Alternative polyadenylation generates transcriptomic diversity, although the physiological impact and regulatory mechanisms involved are still poorly understood. The cell cycle kinase Polo is controlled by alternative polyadenylation in the 3′ untranslated region (3′UTR), with critical physiological consequences. Here, we characterized the molecular mechanisms required for polo alternative polyadenylation.
KEYWORDS: 3′UTR, alternative polyadenylation, Drosophila melanogaster, Heph/PTBP1, USE, cell cycle, cis regulatory sequences, Polo
ABSTRACT
Alternative polyadenylation generates transcriptomic diversity, although the physiological impact and regulatory mechanisms involved are still poorly understood. The cell cycle kinase Polo is controlled by alternative polyadenylation in the 3′ untranslated region (3′UTR), with critical physiological consequences. Here, we characterized the molecular mechanisms required for polo alternative polyadenylation. We identified a conserved upstream sequence element (USE) close to the polo proximal poly(A) signal. Transgenic flies without this sequence show incorrect selection of polo poly(A) signals with consequent downregulation of Polo expression levels and insufficient/defective activation of Polo kinetochore targets Mps1 and Aurora B. Deletion of the USE results in abnormal mitoses in neuroblasts, revealing a role for this sequence in vivo. We found that Hephaestus binds to the USE RNA and that hephaestus mutants display defects in polo alternative polyadenylation concomitant with a striking reduction in Polo protein levels, leading to mitotic errors and aneuploidy. Bioinformatic analyses show that the USE is preferentially localized upstream of noncanonical polyadenylation signals in Drosophila melanogaster genes. Taken together, our results revealed the molecular mechanisms involved in polo alternative polyadenylation, with remarkable physiological functions in Polo expression and activity at the kinetochores, and disclosed a new in vivo function for USEs in Drosophila melanogaster.
INTRODUCTION
Genome-wide studies revealed a correlation between alternative polyadenylation (APA) in the 3′ untranslated region (3′UTR) and specific cellular programs such as cell activation, differentiation, proliferation, and development (1–6), as well as diseases such as myotonic dystrophy type 1 (7) and cancer (3, 8). Some APA regulators have been identified in cancer, such as CstF64, a well-known cleavage and polyadenylation factor (9), and the cleavage factor CFIm25 (10).
Although the physiological relevance of widespread APA is still not completely understood, it has now been demonstrated for several genes. In particular, APA regulates human CD47 membrane protein localization (11), brain-derived neurotrophic factor (BDNF) confinement to dendrites (12), Rac1 function in murine cortical neuron outgrowth (13), ubx mRNAs in central nervous system development (14), and Polo protein production and cell proliferation in Drosophila melanogaster (15).
Polo is the founder member of an important family of serine-threonine protein kinases with key functions in cell cycle regulation (16) and is also involved in human cancer (17, 18). polo contains two poly(A) (pA) signals in the 3′UTR that are differently selected to regulate Polo protein levels. The distal pA signal is mainly responsible for Polo protein production, as the mRNA containing the longest 3′UTR is more efficiently translated into protein, and the proximal pA signal controls Polo protein levels by an autoregulatory feedback mechanism (15).
Poly(A) signals and the protein factors involved in pre-mRNA cleavage and polyadenylation are well conserved between mammals and Drosophila melanogaster (19, 20). Similar to mammals, many functional homologues of cleavage/polyadenylation factors have been characterized (21–27), and the most common pA signal in D. melanogaster is the AAUAAA signal (28). However, about half of human and D. melanogaster pre-mRNAs do not contain a canonical pA signal (AAUAAA) (28–31), and yet they are efficiently cleaved and polyadenylated. In mammals, this is in part achieved by the existence of auxiliary cis elements upstream or downstream of the pA signal in the pre-mRNA, such as upstream sequence elements (USEs) that influence cleavage and polyadenylation in an orientation- and position-dependent manner (32–34). USEs are typically pyrimidine- or U-rich sequences that were initially identified in viruses (35–40) and subsequently described in human genes such as the C2 complement (41), COX-2 (42), MECP2 (43), F2 (44), collagen (45), and ADD1 (46) genes. However, USEs had not been identified in D. melanogaster yet.
Together with the core cleavage and polyadenylation factors, several RNA-binding proteins (RBPs) bind to pre-mRNA cis regulatory sequences to modulate mRNA 3′-end formation. Such an example is human polypyrimidine tract-binding protein (PTB/PTBP1/hnRNPI) (reviewed in references 47 to 50), which is a multifunctional RBP involved in alternative splicing regulation (50), polyadenylation (44, 51–54), mRNA localization in Xenopus (55), and internal ribosome entry site-driven translation (56). In Drosophila melanogaster, Hephaestus (Heph) (the PTBP1 homologue) is involved in alternative splicing (57, 58) and regulates oskar mRNA translation and mRNA levels (57), Gurken protein location (59), embryo dorsoventral patterning, and germ line-soma signaling (59–61), as well as spermatogenesis (58, 62–64). However, a function for Heph in APA had not been identified yet.
Here we characterized the regulatory cis element and RBP responsible for polo APA. We identified a cis regulatory element upstream of the proximal pA signal with a strong conservation with mammalian and viral USEs that have been previously implicated in the regulation of polyadenylation. We also demonstrate the function of the USE in polo APA in vivo, as deletion of this element causes developmental defects in flies and incorrect polo mRNA 3′-end formation, expression, and cell cycle progression. We identified Heph as an RBP that specifically binds to the polo USE RNA and demonstrate that Heph has a function in polo mRNA 3′-end formation and expression, which are impaired in heph mutants. We also show that the USE is present in 5.2% of all 3′UTRs and is most frequently found within 90 nucleotides (nt) upstream of noncanonical pA signals, suggesting a general function for these regulatory elements in Drosophila melanogaster. Collectively, we provide in vivo evidence for the function of USEs in Drosophila melanogaster and the action of Heph in polo APA and expression.
RESULTS
The polo 3′UTR contains a 28-nt-long conserved pyrimidine-rich sequence.
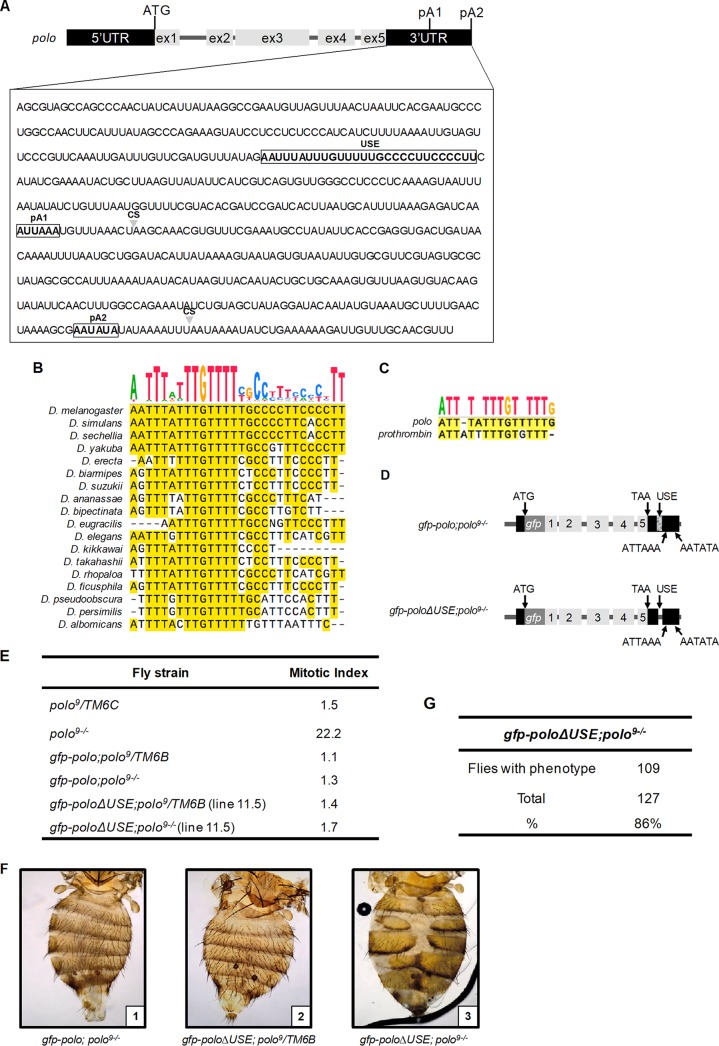
polo contains five exons and two pA signals in the 3′UTR, with the proximal pA signal (pA1, AUUAAA) localized 194 nt upstream of the distal pA signal (pA2, AAUAUA) (Fig. 1A). The usage of these two pA signals produces two mRNAs with 3′UTRs of different lengths that are necessary for correct protein production (15, 16). To identify potential cis regulatory elements in polo APA, we searched for conserved sequences present in the 3′UTR by aligning 18 Drosophila genomes using the SnapGene software (Fig. 1B). We identified a particularly conserved pyrimidine-rich sequence positioned 127 nt upstream of pA1. Notably, the thymidine tract TTGTTTT is conserved between the 50-million-year-distant Drosophila melanogaster and Drosophila albomicans (Fig. 1B). Part of this sequence is clearly similar to the previously described viral and human USEs (39, 42, 45, 65–68), in particular the prothrombin F2 USE (44) (Fig. 1C), and thus we named this sequence USE.
FIG 1.
The Drosophila melanogaster polo 3′UTR has a conserved pyrimidine-rich regulatory upstream sequence element (USE) whose in vivo deletion causes a prevalent abdominal phenotype. (A) Schematic representation of the polo gene. Gray boxes represent polo coding exons. The ATG refers to the translation start site. The upstream sequence element (USE) and the proximal (pA1) and distal (pA2) pA signals are indicated by white boxes. Arrowheads indicate pre-mRNA cleavage sites (CS). (B) Sequence alignments showing the conservation of the polo USE in 18 different Drosophila species. The sequence above the alignment represents the consensus sequence. (C) Sequence alignment and conservation of polo and prothrombin USEs. The sequence above the alignment represents the consensus sequence. (D) Schematic representation of the transgene constructed to generate gfp-poloΔUSE; polo9−/− transgenic flies. The arrow at the beginning of the transgene represents the endogenous polo promoter. Boxes 1 to 5 represent the five polo exons; black boxes represent the 5′UTR and 3′UTR. ATG refers to the translation start site, and TAA refers to the stop codon. The gfp box represents the gfp coding sequence inserted in frame with the polo initiation codon. The hatched box in the 3′UTR represents the USE, which was deleted in gfp-poloΔUSE; polo9−/− flies. ATTAAA and AATATA depict the proximal (pA1) and distal (pA2) pA signals, respectively. (E) Quantification of the mitotic index in gfp-polo and gfp-poloΔUSE third-instar larvae in either a polo9/TM6B or a polo9−/− genetic background. As a control, the mitotic index of the polo hypomorph polo9/TM6C and polo9−/− third-instar larvae was also determined. (F) Dorsal view of abdomen preparations from gfp-polo; polo9−/− (panel 1), gfp-poloΔUSE; polo9/TM6B (panel 2), and gfp-poloΔUSE; polo9−/− (panel 3) adult females. The anterior side is at the top. (G) Prevalence of the abdominal phenotype found in gfp-poloΔUSE; polo9−/− adult flies compared to heterozygous individuals.
The USE controls polo alternative polyadenylation and Polo protein levels in vivo.
To investigate the physiological function of the USE, we generated transgenic flies lacking the polo USE (Fig. 1A, D, and E). We used a transgene containing the polo gene tagged with the green fluorescent protein gene (gfp) to delete the 28-nt-long USE (Fig. 1D) (15, 69). We then studied flies expressing polo mRNAs without the USE in a polo9 heterozygous or homozygous background. Two independent transgenic lines were obtained. Homozygous gfp-poloΔUSE; polo9−/− individuals are viable, indicating that the GFP-Polo levels produced by gfp-poloΔUSE; polo9−/− flies are sufficient to rescue the polo9 lethality which occurs in the third-instar larval stage (70). The mitotic index in gfp-poloΔUSE; polo9−/− (Fig. 1E) larvae is equivalent to that in control individuals expressing gfp-polo (gfp-polo; polo9−/−) and in larvae heterozygous for the insertion (gfp-poloΔUSE; polo9/TM6B) (Fig. 1E), which corroborates the rescue capacity of the transgene. Remarkably, in both lines, gfp-poloΔUSE; polo9−/− adults show the same abdominal phenotype (Fig. 1F, panel 3), characterized by malformation of the abdominal epidermis. In particular, tergite defects similar to those presented by individuals with mutations in the polo proximal pA signal (gfp-poloΔpA1; polo9−/−) (15) are clearly observed. Individuals that still contain one polo allele provided by the TM6B balancer are fully viable and do not show any abnormal phenotype (Fig. 1F, panel 2), as expected. As Polo is required for the rapid histoblast cell divisions that occur during abdominal epidermis formation, the gfp-poloΔUSE; polo9−/− abdominal defects are possibly due to low Polo protein production. The abdominal phenotype is predominant in most gfp-poloΔUSE; polo9−/− adults (Fig. 1G). Notably, we observed that only 20% of homozygous adults are female and, in agreement with a polo loss of function described for the kinase-dead polo1 homozygous females (71, 72), that gfp-poloΔUSE; polo9−/− females are sterile, which further indicates that the USE has a physiological function in polo gene expression.
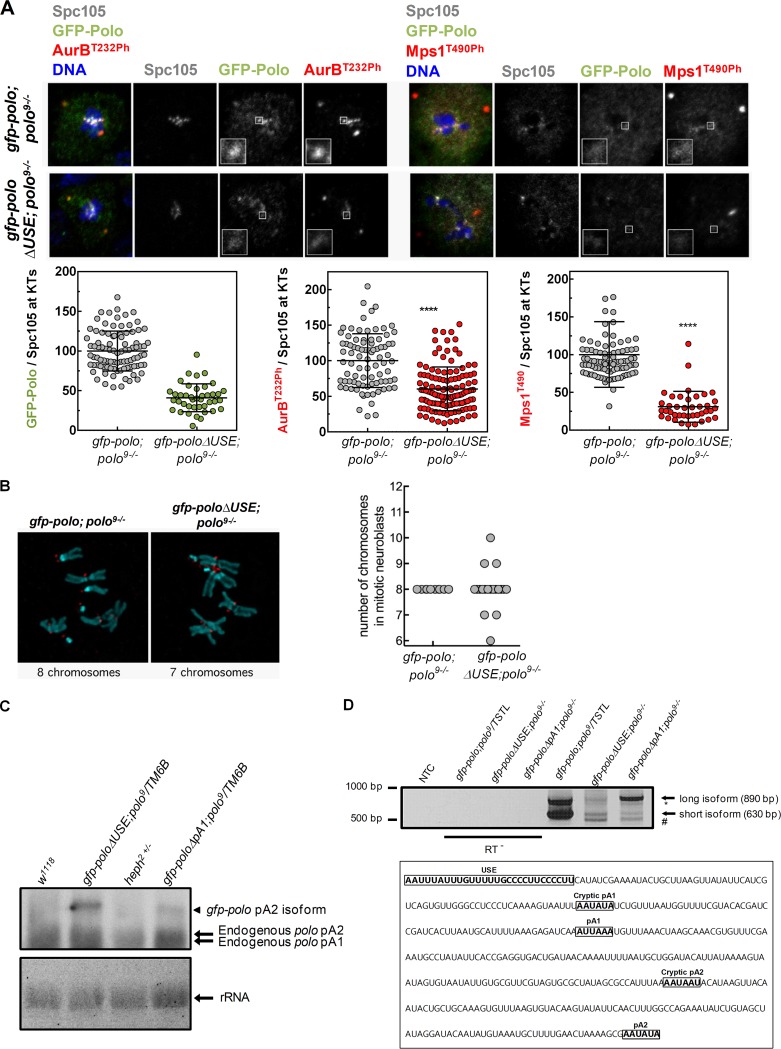
We then asked if the absence of the polo USE affects the biological pathways controlled by Polo. We focused on two well-characterized mitotic kinases, Aurora B and Mps1, which are Polo targets at the kinetochores of dividing neuroblasts (73–75). In gfp-poloΔUSE; polo9−/− kinetochores (Fig. 2A), we observed that GFP-Polo levels decreased 2-fold in comparison to those in the gfp-polo; polo9−/− control (Fig. 2A) (15, 76). Consequently, T-loop phosphorylation of Aurora B and Mps1 shows a similar reduction (Fig. 2A), indicating that kinetochore activation of these kinases is compromised upon deletion of the polo USE. Reduced activity of Mps1 and Aurora B in mitosis often leads to chromosome segregation errors. Accordingly, we detected gfp-poloΔUSE; polo9−/− (Fig. 2B) neuroblasts containing an abnormal number of chromosomes as opposed to the regular karyotype observed in every control neuroblast analyzed (gfp-polo;polo9−/−) (Fig. 2B).
FIG 2.
In vivo deletion of the polo USE impairs Polo activity and polo pA signal selection. (A) Representative immunofluorescence images and respective quantifications of GFP-Polo, Aurora BT232Ph, and Mps1T490Ph levels at kinetochores of dividing third-instar larval neuroblasts expressing gfp-polo; polo9−/− and gfp-poloΔUSE; polo9−/−. GFP-Polo, Aurora BT232Ph, and Mps1T490Ph fluorescence intensities were determined relative to the Spc105 signal, which was used as a kinetochore reference. All values were normalized to the control (gfp-polo; polo9−/−) mean fluorescence intensity, which was set to 100% (n ≥ 3 kinetochores from at least 10 neuroblasts for each condition). Results are expressed as mean values ± SD, and statistical significance was assessed by the Mann-Whitney test. (B) Representative immunofluorescence images of mitotic neuroblasts from squashed gfp-polo; polo9−/− and gfp-poloΔUSE; polo9−/− larval brains. Spc105 was used as a kinetochore reference. The chromosome content is shown for each representative neuroblast. The graph represents the quantification of chromosome numbers in gfp-polo; polo9−/− and gfp-poloΔUSE; polo9−/− mitotic neuroblasts. (C) Northern blot using total RNA from w1118 (lane 1), gfp-poloΔUSE; polo9/TM6B (lane 2), heph2/TM6B (lane 3), and gfp-poloΔpA1; polo9/TM6B (lane 4) third-instar larva brains. The two endogenous polo mRNAs present in every lane (endogenous polo pA1 and pA2 isoforms) are indicated by arrows. Only the gfp-polo pA2 isoform is expressed by the gfp-poloΔUSE; polo9/TM6B and gfp-poloΔpA1; polo9/TM6B strains, as indicated by the arrowheads. rRNA served as loading control. (D) 3′-end mapping of polo in the abdomens of gfp-poloΔUSE; polo9−/− and gfp-poloΔpA1; polo9−/− adults by 3′RACE. gfp-poloΔUSE; polo9−/− and gfp-poloΔpA1; polo9−/− flies produce two additional polo mRNA isoforms (depicted by * and #) other than the expected short (630-bp) and long (890-bp) mRNAs seen in the control (gfp-polo; polo9/TSTL). NTC, no-template control; and RT−, negative control. In the 3′UTR sequence of polo, the positions of the USE, both pA signals (pA1 and pA2), the cryptic pA1 (AAUAUA) 57 bp upstream of pA1, and the cryptic pA2 (AAUAAU) 113 bp upstream of pA2 are highlighted.
To investigate the function of the USE in polo mRNAs production, we performed Northern blot analyses in gfp-poloΔUSE; polo9/TM6B and gfp-poloΔpA1; polo9/TM6B flies (Fig. 2C). As previously described, gfp-poloΔpA1; polo9/TM6B transgenic flies present a single gfp-polo band corresponding to pA2 usage, indicating that polo pA1 mutation affects the shorter polo mRNA isoform production (Fig. 2C) (15). gfp-poloΔUSE; polo9/TM6B flies show an identical pattern of bands, indicating that the USE deletion also affects the production of polo shorter mRNA. The endogenous polo mRNAs are visible in all strains, as expected (Fig. 2C). To precisely map the 3′ ends of all polo mRNA isoforms produced by ΔUSE and ΔpA1 flies, we performed 3′ rapid amplification of cDNA ends (3′RACE) (Fig. 2D) and sequencing analyses in gfp-polo; polo9/TSTL (control), gfp-poloΔUSE; polo9−/−, and gfp-poloΔpA1; polo9−/− flies. As expected, control flies produce the two polo mRNA isoforms due to pA1 and pA2 usage (gfp-polo; polo9/TSTL) (Fig. 2D). gfp-poloΔUSE; polo9−/− and gfp-poloΔpA1; polo9−/− flies show impaired pA signal selection as cryptic pA signals were used, producing previously undescribed polo mRNA 3′ ends (Fig. 2D, * and #). The 3′ end of the new shortest isoform corresponds to the usage of the cryptic pA1 AATATA localized 57 nt upstream of polo pA1, and the novel longer isoform corresponds to the cryptic pA2 AATAAT positioned 113 nt upstream of pA2 (the sequence is shown in Fig. 2D). Although the Northern blot does not allow discrimination between the endogenous polo pA1 and polo cryptic pA1 bands detected by 3′RACE, these results show that USE deletion and pA1 mutation display abnormal mRNA 3′-end formation. Taken together, our data clearly demonstrate that the USE controls polo pA signal selection in vivo, modulating the correct levels of Polo protein at the kinetochores and ultimately ensuring mitotic fidelity.
Heph interacts with polo USE RNA.
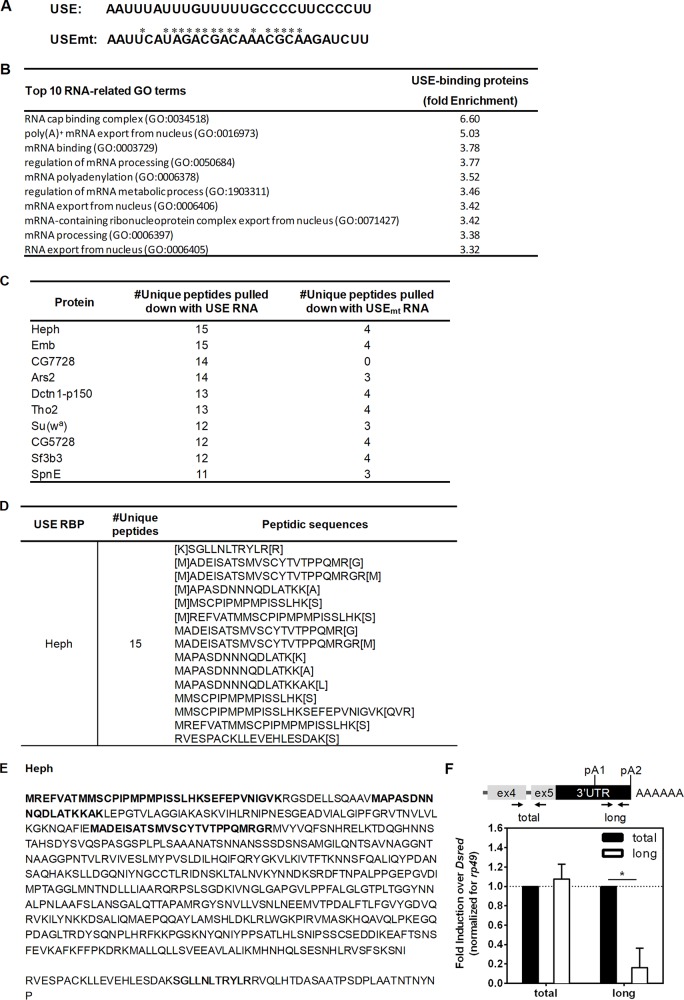
To identify the proteins that specifically bind to the USE RNA, we used an RNA-protein pulldown kit combined with liquid chromatography-tandem mass spectrometry (LC-MS/MS) analysis. We in vitro transcribed the 28-nt-long USE and a USE mutant in which 17 single mutations were introduced to specifically disrupt the most conserved nucleotides of the sequence, including TTGTTTTT (Fig. 3A, USEmt; the mutated nucleotides are marked with asterisks). We normalized both the USE and USEmt eluates to the same total amount of protein before loading the LC-MS/MS, and protein profiles from the eluates of USE and USEmt RNAs before LC-MS/MS separation show clear differences (see Fig. S1 in the supplemental material). The proteins identified by LC-MS/MS were considered positive hits if at least five unique peptides for the USE RNA (number of unique peptidesUSE ≥ 5) and fewer than five unique peptides for the USEmt RNA (number of unique peptidesUSEmt < 5) had been identified. The top ten RNA-related gene ontology (GO) terms of the proteins that bind to the USE but not to USEmt are enriched for several 3′-end processing events, such as poly(A)+ mRNA export from nucleus, regulation of mRNA processing, and mRNA polyadenylation (Fig. 3B). The top ten USE-binding proteins involved in RNA metabolism are shown in Fig. 3C sorted by the number of their unique peptides identified. The RBP Heph and Embargoed (Emb) (involved in actin export [77]) were identified by 15 unique peptides (Fig. 3D), and the Heph peptidic sequences detected are depicted in Fig. 3E in bold.
FIG 3.
Heph binds to polo USE RNA. (A) Sequences of the USE and USEmt RNAs used in the RNA-protein pulldown assay (5′ to 3′). *, point mutation in relation to the USE RNA. (B) Top 10 RNA-related GO terms enriched in the proteins that bind to the USE RNA detected by LC-MS/MS. (C) List of the top 10 RNA-related proteins obtained in LC-MS/MS that bind to the USE (number of unique peptides ≥ 5) and do not bind to USEmt (number of unique peptides < 5) ordered from largest to smallest number of unique peptides identified per protein. (D) Numbers of unique peptides and respective sequences identified by LC-MS/MS for Heph (corresponding to the UniProtKB entries Q7KRS7, A0A0B4K6W9, E1JJ45, Q7KES3, and Q8MT11). (E) Amino acid sequences of Heph with the peptidic sequences identified by LC-MS/MS highlighted in bold. From the LC-MS/MS data, the Heph UniProtKB entry Q7KRS7 sequence is depicted, which also includes the sequences of the A0A0B4K6W9, E1JJ45, and Q7KES3 entries. The Heph UniProtKB entry Q8MT11 sequence is truncated and shown below. (F) The longest polo mRNA levels are decreased upon 96 h of heph knockdown. polo mRNA isoform expression levels were measured by RT-qPCR with specific oligonucleotides targeting both polo mRNA isoforms (total) and the longest mRNA isoform (long). rp49 was used as a housekeeping gene. Fold induction over DsRed is displayed (DsRed was set to 1).
To investigate the function of Heph in polo mRNA expression, we knocked down heph in Drosophila S2 cells and quantified polo mRNA levels by reverse transcription-quantitative PCR (RT-qPCR) (Fig. 3F). We found that the levels of the longest polo isoform are significantly reduced, suggesting that Heph promotes the expression of the longest polo isoform.
Heph controls cell cycle progression through polo distal pA signal selection and Polo kinase activity.
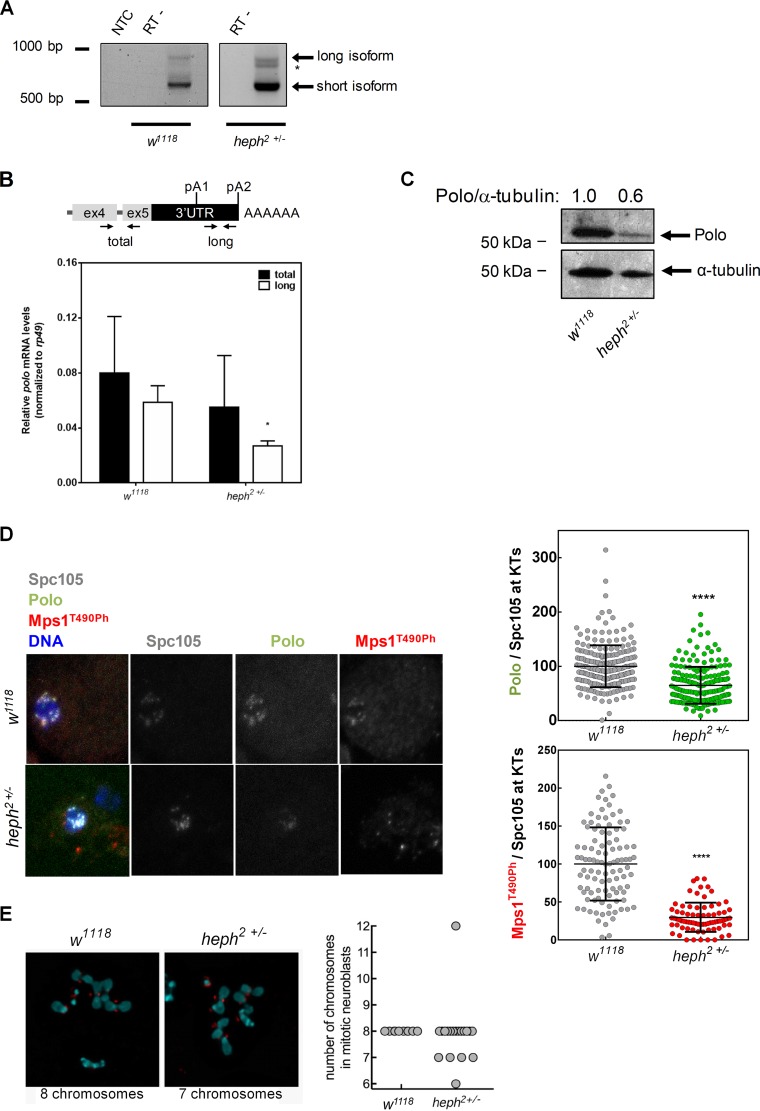
To understand the role of Heph in polo mRNA 3′-end formation, we mapped the 3′ ends of the mRNAs by 3′RACE in the heph2/TM6B hypomorph mutant. This allele had previously been shown to produce low levels of heph mRNA and protein (63, 64), and we also confirmed that heph mRNA levels are reduced (see Fig. S2 in the supplemental material). It was observed that cryptic pA2 is utilized by the heph2/TM6B mutant (heph2+/−) (asterisk in Fig. 4A), which corresponds to the cryptic pA2 used by the gfp-poloΔUSE; polo9−/− strain. We also quantified the levels of both polo mRNA isoforms by RT-qPCR and found that the levels of the longest polo mRNA are significantly decreased in the heph2/TM6B larval brain (Fig. 4B), which is similar to what we observed upon heph knockdown in Drosophila S2 cells (Fig. 3F). Additionally, by Northern blotting heph2/TM6B larval brains display smaller amounts of endogenous polo mRNAs than the w1118 control (Fig. 2C). Taken together, these results clearly indicate that Heph is necessary for correct distal polo pA signal selection.
FIG 4.
Heph regulates polo 3′-end formation and protein production. (A) 3′-end mapping of polo in w1118 and heph2/TM6B 0- to 24-h embryos by 3′RACE. polo pA signal usage in the heph2/TM6B mutant shows an additional mRNA isoform (*) compared to the w1118 control. NTC, no-template control; RT−, negative controls. (B) Expression levels of total polo mRNA in heph2/TM6B third-instar larval brains quantified by RT-qPCR. rp49 was used as a housekeeping gene. Error bars show SD from three independent experiments. *, P < 0.05. (C) Representative Western blot showing that Polo protein levels (anti-Polo MA294, 1:40) are decreased in heph2/TM6B mutant neuroblasts. α-Tubulin (anti-α-tubulin DM1A, 1:20,000) was used as loading control and for the semiquantification of Polo by densitometry depicted above each lane. (D) Representative immunofluorescence images and respective quantifications of Polo and Mps1T490Ph levels at kinetochores of dividing third-instar larval neuroblasts from w1118 (control) and heph2/TM6B mutants. Polo and Mps1T490Ph fluorescence intensities were determined relative to the Spc105 signal, which was used as a kinetochore reference. All values were normalized to the control (w1118) mean fluorescence intensity, which was set to 100% (n ≥ 7 kinetochores from at least 10 neuroblasts for each condition). Results are expressed as mean values ± SD, and statistical significance was assessed by the Mann-Whitney test. (E) Representative immunofluorescence images of mitotic neuroblasts from squashed Drosophila melanogaster larval brains with the w1118 and heph2/TM6B genotypes. Spc105 was used as a kinetochore reference. The chromosome content is shown for each representative neuroblast. The graph represents the quantification of chromosome numbers in w1118 and heph2/TM6B mitotic neuroblasts.
To understand the physiological function of Heph in Polo-dependent pathways, we started by analyzing total Polo protein levels in heph mutants. heph2/TM6B larval brains showed a 40% decrease in total Polo protein in comparison to the w1118 control (Fig. 4C). These results are consistent with a new function for Heph in polo distal pA signal selection (Fig. 4A and B), as polo mRNA containing the longest 3′UTR is responsible for most of the Polo protein production (15). Notably, at the kinetochores of dividing heph2/TM6B neuroblasts, there is a strong decrease in Polo and Mps1 protein and activity levels in comparison to those in the w1118 control (Fig. 4D). The physiological consequences are remarkable, which we confirmed by detecting a higher prevalence of aneuploidy in heph2/TM6B neuroblasts (Fig. 4E). Taken together, these results reveal new regulatory functions for Heph and clearly illustrate the in vivo effect of Heph on Polo protein production and downstream Polo targets that leads to mitotic abnormalities.
Drosophila melanogaster USEs are localized in the vicinity of polyadenylation signals.
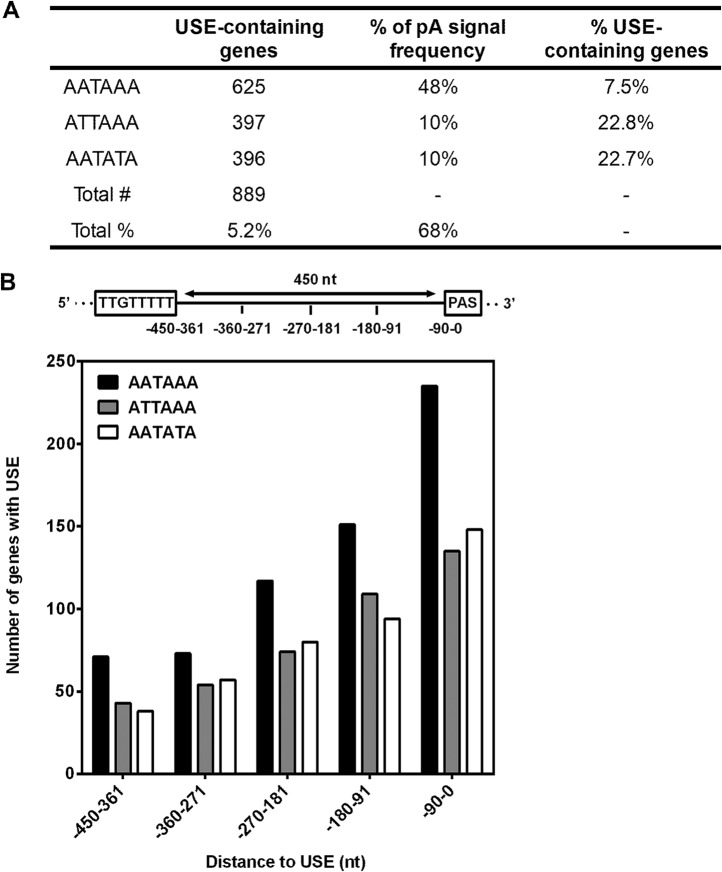
To investigate the prevalence of the USE at the genome-wide level in the fruit fly genome, and human genomes we developed an R-based script (78) to ask whether the most conserved region of the polo USE (TTGTTTTT) is associated with pA signals in the 3′UTR (see Fig. S3 in the supplemental material). As polo pA2 is 387 nt downstream of polo USE, we searched for USEs within a distance of 450 nt upstream of pA signals. We chose three different pA signals: AATAAA (the canonical pA signal in flies and humans [30, 79]), ATTAAA (polo pA1), and AATATA (polo pA2). While ATTAAA is the second-most-efficient pA signal in humans (80), with a 17% genome frequency in the human genome (30) and a 10% frequency in the fly genome (79), AATATA is a particularly weak signal in humans (80), with a 2% genome frequency the human genome (30) and a 10% frequency in the fly genome (79, 81). Importantly, we found that 5.2% of the D. melanogaster 3′UTRs contain a USE upstream of at least one of these pA signals (Fig. 5A, first column). In the human genome, 2.7% of all genes possess a USE upstream of at least one of these pA signals (see Table S1 [first column] in the supplemental material).
FIG 5.
USEs are more prevalent upstream of weak polyadenylation signals. (A) Number of Drosophila melanogaster genes containing the conserved USE upstream of AATAAA, ATTAAA, or AATATA, which corresponds to approximately 5.2% of the fly genome. The percentage of AATAAA-, ATTAAA-, or AATATA-containing genes is also depicted for Drosophila melanogaster (79), which approximately corresponds to 68% of the fly genome. The percentage of Drosophila melanogaster USE-containing genes was normalized to each pA signal frequency. The TTGTTTTT sequence is more common upstream of weaker pA signals in the 3′UTR (ATTAAA and AATATA in comparison to AATAAA). (B) The USE is located near pA signals in Drosophila melanogaster. USE-containing genes were sorted according to the distance (nucleotides) between the USE and each pA signal (AATAAA, ATTAAA, or AATATA). The distance was divided into 90-nt slots: 0 to 90, 91 to 180, 181 to 270, 271 to 360, and 361 to 450.
When the percentages of genes containing the AATAAA, ATTAAA, or AATATA signal across the D. melanogaster 3′UTRs were taken into consideration (79) (Fig. 5A, second column), we observed a higher prevalence of the USE upstream of noncanonical pA signals (ATTAAA and AATATA) compared to the canonical signal (AATAAA) (Fig. 5A, third column). For human genes, we observed a similar trend; i.e., the prevalence of the USE is higher upstream of AATATA in comparison to AATAAA or ATTAAA (Table S1, third column). To investigate the distribution of the USE in the proximity of pA signals, we divided the 450-nt distance evenly into 90-nt bins. Interestingly, we observed that the USE is more prevalent near pA signals regardless of pA signal strength (Fig. 5B). This is similar in humans (see Fig. S4 in the supplemental material), in agreement with previous observations in mammals (44), and indicates a conservation in the genomic architecture of the USE pA signal between Drosophila melanogaster and mammals. Taken together, these results suggest that the USE may act as a regulator of gene expression in Drosophila melanogaster.
DISCUSSION
Polo protein levels are critical for cell cycle progression (16) and are tightly controlled by APA, having a crucial physiological function in fly development (15). Here we identified the regulatory cis element with an in vivo function, USE, and a protein factor responsible for correct polo APA, Heph. We further showed that both the USE and Heph are necessary for correct Polo levels at the kinetochores and cell cycle progression.
USEs were initially described in mammals and viruses as U-rich cis auxiliary elements localized upstream of pA signals that bind RBPs and modulate the efficiency of mRNA 3′-end formation (32, 41, 44, 45, 51, 67, 82–85). We identified the first example of a USE in D. melanogaster, upstream of polo proximal pA signal, which shows strong sequence and functional similarity to USEs in other species, indicating that these elements are widespread. Using gfp-poloΔUSE; polo9−/− transgenic flies, we unequivocally demonstrate an in vivo function for the polo USE. Deletion of this USE results in a phenotype consistent with reduced Polo protein levels, female sterility, low levels of crucial cell cycle kinases at the kinetochores, and high levels of aneuploidy. gfp-poloΔUSE; polo9−/− flies display the same phenotype as flies that are mutated in pA1 (gfp-poloΔpA1; polo9−/−), an abdominal phenotype characterized by malformation of the tergites which was previously shown to correlate with incorrect polo APA (15). Accordingly, mapping of the 3′ ends of the mRNAs and Northern analyses revealed that cryptic pA signals are activated whenever pA1 is mutated or the USE is deleted, demonstrating a role for this cis element in APA in Drosophila melanogaster.
Polo has key functions at the kinetochores, where it promotes the phosphorylation of Mps1 and Aurora B kinases, both of which are essential for faithful cell cycle progression (73). Noticeably, deletion of the USE in gfp-poloΔUSE; polo9−/− flies causes a decrease in Polo levels at the kinetochores that results in reduced activation of Mps1 and Aurora B. The suboptimal activity of these essential mitotic kinases at the kinetochores of gfp-poloΔUSE; polo9−/− neuroblasts compromises mitotic fidelity and ultimately culminates in severe abnormalities, such as aneuploidies. As Polo protein levels are controlled by APA (15), these results further corroborate a physiological function for the USE in pA signal selection in D. melanogaster. We envisage that the polo USE is particularly relevant to enhance Polo levels in highly proliferative tissues.
In humans, USEs have been shown to act on pA signal through recruiting core and auxiliary polyadenylation factors. For instance, the USEs of the COX-2, prothrombin F2, and C2 complement pre-mRNAs are recognized by PTBP1, which affects pA signal efficiency (42, 44, 51). Interestingly, we have identified Heph, the orthologue of PTBP1 in Drosophila, as a regulator of polo APA via binding to polo USE. We show that Heph binds to the USE RNA but not to a mutated USE. We demonstrate that the heph2/TM6B mutant, which has a phenotype in testis (62) and defects in spermatogenesis (58, 63) that ultimately lead to male sterility (64), presents abnormal polo mRNA 3′-end formation with selection of the cryptic pA2, which is also chosen by gfp-poloΔUSE; polo9−/− flies. We also observed that gfp-poloΔUSE; polo9−/− flies use another cryptic pA signal upstream of pA1 (cryptic pA1) which is not used by heph2/TM6B mutants, implying that the in vivo deletion of the polo USE is more severe in pA signal selection than the lack of Heph. Our results are consistent with a new function for Heph in polo distal pA signal selection, which is corroborated by the lower levels of the longest polo mRNA isoform observed in the absence of Heph both in vitro (heph knockdown in Drosophila S2 cells) and in vivo (heph2/TM6B mutants). Accordingly, heph2/TM6B mutants present lower Polo protein levels, as the longest polo mRNA isoform is the main one responsible for protein production (15). Remarkably, at the kinetochores of heph2/TM6B neuroblasts, the activation of both Polo and Mps1 is hindered, thus compromising normal cell cycle progression in a manner similar to that seen in the neuroblasts of gfp-poloΔUSE; polo9−/− flies. Furthermore, we found that neuroblasts with reduced levels of Heph are more prone to missegregate chromosomes and become aneuploid. These results indicate that the absence of Heph or the USE has an identical impact on Polo protein levels and efficient activation of Polo downstream targets and that Heph is responsible for correct distal polo pA signal selection.
The conservation of the USE observed along the Drosophila genus suggests a functional role for this sequence. Using a bioinformatic approach, we showed that 5.2% of all Drosophila melanogaster genes possess the most conserved region of the polo USE (TTGTTTTT) upstream of a pA signal in their 3′UTRs, highlighting the relevance of the USE in D. melanogaster. Interestingly, we also found that the USE is more frequently localized upstream of weak pA signals, which are less efficiently processed than the canonical signals (80) and therefore more likely to be regulated (29). GO analyses show that USE-containing genes are enriched for important physiological roles such as development, neurogenesis, and RNA processing events (see Tables S2 to S4 in the supplemental material).
Collectively, we show for the first time that USEs are conserved and widespread in the 3′UTRs of Drosophila melanogaster. We also demonstrate that the polo USE has a crucial role in vivo, modulating polo APA and Polo activity at the kinetochores. Consequently, the lack of the USE affects key cell cycle kinases that are downstream targets of Polo, ultimately causing mitotic abnormalities, such as aneuploidies. Finally, our results reveal a new physiological function for Heph in polo pA signal selection, adding a new level of complexity to this regulatory mechanism.
MATERIALS AND METHODS
USE conservation analysis.
The polo 3′UTR sequences of 18 Drosophila species were obtained from FlyBase (86) and aligned using the SnapGene software (from GSL Biotech, Chicago, IL). The colored sequences above the alignments in the figures represent the consensus sequence.
Drosophila melanogaster fly stocks.
The heph2 hypomorph mutant flies were obtained from the Bloomington Drosophila Stock Center (Indiana University, Bloomington, IN). These flies were balanced with TM6B. polo9/TM6C flies were kindly provided by David Glover (Department of Genetics, University of Cambridge, Cambridge, United Kingdom). Mz1061-Gal4; gfp-polo; polo9/TSTL (referred to as gfp-polo;polo9/TSTL) and w1118; gfp-poloΔpA1; polo9/TM6B (referred to as gfp-poloΔpA1; polo9/TM6B) flies were previously described (15, 76). All stocks were grown at 25°C with standard culture conditions and media.
Generation of gfp-poloΔUSE; polo9−/− flies.
Transgenic flies carrying pW8-gfp-poloΔUSE1 were obtained by injection of w1118 embryos with the respective transgenes, as described previously (87). Two viable and fertile homozygous lines were obtained with the transgene inserted on the second chromosome. Selection of transgenic lines was performed by mating the transgenic flies with a strain carrying dominant markers and a balancer chromosome, w1118; Sco/SM6. These were then mated with w1118; If/CyO; MKRS/TM6B and w1118; If/CyO; polo9/TM6C flies to generate the w1118; gfp-poloΔUSE; polo9/TM6B line (referred to as gfp-poloΔUSE; polo9/TM6B).
Preparation of adult abdomens.
Flies were dissected between the thorax and the abdomen, carefully removing all appendages. The abdomens were then incubated in a solution of lactic acid and double-distilled water (ddH2O) (3:1) overnight at 60°C, mounted in a fresh lactic acid-ddH2O (3:1) solution, and incubated overnight in a 60°C oven.
gfp-poloΔUSE; polo9−/− abdomen phenotype analysis.
gfp-poloΔUSE; polo9−/− individuals were identified by the Hu marker, and every abdominal defects in comparison to heterozygous individuals was considered an abnormality. These defects included fewer or missing bristles and missing, malformed, or nicked tergites in both males and females.
Determination of mitotic index.
Third-instar larval brains were dissected in phosphate-buffered saline (PBS) and fixed in 45% (vol/vol) acetic acid followed by 60% (vol/vol) acetic acid for 1 min. The fixed brains were squashed and immersed in liquid nitrogen. DNA was counterstained with DAPI (4′,6′-diamidino-2-phenylindole) in Vectashield (Vector Laboratories). For each genotype, eight brains were prepared, and 1,000 cells were scored for each brain. The mitotic index was then determined as the number of mitotic cells present over the total number of cells. All quantifications were performed on an Axioskop upright microscope (Carl Zeiss, Germany) with a black-and-white or color Spot 2 charge-coupled device (CCD) camera (Diagnostic Instruments, USA).
Immunofluorescence in third-instar larval brain squashes.
Dissected third-instar larval brains from each strain were placed in fresh 50 μM colchicine (Sigma-Aldrich) in PBS for 1 h 30 min at 25°C and then fixed for 5 min with 1.8% formaldehyde (Sigma-Aldrich) and 45% acetic acid. Preparations were squashed between a coverslip and a slide and immersed in liquid nitrogen. The coverslip was then flipped off, and brain squashes were incubated with absolute cold ethanol for 10 min and then with PBS–0.1% Triton X-100 10 min for permeabilization, followed by a 10-min wash with PBS. The slides were processed for immunostaining as previously described (88). Fixed brains were blocked with 10% fetal bovine serum (FBS) in PBS–0.05% Tween 20 for 1 h at room temperature and then incubated overnight at 4°C with the following primary antibodies: rat anti-Spc105 (1:150) (73), mouse anti-Polo (1:10; MA294), rabbit anti-P-Thr232-Aurora B (1:500; Rockland Immunochemicals, Limerick, PA), and rabbit anti-P-Thr676-Mps1 (1:2,000; a gift from Geert Kops [89]). This was followed by three 5-min washes in PBS–0.05% Tween 20 and incubation with the following secondary antibodies (Thermo Fisher Scientific) diluted 1:250 in PBS–0.05% Tween 20 for 2 h at room temperature: goat anti-rat antibody–Alexa Fluor 647, goat anti-mouse antibody–Alexa Fluor 488, goat anti-rabbit antibody–Alexa Fluor 568, and goat anti-guinea pig antibody–Alexa Fluor 568. This was followed by three washes. Image acquisition was performed using a laser Leica TCS SP5 II scanning confocal microscope and the LAS 2.6 software (Leica Microsystems, Wetzlar, Germany). Image analyses were performed using Fiji (https://fiji.sc/) (90). Neuroblasts were identified as the larger cells based on the background signal from the different antibodies used that made the cell shape visible. For immunofluorescence quantification, each individual kinetochore was detected by their constitutive marker Spc105 mean fluorescence intensity, and a region of interest (ROI) corresponding to each kinetochore per cell was defined. The same ROIs per cell were then used to detect the mean fluorescence intensity for Polo, P-Aurora, and P-Mps1, and these values were then normalized to Spc105. Background intensity was defined by the mean fluorescence intensity of ROIs inside the cell free of kinetochores and subtracted from each value. Control values were averaged and used for normalization of values determined under the different biological conditions tested.
Aneuploidy immunofluorescence in third-instar larval brain squashes.
Dissected third-instar larval brains with the gfp-polo; polo9−/−, gfp-poloΔUSE; polo9−/−, w1118, and heph2/TM6B genotypes were squashed and incubated with colchicine as described above to obtain chromosome spreads that facilitate the visualization of chromosome content. Spc105 was used as a kinetochore reference.
Total RNA purification.
For each strain, total RNA of 20 third-instar larval brains was extracted with TRIzol (Thermo Fisher Scientific) according to the manufacturer’s protocol. RNA integrity was analyzed either using an Experion RNA StdSens analysis kit with an Experion automated electrophoresis station or by 1 to 1.5% agarose gel electrophoresis.
3′RACE.
cDNAs for 3′ rapid amplification of cDNA ends (3′RACE) were synthesized using SuperScript IV reverse transcriptase (Thermo Fisher Scientific) according to the manufacturer’s instructions, with 2.5 μM 3′RACE adapter primer (sequence shown below) and 0.5 to 1 μg of total RNA isolated as mentioned above from w1118 and heph2/TM6B mutant embryos and gfp-polo; polo9/TSTL, gfp-poloΔUSE; polo9−/−, and gfp-poloΔpA1; polo9−/− female adult abdomens. The 3′RACE PCR was performed using GoTaq DNA polymerase (Promega, Madison, WI) according to the manufacturer’s instructions, an anchor primer (which annealed to the 3′RACE adapter primer [sequence shown below]), and a specific primer on the coding sequence of polo (sequence shown below). Reactions were performed in a Biometra 48-well T-Personal thermocycler (Analytik Jena, Jena, Germany) with the following program: 5 min at 95°C; 35 cycles of 30 s at 95°C, 1 min at 52°C, and 90 s at 72°C; and a final elongation step for 10 min at 72°C. The PCR products were then separated on a 0.8 to 2% agarose gel, and the bands were cut and kept at −80°C overnight. The bands were then incubated at 42°C for 4 min, and the products were purified using Sephadex columns. Each sample was sequenced with specific primers for polo.
RNA-protein pulldown assay.
One-microgram quantities of oligonucleotides containing a T7 anchor (GTAATACGACTCACTATAGGG) followed by the wild-type or mutated USE (NZyTech, Lisbon, Portugal) were used as DNA templates for in vitro transcription using the MEGAscript T7 transcription kit (Thermo Fisher Scientific) and the Magnetic RNA-protein pulldown kit (Thermo Fisher Scientific) according to the manufacturer’s instructions. Briefly, 50 pmol of the in vitro-transcribed RNAs was biotinylated and bound to streptavidin magnetic beads for 30 min with agitation. The complex was incubated with 200 μg of D. melanogaster 0- to 24-h embryo protein extract for 45 min at 4°C with agitation (the RNA-protein binding buffer included 20 mM Tris [pH 7.5], 300 mM NaCl, 2 mM MgCl2, and 0.1% Tween 20) and then washed before the bound proteins were eluted from the RNAs with agitation. Half of the eluate was separated in 12% (resolving) and 5% (stacking) Tris-glycine-sodium dodecyl sulfate (SDS)-polyacrylamide gels at 120 V for 1 h 30 min and stained with BlueSafe (NZyTech) for 1 h to determine protein recovery. The other half was processed for identification by mass spectrometry.
Mass spectrometry and gene ontology analyses of USE RBPs.
Protein eluates were first reduced, alkylated, and digested with trypsin and then separated on a 15-cm liquid chromatography (LC) C18 column with a 2-h run and eluted into an electrospray ionization high-resolution accurate-mass Orbitrap mass spectrometer (Q Exactive; Thermo Fisher Scientific). Results were acquired in the data-dependent (dd) positive acquisition mode alternating between a full scan and subsequent higher-energy collisional dissociation tandem mass spectrometry (MS/MS) of the 10 most intense peaks from the full scan. Protein identification was performed based on the 8,634 Drosophila melanogaster proteins classified as mRNA binding in the UniProt database (91) using the Proteome Discoverer 2.2 software (Thermo Fisher Scientific). The Precursor Ions Quantifier node of the Proteome Discoverer software calculated the protein abundances in the samples, normalized the peptide groups, and scaled them. Normalization was based on total peptide amount by summing the peptide group abundances for each sample and determining the maximum sum within a sample. The sample with the highest abundance is taken as a reference, and the abundance values of the other samples are corrected by a constant factor per sample so that at the end the total abundance is the same for all samples. Proteins identified by five or more unique peptides according to Proteome Discoverer were considered reliable hits, and their accession codes were uploaded onto the PANTHER platform (92, 93) (version 13.1, released on 3 February 2018) to analyze their gene ontology (GO) molecular functions. Peptidic sequences of Heph (corresponding to UniProtKB entries Q7KRS7, A0A0B4K6W9, E1JJ45, Q7KES3, and Q8MT11 identified in LC-MS/MS) were obtained from the UniProt database (91).
Drosophila S2 cell culture.
Semiadherent Drosophila S2 cells were cultured at 25°C in Schneider’s insect medium (Sigma-Aldrich) supplemented with 10% heat-inactivated fetal bovine serum (FBS) (Gibco) without antibiotics. All assays were initiated with cells in exponential growth.
hephaestus RNAi in Drosophila S2 cells.
Double-stranded RNA (dsRNA) for hephaestus knockdown by RNA interference (RNAi) was generated by in vitro transcription using the MEGAscript T7 kit. Briefly, 1 μg of Drosophila S2 cell DNA with opposing T7 promoters at the 5′ end of each strand was used as the template, and a single transcription reaction was performed at 37°C for 16 h. The dsRNA was purified with the PureLink RNA minikit (Ambion) following the manufacturer’s instructions for the purification of RNA from liquid samples. To anneal the RNA strands, the reaction mix was incubated in a 48-well T-Personal thermocycler with the following program: 96°C for 5 min and 1 min for each further cycle, sequentially decreasing the temperature by 1°C until reaching 24°C. Drosophila S2 cells (1 × 106) were plated in Schneider’s medium without FBS, and 3 μg of heph dsRNA was added dropwise. After 1 h at 25°C, 2 ml of regular growth medium was added. dsRNA for DsRed (coding for the red fluorescent protein of Discosoma sp.) was used as control. After 96 h, cells were collected and their RNA was extracted.
Western blotting.
Twenty third-instar larval brains were homogenized in 50 mM Tris-HCl (pH 7.5)–1 mM EDTA–10% glycerol–50 mM NaF–5 mM sodium pyrophosphate–1% Triton X-100–1 mM dithiothreitol (DTT)–0.1 mM phenylmethyl sulfonyl fluoride–1:100 Na3VO4 and protease inhibitor cocktail (Sigma-Aldrich). The extracts were incubated for 20 to 30 min at 4°C with rotation and centrifuged at 8,000 rpm for 5 min to remove debris. Total protein was determined by the Bradford protein assay (Bio-Rad protein assay dye reagent concentrate). Proteins were separated in 7.5% (resolving) and 5% (stacking) Tris-glycine-SDS-polyacrylamide gels at 120 V for 1 h 30 min and transferred to a nitrocellulose membrane (Novex; Thermo Fisher Scientific) with the iBlot gel transfer device (Invitrogen) using the P3 parameters for 7 min. After blocking in 5% nonfat dry milk in Tris-buffered saline and 0.01% Tween 20, membranes were incubated with mouse anti-Polo (MA294, 1:40) and mouse anti-α-tubulin DM1A (1:20,000; Santa Cruz Biotechnology) (loading control) and then incubated with goat anti-mouse immunoglobulin G–horseradish peroxidase (1:20,000, Santa Cruz Biotechnology). Signal was detected with the Amersham ECL Prime Western blotting detection reagent (GE Healthcare), the Amersham Hyperfilm ECL (GE Healthcare), and the Fuji Medical Film Processor FPM-100A together with the Anatomix developer and X-Fix fixer (Fujifilm Europe GmbH). Protein semiquantification was performed using the Molecular Imager GS-800 calibrated densitometer (Bio-Rad) and analyzed with the Quantity One software (Bio-Rad).
Northern blotting.
Probes were generated by PCR using w1118 or gfp-poloΔUSE; polo9/TM6B larval brain cDNA (the oligonucleotides used are shown below) and were labeled with [α-32P]dCTP (10 mCi/ml) (PELSBLU013H250UC; PerkinElmer) according to the manufacturer’s instructions. Twenty micrograms of total RNA from w1118, heph2/TM6B, gfp-poloΔUSE; polo9/TM6B, and gfp-poloΔpA1; polo9/TM6B larval brains was loaded onto a 1% agarose gel and separated by electrophoresis for 6 to 7 h at 75 V. mRNA was transferred to a Hybond-N+ nylon membrane (Amersham Biosciences), which was cross-linked in a Hoefer UVC 500 UV cross-linker (700 J/cm2). The hybridization temperatures were 46.6°C for the polo probe and 51.5°C for the gfp probe. The Northern blot was developed using Fuji Super RX-N medical X-Ray film (Fujifilm).
Oligonucleotide sequences.
The following oligonucleotide sequences (5′ to 3′) were used in this study: 3′RACE adapter primer, GCGAGCACAGAATTAATACGACTCACTATAGGT15VN; anchor primer, GCGAGCACAGAATTAATACGACT; polo small isoform Northern probe, CCGTACAACATGTGCCGTAG (forward) and CTTTAGACACGCCGTTCTCC (reverse); polo large isoform Northern probe, CGGGTTTGCAAAATGTTACG (forward) and AGATTGGCCTTGAGGAAGGT (reverse); gfp Northern probe, GGAGAGGGTGAAGGTGATGC (forward) and TCGAAAGGGCAGATTGTGTG (reverse); polo short RT-qPCR primers, CCGTACAACATGTGCCGTAG (forward) and CTTTAGACACGCCGTTCTCC (reverse); polo long RT-qPCR primers, TACTGCTGCAAAGTGTTTAAGTG (forward) and CGCTTTTAGTCAAAAGCATTTAC (reverse); heph RT-qPCR primers, ATCACACGTATCGGCTTTCC (forward) and CACAGCCATGTCTCACTT (reverse); rp49 RT-qPCR primers, ATCGGTTACGGATCGAACAA (forward) and GACAATCTCCTTGCGCTTCT (reverse).
Sequence motif search and gene ontology enrichment of USE-containing genes.
NCBI RefSeq transcripts of Drosophila melanogaster (assembly dm6) and Homo sapiens (assembly hg38) were retrieved from UCSC Table Browser as .bed files. Using an R-based script (78), we queried the 3′UTR of the Drosophila melanogaster and human genomes for the presence of the conserved TTGTTTTT sequence. The search was restricted to 450 nt upstream of one of three different pA signals: AATAAA, ATTAAA, or AATATA (see Tables S5 and S6 in the supplemental material). Gene ontology (GO) enrichment analysis of the USE-containing genes was performed using the PANTHER platform (92, 93) (version 13.0, released on 12 November 2017).
Frequency and distance-to-pA signal analyses of USE-containing genes.
The genome-wide percentage of the USE-containing genes was calculated using the frequency of each pA signal (AATAAA, ATTAAA, or AATATA) in the Drosophila melanogaster (79) and human (30) genomes. Additionally, the 3′UTRs of USE-containing genes were evenly divided in 90-nt slots up to 450 nt, and the genes were sorted according to distance between the USE and each pA signal.
Statistical analysis.
For each assay, at least three independent experiments were performed. Results were expressed as mean values ± standard deviations (SD) determined by Prism 7.02 (GraphPad Software, La Jolla, CA). Differences in P values below 0.05 calculated via a two-tailed paired Student t test were considered statistically significant. Statistical significance in the immunofluorescence assays was assessed by the Mann-Whitney test in GraphPad Prism 7.02.
Supplementary Material
ACKNOWLEDGMENTS
This work was funded by Fundo Europeu de Desenvolvimento Regional (FEDER) funds through the COMPETE 2020-Operational Program for Competitiveness and Internationalisation (POCI), Portugal 2020, and by Portuguese funds through Fundação para a Ciência e a Tecnologia/Ministério da Ciência (FCT), Tecnologia e Ensino Superior, in the framework of the project Institute for Research and Innovation in Health Sciences (POCI-01-0145-FEDER-007274), and project Norte-01-0145-FEDER-000008-Porto-Neurosciences and Neurologic Disease Research Initiative at i3S, supported by Norte Portugal Regional Operational Program (NORTE 2020) under the PORTUGAL 2020 Partnership Agreement through the European Regional Development Fund (FEDER). M.S.O. was supported by Instituto de Ciências Biomédicas Abel Salazar and funded by the FCT Ph.D. fellowship SFRH/BD/102002/2014; C.C. is supported by an FCT investigator position and funding (IF/01755/2014). Stocks obtained from the Bloomington Drosophila Stock Center (NIH P40OD018537) were used in this study.
We are very grateful to David Glover (Cambridge University) and Geert Kops (Utrecht University, Utrecht, The Netherlands) for fly strains, antibodies, and reagents. We thank all current and past members of the laboratories for helpful suggestions.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/MCB.00581-18.
REFERENCES
- 1.Sandberg R, Neilson JR, Sarma A, Sharp PA, Burge CB. 2008. Proliferating cells express mRNAs with shortened 3′ untranslated regions and fewer microRNA target sites. Science 320:1643–1647. doi: 10.1126/science.1155390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ji Z, Lee JY, Pan Z, Jiang B, Tian B. 2009. Progressive lengthening of 3′ untranslated regions of mRNAs by alternative polyadenylation during mouse embryonic development. Proc Natl Acad Sci U S A 106:7028–7033. doi: 10.1073/pnas.0900028106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mayr C, Bartel DP. 2009. Widespread shortening of 3′UTRs by alternative cleavage and polyadenylation activates oncogenes in cancer cells. Cell 138:673–684. doi: 10.1016/j.cell.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moreira A. 2011. Integrating transcription kinetics with alternative polyadenylation and cell cycle control. Nucleus 2:556–561. doi: 10.4161/nucl.2.6.18064. [DOI] [PubMed] [Google Scholar]
- 5.Lutz CS, Moreira A. 2011. Alternative mRNA polyadenylation in eukaryotes: an effective regulator of gene expression. Wiley Interdiscip Rev RNA 2:22–31. doi: 10.1002/wrna.47. [DOI] [PubMed] [Google Scholar]
- 6.Tian B, Manley JL. 2013. Alternative cleavage and polyadenylation: the long and short of it. Trends Biochem Sci 38:312–320. doi: 10.1016/j.tibs.2013.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Batra R, Charizanis K, Manchanda M, Mohan A, Li M, Finn DJ, Goodwin M, Zhang C, Sobczak K, Thornton CA, Swanson MS. 2014. Loss of MBNL leads to disruption of developmentally regulated alternative polyadenylation in RNA-mediated disease. Mol Cell 56:311–322. doi: 10.1016/j.molcel.2014.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morris AR, Bos A, Diosdado B, Rooijers K, Elkon R, Bolijn AS, Carvalho B, Meijer GA, Agami R. 2012. Alternative cleavage and polyadenylation during colorectal cancer development. Clin Cancer Res 18:5256–5266. doi: 10.1158/1078-0432.CCR-12-0543. [DOI] [PubMed] [Google Scholar]
- 9.Xia Z, Donehower LA, Cooper TA, Neilson JR, Wheeler DA, Wagner EJ, Li W. 2014. Dynamic analyses of alternative polyadenylation from RNA-seq reveal a 3′-UTR landscape across seven tumour types. Nat Commun 5:5274. doi: 10.1038/ncomms6274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Masamha CP, Xia Z, Yang J, Albrecht TR, Li M, Shyu AB, Li W, Wagner EJ. 2014. CFIm25 links alternative polyadenylation to glioblastoma tumour suppression. Nature 510:412–416. doi: 10.1038/nature13261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berkovits BD, Mayr C. 2015. Alternative 3′ UTRs act as scaffolds to regulate membrane protein localization. Nature 522:363–367. doi: 10.1038/nature14321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.An JJ, Gharami K, Liao GY, Woo NH, Lau AG, Vanevski F, Torre ER, Jones KR, Feng Y, Lu B, Xu B. 2008. Distinct role of long 3′ UTR BDNF mRNA in spine morphology and synaptic plasticity in hippocampal neurons. Cell 134:175–187. doi: 10.1016/j.cell.2008.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Braz SO, Cruz A, Lobo A, Bravo J, Moreira-Ribeiro J, Pereira-Castro I, Freitas J, Relvas JB, Summavielle T, Moreira A. 2017. Expression of Rac1 alternative 3′ UTRs is a cell specific mechanism with a function in dendrite outgrowth in cortical neurons. Biochim Biophys Acta Gene Regul Mech 1860:685–694. doi: 10.1016/j.bbagrm.2017.03.002. [DOI] [PubMed] [Google Scholar]
- 14.Rogulja-Ortmann A, Picao-Osorio J, Villava C, Patraquim P, Lafuente E, Aspden J, Thomsen S, Technau GM, Alonso CR. 2014. The RNA-binding protein ELAV regulates Hox RNA processing, expression and function within the Drosophila nervous system. Development 141:2046–2056. doi: 10.1242/dev.101519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pinto PA, Henriques T, Freitas MO, Martins T, Domingues RG, Wyrzykowska PS, Coelho PA, Carmo AM, Sunkel CE, Proudfoot NJ, Moreira A. 2011. RNA polymerase II kinetics in polo polyadenylation signal selection. EMBO J 30:2431–2444. doi: 10.1038/emboj.2011.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Llamazares S, Moreira A, Tavares A, Girdham C, Spruce BA, Gonzalez C, Karess RE, Glover DM, Sunkel CE. 1991. polo encodes a protein kinase homolog required for mitosis in Drosophila. Genes Dev 5:2153–2165. doi: 10.1101/gad.5.12a.2153. [DOI] [PubMed] [Google Scholar]
- 17.Glover DM. 2005. Polo kinase and progression through M phase in Drosophila: a perspective from the spindle poles. Oncogene 24:230–237. doi: 10.1038/sj.onc.1208279. [DOI] [PubMed] [Google Scholar]
- 18.Zitouni S, Nabais C, Jana SC, Guerrero A, Bettencourt-Dias M. 2014. Polo-like kinases: structural variations lead to multiple functions. Nat Rev Mol Cell Biol 15:433–452. doi: 10.1038/nrm3819. [DOI] [PubMed] [Google Scholar]
- 19.Mount SM, Salz HK. 2000. Pre-messenger RNA processing factors in the Drosophila genome. J Cell Biol 150:F37–44. doi: 10.1083/jcb.150.2.F37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Darmon SK, Lutz CS. 2012. mRNA 3′ end processing factors: a phylogenetic comparison. Comp. Funct Genomics 2012:876893. doi: 10.1155/2012/876893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takagaki Y, Manley JL. 1994. A polyadenylation factor subunit is the human homologue of the Drosophila suppressor of forked protein. Nature 372:471–474. doi: 10.1038/372471a0. [DOI] [PubMed] [Google Scholar]
- 22.Bai C, Tolias PP. 1998. Drosophila clipper/CPSF 30K is a post-transcriptionally regulated nuclear protein that binds RNA containing GC clusters. Nucleic Acids Res 26:1597–1604. doi: 10.1093/nar/26.7.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Salinas CA, Sinclair DA, O'Hare K, Brock HW. 1998. Characterization of a Drosophila homologue of the 160-kDa subunit of the cleavage and polyadenylation specificity factor CPSF. Mol Gen Genet 257:672–680. doi: 10.1007/s004380050696. [DOI] [PubMed] [Google Scholar]
- 24.Audibert A, Simonelig M. 1999. The suppressor of forked gene of Drosophila, which encodes a homologue of human CstF-77K involved in mRNA 3′-end processing, is required for progression through mitosis. Mech Dev 82:41–50. doi: 10.1016/S0925-4773(99)00011-8. [DOI] [PubMed] [Google Scholar]
- 25.Benoit B, Nemeth A, Aulner N, Kuhn U, Simonelig M, Wahle E, Bourbon HM. 1999. The Drosophila poly(A)-binding protein II is ubiquitous throughout Drosophila development and has the same function in mRNA polyadenylation as its bovine homolog in vitro. Nucleic Acids Res 27:3771–3778. doi: 10.1093/nar/27.19.3771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murata T, Nagaso H, Kashiwabara S, Baba T, Okano H, Yokoyama KK. 2001. The hiiragi gene encodes a poly(A) polymerase, which controls the formation of the wing margin in Drosophila melanogaster. Dev Biol 233:137–147. doi: 10.1006/dbio.2001.0205. [DOI] [PubMed] [Google Scholar]
- 27.Benoit B, Juge F, Iral F, Audibert A, Simonelig M. 2002. Chimeric human CstF-77/Drosophila Suppressor of forked proteins rescue suppressor of forked mutant lethality and mRNA 3′ end processing in Drosophila. Proc Natl Acad Sci U S A 99:10593–10598. doi: 10.1073/pnas.162191899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Graber JH, Cantor CR, Mohr SC, Smith TF. 1999. In silico detection of control signals: mRNA 3′-end-processing sequences in diverse species. Proc Natl Acad Sci U S A 96:14055–14060. doi: 10.1073/pnas.96.24.14055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Beaudoing E, Freier S, Wyatt JR, Claverie JM, Gautheret D. 2000. Patterns of variant polyadenylation signal usage in human genes. Genome Res 10:1001–1010. doi: 10.1101/gr.10.7.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tian B, Hu J, Zhang H, Lutz CS. 2005. A large-scale analysis of mRNA polyadenylation of human and mouse genes. Nucleic Acids Res 33:201–212. doi: 10.1093/nar/gki158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nunes NM, Li W, Tian B, Furger A. 2010. A functional human poly(A) site requires only a potent DSE and an A-rich upstream sequence. EMBO J 29:1523–1536. doi: 10.1038/emboj.2010.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhao J, Hyman L, Moore C. 1999. Formation of mRNA 3′ ends in eukaryotes: mechanism, regulation, and interrelationships with other steps in mRNA synthesis. Microbiol Mol Biol Rev 63:405–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaufmann I, Martin G, Friedlein A, Langen H, Keller W. 2004. Human Fip1 is a subunit of CPSF that binds to U-rich RNA elements and stimulates poly(A) polymerase. EMBO J 23:616–626. doi: 10.1038/sj.emboj.7600070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Darmon SK, Lutz CS. 2012. Novel upstream and downstream sequence elements contribute to polyadenylation efficiency. RNA Biol 9:1255–1265. doi: 10.4161/rna.21957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.DeZazzo JD, Imperiale MJ. 1989. Sequences upstream of AAUAAA influence poly(A) site selection in a complex transcription unit. Mol Cell Biol 9:4951–4961. doi: 10.1128/MCB.9.11.4951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brown PH, Tiley LS, Cullen BR. 1991. Efficient polyadenylation within the human immunodeficiency virus type 1 long terminal repeat requires flanking U3-specific sequences. J Virol 65:3340–3343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sanfacon H, Brodmann P, Hohn T. 1991. A dissection of the cauliflower mosaic virus polyadenylation signal. Genes Dev 5:141–149. doi: 10.1101/gad.5.1.141. [DOI] [PubMed] [Google Scholar]
- 38.Cherrington J, Ganem D. 1992. Regulation of polyadenylation in human immunodeficiency virus (HIV): contributions of promoter proximity and upstream sequences. EMBO J 11:1513–1524. doi: 10.1002/j.1460-2075.1992.tb05196.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schek N, Cooke C, Alwine JC. 1992. Definition of the upstream efficiency element of the simian virus 40 late polyadenylation signal by using in vitro analyses. Mol Cell Biol 12:5386–5393. doi: 10.1128/MCB.12.12.5386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Graveley BR, Gilmartin GM. 1996. A common mechanism for the enhancement of mRNA 3′ processing by U3 sequences in two distantly related lentiviruses. J Virol 70:1612–1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moreira A, Wollerton M, Monks J, Proudfoot NJ. 1995. Upstream sequence elements enhance poly(A) site efficiency of the C2 complement gene and are phylogenetically conserved. EMBO J 14:3809–3819. doi: 10.1002/j.1460-2075.1995.tb00050.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hall-Pogar T, Liang S, Hague LK, Lutz CS. 2007. Specific trans-acting proteins interact with auxiliary RNA polyadenylation elements in the COX-2 3′-UTR. RNA 13:1103–1115. doi: 10.1261/rna.577707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Newnham CM, Hall-Pogar T, Liang S, Wu J, Tian B, Hu J, Lutz CS. 2010. Alternative polyadenylation of MeCP2: influence of cis-acting elements and trans-acting factors. RNA Biol 7:361–372. doi: 10.4161/rna.7.3.11564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Danckwardt S, Kaufmann I, Gentzel M, Foerstner KU, Gantzert AS, Gehring NH, Neu-Yilik G, Bork P, Keller W, Wilm M, Hentze MW, Kulozik AE. 2007. Splicing factors stimulate polyadenylation via USEs at non-canonical 3′ end formation signals. EMBO J 26:2658–2669. doi: 10.1038/sj.emboj.7601699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Natalizio BJ, Muniz LC, Arhin GK, Wilusz J, Lutz CS. 2002. Upstream elements present in the 3′-untranslated region of collagen genes influence the processing efficiency of overlapping polyadenylation signals. J Biol Chem 277:42733–42740. doi: 10.1074/jbc.M208070200. [DOI] [PubMed] [Google Scholar]
- 46.Nedeljkovic M, Costessi L, Iaconcig A, Porro F, Muro AF. 2013. Long-distance regulation of Add2 pre-mRNA 3′end processing. RNA Biol 10:516–527. doi: 10.4161/rna.23855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Valcarcel J, Gebauer F. 1997. Post-transcriptional regulation: the dawn of PTB. Curr Biol 7:R705–R708. doi: 10.1016/S0960-9822(06)00361-7. [DOI] [PubMed] [Google Scholar]
- 48.Wagner EJ, Garcia-Blanco MA. 2001. Polypyrimidine tract binding protein antagonizes exon definition. Mol Cell Biol 21:3281–3288. doi: 10.1128/MCB.21.10.3281-3288.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Coutinho-Mansfield GC, Xue Y, Zhang Y, Fu XD. 2007. PTB/nPTB switch: a post-transcriptional mechanism for programming neuronal differentiation. Genes Dev 21:1573–1577. doi: 10.1101/gad.1575607. [DOI] [PubMed] [Google Scholar]
- 50.Kafasla P, Mickleburgh I, Llorian M, Coelho M, Gooding C, Cherny D, Joshi A, Kotik-Kogan O, Curry S, Eperon IC, Jackson RJ, Smith CW. 2012. Defining the roles and interactions of PTB. Biochem Soc Trans 40:815–820. doi: 10.1042/BST20120044. [DOI] [PubMed] [Google Scholar]
- 51.Moreira A, Takagaki Y, Brackenridge S, Wollerton M, Manley JL, Proudfoot NJ. 1998. The upstream sequence element of the C2 complement poly(A) signal activates mRNA 3′ end formation by two distinct mechanisms. Genes Dev 12:2522–2534. doi: 10.1101/gad.12.16.2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Castelo-Branco P, Furger A, Wollerton M, Smith C, Moreira A, Proudfoot N. 2004. Polypyrimidine tract binding protein modulates efficiency of polyadenylation. Mol Cell Biol 24:4174–4183. doi: 10.1128/MCB.24.10.4174-4183.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Millevoi S, Decorsiere A, Loulergue C, Iacovoni J, Bernat S, Antoniou M, Vagner S. 2009. A physical and functional link between splicing factors promotes pre-mRNA 3′ end processing. Nucleic Acids Res 37:4672–4683. doi: 10.1093/nar/gkp470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Costessi L, Porro F, Iaconcig A, Nedeljkovic M, Muro AF. 2013. Characterization of the distal polyadenylation site of the ss-adducin (Add2) pre-mRNA. PLoS One 8:e58879. doi: 10.1371/journal.pone.0058879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cote CA, Gautreau D, Denegre JM, Kress TL, Terry NA, Mowry KL. 1999. A Xenopus protein related to hnRNP I has a role in cytoplasmic RNA localization. Mol Cell 4:431–437. doi: 10.1016/S1097-2765(00)80345-7. [DOI] [PubMed] [Google Scholar]
- 56.Sawicka K, Bushell M, Spriggs KA, Willis AE. 2008. Polypyrimidine-tract-binding protein: a multifunctional RNA-binding protein. Biochem Soc Trans 36:641–647. doi: 10.1042/BST0360641. [DOI] [PubMed] [Google Scholar]
- 57.Besse F, Lopez de Quinto S, Marchand V, Trucco A, Ephrussi A. 2009. Drosophila PTB promotes formation of high-order RNP particles and represses oskar translation. Genes Dev 23:195–207. doi: 10.1101/gad.505709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sridharan V, Heimiller J, Robida MD, Singh R. 2016. High throughput sequencing identifies misregulated genes in the Drosophila polypyrimidine tract-binding protein (hephaestus) mutant defective in spermatogenesis. PLoS One 11:e0150768. doi: 10.1371/journal.pone.0150768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.McDermott SM, Davis I. 2013. Drosophila hephaestus/polypyrimidine tract binding protein is required for dorso-ventral patterning and regulation of signalling between the germline and soma. PLoS One 8:e69978. doi: 10.1371/journal.pone.0069978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wesley CS, Guo H, Chaudhry KA, Thali MJ, Yin JC, Clason T, Wesley UV. 2011. Loss of PTB or negative regulation of Notch mRNA reveals distinct zones of Notch and actin protein accumulation in Drosophila embryo. PLoS One 6:e21876. doi: 10.1371/journal.pone.0021876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Heimiller J, Sridharan V, Huntley J, Wesley CS, Singh R. 2014. Drosophila polypyrimidine tract-binding protein (DmPTB) regulates dorso-ventral patterning genes in embryos. PLoS One 9:e98585. doi: 10.1371/journal.pone.0098585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Castrillon DH, Gonczy P, Alexander S, Rawson R, Eberhart CG, Viswanathan S, DiNardo S, Wasserman SA. 1993. Toward a molecular genetic analysis of spermatogenesis in Drosophila melanogaster: characterization of male-sterile mutants generated by single P element mutagenesis. Genetics 135:489–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Robida MD, Singh R. 2003. Drosophila polypyrimidine-tract binding protein (PTB) functions specifically in the male germline. EMBO J 22:2924–2933. doi: 10.1093/emboj/cdg301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Robida M, Sridharan V, Morgan S, Rao T, Singh R. 2010. Drosophila polypyrimidine tract-binding protein is necessary for spermatid individualization. Proc Natl Acad Sci U S A 107:12570–12575. doi: 10.1073/pnas.1007935107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Carswell S, Alwine JC. 1989. Efficiency of utilization of the simian virus 40 late polyadenylation site: effects of upstream sequences. Mol Cell Biol 9:4248–4258. doi: 10.1128/MCB.9.10.4248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sully G, Dean JL, Wait R, Rawlinson L, Santalucia T, Saklatvala J, Clark AR. 2004. Structural and functional dissection of a conserved destabilizing element of cyclo-oxygenase-2 mRNA: evidence against the involvement of AUF-1 [AU-rich element/poly(U)-binding/degradation factor-1], AUF-2, tristetraprolin, HuR (Hu antigen R) or FBP1 (far-upstream-sequence-element-binding protein 1). Biochem J 377:629–639. doi: 10.1042/BJ20031484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hall-Pogar T, Zhang H, Tian B, Lutz CS. 2005. Alternative polyadenylation of cyclooxygenase-2. Nucleic Acids Res 33:2565–2579. doi: 10.1093/nar/gki544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang L, Tanaka S, Ramirez F. 2005. GATA-4 binds to an upstream element of the human alpha2(I) collagen gene (COL1A2) and inhibits transcription in fibroblasts. Matrix Biol 24:333–340. doi: 10.1016/j.matbio.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 69.Moutinho-Santos T, Sampaio P, Amorim I, Costa M, Sunkel CE. 1999. In vivo localisation of the mitotic POLO kinase shows a highly dynamic association with the mitotic apparatus during early embryogenesis in Drosophila. Biol Cell 91:585–596. doi: 10.1016/S0248-4900(00)88523-8. [DOI] [PubMed] [Google Scholar]
- 70.Donaldson MM, Tavares AA, Ohkura H, Deak P, Glover DM. 2001. Metaphase arrest with centromere separation in polo mutants of Drosophila. J Cell Biol 153:663–676. doi: 10.1083/jcb.153.4.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tavares AA, Glover DM, Sunkel CE. 1996. The conserved mitotic kinase polo is regulated by phosphorylation and has preferred microtubule-associated substrates in Drosophila embryo extracts. EMBO J 15:4873–4883. doi: 10.1002/j.1460-2075.1996.tb00868.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sunkel CE, Glover DM. 1988. polo, a mitotic mutant of Drosophila displaying abnormal spindle poles. J Cell Sci 89:25–38. [DOI] [PubMed] [Google Scholar]
- 73.Conde C, Osswald M, Barbosa J, Moutinho-Santos T, Pinheiro D, Guimaraes S, Matos I, Maiato H, Sunkel CE. 2013. Drosophila Polo regulates the spindle assembly checkpoint through Mps1-dependent BubR1 phosphorylation. EMBO J 32:1761–1777. doi: 10.1038/emboj.2013.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Carmena M, Lombardia MO, Ogawa H, Earnshaw WC. 2014. Polo kinase regulates the localization and activity of the chromosomal passenger complex in meiosis and mitosis in Drosophila melanogaster. Open Biol 4:140162. doi: 10.1098/rsob.140162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.von Schubert C, Cubizolles F, Bracher JM, Sliedrecht T, Kops G, Nigg EA. 2015. Plk1 and Mps1 cooperatively regulate the spindle assembly checkpoint in human cells. Cell Rep 12:66–78. doi: 10.1016/j.celrep.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 76.Martins T, Maia AF, Steffensen S, Sunkel CE. 2009. Sgt1, a co-chaperone of Hsp90 stabilizes Polo and is required for centrosome organization. EMBO J 28:234–247. doi: 10.1038/emboj.2008.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Collier S, Chan HY, Toda T, McKimmie C, Johnson G, Adler PN, O'Kane C, Ashburner M. 2000. The Drosophila embargoed gene is required for larval progression and encodes the functional homolog of schizosaccharomyces Crm1. Genetics 155:1799–1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.R Development Core Team. 2008. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. [Google Scholar]
- 79.Retelska D, Iseli C, Bucher P, Jongeneel CV, Naef F. 2006. Similarities and differences of polyadenylation signals in human and fly. BMC Genomics 7:176. doi: 10.1186/1471-2164-7-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wickens M. 1990. How the messenger got its tail: addition of poly(A) in the nucleus. Trends Biochem Sci 15:277–281. doi: 10.1016/0968-0004(90)90054-F. [DOI] [PubMed] [Google Scholar]
- 81.Sanfilippo P, Wen J, Lai EC. 2017. Landscape and evolution of tissue-specific alternative polyadenylation across Drosophila species. Genome Biol 18:229. doi: 10.1186/s13059-017-1358-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Brackenridge S, Proudfoot NJ. 2000. Recruitment of a basal polyadenylation factor by the upstream sequence element of the human lamin B2 polyadenylation signal. Mol Cell Biol 20:2660–2669. doi: 10.1128/MCB.20.8.2660-2669.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Legendre M, Gautheret D. 2003. Sequence determinants in human polyadenylation site selection. BMC Genomics 4:7. doi: 10.1186/1471-2164-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Danckwardt S, Gehring NH, Neu-Yilik G, Hundsdoerfer P, Pforsich M, Frede U, Hentze MW, Kulozik AE. 2004. The prothrombin 3′end formation signal reveals a unique architecture that is sensitive to thrombophilic gain-of-function mutations. Blood 104:428–435. doi: 10.1182/blood-2003-08-2894. [DOI] [PubMed] [Google Scholar]
- 85.Tian B, Graber JH. 2012. Signals for pre-mRNA cleavage and polyadenylation. Wiley Interdiscip Rev RNA 3:385–396. doi: 10.1002/wrna.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gramates LS, Marygold SJ, Santos GD, Urbano JM, Antonazzo G, Matthews BB, Rey AJ, Tabone CJ, Crosby MA, Emmert DB, Falls K, Goodman JL, Hu Y, Ponting L, Schroeder AJ, Strelets VB, Thurmond J, Zhou P, The FlyBase Consortium. 2017. FlyBase at 25: looking to the future. Nucleic Acids Res 45:D663–D671. doi: 10.1093/nar/gkw1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Roberts DB. 1986. Drosophila: a practical approach. IRL Press, Oxford, UK. [Google Scholar]
- 88.Moura M, Osswald M, Leca N, Barbosa J, Pereira AJ, Maiato H, Sunkel CE, Conde C. 2017. Protein phosphatase 1 inactivates Mps1 to ensure efficient spindle assembly checkpoint silencing. Elife 6:e25366. doi: 10.7554/eLife.25366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jelluma N, Brenkman AB, McLeod I, Yates JR 3rd, Cleveland DW, Medema RH, Kops GJ. 2008. Chromosomal instability by inefficient Mps1 auto-activation due to a weakened mitotic checkpoint and lagging chromosomes. PLoS One 3:e2415. doi: 10.1371/journal.pone.0002415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez JY, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A. 2012. Fiji: an open-source platform for biological-image analysis. Nat Methods 9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.The UniProt Consortium. 2017. UniProt: the universal protein knowledgebase. Nucleic Acids Res 45:D158–D169. doi: 10.1093/nar/gkw1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mi H, Muruganujan A, Casagrande JT, Thomas PD. 2013. Large-scale gene function analysis with the PANTHER classification system. Nat Protoc 8:1551–1566. doi: 10.1038/nprot.2013.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mi H, Huang X, Muruganujan A, Tang H, Mills C, Kang D, Thomas PD. 2017. PANTHER version 11: expanded annotation data from Gene Ontology and Reactome pathways, and data analysis tool enhancements. Nucleic Acids Res 45:D183–D189. doi: 10.1093/nar/gkw1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.