Abstract
Purpose
The aim of the present study was to evaluate the prognostic value of magnetic resonance imaging (MRI)‒determined lymph nodal necrosis (LNN) in nasopharyngeal carcinoma (NPC) and explore the feasibility of an N-classification system based on the 8th edition of the American Joint Committee on Cancer (AJCC) system.
Materials and Methods
The MRI scans of 616 patients with newly diagnosed stage T1-4N1-3M0 NPC who were treated with definitive intensity-modulated radiotherapy (IMRT) were reviewed.
Results
Multivariate analysis showed that LNN was an independent negative prognostic predictor of distant metastasis free survival (hazard ratio, 1.634; 95% confidence interval, 1.023 to 2.609; p=0.040) and overall survival (hazard ratio, 2.154; 95% confidence interval, 1.282 to 3.620; p=0.004). Patients of classification N1 disease with LNN were reclassified as classification N2, and classification N2 disease with LNN as classification N3 in the proposed N-classification system. Correlation with death and distant failure was significant, and the total difference between N1 and N3 was wider with the proposed system.
Conclusion
MRI-determined LNN is an independent negative prognostic factor for NPC. The proposed N classification system is powerfully predictive.
Keywords: Nasopharyngeal carcinoma, Lymph nodes, Intensity-modulated radiotherapy, Prognosis, Neoplasm staging
Introduction
Distant metastasis remains the most commonly failure pattern for patients with nasopharyngeal carcinoma (NPC) after intensity-modulated radiotherapy (IMRT) and N classification has been reported to be the most crucial predictor for distant metastasis [1-4]. Magnetic resonance imaging (MRI) is recommended as the preferred modalities for the clinical staging of NPC [5]. However, radiomics features associated with MRI are not included in the N classification by the 8th edition of the American Joint Committee on Cancer (AJCC) system for NPC [6,7].
Lymph nodal necrosis (LNN) is one of the most important imaging features used to distinguish between benign and malignant lymph nodes. At MRI, LNN is seen as a focal area of high signal intensity on T2-weighted images and as an area of low signal intensity on contrast material–enhanced T1-weighted images, with or without a surrounding rim of enhancement [8]. The incidence of LNN in NPC ranges from 22.9% to 44% [8-11]. A recent study conducted by Zhang et al. [11] demonstrated that LNN diagnosed on MRI was a negative prognostic factor for NPC after IMRI. Thus, in this study, we evaluated the prognostic value of MRI-determined LNN in NPC patients treated by IMRT and further explored the feasibility of an N-classification system based on the 8th edition of the AJCC system.
Materials and Methods
1. Patients and work-up
The medical records of consecutive 616 patients with previously untreated, biopsy-proven NPC with regional lymph nodes metastasis and no distant metastasis who were treated by IMRT between January 2007 and February 2012 in our center were retrospectively evaluated. The male (n=418)-to-female (n=198) ratio was 2.1:1, and the median age was 48 years (range, 14 to 81 years). Pathologically, non-keratinizing and keratinizing disease accounted for 99.4% (612 of 616) and 0.6% (4 of 616) of patients, respectively. All patients were restaged according to the 8th edition of the AJCC staging system. Classification T1, T2, T3, and T4 disease accounted for 15.1% (93 of 616), 15.1% (93 of 616), 38.6% (238 of 616), and 31.2% (192 of 616) of patients, respectively, and classification N1, N2, and N3 disease accounted for 49.4% (304 of 616), 34.4% (212 of 616), and 16.2% (100 of 616) of patients, respectively. The pretreatment workup included a complete history and physical examination, hematology, and biochemistry profiles, fiber-optic nasopharyngoscopy, MRI of the head and neck, bone scintigraphy, computed tomography scan of the chest and abdominal region, and dental check.
2. MR imaging
All patients underwent MRI on a 1.5- or 3.0-T system system (Magnetom Symphony/Verio, Siemens Healthcare, Erlangen, Germany) with a head and neck combined coil [12]. The scan range covered from the suprasellar cistern to the inferior margin of the sternoclavicular joint. Non-enhanced series included: axial T1-weighted imaging (T1WI), axial T2-weighted imaging, sagittal T1WI. Contrast-enhanced scan was performed after injection of gadopentetate dimeglumine with a dose of 0.2 mL/kg and rate of 2.0 mL/sec. Two radiologists with more than 10 years of experience in MRI of head and neck cancers independently evaluated all scans, and any disagreements were resolved by consensus. Also, there is a multidisciplinary team of head and neck cancers in our center to confirm the extent of diseases and the treatment of patients with NPC.
According to the previous study, diagnostic criteria for metastatic lymphadenopathy include (1) lateral retropharyngeal lymph node with a minimal axial diameter (MID) in the largest plane of an individual node at least 5 mm and any node seen in the median retropharyngeal group, lymph nodes with a MID of at least 11 mm in the jugulodigastric region and 10 mm for all other cervical nodes, excluding the retropharyngeal group; (2) lymph nodes of any size with central necrosis or a contrast-enhancing rim; (3) the presence of three or more contiguous and confluent lymph nodes, each of which should have a MID of 8 mm or more; and (4) lymph nodes of any size with extracapsular spread, including the presence of indistinct nodal margins, irregular nodal capsular enhancement or infiltration into the adjacent fat or muscle [13]. The criteria for diagnosing LNN at MRI are a focal area of high signal intensity on T2-weighted images or a focal area of low signal intensity on contrast-enhanced T1-weighted images, with or without a surrounding rim of enhancement [8].
3. Treatment
All patients received definitive radiotherapy using IMRT techniques. A detailed description of IMRT has been previously reported [14]. Briefly, the dose prescribed was 69-70.4 Gy, 63-67.2 Gy, 60-60.8 Gy, and 54-54.4 Gy in 30-32 fractions delivered over 6 weeks at the periphery of the planning target volume (PTV) of primary tumor, PTV of metastatic lymph nodes, PTV of high-risk clinical target volume, and PTV of low-risk clinical target volume, respectively, using the simultaneous integrated boost technique. According to the institutional guidelines, concurrent chemotherapy was given for patients with stage II disease, and both neoadjuvant or adjuvant chemotherapy and concurrent chemotherapy were given for patients with locally advanced diseases. Neoadjuvant or adjuvant chemotherapy consisted of cisplatin with 5-fluorouracil or cisplatin with docetaxel and 5-fluorouracil every 3 weeks for three cycles. Concurrent chemotherapy consisted of cisplatin 80 mg/m2 given on days 1 and 22 of radiotherapy. Deviations from the chemotherapy guidelines were allowed for patients aged over 70-year-old and/or patients with organ dysfunction suggesting intolerance to chemotherapy. Most patients (n=601, 97.5%) underwent platinum-based neoadjuvant, concurrent, or adjuvant chemotherapy.
4. Follow-up and statistical analysis
Follow-up was calculated from the day of radiation therapy completion to the date of the event or the last follow-up visit. All patients were followed up after the completion of radiotherapy: 1 month after the completion of radiotherapy, every 3 months in the first 2 years, every 6 months from year 3 to year 5, and annually thereafter.
The SPSS ver. 17.0 (SPSS Inc., Chicago, IL), software was used for statistical analysis. The locoregional failure free survival (LRFFS), distant metastasis free survival (DMFS), and overall survival (OS) were estimated by use of the Kaplan-Meier method. LRFFS, DMFS, and OS were measured from day 1 of treatment to the date of the event. Subgroup analysis of the LNN and non-LNN groups was conducted according to the T classification, N classification, and overall stage. Multivariate analyses with the Cox proportional hazards model were used to test independent significance by using backward elimination of insignificant explanatory variables. Host factors (age and sex) were included as the covariates in all tests. Also, the effect of N classification on risk for death and distant metastasis were performed by using the Cox proportional hazards model. All statistical tests were two sided, and p < 0.05 was considered to be statistically significant.
5. Ethical statement
This study was approved by the Institutional Review Board of Zhejiang Cancer Hospital with a waiver of informed consent and performed in accordance with the principles of the Declaration of Helsinki.
Results
1. Incidence and size of LNN
In patients with positive lymph node metastases, the incidence of LNN was 38.1% (235 of 616) (Fig. 1). Of 235 patients with LNN, 21.3% (50/235) had retropharyngeal lymph node involvement alone, 44.7% (105/235) had cervical lymph node involvement alone, whereas 34.0% (80 of 235) had both retropharyngeal and cervical lymph node involvement. Of 130 patients with retropharyngeal LNN, 83.1% (108/130) had unilateral retropharyngeal lymph node involvement, whereas 16.9% (22 of 130) had bilateral involvement. Of 185 patients with cervical LNN, 80.0% (148/185) had unilateral cervical lymph node involvement, whereas 20.0% (37 of 185) had bilateral involvement. The median maximal axial diameter of cervical positive lymph node was 24 mm (range, 0 to 89 mm) for all patients, 28 mm (range, 0 to 89 mm) for patients with LNN, and 20 mm (range, 0 to 71 mm) for patients without LNN (0 mm indicated patients with retropharyngeal lymph node involvement alone). The difference between the LNN and non-LNN groups in terms of the median maximal axial diameter of cervical positive lymph node was statistically significant (p < 0.001). The T and N classification distribution according to lymph node status is shown in Table 1.
Fig. 1.
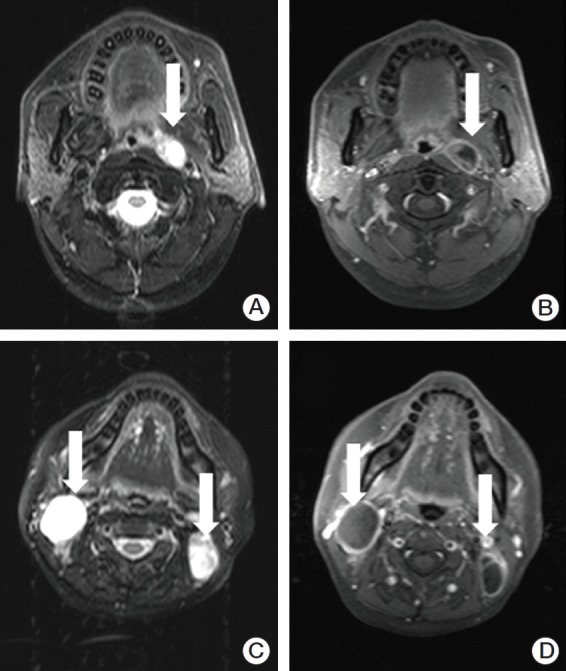
Necrotic lymph nodes in two patients with nasopharyngeal carcinoma. Axial T2-weighted (A) and contrast-enhanced T1-weighted (B) magnetic resonance (MR) images in a 43-year-old woman show the left retropharyngeal lymph node with necrosis (arrows). Axial T2-weighted (C) and contrast-enhanced T1-weighted (D) MR images in a 40-year-old man show necrotic lymph nodes in the bilateral level II area (arrows).
Table 1.
Distribution of T and N classifications with lymph node status
| Non-LNN group (n=381) |
LNN group (n=235) |
|||||||
|---|---|---|---|---|---|---|---|---|
| N1 | N2 | N3 | Total | N1 | N2 | N3 | Total | |
| T1 | 35 (57.4) | 19 (31.1) | 7 (11.5) | 61 (16.0) | 7 (21.9) | 10 (31.3) | 15 (46.9) | 32 (13.6) |
| T2 | 28 (49.1) | 19 (33.3) | 10 (17.5) | 57 (15.0) | 16 (44.4) | 9 (25.0) | 11 (30.6) | 36 (15.3) |
| T3 | 76 (51.4) | 55 (37.2) | 17 (11.5) | 148 (38.8) | 36 (40.0) | 38 (42.2) | 16 (17.8) | 90 (38.3) |
| T4 | 71 (61.7) | 31 (27.0) | 13 (11.3) | 115 (30.2) | 35 (45.5) | 31 (40.3) | 11 (14.3) | 77 (32.8) |
Values are presented as number (%). LNN, lymph nodal necrosis.
2. Treatment outcomes and subgroup analysis
The median follow-up time was 62.6 months (range, 3.4 to 119.4 months); 43 of 616 patients (7.0%) were lost to follow-up. By the last follow-up, 22.1% (136/616) of patients developed treatment failure and more patients developed treatment failure in the LNN group (30.2% vs. 17.1%, p=0.001). The details of treatment failure are listed in Table 2. For all patients, the estimated 5-year LRFFS, DMFS, and OS rates were 87.5%, 86.7%, and 89.2%, respectively, and for the LNN and non-LNN groups, they were 78.8% vs. 91.8%, 78.2% vs. 91.2%, and 78.4% vs. 91.6% (p < 0.05), respectively (Fig. 2).
Table 2.
Patterns of treatment failure for all patients
| Treatment failure pattern | Non-LNN group (n=381) | LNN group (n=235) | p-valuea) |
|---|---|---|---|
| Distant only | 25 (6.6) | 35 (14.9) | 0.001 |
| Bone | 7 (1.8) | 9 (3.8) | |
| Liver | 4 (1.0) | 12 (5.1) | |
| Lung | 11 (2.9) | 8 (3.4) | |
| Bone and liver | 2 (0.5) | 0 | |
| Bone and lung | 0 | 3 (1.3) | |
| Lung and liver | 0 | 2 (0.9) | |
| Other | 1 (0.3)b) | 1 (0.4)c) | |
| Regional and distant | 5 (1.3) | 5 (2.1) | |
| Local and distant | 4 (1.0) | 1 (0.4) | |
| Local, regional, and distant | 1 (0.3) | 2 (0.9) | |
| Local only | 15 (3.9) | 10 (4.3) | |
| Regional only | 9 (2.4) | 16 (6.8)d) | |
| Local and regional | 6 (1.6) | 2 (0.9) | |
| Total | 65 (17.1) | 71 (30.2) |
Values are presented as number (%). LNN, lymph nodal necrosis.
p-values were calculated by using the chi-square test,
Left inguinal lymph nodes,
Include 1 failure in the left parotid region,
Lung, liver, mediastinal and retroperitoneal lymph nodes, and left adrenal gland.
Fig. 2.
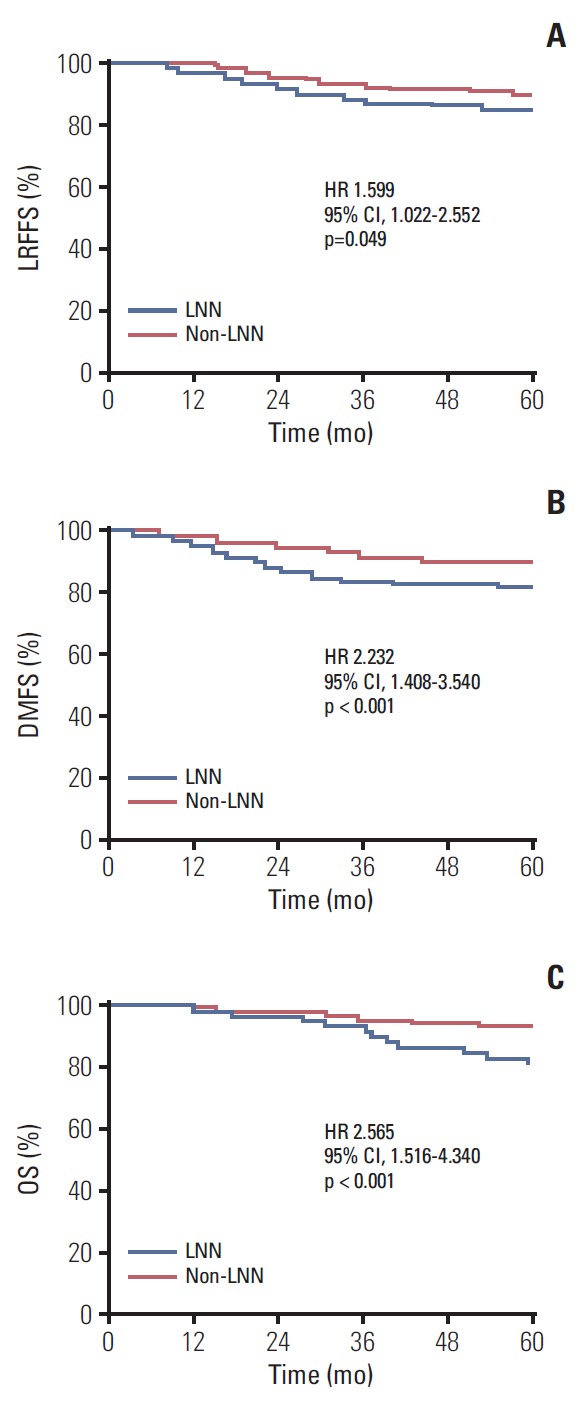
Kaplan-Meier curves show locoregional failure-free survival (LRFFS) (A), distant metastasis-free survival (DMFS) (B), and overall survival (OS) (C) rates for the lymph nodal necrosis (LNN) and non-LNN groups. HR, hazard ratio; CI, confidence interval.
Subgroup analysis of lymph node status according to T classification, N classification, and overall stage are presented in Table 3. The estimated 5-year LRFFS and OS rates for patients with classification N2 disease and LNN were substantially poorer than patients with classification N2 disease without LNN (p < 0.05). The estimated 5-year LRFFS, DMFS, and OS rates for patients with classification N2 disease and LNN were similar to patients with classification N3 disease (5-year LRFFS: 78.1% vs. 84.8%, p=0.112; DMFS: 81.0% vs. 77.5%, p=0.390; OS: 78.1% vs. 84.4%, p=0.367). Although the presence of LNN did not affect the survival outcomes for patients with classification N1 disease (p > 0.05), those with classification N1 disease and LNN had similar survival outcomes as those with stage N2 disease without LNN, with estimated 5-year LRFFS, DMFS, and OS rates of 88.0% vs. 88.1% (p=0.899), 88.9% vs. 87.8% (p=0.942), and 87.9% vs. 94.4% (p=0.070), respectively.
Table 3.
Subgroup analysis of LNN and non-LNN groups between different clinical stage
| 5-Year LRFFS |
5-Year DMFS |
5-Year OS |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Non-LNN group | LNN group | p-value | Non-LNN group | LNN group | p-value | Non-LNN group | LNN group | p-value | |
| T classification | |||||||||
| T1 | 95.8 | 73.5 | 0.008 | 96.6 | 90.6 | 0.021 | 98.2 | 93.5 | 0.273 |
| T2 | 94.4 | 80.2 | 0.050 | 92.0 | 73.5 | 0.012 | 97.4 | 75.7 | 0.002 |
| T3 | 85.8 | 90.1 | 0.553 | 89.9 | 84.3 | 0.156 | 91.9 | 87.6 | 0.456 |
| T4 | 89.2 | 82.1 | 0.200 | 84.6 | 79.0 | 0.196 | 89.4 | 75.5 | 0.047 |
| N classification | |||||||||
| N1 | 92.1 | 88.0 | 0.532 | 91.7 | 88.9 | 0.286 | 93.2 | 87.9 | 0.167 |
| N2 | 88.1 | 78.1 | 0.023 | 87.8 | 81.0 | 0.127 | 94.4 | 78.1 | 0.001 |
| N3 | 83.2 | 85.6 | 0.660 | 86.0 | 70.3 | 0.026 | 89.0 | 80.7 | 0.347 |
| Overall stage (8th AJCC) | |||||||||
| 100 | 85.4 | 0.089 | 94.7 | 91.1 | 0.475 | 98.2 | 88.8 | 0.109 | |
| 87.3 | 84.6 | 0.353 | 91.2 | 87.0 | 0.159 | 94.1 | 87.7 | 0.060 | |
| 88.1 | 82.9 | 0.351 | 86.1 | 76.1 | 0.012 | 89.5 | 77.1 | 0.032 | |
Unless otherwise indicated, numbers are percentages. p-values were calculated by using the log-rank test. LNN, lymph nodal necrosis; LRFFS, locoregional failure free survival; DMFS, distant metastasis-free survival; OS, overall survival; AJCC, American Joint Committee on Cancer.
3. Univariate and multivariate analyses
The value of various potential prognostic factors including age, sex, lymph node status, maximal axial diameter, T classification, N classification, and overall stage on predicting LRFFS, DMFS, and OS were evaluated. Univariate analysis by log-rank test showed that lymph node status (LNN vs. non-LNN) was associated with LRFFS, DMFS, and OS (Table 4). Multivariate analysis by Cox proportional-hazards model showed that the lymph node status (LNN vs. non-LNN), T classification, and N classification were independent prognostic predictors of DMFS. Age, lymph node status (LNN vs. non-LNN), and overall stage were independent prognostic predictors of OS (Table 5).
Table 4.
Univariate analysis of variables correlated with various clinical endpoints
| Characteristic | No. of patients | 5-Year LRFFS | p-valuea) | 5-Year DMFS | p-valuea) | 5-Year OS | p-valuea) |
|---|---|---|---|---|---|---|---|
| Sex | |||||||
| Male | 418 | 86.3 | 0.201 | 85.3 | 0.110 | 88.3 | 0.304 |
| Female | 198 | 90.1 | 89.8 | 91.0 | |||
| Age (yr) | |||||||
| ≥ 48 | 319 | 87.6 | 0.815 | 86.0 | 0.711 | 85.8 | 0.017 |
| < 48 | 297 | 87.5 | 87.4 | 92.5 | |||
| Lymph node status | |||||||
| LNN | 235 | 83.8 | 0.049 | 81.8 | 0.001 | 82.9 | < 0.001 |
| Non-LNN | 381 | 89.7 | 89.8 | 93.0 | |||
| Maximal axial diameter (mm) | |||||||
| ≥ 24 | 310 | 85.5 | 0.061 | 81.6 | < 0.001 | 85.8 | 0.027 |
| < 24 | 306 | 89.6 | 91.8 | 92.6 | |||
| T classification | |||||||
| T1 | 93 | 88.5 | 0.777 | 94.5 | 0.071 | 96.5 | 0.017 |
| T2 | 93 | 89.2 | 84.8 | 88.9 | |||
| T3 | 238 | 87.4 | 87.8 | 90.3 | |||
| T4 | 192 | 86.5 | 82.4 | 83.9 | |||
| N classification | |||||||
| N1 | 304 | 90.8 | 0.124 | 90.9 | < 0.001 | 91.5 | 0.248 |
| N2 | 212 | 84.1 | 85.1 | 87.9 | |||
| N3 | 100 | 84.8 | 77.5 | 84.4 | |||
| Overall stage (8th AJCC) | |||||||
| 86 | 96.1 | 0.111 | 93.6 | 0.001 | 95.8 | 0.002 | |
| 262 | 86.3 | 89.7 | 91.8 | ||||
| 268 | 86.0 | 81.6 | 84.9 |
LRFFS, locoregional failure free survival; DMFS, distant metastasis-free survival; OS, overall survival; LNN, lymph nodal necrosis; AJCC, American Joint Committee on Cancer.
p-values were calculated by using the log-rank test.
Table 5.
Multivariate analysis of variables correlated with various clinical endpoints
| Endpoint | HR | 95% CI | p-value |
|---|---|---|---|
| LRFFS | |||
| Lymph node status | |||
| LNN vs. non-LNN | 1.550 | 0.986-2.438 | 0.058 |
| DMFS | |||
| N classification | 0.041 | ||
| N1 | 1 | ||
| N2 | 1.498 | 0.860-2.610 | 0.154 |
| N3 | 2.288 | 1.204-4.346 | 0.011 |
| T classification | 0.042 | ||
| T1 | 1 | ||
| T2 | 2.231 | 0.887-5.608 | 0.088 |
| T3 | 1.708 | 0.736-3.965 | 0.212 |
| T4 | 2.915 | 1.273-6.674 | 0.011 |
| Lymph node status | |||
| LNN vs. non-LNN | 1.634 | 1.023-2.609 | 0.040 |
| OS | |||
| Age (yr) | |||
| ≥ 48 vs. < 48 | 1.815 | 1.062-3.101 | 0.029 |
| Lymph node status | |||
| LNN vs. non-LNN | 2.154 | 1.282-3.620 | 0.004 |
| Overall stage (8th AJCC) | 0.026 | ||
| 1 | |||
| 2.384 | 0.614-9.263 | 0.210 | |
| 5.055 | 1.332-19.175 | 0.017 |
p-values were calculated by using the Cox proportional hazards model. HR, hazard ratio; CI, confidence interval; LRFFS, locoregional failure free survival; LNN, lymph nodal necrosis; DMFS, distant metastasis-free survival; OS, overall survival; AJCC, American Joint Committee on Cancer.
4. Proposed N classification system
Our data therefore suggested that N classification system could be optimized by adopting the following criteria: reclassified patients of classification N1 disease with LNN as classification N2, and classification N2 disease with LNN as classification N3. Correlation with death and distant failure was significant, and the total difference between N1 and N3 was wider with the proposed system. (Table 6). Furthermore, we used the area under the receiver operator characteristic curve (AUC) to quantify the prognostic performance of the proposed N classification system and the 8th edition of the N classification system. The AUC of the 8th edition of the N classification system was 0.551 (95% confidence interval [CI], 0.473 to 0.629) for death and 0.621 (95% CI, 0.554 to 0.689) for distant failure. The AUC of the proposed N classification system was higher than the 8th edition of the N classification system (0.606 [95% CI, 0.530 to 0.683] for death and 0.633 [95% CI, 0.567 to 0.698] for distant failure).
Table 6.
Effect of N classification for death and distant failure
| Death |
Distant failure |
|||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-value | HR | 95% CI | p-value | |
| Proposed system: N classification | 0.004 | < 0.001 | ||||
| N1 | 1 | 1 | ||||
| N2 | 1.149 | 0.566-2.331 | 0.700 | 1.562 | 0.819-2.978 | 0.175 |
| N3 | 2.549 | 1.346-4.824 | 0.004 | 3.240 | 1.786-5.878 | < 0.001 |
| 8th AJCC system: N classification | 0.254 | < 0.001 | ||||
| N1 | 1 | 1 | ||||
| N2 | 1.299 | 0.732-2.303 | 0.371 | 1.771 | 1.041-3.011 | 0.035 |
| N3 | 1.749 | 0.895-3.419 | 0.102 | 3.163 | 1.795-5.573 | < 0.001 |
Hazard ratios and p-values were calculated by using the Cox proportional hazards model. HR, hazard ratio; CI, confidence interval; AJCC, American Joint Committee on Cancer.
By the proposed system, 34.1% (210 of 616) of the patients were classified as N1, with 35.4% (218 of 616) as N2, and 30.5% (188 of 616) as N3. By the 8th AJCC system, 34.4% (212 of 616) would be classified as N2, and 16.2% (100 of 616) as N3, with the largest portion of patients (49.4%) as N1. We could conclude that the proposed system is obviously less skewed than that of the 8th AJCC system.
Discussion
In this study, we observed a high incidence of LNN in NPC with lymph nodes metastasis and LNN is an independent prognostic factor of DMFS and OS in NPC after IMRT. The proposed N classification system of NPC including LNN based on MRI was more predictive than that of the 8th AJCC system.
The reported incidence of LNN in NPC ranges from 22.9% to 44% [8-11]. In the era of MRI, Mao et al. [9] reported that the incidence of LNN was only 26.5% of 786 NPC patients with positive cervical lymph nodes. However, when the retropharyngeal lymph node status was included, the reported incidence of LNN ranges from 33% to 44% [8,11], which was similar to our results (38.1%).
The important implication of LNN is the presence of hypoxic tumor cells at the interface between necrotic areas and well-aerated cells. Increase in radioresistance of hypoxic tumor cells has been well documented. Also, tumor hypoxia may induce gene amplification associated with drug resistance [10]. IMRT has been accepted as the standard treatment technique of NPC [15]. In 2014, Tang et al. [16] reported that retropharyngeal LNN had a negative effect on disease failure in NPC after IMRT. In another study of 1,423 NPC patients treated by IMRT, multivariate analyses revealed that LNN was an independent negative prognostic factor for OS, DMFS, regional relapse-free survival, local relapse-free survival, and disease-free survival [11]. In the present study, multivariate analyses revealed LNN as a significant negative prognostic factor for OS and DMFS. A marginal significant difference was observed in terms of lymph node necrosis for LRFFS (p=0.058).
Four criteria were defined for a good staging classification system: (1) the different survival rates among the groups; (2) the given group including the subgroups defined by T, N, and M within a grouping system has similar survival rates; (3) the balanced distribution of patients among the groups; and (4) the high prediction of cure [17]. In the previous study, the total difference between classification N2 and N3 was wider with the proposed system which reclassified classification N1 with LNN as classification N2 and classification N2 with LNN as classification N3, and the hazard ratio discrimination between classification N2 and N3 was improved. Although a large cohort of patients (n=1,800) were included, most patients (n=1,064, 59.1%) were treated with conventional techniques and the AJCC 2010 staging system was adopted [8]. The proposed N classification system was proved to be powerfully predictive based on data with full statistical justification in the present study. More importantly, it meets the development of radiotherapy techniques and introduces the MRI-determined LNN to an N classification system for NPC based on the 8th AJCC system.
There are several limitations in the current study, including the retrospective nature of the study design and the inclusion of patients treated at a single center. We predict that patients with LNN may benefit from combined treatment. However, 97.5% patients underwent platinum-based chemotherapy, it would be inappropriate to evaluate the role of chemotherapy for patients with LNN in the present study. Moreover, multiparametric MRI-based radiomics from primary tumor provided improved prognostic ability in NPC [7]. The role of MRI-based radiomics from lymph nodes for NPC should be elucidated in the future.
In conclusion, MRI-determined LNN is an independent prognostic factor for NPC treated by IMRT. The proposed N classification system with MRI-determined LNN based on the 8th AJCC system is powerfully predictive.
Acknowledgments
The present study was supported by Zhejiang Province Medical and Health Science and Technology Project (No. 2017182785, 2018-244679).
Footnotes
Conflict of interest relevant to this article was not reported.
References
- 1.Sun X, Su S, Chen C, Han F, Zhao C, Xiao W, et al. Long-term outcomes of intensity-modulated radiotherapy for 868 patients with nasopharyngeal carcinoma: an analysis of survival and treatment toxicities. Radiother Oncol. 2014;110:398–403. doi: 10.1016/j.radonc.2013.10.020. [DOI] [PubMed] [Google Scholar]
- 2.Lin S, Pan J, Han L, Guo Q, Hu C, Zong J, et al. Update report of nasopharyngeal carcinoma treated with reduced-volume intensity-modulated radiation therapy and hypothesis of the optimal margin. Radiother Oncol. 2014;110:385–9. doi: 10.1016/j.radonc.2014.01.011. [DOI] [PubMed] [Google Scholar]
- 3.Lee AW, Ng WT, Chan LL, Hung WM, Chan CC, Sze HC, et al. Evolution of treatment for nasopharyngeal cancer--success and setback in the intensity-modulated radiotherapy era. Radiother Oncol. 2014;110:377–84. doi: 10.1016/j.radonc.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 4.Cao CN, Luo JW, Gao L, Yi JL, Huang XD, Wang K, et al. Update report of T4 classification nasopharyngeal carcinoma after intensity-modulated radiotherapy: an analysis of survival and treatment toxicities. Oral Oncol. 2015;51:190–4. doi: 10.1016/j.oraloncology.2014.11.009. [DOI] [PubMed] [Google Scholar]
- 5.Chua ML, Wee JT, Hui EP, Chan AT. Nasopharyngeal carcinoma. Lancet. 2016;387:1012–24. doi: 10.1016/S0140-6736(15)00055-0. [DOI] [PubMed] [Google Scholar]
- 6.Amin MB, Edge S, Greene F, Byrd DR, Brookland RK, Washington MK, et al. AJCC cancer staging manual. 8th ed. New York: Springer; 2017. [Google Scholar]
- 7.Zhang B, Tian J, Dong D, Gu D, Dong Y, Zhang L, et al. Radiomics features of multiparametric MRI as novel prognostic factors in advanced nasopharyngeal carcinoma. Clin Cancer Res. 2017;23:4259–69. doi: 10.1158/1078-0432.CCR-16-2910. [DOI] [PubMed] [Google Scholar]
- 8.Lan M, Huang Y, Chen CY, Han F, Wu SX, Tian L, et al. Prognostic value of cervical nodal necrosis in nasopharyngeal carcinoma: analysis of 1800 patients with positive cervical nodal metastasis at MR imaging. Radiology. 2015;276:619. doi: 10.1148/radiol.15154020. [DOI] [PubMed] [Google Scholar]
- 9.Mao YP, Liang SB, Liu LZ, Chen Y, Sun Y, Tang LL, et al. The N staging system in nasopharyngeal carcinoma with radiation therapy oncology group guidelines for lymph node levels based on magnetic resonance imaging. Clin Cancer Res. 2008;14:7497–503. doi: 10.1158/1078-0432.CCR-08-0271. [DOI] [PubMed] [Google Scholar]
- 10.Chua DT, Sham JS, Kwong DL, Choy DT, Leong L, Chan FL. Evaluation of cervical nodal necrosis in nasopharyngeal carcinoma by computed tomography: incidence and prognostic significance. Head Neck. 1997;19:266–75. doi: 10.1002/(sici)1097-0347(199707)19:4<266::aid-hed4>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 11.Zhang LL, Li JX, Zhou GQ, Tang LL, Ma J, Lin AH, et al. Influence of cervical node necrosis of different grades on the prognosis of nasopharyngeal carcinoma patients treated with intensity-modulated radiotherapy. J Cancer. 2017;8:959–66. doi: 10.7150/jca.17998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cao C, Jiang F, Jin Q, Jin T, Huang S, Hu Q, et al. Paranasal sinus invasion in nasopharyngeal carcinoma after intensity-modulated radiotherapy. Cancer Res Treat. 2019;51:73–9. doi: 10.4143/crt.2017.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van den Brekel MW, Stel HV, Castelijns JA, Nauta JJ, van der Waal I, Valk J, et al. Cervical lymph node metastasis: assessment of radiologic criteria. Radiology. 1990;177:379–84. doi: 10.1148/radiology.177.2.2217772. [DOI] [PubMed] [Google Scholar]
- 14.Jin T, Qin WF, Jiang F, Jin QF, Wei QC, Jia YS, et al. Cisplatin and fluorouracil induction chemotherapy with or without docetaxel in locoregionally advanced nasopharyngeal carcinoma. Transl Oncol. 2019;12:633–9. doi: 10.1016/j.tranon.2019.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang TJ, Riaz N, Cheng SK, Lu JJ, Lee NY. Intensity-modulated radiation therapy for nasopharyngeal carcinoma: a review. J Radiat Oncol. 2012;1:129–46. [Google Scholar]
- 16.Tang LL, Guo R, Zhou G, Sun Y, Liu LZ, Lin AH, et al. Prognostic value and staging classification of retropharyngeal lymph node metastasis in nasopharyngeal carcinoma patients treated with intensity-modulated radiotherapy. PLoS One. 2014;9:e108375. doi: 10.1371/journal.pone.0108375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Groome PA, Schulze K, Boysen M, Hall SF, Mackillop WJ. A comparison of published head and neck stage groupings in carcinomas of the oral cavity. Head Neck. 2001;23:613–24. doi: 10.1002/hed.1087. [DOI] [PubMed] [Google Scholar]


