Abstract
Background
Therapeutic plasma exchange (TPE) is used increasingly in small animals to remove circulating large molecular products such as antibodies, pathogenic proteins, and protein‐bound toxins. Specific, efficient, and safe protocols need to be developed.
Hypothesis/Objectives
To describe the technique of membrane‐based TPE, the resulting physiological and metabolic changes, and to define an adequate regional citrate anticoagulation protocol.
Animals
Thirty‐four dogs treated with TPE (2011‐2017).
Methods
Retrospective review of all TPE treatments performed at the Vetsuisse Faculty, University of Bern, identified through a search of the institutional database for extracorporeal treatments.
Results
Sixty‐four treatments were performed, resulting in 1.0 plasma volume exchange (range, 0.4‐1.1). Replacement fluids included fresh frozen plasma (12%‐100% volume), colloids (0%‐52%), human albumin (0%‐41%), and saline (0%‐70%). Anticoagulation was performed with regional citrate (n = 24), systemic heparinization (n = 2), or combined (n = 38). Main relevant laboratory changes included a 24.7% decrease in total proteins (interquartile range, 16.7‐31.4; P < .001), 53% in fibrinogen (−30 to 63; P = .009), 36% in bilirubin (13‐43, P = .02), 9.0% in urea (0.7‐15.7; P < .001), and 4.5% in creatinine (−6.6 to 10.6; P = .006). Citrate accumulation was evidenced in all dogs, more pronounced in those with renal but not with hepatic impairment. Maximal tolerable citrate rates were estimated as 5.5 and 9.0 μmol/kg/min for treatments in dogs with and without renal impairment, respectively. Complications were observed in 22 treatments (34%) and were fatal in 2 dogs.
Conclusions and Clinical Importance
Therapeutic plasma exchange causes metabolic and biochemical alterations. Understanding these effects makes possible to anticipate most complications and to improve safety of the procedure.
Keywords: coagulation, extracorporeal blood purification, plasmapheresis
Abbreviations
- AKI
acute kidney injury
- BW
body weight
- cTPE
centrifugation‐based therapeutic plasma exchange
- FFP
fresh‐frozen plasma
- HAlb
human albumin
- HepCit
heparin‐citrate anticoagulation
- HES
hydroxyethylstarch
- iCa
ionized calcium
- IQR
interquartile range
- mTPE
membrane‐based therapeutic plasma exchange
- niCa
non‐ionized calcium
- PVE
plasma volume exchange
- RCA
regional citrate anticoagulation
- ROC
receiver operating characteristic
- SH
systemic heparinization
- TCa
total calcium
- TCa:iCa
total calcium to ionized calcium ratio
- TPE
therapeutic plasma exchange
1. INTRODUCTION
Therapeutic plasma exchange (TPE) is an extracorporeal blood purification technique aiming at removing large molecular weight compounds from the circulation.1 Main indications include immune‐mediated diseases, hyperviscosity syndrome and toxicosis caused by highly protein‐bound solutes.2 Aside from the older manual centrifugation, 2 main modern techniques have been developed to separate the plasma from the blood circulating in an extracorporeal circuit: continuous flow centrifugation‐based TPE (cTPE) and membrane filtration TPE (mTPE).1, 3 Although the cTPE platforms offer the possibility of other apheresis techniques such as red cell, white cell, or platelet apheresis, they require specific machines that are only available in few specialized institutions. The technique of mTPE, however, has become more widely available, with the development of multifunctional blood purification platforms, allowing a wide spectrum of organ replacement treatments including renal, liver, or pulmonary support. Safe extracorporeal treatments require a good understanding of the physiological, hematological, and biochemical changes expected from the treatments. Although most treatments are initially inspired by human protocols, extrapolation to dogs and cats with their diseases and their specific pathophysiological, cardiovascular, and metabolic responses has limitations. Small animal protocols therefore need to be established based on species‐specific data, but few cases have been published, reporting its use in toxicoses,4, 5, 6 hyperviscosity syndromes,7, 8, 9, 10, 11 immune‐mediated diseases,12, 13, 14, 15, 16, 17 and microangiopathy.18
The mTPE technique uses a continuous extracorporeal circulation and a hollow‐fiber plasma separator with a large pore high cutoff membrane retaining only the cellular blood elements and filtering out the plasma. Replacement fluids are used for isovolemic reconstitution of the blood and for compensation of the discarded plasma components. Fresh‐frozen plasma (FFP) typically is the first‐choice replacement, but most TPE indications do not require 100% FFP and some of the plasma can be replaced by albumin, colloids, or crystalloids, depending on the underlying disease.
For animals at risk of bleeding, systemic heparinization (SH) can be replaced by regional citrate anticoagulation (RCA) for extracorporeal blood purification techniques.19 However, the clearance of solutes in mTPE being limited by a maximal filtration fraction of 20%‐25%, citrate and citrate‐calcium complexes tend to accumulate when their administration rate exceeds the capacity of hepatic metabolism. The resulting citrate toxicosis can lead to ionized hypocalcemia, total hypercalcemia, and metabolic alkalosis, limiting the blood flow rate and the substitution rate with citrate‐containing FFP. Therefore, RCA is considered relatively contraindicated for mTPE in humans.20, 21 For diseases with hemostatic disorders including thrombocytopenia, disseminated intravascular coagulation, pulmonary hemorrhages, or central nervous system bleeding, SH remains an absolute contraindication and RCA has been used tentatively at low administration rates. Data on citrate tolerance in TPE treatments for dogs are therefore needed to improve the safety of the procedure and to widen the spectrum of treatable conditions.
The aims of this retrospective study were therefore to describe the technique of mTPE as used in our hospital and to analyze the clinical and clinicopathological changes associated with the treatment. The tolerance to citrate administration during mTPE was analyzed to define a tentative and potentially safe administration rate for dogs.
2. MATERIALS AND METHODS
2.1. Study design and animals
This study was designed as a retrospective evaluation of data collected from treatments based on prospectively planned protocols. Dogs treated with mTPE between 2011 and 2017 were included in the analysis. Their medical and treatment records were analyzed and following data were extracted: signalment, disease and indication for mTPE treatment, vascular access, treatment prescription, clinicopathological data before and after treatment, and specifics of anticoagulation.
2.2. Specifics of mTPE treatment
Vascular access was provided using a right jugular central venous catheter adapted to the size of the dog and dedicated strictly to the blood purification procedure. Between the individual treatments, the access catheter was locked either with sodium citrate (46.7%, DuraLockc, Medcomp, Euromed Swiss AG, Frauenfeld, Switzerland) or with unfractionated heparin (50‐1000 IU/mL, Heparin Bichsel, Dr G. Bichsel Pharmacy, Interlaken, Switzerland).
The mTPE treatment was performed on a Prismaflex platform, using a TPE 1000 circuit (Baxter Healthcare, Glattpark, Switzerland). This pre‐connected set has a total priming blood volume of 71 mL. The incorporated plasmafilter with a polypropylene membrane has a blood volume of 23 mL and an effective filtration surface area of 0.15 m2. Its sieving coefficient for albumin is 0.97, for IgG 1.00, and for IgM 0.92 (manufacturer's data). Treatments were prescribed based on human protocols and were adjusted individually depending on the actual requirements of the animals, the perceived complication risks, and the response to previous treatments. The basis for the calculation of the mTPE prescription was to provide 1.0 plasma volume exchange (PVE) per treatment. Plasma volume was estimated using the formula: PVE = 0.08 × body weight (BW) × (1 − hematocrit), assuming a blood volume of 8% of the BW.
Replacement fluids included FFP, 5% human albumin (HAlb, as a 1:3 mixture of 20% HAlb [Albumin CSL 20%, CSL Behring, Bern, Switzerland] and 0.9% NaCl), 3% hydroxyethylstarch (HES, as a 1:1 mixture of 6% HES [Voluven balanced 6%, 130/0.4, Fresenius Kabi, Oberdorf, Switzerland] and 0.9% NaCl), and 0.9% saline. The basic composition of the replacement fluid consisted of 35%‐65% FFP, 10%‐30% HAlb, 0%‐10% HES, and 0%‐50% saline, administered sequentially; it was adjusted to the individual needs of the animals, taking in consideration the underlying disease and comorbid disorders, the cardiovascular condition, and the hemostatic status. The plasma was preferentially chosen from the same blood group as the dogs, but this could not always be done because of limited availability concerns. Intravenous test injections of 1 mL HAlb and of 1 mL of each unit FFP were performed sequentially, at a 15 minute interval, immediately before the mTPE was initiated. Specific monitoring was implemented for 14 days to evaluate for potential adverse effects of the administration of HAlb and HES. Daily physical examinations were performed with special attention to body temperature, peripheral and facial edema, skin lesions suggestive of vasculitis, and gastro‐intestinal and respiratory disturbances. Complete blood counts and chemistry profiles were performed every 2‐5 days during hospitalization, and the presence of proteinuria was assessed when clinical or laboratory signs potentially associated with glomerular disease were observed.
Anticoagulation consisted of either SH, RCA, or a combination of both (heparin‐citrate anticoagulation [HepCit]), depending on the perceived risk of hemorrhage or of thrombosis from the underlying disease. For SH, 50 IU/kg of unfractionated heparin (Heparin Bichsel, 1000 U/mL, Dr Bichsel Pharmacy, Interlaken, Switzerland) were administered IV 5 minutes before starting treatment and a constant rate infusion of 50 IU/kg/h was maintained until 15 minutes before the end of the treatment. Monitoring and adjustments were based on the activated coagulation time (Medtronic ACT II Automated Coagulation Timer, Medtronic Switzerland, Munchenbuchsee, Switzerland) maintained between 150 and 200 seconds (reference range, 60‐90 seconds). For RCA, the anticoagulation module of the Prismaflex platform was used with minor adjustments, based on the protocol recently described for intermittent hemodialysis in dogs.19 Trisodium citrate 3% (Dr Bichsel Pharmacy, Interlaken, Switzerland) was administered at an initial flow rate providing 3.5 mmol citrate per liter of blood; calcium chloride dihydrate 5% (Dr Bichsel Pharmacy, Interlaken, Switzerland) was administered with an external syringe pump at an initial flow rate corresponding to 1/10 of the citrate flow rate (0.33 mmol Ca per mmol citrate). Rate adjustments were based on ionized calcium (iCa) monitoring in the dog (goal, 1.0‐1.2 mmol/L) and in the circuit (goal, <0.3 mmol/L) at 30 minutes of treatment start and every 30‐60 minutes thereafter. For HepCit anticoagulation, the same RCA protocol was used, and in addition, a low dose of unfractionated heparin (10‐20 IU/kg) was administered IV at the start of treatment; this injection was repeated if deemed necessary based on changes in the filter pressure gradient and in the transmembrane pressure. In these dogs, the monitoring of anticoagulation was based solely on the monitoring of the iCa, as for pure RCA.
As the most appropriate variables and cutoff values to define citrate toxicosis have not yet been evaluated in dogs, a 3‐step grading system (nonrelevant, mild, severe accumulation) was used, based on a combination of all 4 variables, with cutoff values similar to those used in humans22, 23, 24, 25: decreased iCa by >0.2 mmol/L (mild) or >0.3 mmol/L (severe); increased non‐ionized calcium (niCa) by >1.0 mmol/L (mild) or >1.5 mmol/L (severe); increased total calcium (TCa) by >1 mmol/L (mild) or >1.5 mmol/L (severe); and increased total calcium to ionized calcium (TCa:iCa) ratio by >1 (mild) or >2 (severe). Mild citrate accumulation was defined as the presence of at least 2/4 abnormal variables in the mild range and severe toxicosis by the presence of at least 2/4 abnormal variables in the severe range.
2.3. Data analysis and statistical methods
Data analysis is mostly descriptive. To evaluate the significance of the laboratory changes associated with the treatments, comparisons before and after treatment were however analyzed statistically, if data were available for more than half of the treatments. For the analysis of citrate accumulation and its occurrence, the dog population was stratified as renal versus nonrenal (creatinine cutoff, 1.3 mg/dL, 117 μmol/L) and hepatic versus non‐hepatic (bilirubin cutoff, 1.2 mg/dL, 20 μmol/L).
As most numerical data sets were not normally distributed, they are presented as median and interquartile range (IQR) and analyzed using nonparametric methods. For comparison of numerical data between groups, a Wilcoxon signed‐rank test was used for paired data, a Mann‐Whitney U Test for unpaired data, and a Kruskal‐Wallis 1‐way ANOVA for comparison of more than 2 groups. A chi‐square test or a Fisher exact test was used for comparison of proportions. Odds ratios for development of citrate accumulation were calculated for dogs affected with renal or hepatic dysfunction, compared to their non‐affected counterparts. A receiver‐operating characteristics (ROC) curve analysis was used to determine a tentative cutoff in citrate administration predicting a 20%‐risk of severe citrate accumulation, a risk considered arbitrarily acceptable for this type of treatment. A P‐value <.05 was considered significant. Analyses were performed with the NCSS commercial statistical software (NCSS 9.0.15; NCSS, LLC, Kaysville, Utah).
3. RESULTS
3.1. Animals and diseases
Thirty‐four dogs were treated with mTPE, including 19 males (12 intact, 35%; 7 castrated, 21%) and 15 females (5 intact, 15%; 10 spayed, 29%). Thirty‐two dogs were pure‐bred dogs from 26 different breeds, and 2 were mixed‐breed dogs. Represented breeds included Labrador Retriever (n = 3), Poodle (n = 3), Shi‐Tzu (n = 2), Yorkshire Terrier (n = 2), and 22 further breeds with 1 dog each. Median age was 5.2 years (IQR, 2.5‐8.5) and BW was 13.0 kg (IQR, 8.2‐29.3; range, 3.3‐45.0).
Main indications for mTPE were primary or secondary immune diseases in 29 dogs (immune‐mediated hemolytic anemia, n = 13; immune‐mediated thrombocytopenia, n = 9; leptospirosis‐associated pulmonary hemorrhages, n = 13; and neuromuscular diseases, n = 2). Other indications included polysystemic diseases in 5 dogs (sepsis, n = 4; microangiopathy, n = 2), and hyperviscosity in 3 dogs (leishmaniasis, n = 3). More than 1 indication was present in 7 dogs.
3.2. Specifics of the mTPE treatments
Sixty‐four mTPE treatments were performed with a median of 2 treatments per dog (range, 1‐3). Vascular access was provided with double‐lumen central venous or hemodialysis catheters with diameters (7‐13.5 Fr) and lengths (16‐28 cm) adapted to the dog's size. Catheters included Arrow 7 Fr x 16 cm Blue FlexTip catheters in 4 dogs, Arrow 8 Fr x 20 cm in 13 dogs, Arrow 12 Fr x 20 cm in 4 dogs (Teleflex Medical GmbH, Belp, Switzerland); Medcomp Duo‐Flow 9 Fr x 15 cm in 1 dog, Medcomp HemoCath 11.5 Fr x 20 cm in 6 dogs, Medcomp 11.5 Fr x 24 cm in 2 dogs, Medcomp 13.5 Fr x 24 cm in 1 dog, and Medcomp 13.5 Fr x 28 cm in 3 dogs (Euromed Swiss AG, Frauenfeld, Switzerland).
Nineteen mTPE treatments (30%) were combined sequentially with intermittent hemodialysis in 15 dogs (44%) with acute kidney injury (AKI). The mTPE procedure was performed first in all these combined treatments with the exception of 1 mTPE treatment performed after hemodialysis. The dogs were premedicated with diphenhydramine (1 mg/kg IV) for 14 treatments (22%), dexamethasone (0.2 mg/kg IV) for 1 treatment (2%), and combined dexamethasone‐diphenhydramine for 1 treatment (2%). No premedication was administered for 48 treatments (75%).
The median PVE calculated for the treatment prescription was 742 mL (IQR, 453‐1825), to reach a goal of 1.0 PVE per treatment. The main characteristics of the mTPE treatments and the composition of the replacement fluid are summarized in Table 1. Except for the first 12 treatments in which HES was administered last and 5 treatments in which a change was deemed necessary, the order of administration was typically starting with HAlb, continuing with HES and saline, and finishing with FFP (n = 46). The dose of HAlb administered was 0.64 g/kg (IQR, 0.44‐0.85) but, as HAlb was typically administered first (n = 60), approximately half of it can be estimated to have been removed during the treatment. The average chloride concentration of the replacement fluid was 129 mmol/L (IQR, 123‐138).
Table 1.
Characteristics of 64 membrane‐based therapeutic plasma exchange (mTPE) treatments and composition of the replacement fluid
| Treatment characteristics | ||
| Total blood volume processed | (mL) | 6000 (3450‐10 900) |
| (mL/kg) | 432 (372‐492) | |
| Average blood flow rate | (mL/min) | 45 (30‐70) |
| Average filtration fraction | (%) | 14 (12‐15) |
| Volume of filtered plasma | (mL) | 726 (482‐1801) |
| (mL/kg) | 60 (54‐67) | |
| Plasma volume exchange | 1.00 (0.94‐1.04) | |
| Duration of procedure | (min) | 125 (103‐163) |
| Exchange rate | (mL/min) | 5.8 (4.3‐8.9) |
| Composition of the replacement fluid a | ||
| HAlb (59/63 tx, 94%) | (%) | 24% (17‐29) |
| (mL/kg) | 12.9 (8.9‐17.0) | |
| HES (20/63 tx, 32%) | (%) | 0% (0‐10) |
| (mL/kg) | 0 (0‐5.7) | |
| NaCl (40/63 tx, 63%) | (%) | 15% (0‐46) |
| (mL/kg) | 9.8 (0.0‐26.7) | |
| FFP (63/63 tx, 100%) | (%) | 48% (29‐62) |
| (mL/kg) | 25.2 (17.9‐36.3) | |
Notes: Data are presented as median (interquartile range [IQR]). The fluids used as replacement of plasma included human albumin (HAlb, 5%); hydroxyethylstarch (HES, 3%); saline (NaCl, 0.9%); and fresh‐frozen plasma (FFP).
Data from 63 treatments. In 1 treatment, the exchange could not really be started because of insufficient blood flow rate.
3.3. Physiologic and metabolic response to mTPE
Body weight increased with mTPE a median of 180 g (IQR, 5‐300), corresponding to 1.5% (IQR, 0.1%‐3.0%) of the initial weight (P < .001). Body temperature did not change significantly, with a difference of +0.1°C (−0.2 to 0.4, P = .29). Heart rate increased mildly from 90 bpm (IQR, 69‐116) to 109 bpm (IQR, 83‐134, P < .001), and systolic blood pressure increased mildly from 144 mm Hg (IQR, 130‐156) to 154 mm Hg (IQR, 132‐169, P = .02).
Complete blood counts were performed before and after mTPE treatment in 4 dogs only. The most relevant change was a marked decrease in the platelet count in all 4 dogs (median from 228 to 48 G/L), whereas packed cell volume did not change significantly. Relevant changes in the CBC, chemistry profile, and coagulation variables are summarized in Table 2. With the chosen composition of the replacement fluid, total proteins decreased by 24.7% (IQR, 16.7‐31.4; P < .001), whereas serum albumin did not change significantly with a reduction ratio of 5.1% (IQR, −10.8 to 12.5, P = .13). The azotemia decreased significantly but only minimally with a median urea reduction ratio of 9.0% (IQR, 0.7‐15.7; P < .001) and a creatinine reduction ratio of 4.5% (IQR, −6.6 to 10.6; P = .006). For dogs with markedly increased serum bilirubin pretreatment (>2.9 mg/dL, 50 μmol/L, n = 11), the total bilirubin concentration decreased significantly with a median reduction ratio of 36% (IQR, 13%‐43%, P = .02). The only significant change noted in the coagulation profile was a marked decrease in fibrinogen concentration by 53% (IQR, −30 to 63; P = .009), without relevant changes in the coagulation times. Only minimal changes were noted in the acid‐base variables, including significant but non clinically relevant metabolic acidosis and acidemia (Table 3).
Table 2.
Changes in laboratory variables associated with mTPE treatment
| Variable | n (pre/post) | RR | Pre‐mTPE | Post‐mTPE | P |
|---|---|---|---|---|---|
| PCV (%) | 63/62 | 39‐57 | 24.0 (18.3‐27.0) | 23.0 (19.3‐28.8) | <.001 |
| PLT (103/μL) | 30/4 | 150‐400 | 134 (35‐272) | 48 (37‐62) | n/a |
| WBC (103/μL) | 30/4 | 6.0‐12.0 | 19.0 (12.7‐29.8) | 19.5 (16.1‐24.4) | n/a |
| Na (mmol/L) | 64/63 | 142‐154 | 144 (141‐148) | 151 (147‐155) | <.001 |
| K (mmol/L) | 64/63 | 4.22‐5.43 | 3.8 (3.3‐4.2) | 3.3 (2.9‐4.0) | <.001 |
| Urea (mg/dL) | 63/63 | 19.8‐64.9 | 65.5 (37.2‐232.4) | 54.1 (30.6‐193.4) | <.001 |
| Crea (mg/dL) | 64/63 | 0.6‐1.3 | 0.8 (0.6‐5.4) | 0.9 (0.5‐4.7) | .006 |
| Bili (mg/dL) | 34/20 | 0.03‐0.2 | 2.9 (0.6‐17.9) | 6.1 (1.0‐12.9) | n/a |
| TP (g/dL) | 51/56 | 5.6‐7.3 | 4.8 (4.1‐5.9) | 3.8 (3.1‐4.6) | .01 |
| Alb (g/dL) | 64/63 | 3.0‐4.0 | 2.4 (2.0‐2.7) | 2.3 (2.1‐2.5) | .13 |
| PT (s) | 30/13 | 6.3‐8.5 | 8.8 (7.9‐9.6) | 9.5 (8.8‐9.9) | n/a |
| aPTT (s) | 30/13 | 9.6‐16.1 | 13.4 (12.3‐20.4) | 14.9 (12.4‐17.6) | n/a |
| Fib (mg/dL) | 30/13 | 150‐300 | 276 (167‐480) | 139 (121‐195) | n/a |
Notes: Data are presented as median (interquartile range [IQR]) and P reflects the statistical significance tested with a Wilcoxon signed‐rank test for paired data. Statistical analysis was only performed when data were available for more than half of the treatments.
Abbreviations: Alb, albumin; aPTT, activated partial thromboplastin time; Bili, bilirubin; Crea, creatinine; Fib, fibrinogen; K, potassium; mTPE, membrane‐based therapeutic plasma exchange; Na, sodium; PCV, packed cell volume; PLT, platelet count; PT, prothrombin time; RR, reference range; TP, total protein; WBC, white blood cell count.
Table 3.
Changes in acid‐base related laboratory variables during the mTPE treatment. Venous blood gas parameters were measured before treatment (pre‐mTPE), during the phases of plasma replacement with HAlb, HES, NaCl, and FFP, respectively, and after treatment (post‐mTPE)
| RR | Pre‐mTPE | HAlb | HES | NaCl | FFP | Post‐mTPE | |
|---|---|---|---|---|---|---|---|
| pH | 7.33‐7.51 | 7.40 (7.33‐7.45) | 7.41 (7.32‐7.45) | 7.34 (7.30‐7.40) | 7.42 (7.37‐7.47) | 7.40 (7.35‐7.43) | 7.37* (7.33‐7.39) |
| pCO2 (mm Hg) | 25.2‐45.4 | 34.2 (31.3‐38.3) | 29.9 (26.5‐32.6) | 31.4 (29.4‐31.6) | 27.2 (21.4‐31.0) | 30.2 (27.6‐33.5) | 35.8 (32.7‐39.1) |
| HCO3 − (mmol/L) | 18.4‐26.3 | 21.4 (19.7‐23.1) | 19.7 (16.0‐21.2) | 17.6 (16.5‐19.1) | 20.0 (15.2‐22.0) | 19.7 (18.0‐21.1) | 20.4* (18.5‐22.0) |
| BE (mmol/L) | −2.0 to +2.0 | −3.4 (−5.8 to −1.4) | −5.6 (−10.6 to −3.6) | −8.1 (−9.6 to −6.2) | −5.0 (−11.1 to −2.6) | −5.3 (−7.4 to −3.4) | −4.5* (−6.8 to −2.6) |
| AG (mmol/L) | 10.8‐19.3 | 14.1 (10.7‐17.6) | 16.7 (12.4‐22.1) | 17.5 (14.4‐22.8) | 14.5 (10.8‐19.9) | 17.5 (13.7‐22.2) | 16.4* (13.5‐20.9) |
| Lactate (mmol/L) | <2.0 | 1.3 (0.9‐1.7) | 1.2 (0.8‐1.6) | 1.7 (1.7‐1.7) | 2.1 (0.8‐2.4) | 1.4 (1.1‐1.6) | 1.5 (1.1‐2.0) |
Note: Data are presented as median (interquartile range [IQR]).
Abbreviations: AG, anion gap; BE, base excess; FFP, fresh‐frozen plasma; HAlb, human albumin; HES, hydroxyethylstarch; mTPE, membrane‐based therapeutic plasma exchange; NaCl, saline; RR, reference range.
*P < .001, for paired comparison between pre‐ and post‐mTPE with a Wilcoxon signed‐rank test.
3.4. Treatment complications
Complications were observed in 22/64 treatments (34%). One technical complication because of low blood flow rates in a small‐sized dog caused early discontinuation of the treatment, but all other treatments could be performed to satisfaction and reached >90% of the plasma exchange goal. The following complications were observed: vomiting (7 treatments), hemorrhagic diarrhea (1), sneezing (2), urticaria (1), laryngeal edema (1), chemosis (1), system clotting (2), and technical problem because of auto‐test difficulties related to insufficiently low blood flow rates (5). Two dogs developed progressive dyspnea after completion of their treatments as a worsening of their preexisting leptospirosis‐associated pulmonary hemorrhages and were therefore euthanized.
Five of the 7 episodes of vomiting and the 2 episodes of sneezing were observed during the second third of the plasma transfusions and they were associated with a low iCa of 0.5‐0.7 mmol/L, concentrations otherwise rarely seen (<10%) in these treatments. The dogs improved rapidly after bolus injection of calcium gluconate. The observed transfusion or allergic reactions with urticaria, laryngeal edema, and chemosis occurred in 3 dogs that had not been premedicated. The fast resolution after interruption of the plasma exchange and injection of dexamethasone and diphenhydramine allowed these treatments to be performed to completion without further complication. These complications triggered a change in our protocol, including the IV injection of 1 mg/kg diphenhydramine, 10 minutes before treatment.
Clinical and laboratory signs suggestive of a reaction to the administration of HAlb or HES were not observed during follow‐up in any of the dogs after TPE. The allergic and transfusion reactions mentioned above recovered quickly and uneventfully after temporarily discontinuing the plasma transfusion and administering antihistamines and steroids, suggesting a reaction to FFP rather than to HAlb. The described pulmonary hemorrhages were most likely unrelated to the infusion of HAlb as they were already present before TPE in the affected dogs with leptospirosis. Twelve dogs died before the 14‐day follow‐up could be completed (median 2 days; IQR, 1‐4). In all other dogs, the monitoring could be completed as planned, either during their hospitalization (n = 6) or at rechecks after their discharge from hospital (n = 16).
3.5. Anticoagulation and citrate toxicosis
Three anticoagulation strategies were used in the mTPE treatments: SH for 2 treatments (3%), RCA for 24 treatments (38%), and HepCit for 38 treatments (59%). In the treatments with SH, a total of 148 IU/kg (IQR, 142‐153) unfractionated heparin was administered, corresponding to 63 IU/kg/h (IQR, 62‐65). In HepCit combined anticoagulation, 53 IU/kg (IQR, 29‐72) unfractionated heparin was administered, corresponding to 20 IU/kg/h (IQR, 13‐37).
For citrate anticoagulation, the total citrate load was 1.91 mmol/kg (IQR, 1.57‐2.27) and the average citrate administration rate was 14.6 μmol/kg/min (IQR, 11.1‐19.6). The plasma administration contributed to 28% (IQR, 20%‐35%) of the citrate load in these dogs. Based on the established criteria for iCa monitoring, citrate infusion rate had to be increased 58 times in 35/62 treatments (56%) and decreased 88 times in 49/62 treatments (79%). Calcium infusion rate had to be increased 79 times in 40/62 treatments (65%) and decreased 64 times in 42/62 treatments (68%).
The dogs showed evidence suggestive of citrate accumulation in all treatments, with a decrease in iCa, an increase in niCa, an increase in TCa, and an increase in the TCa:iCa ratio (Table 4 and Figure 1). This citrate accumulation was classified as nonrelevant for 17 treatments (27%), mild for 20 treatments (31%), and severe for 27 treatments (42%). The citrate accumulation was associated with the total citrate load, with 1.59 mmol/kg citrate (IQR, 1.43‐1.96) for dogs with nonrelevant accumulation, 1.78 mmol/kg (IQR, 1.49‐2.21) for mild accumulation, and 2.12 mmol/kg (IQR, 1.81‐2.48) for severe accumulation (P = .002). Corresponding citrate administration rates were 11.1 (IQR, 9.9‐13.5), 12.0 (IQR, 9.4‐17.4), and 17.7 μmol/kg/min (IQR, 14.4‐21.9), respectively (P = .001). Based on the ROC curve analysis, a citrate administration rate of >8.9 μmol/kg/min (citrate load >1.30 mmol/kg) was associated with a >20% risk, and a rate of >11.4 μmol/kg/min (citrate load >1.59 mmol/kg) with a >50% risk of relevant citrate accumulation.
Table 4.
Changes in calcium fractions and evidence of citrate accumulation in dogs treated with mTPE
| Variables | Before tx | After tx | Δ |
|---|---|---|---|
| TCa (mg/dL) | 9.4 (8.5‐10.0) | 14.9 (12.4‐16.8) | 5.1 (3.1‐7.3) |
| TCa (mmol/L) | 2.33 (2.12‐2.50) | 3.71 (3.09‐4.20) | 1.28 (0.77‐1.83) |
| ΔTCa | >0.0 mg/dL >0.0 mmol/L |
61/62 (98%) | |
| >4.0 mg/dL >1.0 mmol/L |
39/62 (63%) | ||
| >6.0 mg/dL >1.5 mmol/L |
23/62 (39%) | ||
| iCa (mmol/L) | 1.17 (1.08 to 1.26) | 0.96 (0.85 to 1.10) | −0.21 (−0.28 to −0.09) |
| ΔiCa | <0.0 | 52/59 (88%) | |
| <−0.2 | 30/59 (47%) | ||
| <−0.3 | 13/59 (22%) | ||
| niCa (mmol/L) | 1.11 (1.00‐1.30) | 2.69 (2.14‐3.27) | 1.50 (0.91‐1.92) |
| ΔniCa | >0.0 | 57/57 (100%) | |
| >1.0 | 39/57 (68%) | ||
| >1.5 | 28/57 (49%) | ||
| TCa:iCa ratio (mmol/L:mmol/L) | 1.93 (1.84‐2.36) | 3.70 (3.17‐4.42) | 1.72 (1.11‐2.34) |
| ΔTCa:iCa | >0.0 | 57/57 (100%) | |
| >1.0 | 45/57 (79%) | ||
| >2.0 | 18/57 (32%) |
Note: Descriptive statistics of the variables before and after the treatments and their respective changes (Δ) are presented as median (interquartile range [IQR]) and the proportions of treatments with the corresponding changes as fractions (%).
Abbreviations: Δ, delta (difference); iCa, ionized calcium; niCa, non‐ionized calcium; TCa, total calcium; TCa:iCa ratio, total to ionized calcium ratio; tx, treatment.
Figure 1.
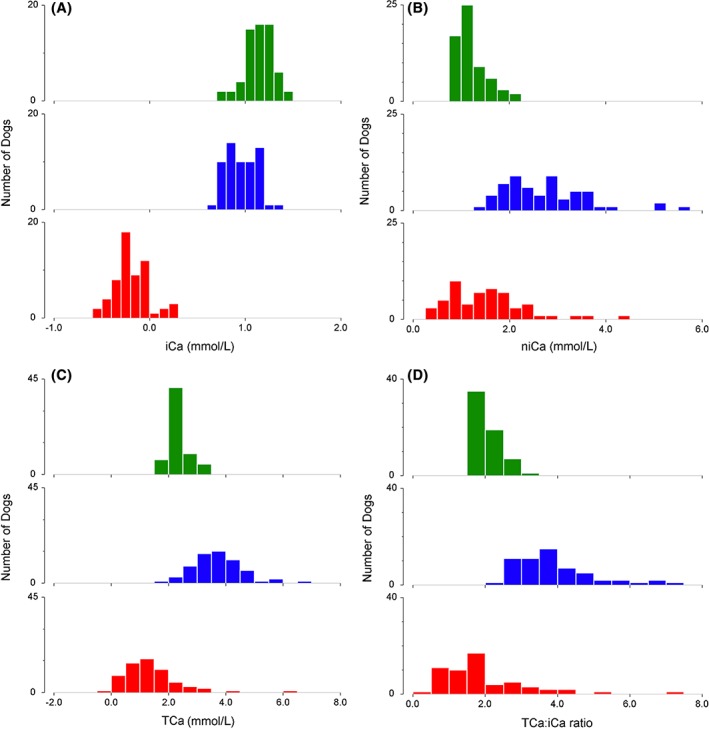
Changes in A, ionized calcium; B, non‐ionized calcium; C, total calcium; and D, total calcium to ionized calcium ratio with membrane‐based therapeutic plasma exchange (mTPE). The histograms represent the data before (green) and after (blue) mTPE treatment, as well as their resulting changes (red). Widths of the categories are 0.1 mmol/L for iCa, 0.25 mmol/L for niCA, and 0.5 mmol/L for TCa and TCa:iCa. iCa, ionized calcium; niCa, non‐ionized calcium; TCa, total calcium; TCa:iCa, total calcium to ionized calcium ratio
Dogs with renal impairment were approximately twice as likely to show citrate accumulation as dogs with normal renal function, despite similar total citrate loads and citrate administration rates (Table 5 and Figure 2). Based on the ROC curve analysis, a total citrate administration rate of ≤5.5 μmol/kg/min (citrate load ≤0.74 mmol/kg) in dogs with renal impairment or ≤9.0 μmol/kg/min (citrate load ≤1.43 mmol/kg) in dogs with normal renal function carried ≤20% risk of severe citrate accumulation. Dogs with impaired liver function and a serum bilirubin concentration of >1.2 mg/dL (20 μmol/L) were not more likely to develop citrate accumulation compared to dogs with a lower bilirubin concentration (Table 5). In these dogs, impaired liver function was defined as the presence of at least 2/5 abnormal liver function tests (bilirubin, albumin, glucose, urea, and cholesterol) and at least 2‐fold increased alanine aminotransferase and aspartate aminotransferase. Dogs with hemolytic anemia and prehepatic hyperbilirubinemia were not included. Even more severe hyperbilirubinemia (>5.8 mg/dL, 100 μmol/L) did not show an increased prevalence of severe citrate accumulation, with 6/16 (38%). Similarly, the combination of renal and liver impairment was not associated with an increased risk of citrate accumulation either.
Table 5.
Citrate accumulation in dogs with renal or hepatic function impairment treated with mTPE
| Variables | Not affected | Affected | P |
|---|---|---|---|
| Renal | n = 38 | n = 26 | |
| Total citrate load (mmol/kg) | 1.80 (1.56‐2.25) | 1.99 (1.53‐2.25) | .61 |
| Citrate rate (μmol/kg/min) | 13.8 (10.1‐19.4) | 15.1 (11.1‐18.5) | .73 |
| Prevalence of all levels CitAcc | 24/38 (63%) | 23/26 (88%) | .04 |
| Prevalence of severe CitAcc | 10/38 (26%) | 17/26 (65%) | .004 |
| OR for all levels CitAcc | ‐ | 2.11 (1.07‐4.20) | .03 |
| OR for severe CitAcc | ‐ | 2.30 (1.34‐3.95) | .002 |
| Hepatic | n = 15 | n = 19 | |
| Total citrate load (mmol/kg) | 1.86 (1.76‐2.24) | 1.95 (1.62‐2.27) | .84 |
| Citrate rate (μmol/kg/min) | 14.0 (10.7‐17.0) | 14.3 (10.0‐17.2) | .86 |
| Prevalence of all levels CitAcc | 9/15 (60%) | 16/19 (84%) | .14 |
| Prevalence of severe CitAcc | 7/15 (47%) | 7/19 (37%) | .73 |
| OR for all levels CitAcc | ‐ | 1.84 (0.84‐4.21) | .12 |
| OR for severe CitAcc | ‐ | 0.81 (0.41‐1.63) | .56 |
| Renal and Hepatic | n = 22 | n = 12 | |
| Total citrate load (mmol/kg) | 1.95 (1.75‐2.37) | 1.86 (1.49‐2.08) | .35 |
| Citrate rate (μmol/kg/min) | 13.0 (10.1‐16.3) | 15.1 (9.5‐18.2) | .65 |
| Prevalence of all levels CitAcc | 15/22 (68%) | 10/12 (83%) | .44 |
| Prevalence of severe CitAcc | 9/22 (41%) | 5/12 (42%) | 1.00 |
| OR for all levels CitAcc | ‐ | 1.57 (0.78‐3.17) | .20 |
| OR for severe CitAcc | ‐ | 1.02 (0.50‐2.07) | .97 |
Note: Numerical data are presented as median (interquartile range [IQR]) and prevalence rates as proportions and fractions (%).
Abbreviations: CitAcc, citrate accumulation; mTPE, membrane‐based therapeutic plasma exchange; OR, odds ratio.
Figure 2.
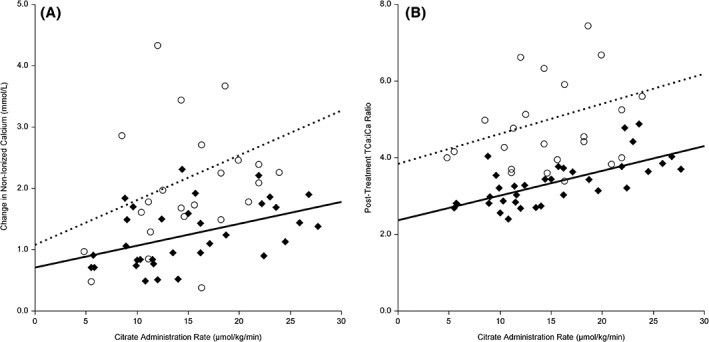
Difference in the relationship of citrate administration to citrate accumulation for dogs with and without renal impairment. The membrane‐based therapeutic plasma exchange (mTPE)‐associated increase of A, non‐ionized calcium (niCa) concentration and B, the post‐mTPE total to ionized calcium (TCa:iCa) ratio are presented and stratified for dogs based on their renal function. The scatter plots represent the data and the regression lines of treatments from dogs with normal renal function (black diamonds, solid line) and from dogs with renal impairment (open circles, dotted line). The citrate administration rate includes both citrate administered for anticoagulation and citrate contained in fresh frozen plasma. The regression analysis denoted weak significant associations only for dogs with normal renal function (niCa: r 2 = .21; P = .008; posttreatment TCa:iCa ratio: r 2 = .46; P < .001). The associations were however not significant for dogs with impaired renal function (niCa: r 2 = .08; P = .17; posttreatment TCa:iCa ratio: r 2 = .07; P = .22)
Clinical problems associated with the anticoagulation procedures included hemorrhagic complications (progression of leptospirosis‐associated pulmonary hemorrhages in 2 dogs and hemorrhagic diarrhea in 1 dog), thrombotic complications (clotting of the filters in 5 treatments: 2 treatments requiring a change of filter and 3 treatments requiring a lower filtration fraction and an extension of the treatment duration), and hypocalcemia‐related complications (sneezing in 2 treatments and vomiting in 5 treatments).
4. DISCUSSION
Based on the results of the present retrospective study and considering the severity of the treated conditions, mTPE can be considered a reasonably safe but not benign procedure for dogs. With a treatment dose of 1 PVE and a combination or replacement fluids including approximately 50% FFP combined with HES, HAlb, and NaCl as used in the study, a stable serum albumin and a moderate decrease in serum fibrinogen concentration can be expected. A substantial decrease in serum bilirubin was however observed in dogs with markedly increased values before treatment. As expected, electrolytes, venous blood gas, and most biochemical variables remained stable, although more complications and changes could have been observed if complete bloodwork had been performed systematically after all treatments.
All dogs treated with mTPE showed laboratory signs of citrate accumulation from the RCA procedure and from the rapid FFP transfusion. The observed laboratory changes indicating citrate accumulation were however only rarely associated with mild clinical signs of hypocalcemia, such as sneezing in 2 dogs and vomiting in 5 dogs. More severe signs commonly associated with hypocalcemia such as facial rubbing, muscle cramping, or seizure‐like activity were not observed in these treatments. The higher citrate accumulation in dogs with renal impairment suggests that dogs with normal renal function likely excrete some of the excess citrate renally. An indirect effect of the uremic state on citrate metabolism has not been reported in other species and seems unlikely. An average total citrate administration rate of ≤5.5 μmol/kg/min in dogs with renal impairment or ≤9.0 μmol/kg/min in dogs with normal renal function carried <20% risk of severe citrate accumulation, and these rates should therefore be viewed as a suggestion for safe RCA in dogs treated with mTPE.
Interestingly, although citrate is metabolized primarily in the liver, even severe liver function impairment was not associated with an increased risk of citrate accumulation. Similar total citrate loads between dogs with and without liver failure suggest that this was not because of a particularly restrictive citrate administration or FFP transfusion rates in the affected dogs. In a multicenter prospective study looking at the safety of RCA in intensive human patients with various degrees of liver failure, a sufficient cellular respiration was suspected to be essential for the liver metabolism of citrate rather than the degree of liver function per se.25 Various types of liver diseases may therefore affect the capacity of hepatic citrate metabolism differently.
Although one third of the treatments were associated with complications, most of those were mild and self‐limiting, and only 1 treatment had to be discontinued early, not reaching its exchange target. In that and 4 other treatments in very small‐sized dogs, the required minimal blood flow rate of 50 mL/min was not reached consistently, causing difficulties with the mandatory auto‐test of the machine, emphasizing the need for a reliable large‐bore venous access. Although most cTPE platforms have larger extracorporeal circuit volumes than mTPE platforms, they can function effectively with inlet blood flow rates as low as 5 mL/min, which can be very helpful for small animals. The allergic‐type reactions with chemosis, urticaria, and laryngeal edema observed in 3 dogs responded quickly to antihistamines and steroids. Nevertheless, we decided to change our protocol and not to rely solely on pretreatment test injections of the albumin solution and the individual plasma units, but also to include systematically a premedication with intravenous diphenhydramine, 10 minutes before the treatment. No allergic reaction was noted in the 22 treatments performed after this change in our protocol. The two dogs with progressive dyspnea and death following the completion of the procedure already had evidence of severe pulmonary hemorrhages associated with leptospirosis and this fatal outcome is unlikely to be because of the treatment itself. Although both dogs have been treated without heparin, an exacerbation of the bleeding associated with the decreased platelet count resulting from the mTPE treatment can however not be ruled out. No evidence of such exacerbation was noticed in 12 other dogs with active leptospirosis‐associated pulmonary hemorrhages. Similarly, no bleeding complication was observed in dogs with severe immune‐mediated thrombocytopenia. Until this is evaluated prospectively, mTPE treatments in dogs at high risk of bleeding should be planned cautiously, carefully weighing the risks and benefits for the animals and avoiding the use of SH.
The choice of replacement fluid is complex and guided mostly by the clinical condition, and also by resource availability and financial concerns. The theoretically ideal replacement with 100% FFP is often cost‐prohibitive, especially in repeated treatments in large‐sized dogs. A full FFP replacement also brings some concerns, including transmission of undiagnosed infectious diseases in an often immunocompromised animal and a high load of citrate limiting the possible plasma exchange rate and prolonging the treatments. In humans, replacement with 100% FFP is also rare and it is reserved for specific conditions. The ideal replacement solution in dogs is unknown at this time. The data reported in the present study therefore bring an important basis for a more differentiated choice of replacement fluid, showing that a 50% FFP replacement does not lead to relevant alterations in the coagulation profiles and therefore can be considered in animals without severe underlying hemostatic disturbances. The inclusion of HES and HAlb was not visibly associated with adverse effects despite the increasing concerns with these solutions potentially associated with the development of AKI26, 27 and Type III hypersensitivity reactions,28, 29 respectively. Neither hospital‐acquired AKI nor progressive proteinuria could be detected in these dogs during hospitalization or follow‐up. However, as not all dogs could be monitored for at least 14 days following administration of HAlb and HES, caution is warranted until the safety of these solutions is better characterized in animals and a systematic long‐term follow‐up should be implemented in dogs receiving these solutions for this or other indications. In the meantime, we decided to minimize the use of these solutions. Human albumin was administered first for transient support of the oncotic pressure, estimating that approximately half of it would be removed in the later phase of the treatment. Hydroxyethylstarch was used only if deemed necessary to support vascular volume and blood pressure. Fresh‐frozen plasma was always administered last to minimize its fraction directly filtered out and lost during the rest of the treatment. Another commonly used replacement fluid prescription is 25% HES, 25% saline, 50% FFP, in that order to avoid the potential risk of HAlb administration. Despite concerns linked to the use of high chloride fluids in dogs with renal impairment, we added saline to our replacement fluid. Calculated average chloride concentration of the pooled replacement fluid was 129 mmol/L. Although saline was only a small part of the replacement fluid, changing it to a lower chloride crystalloid could have decreased this chloride load by approximately 14 mmol/L. Definitive recommendations can certainly not be drawn from the present retrospective study, but the data collected should be used to design prospective studies with specific TPE indications.
The main limitations of the present study include its retrospective nature, even if most data have been collected prospectively and protocols defined in advance. Some of the nonessential laboratory analyses thus were not performed in all dogs. The study results should therefore be considered descriptive and viewed as a step in the development of this important modality in small animals.30 The wide variety of indications and underlying medical conditions represent another limitation, but since the goals of the study were to describe the technical aspects of the treatment, these features add valuable information for situations that are encountered with clinical cases and the conclusions concerning difficulties and complications can be expanded to a wide range of dogs and conditions. The analysis of the effects of kidney and liver involvement on the citrate tolerance should be viewed as an attempt to define medical conditions possibly associated with an increased risk of citrate accumulation. Although a definitive statement on the safety of RCA for mTPE in dogs with renal or hepatic involvement would require a prospective evaluation of a larger number of dogs with various pathomechanisms and more thorough functional evaluation, the results of the present study suggest that RCA can be used for most disease conditions resulting in renal or liver failure, if necessary with individual adaptations of the citrate administration rates.
In conclusion, we described in the present study the technique of mTPE in dogs with various diseases and we reported the expected changes in physiological and laboratory variables. We further determined tentative rates of safe citrate administration for dogs with and without renal impairment, allowing this technique to be used in dogs at risk of bleeding. Altogether this procedure can be performed safely in most dogs even with multiple and severe organ impairment, provided that a close monitoring of the TCa and iCa can be performed.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Authors declare no IACUC or other approval was needed.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
ACKNOWLEDGMENTS
Parts of this study have been presented in abstract form at the 10th Meeting of the International Leptospirosis Society, Palmerston North, New Zealand, 2017, and at the 28th ECVIM‐CA Annual Congress, Rotterdam, the Netherlands, 2018.
Francey T, Schweighauser A. Membrane‐based therapeutic plasma exchange in dogs: Prescription, anticoagulation, and metabolic response. J Vet Intern Med. 2019;33:1635–1645. 10.1111/jvim.15528
Funding information Robmar Foundation for Research and Promotion of the Human‐Animal Bond
REFERENCES
- 1. Gashti CN, Andreoli DC, Patel D. Membrane‐based therapeutic plasma exchange (mTPE): technical and clinical experience. J Clin Apher. 2018;33(1):38‐45. [DOI] [PubMed] [Google Scholar]
- 2. Gashti CN. Membrane‐based therapeutic plasma exchange: a new frontier for nephrologists. Semin Dial. 2016;29(5):382‐390. [DOI] [PubMed] [Google Scholar]
- 3. Cowgill LD, Francey T. Hemodialysis and extracorporeal blood purification In: DiBartola SP, ed. Fluid, Electrolyte, and Acid‐Base Disorders in Small Animal Practice. 4th ed. St. Louis, MO: Elsevier Saunders; 2012:680‐713. [Google Scholar]
- 4. Tovar T, Deitschel S, Guenther C. The use of therapeutic plasma exchange to reduce serum bilirubin in a dog with kernicterus. J Vet Emerg Crit Care. 2017;27(4):458‐464. [DOI] [PubMed] [Google Scholar]
- 5. Walton S, Ryan KA, Davis JL, Acierno M. Treatment of meloxicam overdose in a dog via therapeutic plasma exchange. J Vet Emerg Crit Care. 2017;27(4):444‐450. [DOI] [PubMed] [Google Scholar]
- 6. Walton S, Ryan KA, Davis JL, Acierno M. Treatment of ibuprofen intoxication in a dog via therapeutic plasma exchange. J Vet Emerg Crit Care. 2017;27(4):451‐457. [DOI] [PubMed] [Google Scholar]
- 7. Lippi I, Perondi F, Ross SJ, Marchetti V, Lubas G, Guidi G. Double filtration plasmapheresis in a dog with multiple myeloma and hyperviscosity syndrome. Open Vet J. 2015;5(2):108‐112. [PMC free article] [PubMed] [Google Scholar]
- 8. Perondi F, Brovida C, Ceccherini G, Guidi G, Lippi I. Double filtration plasmapheresis in the treatment of hyperproteinemia in dogs affected by Leishmania infantum . J Vet Sci. 2017;19(3):472‐476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Matus RE, Leifer CE, Gordon BR, MacEwen EG, Hurvitz AI. Plasmapheresis and chemotherapy of hyperviscosity syndrome associated with monoclonal gammopathy in the dog. J Am Vet Med Assoc. 1983;183(2):215‐218. [PubMed] [Google Scholar]
- 10. Matus RE, Leifer CE, Hurvitz AI. Use of plasmapheresis and chemotherapy for treatment of monoclonal gammopathy associated with Ehrlichia canis infection in a dog. J Am Vet Med Assoc. 1987;190(10):1302‐1304. [PubMed] [Google Scholar]
- 11. Boyle TE, Holowaychuk MK, Adams AK, Marks SL. Treatment of three cats with hyperviscosity syndrome and congestive heart failure using plasmapheresis. J Am Anim Hosp Assoc. 2011;47(1):50‐55. [DOI] [PubMed] [Google Scholar]
- 12. Matus RE, Schrader LA, Leifer CE, Gordon BR, Hurvitz AI. Plasmapheresis as adjuvant therapy for autoimmune hemolytic anemia in two dogs. J Am Vet Med Assoc. 1985;186(7):691‐693. [PubMed] [Google Scholar]
- 13. Matus RE, Scott RC, Saal S, Gordon BR, Hurvitz AI. Plasmapheresis‐immunoadsorption for treatment of systemic lupus erythematosus in a dog. J Am Vet Med Assoc. 1983;182(5):499‐502. [PubMed] [Google Scholar]
- 14. Bartges JW, Klausner JS, Bostwick EF, Hakala JE, Lennon VA. Clinical remission following plasmapheresis and corticosteroid treatment in a dog with acquired myasthenia gravis. J Am Vet Med Assoc. 1990;196(8):1276‐1278. [PubMed] [Google Scholar]
- 15. Crump KL, Seshadri R. Use of therapeutic plasmapheresis in a case of canine immune‐mediated hemolytic anemia. J Vet Emerg Crit Care. 2009;19(4):375‐380. [DOI] [PubMed] [Google Scholar]
- 16. Scagnelli AM, Walton SA, Liu C‐C, Acierno MJ. Effects of therapeutic plasma exchange on serum immunoglobulin concentrations in a dog with refractory immune‐mediated hemolytic anemia. J Am Vet Med Assoc. 2018;252(9):1108‐1112. [DOI] [PubMed] [Google Scholar]
- 17. Matus RE, Gordon BR, Leifer CE, Saal S, Hurvitz AI. Plasmapheresis in five dogs with systemic immune‐mediated disease. J Am Vet Med Assoc. 1985;187(6):595‐599. [PubMed] [Google Scholar]
- 18. Skulberg R, Cortellini S, Chan DL, Stanzani G, Jepson RE. Description of the use of plasma exchange in dogs with cutaneous and renal glomerular vasculopathy. Front Vet Sci. 2018;5:161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Francey T, Schweighauser A. Regional citrate anticoagulation for intermittent hemodialysis in dogs. J Vet Intern Med. 2018;32(1):147‐156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ward DM. Conventional apheresis therapies: a review. J Clin Apher. 2011;26(5):230‐238. [DOI] [PubMed] [Google Scholar]
- 21. Shunkwiler SM, Pham HP, Wool G, et al. The management of anticoagulation in patients undergoing therapeutic plasma exchange: a concise review. J Clin Apher. 2018;33:371‐379. [DOI] [PubMed] [Google Scholar]
- 22. Patel S, Wendon J. Regional citrate anticoagulation in patients with liver failure‐‐time for a rethink? Crit Care. 2012;16(5):153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schultheiß C, Saugel B, Phillip V, et al. Continuous venovenous hemodialysis with regional citrate anticoagulation in patients with liver failure: a prospective observational study. Crit Care. 2012;16(4):R162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tolwani A, Wille KM. Advances in continuous renal replacement therapy: citrate anticoagulation update. Blood Purif. 2012;34(2):88‐93. [DOI] [PubMed] [Google Scholar]
- 25. Slowinski T, Morgera S, Joannidis M, et al. Safety and efficacy of regional citrate anticoagulation in continuous venovenous hemodialysis in the presence of liver failure: the Liver Citrate Anticoagulation Threshold (L‐CAT) observational study. Crit Care. 2015;19(1):349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Adamik KN, Yozova ID, Regenscheit N. Controversies in the use of hydroxyethyl starch solutions in small animal emergency and critical care. J Vet Emerg Crit Care. 2015;25(1):20‐47. [DOI] [PubMed] [Google Scholar]
- 27. Sigrist NE, Kälin N, Dreyfus A. Changes in serum creatinine concentration and acute kidney injury (AKI) grade in dogs treated with hydroxyethyl starch 130/0.4 from 2013 to 2015. J Vet Intern Med. 2017;31(2):434‐441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Francis AH, Martin LG, Haldorson GJ, et al. Adverse reactions suggestive of type III hypersensitivity in six healthy dogs given human albumin. J Am Vet Med Assoc. 2007;230(6):873‐879. [DOI] [PubMed] [Google Scholar]
- 29. Powell C, Thompson L, Murtaugh RJ. Type III hypersensitivity reaction with immune complex deposition in 2 critically ill dogs administered human serum albumin. J Vet Emerg Crit Care. 2013;23(6):598‐604. [DOI] [PubMed] [Google Scholar]
- 30. Siami GA. 100 years of plasmapheresis. J Clin Apher. 2014;29(1):70. [Google Scholar]


