Abstract
Background
Appropriate depression care is a cancer‐care priority. However, many cancer survivors live with undiagnosed and untreated depression. Prostate cancer survivors may be particularly vulnerable, but little is known about their access to depression care. The goal of this study was to describe patterns and predictors of clinical diagnosis and treatment of depression in prostate cancer survivors.
Methods
Generalized estimating equations were used to evaluate indicators of self‐reported clinical diagnosis and treatment depression as a function of individual‐level characteristics within a longitudinal dataset. The data were from a population‐based cohort of North Carolinian prostate cancer survivors who were enrolled from 2004 to 2007 on the North Carolina‐Louisiana Prostate Cancer Project (N = 1,031), and prospectively followed annually from 2008 to 2011 on the Health Care Access and Prostate Cancer Treatment in North Carolina (N = 805).
Results
The average rate of self‐reported clinical diagnosis of depression was 44% (95% CI: 39%‐49%), which declined from 60% to 40% between prostate cancer diagnosis and 5‐7 years later. Factors associated with lower odds of self‐reported clinical diagnosis of depression include African‐American race, employment, age at enrollment, low education, infrequent primary care visits, and living with a prostate cancer diagnosis for more than 2 years. The average rate of self‐reported depression treatment was 62% (95% CI: 55%‐69%). Factors associated with lower odds of self‐reported depression treatment included employment and living with a prostate cancer diagnosis for 2 or more years.
Conclusion
Prostate cancer survivors experience barriers when in need of depression care.
Keywords: clinical diagnosis, clinical recognition, depression treatment, predictors, prostate cancer
1. INTRODUCTION
Major and persistent depressive disorders (or depression) affect one in five cancer survivors.1, 2 Adverse effects on the cost, quality, and duration of cancer survivorship justify prevention of depression as a cancer care priority.3, 4, 5, 6, 7, 8, 9, 10, 11 For example, since 2014, the American Society of Clinical Oncology recommends depression screening for all survivors at their initial visit, at appropriate intervals, and as clinically indicated, especially with changes in disease or treatment status (ie, posttreatment, recurrence, progression) and on transition to palliative and end‐of‐life care.10 Since 2015, the American College of Surgeons' Commission on Cancer required cancer centers in the United States to screen survivors for psychosocial distress.11 The 2016 US Preventive Services Task Force statement recommended depression screening and appropriate follow‐up care in all adults and emphasized that cancer survivors face higher risks for depression.12
Despite widespread support for depression care in cancer survivors, many depressed survivors live with undiagnosed or untreated depression due to low‐screening rates (4% in the general population),11, 13 poor clinician recognition,14, 15 and patients’ refusal/inability to get help.16, 17 Available evidence suggests that (a) 2‐3 in four depressed survivors do not receive a clinical diagnosis of depression; (b) 1‐2 in five survivors fail to initiate depression treatment despite having received a clinical diagnosis of depression; and (c) unequal access to depression care persists across sociodemographic groups.18, 19, 20, 21, 22, 23, 24 These gaps along the depression care pathway may impose dire consequences on the health and safety of cancer survivors.25, 26 Moreover, prostate cancer survivors may be the most vulnerable cancer survivor–group since men are usually reluctant to report depressive symptoms or seek mental health care.27, 28, 29 However, little is known about clinical diagnosis and treatment of depression in this patient population.
The overarching goal of this study was to describe rates, patterns and predictors of clinical diagnosis, and treatment of depression among prostate cancer survivors. These descriptions were based on self‐report, and the study was designed to: (a) provide insight into the reported racial difference in the risk of depression among prostate cancer survivors30; (b) describe gaps in depression care in the short‐ to medium‐term after prostate cancer diagnosis; (c) enhance the ability to plan and execute interventions that make depression care more accessible; and (d) respond to the request for “research to evaluate…the diagnostic process, and to identify, learn from and reduce diagnostic error… (including diagnosis that was unintentionally missed)” by the National Academy of Science, Engineering and Medicine's Committee on Diagnostic Error in Medicine.31
The analytic approach was informed by Klinkman's Competing demands in psychosocial care model, which provides a framework for accessing determinants of clinical diagnosis of depression, and depression treatment conditional on clinical diagnosis of depression (ie, depression care).32 The conceptual model and available evidence suggested that patients’ attributes largely determine who receives depression care.32, 33 Relevant patient attributes include (a) sociodemographic characteristics (ie, race,34 age,35 income,21 educational attainment,36 employment status,21 rural or urban residence,36 and marital status)35; (b) clinical characteristics (ie, severity of depression,37 and cancer stage at diagnosis)36; and (c) other individual‐level characteristics (ie, health insurance coverage 38, 39 Charlson comorbidity index,40 and number of annual visits to primary care clinics).41 After reviewing studies in other patient populations, we hypothesized that age, African‐American race, low education, rural residence, being unmarried, unemployment, and low annual income were negatively associated with depression care among prostate cancer survivors.34, 37, 42, 43
2. METHODS
2.1. Study population and procedure
Longitudinal data from a population‐based cohort of North Carolinian prostate cancer survivors who were enrolled from 2004 to 2007 in the North Carolina‐Louisiana Prostate Cancer Project (PCaP, N = 1031) was assessed. In brief, PCaP is a multidisciplinary study of social, individual, and tumor‐level causes of racial differences in prostate cancer aggressiveness. 44, 45 North Carolinian research subjects were incident prostate cancer cases diagnosed on or after July 1, 2004 and identified using records from the North Carolina Central Cancer Registry. Only African‐American and European‐American survivors were enrolled in equal proportions (sampling weight = 1:0.44).45 North Carolinian research subjects were contacted between September 2004 and December 2007 to obtain questionnaire data, biological specimens, and permission to obtain medical records. North Carolinian research subjects also had up to three annual follow‐up interviews on the Health Care Access and Prostate Cancer Treatment in North Carolina Study (HCaP‐NC, 2008‐2011 [N = 805]). Interview questionnaires were completed by regular mail or by phone interview for the first contact (between September 2008 and August 2009), second contact (September 2009 to August 2010) and third contact (September 2010 to August 2011). Data from 804 research subjects were analyzed. The University of North Carolina at Chapel Hill's Office of Human Research Ethics approved this study (Study # 17‐0183).
2.2. Measures
2.2.1. Identifying self‐reported clinical diagnoses and treatment of depression
During enrollment, research subjects self‐reported prior clinical diagnosis of depression, and prior/ongoing depression treatment.45 During follow‐up, research subjects were asked the following questions: if they had ever been told that they had depression or anxiety by a health professional; if they were receiving antidepressants; and if they had received psychotherapy since the prior survey contact. Research subjects’ responses were used to create time–varying binary indicators of any self‐reported clinical diagnosis of depression and self‐reported depression treatment during the index survey wave.
2.2.2. Identifying probable depression
Short Form 12 (SF‐12) is a patient reported outcome measure commonly used to assess physical and mental aspects of health‐related quality of life. SF‐12 is a 12‐item instrument, and responses are scored, weighed, and aggregated to yield physical and mental composite scores that are between 0 and 100 (average scores are 50, and higher scores indicate better health). Villagut and colleagues showed that SF‐12 mental composite scores (SF‐12 MCS) of 48.9 or less are 74% sensitive and 83% specific for identifying the occurrence of major or persistent depressive disorders within the prior 12 months.46 An SF‐12 MCS threshold score of 48.9 was used to create a time‐varying binary indicator of probable depression in the prior 12 months (probable because the threshold score is not diagnostic). This indicator was used as the denominator when estimating the average and annual rates of clinical diagnosis of depression among research subjects.
2.2.3. Predictors
Key explanatory variables included age at enrollment, race, educational attainment (up to high school or beyond high school), rural or urban residence (using the 2010 US Census classification),47 current marital status (currently married, previously married or never married), current employment status (not employed [retired and unemployed] or employed) and current annual income (<$20 000, $20 000‐$40 000, $40 001‐$70 000, or >$70 000). Control covariates included time (in years) since prostate cancer diagnosis, a time‐invariant binary indicator of prostate cancer stage (T1 vs T2/T3),48 and time‐varying indicators of Charlson comorbidity index (0‐1 vs ≥2),49 health insurance status (insured vs uninsured), annual number of primary care visits (≤3 vs >3, [with three being the average number of annual visits to primary care clinics among similarly aged men in the general population during the study period]),50 and depression severity. The indicator of depression severity was created by applying SF‐12 MCS threshold scores suggested by other researchers (ie, <32.8 for moderate or severe depression, 32.8‐43 for mild depression, >43‐48.9 for plausible subthreshold depression, and >48.9 for no depression).51, 52
2.3. Statistical analyses
2.3.1. Assessing predictors and rates of clinical diagnosis of depression
Generalized estimating equations (GEE) with a binomial family, logit link, and independent correlation were used to evaluate the indicator of self‐reported clinical diagnosis of depression as a function of indicated key explanatory variables and control covariates.53 The model predicted the average and annual rates of clinical diagnosis of depression among research subjects with probable depression. Survey sampling weights were applied and an alpha of 0.05 was used to determine statistical significance. Sensitivity analyses used alternative GEE correlation structures (ie, unstructured and exchangeable). Alwhaibi and colleagues have shown that frequent primary care visits are associated with a greater chance of clinical diagnosis of depression.41 Conversely, two studies have shown that patients undergoing depression treatment make frequent primary care visits.54, 55 Reverse causation between self‐reported clinical diagnosis of depression and annual primary care visits was reduced by lagging the indicator of annual primary care visits by one survey wave.
2.3.2. Assessing predictors and rates of depression treatment conditional on clinical diagnosis of depression
A subsample of research subjects reported their first clinical diagnosis of depression during follow‐up. GEE with a binomial family, logit link, and independent correlation was used to evaluate the indicator of self‐reported depression treatment as a function of indicated key explanatory variables and control covariates in the subsample.53 The model predicted average rates of depression treatment using single observations from periods when research subjects reported their first clinical diagnosis. Survey sampling weights were applied and an alpha of 0.05 was used to determine statistical significance. Sensitivity analyses used alternative GEE correlation structures (ie, unstructured and exchangeable). Annual primary care visits were lagged by one survey wave to minimize bias from reverse causation.
2.3.3. Dealing with missing data
400 PCaP research subjects were lost to follow‐up before the end of HCaP‐NC.30 T‐ and Chi‐square tests showed that research subjects lost to follow‐up were more likely to be African American, uninsured, smokers, and low income earners with higher prostate cancer stages.56 Logit regression showed that loss to follow‐up was random conditional on observed variables.56, 57 Survey response rates were about 95% (with respect to analytic variables) during each survey contact. Missing observations from survey nonresponse occurred at random and were addressed via multiple imputation (with 50 imputed datasets for explanatory variables only).58, 59, 60 Details of the imputation process (including specifications and diagnostics) are available on request.
3. RESULTS
3.1. Descriptive statistics
Most research subjects were middle‐aged/elderly, lived in urban areas, and were enrolled shortly after prostate cancer diagnosis (Table 1). Both European‐American and African‐American research subjects had similar distributions of health insurance coverage and cancer stage at diagnosis. However, African‐American research subjects had lower income and less education. Over 10% of research subjects reportedly received a clinical diagnosis of depression prior to enrollment, and an additional 12% reportedly received their first clinical diagnosis of depression during follow‐up. Similarly, about 7% of research subjects reportedly received depression treatment prior to enrollment, and an additional 9% reportedly initiated depression treatment during follow‐up. All research subjects who reportedly received depression treatment also reportedly received a clinical diagnosis of depression.
Table 1.
Descriptive statistics at enrollment (N = 804)
| Characteristics | Size (%) |
|---|---|
| Was diagnosed with depression prior to enrollment | |
| No | 721 (90%) |
| Yes | 83 (10%) |
| Race | |
| European American | 450 (56%) |
| African American | 354 (44%) |
| Marital status | |
| Currently married | 628 (78%) |
| Previously married | 133 (17%) |
| Never married | 42 (5%) |
| Educational attainment | |
| More than high school | 350 (44%) |
| High school or less than high school | 453 (56%) |
| Residence | |
| Urban | 609 (76%) |
| Rural | 195 (24%) |
| Employment status | |
| Retired | 366 (46%) |
| Employed | 399 (50%) |
| Unemployed | 36 (4%) |
| Annual income | |
| >$70 000 | 265 (35%) |
| $40 001‐$70 000 | 208 (27%) |
| $20 001‐$40 000 | 186 (24%) |
| ≤$20 000 | 102 (13%) |
| Age at enrollment | |
| 40‐49 years | 39 (5%) |
| 50‐59 years | 254 (32%) |
| 60‐69 years | 341 (42%) |
| 70‐79 years | 170 (21%) |
| Indicators of depression severity | |
| No depression | 575 (72%) |
| Plausible subthreshold depression | 91 (11%) |
| Mild depression | 86 (11%) |
| Moderate or severe depression | 46 (6%) |
| Health insurance status | |
| Insured | 423 (53%) |
| Uninsured | 381 (47%) |
| Cancer stage at diagnosis | |
| T1(ref) | 493 (62%) |
| T2/T3 | 306 (38%) |
| Charlson comorbidity index | |
| 0‐1 (ref) | 615 (77%) |
| ≥2 | 187 (23%) |
| Visits to primary care clinics | |
| ≤3 visits per year | 428 (61%) |
| >3 visits per year | 271 (39%) |
3.2. Rates of self‐reported clinical diagnosis and treatment of depression
The average rate of self‐reported clinical diagnosis of depression among research subjects with probable depression was 44% (95% CI: 39%‐49%) over the study period (Table 2). The average rate of clinical diagnosis of depression was significantly lower among research subjects with the following characteristics: African American race; below college education; employed; and infrequent primary care visits. The rate of clinical diagnosis of depression among research subject with probable depression significantly declined from about 60% in the first 2 years after cancer diagnosis, to about 40% 3‐5 years later (Figure 1). The average rate of depression treatment conditional on clinical diagnosis of depression was 62% (95% CI: 55%‐69%) over the study period (Table 2). The average rate of depression treatment was similar across categories of race, educational attainment, employment status, and frequency of annual primary care visits.
Table 2.
Estimated rates of self‐reported clinical diagnosis (conditional on probable depression) and treatment of depression (conditional on self‐reported clinical diagnosis of depression) among research subjects
| Group | Outcomes | |||
|---|---|---|---|---|
|
Clinical diagnosis of depression (N = 647 participant‐surveys) |
Treatment of depression (N = 138 participant‐surveys) |
|||
| Estimated rates | P‐Value | Estimated rates | P‐Value | |
| All research subjects | 44.3% (39.2‐49.4%) | — | 62.0% (54.8‐69.2%) | — |
| By race | ||||
| European American | 47.4% (41.2‐53.6%) | Ref | 63.7% (55.0‐72.5%) | Ref |
| African American | 37.7% (31.1‐44.4%) | 0.02 | 56.1% (40.6‐71.6%) | 0.43 |
| By educational attainment | ||||
| ≤High school | 38.9% (32.0‐45.9%) | Ref. | 60.2% (50.7‐69.7%) | Ref. |
| >High school | 48.3% (41.9‐54.7%) | 0.03 | 66.0% (49.6‐82.6%) | 0.59 |
| By employment status | ||||
| Retired/unemployed | 48.6% (42.4‐54.9%) | Ref. | 66.5% (56.4‐76.5%) | Ref. |
| Employed | 40.0% (32.3‐45.7%) | 0.02 | 56.3% (43.9‐68.7%) | 0.24 |
| By annual primary care visits | ||||
| At most three | 39.0% (33.2‐44.8%) | Ref. | 69.7% (58.0‐81.4%) | Ref. |
| Four or more | 49.2% (43.3‐55.1%) | <0.01 | 55.0% (43.9‐66.2%) | 0.10 |
Research subjects with probable depression had Short‐form 12 mental composite scores ≤48.9.46
Abbreviations: dep, depression; Ref, reference group.
Figure 1.
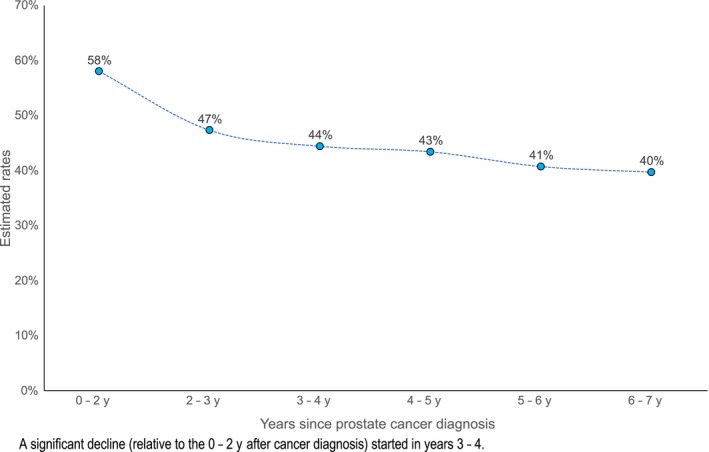
Estimated rates of self‐reported clinical diagnosis of depression during PCaP/HCaP‐NC
3.3. Predictors of self‐reported clinical diagnosis and treatment of depression
Variables with significantly lower odds of self‐reported clinical diagnosis of depression included African‐American race; employment; age at enrollment; living with prostate cancer diagnosis for three or more years; having high school education or less; decreasing depression severity; and infrequent primary care visits. There was no evidence of significant associations between self‐reported clinical diagnosis of depression and any other variable in the model (Table 3). Variables with significantly lower odds of self‐reported depression treatment included decreasing depression severity, being employed and living with a prostate cancer diagnosis for more than 2 years. There was no evidence of any statistically significant association between self‐reported depression treatment and any other variable in the model.
Table 3.
Factors associated with depression care among research subjects. Indicators of care were self‐reported clinical diagnosis of depression (N = 804 [or 2156 participant‐surveys]) and self‐reported depression treatment among research subjects with a self‐reported clinical diagnosis of depression during follow‐up (N = 132 [or 359 participant observations])
| Outcomes | Depression care | |
|---|---|---|
| Variables | Diagnosis | Treatment |
|
Odds ratios
(95% CI) N = 804 |
Odds ratios
(95% CI) N = 132 |
|
| Race | ||
| European American (ref) | — | — |
| African American | 0.63* | 0.70 |
| (0.42‐0.94) | (0.35‐1.42) | |
| Educational attainment | ||
| High school or below | — | — |
| Above high school | 1.56* | 1.14 |
| (1.03‐2.35) | (0.54‐2.41) | |
| Employment status | ||
| Retired/unemployed (ref) | — | — |
| Employed/yet to retire | 0.63* | 0.49* |
| (0.43‐0.93) | (0.25‐0.96) | |
| Health insurance coverage | ||
| Insured (ref) | — | — |
| Uninsured | 0.57 | 2.07 |
| (0.22‐1.50) | (0.44‐9.70) | |
| Cancer stage at diagnosis | ||
| T1: a‐c (ref) | — | — |
| T2/T3: a‐c | 0.82 | 0.64 |
| (0.56‐1.21) | (0.32‐1.26) | |
| Age at enrollment | ||
| 0.95** | 0.99 | |
| (0.93‐0.98) | (0.95‐1.04) | |
| Time since cancer diagnosis | ||
| 13‐24 months (ref) | — | — |
| 25‐36 months | 0.61 | 0.14** |
| (0.33‐1.11) | (0.04‐0.51) | |
| 37‐48 months | 0.53* | 0.22* |
| (0.28‐0.98) | (0.06‐0.84) | |
| 49‐60 months | 0.50* | 0.22* |
| (0.26‐0.98) | (0.05‐0.91) | |
| 61‐72 months | 0.44* | 0.17* |
| (0.22‐0.88) | (0.04‐0.76) | |
| 73‐84 months | 0.42 | 0.27 |
| (0.18‐1.01) | (0.04‐1.83) | |
| Marital status | ||
| Currently married (ref) | — | — |
| Previously/never married | 1.23 | 1.12 |
| (0.78‐1.91) | (0.50‐2.51) | |
| Residence | ||
| Mostly urban (ref) | — | — |
| Mostly rural | 1.24 | 1.42 |
| (0.80‐1.92) | (0.68‐2.93) | |
| Charlson comorbidity index | ||
| 0‐1 (ref) | — | — |
| ≥2 | 1.08 | 0.72 |
| (0.78‐1.49) | (0.42‐1.25) | |
| Visits to primary care clinics | ||
| ≤3 visits per year (ref.) | — | — |
| >3 visits per year | 1.62** | 1.42 |
| (1.24‐2.12) | (0.82‐2.45) | |
| Depression severity | ||
| No depression (ref) | — | — |
| Plausible subthreshold dep. | 2.10** | 2.19 |
| (1.43‐3.09) | (0.97‐4.97) | |
| Mild depression | 5.08** | 3.77** |
| (3.42‐7.55) | (1.72‐8.22) | |
| Moderate/severe depression | 13.04** | 4.93** |
| (7.48‐22.73) | (2.22‐10.96) | |
| Annual income | ||
| >$70 000 (ref) | — | — |
| $40 001‐$70 000 | 0.80 | 1.05 |
| (0.52‐1.22) | (0.44‐2.50) | |
| $20 001‐$40 000 | 0.84 | 0.65 |
| (0.50‐1.42) | (0.25‐1.67) | |
| ≤$20 000 | 0.93 | 0.46 |
| (0.50‐1.71) | (0.14‐1.53) | |
50 imputed datasets were used.
Abbreviations: CI, confidence interval; Dep, depression; ref, referent.
P‐value ≤ 0.05.
P‐value ≤ 0.01.
3.4. Sensitivity analyses
The findings were robust to GEE with exchangeable or unstructured correlations and to complete case analyses. Emerging evidence suggests reverse causation exists between depression severity and clinical diagnosis of depression: Simon and colleagues (1999) argue that depression severity predicts clinical diagnosis of depression61; Hung and colleagues (2017) showed that the duration of undiagnosed depression predicts depression severity.62 Study findings remained robust even after depression severity had been lagged by one survey wave to minimize bias from reverse causation.
4. DISCUSSION
4.1. Study implications
The data demonstrated the existence and nature of barriers to depression care among prostate cancer survivors. These findings have several important implications.
The rate of clinical diagnosis of depression in the general population [ie, 47% [95% CI: 42%‐53%]) is similar to our estimate (ie, 44% [95% CI: 39%‐49%]).18 Additionally, the rate of depression treatment in the general population (ie, 52% [95% CI: 42%‐62%]) is also similar to our estimate (ie, 62% [95% CI: 55%‐69%]).63 These suggest that despite an expected reluctance to seek depression care,27, 28, 29 prostate cancer survivors use depression care resources similarly to the general population. However, considering rates of depression treatment in the cancer literature (eg, 73% by Alwhaibi and colleagues [2017],41 and 76% by Findley and colleagues [2012],36) there seems to be a tendency toward more depression treatment among cancer survivors.
Evidence from this study demonstrated a significant racial difference in self‐reported clinical diagnosis of depression. This racial difference may arise from unequal access to depression care providers: this is consistent with evidence from the general population,34 from cancer survivors at the VA,64 and from research showing that race does not affect providers’ decision to make a clinical diagnosis of depression.33 Furthermore, no association was found between race and self‐reported depression treatment, which also is consistent with evidence from studies on survivors with other cancer types.36, 41 Hence, it is plausible that unequal access to depression treatment in the general population does not extend to prostate (or other) cancer survivors.39, 65 Additionally, the previously reported racial difference in risk of probable depression among research subjects is more likely due to unequal access to depression care providers than to depression treatment after clinical diagnosis of depression.30
The estimated rate of clinical diagnosis of depression among research subjects with probable depression declined from about 60% to 40% between prostate cancer diagnosis and 5‐7 years later. This suggests that lack of clinical recognition of depression (a type of diagnostic error)31 increased over time, and demonstrates a growing unmet need for depression care in prostate cancer survivors. Similarly, Table 3 showed that clinical recognition of depression decreased with advancing age at enrollment, thus depressed elderly survivors may be more likely to remain undiagnosed (as cancer care may gradually “crowd‐out” the clinical attention and quality of care that cancer survivors receive for comorbidities [including depression],22, 23, 24 and uncertainty about who [between primary and cancer care providers] should be responsible for meeting mental health care needs of cancer survivors may be increasing).66, 67 Other predisposing factors to undiagnosed depression include fewer primary care visits (where depression care is usually initiated),66, 67, 68 low education (due to higher mental health stigma than in those with more education),69, 70 and being employed (due to workplace stigma and potential income loss).20, 21, 71
The negative association between employment and self‐reported depression treatment deserves special consideration because of plausible policy implications. Luber and colleagues (2001) showed that adults receiving depression treatment are expected to have more frequent office visits for psychotherapy.55 Depressed employees undergoing psychotherapy for depression may require up to 20 weekly 1‐hour sessions at sites of care.72, 73 Leaving work for psychotherapy sessions creates a potential for income loss, assuming one only gets paid for hours worked.71 Paid Sick Leave laws and policies, as well as short‐ and long‐term disability insurance provide protection against income loss.74, 75 However, Paid Sick Leave laws and policies cover short‐term health needs (ie, between 24 and 72 hours per year),74 and may not protect sicker employees (with moderate/severe or treatment‐resistant depression) or those who need to travel long distances to get psychotherapy.71, 74 Similarly, short‐ and long‐term disability insurance covers 3‐4 in 10 workers and replaces 60%‐80% of one's income.75, 76 Hence, depressed employees with limited or no protection against income loss may have to choose one of two mutually exclusive options: to receive depression treatment and lose income; or to forego depression treatment for a chance at spontaneous remission with or without accompanying productivity and/or job loss (productivity loss being mostly borne by employers).71, 77, 78 Loss aversion under Prospect theory dictates that most people in this situation will choose the latter option,79 which may explain the negative association between employment and depression treatment. If this is the case, providing more protection against income loss may increase depression treatment rates.
4.2. Strengths and limitations
This study has two main strengths. Several clinically relevant factors (eg, depression severity, comorbidities, and cancer stage) and two major contributors to racial differences in access to mental health care (ie, geography in North Carolina and health insurance coverage)] were controlled for in the models.80 Application of sample weights makes these findings generalizable to prostate cancer survivors in North Carolina. This study also has several weaknesses. These findings from up to 13 years ago may not apply to present day prostate cancer survivors: for the Mental Health Parity and Addiction Equity Act (2008) and the Affordable Care Act (2010) were introduced after PCaP and reportedly increased access to depression care (ie, they would have led to higher rates of clinical diagnosis and treatment of depression among research subjects).81, 82, 83 What is unclear is if these Acts significantly reduced sociodemographic differences in access to depression care services for prostate (or all) cancer survivors. Self‐reported clinical recognition and treatment of depression is susceptible to error from misdiagnosis, recall bias and social desirability bias. Additionally, dependent variables in the GEE models may have been mislabeled in research subjects who reportedly received a clinical diagnosis of depression prior to enrollment and who had probable depression in an index wave84; and when assuming that self‐reported depression treatment was for depression rather than for anxiety disorders or chronic pain.85, 86 These errors may bias regression estimates towards the null or increase variance and risk of type 2 error. However, risks of bias and type 2 error are likely to be minimal for three key reasons. First, studies examining clinical diagnosis of depression using self‐reports and medical records demonstrated an 80% positive agreement (range: 51%‐100%) and 90% negative agreement (range: 71%‐100%).87, 88, 89 Second, the estimated rates of clinical diagnosis and treatment of depression in research subjects are consistent with the literature (described above).18, 36, 41, 63 Third, adults experiencing anxiety disorders or chronic pain are likely to be depressed.2, 90 Finally, the sample did not include prostate cancer survivors with late stage cancer at diagnosis—hence our findings do not extend to late stage disease.
5. CONCLUSION
Prostate cancer survivors experience access‐related barriers when in need of depression care. Factors associated with lower odds of clinical diagnosis of depression include African‐American race, being employed, older age at enrollment, low education, infrequent primary care visits and living with a prostate cancer diagnosis for three or more years. Factors associated with lower odds of depression treatment after receiving a clinical diagnosis of depression include being employed, decreasing depression severity, and living with a prostate cancer diagnosis for more than 2 years.
CONFLICT OF INTEREST
None to declare.
AUTHORS CONTRIBUTIONS
Daniel O. Erim was involved in conceptualization, investigation, formal analyses, writing—original draft, project management. Jeannette T. Bensen, James L. Mohler, Elizabeth TH Fontham, Lixin Song, Laura Farnan, Scott E. Delacroix, and Edward S. Peters were involved in investigation, writing—review and editing. Theodora N. Erim and Bradley N. Gaynes were involved in supervision, writing—review and editing. Ronald C. Chen was involve in investigation, supervision, writing—review and editing.
ACKNOWLEDGMENTS
We thank the staff, advisory committees, and research subjects participating in the North Carolina‐Louisiana Prostate Cancer Project (PCaP) and the Health Care Access and Prostate Cancer Treatment in North Carolina (HCaP‐NC) studies for their important contributions. We also acknowledge contributions from the following individuals: Sally C. Stearns PhD, George Pink PhD, Marisa E. Domino PhD, Antonia V. Bennet PhD, Nkechi Conteh MD, MPH, and Adrian Gerstel.
Erim DO, Bensen JT, Mohler JL, et al. Patterns and predictors of self‐reported clinical diagnosis and treatment for depression in prostate cancer survivors. Cancer Med. 2019;8:3648–3658. 10.1002/cam4.2239
Funding information
The North Carolina‐Louisiana Prostate Cancer Project (PCaP) and the Health Care Access and Prostate Cancer Treatment in North Carolina (HCaP‐NC) study are performed as collaborative studies supported by the Department of Defense (contract DAMD 17‐03‐2‐0052) and the American Cancer Society (award RSGT‐08‐008‐01‐CPHPS), respectively.
Data Availability Statement: The data that support the findings of this study are available from PCaP/HCaP‐NC management team. Restrictions apply to the availability of these data, which were used under license for this study.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from PCaP/HCaP‐NC management team. Restrictions apply to the availability of these data, which were used under license for this study.
REFERENCES
- 1. Massie MJ. Prevalence of depression in patients with cancer. J Natl Cancer Inst Monogr. 2004;32:57‐71. [DOI] [PubMed] [Google Scholar]
- 2. Mitchell AJ, Chan M, Bhatti H, et al. Prevalence of depression, anxiety, and adjustment disorder in oncological, haematological, and palliative‐care settings: a meta‐analysis of 94 interview‐based studies. Lancet Oncol. 2011;12(2):160‐174. [DOI] [PubMed] [Google Scholar]
- 3. Colleoni M, Mandala M, Peruzzotti G, Robertson C, Bredart A, Goldhirsch A. Depression and degree of acceptance of adjuvant cytotoxic drugs. Lancet. 2000;356(9238):1326‐1327. [DOI] [PubMed] [Google Scholar]
- 4. Prieto JM, Blanch J, Atala J, et al. Psychiatric morbidity and impact on hospital length of stay among hematologic cancer patients receiving stem‐cell transplantation. J Clin Oncol. 2002;20(7):1907‐1917. [DOI] [PubMed] [Google Scholar]
- 5. Bui Q‐UT, Ostir GV, Kuo Y‐F, Freeman J, Goodwin JS. Relationship of depression to patient satisfaction: findings from the barriers to breast cancer study. Breast Cancer Res Treat. 2005;89(1):23‐28. [DOI] [PubMed] [Google Scholar]
- 6. Pinquart M, Duberstein PR. Depression and cancer mortality: a meta‐analysis. Psychol Med. 2010;40(11):1797‐1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ruo B, Rumsfeld JS, Hlatky MA, Liu H, Browner WS, Whooley MA. Depressive symptoms and health‐related quality of life: the heart and soul study. JAMA. 2003;290(2):215‐221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cuijpers P, Smit F. Excess mortality in depression: a meta‐analysis of community studies. J Affect Disord. 2002;72(3):227‐236. [DOI] [PubMed] [Google Scholar]
- 9. USPTF . Screening for depression in adults: US preventive services task force recommendation statement. Ann Intern Med. 2009;151(11):784. [DOI] [PubMed] [Google Scholar]
- 10. Andersen BL, DeRubeis RJ, Berman BS, et al. Screening, assessment, and care of anxiety and depressive symptoms in adults with cancer: an American Society of Clinical Oncology guideline adaptation. J Clin Oncol. 2014;32(15):1605‐1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bankhead C. Depression in Cancer Common but Untreated. MEDPAGE TODAY, 2014. https://www.medpagetoday.com/psychiatry/depression/47405. Accessed November 6, 2014. [Google Scholar]
- 12. Siu AL, Bibbins‐Domingo K, Grossman DC, et al. Screening for depression in adults: US Preventive Services Task Force recommendation statement. J JAMA. 2016;315(4):380‐387. [DOI] [PubMed] [Google Scholar]
- 13. Akincigil A, Matthews EB. National rates and patterns of depression screening in primary care: results from 2012 and 2013. J Psychiatric Serv. 2017;68(7):660‐666. [DOI] [PubMed] [Google Scholar]
- 14. Cameron IM. Recognition of Depression in Primary Care: Associated Factors and Outcomes [Doctoral dissertation]. University of Aberdeen; 2010. [Google Scholar]
- 15. Cepoiu M, McCusker J, Cole MG, Sewitch M, Belzile E, Ciampi A. Recognition of depression by non‐psychiatric physicians—a systematic literature review and meta‐analysis. J Gen Intern Med. 2008;23(1):25‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Thompson A, Hunt C, Issakidis C. Why wait? reasons for delay and prompts to seek help for mental health problems in an Australian clinical sample. Soc Psychiatry Psychiatr Epidemiol. 2004;39(10):810‐817. [DOI] [PubMed] [Google Scholar]
- 17. Posternak MA, Solomon DA, Leon AC, et al. The naturalistic course of unipolar major depression in the absence of somatic therapy. J Nerv Ment Dis. 2006;194(5):324‐329. [DOI] [PubMed] [Google Scholar]
- 18. Mitchell AJ, Vaze A, Rao S. Clinical diagnosis of depression in primary care: a meta‐analysis. Lancet. 2009;374(9690):609‐619. [DOI] [PubMed] [Google Scholar]
- 19. Ell K, Sanchez K, Vourlekis B, et al. Depression, correlates of depression, and receipt of depression care among low‐income women with breast or gynecologic cancer. J Clin. 2005;23(13):3052‐3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lagomasino IT, Dwight‐Johnson M, Miranda J, et al. Disparities in depression treatment for Latinos and site of care. J Psychiatric Serv. 2005;56(12):1517‐1523. [DOI] [PubMed] [Google Scholar]
- 21. Fukuda Y, Hiyoshi A. Influences of income and employment on psychological distress and depression treatment in Japanese adults. Environ Health Prev Med. 2012;17(1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sarfati D, Koczwara B, Jackson C, Jackson C. The impact of comorbidity on cancer and its treatment. CA Cancer J Clin. 2016;66(4):337‐350. [DOI] [PubMed] [Google Scholar]
- 23. Krishnan KR, Delong M, Kraemer H, et al. Comorbidity of depression with other medical diseases in the elderly. Biol Psychiatry. 2002;52(6):559‐588. [DOI] [PubMed] [Google Scholar]
- 24. Kales HC, Chen P, Blow FC, Welsh DE, Mellow AM. Rates of clinical depression diagnosis, functional impairment, and nursing home placement in coexisting dementia and depression. Am J Geriatr Psychiatry. 2005;13(6):441‐449. [DOI] [PubMed] [Google Scholar]
- 25. Eisenberg JM, Power EJ. Transforming insurance coverage into quality health care: voltage drops from potential to delivered quality. JAMA. 2000;284(16):2100‐2107. [DOI] [PubMed] [Google Scholar]
- 26. Pence BW, O’Donnell JK, Gaynes BN. The depression treatment cascade in primary care: a public health perspective. Curr Psychiatry Rep. 2012;14:328‐335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cochran SV, Rabinowitz FE. Practical resources for the mental health professional. Menand depression: Clinical and empirical perspectives. San Diego, CA: Academic Press; 1999. [Google Scholar]
- 28. Real T. I don't want to talk about it: Overcoming the secret legacy of male depression. New York: Simon and Schuster; 1998. [Google Scholar]
- 29. Addis ME, Mahalik JR. Men, masculinity, and the contexts of help seeking. Am Psychol. 2003;58(1):5. [DOI] [PubMed] [Google Scholar]
- 30. Erim DO. Policy‐Relevant Characteristics of Common Depressive and Anxiety Disorders in Prostate Cancer Survivors [Doctoral dissertation]. Chapel Hill: Department of Health Policy and Management, The University of North Carolina; 2019. [Google Scholar]
- 31. National Academies of Sciences, Engineering, and Medicine . Improving Diagnosis in Health Care. Washington, DC: National Academies Press; 2015. [Google Scholar]
- 32. Klinkman MS. Competing demands in psychosocial care: a model for the identification and treatment of depressive disorders in primary care. Gen Hosp Psychiatry. 1997;19(2):98‐111. [DOI] [PubMed] [Google Scholar]
- 33. Kales HC, Neighbors HW, Valenstein M, et al. Effect of race and sex on primary care physicians' diagnosis and treatment of late‐life depression. J Am Geriatr Soc. 2005;53(5):777‐784. [DOI] [PubMed] [Google Scholar]
- 34. Stockdale SE, Lagomasino IT, Siddique J, McGuire T, Miranda J. Racial and ethnic disparities in detection and treatment of depression and anxiety among psychiatric and primary health care visits, 1995–2005. Med Care. 2008;46(7):668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rane PB, Sambamoorthi U, Madhavan S. Depression treatment in individuals with cancer: a comparative analysis with cardio‐metabolic conditions. Health Psychol Res. 2013;1(1):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Findley PA, Shen C, Sambamoorthi U. Depression treatment patterns among elderly with cancer. Depress Res Treat. 2012;2012:1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kessler RC, Zhao S, Blazer DG, Swartz M. Prevalence, correlates, and course of minor depression and major depression in the National Comorbidity Survey. J Affect Disord. 1997;45(1):19‐30. [DOI] [PubMed] [Google Scholar]
- 38. Unützer J, Katon W, Callahan CM, et al. Depression treatment in a sample of 1,801 depressed older adults in primary care. J Am Geriatr Soc. 2003;51(4):505‐514. [DOI] [PubMed] [Google Scholar]
- 39. Harman JS, Edlund MJ, Fortney JC. Disparities in the adequacy of depression treatment in the United States. Psychiatric Serv. 2004;55(12):1379‐1385. [DOI] [PubMed] [Google Scholar]
- 40. Burnett‐Zeigler I, Zivin K, Ilgen M, Szymanski B, Blow FC, Kales HC. Depression treatment in older adult veterans. Am J Geriatr Psychiatry. 2012;20(3):228‐238. [DOI] [PubMed] [Google Scholar]
- 41. Alwhaibi M, Madhavan S, Bias T, Kelly K, Walkup J, Sambamoorthi U. Depression treatment among elderly Medicare beneficiaries with incident cases of cancer and newly diagnosed depression. Psychiatric Serv. 2017;68(5):482‐489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Alegría M, Chatterji P, Wells K, et al. Disparity in depression treatment among racial and ethnic minority populations in the United States. Psychiatric Serv. 2008;59(11):1264‐1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Skolarus TA, Wolf A, Erb NL, et al. Society prostate cancer survivorship care guidelines. CA Cancer J Clin. 2014;64(4):225‐249. [DOI] [PubMed] [Google Scholar]
- 44. Holmes JA, Bensen JT, Mohler JL, Song L, Mishel MH, Chen RC. Quality of care received and patient‐reported regret in prostate cancer: analysis of a population‐based prospective cohort. Cancer. 2017;123(1):138‐143. [DOI] [PubMed] [Google Scholar]
- 45. Schroeder JC, Bensen JT, Su LJ, et al. The North Carolina‐Louisiana Prostate Cancer Project (PCaP): methods and design of a multidisciplinary population‐based cohort study of racial differences in prostate cancer outcomes. Prostate. 2006;66(11):1162‐1176. [DOI] [PubMed] [Google Scholar]
- 46. Vilagut G, Forero CG, Pinto‐Meza A, et al. The mental component of the short‐form 12 health survey (SF‐12) as a measure of depressive disorders in the general population: results with three alternative scoring methods. Value Health. 2013;16(4):564‐573. [DOI] [PubMed] [Google Scholar]
- 47. https://www.census.gov/geo/reference/ua/urban-rural-2010.html. Accessed February 16, 2018
- 48. Prostate Cancer: Stages and Grades, https://www.cancer.net/cancer-types/prostate-cancer/stages-and-grades Web site. Accessed June 18, 2018.
- 49. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chron Dis. 1987;40(5):373‐383. [DOI] [PubMed] [Google Scholar]
- 50. Petterson SM, Liaw WR, Phillips RL Jr, Rabin DL, Meyers DS, Bazemore AW. Projecting US primary care physician workforce needs: 2010–2025. Ann Family Med. 2012;10(6):503‐509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sanderson K, Andrews G. Prevalence and severity of mental health‐related disability and relationship to diagnosis. Psychiatric Serv. 2002;53(1):80‐86. [DOI] [PubMed] [Google Scholar]
- 52. Santos JF, Ramos‐Cerqueira A, Furegato AF, Lebrão M, Duarte YO. O2–3.5 the short form health survey as an instrument for the screening of depressive symptoms in the elderly population. J Epidemiol Community Health. 2011;65(Suppl 1):A24‐A24. [Google Scholar]
- 53. Pan W. Model selection in estimating equations. Biometrics. 2001;57(2):529‐534. [DOI] [PubMed] [Google Scholar]
- 54. Worley MJ, Trim RS, Tate SR, Hall JE, Brown SA. Service utilization during and after outpatient treatment for comorbid substance use disorder and depression. J Subst Abuse Treat. 2010;39(2):124‐131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Luber MP, Meyers BS, Williams‐Russo PG, et al. Depression and service utilization in elderly primary care patients. Am J Geriatr Psychiatry. 2001;9(2):169‐176. [PubMed] [Google Scholar]
- 56. Miller RB, Wright DW. Detecting and correcting attrition bias in longitudinal family research. J Marriage Family. 1995;57(4):921–929. [Google Scholar]
- 57. Allison PD. Missing Data. Vol 136 Thousand Oaks, CA: Sage publications; 2001. [Google Scholar]
- 58. Lin G, Rodriguez RN, SAS I . Weighted methods for analyzing missing data with the GEE Procedure. Paper presented at: Proceedings of SAS Global Forum, Washington DC (2014 March 23th‐26th); 2015.
- 59. White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med. 2011;30(4):377‐399. [DOI] [PubMed] [Google Scholar]
- 60. Azur MJ, Stuart EA, Frangakis C, Leaf PJ. Multiple imputation by chained equations: what is it and how does it work? Int J Methods Psychiatric Res. 2011;20(1):40‐49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Simon GE, Goldberg D, Tiemens BG, Ustun TB. Outcomes of recognized and unrecognized depression in an international primary care study. Gen Hosp Psychiatry. 1999;21(2):97‐105. [DOI] [PubMed] [Google Scholar]
- 62. Hung C‐I, Liu C‐Y, Yang C‐H. Untreated duration predicted the severity of depression at the two‐year follow‐up point. PLoS ONE. 2017;12(9):e0185119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Wang PS, Lane M, Olfson M, Pincus HA, Wells KB, Kessler RC. Twelve‐month use of mental health services in the United States: results from the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):629‐640. [DOI] [PubMed] [Google Scholar]
- 64. Clary AS. The Association of Hospital and Patient Characteristics with Treatment Initiation among Veterans with Stage I, II, or II Lung Colon or Rectal Cancer [Doctoral dissertation]. Chapel Hill, NC: The University of North Carolina; 2019. [Google Scholar]
- 65. Hankerson SH, Fenton MC, Geier TJ, Keyes KM, Weissman MM, Hasin DS. Racial differences in symptoms, comorbidity, and treatment for major depressive disorder among black and white adults. J Natl Med Assoc. 2011;103(7):576‐584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Balasubramanian BA, Jetelina KK, Lee SC. Oncologist and primary care physician attitudes and practices toward cancer survivor follow‐up care in an integrated health system. J Clin Oncol. 2016;34(3_suppl):105‐105.26573072 [Google Scholar]
- 67. Klabunde CN, Ambs A, Keating NL, et al. The role of primary care physicians in cancer care. J Gen Intern Med. 2009;24(9):1029‐1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Mitchell AJ, Vahabzadeh A, Magruder K. Screening for distress and depression in cancer settings: 10 lessons from 40 years of primary‐care research. Psycho Oncol. 2011;20(6):572‐584. [DOI] [PubMed] [Google Scholar]
- 69. Liu C‐F, Campbell DG, Chaney EF, Li Y‐F, McDonell M, Fihn SD. Depression diagnosis and antidepressant treatment among depressed VA primary care patients. Adm Policy Ment Health Serv Res. 2006;33(3):331‐341. [DOI] [PubMed] [Google Scholar]
- 70. Lopez V, Sanchez K, Killian MO, Eghaneyan BH. Depression screening and education: an examination of mental health literacy and stigma in a sample of Hispanic women. BMC Public Health. 2018;18(1):646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Birnbaum HG, Kessler RC, Kelley D, Ben‐Hamadi R, Joish VN, Greenberg PE. Employer burden of mild, moderate, and severe major depressive disorder: mental health services utilization and costs, and work performance. J Depression Anxiety. 2010;27(1):78‐89. [DOI] [PubMed] [Google Scholar]
- 72. Nieuwsma JA, Trivedi RB, McDuffie J, Kronish I, Benjamin D, Williams JW. Brief psychotherapy for depression: a systematic review and meta‐analysis. Int J Psychiatry Med. 2012;43(2):129‐151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Gelenberg AJ, Freeman MP, Markowitz JC, et al. Practice guideline for the treatment of patients with major depressive disorder third edition. Am J Psychiatry. 2010;167(10):1.20068118 [Google Scholar]
- 74. What’s the Difference? Paid Sick Leave, FMLA, and Paid Family and Medical Leave. In: US Department of Labor; https://www.dol.gov/sites/default/files/PaidLeaveFinalRuleComparison.pdf. Accessed June 12, 2018. [Google Scholar]
- 75. Monaco K. Disability insurance plans: trends in employee access and employer costs. Beyond the Numbers, vol. 4. Washington, DC: Bureau of Labor Statistics; 2015. [Google Scholar]
- 76. Autor D, Duggan M, Gruber J, Maclean C. How Does Access to Short Term Disability Insurance Impact SSDI Claiming? National Bureau of Economic Research; 2013. http://projects.nber.org/projects_backend/drc/papers/odrc13-09.pdf. Accessed November 5, 2018. [Google Scholar]
- 77. Lerner D, Adler DA, Chang H, et al. Unemployment, job retention, and productivity loss among employees with depression. Psychiatric Serv. 2004;55(12):1371‐1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Greenberg PE, Fournier A‐A, Sisitsky T, Pike CT, Kessler RC. The economic burden of adults with major depressive disorder in the United States (2005 and 2010). J Clin Psychiatry. 2015;76(2):155‐162. [DOI] [PubMed] [Google Scholar]
- 79. Kahneman D, Tversky A. Prospect theory: an analysis of decision under risk. Econometrics. 1979;47:263‐291. [Google Scholar]
- 80. McGuire TG, Miranda J. New evidence regarding racial and ethnic disparities in mental health: policy implications. Health Aff. 2008;27(2):393‐403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Beronio K, Glied S, Frank R. How the affordable care act and mental health parity and addiction equity act greatly expand coverage of behavioral health care. J Behav Health Serv Res. 2014;41(4):410‐428. [DOI] [PubMed] [Google Scholar]
- 82. Barry CL, Huskamp HA. Moving beyond parity — mental health and addiction care under the ACA. N Engl J Med. 2011;365(11):973‐975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Beronio K, Po R, Skopec L, Glied S. Affordable Care Act will expand mental health and substance use disorder benefits and parity protections for 62 million Americans. J Mental Health. 2014;2. [Google Scholar]
- 84. Baik S‐Y, Bowers BJ, Oakley LD. The recognition of depression: the primary care clinician's perspective. Ann Family Med. 2005;3(1):31‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Borkovec TD, Ruscio AM. Psychotherapy for generalized anxiety disorder. J Clin Psychiatry. 2001;62:37-42. [PubMed] [Google Scholar]
- 86. Cascade EF, Kalali AH, Thase ME. Use of antidepressants: expansion beyond depression and anxiety. J Psychiatry. 2007;4(12):25. [PMC free article] [PubMed] [Google Scholar]
- 87. Smith B, Chu LK, Smith TC, et al. Challenges of self‐reported medical conditions and electronic medical records among members of a large military cohort. BMC Med Res Methodol. 2008;8(1):37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Sanchez‐Villegas A, Schlatter J, Ortuno F, et al. Validity of a self‐reported diagnosis of depression among participants in a cohort study using the structured clinical interview for DSM‐IV (SCID‐I). BMC Psychiatry. 2008;8(1):43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Stuart AL, Pasco JA, Jacka FN, Brennan SL, Berk M, Williams LJ. Comparison of self‐report and structured clinical interview in the identification of depression. Compr Psychiatry. 2014;55(4):866‐869. [DOI] [PubMed] [Google Scholar]
- 90. Van Hecke O, Torrance N, Smith B. Chronic pain epidemiology and its clinical relevance. J Br J Anaesth. 2013;111(1):13‐18. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from PCaP/HCaP‐NC management team. Restrictions apply to the availability of these data, which were used under license for this study.


