Abstract
Decreasing egg quality following oocyte ageing is a major restricting factor for the breeding programs. The mechanisms behind this process has not yet been clarified. To examine the possible involvement of oxidative stress in the oocyte ageing process, the relative mRNA abundance of specific transcripts were determined in oocytes collected from 6 females and incubated in vitro for 18 hours post stripping at 20 °C in goldfish Carassius auratus. During the 18 hour-post-stripping ageing of the oocytes, relative mRNA levels of candidate transcripts involved in oxidative injury, mitochondrial function and stress response, cell cycles, apoptosis, reproduction and germ line speciation and developmental competence were measured by real-time PCR. None of the relative mRNA abundance of the examined genes were significantly altered through oocyte ageing. In addition, the amount of thiobarbituric acid reactive substances (TBARS), an indicator of lipid peroxidation, did not change over time following stripping. The activity of the antioxidant enzymes also remained constant during oocyte ageing. The results of the current study indicated that oxidative stress unlikely plays a role as an initiator or promotor in the progress of oocyte ageing in goldfish.
Subject terms: Reproductive biology, Ageing, Animal physiology, Transcriptomics
Introduction
The meiosis in female germ cell is accompanied by changes in nucleus and cytoplasm, finally preparing oocyte to be fertilized and subsequently develop into an embryo1. It is known that ovulated oocytes could have successful fertilization when the fusion of sperm and ovum takes place in the optimal period for oocyte fertilization. If post-ovulatory oocytes have prolonged residence in the oviduct or body cavity (in vivo) or in the culture media (in vitro), the ageing of oocyte occur which significantly reduces the oocyte quality and may cause abnormal development of arising embryos1. Oocytes more than the optimal period for fertilization after onset of ovulation show ageing symptoms. The quality and developmental potential of oocyte significantly decreases with increasing time after ovulation. Ageing of oocytes display many functional changes including limited fertilization rate2,3, increased the frequency of ploidy anomalies4,5, and the malformed larvae5–7. For the higher vertebrates, post-ovulatory ageing of oocytes has been reported to display some other functional changes which could be observed as polyspermy, poor development of embryos and higher incidence of abnormalities in offspring probably due to aberrant epigenetic profiles [e.g.8-10,]. Because the oocytes contain valuable information for orchestrating embryogenesis11 and for remodeling the parental genomes12, the quality of embryos and their later developmental process are highly dependent on the oocyte integrity. In fish, oocytes display various optimum time of fertilization depending on the species and storage temperature13.
Once ovulation occurs, the ageing process of the oocytes is the most important factor that could affect egg quality. This has already been shown in several fish species. Maternally provided factors, including maternal mRNAs and proteins largely affect embryo development at the first steps, before zygotic transcription activates14. The levels and processing of maternally provided mRNAs and proteins could be impacted not only by genetic effects but also by non-genetic effects such as environmental variables like oocyte ageing15. Several morphological, physiological, biochemical, cellular and molecular changes occur during the progress of oocyte ageing, that results in decreasing oocyte quality13.
There are only few reports about the molecular changes during fish oocyte ageing15–19. Most reports in this field are from the studies on higher vertebrates. The specific molecular functions that determine egg quality during oocyte ageing in fish and in other vertebrates remain largely unknown. In other vertebrates, it has been suggested that increased Reactive Oxygen Species (ROS) and oxidative stress might be the initiator of oocyte ageing deteriorations [e.g.8,20–22,]. This leads to lowered ATP production and irregular Ca2+ oscillation changes. The consequences are ROS-induced mitochondrial dysfunction, ROS-induced lipid alterations and ROS-induced DNA fragmentation followed by impaired embryonic development and apoptosis23,24. In addition, it is believed that decreased levels of factors which are critical in the cell cycle25 and mitochondrial dysfunction are involved in this process8.
There have always been difficulties involved in the study of oocyte ageing in higher vertebrates, regarding ethical issues as well as the intrinsic nature of their reproduction biology and the difficulty of collecting adequate numbers of the oocytes. Thus, there are advantages to using fish as model animals to analyse oocyte ageing; in contrast to other animals, fish potentially have a larger number of oocytes. In addition, fish have variety modes of reproduction in oocyte ageing studies. The current study therefore, examined the possible involvement of oxidative stress in the oocyte ageing process using goldfish, Carassius auratus, as the model animal. The over ripening time in goldfish oocytes were characterized. Then the possible changes in the activity of antioxidant enzymes; catalase (CAT), superoxide dismutase (SOD), glutathione reductase (GR) and glutathione peroxidase (GPX), as well as TBARS as the marker of lipid oxidation during oocyte ageing were examined. Furthermore, the transcript abundance of genes involved in oxidative damage, stress response and mitochondrial function (hsp70, cox1, sodMn, calmodulin), genes with roles in fertilization (vasa), embryo development (igf2), cell cycling (cyclinA, cyclinA2, cyclinB and jnk) as well as the gene related to the apoptosis (ctpb) were investigated during oocyte ageing.
Although considerable improvements in assisted reproduction technologies are going on, there are still failures due to the oocyte ageing. There are some ethical issues that makes it difficult to study oocyte ageing on the higher vertebrates. Therefore, identifying molecular mechanisms behind the oocyte ageing process in fish and consequent declining egg quality could have important implications both for aspects of basic research as well as for the practical applications to prevent or delay oocyte ageing. The results of this study may provide a better understanding of the mechanisms involved in the oocyte ageing process which might be beneficial to other vertebrates as well.
Results
Embryo survival rates during in vitro oocyte ageing
The embryo survival percentages remained unchanged, at around 60%, for the eggs fertilized up to 3 hours post-stripping (Fig. 1). Then, the survival rates decreased significantly by elapsing the time storage and were 7.5% for the eggs stored in vitro for 12 hours post stripping (HPS). After 18 hours of oocyte storage, the fertilization rates totally lost and no viable embryos were detected.
Figure 1.
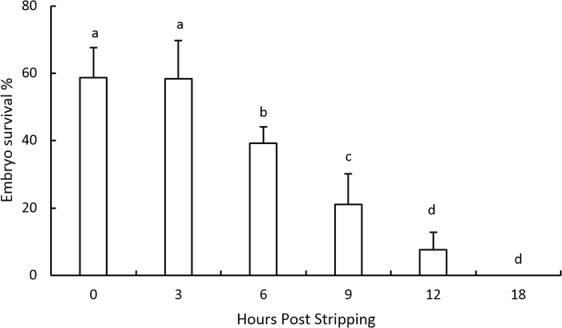
Effect of in vitro oocyte ageing on the embryo survival rates in goldfish (mean ± SD). Means sharing a common alphabetical symbol do not differ significantly.
Relative abundance of mRNAs during in vitro oocyte ageing
The mRNA abundance of 13 specific transcripts were determined and compared in different aged oocytes, usibg b-actin as the reference gene. During 18 hours of post-stripping ova ageing, the mRNA levels in none of the evaluated transcripts exhibited significant changes (Fig. 2). Levels of cyclinA2, cyclinB and jnk1 showed continuous down- and upregulated trends during the time interval between egg stripping and the occurrence of oocyte over-ripening.
Figure 2.
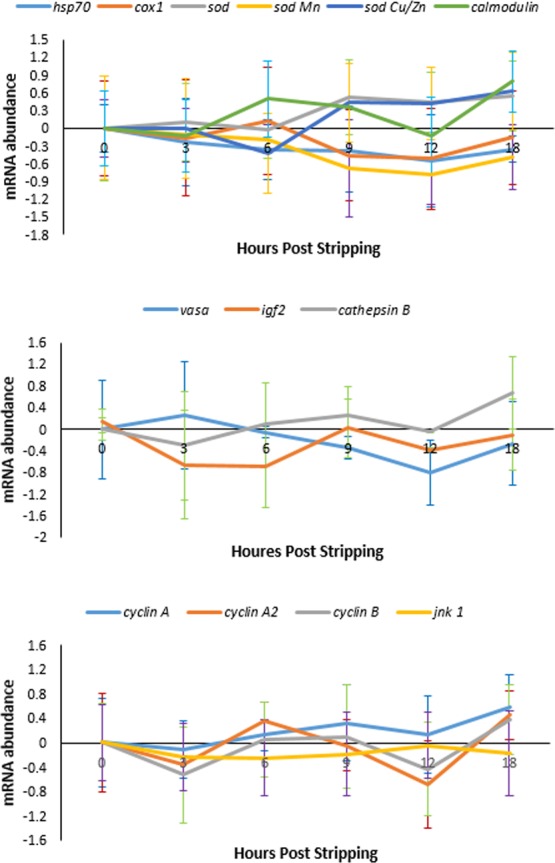
Effect of in vitro oocyte ageing at 20 °C on the mRNA expression levels of the selected genes in goldfish (mean ± SD).
Activity of antioxidant enzymes during in vitro oocyte ageing
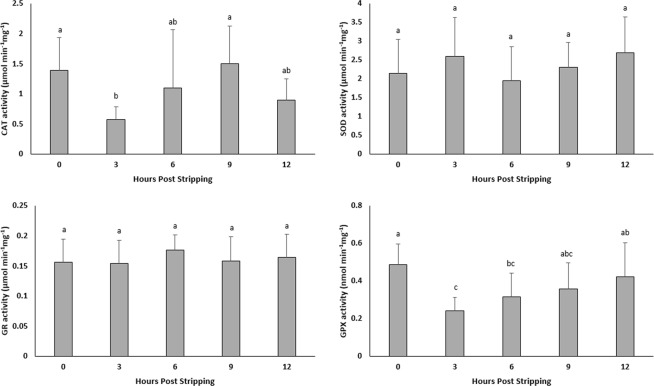
The activities of SOD and GR did not change significantly during oocyte ageing (Fig. 3). The CAT and GPX activities decreased at 3 HPS and thereafter stayed almost constant with elapsing time after ovulation.
Figure 3.
Effect of in vitro oocyte ageing in goldfish on the activities of catalase (CAT) (µmol/min/mg), superoxide dismutase (SOD) (µmol/min/mg), glutathione reductase (GR) (µmol/min/mg) and glutathione peroxidase (GPX) (µmol/min/mg) (mean ± SD).
Lipid oxidation during in vitro oocyte ageing
The level of TBARS did not change significantly and remained at around 4 µg g−1 malondialdehyde (MDA) during time post-stripping (Fig. 4).
Figure 4.
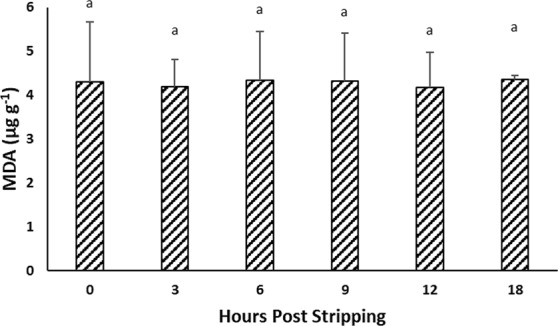
Effect of in vitro oocyte ageing in goldfish on TBARS, expressed as malonaldehyde (MDA) (µg g−1) (mean ± SD).
Discussion
Oocyte ageing in goldfish was associated with decreasing embryo survival rates. While the highest survival rates were obtained for the eggs fertilized up to 3 HPS, the fertilizing ability of eggs totally lost at 18 HPS. Formacion et al.26 also found that the over-ripening of goldfish eggs occurs at approximately 12 hours post ovulation with advanced degeneration by 24 hours. The time window for optimal fertilization rate is a few minutes to a few weeks. This time window highly depends on the fish species as well as the storage temperature (Reviewed by Samarin13).
The molecular mechanisms behind the process of oocyte ageing have not been clarified yet. Previous studies in other vertebrates have suggested that ROS and subsequently oxidative stress in oocytes increase during post-ovulatory ageing [e.g.20–23]. In addition, many genes used to predict egg quality of aged oocytes in other vertebrates are associated with mitochondrial function, metabolism and cell cycle control27–30. Microarray analysis of egg transcriptomic profiles during oocyte ageing in mice have revealed that the genes involved in oxidative stress and mitochondrial function are differentially expressed30. Additionally, this study suggested the alteration of expression patterns for the genes related to chromatin structure, DNA methylation, genome stability and RNA helicases during oocyte ageing. Comparatively, only a few studies have analysed egg transcriptomes during fish oocyte ageing15,17,19.
Our results showed no significant changes in the mRNA abundance of transcripts associated with oxidative stress (hsp70, cox and sod) during post-stripping oocyte ageing in goldfish. Esponda and Diaz31 showed that mRNA abundance of hsp70 increases in aged mice oocytes. Verbeke et al.32 also found that the accumulation of damaged, oxidized and glycerated proteins might result from age-associated defects in the production of heat shock proteins. Hamatani et al.30 compared the mRNA expression profiles of mouse oocytes from young females with those of aged females. The authors reported increased cox1 mRNA levels in oocytes from aged mice. The mitochondrial gene Cox, is related to stress response that is known to catalyse the electron transfer in the respiratory chain. Therefore, mitochondrial respiratory chain in aged oocytes, might be affected by abnormal expression of cox1 and following egg quality defects. Sod is an antioxidant protector in cells33,34 and has an important role in maintaining cellular homeostasis through removing ROS. Sod, an oxidative stress related gene, can protect cells from oxidative injury. Decreased mRNA levels of sod may decrease the capacity to cope with oxidative damage in oocytes with increasing time following ovulation. However, as we did not find such trends, we suggest that oxidative injury is not a major factor in oocyte overripening in goldfish.
Additionally, the level of TBARs which shows the extension of lipid peroxidation, did not change in the oocytes over time following stripping. The results obtained in this study showed that the activity of CAT, SOD, GR and GPX did not change significantly during oocyte ageing in goldfish, confirming that oxidative stress is not likely the main promotor in the ageing process of oocytes in goldfish. Antioxidant enzymes can reduce defective effects of oxidative stress through scavenging ROS. If oxidative stress plays critical role during the progress of oocyte ageing, then alterations in total oxidation status and antioxidant enzymes activity would be expected following ovulation. In African catfish Clarias gariepinus, similar results were obtained24, showing no involvement of oxidative stress on the oocyte ageing process at least until the complete loss of egg fertilizing ability.
The mRNA abundance of calmodulin in the current study showed an upward trend during oocyte ageing, although do not differ significantly. The previous studies show that increase in the ROS levels affects calcium binding in calmodulin and significantly disturb Ca2+ homeostasis and mitochondrial function35,36. A link has already been indicated between the Ca2+ oscillation, egg development and abnormalities suggesting that altered Ca2+ oscillation may lead to the poor embryo development. In other vertebrates, impaired Ca2+ regulation characterized by higher frequency and lower amplitude in aged oocytes [e.g.22,37], has been suggested as one of the molecular mechanisms behind the oocyte ageing process.
Although they do not differ significantly, the relative mRNA abundance of the transcripts related to cell cycling (cyclinA, cyclinA2, cyclinB and jnk1) were continuously decreased and increasde during the time interval between egg collection until the occurrence of oocyte ageing. Oocyte ageing in rainbow trout Oncorhynchus mykiss has been associated with increase in the maternal mRNA concentrations of cyclinA, cyclinA2 and jnk117. The authors concluded that since cyclinA regulates cell cycle progression, it is possible that post-ovulatory increase of cyclinA mRNA stock, participate in building the developmental competence of the egg. jnk, which is belonging to the family of mitogen-activated protein kinase, is an ageing-related and stress stimuli response gene. Additionally, jnk activity is relevant to important intracellular functions regulating cell survival and apoptosis. Previous studies in other vertebrates have suggested that critical cell cycle factors, maturation promoting factor and mitogen-activated protein kinases are decreasing during post-ovulatory ageing of oocytes25,38,39. The study by Xu et al.38 showed that altered levels of the factors which are critical in cell cycle and cytoplasmic changes are involved in the spontaneous activation of oocyte ageing process. In contrast, in Gilthead Sea Bream, Sparus aurata eggs, higher number of protein components and RNA levels involved in cell cycle regulation has been reported in higher quality eggs40. The observed continuous up and downward trends in mRNA abundance of the abovementioned transcripts during oocyte ageing would be interesting to be studied in the future researches.
The downward trend observed in the mRNA abundance of vasa in the current study during in vitro oocyte ageing is in accordance with the study of Hamatani et al.30 who reported that the expression of vasa in mouse oocytes decreases with maternal ageing30.
Vasa is a specific marker for germ cells. During embryogenesis vasa is expressed in the cytoplasm of primordial germ cells and has role in differentiation of the germ cells into gonads41, as well as a role in germ cell function42. Oocyte ageing in human has been suggested to be associated with a distorted secondary sex ratio in favour of males23. Our preliminary results with zebrafish (vasa transgenic strain), indicated that the number of the PGCs are significantly affected by the age of oocytes (unpublished data). Since depletion of PGCs converts the sex differentiation in favour of males in zebrafish43 and other fish species44, oocyte ageing may bias the sex ratio in favour of males or the occurrence of completely sterile individuals. This possibility should be addressed in future studies.
The results of our study indicated that the mRNA abundance of igf2 decreased slightly up to 6 HPS and then showed an increasing trend until the 18 HPS, when no egg viabilities observed. The genes that are directly involved in the pathway of insulin growth factor did not show significant alterations during maternal oocyte ageing in mice30. In contrast, higher mRNA levels of igf2 in more aged oocytes of rainbow trout was reported in comparison with the freshly ovulated ones17. They also reported that igf2 mRNA exhibit 2–4 fold less abundance in oocytes exhibiting low embryonic survival. The IGF axis prevents apoptosis function in the cells.
Therefore, the increased mRNA abundance of igf2 may act as a protection against the loss of fertilizing ability and apoptosis which is the end point of the oocyte ageing process. With elapsing time after stripping, the mRNA expression of cathepsinB also increased. Upregulation of cathepsinB has been involved in cell death45. In African catfish Clarias gariepinus the mRNA abundance of an apoptosis-related gene, cathepsinD, demonstrated an upward trend during in vitro oocyte ageing24. Lysosomal proteases cathepsinD and cathepsinB act as stimulators of apoptosis46. Therefore, the observed increasing trend in the mRNA levels of CathepsinB was not unexpected in the current study. Our recent experiment on common carp indicated the role of apoptotic related genes on the progress of oocyte ageing47.
Additional analysis such as measurement of the total ROS, the indicators of mitochondrial dysfunction, egg ATP content etc., might be helpful tools to clearly understand about the possible involvement of oxidative stress in egg quality defects during oocyte ageing. Genome is transcriptionally silent from ovulation until zygotic genome activation48. Therefore, factors which function in post-transcriptional regulation of gene expression such as miRNA or poly(A) tail length of maternal genes may play important role during early embryo development and thus be responsible for deleterious effects of post-ovulatory ageing on the egg quality. Until zygotic genome activation, protein levels can be regulated by poly(A) tail length of maternal genes49,50. Delayed fertilization can affect this post-transcriptional regulation followed by developmental defects. Recently postovulatory ageing in murine MII oocytes has been associated with shortening of poly(A) tails of maternal effect genes either in vivo or in vitro culture media51. Poly(A) tail shortening can in turn affect the protein translation time of maternal gene transcripts followed by disturbed fertilization and developmental defects. Hence, post-transcriptional regulations and quantification of protein levels will benefit to the study on fish egg quality affected by oocyte ageing.
Epigenetics, the link between the environment and genes, has been recognized as a possible contribution to the ageing phenotype52–54. Some studies on higher vertebrates have already shown that post-ovulatory oocyte ageing induces epigenetic changes9,55. DNA methylation, histone modifications and microRNA changes are epigenetic and regulatory mechanisms that affect the gene expression without changing the original DNA sequence56. Factors like ageing, environment, husbandry practices etc. can be the cause of these epigenetic changes and developmental competence57,58 and they can be inherited by the offspring59,60. In fact, epigenetic mechanisms are the key regulators of gene transcription, with significance in responses to altered environmental signals61. Therefore, epigenetic modifications could be considered as a promising path for the future studies in the field of oocyte aging1. The results of the current study indicated that the activity of antioxidant enzymes as well as the oxidation products remains constant during oocyte ageing. Therefore, oxidative stress unlikely plays a role as an initiator or promotor in the progress of oocyte ageing in goldfish.
Materials and Methods
Ethics
Approval
The expert committee at the Institutional Animal Care and Use Committee (IACUC) of the University of South Bohemia approved the methodological protocol, experimental manipulations and sampling procedures used in this study. The permission for conducting and managing experiments involving animals was previously obtained by the co-author of this study (Certificate No. CZ 01660) according to section 15d paragraph 3 of Act No. 246/1992 Coll. Neither endangered nor protected species involved in the current study. The owner of the site; Research Institute of Aquaculture and Biodiversity of Hydrocenoses, University of South Bohemia, issued the permission for conducting the experiment.
Accordance
The experimental procedures were in accordance with the ethical rules of the EU-harmonized Animal Welfare Act of the Czech Republic. The unit is licensed (No. 53100/2013-MZE-17214) according to the Czech National Directive (the Law against Animal Cruelty, No. 246/1992).
Fish
The study was conducted using 20 male and 20 female of goldfish as experimental animals. Broodfish were captured from an earthen pond at the end of spring when the average daily water temperature reached 17 °C. The captured fish were transferred to indoor cylindrical holding tanks (450 L capacity) provided with water from a recirculating system; females and males were kept separately. The fish were prepared for artificial breeding by adjusting the photoperiod to 14 L: 10 D, and the gradual increasing of water temperature to 20 °C. After 2 weeks of acclimation, the females (58 ± 11.7 g body weight, mean ± SD) and males (41.2 ± 10.6 g body weight, mean ± SD) were subjected to hormonal treatment according to Samarin et al.47. The females were checked for the occurrence of ovulation 6 hours after the second injection and this examination was repeated every 3 hours. The occurrence of ovulation was judged by applying a gentle palpation on the female abdomen. The fish was considered as an ovulated one, if the eggs could be simply stripped by applying a gentle pressure on the abdomen. The inspections were performed every 3 hours thereafter. Among the females ovulated within 3 hours, six of them, were selected arbitrary and used as the experimental fish. Fish were anaesthesized using 0.03 ml/L clove oil water bath when examining for ovulation and stripping their eggs4.
In vitro storage of the eggs
Ova were stripped from 6 females and stored separately in sterile cell culture plates (six-well, each well diameter: 3.5 cm). The ova were stored in the laboratory incubator adjusted at 20 °C for 18 hours after stripping according to Samarin et al.47. Stored ova were fertilized at 0 (immediately after stripping), 3, 6, 9, 12 and 18 HPS.
Artificial fertilization
In total, 20 mature males were used for the experiment. To provide a uniform fertilizing ability for all egg batches, 0.5 ml of milt was collected separately from each of three males at each HPS, pooled prior to fertilization and then used at each fertilization step. Before the egg insemination at each HPS, sperm motility was assessed separately for each male according to Fauvel et al.62. A total volume of 1.5 ml was then mixed gently and used for fertilization. At each fertilization time, ~130 eggs from each female were gently dispersed into a small petri dish and then fertilized by adding 0.15 ml of the mixed milt and 2 ml of the hatchery water. This was continued by shaking the batch for one minute. The eggs were then washed by pouring Five ml of hatchery water into each petri dish. The applied ratio of sperm to egg was previously shown be sufficient to fertilize all the eggs in the preliminary tests.
Incubation and evaluation embryo survival rates
The eggs were washed 4–5 times with the hatchery water after the artificial fertilization, to remove the extra milt. The petri dishes were then left for 5 minutes to get assured about the attachment of the eggs to their surface. Finally, each plate was placed into a separate rectangular-shaped incubator (4.5 L capacity) supplied by recirculating water at 20 ± 0.5 °C and a flow rate of 1 L.min−1. The embryo survival rates were calculated 24 hours after fertilization as the percentage of live embryos to the total number of initially fertilized eggs using a stereomicroscope (Nikon SMZ745T, Japan).
Transcriptome changes during oocyte ageing
RNA isolation and reverse transcription
At all HPSs the unfertilized eggs (1 g, three replicates for each fertilization time point) were snap-frozen in liquid nitrogen prior storage at −80 °C until RNA isolation.
Total RNA was isolated from 20 eggs using TRIzol reagent (Invitrogen) in combination with PureLink RNA Purification Kit (Invitrogen Corporation, Carlsbad, CA) according to the manufacturer’s instructions. The eggs were homogenized in a Precellys24 homogenizer (Bertin Instruments), 5500 rpm for 2 × 20 sec with a 5 sec interval. The RNA was treated with DNaseI to avoid contatmination of genomic DNA using a PureLink DNase Kit (Invitrogen) according to the protocol. The concentration and purtity of the RNA were assessed using a NanoDrop Spectrophotometer (ND-1000, Thermo scientific). cDNA was synthezied from 1000 ng RNA using TaqMan reverse transcription Reagents (Life Technologies) and random hexamers to prime the reaction. Reverse transcription was performed at 25 °C for 10 min, 48 °C for 1 hour and 95 °C for 5 min. Reactions without TaqMan reverse transcriptase were used as negative controls in the real-time PCR study.
qRT-PCR analysis
The primers were designed with Primer3 using nucleotide sequences corresponding to goldfish mRNA (NCBI) and were provided by Invitrogen63,64. Sequences of primers used are listed in Table 1. The relative transcript levels of 16 genes (hsp70, cox1, sod, gpx1, cyclinA, cyclinB, jnkA, jnkB, caspase3A, caspase9, bax, bcl2, cathepsinB, cathepsinZ, vasa and igf2) were determined by real time qPCR. The qPCR reaction was run in parallel in a LightCycler 480 (Roche), and the reaction mix consisted of 4 μl diluted (1:10) cDNA, 1 μl forward and reverse primer (final concentration of 500 nM, Table 1), and 5 μl SYBR Green-I Master (Roche Applied Science, Germany). The efficiency of all primers were evalutated using a standard curve and a melting curve of all reactions were run to control the specificity of the primers. A non-template control with water was included for each primer set. The qPCR reaction was run under the following conditions: preincubation at 95 °C for 5 min, amplification with 45 cycles at 95 °C for 15 sec and 60 °C for 1 min, melting curve at 95 °C for 5 sec and 65 °C for 1 min, and cooling at 40 °C for 10 sec.
Table 1.
qRT-PCR primer sequences.
| Target gene | Forward primer (5′-3′) | Reverse primer (5′-3′) | Genbankaccession no. |
|---|---|---|---|
| hsp 70 | CTGTACGAGGGCATCGACTT | GCTTTCTCCACAGGCTCAAG | DQ872648 |
| cytochrome c oxidase I | CTCATTGGAGGATTCGGAAA | GGAATGATGGGGGAAGAAGT | JF752338 |
| vasa | CAGAGGAGGATCACGAGGAG | TTGGCCTTCATCTTTCCAAC | AY821684 |
| igf 2 | TGTGGAAAGGCAAACAATGA | CTAGTTCTCCACCGCAAAGG | FJ410929 |
| sod Mn | TTATGCAGCTTCACCACAGC | ACACATCACCCTTTGCCAGT | JX477243 |
| cyclinA | TGCATGTCTGTCCTGAGAGG | TCCACTTCCGGAGGATACAC | EU380204 |
| jnk 1 | GACTCCACGTTCACCGTTTT | CGTTCAAGGACATGGTCGTA | EU374209 |
| b-actin | CCCTGTATGCTTCAGGTCGT | ATTGCATGGGGAAGAGCATA | AB039726 |
| calmodulin | TGAAGTGGATGCTGATGGAA | TCTGATCTCCTCCTCGCTGT | JX477193 |
| Sod CuZn | GTCAGACACGTCGGAGACCT | GTATTCCCCAAACAGGGTCA | JX477242 |
| CathepsinB | TTCTGGAGCTCTGACGGTCT | GCCATTCACATGATGCTCAC | JX477223 |
| cyclinB | AAGTTCAGGCTGCTTCAGGA | AAGCTGGAGCTGCTTCTTTG | AF273495 |
| sod | GTCAGACACGTCGGAGACCT | GTATTCCCCAAACAGGGTCA | JQ776518 |
| cyclinA2 | TGGTGAGCTGAGCTTGATTG | CTCCTGCAATTGTGTGGTTG | AF273493 |
The relative gene expression was determined using the comparative CT method (2−∆Ct). To normalize relative mRNA levels, the stability of three reference genes, Glyceraldehyde 3-phosphate dehydrogenase (gapdh), 18S ribosomal RNA (18s) and beta-actin (b-actin), were tested. The b-actin was shown to be the most stable and was selected as reference gene.
Examining the activity of antioxidant enzymes during oocyte ageing
Preparation of post-mitochondrial supernatant
Samples of fish eggs (approx. 400 mg) were homogenized in 0.1 M K-phosphate buffer (pH 7.4) and centrifuged at 15,000 g at 4 °C for 15 min to isolate the post-mitochondrial supernatant. Protein levels in post-mitochondrial supernatant were measured spectrophotometrically using bovine serum albumin as a standard65. The samples were diluted to a protein content of 10 mg/mL. All steps were carried out on ice.
Antioxidant enzyme activity
Catalase (CAT) activity was determined using H2O2 as a substrate to proceed reaction based on the method of Claiborne66. Calculations were made using a molar extinction coefficient of 40 M−1 cm−1. Total superoxide dismutase (SOD) activity was determined as formation of nitro blue tetrazolium from phenazine methosulfonate substrate based on the method of Nishikimi et al.67. Glutathione peroxidase (GPX) activity was assayed based on the rate of NADPH oxidation at 340 nm68 by the coupled reaction with glutathione reductase (GR), which was measured as described in Cribb et al.69.
All biochemical assays were performed spectrophotometrically in triplicate or quadruplicate using microplate reader (Tecan infinite M200, Germany). Reaction activity was linear over time. Enzyme activity was expressed as units (µmol for CAT, SOD and GR; nmol for GPX) of produced product per mg of protein per minute.
Lipid oxydation
Lipid oxidation in oocytes was analysed by evaluation of MDA equivalents using thiobarbituric acid reactive substances (TBARS) method according to Li et al.70. Briefly, samples were incubated in darkness at room temperature (20 °C), overnight (for 15–20 hours) with thiobarbituric acid and the formed MDA was measured with a UV-visual plate reader (AF 2200: Austria) at 530 nm and expressed as µg/g.
Statistical analysis
The normality of the data was identified using SPSS software version 18. ANOVA followed by Duncan’s multiple range test was used to evaluate the differences between the means of the groups for the examined paremeters; embryo survival rates as well as each individual gene, lipid oxidation levels and antioxidant enzyme activities. P < 0.05 was considered to be significant.
Supplementary information
Acknowledgements
This study was financially supported by the Ministry of Education, Youth and Sports of the Czech Republic - projects: “CENAKVA” (LM2018099), Biodiversity (CZ.02.1.01/0.0/0.0/16_025/0007370), NF-CZ07-MOP-3-184-2015 and NF-CZ07-ICP-3-185-2015. We would like to thank the Elsevier Language Editing Service for improving the English of the manuscript.
Author Contributions
Azadeh M.S., Azin M.S. and T.P. designed the experiment. Azadeh M.S., Azin M.S., M.B. and T.P. performed the animal experiment. Azadeh M.S., Azin M.S., T.K.Ø. and B.R. performed the qPCR analyses. S.S. and V.B. performed the enzyme analyses part. Azadeh M.S., T.K.Ø. and B.R. contributed for the statistical evaluation of the obtained data. Azadeh M.S., Azin M.S., wrote the first draft of the manuscript. All authors revised and edited the manuscript and approved to publish the final version.
Data Availability
All data generated and analyzed during this study are included in this article and its Supporting Information files in the public repository data at https://osf.io/wpfxb/quickfiles.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1038/s41598-019-46895-1.
References
- 1.Liang X, Ma J, Schatten H, Sun Q. Epigenetic changes associated with oocyte aging. Sci. China. Life. Sci. 2012;55(8):670–676. doi: 10.1007/s11427-012-4354-3. [DOI] [PubMed] [Google Scholar]
- 2.Lahnsteiner F, Urbanyi B, Horvath A, Weismann T. Bio-markers for egg quality determination in cyprinid fish. Aquaculture. 2001;195:331–352. doi: 10.1016/S0044-8486(00)00550-0. [DOI] [Google Scholar]
- 3.Samarin AM, et al. Effects of post-ovulatory oocyte ageing and temperature on egg quality in kutum Rutilus frisii kutum. World. Appl. Sci. J. 2011;15(1):14–18. [Google Scholar]
- 4.Flajshans M, Kohlmann K, Rab P. Autotriploid tench Tinca tinca (L.) larvae obtained by fertilization of eggs previously subjected to postovulatory ageing in vitro and in vivo. J. Fish Biol. 2007;71:868–876. doi: 10.1111/j.1095-8649.2007.01557.x. [DOI] [Google Scholar]
- 5.Aegerter S, Jalabert B. Effects of post ovulatory oocyte ageing and temperature on egg quality and on the occurrence of triploid fry in rainbow trout Oncorhynchus mykiss. Aquaculture. 2004;231:59–71. doi: 10.1016/j.aquaculture.2003.08.019. [DOI] [Google Scholar]
- 6.Bonnet E, Fostier A, Bobe J. Characterization of rainbow trout egg quality: a case study using four different breeding protocols, with emphasis on the incidence of embryonic malformations. Theriogenology. 2007;67:786–794. doi: 10.1016/j.theriogenology.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 7.Samarin AM, et al. In vitro storage of unfertilized eggs of the Eurasian perch and its effect on egg viability rates and the occurrence of larval malformations. Animal. 2017;11(1):78–83. doi: 10.1017/S1751731116001361. [DOI] [PubMed] [Google Scholar]
- 8.Tarin JJ, Perez-Albala S, Perez-Hoyos P, Cano A. Postovulatory aging of oocytes decreases reproductive fitness and longevity of offspring. Biol. Reprod. 2002;66:495–499. doi: 10.1095/biolreprod66.2.495. [DOI] [PubMed] [Google Scholar]
- 9.Liang XW, et al. The effects of postovulatory aging of mouse oocytes on methylation and expression of imprinted genes at midterm gestation. Mol. Hum. Reprod. 2011;17:562–567. doi: 10.1093/molehr/gar018. [DOI] [PubMed] [Google Scholar]
- 10.Liang XW, et al. Loss of methylation imprint of Snrpn in postovulatory aging mouse oocyte. Biochem. Biophys. Res. Commun. 2008;371:16–21. doi: 10.1016/j.bbrc.2008.03.105. [DOI] [PubMed] [Google Scholar]
- 11.Minami N, Suzuki T, Tsukamoto S. Zygotic gene activation and maternal factors in mammals. J. Reprod. Dev. 2007;53:707–715. doi: 10.1262/jrd.19029. [DOI] [PubMed] [Google Scholar]
- 12.Yoshida N, Brahmajisyula M, Shoji S, Amanai M, Perry AC. Epigenetic discrimination by mouse metaphase II oocytes mediated asymmetric chromatin remodelling independently of meiotic exit. Dev. Biol. 2007;301:464–477. doi: 10.1016/j.ydbio.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 13.Samarin AM, Policar T, Lahnsteiner F. Fish oocyte ageing and its effect on egg quality. Rev. Fish. Sci. Aquac. 2015;23:302–314. doi: 10.1080/23308249.2015.1053560. [DOI] [Google Scholar]
- 14.Zuccotti M, Merico V, Cecconi S, Redi CA, Garagna S. What does it take to make a developmentally competent mammalian egg? Hum. Reprod. Update. 2011;17:525–540. doi: 10.1093/humupd/dmr009. [DOI] [PubMed] [Google Scholar]
- 15.Bonnet E, Fostier A, Bobe J. Microarray-based analysis of fish egg quality after natural or controlled ovulation. BMC Genomics. 2007;8:55. doi: 10.1186/1471-2164-8-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aegerter S, Jalabert B, Bobe J. MessengerRNAstockpile of cyclin B, insulin-like growth factor I, insulin-like growth factor II, insulinlike growth factor receptor Ib, and p53 in the rainbow trout oocyte in relation with developmental competence. Mol. Reprod. Dev. 2004;67:127–135. doi: 10.1002/mrd.10384. [DOI] [PubMed] [Google Scholar]
- 17.Aegerter S, Jalabert B, Bobe J. Large scale real-time PCR analysis of mRNA abundance in rainbow trout eggs in relationship with egg quality and postovulatory ageing. Mol. Reprod. Dev. 2005;72:377–385. doi: 10.1002/mrd.20361. [DOI] [PubMed] [Google Scholar]
- 18.Mommens M, et al. Maternal gene expression in Atlantic halibut (Hippoglossus hippoglossus L.) and its relation to egg quality. BMC Res Notes. 2010;3:138. doi: 10.1186/1756-0500-3-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ma H, et al. MicroRNA expression profiles from eggs of different qualities associated with post-ovulatory ageing in rainbow trout (Oncorhynchus mykiss) BMC Genomics. 2015;16:201. doi: 10.1186/s12864-015-1400-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lord T, Nixon B, Jones KT, Aitken RJ. Melatonin prevents postovulatory oocyte aging in the mouse and extends the window for optimal fertilization in vitro. Biol. Reprod. 2013;88:1–9. doi: 10.1095/biolreprod.112.106450. [DOI] [PubMed] [Google Scholar]
- 21.Lord T, Aitken RJ. Oxidative stress and ageing of the post-ovulatory oocyte. Reproduction. 2013;146:217–227. doi: 10.1530/REP-13-0111. [DOI] [PubMed] [Google Scholar]
- 22.Takahashi T, Takahashi E, Igarashi H, Tezuka N, Kurachi H. Impact of oxidative stress in aged mouse oocytes on calcium oscillations at fertilization. Mol. Reprod. Dev. 2003;66:143–152. doi: 10.1002/mrd.10341. [DOI] [PubMed] [Google Scholar]
- 23.Tarin JJ, Perez-Albala S, Cano A. Consequences on offspring of abnormal function in ageing gametes. Hum. Reprod. Update. 2000;6:532–549. doi: 10.1093/humupd/6.6.532. [DOI] [PubMed] [Google Scholar]
- 24.Samarin AM, et al. mRNA abundance changes during in vitro oocyte ageing in African catfish Clarias gariepinus (Burchell, 1822) Aquac. Res. 2018;49:1037–1045. doi: 10.1111/are.13552. [DOI] [Google Scholar]
- 25.Kikuchi K, Naito K, Noguchi J, Kaneko H, Tojo H. Maturation/M-phase promoting factor regulates aging of porcine oocytes matured in vitro. Cloning Stem Cells. 2002;4:211–222. doi: 10.1089/15362300260339494. [DOI] [PubMed] [Google Scholar]
- 26.Formacion MJ, Venkatesh B, Tan CH, Lam TJ. Overripening of ovulated eggs in goldfish, Carassius auratus: II. Possible involvement of postovulatory follicles and steroids. Fish Physiol. Biochem. 1995;14(3):237–246. doi: 10.1007/BF00004314. [DOI] [PubMed] [Google Scholar]
- 27.Grondahl ML, et al. Gene expression profiles of single human mature oocytes in relation to age. Hum. Reprod. 2010;25(4):957–68. doi: 10.1093/humrep/deq014. [DOI] [PubMed] [Google Scholar]
- 28.Steuerwald NM, Bermudez MG, Wells D, Munne S, Cohen J. Maternal age-related differential global expression profiles observed in human oocytes. Reprod. Biomed. Online. 2007;14:700–708. doi: 10.1016/S1472-6483(10)60671-2. [DOI] [PubMed] [Google Scholar]
- 29.Pan H, O’Brien MJ, Wigglesworth K, Eppig JJ, Schultz RM. Transcript profiling during mouse oocyte development and the effect of gonadotropin priming and develop¬ment in vitro. Dev. Biol. 2005;286:493–506. doi: 10.1016/j.ydbio.2005.08.023. [DOI] [PubMed] [Google Scholar]
- 30.Hamatani T, et al. Age-associated alteration of gene expression patterns in mouse oocytes. Hum. Mol. Genet. 2004;13(19):2263–2278. doi: 10.1093/hmg/ddh241. [DOI] [PubMed] [Google Scholar]
- 31.Esponda, P. & Dıaz, H. Age-induced apoptosis and activation of heat shock protein HSP70 in mammalian oocytes. Signalling and Apoptosis, PP-184a (2006).
- 32.Verbeke P, Fonager J, Clark BF, Rattan SI. Heat shock response and ageing: mechanisms and applications. Cell Biol. Int. 2001;25:845–857. doi: 10.1006/cbir.2001.0789. [DOI] [PubMed] [Google Scholar]
- 33.Sturtz LA, Diekert K, Jensen LT, Lill R, Culotta VC. A fraction of yeast Cu, Zn superoxide dismutase and its metallochaperone, CCS, localize to the intermembrane space of mitochondria: a physiological role for SOD1 in guarding against mitochondrial oxidative damage. J. Biol. Chem. 2001;276:38084–9. doi: 10.1074/jbc.M105296200. [DOI] [PubMed] [Google Scholar]
- 34.Pechenino AS, Brown TR. Superoxide dismutase in the prostate lobes of aging Brown Norway rats. Prostate. 2006;66:522–35. doi: 10.1002/pros.20364. [DOI] [PubMed] [Google Scholar]
- 35.Wesson DE, Elliott SJ. The H2O2-generating enzyme, xanthine oxidase, decreases luminal Ca2+ content of the IP3- sensitive Ca2+ store in vascular endothelial cells. Microcirculation. 1995;2:195–203. doi: 10.3109/10739689509146767. [DOI] [PubMed] [Google Scholar]
- 36.Schallreuter KU, Gibbons NC, Zothner C, Abou Elloof MM, Wood JM. Hydrogen peroxide-mediated oxidative stress disrupts calcium binding on calmodulin: more evidence for oxidative stress in vitiligo. Biochem. Biophys. Res. Commun. 2007;360:70–75. doi: 10.1016/j.bbrc.2007.05.218. [DOI] [PubMed] [Google Scholar]
- 37.Takahashi T, et al. Poor embryo development in mouse oocytes aged in vitro is associated with impaired calcium homeostasis. Biol. Reprod. 2009;80:493–502. doi: 10.1095/biolreprod.108.072017. [DOI] [PubMed] [Google Scholar]
- 38.Xu Z, Abbott A, Kopf GS, Schultz RM, Ducibella T. Spontaneous activation of ovulated mouse eggs: time-dependent effects on M-phase exit, cortical granule exocytosis, maternal messenger ribonucleic acid recruitment, and inositol 1,4,5-triphosphate sensitivity. Biol. Reprod. 1997;57:743–750. doi: 10.1095/biolreprod57.4.743. [DOI] [PubMed] [Google Scholar]
- 39.Ma W, et al. Reduced expression of MAD2, BCL2, and MAP kinase activity in pig oocytes after in vitro aging are associated with defects in sister chromatid segregation during meiosis II and embryo fragmentation after activation. Biol. Reprod. 2005;72:373–383. doi: 10.1095/biolreprod.104.030999. [DOI] [PubMed] [Google Scholar]
- 40.Carnevali O, Mosconi G, Cardinali M, Meiri I, Polzonetti-Magni A. Molecular components related to egg viability in the gilthead sea bream, Sparus aurata. Mol. Reprod. Dev. 2001;58(3):330–5. doi: 10.1002/1098-2795(200103)58:3<330::AID-MRD11>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 41.Raz E. The function and regulation of vasa-like genes in germ-cell development. Genome Biol. 2000;1(3):reviews1017. doi: 10.1186/gb-2000-1-3-reviews1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nikolic, A., Volarevic, V., Armstrong, L., Lako, M. & Stojkovic, M. Primordial germ cells: current knowledge and perspectives. Stem Cells Int. 1741072 (2016). [DOI] [PMC free article] [PubMed]
- 43.Tzung KW, et al. Early Depletion of Primordial Germ Cells in Zebrafish Promotes Testis Formation. Stem Cell Reports. 2015;4(1):61–73. doi: 10.1016/j.stemcr.2014.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu W, et al. Complete depletion of primordial germ cells in an All-female fish leads to Sex-biased gene expression alteration and sterile All-male occurrence. BMC Genomics. 2015;16:971. doi: 10.1186/s12864-015-2130-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Houseweart MK, et al. Cathepsin B but not cathepsins L or S contributes to the pathogenesis of Unverricht-Lundborg progressive myoclonus epilepsy (EPM1) J. Neurobiol. 2003;56(4):315–327. doi: 10.1002/neu.10253. [DOI] [PubMed] [Google Scholar]
- 46.Kågedal K, Johansson U, Öllinger K. The lysosomal protease cathepsin D mediates apoptosis induced by oxidative stress. FASEB J. 2001;15:1592–1594. doi: 10.1096/fj.00-0708fje. [DOI] [PubMed] [Google Scholar]
- 47.Samarin AM, et al. Alteration of mRNA abundance, oxidation products and antioxidant enzyme activities during oocyte ageing in common carp Cyprinus carpio. PLoS One. 2019;14(2):e0212694. doi: 10.1371/journal.pone.0212694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Abe K, Inoue A, Suzuki MG, Aoki F. Global gene silencing is caused by the dissociation of RNA polymerase II from DNA in mouse oocytes. J. Reprod. Dev. 2010;56:502–507. doi: 10.1262/jrd.10-068A. [DOI] [PubMed] [Google Scholar]
- 49.Clarke HJ. Post-transcriptional control of gene expression during mouse oogenesis. Results. Probl. Cell. Differ. 2012;55:1–21. doi: 10.1007/978-3-642-30406-4_1. [DOI] [PubMed] [Google Scholar]
- 50.Weill L, Belloc E, Bava FA, Mendez R. Translational control by changes in poly(A) tail length: recycling mRNAs. Nat. Struct. Mol. Biol. 2012;19:577–585. doi: 10.1038/nsmb.2311. [DOI] [PubMed] [Google Scholar]
- 51.Dankert D, et al. Pre- and postovulatory aging of murine oocytes affect the transcript level and poly(A) tail length of maternal effect genes. PLoS One. 2014;9:e108907. doi: 10.1371/journal.pone.0108907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wilson VL, Jones PA. DNA methylation decreases in aging but not in immortal cells. Science. 1983;220:1055–1057. doi: 10.1126/science.6844925. [DOI] [PubMed] [Google Scholar]
- 53.Crary-Dooley FK, et al. A comparison of existing global DNA methylation assays to low-coverage whole-genome bisulfite sequencing for epidemiological studies. Epigenetics. 2017;12:206–214. doi: 10.1080/15592294.2016.1276680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McCauley BS, Dang W. Histone methylation and aging: lessons learned from model systems. Biochem. Biophys. Acta. 2014;1839(12):1454–62. doi: 10.1016/j.bbagrm.2014.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xu Y, Zhang JJ, Grifo JA, Krey LC. DNA methylation patterns in human tripronucleate zygotes. Mol. Hum. Reprod. 2004;11:167–171. doi: 10.1093/molehr/gah145. [DOI] [PubMed] [Google Scholar]
- 56.Ge ZJ, Schatten H, Zhang CL, Sun QY. Oocyte ageing and epigenetics. Reproduction. 2015;149:R103–R114. doi: 10.1530/REP-14-0242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kjorsvik E, Mangor-Jensen A, Holmefjord I. Egg quality in fishes. Adv. Mar. boil. 1990;26:71–113. doi: 10.1016/S0065-2881(08)60199-6. [DOI] [Google Scholar]
- 58.Brooks S, Tyler CR, Sumpter JP. Egg quality in fish: what makes a good egg? Rev. Fish. Biol. Fisher. 1997;7:387–416. doi: 10.1023/A:1018400130692. [DOI] [Google Scholar]
- 59.Flanagan JM, et al. Intra-and interindividual epigenetic variation in human germ cells. Am. J. Hum. Genet. 2006;79:67–84. doi: 10.1086/504729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Goldberg AD, Allis CD, Bernstein E. Epigenetics: a landscape takes shape. Cell. 2007;128:635–638. doi: 10.1016/j.cell.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 61.Jaenisch R, Bird A. Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nat. Genet. 2003;33:245–254. doi: 10.1038/ng1089. [DOI] [PubMed] [Google Scholar]
- 62.Fauvel C, Suquet M, Cosson J. Evaluation of fish sperm quality. J. Appl. Ichthyol. 2010;26:636–643. doi: 10.1111/j.1439-0426.2010.01529.x. [DOI] [Google Scholar]
- 63.Koressaar T, Remm M. Enhancements and modifications of primer design program Primer3. Bioinformatics. 2007;23(10):1289–1291. doi: 10.1093/bioinformatics/btm091. [DOI] [PubMed] [Google Scholar]
- 64.Untergasser A, et al. Primer3 - new capabilities and interfaces. Nucleic Acids Res. 2012;40(15):e115. doi: 10.1093/nar/gks596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Smith PK, et al. Measurement of protein using bicinchoninic acid. Anal. Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- 66.Claiborne, A. Catalase activity. In Greenwall, R. A. (Ed.), CRC handbook of methods in oxygen radical research (pp. 283–284). Boca Raton, FL: CRC Press (1985).
- 67.Nishikimi M, Rao NA, Yagi K. The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem. Biophys. Res. Commun. 1972;46:849–854. doi: 10.1016/S0006-291X(72)80218-3. [DOI] [PubMed] [Google Scholar]
- 68.Mohandas J, Marshall JJ, Duggin GG, Horvath JS, Tiller DJ. Differential distribution of glutathione and glutathione-related enzymes in rabbit kidney - possible implications in analgesic nephropathy. Biochem. Pharmacol. 1984;33:1801–1807. doi: 10.1016/0006-2952(84)90353-8. [DOI] [PubMed] [Google Scholar]
- 69.Cribb AE, Leeder JS, Spielberg SP. Use of a microplate reader in an assay of glutathione-reductase using 5,5′-dithiobis (2-nitrobenzoic acid) Anal. Biochem. 1989;183:195–196. doi: 10.1016/0003-2697(89)90188-7. [DOI] [PubMed] [Google Scholar]
- 70.Li P, et al. Evaluating the impacts of osmotic and oxidative stress on common carp (Cyprinus carpio L.) sperm caused by cryopreservation techniques. Biol. Reprod. 2010;83:852–858. doi: 10.1095/biolreprod.110.085852. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated and analyzed during this study are included in this article and its Supporting Information files in the public repository data at https://osf.io/wpfxb/quickfiles.