Abstract
Background
FIGHTDIGO study has shown the feasibility of handgrip strength (HGS) measurements in 201 consecutive digestive cancer patients undergoing chemotherapy.
Objective
This study focuses on a secondary aim of FIGHTDIGO study: the relationship between muscle mass and HGS.
Design
Two consecutive bilateral measures of HGS were performed using a Jamar dynamometer before the start of each chemotherapy. The highest value was chosen for final evaluation. Dynapenia (loss of muscle strength) was defined as HGS < 30 kg (men) and < 20 kg (women). Muscle mass was measured at lumbar level (L3) on Computed Tomography (CT) scans performed less than 3 weeks before or after the measurement of HGS. Muscle mass loss was defined by skeletal muscle index (SMI) < 53 cm2/m2 (in men with a body mass index (BMI)> 25 kg/m2), < 43 cm2/m2 (in men with a BMI < 25 kg/m2), and < 41 cm2/m2 (in women regardless of BMI). Sarcopenia was defined by the association of a dynapenia and a loss of muscle mass.
Results
A total of 150 patients were included in this analysis (mean age: 65.6 ± 10.9 years, 87 males (58%), colorectal cancer (47.3%), metastatic stage (76.7%)). A total of 348 CT scans were evaluated. For the 348 measurements, mean SMI and HGS were 41.8 ± 8.7 cm2/m2 and 32.1 ± 11.0 kg, respectively. Muscle mass loss, dynapenia, or sarcopenia were reported at least once, in 120 (80%), 45 (30%), and 30 (20%) patients, respectively. SMI was significantly correlated with HGS (Pearson coefficient = 0.53, P < 0.0001). At concordance analysis, 188 dyad SMI/HGS (54%) were in agreement (Kappa = 0.14 [95% CI, 0.07‐0.21]).
Conclusion
Correlation between the measurements of HGS and SMI is strong but the concordance between dynapenia and muscle mass loss is poor. Further studies should be performed to confirm the diagnostic thresholds, and to study the chronology of dynapenia and loss of muscle mass.
Keywords: dynapenia, sarcopenia, muscle strength, muscle mass, digestive system neoplasms
1. INTRODUCTION
The association between denutrition and cancer has become a major concern worldwide during the last century.1 Digestive cancers are among the ten most diagnosed cancers.2 In this specific population, malnutrition is common, and is present in about 39% of colorectal cancers, 44% of esophagus and/or stomach cancers, and 67% of pancreatic cancers.3 As malnutritrion is responsible for a high morbidity and mortality rate, its screening and treatment are of paramount importance.4
Definition of malnutrition relies on a low body mass index (BMI), and/or on an unintentional weight loss, and/or on hypoalbuminemia without any inflammatory syndrome.5, 6 However, these three criteria do not totally reflect the complex physiopathology of malnutrition and its impact. For many years, more investigations have been carried out in the area of muscle mass loss, which occurs in 80% of patients with cancers, and is a first step towards malnutrition.7
Muscle mass alone cannot be interpreted without taking into account its function, which is represented by muscle strength. Sarcopenia is defined as the association of age‐related loss in skeletal muscle mass, as well as loss of muscle function (dynapenia or performance).8, 9
Handgrip strength (HGS) evaluation using a handgrip dynamometer has been widely studied. Strength physiologically declines with age.10, 11, 12 Weak strength, called dynapenia,13 predicts the risk of mortality from all causes when measured during mid‐life in general population.10 Moreover, dynapenia seems to be a factor of disability,14 nosocomial infection,15 and length of hospital stay 16 in elderly people. In oncological context, low HGS is associated with cancer‐related fatigue,17 poor quality of life,18 postoperative complications,19 chemotherapy toxicity, 20 and high mortality.21 The FIGHTDIGO study has shown the feasibility and acceptability of routine HGS measurements in digestive cancer patients undergoing ambulatory chemotherapy.4 The association between pretherapeutic dynapenia and chemotherapy‐induced dose‐limiting neurotoxicity has also been reported.22
Currently, the relationship between muscle mass and muscle strength remains poorly known in oncology. The second aim of the FIGHTDIGO study was to analyze the relationship between the HGS evaluated using Jamar dynamometer and the muscle mass evaluated on Computed‐Tomography (CT) scan.
2. MATERIALS AND METHODS
2.1. Study design and participants
The prospective monocentric FIGHTDIGO study was conducted in the ambulatory cancer unit (UMA‐CH) in Reims teaching hospital (CHU) in France.4 Study population included patients older than 18 years of age, having a primary digestive cancer regardless of its stage, and undergoing cytotoxic chemotherapy and/or biotherapy. Patients who could not give their consent, did not understand the HGS, had a history of neuro‐muscular disorder and/or had appointed a health care proxy, or whose CT scanner could not be analyzed were excluded. The patients were recruited from May 18, 2016 to November 18, 2016, and then were followed up for 6 months. They were asked to perform the HGS test on each of their appointment to the unit, before the start of their treatment (every week, every two weeks or more, depending on the chemotherapy regimen).
Patients performed CT scans during this period in routine care. In this study, only the CT scans performed less than three weeks before or after a HGS test were analyzed.
2.2. Ethical approval
Informed written consent was obtained for each enrolled patient in the trial. The FIGHTDIGO study was approved by the ethics committee (Committee for the Protection of Person EST I DIJON, March 25.2016) and was registered June 13, 2016 in Clinicaltrials.gov (NCT02797197).
2.3. Outcome
The aims were to evaluate the quantitative correlation between the measurements of HGS using Jamar dynamometer and muscle mass on the CT scans, and also the qualitative concordance between dynapenia, muscle mass loss, and sarcopenia according to consensual classification.9
2.4. HGS measurement
HGS was measured with a hydraulic Jamar dynamometer. Position 2 was used among the 5 possible handle‐positions. Every patient was seated comfortably in a chair. The shoulder of the upper limb holding the dynamometer was in an adduction position, the elbow flexed to 90 degrees, and the forearm and wrist in a neutral position. The other upper limb was placed alongside the body and relaxed. During the examination, patients were verbally encouraged as to try and obtain their best score. Four measurements were determined: each one had to last three seconds; patients had to perform the first two measurements in a row: one with the dominant hand and the other one with the non‐dominant hand; a one‐minute break was then respected before repeating the last two measurements. The highest value was chosen for final evaluation. According to the European Working Group on Sarcopenia, dynapenia was defined as HGS < 30kg (men) and < 20 kg (women).8
2.5. CT scans and muscle mass assessment
Body mass index (BMI) was calculated [weight (kg)/height (m2)]. Skeletal muscle index (SMI) was defined as total muscle area (TMA) measured on an axial section of CT scan through the third lumbar vertebra (L3), at a level where both pedicles were visible using a preestablished density threshold (−29 to + 150 Hounsfield units) for skeletal muscles. SMI was then normalized to height and expressed in cm2/m2.23 The muscles in the L3 region include psoas, erector spinae, quadratus lumborum, transversus abdominis, external oblique, internal obliques, and rectus abdominis muscles.24, 25, 26, 27, 28, 29 After a period of training with a radiologist, a resident in gastroenterology who was unaware of the HGS values, measured TMA (cm2) using manual segmentation on a dedicated posttreatment station. ImageJ software v1.46r, a free public domain software developed by the National Institute of Health (NIH) was used (Figure 1).30, 31 According to the definition proposed by Martin et al, patients were considered as presenting a loss of muscle mass as follows: SMI (TMA at L3 divided by height squared) < 53 cm2/m2 in men with a BMI > 25 kg/m2, < 43 cm2/m2 in men with a BMI < 25 kg/m2, and < 41 cm2/m2 in women regardless of their BMI.23
Figure 1.
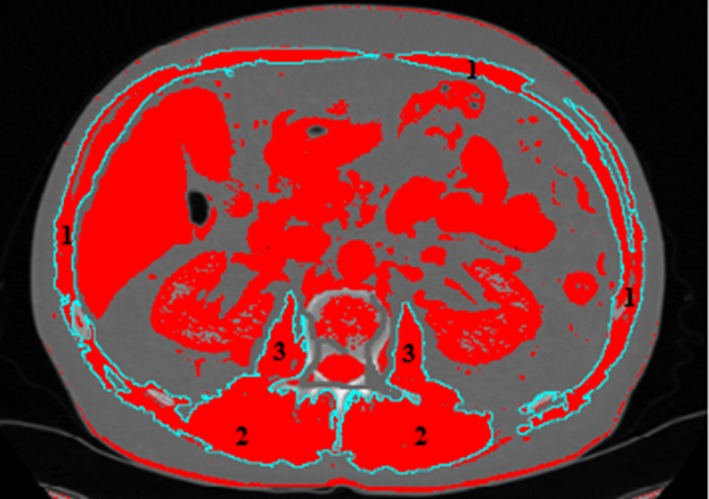
Selection of lumbar muscle areas with specific thresholds at third lumbar vertebra (L3). Regions of interest (inturquoise blue) corresponding to rectus abdominis, quadratus lumborum, transversus abdominis, external and internal oblique (1), paraspinal (2), and psoas muscles (3). Image from ImageJ software
2.6. Association between muscle mass and HGS
To perform this correlation, SMI measurements performed on CT examinations were associated with the HGS measurements using binomial analysis. The delay between the two measurements should be less than 3 weeks. Patients were considered to be sarcopenic in case of an association of both dynapenia and muscle mass loss.
2.7. Statistical analysis
Quantitative variables were expressed as mean ± standard deviation (SD) or as median +[range], and qualitative variables as numbers (percentages).
Patient's characteristics were compared between patients with and without available CT scans in an univariate analysis using Students’ t tests for continuous variables or using Fisher exact tests for qualitative variables.
Association between SMI and HGS was studied using Pearson's correlation coefficient. Concordance between dynapenia and loss of muscle mass was analyzed using Kappa (κ) coefficient with κ values of 0.00‐0.20 indicating "poor", 0.21‐0.40 indicating "fair", 0.41‐0.60 indicating "moderate", 0.61‐0.80 indicating "good", and 0.81‐1.00 indicating "excellent" agreement. A P value < 0.05 was considered statistically significant. All analyses were performed using SAS version 9.4 (SAS Inc, Cary, NC).32
3. RESULTS
3.1. Description of the population
Among the 201 consecutive patients included in FIGHTDIGO study, CT scan examinations were unavailable in 51 patients. Finally, 348 CT scans were analyzed in 150 patients as shown in the patient flow chart (Figure 2). Table 1 shows baseline patient characteristics. Mean age was 65.6 (± 10.9) years. Colo‐rectal cancer was the most frequent cancer (n = 71, 47.3%). The majority of the patients was treated for metastatic tumor (n = 115, 76.7%).
Figure 2.
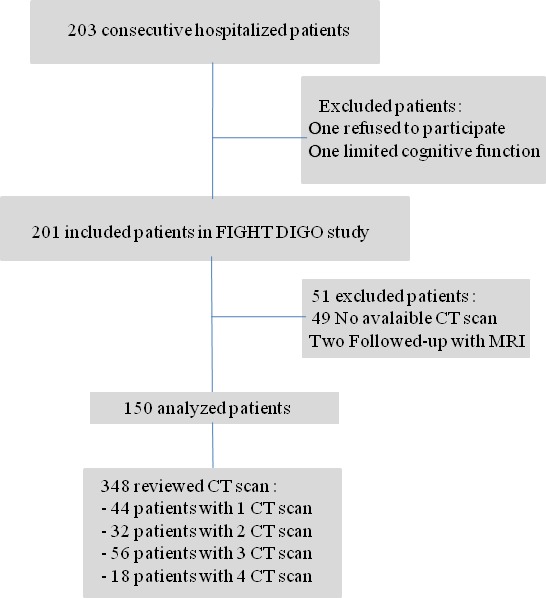
Patient flow chart: patient identification, inclusion, and exclusion steps
Table 1.
Baseline population characteristics
| Characteristics | FIGHTDIGO Population (n=201) | Patients with at least one assessable CT scan (n=150) | No assessable CT scan (n=51) | Univariate Analysis P value |
|---|---|---|---|---|
| Age, mean ± SD | 65.5 ± 10.8 | 65.6 ± 10.9 | 65.1 ± 10.8 | 0.80 |
| Gender, N (%) | 0.92 | |||
| Men | 117 (58.2) | 87 (58.0) | 30 (58.8) | |
| Women | 82 (41.8) | 63 (42.0) | 21 (41.2) | |
| BMI, mean ±SD | 25.0 ± 5.1 | 24.9 ± 4.9 | 25.3 ± 5.5 | 0.56 |
| Leg edema, N (%) | 4 (2.0) | 4 (2.7) | 0 (0.0) | 0.57 |
| Serum albumin, mean ±SD | 38.6 ± 4.5 | 38.4 ± 4.6 | 39.4 ± 4.1 | 0.15 |
| C‐reactive protein, mean ±SD | 15.9 ± 32.8 | 17.8 ± 37.1 | 11.1 ± 15.5 | 0.09 |
| Previous oncologic surgery, N (%) | 128 (63.7) | 87 (58.0) | 41 (80.4) | 0.004 |
| mGPS, N (%) | 0.29 | |||
| 0 | 99 (55.0) | 68 (51.5) | 31 (64.6) | |
| 1 | 68 (37.8) | 54 (40.9) | 14 (29.2) | |
| 2 | 13 (7.2) | 10 (7.6) | 2 (6.2) | |
| G8 score, mean ± SD | 12.4 ± 2.1 | 12.1 ± 2.6 | 13.2 ± 2.2 | 0.12 |
| Primary tumor, N (%) | 0.35 | |||
| Colon and rectum | 103 (51.2) | 71 (47.3) | 32 (62.7) | |
| Oesphagus | 8 (4.0) | 5 (3.3) | 3 (5.9) | |
| Stomach | 22 (10.9) | 16 (10.7) | 6 (11.8) | |
| Cholangiocarcinoma | 11 (5.5) | 9 (6.0) | 2 (3.9) | |
| Pancreas | 44 (21.9) | 38 (25.3) | 6 (11.8) | |
| Small intestine | 3 (1.5) | 2 (1.3) | 1 (2.0) | |
| Neuroendocrine tumor | 8 (4.0) | 7 (4.7) | 1 (2.0) | |
| Adenocarcinoma, unknown primitive tumor | 2 (1.0) | 2 (1.3) | 0 (0.0) | |
| Stage, N (%) | <0.0001 | |||
| Local | 40 (19.9) | 19 (12.7) | 19 (41.2) | |
| Locally advanced | 23 (11.4) | 16 (10.7) | 7 (13.7) | |
| Metastatic | 138 (68.7) | 115 (76.7) | 23 (45.1) | |
| Type of treatment, N (%) | 0.004 | |||
| Chemotherapy alone | 147 (73.1) | 101 (67.3) | 46 (90.2) | |
| Biotherapy alone | 4 (2.0) | 4 (2.7) | 0 (0.0) | |
| Chemotherapy and biotherapy | 50 (24.9) | 45 (30.0) | 5 (9.8) | |
| Indication, N (%) | <0.0001 | |||
| Adjuvant | 47 (23.4) | 23 (15.3) | 24 (47.1) | |
| Neoadjuvant | 10 (5.0) | 7 (4.7) | 3 (5.9) | |
| Palliative | 144 (71.6) | 120 (80.0) | 24 (47.1) | |
| First line, N (%) | 132 (65.2) | 92 (61.3) | 40 (78.4) | 0.03 |
Abbreviations: BMI, body mass index; mGPS, modified Glasgow Prognostic Score; N, number; SD, standard deviation.
Comparison between baseline characteristics of patients with at least one available CT scan (n = 150) and of patients without available CT scan (n = 51) are showed in Table 1. Patients with at least one available CT scan had significantly more metastatic tumors (76.7% versus 45.1%; P < 0.0001), had had previous surgery more often (58.0% versus 80.4%; P = 0.004), were more often treated with biotherapy (32.7% versus 9.8%; P = 0.001), were more often undergoing palliative treatment (80.0% vs 47.1%; P < 0.0001), and were less often undergoing first line treatment (61.3% vs 78.4%; P = 0.03).
3.2. Relationship between HGS and muscle mass.
A total of 348 dyad HGS/CT scan were analyzed. The number of CT scans per patient is shown in Figure 2. Forty‐four patients had only one CT scan and only 18 patients had four CT scans. The median time between the measurements of HGS and SMI was 8 days [0 ; 21].
SMI and HGS quantitative and qualitative results are reported in Table 2. For the 348 measurements, mean SMI and HGS were respectively 41.8 (± 8.7) cm2/m2 and 32.1 (± 11.0) kg. Among the 150 studied patients, muscle mass loss, dynapenia, or sarcopenia were reported at least once, in 120 (80%), 45 (30%), and 30 (20%) patients, respectively.
Table 2.
Skeletal muscle index (SMI) and handgrip muscle strength (HGS) results (348 measurements in 150 patients)
| Dyad CT scan/HGS | |
|---|---|
| SMI, mean± SD | 41.8 ± 8.7 |
| SMI loss, N (%) | 58 (16.7) |
| HGS, mean± SD | 32.1 ± 11.0 |
| HGS loss (dynapenia), N (%) | 192 (55.2) |
| Sarcopenia (HGS and muscle mass loss), N (%) | 50 (14.4) |
Abbreviations: HGS, handgrip strength; SMI, skeletal muscle index.
SMI was correlated with HGS (r = 0.53, P < 0.0001) (Figure 3). At concordance analysis, 188 dyad SMI/HGS (54%) were in agreement (κ = 0.14 [95% CI), 0.07‐0.21]). Dynapenia and loss of muscle mass were present in 45 dyads (12.9% of the 348 dyads). Dynapenia and loss of muscle mass were absent in 143 dyads (41.1% of the 348 dyads). Of the 348 analyzed CT scan, eight patients had edema without any significant influence on SMI (38.7 ± 8.3 cm2/m2 for patients with edema versus 41.9 ± 8.7 cm2/m2 for patients without edema, respectively ( P = 0.24).
Figure 3.
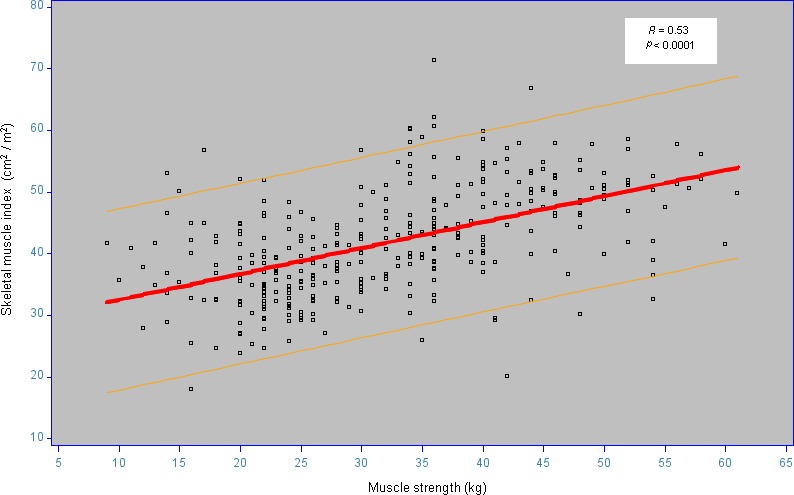
Correlation between handgrip muscle strength (HGS) and skeletal muscle index (SMI). The regression curve is in red. The yellow curves surround 95% of values. The letter r is Pearson coefficient
4. DISCUSSION
To our knowledge, this is the first study to evaluate and show a good correlation between the measurement of HGS and the muscle mass, both pivotal parameters of sarcopenia definition,8, 9 in cancer patients undergoing a cytotoxic chemotherapy. SMI was indeed positively correlated with HGS (r = 0.53, P < 0.0001). In contrast, the concordance was poor between dynapenia and muscle mass loss according to consensual classification. Muscle mass loss was more frequently present than dynapenia.
Another study evaluating the relationship between HGS and muscle mass suggested that high levels of HGS may not be closely related to greater muscle mass,33 nor low levels of HGS related to muscle mass loss. However, HGS was positively associated with the dependent variable (muscle mass).33 The population in that study was different from ours as it involved women survivors of breast cancer in Columbia). Moreover, another technique, namely tetrapolar bioelectrical impedance analysis was used to determine muscle mass 33 as well as the fatty mass (% and kg), the muscle mass (% and kg), and the total mass (kg). Furthermore, no patient had clinically detectable edema, a condition that could have affected resistance and reactance.
The weak agreement between SMI and HGS confirms that the issue of sarcopenia and dynapenia cutoffs and measurement methods, which vary across literature, have not yet been unanimously established.8, 12, 23, 24, 29, 34, 35, 36, 37, 38
Measurement of SMI is only quantitative and does not take into account the quality of the muscle. Edema could overrate SMI measurements. We did not evidence any impact of edema on the SMI though, but the number of patients with edema was very low in our series (8 out of 150). Skeletal muscle density (SMD) reflects muscular quality. Dolan et al showed that low SMI and myosteatosis (low SMD) were significantly associated with survival in colorectal patients undergoing surgery.39 Yet, they did not study muscle strength although it is noteworthy that sarcopenia is defined by a loss of strength and muscle mass.8, 9
Since SMI obtained with CT scan is the gold standard evaluating sarcopenia, but is burdened by its costs and ionizing radiation exposure.40, 41, 42 Alternate methods for measurement of muscle mass may be used including as dual energy X‐ray or tetrapolar bioelectrical impedance analysis.33, 43
Several studies have examined the relationship between evaluation of the different components of the diagnosis of sarcopenia diagnosis,33, 44, 45, 46, 47, 48, 49, 50 yet the relationship and chronology between muscle strength and muscle mass is still poorly known. The analysis of this chronology was not planned to be included in the FIGHTDIGO study because CT examinations were not performed in a systematic way. The time interval between the CT scans were indeed very variable and only 18 patients had 4 CT scans (maximum number of performed CT examination). Moreover, the patients were included at different time points during their follow‐up and were likely to have received several cycles and lines of chemotherapy before the measurements. Muscle mass loss appeared to be far more frequent (80%) than dynapenia (30%) and sarcopenia (20%). Loss of skeletal muscle mass could therefore precede that of muscle strength.
Limitations of this study were the heterogeneity of the population, the sub‐group analysis, and the small number of chemo‐naïve patients undergoing CT follow‐up.
In conclusion, correlation between measurements of HGS and SMI was strong but the concordance between dynapenia and muscle mass loss according to consensual classification was poor. It was very interesting how an easily measurable clinical tool like HGS correlated well with SMI. Muscle mass loss could precede dynapenia. Further studies should be performed so as to confirm diagnostic threshold values of the different modalities and to study the chronology of dynapenia and of muscle mass loss.
Moreau J, Ordan M‐A, Barbe C, et al. Correlation between muscle mass and handgrip strength in digestive cancer patients undergoing chemotherapy. Cancer Med. 2019;8:3677–3684. 10.1002/cam4.2238
REFERENCES
- 1. Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–386. [DOI] [PubMed] [Google Scholar]
- 2. World Health Organization. Cancer [Internet]. http://www.who.int/mediacentre/factsheets/fs297/en/. Accessed Aug 4, 2017
- 3. Hébuterne X, Lemarié E, Michallet M, de Montreuil CB, Schneider SM, Goldwasser F. Prevalence of malnutrition and current use of nutrition support in patients with cancer. JPEN J Parenter Enteral Nutr. 2014;38:196–204. [DOI] [PubMed] [Google Scholar]
- 4. Ordan M‐A, Mazza C, Barbe C, et al. Feasibility of systematic handgrip strength testing in digestive cancer patients treated with chemotherapy: The FIGHTDIGO study. Cancer. 2018;124:1501–1506. [DOI] [PubMed] [Google Scholar]
- 5. Cederholm T, Bosaeus I, Barazzoni R, et al. Diagnostic criteria for malnutrition ‐ An ESPEN Consensus Statement. Clin Nutr Edinb Scotl. 2015;34:335–340. [DOI] [PubMed] [Google Scholar]
- 6. McMillan DC. The systemic inflammation‐based Glasgow Prognostic Score: a decade of experience in patients with cancer. Cancer Treat Rev. 2013;39:534–540. [DOI] [PubMed] [Google Scholar]
- 7. Cohen S, Nathan JA, Goldberg AL. Muscle wasting in disease: molecular mechanisms and promising therapies. Nat Rev Drug Discov. 2015;14:58–74. [DOI] [PubMed] [Google Scholar]
- 8. Cruz‐Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39:412–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cruz‐Jentoft AJ, Landi F, Schneider SM, et al. Prevalence of and interventions for sarcopenia in ageing adults: a systematic review. Report of the International Sarcopenia Initiative (EWGSOP and IWGS). Age Ageing. 2014;43:748–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rantanen T, Harris T, Leveille SG, et al. Muscle strength and body mass index as long‐term predictors of mortality in initially healthy men. J Gerontol A Biol Sci Med Sci. 2000;55:M168–173. [DOI] [PubMed] [Google Scholar]
- 11. Norman K, Stobäus N, Gonzalez MC, Schulzke J‐D, Pirlich M. Hand grip strength: outcome predictor and marker of nutritional status. Clin Nutr Edinb Scotl. 2011;30:135–142. [DOI] [PubMed] [Google Scholar]
- 12. Dodds RM, Syddall HE, Cooper R, Kuh D, Cooper C, Sayer AA. Global variation in grip strength: a systematic review and meta‐analysis of normative data. Age Ageing. 2016;45:209–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Clark BC, Manini TM. Sarcopenia =/= dynapenia. J Gerontol A Biol Sci Med Sci. 2008;63:829–834. [DOI] [PubMed] [Google Scholar]
- 14. Rantanen T, Guralnik JM, Foley D, et al. Midlife hand grip strength as a predictor of old age disability. JAMA. 1999;281:558–560. [DOI] [PubMed] [Google Scholar]
- 15. Cosquéric G, Sebag A, Ducolombier C, Thomas C, Piette F, Weill‐Engerer S. Sarcopenia is predictive of nosocomial infection in care of the elderly. Br J Nutr. 2006;96:895–901. [DOI] [PubMed] [Google Scholar]
- 16. Kerr A, Syddall HE, Cooper C, Turner GF, Briggs RS, Sayer AA. Does admission grip strength predict length of stay in hospitalised older patients? Age Ageing. 2006;35:82–84. [DOI] [PubMed] [Google Scholar]
- 17. Kilgour RD, Vigano A, Trutschnigg B, et al. Cancer‐related fatigue: the impact of skeletal muscle mass and strength in patients with advanced cancer. J Cachexia Sarcopenia Muscle. 2010;1:177–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Norman K, Stobäus N, Smoliner C, et al. Determinants of hand grip strength, knee extension strength and functional status in cancer patients. Clin Nutr Edinb Scotl. 2010;29:586–591. [DOI] [PubMed] [Google Scholar]
- 19. Chen C‐H, Ho‐Chang, Huang Y‐Z, Hung T‐T. Hand‐grip strength is a simple and effective outcome predictor in esophageal cancer following esophagectomy with reconstruction: a prospective study. J Cardiothorac Surg. 2011;6:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Prado C, Baracos VE, McCargar LJ, et al. Sarcopenia as a determinant of chemotherapy toxicity and time to tumor progression in metastatic breast cancer patients receiving capecitabine treatment. Clin Cancer Res. 2009;15:2920–2926. [DOI] [PubMed] [Google Scholar]
- 21. Kilgour RD, Vigano A, Trutschnigg B, Lucar E, Borod M, Morais JA. Handgrip strength predicts survival and is associated with markers of clinical and functional outcomes in advanced cancer patients. Support Care Cancer. 2013;21:3261–3270. [DOI] [PubMed] [Google Scholar]
- 22. Botsen D, Ordan M‐A, Barbe C, et al. Dynapenia could predict chemotherapy‐induced dose‐limiting neurotoxicity in digestive cancer patients. BMC Cancer. 2018;18:955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Martin L, Birdsell L, Macdonald N, et al. Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol. 2013;31:1539–1547. [DOI] [PubMed] [Google Scholar]
- 24. Prado C, Lieffers JR, McCargar LJ, et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population‐based study. Lancet Oncol. 2008;9:629–635. [DOI] [PubMed] [Google Scholar]
- 25. Fearon K, Strasser F, Anker SD, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. 2011;12:489–495. [DOI] [PubMed] [Google Scholar]
- 26. Huang D‐D, Wang S‐L, Zhuang C‐L, et al. Sarcopenia, as defined by low muscle mass, strength and physical performance, predicts complications after surgery for colorectal cancer. Colorectal Dis. 2015;17:O256–264. [DOI] [PubMed] [Google Scholar]
- 27. Moryoussef F, Dhooge M, Volet J, et al. Reversible sarcopenia in patients with gastrointestinal stromal tumor treated with imatinib. J Cachexia Sarcopenia Muscle. 2015;6:343–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Richards CH, Roxburgh C, MacMillan MT, et al. The relationships between body composition and the systemic inflammatory response in patients with primary operable colorectal cancer. PLoS ONE. 2012;7:e41883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gibson DJ, Burden ST, Strauss BJ, Todd C, Lal S. The role of computed tomography in evaluating body composition and the influence of reduced muscle mass on clinical outcome in abdominal malignancy: a systematic review. Eur J Clin Nutr. 2015;69:1079–1086. [DOI] [PubMed] [Google Scholar]
- 30. Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9:671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gomez‐Perez SL, Haus JM, Sheean P, et al. Measuring abdominal circumference and skeletal muscle from a single cross‐sectional computed tomography image: A step‐by‐step guide for clinicians using National Institutes of Health ImageJ. JPEN J Parenter Enteral Nutr. 2016;40:308–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Statistical F. Methods for Rates and Proportions, 2nd edn, vol. 336 New York: Wiley, John and Sons, Incorporated; 1981:p.. [Google Scholar]
- 33. Benavides‐Rodríguez L, García‐Hermoso A, Rodrigues‐Bezerra D, Izquierdo M, Correa‐Bautista JE, Ramírez‐Vélez R. Relationship between handgrip strength and muscle mass in female survivors of breast. Cancer: A Mediation Analysis. Nutrients. 2017;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sampaio R, Sampaio P, Castaño L, et al. Cutoff values for appendicular skeletal muscle mass and strength in relation to fear of falling among Brazilian older adults: cross‐sectional study. Sao Paulo Med J. 2017;135:434–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hanaoka M, Yasuno M, Ishiguro M, et al. Morphologic change of the psoas muscle as a surrogate marker of sarcopenia and predictor of complications after colorectal cancer surgery. Int J Colorectal Dis. 2017;32:847–856. [DOI] [PubMed] [Google Scholar]
- 36. Mourtzakis M, Prado C, Lieffers JR, Reiman T, McCargar LJ, Baracos VE. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metab. 2008;33:997–1006. [DOI] [PubMed] [Google Scholar]
- 37. Baracos VE. Psoas as a sentinel muscle for sarcopenia: a flawed premise. J Cachexia Sarcopenia Muscle. 2017;8:527–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rutten I, Ubachs J, Kruitwagen R, Beets‐Tan R, Olde Damink S, Van Gorp T. Psoas muscle area is not representative of total skeletal muscle area in the assessment of sarcopenia in ovarian cancer. J Cachexia Sarcopenia Muscle. 2017;8:630–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Dolan RD, Almasaudi AS, Dieu LB, Horgan PG, McSorley ST, McMillan DC. The relationship between computed tomography‐derived body composition, systemic inflammatory response, and survival in patients undergoing surgery for colorectal cancer. J Cachexia Sarcopenia Muscle. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Brenner DJ, Hall E. Computed tomography — An increasing source of radiation exposure. N Engl J Med. 2007;357:2277–2284. [DOI] [PubMed] [Google Scholar]
- 41. MacDonald AJ, Greig CA, Baracos V. The advantages and limitations of cross‐sectional body composition analysis. Curr Opin Support Palliat Care. 2011;5:342–349. [DOI] [PubMed] [Google Scholar]
- 42. Malietzis G, Aziz O, Bagnall NM, Johns N, Fearon KC, Jenkins JT. The role of body composition evaluation by computerized tomography in determining colorectal cancer treatment outcomes: a systematic review. Eur J Surg Oncol. 2015;41:186–196. [DOI] [PubMed] [Google Scholar]
- 43. Prado C, Lieffers JR, Bowthorpe L, Baracos VE, Mourtzakis M, McCargar LJ. Sarcopenia and physical function in overweight patients with advanced cancer. Can J Diet Pract Res. 2013;74:69–74. [DOI] [PubMed] [Google Scholar]
- 44. Rier HN, Jager A, Sleijfer S, van Rosmalen J, Kock M, Levin M‐D. Low muscle attenuation is a prognostic factor for survival in metastatic breast cancer patients treated with first line palliative chemotherapy. Breast Edinb Scotl. 2017;31:9–15. [DOI] [PubMed] [Google Scholar]
- 45. Biolo G, Di Girolamo FG, Breglia A, et al. Inverse relationship between “a body shape index” (ABSI) and fat‐free mass in women and men: Insights into mechanisms of sarcopenic obesity. Clin Nutr Edinb Scotl. 2015;34:323–327. [DOI] [PubMed] [Google Scholar]
- 46. Glass DJ. Signaling pathways perturbing muscle mass. Curr Opin Clin Nutr Metab Care. 2010;13:225–229. [DOI] [PubMed] [Google Scholar]
- 47. Yeh SS, Schuster MW. Geriatric cachexia: the role of cytokines. Am J Clin Nutr. 1999;70:183–197. [DOI] [PubMed] [Google Scholar]
- 48. Kalinkovich A, Livshits G. Sarcopenic obesity or obese sarcopenia: A cross talk between age‐associated adipose tissue and skeletal muscle inflammation as a main mechanism of the pathogenesis. Ageing Res Rev. 2017;35:200–221. [DOI] [PubMed] [Google Scholar]
- 49. Sakuma K, Yamaguchi A. Sarcopenia and cachexia: the adaptations of negative regulators of skeletal muscle mass. J Cachexia Sarcopenia Muscle. 2012;3:77–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Biolo G, Cederholm T, Muscaritoli M. Muscle contractile and metabolic dysfunction is a common feature of sarcopenia of aging and chronic diseases: from sarcopenic obesity to cachexia. Clin Nutr Edinb Scotl. 2014;33:737–748. [DOI] [PubMed] [Google Scholar]


