Abstract
Background: There is a continued debate and inconsistent findings in previous literature about the relationship of catechol-O-methyltransferase (COMT) and Parkinson’s disease (PD) susceptibility as well as cognitive dysfunction. To substantiate this existing gap, we comprehensively examine COMT genotype effects on the development of PD and test the hypothesis that the Met158 allele of the COMT gene is associated with cognitive dysfunction by conducting a meta-analysis review.
Methods: PubMed/MEDLINE, Embase, Cochrane databases search (18/30/08) yielded 49 included studies. Data were extracted by two reviewers and included COMT genotype, publication year, diagnostic status, ancestry, the proportion of male participants, and whether genotype frequencies were consistent with Hardy–Weinberg equilibrium. Unadjusted odds ratios (ORs) were used to derive pooled estimates of PD risk overall and in subgroups defined by ethnicity, gender, and onset of disease. Moreover, the association of certain cognitive domains in PD and COMT gene type was explored. Meta-analyses were performed using random-effect models and p value–based methods. All statistical tests were two-sided. The present study was registered with PROSPERO (CRD42018087323).
Results: In the current studies, we found no association between COMT Val158/108Met polymorphism and PD susceptibility. However, the gender-stratified analyses revealed marginally significant effects in heterozygote model analyses in women (P = 0.053). In addition, stratification according to onset of PD also shows significant effects of COMT Val158/108Met polymorphism on late-onset population both in recessive (P = 0.017) and allelic (P = 0.017) genetic models. For the intelligence quotient (IQ) score and Unified Parkinson Disease Rating Scale III (UPDRS III), there was no evidence for genetic association, except in subgroup analyses in Asian populations (IQ score, P = 0.016; UPDRS III, P < 0.001).
Conclusion: The COMT Val158/108Met polymorphism is associated with the risk for PD in female or late-onset PD. Methionine/methionine carriers of Asian population performed significantly worse than the valine allele carriers in IQ score and UPDRS III.
Keywords: catechol-O-methyltransferase, Parkinson’s disease, genetic association, cognitive dysfunction, meta-analysis
Introduction
Parkinson’s disease (PD) is a common neurodegenerative disorder that leads to a syndrome that is related to neurological control of movement as well as other brain functions including cognition (William, 2006). Due to the high prevalence and poor treatment, it is associated with an increasing socio-economic burden occupying a wide spectrum from minimal disability to marked impairment of capabilities with respect to independence, safety, and communication (Gómez-Esteban et al., 2007; LeWitt, 2008). The development of PD is mainly connected with aging. However, there have been other confirmed risk factors that influence the occurrence of PD.
First and foremost, protein pathological hypothesis displays complex and distinctive pathophysiological profiles: accumulation of aberrantly processed and misfolded proteins, such as amyloid- β (Aβ), tau, α-synuclein, TAR DNA-binding protein 43 (TDP43) (Dikic, 2017; Galluzzi et al., 2017), and mutant forms of Huntingtin (Htt) (Boland et al., 2018). The clearance of these proteins is impaired (Ciechanover and Kwon, 2017), which aggravates the pathological accumulation aberrantly and neurotoxic effects (Menzies et al., 2017). Next, oxidative stress and neuro-inflammation cause non-autonomous cell death due to complex multi-factors interaction, including α-synuclein accumulation, ubiquitin-proteasome system dysfunction, and metabolic disturbances (Duyckaerts et al., 2010), such as hyperammonemia (Choudhury and Borah, 2015). There is strong evidence that demonstrates that enteric nervous system is involved in PD pathological progression toward the central nervous system. Gut–brain axis suggests that PD starts in the enteric nervous system and spreads to olfactory bulb or brainstem, a process that antedates degeneration of the dopaminergic nigrostriatal system (Halliday et al., 2012; Goedert et al., 2013). Among the reasons for this are involved in interacting with the microenvironment [outer: microbiota (Mayer et al., 2015), metabolites, and nutrients (Holmqvist et al., 2014); inner: immune cells (Neunlist et al., 2014) and inflammatory cytokines] of the gut and specific living conditions (Gatto et al., 2009). In addition, the above several aspects are ascribed to the root of genetic susceptibility. Genome-wide association studies (GWAS) have identified numerous genetic variants associated with the highly penetrant autosomal dominant or recessive PD (Vila and Przedborski, 2004). Recent studies have shown that genetic variants can influence cognitive abilities. The ensuing molecular insights have contributed to substantial advances in our understanding of the pathogenesis of PD. Further mechanism studies have shown that NUS1 loss can reduce the number of dopaminergic neurons and the dopamine level, and induce apoptosis events in the brain of fruit flies (Guo et al., 2018).
Research on genetics, however, has always been hampered by inadequate sample size. Meanwhile, gene phenotypes might be better targets for exploring the etiology and pathophysiology of PD. Although the diagnosis of PD relies on the presence of motor deficits and clinical effects of dopamine therapy, this disease is closely related with non-motor symptoms and signs before the onset of the classical motor symptoms. Indeed, some evidence has suggested some of gene phenotypes are dominated by non-motor symptoms, such as cognitive impairment (Marras and Chaudhuri, 2016).
Catechol-O-methyltransferase (COMT) is involved in catecholamine degradation (Vidgren et al., 1994). The Val158/108Met single nucleotide polymorphism (SNP) in COMT gene has been extensively investigated in relation to PD, as well as the cognitive dysfunctions, including five aspects: executive function, attention and working memory, language, memory, and visuospatial function (Goetz et al., 2008). It contains a common functional SNP at codon 158/108 and generates a valine (Val)-to-methionine (Met) substitution (Val158Met), which results in a reduction of COMT enzyme activity and an increase in the dopamine level in the prefrontal cortex (Meyer-Lindenberg et al., 2005; Scheggia et al., 2018). The association between COMT genotype and cognition symptoms in subjects with PD has been checked, but the results are controversial. In their results, Barnett et al. (2008) did find a robust association between Val158Met and IQ. Healthy individuals with Val/Val have poorer scores on working memory (Aguilera et al., 2008). However, the other neurocognitive phenotype studies demonstrated substantial between-study heterogeneity. Dennis et al. reported that COMT val108/158met genotype has no effect on cognitive behavioral measures in healthy individuals but has an impact on neural activation patterns. Beyond that, Porter et al. (2019) found no previous associations between COMT Val158Met and cognitive performance in the cohort of cognitively normal older adults. In PD, many similar explorations focusing on genetic factors in cognitive decline were also made. COMT genotype was associated with attention but not with overall cognitive status (Bialecka et al., 2012; Morley et al., 2012). Certainly, some studies asserted that COMT Met/Met genotype could be a predictor of faster cognitive decline in PD (Paul et al., 2016).
To systematically synthesize these disparate studies, we attempted to synthesize the evidence regarding COMT Val158/108Met polymorphism and occurrence of PD, so much as PD with cognitive disorder through meta-analytic techniques. Therefore, the present study aimed to further our understanding of the effect of the COMT Val158/108Met variant on cognition function of PD. It would also be useful to reveal the mixed findings concerning COMT and PD in GWAS correlational research.
Methods and Materials
Systematic review and meta-analysis were performed using the preferred reporting items for systematic review and meta-analysis statement (PRISMA) (Moher et al., 2009). The protocol for the present study was registered with the PROSPERO registry (CRD42018087323).
Search Strategy
We searched major scientific databases including but not limited to PubMed, EMBASE, the Cochrane Library, Web of Science, the WanFang databases, SinoMed databases, and the CNKI up to November 2018 with different combinations of the following words: “Parkinson Disease,” “Idiopathic Parkinson Disease,” “catechol-O-methyltransferase” or “COMT,” “rs4680,” and “cognitive,” “cognition,” “cognition disorders,” “perception,” “memory,” “executive,” “dementia,” and “attention.” The search was restricted to English and Chinese language publications. We also obtained additional articles using reference lists of articles identified in the initial searches.
Inclusion and Exclusion Criteria
Qualified studies in this meta-analysis should satisfy the inclusion criteria:
1) case-control studies that assessed the relationship between COMT Val158/108Met polymorphism and PD susceptibility or reported the association between the COMT Val158/108Met and cognition in PD subjects regarding the association between COMT; 2) there were sufficient data of cases and controls to calculate an odd ratio (OR) with 95% confidence interval (CI); 3) the genotypes distribution in the control group was consistent with Hardy–Weinberg equilibrium (HWE); 4) the study was published in the English or the Chinese language; 5) if there were numerous studies from the same population, only the latest one was entered.
Studies were excluded for the major following reasons: 1) no PD cognitive data reported; 2) sample comprised patients with 22q11 deletion syndrome (who have only one copy of the COMT gene); 3) studies of a different COMT polymorphism, or overlap of reported data between papers; 4) animal trials, review, and the studies that did not report the genotype frequencies were ruled out.
Data Extraction
Data were independently extracted by two authors according to the inclusion and exclusion criteria listed above. Disagreements were resolved through discussion with a third author. The following information from included studies was extracted: first author, published year, country, ethnicity, sample size, average age of sample, number of male and female participants, allele frequency distribution in case and control, sample size, mean, and standard deviation for each cognitive variable by genotype group. COMT genotypes were grouped according to the presence or absence of the Val allele (Val/Val or Val/Met vs. Met/Met). The Newcastle-Ottawa Scale (NOS) was used to assess the methodological quality of each study (Stang, 2010).
Statistical Analyses
The HWE with an exact test was used to evaluate normal heterogeneity of the population. Six separate analyses as the allelic, recessive, dominant, homozygous, heterozygous, and additive genetic models were conducted in the present meta-analysis. Pooled odds ratios (ORs) with 95% confidence intervals (95% CIs) were used to compare the relationship between COMT polymorphism and PD risk, and standardized mean differences (SMDs) with 95% CIs were calculated for continuous variables. The omnibus (Q) tests and I2 test were used to examine the heterogeneity among the studies. If P > 0.10 or I2 < 50%, the fixed-effect model was adopted. Otherwise, the random-effect model would be used. We made a prior assumption that the meta-analysis can be affected by study level variability determined by different inclusion criteria across RCTs, which is more appropriately addressed by a random-effects model over a fixed-effect model. The pooled OR was assessed by Z test and P < 0.05 level was considered as statistically significant. Sensitivity analysis was carried out by sequentially omitting one study at a time to estimate the stability of the result. Subgroup analysis was performed to assess the influence of potential moderators. Potential publication bias was estimated using Egger’s linear regression test by visual inspection of the funnel plot. All analyses were performed using Stata 12.0.
Results
Study Characteristics
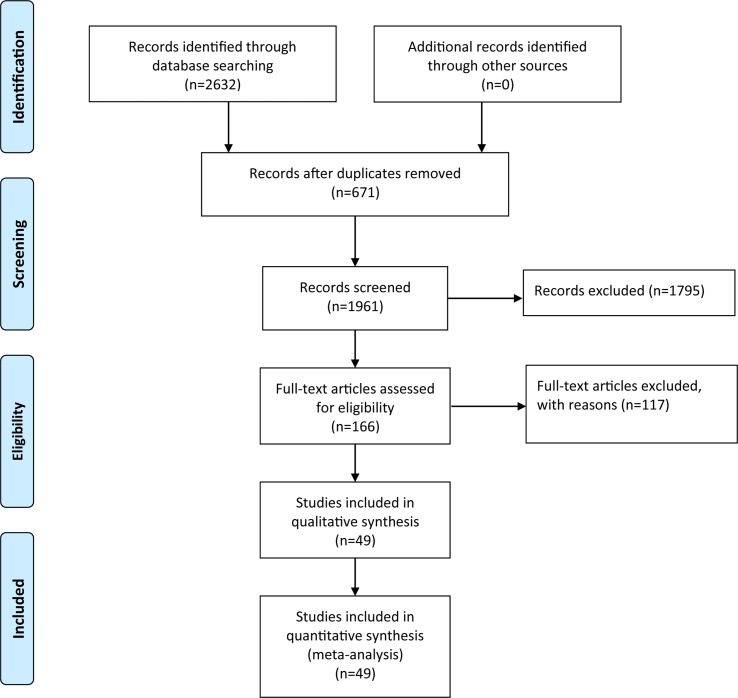
After the retrieval process, 49 relevant studies met the inclusion criteria, 35 (Hoda et al., 1996; Kunugi et al., 1997; Syvänen et al., 1997; Xie et al., 1997; Yoritaka et al., 1997; Mizuta et al., 2000; Lee et al., 2001; Wu et al., 2001; Eerola et al., 2002; Goudreau et al., 2002; Hernán et al., 2002; Xu et al., 2002; Lynch et al., 2003; Watanabe et al., 2003; Zhao et al., 2003; Shao et al., 2005; Bialecka et al., 2008; Kalinderi et al., 2008; Benitez et al., 2010; Rowe et al., 2010; Kiyohara et al., 2011; Bialecka et al., 2012; Torkaman-Boutorabi et al., 2012; Zeng, 2012; Klebe et al., 2013; Qi et al., 2013; Shih et al., 2013; Jin, 2014; Song et al., 2014; Wang, 2014; Song et al., 2015; Zhang et al., 2015; Paul et al., 2016; Qian et al., 2017; Ma et al., 2018) of which reported the association between COMT Val158/108Met polymorphism and the risk of PD, and a total of 11,773 patients and 17,046 controls were included in this section. Four (Bialecka et al., 2012; Wang, 2014; Dai et al., 2015; Li, 2016) studies reported the association between COMT Val158/108Met polymorphism and cognitive dysfunction in PD, whereas the other 10 (Williams-Gray et al., 2007; Williams-Gray et al., 2008; Hoogland et al., 2010; Morley et al., 2012; Wu et al., 2012; Fallon et al., 2015; Paul et al., 2016; Zhang et al., 2016; Xiao et al., 2017; Bäckström et al., 2018) articles mentioned the association between COMT Val158Met polymorphism and cognitive decline in PD including 1,547 PD patients. The selection process was shown in Figure 1 , and the main characteristics of the included studies are listed in Tables 1 – 3 .
Figure 1.
Flow diagram of articles selection process.
Table 1.
Characteristics of the included studies of the association between the COMT Val158Met polymorphism and PD susceptibility.
| First author | Year | Country | Number of | Mean age | Male/female | Case | Controls | HWE | NOS score | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| cases | control | cases | control | cases | control | V-V | V-M | M-M | V-V | V-M | M-M | cases | control | |||||
| Song et al. | 2015 | China | 221 | 229 | 66.88 | 66.74 | 134/87 | 122/107 | 100 | 100 | 21 | 107 | 97 | 25 | 0.577 | 0.669 | 7 | |
| Male | 134 | 122 | − | − | − | − | 63 | 59 | 12 | 57 | 52 | 13 | 0.730 | 0.824 | ||||
| Female | 87 | 107 | − | − | − | − | 37 | 41 | 9 | 50 | 45 | 12 | 0.631 | 0.698 | ||||
| Zhang et al. | 2015 | China | 437 | 530 | 62.4(11.4) | 61.6(8.7) | 230/207 | 318/212 | 251 | 173 | 13 | 291 | 210 | 29 | 0.009 | 0.262 | 6 | |
| Qi et al. | 2013 | China | 90 | 95 | 63.2(10.3) | 64.2(10.1) | 47/43 | 50/45 | 59 | 26 | 5 | 45 | 34 | 16 | 0.356 | 0.040 | 6 | |
| Early | 44 | 51 | − | − | − | − | 28 | 14 | 2 | 24 | 18 | 9 | 0.883 | 0.0004 | ||||
| Late | 46 | 43 | − | − | − | − | 31 | 12 | 3 | 21 | 16 | 7 | 0.246 | 0.206 | ||||
| Jin et al. | 2014 | China | 188 | 111 | 61.3(10.3) | 58.3(9.7) | 98/90 | 54/57 | 108 | 72 | 8 | 59 | 46 | 6 | 0.350 | 0.438 | 7 | |
| Early | 47 | 111 | − | − | − | − | 24 | 22 | 1 | 59 | 46 | 6 | 0.113 | 0.438 | ||||
| Late | 141 | 111 | − | − | − | − | 84 | 50 | 7 | 59 | 46 | 6 | 0.900 | 0.438 | ||||
| Ma et al. | 2018 | China | 152 | 252 | 67.9(9.5) | 67.8(10.8) | 77/75 | 130/122 | 87 | 57 | 8 | 145 | 90 | 17 | 0.734 | 0.553 | 6 | |
| Wang et al. | 2014 | China | 265 | 282 | − | 66.74(7.25) | − | 141/141 | 143 | 107 | 15 | 150 | 117 | 15 | 0.386 | 0.199 | 7 | |
| Zhao et al. | 2003 | China | 144 | 188 | 63.3(14) | 55.8(15.5) | 78/66 | 97/91 | 80 | 54 | 10 | 98 | 81 | 9 | 0.830 | 0.129 | 6 | |
| Song et al. | 2014 | China | 237 | 247 | − | − | 146/91 | 133/114 | 109 | 107 | 21 | 118 | 102 | 27 | 0.466 | 0.486 | 6 | |
| Late | 199 | 196 | − | − | − | − | 95 | 84 | 20 | 91 | 21 | 84 | 0.821 | 0.001 | ||||
| Male | 146 | 133 | − | − | − | − | 67 | 67 | 12 | 64 | 54 | 15 | 0.400 | 0.486 | ||||
| Female | 91 | 114 | − | − | − | − | 42 | 40 | 9 | 54 | 48 | 12 | 0.907 | 0.784 | ||||
| Zen et al. | 2012 | China | 94 | 104 | 65.12(10.2) | 67.06(10.28) | 58/36 | 60/44 | 33 | 45 | 16 | 40 | 50 | 14 | 0.921 | 0.794 | 7 | |
| Male | 58 | 60 | − | − | − | − | 22 | 30 | 6 | 25 | 27 | 8 | 0.362 | 0.868 | ||||
| Female | 36 | 44 | − | − | − | − | 11 | 15 | 10 | 15 | 23 | 6 | 0.319 | 0.546 | ||||
| Xiao et al. | 2017 | China | 143 | 157 | 65.1(8.7) | 65.4(7.2) | 79/64 | 88/69 | 79 | 56 | 8 | 91 | 57 | 9 | 0.637 | 0.985 | 7 | |
| Early | 24 | 157 | − | − | − | − | 8 | 15 | 1 | 91 | 57 | 9 | 0.073 | 0.985 | ||||
| Late | 119 | 157 | − | − | − | − | 71 | 41 | 7 | 91 | 57 | 9 | 0.739 | 0.985 | ||||
| Eerolaa et al. | 2002 | Finland | 147 | 137 | 67.2 | 65.8 | 87/60 | 50/87 | 30 | 71 | 46 | 31 | 71 | 35 | 0.786 | 0.595 | 7 | |
| Watanabea et al. | 2003 | Japan | 121 | 100 | 68.2 | 64(9) | 49/72 | 85/15 | 57 | 47 | 14 | 47 | 49 | 4 | 0.377 | 0.043 | 7 | |
| Toraman-Boutorabi et al. | 2012 | Iran | 108 | 70 | 57.46(10.35) | 55.73(11.69) | 72/31 | 42/28 | 20 | 50 | 33 | 19 | 32 | 19 | 0.892 | 0.473 | 7 | |
| Male | 72 | 50 | − | − | − | − | 13 | 38 | 21 | 12 | 19 | 11 | 0.560 | 0.539 | ||||
| Female | 31 | 28 | − | − | − | − | 7 | 12 | 12 | 7 | 13 | 8 | 0.253 | 0.710 | ||||
| Yoritaka et al. | 1997 | Japan | 176 | 156 | 62.7(8.8) | 57.9(16.2) | − | − | 100 | 62 | 14 | 69 | 77 | 10 | 0.323 | 0.058 | 6 | |
| Benitez et al. | 2010 | Colombia | 104 | 136 | 60.1(12.6) | 62.4(9.5) | 61/43 | 63/73 | 47 | 39 | 17 | 52 | 68 | 13 | 0.800 | 0.171 | 7 | |
| Xie et al. | 1997 | China | 70 | 62 | 64.3(9.96) | 63.9(9.86) | 31/39 | 24/38 | 44 | 21 | 5 | 37 | 19 | 6 | 0.277 | 0.150 | 7 | |
| Syvanen et al. | 1997 | Finland | 158 | 76 | − | − | 87/71 | 36/40 | 39 | 80 | 39 | 14 | 35 | 27 | 0.874 | 0.655 | 6 | |
| Kiyohara et al. | 2011 | Japan | 238 | 369 | 68.5(1.1) | 66.6(0.85) | 91/147 | 140/228 | 98 | 116 | 24 | 179 | 166 | 24 | 0.222 | 0.076 | 6 | |
| Lee et al. | 2001 | Finland | 73 | 49 | 62 | − | 33/40 | − | 40 | 27 | 6 | 26 | 21 | 2 | 0.636 | 0.371 | 6 | |
| Goudreau et al. | 2002 | USA | 319 | 196 | − | − | 197/122 | 74/122 | 59 | 163 | 84 | 50 | 82 | 55 | 0.205 | 0.094 | 7 | |
| Male | 191 | 72 | − | − | − | − | 39 | 99 | 53 | 17 | 37 | 18 | 0.559 | 0.812 | ||||
| Female | 115 | 115 | − | − | − | − | 20 | 64 | 31 | 33 | 45 | 37 | 0.186 | 0.203 | ||||
| Early | 164 | 82 | − | − | − | − | 26 | 89 | 49 | 28 | 37 | 17 | 0.170 | 0.463 | ||||
| Late | 142 | 105 | − | − | − | − | 33 | 74 | 35 | 22 | 45 | 38 | 0.613 | 0.209 | ||||
| Kunugi et al. | 1997 | Japan | 109 | 153 | − | − | 55/54 | 75/78 | 46 | 47 | 16 | 74 | 70 | 9 | 0.485 | 0.149 | 6 | |
| Hoda et al. | 1996 | UK | 139 | 173 | − | − | − | − | 32 | 70 | 37 | 40 | 88 | 45 | 0.920 | 0.811 | 6 | |
| Klebe et al. | 2013 | France | 5886 | 10723 | 57.6(13.8) | 60.0(10.1) | 3532/2354 | 5388/5335 | 1417 | 2911 | 1558 | 2526 | 5303 | 2894 | 0.429 | 0.313 | 8 | |
| Lynch et al. | 2002 | USA | 100 | 66 | 60.6 | 46.5 | 71/29 | 30/36 | 29 | 45 | 25 | 20 | 30 | 16 | 0.374 | 0.477 | 7 | |
| Hernán et al. | 2002 | USA | 213 | 439 | − | − | − | − | 50 | 106 | 57 | 112 | 220 | 107 | 0.958 | 0.960 | 6 | |
| Male | 101 | 203 | − | − | − | − | 26 | 44 | 31 | 50 | 102 | 51 | 0.203 | 0.944 | ||||
| Female | 112 | 236 | − | − | − | − | 24 | 62 | 26 | 62 | 118 | 56 | 0.255 | 0.487 | ||||
| Mizuta et al. | 2000 | Japan | 171 | 199 | 66.0(8.0) | 67.5(11.7) | − | − | 85 | 71 | 15 | 88 | 90 | 21 | 0.975 | 0.776 | 7 | |
| Shih et al. | 2013 | China | 260 | 100 | 71.4(0.787) | − | 137/123 | − | 100 | 90 | 70 | 30 | 46 | 24 | 0.001 | 0.443 | 6 | |
| Kalinderi et al. | 2008 | Greece | 134 | 125 | 61.7 | 71.7 | − | − | 48 | 54 | 32 | 40 | 49 | 36 | 0.035 | 0.016 | 7 | |
| Paul et al. | 2016 | USA | 341 | 483 | − | − | − | − | 83 | 174 | 84 | 127 | 229 | 127 | 0.704 | 0.255 | 7 | |
| Bialecka et al. | 2008 | Poland | 322 | 357 | 64.0(10.2) | 72.5(9.7) | 191/131 | 207/150 | 78 | 160 | 84 | 80 | 166 | 111 | 0.916 | 0.234 | 8 | |
| Early | 101 | 357 | − | − | − | − | 24 | 46 | 31 | 80 | 166 | 111 | 0.394 | 0.234 | ||||
| Late | 221 | 357 | − | − | − | − | 54 | 114 | 53 | 80 | 166 | 111 | 0.637 | 0.234 | ||||
| Białecka et al. | 2012 | Poland | 57 | 64 | − | − | − | − | 38 | 15 | 4 | 38 | 23 | 3 | 0.167 | 0.839 | 6 | |
| Wu et al. | 2001 | China | 222 | 191 | 67.2(9.1) | 65.8(9.2) | 162/62 | 145/52 | 125 | 79 | 18 | 117 | 62 | 12 | 0.277 | 0.336 | 6 | |
| Early | 37 | 34 | − | − | − | − | 25 | 16a | 33 | 15a | − | − | ||||||
| Late | 187 | 163 | − | − | − | − | 100 | 81a | 86 | 57a | − | − | ||||||
| Male | 160 | 140 | − | − | − | − | 32 | 30a | 28 | 23a | − | − | ||||||
| Female | 62 | 61 | − | − | − | − | 93 | 67 | 91 | 49a | − | − | ||||||
| Rowe et al. | 2010 | UK | 50 | 82 | 64.9(9.0) | 66.2(7.3) | − | − | 16 | 18 | 15 | 22 | 36 | 22 | 0.064 | 0.371 | 7 | |
| Shao et al. | 2005 | China | 140 | 144 | − | − | 80/60 | 74/70 | 84 | 41 | 15 | 75 | 62 | 7 | 0.007 | 0.264 | 7 | |
| Xu et al. | 2002 | China | 144 | 201 | − | − | − | − | 80 | 54 | 10 | 105 | 86 | 10 | 0.830 | 0.149 | 7 | |
| Early | 47 | 161 | − | − | − | − | 29 | 17 | 1 | 84 | 68 | 9 | 0.405 | 0.317 | ||||
| Late | China | 97 | 40 | − | − | − | − | 51 | 37 | 9 | 21 | 18 | 1 | 0.547 | 0.206 | |||
HWE, Hardy-Weinberg equilibrium, a; GA plus AA.
Table 3.
Characteristics of the included studies of the association between the COMT Val158Met and neuro-cognition in PD.
| Author | Year | Country | Subjects | Ethnicity | %Male | Age | Met/Met | Val/Met | Val/Val | Phenotype | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M | SD | n | M | SD | n | M | SD | n | ||||||||
| UPDRS | ||||||||||||||||
| Wu | 2012 | UK | PD | Caucasians | 65 | 63 | 25.60 | 8.50 | 10 | — | — | — | 26.80 | 12.10 | 10 | UPDRS |
| Xiao | 2017 | CHINA | PD | Asian | — | 58 | 31.00 | 11.90 | 8 | 22.40 | 12.10 | 56 | 25.80 | 11.90 | 79 | UPDRS |
| Morley | 2012 | USA | PD | Caucasians | 74 | 71 | 24.00 | 11.00 | 56 | — | — | — | 22.00 | 11.00 | 156 | UPDRS |
| Fallon | 2015 | Netherlands | PD | Caucasians | 67 | 64 | 31.00 | 11.00 | 108 | 33.00 | 12.00 | 184 | 31.00 | 9.00 | 80 | UPDRS |
| Williams-Gray | 2007 | UK | PD | Caucasians | 65 | 65 | 26.10 | 9.40 | 16 | — | — | — | 22.50 | 10.50 | 16 | UPDRS |
| Zhang | 2016 | CHINA | PD | Asian | 58 | 60 | 30.47 | 12.28 | 8 | 30.00 | 8.75 | 105 | 27.26 | 14.05 | 137 | UPDRS |
| Williams-Gray | 2008 | UK | PD | Caucasians | 62 | 64 | 26.80 | 10.00 | 13 | — | — | — | 23.00 | 10.80 | 16 | UPDRS |
| Bäckström | 2017 | Sweden | PD | Caucasians | 59 | 69 | 24.94 | 17.65 | 42 | 25.7 | 12.13 | 64 | 30.07 | 10.19 | 26 | UPDRS |
| Paul | 2016 | USA | PD | Caucasians | 55 | 66 | 20.2 | 9.3 | 63 | — | — | — | 18.8 | 9.1 | 168 | UPDRS |
| Hoogland | 2010 | The Netherlands | PD | Caucasians | 54 | 66 | 20.00 | 9.00 | 37 | 20 | 10 | 84 | 18.00 | 9.00 | 32 | UPDRS |
| MMSE | ||||||||||||||||
| Wu | 2012 | UK | PD | Caucasians | 65 | 63 | 29.60 | 0.50 | 10 | — | — | — | 29.60 | 0.70 | 10 | MMSE |
| Fallon | 2015 | Netherlands | PD | Caucasians | 67 | 64 | 28.00 | 2.00 | 108 | 29.00 | 1.00 | 184 | 28.00 | 2.00 | 80 | MMSE |
| Williams-Gray | 2007 | UK | PD | Caucasians | 65 | 65 | 28.70 | 0.80 | 16 | — | — | — | 28.90 | 1.20 | 16 | MMSE |
| Zhang | 2016 | CHINA | PD | Asian | 58 | 60 | 26.75 | 3.45 | 8 | 26.74 | 3.50 | 105 | 26.93 | 3.03 | 137 | MMSE |
| Williams-Gray | 2008 | UK | PD | Caucasians | 62 | 64 | 28.80 | 0.70 | 13 | — | — | — | 28.90 | 1.20 | 16 | MMSE |
| Bäckström | 2017 | Sweden | PD | Caucasians | 59 | 69 | 28.60 | 1.40 | 42 | 28.70 | 1.40 | 64 | 28.70 | 1.30 | 26 | MMSE |
| Paul | 2016 | USA | PD | Caucasians | 55 | 66 | 28.20 | 2.60 | 63 | — | — | — | 28.30 | 1.90 | 168 | MMSE |
| Combined IQ | ||||||||||||||||
| Wu | 2012 | UK | PD | Caucasians | 65 | 63 | 120.20 | 4.40 | 10 | — | — | — | 114.30 | 8.20 | 10 | NART |
| Fallon | 2015 | Netherlands | PD | Caucasians | 67 | 64 | 102.00 | 20.00 | 108 | 103.00 | 18.00 | 184 | 103.00 | 18.00 | 80 | NART |
| Williams-Gray | 2007 | UK | PD | Caucasians | 65 | 65 | 114.70 | 6.20 | 16 | — | — | — | 113.10 | 7.30 | 16 | NART |
| Zhang | 2016 | CHINA | PD | Asian | 58 | 60 | 92.56 | 16.90 | 8 | 97.99 | 14.94 | 105 | 100.88 | 14.84 | 137 | WAIS-VIQ |
| Zhang | 2016 | CHINA | PD | Asian | 58 | 60 | 83.19 | 18.39 | 8 | 94.98 | 14.24 | 105 | 95.71 | 12.93 | 137 | WAIS-PIQ |
| Zhang | 2016 | CHINA | PD | Asian | 58 | 60 | 85.24 | 18.29 | 8 | 95.74 | 14.94 | 105 | 97.68 | 14.16 | 137 | WAIS-FIQ |
| Williams-Gray | 2008 | UK | PD | Caucasians | 62 | 64 | 114.50 | 6.60 | 13 | — | — | — | 114.20 | 6.90 | 16 | NART |
| Hoogland | 2010 | The Netherlands | PD | Caucasians | 54 | 66 | 105.00 | 17.00 | 37 | 100 | 18 | 84 | 102.00 | 20.00 | 32 | NART |
UPDRS, Unified Parkinson’s Disease Rating Scale; MMSE, Mini Mental State Examination; NART, National Adult Reading Test; WAIS, Wechsler Adult Intelligence Scale; VIQ, Verbal Intelligence Quotient; FIQ, Full Intelligence Quotient; PIQ, Performance Intelligence Quotient.
As shown in Table 1 , 15 studies were performed on Caucasians. Of the 35 studies, 20 focused only on Asian. Six studies explored the relationships of alleles or genotypes with age at disease onset. Meanwhile, six studies explored the relationships with gender. Nevertheless, three studies were deviated according to HWE of genotype frequencies among the controls.
As shown in Table 2 , four case-control studies reported the association between COMT polymorphism and cognitive dysfunction in PD, including 890 PD-NC (PD, no cognitive impairment) and 643 PD-CI (PD, cognitive impairment includes mild cognitive impairment and dementia of PD) . Three studies involved the Chinese population. In addition, one was from Poland. The genotype frequencies in all studies fully complied with HWE.
Table 2.
Characteristics of the included studies of the association between the COMT Val158Met polymorphism and cognitive dysfunction in PD.
| First author | Year | Country | Number of | Mean age | Male/female | PD-NC | PD-CI | HWE | NOS score | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PD-NC | PD-CI | V-V | V-M | M-M | V-V | V-M | M-M | PD-CN | PD-CI | ||||||
| Dai | 2015 | China | 702 | 385 | 63.0 | 631/456 | 344 | 291 | 67 | 192 | 162 | 33 | 0.632 | 0.887 | 7 |
| Li | 2014 | China | 68 | 71 | 59.64(11.61) | 78/61 | 35 | 28 | 5 | 40 | 24 | 7 | 0.852 | 0.246 | 7 |
| Wang | 2014 | China | 91 | 75a | 66.39 | —— | 52 | 32 | 7 | 34 | 36 | 5 | 0.510 | 0.264 | 7 |
| Wang | 2014 | China | 91 | 99b | 66.39 | —— | 52 | 32 | 7 | 57 | 39 | 3 | 0.510 | 0.226 | 6 |
| Białecka | 2012 | Poland | 29 | 13 | —— | —— | 20 | 5 | 4 | 9 | 4 | 0 | 0.006 | 0.512 | 7 |
PD-NC, PD with no cognitive impairment; PD-CI, cognitive impairment in PD; a, mild cognitive impairment in PD; b, dementia in PD.
Table 3 identifies 10 studies included in the meta-analytic to compare cognitive effects between groups of Met homozygotes and Val carriers with PD on cognitive scores. The table includes information about sample characteristics (gender distribution, age, genotype frequencies, ethnicity, etc.) and measures administered.
Meta-Analysis of the Association Between COMT Val158/108Met and PD Susceptibility
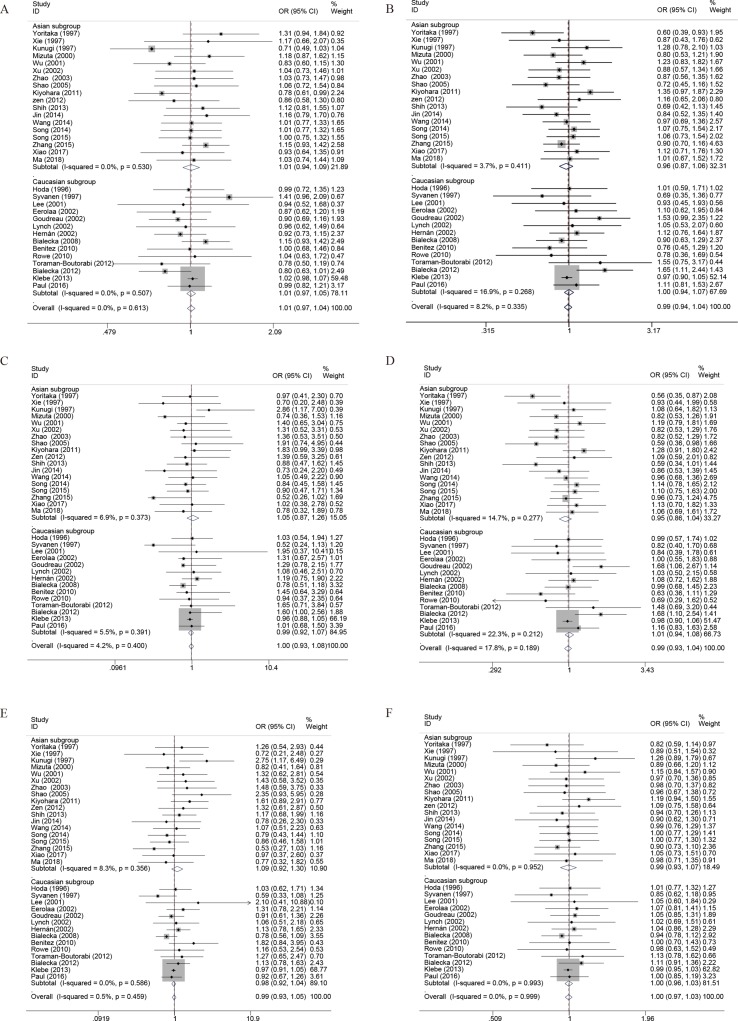
Overall, there were 11,428 cases and 16,726 controls included in the analysis. The ORs and 95% CIs in random- and fixed-effect models were calculated according to the values of Q test and I2. There was no significant association between COMT Val158/108Met polymorphism and PD susceptibility in the whole population under allelic (OR, 1.01; 95% CI, 0.97–1.04; P = 0.667), recessive (OR, 0.99; 95% CI, 0.93–1.05; P = 0.792), dominant (OR, 0.99; 95% CI, 0.94–1.04; P = 0.671), homozygous (OR, 1.00; 95% CI, 0.93–1.08; P = 0.985), heterozygous (OR, 0.99; 95% CI, 0.93–1.04; P = 0.655), and additive genetic models (OR, 1.00; 95% CI, 0.97–1.03; P = 0.790) ( Figure 2 , Table 4 ). To investigate the exact consequence of the relationship between COMT polymorphism and PD susceptibility, subgroup analyses by ethnicity were performed. No significant association was found in any subgroup under different genetic models between PD risk and COMT genotype ( Figure 2 , Table 4 ).
Figure 2.
Forest plots for the association between COMT Val158/108Met polymorphism and PD risk. (A) Addictive genetic model (A vs G). (B) Dominant genetic model (AA +GA vs GG). (C) Homozygous model (AA vs GG). (D) Heterozygous model (GA vs. GG). (E) Recessive genetic model (AA vs. GG + GA). (F) Allelic genetic model (A allele distribution frequency of COMT Val158Met gene polymorphism).
Table 4.
Summary of meta-analysis of association between COMT Val158/108Met polymorphism and PD risk.
| Genetic model | Pooled OR (95% CI) | Z value | P value | PHeterogeneity (I2%) | Publication bias | |
|---|---|---|---|---|---|---|
| Egger”s test(t) | P value | |||||
| Allelic genetic model | ||||||
| Ethnicity | ||||||
| Asian subgroup | 1.01(0.94,1.09) | 0.21 | 0.833 | 0.530(0.0%) | 0.00 | 0.998 |
| Caucasian subgroup | 1.01(0.97,1.05) | 0.38 | 0.708 | 0.507(0.0%) | −0.39 | 0.291 |
| Gender | ||||||
| Male | 0.95(0.80,1.11) | 0.67 | 0.502 | 0.942(0.0%) | −1.12 | 0.396 |
| Female | 0.90(0.75,1.07) | 1.25 | 0.213 | 0.962(0.0%) | −1.12 | 0.112 |
| Onset of PD | ||||||
| Early | 0.87(0.62,1.23) | 0.78 | 0.436 | 0.028(63.1%) | 0.01 | 0.998 |
| Late | 1.20(1.03,1.40) | 2.38 | 0.017 | 0.435(0.0%) | 0.38 | 0.792 |
| Recessive genetic model | ||||||
| Ethnicity | ||||||
| Asian subgroup | 1.09(0.92,1.30) | 0.98 | 0.326 | 0.356(8.3%) | 0.69 | 0.564 |
| Caucasian subgroup | 0.98(0.92,1.04) | 0.63 | 0.527 | 0.586(0.0%) | 0.36 | 0.302 |
| Gender | ||||||
| Male | 1.04(0.78,1.40) | 0.28 | 0.776 | 0.778(0.0%) | −2.09 | 0.076 |
| Female | 1.00(0.74,1.36) | 0.03 | 0.980 | 0.566(0.0%) | 1.94 | 0.107 |
| Onset of PD | ||||||
| Early | 1.07(0.75,1.52) | 0.36 | 0.716 | 0.423(0.0%) | −0.98 | 0.263 |
| Late | 0.71(0.54,0.94) | 2.38 | 0.017 | 0.457(0.0%) | 0.79 | 0.362 |
| Dominant genetic model | ||||||
| Ethnicity | ||||||
| Asian subgroup | 0.96(0.87,1.06) | 0.80 | 0.423 | 0.411(3.7%) | −0.80 | 0.483 |
| Caucasian subgroup | 1.00(0.94,1.07) | 0.03 | 0.973 | 0.268(16.9%) | 0.41 | 0.333 |
| Gender | ||||||
| Male | 1.07(0.86,1.32) | 0.59 | 0.555 | 0.979(0.0%) | 0.99 | 0.148 |
| Female | 1.21(0.97,1.52) | 1.71 | 0.087 | 0.865(0.0%) | −0.52 | 0.613 |
| Onset of PD | ||||||
| Early | 1.28(0.98,1.68) | 1.79 | 0.073 | 0.016(64.0%) | 3.04 | 0.465 |
| Late | 0.91(0.74,1.10) | 0.99 | 0.323 | 0.550(0.0%) | −1.96 | 0.198 |
| Homozygous genetic model | ||||||
| Ethnicity | ||||||
| Asian subgroup | 1.05(0.87,1.26) | 0.53 | 0.594 | 0.373(6.9%) | 0.77 | 0.554 |
| Caucasian subgroup | 0.99(0.92,1.08) | 0.21 | 0.836 | 0.391(5.5%) | 0.56 | 0.134 |
| Gender | ||||||
| Male | 1.07(0.75,1.50) | 0.36 | 0.720 | 0.822(0.0%) | −0.15 | 0.926 |
| Female | 1.26(0.87,1.81) | 1.24 | 0.216 | 0.917(0.0%) | 0.74 | 0.455 |
| Onset of PD | ||||||
| Early | 1.25(0.82,1.92) | 1.04 | 0.297 | 0.064(55.1%) | −0.96 | 0.546 |
| Late | 0.73(0.52,1.02) | 1.87 | 0.062 | 0.493(0.0%) | 0.59 | 0.556 |
| Heterozygous genetic model | ||||||
| Ethnicity | ||||||
| Asian subgroup | 0.95(0.86,1.04) | 1.11 | 0.267 | 0.277(14.7%) | −1.44 | 0.211 |
| Caucasian subgroup | 1.01(0.94,1.08) | 0.23 | 0.819 | 0.212(22.3%) | 0.31 | 0.477 |
| Gender | ||||||
| Male | 1.11(0.86,1.43) | 0.81 | 0.415 | 0.807(0.0%) | 1.77 | 0.196 |
| Female | 1.32(1.00,1.74) | 1.93 | 0.053 | 0.517(0.0%) | −0.93 | 0.569 |
| Onset of PD | ||||||
| Early | 1.30(0.97,1.75) | 1.73 | 0.084 | 0.019(66.0%) | 5.67 | 0.329 |
| Late | 0.89(0.71,1.13) | 0.95 | 0.343 | 0.770(0.0%) | −1.58 | 0.201 |
| Additive genetic model | ||||||
| Ethnicity | ||||||
| Asian subgroup | 0.99(0.93,1.07) | 0.18 | 0.861 | 0.952(0.0%) | −0.05 | 0.958 |
| Caucasian subgroup | 1.00(0.96,1.03) | 0.21 | 0.832 | 0.993(0.0%) | 0.22 | 0.273 |
| Gender | ||||||
| Male | 1.03(0.89,1.19) | 0.38 | 0.702 | 0.995(0.0%) | 0.28 | 0.713 |
| Female | 1.06(0.91,1.24) | 0.75 | 0.453 | 0.997(0.0%) | 0.60 | 0.150 |
| Onset of PD | ||||||
| Early | 1.07(0.90,1.27) | 0.77 | 0.442 | 0.325(14.0%) | 0.03 | 0.989 |
| Late | 0.90(0.79,1.03) | 1.50 | 0.134 | 0.752(0.0%) | −0.47 | 0.614 |
Furthermore, to better explore the role of COMT in PD, we performed the subgroup analysis by the gender and onset of PD. As noted previously, a total of six case-control studies have been reported regarding the association between COMT polymorphism and gender of PD susceptibility. In the gender subgroup, no significant association was detected under all the genetic models, except that a borderline significant association was detected of female PD in the heterozygous genetic models (OR, 1.32; 95% CI, 1.00–1.74; P = 0.053) in the pooled populations ( Figure 3 , Table 4 ). In addition, the pooled analyses indicated that there was a significant association between COMT polymorphism and late onset of PD susceptibility under recessive (OR, 0.71; 95% CI, 0.54–0.94; P = 0.017) and allelic genetic models (OR, 1.20; 95% CI, 1.03–1.40; P = 0.017) ( Figure 4 , Table 4 ).
Figure 3.
Forest plots for the association between COMT Val158/108Met polymorphism and PD risk-stratified by gender. (A) Addictive genetic model (A vs G). (B) Dominant genetic model (AA +GA vs GG). (C) Homozygous model (AA vs GG). (D) Heterozygous model (GA vs. GG). (E) Recessive genetic model (AA vs. GG + GA). (F) Allelic genetic model (A allele distribution frequency of COMT Val158Met gene polymorphism).
Figure 4.
Forest plots for the association between COMT Val158/108Met polymorphism and PD risk-stratified by onset of PD. (A) Addictive genetic model (A vs G). (B) Dominant genetic model (AA +GA vs GG). (C) Homozygous model (AA vs GG). (D) Heterozygous model (GA vs. GG). (E) Recessive genetic model (AA vs. GG + GA). (F) Allelic genetic model (A allele distribution frequency of COMT Val158Met gene polymorphism).
Sensitivity analysis was carried out for this meta-analysis by omitting one study at a time to assess the influence of any single study. When removing one study at the time, there was no significant change in the pooled ORs.
The publication bias of the studies was evaluated by Egger’s test. The results of Egger’s test also suggested that the possibility of publication bias was low ( Table 4 ).
Meta-Analysis of the Association Between COMT Val158/108Met and PD-CI Susceptibility
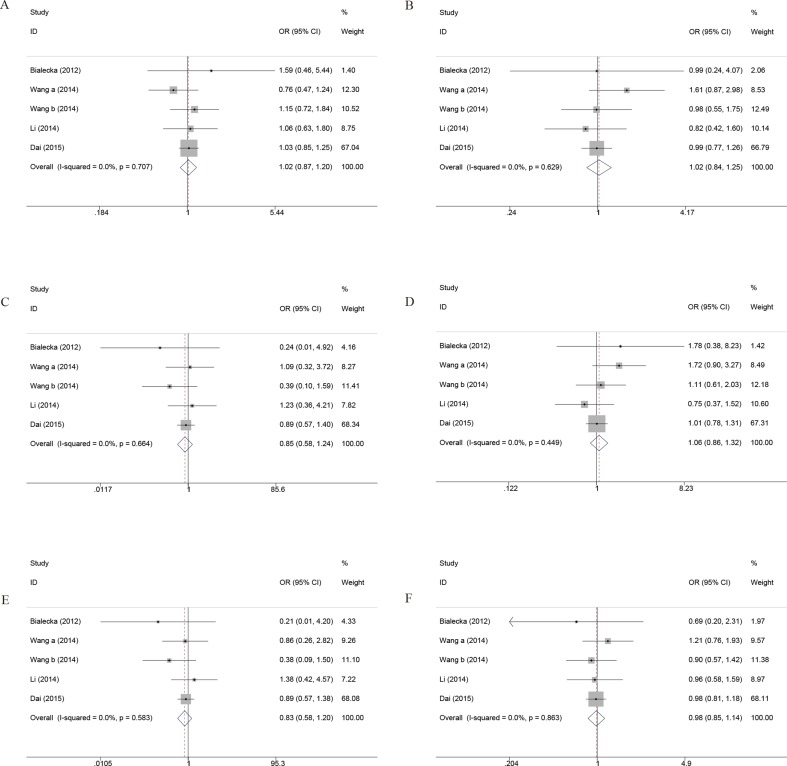
From the four included studies, we pooled data from 1,533 subjects, including 890 PN-NC and 643 PD-CI. There was no significant association between COMT Val158/108Met polymorphism and PD in the whole population under allelic (OR, 1.02; 95% CI, 0.87–1.20; P = 0.788), recessive (OR, 0.83; 95% CI, 0.58–1.20; P = 0.334), dominant (OR, 1.02; 95% CI, 0.84–1.25; P = 0.831), homozygous (OR, 0.85; 95% CI, 0.58–1.24; P = 0.402), heterozygous (OR, 1.06; 95% CI, 0.86–1.32; P = 0.561), and additive genetic models (OR, 0.98; 95% CI, 0.85–1.14; P = 0.836) ( Figure 5 ).
Figure 5.
Forest plots for the association between COMT Val158/108Met polymorphism and cognitive impairment in PD. (A) Addictive genetic model (A vs G). (B) Dominant genetic model (AA +GA vs GG). (C) Homozygous model (AA vs GG). (D) Heterozygous model (GA vs. GG). (E) Recessive genetic model (AA vs. GG + GA). (F) Allelic genetic model (A allele distribution frequency of COMT Val158Met gene polymorphism). a, PD-NC vs PD-MCI; b, PD-NC vs PDD.
Sensitivity analysis was carried out for this meta-analysis by omitting one study at a time to assess the influence of any single study and found no significant change in the pooled ORs. The publication bias of the studies was evaluated by Egger’s test. The results of Egger’s test also suggested that the possibility of publication bias was low (data not shown).
Meta-Analysis of the Cognitive Effects of the COMT Val158/108Met Polymorphism in PD
UPDRS III
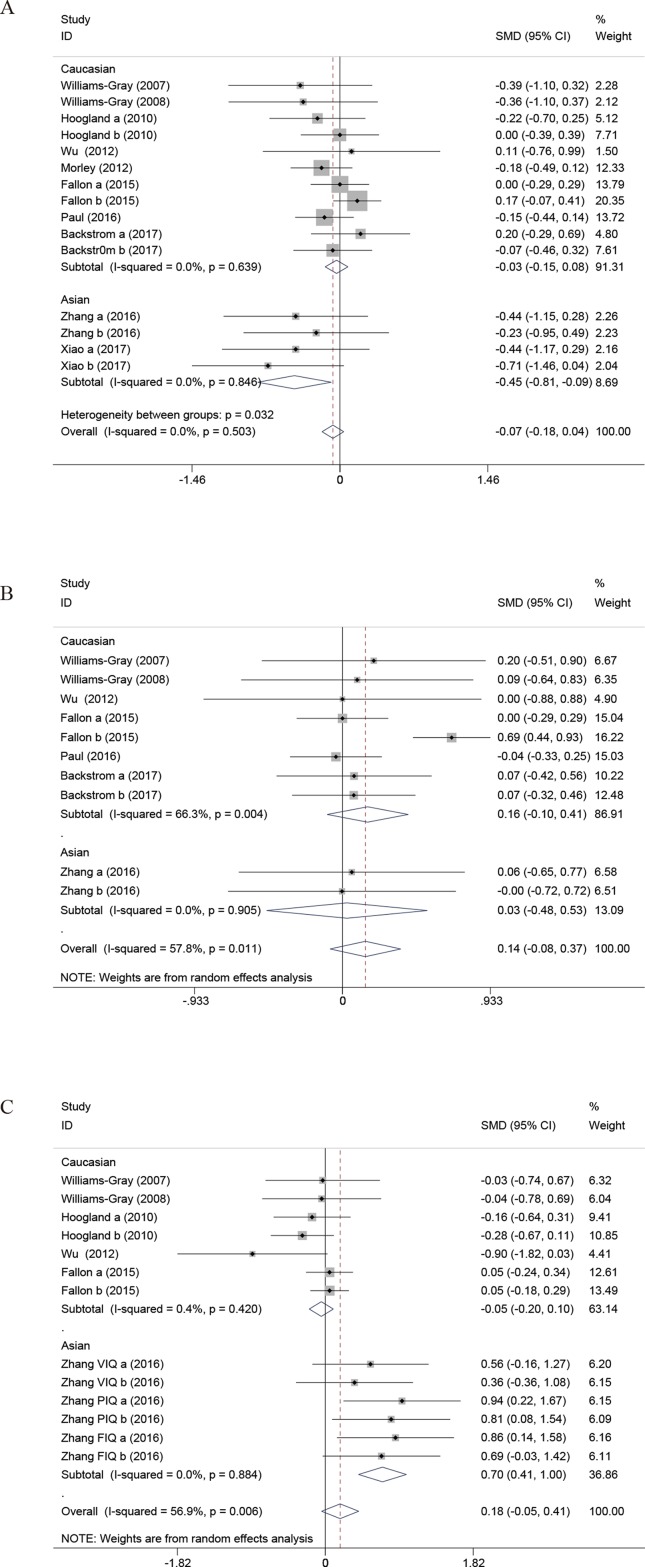
Ten independent studies with 1,574 PD patients contributed to the meta-analysis. There was no evidence of between-study heterogeneity (Q-statistic = 13.30; P = 0.503; I 2 = 0%), and random effects analysis indicated no evidence of association (SMD, −0.07; 95% CI, −0.18; 0.04; P = 0.206) ( Figure 6A ).
Figure 6.
The forest plot for differences between Val carriers and Met homozygotes on the cognitive effects. (A) UPDRS III. (B) MMSE. (C) IQ Score. UPDRS, Unified Parkinson’s Disease Rating Scale; MMSE, Mini Mental State Examination; CI, confidence interval; OR, odds ratio. a, Val/Val vs Met/Met; b, Val/Met vs Met/Met.
The results of Egger’s test also suggested that the possibility of publication bias was high (P = 0.017). However, a subgroup analysis comparing the Asians versus Caucasians was significant, indicating that homozygotes for the Met158 allele have higher UPDRS III scores than the Val allele carriers in Asian population (SMD, −0.45; 95% CI, −0.81 to −0.09; P = 0.016). The publication bias of the studies was evaluated by Egger’s test. The results of Egger’s test also suggested that publication bias was low in Asians (P = 0.169) or Caucasians (P = 0.259).
MMSE
Seven independent samples with 835 PD patients contributed to the meta-analysis. There was evidence of between-study heterogeneity (Q-statistic = 21.32; P = 0.011; I2 = 57.8%), and random effects analysis indicated no evidence of association (SMD, 0.14; 95% CI, −0.08 to 0.37; P = 0.203). The results of Egger’s test also suggested that the possibility of publication bias was low (P = 0.306). In addition, subgroup analysis by ethnicity indicated that no association was detected in Asian population or Caucasian population ( Figure 6B ).
IQ Score
Six independent samples with 856 PD patients contributed to the meta-analysis. Combined IQ score included the National Adult Reading Test (NART) and Wechsler Adult Intelligence Scale (WAIS). There was evidence of between-study heterogeneity (Q statistic = 27.82, P = 0.006, I 2 = 56.9%), and random effects analysis indicated no evidence of association (SMD, 0.18; 95% CI, −0.05 to 0.41; P = 0.116).
The results of Egger’s test also suggested that the possibility of publication bias was low (P = 0.206), although subgroup analysis by ethnicity detected that homozygotes for the Met158 allele have lower IQ scores than the Val allele carriers in Asian population (SMD, 0.70; 95% CI, 0.41–1.00; P < 0.001). Considering that the sample size of Asians is limited, one needs to be cautious of the result and large-scale studies need to clarify the significance of the conclusion ( Figure 6C ).
Discussion
In the present meta-analysis, we did not find any statistical association between the COMT polymorphism with risks of PD in the overall population, even though five different models were utilized. In the new stratified analyses of population-based studies on different ethnicities (Asians and Caucasians), no association with COMT Val158/108Met polymorphism was shown. Intriguingly, subgroup analyses focusing on gender and onset time demonstrated that COMT Val158/108Met polymorphism has a major impact on the female in the heterozygous genetic model and the late onset in recessive and allelic genetic models. In addition, there was also a faint association between genotype and abstract thought (IQ: WAIS, NART). We also found no evidence of significant publication bias.
For the deep analysis, gender should be considered as a main factor to explore the COMT genotype with cognition in PD. However, not enough data could be obtained for accurate analysis. Sannino et al. (2015, 2017) reported that female COMT Met carriers show reduced cortical thickness after puberty and closely correlated with cognitive functions, which indicates that there is a change of brain structure that can increase the risk of PD. These findings suggest that it is important to appreciate the genetic and gender difference in the patient population. In the context of COMT Val158/108Met polymorphism, female PD patients should be assessed for cognitive function alone in future studies.
Our studies indicated that female carriers of the Met allele of COMT may be at an increased risk for developing PD. The question remains: How could the COMT genotype influence PD development in a gender-specific way? The COMT gene is located on chromosome 22, band q11.2 (Winqvist et al., 1992), and its encoded enzyme degrades a broad group of physiological catechols. This is one of the main mechanisms of terminating DA action in the synapse, together with DA reuptake by the DAT and DA diffusion out of the synapse (Axelrod and Weinshilboum, 1972; Meiser et al., 2013). It is reported that Val is a predominant factor that determines higher COMT activity, which presumably leads to lower synaptic dopamine levels (Chen et al., 2004). The Met/Met genotype causes the change in thermos ability of the enzyme, which is often characterized by decreased enzymatic activity even at 37°C (Boudíková et al., 1990). It may be that women’s hormone levels and thermoregulation differ from men, which illustrates a potential reason why COMT genotype is associated with PD in women alone. Some compelling evidence also demonstrated that estrogens may modulate COMT gene expression and protein activity via estrogen receptor function (Schendzielorz et al., 2011). As mentioned above, there is another possibility that female COMT Met carriers may remodel brain structures involved in the neurobiology of PD (Sannino et al., 2015).
Importantly, it was also reported that the Val allele had a significant effect on the age of onset of PD (Jimenez-Jimenez et al., 2014). This genotype may confer a greater risk of PD because of higher catecholaminergic metabolism, leading to an accelerated use of endogenous dopamine reservoirs as well as activating catabolism of COMT substrates (Bialecka et al., 2008). In our analysis, the result was consistent with the previous literature. Carriers of the Val allele of COMT Val158/108Met polymorphism may be at an increased risk for late-onset PD.
Cognitive disorders associated with PD could be so mild that they are under-recognized and do not alter daily living activities (Papagno and Trojano, 2018). A wealth of evidence has shown that the circuitry of dorsolateral prefrontal cortex and the front striatal (Beyer et al., 2007) supports various advanced brain functions (Arnsten, 1998). It has been also shown that regulating the level of DA can improve the specific cognitive function relying on this circuitry (Lewis et al., 2005). Watanabe et al. (1997) also identified that the DA level in prefrontal cortex was strongly related to the performance of working memory. Yerkes–Dodson type U-shape relationship governs the DA modulation of neural function, which means reduction or excessive increases of DA will selectively impair specific cognitive function (Rowe et al., 2008). Further evidence demonstrating the role of DA on cognitive function in PD has been obtained from the study of polymorphisms of COMT, which regulates prefrontal cortical DA turnover, as well as activation in prefrontal–striatal regions (Nombela et al., 2014).
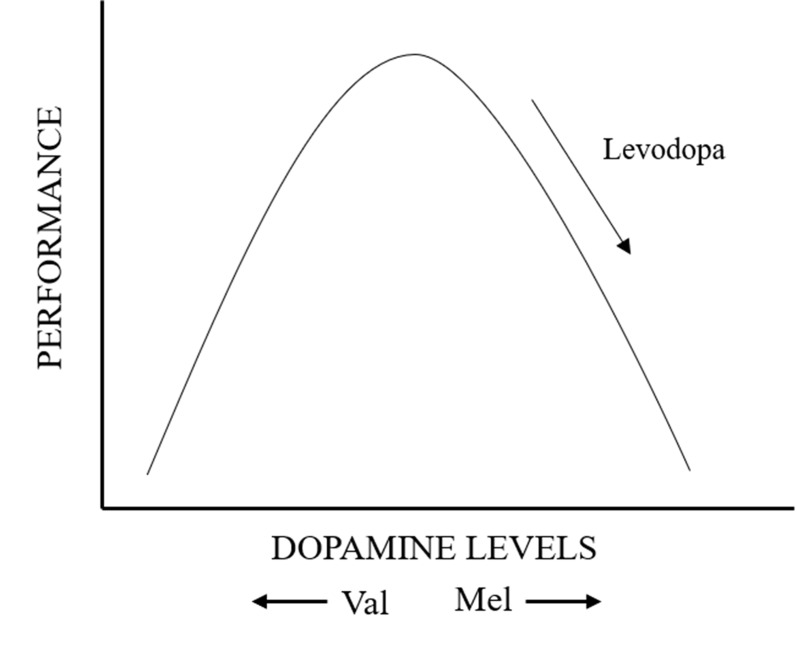
In our analysis of association between cognitive and motor abilities in PD and COMT Val158/108Met polymorphism, there is no positive evidence. During the seriatim heterogeneity test and sensitivity analysis, we found one study that if we removed from the analysis, the results would have been reversed. The eccentric study was about the European population. First and foremost, we made a subgroup analysis of ethnics. The result showed that the relationship between COMT and UPDRS III, IQ was appreciable in Asian, but not Europeans. Asians with Met carrier conferred an increased risk of high UPDRS III score, meaning a more severe motor impairment. Meanwhile, Asian patients with Met/Met genotype performed significantly worse on abstract thought (IQ: WAIS, NART). After further analysis on these two studies, we found that the levodopa dose was lower in subjects with Met/Met genotype than Val/Val group. However, in other studies, almost all PD patients with Met/Met genotype took more LODA. Given the change in the results before and after inclusion, LODA dose was a major factor and concern. It was reported that the relationship between prefrontal function and dopamine levels follows an inverted U-shaped curve ( Figure 7 ) (Williams-Gray et al., 2007). According to this theory, we speculated that, among PD patients, those with low activity COMT (Met/Met alleles) who take more LODA have worse performance in motor and cognition because of excessive DA levels. This is believed to contribute to impaired cognitive performance in prefrontal regions, which explains the reason of disruptive changes when one study was omitted. To sum up, factors about dosage and type of anti-PD medication should account for many reasons. Dopamine supplements may mask some linking of COMT polymorphisms and cognitive function. Studies have confirmed that high doses of dopamine reduce the expression of dopamine transporters in the prefrontal cortex, which could lead to cumulative toxicity of dopamine and make patients appear to have cognitive impairment like the patients with low-activity COMT. In other words, if PD patients with low-activity COMT use large-dose levodopa for a long time, cognitive impairment can be more severe. However, even if the use and types of anti-PD drugs have been taken into account, we could not complete this analysis due to the uncertainty and ambiguity of information.
Figure 7.
Diagram of inverted U-shaped relationship between prefrontal function and dopamine levels.
The Val158/108Met polymorphism remains a plausible candidate that may contribute to cognitive function deficits in PD. Although the results do not meet our expectations, it does not negate the need for scientific rigor in both the analysis and the reporting of results. At the very least, we cannot exclude the possibility that COMT genotype has a small influence on cognitive phenotypes and PD susceptibility in women. These results are partly in agreement with the previous meta-analysis, which reported that there is a significant association between COMT rs4680 and the risk for PD in the total series, as well as homozygosity for the low-activity allele in the Asiatic population (Jimenez-Jimenez et al., 2014). In addition, our analysis also further revealed the relationship between COMT genotype and PD of female and late onset. Meanwhile, in the aspect of cognition of PD, it also gives some risk analysis and discussion.
There are several potential limitations in the present meta-analysis. First, there is marginal significance among female PD patients, which encourages us; further research on the association of gender of PD and COMT Val158/108Met polymorphism should be carried out. Second, sample size limited our ability to investigate the accuracy of association of COMT Val158/108Met polymorphism and cognitive dysfunctions in PD. Additionally, limited data were obtained to explore the COMT genotype and cognition in early or late onset of PD. Due to the uneven nature of population genotype frequencies in different ethnic groups, the association of COMT Val158/108Met polymorphism and cognitive dysfunctions in PD needs further confirmation. The cognition scale indicators were multitudinous, and we were underpowered to test the combined effect in the current sample. Lastly, more studies are desirable in COMT polymorphism studies; also, the type and dose of anti-PD drug should be included specifically.
Our findings highlight the impact that COMT genetic factors can have on the susceptibility of PD and cognitive performance, specifically differences in female, late-onset PD patients, and IQ.
Conclusion
In summary, the results of our meta-analysis indicated that the COMT Val158/108Met polymorphism may be associated with the risk for PD in female or late-onset PD. Meanwhile, Met/Met carriers of Asian population performed significantly worse than the Val allele carriers in IQ score and UPDRS III. Considering the above potential limitation, these conclusions need to be further confirmed in future research.
Author Contributions
All authors listed have made substantial, direct, and intellectual contribution to the work and approved it for publication.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors would like to thank Chuanxi Tang in developing the protocol for the review and overseeing this project from inception to completion; Chuanxi Tang and Wei Wang for the drafting and submission of the manuscript; Mingyu Shi, Chengcheng Ma, and Xue Li for designing and conducting the systematic literature searches; Na Zhang, Xue Li, and Gang Chen for assistance in data acquisition; Na Zhang for organizing pictures and tables; Wei Wang, Mingyu Shi, and Xiaoyu Zhou for statistical analysis; and Jie Xiang and Dianshuai Gao for the program design guidance.
All authors read and approved the manuscript and agree to be accountable for all aspects of the research in ensuring that the accuracy or integrity of any part of the work is appropriately investigated and resolved.
References
- Aguilera M., Barrantes-Vidal N., Arias B., Moya J., Villa H., Ibanez M. I., et al. (2008). Putative role of the COMT gene polymorphism (Val158Met) on verbal working memory functioning in a healthy population. Am. J. Med. Genet. B Neuropsychiatr. Genet. 147B (6), 898–902. 10.1002/ajmg.b.30705 [DOI] [PubMed] [Google Scholar]
- Arnsten A. F. (1998). Catecholamine modulation of prefrontal cortical cognitive function. Trends Cogn. Sci. 2 (11), 436–447. 10.1016/S1364-6613(98)01240-6 [DOI] [PubMed] [Google Scholar]
- Axelrod J., Weinshilboum R. (1972). Catecholamines. N. Engl. J. Med. 287 (5), 237–242. 10.1056/NEJM197208032870508 [DOI] [PubMed] [Google Scholar]
- Bäckström D., Eriksson Domellöf M., Granåsen G., Linder J., Mayans S., Elgh E., et al. (2018). Polymorphisms in dopamine-associated genes and cognitive decline in Parkinson’s disease. Acta Neurol. Scand. 137 (1), 91–98. 10.1111/ane.12812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett J. H., Scoriels L., Munafo M. R. (2008). Meta-analysis of the cognitive effects of the catechol-O-methyltransferase gene Val158/108Met polymorphism. Biol. Psychiatry 64 (2), 137–144. 10.1016/j.biopsych.2008.01.005 [DOI] [PubMed] [Google Scholar]
- Benitez B. A., Forero D. A., Arboleda G. H., Granados L. A., Yunis J. J., Fernandez W., et al. (2010). Exploration of genetic susceptibility factors for Parkinson’s disease in a South American sample. J. Genet. 89 (2), 229–232. 10.1007/s12041-010-0030-1 [DOI] [PubMed] [Google Scholar]
- Beyer M. K., Janvin C. C., Larsen J. P., Aarsland D. (2007). A magnetic resonance imaging study of patients with Parkinson’s disease with mild cognitive impairment and dementia using voxel-based morphometry. J. Neurol. Neurosurg. Psychiatry 78 (3), 254–259. 10.1136/jnnp.2006.093849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bialecka M., Kurzawski M., Klodowska-Duda G., Opala G., Tan E.-K., Drozdzik M. (2008). The association of functional catechol-O-methyltransferase haplotypes with risk of Parkinson’s disease, levodopa treatment response, and complications. Pharmacogenet. Genomics 18 (9), 815–821. 10.1097/FPC.0b013e328306c2f2 [DOI] [PubMed] [Google Scholar]
- Bialecka M., Kurzawski M., Roszmann A., Robowski P., Sitek E. J., Honczarenko K., et al. (2012). Association of COMT, MTHFR, and SLC19A1 (RFC-1) polymorphisms with homocysteine blood levels and cognitive impairment in Parkinson’s disease. Pharmacogenet. Genomics 22 (10), 716–724. 10.1097/FPC.0b013e32835693f7 [DOI] [PubMed] [Google Scholar]
- Boland B., Yu W. H., Corti O., Mollereau B., Henriques A., Bezard E., et al. (2018). Promoting the clearance of neurotoxic proteins in neurodegenerative disorders of ageing. Nat. Rev. Drug Discov. 17 (9), 660. 10.1038/nrd.2018.109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudíková B., Szumlanski C., Maidak B., Weinshilboum R. (1990). Human liver catechol-O-methyltransferase pharmacogenetics. Clin. Pharmacol. Ther. 48 (4), 381–389. 10.1038/clpt.1990.166 [DOI] [PubMed] [Google Scholar]
- Chen J., Lipska B. K., Halim N., Ma Q. D., Matsumoto M., Melhem S., et al. (2004). Functional analysis of genetic variation in catechol-O-methyltransferase (COMT): effects on mRNA, protein, and enzyme activity in postmortem human brain. Am. J. Hum. Genet. 75 (5), 807–821. 10.1086/425589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhury S., Borah A. (2015). Activation of NMDA receptor by elevated homocysteine in chronic liver disease contributes to encephalopathy. Med. Hypotheses 85 (1), 64–67. 10.1016/j.mehy.2015.03.027 [DOI] [PubMed] [Google Scholar]
- Ciechanover A., Kwon Y. T. (2017). Protein quality control by molecular chaperones in neurodegeneration. Front. Neurosci. 11, 185. 10.3389/fnins.2017.00185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai H., Hao H., Shao M., Chen B. (2015). Association of COMT gene polymorphism and related fators with cognitive impairment in Parkinson’s disease in Chinese population. Chin. J. Mult. Organ Dis. Elder. 14 (6), 431–434. 10.11915/j.issn.1671-5403.2015.06.099 [DOI] [Google Scholar]
- Dikic I. (2017). Proteasomal and autophagic degradation systems. Annu. Rev. Biochem. 86, 193–224. 10.1146/annurev-biochem-061516-044908 [DOI] [PubMed] [Google Scholar]
- Duyckaerts C., Sazdovitch V., Seilhean D. (2010). Update on the pathophysiology of Parkinson’disease. Bull. Acad. Natl. Med. 194 (7), 1287–1303. discussion 1303-4. 10.1016/bs.irn.2017.05.013 [DOI] [PubMed] [Google Scholar]
- Eerola J., Launes J., Hellström O., Tienari P. J., Apolipoprotein E. (2002). (APOE), PARKIN and catechol-O-methyltransferase (COMT) genes and susceptibility to sporadic Parkinson’s disease in Finland. Neuroscience Letters 330 (3), 296–298. 10.1016/S0304-3940(02)00819-4 [DOI] [PubMed] [Google Scholar]
- Fallon S. J., Smulders K., Esselink R. A., de Warrenburg B. P., Bloem B. R., Cools R. (2015). Differential optimal dopamine levels for set-shifting and working memory in Parkinson’s disease. Neuropsychologia 77, 42–51. 10.1016/j.neuropsychologia.2015.07.031 [DOI] [PubMed] [Google Scholar]
- Galluzzi L., Baehrecke E. H., Ballabio A., Boya P., Bravo-San Pedro J. M., Cecconi F., et al. (2017). Molecular definitions of autophagy and related processes. EMBO J. 36 (13), 1811–1836. 10.15252/embj.201796697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatto N. M., Cockburn M., Bronstein J., Manthripragada A. D., Ritz B. (2009). Well-water consumption and Parkinson’s disease in rural California. Environ. Health Perspect. 117 (12), 1912–1918. 10.1289/ehp.0900852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goedert M., Spillantini M. G., Del Tredici K., Braak H. (2013). 100 years of Lewy pathology. Nat. Rev. Neurol. 9 (1), 13. 10.1038/nrneurol.2012.242 [DOI] [PubMed] [Google Scholar]
- Goetz C. G., Emre M., Dubois B. (2008). Parkinson’s disease dementia: definitions, guidelines, and research perspectives in diagnosis. Ann. Neurol. 64 Suppl 2, S81–S92. 10.1002/ana.21455 [DOI] [PubMed] [Google Scholar]
- Gómez-Esteban J. C., Zarranz J. J., Lezcano E., Tijero B., Luna A., Velasco F., et al. (2007). Influence of motor symptoms upon the quality of life of patients with Parkinson’s disease. Eur. Neurol. 57 (3), 161–165. 10.1159/000098468 [DOI] [PubMed] [Google Scholar]
- Goudreau J. L., Maraganore D. M., Farrer M. J., Lesnick T. G., Singleton A. B., Bower J. H., et al. (2002). Case-control study of dopamine transporter-1, monoamine oxidase-B, and catechol-O-methyl transferase polymorphisms in Parkinson’s disease. Mov. Disord. 17 (6), 1305–1311. 10.1002/mds.10268 [DOI] [PubMed] [Google Scholar]
- Guo J.-f., Zhang L., Li K., Mei J.-p., Xue J., Chen J., et al. (2018). Coding mutations in NUS1 contribute to Parkinson’s disease. Proc. Natl. Acad. Sci. 115 (45), 11567–11572. 10.1073/pnas.1809969115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliday G., McCann H., Shepherd C. (2012). Evaluation of the Braak hypothesis: how far can it explain the pathogenesis of Parkinson’s disease? Expert Rev. Neurother. 12 (6), 673–686. 10.1586/ern.12.47 [DOI] [PubMed] [Google Scholar]
- Hernán M. A., Checkoway H., O’brien R., Costa–Mallen P., De Vivo I., Colditz G., et al. (2002). MAOB intron 13 and COMT codon 158 polymorphisms, cigarette smoking, and the risk of PD. Neurology 58 (9), 1381–1387. 10.1212/WNL.58.9.1381 [DOI] [PubMed] [Google Scholar]
- Hoda F., Nicholl D., Bennett P., Arranz M., Aitchison K. J., Al-Chalabi A., et al. (1996). No Association between Parkinson’s disease and low-activity alleles of CatecholO-Methyltransferase. Biochem. Biophys. Res. Commun. 228 (3), 780–784. 10.1006/bbrc.1996.1731 [DOI] [PubMed] [Google Scholar]
- Holmqvist S., Chutna O., Bousset L., Aldrin-Kirk P., Li W., Björklund T., et al. (2014). Direct evidence of Parkinson pathology spread from the gastrointestinal tract to the brain in rats. Acta Neuropathol. 128 (6), 805–820. 10.1007/s00401-014-1343-6 [DOI] [PubMed] [Google Scholar]
- Hoogland J., de Bie R. M., Williams-Gray C. H., Muslimović D., Schmand B., Post B. (2010). Catechol-O-methyltransferase val158met and cognitive function in Parkinson’s disease. Mov. Disord. 25 (15), 2550–2554. 10.1002/mds.23319 [DOI] [PubMed] [Google Scholar]
- Jimenez-Jimenez F. J., Alonso-Navarro H., Garcia-Martin E., Agundez J. A. (2014). COMT gene and risk for Parkinson’s disease: a systematic review and meta-analysis. Pharmacogenet. Genomics 24 (7), 331–339. 10.1097/FPC.0000000000000056 [DOI] [PubMed] [Google Scholar]
- Jin Y. The association analys is of COMT genetic polymorphisms with the susceptibility of Parkinson’s disease and levodopa induced dysikinesia, Central South University, 2014. [Google Scholar]
- Kalinderi K., Fidani L., Kourtesi G., Katsarou Z., Mioglou E., Bostantjopoulou S. (2008). No association of the Val158Met COMT polymorphism with Parkinson’s disease in the Greek population. Eur. J. Neurol. 15 (8), e83–e83. 10.1111/j.1468-1331.2008.02186.x [DOI] [PubMed] [Google Scholar]
- Kiyohara C., Miyake Y., Koyanagi M., Fujimoto T., Shirasawa S., Tanaka K., et al. (2011). Genetic polymorphisms involved in dopaminergic neurotransmission and risk for Parkinson’s disease in a Japanese population. BMC Neurol. 11 (1), 89. 10.1186/1471-2377-11-89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebe S., Golmard J.-L., Nalls M. A., Saad M., Singleton A. B., Bras J. M., et al. (2013). The Val158Met COMT polymorphism is a modifier of the age at onset in Parkinson’s disease with a sexual dimorphism. J. Neurol. Neurosurg. Psychiatry 84 (6), 666–673. 10.1136/jnnp-2012-304475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunugi H., Nanko S., Ueki A., Otsuka E., Hattori M., Hoda F., et al. (1997). High and low activity alleles of catechol-O-methyltransferase gene: ethnic difference and possible association with Parkinson’s disease. Neuroscience Letters 221 (2-3), 202–204. 10.1016/S0304-3940(96)13289-4 [DOI] [PubMed] [Google Scholar]
- Lee M. S., Lyoo C. H., Ulmanen I., Syvänen A.-C., Rinne J. O. (2001). Genotypes of catechol-O-methyltransferase and response to levodopa treatment in patients with Parkinson’s disease. Neuroscience Letters 298 (2), 131–134. 10.1016/S0304-3940(00)01749-3 [DOI] [PubMed] [Google Scholar]
- Lewis S., Foltynie T., Blackwell A. D., Robbins T. W., Owen A. M., Barker R. A. (2005). Heterogeneity of Parkinson’s disease in the early clinical stages using a data driven approach. J. Neurol. Neurosurg. Psychiatry 76 (3), 343–348. 10.1136/jnnp.2003.033530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeWitt P. A. (2008). Levodopa for the treatment of Parkinson’s disease. N. Engl. J. Med. 359 (23), 2468–2476. 10.1056/NEJMct0800326 [DOI] [PubMed] [Google Scholar]
- Li Y. The study on the relationship between dopamine pathways gene polymorphisms and Parkinson’s disease executive dysfunction, Shantou University, 2016. [Google Scholar]
- Lynch D. R., Mozley P. D., Sokol S., Maas N. M., Balcer L. J., Siderowf A. D. (2003). Lack of effect of polymorphisms in dopamine metabolism related genes on imaging of TRODAT-1 in striatum of asymptomatic volunteers and patients with Parkinson’s disease. Mov. Disord. 18 (7), 804–812. 10.1002/mds.10430 [DOI] [PubMed] [Google Scholar]
- Ma H., Ma L., Feng T. (2018). Relationship between Val158Met polymorphism in catechol-o-methyltransferase gene and depression in Parkinson’s disease. Chin. J. Rehabil. Theory Pract. 24 (7), 753–756. 10.3969/j.issn.1006-9771.2018.07.001 [DOI] [Google Scholar]
- Marras C., Chaudhuri K. R. (2016). Nonmotor features of Parkinson’s disease subtypes. Mov. Disord. 31 (8), 1095–1102. 10.1002/mds.26510 [DOI] [PubMed] [Google Scholar]
- Mayer E. A., Tillisch K., Gupta A. (2015). Gut/brain axis and the microbiota. J. Clin. Invest. 125 (3), 926–938. 10.1172/JCI76304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meiser J., Weindl D., Hiller K. (2013). Complexity of dopamine metabolism. Cell Commun. Signal 11 (1), 34. 10.1186/1478-811X-11-34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menzies F. M., Fleming A., Caricasole A., Bento C. F., Andrews S. P., Ashkenazi A., et al. (2017). Autophagy and neurodegeneration: pathogenic mechanisms and therapeutic opportunities. Neuron 93 (5), 1015–1034. 10.1016/j.neuron.2017.01.022 [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A., Kohn P. D., Kolachana B., Kippenhan S., Inerney-Leo A., Nussbaum R., et al. (2005). Midbrain dopamine and prefrontal function in humans: interaction and modulation by COMT genotype. Nat. Neurosci. 8 (5), 594–596. 10.1038/nn1438 [DOI] [PubMed] [Google Scholar]
- Mizuta I., Mizuta E., Yamasaki S., Kuno S., Yasuda M., Tanaka C. (2000). Meta-analysis of polymorphism of the catechol-O-methyltransferase gene in relation to the etiology of Parkinson’s disease in Japan. Mov. Disord. 15 (5), 1013–1014. [DOI] [PubMed] [Google Scholar]
- Moher D., Liberati A., Tetzlaff J., Altman D. G., Group P. (2009). Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 339, b2535. 10.1136/bmj.b2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morley J. F., Xie S. X., Hurtig H. I., Stern M. B., Colcher A., Horn S., et al. (2012). Genetic influences on cognitive decline in Parkinson’s disease. Mov. Disord. 27 (4), 512–518. 10.1002/mds.24946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neunlist M., Rolli-Derkinderen M., Latorre R., Van Landeghem L., Coron E., Derkinderen P., et al. (2014). Enteric glial cells: recent developments and future directions. Gastroenterology 147 (6), 1230–1237. 10.1053/j.gastro.2014.09.040 [DOI] [PubMed] [Google Scholar]
- Nombela C., Rowe J. B., Winder-Rhodes S. E., Hampshire A., Owen A. M., Breen D. P., et al. (2014). Genetic impact on cognition and brain function in newly diagnosed Parkinson’s disease: ICICLE-PD study. Brain 137 (10), 2743–2758. 10.1093/brain/awu201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papagno C., Trojano L. (2018). Cognitive and behavioral disorders in Parkinson’s disease: an update. Neurol. Sci. 39 (2), 215–223. 10.1007/s10072-017-3154-8 [DOI] [PubMed] [Google Scholar]
- Paul K. C., Rausch R., Creek M. M., Sinsheimer J. S., Bronstein J. M., Bordelon Y., et al. (2016). APOE, MAPT, and COMT and Parkinson’s disease susceptibility and cognitive symptom progression. J. Parkinsons Dis. 6 (2), 349–359. 10.3233/JPD-150762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter T., Burnham S. C., Milicic L., Savage G., Maruff P., Sohrabi H. R., et al. (2019). COMT val158met is not associated with Abeta-amyloid and APOE epsilon4 related cognitive decline in cognitively normal older adults. IBRO Rep. 6, 147–152. 10.1016/j.ibror.2019.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi D., Dai Y., Chen X., Li J., Zhang C. (2013). Research on relationship between COMT gene exon four and Parkinson’s genetic susceptibility. Clin. J. Chin. Med. 5 (21), 87–88. 10.3969/j.issn.1674-7860.2013.21.051 [DOI] [Google Scholar]
- Qian Y., Liu J., Xu S., Yang X., Xiao Q. (2017). Roles of functional catechol-O-methyltransferase genotypes in Chinese patients with Parkinson’s disease. Transl. Neurodegener. 6 (1), 11. 10.1186/s40035-017-0081-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe J., Hughes L., Ghosh B., Eckstein D., Williams-Gray C., Fallon S., et al. (2008). Parkinson’s disease and dopaminergic therapy—differential effects on movement, reward and cognition. Brain 131 (8), 2094–2105. 10.1093/brain/awn112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe J., Hughes L., Williams-Gray C., Bishop S., Fallon S., Barker R., et al. (2010). The val158met COMT polymorphism’s effect on atrophy in healthy aging and Parkinson’s disease. Neurobiol. Aging 31 (6), 1064–1068. 10.1016/j.neurobiolaging.2008.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sannino S., Gozzi A., Cerasa A., Piras F., Scheggia D., Manago F., et al. (2015). COMT genetic reduction produces sexually divergent effects on cortical anatomy and working memory in mice and humans. Cereb. Cortex 25 (9), 2529–2541. 10.1093/cercor/bhu053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sannino S., Padula M. C., Manago F., Schaer M., Schneider M., Armando M., et al. (2017). Adolescence is the starting point of sex-dichotomous COMT genetic effects. Transl. Psychiatry 7 (5), e1141. 10.1038/tp.2017.109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheggia D., Zamberletti E., Realini N., Mereu M., Contarini G., Ferretti V., et al. (2018). Remote memories are enhanced by COMT activity through dysregulation of the endocannabinoid system in the prefrontal cortex. Mol. Psychiatry 23 (4), 1040–1050. 10.1038/mp.2017.126 [DOI] [PubMed] [Google Scholar]
- Schendzielorz N., Rysa A., Reenila I., Raasmaja A., Mannisto P. T. (2011). Complex estrogenic regulation of catechol-O-methyltransferase (COMT) in rats. J. Physiol. Pharmacol. 62 (4), 483–490. [PubMed] [Google Scholar]
- Shao M., Liu Z., Tao E., Chen B. (2005). Correlation between the genetic polymorphism of dopamine metabolic enzymes and the genetic susceptibility of Parkinson’s disease. Chin. J. Gerontol. 25 (07), 5–7. 10.3969/j.issn.1005-9202.2005.07.001 [DOI] [Google Scholar]
- Shih P.-Y., Er T.-K., Chang J.-G. (2013). An association study between genetic variants at mu-opioid receptor, dopamine transporter, catechol-O-methyltransferase, and dopamine genes and risk of Parkinson’s disease. Neurol. Asia 18 (3), 279–287. [Google Scholar]
- Song Q., Li Y., Zhang X. (2015). Relationship between Xinjiang Region Parkinson’s disease and the interaction between polymorphisms of catechol-o-methyltransferase, monoamine oxidase B, dopamine β-hydroxylase and environmental factors. Chin. J. Clin. Neurosci. 23 (5), 497–508. [Google Scholar]
- Song Q., Zhang X., Li Y. (2014). The correlation research among the polymorphisms of COMT gene, CYP1A1 gene and Parkinson disease in Xinjiang. J. Apoplexy Nerv. Dis. 31 (2), 153–157. [Google Scholar]
- Stang A. (2010). Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 25 (9), 603–605. 10.1007/s10654-010-9491-z [DOI] [PubMed] [Google Scholar]
- Syvänen A.-C., Tilgmann C., Rinne J., Ulmanen I. (1997). Genetic polymorphism of catechol-O-methyltransferase (COMT): correlation of genotype with individual variation of S-COMT activity and comparison of the allele frequencies in the normal population and parkinsonian patients in Finland. Pharmacogenetics 7 (1), 65–71. 10.1097/00008571-199702000-00009 [DOI] [PubMed] [Google Scholar]
- Torkaman-Boutorabi A., Shahidi G. A., Choopani S., Zarrindast M. R. (2012). Association of monoamine oxidase B and catechol-O-methyltransferase polymorphisms with sporadic Parkinson’s disease in an Iranian population. Folia Neuropathol. 50 (4), 382–389. 10.5114/fn.2012.32368 [DOI] [PubMed] [Google Scholar]
- Vidgren J., Svensson L. A., Liljas A. (1994). Crystal structure of catechol O-methyltransferase. Nature 368 (6469), 354–358. 10.1038/368354a0 [DOI] [PubMed] [Google Scholar]
- Vila M., Przedborski S. (2004). Genetic clues to the pathogenesis of Parkinson’s disease. Nat. Med. 10 (7), S58–S62. 10.1038/nm1068 [DOI] [PubMed] [Google Scholar]
- Wang Y. Cognitive impairment and genetic susceptibility in Parkinson’s disease, Central South University, 2014. [Google Scholar]
- Watanabe M., Harada S., Nakamura T., Ohkoshi N., Yoshizawa K., Hayashi A., et al. (2003). Association between catechol-O-methyltransferase gene polymorphisms and wearing-off and dyskinesia in Parkinson’s disease. Neuropsychobiology 48 (4), 190–193. 10.1159/000074637 [DOI] [PubMed] [Google Scholar]
- Watanabe M., Kodama T., Hikosaka K. (1997). Increase of extracellular dopamine in primate prefrontal cortex during a working memory task. J. Neurophysiol. 78 (5), 2795–2798. 10.1152/jn.1997.78.5.2795 [DOI] [PubMed] [Google Scholar]
- William L. J. (2006). The Parkinson’s complex: parkinsonism is just the tip of the iceberg. Ann. Neurol. 59 (4), 591–596. 10.1002/ana.20834 [DOI] [PubMed] [Google Scholar]
- Williams-Gray C. H., Hampshire A., Barker R. A., Owen A. M. (2008). Attentional control in Parkinson’s disease is dependent on COMT val158met genotype. Brain 131 (2), 397–408. 10.1093/brain/awm313 [DOI] [PubMed] [Google Scholar]
- Williams-Gray C. H., Hampshire A., Robbins T. W., Owen A. M., Barker R. A. (2007). Catechol O-methyltransferase Val158Met genotype influences frontoparietal activity during planning in patients with Parkinson’s disease. J. Neurosci. 27 (18), 4832–4838. 10.1523/JNEUROSCI.0774-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winqvist R., Lundström K., Salminen M., Laatikainen M., Ulmanen I. (1992). The human catechol-O-methyltransferase (COMT) gene maps to band q11. Cytogenet. Genome Res. 59 (4), 253–257. 10.1159/000133262 [DOI] [PubMed] [Google Scholar]
- Wu K., O’Keeffe D., Politis M., O’Keeffe G. C., Robbins T. W., Bose S. K., et al. (2012). The catechol-O-methyltransferase Val158Met polymorphism modulates fronto-cortical dopamine turnover in early Parkinson’s disease: a PET study. Brain 135 (8), 2449–2457. 10.1093/brain/aws157 [DOI] [PubMed] [Google Scholar]
- Wu R.-M., Cheng C., Chen K., Lu S., Shan D., Ho Y., et al. (2001). The COMT L allele modifies the association between MAOB polymorphism and PD in Taiwanese. Neurology 56 (3), 375–382. 10.1212/WNL.56.3.375 [DOI] [PubMed] [Google Scholar]
- Xiao Q., Qian Y., Liu J., Xu S., Yang X. (2017). Roles of functional catechol-O-methyltransferase genotypes in Chinese patients with Parkinson’s disease. Transl. Neurodegener. 6, 11. 10.1186/s40035-017-0081-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie T., Ho S., Li L., Ma O. (1997). G/A1947 polymorphism in catechol-O-methyltransferase (COMT) gene in Parkinson’s disease. Mov. Disord. 12 (3), 426–427. 10.1002/mds.870120325 [DOI] [PubMed] [Google Scholar]
- Xu L., Hao Y., Xie H., Tang G., Ren D. (2002). Correlation between catecholamine oxygen level methyltransferase gene G/A polymorphism and Parkinson’s disease in Han population of Shanghai. Chin. J. Med. Genet. 19 (5), 440–441. 10.3760/j.issn:1003-9406.2002.05.022 [DOI] [Google Scholar]
- Yoritaka A., Hattori N., Yoshino H., Mizuno Y. (1997). Catechol-O-methyltransferase genotype and susceptibility to Parkinson’s disease in Japan. J. Neural. Transm. 104 (11-12), 1313–1317. 10.1007/BF01294732 [DOI] [PubMed] [Google Scholar]
- Zeng W. Relationship between polymorphism of monoamine oxidase B,catechol-o-methyltansferase gene and Parkinson disease in Xinjiang uygurs, Shihezi University, 2012. [Google Scholar]
- Zhang Y., Feng S., Nie K., Wang L., Zhu R., Tang H., et al. (2015). Association of the catechol-O-methyltransferase rs4680 polymorphism with Parkinson’s disease in a Han Chinese cohort. Chin. J. Neurol. 48 (1), 18–22. 10.3760/cma.j.issn.1006-7876.2015.01.005 [DOI] [Google Scholar]
- Zhang Y., Feng S., Nie K., Zhao X., Gan R., Wang L., et al. (2016). Catechol-O-methyltransferase Val158Met polymorphism influences prefrontal executive function in early Parkinson’s disease. J. Neurol. Sci. 369, 347–353. 10.1016/j.jns.2016.08.063 [DOI] [PubMed] [Google Scholar]
- Zhao X., Xie H., Tang G., Zhao W., Xu L., Lin D., et al. (2003). Association between four genes involved in dopaminergic neurotransmitter metabolism COMT, DBH, DAT1, MAOB and susceptibility to Parkinson’s disease in Shanghai Hans. Chin. J. Clin. Rehabil. 7 (7), 1126–1127. 10.3321/j.issn:1673-8225.2003.07.040 [DOI] [Google Scholar]