Abstract
Background
Asthma in horses is associated with nonspecific respiratory clinical signs and may be manifested only as exercise intolerance. Its diagnosis relies on bronchoalveolar lavage fluid (BALF) cytology in the presence of compatible clinical signs. The identification of blood biomarkers for this condition would facilitate diagnosis in the field, because there are regional areas where BAL is not routinely performed in clinical practice.
Objective
Identification of blood biomarkers for the diagnosis of asthma in horses.
Animals
Fourteen horses with asthma with increased neutrophil numbers in BALF (neutrophilic asthma), 9 healthy control horses, and 10 horses with other pathologic conditions (pathologic controls).
Methods
Physical examination, clinical score, hematology, and BALF cytology (in a subset of horses) were performed. Serum concentrations of surfactant protein D (SP‐D), haptoglobin, and secretoglobin (SCGB) were measured using commercial ELISA assays.
Results
Serum concentration of SP‐D > 43 ng/mL, serum concentration of haptoglobin >5730 ng/mL, and serum concentration of SCGB <19 ng/mL allowed differentiation of horses with neutrophilic asthma from horses of the control groups (healthy and pathologic) with sensitivity of 55, 95, and 75%, and specificity of 67, 28, and 60%, respectively. Specificity of 100% and sensitivity of 45% were obtained with the combination of SP‐D, haptoglobin, and SCGB at the serum concentrations indicated above. Specificity of 95% and sensitivity of 45% were obtained with the combination of SP‐D and SCGB serum concentrations.
Conclusions and Clinical Importance
Haptoglobin, SCGB, and SP‐D may be diagnostic aids in horses with clinical signs of lower airway disease and neutrophilic pulmonary inflammation.
Keywords: biomarker, equine lungs, haptoglobin, secretoglobin, SP‐D
Abbreviations
- BAL
bronchoalveolar lavage
- BALF
bronchoalveolar lavage fluid
- CI
confidence interval
- EIPH
exercise‐induced pulmonary hemorrhage
- HOARSI
Horse Owner Assessed Respiratory Signs Index
- SCGB
secretoglobin
- SP‐D
surfactant protein D
1. INTRODUCTION
Mild to moderate asthma (also known as inflammatory airway disease) is associated with lower airway obstruction and inflammation with high prevalence in the equine population worldwide.1, 2, 3 The term “mild equine asthma” may be used to describe the condition affecting horses with lower airway inflammation and obstruction, but without obvious clinical signs suggestive of lung disease. The term “moderate equine asthma” then refers to horses with clinical signs (eg, cough) or clinical findings (abnormal lung sounds) of lung disease. Asthma is believed to be underdiagnosed in the field because the clinical signs are nonspecific, and sensitive and portable means of evaluating lung function of horses are not available. Currently, the diagnosis most often is based on the finding of abnormal bronchoalveolar lavage fluid (BALF) cytology (increased numbers of neutrophils, mast cells, eosinophils, or some combination of these) in horses with compatible clinical signs. Because there remain regional areas where BAL is not performed routinely in the field, a need exists for easily accessible sensitive and specific means of diagnosis.
Blood biomarkers of lung inflammation have been studied, but few are pathognomonic for a given condition. Racehorses without respiratory clinical signs but with mildly increased numbers of BALF neutrophils and mast cells have a detectable increase in serum surfactant protein D (SP‐D) concentration, suggesting its possible use for the diagnosis of mild asthma in horses.4 The combined serum concentrations of SP‐D and haptoglobin were reported to differentiate horses with moderate asthma from healthy horses with no overlap between groups (sensitivity and specificity of 100%).5 Serum haptoglobin concentration or other serum acute phase protein concentrations such as serum amyloid A or C‐reactive protein do not appear to be altered in exercise‐intolerant racehorses with mild to moderate asthma.6 None of these 3 studies investigated the specificity of these biomarkers by integrating other conditions responsible for exercise intolerance. Blood biomarkers with high specificity for the lungs would represent ideal molecules for the diagnosis of asthma. Secretoglobin (SCGB) is a novel candidate protein, because it is decreased in BALF of severely asthmatic horses when compared to age‐matched controls because of destruction of club cells.7, 8
We therefore aimed to investigate the concentrations of SP‐D, haptoglobin, and SCGB in the serum of horses with mild to moderate asthma and controls (healthy or pathologic horses). We hypothesized that the combined serum concentrations of these biomarkers would predict mild to moderate asthma in horses.
2. MATERIALS AND METHODS
This prospective study was performed in accordance with the guidelines of the Canadian Council on Animal Care and the protocol was approved by the Ethics Committee of the Université de Montréal (#Rech‐1647).
2.1. Animals
Horses with asthma were selected from cases referred to the Equine Hospital of the Université de Montréal between August 2014 and July 2016. These horses were included in the study if they were presented for evaluation of coughing episodes, exercise intolerance, or both, and had an increased percentage of at least 1 type of granulocyte (neutrophils [>5%], mast cells [≥2%], or eosinophils [>1%]) on evaluation of BALF cytology.1 Healthy control horses were owned by the Université de Montréal or by the personnel of the university. Another control group consisted of horses referred to the Equine Hospital of the Université de Montréal with conditions other than asthma possibly responsible for exercise intolerance. These horses were included in the study if they were presented for evaluation of a musculoskeletal or cardiovascular disease. Horses from both control groups had no evidence of systemic inflammation based on clinical examination, results of a CBC and serum biochemistry or both. Cytology of BALF was performed and found to be within the reference range (neutrophils [≤5%], mast cells [<2%], eosinophils [≤1%], or some combination of these) in all healthy controls and in all the pathologic controls for which this procedure was performed (40%). Bronchoalveolar lavage could not be performed in the other horses because the procedure was declined by the owners or the horses were scheduled for surgery. Racehorses in training or racing were excluded, as were horses with a history of increased respiratory efforts at rest, or horses that had received any drugs within 15 days before admission. All owners signed an informed consent form.
2.2. Questionnaire
The Horse Owner Assessed Respiratory Signs Index (HOARSI) is a validated standardized questionnaire based on the frequency of cough, the presence of nasal discharge after exercise, the presence of abnormal respiration, and an evaluation of the performance of the horse by the owner.9 It is a 4‐point scoring system: 1 for horses without clinical signs of respiratory disease, 2 and 3 for horses with mild or moderate signs, and 4 for horses with severe signs of respiratory disease, compatible with severe asthma.
2.3. Blood collection
Twenty milliliters of blood were collected from a jugular vein by means of 18G needles into sterile tubes (serum tube and EDTA tube [BD Vacutainer, Franklin Lakes, New Jersey]) within the first hour after arrival. Within 2 hours after collection, plasma and serum were separated and stored in 1.5 mL aliquots at −80°C until analysis.
2.4. Protein expression studies
Surfactant protein D, haptoglobin, and SCGB were quantified in serum samples using commercially available ELISA kits validated for horses. The human SP‐D ELISA (Biovendor, Asheville, North Carolina), the equine haptoglobulin ELISA (Cederlane, Burlington, Ontario, Canada) and equine uteroglobin (SCGB1A1, SCGB 1A1) ELISA (MyBioSource, San Diego, California) previously were validated by the manufacturer and used accordingly to their instructions. Plates were washed using an automatic plate washer and absorbance was obtained using a microplate reader. The assay standard curves ranged from 1.56 to 100 ng/mL for SP‐D (serum dilution, 1:10), 9.38 to 600 ng/mL for haptoglobin (serum dilution, 1:32 000), and 1.56 to 100 ng/mL for SCGB. When SCGB concentrations exceeded the assay quantification range, they were attributed the higher or lower limit value of the linear part of the curve.
2.5. Bronchoalveolar lavage
Bronchoalveolar lavage was performed as previously described with 2 × 250 mL of sterile isotonic saline.10 Cytopreparations from unfiltered BALF were stained with May Grünwald Giemsa. Differential counts of 400 cells (excluding epithelial cells) were performed by a board‐certified clinical pathologist (C. Grimes) who was blinded to the clinical status of the horses.
2.6. Experimental protocol
All horses were transported to the hospital on the day of examination. Physical examination and hematology were performed on all horses during the first hour of arrival. The HOARSI questionnaire was administered by 1 author (C. Gy) for all owners. Blood for ELISA also was withdrawn 1 hour after arrival in all horses.
3. STATISTICS
Statistical analyses were performed using Prism 7 (GraphPad Software Inc., La Jolla, California). All data were analyzed after log‐10 transformations. Group size was based on a previous study,5 which showed with statistical significance that, when combined, the serum concentrations of SP‐D and haptoglobin allow differentiation of horses with mild to moderate asthma from healthy horses, with no overlap between groups. The Mantel‐Haenszel test was used for detecting correlations between the HOARSI score and the classification of the horse population based on history and physical examination. Student's t tests or 1‐way analysis of variance with Turkey's post‐tests were used to compare the variables studied in the different groups (BALF cytology, hematology, and serum biochemistry). The asthma group was further subjectively classified based on the severity of neutrophilic inflammation in BALF (≤5%, >5 to <15% versus 15 to 20%) to determine whether the blood biomarkers varied according to the severity of the airway neutrophilic inflammation. Pearson tests were used for detecting correlations between serum biomarker concentrations and BALF cytology. The area under receiver operating characteristic curves was determined for the different biomarkers and combinations. Sensitivity and specificity were calculated for all biomarkers alone, and in combination. Neutrophilic asthmatic horses (>5 to 20%) were compared to the combination of both control groups (healthy and pathologic).
4. RESULTS
Fifty‐four horses were evaluated. Three, 6, and 4 horses of the asthmatic, healthy, and pathologic groups, respectively, were excluded from the study because the BALF cytology inclusion criteria were not met. Two additional horses from the pathologic control group were excluded because of evidence of systemic inflammation based on clinical examination and results of the CBC and serum biochemistry profile. Because of the small number of horses with increased of BALF mast cells and eosinophils, only neutrophilic asthmatic horses were studied.
Fourteen horses with mild to moderate neutrophilic asthma, 9 healthy controls, and 10 pathologic controls were finally included in the study (Table 1).
Table 1.
Description of the 3 groups
| Asthma (n = 14) | Healthy controls (n = 9) | Pathologic controls (n = 10 including 4 with BALF cytology) | ||||
|---|---|---|---|---|---|---|
| Median | Range | Median | Range | Median | Range | |
| Age | 8 | 3‐14 | 9 | 5‐20 | 8 | 2‐16 |
| HOARSI score | 2 | 2 | 1 | 1 | 1 | 1 |
| BALF | ||||||
| Neutrophil (%) | 14 | 6.0‐21 | 2.2 | 1.6‐4.2 | 3.0 | 1.2‐5.0 |
| Mast cell (%) | 0.9 | 0.0‐3.2 | 1.0 | 0.0‐1.8 | 1.5 | 1.0‐1.8 |
| Eosinophil (%) | 0.0 | 0.0‐1.0 | 0.0 | 0.0‐0.8 | 0.0 | 0.0 |
| Lymphocyte (%) | 44 | 29‐71 | 57 | 18‐60 | 41 | 40‐43 |
| Macrophage (%) | 37 | 15‐58 | 40 | 34‐79 | 53 | 48‐57 |
| CBC | ||||||
| Leukocytes (×109/L) | 8.6 | 6.1‐10 | 5.7 | 5.1‐8.2 | 6.4 | 3.6‐10 |
| Neutrophils (×109/L) | 5.5 | 3.3‐8.0 | 3.3 | 2.4‐4.2 | 4.5 | 2.4‐6.8 |
| Lymphocytes (×109/L) | 1.9 | 1.4‐4.1 | 2.3 | 1.9‐4.0 | 1.9 | 0.9‐3.7 |
| Total solids (g/L) | 68 | 61‐76 | 65 | 59‐69 | 68 | 60‐82 |
| Fibrinogen (g/L) | 2.0 | 1.0‐3.0 | 2.0 | 2.0‐3.0 | 2.0 | 1.0‐5.0 |
Abbreviations: BALF, bronchoalveolar lavage fluid (differential cell count performed on 400 cells); HOARSI, Horse Owner Assessed Respiratory Signs Index.
4.1. Animals
The mild to moderate asthma group consisted of 9 Quarter Horses and associated breeds, 4 Warmbloods, and 1 Shire. There were 6 females, 7 geldings, and 1 male. Disease duration was <2 years except for 1 horse (5 years' duration). Horses were fed dry hay while being stabled (n = 10) or kept in a paddock or pasture (n = 4). Horses had been presented with a history of cough (12 horses), exercise intolerance (1 horse), or both clinical signs (1 horse).
The healthy control group consisted of 6 Quarter Horses and associated breeds, 1 Warmblood, 1 Thoroughbred, and 1 Draft horse. There were 4 females and 5 geldings. They were fed hay while being stabled (n = 5) or kept in a paddock or pasture (n = 4). Two horses were part of a teaching herd owned by the Université de Montréal.
The pathologic control group consisted of 3 Quarter Horses and associated breeds, 5 Warmbloods, and 2 cross breeds. There were 5 females, 4 geldings, and 1 male. They were fed hay while being stabled (n = 9) or kept in a paddock or pasture (n = 1). Three horses had osteochondrosis of the hock, 2 had navicular disease in the front limb, 2 had osteoarthritis (hip [n = 1]; hock [n = 1]), 1 had septic tenosynovitis, 1 had motor neuron disease, and 1 had myositis.
4.2. Horse Owner Assessed Respiratory Signs Index
The 14 horses that had a history consistent with asthma had a HOARSI score of 2, the 19 horses that had a history consistent with being controls had a HOARSI score of 1 (Table 1). The HOARSI score showed good agreement with the history and physical examination in this horse population (P < .001).
4.3. Bronchoalveolar lavage fluid cytology
Figure 1 and Table 1 summarize the BALF cytology findings. Only the percentage of neutrophils in BALF was significantly increased in asthmatic horses in comparison to healthy control horses (P = .007) and both control groups combined (P = .004).
Figure 1.
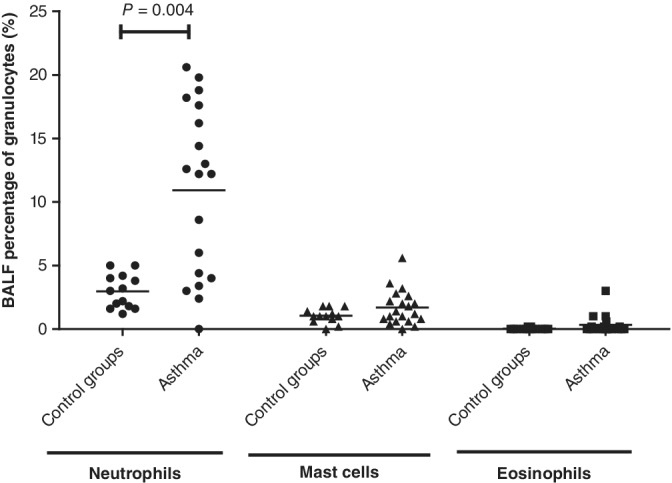
Bronchoalveolar lavage fluid concentration of neutrophils, mast cells, and eosinophils in 20 asthmatic horses and 13 control horses
4.4. Blood analysis
Blood lymphocytes, total solids, and fibrinogen concentrations were similar among the 3 groups of horses. Blood leukocytes (P = .0006) and neutrophils (P < .0001) were within normal limits but were significantly increased in horses with asthma in comparison with healthy horses, but not with the pathological control horses (P = .13 and .33, respectively; Table 1).
4.5. Blood biomarkers
The serum concentration of SP‐D was significantly increased in horses with asthma with ≥15% neutrophils in BALF compared to healthy (P = .006) and pathologic controls (P = .03; Figure 2). No other significant difference was found among groups for the other 2 biomarkers (haptoglobin, P = .5; SCGB, P = .8).
Figure 2.
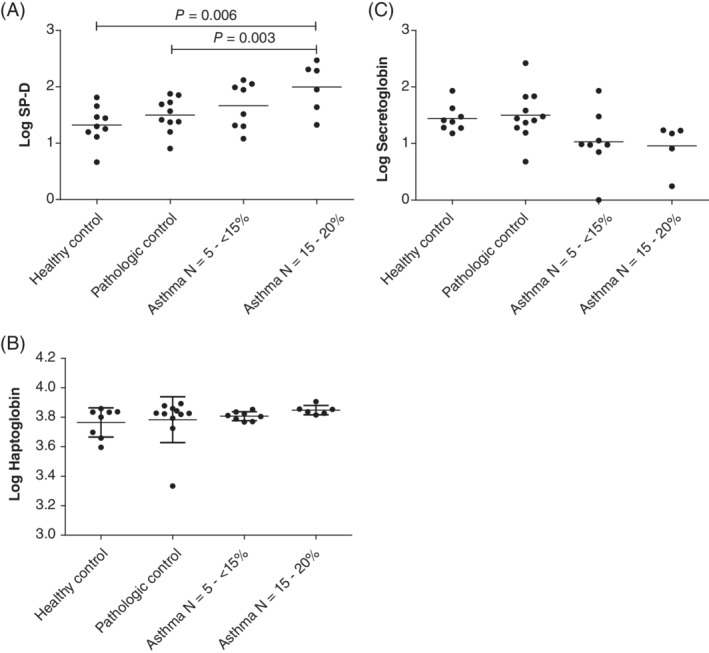
Serum concentration of surfactant protein D (SP‐D) (A), haptoglobin (B), and SCGB (C) in horses with neutrophilic asthma (n = 14) and controls (healthy [n = 9] and pathologic [n = 10]). Values were log transformed for analysis
A cutoff of 43 ng/mL for SP‐D allowed differentiation of horses with neutrophilic asthma from controls (healthy and pathologic) with sensitivity and specificity of 55 and 67%, respectively, and the area under the curve (AUC) was 0.7 (95% confidence interval [CI], 0.55‐0.84; P = .02; Figure 3 and Table 2). Horses with haptoglobin concentration >5730 ng/mL were more likely to have neutrophilic asthma with sensitivity and specificity of 95 and 28%, respectively, and the AUC was 0.55 (95% CI, 0.40‐0.71; P = .5). Horses with SCGB concentration <19 ng/mL were more likely to have neutrophilic asthma, with sensitivity of 75% and specificity of 60% and the AUC was 0.63 (95% CI, 0.47‐0.79; P = .2).
Figure 3.
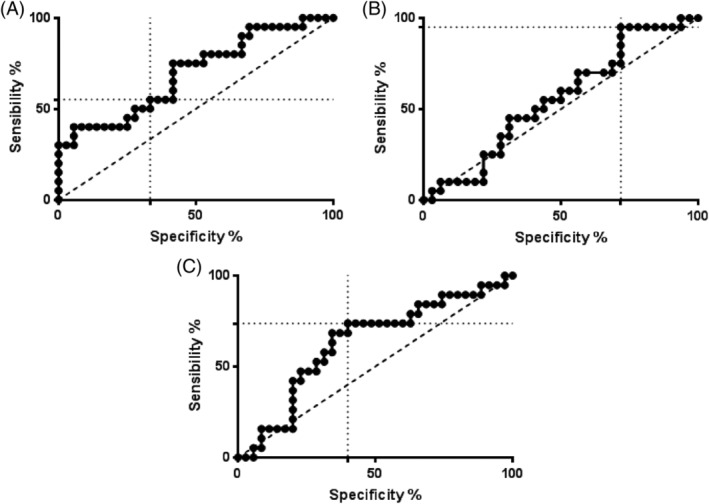
Receiver operating characteristic curves of the biomarkers surfactant protein D (SP‐D) (A), haptoglobin (B), and SCGB (C)
Table 2.
Sensitivity and specificity for all biomarkers and combination. Sensitivity and Specificity were calculated using 14 neutrophilic asthmatic horses, 9 healthy controls, and 10 pathologic controls
| Criteria | Sensitivity (%) | Specificity (%) |
|---|---|---|
| SP‐D > 43 ng/mL | 55 | 67 |
| SP‐D > 43 ng/mL and HOARSI = 2 | 55 | 100 |
| Haptoglobin >5730 ng/mL | 95 | 28 |
| Haptoglobin >5730 ng/mL and HOARSI = 2 | 75 | 100 |
| SCGB <19 ng/mL | 75 | 60 |
| SCGB <19 ng/mL and HOARSI = 2 | 75 | 100 |
| SP‐D > 43 ng/mL and haptoglobin >5730 ng/mL | 55 | 86 |
| SP‐D > 43 ng/mL and haptoglobin >5730 ng/mL and HOARSI = 2 | 55 | 100 |
| SP‐D > 43 ng/mL and SCGB <19 ng/mL | 45 | 95 |
| SP‐D > 43 ng/mL and SCGB <19 ng/mL and HOARSI = 2 | 45 | 100 |
| Haptoglobin >5730 ng/mL and SCGB <19 ng/mL | 70 | 80 |
| Haptoglobin >5730 ng/mL and SCGB <19 ng/mL and HOARSI = 2 | 70 | 100 |
| SP‐D > 43 ng/mL and haptoglobin >5730 ng/mL and SCGB <19 ng/mL | 45 | 100 |
| SP‐D > 43 ng/mL and haptoglobin >5730 ng/mL and SCGB <19 ng/mL and HOARSI = 2 | 45 | 100 |
Abbreviations: HOARSI, Horse Owner Assessed Respiratory Signs Index; SP‐D, Surfactant protein D.
Sensitivity of 45% and specificity of 100% were obtained with the combination of SP‐D >43 ng/mL, haptoglobin >5730 ng/mL, and SCGB <19 ng/mL (Table 2) for the diagnosis of neutrophilic asthma (AUC, 0.6; 95% CI, 0.41‐0.80; P = .3). Sensitivity of 45% and specificity of 95% were obtained with the combination of SP‐D >43 ng/mL and SCGB <19 ng/mL (AUC, 0.69; 95% CI, 0.49‐0.89; P = .07).
Sensitivity and specificity of the biomarker combinations all were increased with inclusion of the criterion HOARSI = 2 (Table 2). The combination of SP‐D >43 ng/mL, haptoglobin >5730 ng/mL, and SCGB <19 ng/mL or the combination of SP‐D >43 ng/mL and SCGB <19 ng/mL showed sensitivity of 45% and specificity of 100%. Haptoglobin >5730 ng/mL or SCGB <19 ng/mL had sensitivity and specificity of 75 and 100%, respectively.
5. DISCUSSION
Evaluation of blood biomarkers specific for lung inflammation could facilitate the diagnosis of asthma in horses. Previous studies have identified blood biomarkers predictive of mild to moderate asthma in horses when compared to healthy control horses. However, to be clinically useful as a diagnostic tool, the biomarkers should have adequate sensitivity and specificity for asthma in horses when compared with other diseases causing exercise intolerance in horses. Our study attempted to identify blood biomarkers capable of accomplishing this goal. Results indicated that the serum concentration of SP‐D was increased in horses with neutrophilic asthma compared to controls (healthy and pathologic). Moreover, the combination of the serum concentrations of SP‐D, haptoglobin, and SCGB was highly suggestive of neutrophilic asthma. Integrating the HOARSI score with these biomarkers further supports the diagnosis of neutrophilic asthma in horses that cannot have BALF analysis performed.
Mainly synthesized in the lungs by alveolar type II cells, SP‐D is a protein that plays an important role in protecting the lungs against various inflammatory processes. In our study, in agreement with previous reports,4, 5 horses with mild to moderate asthma had higher serum concentrations of SP‐D than did healthy controls, but only in horses with neutrophilic inflammation >15%. Moreover, serum SP‐D concentration was significantly increased in neutrophilic asthmatic horses when compared to horses with exercise intolerance from other causes.
Haptoglobin is an acute phase protein produced by the liver, which increases in serum in response to inflammatory stimuli. Circulating haptoglobin concentration increases 1‐10 times in the 12‐24 hours after an injury and peaks 72 hours later.11 In previous studies,4, 5 serum haptoglobin concentration was found to be increased on average 2‐fold in horses with mild to moderate asthma in comparison to that of healthy control horses. In our study, serum haptoglobin concentrations of asthmatic horses and healthy and pathological controls were not significantly different. These divergent results possibly could be explained by the milder airway inflammation in the present population as compared to that of a previous study.4 Indeed, in our study, BALF neutrophil percentage ranged from 6 to 21% (14 ± 4.6%), in comparison with results obtained previously,4 with BALF neutrophils percentage ranging from 6 to 53%. Ideally, the specificity of serum haptoglobin as a biomarker for asthma in horses should be ascertained in the presence of exercise‐induced pulmonary hemorrhage (EIPH), because haptoglobin is a scavenger for hemoglobin.5 The horse population we studied did not allow determining whether or not EIPH was a confounding factor for the diagnosis of asthma. The combined serum concentrations of SP‐D and haptoglobin were reported to differentiate horses with moderate asthma from healthy horses with no overlap between groups (sensitivity and specificity of 100%).5 These biomarkers only showed a sensitivity of 55% and specificity of 86% when combined in our population of horses. As indicated above, the milder airway inflammation and the inclusion of pathological controls in our study may have contributed to the apparent discrepancies between the 2 studies.
Secretoglobin is a small anti‐inflammatory protein mainly secreted by mucosal epithelial cells of the lungs, the so‐called club cells (formerly Clara cells). This protein represents a major constituent of airway surface fluid.7 In previous reports, BALF SCGB expression was significantly decreased in severely asthmatic horses,12, 13 as well as in humans with asthma,14 when compared to healthy controls. We had postulated that combining biomarkers that are both increased and decreased in the blood of asthmatic horses would increase the sensitivity and specificity for diagnosis. Although no significant decrease in the serum concentration of SCGB was found in asthmatic horses compared to our 2 control groups, when combined with a HOARSI score >2, it had high sensitivity and specificity for the diagnosis of asthma, making it possibly a suitable field biomarker.
Blood biomarkers possibly could be directed toward the diagnosis of the phenotype of asthma in horses (neutrophilic, mastocytic, and eosinophilic) given the growing interest in targeted treatment, especially in human medicine. The majority of the horses in our study had an increased percentage of neutrophils in BALF, preventing the examination of such a possible link.
In our study, HOARSI was used to complement the history and clinical signs for disease classification purposes. A previous study found good discrimination only between severely asthma‐affected horses and non‐affected horses using HOARSI, but we found good agreement (P < .001) between HOARSI and classification of horses based on history and physical examination in our population of moderately asthmatic horses.15 This finding was a useful addition, considering that for ethical and logistical considerations, BALF cytology could only be performed in a subset of our pathological controls.
Lastly, a significant difference was found in blood leukocyte and neutrophil counts between asthmatic and healthy horses. These results suggest systemic inflammation, but it remains to be determined if it represents a spillover of lung inflammation into the bloodstream or an intrinsic feature of the disease as suggested for horses with severe asthma.16
6. CONCLUSIONS
Haptoglobin, SCGB, SP‐D, and their combination are potential diagnostic aids for the diagnosis of neutrophilic asthma in horses. More studies are needed to correlate biomarkers with the phenotype of asthma and potentially develop specific treatments for mild to moderate asthma.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
This study was performed in accordance with the guidelines of the Canadian Council on Animal Care and the protocol approved by the Ethics Committee of the Université de Montréal (#Rech‐1647).
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
ACKNOWLEDGMENTS
The authors thank Dorothee Bienzle for her collaboration in the development of the biomarker SCGB and Guy Beauchamp for the statistical analyses. The authors are grateful to the veterinarians and personnel from the Equine Hospital of the Université de Montréal who contributed to this study.
Gy C, Leclere M, Vargas A, Grimes C, Lavoie J‐P. Investigation of blood biomarkers for the diagnosis of mild to moderate asthma in horses. J Vet Intern Med. 2019;33:33:1789–1795. 10.1111/jvim.15505
Funding information Fonds du Centenaire; Fonds en santé équine de l'Université de Montréal; Zoetis
REFERENCES
- 1. Couetil LL, Cardwell JM, Gerber V, Lavoie JP, Léguillette R, Richard EA. Inflammatory airway disease of horses–revised consensus statement. J Vet Intern Med. 2016;30(2):503‐515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lavoie JP. Is the time primed for equine asthma? Equine Vet Educ. 2015;27(5):225‐226. [Google Scholar]
- 3. Pirie RS, Couëtil LL, Robinson NE, Lavoie JP. Equine asthma: an appropriate, translational and comprehendible terminology? Equine Vet J. 2016;48:403‐405. [DOI] [PubMed] [Google Scholar]
- 4. Richard EA, Pitel PH, Christmann U, Lekeux P, Fortier G, Pronost S. Serum concentration of surfactant protein D in horses with lower airway inflammation. Equine Vet J. 2012;44(3):277‐281. [DOI] [PubMed] [Google Scholar]
- 5. Bullone M, de Lagarde M, Vargas A, Lavoie JP. Serum surfactant protein D and haptoglobin as potential biomarkers for inflammatory airway disease in horses. J Vet Intern Med. 2015;29(6):1707‐1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Leclere M, Lavoie‐Lamoureux A, Lavoie JP. Acute phase proteins in racehorses with inflammatory airway disease. J Vet Intern Med. 2015;29(3):940‐945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cote O, Lillie BN, Hayes MA, et al. Multiple secretoglobin 1A1 genes are differentially expressed in horses. BMC Genomics. 2012;13:712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Katavolos P, Ackerley CA, Viel L, Clark ME, Wen X, Bienzle D. Clara cell secretory protein is reduced in equine recurrent airway obstruction. Vet Pathol. 2009;46(4):604‐613. [DOI] [PubMed] [Google Scholar]
- 9. Ramseyer A, Gaillard C, Burger D, et al. Effects of genetic and environmental factors on chronic lower airway disease in horses. J Vet Intern Med. 2007;21(1):149‐156. [DOI] [PubMed] [Google Scholar]
- 10. Jean D, Vrins A, Beauchamp G, Lavoie JP. Evaluation of variations in bronchoalveolar lavage fluid in horses with recurrent airway obstruction. Am J Vet Res. 2011;72(6):838‐842. [DOI] [PubMed] [Google Scholar]
- 11. Hulten C, Grönlund U, Hirvonen J, et al. Dynamics in serum of the inflammatory markers serum amyloid A (SAA), haptoglobin, fibrinogen and alpha2‐globulins during induced noninfectious arthritis in the horse. Equine Vet J. 2002;34(7):699‐704. [DOI] [PubMed] [Google Scholar]
- 12. Cote O, Clark ME, Viel L, et al. Secretoglobin 1A1 and 1A1A differentially regulate neutrophil reactive oxygen species production, phagocytosis and extracellular trap formation. PLoS One. 2014;9(4):e96217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Miskovic Feutz M, Couetil LL, Riley CP, Zhang X, Adamec J, Raskin RE. Secretoglobin and transferrin expression in bronchoalveolar lavage fluid of horses with chronic respiratory disease. J Vet Intern Med. 2015;29(6):1692‐1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Inoue K, Wang X, Saito J, et al. Plasma UGRP1 levels associate with promoter G‐112A polymorphism and the severity of asthma. Allergol Int. 2008;57(1):57‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Laumen E, Doherr MG, Gerber V. Relationship of horse owner assessed respiratory signs index to characteristics of recurrent airway obstruction in two Warmblood families. Equine Vet J. 2010;42(2):142‐148. [DOI] [PubMed] [Google Scholar]
- 16. Lavoie‐Lamoureux A, Leclere M, Lemos K, Wagner B, Lavoie JP. Markers of systemic inflammation in horses with heaves. J Vet Intern Med. 2012;26(6):1419‐1426. [DOI] [PubMed] [Google Scholar]


