Abstract
Background
Lymphocytic‐plasmacytic enteritis is the common form of idiopathic inflammatory bowel disease (IBD) in dogs. In human IBD, disturbances of amino acid metabolism have been demonstrated to be involved in the pathophysiology of IBD. Therefore, plasma amino acid profile might represent a novel marker of human IBD.
Objectives
To determine the plasma amino acid profiles of dogs with IBD and its usefulness as a novel marker of IBD in dogs.
Animals
Fasting blood plasma was obtained from 10 dogs with IBD and 12 healthy dogs.
Methods
All IBD dogs were prospectively included in this study, and heparinized blood samples were collected. The plasma concentrations of 21 amino acids were determined using the ninhydrin method. The relationships among the plasma amino acid concentrations and plasma C‐reactive protein (CRP) concentration, canine chronic enteropathy clinical activity index (CCECAI), and overall World Small Animal Veterinary Association (WSAVA) score were investigated.
Results
Median concentration (nmol/mL) of methionine [46.2; range, 30.0‐59.3], proline [119.4; range, 76.7‐189.2], serine [115.1; range, 61.4‐155.9], and tryptophan [17.4; range, 11.9‐56.3]) were significantly lower than in control dogs [62.6; range, 51.0‐83.6, 199.1; range, 132.5‐376.7, 164.3; range, 124.7‐222.9, and 68.3; range, 35.7‐94.8, respectively]. A negative correlation was identified between the plasma serine concentration and CCECAI (r s = −.67, P = .03), but there were no correlations between plasma amino acid concentrations and CRP concentration or overall WSAVA score.
Conclusions and Clinical Importance
Plasma serine concentration might represent a novel maker of IBD in dogs.
Keywords: canine, chronic enteropathy, gastrointestinal disease, metabolism
Abbreviations
- ALB
albumin
- CCECAI
canine chronic enteropathy clinical activity index
- CRP
C‐reactive protein
- IBD
idiopathic inflammatory bowel disease
- IL
interleukin
- LPE
lymphocytic‐plasmacytic enteritis
- PAR
protease‐activated receptor
- PLE
protein‐losing enteropathy
- TP
total protein
- WSAVA
World Small Animal Veterinary Association
1. INTRODUCTION
Lymphocytic‐plasmacytic enteritis (LPE) is the most common form of inflammatory bowel disease (IBD) in dogs affecting the small intestine, characterized by the diffuse infiltration of lymphocytes and plasma cells into the enteric lamina propria. The clinical features of dogs with IBD are persistent gastrointestinal manifestations such as vomiting, diarrhea, and weight loss. The etiology of IBD is unknown although it has been suggested that dysfunction of the mucosal immune system with loss of tolerance to luminal antigens plays an important role in its pathogenesis.1
The etiology of human IBD also remains to be established.2 In humans, plasma amino acid analysis is used to provide a metabolomic assessment of health status. Moreover, disturbances of metabolic homeostasis are involved in the pathogenesis of metabolic diseases, chronic inflammatory disorders, and cancers.3, 4, 5 Specific disturbances in amino acid metabolism characterize the pathophysiological stage of IBD, and therefore the plasma amino acid profile represents a novel, noninvasive, diagnostic, and staging marker for human IBD.6, 7
In veterinary medicine, the plasma amino acid profile is altered in dogs with mammary gland tumors, critical illness, or superficial necrolytic dermatitis.8, 9, 10 Therefore, plasma amino acid concentrations might provide useful biomarkers in dogs with various disorders. There are alterations in serum amino acid concentrations in dogs with protein‐losing enteropathy (PLE).11 Therefore, the aim of the present study was to characterize the plasma amino acid profile of dogs with IBD.
2. MATERIALS AND METHODS
2.1. Animals and plasma samples
Heparinized plasma samples were obtained from 10 dogs with IBD. Dogs referred to our veterinary hospital between August 2012 and September 2013 for evaluation of chronic gastrointestinal tract disorders were recruited for this study. A diagnosis of IBD was made on the basis of clinical signs (vomiting, diarrhea, and weight loss) of at least 3 weeks' duration, the exclusion of other causes of gastrointestinal manifestations, and the identification of lymphoplasmacytic inflammation on the histopathologic review of duodenal biopsy samples.12, 13, 14
The exclusion criterion was evidence of other causes of gastrointestinal manifestations, including metabolic disease, infection, parasitic disease, and other causes of hypoalbuminemia, including hepatic and renal disease. Such evidence was provided by performing a complete blood count, a serum biochemistry profile including preprandial total bile acids, fecal examination, urinalysis including urinary protein‐to‐creatinine ratio, and abdominal radiography and ultrasonography. Food‐responsive diarrhea was ruled out by the lack of complete remission of clinical signs after 2 weeks of feeding an elimination diet,15 such as a novel antigen diet or a hydrolyzed protein diet. Antibiotics‐responsive diarrhea was ruled out by the lack of a complete response to 2 weeks of administration of metronidazole (10‐15 mg/kg PO, twice daily). Dogs treated with corticosteroids within 2 weeks of presentation were also excluded.
Duodenal mucosal biopsy samples were obtained endoscopically from all 10 dogs with IBD. Before the endoscopic biopsy procedure, the dogs were sedated with midazolam (.1 mg/kg, IV) and butorphanol tartrate (.2 mg/kg, IV). Anesthesia was induced with propofol (4‐6 mg/kg, IV) and maintained with isoflurane in oxygen. Gastroduodenoscopy was performed under anesthesia using a flexible video endoscope (VQ‐8143A, AVS, Tokyo, Japan), and multiple (6‐8) mucosal biopsies were obtained from each of the descending duodenum and caudal duodenal flexure using serrated biopsy forceps (FB‐53Q‐1, AVS). During the endoscopic procedures, electrocardiogram, respiratory rate, rectal temperature, arterial blood pressure, pulse oximetry values, and capnography values were monitored and recorded. All endoscopic procedures were completed within 2 hours. Biopsy samples for histologic examination were fixed in neutral buffered 10% formalin, embedded in paraffin wax, and hematoxylin and eosin‐stained sections were prepared. Histopathologic diagnosis was carried out by an American College of Veterinary Pathologists board‐certified pathologist (Y.K.) and each case was scored according to histopathologic standards established by the World Small Animal Veterinary Association (WSAVA).12 All the dogs diagnosed with IBD were also scored using the canine chronic enteropathy clinical activity index (CCECAI) for assessment of the clinical severity of their disease.16 The total CCECAI score was classified as grade 1 (score 0‐3), grade 2 (score 4‐5), grade 3 (score 6‐8), grade 4 (score 9‐11), or grade 5 (score >12). Written consent from the owner was obtained for the inclusion of each dog in the study.
Control heparinized plasma samples were obtained from 12 healthy control dogs (6 Beagles and 6 Mongrels) that were housed in a research colony and fed a commercial dry diet. These consisted of 5 intact females and 7 intact males, and their median age was 3.5 years (range, 1‐14 years). The median body mass of the control dogs was 12.6 kg (range, 9.2‐14.6 kg). None has shown clinical manifestations of gastrointestinal disease or weight loss for more than 1 year before blood sampling. Hematologic, serum biochemical, fecal, and abdominal ultrasonographic examinations were performed on all the dogs.
This study was approved by the Animal Care and Use Committee and the Laboratory Animal Experimentation Committee of our University (approval no. 08‐0332).
2.2. Plasma amino acids analysis
Blood samples for amino acid analysis were collected in heparinized tubes after at least 8 hours of fasting to minimize the influence of diet on circulating amino acids. Heparinized blood was immediately centrifuged and the plasma separated. Then, the plasma total protein (TP), albumin (ALB), and C‐reactive protein (CRP) concentrations were measured by dry chemistry (Dry‐Chem 7000 Z, Fujifilm Medical, Tokyo, Japan). Plasma samples for amino acid analysis were stored at −80 °C until analysis, then briefly thawed, mixed with same volume of 5% (w/v) trichloroacetic acid, and centrifuged for 15 minutes at 9000g at 4 °C to remove proteins. The supernatant was collected and filtered through a .45‐μm syringe filter (Millipore, Bedford, Massachusetts). Plasma amino acids were separated by cation‐exchange chromatography and identified spectrophotometrically after reaction with ninhydrin reagent. Twenty‐one basic amino acids (alanine, arginine, asparagine, citrulline, glutamic acid, glutamine, glycine, histidine, isoleucine, leucine, lysine, methionine, ornithine, phenylalanine, proline, serine, taurine, threonine, tryptophan, tyrosine, and valine) were assayed using an automated amino acid analyzer (L‐8900, Hitachi, Tokyo, Japan).
2.3. Statistical analysis
The data were analyzed using JMP Pro 12 software (SAS Institute Inc, Cary, North Carolina). Their normality was assessed using the Shapiro‐Wilk W test. Then, Student's t test or the Wilcoxon rank sum (Mann‐Whitney U) test was used. Bonferroni correction of the 21 plasma amino acid concentrations was applied, after which P < .002 was defined as representing statistical significance.
In addition, plasma amino acid concentrations were compared between IBD dogs with hypoalbuminemia (ALB <2.6 g/dL) and normoalbuminemia (ALB ≥2.6 g/dL) using Student's t test or the Wilcoxon rank sum (Mann‐Whitney U) test, with Bonferroni correction.
Fisher's exact test was used to identify differences between values of categorical variables. The relationships among each plasma amino acid concentration, CPR concentration, total CCECAI score, and overall WSAVA score for IBD dogs was evaluated using the Spearman rank correlation coefficient. Values of P < .05 were considered to represent statistical significance.
3. RESULTS
3.1. Inflammatory bowel disease in dogs
Ten dogs with a diagnosis of IBD were included in this study. All of these dogs had inflammation in their intestinal mucosal samples and a histologic diagnosis of LPE. The median age of the dogs was 8.5 years (range, 4.7‐12.8 years) and they comprised 8 males, 2 of which were neutered, and 2 neutered females. Their median body mass was 5.4 kg (range, 2.5‐14.3 kg) and they comprised 3 Miniature Dachshunds, and 1 each of Boston Terrier, Italian Greyhound, Miniature Schnauzer, Papillon, Shih‐Tzu, Toy Poodle, and mixed breed dog. The CCECAI scores of the 10 dogs with IBD were grade 1: 3 dogs, grade 2: 1 dog, grade 3: 2 dogs, grade 4: 3 dogs, and grade 5: 1 dog. The median CCECAI score for the dogs with IBD was 6.5 (range, 1‐18) and the median overall WSAVA score was 2 (range, 1‐5). The medians and ranges of the plasma TP, ALB, and CRP concentrations were 4.9 g/dL (3.1‐7.4 g/dL), 2.7 g/dL (1.5‐3.7 g/dL), and .4 mg/dL (.1‐5.7 mg/dL), respectively. Four of the 10 IBD dogs were hypoalbuminemia (ALB <2.6 g/dL) and 6 were normoalbuminemia (ALB ≥2.6 g/dL). Plasma ALB concentration was significantly lower (P = .02) and plasma CRP concentration was significantly higher (P < .001) in dogs with IBD. There was also a significant difference in body mass (P < .001) between IBD dogs and control dogs. In contrast, the age and sex distribution were not significantly different (P > .06) between the 2 groups.
3.2. Plasma amino acid concentrations in dogs with IBD
In dogs with IBD, the plasma concentrations of 4 amino acids (methionine, proline, serine, and tryptophan) were significantly lower than in control dogs (Table 1). There was a significant negative correlation between plasma serine concentration and total CCECAI scores in dogs with IBD (Figure 1), but none of the plasma amino acid concentrations of dogs with IBD showed correlations with plasma CRP or overall WSAVA scores. In addition, there were no significant differences in any of the plasma amino acid concentrations between the 4 hypoalbuminemic IBD dogs and the 6 normoalbuminemic dogs after Bonferroni correction (P > .04).
Table 1.
Plasma amino acid concentrations in dogs with inflammatory bowel disease (IBD) and healthy control dogs
| Controls | IBD | P‐value | |
|---|---|---|---|
| n | 12 | 10 | |
| Alanine | 506.0 (403.8‐726.5) | 447.1 (244.7‐1190.7) | .64 |
| Arginine | 103.9 (69.9‐158.9) | 91.7 (53.7‐166.2) | .32 |
| Asparagine | 52.4 (40.7‐78.3) | 39.6 (23.9‐87.0) | .008 |
| Citrulline | 58.3 (22.2‐77.7) | 69.3 (27.5‐155.4) | .32 |
| Glutamic acid | 24.9 (14.1‐52.2) | 45.2 (32.6‐78.0) | .009 |
| Glutamine | 819.0 (588.5‐1003.4) | 508.7 (390.5‐1312.5) | .05 |
| Glycine | 233.4 (180.2‐335.3) | 160.7 (96.8‐305.9) | .02 |
| Histidine | 77.9 (67.7‐95.3) | 62.6 (47.6‐84.0) | .008 |
| Isoleucine | 62.7 (37.1‐72.2) | 54.8 (34.8‐90.0) | .84 |
| Leucine | 133.0 (61.4‐187.5) | 106.2 (70.2‐229.1) | .51 |
| Lysine | 127.6 (89.4‐177.9) | 137.7 (83.4‐286.2) | .79 |
| Methionine | 62.6 (51.0‐83.6) | 46.2 (30.0‐59.3) | <.001 |
| Ornithine | 15.7 (10.7‐31.2) | 19.7 (10.7‐33.2) | .51 |
| Phenylalanine | 65.9 (54.5‐75.2) | 59.8 (37.5‐71.7) | .13 |
| Proline | 199.1 (132.5‐376.7) | 119.4 (76.7‐189.2) | .001 |
| Serine | 164.3 (124.7‐222.9) | 115.1 (61.4‐155.9) | .001 |
| Taurine | 115.6 (69.6‐211.8) | 118.4 (53.4‐174.9) | .95 |
| Threonine | 206.2 (147.8‐444.8) | 118.7 (71.0‐215.4) | .005 |
| Tryptophan | 68.3 (35.7‐94.8) | 17.4 (11.9‐56.3) | .001 |
| Tyrosine | 54.6 (41.7‐81.5) | 40.6 (26.6‐60.3) | .003 |
| Valine | 169.7 (97.8‐230.0) | 153.2 (95.6‐378.3) | .74 |
| Total | 2982.8 (2264.6‐3899.3) | 2643.4 (1923.9‐4869.6) | .06 |
Notes: Data are presented as median (range). Amino acid concentrations are in nmol/mL.
Figure 1.
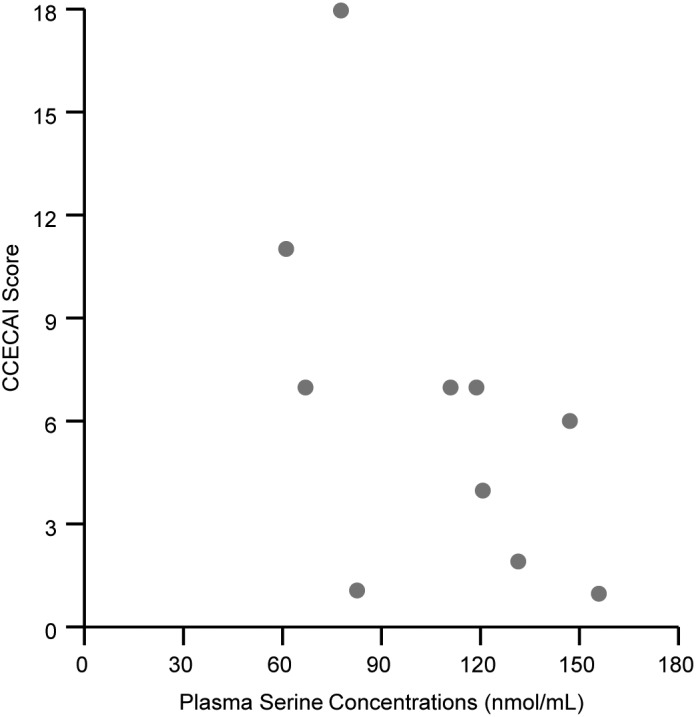
Correlation between plasma serine concentrations (x‐axis) and the canine chronic enteropathy clinical activity index (CCECAI) scores (y‐axis) in dogs with IBD. Plasma serine concentrations were negatively correlated with the CCECAI scores (r s = −.67, P = .03)
4. DISCUSSION
In the present study, we aimed to identify disturbances in amino acid metabolism in dogs with IBD. Significantly lower plasma methionine, proline, serine, and tryptophan concentrations were found in dogs with IBD, and plasma serine concentration was negatively correlated with CCECAI score. However, we did not find any differences in plasma amino acid concentrations between IBD dogs with hypoalbuminemia or normoalbuminemia, although a previous study of dogs with PLE reported that only serum tryptophan concentration was significantly decreased in PLE dogs compared to healthy dogs.11
In this study, we used the post‐column ninhydrin method for plasma amino acid analysis. This method is thought to be a more quantitative and reproducible analytical method than pre‐column derivatization followed by high‐performance liquid chromatography, and less likely to be affected by the nature of matrix containing the biogenic substance, such as serum or plasma. However, 1 major disadvantage is that it has a lower sensitivity than the pre‐column method. Nevertheless, on balance, the post‐column ninhydrin method appears suitable for the analysis of amino acids concentrations in plasma.
In human IBD, plasma histidine and tryptophan concentrations are significantly lower than normal and show negative correlations with disease activity indexes.6 Serum tryptophan concentrations are significantly lower in dogs with PLE than in healthy dogs. Serum tryptophan concentration also correlates with serum ALB concentration in dogs with PLE, but not with clinical disease stage (CCECAI scores).11 Whereas in our study, the concentrations of 4 amino acids including tryptophan and serine were significantly lower than those of control dogs, and only plasma serine concentration showed a negative correlation with CCECAI scores. Thus, plasma serine concentration might represent a novel marker in dogs with IBD.
Serine act on protease‐activated receptor (PAR)‐2, which is expressed widely, but particularly in the intestine.17 Intestinal PAR‐2 and fecal serine protease activity are higher in dogs with IBD and might contribute to intestinal cytokine expression.18 In murine and human cell lines, serine proteases produced by infectious Enterobacteriaceae induce inflammatory responses through PAR‐2.19, 20 Moreover, the high fecal serine protease activity reflects dysbiosis of the intestinal microbiome in human IBD patients.21 Thus, fecal serine proteases might originate in intestinal bacteria under inflammatory conditions. Intestinal dysbiosis, with overrepresentation of fecal Enterobacteriaceae, occurs in dogs with IBD.22 Therefore, it is possible that intestinal serine is consumed by pathogenic bacteria, and lower plasma serine concentrations might be the result of serine protease synthesis by the abnormal intestinal microbiome in dogs with IBD.
Tryptophan is the least prevalent essential amino acid in mammals.23 Tryptophan supplementation ameliorates clinical signs, improves feed conversion ratio, ameliorates histologic signs of colonic inflammation, and reduces gut permeability and the expression of several pro‐inflammatory cytokines in animal models of dextran sodium sulfate‐induced colitis.23, 24 Recently, interest in amino acids, including tryptophan, has been focused on the relationship between intestinal inflammation and their therapeutic potential in IBD.25, 26, 27 Therefore, oral supplementation with tryptophan might represent a novel therapeutic strategy for dogs with IBD, especially in IBD dogs with PLE. Because, similar to the result of the present study, serum tryptophan concentration is low in dogs with PLE.11 One of the mechanisms whereby tryptophan supplementation could be therapeutically effective is through inhibition of reactive oxygen species, such as superoxide.24 Tryptophan and methionine have antioxidant ability.28, 29 Therefore, antioxidant amino acids might be effective at preventing intestinal inflammation.
There were several limitations to the present study. First, the number of cases was small, and the group of IBD dogs studied contained animals with hypoalbuminemia and others with normoalbuminemia. Thus, it is possible that limited number of IBD dogs with hypoalbuminemia made differences in amino acid profiles between 10 dogs with IBD in our study and 30 dogs with PLE in the previous study. In addition, dogs with PLE without intestinal biopsies were still included in the previous study. Therefore, a further study of a larger number of cases is needed to confirm the typical amino acid profile of histopathologically confirmed IBD dogs with and without PLE. Second, we could not completely exclude the presence of liver failure or pancreatic insufficiency, because we did not measure postprandial bile acids or canine trypsin‐like immunoreactivity in all the dogs. Third, we could not completely exclude a diagnosis of antibiotics‐responsive diarrhea, because the IBD dogs were treated with metronidazole but not tylosin. Fourth, there are no accurate species‐specific reference ranges for plasma amino acid concentrations in dogs. Finally, the control dogs were not food, body weight, or breed‐matched to the IBD dogs in the study.
In conclusion, the present study has demonstrated that the plasma concentrations of 4 amino acids are significantly lower in dogs with IBD than in healthy dogs. In particular, plasma serine concentration negatively correlated with CCECAI score. Thus, plasma serine concentration might represent a novel marker of IBD in dogs. However, further studies are required to determine whether correction of abnormal serum serine concentrations might improve the CCECAI scores in dogs with IBD.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
All procedures and use of dogs in this study were approved by the Animal Care and Use Committee and the Laboratory Animal Experimentation Committee, Graduate School of Veterinary Medicine, Hokkaido University.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
ACKNOWLEDGMENTS
This study was presented as abstract form at the 2014 American College of Veterinary Internal Medicine Forum, Nashville, Tennessee. The authors thank N. Takeda at Instrumental Analysis Division, Equipment Management Center, Creative Research Institution, Hokkaido University, for technical support of amino acid analysis.
Tamura Y, Ohta H, Kagawa Y, et al. Plasma amino acid profiles in dogs with inflammatory bowel disease. J Vet Intern Med. 2019;33:1602–1607. 10.1111/jvim.15525
REFERENCES
- 1. German AJ, Hall EJ, Day MJ. Chronic intestinal inflammation and intestinal disease in dogs. J Vet Intern Med. 2003;17:8‐20. [DOI] [PubMed] [Google Scholar]
- 2. Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427‐434. [DOI] [PubMed] [Google Scholar]
- 3. Gu Y, Chen T, Fu S, et al. Perioperative dynamics and significance of amino acid profiles in patients with cancer. J Transl Med. 2015;13:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kamaura M, Nishijima K, Takahashi M, Ando T, Mizushima S, Tochikubo O. Lifestyle modification in metabolic syndrome and associated changes in plasma amino acid profiles. Circ J. 2010;74:2434‐2440. [DOI] [PubMed] [Google Scholar]
- 5. Melchior D, Sève B, Le Floc'h N. Chronic lung inflammation affects plasma amino acid concentrations in pigs. J Anim Sci. 2004;82:1091‐1099. [DOI] [PubMed] [Google Scholar]
- 6. Hisamatsu T, Okamoto S, Hashimoto M, et al. Novel, objective, multivariate biomarkers composed of plasma amino acid profiles for the diagnosis and assessment of inflammatory bowel disease. PLoS One. 2012;7:e31131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hisamatsu T, Ono N, Imaizumi A, et al. Decreased plasma histidine level predicts risk of relapse in patients with ulcerative colitis in remission. PLoS One. 2015;10:e0140716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Azuma K, Osaki T, Tsuka T, Imagawa T, Minami S, Okamoto Y. Plasma free amino acid profiles of canine mammary gland tumors. J Vet Sci. 2012;13:433‐436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chan DL, Rozanski EA, Freeman LM. Relationship among plasma amino acids, C‐reactive protein, illness severity, and outcome in critically ill dogs. J Vet Intern Med. 2009;23:559‐563. [DOI] [PubMed] [Google Scholar]
- 10. Outerbridge CA, Marks SL, Rogers QR. Plasma amino acid concentrations in 36 dogs with histologically confirmed superficial necrolytic dermatitis. Vet Dermatol. 2002;13:177‐186. [DOI] [PubMed] [Google Scholar]
- 11. Kathrani A, Allenspach K, Fascetti AJ, Larsen JA, Hall EJ. Alterations in serum amino acid concentrations in dogs with protein‐losing enteropathy. J Vet Intern Med. 2018;32:1026‐1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Day MJ, Bilzer T, Mansell J, et al. Histopathological standards for the diagnosis of gastrointestinal inflammation in endoscopic biopsy samples from the dog and cat: a report from the World Small Animal Veterinary Association Gastrointestinal Standardization Group. J Comp Pathol. 2008;138(Suppl 1):S1‐S43. [DOI] [PubMed] [Google Scholar]
- 13. Washabau RJ, Day MJ, Willard MD, et al. Endoscopic, biopsy, and histopathologic guidelines for the evaluation of gastrointestinal inflammation in companion animals. J Vet Intern Med. 2010;24:10‐26. [DOI] [PubMed] [Google Scholar]
- 14. Schmitz S, Garden OA, Werling D, Allenspach K. Gene expression of selected signature cytokines of T cell subsets in duodenal tissues of dogs with and without inflammatory bowel disease. Vet Immunol Immunopathol. 2012;146:87‐91. [DOI] [PubMed] [Google Scholar]
- 15. Maeda S, Ohno K, Nakamura K, et al. Increased expression of fractalkine and its receptor CX3CR1 in canine inflammatory bowel disease and their possible role in recruitment of intraepithelial lymphocytes. Vet Immunol Immunopathol. 2012;148:226‐235. [DOI] [PubMed] [Google Scholar]
- 16. Allenspach K, Wieland B, Gröne A, Gaschen F. Chronic enteropathies in dogs: evaluation of risk factors for negative outcome. J Vet Intern Med. 2007;21:700‐708. [DOI] [PubMed] [Google Scholar]
- 17. Rothmeier AS, Ruf W. Protease‐activated receptor 2 signaling in inflammation. Semin Immunopathol. 2012;34:133‐149. [DOI] [PubMed] [Google Scholar]
- 18. Maeda S, Ohno K, Uchida K, et al. Intestinal protease‐activated receptor‐2 and fecal serine protease activity are increased in canine inflammatory bowel disease and may contribute to intestinal cytokine expression. J Vet Med Sci. 2014;76:1119‐1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kida Y, Inoue H, Shimizu T, Kuwano K. Serratia marcescens serralysin induces inflammatory responses through protease‐activated receptor 2. Infect Immun. 2007;75:164‐174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hansen KK, Sherman PM, Cellars L, et al. A major role for proteolytic activity and proteinase‐activated receptor‐2 in the pathogenesis of infectious colitis. Proc Natl Acad Sci U S A. 2005;102:8363‐8368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Midtvedt T, Zabarovsky E, Norin E, et al. Increase of faecal tryptic activity relates to changes in the intestinal microbiome: analysis of Crohn's disease with a multidisciplinary platform. PLoS One. 2013;8:e66074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Minamoto Y, Otoni CC, Steelman SM, et al. Alteration of the fecal microbiota and serum metabolite profiles in dogs with idiopathic inflammatory bowel disease. Gut Microbes. 2015;6:33‐47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kim CJ, Kovacs‐Nolan JA, Yang C, Archbold T, Fan MZ, Mine Y. L‐tryptophan exhibits therapeutic function in a porcine model of dextran sodium sulfate (DSS)‐induced colitis. J Nutr Biochem. 2010;21:468‐475. [DOI] [PubMed] [Google Scholar]
- 24. Shizuma T, Mori H, Fukuyama N. Protective effect of tryptophan against dextran sulfate sodium‐ induced experimental colitis. Turk J Gastroenterol. 2013;24:30‐35. [DOI] [PubMed] [Google Scholar]
- 25. He F, Wu C, Li P, et al. Functions and signaling pathways of amino acids in intestinal inflammation. Biomed Res Int. 2018;2018:9171905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Liu H, Tan B, Huang B, et al. Involvement of calcium‐sensing receptor activation in the alleviation of intestinal inflammation in a piglet model by dietary aromatic amino acid supplementation. Br J Nutr. 2018;120:1321‐1331. [DOI] [PubMed] [Google Scholar]
- 27. Liu Y, Wang X, Hu CA. Therapeutic potential of amino acids in inflammatory bowel disease. Nutrients. 2017;9:920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Saito K, Jin DH, Ogawa T, et al. Antioxidative properties of tripeptide libraries prepared by the combinatorial chemistry. J Agric Food Chem. 2003;51:3668‐3674. [DOI] [PubMed] [Google Scholar]
- 29. Zhang H, Hu CA, Kovacs‐Nolan J, et al. Bioactive dietary peptides and amino acids in inflammatory bowel disease. Amino Acids. 2014;47:2127‐2141. [DOI] [PubMed] [Google Scholar]


