Abstract
Background
After‐hours or out‐of‐clinic crossmatches are often limited by the lack of access to specialized material and technical expertise.
Hypothesis/Objectives
The goal was to adapt a stall‐side crossmatch test for pretransfusion evaluation in horses.
Animals
Twelve healthy mares (plasma and blood donors, teaching mares).
Methods
In a prospective study, blood from 12 mares was used to compare the results of 132 crossmatches performed with a rapid gel assay to crossmatches performed with a microgel column assay, and with predicted compatibilities based on blood types and detection of antibodies at a reference laboratory (microplate assay). The rapid gel assay protocol for dogs was adapted to decrease the formation of rouleaux that initially precluded equine erythrocytes migration through the gel.
Results
There was a good agreement between the rapid gel assay and the microgel assay as well as with the predicted compatibilities (κ > .6 for both). Agreement was higher between the microgel assay and the predicted compatibilities (κ = .8). The rapid gel assay failed to detect 6 predicted Aa incompatibilities (agglutinins‐related), 3 of which were also not detected with the microgel assay.
Conclusions and Clinical Importance
Based on these results, the modified rapid gel assay could be useful in settings when access to the microgel assay is not available. Discrepancies between both gel techniques and predicted compatibilities were most often low‐grade agglutination, which warrants further investigation to assess their clinical importance.
Keywords: agglutination, blood transfusion, compatibility, equine
Abbreviations
- EDTA
ethylenediaminetetraacetic acid
- UC Davis
University of California Davis
1. INTRODUCTION
Equine blood types include 7 systems (A, C, D, K, P, Q, and U), each containing 1 (systems C, K, U) to 15 factors (system D) composed of proteins or carbohydrates,1, 2, 3 giving rise to countless possibilities of different blood types. This complex system makes the likelihood of having 2 unrelated horses from the same breed sharing the exact same blood group less than 1 in 100 000.4 A perfect blood type match is unlikely, especially when most referral centers keep only a few blood donors available for fresh blood transfusions. Fortunately, most horses do not possess natural alloantibodies against erythrocyte antigens they do not carry, and not all horses develop alloantibodies after a single incompatible transfusion.5, 6 Out of 390 and 409 pregnant Standardbred and Thoroughbred mares, 20 and 10%, respectively, had alloantibodies,7 the majority being anti‐Ca antibodies. Therefore, it is estimated that more than 80%‐90% of the general horse population do not have alloantibodies, and the ones that do are more likely to have anti‐Ca antibodies, believed to be less immunogenic than anti‐Aa antibodies. However, horses with incompatible crossmatches are more likely to have transfusion reactions.6 Also, transfused red blood cells to recipients with alloantibodies will have shorter half‐lives, even when the antibodies implicated are anti‐Ca.6 Therefore, crossmatches should ideally be performed before any transfusion, provided that the emergency of the situation allows for it.
Pretransfusion crossmatches for horses are currently performed with a microgel column assay or more often with the traditional tube assay. The microgel column assay was recently validated in horses. It is less time consuming and less operator dependent in its interpretation than the tube assay,8 but it requires access to equipment (specifically designed centrifuge) not always available to untrained after‐hours clinical staff. A simple rapid gel assay was developed for use in small animals9 but has not been validated for use in horses. The hypothesis of this study was that the rapid gel assay would have an acceptable agreement (≥80% or κ > .6) with the microgel column assay and could be used as a pretransfusion test in emergency situations. The specific objectives were (1) to adapt the rapid gel assay for use with equine blood, (2) to evaluate the concordance between the rapid gel assay and the microgel assay, and (3) to compare results with predicted compatibilities based on blood types and known antibodies detected by the microplate assay from a reference laboratory.
2. MATERIALS AND METHODS
2.1. Animals
Twelve healthy mares (9 Standardbreds, 2 Quarter‐Horses, and 1 Paint; 11 ± 5 (standard deviation) years old, estimated 400‐500 kg) belonging to the Centre Hospitalier Universitaire Vétérinaire of the University of Montreal were studied. They were part of the teaching herd and 4 were also plasma or whole blood donors. Donors had not been collected for a minimum of 4 weeks before the study. They were regularly dewormed and vaccinated, and had negative Coggins's tests upon entry in the herd. No information was available on whether they had carried foals or received blood transfusions before their entry in the herd, but some mares had likely been pregnant in the years before the study. All animal manipulations were performed in accordance with the guidelines of the Canadian Council for Animal Care, and the protocol was approved by Animal Care and Use Committee of the University of Montreal (approval number: 17Rech1915).
2.2. Study design
Upon entry in the herd (up to 10 years before the study), these mares had been screened for the presence of anti‐erythrocyte antibodies (including anti‐donkey) and all but 1 were blood typed at a reference veterinary laboratory (Clinical Diagnostic Laboratories at University of California [UC] Davis Veterinary Medical Teaching Hospital, Davis, CA). These historical data were used to select 12 mares, out of the 18 potentially available. Mares were selected to have the highest possible numbers of predicted incompatibilities based on blood typing and previous antibody screening results, that is, a minimum of 25% incompatible minor or major crossmatches, including 8% Aa incompatibilities, and up to 54% incompatible crossmatches because of the presence of antibodies classified as unidentified (Table 1). Once selected, blood typing and screening for alloantibodies was repeated, except for 1 horse for which only antibody screening was done, as she had been blood typed 10 months before the study. All pairs were tested, each mare serving as “donor” and “receiver,” for a total of 144 crossmatches (including auto‐controls) performed with the rapid gel assay (RapidVet‐H, DMS laboratories, Flemington, New Jersey) and with the microgel column assay (ID‐Micro Typing System Cards and reagents, Ortho Clinical Diagnostics, Raritan, New Jersey; Centrifuge and incubator, DiaMed GmbH, Bio‐Rad Laboratories, Cressier sur Morat, Switzerland).
Table 1.
Agreement between predicted outcomes and gel assays
| Antibodies | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Serum/plasma | Agglutinins 2017 | Hemolysins 2017 | Previous screening (if different) | 1 AbCa Ua | 2 AbCa PaUa | 3 AaCaK aQcUa | 4 AaCa PaUa | 5 AaCa Qabc | 6 AaCa KaPa Ua | 7 AbCa Qc | 8 AabKa Ua | 9 AbKa Ua | 10 AbCa KaUa | 11 AaKa Ua | 12 AbCa Ka |
| P‐R‐M | P‐R‐M | P‐R‐M | P‐R‐M | P‐R‐M | P‐R‐M | P‐R‐M | P‐R‐M | P‐R‐M | P‐R‐M | P‐R‐M | P‐R‐M | ||||
| 1 | ‐ | ‐ | Same | C‐C‐C | C‐I‐C | C‐C‐C | C‐C‐C | C‐C‐C | C‐C‐C | C‐C‐C | C‐C‐C | C‐C‐C | C‐I‐C | C‐C‐C | C‐I‐C |
| 2 | Anti‐Aa Anti‐dk | ‐ | No Ab | C‐C‐C | C‐CC | I‐C‐I | I‐C‐C | I‐C‐I | I‐C‐I | C‐C‐C | I‐C‐C | C‐C‐C | C‐C‐C | I‐C‐C | C‐C‐C |
| 3 | Anti‐dk | ‐ | No Ab | C‐C‐C | C‐C‐C | C‐C‐C | C‐I‐C | C‐C‐C | C‐I‐C | C‐I‐C | C‐C‐C | C‐I‐C | C‐I‐C | C‐C‐C | C‐I‐C |
| 4 | ‐ | ‐ | Same | C‐C‐C | C‐C‐C | C‐C‐C | C‐C‐C | C‐C‐C | C‐C‐C | C‐C‐C | C‐C‐C | C‐C‐C | C‐C‐C | C‐C‐C | C‐C‐C |
| 5 | ‐ | ‐ | Anti‐X | C‐C‐C | C‐I‐I | C‐C‐I | C‐C‐I | C‐C‐C | C‐C‐C | C‐C‐I | C‐C‐C | C‐C‐C | C‐C‐I | C‐C‐I | C‐C‐I |
| 6 | Anti‐X | ‐ | Same | U‐C‐C | U‐I‐Ca | U‐C‐C | U‐C‐Ia | U‐C‐C | C‐C‐C | U‐C‐C | U‐C‐C | U‐C‐Ia | U‐C‐C | U‐C‐C | U‐C‐C |
| 7 | ‐ | ‐ | Anti‐Aa Anti‐Ac |
C‐C‐C | C‐C‐C | C‐C‐C | C‐C‐C | C‐C‐C | C‐C‐I | C‐C‐C | C‐C‐C | C‐C‐C | C‐C‐C | C‐C‐C | C‐C‐C |
| 8 | Anti‐Ca Anti‐X |
Anti‐Ca | Same | I‐I‐I | I‐I‐I | I‐I‐I | I‐I‐I | I‐I‐I | I‐I‐I | I‐I‐I | C‐C‐C | U‐C‐Ia | I‐I‐I | U‐C‐C | I‐I‐I |
| 9 | Anti‐Ca Anti‐X |
Anti‐Ca | Same | I‐I‐I | I‐I‐I | I‐I‐I | I‐I‐I | I‐I‐I | I‐I‐I | I‐I‐I | U‐C‐C | C‐C‐C | I‐I‐I | U‐C‐C | I‐I‐I |
| 10 | ‐ | ‐ | Anti‐X | C‐C‐C | C‐C‐C | C‐C‐C | C‐C‐C | C‐C‐C | C‐C‐C | C‐C‐C | C‐C‐C | C‐C‐C | C‐C‐C | C‐C‐C | C‐C‐C |
| 11 | Anti‐Ca | Anti‐Ca | I‐I‐I | I‐I‐I | I‐I‐I | I‐I‐I | I‐I‐I | I‐I‐I | I‐I‐I | C‐C‐C | C‐C‐C | I‐I‐I | C‐C‐C | I‐I‐I | |
1 to 12 indicates horse identification with their respective blood antigens and antibodies. The plasma/serum from horse 12 was excluded from analysis because of consistently positive auto‐controls with the rapid gel assay. ‐ indicates no antibodies. Same indicates that the antibodies in the previous screening were the same as in the 2017 screening. Gray background emphasizes discordant results between rapid gel or microgel assays and predicted results. Superscript letter “a” identifies discordant results between the 2 gel assays for which the prediction was unknown (ie, unidentified antibodies).
Abbreviations: Ab, antibody; Anti‐dk, anti‐donkey antibody; Anti‐X, unidentified antibody; C, compatible; I, incompatible; P‐R‐M, results for predicted compatibility, rapid gel assay, and microgel assay; U, unknown (because of the presence of an unidentified antibody).
2.3. Blood typing and alloantibody screening
Blood was collected in dry tubes and tubes containing ethylenediaminetetraacetic acid (EDTA). Serum and anticoagulated whole blood were sent overnight to the Clinical Diagnostic Laboratories at UC Davis. Blood typing was done for systems A, C, K, P, Q, and U, and serum was screened for the presence of anti‐erythrocyte hemolysin and agglutinin antibodies (against Aa, Ab, Ac, Ca, Ka, Pa, Pb, Qa, Qb, Qc, Ua, and donkey factor) as described before.6 Briefly, screening for antibodies was performed by incubating serial dilutions of a serum sample with a series of equine red blood cells of known blood types. The process was repeated with the addition of complement for the hemolysin assay. The presence of agglutination and hemolysis was assessed visually, and antibodies were reported as present or absent. If antibodies were detected but could not be further identified, that is, not able to determine which red cell antigens they were directed against, they were classified as “unidentified antibodies.” At the time of the study, screening for anti‐D and anti‐Af antibodies (ie, directed against D and Af antigens) was unavailable, and therefore would have been reported as “unidentified.”
2.4. Microgel column assay
Washed erythrocytes and serum were processed following manufacturer's instructions, with minor modifications, and as previously described.8 Briefly, blood with EDTA anticoagulant was centrifuged, plasma was collected, and erythrocytes were resuspended and washed 3 times in isotonic saline, then put in a 1% suspension with low ionic saline. Twenty‐five microliters of plasma and 50 μL of the 1% erythrocyte suspension were incubated together in the chamber over the polypropylene microgel columns for 15 minutes at 37°C (ID‐Incubator, Ortho Clinical Diagnostics). The 6‐microgel column cartridges were then centrifuged for 10 minutes at 80g (ID‐Centrifuge, Ortho Clinical Diagnostics). Agglutination was graded as follows: 0, all erythrocytes passed through the gel and formed a compact pellet at the bottom; 1, most erythrocytes form a pellet at the bottom of the gel, but not compact, with few erythrocytes visible in the lower half of the gel; 2, erythrocytes are predominantly observed in the lower half of the gel column or are dispersed throughout the gel; 3, erythrocytes are dispersed on the top half of the gel with some retained on the gel surface; and 4, all erythrocytes are retained on top of the gel (Figure 1). Grades ≤1 were considered compatible.10, 11 Complement was not used to detect hemolysis. Hemolysis was considered present and result recorded as incompatible if, compared to the auto‐control, red discoloration of the solution was observed in the gel column or reaction chamber.
Figure 1.
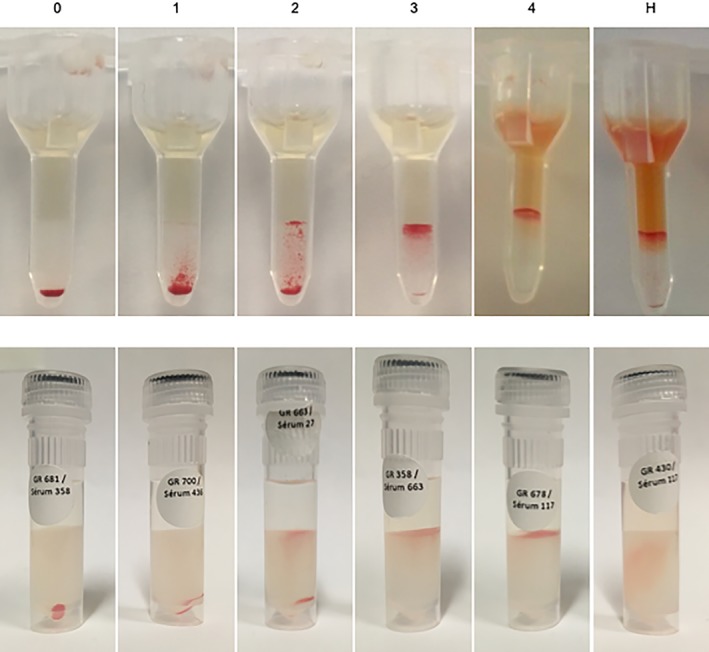
Microgel and rapid gel grades. Agglutination grades for microgel assay (top panel) and rapid gel assay (bottom panel). 0: all erythrocytes passed through the gel and formed a compact pellet at the bottom, 1: most erythrocytes form a pellet at the bottom of the gel, but not compact, with few erythrocytes visible in the lower half of the gel, 2: erythrocytes are predominantly observed in the lower half of the gel column or are dispersed throughout the gel, 3: erythrocytes are dispersed on the top half of the gel with some retained on the gel surface, and 4: all erythrocytes are retained on top of the gel (here with some hemolysis in the microgel). Hemolysis (H) was considered present when red discoloration was observed in the microgel chamber (here with grade 4 agglutination) or in the gel itself for the rapid gel assay. In opposite to this rapid gel assay picture where only hemolysis can be appreciated, all 8 crossmatches in the current study with detectable hemolysis also resulted in positive agglutination (both with the rapid gel and microgel assays). Crossmatches with hemolysis or grades ≥2 agglutination were considered incompatible. For the top panel (microgel) grades 4 and H come from clinical cases of neonatal isoerythrolysis
2.5. Rapid gel assay
In a preliminary study, the manufacturer's protocol for canine blood was used. Briefly, erythrocytes were washed 3 times in isotonic saline then suspended in a 6% solution. Two hundred microliters of plasma and 100 μL of the 6% erythrocyte suspension were incubated together in a tube for 5 minutes at room temperature. Then, 50 μL of the solution were transferred on top of the gel column tube, which was then centrifuged for 5 minutes at 1547g (Clay Adams, Triac Blood model, New York, NY).
After inconclusive preliminary results and multiple attempts using various temperature, incubation time, and dilutions, the protocol was modified as follows to reduce rouleaux formation. A 1% erythrocyte suspension was prepared as described above for the microgel column assay. Then, 75 μL of isotonic saline, 25 μL of serum, and 50 μL of the 1% erythrocyte suspension were incubated in a tube for 5 minutes at room temperature. The solution (150 μL) was then transferred on top of the gel column and the tube was centrifuged for 5 minutes at 1547g. The same agglutination grading system was used as for the microgel column assay. In comparison with the auto‐control, a combination of diffuse red discoloration of the gel and the absence of a distinguishable erythrocyte pellet or agglutination was considered evidence of hemolysis (Figure 1).
2.6. Compatibility assessment
Each crossmatch reaction was read independently by a junior (P. Casenave) and senior evaluator (M.‐C. Blais) on site, and later by consensus (P. Casenave, M.‐C. Blais, M. Leclere) by looking at photographs. To allow for comparison with the auto‐controls, all crossmatches using the same plasma/serum were read simultaneously, but the evaluators remained blinded to the plasma/serum and erythrocytes identification.
2.7. Statistical analysis
Sample size calculations were based on a predicted incompatible reaction percentage of 50% (pi value of .5) and an agreement between 50 and 80% (estimated from Luethy et al8). With a sample size of 144, differences of 10% should be detected 80% of the time.12 Agreement between evaluators and between gel assays was evaluated for grades (0‐4) and binary classification (compatible versus incompatible), with the Cohen's kappa coefficient (κ and weighed κ for grades) using a commercial software (SAS, version 9.3, SAS Institute Inc, Cary, North Carolina). Agreement between the gel columns assays and the predicted results was evaluated for binary classification only, and only for pairs with known outcome (ie, in horses carrying an unidentified antibody, the crossmatch was classified as “unknown” rather than compatible or incompatible). For comparison between assays, grades from the senior evaluator were used. Agreement was considered poor with κ values below .21, slight between .21 and .4, fair between .41 and .6, good between .61 and .80, and very good to excellent above .81.13, 14 Sensitivity and specificity with 95% confidence interval (CI) were calculated for the presence of hemolysis using the presence of hemolytic antibodies as gold standard.
3. RESULTS
3.1. Preliminary study
Crossmatches done with the manufacturer's instructions for canine blood gave highly inconsistent results, including positive auto‐controls. When the solution was put on a slide after incubation, numerous erythrocyte rouleaux were observed. Different protocols were tried with variations in temperature, incubation time, and in the erythrocyte, saline, serum, or plasma ratios. The protocol selected offered adequate reduction in rouleaux formation while keeping the manipulations relatively simple. Figure 2 illustrates positive auto‐controls and extensive rouleaux formation with the initial protocol.
Figure 2.
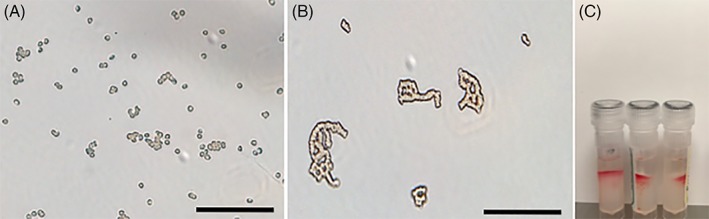
Preliminary study. The incubation solution from the microgel protocol when looked under a microscope showing scattered red blood cells (A). The incubation solution of the rapid gel assay with the original canine protocol showing extensive rouleaux formation (B) and positive (incompatible) auto‐controls (C). Scale bar = 80 μm
3.2. Main study
The plasma/serum from 1 horse was excluded from analysis because of consistently positive auto‐controls with the rapid gel assay, decreasing the number of analyzed crossmatches to 132. Unfortunately, this also decreased the number of expected incompatible crossmatches because of Aa antibodies from 12 to 6.
3.3. Blood typing and alloantibodies screening
For the 10 horses in which blood typing was repeated for the study (1 had never been tested and 1 had been blood typed within a year), there were no discrepancies between the first and second blood typing result, except for the loss of information on groups D and Af. Six, 9, and 0 horses carried the Aa, Ca, and Qa antigens, respectively. One horse had anti‐Aa agglutinating antibodies, 3 had anti‐Ca agglutinating and hemolytic antibodies, and 3 horses had unidentified agglutinating antibodies (2 of them also had anti‐Ca antibodies). Interestingly, alloantibody screening results changed over time for 5 horses (Table 1). One blood donor had anti‐Aa and anti‐donkey antibodies that were not previously detected, and a second horse had newly identified anti‐donkey antibodies. There was no history of gestation or exposure to blood products during that period in either case. Conversely, antibodies previously identified in 3 horses were no longer detected, including unidentified agglutinating antibodies (2 horses), and anti‐Aa and anti‐Ac antibodies (1 horse). Only the most recent antibody screening was used to predict compatibility.
3.4. Agreement between evaluators
For the microgel column assay, agreement between the junior and senior evaluators was excellent (κ = 1 for compatibility, weighted κ = .97 for grades), as was agreement between the senior evaluator and the photo evaluation (κ = .95 for compatibility and grades). For the rapid gel assay, agreement for compatibility between the junior and senior evaluators was excellent (κ = .98), but agreement between grades was lower (weighed κ = .69). Agreement for compatibility between the senior evaluator and the photo evaluation was also very good (κ = .86), and slightly lower for grades (weighed κ = .78).
3.5. Agreement between tests
Agreement between the rapid gel and the microgel assays was good for compatibility (κ = .62) but slight for grades (weighed κ = .40), with the rapid gel assay often having higher grades than the microgel (Figure 3). Agreement for compatibility between the microgel assay and the predicted compatibilities was good (κ = .80), as was agreement between the rapid gel assay and the predicted compatibilities (κ = .69). Despite being categorized as “good agreement,” there were still 15 rapid gel assays and 10 microgel column crossmatches that did not correspond to the predicted compatibilities, 4 of these having matching results between the 2 gel assays (Table 1). Nine of 15 rapid gel/predicted and 7 of 10 microgel/predicted discordant results were incompatible crossmatches that were predicted compatible. The other 6 (rapid gel assay) and 3 (microgel assay) discordant results were crossmatches found compatible but predicted Aa incompatibilities. Twenty‐three of these 25 discrepancies (92%) involved 4 horses that had a change in their antibodies detected between the 2 screenings. Four additional crossmatches, for which the prediction was unknown (ie, unidentified antibodies), were discordant between gel assays.
Figure 3.
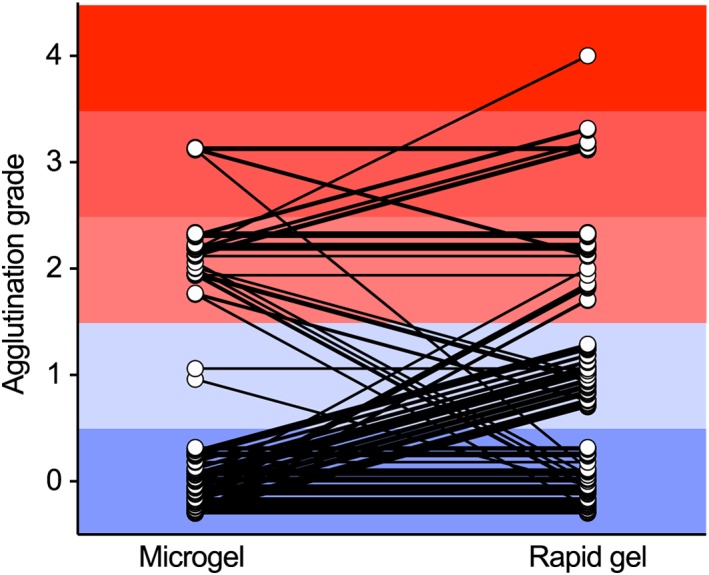
Grade agreement between microgel and rapid gel assays. One hundred thirty‐two crossmatches between the plasma (microgel) or serum (rapid gel) of 11 horses and the erythrocytes of 12 horses. In each section, dots were spread out within the appropriate grade to avoid overlap. Grades highlighted in blue (0 and 1) are considered compatible and grades in red (2‐4) are considered incompatible. Any diagonal line between 2 dots represents a discrepancy of grade and lines crossing between the blue and red sections represent a discrepancy of compatibility between the 2 tests. Agreement between the rapid gel and the microgel assays was good for compatibility (κ = .62) but slight for grades (weighted κ = .40)
3.6. Hemolysis
No hemolysis was detected with the microgel assay. With the rapid gel assay, hemolysis was detected in 10 crossmatches, 8 of them with the serum of the 3 horses with known hemolytic anti‐Ca antibodies. All 8 crossmatches (out of the 27 crossmatches that should have led to hemolysis) also resulted in positive agglutination, both with the rapid gel and microgel assays. The other 2 crossmatches were performed with a serum that contained only anti‐donkey agglutinins. In the absence of hemolysins, they were considered false positives. Overall, when considering the presence of hemolytic antibodies (present in high concentrations upon both screenings), sensitivity for the detection of hemolysis by the rapid gel assay was low (30% [95% CI: .159, .485]), but specificity was high (98% [.933, .997]).
4. DISCUSSION
After adapting the protocol to limit rouleaux formation, the modified rapid gel assay performed satisfactorily when compared to the microgel assay and to the predicted compatibilities.
4.1. Rapid gel assay: technique and interpretation
Rouleaux formation is common with equine erythrocytes and seems to be the main factor preventing the migration of red blood cells through the gel with the rapid gel assay when following the manufacturer's protocol. We were able to circumvent this problem by using serum instead of plasma (removing the possible contribution of fibrinogen to the rouleaux formation), and by modifying the ratio of serum and erythrocytes incubated together. The final dilutions used are roughly half (more diluted) of the ones used in the microgel assay protocol. This made the migration through the gel possible, but also decreased the total number of erythrocytes in the tube. This made the pellet smaller and the reading slightly more difficult, probably contributing to a lower agreement between evaluators. This lower agreement with the rapid gel assay also reflects the greater subjectivity of interpretation with this technique, which was noted to be a major caveat in another study.9 It was not considered a major flaw of the assay in the current study, as discrepancies between evaluators were most often between 2 grades within the same compatibility/incompatibility categories and would overall have little effect on the choice of a donor. Finally, the very simple protocol for cats and dogs became unfortunately more complicated with our modifications as micropipettes are required to prepare the incubation mixture accurately and serum takes longer to prepare than plasma. The rapid gel assay remains nevertheless more convenient for after‐hours use than the microgel assay as there is no need for a customized incubator and centrifuge for the cartridges.
4.2. Microgel assay performed as expected
Microgel column methods are considered highly accurate in human medicine15 and have good sensitivity and specificity when compared to the more time‐consuming and operator‐dependent standard tube technique.8, 16 Excellent agreement between evaluators of different backgrounds reflects the ease of interpretation and the smaller contribution of subjectivity and training required for the reading of the microgel assay. Interpretation is not affected by evaluating images, which is useful when a second opinion is needed or to store data for future use, unlike the conventional tube crossmatch. In the current study and as reported before,8 the microgel column tended to find incompatible crossmatches that were predicted compatible (7 of 10 discordant results), as did the rapid gel assay. Of note, although a previous study8 considered crossmatches with 1+ reactivity incompatible, the definition and images used to describe their 1+ results correspond better to a 2+ reaction in the current and other studies.10, 11
4.3. Discrepancies between tests and the elusive theoretical gold standard
The change in the detection of antibodies over the years in 5 horses was unexpected and made us reconsider the use of the predicted compatibilities as the gold standard. Antibody screening is essentially based on serological tests using the plasma/serum against a panel of erythrocytes from horses and a donkey of known blood types (11 horses and 1 donkey at the Clinical Diagnostic Laboratories at UC Davis). Discrepancies between 2 screenings could be because of a true change in the presence or absence of antibodies, or a change in the capacity of the test to detect antibodies in low concentrations or of low immunoreactivity. It is reported that horses lacking Aa and Ca erythrocyte antigens can develop antibodies against these antigens without known sensitization.17, 18 This could explain the development of anti‐Aa antibodies in horse #2, but does not explain the newly detected anti‐donkey antibodies and the disappearance of anti‐Aa, anti‐Ac, and unidentified antibodies in other horses (Table 1). Together with the fact that horses that had a change in their antibodies were associated with most of the discrepancies between tests, this suggests that there are factors influencing the sensitivity and specificity of the technique itself, particularly when dealing with the detection of weak antibodies. Indeed, 23 of the 25 discordant results between rapid gel and/or microgel assays and predicted results were associated with 3 horses (#2, 3, 5) that had changed antibody profiles over the past years (ie, between the 2 screenings). The UC Davis Clinical Diagnostic Laboratories generously provided the detailed titration results to help interpret this finding. In these 3 horses, antibodies that were either newly detected or no longer detectable could be categorized as weak, that is, only trace/1+ agglutination in 1:2 dilution (which sometime persisted as trace in 1:4 dilution). Change in sensitivity for the detection of antibodies could be due in part to the subjectivity of the reading (although less likely here because of the level of training and the fact that any trace of a positive reaction is considered positive). Inevitable changes over time in the panel of horses and donkeys used may have been associated with different levels of antigen expression, as it is recognized for dog erythrocyte antigen 1 in dogs19 or D antigens in humans,20 which could change the level of detection for some antibodies with weaker affinity. Finally, shipping conditions could affect the detection of less stable antibodies, and despite our best efforts to standardize the process for all research and hospital samples, blood and serum still had to be shipped across borders, which comes with potential delays.
The detection of incompatible crossmatches predicted that compatible could either represent false positive results or a true increased sensitivity of gel‐based crossmatching techniques. In either case, this would not have put the recipient at risk. In opposite, the inability of detecting predicted incompatibilities is of clinical concern, even if related to only weak antibodies. Of clinical interest, questionable mild transfusion reactions, as well as some incompatible microgel crossmatches, had been reported with horse #2 (now retired) presenting newly identified anti‐Aa antibodies (M. Leclere, personal communication).
4.4. Hemolysis
The microgel assay was not designed to detect hemolysis in any species. This was recently investigated by others who demonstrated that the microgel assay had a low sensitivity to detect hemolysis despite the addition of rabbit complement.8 The same was expected with the rapid gel assay and therefore, complement was not included in the protocol. Other reasons not to include rabbit complement are the increased time and complexity of the manipulations and previous data showing that clinically relevant crossmatch incompatibilities in horses (ie, resulting in decreased lifespan of transfused erythrocytes) were detected by the presence of agglutination whether or not hemolysis was detected.6 Despite the absence of exogenous complement, hemolysis was surprisingly observed correctly (ie, as predicted) in 8 crossmatches done with the rapid gel assay, but with low sensitivity (only 8 of 27 predicted). Differences in antigen expression, antibody concentrations, and endogenous complement activity21 could explain why hemolysis was detected in some crossmatches, but not in others. It should be noted that in our data (as in a previous study6), all horses with hemolytic anti‐Ca antibodies also had anti‐Ca agglutinins and that none of the predicted Ca incompatibilities were missed. We cannot extrapolate this finding to antibodies that are usually only hemolytic such as anti‐Qa.17
4.5. Clinical relevance
Approximately 10% of horses have naturally occurring anti‐erythrocyte antibodies, most commonly anti‐Aa and anti‐Ca.2, 18 Based on work in neonatal isoerythrolysis, anti‐Aa and anti‐Qa antibodies are reported to be of greatest clinical relevance, whereas anti‐Ca antibodies may not be clinically important in that context.2, 7 However, it was shown that incompatible crossmatches predicted febrile and tachycardic transfusion reactions (although not life‐threatening) in addition to much shorter erythrocytes half‐life (≈5 days versus ≈30 days), even if the majority of incompatible crossmatches were associated with the presence of Ca antibodies.6 Incompatible crossmatches were also associated with transfusion reactions (urticaria, hemolysis, anaphylactic shock)22 but others did not observe this.23 Although pretransfusion crossmatches are not perfect predictors of transfusion reactions, incompatible crossmatches should preclude transfusion, or warrant washing erythrocytes (or minimally removing plasma) when the minor crossmatch is incompatible. On the contrary, horses with compatible crossmatches have a decreased risk of having a transfusion reaction, but this certainly does not eliminate the need for careful monitoring due to lack of accuracy of the different tests, the possibility that hemolytic antibodies can be missed when complement is not used, as well as other factors contributing to immunologic and non‐immunologic transfusion reactions (ie, other blood components [leukocytes, platelets, plasma proteins], bacterial overgrowth, volume administered, etc.).22
4.6. Limitations
The main limitations of this study are the few Aa incompabilities and the absence of Qa incompabilities. In addition, the Aa incompabilities present had low antibody concentrations, which could have contributed to the inconsistent results obtained with both gel assays. The change in the antibodies detected over time, which were associated with discrepancies between tests for these horses, limited the use of a gold standard to evaluate the rapid gel test. Finally, by adapting the rapid gel protocol to prevent rouleaux formation, we lost some of the appeal of a quick and simple test, that is, which can be performed using a standard plastic pipette.
In conclusion, the modified rapid gel assay could be useful in settings when access to the microgel assay is not available. Discrepancies between both gel techniques and predicted compatibilities most often involved low‐grade agglutination and were associated with horses with antibodies not consistently detected in a reference laboratory, which warrants further investigation to assess their clinical relevance.
CONFLICT OF INTEREST DECLARATION
Since the completion of this study, Dr Blais has started a collaboration with DMS laboratories. This collaboration is non‐financial. Funding was provided by the Equine Health Fund from the Faculty of Veterinary Medicine of the Université de Montréal, supported by Zoetis. Funding sources did not have any involvement in the study design, data analysis and interpretation, or writing and publication of the manuscript.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
All animal manipulations were performed in accordance with the guidelines of the Canadian Council for Animal Care, and the protocol was approved by the Animal Care and Use Committee of the University of Montreal (approval number: 17Rech1915).
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
ACKNOWLEDGMENT
The authors acknowledge Roxane Boivin for her technical assistance, as well as Danielle Carrade Holt and Christina Steckley from the Clinical Diagnostic Laboratories and Veterinary Blood Bank at University of California, Davis Veterinary Medical Teaching Hospital for their collaboration in providing titration data. This work was presented as an oral abstract at the European College of Equine Internal Medicine conference, in Ghent, Belgium, November 7‐10, 2018.
Casenave P, Leclere M, Beauchamp G, Blais M‐C. Modified stall‐side crossmatch for transfusions in horses. J Vet Intern Med. 2019;33:1775–1783. 10.1111/jvim.15519
Present address Pauline Casenave, La Clinique du Cheval, 3910 route de Launac, 31330 Grenade sur Garonne, France
Pauline Casenave and Mathilde Leclere have contributed equally to this work.
REFERENCES
- 1. Dean L. Blood Groups and Red Cell Antigens [Internet] Bethesda (MD): National Center for Biotechnology Information (US); 2005. Chapter 2, Blood group antigens are surface markers on the red blood cell membrane. Available from: https://www.ncbi.nlm.nih.gov/books/NBK2264/. [Google Scholar]
- 2. Sandberg K, Cothran EG. Blood groups and biochemical polymorphisms In: Bowling AT, Ruvinsky A, eds. The Genetics of the Horse. Cambridge, MA: CABI Publishing; 2000:85‐108. [Google Scholar]
- 3. Sandberg K. Guidelines for the interpretation of blood typing tests in horses. Paper presented at: International Society for Animal Genetics 1996:1‐10.
- 4. Sandberg K. Blood typing of horses: current status and application to identification problems. Paper presented at: Proceedings of the 1st Congress on Genetics Applied to Livestock Production, Madrid 1974;253‐265.
- 5. Wong PL, Nickel LS, Bowling AT, Steffey EP. Clinical survey of antibodies against red blood cells in horses after homologous blood transfusion. Am J Vet Res. 1986;47:2566‐2571. [PubMed] [Google Scholar]
- 6. Tomlinson JE, Taberner E, Boston RC, Owens SD, Nolen‐Walston RD. Survival time of cross‐match incompatible red blood cells in adult horses. J Vet Intern Med. 2015;29:1683‐1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bailey E. Prevalence of anti‐red blood cell antibodies in the serum and colostrum of mares and its relationship to neonatal isoerythrolysis. Am J Vet Res. 1982;43:1917‐1921. [PubMed] [Google Scholar]
- 8. Luethy D, Owens SD, Stefanovski D, Nolen‐Walston R, Giger U. Comparison of tube, gel, and immunochromatographic strip methods for evaluation of blood transfusion compatibility in horses. J Vet Intern Med. 2016;30:1864‐1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Villarnovo D, Burton SA, Horney BS, MacKenzie AL, Vanderstichel R. Preliminary evaluation of a gel tube agglutination major cross‐match method in dogs. Vet Clin Pathol. 2016;45:411‐416. [DOI] [PubMed] [Google Scholar]
- 10. Euler CC, Lee JH, Kim HY, Raj K, Mizukami K, Giger U. Survey of two new (Kai 1 and Kai 2) and other blood groups in dogs of North America. J Vet Intern Med. 2016;30:1642‐1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kessler RJ, Reese J, Chang D, Seth M, Hale AS, Giger U. Dog erythrocyte antigens 1.1, 1.2, 3, 4, 7, and Dal blood typing and cross‐matching by gel column technique. Vet Clin Pathol. 2010;39:306‐316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hong H, Choi Y, Hahn S, Park SK, Park BJ. Nomogram for sample size calculation on a straightforward basis for the kappa statistic. Ann Epidemiol. 2014;24:673‐680. [DOI] [PubMed] [Google Scholar]
- 13. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159‐174. [PubMed] [Google Scholar]
- 14. Fleiss JL, Cohen J. The equivalence of weighted kappa and the intraclass correlation coefficient as measures of reliability. Educ Psychol Meas. 1973;33:613‐619. [Google Scholar]
- 15. Noumsi G. The role of automated gel column testing technology in enhancing transfusion safety. MLO Med Lab Obs. 2014;46:34,36. [PubMed] [Google Scholar]
- 16. Garg S, Saini N, Bedi RK, Basu S. Comparison of micro column technology with conventional tube methods for antibody detection. J Lab Physicians. 2017;9:95‐99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Andrews GA, Penedo MCT. Erythrocyte antigens and blood groups In: Weiss DJ, Wardrop KJ, eds. Schalm's Veterinary Hematology. 6th ed. Ames, IA: Wiley‐Blackwell; 2010:711‐724. [Google Scholar]
- 18. Bowling AT. Red blood cell antigens and blood groups in the horse In: Feldman BF, Zinkl JG, Jain NC, eds. Schalm's Veterinary Hematology, 5th. Philadelphia, PA: Lippincott Williams & Wilkins; 2000:774‐777. [Google Scholar]
- 19. Acierno MM, Raj K, Giger U. DEA 1 expression on dog erythrocytes analyzed by immunochromatographic and flow cytometric techniques. J Vet Intern Med. 2014;28:592‐598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Then WL, Aguilar MI, Garnier G. Quantitative detection of weak D antigen variants in blood typing using SPR. Sci Rep. 2017;7:1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sotirov L. Lysozyme and complement concentrations in horses, donkeys and mules. Revue Méd Vét. 2004;155:221‐225. [Google Scholar]
- 22. Hurcombe SD, Mudge MC, Hinchcliff KW. Clinical and clinicopathologic variables in adult horses receiving blood transfusions: 31 cases (1999‐2005). J Am Vet Med Assoc. 2007;231:267‐274. [DOI] [PubMed] [Google Scholar]
- 23. Kallfelz FA, Whitlock RH, Schultz RD. Survival of 59Fe‐labeled erythrocytes in cross‐transfused equine blood. Am J Vet Res. 1978;39:617‐620. [PubMed] [Google Scholar]


