Abstract
Background
Hepatic copper accumulation causes chronic hepatitis in dogs. Mutations in the copper transporters ATP7A and ATP7B were, respectively, associated with attenuation and enhancement of hepatic copper concentrations in Labrador Retrievers. There is a predisposition of Dobermanns to hepatitis with increased hepatic copper concentrations.
Objectives
To investigate whether the ATP7A:c.980C>T and ATP7B:c.4358G>A mutations identified in Labrador Retrievers were associated with hepatic copper concentrations in Dobermanns.
Animals
Dobermanns from the Netherlands (n = 122) and the United States (n = 78).
Methods
In this retrospective study, mutations in ATP7A and ATP7B were investigated as risk factors for hepatic copper accumulation in Dobermanns. Liver biopsies of 200 Dobermanns were evaluated by histochemical copper staining, quantitative copper measurement, or both modalities. ATP7A and ATP7B genotypes were obtained by Kompetitive Allele Specific PCR. A linear regression model was used to investigate an association between genotype and hepatic copper concentrations.
Results
The ATP7A:c.980C>T was identified in both Dutch (2 heterozygous individuals) and American Dobermanns. In the American cohort, the minor allele frequency of the mutation was low (.081) and a possible effect on hepatic copper concentrations could not be established from this data set. A significant association of the ATP7B:c.4358G>A variant with increased hepatic copper concentrations in Dobermanns was observed.
Conclusions and Clinical Importance
The ATP7B:c.4358G>A variant could be a contributor to hepatic copper accumulation underlying the risk of development of copper‐associated hepatitis in breeds other than the Labrador Retriever.
Keywords: canine, copper‐transporting ATPase, hepatitis, liver, Wilson disease
Abbreviations
- Arg
arginine
- ATP7A
copper‐transporting ATPase α
- ATP7B
copper‐transporting ATPase β
- CuQ
quantitative copper
- DH
Dobermann hepatitis
- Gln
glutamine
- MUT
mutation
- RA
rubeanic acid
- WT
wild type
1. INTRODUCTION
A high prevalence of chronic hepatitis, accompanied by increased hepatic copper concentrations was recognized in Dobermanns decades ago.1, 2, 3 Affected dogs generally have abnormally high serum activities of liver enzymes alanine aminotransferase and alkaline phosphatase, and there is a clear female predisposition.1, 2, 4
Histologically, both centrolobular inflammatory infiltrates associated with copper deposits around the central vein, which is typical for primary copper toxicosis,5 and predominant lymphoplasmacellular infiltration and piecemeal necrosis in the portal areas, which is a hallmark for autoimmune hepatitis2 occur in Dobermann hepatitis (DH) cases. Involvement of the immune system in etiology of DH was suggested by the female predisposition, the presence of lymphocyte proliferation, aberrant expression of major histocompatibility complex class II antigens on hepatocytes,6 homozygosity for the risk allele DRB1*00601 of the dog leukocyte antigen system,7 as well as presence of antihistone autoantibodies8 and detection of autoantibodies against the liver related enzymes GAPDH and ADH.9 However, autoimmune hepatitis is not typically accompanied by hepatic copper accumulation in the centrolobular area, and there is no evidence that dogs accumulate copper secondary to chronic liver disease.
Copper homeostasis relies on regulation of uptake of copper in the small intestine, cellular metabolism executed by a variety of copper transporters and chaperones, as well as excretion of excessive copper via the biliary tract. The P‐type copper‐transporting ATPases, ATP7A and ATP7B, are crucial for the regulation of copper homeostasis in mammals.10 ATP7A mainly resides at the basal membrane of enterocytes and facilitates copper transport into the portal circulation.11, 12 ATP7B is located at the Golgi complex in hepatocytes and moves to the endo‐lysosomal cellular compartments upon copper overload. ATP7B has a dual function. In terms of its biosynthetic role, ATP7B facilitates incorporation of copper into apo‐ceruloplasmin within the trans‐Golgi network to form ceruloplasmin. Furthermore, ATP7B facilitates excretion of copper into the bile via the apical membrane of hepatocytes. In humans, mutations (MUTs) of ATP7A and ATP7B result in recessively inherited disorders Menkes disease and Wilson disease, respectively.13, 14, 15 Menkes disease is characterized by copper deficiency14 whereas copper overload is typical for Wilson disease.15, 16 It was previously reported that copper‐associated hepatitis in the Labrador Retriever is a complex disease, involving multiple genes and environmental factors.17, 18, 19 Mutations in 2 genes (ATP7A:c.980C>T and ATP7B:c.4358G>A) leading to amino acid substitutions ATP7A:p.Thr327Ile and ATP7B:p.Arg1453Gln have been identified in Labrador Retrievers.
The amino acid substitution ATP7A:p.Thr327Ile is associated with lower hepatic copper concentrations in Labrador Retrievers. Functional studies in canine dermal fibroblasts showed that cells with the mutant protein accumulated more copper than cells with the wild‐type (WT) protein, probably because of impaired copper efflux in cells harboring the MUT. For ATP7A, this would implicate that copper accumulates in enterocytes and is lost in feces, thereby preventing uptake in the portal circulation.17
In a mutagenized cell model, overexpression of the human ortholog ATP7B:p.Arg1453Gln resulted in mislocalization of the protein in the endoplasmic reticulum and inefficient trafficking of ATP7B to the apical membrane in polarized cells.17 To investigate if the variants ATP7A:c.980C>T and ATP7B:c.4358G>A are present in Dobermanns and whether they are associated with hepatic copper concentrations, we evaluated the distribution of these gene variants in 200 Dobermanns with known copper status from the Netherlands and the United States.
2. MATERIALS AND METHODS
2.1. Dogs
The Dobermanns included in this study were recruited from the Netherlands (n = 122) and the United States (n = 78). The Dutch cohort was previously used to investigate the prevalence of subclinical hepatitis in the Netherlands between 1995 and 1996. Dobermanns were randomly selected from 150 litters and 1 female and 1 male dog were drawn from each litter.3 Besides 113 randomly selected Dobermanns (male 45, female 68), 9 Dobermanns (male 2, female 7) with clinical signs of DH were included in the investigation as well. The American cohort primarily contained dogs that underwent a liver biopsy because they were suspected of liver disease. From all Dobermanns in this study, liver biopsies were available for copper quantitation. From the Dutch dogs, DNA was isolated from blood and from the American dogs DNA was isolated from formalin‐fixed paraffin‐embedded samples.20 All dogs were client‐owned and data were collected after obtaining informed consent of the owners.
2.2. Phenotypes
From the Dutch cohort, histological biopsies were stained with rubeanic acid (RA) and a grade of 0 to 5 was assigned by the pathologist to score the amount of copper in the liver.21 In 91 dogs, additional quantitative copper (CuQ) measurement by instrumental neutron activation analysis was available.22 The correlation between histological grade (RA) and CuQ measurement by instrumental neutron activation analysis was .73 (P‐value <2.2e‐16, 95% confidence interval [.60‐.82]) using Spearman's rank correlation method.
For the American dogs, no histochemical staining scores were available, but copper was quantified, either by atomic absorption spectrometry or by digital image analysis.23
Quantitative copper was reported in mg/kg copper per dry weight liver (mg/kg dwl) as described. A concentration above 400 mg/kg dwl in dogs was considered abnormally high.24
2.3. Genotyping
Genotyping for ATP7A:c.980C>T (XM_005641519.1), corresponding to ATP7A:Thr327Ile and ATP7B:c.4358G>A (XM_005633826.1), corresponding to ATP7B:Arg1453Gln, was carried out by Kompetitive Allele Specific PCR according to the manufacturer's instructions (LGC Genomics, United Kingdom) using 2 allele specific forward primers and 1 common reverse primer for each of the genes.
The primer set for the ATP7A variant was: 5′‐GACCAAGTTCATGC TAGTAAAGTACAATGCAAGCTTAGTCAC‐3′, 5′‐CGGAGTCAACGGATTATAGTAAAGTACAATGCAAGCTTAGTCAT‐3′, and 5′‐CCTGGTGATATGGCCTCTATTGCTT‐3′.
The primer set for the ATP7B variant was as follows: 5′‐GAAGGTGACCAAGTTCATGCTGTCCCAGGGCGTGGCCC‐3′, 5′‐GAAGGTCGGAGTCAACGGATTGGTCCCAGGGCGTGGCCT‐3′, and 5′‐ATTGGCATGGATGACCGGCGGT‐3′.
The fluorescence signals were detected by a CFX384 Touch Real‐Time PCR Detection System (Bio‐Rad, Hercules, California) and the results were analyzed by Bio‐Rad CFX manager 3.0 (Bio‐Rad).
2.4. Statistical analysis
The genotype data were analyzed using RStudio.25 A multivariate linear model was used to assess the association between either CuQ or RA staining score with the genotype of ATP7A and ATP7B. Genotypes were modeled additively, and the effect estimates were estimated for the number of copies of the nonreference allele. In the case of the X‐chromosomal ATP7A gene, hemizygous dogs were scored 0 (CY) or 2 (TY). Sex and age were added to the model as covariables, because copper could accumulate over time, depending on dietary copper intake. R‐squared values for each independent variable in univariate model were summarized in Table 4. A combined analysis for all Dobermanns of both countries was performed for CuQ, and the country of origin was added to the model as a covariable (Table 4).
Validity of the final model was checked by studying the residuals on normality and constancy of variance. Phenotype of CuQ was log transformed to meet the requirements for the residuals. The cutoff value for significance was set as an alpha of .05.
3. RESULTS
Clinical results from dogs are summarized in Table 1. From all Dutch Labrador Retrievers (n = 122; 47 M, 75 F), RA staining scores of liver biopsies were available. Additional CuQ concentrations were available in 91 Dobermanns (37 M, 54 F). From all American Dobermanns (32 M, 46 F), CuQ values were available from measurement by atomic absorption spectrometry or from digital imaging.23
Table 1.
Phenotype summary of Dobermann cohorts from the Netherlands and the United States
| Population | Sex | Agea (y) | Rubeanic acid staining scoreb | Quantitative copperc (mg/kg dwl) | ||
|---|---|---|---|---|---|---|
| The Netherlands | 47 M | 4 (1.1‐10.0) | n = 47 | 1.5 (0‐3.5) | n = 37 | 201 (94‐981) |
| 75 F | 3.6 (2.4‐10.4) | n = 75 | 2.0 (0‐4.0) | n = 54 | 240 (82‐1660) | |
| United States | 32 M | 6.0 (0.2‐11.0) | NA | n = 32 | 735 (195‐2449) | |
| 46 F | 6.0 (1.0‐12.0) | NA | n = 46 | 839 (206‐3488) | ||
Note: NA indicated rubeanic acid staining score not available for American cohort.
Abbreviations: F, female; M, male.
Median age in years (range).
Median rubeanic acid staining score (range), values were available for all dogs.
Median quantitative copper level as mg/kg dry weight (range), number of dogs for which quantitative copper levels were available indicated.
In the Dutch cohort, 27 dogs had normal hepatic copper concentrations (RA staining scores <2, CuQ concentrations <400 mg/kg dwl) and normal histology. In the American cohort, 12 dogs had CuQ concentrations <400 mg/kg. Both the ATP7A:c.980C>T and the ATP7B:c.4358G>A variants were identified in Dobermanns. The ATP7A:c.980C>T variant was identified in 2 heterozygous dogs. In the American cohort, the minor allele frequency of the ATP7A:c.980C>T variant was .081 (8 heterozygous and 1 homozygous female dogs with the ATP7A:c.980C>T variant were present, all other dogs were hemizygous or homozygous for the reference allele). Summary of copper score in relation to ATP7A and ATP7B genotypes in Dutch Dobermanns was shown in Table 2. Summary of CuQ in relation to ATP7A and ATP7B genotypes in American Dobermanns was shown in Table 3.
Table 2.
Copper score in relation to ATP7A and ATP7B genotypes in Dobermanns from the Netherlands
| ATP7A:c.980C>T genotypes | ||||||||
|---|---|---|---|---|---|---|---|---|
| Males (n = 47) | Females (n = 75) | |||||||
| ATP7B:c:4358G>A genotypes | CY (n = 47) | TY (n = 0) | CC (n = 73) | CT (n = 2) | TT (n = 0) | |||
| AA | n = 8 | 1.75 (0‐2.5) | 0 | n = 10 | 2.5 (1.5‐4.0) | 0 | 0 | |
| AG | n = 22 | 2.0 (0‐3.5) | 0 | n = 25 | 2.0 (0‐3.5) | n = 1 | (3)a | 0 |
| GG | n = 17 | 1.5 (0‐3.5) | 0 | n = 38 | 1.5 (0‐3.5) | n = 1 | (2.5)a | 0 |
Note: Copper scores based on rubeanic acid staining were shown in the form of median with their ranges in the brackets and number of dogs was indicated; superscript letter “a” indicated individual dog, corresponding copper score presented in brackets.
Table 3.
Quantitative copper in relation to ATP7A and ATP7B genotypes in Dobermanns from the United States
| ATP7A:c.980C>T genotypes | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Males (n = 32) | Females (n = 46) | ||||||||
| ATP7B:c:4358G>A genotypes | CY (n = 32) | TY (n = 0) | CC (n = 37) | CT (n = 8) | TT (n = 1) | ||||
| AA | n = 5 | 751 (396‐2250) | 0 | n = 2 | 1782 (1163‐2400) | 0 | 0 | ||
| AG | n = 9 | 803 (616‐2449) | 0 | n = 5 | 1099 (222‐3488) | n = 4 | 1467 (721‐3040) | 0 | |
| GG | n = 18 | 650 (195‐2060) | 0 | n = 30 | 705 (206‐2884) | n = 4 | 1230 (351‐2691) | n = 1 | 683a |
Notes: quantitative copper was shown in mg/kg dry weight liver in the form of median (range); n, number of dogs; superscript letter “a” indicated individual dog encountering and the corresponding quantitative copper value was shown.
The ATP7B: c.4358G>A variant had a minor allele frequency of .34 in the Dutch cohort and of .20 in the American cohort.
Two American dogs with normal hepatic copper concentrations harbored the ATP7B:c.4358G>A MUT. Both of them were WT for the ATP7A:c.980C>T MUT. One of them was a young male (1 year old) that was homozygous for ATP7B:c.4358A and had a hepatic copper concentration at the upper concentration of normal (396 mg/kg dwl). The other dog was a female of 6 years old that was heterozygous for ATP7B:c.4358G>A and had a hepatic copper concentration of 222 mg/kg dwl.
The generalized linear model analysis of Dutch Dobermanns showed a significant association between the ATP7B:c.4358 genotype and the copper staining score with a P‐value of .01 (Figure 1). The CuQ values of the Dutch cohort rose with the increase of the number of mutant alleles with a P‐value of .08 (Figure 2A). Female dogs accumulate more CuQ than male dogs with a P‐value of .02. Age was positively associated with CuQ with a P‐value of .001 (Table 4). In the American Dobermanns, there was a significant association between the ATP7B:c.4358 genotype and CuQ with a P‐value of .005 (Table 4). However, sex and age were not significantly associated with CuQ in the American cohort (Table 4).
Figure 1.
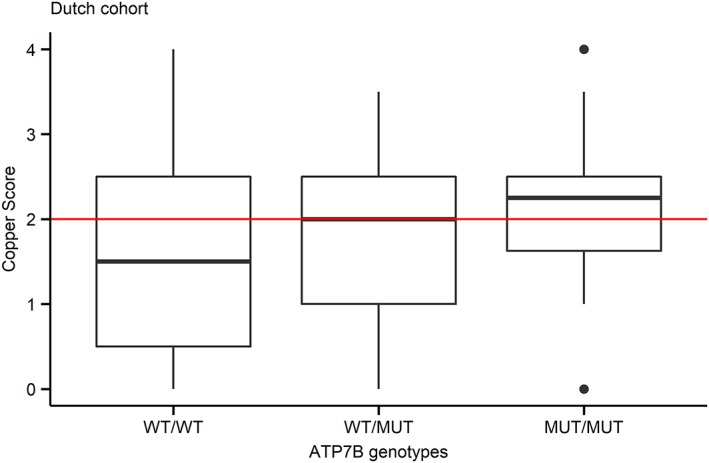
Boxplot of association between ATP7B:c.4358G>A genotype and rubeanic acid staining score of Dobermanns from the Netherlands (n = 122). The copper scores were plotted against the genotypes. The red horizontal line is the threshold of copper score which is 2. The genotypes are wild type (WT/WT), heterozygous (WT/MUT), and homozygous mutant (MUT/MUT)
Figure 2.
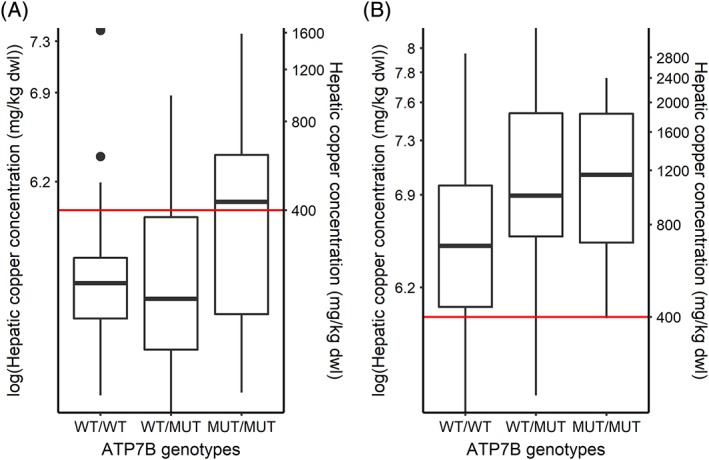
Boxplots of association between the ATP7B:c.4358G>A genotype and quantitative copper concentrations of liver biopsies of Dobermanns. The copper concentrations were plotted against the ATP7B:c.4358 genotype of dogs from the Netherlands (A, n = 91) and the United States (B, n = 78). Dual y‐axes represented log transformation of hepatic copper concentration on the left y‐axis and hepatic copper concentration on the right y‐axis. The genotypes are wild type (WT/WT), heterozygous (WT/MUT), and homozygous mutant (MUT/MUT). The red horizontal line corresponds to a normal hepatic copper level of 400 mg/kg dwl
Table 4.
Results of the generalized linear model analysis for association between covariables and log transformed quantitative copper levels or rubeanic acid staining scores in Dobermann livers
| Dutch cohort | American cohort | Combined cohort | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rubeanic acid staining score | Quantitative copper | Quantitative copper | Quantitative copper | |||||||||||||
| (n = 122 dogs) | (n = 91 dogs) | (n = 78 dogs) | (n = 169 dogs) | |||||||||||||
| Covariables | Effect estimate | SE | R 2 | P value | Effect estimate | SE | R 2 | P value | Effect estimate | SE | R 2 | P value | Effect estimate | SE | R 2 | P value |
| ATP7B:c:4358G>A | .35 | .13 | .05 | .01* | .16 | .1 | .04 | .08 | .36 | .12 | .08 | .005** | .26 | .075 | .0007 | .0004*** |
| ATP7A:c.980C>T | NA | NA | NA | NA | NA | NA | NA | NA | .24 | .22 | .04 | .27 | NA | NA | NA | NA |
| Sex (female = 0, male = 1) | −.21 | .19 | .002 | .27 | −.32 | .14 | .02 | .02* | −.11 | .17 | .001 | .51 | −.18 | .11 | .003 | .07 |
| Age (y) | .07 | .05 | .02 | .12 | .14 | .04 | .13 | .001** | .03 | .03 | .002 | .31 | .049 | .023 | .12 | .03* |
| Country (United States) | 1.14 | .11 | .4 | <2e‐16*** | ||||||||||||
Notes: NA indicated ATP7A:c.980C>T not being included in the calculation because of insufficient number of samples; R 2 values were calculated in a univariate model with corresponding independent variable; asterisks indicated the levels of significance.
4. DISCUSSION
We identified a significant association between the ATP7B: c.4358G>A variant and hepatic copper concentrations in Dobermanns from the Netherlands and the United States. Based on the generalized multivariate linear regression model, which contained independent covariants in the combined data set as shown in Table 4, ATP7B: c.4358G>A variant MUT and age showed a significant positive association with hepatic copper concentrations.
The variant ATP7A:c.980 C>T first identified in Labrador Retrievers in association with a decreased copper concentration17 was identified in Dobermanns as well. We were unable to identify a relationship between the variant ATP7A:c.980 C>T and hepatic copper concentrations in the current Dobermann cohort. We cannot exclude a causal role of this MUT on hepatic copper concentrations, but because of the low minor allele frequency and the size of the cohort, we were unable to draw a conclusion on the effect of the variant on hepatic copper concentrations.
The ATP7B: c.4358G>A variant was associated with higher copper for both quantitative measurement and semiquantitative scoring. In both the Dutch and the American Dobermann population, the ATP7B: c.4358G>A variant was associated with hepatic copper concentrations in Dobermanns as it was in Labrador Retrievers.17 Eleven polymorphisms in the ATP7B gene, including c.4358G>A, have been investigated for association with hepatic copper concentrations in Bedlington Terriers that were not homozygous for the exon 2 deletion in the COMMD1 gene.26 However, there was no significant association between specific ATP7B haplotypes and hepatic copper concentrations.26 Possibly, the effects were too small to be identified because of the limited number of dogs (N = 9) included in this study.
In a study from Spee et al,27 mRNA expression concentrations of both ATP7A and ATP7B were found to be downregulated in DH cases. A similar observation was made in COMMD1‐deficient dogs that were followed over time28 in which expression of both genes was decreased compared to a control group in the end stage of the disease. In the Dutch Dobermann dogs that were used in the study of Spee et al, no dogs with the ATP7A:c.980 C>T variant were present and therefore it can be excluded that this MUT was responsible for the observed decrease in ATP7A mRNA expression. Whether the ATP7B:c.4358G>A variant influences mRNA expression of ATP7B and in this way contributes to hepatocytic copper accumulation in addition to retainment in the endoplasmic reticulum and aberrant trafficking to the apical membrane could not be concluded from the small number of dogs used in the study from Spee et al and requires further investigation.
Our study showed that female Dobermanns had higher copper concentrations than male dogs in both the Dutch and American cohort. A significant difference between hepatic copper concentrations from male and female dogs was observed in the Dutch cohort when analyzing the CuQ scores.
Overall, hepatic copper concentrations in the American cohort were significantly higher than in the Dutch cohort (P‐value <2e‐16), which most likely resulted from the way the dogs were recruited. The Dutch cohort consisted mainly of subclinical Dobermanns recruited through a random population survey that contained both affected and unaffected dogs, in contrast to the American cohort that mainly consisted of dogs with clinical signs and more progressed stages of disease. Other factors, like environment or genetic diversity between Dutch and American Dobermann populations cannot be excluded as cause for the observed difference in hepatic copper concentrations between the cohorts.
Therefore, of the drawbacks of this study is that we could not include data on copper intake of our study population, as this could have influenced the outcome.
Recent studies have shown evidence that the development of chronic hepatitis in Dobermanns has an autoimmune component.6, 7, 8, 9 High hepatic copper concentrations cause or contribute to progression of chronic hepatitis. In Dobermanns with chronic hepatitis, there are increased hepatic copper concentrations in the majority of cases.1, 3 Here, we show that the ATP7B:c.4358G>A MUT is present in the Dobermann and is associated with increased hepatic copper concentrations. Therefore, it will be of great importance for treatment strategies and prognosis to address the different etiologies in the diagnostic process in Dobermanns with chronic hepatitis. This would include careful histopathological examination for characterization of the type and localization of the inflammatory infiltration (copper containing macrophages, lymphocytes, and plasma cells and their portal or centrolobular localization), histochemical localization and quantification of copper, genetic evaluation (genotyping for dog leukocyte antigen risk alleles and ATP7B:c.4358G>A), and testing for the presence of autoantibodies.
To conclude, we identified an association between the ATP7B:c.4358G>A variant and hepatic copper concentrations in Dobermanns from the Netherlands and the United States. This variant could be a contributor to hepatic copper concentrations underlying the risk of development of copper‐associated hepatitis in breeds other than the Labrador Retriever. This finding can help to develop breeding strategies to decrease the incidence of copper‐associated hepatitis in Dobermanns.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
The procedures were approved by the Utrecht University Ethical Committee as required under Dutch legislation.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
ACKNOWLEDGMENTS
This study is supported by a grant to X.W. by the Chinese Scholarship Council (File No.201407090064). The authors thank Sharon Center for making the American Dobermann samples available for this study. Waltham is acknowledged for sponsoring part of this study. The authors thank biostatistician Hans Vernooij for statistical advice.
Wu X, Mandigers PJJ, Watson AL, van den Ingh Ted S. G. A. M., Leegwater PAJ, Fieten H. Association of the canine ATP7A and ATP7B with hepatic copper accumulation in Dobermann dogs. J Vet Intern Med. 2019;33:1646–1652. 10.1111/jvim.15536
Funding information Chinese Scholarship Council grant for Xiaoyan Wu., Grant/Award Number: File No.201407090064; WALTHAM Foundation
REFERENCES
- 1. Speeti M, Ihantola M, Westermarck E. Subclinical versus clinical hepatitis in the Dobermann: evaluation of changes in blood parameters. J Small Anim Pract. 1996;37:465‐470. [DOI] [PubMed] [Google Scholar]
- 2. Speeti M, Eriksson J, Saari S, Westermarck E. Lesions of subclinical Doberman hepatitis. Vet Pathol. 1998;35:361‐369. [DOI] [PubMed] [Google Scholar]
- 3. Mandigers PJ, van den Ingh TS, Bode P, Teske E, Rothuizen J. Association between liver copper concentration and subclinical hepatitis in Doberman pinschers. J Vet Intern Med. 2004;18:647‐650. [DOI] [PubMed] [Google Scholar]
- 4. Crawford MA, Schall WD, Jensen RK, Tasker JB. Chronic active hepatitis in 26 Doberman pinschers. J Am Vet Med Assoc. 1985;187:1343‐1350. [PubMed] [Google Scholar]
- 5. Thornburg LP. Histomorphological and immunohistochemical studies of chronic active hepatitis in Doberman pinschers. Vet Pathol. 1998;35:380‐385. [DOI] [PubMed] [Google Scholar]
- 6. Speeti M, Stahls A, Meri S, Westermarck E. Upregulation of major histocompatibility complex class II antigens in hepatocytes in Doberman hepatitis. Vet Immunol Immunopathol. 2003;96:1‐12. [DOI] [PubMed] [Google Scholar]
- 7. Dyggve H, Kennedy LJ, Meri S, Spillmann T, Lohi H, Speeti M. Association of Doberman hepatitis to canine major histocompatibility complex II. Tissue Antigens. 2011;77:30‐35. [DOI] [PubMed] [Google Scholar]
- 8. Dyggve H, Meri S, Spillmann T, Jarva H, Speeti M. Antihistone autoantibodies in Dobermans with hepatitis. J Vet Intern Med. 2017;31:1717‐1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dyggve H, Jarva H, Spillmann T, Speeti M, Meri S. Identification of glyceraldehyde‐3‐phosphate and alcohol dehydrogenases as autoantigens in Doberman hepatitis. Scand J Immunol. 2017;86:156‐164. [DOI] [PubMed] [Google Scholar]
- 10. La Fontaine S, Mercer JF. Trafficking of the copper‐ATPases, ATP7A and ATP7B: role in copper homeostasis. Arch Biochem Biophys. 2007;463:149‐167. [DOI] [PubMed] [Google Scholar]
- 11. Wang Y, Hodgkinson V, Zhu S, Weisman GA, Petris MJ. Advances in the understanding of mammalian copper transporters. Adv Nutr. 2011;2:129‐137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fieten H, Leegwater PA, Watson AL, Rothuizen J. Canine models of copper toxicosis for understanding mammalian copper metabolism. Mamm Genome. 2012;23:62‐75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kaler SG. ATP7A‐related copper transport disorders In: Pagon RA, Adam MP, Ardinger HH, et al., eds. GeneReviews(R). Seattle, WA: University of Washington; 1993. [PubMed] [Google Scholar]
- 14. Kaler SG. ATP7A‐related copper transport diseases‐emerging concepts and future trends. Nat Rev Neurol. 2011;7:15‐29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tanzi RE, Petrukhin K, Chernov I, et al. The Wilson disease gene is a copper transporting ATPase with homology to the Menkes disease gene. Nat Genet. 1993;5:344‐350. [DOI] [PubMed] [Google Scholar]
- 16. Bull PC, Thomas GR, Rommens JM, Forbes JR, Cox DW. The Wilson disease gene is a putative copper transporting P‐type ATPase similar to the Menkes gene. Nat Genet. 1993;5:327‐337. [DOI] [PubMed] [Google Scholar]
- 17. Fieten H, Gill Y, Martin AJ, et al. The Menkes and Wilson disease genes counteract in copper toxicosis in Labrador Retrievers: a new canine model for copper‐metabolism disorders. Dis Model Mech. 2016;9:25‐38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hoffmann G, van den Ingh TS, Bode P, Rothuizen J. Copper‐associated chronic hepatitis in Labrador Retrievers. J Vet Intern Med. 2006;20:856‐861. [DOI] [PubMed] [Google Scholar]
- 19. Fieten H, Biourge VC, Watson AL, Leegwater PAJ, van den Ingh TSGAM, Rothuizen J. Dietary management of Labrador Retrievers with subclinical hepatic copper accumulation. J Vet Intern Med. 2015;29:822‐827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pikor LA, Enfield KS, Cameron H, Lam WL. DNA extraction from paraffin embedded material for genetic and epigenetic analyses. J Vis Exp 2011;(49). pii: 2763 10.3791/2763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fieten H, Rothuizen J. Web chapter 48: copper‐associated hepatitis In: Bonagura JD, Twedt DC, eds. Kirk's Current Veterinary Therapy. Missouri: Elsevier; 2014:e231. [Google Scholar]
- 22. Bode P. Instrumental neutron activation analysis in a routine way. J Trace Microprobe Tech. 1990;8(1):139‐154. [Google Scholar]
- 23. Center SA, McDonough SP, Bogdanovic L. Digital image analysis of rhodanine‐stained liver biopsy specimens for calculation of hepatic copper concentrations in dogs. Am J Vet Res. 2013;74:1474‐1480. [DOI] [PubMed] [Google Scholar]
- 24. Puls R. Mineral Levels in Animal Health: Diagnostic Data. 2nd ed. Clearbrook, Canada: Sherpa International; 1994. [Google Scholar]
- 25. RStudio Team . RStudio: Integrated Development for R. Boston, MA: RStudio, Inc; 2015. [Google Scholar]
- 26. Coronado VA, O'Neill B, Nanji M, Cox DW. Polymorphisms in canine ATP7B: candidate modifier of copper toxicosis in the Bedlington Terrier. Vet J. 2008;177:293‐296. [DOI] [PubMed] [Google Scholar]
- 27. Spee B, Mandigers PJ, Arends B, et al. Differential expression of copper‐associated and oxidative stress related proteins in a new variant of copper toxicosis in Doberman Pinschers. Comp Hepatol. 2005;4:3‐5926‐4‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Favier RP, Spee B, Fieten H, et al. Aberrant expression of copper associated genes after copper accumulation in COMMD1‐deficient dogs. J Trace Elem Med Biol. 2015;29:347‐353. [DOI] [PubMed] [Google Scholar]


