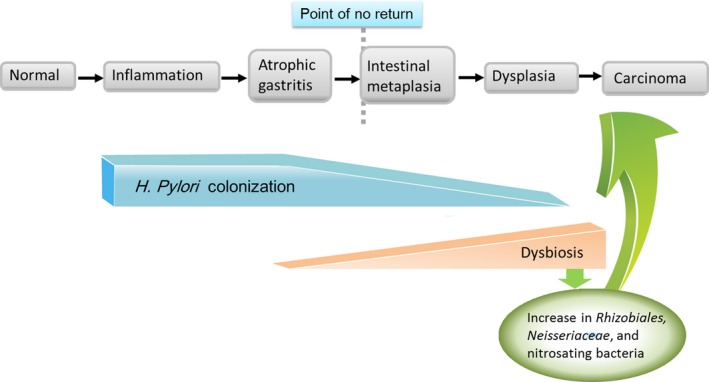
Relationship between stomach microbiota and gastric cancer
4. CONCLUSION
The Japanese strategy of H pylori eradication therapy is unlikely to be able to achieve the predicted reduction in the number of gastric cancer‐related deaths based on the actual data from the last 5 years. Logically, the effect of the strategy might appear in 2023. However, there is a possibility that the risk of developing gastric cancer may increase in some populations due to the influence of PPIs and dysbiosis in the gastric microbiome. To solve these problems, combined therapy with PPIs and aspirin for patients after H pylori eradication should be considered.
CONFLICT OF INTEREST
Uno Y declares no conflicts of interest in relation to the publication of this article.
AUTHOR CONTRIBUTIONS
Uno Y contributed to the writing of the manuscript, creation of theory, reference collection, and creation of the figures.
Supporting information
Uno Y. Prevention of gastric cancer by Helicobacter pylori eradication: A review from Japan. Cancer Med. 2019;8:3992–4000. 10.1002/cam4.2277
REFERENCES
- 1. Uemura N, Okamoto S, Yamamoto S, et al. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med. 2001;345:784‐789. [DOI] [PubMed] [Google Scholar]
- 2. Asaka M, Kato M, Sakamoto N. Roadmap to eliminate gastric cancer with Helicobacter pylori eradication and consecutive surveillance in Japan. J Gastroenterol. 2014;49:1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Asaka M, Kato M, Graham DY. Strategy for eliminating gastric cancer in Japan. Helicobacter. 2010;15:486‐490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Asaka M. Extermination of gastric cancer from Japan. Nihon Shokakibyo Gakkai Zasshi. 2010;107:359‐364. [PubMed] [Google Scholar]
- 5. Japan Health, Labor and Welfare Ministry . Result of national cancer registration in 2016. https://www.mhlw.go.jp/content/10900000/000468976.pdf. Accessed May 1, 2019.
- 6. Cancer Resistry and Statics . 2017 Tokyo, Japan: Cancer Information Service, National Cancer Center. January 21, 2019. https://ganjoho.jp/reg_stat/statistics/stat/summary.html. Accessed May 1, 2019.
- 7. Kamada T, Haruma K, Ito M, et al. Time trends in Helicobacter pylori infection and atrophic gastritis over 40 years in Japan. Helicobacter. 2015;20:192‐198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ueda J, Gosho M, Inui Y, et al. Prevalence of Helicobacter pylori infection by birth year and geographic area in Japan. Helicobacter. 2014;19:105‐110. [DOI] [PubMed] [Google Scholar]
- 9. Asaka M, Sugiyama T, Nobuta A, Kato M, Takeda H, Graham DY. Atrophic gastritis and intestinal metaplasia in Japan: results of a large multicenter study. Helicobacter. 2001;6:294‐299. [DOI] [PubMed] [Google Scholar]
- 10. Fukase K, Kato M, Kikuchi S, et al. Effect of eradication of Helicobacter pylori on incidence of metachronous gastric carcinoma after endoscopic resection of early gastric cancer: an open‐label, randomised controlled trial. Lancet. 2008;372(9636):392‐397. [DOI] [PubMed] [Google Scholar]
- 11. Asaka M, Kimura T, Kudo M, et al. Relationship of Helicobacter pylori to serum pepsinogens in an asymptomatic Japanese population. Gastroenterology. 1992;102:760‐766. [DOI] [PubMed] [Google Scholar]
- 12. Hiroi S, Sugano K, Tanaka S, Kawakami K. Impact of health insurance coverage for Helicobacter pylori gastritis on the trends in eradication therapy in Japan: retrospective observational study and simulation study based on real‐world data. BMJ Open. 2017;7:e015855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tsuda M, Asaka M, Kato M, et al. Effect on Helicobacter pylori eradication therapy against gastric cancer in Japan. Helicobacter. 2017;22:e12415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sugano K, Hiroi S, Yamaki Sugano K, Hiroi S, Yamaoka Y. Prevalence of Helicobacter pylori infection in Asia: remembrance of things past? Gastroenterology. 2018;154:257‐258. [DOI] [PubMed] [Google Scholar]
- 15. Watanabe M, Ito H, Hosono S, et al. Declining trends in prevalence of Helicobacter pylori infection by birth‐year in a Japanese population. Cancer Sci. 2015;106:1738‐1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cancer Resistry and Statics . Center for cancer control and information service, Monitoring of Cancer Incidence in Japan. April 24, 2019. https://ganjoho.jp/reg_stat/statistics/brochure/monitoring.html. Accessed May 1, 2019.
- 17. Kato M, Nishida T, Yamamoto K, et al. Scheduled endiscopic surveillance controls secondery cancer after curative endoscopic resection for early gastric cancer: a multicentre retrospective cohort study by Osaka University ESD study group. Gut. 2013;62:1425‐1432. [DOI] [PubMed] [Google Scholar]
- 18. Maehata Y, Nakamura S, Fujisawa K, et al. Long‐term effect of Helicobacter pylori eradication on the development of metachronous gastric cancer after endoscopic resection of early gastric cancer. Gastrointest Endosc. 2012;75:39‐46. [DOI] [PubMed] [Google Scholar]
- 19. Maehata Y, Nakamura S, Esaki M, et al. Characteristics of primary and metachronous gastric cancers discovered after Helicobacter pylori eradication: a multicenter propensity score–matched study. Gut Liv. 2017;11:628‐634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Uno Y. “Latent gastric cancer” after eradicating Helicobacter pylori . Gut Liv. 2018;12:367‐368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Majima A, Handa O, Naito Y, et al. Early‐stage gastric cancer can be found in improved atrophic mucosa over time from successful Helicobacter pylori eradication. Digestion. 2017;95:194‐200. [DOI] [PubMed] [Google Scholar]
- 22. Ito M, Tanaka S, Takata S, et al. Morphological changes in human gastric tumours after eradication therapy of Helicobacter pylori in a short‐term follow‐up. Aliment Pharmacol Ther. 2005;21:559‐566. [DOI] [PubMed] [Google Scholar]
- 23. Kotachi T, Ito M, Boda T, et al. Clinical significance of reddish depressed lesions observed in the gastric mucosa after Helicobacter pylori eradication. Digestion. 2018;98:48‐55. [DOI] [PubMed] [Google Scholar]
- 24. Nishizawa T, Suzuki H, Arano T, et al. Characteristics of gastric cancer detected within 1 year after successful eradication of Helicobacter pylori . J Clin Biochem Nutr. 2016;59:226‐230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shichijo S, Hirata Y. Characteristics and predictors of gastric cancer after Helicobacter pylori eradication. World J Gastroenterol. 2018;24:2163‐2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Watanabe K, Nagata N, Nakashima R, et al. Predictive findings for Helicobacter pylori‐uninfected, ‐infected and ‐eradicated gastric mucosa: validation study. World J Gastroenterol. 2013;19:4374‐4379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Correa P, Piazuelo MB. The gastric precancerous cascade. J Dig Dis. 2012;13:2‐4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kiriyama Y, Tahara T, Shibata T, et al. Gastric‐and‐intestinal mixed intestinal metaplasia is irreversible point with eradication of Helicobacter pylori . Open J Pathol. 2016;6:93‐104. [Google Scholar]
- 29. Tahara S, Tahara T, Tuskamoto T, et al. Morphologic characterization of residual DNA methylation in the gastric mucosa after Helicobacter pylori eradication. Cancer Med. 2017;6:1730‐1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chen HN, Wang Z, Li X, Zhou ZG. Helicobacter pylori eradication cannot reduce the risk of gastric cancer in patients with intestinal metaplasia and dysplasia: evidence from a meta‐analysis. Gastric Cancer. 2016;19:166‐175. [DOI] [PubMed] [Google Scholar]
- 31. Hwang YJ, Kim N, Lee HS, et al. Reversibility of atrophic gastritis and intestinal metaplasia after Helicobacter pylori eradication– a prospective study for up to 10 years. Aliment Pharmacol Ther. 2018;47:380‐390. [DOI] [PubMed] [Google Scholar]
- 32. Rugge M, Meggio A, Pravadelli C, et al. Gastritis staging in the endoscopic follow‐up for the secondary prevention of gastric cancer: a 5‐year prospective study of 1755 patients. Gut. 2018;68(1):11–17. 10.1136/gutjnl-2017-314600. [DOI] [PubMed] [Google Scholar]
- 33. Schlemper RJ, Itabashi M, Kato Y, et al. Differences in diagnostic criteria for gastric carcinoma between Japanese and western pathologists. Lancet. 1997;349(9067):1725‐1729. [DOI] [PubMed] [Google Scholar]
- 34. Schlemper RJ, Riddell RH, Kato Y, et al. The Vienna classification of gastrointestinal epithelial neoplasia. Gut. 2000;47:251‐255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tsukuma H, Oshima A, Narahara H, Morii T. Natural history of early gastric cancer: a non‐concurrent, long term, follow up study. Gut. 2000;47:618‐621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Leung WK, Wong I, Cheung KS, et al. Effects of Helicobacter pylori treatment on incidence of gastric cancer in older individuals. Gastroenterology. 2018;155:67‐75. [DOI] [PubMed] [Google Scholar]
- 37. Iijima K, Koike T, Shimosegawa T. Reflux esophagitis triggered after Helicobacter pylori eradication: a noteworthy demerit of eradication therapy among the Japanese? Front Microbiol. 2015;6:566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Minatsuki C, Yamamichi N, Shimamoto T, et al. Background factors of reflux esophagitis and non‐erosive reflux disease: a cross‐sectional study of 10,837 subjects in Japan. PLoS ONE. 2013;8:e69891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lee YC, Chen TH, Chiu HM, et al. The benefit of mass eradication of Helicobacter pylori infection: a community–based study of gastric cancer prevention. Gut. 2013;62:676‐682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Nam SY, Choi IJ, Ryu KH, Kim BC, Kim CG, Nam BH. Effect of Helicobacter pylori infection and its eradication on reflux esophagitis and reflux symptoms. Am J Gastroenterol. 2010;105:2153‐2162. [DOI] [PubMed] [Google Scholar]
- 41. Ahn JS, Eom CS, Jeon CY, Park SM. Acid suppressive drugs and gastric cancer: a meta‐analysis of observational studies. World J Gastroenterol. 2013;19:2560‐2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tran‐Duy A, Spaetgens B, Hoes AW, de Wit NJ, Stehouwer C. Use of proton pump inhibitors and risks of fundic gland polyps and gastric cancer: systematic review and meta‐analysis. Clin Gastroenterol Hepatol. 2016;14:1706‐1719. [DOI] [PubMed] [Google Scholar]
- 43. Cheung KS, Leung WK. Long‐term use of proton‐pump inhibitors and risk of gastric cancer: a review of the current evidence. Therap Adv Gastroenterol. 2019;12:1756284819834511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Cheung KS, Chan EW, Wong A, Chen L, Wong I, Leung WK. Long‐term proton pump inhibitors and risk of gastric cancer development after treatment for Helicobacter pylori: a population‐based study. Gut. 2018;67:28‐35. [DOI] [PubMed] [Google Scholar]
- 45. Cheung KS, Chan EW, Wong A, et al. Aspirin and risk of gastric cancer after Helicobacter pylori eradication: a territory‐wide study. J Natl Cancer Inst. 2018;110:743‐749. [DOI] [PubMed] [Google Scholar]
- 46. Cheung KS, Leung WK. Modification of gastric cancer risk associated with proton pump inhibitors by aspirin after Helicobacter pylori eradication. Oncotarget. 2018;9:36891‐36893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Jankowski J, de Caestecker J, Love SB, et al. Esomeprazole and aspirin in Barrett's oesophagus (AspECT): a randomised factorial trial. Lancet. 2018;392(10145):400‐408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. García Rodríguez LA, Martín‐Pérez M, Hennekens CH, Rothwell PM, Lanas A. Bleeding risk with long‐term low‐dose aspirin: a systematic review of observational studies. PLoS ONE. 2016;11:e0160046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Chen S, Ying L, Kong M, Zhang Y, Li Y. The prevalence of Helicobacter pylori infection decreases with older age in atrophic gastritis. Gastroenterol Res Pract. 2013;2013:494783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Mizuno S, Miki I, Ishida T, et al. Prescreening of a high‐risk group for gastric cancer by serologically determined Helicobacter pylori infection and atrophic gastritis. Dig Dis Sci. 2010;55:3132‐3137. [DOI] [PubMed] [Google Scholar]
- 51. Watabe H, Mitsushima T, Yamaji Y, et al. Predicting the development of gastric cancer from combining Helicobacter pylori antibodies and serum pepsinogen status: a prospective endoscopic cohort study. Gut. 2005;54:764‐768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bik EM, Eckburg PB, Gill SR, et al. Molecular analysis of the bacterial microbiota in the human stomach. Proc Natl Acad Sci USA. 2006;103:732‐737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Liu X, Shao L, Liu X, et al. Alterations of gastric mucosal microbiota across different stomach microhabitats in a cohort of 276 patients with gastric cancer. EBioMedicine. 2019;40:336-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Castaño‐Rodríguez N, Goh KL, Fock KM, Mitchell HM, Kaakoush NO. Dysbiosis of the microbiome in gastric carcinogenesis. Sci Rep. 2017;7:15957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ferreira RM, Pereira‐Marques J, Pinto‐Ribeiro I, et al. Gastric microbial community profiling reveals a dysbiotic cancer‐associated microbiota. Gut. 2018;67:226‐236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Park CH, Lee AR, Lee YR, Eun CS, Lee SK, Han DS. Evaluation of gastric microbiome and metagenomic function in patients with intestinal metaplasia using 16S rRNA gene sequencing. Helicobacter. 2018;24(1):e12547. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


