Abstract
To determine whether radiotherapy (RT) can increase pelvic fracture risk in rectal cancer survivors. Rectal cancer patients who underwent curative surgery between 1996 and 2011 in Taiwan were retrospectively studied using the National Health Insurance Research Database (NHIRD) of Taiwan. ICD‐9 Codes 808, 805.4‐805.7, 806.4‐806.7, and 820 (including pelvic, sacrum, lumbar, and femoral neck fracture) were defined as pelvic fracture. Propensity scores for RT, age, and sex were used to perform one‐to‐one matches between the RT and non‐RT group. Risks of pelvic and arm fractures were compared by multivariable Cox regression. Of the 32 689 patients, 7807 (23.9%) received RT, and 1616 suffered from a pelvic fracture (incidence rate: 1.17/100 person‐years). The median time to pelvic fracture was 2.47 years. After matching, 6952 patients each in the RT and non‐RT groups were analyzed. RT was associated with an increased risk of pelvic fractures in the multivariable Cox model (hazard ratio (HR): 1.246, 95% confidence interval (CI): 1.037‐1.495, P = 0.019) but not with arm fractures (HR: 1.013, 95% CI: 0.814‐1.259, P = 0.911). Subgroup analyses revealed that RT was associated with a higher pelvic fracture rate in women (HR: 1.431, 95% CI: 1.117‐1.834) but not in men, and the interaction between sex and RT was significant (P = 0.03). The HR of pelvic fracture increased 2‐4 years after RT (HR: 1.707, 95% CI: 1.150‐2.534, P = 0.008). An increased risk of pelvic fracture is noted in rectal cancer survivors, especially women, who receive RT.
Keywords: pelvic fracture, pelvic insufficiency fracture, radiotherapy, rectal cancer
1. INTRODUCTION
The incidence rates of rectal cancer have been increasing, and the risk of the disease is shifting to younger populations gradually.1 Due to improvements in medical care, more rectal cancer patients are surviving and experiencing the sequelae of cancer treatment.2
Radiotherapy (RT), chemotherapy (C/T), and surgical resection are important treatments for rectal cancer patients. Acute side effects after treatment, such as gastrointestinal and genitourinary toxicities, have been discussed thoroughly in previous studies.3 However, few studies have discussed late side effects of pelvic irradiation.4
Pelvic fracture may be one of the late side effects of pelvic irradiation.5, 6 Approximately 50% of previously ambulatory women become confined to bed after a pelvic fracture,7 and pelvic fracture significantly increases mortality by 12%‐20% in the first year of follow‐up.8 The importance of early detection and prevention of pelvic fracture should be emphasized.
Most studies have discussed pelvic fracture after irradiation for gynecological and prostate cancers.5, 9 Very few studies have discussed pelvic fractures in rectal cancer patients.4, 10 This study aimed to evaluate the pelvic fracture risk of rectal cancer patients who received RT.
2. METHODS
2.1. Data source
We used Taiwan's National Health Insurance Database (NHIRD) as our data source. Taiwan's National Health Insurance was founded in 1995 via the Taiwanese government. More than 99% of Taiwanese are enrolled in this national insurance and receive its comprehensive medical care. Taiwan's NHIRD collects nationwide medical information in detail, such as inpatient and outpatient diagnoses, medical procedures, drug prescriptions, medical treatment duration, and medical costs. The specialists in the Registry of Catastrophic Illness Database (RCID), a subpart of the NHIRD, review the records of those who are newly diagnosed as cancer patients by reviewing medical records and pathological tissue confirmation. Patients who pass the peer review and receive a Catastrophic Illness Card will have better social benefits and financial support by Taiwan's government. This study was approved by the Institutional Review Board of Taipei Veterans General Hospital (2016‐05‐007BC).
2.2. Cohort selection
The cohort was composed of patients aged 20 years or older who were diagnosed as having a first primary rectal cancer (ICD‐9‐CM 154.0 and 154.1) from the NHIRD between 1 January 1996 and 31 December 2011, including inpatient and outpatient information. We enrolled rectal cancer patients who had received radical rectal surgery, such as abdominoperineal resection of the rectum, low anterior resection, local excision, transsacral rectosigmoidectomy, or posterior resection of the rectum. To decrease any interference from other treatments and patients with a poor prognosis, we excluded patients with a history of HIV infection, previous malignancy, and pathological fracture of any bone. In clinical practice, it is still difficult for doctors to differentiate from pathological fracture to insufficient fracture. Patients with pathological fracture may have poor prognosis and disease control,11 and those patients may also have higher chance of pathological fractures risk over other part of the body, including pelvis. Therefore, we excluded patients who already have diagnosis of pathological fracture of any place to decrease the potential interference. Because the study entry point is half year after radical rectal surgery, we excluded patients whose observation interval was less than 6 months after curative rectal surgery (before our study entry point starts).
We collected RT information from our cohort. Preoperative and postoperative RT in this study are defined as patients received RT within 6 months before and after radical rectal surgery, respectively. The total portal numbers for the entire RT course were recorded completely in the NHIRD. We defined our long‐ and short‐course RT using portal numbers (radiation portal number per fraction × fraction numbers), which has been used in previous studies.12, 13 The typical radiation regimen for preoperative long‐course RT and postoperative RT is 45‐50.4 Gy in 25‐28 fractions (usually more than three portals per day). Therefore, we included patients who had received more than 75 (3 × 25) portals within 6 months before and after radical rectal surgery as the long‐course RT group. Similarly, the typical radiation regimen for preoperative short‐course RT is 25 Gy in five fractions (usually four portals per day). Thus, we included patients who had received 18‐22 portals (approximately 20 portals (4 × 5)) within 6 months before radical rectal surgery as the short‐course RT group. In this study, we want to evaluate pelvic radiotherapy directly related to rectal cancer (such as neoadjuvant radiotherapy and postoperative pelvic radiotherapy). Therefore, patients who received miscellaneous RT portals, with a portal number not in the range of short‐course or long‐course definition, were excluded, which radiotherapy may relate to other reasons or palliative intention. The non‐RT group comprised patients who underwent radical rectal surgery but never received any RT portals.
The follow‐up time for each patient is ended on the date of diagnosis of any pelvic fracture, death, or the end of the study (31 December 2011), whichever occurred first.
Pelvic fracture is defined as patients with International Classification of Diseases, Ninth Revision, Clinical Modification (ICD‐9‐CM) Codes 808, 805.4‐805.7, 806.4‐806.7, and 820 (including pelvic, sacrum, lumbar vertebral fracture, and femoral neck fracture) during the follow‐up time.14, 15, 16, 17 The radiation field of rectal cancer is usually designed to cover tumor or tumor bed, mesorectal, presacral, and internal iliac bones, and sometimes also included external iliac nodes in some cases.18 In contrast to pelvic bone, arm would not be irradiated and can be used as a comparison to evaluate the effect of radiation. This kind of study design has been used in previous study.19 Arm fracture was defined as patients with ICD‐9‐CM Codes with 812, 813, and 814 (including fractures of the humerus, radius or ulna, and carpal bones) during the follow‐up.17
2.3. Treatment factors and comorbidities
The C/T agents were classified and collected by their Anatomical Therapeutic Chemical (ATC) code. Other demographic data such as age at diagnosis, sex, and comorbidities, including autoimmune diseases, chronic obstructive pulmonary disease, diabetes mellitus, dyslipidemia, end‐stage renal disease, liver cirrhosis, and hypertension were also collected from the NHIRD. The Charlson comorbidity index score for each patient was calculated according to each patient's comorbidities. Osteoporosis was defined as patients with ICD‐9 Codes 733.00‐733.03 and 733.9 diagnosed before the radical rectal surgery.
2.4. Statistical methods
We analyzed the pelvic fracture incidence of the whole cohort and evaluated other demographic features, including age, sex, Charlson comorbidity index score, osteoporosis, and C/T, comparing the RT and non‐RT groups. Pelvic fracture risk between short‐course and long‐course RT were analyzed and compared.
To reduce the potential influence of confounding variables, we used a propensity score matching. Propensity scores for RT for each patient were calculated using age, sex, Charlson comorbidity index score, and C/T. One‐to‐one exact matching using propensity scores, age, and sex were performed to classify them into an RT group and a non‐RT group. We compared the pelvic fracture incidence rate between the RT and non‐RT group, using person‐years as the denominator.
We used univariable and multivariable Cox regression to identify risk factors for pelvic fracture, including age, sex, Charlson comorbidity index score, RT, C/T, and osteoporosis. The same analysis was repeated for arm fracture. To further explore the time‐varying effect of RT, we calculated the hazard ratio and P value of each factor in multivariable Cox model in matched cohort with RT divided into 2‐year interval. Cox regression with age as the time scale (adjusted for follow‐up time) was also calculated. Subgroup analyses were used to assess the relative risk of pelvic fracture across possible risk factors. Interaction examinations of RT and each subgroup factors were performed using multivariable Cox models. We also calculated the hazard ratio of pelvic fracture in the RT group every 2 years after RT during the 10‐year follow‐up. The time‐varying hazard ratios were estimated, which means that the hazard ratios would be modeled as step functions, that is, different coefficients over different time intervals.20
The data processing was performed with Microsoft SQL Server 2012 (Microsoft Corp., Redmond, WA). All analyses were computed in R (version R‐3.4.3; http://www.r-project.org). A two‐sided P value less than 0.05 was considered statistically significant. The cox.zph function shipped with the survival package in R was used to examine the correlation between Schoenfeld residuals and time.21
3. RESULTS
3.1. Population demographics
We collected the records of 39 750 rectal cancer patients from NHIRD who had received curative rectal surgery from 1996 to 2011. After excluding patients with previous malignancies (1371 patients), patients with pathological fractures (1434 patients), patients with HIV infection (19 patients), patients whose observation interval was less than 6 months after curative rectal surgery (before our study entry point starts) (3393 patients) and patients receiving miscellaneous RT portal regiments (934 patients), 32 689 patients were selected (Figure 1).
Figure 1.
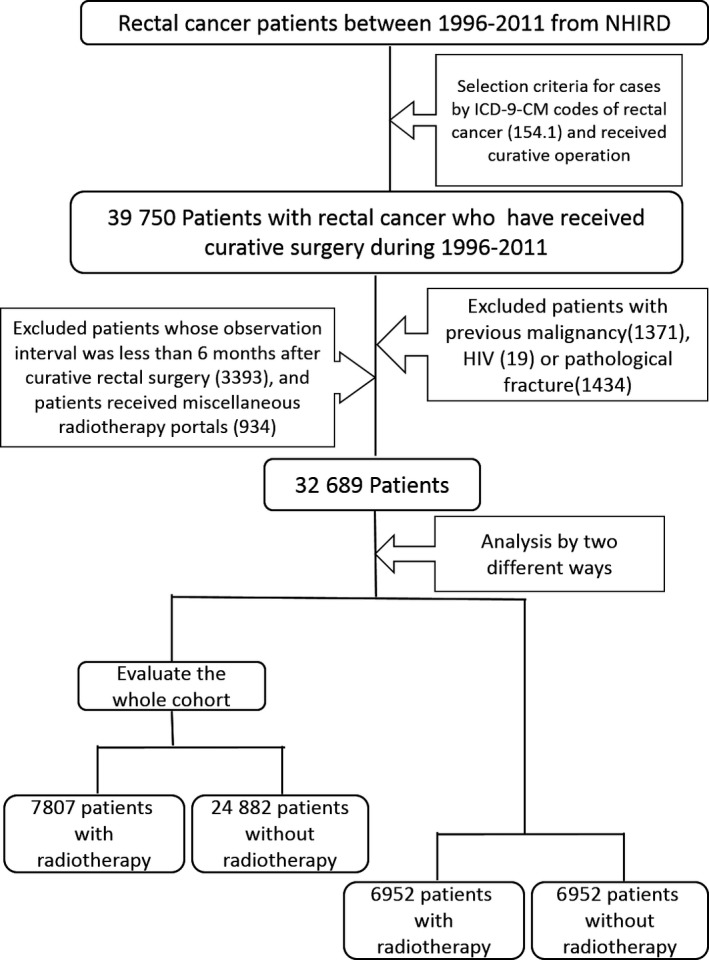
Research Flowchart (NHIRD: National Health Insurance Research Database)
Of the 32 689 patients who we studied, 7807 patients (23.9%) received RT, and 24 882 patients (76.1%) did not. There were 1616 patients who suffered from a pelvic fracture, an incidence of 1.17/100 person‐years among the whole cohort. Most patients received long‐course RT (6679 patients; 85.6%).
Before matching, we noticed that the distributions of the patient's sex and age were significantly different between patients who had received RT and those who did not. The RT group was younger than the non‐RT group (mean age 60.96 vs 65.06 years old, P < 0.001). The sex distribution was also significantly different. The male to female ratio was 63.1% to 36.9% in the RT group but 58.3% to 41.7% in the non‐RT group (P < 0.001). Therefore, propensity scores, age, and sex were used to perform one‐to‐one matches between the RT group and non‐RT group. After matching, 6952 patients in the RT group and 6952 patients in the non‐RT group were selected.
The median follow‐up time for all patients was 3.18 years (range from 1 day to 14.7 years). The percentages according to sex, osteoporosis diagnosis, C/T, average age at diagnosis, and the Charlson comorbidity index score of the cohort and matched populations were also recorded (Table 1).
Table 1.
Patient characteristics
| Factors | RT (Cohort) (N = 7807) | Non‐RT (Cohort) (N = 24882) | P value | RT (matched) (N = 6952) | Non‐RT (matched) (N = 6952) | P value |
|---|---|---|---|---|---|---|
| Age | <0.001 | 1.000 | ||||
| Mean (years) | 60.96 | 65.06 | 61.83 | 61.83 | ||
| SD | 12.50 | 12.52 | 11.70 | 11.70 | ||
| Range | 8‐89 | 16‐91 | 24‐94 | 24‐94 | ||
| Sex | <0.001 | 1.000 | ||||
| Male | 4923 (63.1%) | 14494 (58.3%) | 4352 (62.6%) | 4352 (62.6%) | ||
| Female | 2884 (36.9%) | 10388 (41.7%) | 2600 (37.4%) | 2600 (37.4%) | ||
| Charlson Comorbidity Index score | 0.108 | 1.000 | ||||
| Mean | 6.47 | 6.42 | 6.56 | 6.56 | ||
| SD | 2.59 | 2.72 | 2.48 | 2.48 | ||
| Range | 2‐16 | 0‐19 | 2‐15 | 2‐15 | ||
| Osteoporosis | <0.001 | 0.003 | ||||
| Yes | 3138 (12.6%) | 860 (11.0%) | 789 (11.3%) | 679 (9.8%) | ||
| No | 21744 (87.4%) | 6947 (89.0%) | 6163 (88.7%) | 6273 (90.2%) | ||
| Chemotherapy | <0.001 | <0.001 | ||||
| Yes | 7206 (92.3%) | 13091 (52.6%) | 6367 (91.6%) | 5405 (77.7%) | ||
| No | 601 (7.7%) | 11791 (47.4) | 585 (8.4%) | 1547 (22.3%) |
RT, Radiotherapy; SD, standard deviation.
3.2. Pelvic fracture and arm facture results
After matching, there were 241 patients who suffered from pelvic fracture over 23 747.45 person‐years in the RT group (incidence rate: 1.01/100 person‐year), while 252 patients suffered from pelvic fractures over 30 609.78 person‐years in the non‐RT group (incidence rate: 0.82/100 person‐year). The pelvic fracture incidence was significantly higher in the RT group than in the non‐RT group (P = 0.020).
In multivariable Cox regression, RT was associated with an increased risk of pelvic fracture (HR: 1.246, 95% confidence interval (CI): 1.037‐1.495, P = 0.019), but it was not associated with an increased risk of arm fracture (HR: 1.013, 95% CI: 0.814‐1.259, P = 0.911). Older age (≥60 years old), female sex, osteoporosis, and a high Charlson comorbidity index score was correlated with an increased risk of pelvic fracture. The HR and P values of each factor in the univariable and multivariable Cox models are summarized in Table 2. Cox regression with age as the time scale (adjusted for follow‐up time) was calculated, which showed similar result, and was provided in the Table S2.
Table 2.
The hazard ratio and P value of each factor in single variate and multivariable Cox model in matched cohort
| Univariable analysis | Multivariable analysis | |||
|---|---|---|---|---|
| Pelvic fracture | Arm fracture | Pelvic fracture | Arm fracture | |
| HR (95% CI); P value | HR (95% CI); P value | |||
| Radiotherapy | 1.209 (1.013‐1.444); 0.036 | 1.018 (0.824‐1.257); 0.87 | 1.246 (1.037‐1.495); 0.019 | 1.013 (0.814‐1.259); 0.911 |
| Osteoporosis | 2.898 (2.339‐3.591); <0.001 | 1.893 (1.418‐2.527); <0.001 | 1.426 (1.130‐1.800); 0.003 | 1.125 (0.826‐1.533); 0.454 |
| Chemotherapy | 0.773 (0.626‐0.955); 0.017 | 0.975 (0.744‐1.276); 0.852 | 0.947 (0.759‐1.181); 0.629 | 1.031 (0.7746‐1.372); 0.834 |
| Age (>=60 vs <60 years) | 1.090 (1.079‐1.100); <0.001 | 1.024 (1.014‐1.034); <0.001 | 1.086 (1.076‐1.097); <0.001 | 1.024 (1.014‐1.034); 630 < 0.001 |
| Sex (Male vs female) | 0.513 (0.429‐ 0.613); <0.001 | 0.401 (0.324‐0.497); <0.001 | 0.473 (0.390‐0.573); <0.001 | 0.384 (0.306‐0.482); <0.001 |
| Charlson comorbidity index score | 1.152 (1.113‐1.193); <0.001 | 1.084 (1.040‐ 1.130); <0.001 | 1.085 (1.050‐1.122); <0.001 | 1.068 (1.024‐1.115); 0.002 |
HR, hazard ratio; CI, confidence interval.
To identify differences in pelvic fracture risk among the different categories of patients, subgroup analyses were performed. Subgroup analyses revealed that RT was associated with a higher risk of pelvic fracture in patients whose ages were over 60 years (HR: 1.267, 95% CI: 1.042‐1.541), those who received C/T (HR: 1.284, 95% CI: 1.042‐1.571), and those whose Charlson comorbidity index scores were more than 7 (HR: 1.264, 95% CI: 1.003‐1.592). Subgroup analyses also showed that RT was associated with a higher pelvic fracture risk in women (HR: 1.431, 95% CI: 1.117‐1.834) but not in men (HR: 1.019, 95% CI: 0.776‐1.339) (Figure 2). The P values of interaction examination with RT for each subgroup factor in the multivariable Cox model were shown in figure 2, and sex and RT had a significant interaction (P = 0.03).
Figure 2.
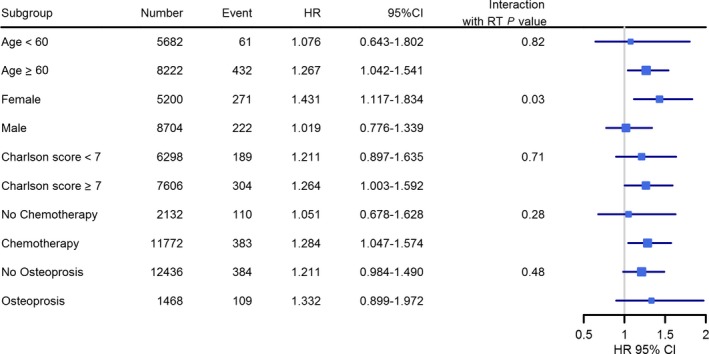
Subgroup analyses of different factors (age, sex, osteoporosis, chemotherapy, and Charlson comorbidity index score) of pelvic fracture risk in radiotherapy group and the P values of interactions with radiotherapy (HR, hazard ratio; CI, confidence interval)
3.3. Other analysis results
In the multivariable Cox regression analysis of the cohort, there was no significant difference in pelvic fracture risk between long‐course and short‐course RT (P = 0.972). We used a multivariable Cox model to evaluate the risk of pelvic fracture in the RT group every 2 years after RT during the 10 years of follow‐up. The hazard ratio of the pelvic fracture risk was significantly increased during follow‐up 2‐4 years after RT (HR: 1.707, 95% CI: 1.150‐2.534, P = 0.008) (Figure 3). The pelvic fracture risk in the RT group during the first 2 years of follow‐up also tended to be higher, but the difference was not statistically significant (HR: 1.231, 95% CI: 0.948‐1.598, P = 0.119). Further detailed HR and P value of each factor in multivariable Cox model in matched cohort with radiotherapy group divided into every 2 years during 10 years of follow‐up were provided in the Table S1. Proportional hazards assumption examination was done, and is not violated for radiotherapy (P = 0.745). Even if the assumption is violated for radiotherapy, the coefficient that we estimate for radiotherapy is a sort of average effect over the range of times observed in our study, which has been described in other reference.22
Figure 3.
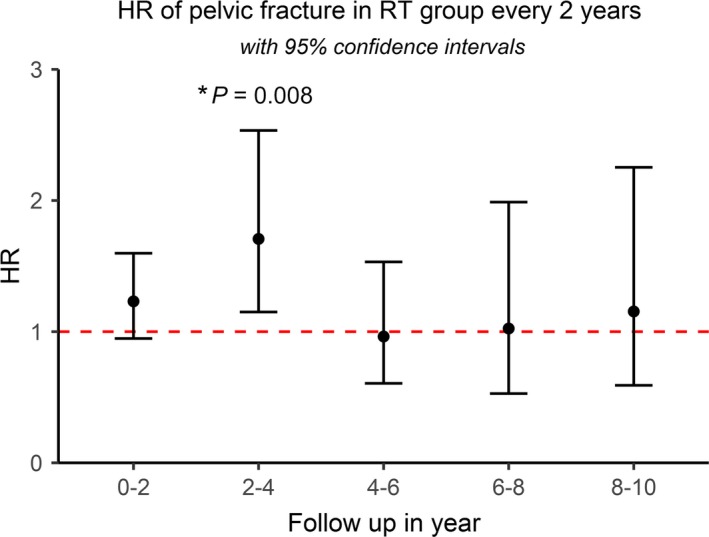
Hazard ratio of pelvic fracture in radiotherapy group every 2 years during 10 years of follow‐up. The hazard ratio of pelvic fracture risk is significantly increased during follow‐up 2‐4 years after radiotherapy (HR: 1.66, 95% CI:1.119‐2.468, P = 0.012) (HR, hazard ratio; CI, confidence interval)
4. DISCUSSION
Post‐RT pelvic fracture has been discussed thoroughly for gynecological and prostate cancer,9, 23 but few studies have discussed it for rectal cancer.4 The survival rate of rectal cancer has been improved due to advances in treatment, and thus, late side effects among survivors have become extremely important.3 The morbidity and mortality risks after pelvic fracture are both high. It has been reported that 17%‐32% of all deaths are related to pelvic fractures,24 and more than half of patients cannot regain mobility within a year after the fracture.25 Pelvic fractures are also associated with infection, depression, and high medical costs.26, 27 It is a serious problem that needs to receive more attention. To our knowledge, this study has the largest patient population ever enrolled to evaluate pelvic fracture after irradiation among rectal cancer patients, with most previous studies only enrolling a few hundred patients or fewer.2, 4, 28
The mechanism through which RT causes fracture is as follows: radiation can reduce osteoblast numbers, arrest their cell cycles, cause apoptosis and result in perivascular damage.5 Preclinical data have shown that irradiation of the tibia of the rat results in osteoblast apoptosis and small trabeculae destruction and causes a 50% reduction in trabecular bone.29 A similar effect also occurs in humans, and in irradiated gynecological cancer patients, bone mineral density is lost by 11% over the L2 spine and 15.8% over the femoral neck after pelvic RT30; moreover, pelvic RT may cause cortical thinning of the femoral neck.31
Pain is the most common symptom of post‐RT pelvic fracture,32 although around 20%‐50% of people are asymptomatic.33 Pelvic insufficiency fracture (PIF) after radiation can be noted as quickly as 2 months or as long as 8 years after RT.28 The most common duration between treatment and fracture is approximately 6‐20 months.32, 34, 35 In our study, we noted that the pelvic fracture risk is higher after 2‐4 years of follow‐up after RT. The pelvic fracture risk in the RT group in the first 2 years of follow‐up tended to be higher but was not statistically significant. Therefore, we suggest that physicians pay more attention to pelvic fractures during the first 4 years of follow‐up among rectal cancer patients who receive pelvic RT. Surgery is an important treatment option for the post‐RT pelvic fracture, but most patients prefer to choose conservative treatments such as pain killers, analgesia, and bed rest.5, 36 Symptoms of pelvic fracture usually resolve after 1‐11 months of bed rest and conservative therapy.37
There are several factors discussed in previous studies that might increase the pelvic fracture rate among patients who received pelvic irradiation. Osteoporosis is one of the major risk factors for pelvic fracture. Patients with osteoporosis have a higher 5‐year PIF rate, approximately 15.6% compared with 2.9% for patients without osteoporosis (P = 0.01).32 Another study evaluated rectal cancer patients and reported that osteoporosis is a risk factor for sacral fracture (HR: 3.23, P = 0.02).4 Osteoporosis is also associated with a higher pelvic fracture rate in the multivariable Cox model in this study.
Whether C/T can result in a higher pelvic fracture rate or not is still uncertain. One study reported that the reduction rate in volumetric bone mineral density among patients who received C/T after 1 year was 15.9% over the L1‐L2 spine and 10.4% over the femoral neck.30 However, C/T was not a significant risk factor for pelvic fracture in several studies.30, 32, 38 Combining C/T with RT may cause a higher pelvic fracture rate based on a study of gynecology malignancy.39 In our study, C/T was a significant factor in single variable analysis but become insignificant after multivariable adjustment. In the subgroup analyses of the RT group, patients with C/T had a higher risk of pelvic fracture compared to the control.
Aging is also an important risk factor. A higher pelvic fracture risk after RT has been reported in many different studies in patients who are older than 50, 55, 60, 65, or 70.36, 38, 40, 41 Our study also proved that in a multivariable Cox model, patients older than 60 years had a higher pelvic fracture risk (HR: 1.086, CI: 1.076‐1.097, P < 0.001).
Women also had a significantly higher risk of pelvic fracture after RT. One study reported on 582 rectal patients and showed that women had a higher risk of sacral fracture after chemoradiation (HR: 2.64, P = 0.008).4 Another study reviewed 284 anal cancer patients and reported that all 4 patients experiencing pelvic fractures were women.41 Our study showed that women were at significantly higher risk for a pelvic fracture than men in the multivariable analysis (P < 0.001). In addition, we found that RT exposure was a significant risk factor in women but not in men. In the International Commission on Radiological Protection Publication (ICRP) in 2007, after a similar dose of radiation exposure, women have a higher risk of cancer and death than men.42 One study reported that women and men differ significantly in radiosensitivity (P = 0.004), which is associated with variations in single nucleotide polymorphisms.43 A group from Germany reported that estradiol treatment can increase the intrinsic radiosensitivity of breast cancer cells.44 Women generally have higher osteoporosis rates than men.45 Because of the lower baseline of bone health in women, the same degree of radiation–induced bone injury may have more of an impact on women than men. Currently, there is no sufficient evidence as to whether RT may cause more toxicity in women's bone health, and it is an important issue that needs additional studies.
In rectal cancer, recommended doses of radiation to the pelvis are typically 45‐50 Gy in 25‐28 fractions for long‐course RT46 and 25 Gy in five fractions for short‐course RT.47 There is only one study that directly compares the pelvic fracture risk between long‐course and short‐course RT,48 and there is no clear answer due to its limited patient number. One early study reported a relatively low fracture incidence of 2.6% in patients who received short‐course RT compared to long‐course RT, which showed a pelvic fracture cumulative incidence of approximately 3.1%‐7.1%.4, 28, 49 However, this result may have been affected by the different study design. In the current study, there was no significant difference in the pelvic fracture risk between the long‐course and short‐course RT groups (P = 0.972).
Thanks to improvements in the RT technique, the use of intensity‐modulated radiation therapy has been proven to reduce pelvic complications including pelvic fracture,50 and image‐guided radiation therapy can reduce the margins of the clinical target volume to planning target volume.51, 52 Using both techniques, the radiation volume and dose to the pelvic bones may be reduced and may reduce the risk of PIF in rectal cancer survivors.
There are several limitations of our study. First, the body mass index and lifestyle information of the patients are not available in the NHIRD, which makes it difficult to analyze those risk factors. Second, because we could not obtain the actual irradiation dose and volume via NHIRD, we could only use radiation portals as a surrogate of RT regimens. Third, we used the ICD‐9‐CM code to define osteoporosis and fracture events, which can sometimes underestimate the pelvic fracture risk if physicians do not record the proper diagnosis with an ICD‐9‐CM code.
In conclusion, an increased risk of pelvic fracture is noted in rectal cancer survivors, especially women, who receive RT.
CONFLICT OF INTEREST
There is no funding disclosures in this article. There is no conflict of interest disclosures from any authors.
Supporting information
ACKNOWLEDGMENTS
We are grateful to Y. Chao and Y.‐M. Liu for their support and encouragement for this research. This article’s abstract has been previously published as a poster in American Society for Radiation Oncology meeting 2017: https://www.redjournal.org/article/S0360-3016(17)32034-5/fulltext.
Kang Y‐M, Chao T‐F, Wang T‐H, Hu Y‐W. Increased risk of pelvic fracture after radiotherapy in rectal cancer survivors: A propensity matched study. Cancer Med. 2019;8:3639‐3647. 10.1002/cam4.2030
Contributor Information
Ti‐Hao Wang, Email: thothwang@gmail.com.
Yu‐Wen Hu, Email: ywhu@vghtpe.gov.tw.
REFERENCES
- 1. Siegel RL, Fedewa SA, Anderson WF, et al. Colorectal cancer incidence patterns in the United States, 1974‐2013. J National Cancer Inst. 2017;109 10.1093/jnci/djw322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pollack J, Holm T, Cedermark B, et al. Late adverse effects of short‐course preoperative radiotherapy in rectal cancer. Br J Surg. 2006;93:1519‐1525. [DOI] [PubMed] [Google Scholar]
- 3. Joye I, Haustermans K. Early and late toxicity of radiotherapy for rectal cancer. Recent Results Cancer Res. 2014;203:189‐201. [DOI] [PubMed] [Google Scholar]
- 4. Kim HJ, Boland PJ, Meredith DS, et al. Fractures of the sacrum after chemoradiation for rectal carcinoma: incidence, risk factors, and radiographic evaluation. Int J Radiat Oncol Biol Phys. 2012;84:694‐699. [DOI] [PubMed] [Google Scholar]
- 5. Higham CE, Faithfull S. Bone health and pelvic radiotherapy. Clin Oncol (R Coll Radiol). 2015;27:668‐678. [DOI] [PubMed] [Google Scholar]
- 6. Schmeler KM, Jhingran A, Iyer RB, et al. Pelvic fractures after radiotherapy for cervical cancer: implications for survivors. Cancer. 2010;116:625‐630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Miller CW. Survival and ambulation following hip fracture. J Bone and Joint Surg Am Vol. 1978;60:930‐934. [PubMed] [Google Scholar]
- 8. Marottoli RA, Berkman LF, Leo‐Summers L, Cooney LM. Predictors of mortality and institutionalization after hip fracture: the New Haven EPESE cohort. Established Populations for Epidemiologic Studies of the Elderly. Am J Public Health. 1994;84:1807‐1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Elliott SP, Jarosek SL, Alanee SR, Konety BR, Dusenbery KE, Virnig BA. Three‐dimensional external beam radiotherapy for prostate cancer increases the risk of hip fracture. Cancer. 2011;117:4557‐4565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chan S, Rowbottom L, McDonald R, et al. Pelvic insufficiency fractures in women following radiation treatment: a case series. Ann Palliat Med. 2016;5:233‐237. [DOI] [PubMed] [Google Scholar]
- 11. Sun L, Li Y, Zhang J, Li H, Li B, Ye Z. Prognostic value of pathologic fracture in patients with high grade localized osteosarcoma: a systemic review and meta‐analysis of cohort studies. J Orthop Res. 2015;33:131‐139. [DOI] [PubMed] [Google Scholar]
- 12. Wang TH, Liu CJ, Chao TF, Chen TJ, Hu YW. Second primary malignancy risk after radiotherapy in rectal cancer survivors. World J Gastroenterol. 2018;24:4586‐4595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lai T‐Y, Wang T‐H, Liu C‐J, Chao T‐F, Chen T‐J, Hu Y‐W. Risk factors for osteonecrosis of the jaw in oral cancer patients after surgery and eventual adjuvant treatment: the potential role of chemotherapy. Radiother Oncol. 2017;123:406‐411. [DOI] [PubMed] [Google Scholar]
- 14. Shen HN, Lin WT, Lu CL, Li CY. Older male physicians have lower risk of trochanteric but not cervical hip fractures. Int J Environ Res Public Health. 2015;12:2249‐2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Huang KC, Huang TW, Yang TY, Lee MS. Chronic NSAIDs use increases the risk of a second hip fracture in patients after hip fracture surgery: evidence from a STROBE‐compliant population‐based study. Medicine. 2015;94:e1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shen SH, Huang KC, Tsai YH, et al. Risk analysis for second hip fracture in patients after hip fracture surgery: a nationwide population‐based study. J Am Med Dir Assoc. 2014;15:725‐731. [DOI] [PubMed] [Google Scholar]
- 17. Baxter NN, Habermann EB, Tepper JE, Durham SB, Virnig BA. Risk of pelvic fractures in older women following pelvic irradiation. JAMA. 2005;294:2587‐2593. [DOI] [PubMed] [Google Scholar]
- 18. Halperin EC, Perez CA. Perez and Brady's principles and practice of radiation oncology. 2013.
- 19. Kye BH, Cho HM. Overview of radiation therapy for treating rectal cancer. Ann Coloproctol. 2014;30:165‐174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Therneau T, Crowson C, Clinic M. Using time dependent covariates and time dependent coefficients in the Cox Model. 2014.
- 21. Grambsch PM, Therneau TM. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81:515‐526. [Google Scholar]
- 22. Allison PD, Institute SAS. Survival Analysis using SAS: a Practical Guide. Cary, N.C.: SAS Institute; 2012. [Google Scholar]
- 23. Igdem S, Alco G, Ercan T, et al. Insufficiency fractures after pelvic radiotherapy in patients with prostate cancer. Int J Radiat Oncol Biol Phys. 2010;77:818‐823. [DOI] [PubMed] [Google Scholar]
- 24. Kanis JA, Oden A, Johnell O, De Laet C, Jonsson B, Oglesby AK. The components of excess mortality after hip fracture. Bone. 2003;32:468‐473. [DOI] [PubMed] [Google Scholar]
- 25. Vochteloo AJ, Moerman S, Tuinebreijer WE, et al. More than half of hip fracture patients do not regain mobility in the first postoperative year. Geriatr Gerontol Int. 2013;13:334‐341. [DOI] [PubMed] [Google Scholar]
- 26. Aprato A, Joeris A, Tosto F, Kalampoki V, Stucchi A, Masse A. Direct and indirect costs of surgically treated pelvic fractures. Arch Orthop Trauma Surg. 2016;136:325‐330. [DOI] [PubMed] [Google Scholar]
- 27. Phillips AC, Upton J, Duggal NA, Carroll D, Lord JM. Depression following hip fracture is associated with increased physical frailty in older adults: the role of the cortisol: dehydroepiandrosterone sulphate ratio. BMC Geriatr. 2013;13:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Herman MP, Kopetz S, Bhosale PR, et al. Sacral insufficiency fractures after preoperative chemoradiation for rectal cancer: incidence, risk factors, and clinical course. Int J Radiat Oncol Biol Phys. 2009;74:818‐823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chandra A, Lin T, Tribble MB, et al. PTH1‐34 alleviates radiotherapy‐induced local bone loss by improving osteoblast and osteocyte survival. Bone. 2014;67:33‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hui SK, Khalil A, Zhang Y, et al. Longitudinal assessment of bone loss from diagnostic computed tomography scans in gynecologic cancer patients treated with chemotherapy and radiation. Am J Obstet Gynecol. 2010;203:353.e351‐353.e357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Okoukoni C, Randolph DM, McTyre ER, et al. Early dose‐dependent cortical thinning of the femoral neck in anal cancer patients treated with pelvic radiation therapy. Bone. 2017;94:84‐89. [DOI] [PubMed] [Google Scholar]
- 32. Shih KK, Folkert MR, Kollmeier MA, et al. Pelvic insufficiency fractures in patients with cervical and endometrial cancer treated with postoperative pelvic radiation. Gynecol Oncol. 2013;128:540‐543. [DOI] [PubMed] [Google Scholar]
- 33. Ogino I, Okamoto N, Ono Y, Kitamura T, Nakayama H. Pelvic insufficiency fractures in postmenopausal woman with advanced cervical cancer treated by radiotherapy. Radiother Oncol. 2003;68:61‐67. [DOI] [PubMed] [Google Scholar]
- 34. Park SH, Kim JC, Lee JE, Park IK. Pelvic insufficiency fracture after radiotherapy in patients with cervical cancer in the era of PET/CT. Radiat Oncol J. 2011;29:269‐276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kwon JW, Huh SJ, Yoon YC, et al. Pelvic bone complications after radiation therapy of uterine cervical cancer: evaluation with MRI. AJR Am J Roentgenol. 2008;191:987‐994. [DOI] [PubMed] [Google Scholar]
- 36. Ramlov A, Pedersen EM, Rohl L, et al. Risk factors for pelvic insufficiency fractures in locally advanced cervical cancer following intensity modulated radiation therapy. Int J Radiat Oncol Biol Phys. 2017;97:1032‐1039. [DOI] [PubMed] [Google Scholar]
- 37. Huh SJ, Kim B, Kang MK, et al. Pelvic insufficiency fracture after pelvic irradiation in uterine cervix cancer. Gynecol Oncol. 2002;86:264‐268. [DOI] [PubMed] [Google Scholar]
- 38. Bazire L, Xu H, Foy JP, et al. Pelvic insufficiency fracture (PIF) incidence in patients treated with intensity‐modulated radiation therapy (IMRT) for gynaecological or anal cancer: single‐institution experience and review of the literature. Br J Radiol. 2017;90:20160885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mehmood Q, Beardwood M, Swindell R, et al. Insufficiency fractures in patients treated with pelvic radiotherapy and chemotherapy for uterine and cervical cancer. Eur J Cancer Care (Engl). 2014;23:43‐50. [DOI] [PubMed] [Google Scholar]
- 40. Tokumaru S, Toita T, Oguchi M, et al. Insufficiency fractures after pelvic radiation therapy for uterine cervical cancer: an analysis of subjects in a prospective multi‐institutional trial, and cooperative study of the Japan Radiation Oncology Group (JAROG) and Japanese Radiation Oncology Study Group (JROSG). Int J Radiat Oncol Biol Phys. 2012;84:e195‐e200. [DOI] [PubMed] [Google Scholar]
- 41. Tomaszewski JM, Link E, Leong T, et al. Twenty‐five‐year experience with radical chemoradiation for anal cancer. Int J Radiat Oncol Biol Phys. 2012;83:552‐558. [DOI] [PubMed] [Google Scholar]
- 42. The 2007 recommendations of the international commission on radiological protection. ICRP publication 103. Ann ICRP. 2007;37:1‐332. [DOI] [PubMed] [Google Scholar]
- 43. Alsbeih G, Al‐Meer RS, Al‐Harbi N, et al. Gender bias in individual radiosensitivity and the association with genetic polymorphic variations. Radiother Oncol. 2016;119:236‐243. [DOI] [PubMed] [Google Scholar]
- 44. Schmidberger H, Hermann RM, Hess CF, Emons G. Interactions between radiation and endocrine therapy in breast cancer. Endocr Relat Cancer. 2003;10:375‐388. [DOI] [PubMed] [Google Scholar]
- 45. Cawthon PM. Gender differences in osteoporosis and fractures. Clin Orthop Relat Res. 2011;469:1900‐1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sauer R, Becker H, Hohenberger W, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351:1731‐1740. [DOI] [PubMed] [Google Scholar]
- 47. Ngan SY, Burmeister B, Fisher RJ, et al. Randomized trial of short‐course radiotherapy versus long‐course chemoradiation comparing rates of local recurrence in patients with T3 rectal cancer: trans‐tasman radiation oncology group trial 01.04. J Clin Oncol. 2012;30:3827‐3833. [DOI] [PubMed] [Google Scholar]
- 48. Bujko K, Nowacki MP, Nasierowska‐Guttmejer A, Michalski W, Bebenek M, Kryj M. Long‐term results of a randomized trial comparing preoperative short‐course radiotherapy with preoperative conventionally fractionated chemoradiation for rectal cancer. Br J Surg. 2006;93:1215‐1223. [DOI] [PubMed] [Google Scholar]
- 49. Holm T, Singnomklao T, Rutqvist LE, Cedermark B. Adjuvant preoperative radiotherapy in patients with rectal carcinoma. Adverse effects during long term follow‐up of two randomized trials. Cancer. 1996;78:968‐976. [DOI] [PubMed] [Google Scholar]
- 50. Ioffe YJ, Hillen TJ, Zhou G, et al. Postradiation damage to the pelvic girdle in cervical cancer patients: is intensity‐modulated radiation therapy safer than conventional radiation? Int J Gynecol Cancer. 2014;24:806‐812. [DOI] [PubMed] [Google Scholar]
- 51. Engels B, Platteaux N, Van den Begin R, et al. Preoperative intensity‐modulated and image‐guided radiotherapy with a simultaneous integrated boost in locally advanced rectal cancer: report on late toxicity and outcome. Radiother Oncol. 2014;110:155‐159. [DOI] [PubMed] [Google Scholar]
- 52. Nguyen NP, Ceizyk M, Almeida F, et al. Effectiveness of image‐guided radiotherapy for locally advanced rectal cancer. Ann Surg Oncol. 2011;18:380‐385. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


