Abstract
The treatment of pediatric myocarditis is controversial, and the benefits of intravenous immunoglobulin (IVIG) are inconclusive due to limited data. We searched studies from PubMed, MEDLINE, Embase, and Cochrane Library databases since establishment until October 1st, 2018. Thirteen studies met the inclusion criteria. We included a total of 812 patients with IVIG treatment and 592 patients without IVIG treatment. The meta-analysis showed that the survival rate in the IVIG group was higher than that in the non-IVIG group (odds ratio = 2.133, 95% confidence interval (CI): 1.32–3.43, p = 0.002). There was moderate statistical heterogeneity among the included studies (I2 = 35%, p = 0.102). However, after adjustment using Duval and Tweedie’s trim and fill method, the point estimate of the overall effect size was 1.40 (95% CI 0.83, 2.35), which became insignificant. Moreover, the meta-regression revealed that age (coefficient = −0.191, 95% CI (−0.398, 0.015), p = 0.069) and gender (coefficient = 0.347, 95% CI (−7.586, 8.279), p = 0.93) were not significantly related to the survival rate. This meta-analysis showed that IVIG treatment was not associated with better survival. The use of IVIG therapy in acute myocarditis in children cannot be routinely recommended based on current evidence. Further prospective and randomized controlled studies are needed to elucidate the effects of IVIG treatment.
Subject terms: Cardiology, Cardiomyopathies, Viral infection
Introduction
Myocarditis is defined as inflammation of the myocardium with variable clinical presentation ranging from subclinical disease to heart failure, arrhythmia, fulminant hemodynamic collapse, and mortality1. Although myocarditis and idiopathic dilated cardiomyopathy (DCM) are considered distinct diseases, myocarditis frequently presents with a phenotype of new-onset DCM2. The predicted annual incidence of myocarditis is 1 to 2 cases per 100,000 children3.
Pediatric patients with myocarditis are stratified into 40 to 50% with the acute type and 30 to 40% with the fulminant type4. Acute myocarditis is defined as presenting with a less distinct onset of illness, established ventricular systolic dysfunction, and possible progression to DCM. Although the outcome of acute myocarditis is favorable in about 50% of cases, sequelae and chronic evolution occur in about 20% of cases, with 80% of cases of chronic cardiomyopathy leading to heart transplantation or death5. On the other hand, a subset of patients develop fulminant myocarditis (FM) presenting with severe cardiovascular compromise within two weeks since the onset of symptoms after a distinct viral infection prodrome6. Despite the severity of illness, most patients with FM regain native ventricular function if the cardiorespiratory and end-organ functions can be adequately supported until myocardial recovery. The survival rate of FM is around 51.6–80% which indicates the importance of prompt adoption of mechanical circulatory support to prevent rapid clinical deterioration and to reduce mortality rate7–9.
The majority of children with myocarditis present with an acute or fulminant disease, and infectious etiologies, particularly viral, are most common. Ventricular systolic dysfunction often normalize in patients surviving the acute illness6. Damage to the myocardium in acute myocarditis may be mediated by predominantly immunological mechanisms rather than by the direct effect of viral infection and replication10. High-dose intravenous immunoglobulin (IVIG) has shown potential in the treatment of myocarditis, hypothetically due to its antiviral, antibacterial, and immunosuppressant properties11. In one randomized multi-center trial, 41 adults (age 19–80 years) had improved survival with IVIG treatment12. However, another randomized controlled study of adults reported that IVIG did not improve the left ventricular ejection fraction (LVEF) or event-free survival13.
The treatment of pediatric myocarditis remains controversial, and the benefits of IVIG are inconclusive due to limited data14. Multivariable analysis in Pediatric Cardiomyopathy Registry (PCMR) study found no association of IVIG or corticosteroids with survival nor left ventricle normalization15. However, several studies have shown that IVIG treatment in children can be effective in improving LVEF16–18 and is beneficial for survival in children19–21.
Despite these discrepancies, IVIG is frequently used in current practice to treat acute myocarditis in adult and pediatric populations. Ghelani et al. conducted a multi-institutional study in the United States and found more than 70% of the pediatric patients were treated with IVIG5. Therefore, we performed this evidence-based meta-analysis and systematic literature review of survival outcomes of children with acute myocarditis after IVIG treatment.
Methods
Data sources and search strategy
We identified studies from PubMed, MEDLINE, Embase, and Cochrane Library databases since establishment until October 1st, 2018 (the date of the last literature search). All articles included in the present study involved human clinical studies published in English. The search parameters included the terms “myocarditis or cardiomyopathy” combined with “IVIG or immunoglobulin” and “children or pediatric”. This meta-analysis was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analyses (http://www.prisma-statement.org/).
Eligibility criteria and study selection
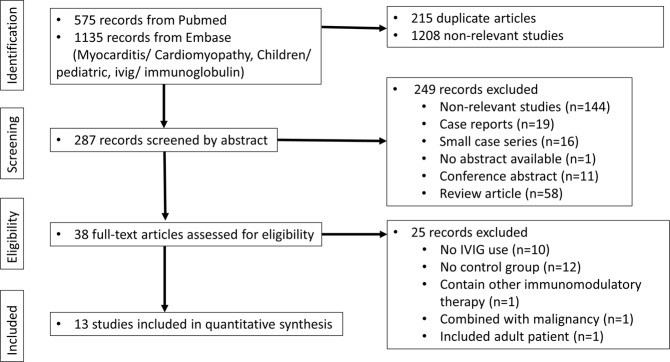
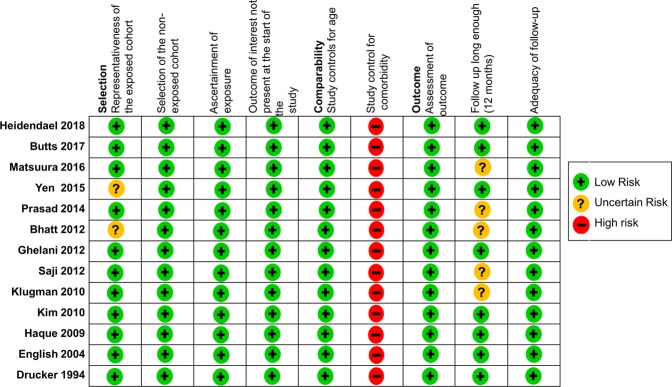
We initially focused the literature search on randomized controlled trials (RCTs), and prospective and retrospective cohort studies. With the broad keyword search, 1710 studies were found. After a detailed inspection, 215 duplicates and 1208 non-relevant studies were found. Non-relevant studies, case reports, case series, conference abstracts, and review articles were all excluded. We also excluded studies in which the participants (1) were older than 18 years of age; (2) had received other immunomodulatory therapy; (3) had concurrent malignancies, and (4) were reported to have chronic myocarditis (Fig. 1). Finally, we only identified one quasi-randomized study and twelve retrospective cohort studies that were eligible. The risk of bias assessments of the included articles was rated using an adapted version of the Newcastle-Ottawa Scale for cohort studies, with a maximum score of 9 points (Fig. 2).
Figure 1.
Selection of studies included in the analysis.
Figure 2.
Risk of bias assessments for cohort studies.
Outcomes
The primary outcome of the present study was the survival rate following IVIG therapy. The endpoint of survival rate differed in each study from the time of discharge to 15 years. The secondary outcome was the recovery of ventricular function. However, five studies did not provide related information. The measurements of ventricular function were different in the studies (mean time to recovery, LVEF recovery percentage, LVEF recovery rate, etc.). Therefore, we only analyzed the primary outcome in our study.
Data extraction
Data were independently extracted by two authors (Dr. Yen and Dr. Wu). Any disagreement was resolved by consensus. Data on the following measures were extracted: study design, diagnosis, sample size, treatment regimen, and authors’ conclusions (Table 1). We also extracted the mean age, gender, disease duration, supportive therapy, initial heart condition, statistical results, and survival rate from the enrolled studies (Table 2). There was no available adjusted odds ratio for the survival rate of IVIG treatment provided in the included studies.
Table 1.
Summary of the included studies.
| Study (year) | Study design | Diagnosis | Sample size (IVIG/Non-IVIG) |
IVIG regimen | Author’s conclusion |
|---|---|---|---|---|---|
| Heidendael, Den Boer et al.17 | Retrospective cohort study | Biopsy-proven or clinically diagnosed viral myocarditis or dilated cardiomyopathy due to viral infection | 94 children: 21/73 | 2 g/kg |
New onset dilated cardiomyopathy (either viral or idiopathic origin) ◎ Did not influence transplant-free survival ◎ Better improvement in LVEF ◎ Better recovery |
| Butts, Boyle et al.26 | Retrospective cohort study | Newly confirmed myocarditis and clinically diagnosed myocarditis | 55 children: 44/11 | No dosing data |
◎ Not associated with mortality ◎ Not associated with heart transplantation, shortening fraction at discharge |
| Matsuura, Ichida et al.4 | Nationwide survey |
Biopsy-proven in 19.2% of cases Acute myocarditis (65.6%), Fulminant myocarditis (33.5%) |
237 children: 142/75 | No dosing data |
◎ Not affect the survival in the whole study population ◎ Better survival in fulminant myocarditis subgroup |
| Yen, Huang et al.19 | Retrospective cohort study | Culture-confirmed enterovirus infection, Clinical evident myocarditis | 15 neonate: 7/8 | 2–2.5 g/kg |
In defined severe neonatal enterovirus infections ◎ Beneficial for survival |
| Prasad and Chaudhary18 | Retrospective cohort study | Clinically diagnosed acute myocarditis | 28 children: 12/16 | 1 g/kg/day (for 2 days) |
◎ Beneficial for survival ◎ Improved recovery of LVEF ◎ Reduction in the episodes of fulminant arrhythmias |
| Bhatt, Sankar et al.20 | Quasi-randomized control study | Acute encephalitis syndrome complicated by clinically diagnosed myocarditis | 83 children: 26/57 | 400 mg/kg/day (for 5 days) |
Children with AES complicated by myocarditis ◎ Beneficial for survival ◎ Improved recovery of LVEF |
| Ghelani, Spaeder et al.5 | Retrospective cohort study | Biopsy-proven or MRI diagnosed acute myocarditis | 514 children: 359/155 | No dosing data | ◎ No difference in transplant-free survival |
| Saji, Matsuura et al.25 | Nationwide survey |
1 Biopsy-proven in 33.1% of cases 2 Acute myocarditis (58%), Fulminant myocarditis (42%) |
44 children: 29/15 | 1–2 g/kg/day (for 1–2 days) | ◎ No difference in survival |
| Klugman, Berger et al.27 | Retrospective cohort study | Clinically diagnosed myocarditis | 216 children: 98/118 | No dosing data | ◎ No difference in survival |
| Kim, Yoo et al.28 | Retrospective cohort study | Clinically diagnosed myocarditis | 33 children: 23/10 | 2 g/kg |
◎ No difference in recovery of LVEF ◎ No difference in survival |
| Haque, Bhatti et al.21 | Retrospective cohort study | Clinically diagnosed myocarditis | 25 children: 12/13 | 2 g/kg/day (for 1 day) |
◎ No difference in recovery of LVEF ◎ Beneficial for survival |
| English, Janosky et al.24 | Retrospective cohort study | Biopsy-proven or clinically diagnosed viral myocarditis | 34 children: 18/16 |
16 patients: 2 g/kg patients: 1 g/kg |
◎ No difference in time to recovery of normal LVEF ◎ No difference in survival |
| Drucker, Colan et al.16 | Retrospective cohort study | Biopsy-proven or clinically diagnosed viral myocarditis | 46 children: 21/25 | 2 g/kg/day (for 1 day) |
◎ Improved recovery of LVEF ◎ Better survival |
IVIG: intravenous immunoglobulin, LVEF: left ventricular ejection fraction.
Table 2.
Summary of the clinical and statistical data of the included studies.
| Study (year) | Mean age (IVIG/Non-IVIG) | Gender (Male%) (IVIG/Non-IVIG) | Disease duration | Initial heart condition (IVIG/Non-IVIG) |
Supportive therapy | Statistical result (IVIG/Non-IVIG) |
Survival rate (IVIG/Non-IVIG) (F/u duration) |
|---|---|---|---|---|---|---|---|
| Heidendael, Den Boer et al.17 | 10/18 months | 48%/56% | Acute (43% < 1 weeks) | No difference in echocardiography & MRI |
1. No difference in inotropic therapy, ICU admission, ECMO rate 2. Mechanical ventilation: 62%/36% (p = 0.03) |
Recovery rate within 5 years: 70%:43% (p = 0.045) |
Transplant-free survival (5 years): 90%:71% (p = 0.24) |
| Butts, Boyle et al.26 | 10.6/16.1 years | 50%/27.3% | N/A | Shortening fraction: 17.8%:33.6% (p < 0.01) | Steroids, inotropes, mechanical circulatory support used |
1. Length of admission: 16.5 days: 4 days (p < 0.01) 2. Transplantation: 18.2%:0% (p = 0.13) |
95.5%:100% (1 year) (p = 0.47) |
| Matsuura, Ichida et al.4 | 6.5 ± 5.3 years | 51% | Median: 3 days (range: 3 to 60 days) | N/A | Steroids used | N/A | 78.9%:72% (At discharge) (p = NS) |
| Yen, Huang et al.19 | Neonate | N/A | N/A | N/A | N/A | N/A | 57%:12% (15 years) (p = 0.089) |
| Prasad and Chaudhary18 | <12 years | 58%/56% | Acute (<3 months) | LVEF: 35.3%:33.5% (p > 0.05) | N/A |
1. LVEF 6 months post-treatment: 62.2%:43.3% (p < 0.01) 2. VT/VF recovered: 2/3: 1/3 (p < 0.01) 3. AV block recovered: 4/5: 1/3 (p < 0.01) |
83%:56% (6 months) (p = 0.032) |
| Bhatt, Sankar et al.20 | 4.4/ 4.7 years | Male in majority | Mean 50 days (between encephalitis and myocarditis | LVEF: 32.8: 33.2% (p = 0.78) | N/A | LVEF at discharge: 49.5: 35.9 (p = 0.001) | 96%:77% (At discharge) (p = 0.061) |
| Ghelani, Spaeder et al.5 | 9.2 ± 6.8 years | 64% | Median: 7 days (interquartile: 3–19 days) | N/A | Steroids, inotropes, mechanical circulatory support used | N/A | 88%:89% (p = 0.65) |
| Saji, Matsuura et al.25 | 1 month- 17 years | 47% | N/A | N/A | Steroids, mechanical circulatory support used | N/A | 86%:46% (p = 0.008) |
| Klugman, Berger et al.27 | <18 years | N/A | N/A | N/A | Inotropes, mechanical circulatory support used | N/A | 93%:90% (At discharge) (p = 0.38) |
| Kim, Yoo et al.28 | 41/60 months | 52.1%/60% | Acute (<2 weeks) | N/A | No difference in inotropic therapy, the use of ACEI, or ventilator care | Mean time to recovery of function: 68 days: 33 days (p = 0.485) | 86%:80% (1 year) (p = 0.607) |
| Haque, Bhatti et al.21 | 7.3/12 months | 50%:46% | N/A | LVEF: 17.5%:22.5% (p = 0.17) | Inotropes: 3: 1.5 (p = 0.001) | Recovery of left ventricular function: 49%:46% (p = 0.13) | 91%:53% (At discharge) (p = 0.04) |
| English, Janosky et al.24 | 85.1/34 months | 44%:56% | Acute (<2 weeks) | Dilated left ventricle: 38%:56% (p = NS) |
1. Inotropes: 14: 12 (p = NS) 2. Intubation: 8: 11 (p = NS) 3. LVAD/ECMO: 2: 4 (p = NS) 4. Steroid used in both group |
Mean time to recovery of function: 2: 2.8 months (p = NS) | 70%:75% (5 years) (p = 0.85) |
| Drucker, Colan et al.16 | <2 years | 47%:56% | Acute (<3 months) | Cardiac index: 3.1: 3.39 (p = NS) |
1. Inotropes: 90%:52% (p < 0.01) 2. ACEI: 88%:53% (p = 0.02) |
Recovery of LV function at 12 months: 100%:37% (p < 0.001) | 84%:60% (1 year) (p = 0.069) |
IVIG: intravenous immunoglobulin, MRI: Magnetic resonance imaging, ICU: intensive care unit, N/A: not available, LVEF: left ventricular ejection fraction, NS: not significant, LVAD: left ventricular assist device, ECMO: extracorporeal membrane oxygenation, VT/VF: ventricular tachycardia/fibrillation, AV: atrioventricular, ACEI: angiotensin-converting enzyme inhibitors.
Data analysis
We performed a meta-analysis on all studies that provided survival rates. Sensitivity analysis was conducted for each study. Heterogeneity was tested using the I2 test, and a fixed-effects model was used if heterogeneity was lacking. Trim and fill with funnel-plot-based method was tested for publication bias. All of these analyses were performed using Comprehensive Meta-Analysis Software version 3 (Biostat Incorporated, Englewood, New Jersey). The quality of evidence and grading of the strength of recommendations was assessed using Grades of Recommendation, Assessment, Development and Evaluation (GRADE)22. The GRADE approach categorizes evidence into high, moderate, low or very low quality, taking into account the limitations of trial design, the risk of bias, inconsistency, indirectness, imprecision, and publication bias23.
Results
Search results and study characteristics
Of 575 records from PubMed and 1135 records from Embase, thirteen studies met the inclusion criteria, including one quasi-randomized study and twelve retrospective cohort studies (Fig. 1). A summary of the study characteristics is presented in Table 1. All studies met the diagnosis of myocarditis by clinical symptoms and echocardiographic evidence of new left-ventricular dysfunction, and six of the thirteen studies also performed endo-myocardial biopsies4,5,16,17,24,25. Only two studies from a nationwide survey by the Japanese Society of Pediatric Cardiology and Cardiac Surgery (JPCCS) reported the effect of IVIG between two clinical types of acute and fulminant myocarditis4,25. We enrolled a total of 812 patients who received IVIG treatment and 592 who did not. Nine studies used a high-dose IVIG regimen with a dosage of 2 g/kg for 1 day, 1 g/kg for 2 days, or 400 mg/kg/day for 5 days. Four studies did not provide information regarding the dosage4,5,26,27. Two patients in the study by English et al. received a single dose of 1 g/kg24. Five studies showed that the IVIG group had a better survival rate, and four studies showed that the IVIG group was associated with better recovery of cardiac function16–18,20.
A summary of the clinical and statistical data is presented in Table 2. The age of the patients in all of the studies were <18 years. There was no statistical difference in the initial heart function between the IVIG and non-IVIG group in six studies16–18,20,21,24, but the patients in the IVIG group had poorer initial heart function in the study by Butts et al. Three studies reported no difference between the IVIG and non-IVIG groups in the requirement of inotropic drugs use, and two studies reported that the IVIG group had a higher rate of requiring inotropic drugs16,21. Corticosteroid drugs were used in five studies and there was no further statistic data to compare the IVIG and control group4,5,24–26. The IVIG group had a higher rate of angiotensin-converting enzyme inhibitors (ACEI) use in Drucker et al. study16. Six studies reported the etiology of myocarditis. Coxsackie B virus was the most common pathogen in four studies, followed by adenovirus and cytomegalovirus17,20,24,25,28. Yen et al. only investigated myocarditis in neonates with severe enterovirus infections19.
Statistical analysis results
The primary outcome of the current study was survival rate. The secondary outcome cannot be analyzed due to variations of the measurement method. The results of the sensitivity analysis are presented in Fig. 3. P-values remained significant throughout the leave-one-out sensitivity analysis. There was moderate statistical heterogeneity among included studies (I2 = 35%, p = 0.102). Additionally, each study had prominent heterogeneity including patient groups, disease etiology, the dosage of medication, and the tracking time, which may cause a substantial difference in the therapeutic result. Random-effects model was used. Compared with the control group, the patients who received IVIG treatment had a significantly higher survival rate (odds ratio = 2.133, 95% confidence interval (CI): 1.32, 3.43), p = 0.002) (Fig. 4). The meta-regression could only be performed in seven studies because age and gender were provided4,5,17,21,24,26,28. The meta-regression revealed that years of age (coefficient = −0.191, 95% CI (−0.398, 0.015), p = 0.069) and gender (coefficient = 0.347, 95% CI (−7.586, 8.279), p = 0.931) were not significantly related to the survival rate.
Figure 3.
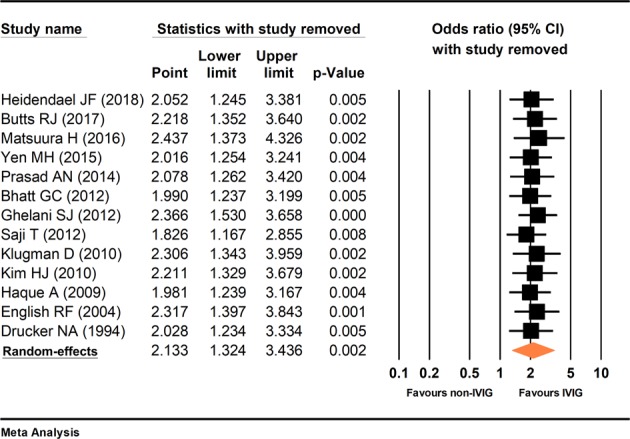
Sensitivity analysis. Forest plot showed each pooled result, having excluded a study, compared to the pooled result including all studies.
Figure 4.
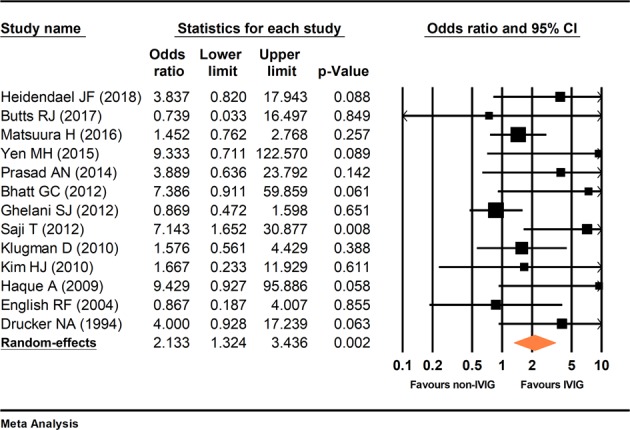
Forest plot. Comparison of survival rate between patients with IVIG and the control group.
The funnel plot (Fig. 5) for changes in the survival rate between the IVIG and non-IVIG group in 13 studies disclosed asymmetry (Egger test, p = 0.01 (2-tailed)) and visual funnel plot showed publication bias. In estimating the true effect size, the Duval and Tweedie’s trim-and-fill method was used. After running the algorithm using Comprehensive Meta-Analysis software, five missing studies were estimated. We found that the corrected point estimate of the overall effect size was 1.40 (95% CI 0.83, 2.35), which was statistically insignificant.
Figure 5.
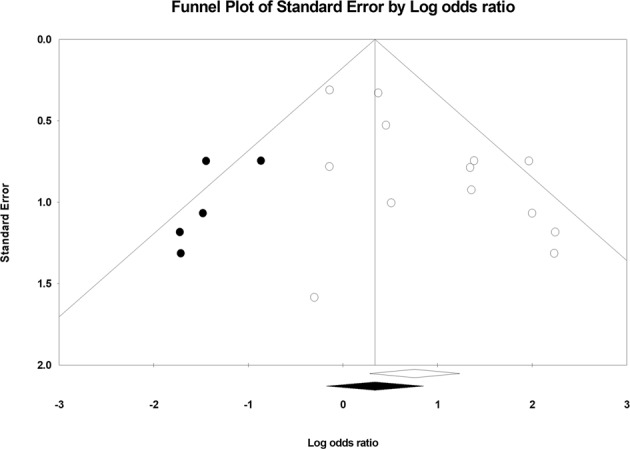
Funnel plot. Funnel plot for odds ratio of survival rate between patients with IVIG and the control group showed asymmetry, which meant publication bias. After running Duval and Tweedie’s trim-and-fill method by Comprehensive Meta-Analysis software, five missing studies were estimated.
The quality of the evidence from the outcome evaluated by the GRADE system was assessed as low since the majority of included studies were retrospective cohort studies and the risk of bias was serious (Table 3). Hence, our confidence in the effect estimate is limited.
Table 3.
GRADE assessment.
| Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Publication bias | Large effect | Dose-response | Reduce all confounders | Quality | Importance |
|---|---|---|---|---|---|---|---|---|---|---|
| One quasi-randomized, twelve retrospective studies | Serious (−1)† | Low to Moderate‡ | Not serious | Not serious |
Detected (−1) |
Nil§ | Unknown | Nil |
Low ⊕⊕○○¶ |
Critical |
†Lack of randomization, blinding, allocation concealment, intention-to-treat analysis.
‡I2 = 35%, p = 0.102 (I2 < 40% may be low; 30–60% may be moderate; 50–90% may be substantial; 75–100% may be considerable).
§Odds ratio < 2 (95% CI 1.21, 2.36).
¶This research provides some indication of the likely effect. However, the likelihood that it will be substantially different (a large enough difference that it might have an effect on a decision) is high.
Discussion
In this systematic review and meta-analysis, our result before trim and fill adjustment demonstrated that children who received IVIG treatment had a significantly higher survival rate than those who did not. Acute myocarditis can be caused by viral infections, but subsequent myocardial damage appears to be mediated by autoimmune mechanisms29. Previous studies have reported the therapeutic effects of high-dose IVIG in acute myocarditis16,17,20. The beneficial effect of IVIG leads to a more rapid clearance of myocardial viral infection via targeting specific antibodies to viruses30. IVIG can also modulate immune responses, which potentially result in decreased cardiac inflammation31,32. In patients with active lymphocytic myocarditis, those with circulating cardiac autoantibodies and no viral genome in the myocardium are the most likely to benefit from immunosuppression33.
IVIG has many immunomodulatory and anti-inflammatory effects at higher doses (2 g/kg over 2–5 days for adults or 2 days for children). Currently, more than 100 inflammatory and autoimmune disorders are treated with IVIG34,35. Eight studies used high-dose IVIG regimens (total of 2 g/kg) to treat acute myocarditis, including one study using 400 mg/kg/day for 5 days. The doses of the IVIG regimens were different in the enrolled studies and four studies did not report the dosage; thus subgroup meta-analysis could not be performed. Therefore, it is still unclear whether the duration of high dose IVIG affects the survival of patients with myocarditis.
Evidence from the Cochrane Collaboration Review of 2015, which was based on one trial, did not support the use of IVIG for the treatment of adults with presumed viral myocarditis due to lack of evidence of improved survival. In addition, the only pediatric trial included had a high risk of bias, but suggested that benefits may be seen in children with acute encephalitis syndrome (AES) complicated by myocarditis36. Bhatt et al. included patients aged 2 to 12 years with the mean age of 4.6 years20. The suspected pathogen was enterovirus, but definitive diagnosis could only be established in 14 children. Therefore, it is not clear whether the benefit of IVIG treatment extends to neonates or older children with myocarditis alone, as well as for other viral etiologies. It is possible that the contrasting effects of IVIG in children compared with adults may be due to the greater role of the immune response in myocarditis and enhanced viral etiology in children. It is also possible that children have a better potential for recovery of ventricular function and higher rates of survival37.
The variation in the efficacy of IVIG across different settings may be due to differences in the specific immunoglobulin components in each bottle, which is dependent on the protocols used in preparing IVIG11. Over 25 IVIG preparations are currently available worldwide, and each is derived from plasma pooled from at least 1000 donors. The characteristics of the various products may differ in immunoglobulin types, subclass distribution and antibody content, which may result in variable efficacy of IVIG38,39. In addition, the efficacy of IVIG may also be influenced by the heterogeneity of the disease etiology and the age of the patients, as most studies did not have details of the disease etiology. The onset of myocarditis is frequently triggered by viral infections caused by common respiratory viruses, enteroviruses and herpesviruses40. There is a growing list of viruses that may cause myocarditis with very few virus-targeted treatment options. Since most patients with acute myocarditis are diagnosed weeks after viral infection, it is relatively late to provide antiviral therapy upon the onset of myocarditis by when viral replication has often been cleared41. Furthermore, the confirmed diagnosis of viral etiology remains difficult to establish17.
The initial heart function may influence the survival outcome. In the study by Haque et al., even though the children in the IVIG group had a worse LVEF and increased need for inotropic drugs, the difference in survival rates between the groups persisted to be significant to demonstrate better outcomes in the IVIG group21. Similarly, the survival rate of the IVIG group in Drucker et al.’s study, in which the children also had increased usage of inotropic therapy, was significant as well16. However, in the study by Butts et al., the IVIG group did not have a better survival rate, and this finding may have been influenced by the poorer initial heart function in this group26. Data of initial heart condition was insufficient and not uniformly assessed.
Four studies revealed that the administration of IVIG was associated with significantly greater improvements in left ventricular function in the children16–18,20, although three studies reported conflicting results24,26,28. Nevertheless, different parameters of ventricular function were assessed in the studies (mean time to recovery, LVEF recovery percentage, LVEF recovery rate, etc.), and thus meta-analysis was not possible. The definite mechanism by which IVIG potentially improves myocardial dysfunction in myocarditis is still unknown. The anti-viral and anti-inflammatory effects of IVIG may play a role in improving myocardial dysfunction.
Fulminant myocarditis is characterized by acute hemodynamic collapse, fatal arrhythmias, or low cardiac output42. Interestingly, IVIG administration correlated with significantly better survival in patients with FM (59.6% vs. 15.0%, P < 0.005) than acute myocarditis (90% vs. 92.7%, P = not significant)4. The same result could be found in the study done by Saji et al.25. In patients with FM, the survival rate tended to be higher in those receiving either IVIG, steroids or mechanical support, in comparison to those who did not receive these treatments. Therefore, there is some argument that fulminant myocarditis should be analyzed separately. However, the other eleven studies did not provide information regarding a further clinical type of myocarditis. Therefore, subgroup meta-analysis comparing the acute myocarditis and FM cannot be analyzed.
Additionally, concomitant administration of medication can affect the survival rate between the study groups. It has been established in the PCMR analysis study that children with dilated cardiomyopathy have improved survival in the more recent era (1990 to 1999). This appears to be associated with the comprehensive treatment of inotropic support, mechanical ventilation, and mechanical circulatory support other than heart transplantation. Medication such as diuretics, beta-blockers and angiotensin-converting enzyme inhibitors may also play a role9. Several studies have demonstrated ACEI can slow progression of reverse remodeling and reduce mortality rates in heart failure patients43. In Drucker et al. study, the IVIG group had a higher rate of ACEI prescription. Therefore, the result of improved survival in this study may be associated with medications apart from IVIG. A review from the Cochrane Database found that corticosteroids treatment did not reduce mortality in myocarditis patients44; nevertheless, the role of corticosteroid in myocarditis remains controversial. Corticosteroids were administered in five included studies, though dosage details between the IVIG and control groups were not recorded4,5,24–26. The influence of corticosteroids on both groups was difficult to assess.
There were only seven studies providing detailed information of both age and gender4,5,17,21,24,26,28, which could be potentially important variables. We therefore examined the impact of age and gender on IVIG effect size by using the meta-regression models. As a result, gender was not associated with survival rate apparently (p = 0.93). Notably, the beneficial effect of IVIG on survival was more evident in infants and young children19–21,25, in which age group enterovirus infection is common45. However, the efficacy of IVIG use in neonatal enterovirus infection remains uncertain. Furthermore, the meta-regression result revealed that age was not significantly related to the survival rate (coefficient = −0.191, 95% CI (−0.398, 0.015), p = 0.069).
Compared with the previous study published in Cochrane Reviews which stated IVIG should not be provided as routine practice36, we added twelve retrospective cohort studies to the meta-analysis in order to strengthen the level of evidence. Since Egger test showed publication bias, we used Duval and Tweedie’s trim-and-fill method, a funnel plot-derived and two-step method, to estimate the number of unpublished counterparts and provide a summary effect adjusted for publication bias46. As a result of adjustment, IVIG treatment was not associated with better survival. Therefore, evidence for IVIG therapy in acute myocarditis remains limited.
Study limitations and considerations
There are several limitations to this study. Firstly, only one RCT was available for analysis. Currently, no prospective randomized controlled trials in literature demonstrated the efficacy of IVIG in children with acute myocarditis. The key reason is that acute myocarditis is rare, with an estimated annual incidence of 0.26 per 1000,000 children from a nationwide survey by JPCCS25. Moreover, pediatric myocarditis is commonly associated with rapid progression to heart failure47, and thus raises the difficulty of conducting a large-scale, randomized trial of IVIG. Hence, the power of this study is limited by the availability of therapeutic information from a small population of patients without random assignment to treatments. However, we included a total of thirteen studies to improve the power of evidence.
Secondly, there was high inter-study variability between the included studies. The diagnostic criteria of myocarditis, the etiologies, IVIG regimens, and the combination of other treatment in these studies were different. We therefore performed heterogeneity testing and sensitivity analysis. The result of I2 test was 35% with p-value = 0.102, implying that the heterogeneity of the studies was moderate. The strength of pooled estimate did not significantly differ according to the characteristics of individual studies in the leave-one-out sensitivity analysis (Fig. 3). In consideration of moderate heterogeneity, the meta-analysis was performed using a random-effects model.
Conclusion
In conclusion, this meta-analysis demonstrated that IVIG treatment for acute myocarditis in children was not associated with better survival rate. The quality of evidence is low and the strength of recommendation according to the GRADE system is weak. Taken together, the use of IVIG therapy in acute myocarditis in children cannot be routinely recommended based on current evidence. However, given the variability and limited number of the included studies, further prospective, randomized controlled studies are needed to elucidate the effects of IVIG treatment.
Author Contributions
K.W., C.L. and M.H. proposed the idea of this study, reviewed and revised the manuscript. C.Y. designed the study, performed the data screening, quality assessment, statistical analysis and drafted the initial manuscript. C.C. and Y.W. polished the English language. The data was interpreted by all authors. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Magnani JW, Dec GW. Myocarditis: current trends in diagnosis and treatment. Circulation. 2006;113:876–890. doi: 10.1161/CIRCULATIONAHA.105.584532. [DOI] [PubMed] [Google Scholar]
- 2.Cooper LT., Jr. Myocarditis. N Engl J Med. 2009;360:1526–1538. doi: 10.1056/NEJMra0800028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lipshultz SE, et al. The incidence of pediatric cardiomyopathy in two regions of the United States. N Engl J Med. 2003;348:1647–1655. doi: 10.1056/NEJMoa021715. [DOI] [PubMed] [Google Scholar]
- 4.Matsuura H, et al. Clinical features of acute and fulminant myocarditis in children – 2nd nationwide survey by Japanese society of pediatric cardiology and cardiac surgery. Circulation Journal. 2016;80:2362–2368. doi: 10.1253/circj.CJ-16-0234. [DOI] [PubMed] [Google Scholar]
- 5.Ghelani SJ, Spaeder MC, Pastor W, Spurney CF, Klugman D. Demographics, trends, and outcomes in pediatric acute myocarditis in the United States, 2006 to 2011. Circ Cardiovasc Qual Outcomes. 2012;5:622–627. doi: 10.1161/CIRCOUTCOMES.112.965749. [DOI] [PubMed] [Google Scholar]
- 6.McCarthy RE, III., et al. Long-term outcome of fulminant myocarditis as compared with acute (nonfulminant) myocarditis. N Engl J Med. 2000;342:690–695. doi: 10.1056/NEJM200003093421003. [DOI] [PubMed] [Google Scholar]
- 7.Amabile N, Fraisse A, Bouvenot J, Chetaille P, Ovaert C. Outcome of acute fulminant myocarditis in children. Heart. 2006;92:1269–1273. doi: 10.1136/hrt.2005.078402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Teele SA, et al. Management and outcomes in pediatric patients presenting with acute fulminant myocarditis. The Journal of pediatrics. 2011;158:638–643.e631. doi: 10.1016/j.jpeds.2010.10.015. [DOI] [PubMed] [Google Scholar]
- 9.Singh RK, et al. Survival Without Cardiac Transplantation Among Children With Dilated Cardiomyopathy. J Am Coll Cardiol. 2017;70:2663–2673. doi: 10.1016/j.jacc.2017.09.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O’Connell JB, Robinson JA, Henkin RE, Gunnar RM. Immunosuppressive therapy in patients with congestive cardiomyopathy and myocardial uptake of gallium-67. Circulation. 1981;64:780–786. doi: 10.1161/01.CIR.64.4.780. [DOI] [PubMed] [Google Scholar]
- 11.Boros P, Gondolesi G, Bromberg JS. High dose intravenous immunoglobulin treatment: mechanisms of action. Liver Transpl. 2005;11:1469–1480. doi: 10.1002/lt.20594. [DOI] [PubMed] [Google Scholar]
- 12.Kishimoto C, et al. Therapy with immunoglobulin in patients with acute myocarditis and cardiomyopathy: analysis of leukocyte balance. Heart Vessels. 2014;29:336–342. doi: 10.1007/s00380-013-0368-4. [DOI] [PubMed] [Google Scholar]
- 13.McNamara DM, et al. Controlled trial of intravenous immune globulin in recent-onset dilated cardiomyopathy. Circulation. 2001;103:2254–2259. doi: 10.1161/01.CIR.103.18.2254. [DOI] [PubMed] [Google Scholar]
- 14.Hia CP, Yip WC, Tai BC, Quek SC. Immunosuppressive therapy in acute myocarditis: an 18 year systematic review. Arch Dis Child. 2004;89:580–584. doi: 10.1136/adc.2003.034686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Foerster SR, et al. Ventricular remodeling and survival are more favorable for myocarditis than for idiopathic dilated cardiomyopathy in childhood: an outcomes study from the Pediatric Cardiomyopathy Registry. Circ Heart Fail. 2010;3:689–697. doi: 10.1161/CIRCHEARTFAILURE.109.902833. [DOI] [PubMed] [Google Scholar]
- 16.Drucker NA, et al. Gamma-globulin treatment of acute myocarditis in the pediatric population. Circulation. 1994;89:252–257. doi: 10.1161/01.CIR.89.1.252. [DOI] [PubMed] [Google Scholar]
- 17.Heidendael JF, et al. Intravenous immunoglobulins in children with new onset dilated cardiomyopathy. Cardiol Young. 2018;28:46–54. doi: 10.1017/S1047951117001561. [DOI] [PubMed] [Google Scholar]
- 18.Prasad AN, Chaudhary S. Intravenous immunoglobulin in children with acute myocarditis and/or early dilated cardiomyopathy. Indian Pediatr. 2014;51:583–584. doi: 10.1007/s13312-014-0456-2. [DOI] [PubMed] [Google Scholar]
- 19.Yen MH, et al. Effect of intravenous immunoglobulin for neonates with severe enteroviral infections with emphasis on the timing of administration. J Clin Virol. 2015;64:92–96. doi: 10.1016/j.jcv.2015.01.013. [DOI] [PubMed] [Google Scholar]
- 20.Bhatt GC, Sankar J, Kushwaha KP. Use of intravenous immunoglobulin compared with standard therapy is associated with improved clinical outcomes in children with acute encephalitis syndrome complicated by myocarditis. Pediatr Cardiol. 2012;33:1370–1376. doi: 10.1007/s00246-012-0350-4. [DOI] [PubMed] [Google Scholar]
- 21.Haque A, Bhatti S, Siddiqui FJ. Intravenous immune globulin for severe acute myocarditis in children. Indian Pediatr. 2009;46:810–811. [PubMed] [Google Scholar]
- 22.Balshem H, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64:401–406. doi: 10.1016/j.jclinepi.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 23.Schünemann, H., Bousquet, J., Guyatt, G. & Oxman, A. GRADE handbook for grading quality of evidence and strength of recommendations. The GRADE Working Group Available from, www.guidelinedevelopment.org/handbook (October 2013).
- 24.English RF, Janosky JE, Ettedgui JA, Webber SA. Outcomes for children with acute myocarditis. Cardiol Young. 2004;14:488–493. doi: 10.1017/S1047951104005049. [DOI] [PubMed] [Google Scholar]
- 25.Saji T, et al. Comparison of the clinical presentation, treatment, and outcome of fulminant and acute myocarditis in children. Circulation journal: official journal of the Japanese Circulation Society. 2012;76:1222–1228. doi: 10.1253/circj.CJ-11-1032. [DOI] [PubMed] [Google Scholar]
- 26.Butts RJ, et al. Characteristics of Clinically Diagnosed Pediatric Myocarditis in a Contemporary Multi-Center Cohort. Pediatr Cardiol. 2017;38:1175–1182. doi: 10.1007/s00246-017-1638-1. [DOI] [PubMed] [Google Scholar]
- 27.Klugman D, et al. Pediatric patients hospitalized with myocarditis: a multi-institutional analysis. Pediatr Cardiol. 2010;31:222–228. doi: 10.1007/s00246-009-9589-9. [DOI] [PubMed] [Google Scholar]
- 28.Kim HJ, Yoo GH, Kil HR. Clinical outcome of acute myocarditis in children according to treatment modalities. Korean J Pediatr. 2010;53:745–752. doi: 10.3345/kjp.2010.53.7.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kishimoto C, Abelmann WH. In vivo significance of T cells in the development of Coxsackievirus B3 myocarditis in mice. Immature but antigen-specific T cells aggravate cardiac injury. Circ Res. 1990;67:589–598. doi: 10.1161/01.RES.67.3.589. [DOI] [PubMed] [Google Scholar]
- 30.Yen MH, et al. Viral load in blood is correlated with disease severity of neonatal coxsackievirus B3 infection: early diagnosis and predicting disease severity is possible in severe neonatal enterovirus infection. Clin Infect Dis. 2007;44:e78–81. doi: 10.1086/515399. [DOI] [PubMed] [Google Scholar]
- 31.Mofenson, L. M., Shearer, W. T., Moye, J., Nugent, R. & Willoughby, A. Manipulating the immune system with immune globulin. The National Institute of Child Health and Human Development. Intravenous Immunoglobulin Study Group. N Engl J Med326, 1636–1637; author reply 1637–1638 (1992). [PubMed]
- 32.Leung DY, Burns JC, Newburger JW, Geha RS. Reversal of lymphocyte activation in vivo in the Kawasaki syndrome by intravenous gammaglobulin. J Clin Invest. 1987;79:468–472. doi: 10.1172/JCI112835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Frustaci A, et al. Immunosuppressive therapy for active lymphocytic myocarditis: virological and immunologic profile of responders versus nonresponders. Circulation. 2003;107:857–863. doi: 10.1161/01.CIR.0000048147.15962.31. [DOI] [PubMed] [Google Scholar]
- 34.Orange JS, et al. Use of intravenous immunoglobulin in human disease: a review of evidence by members of the Primary Immunodeficiency Committee of the American Academy of Allergy, Asthma and Immunology. J Allergy Clin Immunol. 2006;117:S525–553. doi: 10.1016/j.jaci.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 35.Leong H, Stachnik J, Bonk ME, Matuszewski KA. Unlabeled uses of intravenous immune globulin. Am J Health Syst Pharm. 2008;65:1815–1824. doi: 10.2146/ajhp070582. [DOI] [PubMed] [Google Scholar]
- 36.Robinson, J., Hartling, L., Vandermeer, B. & Klassen, T. P. Intravenous immunoglobulin for presumed viral myocarditis in children and adults. Cochrane Database Syst Rev, CD004370, 10.1002/14651858.CD004370.pub3 (2015). [DOI] [PubMed]
- 37.Lee KJ, et al. Clinical outcomes of acute myocarditis in childhood. Heart. 1999;82:226–233. doi: 10.1136/hrt.82.2.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hooper JA. Intravenous immunoglobulins: evolution of commercial IVIG preparations. Immunol Allergy Clin North Am. 2008;28:765–778, viii. doi: 10.1016/j.iac.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Luzi G, Bongiorno F, Paparo Barbaro S, Bruno G. Intravenous IgG: biological modulating molecules. J Biol Regul Homeost Agents. 2009;23:1–9. [PubMed] [Google Scholar]
- 40.Pankuweit S, Klingel K. Viral myocarditis: from experimental models to molecular diagnosis in patients. Heart Fail Rev. 2013;18:683–702. doi: 10.1007/s10741-012-9357-4. [DOI] [PubMed] [Google Scholar]
- 41.Jensen LD, Marchant DJ. Emerging pharmacologic targets and treatments for myocarditis. Pharmacol Ther. 2016;161:40–51. doi: 10.1016/j.pharmthera.2016.03.006. [DOI] [PubMed] [Google Scholar]
- 42.Group JCSJW. Guidelines for diagnosis and treatment of myocarditis (JCS 2009): digest version. Circulation journal: official journal of the Japanese Circulation Society. 2011;75:734–743. doi: 10.1253/circj.CJ-88-0008. [DOI] [PubMed] [Google Scholar]
- 43.Group CTS. Effects of enalapril on mortality in severe congestive heart failure. Results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS) N Engl J Med. 1987;316:1429–1435. doi: 10.1056/NEJM198706043162301. [DOI] [PubMed] [Google Scholar]
- 44.Chen, H. S., Wang, W., Wu, S. N. & Liu, J. P. Corticosteroids for viral myocarditis. Cochrane Database Syst Rev, CD004471, 10.1002/14651858.CD004471.pub3 (2013). [DOI] [PMC free article] [PubMed]
- 45.Verboon-Maciolek MA, et al. Epidemiological survey of neonatal non-polio enterovirus infection in the Netherlands. Journal of medical virology. 2002;66:241–245. doi: 10.1002/jmv.2136. [DOI] [PubMed] [Google Scholar]
- 46.Mavridis D, Salanti G. Exploring and accounting for publication bias in mental health: a brief overview of methods. Evid Based Ment Health. 2014;17:11–15. doi: 10.1136/eb-2013-101700. [DOI] [PubMed] [Google Scholar]
- 47.Canter CE, Simpson KE. Diagnosis and treatment of myocarditis in children in the current era. Circulation. 2014;129:115–128. doi: 10.1161/CIRCULATIONAHA.113.001372. [DOI] [PubMed] [Google Scholar]