Abstract
Background
Chagas disease, caused by the protozoan parasite Trypanosoma cruzi, causes sudden death and chronic heart disease with no currently approved treatment.
Objective
To report epidemiologic and select cardiac characteristics associated with T. cruzi infection in dogs presenting to a teaching hospital in Texas.
Animals
Three hundred seventy‐five client‐owned dogs.
Methods
A retrospective search of medical records identified dogs tested for T. cruzi antibodies or with histologic T. cruzi parasites. Data retrieved included signalment, location of residence, reported reason for testing, cardiac troponin I (cTnI) concentration, and ECG abnormalities.
Results
Trypanosoma cruzi‐infected dogs (N = 63, 16.8%) were significantly younger than negative dogs (N = 312) (mean, 5.9 ± 3.8 versus 7.4 ± 4.0 years; P = .007) with no difference by sex or breed. Ninety‐one breeds were tested; the highest percent infected were non‐sporting (10/35; 29%) and toy breed (10/42; 24%) groups. The odds of infection were 13 times greater among dogs with an infected housemate or littermate (95% confidence interval [CI], 3.94‐50.45; P < .001). Infected dogs were more likely to have ventricular arrhythmias (odds ratio [OR], 2.19; 95% CI, 1.15‐4.33, P = .02), combinations of ECG abnormalities (OR, 2.91; 95% CI, 1.37‐5.99; P = .004), and cTnI >0.129 ng/mL (ADVIA; OR, 10.71; 95% CI, 1.60‐212.21; P = .035).
Conclusions and Clinical Importance
Dogs infected with T. cruzi were identified in Texas in many breed groups including breeds affected by well‐described heart diseases that mimic Chagas disease suggesting a need for increased awareness, including knowledge of when to consider testing.
Keywords: canine, Chagas disease, ECG, epidemiology, heart, troponin
Abbreviations
- AKC
American Kennel Club
- AV
atrioventricular
- AVB
atrioventricular block
- CHF
congestive heart failure
- cTnI
cardiac troponin I
- IFA
indirect fluorescent antibody
- IgG
immunoglobulin G
- IQR
interquartile range
- OR
odds ratio
- TVMDL
Texas A&M Veterinary Medical Diagnostic Laboratory
- VMTH
Veterinary Medical Teaching Hospital
1. INTRODUCTION
Infection with the protozoan parasite Trypanosoma cruzi causes Chagas disease in humans and animals across the Americas and is the leading cause of infectious myocarditis in humans worldwide.1 Trypanosoma cruzi infection can occur by vector‐mediated transmission by triatomine insects after the introduction of infected bug feces into the bite site or mucous membrane, through the ingestion of infected bugs or their feces, or through congenital transmission.2, 3 In both dogs and humans, clinical outcome is variable, ranging from sudden death to chronic heart disease but also including asymptomatic infections. In some ecological settings, dogs can be sentinels of human disease risk.3, 4 Chagas disease is increasingly recognized in the southern United States, where the parasite is maintained among diverse wildlife in sylvatic transmission cycles.2, 5 The seroprevalence of T. cruzi in dogs is particularly high in the state of Texas, with seroprevalence estimates of 6%‐13% in shelter dogs across the state, 6%‐18% in government working dogs along the Texas‐Mexico border, and seroprevalence exceeding 50% in some multi‐dog kennel environments with triatomine infestations.6, 7, 8 Seropositive dogs occur in Louisiana, Oklahoma, Tennessee, Georgia, and Virginia.9, 10, 11, 12, 13, 14, 15
Clinical presentation of Chagas heart disease varies widely depending on the extent of myocardial damage that results in a combination of arrhythmias, conduction abnormalities, heart enlargement, and heart failure.16, 17 There are 4 recognized pathogenic mechanisms for the development of Chagas heart disease in humans: (1) parasite‐induced damage to the cardiac tissue; (2) cardiac dysautonomia, microvascular circulation disturbances; (3) immune‐mediated myocardial injury; and (4) neurogenic disorders.18, 19 In the limited experimental models of Chagas disease in dogs, acute clinical signs were detected between 14 and 21 days post‐exposure.20 Acute signs are nonspecific and include lethargy, generalized lymphadenopathy, pale mucous membrane, slow capillary refill time, ascites, weak pulse, enlarged liver, enlarged spleen, and sudden death.20 During this stage, arrhythmia and conduction abnormalities can be detected in infected dogs.18, 21 Both human and dogs can then enter an indeterminate period where clinical signs are not evident.18, 21 Chronic Chagas disease in dogs is associated with ventricular arrhythmias, predominantly premature contractions, which can be multiform and progress to ventricular tachycardia.21 As chronic Chagas disease progresses, the dogs could develop myocardial dysfunction and heart failure.18, 21, 22, 23 Experimental treatments, such as benznidazole, in dogs can cause a temporary reduction in parasite load, but the treatment does not prevent myocardial lesions or dysfunction.24, 25 In the United States, there are currently no vaccinations or approved antiparasitic treatments for T. cruzi infections in dogs, and infected dogs are typically treated symptomatically.
Limited studies have been conducted in dogs naturally infected with T. cruzi, and a better understanding of Chagas disease in dogs is important for veterinarian medicine. Trypanosoma cruzi infection is considered to be lifelong, with self‐cure highly unlikely2, 3; therefore, dogs with indirect fluorescent antibody (IFA) test immunoglobulin G (IgG) antibody titers are considered infected.7, 8 In this study, dogs were counted as infected if their IFA IgG antibody titer was ≥20 or they had a histopathologic diagnosis of Chagas disease. The objective of this study was to report epidemiologic, cardiac troponin I (cTnI) and electrocardiographic characteristics, including the presence of arrhythmia or conduction abnormality and approximate anatomic origin of the abnormality within the heart, associated with T. cruzi infection status in dogs presenting to a teaching hospital in Texas.
2. MATERIALS AND METHODS
A retrospective review of electronic medical records was performed to identify all dogs evaluated at Texas A&M University's Small Animal Veterinary Medical Teaching Hospital (VMTH) in College Station, Texas, which were tested for anti‐T. cruzi antibodies or for which T. cruzi was detected using histology of cardiac tissue. The approximately 7‐year period of analysis was January 21, 2010, through December 20, 2016. Data collected from the medical records included signalment (age, sex, breed), geographic location of residence, year presented, reason tested for T. cruzi, IFA test result, histopathology findings of T. cruzi, and as available, cTnl concentration, and ECG abnormalities (listed below).
In the case of multiple T. cruzi serological results from different dates for the same dog, results were compared to assess concordance or discordance. For dogs with concordant results, clinical data taken at the time of the initial appointment were used in the analysis to determine risk factors and clinical outcomes. For dogs with discordant results (ie, at least 1 positive and 1 negative test result from 2 different blood samples), data from the appointment with a positive titer was used for analysis.
The method of serological testing for T. cruzi in these dogs was an IFA performed by the Texas A&M Veterinary Medical Diagnostic Laboratory (TVMDL, College Station, Texas), and samples with IgG titers of ≥20 were considered positive per TVMDL standard protocol; this cutoff has been used in dog and human studies.7, 26 In 3 dogs, no serologic testing was performed, but dogs were included in the analysis due to necropsy records indicating histopathological confirmation of intramyositic T. cruzi amastigotes. A positive serology result or histopathology diagnosis of Chagas disease qualified dogs as naturally infected for purposes of the analysis.
Cardiac troponin I concentrations were measured using 1 of 2 commercially available assays. For dogs admitted from 2010 to 2013, cTnI analysis was performed using a 2‐site chemiluminescent immunometric assay (Immulite 2000; Siemens Healthcare Diagnostics, Los Angeles, California) at the Gastrointestinal Laboratory at Texas A&M University. The Immulite has a lower detection limit of 0.2 ng/mL and has been validated in dogs.27, 28 Since 2013, the Gastrointestinal Laboratory measures serum cTnI concentrations with the ADVIA Centaur CP immunoassay (Ultra‐TnI; Siemens Medical Solutions USA, Inc, Malvern, Pennsylvania). The reported range for cTnI by the manufacturer is 0.006 to 50.0 ng/mL and has been validated in dogs.29
Electrocardiogram reports and traces were reviewed, and abnormalities were recorded and categorized as follows: presence of an abnormality (none detected, arrhythmia, conduction abnormality, or arrhythmia and conduction abnormality) and approximate anatomic location of the abnormality when present (atria including sinus node, atrioventricular [AV] node, ventricles, or combination). Conduction abnormalities included atrioventricular block (AVB), bundle branch block, and sinus node dysfunction.
To evaluate potential risk factors of T. cruzi infection, data were imported into RStudio 1.0.136 software.30 Putative risk factors with respect to signalment were age at the time of serology or histopathology testing, sex, American Kennel Club (AKC) breed group, year of evaluation, and geographic location of residence (dogs from Texas were categorized into 10 ecoregions31; dogs from other states were grouped as “outside Texas” including Connecticut, Washington, Arkansas, Montana, Michigan, Oklahoma, California, and Louisiana). For analysis, 3 ecoregions had less than 4 dogs each, so these dogs were grouped with the nearest ecoregion (Figure 1). Reasons tested included AVB, ventricular arrhythmias; other arrhythmias and conduction abnormalities (including supraventricular premature contractions, atrial fibrillation, atrial standstill, sinus arrest, sinus bradycardia, sick sinus syndrome, and right bundle branch block), congestive heart failure (CHF) (including left sided, right sided, or both), littermate/housemate tested or affected, ventricular enlargement and systolic dysfunction, or other. Other included blood donor, esophageal disease, systemic illness, or infectious disease. For cTnI concentration, the 2 assays (Immulite 2000 and ADVIA Centaur CP immunoassay) were analyzed independently and divided into 3 categories: within the reference range of healthy dogs,32, 33 1‐2 times the upper reference limit, and ≥2 times the upper reference limit. For the Immulite 2000, these values were ≤0.5 ng/mL, 0.51‐1.0 ng/mL, and ≥1.01 ng/mL, respectively.28 In 2 studies in dogs without cardiac disease, cTnI concentration was not detectable in the majority (90%‐100%) of dogs at baseline evaluation using the Immulite assay.32, 33 Dogs with a cTnI concentration above 1.01 ng/mL have more severe cardiac disease including arrhythmogenic right ventricular cardiomyopathy, ventricular tachycardia, AVB or neoplasia/myocardial infiltration, and a significantly shorter median survival time compared to dogs with cTnI ≤0.15 ng/mL using the Immulite Troponin I assay.34 For the ADVIA Centaur CP immunoassay, these values were ≤0.128 ng/mL, 0.129‐0.255 ng/mL, and ≥0.256 ng/mL, respectively.29 A cohort of healthy dogs had an average cTnI concentration of 0.017 ng/mL with a range of 0.006‐0.128 ng/mL using the ADVIA Centaur CP immunoassay.29 Bivariable analysis using the chi‐squared or Fisher's exact and t test were used to identify factors with a P ≤ .25; these factors were used in a logistic regression model. Additionally, linear regression was run on the number of dogs tested that were presented to the VMTH (dependent) per year (explanatory) to determine if the number of dogs tested varied by year. Logistic and linear regression models were calculated, and factors with values of P < .05 were considered significant. Odds ratios (ORs) and 95% confidence intervals were calculated for logistic regression models.
Figure 1.
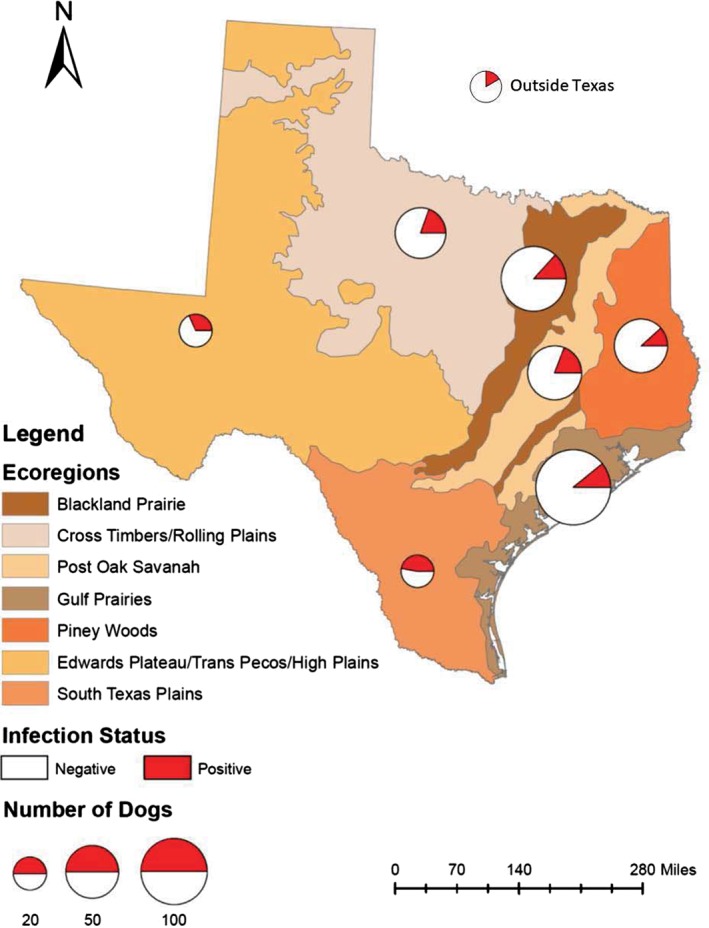
Distribution of Trypanosoma cruzi infection across Texas ecoregions in 375 dogs presenting to Texas A&M University's Small Animal Veterinary Medical Teaching Hospital between 2010 and 2016 that received T. cruzi serology testing or had T. cruzi histological findings. Twelve dogs resided outside Texas, and percent positive are depicted by a pie chart. Circles are proportional to the number of dogs sampled per ecoregion. Map was created using ArcGIS and the base layer is from Gould Ecological Regions created by Texas Parks and Wildlife Department GIS laboratory, downloaded from http://aampo‐mpo.opendata.arcgis.com/
3. RESULTS
3.1. Study population
A total of 375 dogs had serological testing for anti‐T. cruzi antibodies or T. cruzi‐positive histology findings during the approximately 7‐year study period. Dogs were considered to be infected if they had an IgG titer ≥20 or histologic evidence of T. cruzi. Of the 375 dogs, 63 (16.8%) were considered to be infected, including 59 which had a positive serology test result only and 4 with a histological diagnosis of Chagas disease. Of the 4 dogs with a histologic diagnosis, 1 had a negative IgG titer which is most likely a consequence of an acute infection and insufficient time for seroconversion.35 Thirty dogs had multiple visits with repeat serological testing, including 20 with negative serology at both evaluations, 5 with positive titers at the first appointment and negative results at the second appointment (ie, seroreversion), and 5 with negative titers at the first appointment and positive titers at the second appointment (ie, seroconversion).
In the total population tested, age ranged from 0.2 to 16 years (median, 7.6 years; mean, 7.1 years), and 91 dog breeds were represented. The most common breeds tested were Labrador Retrievers (14.4%) and Boxers (10.6%). Negative dogs had a higher mean age (7.4 years) than infected dogs (5.9 years; P = .006), in which the odds of infection are 0.91 times lower for each year increase in age (OR, 0.91; 95% CI, 0.85‐0.97; P = .007). In this referral population, the breeds with the highest percent of infected dogs were Bulldogs (English and French) (7/14), German Shepherd (3/10), Chihuahua and Australian Heeler (both 2/7), and Doberman Pinscher (2/8) (Table 1). The most common AKC breed group tested was sporting dogs, which comprised 26% of the sampled dogs (Table 2). The non‐sporting breed group had the highest percentage of dogs that were infected (29%) followed by the toy breed group (24%), although there were no statistically significant associations between breed group and T. cruzi infection status. The most frequent reason why dogs in the toy and non‐sporting breed group were tested for T. cruzi was the presence of AV block (toy; 60%, non‐sporting; 49%) and ventricular arrhythmias (toy; 33%, non‐sporting; 31%). There were 207 males (55.2%) and 168 females (44.8%) tested with no difference in the proportion of infected dogs by sex (P = .69; Table 2).
Table 1.
Breed characteristics for 375 dogs that received Trypanosoma cruzi serology testing or had T. cruzi histological findings between 2010 and 2016
| Breed | Breed group | Sample size, no. (%) | Positive T. cruzi infection, no. (%) | Positive dogs with ECG abnormalities at the level of ventricles (%) |
|---|---|---|---|---|
| Bulldog—English/French | Non‐sporting | 14 (3.7) | 7 (53.9) | 5 (83.3)* |
| German Shepherd | Herding | 10 (2.7) | 3 (30.0) | 3 (100.0) |
| Chihuahua | Toy | 7 (1.9) | 2 (28.6) | 1 (50.0) |
| Australian Heeler | Herding | 7 (1.9) | 2 (28.6) | 1 (50.0) |
| Doberman Pinscher | Working | 8 (2.1) | 2 (25.0) | 1 (50.0) |
| Border Collie | Herding | 9 (2.4) | 2 (22.2) | 1 (100.0)* |
| German Short Haired Pointer | Sporting | 10 (2.7) | 2 (20.0) | * |
| Dachshund | Hound | 5 (1.3) | 1 (20.0) | 1 (100.0) |
| Spaniel‐Cavalier | Toy | 10 (2.7) | 2 (20.0) | 1 (100.0)* |
| American Pit Bull Terrier | Terrier | 12 (3.2) | 2 (16.7) | 1 (100.0)* |
| Australian Shepherd | Herding | 12 (3.2) | 2 (16.7) | 2 (100.0) |
| Labrador Retriever | Sporting | 54 (14.4) | 9 (16.7) | 4 (50.0)* |
| Yorkshire Terrier | Toy | 7 (1.9) | 1 (14.3) | * |
| Mixed | NA | 26 (6.9) | 3 (11.4) | 3 (100.0) |
| Golden Retriever | Sporting | 9 (2.4) | 1 (11.1) | 0 (0.0) |
| Boxer | Working | 41 (10.9) | 3 (7.3) | 1 (50.0)* |
| Rhodesian Ridgeback | Hound | 6 (1.6) | 0 (0.0) | |
| Boston Terrier | Non‐sporting | 6 (1.6) | 0 (0.0) | |
| Other | NA | 122 (32.5) | 19 (15.6) | 9 (64.3) |
Breeds with less than 5 dogs total are grouped into “other.”
Not all positive dogs had an ECG performed.
Table 2.
Results of bivariable and logistic regression analysis of signalment, year of evaluation, and geographic location of residence for 375 dogs that received Trypanosoma cruzi serology testing or had T. cruzi histological findings between 2010 and 2016
| Logistic regression | ||||||
|---|---|---|---|---|---|---|
| Variable | Sample size, no. (%) | Positive T. cruzi infection, no. (%) | Bivariable P‐value | Odds ratio | 95% confidence interval | P‐value |
| Sex | .69 | |||||
| Male | 207 (55.2) | 38 (18.4) | … | … | … | |
| Female | 168 (44.8) | 25 (14.9) | ||||
| AKC breed group | .29* | |||||
| Stock Service | 2 (0.5) | 0 (0.0) | … | … | … | |
| Herding | 48 (12.8) | 10 (20.8) | … | … | … | |
| Hound | 20 (5.3) | 3 (15.0) | … | … | … | |
| Mixed | 26 (6.9) | 3 (11.5) | … | … | … | |
| Non‐sporting | 35 (9.3) | 10 (28.6) | … | … | … | |
| Sporting | 96 (25.6) | 16 (16.7) | … | … | … | |
| Terrier | 30 (8.0) | 2 (6.7) | … | … | … | |
| Toy | 42 (11.2) | 10 (23.8) | … | … | … | |
| Working | 76 (20.3) | 9 (11.8) | … | … | … | |
| Ecoregion | .01* | |||||
| Outside Texas | 12 (3.2) | 2 (16.7) | Reference | |||
| Blackland Prairie | 75 (20.0) | 10 (13.3) | 0.79 | 0.17‐5.73 | .79 | |
| Cross Timbers | 46 (12.3) | 9 (19.6) | 1.34 | 0.28‐9.92 | .73 | |
| Edwards Plateau | 19 (5.1) | 6 (31.6) | 2.33 | 0.41‐18.72 | .36 | |
| Gulf Prairie | 101 (26.9) | 11 (10.9) | 0.67 | 0.14‐4.79 | .63 | |
| Piney Woods | 51 (13.6) | 6 (11.8) | 0.74 | 0.14‐5.63 | .74 | |
| Post Oak Savannah | 52 (13.9) | 10 (19.2) | 1.41 | 0.30‐10.31 | .69 | |
| South Texas Plains | 19 (5.1) | 9 (47.4) | 4.70 | 0.88‐37.28 | .09 | |
| Year | .04 | |||||
| 2010 | 60 (16.1) | 14 (23.3) | Reference | |||
| 2011 | 59 (15.7) | 10 (16.9) | 0.67 | 0.26‐1.70 | .41 | |
| 2012 | 42 (11.2) | 7 (16.7) | 0.64 | 0.22‐1.79 | .41 | |
| 2013 | 48 (12.8) | 13 (27.1) | 1.17 | 0.47‐2.93 | .73 | |
| 2014 | 57 (15.2) | 11 (19.3) | 0.68 | 0.26‐1.71 | .41 | |
| 2015 | 56 (14.9) | 3 (5.4) | 0.20 | 0.04‐0.68 | .02 | |
| 2016 | 53 (14.1) | 5 (9.4) | 0.30 | 0.09‐0.90 | .04 | |
Expected cell count in the contingency table <5; Fisher's exact test reported instead of chi square.
Twelve dogs presented for evaluation from outside of Texas, whereas the remaining 363 dogs resided in 70 Texas counties spanning all ecoregions.31 Infected dogs were from 33 counties and 8 ecoregions. Bivariable analysis demonstrated a significant difference in infection status across ecoregion (P = .01), in which the highest proportion (47.4%) of infected dogs came from the South Texas Plains ecoregion followed by Edwards Plateau (31.6%; Table 2).
Between 2010 and 2016, 42 to 60 dogs per year had serological tests or histological findings of T. cruzi and the proportion of infected dogs varied across years from 5% to 27% (P = .04; Table 2). The odds of infection in dogs in the last 2 years of the study decreased and were 3.3 (2016) to 5.0 (2015) times lower than the initial year of study in 2010 (OR, 0.20‐0.30; 95% CI, 0.04‐0.9; P = .02‐0.04; Table 2). In a linear regression analysis, the overall number of dogs tested did not vary significantly across year (P = .76).
3.2. Reason for testing
Overall, ventricular arrhythmias were the primary reason for testing for T. cruzi infection in the total population (175/375, 46.7% of dogs; Table 3). Among dogs that tested positive, the predominant reasons for testing were the presence of an infected littermate or housemate (8/12, 67%), CHF (left sided, right sided, or both; 10/25, 40%), and ventricular enlargement with systolic dysfunction (11/40, 28%). Overall, 8 dogs had both CHF and ventricular enlargement with systolic dysfunction, and 5 of them were considered infected. Trypanosoma cruzi infection was significantly higher in dogs with CHF as the reason for testing (10/25, 40%; P = .004) than those that did not report CHF (53/350, 15.1%) in which the odds of being infected with T. cruzi were over 4 times greater in dogs with CHF (OR, 4.34; 95% CI, 1.79‐10.17; P < .001; Table 3). Trypanosoma cruzi infection was significantly different between dogs with a known infected house or littermate (8/12, 67%; P < .001) and dogs that did not report infected house or littermates as the reason for testing (55/363, 15.7%). The odds of infection were 13 times higher in dogs that had a known infected house or littermate than among dogs that did not report an infected house or littermate (OR, 13.0; 95% CI, 3.94‐50.45; P < .001; Table 3). Overall, there was no significant difference between infected and negative dogs when reason for testing included the presence of arrhythmias (AVB, ventricular, and “other” arrhythmias) or “other” reason for testing.
Table 3.
Results of bivariable and logistic regression analysis of reason tested for Trypanosoma cruzi infection in 375 dogs that had T. cruzi serology performed or T. cruzi histological findings between 2010 and 2016
| Logistic regression | ||||||
|---|---|---|---|---|---|---|
| Variable | Sample size, no. (%) | Positive T. cruzi infection, no. % | Bivariable P‐value | Odds ratio | 95% confidence interval | P‐value |
| hyphen;AVB | .28 | … | … | … | ||
| Present | 120 (32.0) | 16 (13.3) | ||||
| Absent | 255 (68.0) | 47 (18.4) | ||||
| Ventricular arrhythmias | .39 | … | … | … | ||
| Present | 175 (46.7) | 33 (18.9) | ||||
| Absent | 200 (53.3) | 30 (15.0) | ||||
| Other arrhythmias/conduction abnormalities | .24 | … | … | … | ||
| Present | 57 (15.2) | 6 (10.5) | ||||
| Absent | 318 (84.8) | 57 (17.9) | ||||
| Congested heart failure | .004* | |||||
| Present | 25 (6.6) | 10 (40.0) | 4.34 | 1.79–10.17 | <.001 | |
| Absent | 350 (93.3) | 53 (15.1) | Reference | |||
| Littermate/housemate tested | <.001* | |||||
| Present | 12 (3.2) | 8 (66.7) | 13.00 | 3.94–50.45 | <.001 | |
| Absent | 363 (96.8) | 55 (15.2) | Reference | |||
| Heart enlargement/systolic dysfunction | .09 | |||||
| Present | 40 (10.7) | 11 (27.5) | ||||
| Absent | 335 (89.3) | 52 (15.5) | ||||
| Other | .52 | … | … | … | ||
| Present | 48 (12.8) | 6 (12.5) | ||||
| Absent | 327 (87.2) | 57 (17.4) | ||||
Expected cell count in the contingency table <5, Fisher's exact test reported instead of chi square.
3.3. Electrocardiographic and cardiac troponin I findings
Data assessed included ECG abnormalities and cTnI concentration. Electrocardiographic abnormalities were based on approximate anatomic location in the heart defined as (1) atria that includes sinus node, (2) AV node, or (3) ventricles and type of abnormality. In bivariable analysis, the T. cruzi infection status was not significantly associated (P = .73) with the presence or absence of an ECG abnormality (Table 4). Dogs with ECG abnormalities (N = 289) were analyzed using bivariable analysis as either having an abnormality at a specified anatomic location or not. For those dogs with abnormalities, infection with T. cruzi was significantly higher in dogs with an abnormality that originated from the ventricles (21.8%; P = .03; Table 4) than from the AV node or atria (11.3%). The odds of T. cruzi infection were approximately 2 times greater (OR, 2.19; 95% CI, 1.15‐4.33; P = .02) among dogs that had ventricular arrhythmias than among dogs that had arrhythmias at other anatomic locations. Trypanosoma cruzi‐infected dogs had significantly more anatomic locations (2 or 3 out of 3) in which an ECG abnormality was present (P = .006; Table 4). The odds of being infected were approximately 3 times greater (OR, 2.91; 95% CI, 1.37‐5.99; P = .004) among dogs that had abnormalities at 2 or more anatomic locations than among dogs that had abnormalities at only 1 location. Only 1 T. cruzi‐negative dog had ECG abnormalities at all 3 anatomic locations. In bivariable analysis, significant association (P = .01) between T. cruzi infection status and the type of ECG abnormality (none, conduction, arrhythmia, or both) was found, with infection being the highest in dogs with both a conduction abnormality and arrhythmia (32%) and infection being the lowest in dogs having only a conduction abnormality (8.8%; Table 4). Overall, the percent of infected dogs is significantly lower in the population of dogs that have only conduction abnormalities without arrhythmias (8.8%) than in the population of dogs without any type of abnormality (20%; OR, 0.39; 95% CI, 0.15‐0.98; P = .05).
Table 4.
Results of bivariable and logistic regression analysis of ECG findings and cardiac troponin I concentrations for dogs that had Trypanosoma cruzi serology performed or T. cruzi histological findings between 2010 and 2016
| Variable | Sample size, no. (%) | Positive T. cruzi infection, no. % | Bivariable, P‐value | Odds ratio | 95% confidence interval | P‐value |
|---|---|---|---|---|---|---|
| Troponin I concentration | ||||||
| Immulite, N = 107 | .19* | |||||
| ≤0.5 ng/mL | 58 (15.5) | 14 (24.1) | Reference | |||
| 0.51–1.0 ng/mL | 16 (4.3) | 3 (18.8) | 0.73 | 0.15‐2.66 | .65 | |
| >1.01 ng/mL | 33 (8.8) | 3 (9.1) | 0.31 | 0.068‐1.06 | .09 | |
| Advia, N = 160 | .001* | |||||
| ≤0.128 ng/mL | 46 (12.3) | 1 (2.2) | Reference | |||
| 0.129‐0.255 ng/mL | 26 (6.9) | 5 (19.2) | 10.71 | 1.60–212.21 | .04 | |
| >0.256 ng/mL | 88 (23.5) | 22 (25.0) | 14.99 | 2.98–273.40 | .009 | |
| Not tested or either assay | 108 (28.8) | 15 (13.9) | ||||
| Electrocardiographic results | ||||||
| Abnormality, N = 375 | .73 | … | … | … | ||
| Present | 289 (77.1) | 49 (17.0) | ||||
| Absent | 66 (17.6) | 13 (19.7) | ||||
| Not tested | 20 (5.3) | 1 (5.0) | ||||
| Location of abnormality, N = 289† | ||||||
| Atria | 53 (18.3) | 12 (22.6) | .31 | … | … | … |
| Other location | 236 (81.6) | 37 (15.7) | ||||
| AV node | 124 (42.9) | 17 (13.7) | .26 | … | … | … |
| Other location | 165 (57.1) | 32 (19.4) | ||||
| Ventricles | 156 (54.0) | 34 (21.8) | .03 | 2.19 | 1.15–4.33 | .02 |
| Other location | 133 (46.0) | 15 (11.3) | Reference | |||
| No. of locations abnormalities present, N = 289 | ||||||
| 1 | 246 (85.1) | 35 (14.2) | .006 | Reference | ||
| ≥2 | 43 (14.9) | 14 (32.5) | 2.91 | 1.37–5.99 | .004 | |
| Type of abnormality, N = 355 | .01 | |||||
| None | 66 (18.6) | 13 (19.7) | Reference | |||
| Conduction | 102 (28.7) | 9 (8.8) | 0.39 | 0.15–0.98 | .05 | |
| Arrhythmia | 159 (44.8) | 31 (19.5) | 0.98 | 0.49‐2.09 | .97 | |
| Both present | 28 (7.9) | 9 (32.1) | 1.93 | 0.70‐5.24 | .20 | |
The lowest range of cardiac troponin I was used as the referent in logistic regression.
Expected cell count in the contingency table <5, Fisher's exact test reported instead of chi square.
When dogs had abnormalities present at more than 1 location, it was counted in both locations.
Of the 375 dogs, 267 dogs had cTnI testing performed, with 107 tested on the Immulite 2000 and 160 tested on the ADVIA Centaur CP immunoassay. For the dog samples run on the Immulite, the cTnI concentration ranged from 0.19 to 104.0 ng/mL with an overall median of 0.45 ng/mL, mean of 3.95 ng/mL, and interquartile range (IQR) 0.19‐1.79 ng/mL. Twenty‐eight of these dogs had cTnI concentrations below the limit of detection. With bivariable analysis, no significant difference was found between T. cruzi‐infected dogs and the 3 predefined ranges of cTnI concentration (≤0.5 ng/mL, 0.51‐1.0 ng/mL, and ≥1.01 ng/mL; P = .19). No significant difference was found between T. cruzi‐infected and T. cruzi‐negative dogs with cTnI concentrations of 0.51‐1.0 ng/mL and ≤0.5 ng/mL. On the dog samples analyzed on the ADVIA Centaur CP immunoassay, cTnI concentration ranged from <0.006 to 51.0 ng/mL with an overall median of 0.31 ng/mL, mean of 2.51 ng/mL, and IQR of 0.11‐1.48 ng/mL. Three dogs had concentrations below the limit of detection. Bivariable analysis demonstrated a significant difference between the 3 ranges of cTnI concentration (≤0.128 ng/mL, 0.129‐0.255 ng/mL, and ≥0.256 ng/mL) and T. cruzi infection (P = .001). Furthermore, the odds of being infected were approximately 11 and 15 times greater in dogs with cTnI concentrations of 0.129‐0.255 ng/mL and ≥0.256 ng/mL, respectively (OR, 10.71; 95% CI, 1.60‐212.21; P = .04; and OR, 14.99; 95% CI, 2.98‐273.40; P = .009) than among dogs that had cTnI concentrations of ≤0.128 ng/mL.
4. DISCUSSION
This study demonstrated that dogs infected with T. cruzi are found in all ecoregions in Texas and in many breed groups including breeds affected by well‐described heart diseases that mimic Chagas disease. Furthermore, a clinical index of suspicion and risk factors are not well defined in dogs with Chagas disease, but in this population, infected dogs were more likely to have ventricular arrhythmias (OR, 2.19; P = .02), combinations of ECG abnormalities (OR, 2.91; P = .004), and cTnI >0.129 ng/mL (ADVIA assay; OR, 10.71; P = .04).
Trypanosoma cruzi infection has been found in over 48 different breeds in the United States with a high prevalence in the AKC sporting and working breed groups.14, 36, 37 In this study, we found the highest prevalence in non‐sporting (29%), toy (24%), and herding (21%) breed groups, and in multiple breeds with a predisposition for acquired heart diseases that result in a similar phenotype as Chagas cardiomyopathy. For example, German Shepherds (30% positive in this population) that can develop breed‐related inherited ventricular arrhythmias and sudden death at a very young age; Doberman Pinschers (25% positive) predisposed to idiopathic dilated cardiomyopathy characterized by cardiac enlargement, ventricular myocardial dysfunction, ventricular arrhythmias, CHF, and sudden death; and Bulldogs (54% positive) and Boxers (7% positive) that can develop arrhythmogenic right ventricular cardiomyopathy.38, 39, 40, 41 This is an important point to consider when making a diagnosis of a heart disease that can mimic Chagas disease in a region known to have Chagas disease, as discrimination between Chagas disease and other etiologies of arrhythmias and ventricular systolic dysfunction could be difficult. Because T. cruzi infection is widespread and all breeds are at risk, it is important for veterinarians to consider T. cruzi testing in any dog when there is a clinical index of suspicion, including dogs with known risks for breed‐related heart diseases.
Trypanosoma cruzi‐positive dogs were reported from all 10 ecoregions present in Texas, demonstrating that T. cruzi is widespread across Texas. Occurrence of T. cruzi infection in dogs in Texas is well documented in the United States, and Texas could be a hotspot for infection with 7 triatomines capable of transmission, the highest nationwide.2, 7, 8, 36, 37, 42, 43, 44, 45, 46 The high prevalence of dogs infected with T. cruzi likely demonstrates an established enzootic transmission cycle, as Texas harbors the highest species diversity of triatomine vectors that can transmit T. cruzi and a diversity of wildlife reservoirs.2, 5, 47 The South Texas Plains ecoregion had the highest percent of infected dogs (47%), whereas the next highest region was the Edwards Plateau (32%). There is a greater than expected density of Triatoma gerstaeckeri in south central Texas, which is where we found the highest percent of infected dogs.48 There is a significant difference between the percent of infection in triatomines collected from different ecoregions (P < .001), where triatomines collected from the Edwards Plateau had the highest infection prevalence of 65.0% (n = 562).47 The high density of infected vectors in these areas could increase exposure and infection in dogs. Furthermore, there is a high T. cruzi infection rate in dogs from south central Texas which includes the border with Mexico (this is the south Texas Plains ecoregion).7, 8, 36, 43 In a population of dogs with T. cruzi testing from 1993 to 2007, more than 60% of the cases came from central and southern Texas.36 Similarly, working hound dogs in south central Texas have a seroprevalence of 57.6% (n = 85).7 Finally, dogs in the Lower Rio Grande Valley have a seroprevalence of 19.6% (n = 209).43 This information suggests that all regions of Texas and surrounding states should be involved in future efforts for increasing veterinary and public awareness for Chagas disease. It is important to note that specific ecoregions can be even broader than reported here because dogs were not confirmed to have lived in only 1 site for the entirety of their lives and dogs are known to move and travel.
The overall number of dogs tested stayed consistent over the years (P = .76). This temporal difference could reflect differences in the referral population over time or could reflect the biology of the disease system. For example, in a multi‐year (2013‐2016) citizen science initiative to collect triatomines across Texas, the infection prevalence in vectors was highest in 2013 and decreased thereafter; authors suggested that the decline in infection was due to climate variability.47 Such fluctuations in the population of infected vectors in nature could account for temporal variation in infection in dogs.
The principal reason reported for testing in T. cruzi‐infected dogs was the presence of an infected littermate or housemate (67%), and the odds of being infected were 13 times greater among dogs that had an infected litter or housemate. Trypanosoma cruzi can be transmitted congenitally from dam to pup and has been shown to infect multiple littermates.49, 50 Although direct dog‐to‐dog transmission through blood contact is unlikely, an infected housemate typically indicates that infected vectors are in the dog's environment in the absence of travel history. In Argentina, the presence of at least 1 infected dog in a household is significantly associated with more infected dogs in the same household.51 Reasons for testing for T. cruzi infection including CHF (P = .004), ventricular enlargement, and systolic dysfunction, and a variety of ECG abnormalities including AVB and sinus node dysfunction are based on the clinical index of suspicion and include consideration of other factors such as age of the dog or unusual breed in context in an individual case.
Overall, T. cruzi infection status was not significantly associated (P = .73) with the presence or absence of an ECG abnormality, demonstrating that T. cruzi infection is 1 of the many reasons for ECG abnormalities in tested dogs. Although infected dogs were more likely to have ventricular arrhythmias and had significantly more combinations of ECG abnormalities, there were 13 infected dogs (20%) without an ECG abnormality detected. When abnormalities were analyzed based on the location (atria, AV node, or ventricles), the odds of a T. cruzi‐infected dog having ventricular arrhythmias were 2 times greater (P = .02) than other anatomic locations. In an experimental infection of dogs with T. cruzi, the chronic stage of Chagas disease was characterized by the development of ventricular premature contractions and ventricular tachycardia.21 Similarly, in humans with Chagas disease, ventricular premature contractions are 1 of the most common ECG abnormalities.52 Mechanisms of myocardial damage in T. cruzi‐infected dog and human patients include focal inflammation, diffuse fibrosis, edema, and scarring of the myocardium and conduction system.35, 53, 54 This destruction can lead to a combination of arrhythmias, conduction disturbances, myocardial dysfunction, and heart failure in patients with Chagas disease.
Trypanosoma cruzi tissue tropism is primarily in the myocardium and can cause an inflammatory response that results in myocyte damage. Cardiac troponin I is exclusively in cardiac myocytes and is released immediately after cardiac injury making it a sensitive and specific marker of myocardial injury. Cardiac troponin I is an indicator of myocardial damage, and increased cTnI concentrations occur in dogs and humans with Chagas cardiomyopathy.35, 55 Serum cTnI levels slowly increase in infected dogs and spike at 10‐30 mg/mL at approximately 21 days after infection.56 Furthermore, cTnI serves as a rigorous biomarker because healthy individuals have negligible levels, and it circulates for days to weeks after cardiac injury57 and is a useful biomarker for Chagas disease development because it is minimally invasive. Paired with ECG results and serology, cTnI can help quantify infection and provide a more thorough prognosis. For dogs that were evaluated between 2010 and 2013 and cTnI concentrations were tested on the Immulite 2000, 24% of T. cruzi‐positive dogs fell into the lowest concentration of cTnI (≤0.5 ng/mL), whereas only 9.1% of the dogs in the highest group (≥1.01 ng/mL) were T. cruzi positive. The odds of having cTnI concentration of ≥1.01 ng/mL were 0.31 times lower in the T. cruzi‐infected dogs than in the negative dogs. Because this population presented to a VMTH, many of the dogs had abnormalities which can also cause increased cTnI. Starting in 2013, cTnI concentration was tested on a more sensitive assay with a lower limit of detection of <0.006 ng/mL. With this assay, the majority of dogs had detectable cTnI, and T. cruzi‐infected dogs were significantly more likely to have cTnI concentrations in the increased ranges of 0.129‐0.255 ng/mL (19%) and ≥0.256 ng/mL (25%). Approximately 18% of dogs on both the Immunite 2000 and ADVIA assay fell into the middle concentration of cTnI and were T. cruzi positive. Chagas disease can affect cardiac health acutely or over a long period.18, 20, 21 This finding of infected dogs with cTnI concentrations in the middle range could indicate low levels of cardiac damage over time and even damage as histopathology demonstrated in an infected litter of Boxer puppies.35 Chagas disease is 1 potential cause of increased cTnI concentrations when considered in conjunction with other signs and should be taken into consideration while evaluating the overall health of the dog.
Regarding signalment as a risk factor for infection, T. cruzi in this population was widespread and not disproportionately associated with any breed group or sex. Older dogs have a higher prevalence of T. cruzi infection than younger dogs, likely because older dogs have had more opportunities for exposure.5, 8, 14, 49 In our study population, infected dogs were significantly younger (mean = 5.9 years; P = .006) than negative dogs (mean = 7.4 years). Dogs presenting to a teaching hospital could be more likely to have clinical disease. During an experimental infection of dogs, clinical signs were more severe in younger infected dogs then older dogs, and dogs inoculated at an earlier age had higher parasitemia than older dogs.20
Five dogs had positive titers ranging from 1 : 20 to 1 : 160 at the first appointment and had negative results at the second appointment. Seroreversion in the absence of treatment occurs in mice, humans, and dogs.3, 49, 58, 59, 60, 61, 62 Few studies have reported on seroreversion in dogs. Seroreversion has been documented in 5%‐6% (1/21 and 2/36) of dogs, 1 of which was concurrently positive by xenodiagnoses and then became seropositive again 2 years later.3, 49 In 2 dogs with an IFA titer of 1 : 20 in the present study, it is possible that the change in result was due to interobserver variability or test cross‐reaction.63 Host biological factors such as exposure history, coinfection, and genetic makeup could have an effect on reaction variability of the IFA. Further characterization of IFA test variability in dogs is needed, as IFA is the only available test for diagnosis in dogs and test variability is not established.
This study is limited in that it is retrospective and the quality of the data could vary, and in some cases, data was missing. Despite missing data for some variables, the sample sizes for each variable were relatively large and allowed for reliable statistical analysis (smallest sample size, n = 267). Furthermore, this study is limited in that the majority of dogs were referred to the hospital causing a selection bias. This limits the ability to apply the findings to the general dog population; however, this study still provides important insight into risk factors associated with T. cruzi infection. This study is further limited by being centered on dogs from Texas (n = 363); however, dogs from 8 other states were included, and Texas has a high prevalence of Chagas disease in dogs making it ideal for a retrospective study.2 Other limitations include changes in the cTnI assay, from the Immulite 2000 with an analytical sensitivity of 0.2 ng/mL to the more sensitive ADVIA Centaur CP Ultra‐TnI immunoassay which detects cTnI to 0.006 ng/mL. Due to the change in assay during the 7‐year study, we analyzed the concentrations of cTnI separately, and the results cannot be directly compared between assays. One limitation to T. cruzi testing in dogs that there is no gold standard for diagnosis and discordant tests results are common.8, 10 The use of multiple serology assays to determine positivity can be useful7, 8, 37, 64, 65, 66, 67 and is in accordance with the WHO guidelines for human medicine.68 Unfortunately, using multiple serology assays is not possible for veterinary medicine because the IFA is the only test approved for use in dogs. Therefore, the true infection rate in dogs is not known. Although other tests have been validated in dogs, they are not clinically available for diagnosis.10 In contrast, human medicine relies on a suite of serology assays to determine if a patient is positive. The ECG abnormalities reported here did not include 24‐hour ambulatory ECG analysis which could improve detection of abnormalities. Finally, detailed classification of echocardiographic abnormalities was not specifically assessed in this study because of the wide variety of diseases present in this population.
In summary, dogs infected with T. cruzi based on a titer ≥20 or histologic evidence were identified in all ecoregions in Texas and in a variety of breed groups. Dogs from breeds affected by well‐described heart diseases that mimic Chagas heart disease can test positive for Chagas disease. The most common reasons for testing were an affected litter or housemate and the presence of cardiac abnormalities (CHF, ventricular enlargement with systolic dysfunction). Infected dogs were more likely to have ventricular arrhythmias and combinations of ECG abnormalities were commonly diagnosed. These findings suggest a need for an increased awareness of Chagas disease in dogs including the knowledge of when to consider testing for it.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Authors declare no IACUC or other approval was needed.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
ACKNOWLEDGMENT
The authors acknowledge and thank Jill VanWhy for assistance in retrieving and compiling medical records.
Meyers AC, Hamer SA, Matthews D, Gordon SG, Saunders AB. Risk factors and select cardiac characteristics in dogs naturally infected with Trypanosoma cruzi presenting to a teaching hospital in Texas. J Vet Intern Med. 2019;33:1695–1706. 10.1111/jvim.15516
Funding information National Science Foundation Graduate Research Fellowship Program, Grant/Award Number: 2015110826; Texas A&M University Open Access to Knowledge Fund
REFERENCES
- 1. Bonney K, Engman D. Chagas heart disease pathogenesis: one mechanism or many? Curr Mol Med. 2008;8:510‐518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bern C, Kjos S, Yabsley M, Montgomery S. Trypanosoma cruzi and Chagas' disease in the United States. Clin Microbiol Rev. 2011;24:655‐681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gürtler RE, Cecere MC, Lauricella MA, et al. Domestic dogs and cats as sources of Trypanosoma cruzi infection in rural northwestern Argentina. Parasitology. 2006;134:69‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gürtler RE, Cecere MC, Lauricella MA, et al. Incidence of Trypanosoma cruzi infection among children following domestic reinfestation after insecticide spraying in rural northwestern Argentina. Am J Trop Med Hyg. 2005;73:95‐103. [PMC free article] [PubMed] [Google Scholar]
- 5. Curtis‐Robles R, Lewis BC, Hamer SA. High Trypanosoma cruzi infection prevalence associated with minimal cardiac pathology among wild carnivores in Central Texas. Int J Parasitol Parasites Wildl. 2016;5:117‐123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bern C, Montgomery SP. An estimate of the burden of Chagas disease in the United States. Clin Infect Dis. 2009;49:e52‐e54. [DOI] [PubMed] [Google Scholar]
- 7. Curtis‐Robles R, Snowden K, Dominguez B, et al. Epidemiology and molecular typing of Trypanosoma cruzi in naturally‐infected hound dogs and associated Triatomine vectors in Texas, USA. PLoS Negl Trop Dis. 2017;11:e0005298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Meyers AM, Meinders M, Hamer SA. Widespread Trypanosoma cruzi infection in government working dogs along the Texas‐Mexico border: discordant serology, parasite genotyping and associated vectors. PLoS Negl Trop Dis. 2017;11:e0005819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Barr SC, Van Beek O, Carlisle‐Nowak MS, et al. Trypanosoma cruzi infection in Walker hounds from Virginia. Am J Vet Res. 1995;56:1037‐1044. [PubMed] [Google Scholar]
- 10. Nieto PD, Boughton R, Dorn PL, et al. Comparison of two immunochromatographic assays and the indirect immunofluorscence antibody test for diagnosis of Trypanosoma cruzi infection in dogs in south Central Louisiana. Vet Parasitol. 2009;165:241‐247. [DOI] [PubMed] [Google Scholar]
- 11. Snider TG, Yaeger RG, Dellucky J. Myocarditis caused by Trypanosoma cruzi in a native Louisiana dog. J am Vet Med Assoc. 1980;177:247‐249. [PubMed] [Google Scholar]
- 12. Fox JC, Ewing SA, Buckner RG, Whitenack D, Manley JH. Trypanosoma cruzi infection in a dog from Oklahoma. J am Vet Med Assoc. 1986;189:1583‐1584. [PubMed] [Google Scholar]
- 13. Bradley KK, Bergman DK, Woods JP, Crutcher JM, Kirchhoff LV. Prevalence of American trypanosomiasis (Chagas disease) among dogs in Oklahoma. J am Vet Med Assoc. 2000;217:1853‐1857. [DOI] [PubMed] [Google Scholar]
- 14. Rowland ME, Maloney J, Cohen S, et al. Factors associated with Trypanosoma cruzi exposure among domestic canines in Tennessee. J Parasitol. 2010;96:547‐551. [DOI] [PubMed] [Google Scholar]
- 15. Tomlinson MJ, Chapman WL Jr, Hanson WL, Gosser HS. Occurrence of antibody to Trypanosoma cruzi in dogs in the southeastern United States. Am J Vet Res. 1981;42:1444‐1446. [PubMed] [Google Scholar]
- 16. Viotti RJ, Vigliano C, Laucella S, et al. Value of echocardiography for diagnosis and prognosis of chronic Chagas disease cardiomyopathy without heart failure. Heart. 2004;90:655‐660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Biolo A, Ribeiro AL, Clausell N. Chagas cardiomyopathy—where do we stand after a hundred years? Prog Cardiovasc Dis. 2010;52:300‐316. [DOI] [PubMed] [Google Scholar]
- 18. Bocchi EA, Bestetti R, Scanavacca MI, et al. Chronic Chagas heart disease management from etiology to cardiomyopathy treatment. J Am Coll Cardiol. 2017;70:1510‐1524. [DOI] [PubMed] [Google Scholar]
- 19. Marin‐Neto JA, Cunha‐Neto E, Maciel BC, Simões MV. Pathogenesis of chronic Chagas heart disease. Circulation. 2007;115:1109‐1123. [DOI] [PubMed] [Google Scholar]
- 20. Barr SC, Gossett KA, Klei TR. Clinical, clinicopathologic, and parasitologic observations of trypanosomiasis in dogs infected with north American Trypanosoma cruzi isolates. Am J Vet Res. 1991;52:954‐960. [PubMed] [Google Scholar]
- 21. Barr SC, Holmes RA, Klei TR. Electrocardiographic and echocardiographic features of trypanosomiasis in dogs inoculated with north American Trypanosoma cruzi isolates. Am J Vet Res. 1992;53:521‐527. [PubMed] [Google Scholar]
- 22. Barr SC, Dennis VA, Klei TR, Norcross NL. Antibody and lymphoblastogenic responses of dogs experimentally infected with Trypanosoma cruzi isolates from north American mammals. Vet Immunol Immunopathol. 1991;29:267‐283. [DOI] [PubMed] [Google Scholar]
- 23. Barr SC, Simpson RM, Schmidt SP, et al. Chronic dilatative myocarditis caused by Trypanosoma cruzi in two dogs. J Vet Med Assoc. 1989;195:1237‐1241. [PubMed] [Google Scholar]
- 24. Santos F, Lima W, Gravel A, et al. Cardiomyopathy prognosis after benznidazole treatment in chronic canine Chagas' disease. J Antimicrob Chemoth. 2012;67:1987‐1995. [DOI] [PubMed] [Google Scholar]
- 25. Santos F, Mazzeti A, Caldas S, et al. Chagas cardiomyopathy: the potential effect of benznidazole treatment on diastolic dysfunction and cardiac damage in dogs chronically infected with Trypanosoma cruzi . Acta Trop. 2016;161:44‐54. [DOI] [PubMed] [Google Scholar]
- 26. Leiby DA, Wendel S, Takaoka DT, et al. Serologic testing for Trypanosoma cruzi: comparison of radioimmunoprecipitation assay with commercially available indirect immunofluorescence assay, indirect hemagglutination assay, and enzyme‐linked immunosorbent assay kits. J Clin Microbiol. 2000;38:639‐642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Spratt DP, Mellanby RJ, Drury N, Archer J. Cardiac troponin I: evaluation of a biomarker for the diagnosis of heart disease in the dog. J Small Anim Pract. 2005;46:139‐145. [DOI] [PubMed] [Google Scholar]
- 28. O'Brien PJ, Smith DE, Knechtel TJ, et al. Cardiac troponin I is a sensitive, specific biomarker of cardiac injury in laboratory animals. Lab Anim. 2006;40:153‐171. [DOI] [PubMed] [Google Scholar]
- 29. Winter RL, Saunders AB, Gordon SG, et al. Analytical validation and clinical evaluation of a commercially available high‐sensitivity immunoassay for the measurement of troponin I in humans for use in dogs. J Vet Cardiol. 2014;16:81‐89. [DOI] [PubMed] [Google Scholar]
- 30. R Development Core Team . R: A language and environment for statistical computing. www.r-project.org; 2008.
- 31. U.S. Environmental Protection Agency . U.S. environmental protection agency level III and IV ecoregions; 2012. https://www.epa.gov/eco-research/ecoregion-download-files-state-region-6#pane-41. Accessed October 30, 2017.
- 32. Singletary G, Saunders A, Saunders B, et al. Cardiac troponin I concentrations following medetomidine‐butorphanol sedation in dogs. Vet Anaesth Analg. 2010;37:342‐346. [DOI] [PubMed] [Google Scholar]
- 33. Saunders A, Hanzlicek A, Martinez E, et al. Assessment of cardiac troponin I and C‐reactive protein concentrations associated with anesthetic protocols using sevoflurane or a combination of fentanyl, midazolam, and sevoflurane in dogs. Vet Anaesth Analg. 2009;36:449‐456. [DOI] [PubMed] [Google Scholar]
- 34. Fonfara S, Loureiro J, Swift S, James R, Cripps P, Dukes‐McEwan J. Cardiac troponin I as a marker for severity and prognosis of cardiac disease in dogs. Vet J. 2010;184:334‐339. [DOI] [PubMed] [Google Scholar]
- 35. Vitt JP, Saunders AB, O'Brien MT, Mansell J, Ajithdoss DK, Hamer SA. Diagnostic features of acute Chagas myocarditis with sudden death in a family of boxer dogs. J Vet Intern Med. 2016;30:1210‐1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kjos SA, Snowden KF, Craig TM, Lewis B, Ronald N, Olson JK. Distribution and characterization of canine Chagas disease in Texas. Vet Parasitol. 2008;152:249‐256. [DOI] [PubMed] [Google Scholar]
- 37. Tenney TD, Curtis‐Robles R, Snowden KF, Hamer SA. Shelter dogs as sentinels for Trypanosoma cruzi transmission across Texas. Emerg Infect Dis. 2014;20:1323‐1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Moïse NS. Inherited arrhythmias in the dog potential experimental models of cardiac disease. Cardiovasc Res. 1999;44:37‐46. [DOI] [PubMed] [Google Scholar]
- 39. Tidholm A, Häggström J, Borgarelli M, Tarducci A. Canine idiopathic dilated cardiomyopathy. Part I: aetiology, clinical characteristics, epidemiology and pathology. Vet J. 2001;162:92‐107. [DOI] [PubMed] [Google Scholar]
- 40. Meurs KM, Stern JA, Reina‐Doreste Y, Spier AW, Koplitz SL, Baumwart RD. Natural history of arrhythmogenic right ventricular cardiomyopathy in the boxer dog: a prospective study. J Vet Intern Med. 2014;28:1214‐1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Santilli RA, Bontempi LV, Perego M, Fornai L, Basso C. Outflow tract segmental arrhythmogenic right ventricular cardiomyopathy in an English bulldog. J Vet Cardiol. 2009;11:47‐51. [DOI] [PubMed] [Google Scholar]
- 42. Hotez PJ. Ten global ‘hotspots’ for the neglected tropical diseases. PLoS Negl Trop Dis. 2014;8:e2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Curtis‐Robles R, Zecca IB, Roman‐Cruz V, et al. Trypanosoma cruzi (agent of Chagas disease) in sympatric human and dog populations in ‘Colonias’ of the lower Rio Grande Valley of Texas. Am J Trop Med Hyg. 2017;96:805‐814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Beard CB, Pye G, Steurer FJ, et al. Chagas disease in a domestic transmission cycle, southern Texas, USA. Emerg Infect Dis. 2003;9:103‐105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wozniak EJ, Lawrence G, Gorchakov R, et al. The biology of the triatomine bugs native to south Central Texas and assessment of the risk they pose for autochthonous Chagas disease exposure. J Parasitol. 2015;101:520‐528. [DOI] [PubMed] [Google Scholar]
- 46. Kjos SA, Snowden KF, Olson JK. Biogeography and Trypanosoma cruzi infection prevalence of Chagas disease vectors in Texas, USA. Vector Borne Zoonotic Dis. 2009;9:41‐50. [DOI] [PubMed] [Google Scholar]
- 47. Curtis‐Robles R, Auckland LD, Snowden KF, Hamer GL, Hamer SA. Analysis of over 1500 triatomine vectors from across the US, predominantly Texas, for Trypanosoma cruzi infection and discrete typing units. Infect Genet Evol. 2018;58:171‐180. [DOI] [PubMed] [Google Scholar]
- 48. Curtis‐Robles R, Hamer G, Levy M, Lane S, Hamer S. Bionomics and spatial distribution of triatomine vectors of Trypanosoma cruzi in Texas and other southern states, USA. Am J Trop Med Hyg. 2017;98:113‐121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Castañera MB, Lauricella MA, Chuit R, Gürtler RE. Evaluation of dogs as sentinels of the transmission of Trypanosoma cruzi in a rural area of North‐Western Argentina. Ann Trop Med Parasitol. 1998;92:671‐683. [DOI] [PubMed] [Google Scholar]
- 50. Rodríguez‐Morales O, Ballinas‐Verdugo MA, Alejandre‐Aguilar R, Reyes PA, Arce‐Fonseca M. Trypanosoma cruzi connatal transmission in dogs with Chagas disease: experimental case report. Vector Borne Zoonotic Dis. 2011;11:1365‐1370. [DOI] [PubMed] [Google Scholar]
- 51. Gürtler RE, Cécere MC, Rubel DN, et al. Chagas disease in north‐West Argentina: infected dogs as a risk factor for the domestic transmission of Trypanosoma cruzi . Trans R Soc Trop Med Hyg. 1991;85:741‐745. [DOI] [PubMed] [Google Scholar]
- 52. Prata A. Clinical and epidemiological aspects of Chagas disease. Lancet Infect Dis. 2001;1:92‐100. [DOI] [PubMed] [Google Scholar]
- 53. Rassi A Jr, Rassi A, Little WC. Chagas' heart disease. Clin Cardiol. 2000;23:883‐889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Andrade ZA. Mechanisms of myocardial damage in Trypanosoma cruzi infection. Ciba Found Symp. 1983;99:214‐233. [DOI] [PubMed] [Google Scholar]
- 55. Aras R, Bastos C, Mota G, et al. Troponin in Chagas disease. Braz J Infect Dis. 2003;7:358‐359. [DOI] [PubMed] [Google Scholar]
- 56. Barr SC. Canine Chagas' disease (American Trypanosomiasis) in North America. Vet Clin North Am Small Anim Pract. 2009;39:1055‐1064. [DOI] [PubMed] [Google Scholar]
- 57. Langhorn R, Willesen JL. Cardiac troponins in dogs and cats. J Vet Intern Med. 2016;30:36‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Francolino SS, Antunes AF, Talice R, et al. New evidence of spontaneous cure in human Chagas' disease. Rev Soc Bras Med Trop. 2003;36:103‐107. [DOI] [PubMed] [Google Scholar]
- 59. Zeledón R, Dias JC, Brilla‐Salazar A, et al. Does a spontaneous cure for Chagas' disease exist? Rev Soc Bras Med Trop. 1988;21:15‐20. [DOI] [PubMed] [Google Scholar]
- 60. Dias JC, Dias E, Martins‐Filho O, et al. Further evidence of spontaneous cure in human Chagas disease. Rev Soc Bras Med Trop. 2008;41:505‐506. [DOI] [PubMed] [Google Scholar]
- 61. Tarleton R. The role of immunology in combating Trypanosoma cruzi infection and Chagas disease. Rev Esp Salud Publica. 2013;87:33‐39. [Google Scholar]
- 62. Bertocchi G, Vigliano C, Lococo B, Petti M, Viotti R. Clinical characteristics and outcome of 107 adult patients with chronic Chagas disease and parasitological cure criteria. Trans R Soc Trop Med Hyg. 2013;107:372‐376. [DOI] [PubMed] [Google Scholar]
- 63. Partel CD, Rossi CL. A rapid, quantitative enzyme‐linked immunosorbent assay (ELISA) for the immunodiagnosis of Chagas' disease. Immunol Invest. 1998;27:89‐96. [DOI] [PubMed] [Google Scholar]
- 64. Castillo‐Neyra R, Chou Chu L, Quispe‐Machaca V, et al. The potential of canine sentinels for reemerging Trypanosoma cruzi transmission. Prev Vet Med. 2015;120:349‐356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Pineda V, Saldaña A, Monfante I, et al. Prevalence of trypanosome infections in dogs from Chagas disease endemic regions in Panama, Central America. Vet Parasitol. 2011;178:360‐363. [DOI] [PubMed] [Google Scholar]
- 66. Montenegro VM, Jimenez M, Dias JC, Zeledon R. Chagas disease in dogs from endemic areas of Costa Rica. Mem Inst Oswaldo Cruz. 2002;97:491‐494. [DOI] [PubMed] [Google Scholar]
- 67. Jimenez‐Coello M, Poot‐Cob M, Ortega‐Pacheco A, et al. American Trypanosomiasis in dogs from an urban and rural area of Yucatan, Mexico. Vector Borne Zoonotic Dis. 2008;8:755‐762. [DOI] [PubMed] [Google Scholar]
- 68. World Health Organization (WHO) . Control of Chagas disease. World Health Organ Tech Rep Ser. 2002;905:1‐109. [PubMed] [Google Scholar]


