Abstract
Background
Steroid‐responsive meningitis‐arteritis (SRMA) is a common inflammatory neurologic disorder of dogs for which certain breeds are predisposed.
Objectives
To determine whether breed differences exist in clinical features, treatment response, and relapse in a population of North American dogs with SRMA, and to evaluate the effect of disease on dogs' quality of life (QoL).
Animals
Sixty‐one client‐owned dogs with SRMA: 29 dogs identified through an American Kennel Club‐Canine Health Foundation survey and 32 dogs from North Carolina (NC) State Veterinary Hospital.
Methods
Retrospective case series. Caregivers completed an online survey to assess QoL.
Results
Breeds represented most often included the Golden Retriever (n = 12), Bernese Mountain Dog (10), Wirehaired Pointing Griffon (9), Boxer (9), and Beagle (6). No breed differences were identified with respect to clinical severity, diagnostic findings, or outcome. Twenty‐nine dogs (48%) had ≥1 disease relapse. There was a significant effect of cerebrospinal fluid nucleated cell count on the frequency of disease relapse (P = .003), but no relationship was identified between treatment protocol and relapse. Dogs' QoL was associated with the severity of corticosteroid‐related adverse effects (P = .03), which were dose‐related (r = .24, P = .02) and more prevalent in Wirehaired Pointing Griffons than in other breeds (P = .04).
Conclusion and Clinical Importance
Golden Retrievers and Wirehaired Pointing Griffons should be considered among the breeds recognized to develop SRMA. Treatment with higher corticosteroid dosages is correlated with more severe adverse effects and worse QoL, but it may not improve clinical outcome.
Keywords: aseptic meningitis, CSF analysis, immune‐mediated disease, treatment response
Abbreviations
- AKC‐CHF
American Kennel Club‐Canine Health Foundation
- CRP
C‐reactive protein
- CSF
cerebrospinal fluid
- MRI
magnetic resonance imaging
- NC
North Carolina
- NCC
nucleated cell count
- QoL
quality of life
- SRMA
steroid‐responsive meningitis‐arteritis
- VH
veterinary hospital
1. INTRODUCTION
Steroid‐responsive meningitis‐arteritis (SRMA) is a systemic immune‐mediated disease characterized by inflammation of the meninges and associated arteries that typically affects dogs 6‐18 months of age.1, 2, 3 Any breed can develop the disease, although a predisposition has been reported in Beagles, Bernese Mountain Dogs, Border Collies, Boxers, English Springer Spaniels, Jack Russell Terriers, Nova Scotia Duck Tolling Retrievers, Weimaraners, and Whippets.2, 4, 5, 6 Acute and chronic forms of SRMA have been described.2, 7 The acute form is manifested by clinical signs of pyrexia, cervical hyperesthesia and lethargy, marked neutrophilic pleocytosis and increased protein concentration in cerebrospinal fluid (CSF), and resolution of clinical signs with corticosteroid treatment.2, 4, 7 The chronic form of SRMA is characterized by repeated episodes of cervical pain, the presence of neurologic deficits, and a predominantly mononuclear or mixed cell CSF pleocytosis with normal or mildly increased protein concentration.2, 7 The etiology of SRMA remains uncertain. Although it is known to be immune‐mediated in nature,8, 9, 10, 11, 12, 13 no environmental, infectious, or neoplastic triggers have been identified to date.3, 5 Because a definitive antemortem diagnostic test for SRMA is not available, a presumptive diagnosis is based on clinical presentation, laboratory findings, exclusion of other diseases, and response to corticosteroids.2, 3, 4 Treatment consists of immunosuppressive dosages of corticosteroids,2, 4, 7 with most dogs responding to treatment, although relapses are frequent.14
Most studies of SRMA have originated from Europe. The clinical characteristics, diagnostic findings, and response to treatment have been described.1, 2, 3, 4, 5, 7, 14 However, there are few reports on SRMA in North America, and any potential geographical differences in the disease have not been explored. In addition, there are characteristics of the disorder that warrant further investigation, including the influence of breed on disease severity and clinical course, and the impact of the disorder on quality of life (QoL).
Our aim was to characterize the acute form of SRMA in a population of dogs in North America. The specific objectives were to determine whether breed differences exist in clinical disease course and treatment response, to identify any factors associated with disease relapse, and to evaluate the caregiver's perception of the effect of SRMA and its treatment on the dog's QoL.
2. MATERIALS AND METHODS
2.1. Case selection criteria
Medical records of cases admitted to North Carolina (NC) State Veterinary Hospital (VH) from July 1, 2003, through June 30, 2017, were searched to identify dogs with a diagnosis of SRMA. In addition, dogs were identified through a survey on SRMA that was developed by the American Kennel Club‐Canine Health Foundation (AKC‐CHF) and distributed to national breed clubs via email and through social media groups related to SRMA in 2016. Medical records of dogs identified through the survey were obtained and reviewed after receipt of owner consent. Inclusion criteria for the study were a diagnosis of the acute form of SRMA based on history of neck pain and absence of other neurologic deficits, CSF analysis indicating a neutrophilic pleocytosis with no evidence of degenerate neutrophils or pathologic organisms, and resolution of clinical signs with corticosteroid treatment. A neutrophilic pleocytosis was defined as CSF nucleated cell count (NCC) >5 cells/μL with >40% of the NCC being neutrophils, but dogs were included in the study with CSF NCC ≤5 cells/μL if increased cellularity was demonstrated on cytology and a diagnosis of neutrophilic pleocytosis was made by a board‐certified veterinary clinical pathologist. For cases with CSF red blood cell (RBC) counts >500 cells/μL, the NCC was adjusted by calculating and subtracting the number of leukocytes expected from peripheral blood contamination, using the ratio of 1 leukocyte to 500 RBCs. Cases were excluded from the study if medical records were incomplete, if the dog had neurologic deficits in addition to neck pain, or if the dog resided outside North America.
2.2. Medical records review
Information retrieved from the medical records included age, breed, sex, neuter status, body weight, duration between onset of clinical signs and initiation of treatment, duration of treatment with corticosteroids, and results of the following additional diagnostic tests when performed: CBC, C‐reactive protein (CRP) concentration, cervical radiographs, magnetic resonance imaging (MRI), arthrocentesis, and infectious disease testing. Dates and dosages of initial and subsequent changes in treatment with corticosteroids and other medications were recorded.
A relapse was defined as recurrent clinical signs that resolved completely after treatment with an increased dosage of corticosteroids, the addition of a second immunomodulatory drug or both. The following data regarding relapses were collected if available: duration between dosage reduction or discontinuation and relapse, clinical signs, diagnostic tests performed, treatment, and total number of relapses. Clinical remission was defined as absence of clinical signs after commencing corticosteroid treatment. Clinical resolution was defined as the absence of clinical signs after completion of the prescribed course of corticosteroids in dogs not receiving any treatment for SRMA at the time of follow‐up, such that the duration of clinical resolution was the time frame between corticosteroid cessation and follow‐up.
Outcome was categorized as (1) clinical resolution, (2) relapse at last contact, (3) clinical remission, (4) deceased because of SRMA, or (5) deceased for reasons unrelated to SRMA.
2.3. Survey and follow‐up data collection
Caregivers were asked to complete an online survey to evaluate the impact of SRMA on QoL, and to provide case follow‐up. The survey comprised a series of closed‐ended questions created with online survey software (©2018 Qualtrics, Provo, Utah) using an interval rating with a Likert‐type scale of 1‐5 or 1‐10 (Supporting Information S1). Although the survey was not formally validated for the study, it was designed to evaluate QoL‐associated themes and questions specific for SRMA in reference to published QoL questionnaires for dogs with other neurological disorders.15, 16, 17, 18
2.4. Statistical analysis
Continuous and ordinal data were reported as mean ± SD, or median and range, respectively. Categorical data were summarized as percentages and fractions. Differences among breeds were analyzed using the 1‐way analysis of variance for continuous variables and the Kruskal‐Wallis test for ordinal outcomes. The Wilcoxon signed rank test was used to compare caregivers' rating of QoL over time. For the QoL data, responses relating to adverse effects of corticosteroids (Supporting Information S1, question 4) were adjusted, such that scores from related items (increased appetite and weight gain; increased thirst and urination) were averaged before being combined to generate a composite score of 14 to 56. The ranking analysis of covariance was used to study the effect of breed on response variables, while adjusting for other covariates of relapse frequency and composite corticosteroid adverse effect score. Analyses of correlations between paired variables were performed using the Pearson correlation (r) and Spearman correlation (r s) for continuous and ordinal variables, respectively. Linear regression was applied to study the effect of clinical factors on response variables of QoL, severity of relapse and number of relapse episodes. A P value <.05 was considered significant for all comparisons. Statistical analyses were performed using SAS 9.4 (SAS Institute, Cary, North Carolina).
3. RESULTS
3.1. Animals
Ninety‐six cases initially were identified, including 57 cases from the AKC‐CHF survey and 39 cases from the NC State VH. Owner's consent to participate in the study was obtained for 37 cases from the AKC‐CHF survey and for all cases from the NC State VH. Eight cases identified through the AKC‐CHF survey were excluded from the study because CSF analysis was not performed (n = 6), residence was outside of North America (1), and CSF findings were not supportive of a diagnosis of the acute form of SRMA (1). Four cases identified at the NC State VH were excluded because of resolution of clinical signs after treatment with antimicrobials in the absence of corticosteroids, and 3 were excluded because of the presence of other neurologic deficits.
Sixty‐one cases met the inclusion criteria (29 cases from the AKC‐CHF survey and 32 cases from the NC State VH). The median age at time of SRMA diagnosis was 8.5 months (range, 2.1‐81.3 months), with 58/61 dogs (95.1%) <24 months of age. There were 34 males (18 sexually intact and 16 neutered), and 27 females (6 sexually intact and 21 spayed). Breeds represented included the Golden Retriever (n = 12), Bernese Mountain Dog (10), Wirehaired Pointing Griffon (9), Boxer (9), Beagle (6), American Staffordshire Terrier (3), German Shepherd Dog (2), Labrador Retriever (2), and 1 each of English Bulldog, Chesapeake Bay Retriever, Pembroke Welsh Corgi, Nova Scotia Duck Tolling Retriever, Giant Schnauzer, Stabyhoun, Weimaraner, and mixed breed. Approximately half of the dogs (31/61) were from North Carolina, with 17 other states represented, including New York (n = 4), Texas (4), California (2), Colorado (2), Illinois (2), Pennsylvania (2), Virginia (2), Connecticut (1), Florida (1), Louisiana (1), Maine (1), Maryland (1), Massachusetts (1), Michigan (1), Minnesota (1), New Jersey (1), and Ohio (1). Two dogs were from Canada; 1 from British Columbia, and 1 from Manitoba.
3.2. Clinical signs
The median time from onset of clinical signs to diagnosis was 5 days (range, 1‐257 days). The primary presenting complaint in all dogs was neck pain and lethargy. Additional clinical signs included reluctance to rise or walk (52/61, 85.2%), stiff gait (43/61, 70.5%), decreased appetite (39/61, 63.9%), kyphotic posture (16/61, 26.2%), tremors (9/61, 14.8%), diarrhea (9/61, 14.8%), vomiting (4/61, 6.6%), and inappropriate urination (3/61, 4.9%). On initial hospital admission, 40 dogs (65.6%) were febrile, with rectal temperature ≥103°F. The median temperature for all dogs was 103.6°F (range, 100.4°F‐106.2°F). Thoracolumbar pain was elicited on spinal palpation in 21 (34.4%) dogs.
3.3. Diagnostic findings
A CBC was available for review in 54 (88.5%) dogs. Of these, neutrophilic leukocytosis was present in 48 dogs (88.9%), with a median neutrophil count of 18.1 × 103/μL (range, 6.6‐48.9 × 103/μL; reference range, 2.8‐9.11 × 103/μL). Cerebrospinal fluid was collected from the cerebellomedullary cistern for analysis in all dogs. The median CSF NCC was 735/μL (range, 4‐7893/μL). The CSF NCC was <50/μL in 11 (18%) dogs, 50‐100/μL in 4 (6.6%) dogs, 101‐1000/μL in 18 (29.5%) dogs, and >1000/μL in 26 (42.6%) dogs. The median CSF protein concentration was 97.7 mg/dL (range, 10.1‐431.6 mg/dL; reference range, 0‐25 mg/dL). The percentage of neutrophils identified on cytologic evaluation was 42%‐97% (median, 81%), with neutrophils reported as nondegenerate and no pathologic organisms noted.
The CRP concentration was increased in 7 of 9 (77.8%) dogs in which it was measured (median, >60 mg/L; range, <2.5 to >60 mg/L; reference range, 0‐7.6 mg/L). Arthrocentesis was performed in 14 (23.0%) dogs, and indicated suppurative inflammation of multiple joints in the absence of infectious agents in 2 dogs, consistent with immune‐mediated polyarthritis. Fifty‐four (88.5%) dogs underwent infectious disease testing (Table 1) that failed to identify a cause for the clinical signs in all dogs.
Table 1.
Infectious disease testing performed
| Type of test | Samples | Organism | Number |
|---|---|---|---|
| Bacterial culture | Blood | Aerobic bacteria | 16 |
| CSF | Aerobic and anaerobic bacteria | 4 | |
| Urine | Aerobic bacteria | 4 | |
| Indirect fluorescent antibody | Serum | Babesia canis | 37 |
| Babesia gibsoni | 37 | ||
| Bartonella henselae | 37 | ||
| Bartonella vinsoni | 37 | ||
| Ehrlichia canis | 37 | ||
| Rickettsia rickettsii | 37 | ||
| Neospora caninum | 15 | ||
| Toxoplasma gondii | 13 | ||
| Latex cryptococcal antigen agglutination test | Serum or CSF | Cryptococcal neoformans | 16 |
| PCR | Pooled whole blood and CSF | Borrelia burgdorferi | 31 |
| Canine distemper virus | 31 | ||
| Neospora hughesi and caninum | 31 | ||
| Toxoplasma gondii | 31 | ||
| SNAP 4DX Plus | Serum | Anaplasma phagocytophilim | 37 |
| Anaplasma platys | 37 | ||
| Borrelia burgdorferi | 37 | ||
| Dirofilaria immitis | 37 | ||
| Ehrlichia canis or Ehrlichia ewingii | 37 |
Abbreviations: CSF, cerebrospinal fluid; PCR, polymerase chain reaction.
Cervical radiographs were performed in 41 (67.2%) dogs, with unremarkable findings. Reports were available for review from 23 (37.8%) dogs that underwent MRI of the cervical spine. A normal study was reported in 6 dogs. Abnormalities included meningeal contrast enhancement in 11 dogs, with this being the sole finding in 6 dogs; signal changes in the cervical paraspinal musculature in 6 dogs, consisting of T2 hyperintensity (n = 1), contrast enhancement (2) or both (3); and, multifocal ill‐defined, T2‐hyperintense lesions within the cervical spinal cord with variable contrast enhancement in 5 dogs.
3.4. Initial treatment
All dogs were treated initially with corticosteroids after diagnosis. Treatment was initiated with prednisone in all but 2 dogs that were initially treated with dexamethasone and then changed to prednisone. The median time from onset of clinical signs to initiation of treatment was 5 days (range, 1‐257 days). The initial prednisone dosage ranged from .5 to 4.2 mg/kg/d (median, 1.7 mg/kg/d). Of the 28 dogs that were placed on immunosuppressive dosages of prednisone (≥2 mg/kg/d), the duration of immunosuppression ranged from 5 to 111 days (median, 22 days). Five (8.5%) dogs had a second immunomodulatory drug administered concurrently with prednisone at the time of initial diagnosis, whereas an additional 4 (6.5%) dogs started a second immunomodulatory drug at 5 days, 3 weeks, 2 months, and 5 months after commencing initial treatment with prednisone. Drugs administered included azathioprine (n = 4), cytarabine (2), cyclosporine A (1), mycophenolate mofetil (1), and leflunomide (1). Antimicrobials were prescribed in 43 (70.5%) dogs, including doxycycline (n = 30), clindamycin (21), amoxicillin‐clavulanic acid (5), enrofloxacin (4), metronidazole (4), minocycline (3), and trimethoprim‐sulfamethoxazole (2). All dogs were noted to have improvement in clinical signs after initiation of prednisone treatment.
3.5. Outcome
The median follow‐up for all dogs was 2 years (range, 10 days to 12.5 years). Three dogs had <1 month of follow‐up at the time of data collection, all of which were in clinical remission. Twenty‐nine (47.5%) dogs suffered at least 1 relapse during the follow‐up period. Nine dogs (14.8%) had 1 relapse, 7 dogs (11.5%) had 2 relapses, 6 dogs (9.8%) had 3 relapses, 2 dogs (3.3%) had 4 relapses, and 5 dogs (8.2%) had ≥5 relapses. Five dogs had repeat CSF analysis performed at the time of clinical relapse. Results were normal in 1 dog (1/μL NCC, 17 mg/dL protein), whereas the remaining 4 dogs had neutrophilic pleocytosis with NCC of 8, 30, 2530, and 2830/μL, and neutrophil percentages on cytology of 50, 73, 77, and 95, respectively. All relapses were treated with prednisone at a median dosage of 2.0 mg/kg/d (range, .2‐6.0 mg/kg/d). A second immunomodulatory drug was commenced in 13/29 (44.8%) dogs, including 4/5 (80%) dogs treated with <1.0 mg/kg/d of prednisone during relapse. Drugs utilized included cyclosporine A (n = 7), azathioprine (5), and mycophenolate mofetil (1).
Twenty‐two (75.9%) dogs had their first relapse after treatment for SRMA was discontinued, occurring a median of 139.5 days after cessation of prednisone treatment (range, 12‐2176 days). The time between completion of prednisone treatment and first relapse was <3 months in 4 dogs, 3‐6 months in 8 dogs, and >6 months in 10 dogs. In 7/29 (24.1%) dogs, relapse occurred while the prednisone dosage was being tapered, with a median time period of 7 days (range, 3‐15 days) from the previous dosage reduction. The median age at first relapse was 19.2 months (range, 3.3‐84.4 months).
Analysis was performed to identify factors associated with frequency and caregiver's assessment of severity of relapse in the 29 dogs. The variables evaluated included age at onset of clinical signs, CSF NCC, CSF neutrophil percentage, CSF protein concentration, prednisone dosage, prednisone treatment duration, treatment with a second immunomodulatory drug at initial diagnosis, and duration between onset of clinical signs and initiation of treatment. A relationship was found between CSF NCC and frequency of disease relapse (P = .003). No other associations were identified.
Twenty‐nine (47.5%) dogs were reported to have resolution of clinical signs after completing the prescribed course of corticosteroids at the time of last follow‐up, with a median duration of resolution of clinical signs of 37 months (range, 1‐143 months) and a median total duration of prednisone treatment of 247 days (range, 45‐1419 days). Two (3.3%) dogs achieved clinical resolution for 64 and 139 days, but were reported to have relapsed at last communication and were lost to follow‐up. Twenty‐five (41.0%) dogs were in remission and undergoing a tapering schedule of prednisone administration at follow‐up, with a median duration of prednisone treatment of 435 days (range, 10‐2174 days). Overall, 5 (8.2%) dogs died during the follow‐up period, of which 4 (80%) died because of reasons unrelated to SRMA. One dog (1.6%) was euthanized because of relapse and development of neurologic deficits.
3.6. Quality of life survey
Fifty‐two (85.2%) caregivers completed the online survey. Caregivers were asked to assess the severity of neck pain at onset, and the majority of respondents (76.9%) noted improvement in clinical signs within 1‐2 days of starting prednisone. Adverse effects of prednisone reported by the caregiver included polydipsia (n = 47, 90.4%), polyuria (46, 88.5%), polyphagia (45, 86.5%), panting (39, 75.0%), weight gain (39, 75.0%), thinning of the hair coat (36, 69.2%), restlessness (29, 55.8%), sleeping more than usual (29, 55.8%), inappropriate urination (25, 48.1%), “pot belly” appearance (25, 48.1%), development of nondermatological infections (20, 38.5%), diarrhea (19, 36.5%), dermatitis (18, 34.6%), and vomiting (7, 13.5%). Adverse effects that were reported by caregivers as being severe included polyphagia (23, 44.3%), polydipsia (22, 42.3%), polyuria (20, 38.5%), and thinning of the hair coat (13, 25.0%). Forty‐eight percent (25/52) of respondents reported that their dog's core vaccinations were not up‐to‐date since being diagnosed with SRMA.
Caregivers were asked to rate their dog's QoL on a scale of 1‐10, with 1 being poor and 10 being excellent. The mean QoL for dogs during treatment (5.3, SD .4) was significantly worse than the QoL during clinical resolution (8.8, SD 1.8; P < .001) and since diagnosis of SRMA (8.2, SD 1.2; P < .001). The QoL during treatment was associated with the severity of prednisone adverse effects (r s = −.52, P = .03; Figure 1). This association persisted when adverse effects were evaluated separately based on the following categories: polyphagia and weight gain (r s = .68, P < .0001), polyuria and polydipsia (r s = .65, P < .0001), urination in inappropriate places (r s = .84, P < .0001), sleeping more (r s = .78, P < .0001), restlessness and pacing (r s = .82, P < .0001), increased panting (r s = 0.83, P < .0001), and change in appearance (r s = .59, P < .0001). A higher prednisone dosage was associated with more severe adverse effects (r = .24, P = .02), but neither the duration of immunosuppression nor the duration of prednisone treatment was associated with the severity of adverse effects.
Figure 1.
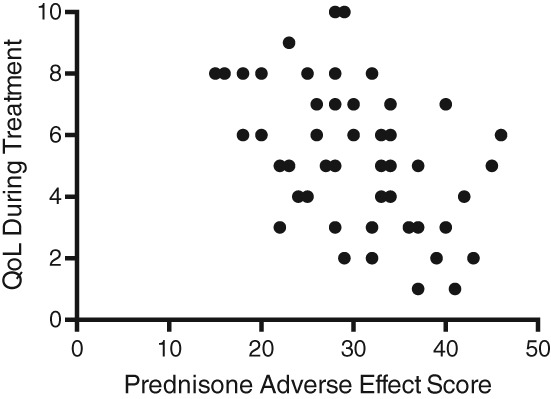
Scatter plot of the correlation between quality of life (QoL) during treatment and combined prednisone adverse effect score. r s = −.52; P = .03
3.7. Breed differences
For analysis of breed differences, breeds represented by ≥6 dogs were included separately, with dogs grouped into the following categories: Beagle, Bernese Mountain Dog, Boxer, Golden Retriever, Wirehaired Pointing Griffon, and others. Variables that were evaluated for differences among breeds are summarized in Table 2. No differences were identified with respect to clinical presentation, CSF findings, treatment response, and relapse. However, a significant difference in the number of reported prednisone adverse effects among breeds was identified (P = .04). Analysis of the difference in least squares means among breeds identified Wirehaired Pointing Griffons as having a significantly higher number of total observed adverse effects compared with other breeds (P = .04). The difference in the severity of prednisone adverse effects among breeds was not statistically significant (P = .06). A significant difference also was identified in the maximal prednisone dosage among breeds (P = .001), with the prednisone dosage administered to Wirehaired Pointing Griffons (mean ± SD, 2.38 ± .46 mg/kg/d) being significantly higher compared to the dosage administered to Beagles (mean ± SD, 1.88 ± .13 mg/kg/d; P = .04), Bernese Mountain Dogs (mean ± SD, 1.55 ± 1.10 mg/kg/d; P = .01), and Golden Retrievers (mean ± SD, 1.86 ± .23 mg/kg/d; P = .005). Boxers (mean ± SD, 3.11 ± 1.07 mg/kg/d) also were treated with a significantly higher prednisone dosage compared to Beagles (P = .02), Bernese Mountain Dogs (P = .008), Golden Retrievers (P = .002), and other breeds (P = .03).
Table 2.
Variables evaluated for differences among breeds (Beagle, Bernese Mountain Dog, Boxer, Golden Retriever, Wirehaired Pointing Griffon, and others)
| Category | Specific variable | Statistical test | P value |
|---|---|---|---|
| Clinical presentation | Severity of neck pain | Kruskal‐Wallis | .71 |
| Age of onset of clinical signs | Kruskal‐Wallis | .12 | |
| CSF findings | NCC | ANOVA | .69 |
| Neutrophil percentage | ANOVA | .53 | |
| Protein concentration | ANOVA | .20 | |
| Treatment | Prednisone dosage | Kruskal‐Wallis | .001 |
| Time to clinical improvement | Kruskal‐Wallis | .44 | |
| Time to return to normal | Kruskal‐Wallis | .71 | |
| Number of adverse effects | ANOVA | .04 | |
| Combined prednisone score | ANOVA | .06 | |
| Relapse | Time to relapse | Kruskal‐Wallis | .86 |
| Relapse frequency | Kruskal‐Wallis | .61 | |
| Severity of clinical signs at relapse | Kruskal‐Wallis | .64 | |
| Quality of life | Before diagnosis | Kruskal‐Wallis | .22 |
| During treatmenta | |||
| • Combined prednisone score | Linear regression | .03 | |
| • Relapse frequency | Linear regression | .84 | |
| During remissiona | |||
| • Combined prednisone score | Linear regression | .24 | |
| • Relapse frequency | Linear regression | .66 | |
| Since diagnosisa | |||
| • Combined prednisone score | Linear regression | .24 | |
| • Relapse frequency | Linear regression | .34 |
Abbreviations: ANOVA, Analysis of variance; CSF, cerebrospinal fluid; NCC, nucleated cell count.
Adjusted for listed covariates.
4. DISCUSSION
To the best of our knowledge, this report is the first study of SRMA involving client‐owned dogs in North America. The breeds represented most often, contributing ≥10% to the study population, were the Golden Retriever, Bernese Mountain Dog, Wirehaired Pointing Griffon, Boxer, and Beagle. Beagles, Bernese Mountain Dogs, and Boxers have been overrepresented in previous studies of SRMA.2, 3, 7, 19 Our findings suggest that Golden Retrievers and Wirehaired Pointing Griffons also should be included among the breeds recognized to develop SRMA. Golden Retrievers have been included in the population of affected dogs in a previous study of SRMA,14 whereas SRMA has not been described previously in the Wirehaired Pointing Griffon. A recent retrospective case control study also identified the Border Collie, Jack Russell Terrier, and Whippet as having increased odds of developing SRMA.3 These newly recognized breeds may have a genetic predisposition to develop SRMA, and provide the opportunity for further investigation into the pathogenesis of the disorder.
Study findings regarding clinical presentation and response to treatment were in agreement with published information on SRMA. The median age of onset was 8.5 months, with 95% of dogs <2 years old at the time of diagnosis, consistent with the previously reported age of onset of 6‐18 months.1, 2 Approximately two thirds of dogs in our study were pyrexic on presentation, with temperature ≥103.0°F. A recent retrospective study of pyrexia in dogs aged 1‐18 months identified SRMA as the most common diagnosis, suggesting that SRMA should be considered in the differential diagnosis of juvenile dogs with persistent pyrexia that is not responsive to antimicrobials and nonsteroidal anti‐inflammatory drugs.20 Our findings are in agreement with a previous prospective study on SRMA that reported pyrexia in 12/20 (60%) dogs,4 indicating that pyrexia can wax and wane, and a normal temperature does not exclude SRMA as a differential diagnosis. Two dogs in our study had concurrent immune‐mediated polyarthritis. An association between SRMA and immune‐mediated polyarthritis has been described previously, supporting the premise that SRMA is a systemic inflammatory disease.3, 21
The majority of dogs (72%) had marked CSF neutrophilic pleocytosis of >100 cells/μL. Marked CSF neutrophilic inflammation is characteristic of few disorders in dogs, including SRMA, bacterial meningitis, and certain fungal infections.22 Cytologic evaluation of CSF is essential to evaluate for underlying infectious etiologies, but additional testing (culture, serology, and polymerase chain reaction) is often required to rule out an infectious cause.
Dogs with SMRA have increases in serum and CSF IgA attributed to dysregulation of the immune system, and measuring IgA concentrations is used as an additional diagnostic tool for SRMA in Europe.1, 2, 4, 7, 10, 11 However, only 3 dogs in our study had IgA concentrations measured, which likely reflects limited availability of this test in North America.
Cervical MRI disclosed abnormalities in most dogs that underwent imaging. Published reports of the MRI characteristics of SRMA are limited.2, 23 Changes included abnormal signal intensity in the cervical musculature, as can be seen with myositis, denervation, and muscle spasm.24, 25, 26 This finding was noted alone or in combination with imaging findings consistent with radiculitis or meningitis. In the latter group of dogs, the muscle groups that were affected were in close proximity to the nerve root and meningeal changes, leading to speculation that meningeal disease might extend into adjacent nerve roots and muscles.26 Alternatively, the observed changes could be secondary to denervation.25, 27 Denervation can cause diffuse edema throughout an affected muscle, because of shifting of water from intracellular to extracellular spaces.24, 25 In addition, enlargement of the vascular bed and increased perfusion in denervated muscle can lead to signal changes.24, 25 However, MRI changes caused by denervation typically are not evident for 2‐4 weeks. This cause is unlikely in dogs that were imaged early in the course of disease, but a subacute process remains possible.24, 25 Overall, the MRI changes of meningeal inflammation and possible cervical muscular involvement seen with SRMA are relatively nonspecific. Although imaging may help rule out other causes of cervical pain, it is not required for a diagnosis.
Our results suggest that SRMA carries an excellent prognosis for remission and a fair to good prognosis for resolution, with remission and resolution rates of 98.4% and 54%, respectively. Most dogs experience improvement within 48 hours of commencing corticosteroid treatment. However, the disease is associated with a high rate of relapse. The 47.5% relapse rate in our study is higher than the published relapse rate of 16%‐34%.4, 7, 14, 28 This finding could be a consequence of differences in study design, in that our relapse frequency was based on owner recall, whereas other studies required confirmation of relapse by repeat examination and diagnostic testing (eg, CSF analysis, CRP). Therefore, the relapse rate identified in our study may be falsely high. However, our relatively long follow‐up period also could have contributed to identifying relapse episodes beyond the study periods of previous studies. Only 1 dog (1.6%) in our study died as a direct consequence of SRMA, which is lower than the previously reported mortality rate of 5%‐8%.7, 14 This finding may be a result of our study being restricted to the acute form of SRMA, whereas previous studies included both acute and chronic forms of disease. The chronic form of SRMA can occur after relapses of acute disease or with inadequate treatment.2, 7 Untreated or inadequately treated SRMA is hypothesized to lead to chronic lesions with meningeal fibrosis and arterial stenosis, which obstruct CSF flow or occlude the vasculature, causing ischemia of the CNS parenchyma and resultant neurological signs.2, 29 Chronic SRMA carries a guarded prognosis, and is associated with a higher mortality rate than the acute form of disease.
Prednisone dosage or duration was not found to be associated with the frequency of relapse in our study, which is contrary to some previous reports,1, 2, 7, 28 but in agreement with a recent retrospective study of 24 dogs with SRMA that failed to demonstrate that anti‐inflammatory dosages of corticosteroids are associated with higher relapse rates compared to immunosuppressive dosages.30 Long‐term immunosuppressive treatment has been widely recommended in SRMA to achieve long‐term remission.1, 4, 7 Monitoring CSF NCC or serum CRP concentration as indicators of disease remission before decreasing the corticosteroid dosage has been advocated, because inadequate corticosteroid dosage and duration of treatment potentially can lead to relapse and result in a chronic protracted form of the disease.1, 2, 7, 8 In our study, time from onset of clinical signs to initiation of treatment also did not affect the frequency of relapse, contrary to previous reports.2 Our study supports the use of corticosteroids as the initial treatment of choice for SRMA. A prospective case series of 20 dogs with SRMA reported full remission in all dogs with prednisolone monotherapy.4 In addition, treatment with a second immunomodulating agent was not shown to affect clinical outcome in our study, but the power of the comparison was limited by low case numbers.
The majority of dogs in our study relapsed after treatment was discontinued rather than during treatment, in contrast to the 60% in‐protocol relapse rate that has been reported previously.1, 4, 7, 14, 28 This finding might be because of differences in study design. The treatment protocol used in previous prospective studies was guided by CSF analysis, with the steroid dosage decreased when clinical examination and CSF analysis were normal. Because repeat CSF analysis was not common in our study, treatment course was based on resolution of clinical signs. Nonetheless, given that our study had a similar resolution rate as previous studies,1, 4 adjusting treatment course based on clinical signs may be appropriate. Recently, monitoring of serum CRP concentration has emerged as a practical and cost‐effective clinical tool in the management of SRMA that can provide information on clinical remission and aid in decision making regarding corticosteroid dosage reduction.
Relapses can occur over a relatively long period of time after the initial diagnosis of SRMA, and approximately one‐third of dogs in our study suffered a relapse after 2 years of age. This finding is in agreement with a previous study (Whitworth F, Adamantos S, Black V. Long‐term follow up of 24 cases of steroid‐responsive meningitis arteritis (SRMA) in dogs. Proceedings British Small Animal Veterinary Congress 2015). Although less common, relapse can occur beyond 2 years of age and at any time period after cessation of treatment. However, information on relapse was obtained retrospectively based on owner recall, and therefore is subject to potential bias.
Our study identified a weak positive correlation between CSF NCC and frequency of relapse. Serum CRP concentration has been evaluated as a biomarker for monitoring SRMA treatment response, particularly in relation to identifying potential relapse.2, 4, 28 Serum CRP concentration appears to be more sensitive than CSF analysis in identifying SRMA relapse, in that serum CRP concentrations were 7‐10 times higher than the reference range in 7 dogs with presumed relapse, whereas 6 of these 7 dogs had normal CSF NCC and protein concentration.4, 28 Although serum CRP concentration has not been shown to be predictive of relapse,4 the associations between serum CRP concentration and CSF NCC, and CSF NCC and disease course warrant further exploration. Should the relationship be confirmed, then a treatment protocol guided by CSF NCC on initial diagnosis could be pursued.
Our study did not identify breed differences in the clinical course of SRMA with respect to overall disease severity, CSF findings, or treatment response. However, the Wirehaired Pointing Griffon had a significantly higher number of prednisone‐related adverse effects when compared to other breeds. This finding is likely a result of Wirehaired Pointing Griffons being treated with substantially higher prednisone dosages when compared to Beagles, Bernese Mountain Dogs, and Golden Retrievers. Other potential causes may include the difference in lifestyle of the Wirehaired Pointing Griffon as a hunting breed, or could reflect an increased genetic susceptibility to corticosteroid‐related adverse effects. Interestingly, treatment with higher prednisone dosages was correlated with more severe adverse effects and worse QoL in our study, but not an improvement in clinical outcome. Because SRMA is believed to be an immune‐mediated disease, immunosuppressive dosages of corticosteroids have been recommended, with prednisone initiated at dosages of 2‐4 mg/kg/d and gradually tapered over 6 months depending on clinical progress.4 We did not identify a difference in treatment response based on corticosteroid dosage, and it is notable that approximately half of the dogs in our study were treated with anti‐inflammatory dosages of corticosteroids. A multicenter randomized prospective case‐control study with prednisone treatment groups at various dosages is needed to determine the optimal treatment regimen for SRMA, in order to maximize clinical response with minimal treatment‐related adverse effects.
Half of the dogs in our study were not up‐to‐date on their core vaccinations. The controversy regarding vaccination likely stems from data suggesting that vaccines may be potential triggers for immune‐mediated hemolytic anemia in some dogs,30, 31 as well as the association between vaccination and certain immune‐mediated neurological disorders of humans.32, 33 However, no relationship between vaccination and development of immune‐mediated hemolytic anemia or SRMA has been proven to date,3, 5, 30, 31 and clinicians should remain prudent in making recommendations regarding vaccination based on evidence‐based literature. The potential risks of not administering vaccines should be evaluated on a case‐by‐case basis, and antibody titers can help determine whether vaccination is indicated.
Our study had several limitations. As with any retrospective study, the accuracy and completeness of the data collected are dependent on the information available in the medical record. The disparity in treatment approach among dogs reflects the nationwide study recruitment, and likely placed limitations on the interpretation of data. Half of the cases were recruited from 1 state, resulting in a biased study population that may not be representative of the North American population. Our low case numbers, particularly in the breed groupings, also restricts the power of the study. It is possible that increased clinical awareness of the disease among veterinarians is leading to more dogs being treated with corticosteroids based on a presumptive diagnosis of SRMA without CSF analysis being performed. Objective evaluation of severity of clinical disease also was complicated by lack of a consistent neck pain grading system for all dogs on initial presentation as well as during relapse. The QoL survey may have been affected by recall bias, especially for caregivers who previously had completed the initial AKC‐CHF survey on SRMA. Moreover, bias exists because participants enrolled through the initial AKC‐CHF survey were not randomly selected, with certain breed groups potentially more interested in participating than others.
In conclusion, our study described the clinical characteristics of SRMA in a population of dogs in North America. Breed differences were not identified in the course of disease. Study findings suggest that corticosteroid dosage may not be associated with treatment response, but adverse effects of corticosteroids negatively impacted QoL. Randomized clinical trials are needed to determine the optimal treatment regimen for SRMA.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Authors declare no IACUC or other approval was needed.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
Supporting information
Appendix S1: Supplementary Material ‐ Quality of life survey.
ACKNOWLEDGMENTS
This study was funded by the American Kennel Club Canine Health Foundation (AKC‐CHF) Clinician Scientist Fellowship. This article was presented in abstract form at the 2018 American College of Veterinary Internal Medicine Forum, Seattle, Washington. The authors thank Diane Brown and Gayle Watkins for performing the initial steroid‐responsive meningitis‐arteritis survey, Hongyu Ru for statistical support, and Ashley Hershey for technical assistance.
Lau J, Nettifee JA, Early PJ, Mariani CL, Olby NJ, Muñana KR. Clinical characteristics, breed differences, and quality of life in North American dogs with acute steroid‐responsive meningitis‐arteritis. J Vet Intern Med. 2019;33:1719–1727. 10.1111/jvim.15543
Funding information American Kennel Club Canine Health Foundation (AKC‐CHF) Clinician Scientist Fellowship
REFERENCES
- 1. Cizinauskas S, Jaggy A, Tipold A. Long‐term treatment of dogs with steroid‐responsive meningitis‐arteritis: clinical, laboratory and therapeutic results. J Small Anim Pract. 2000;41(7):295‐301. [DOI] [PubMed] [Google Scholar]
- 2. Tipold A, Schatzberg SJ. An update on steroid responsive meningitis‐arteritis. J Small Anim Pract. 2010;51(3):150‐154. [DOI] [PubMed] [Google Scholar]
- 3. Rose JH, Harcourt‐Brown TR. Screening diagnostics to identify triggers in 21 cases of steroid‐responsive meningitis‐arteritis. J Small Anim Pract. 2013;54(11):575‐578. [DOI] [PubMed] [Google Scholar]
- 4. Lowrie M, Penderis J, McLaughlin M, et al. Steroid responsive meningitis‐arteritis: a prospective study of potential disease markers, prednisolone treatment, and long‐term outcome in 20 dogs (2006‐2008). J Vet Intern Med. 2009;23(4):862‐870. [DOI] [PubMed] [Google Scholar]
- 5. Rose JH, Kwiatkowska M, Henderson ER, et al. The impact of demographic, social, and environmental factors on the development of steroid‐responsive meningitis‐arteritis (SRMA) in the United Kingdom. J Vet Intern Med. 2014;28(4):1199‐1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hansson‐Hamlin H, Lilliehook I. Steroid‐responsive meningitis‐arteritis in Nova Scotia duck tolling retrievers. Vet Rec. 2013;173(21):527. [DOI] [PubMed] [Google Scholar]
- 7. Tipold A, Jaggy A. Steroid responsive meningitis‐arteritis in dogs: long‐term study of 32 cases. J Small Anim Pract. 1994;35(6):311‐316. [Google Scholar]
- 8. Schwartz M, Moore PF, Tipold A. Disproportionally strong increase of B cells in inflammatory cerebrospinal fluid of dogs with steroid‐responsive meningitis‐arteritis. Vet Immunol Immunopathol. 2008;125(3‐4):274‐283. [DOI] [PubMed] [Google Scholar]
- 9. Schwartz M, Carlson R, Tipold A. Selective CD11a upregulation on neutrophils in the acute phase of steroid‐responsive meningitis‐arteritis in dogs. Vet Immunol Immunopathol. 2008;126(3‐4):248‐255. [DOI] [PubMed] [Google Scholar]
- 10. Schwartz M, Puff C, Stein VM, et al. Pathogenetic factors for excessive IgA production: Th2‐dominated immune response in canine steroid‐responsive meningitis‐arteritis. Vet J. 2011;187(2):260‐266. [DOI] [PubMed] [Google Scholar]
- 11. Maiolini A, Carlson R, Schwartz M, et al. Determination of immunoglobulin A concentrations in the serum and cerebrospinal fluid of dogs: an estimation of its diagnostic value in canine steroid‐responsive meningitis‐arteritis. Vet J. 2012;191(2):219‐224. [DOI] [PubMed] [Google Scholar]
- 12. Maiolini A, Otten M, Hewicker‐Trautwein M, et al. Interleukin‐6, vascular endothelial growth factor and transforming growth factor beta 1 in canine steroid responsive meningitis‐arteritis. BMC Vet Res. 2013;9:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Freundt‐Revilla J, Maiolini A, Carlson R, et al. Th17‐skewed immune response and cluster of differentiation 40 ligand expression in canine steroid‐responsive meningitis‐arteritis, a large animal model for neutrophilic meningitis. J Neuroinflammation. 2017;14(1):20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Biedermann E, Tipold A, Flegel T. Relapses in dogs with steroid‐responsive meningitis‐arteritis. J Small Anim Pract. 2016;57(2):91‐95. [DOI] [PubMed] [Google Scholar]
- 15. Wessmann A, Volk HA, Packer RM, et al. Quality‐of‐life aspects in idiopathic epilepsy in dogs. Vet Rec. 2016;179(9):229. [DOI] [PubMed] [Google Scholar]
- 16. De Risio L, Freeman J, Shea A. Evaluation of quality of life of carers of Italian spinoni with idiopathic epilepsy. Vet Rec Open. 2016;3(1):e000174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wessmann A, Volk HA, Parkin T, et al. Evaluation of quality of life in dogs with idiopathic epilepsy. J Vet Intern Med. 2014;28(2):510‐514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Budke CM, Levine JM, Kerwin SC, et al. Evaluation of a questionnaire for obtaining owner‐perceived, weighted quality‐of‐life assessments for dogs with spinal cord injuries. J Am Vet Med Assoc. 2008;233(6):925‐930. [DOI] [PubMed] [Google Scholar]
- 19. Tipold A, Stein VM. Inflammatory diseases of the spine in small animals. Vet Clin North Am Small Anim Pract. 2010;40(5):871‐879. [DOI] [PubMed] [Google Scholar]
- 20. Black VL, Whitworth FJS, Adamantos S. Pyrexia in juvenile dogs: a review of 140 referred cases. J Small Anim Pract. 2018;60(2):116‐120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Webb A, Taylor S, Muir G. Steroid‐responsive meningitis‐arteritis in dogs with noninfectious, nonerosive, idiopathic immune‐mediated polyarthritis. J Vet Intern Med. 2002;16:269‐273. [DOI] [PubMed] [Google Scholar]
- 22. Chrisman CL. Cerebrospinal fluid analysis. Vet Clin North Am Small Anim Pract. 1992;22(4):781‐810. [DOI] [PubMed] [Google Scholar]
- 23. O'Neill JJ, Hammond JJ, Glass EN, et al. What is your diagnosis? J Am Vet Med Assoc. 2013;242(1):29‐31. [DOI] [PubMed] [Google Scholar]
- 24. May DA, Disler DG, Jones EA, et al. Abnormal signal intensity in skeletal muscle at MR imaging: patterns, pearls, and pitfalls. Radiographics. 2000;20:S295‐315. [DOI] [PubMed] [Google Scholar]
- 25. Platt SR, McConnell JF, Garosi LS, et al. Magnetic resonance imaging in the diagnosis of canine inflammatory myopathies in three dogs. Vet Radiol Ultrasound. 2006;47(6):532‐537. [DOI] [PubMed] [Google Scholar]
- 26. Eminaga S, Cherubini GB, Villiers E, et al. STIR muscle hyperintensity in the cervical muscles associated with inflammatory spinal cord disease of unknown origin. J Small Anim Pract. 2013;54(3):137‐142. [DOI] [PubMed] [Google Scholar]
- 27. Trampus P, Goepfert C, Welle M, et al. Magnetic resonance imaging signal alterations in paraspinal muscles in dogs with acute thoracolumbar intervertebral disk extrusion. Front Vet Sci. 2018;5:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bathen‐Noethen A, Carlson R, Menzel D, et al. Concentrations of acute‐phase proteins in dogs with steroid responsive meningitis‐arteritis. J Vet Intern Med. 2008;22(5):1149‐1156. [DOI] [PubMed] [Google Scholar]
- 29. Summers BA, Cummings JF, De LaHunta A. Inflammatory diseases of the central nervous system In: Summers BA, Cummings JF, De LaHunta A, eds. Veterinary Neuropathology. St. Louis, MO: Mosby; 1995:95‐188. [Google Scholar]
- 30. Duval D, Giger U. Vaccine‐associated immune‐mediated hemolytic anemia in the dog. J Vet Intern Med. 1996;10(5):290‐295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Moon A, Veir J. Vaccination and associated adverse events in dogs previously treated with primary immune‐mediated hemolytic anemia. J Am Anim Hosp Assoc. 2019;55(1):29‐34. [DOI] [PubMed] [Google Scholar]
- 32. Martin Arias LH, Sanz R, Sainz M, Treceño C, Carvajal A. Guillain‐Barre syndrome and influenza vaccines: a meta‐analysis. Vaccine. 2015;33(31):3773‐3778. [DOI] [PubMed] [Google Scholar]
- 33. Vellozzi C, Iqbal S, Broder K. Guillain‐Barre syndrome, influenza, and influenza vaccination: the epidemiologic evidence. Clin Infect Dis. 2014;58(8):1149‐1155. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Supplementary Material ‐ Quality of life survey.


