Abstract
Background
The hypothalamic‐pituitary‐adrenal axis regulates the response to sepsis‐associated stress. Relative adrenal insufficiency or adrenocorticotropic hormone (ACTH):cortisol imbalance, defined as a poor cortisol response to administration of ACTH, is common and associated with death in hospitalized foals. However, information on other adrenal steroid response to ACTH stimulation in sick foals is minimal.
Objective
To investigate the response of multiple adrenocortical steroids to administration of ACTH in foals.
Animals
Hospitalized (n = 34) and healthy (n = 13) foals.
Methods
In this prospective study, hospitalized foals were categorized into 2 groups using cluster analysis based on adrenal steroids response to ACTH stimulation: Cluster 1 (n = 11) and Cluster 2 (n = 23). After baseline blood sample collection, foals received 10 μg of ACTH with additional samples collected at 30 and 90 minutes after ACTH. Steroid and ACTH concentrations were determined by immunoassays. The area under the curve (AUC) and Delta0‐30 were calculated for each hormone.
Results
The AUC for cortisol, aldosterone, androstenedione, pregnenolone, 17α‐OH‐progesterone, and progesterone were higher in critically ill (Cluster 1) compared to healthy foals (P < .01). Delta0‐30 for cortisol and 17α‐OH‐progesterone was lower in Cluster 1 (24%, 26.7%) and Cluster 2 (16%, 11.2%) compared to healthy foals (125%, 71%), respectively (P < .05). Foals that died had increased AUC for endogenous ACTH (269 versus 76.4 pg/mL/h, P < .05) accompanied by a low AUC for cortisol (5.5 versus 15.5 μg/dL/h, P < .05), suggesting adrenocortical dysfunction.
Conclusion and Clinical Importance
The 17α‐OH‐progesterone response to administration of ACTH was a good predictor of disease severity and death in hospitalized foals.
Keywords: adrenal insufficiency, cortisol, equine, sepsis
Abbreviations
- ACI
ACTH:cortisol imbalance
- ACTH
adrenocorticotropic hormone
- AUC
area under the curve
- CRH
corticotropin‐releasing hormone
- DHEAS
dehydroepiandrosterone sulfate
- HPAA
hypothalamic‐pituitary‐adrenal axis
- IgG
immunoglobulin G
- RAI
relative adrenal insufficiency
- ROC
receiver operating characteristic
1. INTRODUCTION
Sepsis remains the main cause of death in critically ill human and equine neonates.1, 2, 3, 4 In response to sepsis‐associated stress, the hypothalamic‐pituitary‐adrenal axis (HPAA) is activated, releasing adrenocorticotropic hormone (ACTH), cortisol and aldosterone to modulate metabolic, cardiovascular, and immune functions.5, 6 However, the secretion of cortisol can be relatively insufficient for the severity of disease, reducing survival.3, 4, 7 Relative adrenal insufficiency (RAI) or critical illness‐related corticosteroid insufficiency is defined as an impaired glucocorticoid response to endogenous or exogenous ACTH.1, 3, 5, 7, 8 Recently, the concept of RAI was challenged, suggesting that hypercortisolemia from reduced cortisol biodegradation might lead to endogenous ACTH suppression in the absence of adrenocortical dysfunction.9 Therefore, a new term, ACTH to cortisol dissociation or ACTH:cortisol imbalance (ACI), has been introduced in critically ill human patients and equine neonates.10, 11, 12 Regardless of the exact pathogenesis, RAI or ACI has been associated with disease severity and death in children and newborn foals, and it is therefore possible that hormonal replacement treatment might decrease hospitalization durations and increase patient survival.1, 2, 3, 4, 11, 13 Although adrenocortical hypofunction has been extensively investigated in people and other species, there is still a considerable debate regarding its clinical importance and what the best diagnostic tests are to assess it.1, 2, 3, 4
In addition to cortisol and aldosterone, a multitude of steroid precursors and neuroactive steroids (pregnenolone, progesterone, androstenedione, and dehydroepiandrosterone sulfate [DHEAS]) are also produced by the adrenal cortex during steroidogenesis. High baseline concentration of multiple adrenal steroids, including progestogens and androgens, is associated with poor outcome and severity of disease in critically ill foals.11
Neuroactive steroids (neurosteroids) can be synthesized from cholesterol or steroid precursors (progestogens in particular) in the central and peripheral nervous systems.14, 15 Neurosteroids modulate neuronal activity, including hypothalamic neurons via gamma‐aminobutyric acid receptors (GABAA), and therefore influence HPAA function. GABAergic neurons can inhibit the hypothalamic secretion of corticotropin‐releasing hormone (CRH), which in turn results in less ACTH and cortisol secretion in acute stress.14
Traditionally, HPAA function has been evaluated by measuring baseline ACTH and cortisol concentrations (ACTH/cortisol ratio) or the cortisol response to exogenous ACTH administration (ACTH stimulation test).5, 7 Cortisol concentration provides valuable information in sick patients; however, in some instances it might not reflect the integrity of the HPAA. For example, reduced cortisol metabolism can increase circulating cortisol in the absence of HPAA upregulation; this in turn could also suppress ACTH secretion.9 Conversely, high concentrations of an inactive isoform of ACTH could fail to stimulate adrenocortical cortisol production, inaccurately suggesting adrenocortical failure or suppression.12, 16 Of interest, the DHEAS response to exogenous ACTH stimulation has been proposed as an alternative and perhaps better method of evaluating HPAA integrity and diagnosing central adrenal insufficiency in hospitalized people.17, 18, 19
Dynamic testing of adrenal insufficiency in foals has focused on the cortisol response to exogenous ACTH.3, 13 However, the response of other adrenal steroids and their precursors to the ACTH stimulation test has not been evaluated in critically ill equine neonates. Therefore, the goal of our study was to investigate the response of multiple adrenocortical steroids to exogenous ACTH in healthy and hospitalized foals. We hypothesized that hospitalized and healthy foals will have a distinct adrenocortical steroid profile after ACTH administration. We also proposed that a poor adrenocortical steroid and steroid precursor response to ACTH will be associated with disease severity and death in critically ill foals.
2. MATERIALS AND METHODS
2.1. Animals
This prospective clinical study was carried out at The Ohio State University (Columbus, OH), Rood and Riddle Equine Hospital (Lexington, KY), the University of Georgia (Athens, GA), and equine farms in Kentucky and Ohio. Adrenocorticotropic hormone stimulation tests were performed in 34 hospitalized and 13 healthy foals. Hospitalized foals were classified into 1 of 2 groups (clusters): Cluster 1 (n = 11) and Cluster 2 (n = 23), based on a cluster analysis using the area under the curve (AUC) for circulating steroids.20 Foals were determined to be healthy by lack of abnormalities identified on physical examination, normal CBC (ADVIA 2120i Hematology System, Siemens Medical Solutions, Malvern, Pennsylvania) and serum biochemical profile (Roche COBAS c501 Roche Diagnostics, Indianapolis, Indiana), and immunoglobulin G (IgG) concentrations >800 mg/dL (Roche COBAS c501, Roche Diagnostics).
Foals discharged from the hospital were considered survivors. Foals that died or were euthanized because of a grave medical prognosis were defined as non‐survivors, whereas those euthanized for nonmedical reasons (eg, financial limitations) were not included in this study.
This study was approved by the Institutional Animal Care and Use Committee and the Veterinary Clinical Research Advisory Committees of The Ohio State University and the University of Georgia, and adhered to the principles for the humane treatment of animals in veterinary clinical investigations as stated by the American College of Veterinary Internal Medicine and National Institutes of Health guidelines. Owner consent was obtained before inclusion in the study.
2.2. Clinical data
History obtained from medical records included medications administered, duration of pregnancy, maternal illness, premature lactation, assisted parturition, dystocia, passing and appearance of fetal membranes, and parity. Foal clinical information included signalment, physical examination, CBC, serum biochemistry, and IgG concentrations. For consistency, the sepsis scores were calculated by the first author for each foal, based on clinical history, physical examination, and laboratory findings.
2.3. Study design
After the baseline blood sample collection on admission (Time 0), each foal received 10 μg of ACTH (cosyntropin) (Novaplus, Rockford, Illinois) IV. Additional blood samples were collected at 30 (Time 30) and 90 (Time 90) minutes post‐ACTH administration. Adrenocorticotropic hormone stimulation tests in healthy foals were performed at the farms after routine newborn foal examination. Blood samples were placed in plain serum and chilled aprotinin‐EDTA tubes. Aprotinin (500 kU/mL of blood) was added to preserve peptide hormone integrity. Samples were centrifuged at 2000g at 4°C for 10 minutes. Serum and plasma were aliquoted and stored at −80°C until analyzed. Blood samples for blood culture, CBC, serum biochemistry, and IgG concentrations were processed immediately.
2.4. Hormone concentrations
Serum aldosterone, androstenedione, cortisol, DHEAS, pregnenolone, progesterone, and 17α‐hydroxyprogesterone concentrations were determined using human‐specific immunoassays according to the manufacturer's instructions (Coat‐A‐Count Radioimmunoassays, Siemens Healthcare Diagnostics, Los Angeles, California; Pregnenolone ELISA kit, Abnova, Walnut, California), and previously validated for equine samples.2, 8, 11 Adrenocorticotropic hormone concentrations were measured with a human‐specific immunochemiluminometric assay previously validated for horses (Immulite, Siemens, Los Angeles, California).8
2.5. Data analysis
Data sets were tested for normality by the Shapiro‐Wilk statistic and results were presented as median and interquartiles or median and ranges. Considering that hormone concentrations were non‐normally distributed, nonparametric tests were used for statistical analysis. Differences in endogenous ACTH and ACTH‐stimulated steroid concentrations were analyzed by the 2‐way (time and group of foals) Friedman repeated measures analysis of variance on ranks, with Student‐Newman‐Keuls post hoc test for multiple comparisons.
The AUC is used in endocrinology as a method to summarize longitudinal hormone information.21, 22 Area under the curve for each hormone was generated by the trapezoidal method.21 These variables (AUCs) were then applied in a cluster analysis using the k‐means method to define groups (clusters) of foals with similar endocrine patterns (adrenocortical steroid response to ACTH stimulation).20 Cluster analysis aims at categorizing different objects into groups in a way that the degree of association between 2 objects is maximal if they belong to the same group and minimal otherwise.20
The delta hormone was defined as the percent change in hormone concentration between Times 0 and 30 (Delta0‐30), and Times 0 and 90 (Delta0‐90) post ACTH stimulation. Blood concentration of all adrenal steroids measured returned to the baseline at 90 minutes post ACTH stimulation. Therefore, the (Delta0‐90) was considered not clinically relevant.
Kruskal‐Wallis statistics with Dunn's post hoc test were used to compare 3 groups of foals. When comparing 2 groups of foals, the Mann‐Whitney rank sum test was used. Relationships between categorical variables were analyzed using contingency tables and chi‐square analysis.
Receiver operating characteristic (ROC) curves and AUC were constructed to determine the predictive abilities and cutoff values of Delta0‐30 17α‐OH‐progesterone for non‐survival. The optimal cutoff value was defined by the points representing the highest sensitivity and specificity to predict non‐survival. Statistical analysis was performed using statistical software (SPSS 21, IBM Corporation, New York, New York).
3. RESULTS
3.1. Study population
A total of 47 foals (≤48 hours old) were included in the study: 72% (34/47) were hospitalized and 28% (13/47) were healthy. Within hospitalized foals, 32% (11/34) were classified in Cluster 1 and 68% (23/34) in Cluster 2. Forty‐six percent (22/47) were fillies and 54% (25/47) were colts. The median age of foals in Clusters 1, 2, and healthy foals was 4 hours (range: 1‐48 hours), 3.5 hours (range: 1‐48 hours), and 16 hours (range: 12‐24 hours), respectively (P < .01). Breeds represented included Thoroughbred (n = 23), Quarter Horse (n = 8), Standardbred (n = 5), Saddlebred (n = 3), Appaloosa (n = 3), Warmblood (n = 3), Arabian (n = 1), and Belgian (n = 1).
In hospitalized foals, 27% had a positive blood culture in Cluster 1 and 17% in Cluster 2 (P > .05).
The overall death rate in neonatal foals was 17% (8/47). In hospitalized foals, the death rate was 22% (2/9) in Cluster 1 and 27% (6/22) in Cluster 2 (P > .05).
3.2. Adrenocortical steroid response to ACTH stimulation in hospitalized and healthy foals
Clusters 1 and 2 were different in their response to exogenous ACTH stimulation (Figure 1). Foals in Cluster 1 had higher concentrations of aldosterone, cortisol, 17α‐OH‐progesterone, and androstenedione compared to Cluster 2 and healthy foals at all 3 time points (P < .01). Progesterone and pregnenolone concentrations were higher in Clusters 1 and 2 than in healthy foals at all 3 time points (P < .01). Endogenous ACTH decreased from baseline to 30 minutes after ACTH stimulation in Clusters 1 and 2 (P < .05). There were no differences in DHEAS concentrations between groups at any time point.
Figure 1.
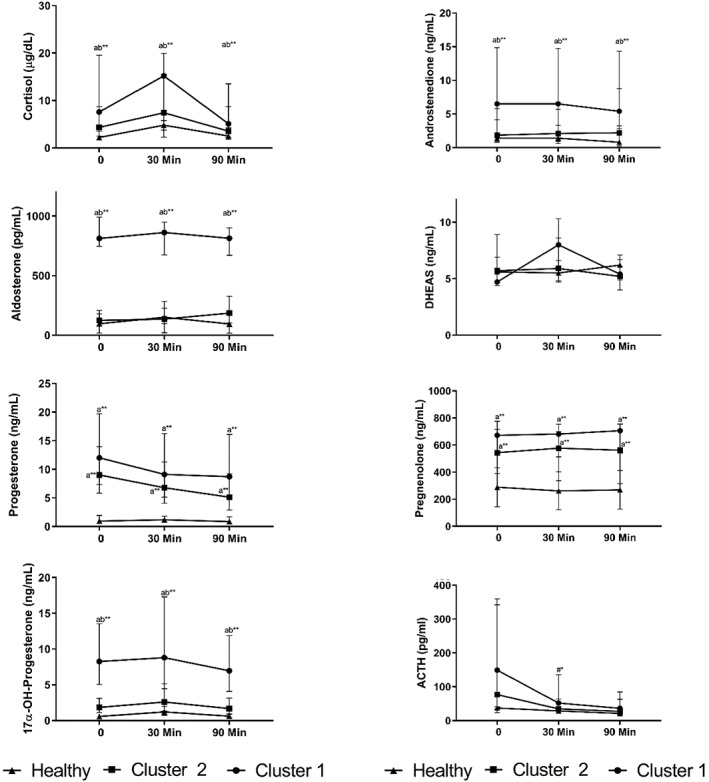
Hormone concentrations in Clusters 1 (n = 11), 2 (n = 23), and healthy (n = 13) foals at 0, 30, and 90 minutes after ACTH stimulation (median and interquartiles). Letters “a” indicates compared to healthy and “b” indicates compared to Cluster 2; “#” indicates compared to Time 0. ACTH, adrenocorticotropic hormone; DHEAS, dehydroepiandrosterone sulfate. *P < .05; **P < .01
3.3. Laboratory findings, AUC, and Delta0‐30 for adrenocortical steroids in hospitalized and healthy foals
The AUC and Delta0‐30 for adrenocortical steroids in hospitalized and healthy foals are presented in Table 1. Cluster 1 had higher AUC for aldosterone, cortisol, androstenedione, pregnenolone, 17α‐OH‐progesterone, and progesterone compared to healthy foals (P < .01). The AUC for pregnenolone and progesterone were higher in Cluster 2 than healthy foals (P < .01). Cluster 1 had higher AUC for aldosterone and 17α‐OH‐progesterone than Cluster 2 (P < .05). Delta0‐30 for cortisol and 17α‐OH‐progesterone were lower in Clusters 1 and 2 compared to healthy foals (P < .05). However, Delta0‐30 DHEAS was higher in Cluster 2 compared to healthy foals (P < .05). There were no differences in Delta0‐30 for other steroids in hospitalized and healthy foals.
Table 1.
Area under the curve, Delta0‐30 for adrenocortical steroids, ACTH/cortisol ratio, and laboratory findings in Clusters 1 and 2, and healthy foals (median and ranges)
| Cluster 1 (n = 11) | Cluster 2 (n = 23) | Healthy (n = 13) | |
|---|---|---|---|
| Sepsis score | 13 (5 to 16) * , a | 11 (3 to 22) * , a | 1 (0 to 3) |
| WBC (103/μL) | 7.1 (4.3 to 14.8) | 6.9 (2.1 to 21.1) | 8.2 (8.1 to 12.5) |
| IgG (mg/dL) | 417 (100 to 1596) * , a | 598 (80 to 1600) | 1617 (1054 to 2342) |
| L‐lactate (mmol/L) | 12.8 (7 to 16.6) * , a | 6.1 (2.5 to 14.6) * , a | 1.8 (0.9 to 2.4) |
| Neutrophil count (103/μL) | 3.5 (2.1 to 12.3) * , a | 5.6 (0.25 to 18.5) | 7.8 (6.9 to 11.3) |
| Glucose (mg/dL) | 109 (44 to 203) * , a | 101 (21 to 280) * , a | 167 (159 to 192) |
| Creatinine (mg/dL) | 7.8 (2.6 to 20.9) * , a b | 3.9 (1 to 9.4) * , a | 1.3 (0.8 to 2.6) |
| AUC aldosterone (pg/mL/h) | 1251 (871 to 1555) ** , a b | 251.4 (37.4 to 755) | 184 (22.7 to 883) |
| AUC cortisol (μg/dL/h) | 18.7 (3.8 to 35) * , a | 9.5 (3 to 34.5) | 5.8 (3 to 11.7) |
| AUC androstenedione (ng/mL/h) | 9.2 (1.6 to 34.8) * , a | 3.4 (0.5 to 17.8) | 1.7 (0.4 to 19.8) |
| AUC ACTH (pg/mL/h) | 108 (35.6 to 643) | 79 (18.2 to 583) | 47 (26 to 94) |
| AUC pregnenolone (ng/mL/h) | 1045 (654 to 1335) ** , a | 850 (16 to 1188) ** , a | 295 (32 to 672) |
| AUC 17α‐OH‐progesterone (ng/mL/h) | 15.8 (2.6 to 26.2) ** , a b | 7.3 (0.3 to 39.2) | 1.4 (0.4 to 4) |
| AUC progesterone (ng/mL/h) | 10 (4.8 to 43.8) ** , a | 13.5 (0.73 to 35.9) ** , a | 1.3 (0.42 to 5.23) |
| AUC DHEAS (ng/mL/h) | 10.4 (4.4 to 21.5) | 8.9 (1.1 to 203) | 8.1 (3.6 to 26.5) |
| Delta0‐30 aldosterone (%) | −1.4 (−31.6 to 52) | 33.8 (−43.7 to 125) | 17 (−16 to 124) |
| Delta0‐30 cortisol (%) | 24 (−88 to 345.8) * , a | 16 (−91 to 295) * , a | 125 (1.3 to 316) |
| Delta0‐30 androstenedione (%) | −12.4 (−47.5 to 57) | 0.2 (−90 to 972) | −5 (−85 to 382) |
| Delta0‐30 ACTH (%) | −47 (−93 to 60) | −41 (−97 to 61) | −22 (−100 to 70) |
| Delta0‐30 pregnenolone (%) | 4 (−33 to 24) | 2.2 (−36 to 144) | −7.7 (−34 to 3.2) |
| Delta0‐30 17α‐OH‐progesterone (%) | 26.7 (−79 to 195) * , a | 11.2 (−61 to 763) * , a | 71 (−1.4 to 528) |
| Delta0‐30 progesterone (%) | −4 (−43 to 5) | −10 (−73 to 335.5) | 7.6 (−50 to 439) |
| Delta0‐30 DHEAS (%) | 25 (7.5 to 131) * , a | 4 (−47 to 90) | −4 (−25 to 45) |
| Non‐survival (%) | 22 (2/9) | 27 (6/22) | N/A |
| Positive blood culture (%) | 27 (3/11) | 17 (4/23) | N/A |
| ACTH/cortisol ratio | 38.5 (3 to 235) | 36 (1.7 to 140) | 19 (7.2 to 57) |
| Hospitalization duration (days) | 6 (2 to 19) * , b | 4 (0 to 8) | N/A |
Abbreviations: ACTH, adrenocorticotropic hormone; AUC, area under the curve; DHEAS, dehydroepiandrosterone sulfate; IgG, immunoglobulin G; N/A, non‐applicable; WBC, white blood count.
P < .05; **P < .01.
Compared to healthy.
Compared to Cluster 2.
Regarding laboratory and clinical findings, foals in Cluster 1 had lower blood glucose and IgG concentrations as well as neutrophil count and higher L‐lactate concentrations than healthy foals (P < .05). Blood L‐lactate was higher and glucose concentration was lower in Cluster 2 compared to healthy foals (P < .05). The sepsis score was higher in Clusters 1 and 2 compared to healthy foals (P < .05). However, there was no difference in the sepsis score between Clusters 1 and 2. Foals in Cluster 1 had higher creatinine concentrations and prolonged hospital stay compared to Cluster 2 and healthy foals (P < .05). There was no difference in ACTH/cortisol ratio between clusters and healthy foals.
3.4. Laboratory findings, AUC, and Delta0‐30 for adrenocortical steroids in surviving and non‐surviving hospitalized foals
The AUC and Delta0‐30 for adrenocortical steroids in surviving and non‐surviving foals are presented in Table 2. Foals that did not survive had lower AUC for cortisol but higher AUC for ACTH (P < .05). Delta0‐30 17α‐OH‐progesterone was lower in non‐surviving than surviving foals (P < .05).
Table 2.
Area under the curve and Delta0‐30 for adrenocortical steroids, ACTH/cortisol ratio, and laboratory findings in surviving and non‐surviving hospitalized foals (median and ranges)
| Non‐survivors (n = 8) | Survivors (n = 26) | |
|---|---|---|
| Sepsis score | 16 (13 to 22)** | 10 (3 to 16) |
| WBC (103/μL) | 3.7 (2.1 to 7)** | 8.1 (2.8 to 21.1) |
| IgG (mg/mL) | 250 (100 to 811)* | 645 (161 to 1600) |
| L‐lactate (mmol/L) | 8.75 (4.1 to 14.6) | 7.5 (2.5 to 16.6) |
| Glucose (mg/mL) | 46.5 (24 to 203)* | 111 (21 to 280) |
| Neutrophil count (103/μL) | 1.48 (0.25 to 5.4)** | 6.43 (1.85 to 18.5) |
| Creatinine (mg/dL) | 5.2 (2.6 to 7.1) | 3.7 (1 to 20.9) |
| AUC aldosterone (pg/mL/h) | 458 (196 to 1555) | 335 (37 to 1583) |
| AUC cortisol (μg/dL/h) | 5.5 (3 to 23.8)* | 15.5 (3.8 to 35) |
| AUC androstenedione (ng/mL/h) | 5 (1.6 to 11) | 8.5 (0.5 to 34.8) |
| AUC ACTH (pg/mL/h) | 269 (70.5 to 584)* | 76.4 (18.2 to 643.2) |
| AUC pregnenolone (ng/mL/h) | 1029 (430 to 1335) | 868 (16.7 to 1221) |
| AUC 17α‐OH‐progesterone (ng/mL/h) | 8 (0.33 to 23.5) | 9.6 (0.3 to 39.2) |
| AUC progesterone (ng/mL/h) | 16 (10.6 to 34.3) | 11 (0.73 to 44) |
| AUC DHEA (ng/mL/h) | 7.6 (4.4 to 204) | 11.4 (1.11 to 925) |
| Delta0‐30 aldosterone (%) | 16 (−21.7 to 105) | 11.5 (−44 to 128) |
| Delta0‐30 cortisol (%) | 0.6 (−88 to 157) | 24 (−91 to 345) |
| Delta0‐30 androstenedione (%) | −16 (−46 to 15) | −3 (−90 to 121) |
| Delta0‐30 ACTH (%) | −19.5 (−93 to 60.5) | −53 (−100 to 61) |
| Delta0‐30 pregnenolone (%) | 1.1 (−19 to 29) | 3.4 (−36 to 144) |
| Delta0‐30 17α‐OH‐progesterone (%) | −7 (−61 to 36)* | 58 (−79 to 762) |
| Delta0‐30 progesterone (%) | −32 (−52 to 335) | 1.2 (−72 to 440) |
| Delta0‐30 DHEAS (%) | 7.5 (−27 to 77.2) | 15 (−47 to 132) |
| ACTH/cortisol ratio | 52 (3 to 140) | 32.6 (1 to 235) |
| Hospitalization duration (days) | 2 (1 to 8) | 3 (0 to 19) |
Abbreviations: ACTH, adrenocorticotropic hormone; AUC, area under the curve; DHEAS, dehydroepiandrosterone sulfate; IgG, immunoglobulin G; WBC, white blood count.
P < .05; **P < .01.
Foals that died during hospitalization had lower white blood cell and neutrophil count, as well as lower IgG and glucose concentrations than surviving foals (P < .01). The sepsis score was higher in foals that died compared to those that survived (P < .01). There were no differences in creatinine concentrations, ACTH/cortisol ratios, and hospitalization stay between these 2 groups of foals.
3.5. Receiver operating characteristic curves and cutoff value for Delta0‐30 17α‐OH‐progesterone to predict non‐survival
Of the calculated Delta0‐30, only 17α‐OH‐progesterone was significantly lower in foals that died compared to survivors. In order to determine the cutoff value below which non‐survival could be most reliably predicted by the Delta0‐30 of 17α‐OH‐progesterone, sensitivity and specificity were calculated based on the ROC curve (Figure 2). The optimal cutoff value was −19% with sensitivity, specificity, positive, and negative predictive values of 87, 70, 93, and 52%, respectively. The AUC for ROC Delta0‐30 of 17α‐OH‐progesterone was 0.83 showing good discrimination between foals that died and those that survived.
Figure 2.
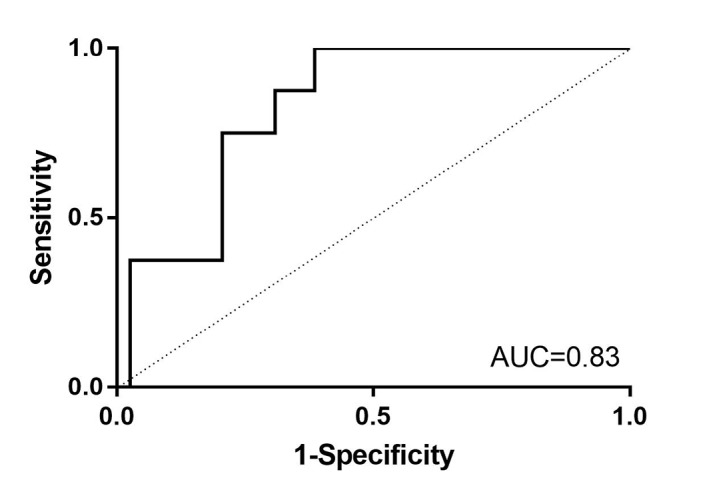
Receiver operating characteristic curve for Delta0‐30 17α‐OH‐progesterone to predict non‐survival in neonatal foals. A cutoff value of (−19%) maximized sensitivity (87%) and specificity (70%) to predict death. AUC, area under the curve
4. DISCUSSION
This study showed that concentrations of multiple adrenocortical steroids and steroid precursors were altered in hospitalized newborn foals and their response to exogenous ACTH was variable. In addition, a lower 17α‐OH‐progesterone response to ACTH stimulation was the most consistent finding associated with non‐survival in critically ill foals. We demonstrated that AUCs for adrenocortical steroids after ACTH stimulation was associated with some clinical variables of disease severity in hospitalized foals. This study shows that adrenocortical dysfunction can be assessed by measuring the response of multiple steroids to administration of exogenous ACTH in critically ill equine neonates.
The AUC has been used in endocrine research as a method to assess ultradian and circadian hormone dynamics as well as overall hormone secretion over a specific time period.21, 22, 23 In the current study, the AUC for each hormone were used in cluster analysis to identify 2 clusters of foals with similar adrenocortical steroid response to ACTH administration within group.20, 21 Cluster 1 included ill foals presented with lower neutrophil count, IgG, and glucose concentrations compared to healthy foals. These foals had higher creatinine concentrations and prolonged hospital stay compared to those of foals from Cluster 2. Aforementioned clinical and laboratory variables such as neutropenia, azotemia, low IgG, and glucose concentrations are well recognized risk factors of sepsis in hospitalized foals.3, 8, 24, 25 In addition, foals in Cluster 1 had higher AUC for cortisol, but also for aldosterone, 17α‐OH‐progesterone, and androstenedione compared to foals in Cluster 2. High concentrations of these steroids before and after ACTH stimulation are consistent with a proper response to stress from critical illness. Other causes for increased steroid concentrations over time include reduced biodegradation and clearance.11, 12 Recently, it was proposed that impaired cortisol clearance from suppressed activity of cortisol‐metabolizing enzymes (A‐ring reductase and 11β‐hydroxysteroid dehydrogenase 1) contributes to hypercortisolemia in critically ill human patients.9 Reduced metabolism of adrenocortical steroids might be involved in adrenal hypofunction in foals and deserve further investigation.
Our results are in line with other studies, which demonstrated high basal cortisol, aldosterone, progestogens, and androgens concentrations in hospitalized foals.2, 3, 11, 26 Elevated steroid secretion in response to critical illness was considered appropriate for most of the foals of this study, whereas concentrations in the reference range indicated a poor response. The traditional definition of RAI or ACI has been based on an inadequate cortisol response to exogenous ACTH stimulation in critically ill patients with septic shock and multiple organ dysfunction, and similar criteria have been applied to severely ill foals.1, 3, 13 In addition to cortisol, the synthesis of other adrenal steroids and steroid precursors might also be altered because of severity of disease. In such situations, the administration of ACTH can be useful at assessing function of all 3 adrenocortical layers.2, 17
The key finding in our study was the impaired response of 17α‐OH‐progesterone and cortisol (low Delta0‐30) to ACTH stimulation in critically ill and non‐surviving foals. This is in agreement with other studies in sick equine neonates that have shown a reduced cortisol secretion in response to exogenous ACTH administration.3, 13 High baseline 17α‐OH‐progesterone and progesterone concentrations are associated with severity of disease and death in septic foals.11 There are increased progesterone and 17α‐OH‐progesterone responses to ACTH stimulation in dogs with adrenal‐ and pituitary‐dependent hyperadrenocorticism, in children with congenital adrenal hyperplasia, and in healthy intact and ovariohysterectomized cats and cows.27, 28, 29, 30 In the current study, the progestogen response to ACTH was lower in foals that died. The reason for the lower 17α‐OH‐progesterone secretion in non‐surviving foals after ACTH administration remains unclear. Both progesterone and 17α‐OH‐progesterone are adrenocortical precursors of cortisol; progesterone is synthesized from pregnenolone by 3β‐hydroxysteroid dehydrogenase to be further converted into 17α‐OH‐progesterone by 17‐α‐hydroxylase, and ultimately to cortisol.28, 29, 31, 32 A deficiency of these enzymes or a shift in adrenal steroidogenesis might reduce progesterone and 17α‐OH‐progesterone concentrations, indirectly resulting in inappropriate glucocorticoid synthesis.28 Although the cortisol response to ACTH stimulation was lower, DHEAS concentrations increased after ACTH stimulation in critically ill foals in our study, further supporting a dissociation in steroidogenesis.11
Of the steroids measured before and after ACTH stimulation in the foals of this study, only Delta0‐3017α‐OH‐progesterone was associated with death. The 17α‐OH‐progesterone response to ACTH stimulation had the best ability to discriminate surviving from non‐surviving foals. This study demonstrates an association between a progestogen response to ACTH and outcome in hospitalized foals. The mechanisms by which the secretion of this steroid is altered in foals with severe disease remain to be elucidated. Inflammatory factors such as IL‐1, TGF‐β, and TNF‐α can modulate the biosynthesis of adrenal steroids in bovine and ovine adrenocortical cells,33, 34, 35 and could have potentially altered progestogen synthesis and secretion in sick foals.
Maturation of the equine fetal HPAA in terms of cortisol secretion occurs late in gestation compared to other species.36 Fetal cortisol concentrations increase rapidly 4‐7 days before delivery when 17α‐hydroxylase expression rises in the zona fasciculata.36, 37 Altered HPAA maturation with decreased 17α‐hydroxylase activity could be an explanation for reduced 17α‐OH‐progesterone production in the non‐surviving foals in our study. Moreover, the median age between healthy and hospitalized foals was different in this study, and ideally, the age of foals should have been similar between groups. This could potentially affect adrenal steroids synthesis and metabolism in hospitalized foals. A possible link between adrenocortical steroid enzyme activity, adrenal insufficiency, age, and outcome in hospitalized equine neonates remains to be evaluated.
The decrease in endogenous ACTH concentrations in response to cosyntropin in the hospitalized foals of this study was expected.5, 38 This indicates that pituitary corticotropes and hypothalamic neurons remain responsive to negative glucocorticoid feedback. It also suggests that adrenocortical dysfunction is the main cause of adrenal insufficiency (primary adrenal insufficiency) in foals.5, 38 Foals that died had increased AUC for ACTH but decreased AUC for cortisol, further supporting the concept of adrenal hypofunction. This ACTH:cortisol dissociation can be explained by adrenocortical resistance to ACTH, low concentration of bioactive ACTH, impaired biodegradation of ACTH, or adrenocortical insufficiency.9, 11, 16 In contradiction to previous studies, the ACTH/cortisol ratio was not different between groups of foals in the current study.3, 11, 25 This difference could be because of the size of the foal population included in studies, variable death rate, and severity of disease. The association of AUCs and death in foals from our study might also suggest that AUC is a more accurate indication of adrenal hypofunction than a hormone ratio on admission. Administration of exogenous CRH and AVP could provide further clarification on mechanisms involved in HPAA dysfunction in critically ill foals.
5. CONCLUSIONS
In summary, our study demonstrated that adrenal dysfunction (RAI/ACI) is a complex process that involves multiple adrenocortical steroids beyond the cortisol response to both exogenous and endogenous ACTH. The 17α‐OH‐progesterone response to ACTH might offer prognostic value for severity of disease and death in critically ill equine neonates. Future studies evaluating ACTH stimulation testing in a larger number of non‐surviving foals, measuring multiple adrenocortical steroids in concert, will likely offer the most value for further dissection of how severe illness and sepsis influence the global adrenocortical function.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
This study was approved by the IACUC and the Veterinary Clinical Research Advisory Committee of The Ohio State University and University of Georgia and adhered to the principles for the humane treatment of animals in veterinary clinical investigations as stated by the American College of Veterinary Internal Medicine and National Institutes of Health guidelines.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
ACKNOWLEDGMENTS
Results of this work were partially presented as an oral abstract at the 2015 American College of Veterinary Internal Medicine Forum, Indianapolis, Indiana. The authors thank Caroline Brown and Sarah Scott for their assistance with laboratory techniques. Special thanks to clinicians and technicians from Rood and Riddle Equine Hospital, University of Georgia, and The Ohio State University Galbreath Equine Center for samples collection. The authors also thank Ashley Lansaw at Rood and Riddle Equine Hospital for samples processing. The authors gratefully acknowledge the support of Morris Animal Foundation Fellowship Training Grant and Grayson‐Jockey Club Research Foundation.
Dembek KA, Johnson LM, Timko KJ, et al. Multiple adrenocortical steroid response to administration of exogenous adrenocorticotropic hormone to hospitalized foals. J Vet Intern Med. 2019;33:1766–1774. 10.1111/jvim.15527
Funding information Grayson‐Jockey Club Research Foundation; Morris Animal Foundation, Grant/Award Number: Fellowship Training Grant
REFERENCES
- 1. Dellinger RP, Levy MM, Rhodes A, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med. 2013;39:165‐228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dembek KA, Onasch K, Hurcombe SD, et al. Renin‐angiotensin‐aldosterone system and hypothalamic‐pituitary‐adrenal axis in hospitalized newborn foals. J Vet Intern Med. 2013;27:331‐338. [DOI] [PubMed] [Google Scholar]
- 3. Hart KA, Slovis NM, Barton MH. Hypothalamic‐pituitary‐adrenal axis dysfunction in hospitalized neonatal foals. J Vet Intern Med. 2009;23:901‐912. [DOI] [PubMed] [Google Scholar]
- 4. Marik PE, Pastores SM, Annane D, et al. Recommendations for the diagnosis and management of corticosteroid insufficiency in critically ill adult patients: consensus statements from an international task force by the American College of Critical Care Medicine. Crit Care Med. 2008;36:1937‐1949. [DOI] [PubMed] [Google Scholar]
- 5. Hart KA, Barton MH. Adrenocortical insufficiency in horses and foals. Vet Clin North Am Equine Pract. 2011;27:19‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Toribio RE. Endocrine dysregulation in critically ill foals and horses. Vet Clin North Am Equine Pract. 2011;27:35‐47. [DOI] [PubMed] [Google Scholar]
- 7. Annane D. Adrenal insufficiency in sepsis. Curr Pharm Des. 2008;14:1882‐1886. [DOI] [PubMed] [Google Scholar]
- 8. Hurcombe SD, Toribio RE, Slovis N, et al. Blood arginine vasopressin, adrenocorticotropin hormone, and cortisol concentrations at admission in septic and critically ill foals and their association with survival. J Vet Intern Med. 2008;22:639‐647. [DOI] [PubMed] [Google Scholar]
- 9. Boonen E, Vervenne H, Meersseman P, et al. Reduced cortisol metabolism during critical illness. N Engl J Med. 2013;368:1477‐1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Boonen E, Bornstein SR, Van den BG. New insights into the controversy of adrenal function during critical illness. Lancet Diabetes Endocrinol. 2015;3:805‐815. [DOI] [PubMed] [Google Scholar]
- 11. Dembek KA, Timko KJ, Johnson LM, et al. Steroids, steroid precursors, and neuroactive steroids in critically ill equine neonates. Vet J. 2017;225:42‐49. [DOI] [PubMed] [Google Scholar]
- 12. Peeters B, Boonen E, Langouche L, Van den Berghe G. The HPA axis response to critical illness: new study results with diagnostic and therapeutic implications. Mol Cell Endocrinol. 2015;408:235‐240. [DOI] [PubMed] [Google Scholar]
- 13. Wong DM, Vo DT, Alcott CJ, Peterson AD, Sponseller BA, Hsu WH. Baseline plasma cortisol and ACTH concentrations and response to low‐dose ACTH stimulation testing in ill foals. J Am Vet Med Assoc. 2009;234:126‐132. [DOI] [PubMed] [Google Scholar]
- 14. Crowley SK, Girdler SS. Neurosteroid, GABAergic and hypothalamic pituitary adrenal (HPA) axis regulation: what is the current state of knowledge in humans? Psychopharmacology (Berl). 2014;231:3619‐3634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gunn BG, Cunningham L, Mitchell SG, Swinny JD, Lambert JJ, Belelli D. GABAA receptor‐acting neurosteroids: a role in the development and regulation of the stress response. Front Neuroendocrinol. 2015;36:28‐48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jellyman JK, Valenzuela OA, Allen VL, Forhead AJ, Holdstock NB, Fowden AL. Neonatal glucocorticoid overexposure programs pituitary‐adrenal function in ponies. Domest Anim Endocrinol. 2015;50:45‐49. [DOI] [PubMed] [Google Scholar]
- 17. Beishuizen A, Thijs LG, Vermes I. Decreased levels of dehydroepiandrosterone sulphate in severe critical illness: a sign of exhausted adrenal reserve? Crit Care. 2002;6:434‐438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Oberbeck R, Kobbe P. Dehydroepiandrosterone (DHEA): a steroid with multiple effects. Is there any possible option in the treatment of critical illness? Curr Med Chem. 2010;17:1039‐1047. [DOI] [PubMed] [Google Scholar]
- 19. Sayyed KL, El SK, Chaiban J, et al. Measurements of serum DHEA and DHEA sulphate levels improve the accuracy of the low‐dose cosyntropin test in the diagnosis of central adrenal insufficiency. J Clin Endocrinol Metab. 2012;97:3655‐3662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wishart D. Efficient hierarchical cluster analysis for data mining and knowledge discovery. Comput Sci Stat. 1998;30:257‐263. [Google Scholar]
- 21. Fekedulegn DB, Andrew ME, Burchfiel CM, et al. Area under the curve and other summary indicators of repeated waking cortisol measurements. Psychosom Med. 2007;69:651‐659. [DOI] [PubMed] [Google Scholar]
- 22. Pruessner JC, Gaab J, Hellhammer DH, Lintz D, Schommer N, Kirschbaum C. Increasing correlations between personality traits and cortisol stress responses obtained by data aggregation. Psychoneuroendocrinology. 1997;22:615‐625. [DOI] [PubMed] [Google Scholar]
- 23. Keenan DM, Alexander S, Irvine C, Veldhuis JD. Quantifying nonlinear interactions within the hypothalamo‐pituitary‐adrenal axis in the conscious horse. Endocrinology. 2009;150:1941‐1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dembek KA, Hurcombe SD, Frazer ML, Morresey PR, Toribio RE. Development of a likelihood of survival scoring system for hospitalized equine neonates using generalized boosted regression modeling. PLoS One. 2014;9:e109212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gold JR, Divers TJ, Barton MH, et al. Plasma adrenocorticotropin, cortisol, and adrenocorticotropin/cortisol ratios in septic and normal‐term foals. J Vet Intern Med. 2007;21:791‐796. [DOI] [PubMed] [Google Scholar]
- 26. Aleman M, Pickles KJ, Conley AJ, et al. Abnormal plasma neuroactive progestagen derivatives in ill, neonatal foals presented to the neonatal intensive care unit. Equine Vet J. 2013;45:661‐665. [DOI] [PubMed] [Google Scholar]
- 27. Chatdarong K, Ponglowhapan S, Karlsson A, Linde‐Forsberg C. The effect of ACTH stimulation on cortisol and progesterone concentrations in intact and ovariohysterectomized domestic cats. Theriogenology. 2006;66:1482‐1487. [DOI] [PubMed] [Google Scholar]
- 28. Honour JW. 17‐Hydroxyprogesterone in children, adolescents and adults. Ann Clin Biochem. 2014;51:424‐440. [DOI] [PubMed] [Google Scholar]
- 29. Monroe WE, Panciera DL, Zimmerman KL. Concentrations of noncortisol adrenal steroids in response to ACTH in dogs with adrenal‐dependent hyperadrenocorticism, pituitary‐dependent hyperadrenocorticism, and nonadrenal illness. J Vet Intern Med. 2012;26:945‐952. [DOI] [PubMed] [Google Scholar]
- 30. Yoshida C, Nakao T. Plasma cortisol and progesterone responses to low doses of adrenocorticotropic hormone in ovariectmized lactating cows. J Reprod Dev. 2006;52:797‐803. [DOI] [PubMed] [Google Scholar]
- 31. Khashana A, Ojaniemi M, Leskinen M, Saarela T, Hallman M. Term neonates with infection and shock display high cortisol precursors despite low levels of normal cortisol. Acta Paediatr. 2015;105:154‐158. [DOI] [PubMed] [Google Scholar]
- 32. McManus F, Fraser R, Davies E, Connell JM, Freel EM. Plasma steroid profiling and response to trophins to illustrate intra‐adrenal dynamics. J Endocrinol. 2015;224:149‐157. [DOI] [PubMed] [Google Scholar]
- 33. Perrin A, Pascal O, Defaye G, et al. Transforming growth factor beta 1 is a negative regulator of steroid 17 alpha‐hydroxylase expression in bovine adrenocortical cells. Endocrinology. 1991;128:357‐362. [DOI] [PubMed] [Google Scholar]
- 34. Rainey WE, Naville D, Saez JM, et al. Transforming growth factor‐beta inhibits steroid 17 alpha‐hydroxylase cytochrome P‐450 expression in ovine adrenocortical cells. Endocrinology. 1990;127:1910‐1915. [DOI] [PubMed] [Google Scholar]
- 35. Straub RH, Gluck T, Cutolo M, et al. The adrenal steroid status in relation to inflammatory cytokines (interleukin‐6 and tumour necrosis factor) in polymyalgia rheumatica. Rheumatology (Oxford). 2000;39:624‐631. [DOI] [PubMed] [Google Scholar]
- 36. Fowden AL, Forhead AJ, Ousey JC. Endocrine adaptations in the foal over the perinatal period. Equine Vet J. 2012;44(Suppl 41):130‐139. [DOI] [PubMed] [Google Scholar]
- 37. Ousey JC, Rossdale PD, Fowden AL, Palmer L, Turnbull C, Allen WR. Effects of manipulating intrauterine growth on post natal adrenocortical development and other parameters of maturity in neonatal foals. Equine Vet J. 2004;36:616‐621. [DOI] [PubMed] [Google Scholar]
- 38. Hurcombe SD. Hypothalamic‐pituitary gland axis function and dysfunction in horses. Vet Clin North Am Equine Pract. 2011;27:1‐17. [DOI] [PubMed] [Google Scholar]


