Abstract
Acetaldehyde is known to be carcinogenic and produced by oral bacteria. Thus, bacterial acetaldehyde production might contribute to oral cancer. Therefore, we examined bacterial acetaldehyde production from ethanol and glucose under various conditions mimicking the oral cavity and clarified the metabolic pathways responsible for bacterial acetaldehyde production. Streptococcus mitis, S. salivarius, S. mutans, Neisseria mucosa and N. sicca were used. The bacterial metabolism was conducted at pH 5.0–8.0 under aerobic and anaerobic conditions. The production of acetaldehyde and organic acids was measured with gas chromatography and HPLC, respectively. Bacterial enzymes were also assessed. All of the bacteria except for S. mutans exhibited their greatest acetaldehyde production from ethanol at neutral to alkaline pH under aerobic conditions. S. mutans demonstrated the greatest acetaldehyde from glucose under anaerobic conditions, although the level was much lower than that from ethanol. Alcohol dehydrogenase and NADH oxidase were detected in all of the bacteria. This study revealed that oral indigenous bacteria, Streptococcus and Neisseria can produce acetaldehyde, and that such acetaldehyde production is affected by environmental conditions. It was suggested that alcohol dehydrogenase and NADH oxidase are involved in ethanol-derived acetaldehyde production and that the branched-pathway from pyruvate is involved in glucose-derived acetaldehyde production.
Subject terms: Pathogens, Oxidoreductases
Introduction
Many epidemiological studies have reported that chronic and heavy alcohol consumption, as well as poor oral hygiene, are strongly correlated with oral cancer1–4. However, in vitro studies have indicated that ethanol itself is not carcinogenic5,6. The mechanism by which poor oral hygiene contributes to the pathogenesis of oral cancer remains unclear. However, a positive association between poor oral hygiene and the occurrence of head and neck cancer was observed in alcohol drinkers7, suggesting that alcohol alone is associated with a low risk of cancer, but the co-existence of bacteria and alcohol increases the risk of cancer. When ethanol was added to saliva, greater acetaldehyde production was detected in patients with poor oral hygiene1, supporting the assertion that oral bacteria are involved in acetaldehyde production in the oral cavity.
Acetaldehyde is produced from ethanol through the oxidation of ethanol by alcohol dehydrogenase in the liver8, and it is widely accepted that the accumulation of acetaldehyde is involved in “hangovers”. Furthermore, acetaldehyde is known to possess carcinogenicity, and the International Agency for Research on Cancer of the World Health Organization classified it as a chemical substance whose carcinogenicity to humans was doubted (group 2B). Many basic studies about the carcinogenicity of acetaldehyde have been performed. In human cells, acetaldehyde can cause sister-chromatid exchanges, gene mutations, and DNA-strand breaks5,9,10. In rat hematopoietic stem cells, acetaldehyde damaged chromosomes and caused stem cell mutations11. In animal experiments, the oral administration and inhalation of acetaldehyde were suggested to be carcinogenic12.
The findings described above suggest that the accumulation of oral bacteria due to poor oral hygiene might increase bacterial acetaldehyde production and subsequently contribute to the development of oral cancer. Recently, oral bacteria, such as Neisseria, Streptococcus, Actinomyces, and Prevotella species, and Candida species were reported to be able to produce acetaldehyde from ethanol or glucose13,14; however, no bacterial species that are specifically associated with oral cancer have ever been found15–17. Therefore, other factors might affect the acetaldehyde production of such bacteria in the oral cavity. Environmental factors in the oral cavity, such as the local oxygen concentration, pH, and the types and concentrations of metabolic substrates, are affected by oral hygiene, dietary nutrients, and salivary secretion. Among such environmental factors, the oxygen concentration and pH are known to particularly affect the metabolic activity of oral bacteria18–20. Therefore, in order to estimate bacterial pathogenicity, such as the acetaldehyde production of the oral biofilm, it is important to examine not only the bacterial composition of the oral biofilm, but also the metabolic activity of these bacteria while considering the effects of environmental factors on bacterial metabolism20–22.
In the present study, we aimed to examine acetaldehyde production by representative examples of the dominant indigenous acetaldehyde-producing bacteria in the oral cavity; i.e., Streptococcus and Neisseria species, and the effects of oral environmental factors; i.e., pH and the concentrations of oxygen and metabolic substrates, on the metabolic activity of such bacteria. Furthermore, we attempted to suggest the metabolic properties of bacterial acetaldehyde production by detecting the enzymes involved in acetaldehyde production as well as the associated metabolic end-products.
Results
Acetaldehyde production from ethanol and the effect of pH and oxygen levels on it
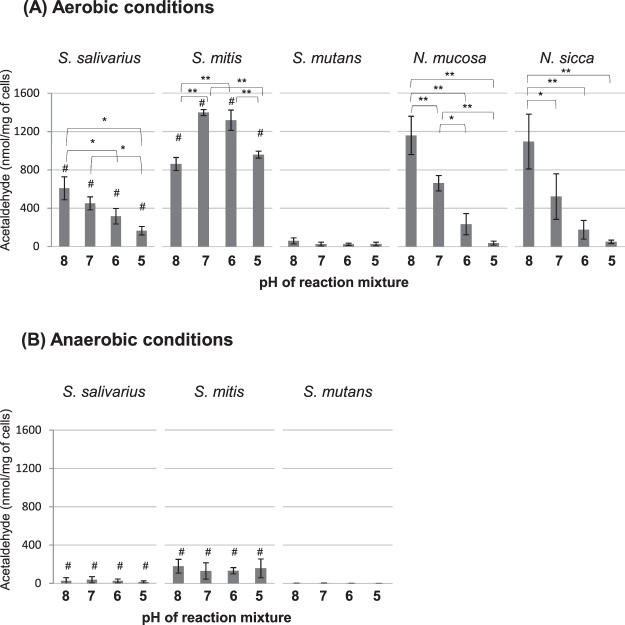
All of the bacterial strains, except for S. mutans, produced acetaldehyde from ethanol (Fig. 1A,B). S. mitis and S. salivarius produced more acetaldehyde under aerobic conditions than under anaerobic conditions (only 4 to 21% of the levels seen during aerobic production). Acetaldehyde production peaked at pH 8.0 in all of the acetaldehyde-producing strains, except for S. mitis, and it decreased as the pH was lowered (Fig. 1A). The acetaldehyde production of S. mitis was high at pH 7.0 and 6.0. The effect on pH on acetaldehyde production under anaerobic conditions was unclear (Fig. 1B).
Figure 1.
Acetaldehyde production from ethanol for 30 min at 37 °C under aerobic (A) or anaerobic (B) conditions. Bars, standard deviation; *significant difference (p < 0.05); **significant difference (p < 0.01); #significant difference between aerobic and anaerobic conditions (p < 0.05).
Acetaldehyde production from glucose and the effect of pH and oxygen levels on it
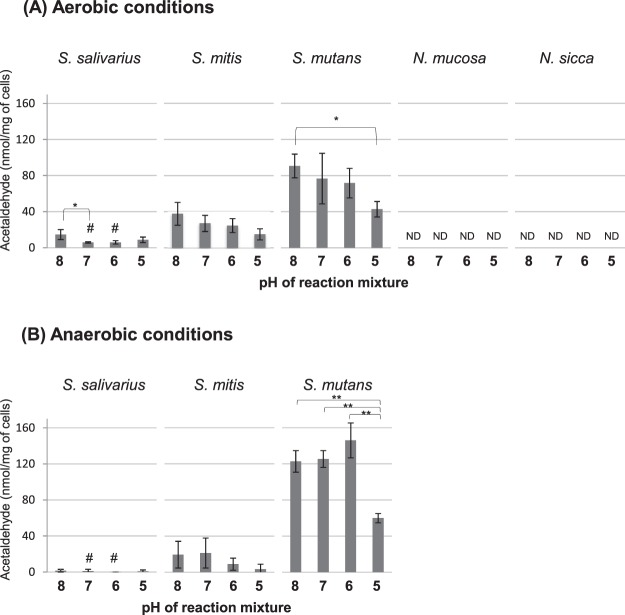
The streptococcal strains produced acetaldehyde from glucose and tended to exhibit high activity at pH 8.0, while the Neisseria strains did not (Fig. 2A,B). The amounts of acetaldehyde produced from glucose by the streptococcal strains were much lower than those produced from ethanol (1.3 to 5.3%) (Figs 1, 2). Contrary to the findings regarding acetaldehyde production from ethanol, S. mutans produced more acetaldehyde than the other strains, and greater acetaldehyde production was seen under anaerobic conditions than under aerobic conditions (Fig. 2A,B).
Figure 2.
Acetaldehyde production from glucose for 30 min at 37 °C under aerobic (A) or anaerobic (B) conditions. Bars, standard deviation; ND, below detection limit; *significant difference (p < 0.05); **significant difference (p < 0.01); #significant difference between aerobic and anaerobic conditions (p < 0.05).
Alcohol dehydrogenase and NADH oxidase activity
Alcohol dehydrogenase and NADH oxidase activity were detected in all of the strains (Table 1). The Neisseria strains exhibited higher alcohol dehydrogenase activity, while the streptococcal strains displayed higher NADH oxidase activity.
Table 1.
The enzymatic activity of alcohol dehydrogenase and NADH oxidase of Streptococcus and Neisseria species.
| Enzyme | Enzymatic Activity (mU/mg of protein)a | ||||
|---|---|---|---|---|---|
| S. mutans | S. salivarius | S. mitis | N. mucosa | N. sicca | |
| Alcohol dehydrogenase | 90.2 ± 17.7 | 9.2 ± 8.7 | 2.5 ± 0.4 | 626 ± 384 | 342 ± 147 |
| NADH oxidase | 235 ± 128 | 198 ± 175 | 769 ± 690 | 28.6 ± 17.5 | 22.8 ± 16.8 |
aMean ± standard deviation obtained from three independent experiments.
End-products of ethanol and glucose metabolism by Streptococcus strains
In addition to acetaldehyde, the streptococcal strains produced lactate, acetate, and formate from ethanol as metabolic end-products. S. mutans produced the highest amounts of end-products under aerobic conditions, with acetate being the main end-product (Fig. 3A). However, these amounts were much smaller than those of acetaldehyde (Fig. 1).
Figure 3.
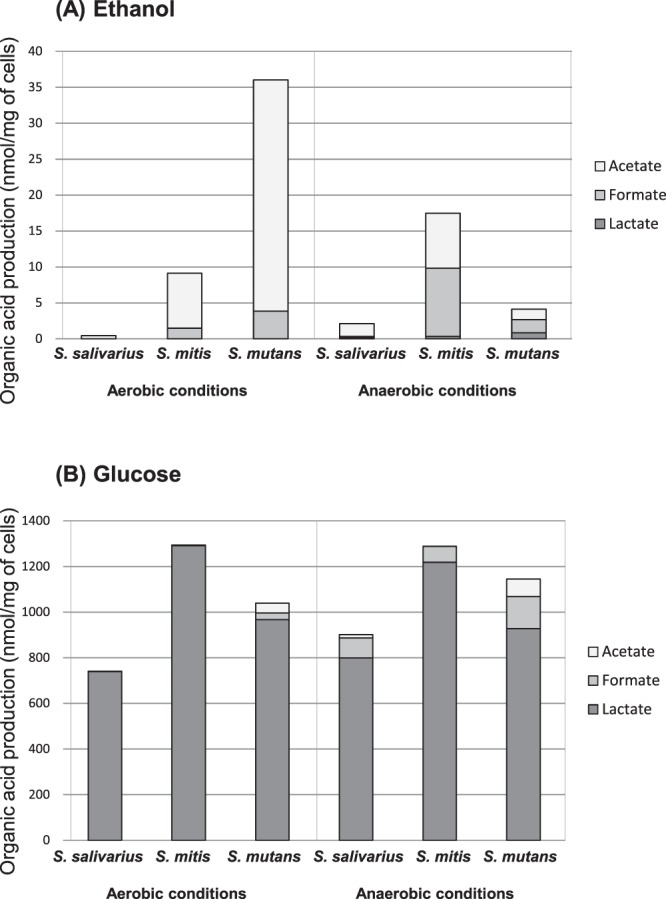
Metabolic end-products produced from ethanol (A) or glucose (B) by Streptococcus species after incubation for 30 min at 37 °C and pH 7.0 under aerobic or anaerobic conditions.
The streptococcal strains mainly produced lactate from glucose, with small amounts of formate and acetate (Fig. 3B). The amounts of these end-products were much higher than the amount of acetaldehyde produced (Fig. 2). S. mutans produced more formate under anaerobic conditions than the other streptococci.
Discussion
S. salivarius, S. mitis, N. mucosa, and N. sicca exhibited greater acetaldehyde production from ethanol under aerobic conditions than under anaerobic conditions (Fig. 1), indicating that aerobic conditions are preferable for acetaldehyde production from ethanol. Only S. salivarius and S. mitis produced acetaldehyde from glucose, although the amounts of acetaldehyde produced were much smaller than those produced from ethanol (Fig. 2), confirming that ethanol is the preferred substrate for acetaldehyde production, as reported previously14. On the other hand, S. mutans only produced a small amount of acetaldehyde from ethanol, whereas it produced more acetaldehyde from glucose than the other streptococci (Figs 1, 2). These metabolic properties of S. mutans will be discussed later. As for the environmental pH, in general bacterial acetaldehyde production was highest at pH 8.0 and decreased as the pH was lowered (Figs 1, 2) (with some exceptions, such as aerobic production from ethanol by S. mitis and anaerobic production from glucose by S. mutans).
These results imply that the dominant indigenous oral bacteria, such as non-mutans streptococci and Neisseria species23, are among those responsible for acetaldehyde production from ethanol in the oral cavity. In addition, the fact that ethanol-derived acetaldehyde production was increased under aerobic conditions and at neutral to weakly alkaline environmental pH suggests that acetaldehyde can be produced in the thin oral biofilm and saliva, even in healthily maintained oral cavities, where oxygen is available enough and pH is neutral to weakly alkaline due to salivary flow. An epidemiological study found a relationship between poor oral hygiene and oral cancer7,24, suggesting that thick biofilms might contribute to carcinogenesis. Thick biofilms contain more bacteria than thin biofilms and saliva, and the surfaces of biofilms are exposed to aerobic conditions and are considered to be able to produce acetaldehyde efficiently from ethanol. Therefore, in general poor oral hygiene is a risk factor for acetaldehyde production. In addition, the interior of thick oral biofilms is anaerobic25, and thus, acetaldehyde might be produced from glucose by S. mutans (Fig. 2), which is known to be present in greater numbers in mature and aciduric biofilms26,27.
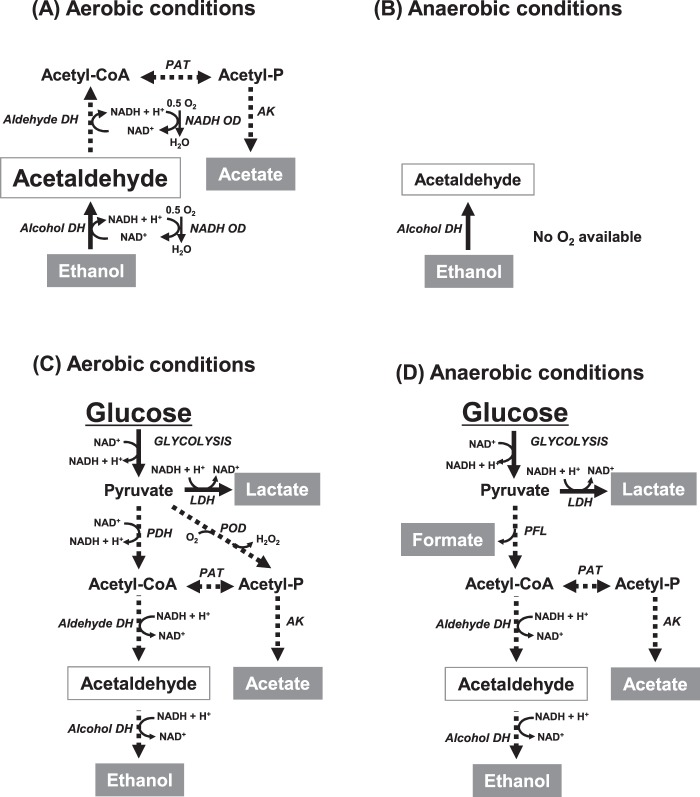
All of the strains examined in this study exhibited alcohol dehydrogenase activity (Table 1), suggesting that bacterial acetaldehyde production from ethanol can be a one-step reaction catalyzed by alcohol dehydrogenase (ethanol + NAD+ → acetaldehyde + NADH + H+, Fig. 4A,B). In addition, to ensure the efficient continuation of this reaction, the NADH produced seems to be recycled to NAD+ by oxidation. All of the strains examined in the current study displayed NADH oxidase activity (Table 1), suggesting that NADH can be oxidized to NAD+ by NADH oxidase in the presence of oxygen molecules, and thus, aerobic conditions seem to be necessary for efficient acetaldehyde production from ethanol. Pavlova et al.28 have reported that alcohol dehydrogenase is responsible to produce acetaldehyde from ethanol in most oral streptococci by using molecular biological methods. Our results support their results.
Figure 4.
Proposed metabolic pathways related to acetaldehyde production from ethanol under aerobic (A) or anaerobic (B) conditions or from glucose under aerobic (C) or anaerobic (D) conditions. Solid arrowed lines, metabolic pathways common for Streptococcus and Neisseria strains (A,B) and Streptococcus strains (C,D); Dash arrowed lines, metabolic pathways specific to certain bacteria (see text). LDH, lactate dehydrogenase; PDH, pyruvate dehydrogenase; POD, pyruvate oxidase; PAT, phosphate acetyltransferase; AK, acetate kinase; Aldehyde DH, aldehyde dehydrogenase (acylating); Alcohol DH, alcohol dehydrogenase; NADH OD, NADH oxidase; PFL, pyruvate formate-lyase.
The optimal pH for alcohol dehydrogenase activity is known to range from 7.5–9.229, which probably explains why greater acetaldehyde production was seen at neutral to weakly alkaline pH (Fig. 1), since the intracellular pH of oral streptococci reflects the environmental pH30,31. Acetaldehyde production by S. mitis was high at pH 7.0 and 6.0, suggesting that the optimal pH for alcohol dehydrogenase activity might differ in this strain, but further studies are needed to examine this.
Despite exhibiting relatively high alcohol dehydrogenase activity, S. mutans only produced a small amount of acetaldehyde from ethanol (Fig. 1 and Table 1) with trace amounts of acetate and formate (Fig. 3A). The production of acetate suggests that the acetaldehyde produced is further metabolized to acetate via acetyl-CoA and acetyl-phosphate (Fig. 4A) as previously reported32, but it seems to be a minor reaction since the amount of acetate was smaller than that of acetaldehyde (Figs 1A, 3A). Over all, these observations suggest that S. mutans cannot incorporate ethanol efficiently or that ethanol cannot penetrate the cell membrane of S. mutans, in other words, S. mutans can be durable against ethanol.
Streptococcus strains are known to metabolize glucose to pyruvate through glycolysis, and in the current study they produced various end-products depending on the environment (Fig. 4C,D). Along with acetaldehyde production (Fig. 2), the Streptococcus strains mainly produced lactate, with small amounts of acetate and formate (Fig. 3B). Acetaldehyde can be produced via the branched-pathways from pyruvate, which are catalyzed by pyruvate dehydrogenase33, pyruvate oxidase34, or pyruvate formate-lyase35 (Fig. 4C,D). S. mutans, which produced the highest amounts of formate and acetate from glucose, is suggested to operate these branched-pathways efficiently, and acetaldehyde, a metabolic intermediate of these branched-pathways, might leak out of these bacterial cells, resulting in the observed acetaldehyde production from glucose.
The Neisseria strains did not produce acetaldehyde from glucose (Fig. 2A). According to the Kyoto Encyclopedia of Genes and Genomes database, both N. sicca and N. mucosa possess gene sequences associated with enzymes related to glycolysis, as well as acid productivity from glucose36. However, Muto et al.37 reported that no aldehyde dehydrogenase activity was detected in these species. Therefore, it is considered that N. sicca and N. mucosa can metabolize glucose, but cannot convert it to acetaldehyde.
In conclusion, oral indigenous bacteria, Streptococcus and Neisseria can produce acetaldehyde, and such acetaldehyde production is affected by environmental conditions. It was suggested that alcohol dehydrogenase and NADH oxidase are involved in ethanol-derived acetaldehyde production and that the branched-pathway from pyruvate is involved in glucose-derived acetaldehyde production.
Methods
Bacterial strains
Streptococcus mitis (JCM 12971), Streptococcus salivarius (JCM 5707), Neisseria mucosa (JCM 12292), and Neisseria sicca (ATCC 29256) were used in this study. Streptococcus mutans (NCTC 10449) was used as a well-studied control.
Bacterial growth conditions and preparation of the cell suspensions
The Streptococcus strains were grown and maintained on blood agar plates (CDC anaerobe 5% sheep blood agar, BD Japan, Japan) or TYG agar plates (agar plates containing 1.7% tryptone, 0.3% yeast extract, 0.5% NaCl, 50 mM potassium phosphate buffer (PPB) [pH 7.0], and 0.5% glucose) and were stored at 4 °C in the air or an anaerobic chamber (80% N2, 10% H2, and 10% CO2; ANB-18-2E, Hirasawa Works, Japan). Glucose was added through a sterile membrane filter after autoclaving. In the anaerobic growth experiments, the culture media and other reagents were kept under anaerobic conditions for at least 3 days in order to remove any oxygen. The Streptococcus strains were cultured in medium at 37 °C, and then 100 μL of the culture were transferred to new medium (40 mL) and further incubated at 37 °C until the logarithmic growth phase. The bacterial cells were harvested via centrifugation (10,000 rpm for 7 min at 4 °C) and washed 3 times with washing buffer (2 mM PPB [pH 7.0] containing 75 mM KCl, 5 mM MgCl2, and 75 mM NaCl), before being suspended in the same buffer. The bacterial cell concentrations of the suspensions were adjusted on the basis of their optical density (OD) at a wavelength of 660 nm (to an OD of 5.0). The anaerobically cultured cells remained in the anaerobic chamber throughout the culture procedure, while the aerobically cultured cells were prepared in the air throughout the culture procedure.
The Neisseria strains were grown and maintained on blood agar plates (Columbia 5% sheep blood agar, BD Japan, Japan) and stored at 4 °C in the air. They were cultured on blood agar plates at 37 °C, transferred to new blood agar plates, and then further incubated at 37 °C for 12 hours in the air. The bacterial cells were harvested via centrifugation (10,000 rpm for 7 min at 4 °C) and washed 3 times with washing buffer (2 mM PPB [pH 7.0] containing 75 mM KCl, 5 mM MgCl2, and 75 mM NaCl), before being suspended in the same buffer. The cell suspensions were adjusted to OD of 7.5 for N. mucosa and 6.2 for N. sicca, as described above. The aerobically cultured cells were prepared in the air throughout the culture procedure.
Measurement of bacterial acetaldehyde production
The reaction mixture used to assess bacterial acetaldehyde production contained 850 μL of the bacterial cell suspension, 100 μL of substrate (100 mM glucose or 11 mM ethanol), and 50 μL of PPB (pH: 8.0, 7.0, 6.0, or 5.0). Each reaction mixture was collected in a tube with a silicone cap, and the tube was completely closed off. The reaction was started via the addition of the metabolic substrate and incubated at 37 °C for 30 min. The reaction was stopped via the injection of 100 μL of 7 M phosphoric acid using a sterile needle (Terumo injection needle 27 G × 3/4, Terumo Corporation, Japan) and syringe (1 mL Terumo syringe for tuberculin, Terumo Corporation, Japan) through the silicone cap, and the mixture was shaken vigorously. The headspace gas of the tube was collected using a gas-tight syringe (10 ml NORM-JECT, HENKE- SASS WOLF, Tuttlingen, Germany) and was diluted with nitrogen gas. Then, the concentrations of acetaldehyde were measured using a sensor gas chromatograph (SGEA-P2, FIS Inc., Japan). The reaction mixture was stored at 4 °C until it was used to measure the levels of organic acids, as described below. The aerobic production of acetaldehyde in air was measured using aerobically cultured cells, while the anaerobic production of this molecules was measured in an anaerobic chamber (80% N2 and 10% H2; ANB-180R, Hirasawa Works, Japan) using anaerobically cultured cells.
Measurement of bacterial alcohol dehydrogenase and NADH oxidase activity
The aerobically cultured bacteria were harvested as described above and stored as pellets at −30 °C until they were used. Each pellet was thawed and suspended in a buffer containing 75 mM KCl, 75 mM NaCl, 2 mM MgCl2, and 2 mM PPB (pH 7.0), before being oscillated with an ultrasonic disruption apparatus (Insonator 201 M, KUBOTA CORPORATION, Japan) (2 A, 190 W, at 4 °C for 7 min) and centrifuged to remove any cell membranes or intact cells. The prepared cell-free extracts were used to measure enzymatic activity. NAD+-dependent alcohol dehydrogenase activity was assessed using an assay kit (alcohol dehydrogenase activity assay kit, K787-100, Funakoshi, Tokyo, Japan), according to the manufacturer’s instructions. The enzymatic reactions were performed in an anaerobic chamber (80% N2 and 10% H2; ANB-180R) in order to avoid masking by NADH oxidase activity.
NADH oxidase activity was measured according to the method of Reusch and Burger38, with minor modifications. In a quartz cell, 870 μL of 50 mM PPB (pH 7.0) containing 2 mM NADH was prepared, and the reaction was started via the addition of 30 μL of the cell-free extract. The activity was monitored with a spectrophotometer (UV SPECTROPHOTOMETER UV-1800, Shimadzu Co. Ltd., Japan) at 340 nm and 30 °C in the air.
The protein concentrations of the cell-free extracts were measured using an assay kit (Takara BCA protein assay kit T9300, Takara Bio Inc., Japan), according to the manufacturer’s instructions.
Measurement of bacterial organic acid production
The reaction mixtures used to assess acetaldehyde production from ethanol or glucose at pH 7.0 were centrifuged at 10,000 rpm at 4 °C for 7 min, and the supernatants were collected. The organic acid (acetate, lactate, and formate) levels of the samples were then measured as described previously39,40. After the bacteria were removed using a filter (pore size: 0.20 µm; polypropylene; Toyo Roshi Ltd., Japan), the organic acid levels of the samples were determined using high-performance liquid chromatography (Shimadzu Prominence LC-20AD, Shimadzu Co. Ltd., Japan).
Statistical analysis
In the statistical analyses, the significance of the differences among multiple groups were analyzed using Tukey’s test, whereas the significance of the differences between pairs of groups were analyzed using the paired t-test, and p values of <0.05 were considered statistically significant (StatFlex Ver. 6 and Microsoft® Excel® Ver. 14).
Acknowledgements
This work was partly supported by KAKENHI (No. 17H04420, 18K19629, 17K12003). The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
Author Contributions
J.W. and N. Tak. conceived the experiments; R.T., J.W., Y.A and N. Tan. conducted the experiments; R.T., J.W., Y.A., K.S. and N. Tak. analyzed the results; R.T., J.W. and N. Tak wrote the manuscript text, and prepared figures and tables;. All authors reviewed the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Homann N, et al. Poor dental status increases acetaldehyde production from ethanol in saliva: A possible link to increased oral cancer risk among heavy drinkers. Oral Oncol. 2001;37:153–158. doi: 10.1016/S1368-8375(00)00076-2. [DOI] [PubMed] [Google Scholar]
- 2.Scoccianti C, et al. European code against cancer 4th edition: Alcohol drinking and cancer. Cancer Epidemiol. 2016;45:181–188. doi: 10.1016/j.canep.2016.09.011. [DOI] [PubMed] [Google Scholar]
- 3.Al-Hebshi NN, et al. Inflammatory bacteriome featuring fusobacterium nucleatum and pseudomonas aeruginosa identified in association with oral squamous cell carcinoma. Sci Rep. 2017;7:1834. doi: 10.1038/s41598-017-02079-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singhvi HR, Malik A, Chaturvedi P. The role of chronic mucosal trauma in oral cancer: A review of literature. Indian J Med Paediatr Oncol. 2017;38:44–50. doi: 10.4103/0971-5851.203510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Obe G, Jonas R, Schmidt S. Metabolism of ethanol in vitro produces a compound which induces sister-chromatid exchanges in human peripheral lymphocytes in vitro: Acetaldehyde not ethanol is mutagenic. Mutat Res. 1986;174:47–51. doi: 10.1016/0165-7992(86)90075-8. [DOI] [PubMed] [Google Scholar]
- 6.Kayani MA, Parry JM. The in vitro genotoxicity of ethanol and acetaldehyde. Toxicol In Vitro. 2010;24:56–60. doi: 10.1016/j.tiv.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 7.Chang JS, et al. Investigating the association between oral hygiene and head and neck cancer. Oral Oncol. 2013;49:1010–1017. doi: 10.1016/j.oraloncology.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 8.Agarwal DP. Genetic polymorphisms of alcohol metabolizing enzymes. Pathol Biol (Paris). 2001;49:703–709. doi: 10.1016/S0369-8114(01)00242-5. [DOI] [PubMed] [Google Scholar]
- 9.Obe G, Ristow H. Mutagenic, cancerogenic and teratogenic effects of alcohol. Mutat Res. 1979;65:229–259. doi: 10.1016/0165-1110(79)90004-6. [DOI] [PubMed] [Google Scholar]
- 10.Singh NP, Khan A. Acetaldehyde: Genotoxicity and cytotoxicity in human lymphocytes. Mutat Res. 1995;337:9–17. doi: 10.1016/0921-8777(95)00006-6. [DOI] [PubMed] [Google Scholar]
- 11.Garaycoechea JI, et al. Alcohol and endogenous aldehydes damage chromosomes and mutate stem cells. Nature. 2018;553:171–177. doi: 10.1038/nature25154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Soffritti M. Results of long-term experimental studies on the carcinogenicity of formaldehyde andacetaldehyde in rats. Ann N Y Acad Sci. 2002;982:87–105. doi: 10.1111/j.1749-6632.2002.tb04926.x. [DOI] [PubMed] [Google Scholar]
- 13.Kurkivuori J, et al. Acetaldehyde production from ethanol by oral streptococci. Oral Oncol. 2007;43:181–186. doi: 10.1016/j.oraloncology.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 14.Moritani K, et al. Acetaldehyde production by major oral microbes. Oral Dis. 2015;21:748–754. doi: 10.1111/odi.12341. [DOI] [PubMed] [Google Scholar]
- 15.Alnuaimi AD, Wiesenfeld D, O’Brien-Simpson NM, Reynolds EC, McCullough MJ. Oral candida colonization in oral cancer patients and its relationship with traditional risk factors of oral cancer: A matched case-control study. Oral Oncol. 2015;51:139–145. doi: 10.1016/j.oraloncology.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 16.Peters BA, et al. Oral microbiome composition reflects prospective risk for esophageal cancers. Cancer Res. 2017;77:6777–6787. doi: 10.1158/0008-5472.CAN-17-1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Perera M, et al. Inflammatory bacteriome and oral squamous cell carcinoma. J Dent Res. 2018;97:725–732. doi: 10.1177/0022034518767118. [DOI] [PubMed] [Google Scholar]
- 18.Horiuchi M, Washio J, Mayanagi H, Takahashi N. Transient acid‐impairment of growth ability of oral Streptococcus, Actinomyces, and Lactobacillus: A possible ecological determinant in dental plaque. Oral Microbiol Immunol. 2009;24:319–324. doi: 10.1111/j.1399-302X.2009.00517.x. [DOI] [PubMed] [Google Scholar]
- 19.Washio J, Shimada Y, Yamada M, Sakamaki R, Takahashi N. Effects of pH and lactate on hydrogen sulfide production by oral Veillonella spp. Appl Environ Microbiol. 2014;80:4184–4188. doi: 10.1128/AEM.00606-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takahashi N. Oral microbiome metabolism: From “who are they?” to “what are they doing?”. J Dent Res. 2015;94:1628–1637. doi: 10.1177/0022034515606045. [DOI] [PubMed] [Google Scholar]
- 21.Ishiguro K, Washio J, Sasaki K, Takahashi N. Real-time monitoring of the metabolic activity of periodontopathic bacteria. J Microbiol Methods. 2015;115:22–26. doi: 10.1016/j.mimet.2015.05.015. [DOI] [PubMed] [Google Scholar]
- 22.Washio Jumpei, Takahashi Nobuhiro. Metabolomic Studies of Oral Biofilm, Oral Cancer, and Beyond. International Journal of Molecular Sciences. 2016;17(6):870. doi: 10.3390/ijms17060870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aas JA, Paster BJ, Stokes LN, Olsen I, Dewhirst FE. Defining the normal bacterial flora of the oral cavity. J Clin Microbiol. 2005;43:5721–5732. doi: 10.1128/JCM.43.11.5721-5732.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gupta B, Bray F, Kumar N, Johnson NW. Associations between oral hygiene habits, diet, tobacco and alcohol and risk of oral cancer: A case-control study from india. Cancer Epidemiol. 2017;51:7–14. doi: 10.1016/j.canep.2017.09.003. [DOI] [PubMed] [Google Scholar]
- 25.Marsh, P. D., Martin, M. V., Lewis, M. A. O. & Williams, D. W. Oral microbiology fifth edition. 74–102 (Elsevier, 2009).
- 26.Ritz H. Microbial population shifts in developing human dental plaque. Arch Oral Biol. 1967;12:1561–1568. doi: 10.1016/0003-9969(67)90190-2. [DOI] [PubMed] [Google Scholar]
- 27.Matsumoto-Nakano M. Role of Streptococcus mutans surface proteins for biofilm formation. Jpn Dent Sci Rev. 2018;54:22–29. doi: 10.1016/j.jdsr.2017.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pavlova SI, Jin L, Gasparovich SR, Tao L. Multiple alcohol dehydrogenases but no functional acetaldehyde dehydrogenase causing excessive acetaldehyde production from ethanol by oral streptococci. Microbiology. 2013;159:1437–1446. doi: 10.1099/mic.0.066258-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bergmeyer, H. U., Bergmeyer, J. & Gramßl, M. Methods of Enzyme Analysis Third edition volume II Samples, Reagents, Assessment of Results, 142 (Verlag Chemie, 1983).
- 30.Iwami Y, Abbe K, Takahashi-Abbe S, Yamada T. Acid production by streptococci growing at low pH in a chemostat under anaerobic conditions. Oral Microbiol Immunol. 1992;7(5):304–8. doi: 10.1111/j.1399-302X.1992.tb00593.x. [DOI] [PubMed] [Google Scholar]
- 31.Nakajo K, et al. Resistance to acidic and alkaline environments in the endodontic pathogen Enterococcus faecalis. Oral Microbiol Immunol. 2006;21(5):283–8. doi: 10.1111/j.1399-302X.2006.00289.x. [DOI] [PubMed] [Google Scholar]
- 32.Fukui K, et al. Kinetic study of a change in intracellular atp level associated with aerobic catabolism of ethanol by Streptococcus mutans. J Bacteriol. 1988;170:4589–4593. doi: 10.1128/jb.170.10.4589-4593.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carlsson J, Kujala U, Edlund MB. Pyruvate dehydrogenase activity in Streptococcus mutans. Infect Immun. 1985;49:674–678. doi: 10.1128/iai.49.3.674-678.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Taniai H, et al. Concerted action of lactate oxidase and pyruvate oxidase in aerobic growth of Streptococcus pneumoniae: Role of lactate as an energy source. J Bacteriol. 2008;190:3572–3579. doi: 10.1128/JB.01882-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Takahashi‐Abbe S, Abe K, Takahashi N. Biochemical and functional properties of a pyruvate formate‐lyase (PFL)‐activating system in Streptococcus mutans. Oral Microbiol Immunol. 2003;18:293–297. doi: 10.1034/j.1399-302X.2003.00081.x. [DOI] [PubMed] [Google Scholar]
- 36.Brenner, D. J., Krieg, N. R., Staley, J. T. & Garrity, G. M. Bergey’s Manual of Systematic Bacteriology second edition volume two The Proteobacteria 775–776 (Springer, 1984).
- 37.Muto M, et al. Acetaldehyde production by non-pathogenic neisseria in human oral microflora: Implications for carcinogenesis in upper aerodigestive tract. Int J Cancer. 2000;88:342–350. doi: 10.1002/1097-0215(20001101)88:3<342::AID-IJC4>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 38.Reusch VJ, Burger M. Distribution of marker enzymes between mesosomal and protoplast membranes. J Biol Chem. 1974;249:5337–5345. [PubMed] [Google Scholar]
- 39.Takahashi N, Abbe K, Takahashi-Abbe S, Yamada T. Oxygen sensitivity of sugar metabolism and interconversion of pyruvate formate-lyase in intact cells of Streptococcus mutans and Streptococcus sanguis. Infect Immun. 1987;55:652–656. doi: 10.1128/iai.55.3.652-656.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Norimatsu Y, Kawashima J, Takano-Yamamoto T, Takahashi N. Nitrogenous compounds stimulate glucose-derived acid production by oral Streptococcus and Actinomyces. Microbiol Immunol. 2015;59:501–506. doi: 10.1111/1348-0421.12283. [DOI] [PubMed] [Google Scholar]