Vaccination is the most effective method to protect older adults against viral infections. However, the immunogenicity of viral vaccines in older adults is notoriously poor. The live attenuated zoster vaccine (ZVL) provides the best example of a gradual decrease of vaccine immunogenicity with every 10-year age increase above 50 years. Here we show that the abundance of regulatory T cells before vaccine administration to older adults has a significant inhibitory effect on immune responses to ZVL and, together with baseline immunity to varicella-zoster virus, explains the effect of age on the immunogenicity of ZVL. Moreover, in vitro blockade of regulatory T cell mechanisms of action with biologic modulators restores immune responses to varicella-zoster virus in vaccinees. Collectively, these observations suggest that immune modulators that block regulatory T cell activity may increase responses to viral attenuated vaccines in older adults.
KEYWORDS: cell-mediated immunity, herpes zoster, zoster vaccine live
ABSTRACT
Older age is associated with increased infectious morbidity and decreased immune responses to vaccines, but the mechanisms that mediate this effect are incompletely understood. The efficacy and immunogenicity of the live attenuated zoster vaccine (ZVL) have a very-well-described negative association with the age of the vaccinee. In a study of 600 ZVL recipients 50 to >80 years of age, we investigated immunological factors that might explain the effect of age on the immunogenicity of ZVL. Using FluoroSpot assays and flow cytometry, we determined that varicella-zoster virus (VZV)-specific peak T helper 1 (VZV-Th1) responses to ZVL were independently predicted by prevaccination VZV-Th1 responses, regulatory T cells (Treg), and PD1-expressing immune checkpoint T cells (Tcheck) but not by the age of the vaccinee. Persistence of VZV-Th1 1 year after vaccination was independently predicted by the factors mentioned above, by peak VZV-Th1 responses to ZVL, and by the age of the vaccinee. We further demonstrated by ex vivo blocking experiments the mechanistic role of PD1 and CTLA4 as modulators of decreased VZV-Th1 responses in the study participants. VZV-specific cytotoxic T cell (VZV-CTL) and T follicular helper responses to ZVL did not correlate with age, but similar to other Th1 responses, VZV-CTL peak and baseline responses were independently correlated. These data expand our understanding of the factors affecting the magnitude and kinetics of T cell responses to ZVL in older adults and show the importance of prevaccination Treg and Tcheck in modulating the immunogenicity of ZVL. This presents new potential interventions to increase vaccine responses in older adults.
IMPORTANCE Vaccination is the most effective method to protect older adults against viral infections. However, the immunogenicity of viral vaccines in older adults is notoriously poor. The live attenuated zoster vaccine (ZVL) provides the best example of a gradual decrease of vaccine immunogenicity with every 10-year age increase above 50 years. Here we show that the abundance of regulatory T cells before vaccine administration to older adults has a significant inhibitory effect on immune responses to ZVL and, together with baseline immunity to varicella-zoster virus, explains the effect of age on the immunogenicity of ZVL. Moreover, in vitro blockade of regulatory T cell mechanisms of action with biologic modulators restores immune responses to varicella-zoster virus in vaccinees. Collectively, these observations suggest that immune modulators that block regulatory T cell activity may increase responses to viral attenuated vaccines in older adults.
INTRODUCTION
Aging is associated with a decline in multiple components of the immune system (termed immune senescence) (1). This is clinically apparent from the increases in the frequency and severity of common infections with increasing age, such as bacterial pneumonia and influenza, and also from the blunted immune responses to vaccination that are characteristic of advancing age. For example, among nonlive vaccines recommended for older individuals (influenza, pneumococcal, and tetanus-diphtheria-pertussis vaccines), the frequency with which protective antibody responses are reached and the magnitude and persistence of these responses are diminished by increasing age (2–4). This is also readily apparent after administration of the live attenuated zoster vaccine (ZVL). ZVL prevents herpes zoster (HZ) in 70% of vaccinees 50 to 59 years of age; this declines to 64% of those 60 to 69 years of age and to 38% at ≥70 years of age (5, 6). In addition, protection progressively wanes as the interval after the administration of ZVL increases (7, 8). To address the waning of protective immunity, we undertook an open-label study to evaluate the antibody and varicella-zoster virus (VZV)-specific T cell-mediated immune responses to a second dose of ZVL administered to prior ZVL recipients. This study (Merck V211-029; ClinTrials.gov identifier NCT01245751) compared adults ≥70 years of age who received a second dose of ZVL, ≥10 years after the first dose, to age-matched individuals receiving their first dose and to younger individuals between 50 and 70 years of age receiving a first dose (9, 10). Here we present additional immunological investigations performed on samples from the parent study in order to identify T cell characteristics, pre- and postvaccination, that might explain the age-related decline in ZVL-induced responses.
RESULTS
Demographic characteristics of the study population.
The study enrolled 600 participants (Table 1). Almost all were white and non-Hispanic; 57% were women. Group 1 included 201 adults 70 to 94 years of age who received a second dose of ZVL (boosted cohort). The other 3 groups included 199 adults 70 to 89 years of age (group 2), 100 adults 60 to 69 years of age (group 3), and 100 adults 50 to 59 years of age (group 4), all of whom received ZVL for the first time (primary cohort). Responses of primary and boosted cohorts were analyzed separately.
TABLE 1.
Demographic characteristicsa
| Characteristic | Value |
||||
|---|---|---|---|---|---|
| Group 1 (booster ZVL recipients ≥70 yr old) | Group 2 (first-time ZVL recipients ≥70 yr old) | Group 3 (first-time ZVL recipients 60–69 yr old) | Group 4 (first-time ZVL recipients 50–59 yr old) | Total | |
| No. of participants/group | 201 | 199 | 100 | 100 | 600 |
| No. (%) of patients of gender | |||||
| Male | 94 (46.8) | 105 (52.8) | 35 (35.0) | 23 (23.0) | 257 (42.8) |
| Female | 107 (53.2) | 94 (52.8) | 65 (65.0) | 77 (77.0) | 343 (57.2) |
| Mean age (yr) (SD) | 77.1 (4.5) | 76.3 (5.1) | 64.1 (3.1) | 55.5 (2.8) | 71.1 |
| Median age (yr) | 76.0 | 75.0 | 63.0 | 56.0 | 73.0 |
| No. (%) of patients of race | |||||
| Native American or Asian | 0 (0.0) | 4 (2.0) | 1 (1.0) | 0 (0.0) | 2 (0.3) |
| Black | 1 (0.5) | 5 (2.5) | 8 (8.0) | 6 (6.0) | 20 (3.3) |
| White | 200 (99.5) | 188 (94.5) | 90 (90.0) | 93 (93.0) | 571 (95.2) |
| No. (%) of patients of ethnicity | |||||
| Hispanic | 2 (1.0) | 2 (1.0) | 0 (0.0) | 4 (4.0) | 8 (1.3) |
| Not Hispanic | 198 (98.5) | 196 (98.5) | 100 (100.0) | 96 (96.0) | 590 (98.3) |
Abbreviations: ZVL, live zoster vaccine; SD, standard deviation.
Kinetics of cell-mediated immune responses to ZVL.
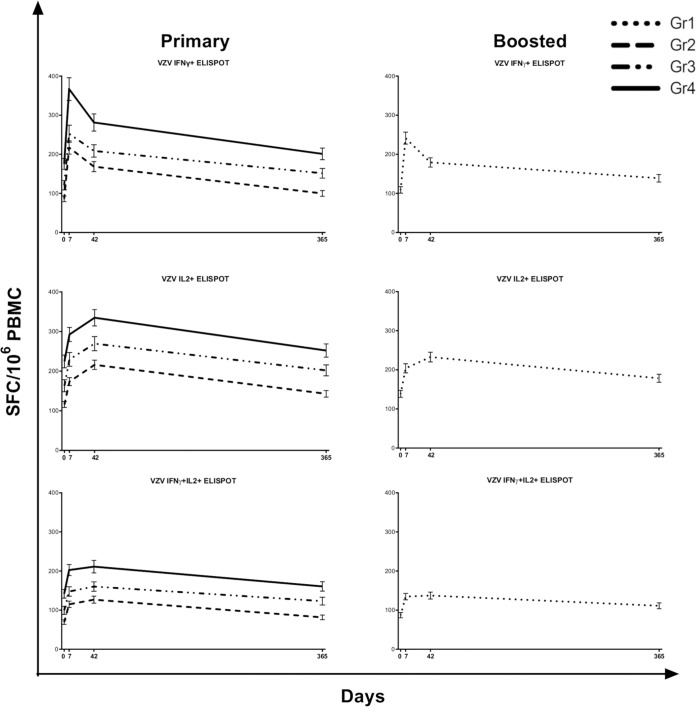
The primary outcome measures for VZV-specific T cell responses to ZVL were VZV-specific T helper 1 (Th1) responses measured by an interleukin-2 (IL-2) and interferon gamma (IFN-γ) dual-color FluoroSpot assay, an assay that primarily measures CD4+ T cell responses (11). The FluoroSpot assay was performed on samples from all participants prevaccination and at 7, 42, and 365 days after vaccination. Figure 1 shows the kinetics of VZV-specific IFN-γ-positive (IFN-γ+), IL-2+, and IFN-γ+ IL-2+ spot-forming cells (SFC)/106 peripheral blood mononuclear cells (PBMC) in the primary and boosted cohorts. This demonstrates the lower VZV-specific Th1 responses occurring with advancing age, both at baseline and after vaccination. VZV-specific Th1 SFC persist above prevaccination levels 1 year after immunization.
FIG 1.
Kinetics of Th1 responses measured by FluoroSpot assays. Data derived from 600 participants who received ZVL at day 0 are presented as means and standard errors of the means (SEM) at each time point. Increases after vaccination were significant in all groups, for all parameters, at all the time points shown. Differences among groups 2, 3, and 4 (primary cohort [≥70 years old, 60 to 69 years old, and 50 to 59 years old, respectively]) were significant at baseline and after vaccination. Group 1 (booster cohort [≥70 years]) had higher responses than the age-matched primary cohort group 2 at baseline and after vaccination (9). ELISPOT, enzyme-linked immunosorbent spot.
The number of IFN-γ+ SFC/106 PBMC peaked at 7 days postimmunization, followed by a rapid decline, displaying kinetics typical of effector T cells (Teff), which allowed us to approximate VZV-specific Teff responses using the IFN-γ SFC. In contrast, the number of IL-2+ SFC/106 PBMC peaked 42 days after vaccination, followed by a slow decline, which is typical of memory cells and was used to approximate VZV-specific memory T cell (Tmem) responses to ZVL. IFN-γ+ IL-2+ SFC/106 PBMC peaked at 7 days, followed by a plateau up to 42 days and a slow decline to 365 days. Their intermediate kinetics prompted us to use them as an approximation of VZV-specific effector memory T cells (Tem).
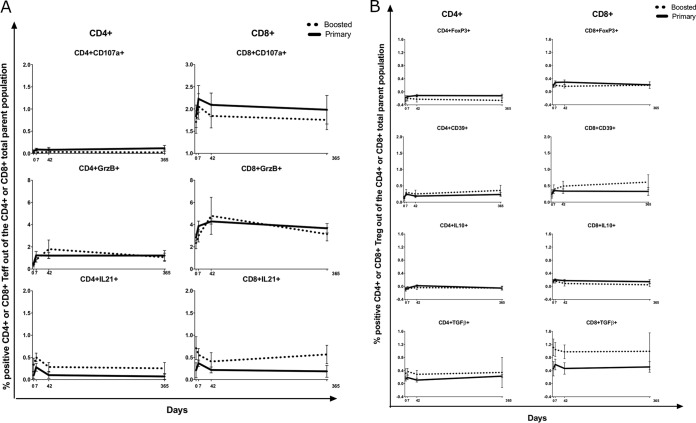
In a subset of 30 to 34 randomly chosen participants in each group (VZV flow cytometry subset), we analyzed the kinetics of VZV-specific cytotoxic CD4+ and CD8+ T cells (cytotoxic T lymphocytes [CTL]), characterized by the expression of CD107a or granzyme B (GrzB); T follicular helper cells (Tfh), characterized by the expression of IL-21; and regulatory T cells (Treg), characterized by the expression of FOXP3, IL-10, transforming growth factor β (TGF-β), or CD39, after ex vivo VZV restimulation (Fig. 2). FOXP3 was used as a marker of Treg after verifying with staining optimization experiments that there was no overlap between FOXP3 expression and Th1 responses as represented by IFN-γ secretion in stimulated or unstimulated cells (see Fig. S1 in the supplemental material). Significant increases from baseline to day 7 after vaccination were observed only for percentages of VZV-specific CD8+ CD107a+, CD4+ GrzB+, and CD8+ GrzB+ (CD4+/CD8+ GrzB+) CTL and for the percentage of VZV-specific CD4+/CD8+ IL-21+ Tfh (P ≤ 0.02) (Fig. 2A). VZV-specific CTL returned to baseline values at day 42, whereas Tfh+ cells plateaued at between days 7 and 42 and returned to baseline at day 365, with Tmem kinetics. Treg subsets did not change significantly after vaccination compared with the baseline (Fig. 2B).
FIG 2.
Kinetics of VZV-specific responses to ZVL. Data derived from 95 participants in the primary cohort and 33 participants in the boosted cohort are presented as means and SEM at each time point. (A) Teff. There were significant increases at day 7 compared with the baseline in percentages of CD8+ CD107a+, CD4+/CD8+ GrzB+, and CD4+/CD8+ IL-21+ cells (P ≤ 0.02 measured by regression analysis). There were no significant differences between primary and boosted cohorts at any time point. (B) Treg. There were no significant changes over time or between cohorts.
Kinetics of circulating T cell subsets in ZVL recipients.
At baseline, we measured the frequencies of CD4+/CD8+ Treg characterized by the expression FOXP3, CD25, IL-10, or TGF-β; CD4+/CD8+ PD1+ immune checkpoint-expressing T cells (Tcheck); CD4+/CD8+ CD28− CD57+ senescent T cells (Tsen); and CD4+/CD8+ CD31+ naive T cells (Tnaive) (Fig. 3). After vaccination, there were no significant changes in Treg (Fig. 3). Only the percentage of CD8+ PD1+ Tcheck significantly increased on day 7 postvaccination compared to the baseline (P < 0.0001) (Fig. 3); these returned to the baseline at between days 42 and 365. Tsen and Tnaive were examined only at baseline.
FIG 3.
Kinetics of T cell subsets associated with immune senescence. Data were derived from all participants, including 399 who received ZVL for the first time in this study and 201 who received a 2nd dose of ZVL ≥10 years after the initial vaccination. Data are presented as means and SEM.
The effect of age on peak VZV-specific Th1 responses to ZVL is explained by baseline VZV-specific Th1 cells, Treg, and Tcheck.
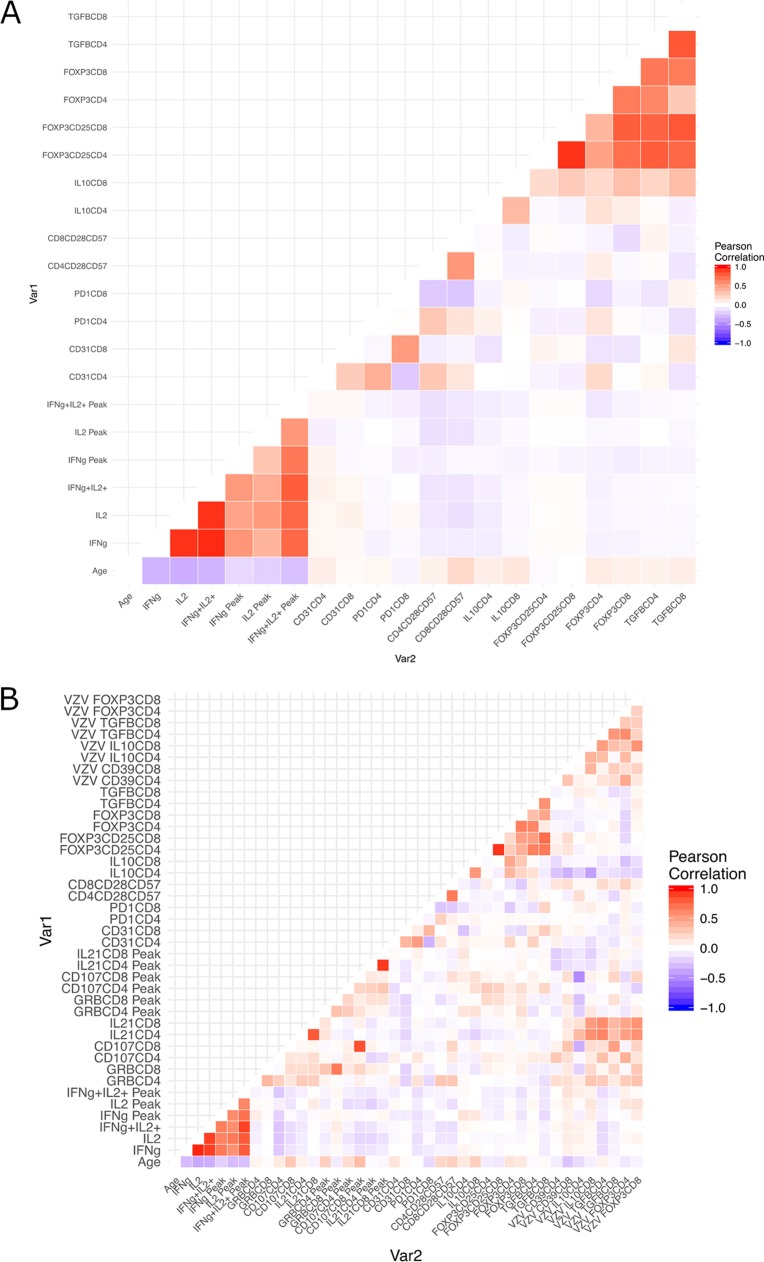
In the primary cohort, we first performed univariate correlation analyses of the peak VZV-specific Tmem (day 42), Teff (day 7), and Tem (day 7) responses to ZVL with respect to the following baseline characteristics: age; VZV-specific Tmem, Teff, and Tem; nonspecific circulating Treg, Tsen, Tnaive, and Tcheck; and VZV-specific Treg (Fig. 4). Overall, older age correlated with lower baseline and peak VZV-specific Th1 cells measured by a FluoroSpot assay (P < 0.0001) (Fig. 4A). Older age was also significantly or moderately correlated with higher percentages of CD4+ FOXP3+ and CD4+/CD8+ IL-10+ Treg and naive CD4+ CD31+ cells at baseline (0.07 > P > 0.01). In the VZV flow cytometry subset, older age was moderately associated with higher baseline and peak VZV-specific percentages of CD8+ CD107a+ CTL (P < 0.02) (Fig. 4B).
FIG 4.
Heat maps of the associations of age, baseline VZV functional responses, immune senescence T cell profile, and baseline VZV Treg with peak VZV functional responses to ZVL. (A) Data derived from 399 participants in the primary cohort, including participants who contributed data to the VZV flow cytometry subset and those who did not. (B) Data derived from 95 participants in the VZV flow cytometry subset in the primary cohort.
In addition, Fig. 4A shows that baseline and peak VZV-specific Tmem, Teff, and Tem and baseline CD4+/CD8+ Treg subsets formed clusters of positive associations, and Fig. 3B shows that ex vivo-restimulated T cell subsets generally formed weakly positively associated clusters regardless of function. There were no appreciable associations between VZV-specific Th1 responses measured by FluoroSpot assays and VZV-specific CTL.
We next performed multivariable analyses. In 399 participants in the primary cohort, higher baseline frequencies of VZV-specific Teff, Tmem, and Tem had independent positive effects on peak VZV-specific Teff, Tmem, and Tem responses to ZVL, respectively, while higher baseline percentages of CD4+ FOXP3+ and CD8+ FOXP3+ CD25+ Treg and CD8+ PD1+ Tcheck had independent negative effects on select VZV-specific Th1 responses to vaccination (Table 2). Age did not have an independent effect on peak VZV-specific Th1 responses to ZVL.
TABLE 2.
Multivariable regression analysis of the effects of age and baseline immune parameters on the magnitude of peak VZV-specific Th1 immune responses to ZVL in the primary cohort (n = 399)a
| Explanatory variable | Peak IL-2+ SFC |
Peak IFN-γ+ SFC |
Peak IFN-γ+ IL-2+ SFC |
|||
|---|---|---|---|---|---|---|
| RCE | P | RCE | P | RCE | P | |
| Age | 0.03 | 0.96 | −0.41 | 0.69 | −0.19 | 0.7 |
| Baseline responses | 1.01 | <0.0001 | 1.46 | <0.0001 | 1.08 | <0.0001 |
| CD4+ IL-10+ | −15.1 | 0.32 | −6.64 | 0.78 | −7.14 | 0.52 |
| CD8+ IL-10+ | 10.03 | 0.43 | −2.43 | 0.90 | −0.06 | 0.99 |
| CD4+ FOXP3+ CD25+ | 82.71 | 0.15 | 193.7 | 0.03 | 56.17 | 0.19 |
| CD8+ FOXP3+ CD25+ | −60.1 | 0.17 | −159 | 0.02 | −37.5 | 0.24 |
| CD4+ FOXP3+ | −36.8 | <0.01 | −35.7 | 0.09 | −20.7 | 0.04 |
| CD8+ FOXP3+ | 26.77 | 0.12 | 27.56 | 0.30 | 13.86 | 0.28 |
| CD4+ TGF-β+ | 19.06 | 0.09 | 9.95 | 0.56 | 6.16 | 0.46 |
| CD8+ TGF-β+ | −12.7 | 0.08 | 1.89 | 0.86 | −3.67 | 0.49 |
| CD4+ CD28− CD57+ | −1.84 | 0.09 | −3.03 | 0.07 | −1.03 | 0.19 |
| CD8+ CD28− CD57+ | −0.31 | 0.54 | 0.27 | 0.73 | −0.15 | 0.67 |
| CD4+ PD1+ | 8.87 | 0.16 | 2.99 | 0.76 | 2.74 | 0.56 |
| CD8+ PD1+ | −19.9 | <0.01 | −1.34 | 0.87 | −11.4 | <0.01 |
| CD4+ CD31+ | −18.3 | 0.09 | 27.94 | 0.09 | −5.73 | 0.47 |
| CD8+ CD31+ | 13.21 | 0.21 | −29.1 | 0.07 | 9.78 | 0.21 |
Boldface type indicates statistically significant effects defined by a P value of <0.02 to adjust for multiple comparisons. Abbreviations: VZV, varicella-zoster virus; Th1, T helper 1; CTL, cytotoxic T lymphocytes; RCE, regression coefficient estimate (indicates the magnitude of the effect of the explanatory variable on the outcome measure); SFC, spot-forming cells/106 PBMC; baseline responses, prevaccination responses in the same category as the outcome measure (e.g., prevaccination IFN-γ SFC as an explanatory variable for peak IFN-γ SFC or prevaccination IL-2 SFC as an explanatory variable for peak IL-2 SFC, etc.).
In 95 participants in the VZV flow cytometry subset (Table 3), age did not have an independent effect on peak VZV-specific Th1 responses, but higher baseline VZV-specific Th1 responses had independent positive effects on VZV-specific peak Th1 responses to ZVL, while higher baseline VZV-specific percentages of CD4+ CD39+ Treg had an independent negative effect on peak VZV-specific Th1 cells.
TABLE 3.
Multivariable regression analysis of the effects of age and baseline immune parameters on the magnitude of peak VZV-specific Th1 immune responses to ZVL in the VZV-specific flow cytometry subset of the primary cohort (n = 95)a
| Explanatory variable | Peak IL-2+ SFC |
Peak IFN-γ+ SFC |
Peak IFN-γ+ IL-2+ SFC |
|||
|---|---|---|---|---|---|---|
| RCE | P | RCE | P | RCE | P | |
| Age | 1.75 | 0.14 | −0.69 | 0.67 | 1.20 | 0.15 |
| Baseline responses | 1.18 | <0.0001 | 1.64 | <0.0001 | 1.21 | <0.0001 |
| VZV-specific CD4+ CD39+ | −76.5 | 0.02 | −71.7 | 0.09 | −51.2 | 0.03 |
| VZV-specific CD8+ CD39+ | 18.3 | 0.32 | 4.22 | 0.86 | 23.9 | 0.07 |
| VZV-specific CD4+ FOXP3+ | 107.4 | 0.04 | 141.6 | 0.04 | 86.5 | 0.021 |
| VZV-specific CD8+ FOXP3+ | 1.18 | 0.96 | 48.2 | 0.17 | 10.8 | 0.57 |
| VZV-specific CD4+ IL-10+ | −23.5 | 0.68 | −122 | 0.1 | 9.6 | 0.81 |
| VZV-specific CD8+ IL-10+ | 30.3 | 0.41 | 73.8 | 0.12 | 0.46 | 0.99 |
| VZV-specific CD4+ TGF-β+ | −17.9 | 0.46 | 9.9 | 0.75 | −6.0 | 0.73 |
| VZV-specific CD8+ TGF-β+ | 1.3 | 0.92 | −2.1 | 0.91 | −1.8 | 0.85 |
Boldface type indicates statistically significant effects defined by a P value of <0.02 to adjust for multiple comparisons. Abbreviations: VZV, varicella-zoster virus; Th1, T helper 1; CTL, cytotoxic T lymphocytes; RCE, regression coefficient estimate (indicates the magnitude of the effect of the explanatory variable on the outcome measure); SFC, spot-forming cells/106 PBMC; baseline responses, prevaccination responses in the same category as the outcome measure (e.g., prevaccination IFN-γ SFC as an explanatory variable for peak IFN-γ SFC or prevaccination IL-2 SFC as an explanatory variable for peak IL-2 SFC, etc.).
Higher baseline percentages of VZV-specific CD4+ CD39+ Treg also had an independent negative effect on peak Tfh responses to ZVL (Table 4). Older age was independently associated with higher peak percentages of VZV-specific CD4+ GrzB+ cells after vaccination but not with other CTL responses. In contrast, higher baseline VZV-specific CTL responses were associated with higher peak VZV-specific CTL responses, with the exception of the percentage of VZV-specific CD4+ GrzB+ cells. In addition, baseline percentages of VZV-specific CD4+ IL-10+ and CD8+ TGF-β+ Treg were independently associated with lower peak percentages of VZV-specific CD8+ CD107a+ CTL.
TABLE 4.
Multivariable regression analysis of the effects of age and baseline immune parameters on the magnitude of peak VZV-specific CTL and Tfh responses to ZVL in the VZV-specific flow cytometry subset of the primary cohort (n = 95)a
| Explanatory variable | VZV CD4+ GrzB+ |
VZV CD8+ GrzB+ |
VZV CD4+ CD107+ |
VZV CD8+ CD107a+ |
VZV CD4+ IL-21+ |
VZV CD8+ IL-21+ |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RCE | P | RCE | P | RCE | P | RCE | P | RCE | P | RCE | P | |
| Age | 0.12 | 0.02 | −0.04 | 0.31 | 0.001 | 0.98 | 0.03 | 0.08 | −0.001 | 0.90 | −0.01 | 0.51 |
| Baseline responses | 0.24 | 0.65 | 0.76 | <0.01 | 0.23 | 0.02 | 0.90 | <0.01 | −0.16 | 0.56 | −0.02 | 0.91 |
| CD4+ IL10+ | 1.02 | 0.28 | −0.06 | 0.94 | 0.01 | 0.90 | 0.51 | 0.13 | 0.07 | 0.76 | 0.33 | 0.25 |
| CD8+ IL10+ | −0.14 | 0.93 | 0.76 | 0.50 | 0.04 | 0.73 | 0.07 | 0.88 | −0.19 | 0.59 | −0.31 | 0.49 |
| CD4+ FOXP3+ CD25+ | −2.72 | 0.72 | 6.68 | 0.26 | −0.04 | 0.90 | 1.13 | 0.67 | 1.02 | 0.59 | 2.3 | 0.32 |
| CD8+ FOXP3+ CD25+ | 5.57 | 0.38 | −0.58 | 0.90 | 0.06 | 0.85 | −0.26 | 0.91 | −0.85 | 0.59 | −2.04 | 0.29 |
| CD4+ FOXP3+ | −0.89 | 0.52 | 0.7 | 0.51 | 0.03 | 0.77 | 0.15 | 0.76 | 0.15 | 0.66 | 0.23 | 0.58 |
| CD8+ FOXP3+ | 1.2 | 0.40 | −0.09 | 0.93 | 0.01 | 0.96 | −0.65 | 0.19 | −0.09 | 0.81 | −0.13 | 0.76 |
| CD4+ TGF-β+ | 0.04 | 0.97 | −0.77 | 0.38 | −0.03 | 0.72 | −0.17 | 0.66 | −0.19 | 0.51 | −0.45 | 0.20 |
| CD8+ TGF-β+ | −0.85 | 0.42 | −0.45 | 0.57 | 0.02 | 0.74 | 0.37 | 0.32 | 0.18 | 0.49 | 0.39 | 0.23 |
| CD4+ CD28− CD57+ | 0.01 | 0.86 | −0.06 | 0.22 | −0.02 | 0.29 | −0.01 | 0.64 | 0.001 | 0.98 | −0.01 | 0.78 |
| CD8+ CD28− CD57+ | −0.02 | 0.52 | 0.025 | 0.35 | 0.001 | 0.93 | −0.01 | 0.71 | −0.01 | 0.78 | −0.01 | 0.91 |
| CD4+ PD1+ | 0.205 | 0.54 | −0.03 | 0.91 | 0.01 | 0.11 | 0.11 | 0.34 | 0.04 | 0.65 | 0.05 | 0.60 |
| CD8+ PD1+ | −0.01 | 0.98 | 0.14 | 0.59 | −0.01 | 0.15 | −0.01 | 0.99 | 0.001 | 0.99 | −0.05 | 0.65 |
| CD4+ CD31+ | −1.01 | 0.30 | −0.27 | 0.71 | −0.01 | 0.63 | 0.08 | 0.81 | 0.01 | 0.99 | 0.06 | 0.83 |
| CD8+ CD31+ | 0.141 | 0.89 | 0.071 | 0.93 | 0.01 | 0.23 | −0.31 | 0.41 | −0.21 | 0.44 | −0.05 | 0.88 |
| VZV CD4+ CD39+ | −1.00 | 0.31 | −0.24 | 0.76 | −0.06 | 0.49 | −0.27 | 0.40 | −0.41 | 0.10 | −0.73 | 0.01 |
| VZV CD8+ CD39+ | 0.17 | 0.76 | 0.24 | 0.58 | −0.03 | 0.46 | −0.19 | 0.31 | −0.17 | 0.20 | −0.18 | 0.25 |
| VZV CD4+ FOXP3+ | 1.23 | 0.39 | 0.73 | 0.52 | 0.04 | 0.68 | 0.21 | 0.65 | 0.38 | 0.31 | 1.03 | 0.03 |
| VZV CD8+ FOXP3+ | −0.26 | 0.73 | −1.26 | 0.04 | −0.14 | 0.08 | 0.31 | 0.20 | −0.06 | 0.75 | 0.08 | 0.73 |
| VZV CD4+ IL-10+ | −3.47 | 0.04 | −2.19 | 0.09 | −0.13 | 0.43 | −1.96 | <0.01 | −0.09 | 0.83 | −0.17 | 0.72 |
| VZV CD8+ IL-10+ | 2.10 | 0.07 | 0.11 | 0.90 | 0.07 | 0.49 | 0.03 | 0.94 | −0.14 | 0.62 | −0.40 | 0.23 |
| VZV CD4+ TGF-β+ | −0.54 | 0.45 | 0.07 | 0.90 | −0.03 | 0.63 | 0.30 | 0.19 | −0.20 | 0.27 | −0.24 | 0.25 |
| VZV CD8+ TGF-β+ | −0.19 | 0.62 | 0.09 | 0.78 | 0.01 | 0.78 | −0.53 | <0.01 | 0.20 | 0.03 | 0.20 | 0.08 |
Boldface type indicates statistically significant effects defined by a P value of <0.02 to adjust for multiple comparisons. Abbreviations: VZV, varicella-zoster virus; Tfh, T follicular helper; GrzB, granzyme B; RCE, regression coefficient estimate (indicates the magnitude of the effect of the explanatory variable on the outcome measure); CTL, cytotoxic T lymphocytes; baseline responses, prevaccination responses in the same category as the outcome measure (e.g., prevaccination IFN-γ SFC as an explanatory variable for peak IFN-γ SFC or prevaccination IL-2 SFC as an explanatory variable for peak IL-2 SFC, etc.).
Effect of age and VZV-specific and phenotypic T cell characteristics on VZV-specific Th1 responses 1 year after ZVL administration.
The multivariable analysis in the primary cohort that included only baseline measures as determining variables showed that age and baseline percentages of CD4+ FOXP3+ Treg and CD8+ PD1+ Tcheck had a negative effect, while baseline VZV-specific Th1 responses had a positive effect on the magnitude of persistent VZV-specific Th1 responses to ZVL at 1 year (Table 5). In the VZV flow cytometry group, the percentage of VZV-specific CD4+ CD39+ Treg also had an independent negative effect on VZV-specific Th1 persistence (Table 6). However, when peak VZV Th1 values were added to the determining variables, Treg or Tcheck no longer had independent effects on VZV-specific Th1 persistence, while the peak VZV-specific Th1 response had a strong independent positive effect on persistence (Tables 7 and 8).
TABLE 5.
Multivariable regression analysis of the effects of age and baseline immune parameters on the magnitude of persistent VZV-specific Th1 cells 1 year after ZVL administration in the primary cohort (n = 399)a
| Explanatory variable | 1-yr IL-2+ SFC |
1-yr IFN-γ+ SFC |
1-yr IFN-γ+ IL-2+ SFC |
|||
|---|---|---|---|---|---|---|
| RCE | P | RCE | P | RCE | P | |
| Age | −1.35 | 0.02 | −1.50 | 0.001 | −1.09 | 0.003 |
| Baseline responses | 0.74 | <0.001 | 0.80 | <0.001 | 0.79 | <0.001 |
| CD4+ IL-10+ | −1.46 | 0.9 | 2.72 | 0.78 | 0.77 | 0.92 |
| CD8+ IL-10+ | 9 | 0.38 | 1.26 | 0.88 | 3.56 | 0.6 |
| CD4+ FOXP3+ CD25+ | 19.11 | 0.68 | −25.43 | 0.50 | −7.93 | 0.79 |
| CD8+ FOXP3+ CD25+ | −37.26 | 0.28 | −6.18 | 0.83 | −10.35 | 0.65 |
| CD4+ FOXP3+ | −24.33 | 0.02 | −10.63 | 0.23 | −10.89 | 0.12 |
| CD8+ FOXP3+ | 8.29 | 0.55 | 0.51 | 0.96 | 0.23 | 0.98 |
| CD4+ TGF-β+ | 15.38 | 0.08 | 6.99 | 0.34 | 8.1 | 0.17 |
| CD8+ TGF-β+ | −1.60 | 0.78 | 3.13 | 0.51 | 1.07 | 0.78 |
| CD4+ CD28− CD57+ | −0.249 | 0.77 | −0.02 | 0.98 | −0.01 | 0.98 |
| CD8+ CD28− CD57+ | −0.72 | 0.07 | −0.44 | 0.18 | −0.41 | 0.12 |
| CD4+ PD1+ | 5.15 | 0.31 | 5.74 | 0.17 | 3.82 | 0.26 |
| CD8+ PD1+ | −10.34 | 0.02 | −6.96 | 0.05 | −6.19 | 0.03 |
| CD4+ CD31+ | −15.97 | 0.06 | −14.02 | 0.05 | −11.74 | 0.04 |
| CD8+ CD31+ | 6.69 | 0.42 | 7.83 | 0.25 | 5.85 | 0.29 |
Boldface type indicates statistically significant effects defined by a P value of <0.02 to adjust for multiple comparisons. Abbreviations: VZV, varicella-zoster virus; Th1, T helper 1; SFC, spot-forming cells/106 PBMC; RCE, regression coefficient estimate (indicates the magnitude of the difference); baseline responses, prevaccination responses in the same category as the outcome measure (e.g., prevaccination IFN-γ SFC as an explanatory variable for peak IFN-γ SFC or prevaccination IL-2 SFC as an explanatory variable for peak IL-2 SFC, etc.).
TABLE 6.
Multivariable regression analysis of the effect of age and baseline immune parameters on the magnitude of persistent VZV-specific Th1 cells 1 year after ZVL in the VZV-specific flow cytometry subset of the primary cohort (n = 95)a
| Explanatory variable | IL-2+ SFC |
IFN-γ+ SFC |
IFN-γ+ IL-2+ SFC |
|||
|---|---|---|---|---|---|---|
| RCE | P | RCE | P | RCE | P | |
| Age | −1.65 | 0.19 | −1.52 | 0.12 | −1.41 | 0.09 |
| Baseline value | 0.81 | <0.001 | 0.88 | <0.001 | 0.82 | <0.001 |
| VZV CD4+ CD39+ | −70.72 | 0.03 | −67.78 | 0.01 | −54.52 | 0.01 |
| VZV CD8+ CD39+ | 12.06 | 0.51 | 13.94 | 0.33 | 12.45 | 0.3 |
| VZV FOXP3+ CD4+ | 64.9 | 0.16 | 59.23 | 0.11 | 44.49 | 0.15 |
| VZV FOXP3+ CD8+ | 7.9 | 0.74 | 13.10 | 0.5 | 11.80 | 0.47 |
| VZV CD4+ IL-10+ | 64.9 | 0.16 | 59.23 | 0.11 | 44.49 | 0.15 |
| VZV CD8+ IL-10+ | 7.9 | 0.74 | 13.1 | 0.5 | 11.80 | 0.47 |
| VZV CD4+ TGF-β+ | 3.16 | 0.95 | 8.14 | 0.85 | 9.05 | 0.80 |
| VZV CD8+ TGF-β+ | −37.46 | 0.32 | −29.86 | 0.32 | −30.03 | 0.24 |
Boldface type indicates statistically significant effects defined by a P value of <0.02 to adjust for multiple comparisons. Abbreviations: VZV, varicella-zoster virus; Th1, T helper 1; SFC, spot-forming cells/106 PBMC; RCE, regression coefficient estimate (indicates the magnitude of the difference); baseline responses, prevaccination responses in the same category as the outcome measure (e.g., prevaccination IFN-γ SFC as an explanatory variable for peak IFN-γ SFC or prevaccination IL-2 SFC as an explanatory variable for peak IL-2 SFC, etc.).
TABLE 7.
Multivariable regression analysis of the effects of age and baseline and peak immune parameters on the magnitude of persistent VZV-specific Th1 cells 1 year after ZVL administration in the primary cohort (n = 399)a
| Explanatory variable | IL-2+ SFC |
IFN-γ+ SFC |
IFN-γ+ IL-2+ SFC |
|||
|---|---|---|---|---|---|---|
| RCE | P | RCE | P | RCE | P | |
| Age | −1.36 | 0.008 | −1.45 | 0.001 | −1.05 | 0.003 |
| Baseline value | 0.42 | <0.001 | 0.63 | <0.001 | 0.52 | <0.001 |
| Peak value | 0.32 | <0.001 | 0.11 | <0.001 | 0.24 | <0.001 |
| CD4+ IL-10+ | 3.33 | 0.76 | 3.46 | 0.72 | 2.5 | 0.74 |
| CD8+ IL-10+ | 5.82 | 0.53 | 1.53 | 0.85 | 3.58 | 0.58 |
| CD4+ FOXP3+ CD25+ | −7.16 | 0.86 | −47.14 | 0.2 | −21.52 | 0.45 |
| CD8+ FOXP3+ CD25+ | −18.18 | 0.56 | 11.64 | 0.67 | −1.28 | 0.95 |
| CD4+ FOXP3+ | −12.65 | 0.2 | −6.63 | 0.44 | −5.9 | 0.39 |
| CD8+ FOXP3+ | −0.22 | 0.99 | −2.58 | 0.81 | −3.12 | 0.72 |
| CD4+ TGF-β+ | 9.32 | 0.25 | 5.87 | 0.4 | 6.61 | 0.24 |
| CD8+ TGF-β+ | 2.44 | 0.64 | 2.92 | 0.52 | 1.96 | 0.59 |
| CD4+ CD28− CD57+ | 0.33 | 0.67 | 0.32 | 0.64 | 0.24 | 0.66 |
| CD8+ CD28− CD57+ | −0.62 | 0.09 | −0.47 | 0.14 | −0.37 | 0.14 |
| CD4+ PD1+ | 2.34 | 0.61 | 5.4 | 0.18 | 3.16 | 0.32 |
| CD8+ PD1+ | −3.99 | 0.32 | −6.81 | 0.05 | −3.43 | 0.2 |
| CD4+ CD31+ | −10.16 | 0.2 | −17.15 | 0.01 | −10.34 | 0.06 |
| CD8+ CD31+ | 2.5 | 0.74 | 11.09 | 0.1 | 3.48 | 0.51 |
Boldface type indicates statistically significant effects. Abbreviations: VZV, varicella-zoster virus; Th1, T helper 1; RCE, regression coefficient estimate (indicates the magnitude of the effect of the explanatory variable on the outcome measure); SFC, spot-forming cells/106 PBMC; baseline responses, prevaccination responses in the same category as the outcome measure (e.g., prevaccination IFN-γ SFC as an explanatory variable for peak IFN-γ SFC or prevaccination IL-2 SFC as an explanatory variable for peak IL-2 SFC, etc.).
TABLE 8.
Multivariable regression analysis of the effects of age and baseline and peak immune parameters on the magnitude of persistent VZV-specific Th1 cells 1 year after ZVL administration in the flow cytometry group of the primary cohort (n = 95)a
| Explanatory variable | IL-2+ SFC |
IFN-γ+ SFC |
IFN-γ+ IL-2+ SFC |
|||
|---|---|---|---|---|---|---|
| RCE | P | RCE | P | RCE | P | |
| Age | −2.46 | 0.04 | −1.45 | 0.14 | −1.83 | 0.02 |
| Baseline value | 0.27 | 0.07 | 0.72 | <0.001 | 0.39 | 0.01 |
| Peak value | 0.46 | <0.001 | 0.10 | 0.15 | 0.35 | <0.001 |
| VZV CD4+ CD39+ | −28.85 | 0.33 | −47.73 | 0.11 | −26.63 | 0.20 |
| VZV CD8+ CD39+ | −1.36 | 0.94 | 12.98 | 0.43 | −2.02 | 0.86 |
| VZV CD4+ FOXP3+ | 7.74 | 0.86 | 36.91 | 0.38 | 7.44 | 0.81 |
| VZV CD8+ FOXP3+ | 12.18 | 0.59 | 7 | 0.75 | 10.36 | 0.51 |
| VZV CD4+ IL-10+ | 16.61 | 0.73 | 44.86 | 0.35 | 0.41 | 0.99 |
| VZV CD8+ IL-10+ | −50.42 | 0.15 | −42.25 | 0.23 | −24.24 | 0.33 |
| VZV CD4+ TGF-β+ | 8.93 | 0.68 | −10.27 | 0.63 | −0.51 | 0.97 |
| VZV CD8+ TGF-β+ | 4.41 | 0.7 | 6.16 | 0.59 | 4.39 | 0.59 |
Boldface type indicates statistically significant effects. Abbreviations: VZV, varicella-zoster virus; Th1, T helper 1; RCE, regression coefficient estimate (indicates the magnitude of the effect of the explanatory variable on the outcome measure); SFC, spot-forming cells/106 PBMC; baseline responses, prevaccination responses in the same category as the outcome measure (e.g., prevaccination IFN-γ SFC as an explanatory variable for peak IFN-γ SFC or prevaccination IL-2 SFC as an explanatory variable for peak IL-2 SFC, etc.).
Effect of a prior dose of ZVL on the association of age and functional and phenotypic characteristics with VZV-specific Th1 responses to ZVL.
To determine if booster administration of ZVL changed the effect of age and baseline T cell functional and phenotypic profiles on peak VZV-specific Th1 responses to the vaccine, we used multivariable regression analyses of peak VZV-specific Th1 responses of all 600 participants, including booster status as one of the determining variables (Tables 7 and 8). Booster status did not have an independent effect on these outcome measures. Furthermore, the inclusion of booster status in the analysis did not appreciably change the associations of peak VZV-specific Th1 responses with the baseline VZV-specific Th1 or phenotypic T cell profile, except for revealing an independent positive effect of the percentage of CD4+ CD31+ Tnaive on peak VZV-specific Teff.
Mechanistic relevance of the negative associations of Treg with functional VZV-specific immune responses to ZVL.
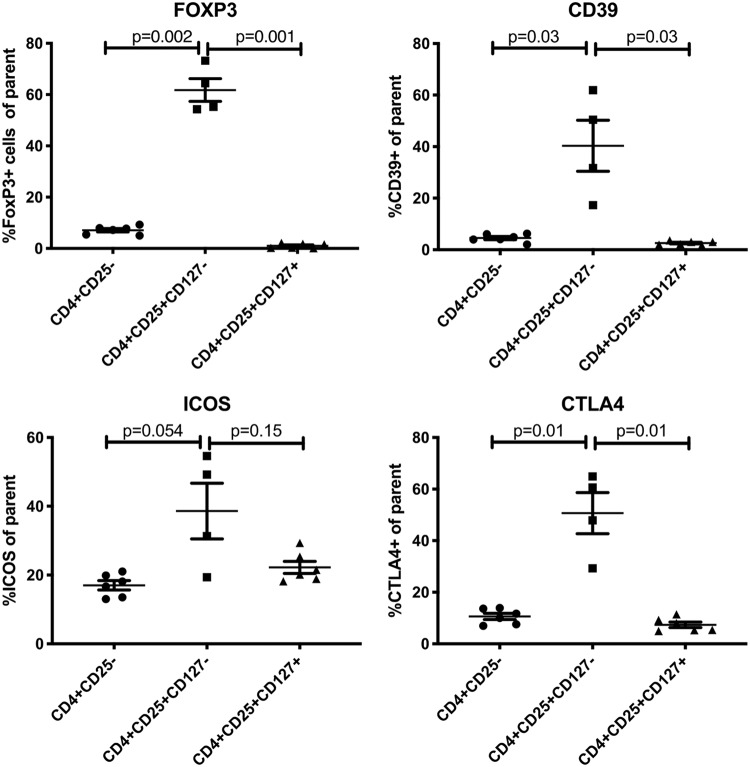
To determine the potential mechanism utilized by Treg to inhibit the memory responses to ZVL, we first identified the inhibitory ligands expressed by circulating Treg in our study population (Fig. 5). Since Treg may sometimes downregulate FOXP3 expression, we defined the Treg as CD4+ CD25+ CD127− T cells and compared their expression of FOXP3, CTLA4, CD39, and ICOS with those of the entire CD4+ parent population and CD4+ CD25+ CD127+ activated T cells (Tact). The data showed significantly higher expression levels of FOXP3, PD1, CTLA4, and CD39 on Treg than on total CD4+ T cells and Tact. ICOS was not significantly different (P values of 0.054 and 0.15 for comparisons with parent CD4+ and with activated CD4+ CD25+ CD127+ T cells).
FIG 5.
Expression of FOXP3 and inhibitory ligands on CD4+ CD25+ CD127− Treg. Data were derived from 6 adults of all ages. The parent population is indicated on the abscissa. Horizontal bars indicate paired comparisons and P values.
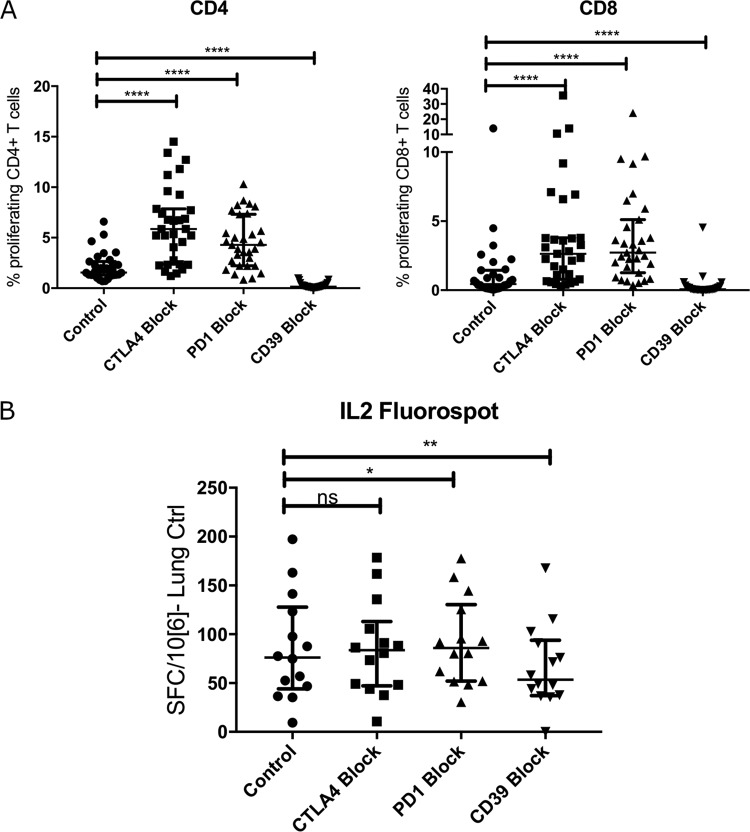
We further analyzed the associations between the proportions of circulating Treg and peak memory Th1 responses to ZVL by performing proliferation-blocking experiments with neutralizing antibodies against PD1, CD39, and CTLA4. PBMC from 31 study participants collected 6 weeks after immunization were stimulated with live VZV to stimulate the proliferation of both CD4+ and CD8+ T cells. Figure 6A shows significantly higher levels of proliferation of both CD4+ and CD8+ T cells when anti-PD1 and anti-CTLA4 were present than in untreated PBMC. In contrast, the addition of anti-CD39 neutralizing antibodies decreased proliferation compared with that of untreated controls. In a subset of 14 participants, we also measured IL-2 production by a FluoroSpot assay under these conditions (Fig. 6B). The data showed a significant increase in IL-2 SFC with anti-PD1 and a significant decrease with anti-CD39, corroborating the proliferation findings.
FIG 6.
Effect of blocking Treg-inhibitory molecules on T cell responses to ex vivo VZV restimulation. (A) Proliferation. Data were derived from 31 adults, including 19 participants >70 years of age, 6 participants 60 to 70 years of age, and 6 participants 50 to 60 years of age. PBMC from day 42 after vaccination corresponding to peak memory responses were stimulated with live VZV vOKA (control), supplemented with either anti-CTLA4 mAb (CTLA4 block), anti-PD1 mAb (PD1 block), or anti-CD39 mAb (CD39 block). (B) IL-2 SFC measured by a FluoroSpot assay. Data were derived from 14 older adults. PBMC from day 42 after vaccination corresponding to peak memory responses were stimulated with the VZV lysate in growth medium (control) or growth medium supplemented with anti-CTLA4 mAb (CTLA4 block), with anti-PD1 mAb (PD1 block), or with anti-CD39 mAb (CD39 block). Horizontal bars indicate comparisons between control and treated conditions. **** indicates a P value of <0.0001, calculated with a Wilcoxon matched-pairs signed-rank test. *, P < 0.05 and ≥ 0.01; **, P < 0.01 and ≥ 0.001; ns, not significant.
DISCUSSION
The primary objective of this analysis was to identify immunological factors that prevent older adults from mounting robust VZV-specific functional immune responses after ZVL. Although a mechanistic immune correlate of protection against HZ has not been identified, there is accumulating evidence of the importance of VZV-specific Th1 and CTL responses for protection against HZ (12, 13). These responses increased in all age groups after ZVL administration, but there was a pronounced negative effect of age, such that every decade increase in age was associated with significantly lower responses. This has been previously reported by us and others (14–16) but not in all studies (17, 18). It is important to note that only large studies were able to demonstrate the negative effect of age on responses to ZVL. Chronologically older adults form a heterogeneous group with respect to biological age, and previous studies have shown more robust correlations of vaccine responses with biological rather than chronological age in older adults (19). This explains why relatively large numbers of observations are needed to avoid biases introduced by biological and chronological age discordances.
An important finding of this study was revealed by the multivariable analyses that showed that the negative effect of age on peak VZV-specific Th1 responses to ZVL could be explained by a composite of immunological characteristics, which included baseline VZV-specific Th1 responses and high proportions of Treg and Tcheck. Lelic et al. also showed a negative effect of Treg on responses to ZVL (16). Using monoclonal antibodies (mAb) that block signaling through PD1 and CTLA4, we showed the mechanistic role of Treg and Tcheck in attenuating responses to ZVL and the potential of using anti-PD1 and anti-CTLA4 to increase vaccine immunogenicity. In contrast, CD39 blockade decreased VZV-specific responses. Anti-PD1 and anti-CTLA4 blocking agents have been licensed for use in cancer therapy. While anti-PD1 and anti-CTLA4 have been associated with a high frequency of severe adverse reactions, anti-PDL1 has a more favorable safety profile and might be an option for future use as an adjuvant for vaccination of older adults (20, 21). Moreover, immune modulators used for this purpose would be applied locally, and the exposure would be limited to a much shorter time than the duration of immune modulation associated with adverse events in cancer patients (21).
The magnitude of peak responses has been used to assess the immunogenicity of vaccines. We found that peak VZV-specific Th1 responses to ZVL had an independent positive effect on the persistence of the responses at 1 year, which is in accordance with our previous data (14). Furthermore, in a comparison of immune responses to ZVL with the recombinant HZ vaccine, which has a higher efficacy than ZVL, peak memory responses to vaccination mediated the difference in the persistence of immunogenicity between the two vaccines (17). This highlights the value of peak responses for predicting persistence.
The mechanism that leads to the accumulation of Tcheck and Treg with advancing age remains largely unexplained. Cytomegalovirus infection has been shown to increase the proportion of senescent T cells in older adults, presumably through repeated reactivations and consequent immune stimulation (22). Other chronic infections, such as tuberculosis and HIV, are associated with increases in Treg and Tcheck in the systemic circulation (23, 24). Moreover, it has been proposed that immune senescence is a consequence of repeated exposure to stressors, including antigens (25, 26). Before this study, it was not known if vaccine-generated immune stimulation led to an accumulation of Treg, Tcheck, or Tsen. In this study, we showed that vaccination did not result in persistent increases of these cell subsets.
It is important to note that while age did not affect peak immune responses to ZVL, older age had an independent negative effect on the persistence of these responses. This suggests that in addition to Treg and Tcheck, which maintained an independent negative effect on persistent Th1 responses, other factors, not included in our study, contribute to the age-associated erosion of VZV-specific Th1 responses. Older age was also negatively associated with baseline VZV-specific Th1, which is in accordance with the negative association between age and VZV-specific Th1 persistence (27).
We did not observe significant negative associations between age and baseline or peak VZV-specific functional responses measured by flow cytometry. This might be explained by the smaller number of participants with flow cytometry observations combined with the discordance between chronological and biological age mentioned above. However, the lack of an age effect on VZV-specific Tfh responses is in accordance with the insignificant variation of VZV-specific antibody responses to ZVL with age (14). Likewise, the lack of an association between age and the proportion of VZV-specific CTL responses to ZVL confirms previous findings that older adults have CTL responses to ZVL comparable to those of younger adults (28). However, the VZV-specific CTL from older adults express an excess of exhaustion and senescence markers compared with VZV-specific CTL of younger adults. This is likely to result in lower CTL functionality in older adults.
Responses to a second dose of ZVL administered ≥10 years after the first dose showed similar associations with age, Treg, and Tcheck. However, the responses to the second dose were higher than the responses to the first dose in age-matched older adults (9).
In conclusion, the decreased immunogenicity in older adults of this and other vaccines that show an age effect is likely to involve multiple mechanisms and cell populations. Here we identified Treg and Tcheck as potential mediators of the effect of age on vaccine responses. The significance of this finding could lead to novel strategies to immunize older adults, including immune modulators.
MATERIALS AND METHODS
Clinical trial design.
Participants were enrolled at the University of Colorado and Duke University Medical Center into a parent study that contained 4 study groups that were administered ZVL (Table 1) (9). Group 1 included 200 subjects ≥70 years of age who received ZVL ≥10 years previously, group 2 included 200 subjects ≥70 years of age who had never received ZVL, group 3 included 100 subjects 60 to <70 years of age who had never received ZVL, and group 4 included 100 subjects ≥50 to <60 years of age who had never received ZVL. Participants had no immunocompromising condition, had stable chronic illnesses characteristic of their age, and had never had HZ. At entry, they had a baseline blood sample obtained and received a single subcutaneous injection of ZVL. Subsequent blood samples were obtained at weeks 1, 6, and 52 thereafter.
Immunological assessments.
(i) IFN-γ and IL-2 FluoroSpot assays. Peripheral blood mononuclear cells (PBMC) from heparinized blood, separated by Ficoll-Hypaque density gradients (Sigma Diagnostics), were obtained from all study participants and frozen at the study sites, as previously described (29). Cells frozen at the Duke site were sent to Denver in liquid nitrogen Dewars. Dual-color IFN-γ and IL-2 FluoroSpot assays were performed using MabTech kits according to the manufacturer’s instructions. PBMC with a viability of ≥70% were added at 250,000 cells/well in 100 μl of RPMI 1640 with glutamine (Gibco) that contained 10% human AB serum (Nabi) and 1% antibiotics (Gibco). Cells were stimulated in duplicate wells with the UV-inactivated VZV-infected cell lysate of mock-infected controls at a 1:20 (vol/vol) concentration (corresponding to a final concentration of ∼10,000 PFU/ml) or 10 μg of phytohemagglutinin A (PHA)/well. The VZV-infected cell lysate was prepared by infecting 75% confluent human lung fibroblasts in T-175 flasks (Corning) with the VZV vOKA strain at a multiplicity of infection of 0.1 PFU per cell for 4 days at 37°C in a 5% CO2 humidified atmosphere. At the end of the incubation, 27 ml of culture medium was discarded, and the cells were scraped off the flask and dispersed in the remaining 3 ml of culture medium using a Dounce homogenizer. The solution was clarified of cellular debris by centrifugation, after which the virus was inactivated by placing the solution in a petri dish 10 cm from a UV light for 20 min. The solution was aliquoted and stored at −80°C until use. A mock-infected control was prepared according to the same procedure performed on uninfected cells. For blocking experiments, anti-CTLA4 (clone L3D10, catalog number 349904; BioLegend), anti-PD1 (clone EH12.2H7, catalog number 329912; BioLegend), anti-CD39 (clone A1, catalog number 328202; BioLegend), and a nonspecific antibody control were added at 5 μg/ml to duplicate wells. After 48 h at 37°C in a 5% CO2 humidified atmosphere, plates were washed, bound IFN-γ was detected with 7-B6-1-FS fluorescein isothiocyanate (FITC), and bound IL-2 was detected with 11-biotin. Spots were revealed using a mixture of anti-FITC-green fluorochrome (IFN-γ) and SA-red fluorochrome (IL-2) and analyzed with an Immunospot II plate reader (CTL). Results are reported as mean SFC/106 PBMC in VZV-stimulated wells after subtraction of the mean SFC in control wells. An assay control consisting of PBMC from a single leukopack was included in each run. All assays were valid as defined by leukopack control wells containing 280 to 850 IFN-γ SFC/106 PBMC (corresponding to the mean ± 2 standard deviations [SD]) and PHA-stimulated wells having ≥400 IFN-γ and ≥400 IL-2 SFC/106 PBMC.
(ii) T cell phenotypic profile. Unstimulated regulatory, senescent, checkpoint-expressing, and naive T cells (Treg, Tsen, Tcheck, and Tnaive) were measured in freshly thawed cryopreserved PBMC from all participants. Cells were washed, and viable PBMC were counted using Guava easyCyte (Millipore) and ViaCount staining (Millipore). Cells were stained using 2 panels at 105 to 106 PBMC/panel. In all panels, PBMC were surface stained using staining buffer (BD Biosciences) and the following conjugated monoclonal antibodies (mAbs): anti-CD3-phycoerythrin-Cy7 (PECy7) (clone SK7; BD Biosciences) and anti-CD4-peridinin chlorophyll protein (PerCP)-Cy5.5 (clone SK3; BD Biosciences). In addition, in panel 1, PBMC were surface stained with anti-CD28-FITC (clone CD28.2; BD Biosciences), anti-CD31-allophycocyanin (APC) (clone WM-59; eBioscience), anti-CD57-PE (clone NK-1; BD Biosciences), and anti-PD1-PECy7 (clone EH12.1; BD Biosciences). In panel 2, PBMC were also surface stained with anti-CD25-PECy7 (clone BC96; BioLegend), after which cells were fixed and permeabilized with Cytofix/Cytoperm (BD Biosciences) and stained with anti-IL-10–APC (catalog number 127107; R&D Systems), anti-FOXP3-PE (clone 206D; BioLegend), and anti-TGF-β–PerCP–Cy5.5 (clone TW4-2F8; BioLegend). Total T cells and subpopulations were characterized and counted on Guava easyCyte 8HT (Millipore) and analyzed with FlowJo (TreeStar). Subsets were expressed as a percentage of the CD3+ CD4+ or CD3+ CD8+ parent populations.
For Treg characterization, thawed PBMC were separated into CD4+ CD25+ CD127−, CD4+ CD25+ CD127+, and CD4+ CD25− CD127+ populations using fluorescence-activated cell sorting (FACS). Cells were stained with viability dye (catalog number 423104; BioLegend) and then surface stained with anti-ICOS Ax488 (Alexa Fluor 488) (clone C398.4A, catalog number 313514; BioLegend), anti-CTLA4 PE-CF594 (cyanine-based fluorescent 594) (clone BNI3, catalog number 562742; BD), anti-LAG3 PerCP-Cy5.5 (clone 11C3C65, catalog number 369312; BioLegend), anti-CD25 PECy7 (clone 2A3, catalog number 335807; BD), anti-CD39 APC (clone TU66, catalog number 560239; BD), anti-CD4 Ax700 (clone RPA-T4, catalog number 557922; BD), anti-CD3 APC-H7 (clone SK7, catalog number 560176; BD), and anti-CD127 BV421 (clone A019D5, catalog number 351310; BioLegend). Intracellular staining was performed with the FoxP3 transcription factor staining set (eBioscience), and anti-FoxP3 PE (clone PCH101, catalog number 12-4776-42; eBioscience) was added. Population purity and characterization were acquired using a Gallios flow cytometer and analyzed using FlowJo analysis software (TreeStar). Figure S1A in the supplemental material shows the gating strategy.
(iii) T cell functional profile. Cryopreserved PBMC from 128 participants were thawed, washed, and counted as described above. VZV-stimulated effector T cells (Teff) and Treg were measured after 48 h of in vitro stimulation with live VZV strain vOKA at 60,000 PFU/cell. In contrast to the FluoroSpot assays, which used an inactivated VZV cell lysate, which is almost exclusively presented in the context of major histocompatibility complex (MHC) class II, therefore stimulating only CD4+ T cells, in the flow cytometry experiments, we used live virus presented in the context of MHC class I and II, therefore stimulating both CD4+ and CD8+ T cells. For the last 12 to 15 h of incubation, anti-CD107a-AF700 (clone H4A3; BD Biosciences) mAb and brefeldin A (at 10 μg/ml; Sigma-Aldrich) were added. Cells were then surface stained using the conjugated mAbs anti-CD3-PECy7 (clone SK7; BD Biosciences), anti CD4-PC5 (clone 13B8.2; Beckman Coulter), and anti-CD39-FITC (clone TU66; BD Biosciences) and fixed and permeabilized with Cytofix/Cytoperm (BD Biosciences), followed by staining with anti-IL-10–APC (clone JES3-19F1; BD Biosciences), anti-FOXP3-PECy7 (clone PCH101; eBioscience), anti-TGF-β–BV421 clone (TW4-2F8; BioLegend), anti-IL-21–PE (clone 3A3-N21; BD Biosciences), and anti-GrzB-PE-CF594 (clone GB11; BD Biosciences). Zombie-Yellow (BioLegend) was used for viability staining. Total T cells and subpopulations were analyzed with a Gallios A94303 10-color flow cytometer (Beckman Coulter) and Kaluza (Beckman Coulter) or FlowJo software (Fig. S1B). Subsets were expressed as a percentage of the parent CD3+ CD4+ or CD3+ CD8+ cell populations.
(iv) T cell proliferation and blocking experiments. Thawed PBMC stained with CellTrace Violet (catalog number C34557; BioLegend) were cultured in the presence of the live VZV vOKA inactivated cell lysate (1:200) for 5 days at 106 PBMC/ml. Blocking anti-CTLA4 (clone L3D10, catalog number 349904; BioLegend), anti-PD1 (clone EH12.2H7, catalog number 329912; BioLegend), or anti-CD39 (clone A1, catalog number 328202; BioLegend) or a nonspecific antibody control was added at 5 μg/ml to the cultures for the full 5 days. At the end of the incubation, PBMC were washed with phosphate-buffered saline (PBS) and stained with Zombie-Yellow viability stain. PBMC were then washed; stained with anti-CD3-Ax700 (clone UCHT1, catalog number 557943; BD), anti-CD4-PC5.5 (clone 13B8.2, catalog number B16491; Beckman Coulter), and anti-CD8-PE-CF594 (clone RPA-T8, catalog number 562282; BD); and analyzed on the Gallios instrument. Proliferation was assessed by CellTrace-dim populations using FlowJo software (Fig. S1C).
Statistical analysis.
Unadjusted Pearson correlation coefficients were calculated to explore the relationship between all the data points. Multiple-linear-regression analysis with log transformation on the response variables was conducted, with different sets of covariate variables, i.e., stimulated/unstimulated flow cytometric variables predefined as senescence, regulatory, exhausted, or naive markers. A criterion of a P value of <0.02 was employed to partially adjust for the multiplicity of comparisons and screen possible significant correlations. All statistical analyses were performed using SAS v9.3, with the exception of Treg effector mechanism experiments, which were analyzed with Prism v8 (GraphPad).
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by Merck & Co., Inc., Kenilworth, NJ.
A.W. received research funds and travel support from Merck & Co. and received research funds from GlaxoSmithKline. M.J.L. has served on an advisory board for and received research funds from Merck Sharp & Dohme. He has served on advisory boards for and received research funds from GlaxoSmithKline. L.P. and Z.P. are employees of Merck & Co., Inc., and may hold stock options. All other authors report no potential conflicts of interest.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/JVI.00305-19.
REFERENCES
- 1.Boraschi D, Del Giudice G, Dutel C, Ivanoff B, Rappuoli R, Grubeck-Loebenstein B. 2010. Ageing and immunity: addressing immune senescence to ensure healthy ageing. Vaccine 28:3627–3631. doi: 10.1016/j.vaccine.2010.03.035. [DOI] [PubMed] [Google Scholar]
- 2.Goodwin K, Viboud C, Simonsen L. 2006. Antibody response to influenza vaccination in the elderly: a quantitative review. Vaccine 24:1159–1169. doi: 10.1016/j.vaccine.2005.08.105. [DOI] [PubMed] [Google Scholar]
- 3.Ronne T, Valentelis R, Tarum S, Griskevica A, Wachmann CH, Aggerbeck H, Plesner AM, Hansen KG, Ricks P. 2000. Immune response to diphtheria booster vaccine in the Baltic states. J Infect Dis 181(Suppl 1):S213–S219. doi: 10.1086/315560. [DOI] [PubMed] [Google Scholar]
- 4.Shapiro ED, Berg AT, Austrian R, Schroeder D, Parcells V, Margolis A, Adair RK, Clemens JD. 1991. The protective efficacy of polyvalent pneumococcal polysaccharide vaccine. N Engl J Med 325:1453–1460. doi: 10.1056/NEJM199111213252101. [DOI] [PubMed] [Google Scholar]
- 5.Oxman MN, Levin MJ, Johnson GR, Schmader KE, Straus SE, Gelb LD, Arbeit RD, Simberkoff MS, Gershon AA, Davis LE, Weinberg A, Boardman KD, Williams HM, Zhang JH, Peduzzi PN, Beisel CE, Morrison VA, Guatelli JC, Brooks PA, Kauffman CA, Pachucki CT, Neuzil KM, Betts RF, Wright PF, Griffin MR, Brunell P, Soto NE, Marques AR, Keay SK, Goodman RP, Cotton DJ, Gnann JW Jr, Loutit J, Holodniy M, Keitel WA, Crawford GE, Yeh SS, Lobo Z, Toney JF, Greenberg RN, Keller PM, Harbecke R, Hayward AR, Irwin MR, Kyriakides TC, Chan CY, Chan IS, Wang WW, Annunziato PW, Silber JL, Shingles Prevention Study Group. 2005. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med 352:2271–2284. doi: 10.1056/NEJMoa051016. [DOI] [PubMed] [Google Scholar]
- 6.Schmader KE, Levin MJ, Gnann JW Jr, McNeil SA, Vesikari T, Betts RF, Keay S, Stek JE, Bundick ND, Su SC, Zhao Y, Li X, Chan IS, Annunziato PW, Parrino J. 2012. Efficacy, safety, and tolerability of herpes zoster vaccine in persons aged 50-59 years. Clin Infect Dis 54:922–928. doi: 10.1093/cid/cir970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morrison VA, Johnson GR, Schmader KE, Levin MJ, Zhang JH, Looney DJ, Betts R, Gelb L, Guatelli JC, Harbecke R, Pachucki C, Keay S, Menzies B, Griffin MR, Kauffman CA, Marques A, Toney J, Boardman K, Su S-C, Li X, Chan ISF, Parrino J, Annunziato P, Oxman MN, Shingles Prevention Study Group. 2015. Long-term persistence of zoster vaccine efficacy. Clin Infect Dis 60:900–909. doi: 10.1093/cid/ciu918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tseng HF, Harpaz R, Luo Y, Hales CM, Sy LS, Tartof SY, Bialek S, Hechter RC, Jacobsen SJ. 2016. Declining effectiveness of herpes zoster vaccine in adults aged >/=60 Years. J Infect Dis 213:1872–1875. doi: 10.1093/infdis/jiw047. [DOI] [PubMed] [Google Scholar]
- 9.Levin MJ, Schmader KE, Pang L, Williams-Diaz A, Zerbe G, Canniff J, Johnson MJ, Caldas Y, Cho A, Lang N, Su SC, Parrino J, Popmihajlov Z, Weinberg A. 2016. Cellular and humoral responses to a second dose of herpes zoster vaccine administered 10 years after the first dose among older adults. J Infect Dis 213:14–22. doi: 10.1093/infdis/jiv480. [DOI] [PubMed] [Google Scholar]
- 10.Weinberg A, Popmihajlov Z, Schmader KE, Johnson MJ, Caldas Y, Salazar AT, Canniff J, McCarson BJ, Martin J, Pang L, Levin MJ. 2019. Persistence of varicella-zoster virus cell-mediated immunity after the administration of a second dose of live herpes zoster vaccine. J Infect Dis 219:335–338. doi: 10.1093/infdis/jiy514. [DOI] [PubMed] [Google Scholar]
- 11.Smith JG, Liu X, Kaufhold RM, Clair J, Caulfield MJ. 2001. Development and validation of a gamma interferon ELISPOT assay for quantitation of cellular immune responses to varicella-zoster virus. Clin Diagn Lab Immunol 8:871–879. doi: 10.1128/CDLI.8.5.871-879.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weinberg A, Huang S, Song LY, Fenton T, Williams P, Patterson J, Tovar-Salazar A, Levin MJ. 2012. Immune correlates of herpes zoster in HIV-infected children and youth. J Virol 86:2878–2881. doi: 10.1128/JVI.06623-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weinberg A, Levin MJ. 2010. VZV T cell-mediated immunity. Curr Top Microbiol Immunol 342:341–357. doi: 10.1007/82_2010_31. [DOI] [PubMed] [Google Scholar]
- 14.Levin MJ, Oxman MN, Zhang JH, Johnson GR, Stanley H, Hayward AR, Caulfield MJ, Irwin MR, Smith JG, Clair J, Chan IS, Williams H, Harbecke R, Marchese R, Straus SE, Gershon A, Weinberg A, Veterans Affairs Cooperative Studies Program Shingles Prevention Study Investigators. 2008. Varicella-zoster virus-specific immune responses in elderly recipients of a herpes zoster vaccine. J Infect Dis 197:825–835. doi: 10.1086/528696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weinberg A, Lazar AA, Zerbe GO, Hayward AR, Chan IS, Vessey R, Silber JL, MacGregor RR, Chan K, Gershon AA, Levin MJ. 2010. Influence of age and nature of primary infection on varicella-zoster virus-specific cell-mediated immune responses. J Infect Dis 201:1024–1030. doi: 10.1086/651199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lelic A, Verschoor CP, Lau VW, Parsons R, Evelegh C, Bowdish DM, Bramson JL, Loeb MB. 2016. Immunogenicity of varicella vaccine and immunologic predictors of response in a cohort of elderly nursing home residents. J Infect Dis 214:1905–1910. doi: 10.1093/infdis/jiw462. [DOI] [PubMed] [Google Scholar]
- 17.Levin MJ, Kroehl ME, Johnson MJ, Hammes A, Reinhold D, Lang N, Weinberg A. 2018. Th1 memory differentiates recombinant from live herpes zoster vaccines. J Clin Invest 128:4429–4440. doi: 10.1172/JCI121484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vukmanovic-Stejic M, Sandhu D, Seidel JA, Patel N, Sobande TO, Agius E, Jackson SE, Fuentes-Duculan J, Suarez-Farinas M, Mabbott NA, Lacy KE, Ogg G, Nestle FO, Krueger JG, Rustin MHA, Akbar AN. 2015. The characterization of varicella zoster virus-specific T cells in skin and blood during aging. J Invest Dermatol 135:1752–1762. doi: 10.1038/jid.2015.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fourati S, Cristescu R, Loboda A, Talla A, Filali A, Railkar R, Schaeffer AK, Favre D, Gagnon D, Peretz Y, Wang IM, Beals CR, Casimiro DR, Carayannopoulos LN, Sekaly RP. 2016. Pre-vaccination inflammation and B-cell signalling predict age-related hyporesponse to hepatitis B vaccination. Nat Commun 7:10369. doi: 10.1038/ncomms10369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Naidoo J, Page DB, Li BT, Connell LC, Schindler K, Lacouture ME, Postow MA, Wolchok JD. 2015. Toxicities of the anti-PD-1 and anti-PD-L1 immune checkpoint antibodies. Ann Oncol 26:2375–2391. doi: 10.1093/annonc/mdv383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spain L, Diem S, Larkin J. 2016. Management of toxicities of immune checkpoint inhibitors. Cancer Treat Rev 44:51–60. doi: 10.1016/j.ctrv.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 22.Derhovanessian E, Maier AB, Hahnel K, Beck R, de Craen AJ, Slagboom EP, Westendorp RG, Pawelec G. 2011. Infection with cytomegalovirus but not herpes simplex virus induces the accumulation of late-differentiated CD4+ and CD8+ T-cells in humans. J Gen Virol 92:2746–2756. doi: 10.1099/vir.0.036004-0. [DOI] [PubMed] [Google Scholar]
- 23.Chevalier MF, Weiss L. 2013. The split personality of regulatory T cells in HIV infection. Blood 121:29–37. doi: 10.1182/blood-2012-07-409755. [DOI] [PubMed] [Google Scholar]
- 24.Larson RP, Shafiani S, Urdahl KB. 2013. Foxp3(+) regulatory T cells in tuberculosis. Adv Exp Med Biol 783:165–180. doi: 10.1007/978-1-4614-6111-1_9. [DOI] [PubMed] [Google Scholar]
- 25.Franceschi C, Bonafe M, Valensin S, Olivieri F, De Luca M, Ottaviani E, De Benedictis G. 2000. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann N Y Acad Sci 908:244–254. [DOI] [PubMed] [Google Scholar]
- 26.De Martinis M, Franceschi C, Monti D, Ginaldi L. 2005. Inflamm-ageing and lifelong antigenic load as major determinants of ageing rate and longevity. FEBS Lett 579:2035–2039. doi: 10.1016/j.febslet.2005.02.055. [DOI] [PubMed] [Google Scholar]
- 27.Qi Q, Cavanagh MM, Le Saux S, Wagar LE, Mackey S, Hu J, Maecker H, Swan GE, Davis MM, Dekker CL, Tian L, Weyand CM, Goronzy JJ. 2016. Defective T memory cell differentiation after varicella zoster vaccination in older individuals. PLoS Pathog 12:e1005892. doi: 10.1371/journal.ppat.1005892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weinberg A, Canniff J, Rouphael N, Mehta A, Mulligan M, Whitaker JA, Levin MJ. 2017. Varicella-zoster virus-specific cellular immune responses to the live attenuated zoster vaccine in young and older adults. J Immunol 199:604–612. doi: 10.4049/jimmunol.1700290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weinberg A, Song LY, Wilkening CL, Fenton T, Hural J, Louzao R, Ferrari G, Etter PE, Berrong M, Canniff JD, Carter D, Defawe OD, Garcia A, Garrelts TL, Gelman R, Lambrecht LK, Pahwa S, Pilakka-Kanthikeel S, Shugarts DL, Tustin NB. 2010. Optimization of storage and shipment of cryopreserved peripheral blood mononuclear cells from HIV-infected and uninfected individuals for ELISPOT assays. J Immunol Methods 363:42–50. doi: 10.1016/j.jim.2010.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.